Abstract
In the silkmoth Bombyx mori, the role of male sensilla trichodea in pheromone detection is well established. Here we study the corresponding female sensilla, which contain two olfactory sensory neurons (OSNs) and come in two lengths, each representing a single physiological type. Only OSNs in medium trichoids respond to the scent of mulberry, the silkworm's exclusive host plant, and are more sensitive in mated females, suggesting a role in oviposition. In long trichoids, one OSN is tuned to (+)-linalool and the other to benzaldehyde and isovaleric acid, both odours emitted by silkworm faeces. While the significance of (+)-linalool detection remains unclear, isovaleric acid repels mated females and may therefore play a role in avoiding crowded oviposition sites. When we examined the underlying molecular components of neurons in female trichoids, we found non-canonical co-expression of Ir8a, the co-receptor for acid responses, and ORco, the co-receptor of odorant receptors, in long trichoids, and the unexpected expression of a specific odorant receptor in both trichoid sensillum types. In addition to elucidating the function of female trichoids, our results suggest that some accepted organizational principles of the insect olfactory system may not apply to the predominant sensilla on the antenna of female B. mori.
Keywords: olfactory, sensilla, Bombyx mori, pheromone, oviposition, receptor
1. Introduction
The antenna of male moths is dedicated to the detection of female sex pheromones, because most of the hair-like olfactory sensilla that cover it are of one type—long sensilla trichodea—and house pheromone-specific olfactory sensory neurons (OSNs). In many moth species, females lack this sensillum type, resulting in a striking sexual dimorphism of the antenna [1,2]. In the domesticated silkmoth Bombyx mori, however, the antennae of both sexes are morphologically similar (electronic supplementary material, figure S1) and possess identical sets of olfactory sensilla, including two types of sensilla trichodea (long and medium) [3]. Both trichoid types together make up the largest proportion of antennal olfactory sensilla in both male (77%) and female (66%) silkmoths [3,4]. Female B. mori are anosmic to their own pheromone [2]. We therefore asked whether female trichoids might be dedicated to the detection of mulberry leaves, the silkworm's exclusive food, since this plant bouquet is particularly important for females when searching for an oviposition site. Female trichoids may also be involved in other behavioural contexts, such as pheromonal communication.
Each long trichoid and most of the medium trichoids of both male and female B. mori house two OSNs [5]. While OSNs in male long trichoids are narrowly tuned to the female pheromone bombykol and the behavioural antagonist bombykal, respectively [6], the two OSNs in female long trichoids have been described to be best activated by the terpenes (±)-linalool and α-terpineol (‘terpene cell’, A-neuron with larger spike amplitude), or by benzoic acid and benzaldehyde (‘benzoic acid cell’, B-neuron with smaller spike amplitude) [2,7,8]. However, these previous physiological studies lacked screening of female long trichoids with a wider range of odorants; and the receptive range of OSNs in medium trichoids was not investigated. We therefore recorded the response of OSNs in both long and medium trichoids to 76 monomolecular odorants from different chemical classes. We also tested complex mixtures emitted from natural sources of potential importance to female silkmoths, such as the headspace of mulberry leaves, which contains (±)-linalool among other compounds [9]. In addition to being released from plants, terpenes, i.e. possible ligands for the ‘terpene cell’, are widespread body volatiles produced by various insects, e.g. butterflies [10], and may play a role in precopulatory behaviour [11]. We therefore included headspace from female and male silkmoths as stimuli for female sensilla trichodea. We also re-examined the response of the ‘benzoic acid cell’ to stimulation with meconium [12], the liquid waste excreted by moths during eclosion, and tested the headspace of silkworm faeces, which may play a role in the choice of oviposition sites, as has been found in the hawkmoth Manduca sexta [13]. Although domesticated silkmoths cannot fly, B. mori males immediately begin to fan their wings upon detecting bombykol and run towards the pheromone-releasing female [14,15], making them a useful model organism for studying odour-guided behaviour. By contrast, B. mori females are immobile for most of their lives. Therefore, the behavioural relevance of female trichoid ligands is unknown.
The molecular basis of odour-evoked responses is in olfactory receptor genes expressed by OSNs. There are two major families of olfactory receptors in insects, odorant receptors (ORs) and ionotropic receptors (IRs) [16–18], which differ in their molecular receptive range. Members of the larger family of ORs are co-expressed with their obligate OR-co-receptor ORco [19], and detect a wide range of chemically diverse molecules, including insect-emitted volatiles that act as pheromones and plant-emitted volatiles such as terpenes. IRs, on the other hand, have a more restricted spectrum, but also require the co-expression of co-receptors that specify the function of a given IR: IRs co-expressed with Ir8a are tuned to acids, while IRs co-expressed with Ir25a and Ir76b detect amines and aldehydes [20–22]. Typically, each OSN expresses only one olfactory receptor, with neurons in sensilla trichodea and basiconica expressing ORs and neurons in sensilla coeloconica expressing IRs, i.e. ORs and IRs are mostly expressed in separate chemosensory subsystems [16,23,24]. So far, 71 ORs and 30 IRs have been identified in B. mori [9,25,26]. Three ORs (BmorOr19, 45 and 47) are mainly present in the female antenna [27], and BmorOr19-expressing cells co-localize with BmorOr45-expressing cells in the same sensillum [28]. Furthermore, BmorOr19 is tuned to detect (±)-linalool, whereas both BmorOr45 and 47 are best activated by benzoic acid and benzaldehyde out of 26 odours tested [28], elucidating BmorOr19 as the receptor expressed in the ‘terpene cell’ and BmorOr45 and/or 47 as the receptor(s) expressed in the ‘benzoic acid cell’. However, the expression of these ORs has not been assigned to a specific sensillum type and other candidate receptors have not been tested, leaving our knowledge of the molecular basis of olfactory responses by OSNs in trichoid sensilla incomplete.
2. Results
(a) . Receptive range of long sensilla trichodea
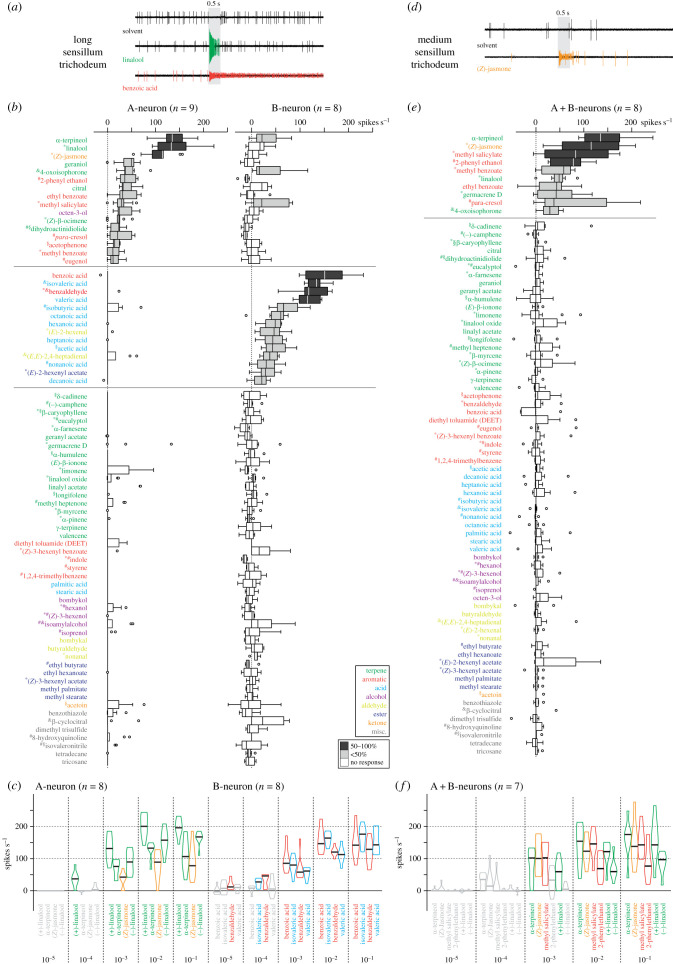
Using single sensillum recordings (SSR), we found two physiological types of sensilla trichodea that occurred in similar numbers on the female antenna, corresponding to long and medium trichoids [3,5]. The two OSNs in one of the types differed in spike amplitude and spontaneous activation frequency, and appeared to be identical to previously described OSNs in long trichoids with comparable characteristics [12]. These neurons not only had different spike amplitudes and spontaneous activity (figure 1a), but also had almost exclusive response spectra (figure 1b). Of the 76 monomolecular stimuli tested, A-neurons responded strongly (≥50% of the maximum response) to only three odours: (±)-linalool and α-terpineol, as earlier reported [2], and (Z)-jasmone, a previously unknown ligand for A-neurons. Thirteen other odours elicited a significant but smaller response (less than 50% of the maximum response). Although (±)-linalool and (Z)-jasmone are among the volatiles emitted by mulberry leaves, A-neurons did not respond to mulberry headspace (table 1), probably owing to the low concentration of these odours (electronic supplementary material, figure S2a). Similarly, neither silkmoth headspace (containing mainly terpenes; electronic supplementary material, figure S2b) nor their meconium (containing molecules of different chemical classes such as terpenes, aromatics, alcohols and acids; electronic supplementary material, figure S2c) elicited a response (table 1). Only headspace from silkworm faeces (electronic supplementary material, figure S2d) containing the minor ligands 4-oxoisophorone and dihydroactinidiolide activated A-neurons.
Figure 1.
Response profile of female sensilla trichodea. (a) Example recordings from long sensillum trichodeum stimulated with the solvent hexane, (±)-linalool (green, large spikes, A-neuron) and benzoic acid (red, small spikes, B-neuron); diluted 10−2 in hexane; grey background, odour exposure. (b) Responses of A-neurons (left) and B-neurons (right) in long trichoids. Boxplots, net, solvent-subtracted maximum spike frequencies; filled boxplots, data differ from zero (p < 0.05, Wilcoxon rank sum test); shades of grey, strength of response (dark grey, ≥half maximal response; light grey, <half maximal response). Stimuli sorted according to median response first in A-neurons, then in B-neurons; stimuli eliciting no response in either neuron sorted alphabetically according to chemical class. Symbols next to odour names, presence in headspace from mulberry leaves (*), silkmoths (§), their meconium (#), or silkworm faeces (&); for chemical composition of headspaces see electronic supplementary material, figure S1. (c) Dose–response experiments with best ligands of long trichoids; violin plots, net maximum spike frequencies (no. spikes s−1) to five (v/v) odour concentrations corresponding to 60 ng, 600 ng, 6 µg, 60 µg, 600 µg of pure substance on filter paper; horizontal line, median; grey violin plots, data not different from zero (p > 0.05, Wilcoxon rank sum test). (d) Example recordings from medium sensillum trichodeum upon stimulation with hexane and (Z)-jasmone (orange, A + B-neurons). (e) Pooled responses of A + B-neurons in medium trichoids. (f) Dose–response experiments with best ligands of medium trichoids and both enantiomers of linalool.
Table 1.
Activation of OSNs in female sensilla trichodea by natural mixtures. ‘X’ depicts difference of solvent-subtracted maximum spike frequency from zero, p < 0.05, Wilcoxon rank sum test.
| odour source | long trichoids (A) | long trichoids (B) | medium trichoids (A + B) |
|---|---|---|---|
| mulberry leaves | − | − | x |
| silkmoth body odour (♀/♂) | −/− | −/− | −/− |
| meconium (♀/♂) | −/− | x/x | −/− |
| silkworm faeces | x | x | − |
B-neurons showed a similar narrow tuning (figure 1b), responding strongly to only four odours: benzoic acid and benzaldehyde, confirming previous results [7,8], and the newly identified ligands isovaleric acid and valeric acid. Thirteen other odours, mostly carboxylic acids, were minor ligands. Consistent with a previous report [12], silkmoth meconium activated B-neurons (table 1), although none of their best ligands was present in the mixture. However, some acids were released from the meconium samples (electronic supplementary material, figure S2c) and may have led to the observed activation of B-neurons in long trichoids. In addition, B-neurons responded to the headspace of silkworm faeces (figure 1d and table 1), which contained two of the best ligands (benzaldehyde, isovaleric acid) and two other minor ligands.
The pheromones bombykol and bombykal did not activate either OSN in female long trichoids, as had been found in previous studies [2].
The high specificity of OSNs housed in female long trichoids was illustrated by high values for lifetime sparseness (S = 0.89 (A-neuron), S = 0.82 (B-neuron); electronic supplementary material, figure S3a), a measure of selectivity that can take values between 0 (response to any odour) and 1 (response to only one odour) [29]. Next, we wanted to know whether these neurons would be even more selective when tested at lower odour concentrations, so we performed dose–response experiments with the best ligands. The two enantiomers of linalool were tested separately to detect a possible enantioselective response. We found that at a threshold dilution of 10−4, A-neuron firing increased only in the presence of (+)-linalool, whereas (−)-linalool, α-terpineol and (Z)-jasmone (which have similar vapour pressures to (+)-linalool) were not active at this low odour concentration (figure 1c). However, at higher doses, the neuron also responded to (−)-linalool, although to a lesser extent. This response was due to a 2% impurity of the opposite enantiomer (electronic supplementary material, figure S4), which inevitably occurs during synthesis. For example, at a dilution of 10−2, the amount of (+)-linalool (1 µg) present in the (−)-linalool stimulus is equivalent to a stimulation with (+)-linalool at a dilution between 10−3 (5 µg) and 10−4 (0.5 µg). The observed response of A-neurons to higher doses of (−)-linalool could therefore be explained by the low contamination with (+)-linalool. Taken together, our results show a clear enantioselective tuning of A-neurons in female long trichoids to (+)-linalool.
At a 10−5 dilution, only benzaldehyde activated B-neurons, whereas the threshold dilution for isovaleric acid was 10−4. The other two best ligands, benzoic acid and valeric acid, did not elicit a response below a dilution of 10−3 (figure 1c). However, benzoic acid has a vapour pressure a thousand times lower than the other three odours, which are comparable in volatility. This means that the probability of benzoic acid molecules reaching the antenna is much lower than for the other stimuli. Our results thus confirmed benzoic acid as one of the best ligands for B-neurons [7], and showed that isovaleric acid is similarly active to benzaldehyde.
(b) . Receptive range of medium sensilla trichodea
The two OSNs in the second physiological type of sensilla trichodea regularly had very similar spike amplitudes (figure 1d). We referred to this type as medium trichoids, which have been described to often house OSNs of similar diameter and consequently similar spike amplitudes [5,30]. As spike sorting was therefore difficult, we analysed the pooled response of both neurons (figure 1e). Interestingly, the four best ligands α-terpineol, (Z)-jasmone, methyl salicylate and 2-phenyl ethanol were also ligands of A-neurons in long trichoids. A further five odours, including (±)-linalool, were shared between OSNs in medium trichoids and A-neurons in long trichoids. The remaining seven A-neuron ligands did not elicit a response from medium trichoids. The sesquiterpene germacrene D and the headspace of mulberry leaves (table 1) were minor but specific ligands for medium trichoids. As with long trichoids, bombykol and bombykal did not elicit a response. The tuning width of OSNs in medium trichoids (S = 0.87) was in the same range as that of OSNs in long trichoids (electronic supplementary material, figure S3b). Dose–response experiments using the four best ligands and both enantiomers of linalool showed a tenfold higher threshold dose for medium trichoids (10−3; figure 1f) than for long trichoids (10−4; figure 1c). However, OSNs in medium trichoids were again enantioselective as they were more sensitive to stimulation with (+)-linalool. This enantiomer elicited a response at a dilution of 10−3, whereas for (−)-linalool the threshold dose was at a dilution of 10−2 (figure 1f). Although not completely overlapping, OSNs in medium trichoids and A-neurons in long trichoids had a similar response profile, particularly with respect to their enantioselective response to (+)-linalool.
(c) . Response spectra of wild and domesticated silkmoths are similar
Bombyx mori silkmoths have been bred under human care for more than 5000 years, resulting in a reduced number of olfactory and gustatory sensilla compared with their extant wild ancestors B. mandarina [31,32]. As the receptive range and/or sensitivity of OSNs in trichoids of the domesticated female may also have changed, we tested active ligands from domesticated females in wild females. We found that the overall response profiles of OSNs were very similar between the two species (electronic supplementary material, figure S5a). This was particularly true for A- and B-neurons in long trichoids, whereas OSNs in ‘wild’ medium trichoids showed a broader tuning than the corresponding ‘domesticated’ neurons. Furthermore, the same characteristic enantioselective response to (+)-linalool was present in both species for A-neurons in long trichoids and for OSNs in medium trichoids, with the only difference that ‘wild’ neurons were more sensitive to this odour (electronic supplementary material, figure S5b). In addition to the reduced number of olfactory sensilla, this attenuation of OSN sensitivity may contribute to the reduced antennal response to stimulation with (±)-linalool in female B. mori [32].
(d) . Behavioural relevance of odours detected by sensilla trichodea
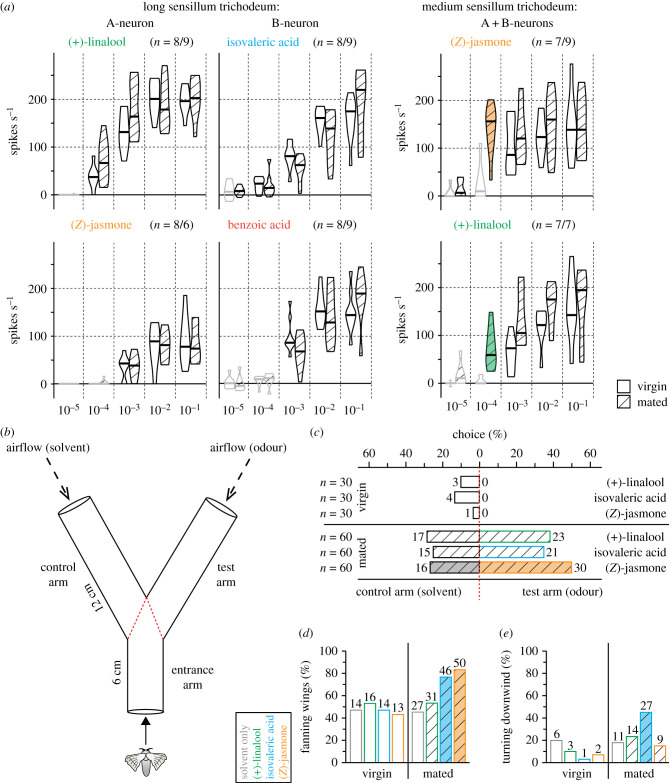
Neuronal sensitivity could be increased after mating if OSNs were critical for host plant location. We found that only OSNs in medium trichoids were more sensitive in mated females than in virgin females (figure 2a), demonstrating that medium trichoids may be involved in the selection of oviposition sites. We next established an assay to test the odour-evoked behaviour of individual females to the major ligands of sensilla trichodea. Since pulses of pheromone filaments are more efficient at attracting male moths than a continuous stream of pheromone [33], we constructed a Y-maze with pulsed olfactory stimulation (figure 2b), and tested this setup with male silkmoths and their response to bombykol (1 : 104). Out of 30 males tested, 29 made a choice, with 27 males choosing the pheromone arm and 2 males choosing the control arm (p < 0.0001, χ2 goodness of fit test). Having confirmed that our assay was suitable for studying odour-guided behaviour in silkmoths, we tested virgin and mated females with (+)-linalool, isovaleric acid or (Z)-jasmone versus solvent (figure 2c). Regardless of the odour tested, virgin females rarely entered the test or control arm (3–13% for each odour), and none of those that made a choice chose the test arm. Interestingly, mating had a strong effect on silkmoths' motivation to move, with around two-thirds of mated females crossing the decision line (p < 0.0001 for each of the three odours, Fisher's exact test). When tested with (+)-linalool or isovaleric acid, females randomly chose one of the arms of the Y-maze. However, in experiments with (Z)-jasmone, females preferred the test arm, demonstrating that (Z)-jasmone is an attractive signal for mated female silkmoths.
Figure 2.
Mating-dependent sensitivity and odour-guided behaviour of female silkmoths. (a) Comparison between single sensillum recording responses of virgin and mated females. Violin plots, net maximum spike frequencies (spikes s−1) to five (v/v) odour concentrations corresponding to 60 ng, 600 ng, 6 µg, 60 µg, 600 µg pure substance on filter paper; horizontal line, median; grey violin plots, data not different from zero (p > 0.05, Wilcoxon rank sum test); filled violin plots, data from virgin and mated females differ (p ≤ 0.003, Mann–Whitney U-test). (b) Experimental setup (Y-maze) with pulsed airstreams. Test arm, 10 µl odour (1 : 104); control arm, 10 µl solvent; red dotted line, virtual decision line. Experiments ended when the female crossed the decision line with its thorax or after 10 min. (c) Outcome of Y-maze assay. Bars, proportion of females that chose control or test arm; filled bar, difference from equal distribution (p = 0.039, χ2 goodness of fit test). (d) Proportion of females fanning their wings in entrance arm. Filled bars, difference from tests with solvent only (p ≤ 0.0007, Fisher's exact test with Bonferroni–Holm correction). (e) Proportion of females turning downwind in entrance arm. Filled bar, difference from tests with solvent only (p = 0.003, Fisher's exact test with Bonferroni–Holm correction). Absolute numbers next to bars in (c–e).
While in the entrance arm, females sometimes fanned their wings intensely or turned 180° downwind. These behaviours could be related to attraction (wing fanning) or aversion (downwind turning). To test whether the frequency of these behaviours was dependent on the presence of odour, we performed additional tests with virgin (n = 30) and mated (n = 60) females, where both arms of the Y-maze were control (solvent) arms. About half of the virgin females fanned their wings, regardless of whether an odour was present or not. However, after mating, the attractive (Z)-jasmone and the supposedly neutral isovaleric acid increased the proportion of wing-fanning females to about 80% (figure 2d). We then counted the number of females that turned 180° downwind. This behaviour was rarely observed in virgin females and was independent of the presence or absence of odour. Remarkably, a higher proportion of mated females turned downwind in tests with isovaleric acid—but not with (+)-linalool or (Z)-jasmone—than in tests with solvent alone (figure 2e). These results suggest that odour-induced wing fanning in mated females may be a sign of both attraction (to (Z)-jasmone) and aversion (away from isovaleric acid).
(e) . Non-canonical expression of olfactory receptors
To elucidate the molecular basis of the observed response spectra, we examined the expression of five ORs with a known female bias in the antenna [27], the (Z)-jasmone receptor BmorOr56 [9] and ORco. As the acid response of B-neurons in long trichoids indicates the expression of acid-sensing IRs, we also investigated the presence of the corresponding IR-co-receptor Ir8a [20]. We qualitatively assessed the presence of each receptor and, where possible, assigned the receptor to a sensillum type (table 2).
Table 2.
Expression of candidate ORs, ORco and Ir8a in the female antenna.
| receptor | frequency | long trichoids | medium trichoids | sensilla basiconica | sensilla coeloconica |
|---|---|---|---|---|---|
| ORco | ++++++++++ | x | x | x | − |
| BmorOr19 | +++++ | x | x | − | − |
| BmorOr45 | +++++ | x | − | − | − |
| BmorOr47 | +++++ | x | − | − | − |
| BmorIr8a | ++++ | x | − | − | x |
| BmorOr12 | ++ | − | − | x | − |
| BmorOr56 | ++ | ? | ? | ? | − |
| BmorOr30 | + | x | x | − | − |
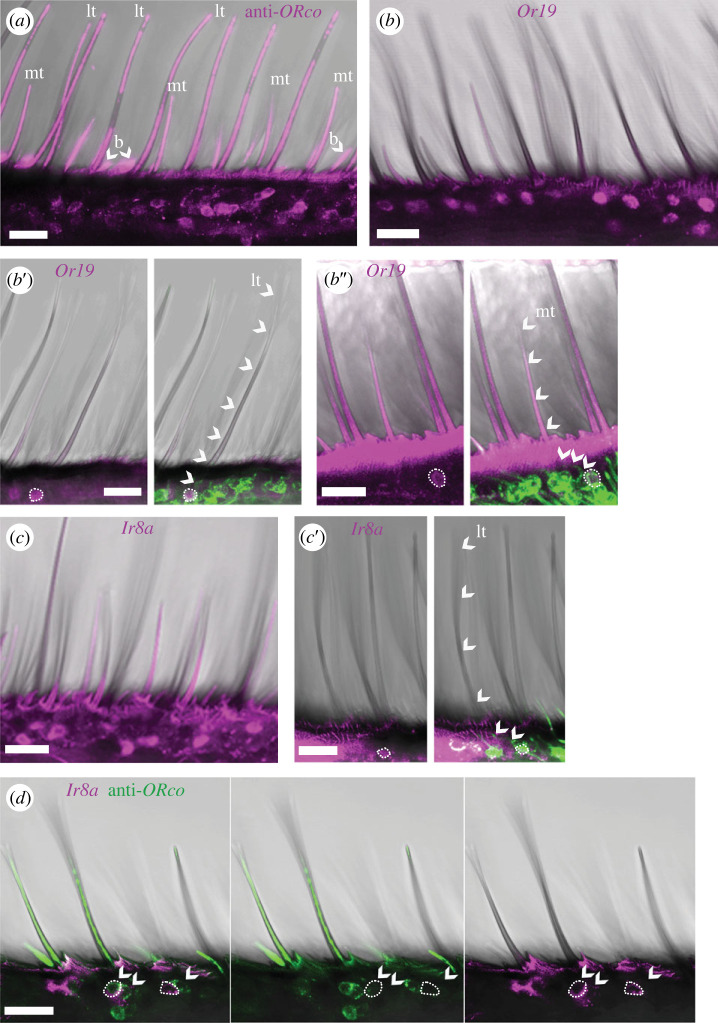
As expected, ORco had by far the highest expression in the female antenna and was present in sensilla trichodea and basiconica (figure 3a) but not in sensilla coeloconica (electronic supplementary material, figure S6a). BmorOr19 was also highly abundant (figure 3b) and expressed by neurons in long trichoids (figure 3b’). Interestingly, BmorOr19 was also expressed by neurons associated with medium trichoids (figure 3b’’). BmorOr45 and 47 had similarly high expression (electronic supplementary material, figure S6b,c) with dendrites in long trichoids (electronic supplementary material, figure S6b’,c’). The next most frequently labelled receptor was BmorIr8a (figure 3c), which was associated with neurons located in long trichoids (figure 3c’) and in sensilla coeloconica (electronic supplementary material, figure S6d). In long trichoids, BmorIr8a mainly occurred in cells co-labelled with an antibody against ORco, but occasionally we observed a BmorIr8a-positive cell without co-expression of ORco. Adjacent to a BmorIr8a-positive cell (with or without ORco co-expression), we typically found an ORco-positive cell (figure 3d).
Figure 3.
Expression of olfactory receptors in the female antenna. Fluorescent RNA in situ hybridization (FISH) and fluorescent immunohistochemistry (FIHC) alone or in combination. Fluorescence channels overlaid with the transmitted light channel. Scale bars, 20 µm. (a) FIHC with anti-ORco antibody [34] (magenta) labelling somata and dendrites of OSNs that innervate medium trichoid (mt), long trichoid (lt) and basiconic (b) sensilla. (b) FISH with Or19-specific RNA probe (magenta) combined in (b’,b’’) with FIHC using anti-HRP antibody (green, neuronal marker), showing innervation (arrowheads) of long trichoids (b’) and medium trichoids (b’’) by Or19-expressing neurons (encircled somata). (c) FISH with Ir8a-specific RNA probe (magenta) combined in (c’) with FIHC using anti-HRP antibody (green), showing innervation (arrowheads) of a long trichoid by an Ir8a-expressing neuron (encircled soma). (d) FISH with Ir8a-specific RNA probe (magenta) combined with FIHC using anti-ORco antibody (green). Encircled, Ir8a-positive somata; arrowheads, ORco-positive somata.
BmorOr12 expression in the antenna was sparse and BmorOr12-positive cells had dendrites in sensilla basiconica (electronic supplementary material, figure S6e). Similarly, BmorOr56 had a sparse expression pattern (electronic supplementary material, figure S6f). However, we could not assign BmorOr56 to any sensillum type. BmorOr30 was rarely detected in the antenna (electronic supplementary material, figure S6g) but was present in both long and medium trichoids (electronic supplementary material, figure S6g’,g’’), as was BmorOr19. Owing to its low expression, BmorOr30 may have only a small contribution to the response profile of sensilla trichodea.
3. Discussion
We investigated the biological role of long and medium sensilla trichodea, the most frequent sensillum types on the antenna of female B. mori. Since A-neurons in long trichoids respond to the mulberry component (±)-linalool [2], it has been postulated that female A-neurons may be responsible for host plant detection [8,28]. However, as none of the OSNs in long trichoids responded to the scent of mulberry leaves (table 1), this hypothesis does not seem to be correct. In the same line, long trichoids of female M. sexta do not respond to the mixture of volatiles released by leaves of typical host plants [35], and host plant bouquets do not activate regions in the first olfactory processing centre in the brain of M. sexta that are presumably targeted by OSNs housed in long trichoids [36]. Taken together, these and our results do not support the hypothesis that A-neurons in long trichoids of female moths are involved in oviposition behaviour.
We found that A-neurons responded with high sensitivity only to (+)-linalool (figure 1c). Enantiomer-specific physiological or behavioural responses to linalool are known from females of other moth species such as M. sexta [37], Mamestra brassicae [38] and Trichoplusia ni [39]. Examples of sensitive, enantioselective and narrowly tuned receptors have typically been found among pheromone receptors [40], suggesting that A-neurons in female long trichoids may be involved in pheromone communication, comparable to sex pheromone-detecting A-neurons in male long trichoids [6]. This hypothesis raises the question of whether (+)-linalool might be a male-produced pheromone, as in T. ni. Males of this species emit a pheromone mixture that is attractive to females and consists of more than 80% (+)-linalool [39]. Accordingly, female T. ni have trichoids that are specifically tuned to (+)-linalool [41]. Male pheromones are typically released from eversible, male-specific scales (hair pencils) on the abdomen of many moth species [42]. Although B. mori males also possess tiny hair pencils, no volatile compounds have been found to emanate from these scales [28]. In our study, we were able to show that the headspace emanating from the body of live silkmoths contained numerous compounds, including many terpenes (electronic supplementary material, figure S2b). However, neither male-specific odours nor linalool were present, and OSNs in female trichoids did not respond to silkmoth body odour (table 1). Trichoplusia ni males release higher levels of pheromones when exposed to the female sex pheromone together with the odour of a host plant [43]. Thus, collecting the headspace of B. mori males under similar conditions—exposure to bombykol and the odour of mulberry leaves—could increase the emission rate of a putative male pheromone above the neuronal detection limit. In this context, it is noteworthy that volatiles emitted by a single male silkmoth were able to attract some virgin females in a Y-maze (electronic supplementary material, figure S7), whereas (+)-linalool alone did not attract any female (figure 2c). Therefore, a potential male sex pheromone could contain a mixture of (+)-linalool and other odours that are only behaviourally active when presented together. However, the reliable and strong attraction of male silkmoths to bombykol [14] is not matched by a similar response of females to (+)-linalool.
In addition to the known ligands for B-neurons in long trichoids, we identified isovaleric acid as a potent activator (figure 1c). When tested in our Y-maze, this compound appeared to induce downwind movement (figure 2d,e), a behaviour interpreted as olfactory aversion [44]. What might be the ecological significance of this aversion to acids? In M. sexta, hexanoic acid and 3-methylvaleric acid are present in the headspace of conspecific larval faeces. Each of these acids alone is capable of deterring females from a host plant to avoid larval competition [13]. Similar results have been reported for noctuid moths [45,46]. In our study, an acid released from silkworm faeces was also repellent to mated silkmoth females and could therefore deter them from crowded oviposition sites.
OSNs co-located in a sensillum often convey information of opposite valence in the same behavioural context, with A-neurons having a positive meaning and mediating approach behaviour, whereas B-neurons often have a negative meaning, leading to aversive behaviour in many insect species [47]. For example, in B. mori males, A-neurons detect the attractive sex pheromone bombykol, whereas B-neurons respond to the sex pheromone of other bombycid moth species (bombykal), which acts as a behavioural antagonist [6,48]. It is tempting to assume that the same principle applies to female long trichoids. However, although the best ligand for A-neurons in female long trichoids was behaviourally neutral, one of the best ligands for B-neurons produced a negative signal, consistent with the valence opponency hypothesis.
Response profiles of OSNs housed in medium trichoids and A-neurons in long trichoids were largely overlapping (figure 1b,e). In particular, neurons in both sensillum types showed a characteristic, enantioselective response to (+)-linalool, suggesting a common molecular basis for these similar olfactory responses. However, OSNs in medium trichoids, but not those in long trichoids, responded to mulberry leaf headspace (table 1), revealing the expression of at least one additional OR specific to neurons in medium trichoids. Among the compounds emitted by mulberry leaves that activated female trichoids, only the sesquiterpene germacrene D was detected by OSNs present in medium trichoids but not in long trichoids, suggesting that silkmoths possess a specialized receptor for germacrene D. Interestingly, about 80% of the OSNs tested in female Heliothis virescens responded with high selectivity and sensitivity to germacrene D [49], and OSNs with an identical receptive range were found in two other noctuid species [50]. Germacrene D therefore appears to be an important plant-related olfactory signal in moths belonging to taxonomically distant families, and could be detected by related and so far unknown ORs.
In addition to the response to mulberry, only OSNs in medium trichoids showed increased sensitivity after mating (figure 2a), further supporting the hypothesis that they may play a role in the context of oviposition. In a Y-maze, mated females were attracted to the mulberry scent (Z)-jasmone at a dilution of 10−4. At this dose, OSNs in medium trichoids of mated females appeared saturated (median response of 156 spikes s−1), whereas A-neurons in long trichoids did not respond at all to (Z)-jasmone (figure 2a). Therefore, the attraction of mated females to (Z)-jasmone could be attributed to the activation of OSNs in medium trichoids, although putative (Z)-jasmone-sensing OSNs in other sensillum types may also be involved.
Several response characteristics of sensilla trichodea of female silkmoths suggest a non-canonical expression of olfactory receptors (summarized in figure 4). We show that OSNs in long and medium trichoids respond with high sensitivity and selectivity to (+)-linalool (figure 1c,f). This is reminiscent of the response to the same given pheromone component in different trichoid sensillum types of male moths described for B. mori [5], M. sexta [51], H. virescens and Antheraea spp. [52,53]. Other insects such as tsetse flies [54] or ambrosia beetles [55] have also been found to have OSNs housed in different sensillum types but with highly correlated response profiles. These observations suggest that a given OR may be expressed by OSNs located in different sensillum types and that these OSNs may therefore co-localize with different OSNs. This expression pattern differs from the fixed pairing paradigm of OSNs in the periphery shown in D. melanogaster [16,23,56]. In our study, we found that OSNs in both long and medium trichoids of B. mori females do indeed express the linalool-detecting BmorOr19 (figure 3b) and most likely co-localize with neurons expressing a different receptor depending on the sensillum type. We were thus able to reveal the molecular basis of physiological results previously obtained in a wide range of insect species, indicating a consistent violation of the stereotypical pairing rule of OSNs established in D. melanogaster.
Figure 4.
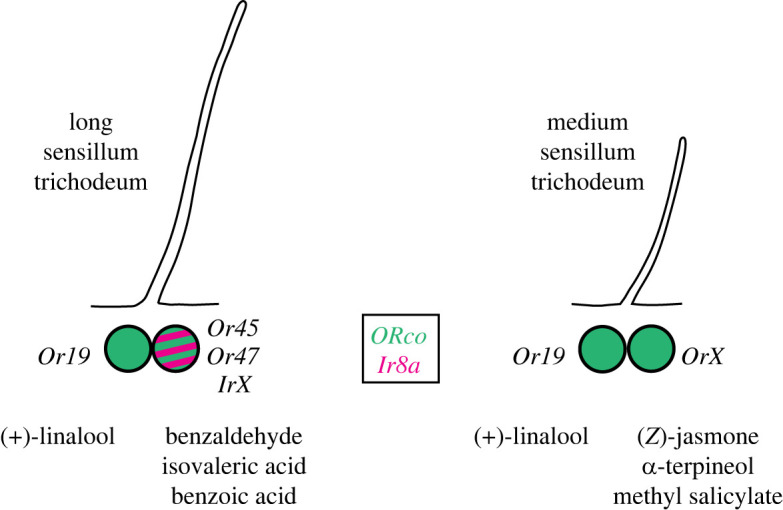
Proposed expression of olfactory receptors and their best ligands in female sensilla trichodea. Green circle, soma of ORco-positive OSN, green circle with magenta stripes, soma of ORco/IR8a-positive OSN. IrX, OrX, unidentified IRs or ORs.
Since B-neurons in long trichoids respond to acids (figure 1), we hypothesized that B-neurons might express Ir8a, the co-receptor of acid-sensing IRs in several insects such as vinegar flies, mosquitoes and moths [13,20,57,58]. Indeed, we found that BmorIr8a was highly expressed in neurons in female trichoids (figure 3c), a sensillum type that typically houses OSNs that express ORs but not IRs [16]. Our result is in agreement with a recent study showing by RT-PCR that BmorIr8a is expressed in the antenna of both sexes of B. mori [59]. In the same paper, three female-biased IRs with similar expression levels to BmorIr8a were described (BmorIr31a, 75p.2, 75q.1), which could be promising candidates to be expressed with BmorIr8a in female long trichoids. In addition, we found that BmorIr8a is also abundantly expressed in the antenna of males (electronic supplementary material, figure S8), suggesting that acid sensing may play a prominent role in silkmoths in general. Another example of the presence of Ir8a in non-coeloconic sensilla of male and female moths is Agrotis segetum [60]. However, in this study it was not possible to decide whether AsegIr8a was expressed in sensilla trichodea or basiconica or in both types of sensilla.
A BmorIr8a-positive cell was usually found in close proximity to an ORco-positive cell, suggesting that both cells were neighbours in the same sensillum, and thus representing an exception to the rule that OR- and IR-expressing neurons are located in mutually exclusive sensillum types [16,20]. Similarly, trichoids on the antenna of the cockroach Periplaneta americana house a PamORco-positive A-neuron and a PamORco-negative B-neuron that responds to acids [61]. Thus, in this distantly related hemimetabolous insect, there is a pairing of OR- and presumably IR-expressing neurons in the same sensillum trichodeum, parallel to our results in B. mori. In the silkmoth antenna, however, we found mostly BmorIr8a-positive cells that were also ORco-positive, indicating co-expression of acid-sensing IRs together with ORs. We also found both types of Ir8a-expressing neurons (Ir8a-positive or Ir8a/ORco-positive) in the male silkmoth antenna. More recently, ORco and one or more of the IR-co-receptors Ir8a, Ir25a and Ir76b have been found to be frequently co-expressed in OSNs of vinegar flies [62] and mosquitoes [63]. Furthermore, co-expression of co-receptors could be mapped to specific sensillum types in the fly, revealing co-expression of ORco/Ir8a in sensilla coeloconica and basiconica, whereas it was absent in sensilla trichodea [62]. In both flies and mosquitoes, ORco/Ir8a was the rarest of all combinations of co-expressed co-receptors. By contrast, our study shows that ORco and Ir8a are co-expressed in the most common sensillum type on the antenna of silkmoths.
Taken together, our results suggest a role for medium trichoids of female silkmoths in the detection of host plants for oviposition. The behavioural significance of A-neurons in female long trichoids, which correspond to bombykol-detecting neurons in the male antenna, has not been elucidated, whereas female B-neurons in the same sensillum convey a repellent signal, as in males. While investigating the molecular basis of these observed olfactory responses, we found an unexpected but consistent co-occurrence of ORco and Ir8a not only in the same sensillum trichodeum but even in a single neuron, and the presence of the female-biased linalool receptor BmorOr19 in both trichoid sensillum types. These expression patterns extend the receptive range of individual neurons and sensilla and may allow combinatorial olfactory coding already in the periphery.
4. Material and methods
Methods regarding headspace collection, WM-FIHC (whole-mount fluorescence immunohistochemistry) and WM-FISH (whole-mount fluorescence in situ hybridization) can be found in the electronic supplementary material.
(a) . Animals
Bombyx mori (Kinshu x Showa) pupae were purchased from Aseptic Sericulture System Laboratory (Kyoto, Japan) and Laboratorio di sericoltura, Centro di Ricerca Agricoltura e Ambiente (Padova, Italy). Bombyx mandarina pupae were provided by Toru Shimada (University of Tokyo, Japan). Male and female pupae were kept in separate boxes at room temperature. After eclosion, moths were stored at 8–10°C until they were used for experiments at days 1 to 8 after eclosion. To obtain mated females, a B. mori couple was placed in a small plastic box at room temperature. Copulation usually started immediately and lasted for several hours. After 7 to 13 h, couples were separated and females used for experiments.
(b) . Odorants
Odorants were from Sigma-Aldrich (http://www.sigma-aldrich.com), Chem Faces (http://www.chemfaces.com) and BOC Sciences (https://www.bocsci.com). The enantiomers of linalool were synthesized by Wittko Francke (University of Hamburg, Germany), and bombykal was synthesized from bombykol (Pherobank, https://www.pherobank.com) by Jerrit Weissflog (MPI for Chemical Ecology Jena, Germany). For screening odorants (n = 76; electronic supplementary material, table S1) in SSR, odorants were diluted in hexane (10−2) or acetone (benzoic acid, 10−2); for dose–response experiments, serial dilutions of odorants were made (10−5, 10−4, 10−3, 10−2 and 10−1). For behavioural assays, odorants were diluted in mineral oil (10−4).
(c) . Single sensillum recordings
For SSR, we performed cut-tip single sensillum recordings [64]. The antenna of a female was cut at the base. The glass capillary of the reference electrode filled with haemolymph Ringer (6.4 mmol l−1 KCl, 20 mmol l−1 KH2PO4, 12 mmol l−1 MgCl2, 1 mmol l−1 CaCl2, 9.6 mmol l−1 KOH, 354 mmol l−1 glucose, 12 mmol l−1 NaCl, pH 6.5) [65] was introduced into the base of the antenna and sealed with Vaseline. As it was impossible to target only one sensillum, several neighbouring sensilla trichodea—long and medium—were cut at the same time with custom-sharpened forceps. Once cut, it was difficult to decide which trichoid subtype was contacted in a given recording. However, the physiological characteristics of both sensillum types were clearly different, so that long and medium trichoids could be distinguished unambiguously. The glass capillary of the recording electrode was filled with sensillum Ringer (171.9 mmol l−1 KCl, 9.2 mmol l−1 KH2PO4, 10.8 mmol l−1 K2HPO4, 3 mmol l−1 MgCl2, 1 mmol l−1 CaCl2, 1.5 mmol l−1 HCl, 22.5 mmol l−1 glucose, 25 mmol l−1 NaCl, pH 6.5) [65]. The antenna was placed under a microscope, where a PEEK tube, providing a constant, humidified charcoal-filtered air stream (0.5 l min−1), was directed to the recording site with outlet at 2 cm distance from antenna. When inserting an odour stimulus (0.4 l min−1) into the air stream, it switched automatically to an additional compensatory air flow (Syntech CS-55 Stimulus Controller, https://www.ockenfels-syntech.com). The recording electrode was directed to cover the tip of a cropped sensillum trichodeum. Filter papers loaded with odorants were freshly prepared before each experiment. Six microlitres of diluted odorants, 10 µl of eluted headspace (mulberry leaves, silkmoths), 10 µl diluted meconium or 10 µl silkworm faeces suspension were pipetted on a filter paper placed in a glass pipette. AutoSpike32 (v3.7) measured changes in extracellular potentials. Signals were 10× amplified (Syntech AC/DC probe), sampled with 48 000 Hz, and filtered (300–3 kHz with 50/60 Hz suppression). Neuronal activity was recorded 3 s before and 20 s after the stimulus pulse (duration: 0.5 s). Each sensillum trichodeum type (long or medium) was recorded only once per antenna. We analysed action potential frequency (spikes s−1) during the recording interval using a bin width of 25 ms. In long trichoids, two neurons could be differentiated based on their different spike amplitudes, while the two neurons in medium trichoids mostly had similar spike amplitudes. Very rarely, we encountered trichoids showing activity from one or three neurons; these were excluded from the present study. The odour-evoked response of OSNs was quantified by subtracting the maximum spiking frequency during 1 s before stimulus onset from the maximum spiking frequency during 1 s after stimulus onset.
(d) . Behavioural assay
We established a two-choice assay for female silkmoths with each experimental arm of the Y-maze (diameter 2.8 cm, length 12 cm) connected to a 100 ml glass bottle containing 1 ml of the solvent mineral oil (control arm) or 1 ml of the diluted odorant (10−4, test arm). To prevent side-biased effects, we switched the position of control and test arm after each experiment. Humidified air was pulsed for 2 s (interstimulus interval 2 s) through the glass bottles into the experimental arms of the Y-maze (0.3 l min−1). Air was pulled out through the entrance arm (diameter 2.8 cm, length 6 cm) of the Y-maze at 0.9 l min−1 to ensure the odour flow through the setup. A camera mounted above the setup recorded the moth's behaviour. Silkmoths were placed in the experimental room (25°C, 70% relative humidity) 30 min before testing. Experiments started at the end of the photo phase (12 h light : 12 h dark). A single moth was placed in the entrance arm of the Y-maze and was observed until it crossed a virtual decision line with its thorax, or until the end of the experiment after 10 min, respectively.
(e) . Statistical analysis
Sample sizes and statistical tests used are given in the text and figure legends. Statistical tests were performed using GraphPad InStat (v3.10) and Social Science Statistics (https://www.socscistatistics.com/).
All data related to figures 1 and 2, electronic supplementary material, figure S5 and table 1 can be found in Raw data file.xlsx in the electronic supplementary material.
Acknowledgements
We thank: Daniel Veit for technical support and building the Y-maze; Gabriel Walther and Maximilian Fraulob for help with behavioural experiments; Carolin Hoyer for help with cloning odorant receptors; Jerrit Weissflog for the synthesis of bombykal; Wittko Francke for synthesizing the enantiomers of linalool; and Toru Shimada for providing pupae of Bombyx mandarina.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Supplementary material is available online [66].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
E.S.: formal analysis, investigation, writing—review and editing; S.C.: formal analysis, investigation, visualization, writing—review and editing; A.R.I.: formal analysis, investigation; R.S.: resources; J.K.: formal analysis, supervision, writing—review and editing; B.S.H.: conceptualization, writing—review and editing; S.S.: conceptualization, funding acquisition, supervision, writing—review and editing; S.B.: conceptualization, formal analysis, investigation, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was supported by the Max Planck Society, by the Deutsche Forschungsgemeinschaft within the priority programme ‘Integrative analysis of olfaction’ (SPP 1392, SA909/3-2) to E.S. and S.S. and by the Ministry of Higher Education and Scientific Research (Egypt) to A.R.I.
References
- 1.Sanes JR, Hildebrand JG. 1976. Structure and development of antennae in a moth, Manduca sexta. Dev. Biol. 51, 282-299. ( 10.1016/0012-1606(76)90144-5) [DOI] [PubMed] [Google Scholar]
- 2.Boeckh J, Kaissling KE, Schneider D. 1965. Insect olfactory receptors. Cold Spring Harb. Symp. Quant. Biol. 30, 263-280. ( 10.1101/SQB.1965.030.01.028) [DOI] [PubMed] [Google Scholar]
- 3.Steinbrecht RA. 1970. Morphometric studies on the antenna of the silk moth, Bombyx mori L.: number and distribution of the olfactory sensilla. Z. Morphol. Tiere. 68, 93-126. ( 10.1007/BF00277500) [DOI] [Google Scholar]
- 4.Pophof B. 1997. Olfactory responses recorded from sensilla coeloconica of the silkmoth Bombyx mori. Physiol. Entomol. 22, 239-248. ( 10.1111/j.1365-3032.1997.tb01164.x) [DOI] [Google Scholar]
- 5.Steinbrecht RA. 1973. The fine-structure of olfactory sensilla in the silk moth (Insecta, Lepidoptera): receptor processes and stimulus conduction apparatus. Zeitschrift fuer Zellforschung und mikroskopische Anatomie. 139, 533-565. ( 10.1007/BF02028392) [DOI] [PubMed] [Google Scholar]
- 6.Kaissling KE, Kasang G. 1978. A new pheromone of the silkworm moth Bombyx mori. Naturwissenschaften. 65, 382-384. ( 10.1007/BF00439702) [DOI] [Google Scholar]
- 7.De Brito Sanchez MG, Kaissling KE. 2005. The antennal benzoic acid receptor cell of the female silk moth Bombyx mori L.: structure-activity relationship studies with halogen substitutes. J. Comp. Physiol. A. 191, 189-196. ( 10.1007/s00359-004-0588-2) [DOI] [PubMed] [Google Scholar]
- 8.Priesner E. 1979. Progress in the analysis of pheromone receptor systems. Annales de Zoologie Ecologie Animale. 11, 533-546. [Google Scholar]
- 9.Tanaka K, Uda Y, Ono Y, Nakagawa T, Suwa M, Yamaoka R, Touhara K. 2009. Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr. Biol. 19, 881-890. ( 10.1016/j.cub.2009.04.035) [DOI] [PubMed] [Google Scholar]
- 10.Francke W, Bartels J, Krohn S, Schulz S, Baader E, Tengo J, Schneider D. 1989. Terpenoids from bark beetles, solitary bees and Danaine butterflies. Pure Appl. Chem. 61, 539-542. ( 10.1351/pac198961030539) [DOI] [Google Scholar]
- 11.Li CZ, Wang H, Chen XM, Yao J, Deng JY. 2022. Visual cues and body volatile beta-ocimene are used by the blue tiger butterfly Tirumala limniace to identify conspecifics during courtship. Behav. Ecol. Sociobiol. 76, 163. ( 10.1007/s00265-022-03266-7) [DOI] [Google Scholar]
- 12.Heinbockel T, Kaissling KE. 1990. Sensitivity and inhibition of antennal benzoic-acid receptor cells of female silkmoth Bombyx mori L. Verhandlungen der Deutschen Zoologischen Gesellschaft 83, 411. [Google Scholar]
- 13.Zhang J, Bisch-Knaden S, Fandino RA, Yan S, Obiero GF, Grosse-Wilde E, Hansson BS, Knaden M. 2019. The olfactory coreceptor IR8a governs larval feces-mediated competition avoidance in a hawkmoth. Proceedings of the National Academy of Sciences of the United States of America. 116, 21 828-21 833. ( 10.1073/pnas.1913485116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obara Y. 1979. Bombyx mori mating dance: an essential in locating the female. Applied Entomology and Zoology. 14, 130-132. ( 10.1303/aez.14.130) [DOI] [Google Scholar]
- 15.Kellog VL. 1907. Some silkworm moth reflexes. Biological Bulletin. 12, 152-154. ( 10.2307/1535862) [DOI] [Google Scholar]
- 16.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 136, 149-162. ( 10.1016/j.cell.2008.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. 1999. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 96, 725-736. ( 10.1016/S0092-8674(00)80582-6) [DOI] [PubMed] [Google Scholar]
- 18.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim JH, Carlson JR. 1999. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327-338. ( 10.1016/S0896-6273(00)81093-4) [DOI] [PubMed] [Google Scholar]
- 19.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703-714. ( 10.1016/j.neuron.2004.08.019) [DOI] [PubMed] [Google Scholar]
- 20.Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GSXE, Benton R. 2011. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13 357-13 375. ( 10.1523/JNEUROSCI.2360-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. 2011. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44-60. ( 10.1016/j.neuron.2010.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vulpe A, Menuz K. 2021. Ir76b is a co-receptor for amine responses in Drosophila olfactory neurons. Front. Cell. Neurosci. 15, 759238. ( 10.3389/fncel.2021.759238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couto A, Alenius M, Dickson BJ. 2005. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535-1547. ( 10.1016/j.cub.2005.07.034) [DOI] [PubMed] [Google Scholar]
- 24.Fishilevich E, Vosshall LB. 2005. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548-1553. ( 10.1016/j.cub.2005.07.066) [DOI] [PubMed] [Google Scholar]
- 25.Liu NY, Xu W, Dong SL, Zhu JY, Xu YX, Anderson A. 2018. Genome-wide analysis of ionotropic receptor gene repertoire in Lepidoptera with an emphasis on its functions of Helicoverpa armigera. Insect Biochem. Mol. Biol. 99, 37-53. ( 10.1016/j.ibmb.2018.05.005) [DOI] [PubMed] [Google Scholar]
- 26.Qiu C-Z, et al. 2018. Evidence of peripheral olfactory impairment in the domestic silkworms: insight from the comparative transcriptome and population genetics. BMC Genom. 19, 788. ( 10.1186/s12864-018-5172-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanner KW, Anderson AR, Trowell SC, Theilmann DA, Robertson HM, Newcomb RD. 2007. Female-biased expression of odourant receptor genes in the adult antennae of the silkworm, Bombyx mori. Insect Mol. Biol. 16, 107-119. ( 10.1111/j.1365-2583.2007.00708.x) [DOI] [PubMed] [Google Scholar]
- 28.Anderson AR, Wanner KW, Trowell SC, Warr CG, Jaquin-Joly E, Zagatti P, Robertson H, Newcomb RD. 2009. Molecular basis of female-specific odorant responses in Bombyx mori. Insect Biochem. Mol. Biol. 39, 189-197. ( 10.1016/j.ibmb.2008.11.002) [DOI] [PubMed] [Google Scholar]
- 29.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. 2007. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 10, 1474-1482. ( 10.1038/nn1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettersen KH, Einevoll GT. 2008. Amplitude variability and extracellular low-pass filtering of neuronal spikes. Biophys. J. 94, 784-802. ( 10.1529/biophysj.107.111179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takai H, Asaoka K, Ishizuna F, Kiuchi T, Katsuma S, Shimada T. 2018. Morphological and electrophysiological differences in tarsal chemosensilla between the wild silkmoth Bombyx mandarina and the domesticated species Bombyx mori. Arthropod Struct. Dev. 47, 238-247. ( 10.1016/j.asd.2018.03.001) [DOI] [PubMed] [Google Scholar]
- 32.Bisch-Knaden S, Daimon T, Shimada T, Hansson BS, Sachse S. 2014. Anatomical and functional analysis of domestication effects on the olfactory system of the silkmoth Bombyx mori. Proc. Biol. Sci. 281, 20132582. ( 10.1098/rspb.2013.2582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker TC, Willis MA, Haynes KF, Phelan PL. 1985. A pulsed cloud of sex-pheromone elicits upwind flight in male moths. Physiol. Entomol. 10, 257-265. ( 10.1111/j.1365-3032.1985.tb00045.x) [DOI] [Google Scholar]
- 34.Nolte A, Gawalek P, Koerte S, Wei HY, Schumann R, Werckenthin A, Krieger J, Stengl M. 2016. No evidence for ionotropic pheromone transduction in the hawkmoth Manduca sexta. PLoS ONE. 11, e0166060. ( 10.1371/journal.pone.0166060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields VDC, Hildebrand JG. 2001. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J. Comp. Physiol. A 186, 1135-1151. ( 10.1007/s003590000165) [DOI] [PubMed] [Google Scholar]
- 36.Bisch-Knaden S, Rafter MA, Knaden M, Hansson BS. 2022. Unique neural coding of crucial versus irrelevant plant odors in a hawkmoth. eLife 11, e77429. ( 10.7554/eLife.77429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reisenman CE, Christensen TA, Francke W, Hildebrand JG. 2004. Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J. Neurosci. 24, 2602-2611. ( 10.1523/JNEUROSCI.5192-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulland S, Ian E, Borg-Karlson AK, Mustaparta H. 2006. Discrimination between enantiomers of linalool by olfactory receptor neurons in the cabbage moth Mamestra brassicae (L.). Chem. Senses 31, 325-334. ( 10.1093/chemse/bjj036) [DOI] [PubMed] [Google Scholar]
- 39.Heath RR, Landolt PJ, Dueben BD, Murphy RE, Schneider RE. 1992. Identification of male cabbage looper sex-pheromone attractive to females. J. Chem. Ecol. 18, 441-453. ( 10.1007/BF00994243) [DOI] [PubMed] [Google Scholar]
- 40.Masson C, Mustaparta H. 1990. Chemical information-processing in the olfactory system of insects. Physiol. Rev. 70, 199-245. ( 10.1152/physrev.1990.70.1.199) [DOI] [PubMed] [Google Scholar]
- 41.Todd JL, Baker TC. 1993. Response of single antennal neurons of female cabbage loopers to behaviorally active attractants. Naturwissenschaften 80, 183-186. ( 10.1007/BF01226381) [DOI] [Google Scholar]
- 42.Birch MC, Poppy GM, Baker TC. 1990. Scents and eversible scent structures of male moths. Annu. Rev. Entomol. 35, 25-58. ( 10.1146/annurev.en.35.010190.000325) [DOI] [Google Scholar]
- 43.Landolt PJ, Heath RR. 1990. Sexual role reversal in mate-finding strategies of the cabbage looper moth. Science 249, 1026-1028. ( 10.1126/science.249.4972.1026) [DOI] [PubMed] [Google Scholar]
- 44.Steck K, Veit D, Grandy R, Badia SB, Mathews Z, Verschure P, Hansson BS, Knaden M. 2012. A high-throughput behavioral paradigm for Drosophila olfaction - the Flywalk. Scient. Rep. 2, 361. ( 10.1038/srep00361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li GQ, Ishikawa Y. 2004. Oviposition deterrents in larval frass of four Ostrinia species fed on an artificial diet. J. Chem. Ecol. 30, 1445-1456. ( 10.1023/B:JOEC.0000037750.64844.4b) [DOI] [PubMed] [Google Scholar]
- 46.Xu HY, Li GQ, Liu ML, Xing GN. 2006. Oviposition deterrents in larval frass of the cotton boll worm, Helicoverpa armigera (Lepidoptera, Noctuidae): chemical identification and electroantennography analysis. J. Insect Physiol. 52, 320-326. ( 10.1016/j.jinsphys.2005.11.011) [DOI] [PubMed] [Google Scholar]
- 47.Wu ST, Chen JY, Martin V, Ng R, Zhang Y, Grover D, Greenspan RJ, Aljadeff J, Su CY. 2022. Valence opponency in peripheral olfactory processing. Proc. Natl Acad. Sci. USA 119, e2120134119. ( 10.1073/pnas.2120134119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daimon T, Fujii T, Yokoyama T, Katsuma S, Shinoda T, Shimada T, Ishikawa Y. 2012. Reinvestigation of the sex pheromone of the wild silkmoth Bombyx mandarina: the effects of bombykal and bombykyl acetate. J. Chem. Ecol. 38, 1031-1035. ( 10.1007/s10886-012-0164-0) [DOI] [PubMed] [Google Scholar]
- 49.Rostelien T, Borg-Karlson AK, Faldt J, Jacobsson U, Mustaparta H. 2000. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neuron of the tobacco budworm moth Heliothis virescens. Chem. Senses 25, 141-148. ( 10.1093/chemse/25.2.141) [DOI] [PubMed] [Google Scholar]
- 50.Stranden M, Liblikas I, Konig WA, Almaas TJ, Borg-Karlson AK, Mustaparta H. 2003. (–)-Germacrene D receptor neurones in three species of heliothine moths: structure-activity relationships. J. Comp. Physiol. A 189, 563-577. ( 10.1007/s00359-003-0434-y) [DOI] [PubMed] [Google Scholar]
- 51.Kalinova B, Hoskovec M, Liblikas I, Unelius CR, Hansson BS. 2001. Detection of sex pheromone components in Manduca sexta (L.). Chem. Senses 26, 1175-1186. ( 10.1093/chemse/26.9.1175) [DOI] [PubMed] [Google Scholar]
- 52.Meng LZ, Wu CH, Wicklein M, Kaissling KE, Bestmann HJ. 1989. Number and sensitivity of three types of pheromone receptor cells in Antheraea pernyi and Antheraea polyphemus. J. Comp. Physiol. A 165, 139-146. ( 10.1007/BF00619188) [DOI] [Google Scholar]
- 53.Almaas TJ, Mustaparta H. 1991. Heliothis virescens: response characteristics of receptor neurons in sensilla trichodea type 1 and type 2. J. Chem. Ecol. 17, 953-972. ( 10.1007/BF01395602) [DOI] [PubMed] [Google Scholar]
- 54.Soni N, Chahda JS, Carlson JR. 2019. Odor coding in the antenna of the tsetse fly Glossina morsitans. Proc. Natl Acad. Sci. USA 116, 14 300-14 308. ( 10.1073/pnas.1907075116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas T, Yuvaraj JK, Hansson BS, Löfstedt C, Anderbrant O, Andersson MN. 2023. Characterization of olfactory sensory neurons in the striped ambrosia beetle Trypodendron lineatum. Front. Physiol. 14, 1155129. ( 10.3389/fphys.2023.1155129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Bruyne M, Foster K, Carlson JR. 2001. Odor coding in the Drosophila antenna. Neuron 30, 537-552. ( 10.1016/S0896-6273(01)00289-6) [DOI] [PubMed] [Google Scholar]
- 57.Raji JI, Melo N, Castillo JS, Gonzalez S, Saldana V, Stensmyr MC, Degennaro M. 2019. Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 29, 1253-1263. ( 10.1016/j.cub.2019.02.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang R, Jiang NJ, Ning C, Li GC, Huang LQ, Wang CZ. 2020. The olfactory reception of acetic acid and ionotropic receptors in the oriental armyworm, Mythimna separata Walker. Insect Biochem. Mol. Biol. 118, 103312. ( 10.1016/j.ibmb.2019.103312) [DOI] [PubMed] [Google Scholar]
- 59.Yin NN, Nuo SM, Xiao HY, Zhao YJ, Zhu JY, Liu NY. 2021. The ionotropic receptor gene family in Lepidoptera and Trichoptera: annotation, evolutionary and functional perspectives. Genomics 113, 601-612. ( 10.1016/j.ygeno.2020.09.056) [DOI] [PubMed] [Google Scholar]
- 60.Hou XQ, Zhang DD, Powell D, Wang HL, Andersson MN, Loefstedt C. 2022. Ionotropic receptors in the turnip moth Agrotis segetum respond to repellent medium-chain fatty acids. BMC Biol. 20, 34. ( 10.1186/s12915-022-01235-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tateishi K, Watanabe T, Nishino H, Mizunami M, Watanabe H. 2022. Silencing the odorant receptor co-receptor impairs olfactory reception in a sensillum-specific manner in the cockroach. iScience. 25, 104272. ( 10.1016/j.isci.2022.104272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Task D, et al. 2022. Chemoreceptor co-expression in Drosophila melanogaster olfactory neurons. eLife 11, e72599. ( 10.7554/eLife.72599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herre M, et al. 2022. Non-canonical odor coding in the mosquito. Cell 185, 3104-3123. ( 10.1016/j.cell.2022.07.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaissling KE, Meng LZ, Bestmann HJ. 1989. Responses of bombykol receptor-cells to (Z,E)-4,6-hexadecadiene and linalool. J. Comp. Physiol. A 165, 147-154. ( 10.1007/BF00619189) [DOI] [Google Scholar]
- 65.Kaissling K-E, Thorson J. 1980. Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organization. In Receptors for neurotransmitters, hormones and pheromones in insects (eds Satelle DB, Hall LM, Hildebrand JG), pp. 261-282. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 66.Schuh E, Cassau S, Ismaieel AR, Stieber R, Krieger J, Hansson BS, Sachse S, Bisch-Knaden S. 2024. Females smell differently: characteristics and significance of the most common olfactory sensilla of female silkmoths. Figshare. ( 10.6084/m9.figshare.c.7005726) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
Data Availability Statement
Supplementary material is available online [66].