Abstract
Ferroptosis is a type of programmed cell death resulting from iron overload-dependent lipid peroxidation, and could be promoted by activating transcription factor 3 (ATF3). SIRT1 is an enzyme accounting for removing acetylated lysine residues from target proteins by consuming NAD+, but its role remains elusive in ferroptosis and activating ATF3. In this study, we found SIRT1 was activated during the process of RSL3-induced glioma cell ferroptosis. Moreover, the glioma cell death was aggravated by SIRT1 activator SRT2183, but suppressed by SIRT inhibitor EX527 or when SIRT1 was silenced with siRNA. These indicated SIRT1 sensitized glioma cells to ferroptosis. Furthermore, we found SIRT1 promoted RSL3-induced expressional upregulation and nuclear translocation of ATF3. Silence of ATF3 with siRNA attenuated RSL3-induced increases of ferrous iron and lipid peroxidation, downregulation of SLC7A11 and GPX4 and depletion of cysteine and GSH. Thus, SIRT1 promoted glioma cell ferroptosis by inducting ATF3 activation. Mechanistically, ATF3 activation was reinforced when RSL3-induced decline of NAD+ was aggravated by FK866 that could inhibit NAD + synthesis via salvage pathway, but suppressed when intracellular NAD+ was maintained at higher level by supplement of exogenous NAD+. Notably, the NAD + decline caused by RSL3 was enhanced when SIRT1 was further activated by SRT2183, but attenuated when SIRT1 activation was inhibited by EX527. These indicated SIRT1 promoted ATF3 activation via consumption of NAD+. Finally, we found RSL3 activated SIRT1 by inducing reactive oxygen species-dependent upregulation of AROS. Together, our study revealed SIRT1 activated by AROS sensitizes glioma cells to ferroptosis via activation of ATF3-dependent inhibition of SLC7A11 and GPX4.
Keywords: Ferroptosis, SIRT1, AROS, ATF3, Glioma, NAD+, Oxidative stress
List of abbreviations
- SLC7A11
solute carrier family 7 member 11
- GPX4
glutathione peroxidase 4
- ATF3
activating transcription factor 3
- SIRT1
silent information regulator 1
- NAD+
nicotinamide adenine dinucleotide
- RSL3
RAS-selective lethal compounds 3
- P53
cellular tumor antigen p53
- NF-κB
nuclear factor-kappa B
- PARP1
poly(ADP-Ribose) Polymerase 1
- NRF2
nuclear factor erythoid-2-like 2
- AMPK
AMP-activated protein kinase
- DPT
deoxypodophyllotoxin
- NAT10
N-acetyltransferase 10
- TF
transferrin
- TFR
transferrin Receptor
- FT
ferritin light chain
- FPN
ferroportin
- NAC
N-Acetylcysteine
- ROS
reactive oxygen species
- FER-1
ferrostatin-1
- GSH
glutathione
- MDA
malonaldehyde
- FAC
ferric ammonium citrate
- DFO
deferoxamine
- AROS
active regulator of SIRT1
- DBC-1
cell cycle and apoptosis regulator protein 2
- H2A
histone H2A
1. Introduction
Ferroptosis is a type of regulated cell death resulting from lipid peroxidation provoked by iron overload [1]. Induction of ferroptosis is emerging as a new strategy to kill cancer cells with or without drug resistance. As a type of malignant brain tumor, glioma cells could also be eliminated via activation of ferroptosis pathway, and natural chemicals such as pseudolaric acid B and brucine were reported to kill glioma cells by triggering ferroptosis [2,3]. Downregulation of SLC7A11 and inactivation of GPX4 are regarded as two canonical pathways triggering ferroptosis in both cancerous and non-cancerous cells [4,5]. Mounting evidences have shown that both erastin and RSL3, which are regarded as classical ferroptosis inducers, could initiate ferroptosis by targeting SLC7A11 and GPX4 [6,7]. As a member of ATF/CREB family of transcription factors, activating transcription Factor 3 (ATF3) functions as a transcriptional activator or repressor to regulate apoptotic, necroptotic and autophagic cell death [[8], [9], [10]]. Further studies show that ATF3 also contributes to ferroptosis by suppressing the transcription of SLC7A11 and GPX4 and could be activated via endoplasmic reticulum pathway [3,11,12], but it is still unclear whether other factors are involved in ATF3 activation.
Sirtuin 1 (SIRT1) is a deacetylase by consuming NAD + to remove acetylated lysine residues from its target proteins such as p53 and PARP1, and involved in modulation of glioma cell progression, invasion and treatment response [[13], [14], [15]]. Mounting evidences have shown activated SIRT1 protects non-cancerous cell against ferroptosis caused by various detrimental factors. It inhibited lung epithelial cell ferroptosis induced by heat stress, neuron ferroptosis due to hypoxia and ischemia, and cardiomyocyte ferroptosis triggered by doxorubin [[16], [17], [18]]. Mechanistically, SIRT1-mediated deacetylation exerts inhibitory effect on ferroptosis primarily via two pathways, one is deacetylating p53 to suppress p53-dependent SLC7A11 downregulation [19], and the other is deacetylating Nrf2 to drive Nrf2-mediated GPX4 upregulation [20]. However, SIRT1-dependent deacetylation of targets proteins theoretically accompanied with depletion of its substrate NAD+. Although NAD + could mediate multiple cellular functions such as calcium homeostasis, energy generation and gene expression [21], its role is still unclear in SIRT1-mediated ferroptosis.
Distinct with protecting non-cancerous cells against various exterior and interior stresses, SIRT1 generates detrimental effect on cancer cells via multiple pathways. It exacerbated metformin-induced pyroptosis in breast cancer cells via aggravating nuclear translocation NF-κB, aggravated quercetin-triggered autophagic death in human lung cancer cells by activating AMPK, and promoted DPT-induced glioma cell parthanatos through upregulating acetyltransferase NAT10 [[22], [23], [24]]. However, it remains elusive whether SIRT1 activation could drive ferroptosis in cancer cells. Therefore, in the present study, we investigated the role of SIRT1 in RSL3-induced glioma cell ferroptosis and its underlying mechanism, and found that SIRT1 sensitized glioma cells to ferroptosis not via deacetylation of its target p53, but by depletion of its substrate NAD+, which provoked ATF3-mediated inhibition of SLC7A11 and GPX4. This reveals a new role of SIRT1 in ferroptosis and a new pathway leading to ATF3 activation.
2. Materials and methods
2.1. Reagents
RSL3, SRT2183, antioxidant N-Acetylcysteine (NAC) and lipid ROS inhibitor ferrostatin-1(Fer-1) were all purchased from Selleck Chemicals (Shanghai, China). FK866, EX527 and NAD+ were all from MedChemExpress company (Shanghai, China). Ferric ammonium citrate (FAC) was from Sigma-Aldrich (Saint Louis, MO). Deferoxamine and antibodies against acetyl-p53 at K382 (ab75754), SIRT1 (ab32441), ATF3 (ab254268), GPX4 (ab125066), SLC7A11 (ab175186), ferritin light chain (ab69090), Histone H2A(ab18255), ferroportin (ab78066), transferrin (ab82411), and transferrin receptor (ab1086) were all from Abcam (Cambridge, UK). AROS antibody (A13231) was from Abclonal technology company (Wuhan, China) and DBC1 antibody (22638-1-AP) was from Proteintech Company (Wuhan, China). Ferrous iron probe FerroOrang was obtained from Dojindo Laboratories (Shanghai, China). The other reagents were from Sigma-Aldrich.
2.2. Cell culture
Three human glioma cell lines U118, U251 and U87 obtained from Shanghai institute of cell biology, Chinese Academy of Sciences were cultured at 37 °C in DMEM medium added with high glucose, 10 % fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 μg/mL) in a humid environment containing 5 % CO2.
2.3. Small interfering RNA transfection
GoldenTrans R from Golden Transfer Technology (Changchun, China) or Lipofectamine 3000 (Invitrogen) were used to transfer siRNA into cells. siRNA SIRT1 (5′-GGATGAAAGTGAAATTGAA-3′) was from RiboBio company (Guangzhou, China), siRNA AROS (5′-CCAAGGGAAAGGUGCCCAATT-3′) was from JTS scientific company (Wuhan, China), siRNA ATF3(GAUGAGAGAAACCUCUUUATT-3′) and scrambled siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) were both from GenePharma company (Suzhou, China)
2.4. Cell death assay
Cell death was assayed by using lactate dehydrogenase (LDH) release assay kit obtained from Beyotime Biotechnology (Nanjing, China). Cell death ratio was calculated by using the formula: cell death ratio (%) = (A sample – A control)/(A max – A control) × 100, in which A represented the absorbance value acquired by a microplate reader. A max represented the absorbance value of the positive group.
2.5. Immunocytochemical staining and ferro-Orange staining
For immunocytochemical staining, the cells treated with RSL3 were incubated with 1 % Triton X-100 for 10 min. After the cells were fixed in ethanol and washed with PBS, the nonspecific binding sites were blocked with BSA. Then, the cells incubated overnight with the antibodies against SIRT1 (1:100), ATF3 (1:100) or AROS (1:100), followed by incubation 1 h with goat anti-rabbit IgG (1:200) conjugated with Cy3 or Fluor 488 and counterstained 5 min with Heochst 33342. For Ferro-Orange staining, the U87 cells seeded on a culture dish were washed three times with DMEM medium and then incubated with 1 μmol/mL FerroOrange working solution at 37 °C for 30 min. Eventually, the cells were observed by a confocal microscope (Olympus FV1000, Tokyo, Japan).
2.6. Measurement of GSH and cysteine
The intracellular levels of GSH and cysteine were analyzed respectively by using the GSH assay kit from Beyotime Biotechnology (Nanjing, China) and cysteine assay kit from Jiancheng Bioengineering Institute (Nanjing, China) as described by us previously [3]. The content of GSH was expressed as a ratio to the absorbance value at 412 nm of the control cells and cysteine content was expressed as a ratio to the absorbance value at 600 nm of the control cells.
2.7. Assay of MDA, ROS and NAD+
MDA was assayed with a kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to manufacturer protocols and its content was expressed as a ratio to the absorbance value at 532 nm of the control cells.
ROS levels were examined by using probe DCFH-DA from Beyotime Biotechnology company (China) as reported by us. The ROS levels were expressed as the percentage of control cells.
NAD+ was measured using NAD+/NADH assay kit (Beyotime Biotechnology, China). Briefly, the collected cells were suspended in NAD+/NADH extraction buffer and then homogenized. When measuring total NAD+ and NADH level, 20 μL homogenate was incubated 10 min with 90 μL alcohol dehydrogenase, followed by incubation 30 min with 10 μL chromogenic solution. The absorbance value at 450 nm was used to calculate the total level of NAD+ and NADH according to a standard concentration curve. For measuring NADH level, the homogenate was heated at 60 °C for 30 min followed by incubation with dehydrogenase and chromogenic solution. NAD + content was calculated by subtracting NADH from total NAD+ and NADH and expressed as a ratio to control group.
2.8. Western blotting and Co-immunoprecipitation
Western blotting and Co-immunoprecipitation were performed as described by us previously [25].
2.9. Statistical analyses
The obtained data were expressed as mean ± SD. One-way ANOVA was used to make statistical comparisons. P values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. RSL3 triggered ferroptosis in glioma cells
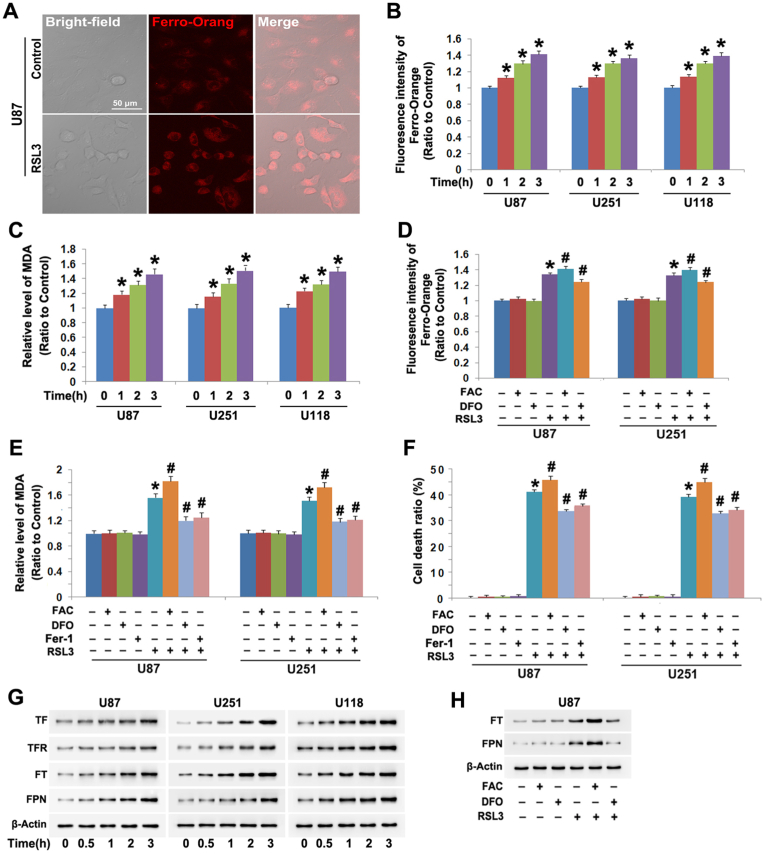
GPX4 inhibitor RSL3 was found by us to cause glioma cell death via activation of lethal autophagy [26]. In the present study, we tested whether RSL3 could provoke ferroptosis in glioma cells. Thus, human U87, U251 and U118 glioma cells were incubated with RSL3 at the dosage 10 μmol/L as described by us [26]. By using probe FerroOrange which could detect intracellular ferrous iron levels, we found that the cells incubated with RSL3 displayed stronger fluorescence at 3 h under confocal microscope than that of control cell (Fig. 1A). Furthermore, statistical analysis of the fluorescence intensity at incubation 1 h, 2 h and 3 h revealed that the fluorescence was improved time-dependently by RSL3 in all the three tested cell lines (Fig. 1B). Moreover, lipid peroxidation product MDA was also significantly increased by RSL3 at each corresponding time (Fig. 1C). These indicated that RSL3 not only induced upregulation of intracellular ferrous iron, but also triggered lipid peroxidation.
Fig. 1.
RSL3 triggered ferroptosis in glioma cells. (A) Confocal microscopy combined with ferrous iron probe FerroOrang staining showed the fluorescence exhibited by FerroOrang was much stronger in the U87 cells treated with RSL3 (10 μmol/L) for 3 h than that in control cells. (B) Statistical analysis proved that the fluorescence intensity was significantly elevated by RSL3 (10 μmol/L) in U87, U251 and U118 glioma cells at incubation 1 h, which became more apparent when incubation time was extended to 2 h and 3 h. (C) RSL3 (10 μmol/L) triggered generation of lipid peroxidation product MDA in U87, U251 and U118 glioma cells in a time-dependent manner. (D) Pretreatment with FAC (500 μmol/L) for 1 h enhanced, but with FAC (500 μmol/L) inhibited RSL3-induced elevation in the fluorescence exhibited by FerroOrange. (E) The elevation of MDA induced by RSL3 (10 μmol/L) at 3 h was significantly enhanced by FAC, but apparently alleviated by DFO or Fer-1 (30 μmol/L). (F) LDH release assay revealed the glioma cell death provoked by RSL3 (10 μmol/L) was exacerbated by FAC, but inhibited by DFO or Fer-1. (G) Western blotting demonstrated RSL3 (10 μmol/L) upregulated the protein levels of transferrin (TF), transferrin receptor (TFR), ferritin (FT) and ferroportin (FPN). (H) Western blotting showed that FAC reinforced, but DFO attenuated RSL3-indcued upregulation of ferritin (FT) and ferroportin (FPN). *: p < 0.01 versus control group; #: p < 0.01 versus RSL3 group. The values are expressed as mean ± SD (n = 5 per group).
To test whether the generation of MDA resulted from the increased ferrous iron, ferric ammonia citrate (FAC) and iron chelator deferoxamine (DFO) were respectively used to improve and decrease intracellular iron level. It was found the MAD generation and the cell death were both aggravated when the increase of ferrous iron was enhanced by FAC, but suppressed when the increase of ferrous iron was attenuated by DFO (Fig. 1D–F). These indicated that RSL3 induced lipid peroxidation and glioma cell death via increasing intracellular ferrous iron.
To elucidate the relationship between lipid peroxidation and RSL3 toxicity in glioma cells, we used Fer-1 to inhibit RSL-induced MDA and then evaluated the cell death. It was found Fer-1 not only apparently inhibited RSL3-provoked MDA generation, but also attenuated glioma cell death (Fig. 1E and F). This indicated lipid peroxidation accounted for RSL3-induced glioma cell death. Thus, RSL3 triggered ferroptosis in glioma cells.
Intracellular homeostasis of iron is primarily modulated by transferrin receptor (TFR) which imports extracellular ferric iron-transferrin (TF) complex into cells, ferritin (FT) which could directly bind with iron and ferroportin (FPN) which accounts for exporting iron out of cells [3]. Thus, we used western blotting to analyze the effect of RSL3 on these proteins and found RSL3 provoked time-dependent upregulation of TFR and TF (Fig. 1G). This indicated that RSL3 increased intracellular iron by upregulating TFR-mediated uptake of extracellular iron. Although FT and FPN were both upregulated by RSL3 as well (Fig. 1G), we found that their increases were reinforced by FAC, but inhibited by DFO (Fig. 1H). Thus, we thought the upregulation of FT and FPN might be a cellular response to the increased iron.
3.2. SIRT1 sensitized glioma cells to RSL3-induced ferroptosis
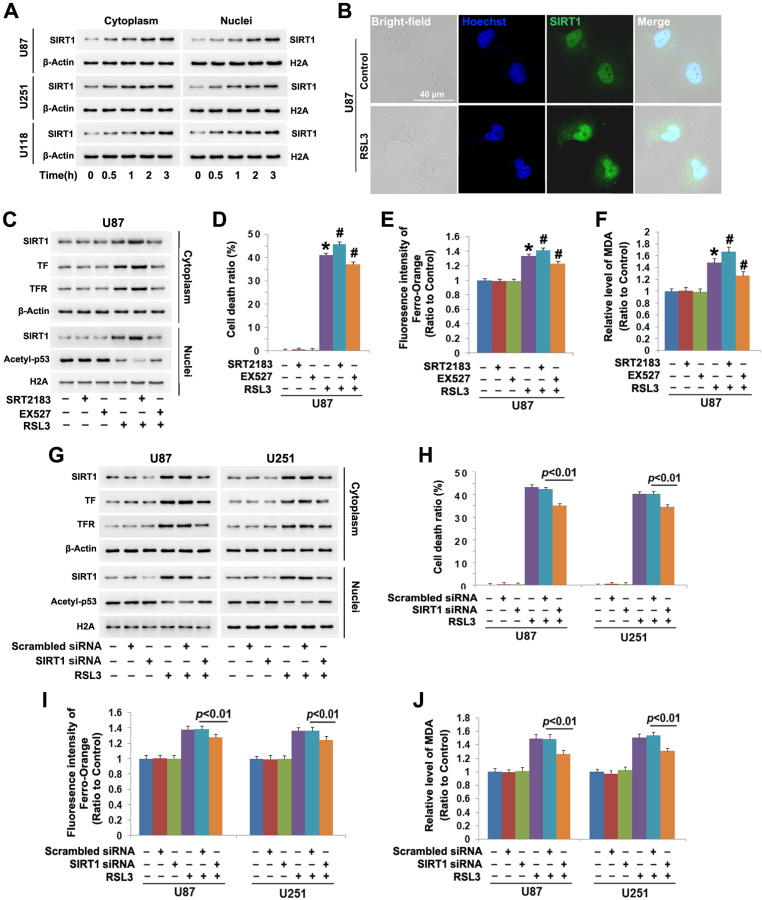
Previous reports showed that SIRT1 activation prevented ferroptosis in on-cancerous cells [[16], [17], [18]], but its role remains elusive in cancer cell ferroptosis. To address this issue, we used western blotting to examine its level in all the three glioma cell lines treated with and without RSL3. It was found cytoplasmic and nuclear levels of SIRT1 were both obviously at higher levels in the cells treated with RSL3 than those in control cells (Fig. 2A). Moreover, the increase of SIRT1 in either cytoplasm or nuclei exhibited a time-dependent manner (Fig. 2A). Furthermore, SIRT1 was shown by confocal microscopy to accumulate more apparently at 3 h in the nuclei of RSL3-treated cells than that of control cells (Fig. 2B). These suggested RSL3 promoted SIRT1 distribution into nuclei, as well as upregulated its expression.
Fig. 2.
SIRT1 sensitized glioma cells to RSL3-induced ferroptosis. (A)Western blotting showed the protein level of SIRT1 was time-dependently upregulated in both cytoplasmic and nuclear fractions isolated from U87, U251 and U118 glioma cells treated with RSL3 (10 μmol/L), when compared with that in control cells. (B) Confocal microscopy combined with immunocytochemical staining showed that SIRT1 accumulated more apparently in the nuclei of the U87 cells treated with RSL3 for 3 h than that in control cells. (C) Western blotting revealed that the upregulation of SIRT1, transferrin (TF) and transferrin receptor (TFR) and the downregulation of acetyl-p53 caused by RSL3 were all reinforced in the cells pretreated 1 h with SIRT1 activator SRT2183 (20 μmol/L), but suppressed by SIRT1 inhibitor EX527 (200 μmol/L). (D) LDH release assay proved that SRT2183 exacerbated, but EX527 inhibited the glioma cell death caused by RSL3 (10 μmol/L). (E) Statistical analysis of the fluorescence exhibited by FerroOrange revealed that the elevated ferrous iron at 3 h due to RSL3 (10 μmol/L) treatment was aggravated by SRT2183, but prevented by EX527. (F) MDA assay showed the lipid peroxidation triggered by RSL3 (10 μmol/L) was exacerbated by SRT2183, but attenuated by EX527. (G) Western blotting proved that SIRT1 knockdown with siRNA not only obviously inhibited RSL3-induced upregulation of SIRT1, transferrin (TF) and transferrin receptor (TFR), but also prevented the downregulation of acetyl-p53. (H) LDH release assay showed that knockdown of SIRT1 with siRNA inhibited RSL3-induced glioma cell death. (I and J) RSL3-trigged ferrous iron increase and MDA elevation were both suppressed when SIRT1 was silenced by siRNA. *: p < 0.01 versus control group; #: p < 0.01 versus RSL3 group. The values are expressed as mean ± SD (n = 5 per group).
To test whether SIRT1 modulated the toxicity of RSL3 in glioma cells, we used SRT2183 to stimulate SIRT1 activity and EX527 to inhibit SIRT1 activation and then examined their impacts on the glioma cell ferroptosis caused by RSL3. As revealed by western blotting, SRT2183 significantly aggravated, but EX527 alleviated RSL3-provoked upregulation of SIRT1 and downregulation of acetyl-p53 (Fig. 2C). Since acetyl-p53 downregulation is an indicator of SIRT1 activation [13], these results suggested that RSL3 induced SIRT1 activation in glioma cells. Correspondingly, LDH release assay proved SRT2183 reinforced, whereas EX527 inhibited RSL3-induced cell death (Fig. 2D). Notably, the increases of ferrous iron and MDA caused by RSL3 were both enhanced by SRT2183, but suppressed by EX527 (Fig. 2E and F). This indicated SIRT1 sensitized glioma cells to RSL3-provoked ferroptosis. Furthermore, the upregulated transferrin receptor and transferrin caused by RSL3 were more apparent in the presence of SRT2183, but alleviated by EX527 (Fig. 2G). This indicated SIRT1 contributed to RSL3-induced iron increase by promoting iron uptake.
To further verify SIRT1 sensitized glioma cells to ferroptosis, siRNA was used to genetically inhibit SIRT1. It was found inhibition of SIRT1 upregulation with SIRT1 siRNA effectively prevented RSL3-induced acetyl-p53 downregulation, elevation of iron and MDA and glioma cell death (Fig. 2G–J). Moreover, RSL3-induced upregulation of transferrin receptor and transferrin were both inhibited when SIRT1 was silenced (Fig. 2G). This further demonstrated SIRT1 promoted glioma cell ferroptosis.
3.3. SIRT1 promoted RSL3-induced downregulation of SLC7A11 and GPX4
Blocking either GPX4 or SLC7A11 is regarded as canonical pathway to initiate ferroptosis by increasing intracellular iron [4,5]. Thus, we examined the influence of RSL3 on the protein levels of SLC7A11 and GPX4. As western blotting showed, the protein levels of GPX4 and SLC7A11 were obviously downregulated by RSL3 at each indicated time in all the three tested glioma cell lines (Fig. 3A). Considering SLC7A11 is a component of glutamate/cystine antiporter which exports glutamate in exchange for cystine uptake and then the imported cystine transforms into cysteine to be used to synthesize GSH [4], we assayed the influence of RSL3 on the level of cysteine and GSH and found they were both significantly decreased by RSL3 at 3 h (Fig. 3B and C). These indicated RSL3 not only induced downregulation of SLC7A11 and GPX4, but also depleted intracellular cysteine and GSH. Notably, we also found that SIRT1 activator SRT2183 exacerbated, whereas its inhibitor EX527 or transfection with SIRT1 siRNA prevented RSL3-triggered downregulation of SLC7A11 and GPX4 and declines of cysteine and GSH (Fig. 3B–G). This indicated SIRT1 promoted ferroptosis by inhibiting the expression of SLC7A11 and GPX4.
Fig. 3.
SIRT1 promoted RSL3-induced downregulation of SLC7A11 and GPX4. (A) Western blotting showed that RSL3 (10 μmol/L) induced time-dependent downregulation of SLC7A11 and GPX4 in U87, U251 and U118 glioma cells. (B and C) RSL3-triggered declines of cysteine and GSH became more apparent in the cells pretreated 1 h with SRT2183 (20 μmol/L), but suppressed in the cells pretreated with EX527 (200 μmol/L). (D) Western blotting revealed SRT2183 exacerbated, but EX527 attenuated RSL3-induced downregulation of SLC7A11 and GPX4. (E)Western blotting demonstrated silence of SIRT1 with siRNA prevented the downregulation of SLC7A11 and GPX4 caused by RSL3 (10 μmol/L). (F and G) Knockdown of SIRT1 with siRNA partially reversed RSL3-indcued declines of cysteine and GSH. (H) Western blotting showed treatment with SRT2183 (40 μmol/L) could obviously upregulate SIRT1 in both cytoplasmic and nuclear fractions, but downregulate acetyl-p53, SLC7A11 and GPX4 in a time-dependent manner. (I) LDH release assay showed the glioma cell death caused by SRT2183 (40 μmol/L) at 24 h was attenuated when the cells were pretreated 1 h with EX527 (200 μmol/L) or supplemented with exogenous NAD+ (2 mmol/L). (J and K) The declines of cysteine and GSH induced by SRT2183 (40 μmol/L) at 24 h were both suppressed by pretreating the cells 1 h with EX527 or supplementing exogenous NAD+. (L) Western blotting proved pretreatment with EX527 or supplement of exogenous NAD + for 1 h not only alleviated SIRT1 upregulation in both cytoplasmic and nuclear fractions, but also inhibited the downregulation of acetyl-p53, SLC7A11 and GPX4 provoked by SRT2183 (40 μmol/L) at 24 h. (M) NAD + assay showed that NAD + decline triggered by SRT2183 (40 μmol/L) at 24 h was inhibited in the cells pretreated 1 h with EX527 or supplemented with exogenous NAD+. *: p < 0.01 versus control group; #: p < 0.01 versus RSL3 group; &: p < 0.01 versus SRT2183 group The values are expressed as mean ± SD (n = 5 per group).
To further verify the influence of activated SIRT1 on GPX4 and SLC7A11, SRT2183 dosage was increased to 40 μmol/L and then used to incubate U87 cells for indicated time. We found SRT2183 apparently upregulated SIRT1 levels in both cytoplasmic and nuclear fractions, but downregulated acetyl-p53, SLC7A11 and GPX4 in a time-dependent manner (Fig. 3H). However, SRT2183-induced glioma cell death, declines of GSH and cysteine, SIRT1 upregulation and downregulation of acetyl-p53, SLC7A11 and GPX4 were all significantly weakened by EX527 (Fig. 3I-L). Given that p53 is inactivated when being deacetylated by SIRT1 [13], these results indicated activated SIRT1 inhibited the expression of GPX4 and SLC7A11 not via p53 pathway.
3.4. SIRT1 triggered downregulation of SLC7A11 and GPX4 by depletion of NAD+
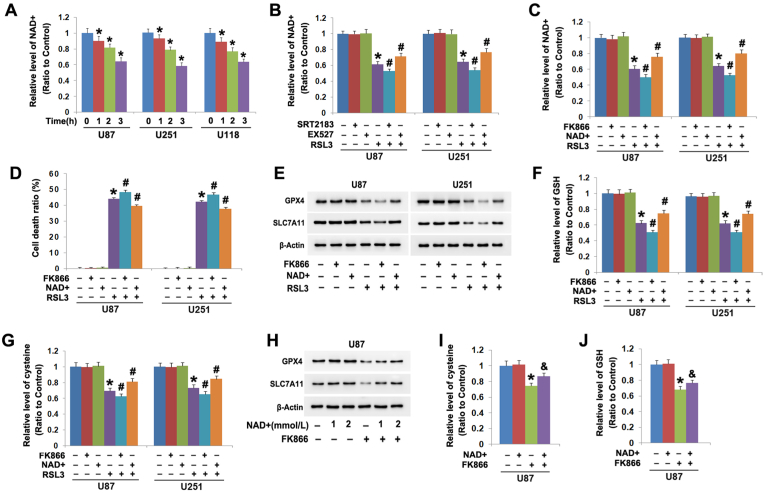
Besides deacetylation of p53, SIRT1 activation also results in decline of NAD + [24]. We thus speculated that NAD + decline might play a pivotal role in SIRT1-dependent downregulation of SLC7A11 and GPX4. Therefore, we assayed the impact of SIRT1 on RSL3-induced changes in NAD+, and found NAD+ was decreased time-dependently by RSL3 in all the three tested glioma cell lines (Fig. 4A). Moreover, the NAD + decline caused by RSL3 was exacerbated by SRT2183, but prevented by EX527 (Fig. 4B). This indicated RSL3 decreased NAD + through activation of SIRT1.
Fig. 4.
SIRT1 triggered downregulation of SLC7A11 and GPX4 by depletion of NAD+. (A) RSL3 (10 μmol/L) triggered time-dependent decline of NAD+ in U87, U251 and U118 cells. (B)The NAD + decline caused by RSL3 (10 μmol/L) at 3 h was aggravated by pretreating the cells 1 h with SRT2183 (20 μmol/L), but attenuated by EX527 (200 μmol/L). (C) Pretreatment with FK866 (500 μmol/L) for 1 h exacerbated, but supplement of exogenous NAD+ (2 mmol/L) prevented NAD + decline in the cells treated with RSL3 (10 μmol/L) for 3 h. (D) LDH release assay showed RSL3-induced glioma cell death was aggravated by FK866, but inhibited by supplement of exogenous NAD+. (E) Western blotting revealed RSL3-induced downregulation of SLC7A11 and GPX4 was enhanced by FK866, but attenuated by exogenous NAD + supplement. (F and G) Pretreatment with FK866 reinforced, but NAD + supplement attenuated RSL3-triggered declines of cysteine and GSH. (H) Western blotting showed that the downregulation of SLC7A11 and GPX4 at 24 h in the cells treated with FK866 (500 μmol/L) alone was inhibited by supplement of 1 mmol/L NAD+, and the inhibition became more apparent when NAD + dosage was increased to 2 mmol/L (I and J) The declines of cysteine and GSH in the cells treated with FK866 (500 μmol/L) was attenuated significantly by supplement of exogenous NAD + at 2 mmol/L *: p < 0.01 versus control group; #: p < 0.01 versus RSL3 group; &: p < 0.01 versus FK866 group. The values are expressed as mean ± SD (n = 5 per group).
To test the influence of NAD + decline on RSL3 toxicity in glioma cells, the cells were incubated with RSL3 following supplement with exogenous NAD + or being treated with FK866. FK866 is a specific inhibitor of NAMPT, which is a rate-limiting enzyme regulating NAD + synthesis via salvage pathway [24]. It was found the NAD + decline and the cell death due to RSL3 treatment were both aggravated by FK866, whereas attenuated by NAD + supplement (Fig. 4C and D). This suggested RSL3 provoked glioma cell death by decline of NAD+. Moreover, western blotting revealed FK866 promoted, but NAD + supplements alleviated RSL3-induced downregulation of GPX4 and SLC7A11 and declines of GSH and cysteine (Fig. 4E–G). These indicated that NAD + depletion promoted RSL3-inducd inhibition of GPX4 and SLC7A11.
To further demonstrate NAD + decline is a crucial factor inhibiting GPX4 and SLC7A11, the U87 cells were treated with exogenous NAD + at indicated dosage for 1 h following incubation 24 h with 500 μmol/L FK866. We found NAD + supplement significantly prevented FK866-induced downregulation of GPX4 and SLC7A11 and declines of cysteine and GSH (Fig. 4H–J), indicating that NAD + depletion inhibited the expression of GPX4 and SLC7A11 in glioma cells.
Furthermore, the decline of NAD+ in the cells treated with 40 μmol/L SRT2183 for 24 h was prevented in the presence of EX527 (Fig.3M). Additionally, exogenous NAD + supplement not only attenuated SRT2183-induced NAD + decline and glioma cell death (Fig. 3 M, I), but also attenuated SRT2183-triggered downregulation of SLC7A11 and GPX4 and declines of cysteine and GSH (Fig. 3J-L). This further verified that activated SIRT1 inhibited SLC7A11 and GPX4 in glioma cells by depletion of NAD+.
3.5. NAD + depletion accounted for SIRT1-dependent activation of ATF3
Given that ATF3 activation inhibits the transcriptions of SLC7A11 and GPX4 during the process of ferroptosis [11,12], we speculated that the inhibitory effect of NAD + decline on SLC7A11 and GPX4 might be dependent on ATF3 activation. We thus used western blotting to analyze expressional differences of ATF3 between the cells treated with and without RSL3. It was found cytoplasmic and nuclear levels of ATF3 were both upregulated in all the three tested glioma cell lines treated with 10 μmol/L RSL3 in a time-dependent manner (Fig. 5A). This was also verified by observing U87 cells with confocal microscope, showing that ATF3 accumulated more obviously at 3 h in the nuclei of RSL3-treated cells than that in control cells (Fig. 5B). Thus, RSL3 triggered ATF3 activation in glioma cells. Moreover, the cell death due to RSL3 treatment, as well as the upregulated ATF3, was also significantly attenuated in the cells transfected with siRAN targeting ATF3 (Fig. 5C and D). Furthermore, ATF3 siRNA transfection not only prevented the inhibitory effect of RSL3 on the expression of SLC7A11 and GPX4 (Fig. 5C), but also alleviated the declines of cysteine and GSH (Fig. 5E and F). These indicated that RSL3 induced downregulation of SLC7A11 and GPX4 by activation of ATF3.
Fig. 5.
NAD + depletion accounted for SIRT1-dependent activation of ATF3 (A) Western blotting revealed the protein level of ATF3 was time-dependently upregulated in both cytoplasmic and nuclear fractions isolated from the U87, U251 and U118 glioma cells treated with RSL3 (10 μmol/L), when compared with that in control cells. (B) Confocal microscopy combined with immunocytochemical staining showed that ATF3 accumulated more apparently at 3 h in the nuclei of the U87 cells treated with RSL3 (10 μmol/L) than that in control cells. (C) Knockdown of ATF3 with siRNA prevented RSL3-induced downregulation of SLC7A11 and GPX4. (D) LDH release assay showed ATF3 knockdown with siRNA suppressed RSL3-induced glioma cell death. (E and F) The declines of cysteine and GSH caused by RSL (10 μmol/L) at 3 h were both partially reversed when ATF3 was knocked down with siRNA. (G) Pretreatment with FK866 (500 μmol/L) or supplement of exogenous NAD + at 2 mmol/L for 1 h prevented RSL3-induced upregulation of ATF3 in both cytoplasmic and nuclear fractions. (H) Supplement of exogenous NAD + at 1 mmol/L could inhibit ATF3 upregulation in the cells treated with FK866 (500 μmol/L) for 24 h, and this inhibition became more apparent by increasing NAD + dosage to 2 mmol/L. (I) The upregulation of ATF3 caused by RSL3 was aggravated by SRT2183, but suppressed by EX527. (J) Pretreatment with EX527 (200 μmol/L) or supplement of exogenous NAD + at 2 mmol/L for 1 h prevented ATF3 upregulation in the cells treated with SRT2183 (40 μmol/L) for 24 h. The values are expressed as mean ± SD (n = 5 per group).
To address NAD + decline is an upstream signal regulating ATF3 activation, the cells were treated with FK866 or exogenous NAD+ and then incubated with RSL3. It was found FK866 enhanced, but supplement of exogenous NAD + alleviated RSL3-triggered upregulation of ATF3 in both cytoplasm and nuclear fractions (Fig. 5G). This suggested that RSL3 activated ATF3 by decreasing NAD+. To further verify the role of NAD + decline in ATF3 activation, intracellular NAD + level was decreased by treating the cells with 500 μmol/L FK866 for 24 h, and then we used western blotting to analyze its effect on ATF3 expression and distribution. It was found FK866 induced obvious increases of ATF3 in both cytoplasmic and nuclear fractions, which were effectively suppressed when the cells were pretreated 1 h with NAD + at indicated dosage (Fig. 5H). This proved NAD + depletion is the upstream signal leading to ATF3 activation.
Because NAD + decline caused by RSL3 was from SIRT1 activation, we examined the impact of SIRT1 on ATF3 activation. It was found RSL3-induced ATF3 upregulation was enhanced when the cells were pretreated with SRT2183, but inhibited by EX527 (Fig. 5I). This indicated that SIRT1 activation promoted RSL3-induced activation of ATF3. To further verify SIRT1 activates ATF3 by decreasing NAD+, the U87 cells were treated with SIRT1 activator SRT2183 at 40 μmol/L for 24 h following incubation with SIRT1 inhibitor EX527 and exogenous NAD+, and then we analyzed ATF3 expression and distribution by western blotting. It was found both cytoplasmic and nuclear ATF3 were upregulated in the cells treated with SRT2183, but this was apparently attenuated by incubating cells with EX527 or exogenous NAD+ (Fig. 5J). This demonstrated as well that SIRT1 promoted ATF3 expression and its translocation into nuclei by depletion of NAD+.
3.6. AROS contributed RSL3-induced activation of SIRT1
NAD+ is not only a substrate of SIRT1, but also acts as an activator of SIRT1 [16]. NAD + depletion caused by SIRT1 might reversely inhibit SIRT1 activation. To address why SIRT1 could be sustainably activated by RSL3, we analyzed the impact of RSL3 on AROS and DBC-1 using western blotting, because SIRT1 could be activated by AROS, but inhibited by DBC-1 [16]. It was found RSL3 triggered time-dependent upregulation of AROS, but downregulation of DBC-1 in all the three tested cell lines (Fig. 6A). Confocal microscopy also demonstrated the nuclear accumulation of AROS was much more obvious in the cells treated with RSL3 than that in control cells (Fig. 6B). Moreover, the upregulation of AROS and the downregulation of DBC-1 caused by RSL3 were both apparently reinforced when the cells were pretreated with SRT2183, but attenuated by EX527 (Fig. 6C). This indicated RSL3 might activate SIRT1 by upregulating AROS. To test whether RSL treatment induce interaction between SIRT1 and AROS, we immunoprecipitated SIRT1 by its antibody, analyzed AROS level in precipitated fractions, and found the co-immunoprecipidated level of AROS was increased at 2 h and further elevated at 3 h in RSL3-treated cells (Fig. 6D). This indicated that the interaction between AROS and SIRT1 was enhanced by RSL3.
Fig. 6.
AROS contributed RSL3-induced activation of SIRT1. (A) Western blotting showed AROS and DBC-1 were both upregulated in the U87, U251 and U118 cells treated with RSL3 (10 μmol/L) in a time-dependent manner. (B) Confocal microscopy combined with immunocytochemical staining showed AROS was increased more apparently at 3 h in the U87 cells treated with RSL3 (10 μmol/L) than that in control cells. (C) Western blotting showed that the upregulation of AROS and downregulation of DBC-1 caused by RSL3 were both aggravated in the cells pretreated 1 h with SRT2183 (20 μmol/L), but inhibited by EX527 (200 μmol/L). (C) Co-immunoprecipitation combined with western blotting revealed that the AROS level co-immunoprecipitated with SIRT1 was elevated with the extension of RSL3 treatment time. (D) Knockdown of AROS with siRNA apparently prevented RSL3-induced upregulation of SIRT1 and ATF3 and downregulation of acetyl-p53, SLC7A11 and GPX4. (F) AROS knockdown with siRNA prevented RSL-induced decline of NAD+. (G) RSL3-triggered increase of ferrous iron was inhibited when AROS was silenced with siRNA. (H and I) Knockdown of AROS suppressed RSL3-induced decline of cysteine and GSH. (J) LDH release assay proved the glioma cell death caused by RSL3 was attenuated by knocking AROS down with siRNA. The values are expressed as mean ± SD (n = 5 per group).
To clarify the role of AROS in RSL3-indcued SIRT1 activation, we used siRNA to knock down AROS and examined the protein levels of SIRT1 and acetyl-p53 by western blotting. It was found RSL3-iduced downregulation of SIRT1 target protein acetyl-p53 and SIRT1 substrate NAD+, as well as upregulation of SIRT1, was all alleviated by knocking AROS down with siRNA (Fig. 6E and F). This indicated that AROS contributed to RSL3-induced sustained activation of SIRT1. Consistently, SIRT1-dependent ATF3 activation, downregulation of SLC7A11 and GPX4 (Fig. 6E), increase of iron (Fig. 6G), declines of GSH and cysteine (Fig. 6 H, I), and glioma cell death were all suppressed as well when AROS was knocked down with siRNA (Fig. 6J). These demonstrated that RSL3 induced SIRT1 activation by upregulation of AROS.
3.7. ROS contributed to AROS-dependent activation of SIRT1
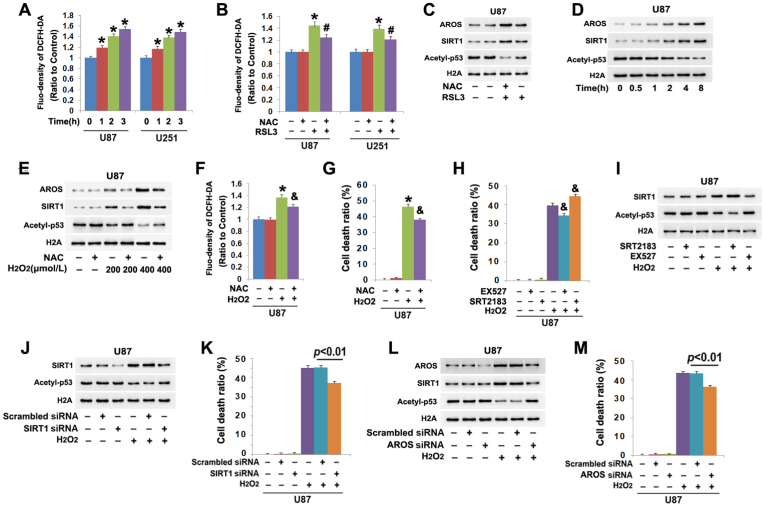
To elucidate why RSL3 could upregulate AROS, we analyzed RSL3-induced changes in intracellular reactive oxygen species (ROS) by using probe DCFH-DA because RSL3 is a specific inhibitor of GPX4 which could clear ROS [26]. We found intracellular ROS were time-dependently improved by RSL3 (Fig. 7A). In contrast, prior incubation of the cells with antioxidant NAC not only prevented RSL3-induced ROS increases, but also inhibited AROS upregulation (Fig. 7B and C). Concomitantly, SIRT1 upregulation and acetyl-p53 downregulation caused by RSL3 were both mitigated by NAC as well (Fig. 7C). Considering that AROS is an activator of SIRT1 and acetyl-p53 is a substrate of SIRT1, these results indicated that RSL3 induced AROS-dependent SIRT1 activation by improving ROS.
Fig. 7.
ROS contributed to AROS-dependent activation of SIRT1. (A) Treatment with RSL3 (10 μmol/L) provoked time-dependent increases of intracellular ROS in the U87 and U251 cells. (B) Pretreatment with antioxidant NAC (5 mmol/L) for 1 h significantly prevented the increase of ROS at 3 h in the cells treated with RSL3 (10 μmol/L). (C) Western blotting showed that NAC obviously suppressed RSL3-induced upregulation of AROs and SIRT1 and downregulation of acetyl-p53. (D) Western blotting revealed AROS and SIRT were both upregulated, but acetyl-p53 was downregulated time-dependently in the U87 cells treated with 400 μmol/L H2O2. (E)Western blotting showed H2O2 triggered dosage-dependent upregulation of AROS and SIRT1 and downregulation of acetyl-p53, which were effectively inhibited by NAC. (F and G) Pretreatment 1 h with NAC (5 mmol/L) obviously prevented H2O2-induced increases of ROS and the glioma cell death. (H) LDH release assay showed that EX527 inhibited, but SRT2183 aggravated the glioma cell death provoked by H2O2. (I) SRT2183 enhanced, but EX527 inhibited H2O2-induced upregulation of SIRT1 and the downregulation of acetyl-p53. (J and K) Knockdown of SIRT1 with siRNA prevented H2O2-induced acetyl-p53 downregulation and glioma cell death. (L and M) Silence of AROS with siRNA suppressed H2O2-induced SIRT1 upregulation, acetyl-p53 downregulation and glioma cell death. *: p < 0.01 versus control group; #: p < 0.01 versus RSL3 group; &: p < 0.01 versus H2O2 group. The values are expressed as mean ± SD (n = 5 per group).
To further clarify the role of ROS in regulating AROS-dependent activation of SIRT1, the cells were treated with 400 μmol/L H2O2 following incubation 1 h with and without 5 mmol/L NAC and then we analyzed its impact on AROS and SIRT1 using western blotting. When compared with control cells, AROS and SIRT1 were both elevated by H2O2 at 1 h, and further upregulated when incubation time was extended to 2 h, 4 h and 8 h. In contrast, acetyl-p53 was decreased correspondingly at each indicated time (Fig. 7D). Moreover, the upregulation of AROS and SIRT1 and the downregulation of acetyl-p53 became more apparent when H2O2 dosage was increased from 200 μmol/L to 400 μmol/L (Fig. 7E). Furthermore, inhibition of H2O2-induced ROS by treating the cells with NAC obviously prevented glioma cell death (Fig. 7 F, G), as well as inhibited AROS upregulation (Fig. 7 E). Correspondingly, SIRT1 upregulation and acetyl-p53 downregulation which are downstream of AROS were also obviously suppressed by NAC (Fig. 7E). These suggested that ROS contributed to AROS-dependent activation of SIRT1 in glioma cells.
To study whether acetyl-p53 downregulation caused by H2O2 was due to SIRT1 activation, the cells were treated respectively with SRT2183 and EX527 prior to incubation with H2O2 for 8 h. It was found H2O2-induced SIRT1 upregulation, acetyl-p53 downregulation and glioma cell death were all enhanced by SRT2183, but attenuated by EX527(Fig. 7 H, I). Consistently, knockdown of SIRT1 with siRNA obviously prevented H2O2-induced acetyl-p53 decrease and glioma cell death (Fig. 7J and K). These indicated SIRT1 was activated in the cells treated with H2O2. Furthermore, we found that knockdown of AROS with siRNA could also prevent H2O2-induced SIRT1 upregulation, acetyl-p53 downregulation and glioma cell death (Fig.7L, M). This further verified that SIRT1 activation triggered by H2O2 was dependent on AROS.
4. Discussion
Summarily, we found in the study that SIRT1 and ATF3 were both activated during the process of RSL3-induced glioma cell ferroptosis. The glioma cell death was aggravated by SIRT1 activator SRT2183, but suppressed by SIRT1 inhibitor EX527 or when SIRT1 was silenced with siRNA. These indicated that SIRT1 sensitized glioma cells to ferroptosis. Furthermore, SIRT1 promoted RSL3-induced expressional upregulation and nuclear translocation of ATF3. Silence of ATF3 with siRNA attenuated RSL3-induced increases of ferrous iron and lipid peroxidation, downregulation of SLC7A11 and GPX4 and depletion of cysteine and GSH. Thus, SIRT1 promoted the occurring of ATF3-dependent ferroptosis. Mechanistically, ATF3 activation was reinforced when RSL3-induced decline of NAD+ was aggravated by inhibiting NAD + synthesis with FK866, but suppressed when intracellular level of NAD+ was maintained by supplement of exogenous NAD+. Notably, we found that the NAD + decline was enhanced when SIRT1 was further activated by SRT2183, but attenuated by when SIRT1 activation was inhibited by EX527. These indicated that SIRT1 promoted ATF3 activation via depletion of NAD+. Finally, we found that RSL3 activated SIRT1 by inducing reactive oxygen species-dependent upregulation of AROS. Together, our study demonstrated that SIRT1 activated by AROS sensitizes glioma cells to ferroptosis via activation of ATF3-dependent inhibition of SLC7A11 and GPX4 (Fig. 8).
Fig. 8.
Schematic diagram for the role of SIRT1 in glioma cell ferroptosis. As a specific inhibitor of GPX4, RSL3 not only induces glioma cell ferroptosis, but also improves intracellular ROS levels. The increased ROS activates SIRT1 by upregulation of SIRT1 activator AROS and downregulation of SIRT1 inhibitor DBC-1. Then, the activated SIRT1 consumes its substrate NAD + excessively, which results in ATF3 accumulation in nuclei. Within nuclei, ATF3 acts as a transcription repressor to inhibit SLC7A11 transcription and expression, which decreases intracellular levels of cysteine and GSH. GSH depletion not only aggravates glioma cell ferroptosis via promoting lipid peroxidation, but also further improves ROS levels. Thus, SIRT1 activated by AROS sensitize RSL3-induced glioma cell ferroptosis by induction of NAD + depletion-dependent activation of ATF3.
RSL3 is often used to induce ferroptosis in cancerous and non-cancerous cells by increasing intracellular ferrous iron (an active form of iron) [27,28]. Generally, extracellular ferric iron-transferrin complex is transported into cells via transferrin receptor located at cellular surface. Then, the complex is delivered via receptor-mediated endocytosis into acid endosomes (pH < 5.5), in which the ferric iron was released from transferrin due to the acid environment and reduced by transmembrane ferrireductase STEAP3 into ferrous iron. After being pump out of endosomes through solute carrier family 11 member 2 (SLC11A2), the ferrous iron forms liable iron pool in cytosol [29]. Thus, intracellular ferrous iron would be increased when transferrin receptor is upregulated. Upregulation of transferrin receptor was reported to improve intracellular iron level and lead to ferroptosis [30], but genetic silence of transferrin receptor prevented the occurrence of ferroptosis [31]. In this study, we found RSL3 treatment not only increased the level of ferrous iron that could be detected by ferro-Orange, but also upregulated the expression of transferrin receptor. Therefore we thought RSL3 increased ferrous iron in glioma cells via upregulation of transferrin receptor.
ATF3 is proven by accumulating evidences to play dual roles in regulating the occurrence of ferroptosis. Different with transcription factor p53 whose activation leads to ferroptosis in both cancer cells and non-cancerous cells [32,33], ATF3 inhibits ferroptosis in non-cancerous cells but promotes cancer cell ferroptosis. It was reported that ATF3 activation obviously attenuated cardiomyocyte ferroptosis provoked by erastin or RSL3, as well as suppressed neuron ferroptosis triggered by ischemic stroke [34,35]. In contrast, ATF3 was found to promote hepatocellular carcinoma cell ferroptosis triggered by saikosaponin A and lung cancer cell ferroptosis induced by shikonin [36,37]. Thus, ATF3-mediated ferroptosis produces opposite effect between cancer cells and non-cancerous cells. In cancer cells, ATF3 activation was found to elevate intracellular iron by increasing the expression of transferrin receptor or inducting autophagy to degrade iron-storage protein ferritin [3,38,39]. Additionally, ATF3 could also upregulate NOX4 to generate hydrogen peroxide, which exacerbates lipid peroxidation after being transformed to potent lipid oxidant hydroxyl radicals via reaction with ferrous iron [3]. Further studies revealed that activated ATF3 suppressed the expression of SLC7A11 or GPX4 at transcriptional level [11,12], both of which were demonstrated extensively to play pivotal roles in initiating ferroptosis when being inhibited or downregulated. Consistently, we found in the study that RSL3 provoked glioma cell ferroptosis through activating ATF3, which resulted in downregulation of SLC7A11 and GPX4. Therefore, RSL3 could not only inhibit the activity of GPX4 as reported previously, but also downregulate GPX4 expression of via activation of ATF3.
ATF3 is activated via endoplasmic reticulum stress pathway in the process of ferroptosis. In glioma cells, the contribution of ATF3 to brucine-provoked ferroptosis was found to depend on endoplasmic reticulum stress-mediated ATF4 upregulation [3]. It was also reported endoplasmic reticulum stress was responsible for saikosaponin A-stimulated ATF3-dependent ferroptosis in hepatocellular carcinoma cells [36]. Furthermore, increasing evidences proved that SIRT1 could also promote endoplasmic reticulum stress and ATF3 activation in both cancer cells and non cancerous cells. As an activator of SIRT1, SRT2183 attenuated glioma cell growth via activation of endoplasmic reticulum stress pathway [40]. However, SIRT1 activator resveratrol promoted renal NRK-52E cells to express anti-aging Klotho gene through activating ATF3 [41]. Similarly, hypoxia-induced ANF secretion in rat atria was decided by SIRT1-reguated ATF3 activation [42]. Therefore, these reports suggest ATF3 plays a pivotal role in SIRT1-regulated inhibition of non-cancerous cell ferroptosis and promotion of cancer cell ferroptosis. Although we did not examine in the present study whether SIRT1 activation trigger ER stress in glioma cells, we found SIRT1 activation obviously reinforced RSL3-induced elevation in nuclear ATF3. Besides activation of ATF3, SIRT1 could also promote cancer cell ferroptosis via activating autophagy. It was reported SIRT1 activated autophagy in human lung cancer cells via AMPK pathway, and autophagy could increase intracellular iron level by degradation of ferritin [26,39]. Therefore, activated SIRT1 could sensitize cancer cells to ferroptosis.
SIRT1-dependent p53 deacetylation is regarded as a crucial pathway to inhibit ferroptosis via regulation of SLC7A11 [43], but NAD + depletion caused by activated SIRT1 remains elusive in modulating ferroptosis. As an abundant metabolite, NAD+ is not only consumed by SIRT1 to deacetylate intracellular proteins, but also essential in maintenance of oxidative phosphorylation, fatty acid oxidation, and the TCA cycle [44]. NAD + depletion could lead to several types of regulated cell death such as parthanatos, autophagic cell death and apoptosis [26,45,46], despite its remains elusive whether NAD + decline could drive ferroptosis. Notably, kynurenine which is often used to synthesize NAD + via de novo pathway was reported to inhibit HeLa cell ferroptosis [47], and this might be associated with maintaining intracellular NAD+. Moreover, cellular susceptibility to ferroptosis was reinforced when CD38, an NAD + consuming enzyme, was over-expressed [48]. Further study showed that NAD + depletion caused by SIRT1 could elevate intracellular ROS in glioma cells by enhancing superoxide generation via mitochondrial electronic transmission chain complex I [26]. In contrast, elevating intracellular NAD + by activation of AMPK protected human MRC‐5 fibroblast cells from oxidative stress-induced senescence [49]. Interestingly, nuclear ATF3 was apparently elevated when glioma cells were treated with exogenous H2O2 alone [3]. Therefore, these previous reports suggested that NAD + decline might activate ATF3 by induction of oxidative stress. In the present study, we found that NAD + depletion enhanced ATF3 activation in glioma cells. Thus, NAD+ is a new factor regulating ATF3 activation.
Mounting evidences have shown that activated SIRT1 results in NAD + depletion not only via consuming it to deacetylate target proteins, but also via other pathways. One is promoting NAD + consumption by activation of other enzymes. PARP1 is an enzyme using NAD + to synthesize PAR polymers after being activating when DNA double stand breaks occur [24]. The activity of PARP1 is also upregulated in glioma cells when NAT10 expression was stimulated by activated SIRT1 [24]. The other way is inhibition of NAD + synthesis. It was found SIRT1 constitutively repressed the expression of NMNAT1 and NMNAT3 during 3T3-L1 differentiation, both of which are responsible for NAD + synthesis [50]. Different with previous reports showing that SIRT1 inhibits ferroptosis in non-cancerous cells by deacetylation of p53, we found that SIRT1 promotes RSL3-induced glioma cell ferroptosis via depletion of NAD+.
Both AROS and DBC1 are intracellular proteins that could directly interact with SIRT1, of which AROS stimulates SIRT1 activity but DBC1 suppress SIRT1 activation [51]. Thus, AROS and DBC1 are respectively regarded as endogenous activator and inhibitor of SIRT1. When compared with non-cancerous cells, the interaction between AROS and SIRT1 is much stronger in cancer cells and could be reinforced when AROS is methylated by methyltransferase NSD2 [52]. Further studies reveal that SIRT1 binding with AROS or DBC1 is decided by the phosphorylated sites within SIRT1. It was reported that phosphorylation of SIRT1 at Ser682 by HIPK2 disrupted the interaction between SIRT1 and AROS and suppressed SIRT1 activity [53]. Phosphorylation of SIRT1 by AMPK at Thr344 makes SIRT1 release from DBC1 and improved its activity [54]. Moreover, phosphorylation by JNK at ser25 reinforces its distribution into nuclei, as well as increasing its activity [24]. In this study, we found RSL3 not only induced AROS upregulation and DBC1 downregulation, but also enhanced AROS binding with SIRT1. Inhibition of AROS expression by siRNA apparently inhibited RSL3-provoked SIRT1-dependent NAD + depletion and acetyl-p53 decrease. Thus, RSL3 induced SIRT1 activation via AROS pathway.
As the activators of SIRT1, both AMPK and JNK are not only activated by intracellular ROS, but also by RSL3. Previous reports showed that RSL3 triggered AMPK activation in human glioma U87 cells and activation JNK in mouse hippocampal HT22 Cells [23,55]. Thus, RSL3 might induce SIRT1 activation by influencing the activation of AMPK or JNK. In the present study, we found that mitigation of RSL3-induced increases of ROS with antioxidant NAC not only obviously prevented AROS upregulation and DBC1 downregulation, but also attenuated SIRT1 upregulation and acetyl-p53 downregulation. We found as well that the protein level of AROS was increase, but DBC1 was decreased in the glioma cells treated with hydrogen peroxide alone. Inhibition of AROS expression by siRNA obviously prevented hydrogen peroxide-induced SIRT1-regualted acetyl-p53 downregulation. Thus, RSL3 activated SIRT1 by inducing ROS-dependent upregulation of AROS and downregulation of DBC1.
In conclusion, we demonstrated in the present study that RSL3-medicated GPX4 inhibition improved intracellular ROS, which activated SIRT1 by upregulation of AROS and downregulation of DBC-1. Then, SIRT1-dependent NAD + depletion exacerbated glioma cell ferroptosis via promoting expressional upregulation and nuclear translocation of ATF3. Our study identified SIRT1 activation as a new pathway to sensitize glioma cells to ferroptosis.
CRediT authorship contribution statement
Xi chen: Writing – original draft, Investigation, Data curation, Conceptualization. Zhenchuan Wang: Writing – original draft, Investigation, Formal analysis, Data curation. Chen Li: Writing – review & editing, Investigation, Formal analysis. Zhao Zhang: Writing – review & editing, Investigation, Formal analysis, Data curation. Shan Lu: Writing – review & editing, Investigation, Formal analysis, Data curation. Xuanzhong Wang: Writing – review & editing, Investigation, Formal analysis. Qi Liang: Writing – review & editing, Investigation. Xiaoxi Zhu: Investigation, Formal analysis. Chengliang Pan: Investigation, Formal analysis. Qingxuan Wang: Writing – review & editing, Investigation, Formal analysis. Zhilin Ji: Writing – review & editing, Investigation. Yubo Wang: Writing – review & editing, Writing – original draft, Supervision, Formal analysis. Meihua Piao: Supervision, Software, Methodology, Data curation. Guangfan Chi: Writing – review & editing, Supervision, Software, Methodology, Data curation. Pengfei Ge: Writing – review & editing, Writing – original draft, Validation, Resources, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
We declare that we have no conflict of interests.
Acknowledgement
This work was supported by National Nature and Science Foundation of China (81972346 and 82173027), Scientific Research Foundation of Jilin province (20230508060RC, 20200201549JC), and Collaboration Fund of the First Hospital of Jilin University and Changchun institute of applied chemistry Chinese academy of sciences (2022YYGFZJC011).
Data availability
Data will be made available on request.
References
- 1.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z., Ding Y., Wang X., Lu S., Wang C., He C., et al. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 2018;428:21–33. doi: 10.1016/j.canlet.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Lu S., Wang X.Z., He C., Wang L., Liang S.P., Wang C.C., et al. ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol. Sin. 2021;42:1690–1702. doi: 10.1038/s41401-021-00700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X., Chen C., Han D., Zhou W., Cui Y., Tang X., et al. SLC7A11/GPX4 inactivation-mediated ferroptosis contributes to the pathogenesis of triptolide-induced cardiotoxicity. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/3192607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X.Y., Wei D.G., Li R.S. Capsaicin induces ferroptosis of NSCLC by regulating SLC7A11/GPX4 signaling in vitro. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-16372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan R., Xie E., Li Y., Li J., Zhang Y., Chi X., et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022;32:687–690. doi: 10.1038/s41422-022-00642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q., Hu B., Yang C., Zhao L., Wu J., Qi N. ATF3 promotes arsenic-induced apoptosis and oppositely regulates DR5 and bcl-xL expression in human bronchial epithelial cells. Int. J. Mol. Sci. 2021;22:4223. doi: 10.3390/ijms22084223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Fu L., Zhang S., Zhang J., Zhao Y., Zheng Y., et al. Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chem. Sci. 2017;8:2687–2701. doi: 10.1039/c6sc05368h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba Y., Hashiuchi E., Watanabe H., Kimura K., Oshima Y., Tsuchiya K., et al. The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice. Nat. Commun. 2023;14:167. doi: 10.1038/s41467-023-35804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Liu Y., Du T., Yang H., Lei L., Guo M., et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 2020;27:662–675. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao C.J., Zhou H.L., Gao X.Z., Xu C.F. Downregulation of miR-221-3p promotes the ferroptosis in gastric cancer cells via upregulation of ATF3 to mediate the transcription inhibition of GPX4 and HRD1. Transl. Oncol. 2023;32 doi: 10.1016/j.tranon.2023.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C.W., Wong L.L., Tse E.Y., Liu H.F., Leong V.Y., Lee J.M., et al. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012;72:4394–4404. doi: 10.1158/0008-5472.CAN-12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajamohan S.B., Pillai V.B., Gupta M., Sundaresan N.R., Birukov K.G., Samant S., et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol. Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B., Li B., Si T. Knockdown of circ0082374 inhibits cell viability, migration, invasion and glycolysis in glioma cells by miR-326/SIRT1. Brain Res. 2020;1748 doi: 10.1016/j.brainres.2020.147108. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Lin X., Yi X., Liu X., Yu R., Fan W., et al. SIRT1-mediated p53 deacetylation inhibits ferroptosis and alleviates heat stress-induced lung epithelial cells injury. Int. J. Hyperther. 2022;39:977–986. doi: 10.1080/02656736.2022.2094476. [DOI] [PubMed] [Google Scholar]
- 17.Ma S., Sun L., Wu W., Wu J., Sun Z., Ren J. USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.551318. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Li D., Liu X., Pi W., Zhang Y., Yu L., Xu C., et al. Fisetin attenuates doxorubicin-induced cardiomyopathy in vivo and in vitro by inhibiting ferroptosis through SIRT1/nrf2 signaling pathway activation. Front. Pharmacol. 2022;12 doi: 10.3389/fphar.2021.808480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q., Liu Y., Li Y., Hong Z., Li S., Liu C. PUM2 aggravates the neuroinflammation and brain damage induced by ischemia-reperfusion through the SLC7A11-dependent inhibition of ferroptosis via suppressing the SIRT1. Mol. Cell. Biochem. 2023;478:609–620. doi: 10.1007/s11010-022-04534-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C., Wu Z., Xue H., Gao Q., Zhang Y., Wang C., et al. Ferroptosis contributes to hypoxic-ischemic brain injury in neonatal rats: role of the SIRT1/Nrf2/GPx4 signaling pathway. CNS Neurosci. Ther. 2022;28:2268–2280. doi: 10.1111/cns.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covarrubias A.J., Perrone R., Grozio A., Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z., Bian Y., Zhang Y., Ren G., Li G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19:1089–1104. doi: 10.1080/15384101.2020.1743911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H., Ding H., Tang X., Liang M., Li S., Zhang J., et al. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer. 2021;12:1415–1422. doi: 10.1111/1759-7714.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang S.P., Wang X.Z., Piao M.H., Chen X., Wang Z.C., Li C., et al. Activated SIRT1 contributes to DPT-induced glioma cell parthanatos by upregulation of NOX2 and NAT10. Acta Pharmacol. Sin. 2023;44:2125–2138. doi: 10.1038/s41401-023-01109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X.Z., Liang S.P., Chen X., Wang Z.C., Li C., Feng C.S., et al. TAX1BP1 contributes to deoxypodophyllotoxin-induced glioma cell parthanatos via inducing nuclear translocation of AIF by activation of mitochondrial respiratory chain complex I. Acta Pharmacol. Sin. 2023;44:1906–1919. doi: 10.1038/s41401-023-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X., Lu S., He C., Wang C., Wang L., Piao M., et al. RSL3 induced autophagic death in glioma cells via causing glycolysis dysfunction. Biochem. Biophys. Res. Commun. 2019;518:590–597. doi: 10.1016/j.bbrc.2019.08.096. [DOI] [PubMed] [Google Scholar]
- 27.Sui X., Zhang R., Liu S., Duan T., Zhai L., Zhang M., et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front. Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang Y., Zhu R., Li S., Qin K.P., Tang H., Shan W.S., et al. Erythropoietin inhibits ferroptosis and ameliorates neurological function after spinal cord injury. Neural Regen Res. 2023;18:881–888. doi: 10.4103/1673-5374.353496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Yu C., Kang R., Tang D. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooko E., Saeed M.E., Kadioglu O., Sarvi S., Colak M., Elmasaoudi K., et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22:1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D., Lu X., Xu G., Liu S., Gong Z., Lu F., et al. Dihydroorotate dehydrogenase regulates ferroptosis in neurons after spinal cord injury via the P53-ALOX15 signaling pathway. CNS Neurosci. Ther. 2023;29:1923–1939. doi: 10.1111/cns.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q., Xu Y., Yuan F., Qi Y., Wang Y., Chen Q., et al. Rho family GTPase 1 (RND1), a novel regulator of p53, enhances ferroptosis in glioblastoma. Cell Biosci. 2022;12:53. doi: 10.1186/s13578-022-00791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H., Mo H., Yang C., Mei X., Song X., Lu W., et al. A novel function of ATF3 in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion. Free Radic. Biol. Med. 2022;189:122–135. doi: 10.1016/j.freeradbiomed.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Jin Z.L., Gao W.Y., Guo F., Liao S.J., Hu M.Z., Yu T., et al. Ring finger protein 146-mediated long-chain fatty-acid-coenzyme a ligase 4 ubiquitination regulates ferroptosis-induced neuronal damage in ischemic stroke. Neuroscience. 2023;529:148–161. doi: 10.1016/j.neuroscience.2023.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Lan T., Wang W., Zeng X.X., Tong Y.H., Mao Z.J., Wang S.W. Saikosaponin A triggers cell ferroptosis in hepatocellular carcinoma by inducing endoplasmic reticulum stress-stimulated ATF3 expression. Biochem. Biophys. Res. Commun. 2023;674:10–18. doi: 10.1016/j.bbrc.2023.06.086. [DOI] [PubMed] [Google Scholar]
- 37.Qian X., Zhu L., Xu M., Liu H., Yu X., Shao Q., et al. Shikonin suppresses small cell lung cancer growth via inducing ATF3-mediated ferroptosis to promote ROS accumulation. Chem. Biol. Interact. 2023;382 doi: 10.1016/j.cbi.2023.110588. [DOI] [PubMed] [Google Scholar]
- 38.Liang Y., Jiang Y., Jin X., Chen P., Heng Y., Cai L., et al. Neddylation inhibition activates the protective autophagy through NF-κB-catalase-ATF3 Axis in human esophageal cancer cells. Cell Commun. Signal. 2020;18:72. doi: 10.1186/s12964-020-00576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park E., Chung S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye T., Wei L., Shi J., Jiang K., Xu H., Hu L., et al. Sirtuin1 activator SRT2183 suppresses glioma cell growth involving activation of endoplasmic reticulum stress pathway. BMC Cancer. 2019;19:706. doi: 10.1186/s12885-019-5852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu S.C., Huang S.M., Chen A., Sun C.Y., Lin S.H., Chen J.S., et al. Resveratrol increases anti-aging Klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int. J. Biochem. Cell Biol. 2014;53:361–371. doi: 10.1016/j.biocel.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Li Z.Y., Liu Y., Wang Y.Y., Li X., Han Z.N., Hong L., et al. NOX4 stimulates ANF secretion via activation of the Sirt1/Nrf2/ATF3/4 axis in hypoxic beating rat atria. Mol. Med. Rep. 2022;25:84. doi: 10.3892/mmr.2022.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W., Qian S., Tang B., Kang P., Zhang H., Shi C. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J. Cell Mol. Med. 2023;27:3075–3089. doi: 10.1111/jcmm.17874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navas L.E., Carnero A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct. Targeted Ther. 2021;6:2. doi: 10.1038/s41392-020-00354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cea M., Cagnetta A., Patrone F., Nencioni A., Gobbi M., Anderson K.C. Intracellular NAD(+) depletion induces autophagic death in multiple myeloma cells. Autophagy. 2013;9:410–412. doi: 10.4161/auto.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolandghamat Pour Z., Nourbakhsh M., Mousavizadeh K., Madjd Z., Ghorbanhosseini S.S., Abdolvahabi Z., et al. Up-regulation of miR-381 inhibits NAD+ salvage pathway and promotes apoptosis in breast cancer cells. EXCLI J. 2019;18:683–696. doi: 10.17179/excli2019-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiore A., Zeitler L., Russier M., Groß A., Hiller M.K., Parker J.L., et al. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol. Cell. 2022;82:920–932. doi: 10.1016/j.molcel.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y., Yi M., Wang W., Liu X., Wang Q., Liu C., et al. Oxidative degradation of dihydrofolate reductase increases CD38-mediated ferroptosis susceptibility. Cell Death Dis. 2022;13:944. doi: 10.1038/s41419-022-05383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han X., Tai H., Wang X., Wang Z., Zhou J., Wei X., et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell. 2016;15:416–427. doi: 10.1111/acel.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majeed Y., Halabi N., Madani A.Y., Engelke R., Bhagwat A.M., Abdesselem H., et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021;11:8177. doi: 10.1038/s41598-021-87759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raynes R., Pombier K.M., Nguyen K., Brunquell J., Mendez J.E., Westerheide S.D. The SIRT1 modulators AROS and DBC1 regulate HSF1 activity and the heat shock response. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Song D., Chen Y., Huang C., Liu A., Wu Q., et al. NSD2 methylates AROS to promote SIRT1 activation and regulates fatty acid metabolism-mediated cancer radiotherapy. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.113126. [DOI] [PubMed] [Google Scholar]
- 53.Conrad E., Polonio-Vallon T., Meister M., Matt S., Bitomsky N., Herbel C., et al. HIPK2 restricts SIRT1 activity upon severe DNA damage by a phosphorylation-controlled mechanism. Cell Death Differ. 2016;23:110–122. doi: 10.1038/cdd.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau A.W., Liu P., Inuzuka H., Gao D. SIRT1 phosphorylation by AMP-activated protein kinase regulates p53 acetylation. Am. J. Cancer Res. 2014;4:245–255. [PMC free article] [PubMed] [Google Scholar]
- 55.Peng W., Ouyang Y., Wang S., Hou J., Zhu Z., Yang Y., et al. L-F001, a multifunctional fasudil-lipoic acid dimer prevents RSL3-induced ferroptosis via maintaining iron homeostasis and inhibiting JNK in HT22 cells. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.774297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.