Abstract
The ctxAB operon, which encodes cholera toxin, resides in the genome of CTXφ, a filamentous bacteriophage. Within Vibrio cholerae cells, the CTXφ genome can exist either as a replicating plasmid or as a prophage integrated into the chromosome. Previous work established that ToxR is required for chromosomal ctxAB expression. We have learned that strains harboring the CTXφ replicative form produce cholera toxin under all conditions tested, independently of ToxR. During passage of CTXφ lysogens through the infant mouse intestine, transduction of CTXφ to a recipient strain can be detected, indicating that phage excision and replication occur in vivo. These results suggest that phage induction might provide a novel mechanism for the regulation of cholera toxin production.
Vibrio cholerae secretes cholera toxin (CT), an A-B type toxin, into the host small intestine to cause diarrhea (17). The A and B subunits of CT are encoded by the ctxAB operon (11), which along with 6 kb of flanking chromosomal DNA was originally termed the CTX genetic element (15, 16). Recent studies have demonstrated that the CTX element corresponds to the genome of CTXφ, a lysogenic filamentous bacteriophage (Fig. 1) (20, 22).
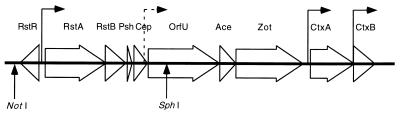
FIG. 1.
Map of the CTXφ genome. The locations of known promoters are shown with solid lines, and the postulated promoter upstream of orfU is designated with a broken line. The NotI site within the intergenic region of the phage genome where the kanamycin cassette was inserted to generate pIG is shown. The orientation of the Kmr gene is the same as that of rstR. The SphI site mutated to create pSL1 is also marked.
Studies of the environmental and genetic factors that govern the production of CT by V. cholerae suggest that the expression of CT is coordinately regulated with the expression of many of the other V. cholerae virulence factors (18). Environmental signals are believed to activate transcriptional regulators ToxR and ToxT to activate the transcription of ctxAB as well as that of other genes in the ToxR regulon (18). Both ToxR and ToxT have been shown to be required for the transcription of ctxAB when CTXφ resides on the V. cholerae chromosome as a prophage (2). Although both ToxR and ToxT are required for expression of CT in both the V. cholerae O1 classical and El Tor biotypes, the in vitro growth conditions that lead to the production of CT by the two biotypes are considerably different (9, 12). The ToxR-regulated toxin-coregulated pilus (TCP) (19) is the receptor for CTXφ (20). TCP is not efficiently expressed by El Tor strains in vitro, and therefore El Tor strains cannot be efficiently infected by CTXφ in vitro.
CTXφ was discovered by finding that the El Tor chromosomal CTX element could be transferred to classical strains, where it was recoverable as a plasmid (20). This plasmid form of the CTX element corresponds to the replicative form (RF) of CTXφ. Cells harboring the RF produce high titers of CTXφ virions in their supernatants. CTXφ integrates at a particular site, designated attRS, on the El Tor chromosome (22). Following infection of classical strains or electroporation of El Tor strains lacking CTXφ and attRS, CTXφ does not integrate into the chromosome but replicates extrachromosomally as a plasmid (22).
The discovery of CTXφ suggested the possibility that CT production might differ depending on the replicative state of the phage. To address this question, CT production by cells in which the phage exists as a stable lysogen was compared with that by cells in which it exists solely as a plasmid in the RF. Furthermore, we designed experiments to detect the induction of CTXφ from lysogens within the mammalian intestine. These experiments demonstrate that the expression of CT differs dramatically depending on the form of the phage and that phage induction can occur within the host intestine.
The wild-type CTXφ RF produces CT.
The CTXφ derivative originally isolated contained a kanamycin resistance gene in place of ctxAB (20). Thus, it was impossible to use this phage derivative to determine whether CT is produced by the RF of CTXφ. We therefore isolated wild-type CTXφ (ctxAB+) from El Tor clinical isolate E7946, a CTXφ lysogen that contains two tandemly arranged copies of CTXφ on its chromosome (10). Filtered supernatants from UV-irradiated E7946 were mixed with cells of classical recipient strain O395 (10). The wild-type phage was then recovered as a plasmid (RF) in O395. The identity of the wild-type phage was confirmed by restriction digestion and Southern analyses (data not shown).
We tested whether the RF of the wild-type phage (pWT) produces CT in a classical V. cholerae strain. The wild-type phage was transduced into O395-NT, a classical strain with ctxAB deleted (11), where it is recoverable as a plasmid (data not shown). The amount of CT present in supernatants of O395-NT (pWT) was measured by a Gm1 enzyme-linked immunosorbent assay (ELISA) (8) after growth under conditions that are optimal for CT and TCP expression in vitro (30°C, pH 6.5, in Luria broth [LB]) (Table 1). Strain O395-NT (pWT) produced amounts of CT similar to those produced by O395, indicating that the RF is capable of producing CT.
TABLE 1.
CT produced by pWT
| Strain [relevant phenotype] | CT productiona at pH:
|
|
|---|---|---|
| 6.5 | 8.5 | |
| O395 [wild type] | 7.7 | 0.03 |
| O395-NT [CT−] | <0.01 | <0.01 |
| O395-NT (pWT) | 8.2 | 0.2 |
Cells were incubated in LB medium at 30°C. Toxin amounts are expressed in micrograms per unit of optical density at 600 nm. Values represent the averages of at least three independent experiments.
To determine whether the RF could produce toxin under conditions that inhibit chromosomal CT production, O395-NT (pWT) was also grown in LB at pH 8.5, a condition nonpermissive for CT and TCP expression from classical strains (12). At pH 8.5 the amounts of CT produced by both O395 and O395-NT (pWT) were significantly less than the amounts produced at pH 6.5 (Table 1). However, the amount produced by the strain harboring the RF was approximately eightfold greater at pH 8.5 than the amount produced by a lysogenic strain. Thus, pWT produces a small amount of CT under conditions that are nonpermissive for chromosomal CT production. Since TCP is made by classical strains at pH 6.5, CTXφ virions produced from the RF in O395-NT (pWT) can continuously reinfect cells, and this leads to stable maintenance of the RF in the population. However, no RF is detectable on ethidium-stained agarose gels of plasmid preparations from O395-NT (pWT) grown under conditions that do not lead to TCP expression (e.g., pH 8.5), indicating that the maintenance of pWT is unstable during overnight incubation in the absence of TCP (data not shown). We hypothesize that this is the primary reason for the large difference in CT production detected in overnight cultures of O395-NT (pWT) at pH 6.5 and 8.5.
Regulation of CT in El Tor strains.
To facilitate transmission and maintenance of the ctxAB+ phage, a kanamycin resistance gene was inserted into the NotI site of an intergenic region of pWT (Fig. 1). This construct was designated pIG (for intergenic). This construct can be introduced into El Tor strains, which are not efficiently transduced in vitro, by electroporation and can be maintained in all cells under a variety of growth conditions by continuous selection for Kanamycin resistance.
We compared CT production by El Tor strains harboring the lysogenic and replicative forms of the phage. Strain Bah-2 is a derivative of E7946 which lacks both CTXφ and attRS (16). In Bah-2 (pIG) the phage does not integrate and exists solely in the RF. After growth of the strains in LB, CT was not detectable in supernatants from lysogen E7946 but was measurable in those from Bah-2 (pIG) (Table 2). This indicates that in El Tor strains CT can be produced by the RF and that the regulation of CT expression depends upon the state of the phage.
TABLE 2.
CT produced by pIG in El Tor strains
| Strain [relevant genotype] | CT productiona in:
|
|
|---|---|---|
| LB | AKI medium | |
| E7946 [wild-type] | <0.01 | 2.3 |
| Bah-2 (pIG) [RF] | 0.7 | 0.8 |
| Bah-2 [CTXφ−] | <0.01 | <0.01 |
| Bah-2-55 (pIG) [toxR]b | 0.8 | 0.8 |
| E7946-55 [toxR]b | <0.01 | <0.01 |
| Bah-2 (pSL1) [ΔSphI]c | 0.01 | 0.08 |
Cells were incubated in the medium indicated (supplemented with kanamycin for strains harboring pIG or pSL1) at 37°C. Toxin amounts are expressed in micrograms per unit of optical density at 600 nm. Values represent the averages of at least three independent experiments.
[toxR], inactivated toxR gene.
pSL1 is a derivative of pIG which contains a deletion of the SphI site in orfU.
In El Tor strains the genes of the ToxR regulon are not expressed in LB but are expressed in AKI medium (9). The production of CT by the RF in Bah-2 (pIG) in LB was therefore surprising. We next compared the levels of CT produced in AKI medium. The amounts of CT produced by the RF in both LB and AKI medium are similar (Table 2). This result suggests that ctxAB residing on the RF can be expressed independently of ToxR. To confirm this, toxR was insertionally inactivated in Bah-2 (pIG) and E7946 with pVM55 (12), resulting in strains Bah-2-55 (pIG) and E7946-55. CT production was unchanged in Bah-2-55 (pIG) but was almost completely abolished in E7946-55 after growth in AKI medium (Table 2). This confirms that, while ctxAB resides on the RF, CT expression is largely independent of ToxR in El Tor strains.
To begin to address the possibility that the ToxR-ToxT-independent expression of CT is due to read-through from a promoter upstream of the known ctxAB promoter, a polar frameshift mutation was introduced into an open reading frame upstream of ctxA on pIG (Fig. 1). This was accomplished by digesting pIG with SphI, blunting the resultant ends with T4 polymerase, and then religating the plasmid. The resulting plasmid, pSL1, replicates similarly to pIG in Bah-2 (data not shown). CT production in Bah-2 (pSL1) was reduced 10-fold compared to that in Bah-2 (pIG) in AKI medium, suggesting that ToxR-independent CT production may result from read-through from an upstream promoter (Table 2). In LB, Bah-2 (pSL1) produced 80-fold-less CT than Bah-2 (pIG). Presumably, in LB only the ToxR-ToxT-independent promoter is active on the RF, accounting for the greater decrease in CT expression from Bah-2 (pSL1) after growth in LB. One alternative but less likely explanation of the reduction in CT production by pSL1 is that OrfU, Ace, or Zot may be required for ToxR-ToxT-independent CT expression.
Regulation of CT in classical strains.
Unlike its expression in El Tor strains, the expression of the ToxR regulon in classical strains is easily manipulated in rich medium by changing incubation conditions. In LB CT expression is maximal after growth at a starting pH of 6.5 and 30°C (12). Changing the initial pH to 8.5 decreases CT levels dramatically. To determine whether these environmental conditions can affect expression of CT by the RF, pIG was introduced into O395, a classical strain of V. cholerae. CT levels in O395 with and without pIG were measured under conditions that are known to alter chromosomal CT expression (Table 3).
TABLE 3.
CT produced from pIG in classical strains
| Strain [relevant genotype] | CT productiona at pH:
|
|
|---|---|---|
| 6.5 | 8.5 | |
| O395 [wild type] | 7.1 | 0.03 |
| O395 (pIG) | 7.8 | 0.8 |
| JJM43 [O395 ΔtoxR] | <0.01 | <0.01 |
| JJM43 (pIG) | 0.5 | 0.8 |
| VJ740 [O395 ΔtoxT] | <0.01 | <0.01 |
| VJ740 (pIG) | 0.3 | 1.5 |
Cells were incubated in LB medium at 30°C at a starting pH of either 6.5 or 8.5. Toxin amounts are expressed in micrograms per unit of optical density at 600 nm. Values represent the averages of at least three independent experiments.
Under CT-permissive conditions (pH 6.5), there is slightly more toxin produced by strains containing the RF than by strains lacking the RF. Since CT is produced by the chromosomal ctxAB operon under these conditions, it is difficult to determine the exact contribution of the RF to the total amount of CT produced in O395 (pIG), where CT is produced both by the chromosomal ctxAB and pIG. After growth of O395 under nonpermissive conditions (pH 8.5), expression by the chromosomal copies of ctxAB is almost completely abolished, as expected. Although production of CT also decreased in O395 (pIG), the amount of CT produced was over 10-fold greater than that produced by strains lacking the RF (Table 3). This indicates that the environmental conditions that ordinarily regulate CT production from the chromosome do not completely regulate CT production from the RF. The difference in toxin levels produced by O395 (pIG) after growth at pH 6.5 or 8.5 was not due to a change in the amount of RF DNA present within the cells (data not shown).
The altered expression of the ToxR regulon in response to changes in pH is dependent on ToxR and ToxT (18). To further investigate the effects of the corresponding genes on CT expression by the RF, the RF was introduced into toxR or toxT mutant derivatives of O395 (JJM43 [7] and VJ740 [2], respectively) (Table 3). As expected, neither JJM43 nor VJ740 produced detectable levels of CT. However, both JJM43 (pIG) and VJ740 (pIG) produced low but detectable levels of CT. The amount of CT produced by either VJ740 (pIG) or JJM43 (pIG) after growth at pH 6.5 or 8.5 is considerably less than the amount of CT produced by O395-NT (pWT) after growth at pH 6.5. This suggests that in classical strains, although the ToxR-ToxT-regulated promoter in the RF still responds to these activators, some CT can be made independently of ToxR and ToxT by the RF.
Recently it was reported that ctxB has its own promoter and that this newly defined promoter appears to be ToxR independent (4). As the antiserum used to detect CT in the ELISA recognizes predominantly the B subunit of CT (CT-B), it was possible that only CT-B was being produced by the RF in our studies. To test this possibility, antiserum which specifically recognizes the A subunit of CT was used in the ELISA to measure CT expression. CT-A produced by the RF was detectable whether ToxR was present or not, indicating that it is not only the ctxB promoter that is active in the absence of ToxR (data not shown).
Lysogens produce infectious CTXφ within the intestine.
To determine whether phage induction from a lysogen can occur in the host small intestine, we measured the transfer of a kanamycin resistance gene-marked CTXφ from El Tor lysogen SM115 (5) into a kanamycin-sensitive recipient strain in vivo. SM115, which carries one copy of the kanamycin resistance gene-marked CTXφ on its chromosome, and classical recipient strain AC-V87 (Lac− Tcr Kms) (1) were mixed together and then inoculated into the stomachs of suckling CD1 mice (Charles River) (21). No transducing phage were detectable in the supernatant of the initial overnight culture of donor strain SM115. After 20 h of intraintestinal incubation, the percentage of AC-V87 recipients that were transduced in the small intestine was determined. Ten percent of the total potential recipient AC-V87 cells were transduced to kanamycin resistance, indicating that CTX-Kmφ virions had been induced from the SM115 lysogen donor strain while the cells were in the mouse intestine. Thus, CTXφ RF intermediates are present in donor strain SM115 during intestinal infection. This suggests that induction of the CTXφ RF may serve as an alternate means to regulate production of CT in the host.
We have investigated the regulation of CT production from CTXφ. Although CT production is completely dependent on ToxR and ToxT when CTXφ is integrated in the chromosome, production of CT appears to be largely independent of these regulators when CTXφ is present as a plasmid. This suggests that phage induction and replication might provide a novel mechanism for the regulation of CT expression during human infection.
More than one mechanism may account for the ToxR-ToxT-independent production of CT from the CTXφ RF. Our finding that the polar mutation in pSL1, which lies 2.5 kb upstream of the ToxR-ToxT-regulated ctxAB promoter, significantly reduces the production of CT by the CTXφ RF suggests that there is a second promoter that only activates ctxAB expression in the RF. A promoter regulating rstA has been identified, and it is possible that this is also the promoter that transcribes the core genes of the phage (Fig. 1) (22). Alternatively, sequence analysis suggests that a promoter exists just upstream of orfU. A homologous promoter in M13 has also been described (13). These promoters may also transcribe ctxAB in the replicative form of the phage. In the lysogenic state the rstA promoter is repressed by RstR (9a), which may account for the absence of ToxR-ToxT-independent CT expression in lysogens. Upon the excision and circularization of CTXφ, changes in DNA topology might prevent RstR repression of the upstream ToxT-ToxR-independent promoter, thereby allowing CT production. Alternatively, upon circularization of the phage the promoter just upstream of ctxB (4) might be able to read through the entire phage to produce both subunits of CT.
We have demonstrated CTXφ induction within the host intestine. This finding may be relevant to the intraintestinal production of CT in several ways. First, prophage induction and RF replication may constitute a mechanism for the amplification of CT production in the intestine. This increased production of CT by phage excision and replication could be mediated both by increased gene dosage of ctxAB and by altered regulatory requirements for ctxAB expression by the RF. Second, since CT production by the RF appears to be largely independent of ToxR and ToxT in El Tor strains, intestinal CTXφ induction may be a mechanism to facilitate CT production by V. cholerae strains residing in intestinal microenvironments that are not conducive for activation of the ToxR regulon. The recent finding that bile inhibits activation of the ToxR regulon suggests that such a microenvironment might exist (6).
Several pathogens are known to be lysogenized with phages carrying toxin genes (3). The potential for these phages to be induced within the mammalian host has not yet been explored. However, it has been shown that in vitro induction of the 933W phage, which encodes Shiga toxin 2 (Stx 2) in Escherichia coli strains, results in significant amplification of Stx 2 production (14). It is possible that the host environment commonly leads to converting phage induction. If so, prophage induction may constitute a general mechanism of virulence gene regulation.
Acknowledgments
We thank M. Russel, H. Kimsey, S. Finkel, D. Acheson, and A. Camilli for critical reading of the manuscript. We thank J. J. Mekalanos for his advice and for the gift of primary antisera against CT and CT-A and V. J. DiRita for strain VJ740.
This work was supported by NIAID grant AIO1321, the Tupper Research Fund, the NEMC Young Investigator Award, and the NEMC GRASP Digestive Disease Center (NIDDK, P30 DK34928). S.L. was supported by a NRSA (AIO7329).
REFERENCES
- 1.Camilli A, Mekalanos J J. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol Microbiol. 1995;18:671–683. doi: 10.1111/j.1365-2958.1995.mmi_18040671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheetham B F, Katz M E. A role for bacteriophages in the evolution and transfer of bacterial virulence determinants. Mol Microbiol. 1995;18:201–208. doi: 10.1111/j.1365-2958.1995.mmi_18020201.x. [DOI] [PubMed] [Google Scholar]
- 4.Fando R, Pérez J L, Rodriguez B L, Campos J, Robert A, García L, Silva A, Benitez J A. Promoter activities in Vibrio cholerae ctxΦ prophage. Infect Immun. 1997;65:1561–1565. doi: 10.1128/iai.65.4.1561-1565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg I, Mekalanos J J. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986;165:723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973;8:851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 9a.Kimsey, H. Unpublished data.
- 10.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 11.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, deWilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 12.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Model P, Russel M. Filamentous bacteriophage. In: Calendar R, editor. The bacteriophages. Vol. 2. London, United Kingdom: Plenum Press; 1988. pp. 375–456. [Google Scholar]
- 14.Mühldorfer I, Hacker J, Keusch G T, Acheson D W, Tschäpe H, Kane A V, Ritter A, Ölschläger T, Donohue-Rolfe A. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect Immun. 1996;64:495–502. doi: 10.1128/iai.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson G D N. Ph.D. thesis. Cambridge, Mass: Harvard University; 1989. [Google Scholar]
- 16.Pearson G D N, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sears C L, Kaper J L. Enteric bacterial toxins: mechanism of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 19.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 21.Waldor M K, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldor M K, Rubin E J, Pearson G D N, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]