Abstract
Since reservoirs perform many important functions, they are exposed to various types of unfavorable phenomena, e.g., eutrophication which leads to a rapid growth of algae (blooms) that degrade water quality. One of the solutions to combat phytoplankton blooms are effective microorganisms (EM). The study aims to evaluate the potential of EM in improving the water quality of the Turawa reservoir on the Mała Panew River in Poland. It is one of the first studies providing insights into the effectiveness of using EM in the bioremediation of water in a eutrophic reservoir. Samples for the study were collected in 2019–2021. The analysis showed that EM could be one of the most effective methods for cleaning water from unfavorable microorganisms (HBN22, HBN36, CBN, FCBN, FEN) — after the application of EM, a reduction in their concentration was observed (from 46.44 to 58.38% on average). The duration of their effect ranged from 17.6 to 34.1 days. The application of EM improved the trophic status of the Turawa reservoir, expressed by the Carlson index, by 7.78%. As shown in the literature review, the use of other methods of water purification (e.g., constructed wetlands, floating beds, or intermittent aeration) leads to an increase in the effectiveness and a prolongation of the duration of the EM action. The findings of the study might serve as a guide for the restoration of eutrophic reservoirs by supporting sustainable management of water resources. Nevertheless, further research should be conducted on the effectiveness of EM and their application in the remediation of eutrophic water reservoirs.
Keywords: Effective microorganisms, Eutrophication, Biological water treatment, Water quality, Reservoir restoration
Introduction
Water, as a resource necessary for the functioning of the environment, economy, and society (Cosgrove and Loucks 2015; Hapich et al. 2022; Wiatkowski et al. 2010), is exposed to various types of pollutants that degrade its quality (Arenas-Sánchez et al. 2016; Lin et al. 2020; Myronidis et al. 2018), such as nutrients (Lu and Tian 2017; Schoumans et al. 2014), heavy metals (Tomczyk et al. 2022; Vardhan et al. 2019), specific pollutants (Kanzari et al. 2012; Rivoira et al. 2022), and microbiological contamination (Páll et al. 2013; Paruch et al. 2019). According to the analysis of Zhang et al. (2022a), the main sources of water pollution at the global level are industrial wastewater (35.7%), rural wastewater (25.1%), municipal wastewater (18.7%), algal blooms, and agricultural activities (15.1%).
Water accumulates in various forms, but only 1% is suitable for direct human use (Oki and Kanae 2006; Khatri and Tyagi 2015). Therefore, an important issue is the purification of water from pollutants of all kinds to ensure its suitable properties for various uses (Koundouri et al. 2016; Price and Heberling 2018). Reservoirs are particularly important because they are often multifunctional and, at the same time, are a subject to various types of pollution that prevent or limit the fulfillment of their intended functions (Suwal et al. 2020; Wiatkowski et al. 2021). One of the unfavorable phenomena resulting from pollution is eutrophication caused by the influx of nutrients. It leads to a rapid growth of algae that degrade water quality (Khan and Mohammad 2014), for example, in terms of physicochemical parameters (a reduction in dissolved oxygen content) (Aguiar et al. 2011) and microbiological parameters (an increase in the number of anaerobic organisms) (Smolders et al. 2007).
Chemical, physical, and biological water treatment methods limit or prevent phytoplankton blooms in water bodies (Anawar and Chowdhury 2020; Paul et al. 2021; Lürling and Mucci 2020; Wang et al. 2019; Yin et al. 2019; Bormans et al. 2016; Jilbert et al. 2020; Bartoszek et al. 2015; Zhang et al. 2016; Ting et al. 2018).
Effective microorganisms (EM) are an example of biological (bioremediation) methods used to restore the appropriate ecological balance of the ecosystem and the ability of water to self-purify (Ateia 2016; Escudero-López et al. 2022; Wdowczyk and Szymańska-Pulikowska 2023). EM use mixtures of bacteria, actinomycetes, yeasts, and fungi with a synergistic effect to inhibit harmful bacteria by excluding competition to achieve a dominance of the effective species (Olle and Williams 2015). The term was introduced in Japan in the 1990s. EM has been studied for soil and foliar application in horticulture as an alternative to more sustainable organic agriculture that does not require fertilizers or pesticides (Talaat et al. 2015). The main advantages of using EM are their cost-effectiveness and environmental friendliness (Sharip et al. 2020). Depending on the application, EM have different forms such as loess balls (Ekpeghere et al. 2012), soil balls (Park et al. 2016), mud balls (Nugroho et al. 2017), or a liquid solution (Tommonaro et al. 2021).
Currently, EM are used not only for the treatment of eutrophic waters but also for sludge treatment, wastewater treatment, composting, medicine, livestock, forestry, and agriculture (Safwat and Matta 2021; Kour et al. 2021). Thanks to their use, it is possible, for example, to reduce sludge volume and shorten aeration time (Grabas et al. 2016; Safwat 2018), as well as to reduce odor emissions from waste and support its humification (Fan et al. 2018). Studies also found reduced nutrients and organic matter content when treating dairy wastewater (Boruszko 2019). In addition, the application of EM contributed to the improvement of vegetable production in agriculture (Olle and Williams 2015).
To date, EM have been studied in bottom sediments, in surface water of reservoirs and rivers, or in industrial waters, concerning the effects on the oxygen properties, nutrients, physical parameters, and specific pollutants of these matrices (Safwat and Matta 2021). For instance, the following phenomena were recorded after the application of EM: (i) the degradation of about 80% of microcystins in the eutrophic lakes Tsukui and Sagami in Japan (Tsuji et al. 2006); (ii) the reduction of the concentration of nitrogen and phosphorus compounds causing eutrophication in reservoirs in Hungary and India (Kamath 2007) and Poland (Jóźwiakowski et al. 2009); and (iii) the reduction of the biochemical oxygen demand (BOD), chemical oxygen demand (COD), and nitrate nitrogen (–N) in river waters (Sharip et al. 2020). On the other hand, some studies have not demonstrated significant effectiveness of the EM technology in reducing heavy metal concentrations (Ting et al. 2013), increasing the oxygenation of water bodies (Dunalska et al. 2015), or reducing the turbidity in reservoirs (Lürling et al. 2016). In this context, detailed analyses should be conducted on the extent and potential of using EM in surface water remediation projects (Mrozińska et al. 2018).
The objectives of the article are (i) an evaluation of the potential of EM to improve the water quality of the multifunctional Turawa reservoir in a separate part of the reservoir (i.e., microbiological and physicochemical parameters) located on the Mała Panew River in the Odra River basin (Southern Poland, Opole Voivodeship); (ii) an analysis of the compliance with the requirements for bathing purposes, ecological status, and trophic status of the water before and after the application of EM; and (iii) an assessment of the influence and similarity of the analyzed water parameters.
To the best of our knowledge, the study is one of a few case studies conducted in this field, providing insights into the effectiveness of using EM as a solution and Bokashi balls in the bioremediation of the multifunctional Turawa reservoir. The main challenge in the studied water body is the ongoing eutrophication process which deteriorates the reservoir water’s microbiological and physicochemical properties (Rajfur et al. 2011). To date, research on reservoir restoration has focused on the effectiveness of EM in terms of its effects on concentrations of nitrogen and phosphorus compounds. However, this manuscript focused primarily on the effects on microbiological parameters of reservoir water. On the other hand, an analysis of the relationship between the affected parameters can help develop more effective water purification strategies within specific parameter groups and develop recommendations consistent with rational water management and sustainable development goals in individual parts of large reservoirs. Pollution of reservoirs (e.g., eutrophication) is a global problem, so the issue of improving the quality of these facilities is the subject of worldwide research.
Nevertheless, in this part of Europe, there are no studies on the effectiveness of EM in improving the water quality of large reservoirs. An innovative element is the separation of a part of the reservoir used for pilot studies, which can serve different functions (e.g., recreation) for the local community. The proposed EM application method can be used as an element that supports the process of improving water quality in the reservoir.
The research on the effectiveness of EM in water treatment is a part of the project “An innovative method for improving water quality in multifunctional retention basins,” used as one of the methods for the reconstruction of the eutrophic Turawa reservoir, carried out as a part of a project awarded by the National Center for Research and Development in Poland under grant number BIOSTRATEG3/343733/15/NCBR/2018. This task was carried out in the period 01/06/2019–30/09/2021. The aim is to eliminate microbiological and chemical hazards in the water of Turawa Reservoir by using one of the innovative biological methods for lake and reservoir remediation. In connection with other tasks of the project, the following works were carried out: analyses of the microbiological parameters of the water, application of EM in the form of Bokashi balls and a liquid solution, installation of a floating dam separating part of the reservoir, and physicochemical analysis of the water.
Materials and methods
Field research
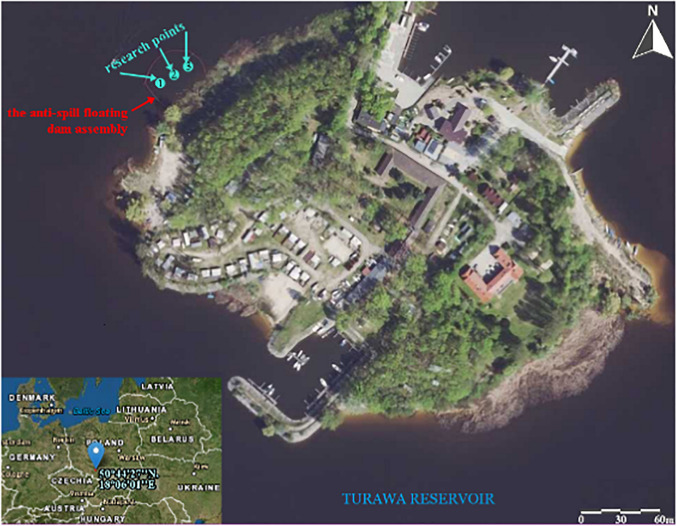
The study sites were located in the Turawa reservoir with an area of 20.80 km2 and a maximum capacity of 107.6 million m3, constructed in 1933–1939 on the Mała Panew River, a right tributary of the Odra River (southern part of Poland, Opole Voivodeship) (Gruss et al. 2021; Wiatkowski and Wiatkowska 2019). Samples were collected in 2019–2021, in three replicates, from a depth of 30 and 100 cm at three study sites in the FINBOOM-type floating dam constructed in the northern part of the reservoir with an area of about 750 m2 (Fig. 1) (Dobrzyński et al. 2022). The location of the floating dam was determined based on recommendations of the administrator of the Turawa reservoir (area excluded from navigation), and its construction made it possible to separate a part of the reservoir (no water exchange with the rest of the reservoir) where pilot studies on the effectiveness of using EM in the surface water treatment could be conducted.
Fig. 1.
Location of research points on the Turawa reservoir (Odra river basin, southern Poland).
Source: https://earthexplorer.usgs.gov/
In each year of the study, microbiological and physicochemical elements were determined in three series of measurements (2 July 2019, 16 September 2019, 20 November 2019, 28 July 2020, 16 September 2020, 23 November 2020, 9 August 2021, 16 August 2021, 23 August 2021).
The application of EM took place on 9 August 2021 in two forms: Bokashi balls (Fig. 2; Table 1) under the water and in the form of liquid solution on and under the water surface. The Bokashi balls were a combination of clay, bottom sediment with a microbiological preparation SD ProBio Original and the addition of wheat bran weighing 270 g. Then, they were thrown into the separated part of Turawa reservoir at a density of 2 pieces per 1 m2 of water surface. As for the liquid solution, it was a stock solution of ProBio Sanit with water in a 1:10 ratio, applied with a sprayer on the water surface at a depth of 0.5 m in the amount of 10 L of the preparation per 1 m2 of water (area = 750 m2). These biopreparations contained about 80 species of bacteria — actinomycetes, yeasts, and fungi (Lactobacillus, Bifidobacterium, Pediococcus, Lactococcus, Streptococcus, Rhodopseudomonas, Aspergillus, Mucor, and Streptomyces). Their numbers in both biopreparations ranged from 108 to 1010 colony-forming units (CFU) in 1 g (Dobrzyński et al. 2022).
Fig. 2.
Bokashi ball application procedure in the Turawa reservoir (M. Prycik)
Table 1.
Taxonomic composition of the preparation with effective microorganisms, used in an application with Bokashi balls and liquid solution
| Taxonomic group | Composition |
|---|---|
| Actinomycetes | Streptomyces and others |
| Aerobic fermenting fungi | Aspergillus, Mucor hiemalis, and others |
| Purple non-sulfur (photosynthetic) bacteria | Rhodopseudomonas palustris, Rhodopseudomonas sphaerorides |
| Lactic fermentation bacteria | Bifidobacterium animalis, Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus buchneri, Lactobacillus delbrueckii, Lactobacillus plantarum, Lactococcus diacetylactis, Lactococcus lactis, Streptococcus thermophilus, Bacillus subtilis var. natto |
| Yeast | Saccharomyces cerevisiae |
Other, not mentioned above; the number of individual microorganisms and the composition is a know-how secret (mother compositions of effective microorganisms developed by Matthew Wood)
Laboratory tests
Water samples were analyzed for six microbiological parameters, including:
• The number of heterotrophic bacteria (HBN22 and HBN36) determined by the serial dilution method at 22 °C and 36 °C according to ISO 6222:1999 (ISO, 1999);
• The number of coliform bacteria (CBN) and fecal coliform bacteria (FCBN) determined using the multiple tube fermentation technique and calculated as the most probable number in 100 mL of the water tested (Bachtiar 2002);
• Fecal enterococci count (FEN) determined by membrane filtration method according to ISO 7899–1 (ISO 2000);
• Salmonella spp. in 1 L of water determined using the membrane filtration method described in ISO 19250:2010 (ISO 2010).
The study also analyzed selected physicochemical parameters of water, including pH (potentiometric method), electrolytic conductivity (EC; conductometric method), total nitrogen (TN; spectrophotometric method), nitrate nitrogen (NO3–N; spectrophotometric method), phosphate phosphorus (PO4–P; spectrophotometric method), total phosphorus (TP; spectrophotometric method), dissolved oxygen (DO; electrochemical sensor), 5-day biochemical oxygen demand (BOD5; dilution method), and chemical oxygen demand (COD; permanganate method). Each water determination was repeated three times (Szymańska-Pulikowska et al. 2023; Puchlik et al. 2022; MIP 2021; MHP 2019).
Data analysis
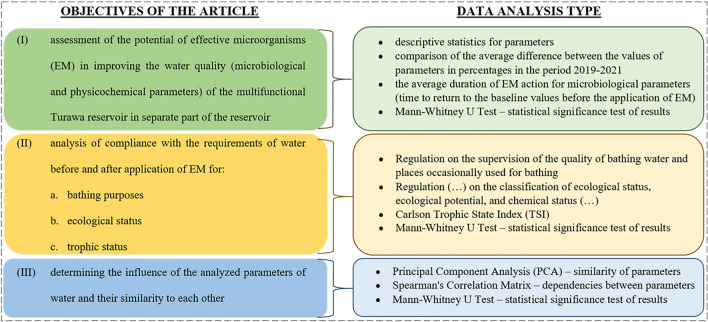
The article includes the following analysis of the results (statistical significance for p < 0.05) (Tomczyk et al. 2022; Wiatkowski et al. 2021; Tomczyk and Wiatkowski 2021; MIP 2021; MHP 2019; Ji et al. 2020):
• Descriptive statistics for microbiological and physicochemical parameters — a comparison of the results before and after application of EM (minimum, maximum, standard deviation, median, mean) — Objective I (see Fig. 3), “Descriptive statistics and statistical significance test of results” section;
• Mann–Whitney U test — for two groups of measurement series, i.e., before and after the application of EM (for the entire research period 2019–2021, as well as a comparison of the results for the series from 2021 — the first series immediately before the application, the second — 1 and 2 weeks after the application, i.e., on 9, 16, and 23 August); the test compares the medians for the selected groups and the data have a non-linear distribution — Objectives I, II, and III, “Descriptive statistics and statistical significance test of results,” “Comparison of the effectiveness of effective microorganisms before and after their application,” and “Correlations between microbiological and physicochemical parameters” sections;
• An evaluation of the effectiveness of EM on microbiological and physicochemical parameter values (a comparison of the average difference between parameter values in percent over the period 2019–2021, the average duration of the effect of EM for microbiological parameters — August 2021 — time to return to baseline values before application of EM as of 9 August 2021, assuming it is proportional to the rate of change between parameter values when comparing August 2021 series before and after application of EM) — Objective I, “Influence on microbiological parameters immediately before and after application” section;
• An assessment of compliance with microbiological requirements for bathing waters — based on the Regulation on monitoring the quality of bathing waters and places occasionally used for bathing (MHP 2019); microbiological parameters (CBN and FEN) are assessed on a 2-point scale, i.e., compliance — 1 point, non-compliance — 0 point; final rating is expressed on a scale from 0 to 1 and indicates the percentage of samples for which bathing water quality requirements were met (average 0.00–0.50 point — non-compliance, 0.51–1.00 — compliance); survey series for the period 2019–2021 and three series from 2021 were compared — Objective IIa, “Ecological status of physicochemical parameters and meeting the microbiological requirements of water for bathing purposes” section;
• A determination of the ecological status of physicochemical parameters — following the Regulation of the Minister of Infrastructure of 25 June 2021 on the classification of ecological status, ecological potential, and chemical status and on the method of classifying the status of surface water bodies and on environmental quality standards for priority substances (MIP 2021); the physicochemical elements are assessed on a 3-point scale, depending on the abiotic type of the water body (in this case, type 0, i.e., the stream of undetermined type — reservoir), and for each of the results from 2019–2021 they were weighted accordingly, i.e., Class I (very good physical–chemical condition) — 1 point, Class II (good condition) — 2 points, Class III (less than good condition) — 3 points, and then the average was calculated for the results from the entire study period; the final classification of the results is as follows: 1.00–1.66 points — 1st class, 1.67–2.33 points — class II, 2.34–3.00 points — 3rd class — Objective IIb, “Ecological status of physicochemical parameters and meeting the microbiological requirements of water for bathing purposes” section;
- • A determination of trophic status (Wiatkowski et al. 2021; Ji et al. 2020) before and after the application of EM by the Carlson trophic state index (TSI) calculated from the average concentrations of TN and TP according to Formulas 1–3 — Objective IIc, “Ecological status of physicochemical parameters and meeting the microbiological requirements of water for bathing purposes” section:
1 2 3 • A principal component analysis (PCA) for all parameters analyzed; additionally, Kaiser–Meyer–Olkin measure of sampling adequacy, Bartlett’s test of sphericity, communalities, explained total variance, Scree plot, and component matrix-destination — Objective III, “Correlations between microbiological and physicochemical parameters” section;
• Spearman’s correlation matrix — a comparison of the direction and strength of correlation between each parameter for a non-linear distribution — Objective III, “Correlations between microbiological and physicochemical parameters” section.
Fig. 3.
Associating article objectives with types of data analysis
The data obtained were analyzed using the following software: IBM SPSS Statistics 26, Statistica 13, Origin 2021b, and Excel 2013.
Figure 3 compares the formulated goals of the article with the data analyses described above.
Results
Descriptive statistics and statistical significance test of results
Data analysis for the period before EM application (2019, 2020, and 9 August 2021) and after EM application (16 and 23 August 2021) is presented in Table 2. It shows that the long-term average outcome difference between the periods ranged from − 72.04% (HBN22) to 137.04% (FEN). Statistical significance of the results, performed with the Mann–Whitney U test, was recorded for 9 of 14 parameters, i.e., HBN22 (− 72.04%), HBN36 (− 71.20%), NO3–N (− 57.19%), PO4–P (− 49.43%), COD (− 12.23%), pH (5.67%), DO (20.29%), FCBN (33.33%), CBN (34.25%), and FEN (137.04%). Throughout the study period, no Salmonella bacteria were found in the water and the parameter did not change over time, so it was not included in further analyses.
Table 2.
Descriptive statistics for microbiological and physicochemical parameters before and after EM application in the Turawa reservoir
| Statistics | Period | Parameter (unit) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBN22 (CFU∙mL−1) | HBN36 (CFU∙mL−1) | CBN (CFU∙mL−1) | FCBN (CFU∙mL−1) | FEN (CFU∙mL−1) | pH (-) | EC (µS∙cm−1) | TN (mg∙L−1) | NO3–N (mg∙L−1) | PO4–P (mg∙L−1) | TP (mg∙L−1) | DO (mg∙L−1) | BOD5 (mg∙L−1) | COD (mg∙L−1) | ||
| Min | Before EM | 180 | 50 | 23 | 15 | 3 | 6.70 | 202 | 1.31 | 0.060 | 0.000 | 0.03 | 0.00 | 0.70 | 1.20 |
| After EM | 2100 | 1500 | 75 | 75 | 9 | 6.88 | 203 | 1.48 | 0.110 | 0.009 | 0.09 | 7.30 | 1.70 | 6.70 | |
| Max | Before EM | 985,000 | 755,000 | 2420 | 2420 | 460 | 9.90 | 491 | 12.30 | 4.210 | 0.390 | 11.50 | 13.10 | 54.00 | 320.10 |
| After EM | 110,000 | 71,000 | 2420 | 2420 | 460 | 10.30 | 507 | 12.09 | 1.710 | 0.100 | 1.07 | 12.30 | 9.80 | 30.80 | |
| Std. deviation | Before EM | 192,694 | 181,379 | 920 | 893 | 86 | 0.65 | 39 | 2.04 | 0.824 | 0.052 | 1.52 | 2.10 | 6.32 | 38.81 |
| After EM | 24,667 | 18,005 | 723 | 709 | 126 | 0.92 | 42 | 2.29 | 0.437 | 0.017 | 0.25 | 1.33 | 2.20 | 6.55 | |
| Mean | Before EM | 79,013 | 77,696 | 760 | 677 | 54 | 8.51 | 403 | 3.78 | 1.140 | 0.075 | 0.65 | 8.30 | 5.27 | 17.47 |
| After EM | 22,093 | 22,374 | 1020 | 902 | 128 | 8.99 | 419 | 3.89 | 0.488 | 0.038 | 0.39 | 9.98 | 4.89 | 15.34 | |
| Mean difference after and before EM | − 72.04% | − 71.20% | 34.25% | 33.33% | 137.04% | 5.67% | 3.88% | 2.84% | − 57.19% | − 49.4% | − 40.58% | 20.29% | − 7.11% | − 12.23% | |
| Mann Whitney U Test | U-value | 1227 | 1113 | 1375 | 1321.5 | 1212 | 787 | 870 | 1039.5 | 1897.5 | 1764 | 1356.5 | 537.5 | 961.5 | 755.5 |
| z-score | − 4.19585 | − 4.65108 | − 3.59558 | − 3.81111 | − 4.25225 | − 2.8597 | − 1.65712 | 0.07906 | 3.96203 | 3.81245 | 0.65932 | − 4.3344 | − 0.9678 | − 2.42999 | |
| p | < 0.00001 | < 0.00001 | 0.00032 | 0.00014 | < 0.00001 | 0.00424 | 0.09692 | 0.93624 | 0.00008 | 0.00014 | 0.50926 | < 0.00001 | 0.33204 | 0.0151 | |
Designations in the table: italics — values statistically significant for p ≤ 0.05
Comparison of the effectiveness of effective microorganisms before and after their application
Influence on microbiological parameters immediately before and after application
The comparison of the results immediately before and after the application of EM (before application — 9 August 2021, after application — 16 and 23 August) is summarized in Table 3. All are statistically significant according to the Mann–Whitney U test. In most cases, there was a decrease in parameter values (exceptions: CBN in point 1 at a depth of 30 cm; HBN36, FCBN, and FEN in point 1 at a depth of 100 cm; and HBN22 and HBN36 in point 2 at a depth of 100 cm — an increase from 17.96 to 99.16%). This reduction ranged from 19.39% (FCBN, point 1 at 30-cm depth) to 88.97% (CBN, point 3 at 100-cm depth). The overall reduction of parameters for all points was decreasing: 58.38% — HBN22, 55.77% — FEN, 48.88% — HBN36, 47.22% — FCBN, and 46.44% — CBN. This is also reflected in the average duration of the action of EM, which ranges from 423 to 819 h (FEN and CBN, respectively). The results obtained demonstrate the effectiveness of EM in most cases, lasting up to more than 1 month after application, with an average reduction effect of at least 45%.
Table 3.
The average percentage effectiveness of EM activity and the average time of EM activity in reducing the value of microbiological parameters in the analyzed water test points of the Turawa reservoir
| Parameters | Description | Microbiological parameters | ||||
|---|---|---|---|---|---|---|
| HBN22 | HBN36 | CBN | FCBN | FEN | ||
| Average percentage effectiveness of EM at test points | 1 (30 cm) | − 60.55% | − 38.21% | 40.00% | − 19.39% | − 37.91% |
| 1 (100 cm) | − 73.13% | 17.96% | 0.00% | 28.57% | 89.96% | |
| 2 ( 30 cm) | − 47.50% | − 92.13% | − 72.18% | − 65.99% | − 86.38% | |
| 2 (100 cm) | 99.16% | 62.45% | − 76.77% | − 72.18% | − 79.23% | |
| 3 (30 cm) | − 63.76% | − 67.73% | − 13.89% | − 79.59% | − 37.74% | |
| 3 (100 cm) | − 71.22% | − 60.71% | − 88.97% | − 73.16% | − 80.66% | |
| Overall1 | − 58.38% | − 48.88% | − 46.44% | − 47.22% | − 55.77% | |
| Average duration of action of EM in total in reducing parameter values2 | Days | 19.52 | 19.67 | 34.13 | 30.47 | 17.64 |
| Hours | 468.5 | 472.1 | 819.2 | 731.3 | 423.4 | |
| Minutes | 28,110 | 28,323 | 49,152 | 43,878 | 25,401 | |
| Mann–Whitney U test | U-value | 65.5 | 84.0 | 77.5 | 74.5 | 55.0 |
| z-score | 3.03731 | 2.35199 | 2.65764 | 2.75256 | 3.36951 | |
| P | 0.00236 | 0.01428 | 0.00782 | 0.00596 | 0.00076 | |
Italics — statistically significant values for p ≤ 0.05
1Mean value for individual parameters from the series immediately before and after the application of EM
Ecological status of physicochemical parameters and meeting the microbiological requirements of water for bathing purposes
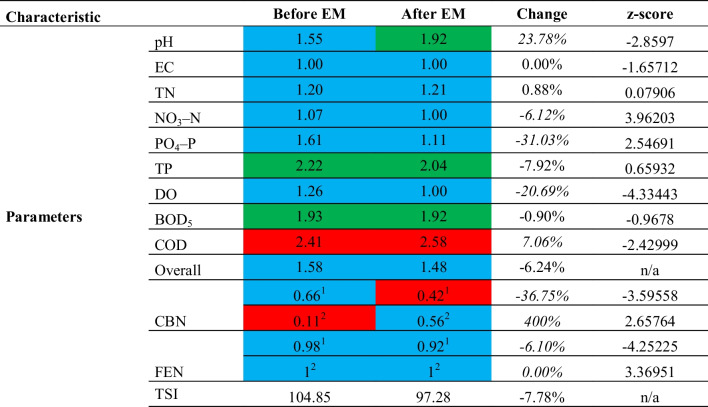
Table 4 presents the standardized results of the ecological status of physicochemical parameters concerning meeting the microbiological requirements of water for bathing purposes and the trophic status before and after the application of EM.
Table 4.
Summary of the ecological status results for physicochemical parameters, microbiological requirements compliance, and trophic status of Turawa reservoir water before and after application of EM
Designations in the table: blue color — class I of physicochemical condition (very good condition) or compliance with microbiological requirements, green color — class II of physicochemical condition, and red color — less than good condition or non-compliance with microbiological requirements
Italic — statistically significant values for p ≤ 0.05, n/a — not applicable
1Values for the whole series of measurements (2019–2021)
2Values for the series immediately before and after EM application
As for the physicochemical parameters for the whole study period 2019–2021, the statistical significance of the results for pH, NO3–N, PO4–P, DO, and COD was found. Among the mentioned parameters, after the use of EM, the ecological status improved for NO3–N (by 6.12%), PO4–P (by 31.03%), and DO (by 20.69%), and worsened for pH (by 23.78%) and COD (by 7.06%). Within the classification of average ecological condition, the class change was observed only in the case of pH (from class I to II, i.e., from very good to good condition). In contrast, in the other cases, the results had the same class both before and after the application of EM. The factor that worsened the results was the COD — physicochemical condition below good. Good status was found for TP and BOD5, and pH — very good status before EM application and good status after application (the objective of the Water Framework Directive is to achieve at least good status in surface waters) until the end of a certain planning period, so these results have no impact. The remaining elements assessed are within the allowable limits for 1st-class ecological status. An overall comparison of the physicochemical results shows that the ecological status improved by only 6.24% after using EM.
For the evaluated microbiological parameters (CBN and FEN), the statistical significance of the results was established, taking into account both the compliance with the requirements for bathing purposes in the series immediately before and after the application of EM (August 2021) and in the series for the entire analyzed research period (2019–2021). It is worth noting that the results for CBN in the series immediately before and after the application of EM improved by 400% in terms of requirements (compliance in 11% before application and 56% after application); in the case of FEN, there were no differences between the results (100% compliance). Considering the whole series of measurements, the results for CBN deteriorated by 36.75% (fulfillment of requirements in 66% of samples before application and 42% after application), and for FEN — by 6.10% (98% before application and 92% after application).
The general trophic condition improved after the treatment with EM — the Carlson index (TSI) decreased by 7.78%. Due to the high concentrations of TP and TN, the waters of Turawa reservoir are eutrophic or hypereutrophic, depending on the classification (Wiatkowski et al. 2021). The TSI values before and after the application of EM equaled: for TN — 115.37 and 116.00 (eutrophic or hypereutrophic) and TP — 94.34 and 78.55 (eutrophic).
Correlations between microbiological and physicochemical parameters
The analysis of the communalities shows that the values for the analyzed parameters after extraction using principal component analysis range from 0.610 (BOD5) to 0.949 (NO3–N). For BOD5, FEN (0.633), pH (0.674), and TP (0.728) values are below 0.800. All parameters were included in the principal component analysis.
The analysis revealed four components with eigenvalues greater than 1. The first principal component (PC1) explained 36.01% of the variance, the second (PC2) — 18.74%, the third (PC3) — 17.88%, and the fourth (PC4) — 9.16% (81.79% of the cases).
The component matrix shows that 6 parameters are most strongly correlated with the PC1 — HBN22, HBN36, CBN, FCBN, FEN, and EC (0.910. 0.928, 0.857, 0.891, 0.759, and 0.717); with the PC2 — 5 parameters: pH, NO3–N, DO, BOD5, and COD (0.644, 0.713, 0.576, 0.666, and 0.710); with the PC3 — 2 parameters: PO4–P and TP (0.931 and 0.819); and with the PC4 — 1 parameter: TN (0.848). These results are shown for the first and second components in Fig. 4.
Fig. 4.
Principal component analysis for the analyzed physicochemical and microbiological parameters of the Turawa reservoir in 2019–2021
The results for microbiological parameters are most similar (FEN, CBN, FCBN, HBN22, and HBN36; the maximum difference in the distance between the points on the axes: X = 0.169, Y = 0.127) for selected nutrients (TP, PO4–P, and TN; difference X = 0.006, Y = 0.228) and some oxygen parameters (COD and BOD5; difference X = 0.080, Y = 0.039). pH, DO, EC, and NO3–N differ from the other results for the first and second components.
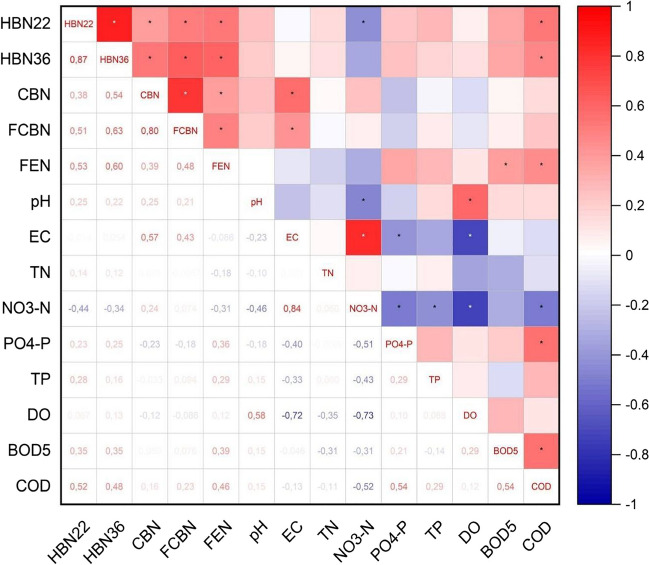
Spearman correlation matrix (Fig. 5) was performed to investigate possible relationships between parameters. Statistical significance was found between all microbiological parameters (HBN22, HBN36, CBN, FCBN, FEN) and also between EC/CBN, EC/FCBN, NO3–N/HBN22, NO3–N/pH, NO3–N/EC, PO4–P/EC, PO4–P/NO3–N, TP/NO3–N, DO/pH, DO/EC, DO/NO3–N, BOD5/FEN, COD/HBN22, COD/HBN36, COD/FEN, COD/NO3–N, COD/PO4–P, and COD/BOD5.
Fig. 5.
Spearman correlation matrix for microbiological and physicochemical water parameters from Turawa reservoir (asterisk — results significant for p ≤ 0.05)
Assuming that R ≥ ± 0.70 means a strong correlation; it occurred in the following cases: HBN36/HBN22 (0.87), FCBN/CBN (0.80), NO3–N/EC (0.84), DO/EC (− 0.72), and DO/NO3–N (− 0.73). It means that there was a strong statistically significant correlation for only five pairs of parameters — directly proportional for positive values and inversely proportional for negative values.
Discussion
To date, few microbiological and physicochemical analyses of surface water have been conducted using EM for river or reservoir remediation (Park et al. 2016; Li et al. 2020; Zhao et al. 2013; Sitarek et al. 2017; Dondajewska et al. 2019). As shown in the summarized results in Table 5, when comparing the results before and after the application of EM, the similarity between the results from this study and literature was observed in 6 out of 9 microbiological and physicochemical parameters in terms of changes in the values of specific parameters (CBN, FEN, TP, PO4–P, NO3–N, pH). The highest number of results in all studies was recorded for TP — conducted in China in artificial wetlands in Kunshan in Jiangsu Province (Li et al. 2020) and artificial lake in Guilin City in Guangxi Province (Zhao et al. 2013)), in Poland in Muchawka reservoir in Siedlce City (Sitarek et al. 2017) and Konin lake near Konin City (Dondajewska et al. 2019), and in South Korea in Dalseong wetland in Daegu City (Park et al. 2016) — the reduction after using EM ranged from 13.75 to 86.87% (in this study: 45.08%). For TN, slightly fewer studies were performed, and the changes in the values of this parameter after using EM ranged from − 57.36 to 2.84% (decrease in 4 studies, increase in 1). The decrease in the parameter values after the application of EM was visible in the case of CBN, FEN, NO3–N, and PO4–P (respectively, by 58.83%, 67.89%, 22.47%, and 37.70%). In the case of pH, there was a 1.31% increase in the average value. Divergent results between the cited test results and those of other conducted studies were found for EC and DO (in each case, a decrease in values in the other study and an increase in this one — with the direction of change being negative for EC in the studies conducted, and positive for DO; the differences between the values before and after the application of EM in this study were at the margin of error of the measurement methods, i.e., up to 5%, so they are most likely not significant).
Table 5.
Comparison of the effectiveness of EM activity concerning selected microbiological and physicochemical parameters of water, based on a literature review
| Parameter (unit) | Before EM | After EM | % difference | References |
|---|---|---|---|---|
| CBN (CFU·mL−1) | 290 | 83.5 | − 71.21% | Sitarek et al. (2017) |
| 16771 | 8981 | − 46.45% | This study | |
| FEN (CFU·mL−1) | 462.5 | 91 | − 80.32% | Sitarek et al. (2017) |
| 1191 | 531 | − 55.46% | This study | |
| pH (-) | 8.37 | 8.67 | 3.59% | Sitarek et al. (2017) |
| 7.231 | 7.301 | 0.98% | This study | |
| EC (μS/cm) | 388 | 311.5 | − 19.72% | Dondajewska et al. (2019) |
| 2071 | 2091 | 0.97% | This study | |
| NO3–N (mg·L−1) | - | - | − 34.50% | Li et al. (2020) |
| 0.5151 | 0.4611 | − 10.43% | This study | |
| TN (mg·L−1) | 3.68 | 3.75 | 1.81% | Dondajewska et al. (2019) |
| 4.50 | 3.30 | − 26.67% | Park et al. (2016) | |
| - | - | − 21.37% | Li et al. (2020) | |
| - | - | − 57.36% | Zhao et al. (2013) | |
| 3.841 | 3.421 | − 10.94% | This study | |
| PO4–P (mg·L−1) | 0.11 | 0.055 | − 50.00% | Dondajewska et al. (2019) |
| 0.0631 | 0.0471 | − 25.40% | This study | |
| TP (mg·L−1) | 0.19 | 0.16 | − 17.24% | Dondajewska et al. (2019) |
| 0.27 | 0.17 | − 38.27% | Sitarek et al. (2017) | |
| 0.55 | 0.23 | − 58.18% | Park et al. (2016) | |
| - | - | − 13.74% | Li et al. (2020) | |
| - | - | − 86.87% | Zhao et al. (2013) | |
| 0.251 | 0.141 | − 45.08% | This study | |
| DO (mg·L−1) | 9.8 | 9.6 | − 2.04% | Dondajewska et al. (2019) |
| 7.891 | 8.291 | 5.07% | This study |
1Value for the series directly before and after EM application
Regarding the duration of the action of EM, in the studies conducted at Kunshan Irrigation and Drainage Experimental Station (Li et al. 2020), a reduction of pollutants in the treated water was observed for each of the parameters considered below (NO3–N, TN, TP), throughout the study period (8 days) after the application of EM. However, in a liquid form (80% water, 10% nutrient solution, and 10% basic EM), the greatest and most permanent reduction of nutrients was achieved in combination with constructed wetlands. In this case, 8 days after the application of these solutions, there was even more than 92% of reduction (for comparison for NO3–N: EM application — reduction by a maximum of 66%, constructed wetlands — by 83%).
In Chinese studies conducted in an artificial lake (polluted by municipal wastewater) in Guilin City, Guangxi Province (Zhao et al. 2013), a pollution reduction was observed after the application of a comprehensive treatment, i.e., a combination of the application of EM in liquid form (concentration 0.2%), the use of phytoplankton-feeding fish, floating islands with macrophytes, and intermittent aeration of the reservoir. A reduction of TN and TP (by 54.18–60.54% and 85.76–87.98%, respectively) was observed during most of the study period (i.e., from 25 February 2012 to 2 June 2012). The only factor that disrupted the process was rainfall with a certain pollutant load.
In a study conducted in the constructed wetland in Kunshan, Jiang Province, China (Li et al. 2020), a decrease in the concentrations of TN and TP was observed throughout the measurement period (30 days) — water with EM-enriched soil balls (Bokashi balls) with 0.75% hardener was used.
In analyses conducted at Lake Konin in Poland (Dondajewska et al. 2019), after the application of EM (in the form of mud balls) in 2014, a gradual decrease of TP was observed compared to the values of 2011–2013 (by about 17.2%), while the median concentration of the parameter remained unchanged in 2015. For TN, the median value was the lowest in 2014 (by 10.4% on average, compared to 2011–2013), but higher concentrations of the parameter were recorded in 2015 (27.3% compared to 2014). It should be noted that the greatest changes observed after the deployment of EM were in species composition and phytoplankton abundance, which underwent a positive change in 2014. Such an effect lasted from April 2014 to June 2015 and, from July 2015, a return to the previous taxonomic structure characteristic of eutrophic waters was observed (however, the number of these taxa was lower than before the reconstruction of the lake).
A decrease in the content of microbiological parameters in the tests at the Mukhavka reservoir in Poland (Dondajewska et al. 2019) was observed only in the first series of measurements after the application of EM in liquid form (3 June 2013). In subsequent series of tests (from 9 July 2013), the content of both CBN and FEN fluctuated, indicating a short-term effect of the application of EM on microbiological parameters.
As shown by the above and other studies on using EM as a biological method to improve surface water quality, their main activity is related to their role in aquatic ecosystems. Since most of these organisms are decomposers that break down organic matter contained in bottom sediments, the direct effect of their use in water reservoirs or rivers is a reduction in the thickness of bottom sediments (Mazur 2020), as well as a reduction in the levels of various forms of nitrogen and phosphorus in the water column. It results from the conversion of the organic compounds used to inorganic compounds and the consumption of oxygen needed to carry out this process (Park et al. 2016). According to research, an additional, further effect of EM is also a change in the species composition of the flora of water reservoirs, especially an increase in the richness of phytoplankton, which is a positive phenomenon (Mazurkiewicz et al. 2020). Some studies have confirmed that immobilized EM can effectively remove microcystins (cyanobacterial toxins) up to 2 months after their application (Tsuji et al. 2006; Zhang et al. 2022b). However, as research points out, EM alone cannot improve water quality. They do not produce the desired long-term effects in terms of reducing nutrients and increasing the transparency and oxygen content of water (Dondajewska et al. 2019; Li et al. 2020). They are recommended as supporting methods for other biological, physical, or chemical methods of surface water restoration (e.g., phytoremediation, use of synthetic zeolites, or injection of salt into bottom sediments) (Gao et al. 2023; Tarczyńska et al. 2001). A particularly recommended biological method for controlling water blooms in reservoirs is the creation of ecotone zones consisting of macrophytes that prevent the resuspension of sediments (containing, among other things, nitrogen and phosphorus compounds that are food for algae that cause eutrophication) that accumulate harmful substances in their tissues (e.g., heavy metals) and that perform important ecological functions (e.g., as shelter for other groups of organisms) (Chen et al. 2019; O’Hare et al. 2018; Wiatkowski 2015).
The results presented in this article demonstrate the described process of decomposition of organic matter by EM, which leads to lower levels of PO4–P, TP, NO3–N, and TN immediately after application. In addition, the microbiological structure was rebuilt, as evidenced by the reduction in the number of analyzed bacteria in most of the collected water samples, which, among others, pose a risk to human health after consumption (e.g., diseases of the digestive and urinary systems) (Braiek and Smaoui 2019; Zhi et al. 2020).
The previously presented strong correlation between HBN36 and HBN22 and FCBN and CBN is related to the identical method of determination between the pairs of parameters, which considered the same groups of bacteria but cultured at different temperatures (HBN36 and HBN22) or similarly (CBN — all coliforms, FCBN — fecal coliforms only) (ISO 1999; Bachtiar 2002). As for the correlation between EC and NO3–N and EC and DO, a higher EC value indicates more saturation of water with ions and often more water pollution. For this reason, the correlation is directly proportional for the first pair and inversely proportional for the second (Zhang et al. 2022c). The correlation between DO and NO3–N results from the decomposition of organic matter, which is mainly carried out by algae as a part of the eutrophication process — when water highly enriched in NO3–N and DO is consumed (especially in the summer months when temperature is high and light is abundant). Thus, there is an inverse correlation (Lv et al. 2022; Sun et al. 2022). It is worth noting that, as shown in other studies on the eutrophic Turawa reservoir (Czerniawska-Kusza and Kusza 2011; Buta et al. 2023), nitrogen compounds in the water come mainly from external sources of agricultural origin, while phosphorus compounds come from internal sources, i.e., resuspension of sediments.
Moreover, according to research results for the period 1998–2020 (Buta et al. 2023), the trophic status of the Turawa reservoir, expressed by the Carlson index (Carlson 1977; Carlson & Simpson 1996) and the Vollenweider and Kajak criterion (Vollenweider 1965; Kajak 2001) is classified as eutrophic, and according to the Trophic Level Index (Abell et al. 2020; Ding et al. 2021) — as supertrophic. It is worth adding that TN was on average 10 times more responsible for eutrophication than TP, taking into account the values of dangerous loads of both parameters according to the Vollenweider and Kajak method (Vollenweider 1965; Kajak 2001). As indicated, the causes of water blooms in the area were municipal sources, i.e., waste (thrown away by tourists and residents) and agriculture (surface runoff of fertilizers and plant protection products from fields) (Buta et al. 2023; Smith et al. 1999).
Conclusions
In the context of water restoration, the use of EM in separate parts of water reservoirs, as presented in the article, can be a more effective solution than their application on the entire surface of the reservoir. It results from increased eutrophication in shallow parts of such hydrographic objects. In this type of a separated ecosystem, the application of only EM, without other methods of renaturation, may prove to be long-term and effective in the context of the desired improvement of water quality parameters (both microbiological and physicochemical). Therefore, in the future, more comprehensive research should be conducted on this topic. Also, a larger number of similar research objects should be analyzed over a longer period of time. EM can be one of the effective methods to purify water from unfavorable microorganisms (HBN22, HBN36, CBN, FCBN, FEN). After the application of EM, a decrease in their concentration was found at an average level of 46.44 to 58.38%, consistent with other studies in this field. Statistical analysis showed the significance of the results for p ≤ 0.05. The following conclusions were drawn from the analyses described in this article:
Based on the tests performed, the average EM action duration ranged from 17.64 to 34.13 days, depending on the microbiological parameter considered.
After the application of EM, the average ecological status improved in terms of NO3–N, PO4–P, TP, and DO (by 6.12%, 31.03%, 7.92%, and 20.69%, respectively) and worsened in terms of pH and COD (by 23.78% and 7.06%, respectively). These results are statistically significant (p ≤ 0.05).
Comparing the series immediately before and after the application of EM, the percentage of samples meeting bathing water standards (defined by Polish regulations) increased by 400% concerning CBN. No differences were found for FEN.
The application of EM improved the trophic status of Turawa reservoir, expressed by the Carlson index, by 7.78%.
Principal component analysis showed that results were most similar for microbiological parameters (FEN, CBN, FCBN, HBN22, and HBN36), selected nutrients (TP, PO4–P, and TN), and some oxygen parameters (COD and BOD5).
According to the Spearman correlation matrix, a statistically significant, strong correlation (R ≥ ± 0.70 at p ≤ 0.05) occurred between the following parameters: HBN36/HBN22 (0.87), FCBN/CBN (0.80), NO3–N/EC (0.84), DO/EC (− 0.72), and DO/NO3–N (− 0.73).
The literature search showed that after applying EM, the CBN, FEN, NO3–N, PO4–P, and TP values decreased, and the pH increased. No consistent results were found for TN, EC, and DO.
There is a need for more research on the effectiveness of EM and its application in restoring eutrophic water reservoirs. The scope of research should include more physicochemical and microbiological parameters related to priority substances (e.g., heavy metals, specific organic pollutants) and biological parameters (e.g., ichthyofauna, plankton, benthos, macrophytes). In addition to water testing, it is also recommended to focus on testing bottom sediments which have strong sorbent properties for contaminants. Technically, it is possible to compare the effectiveness of EM applied at different times to different matrices (e.g., water, bottom sediments, plants) in different forms (e.g., as a liquid solution, loess balls, soil balls, mud balls, or Bokashi balls), at different concentrations and compositions, or with the use of other solutions to assist in water purification (physical, chemical, biological methods).
Author contribution
Conceptualization, J.D., P.T., M.W., and P.S.W.; methodology, J.D., I.K., P.T., and M.W.; software, J.D., P.T., and B.W.; validation, J.D., A.K., I.K., P.T., and P.S.W.; formal analysis, P.T.; investigation, J.D., T.K., A.K., I.K., M.P., and P.S.W.; resources, J.D., I.K., and P.T.; data curation, J.D., I.K., and P.T.; writing—original draft preparation, P.T.; writing—review and editing, J.D., J. Dr., Ł.G., A.K., I.K., W.S., P.T., M.W., P.S.W., and B.W.; visualization, P.T.; supervision, M.W. and B.W.; project administration, W.S., M.W., and B.W.; funding acquisition, W.S., M.W., and B.W. All authors read and approved the final manuscript.
Funding
The National Centre funded this research for Research and Development in Poland, grant number BIOSTRATEG3/343733/15/NCBR/2018. Alban Kuriqi is grateful for the Foundation for Science and Technology’s support through funding UIDB/04625/2020 from the research unit CERIS.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abell JM, van Dam-Bates P, Özkundakci D, Hamilton DP. Reference and current trophic level index of New Zealand lakes: benchmarks to inform lake management and assessment. NZ J Mar Freshwat Res. 2020;54(4):636–657. doi: 10.1080/00288330.2020.1726974. [DOI] [Google Scholar]
- Aguiar VM, de Neto CJAB, Rangel CM. Eutrophication and hypoxia in four streams discharging in Guanabara Bay RJ Brazil: a case study. Mar Pollut Bull. 2011;62(8):1915–1919. doi: 10.1016/J.MARPOLBUL.2011.04.035. [DOI] [PubMed] [Google Scholar]
- Anawar HM, Chowdhury R. Remediation of polluted river water by biological chemical ecological and engineering processes. Sustainability. 2020;12(17):7017. doi: 10.3390/SU12177017. [DOI] [Google Scholar]
- Arenas-Sánchez A, Rico A, Vighi M. Effects of water scarcity and chemical pollution in aquatic ecosystems: state of the art. Sci Total Environ. 2016;572:390–403. doi: 10.1016/J.SCITOTENV.2016.07.211. [DOI] [PubMed] [Google Scholar]
- Ateia M (2016) In-situ biological water treatment technologies for environmental remediation: a review. J Bioremediat Biodegrad 07(03). 10.4172/2155-6199.1000348
- Bachtiar T. Preliminary study on potency of coprostanol and coliform bacteria in Semarang coastal area. J Coast Dev. 2002;6(1):47–54. [Google Scholar]
- Bartoszek L, Koszelnik P, Gruca-Rokosz R. Assessment of agricultural use of the bottom sediments from eutrophic Rzeszów reservoir. Ann Set Environ Protect. 2015;17(1):396–409. [Google Scholar]
- Bormans M, Maršálek B, Jančula D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review. Aquat Ecol. 2016;50(3):407–422. doi: 10.1007/S10452-015-9564-X. [DOI] [Google Scholar]
- Boruszko D. Research of effective microorganisms on dairy sewage sludge stabilization. J Ecol Eng. 2019;20(3):241–252. doi: 10.12911/22998993/99787. [DOI] [Google Scholar]
- Braiek BO, Smaoui S (2019) Enterococci: between emerging pathogens and potential probiotics. BioMed Res Int 2019. 10.1155/2019/5938210 [DOI] [PMC free article] [PubMed]
- Buta B, Wiatkowski M, Gruss Ł, et al. Spatio-temporal evolution of eutrophication and water quality in the Turawa dam reservoir. Poland Sci Rep. 2023;13:9880. doi: 10.1038/s41598-023-36936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RE. A trophic status index for lakes. Limnol Oceanogr. 1977;22(2):361–369. doi: 10.4319/lo.1977.22.2.0361. [DOI] [Google Scholar]
- Carlson RE, Simpson JA (1996) Coordinator’s guide to volunteer lake monitoring methods. (North American Lake Management Society).
- Chen F, Lu S, Hu X, He Q, Feng C, Xu Q, Guo H. Multi-dimensional habitat vegetation restoration mode for lake riparian zone, Taihu, China. Ecol Eng. 2019;134:56–64. doi: 10.1016/j.ecoleng.2019.05.002. [DOI] [Google Scholar]
- Cosgrove WJ, Loucks DP. Water management: current and future challenges and research directions. Water Resour Res. 2015;51(6):4823–4839. doi: 10.1002/2014WR016869. [DOI] [Google Scholar]
- Czerniawska-Kusza I, Kusza G. The potential of the Phytotoxkit microbiotest for hazard evaluation of sediments in eutrophic freshwater ecosystems. Environ Monit Assess. 2011;179:113–121. doi: 10.1007/s10661-010-1722-y. [DOI] [PubMed] [Google Scholar]
- Ding Y, Zhao J, Peng W, Zhang J, Chen Q, Fu Y, Duan M. Stochastic trophic level index model: a new method for evaluating eutrophication state. J Environ Manage. 2021;280:111826. doi: 10.1016/J.JENVMAN.2020.111826. [DOI] [PubMed] [Google Scholar]
- Dobrzyński J, Kulkova I, Wierzchowski PS, Wróbel B (2022) Response of physicochemical and microbiological properties to the application of effective microorganisms in the water of the Turawa reservoir. Water 14(12). 10.3390/W14010012
- Dondajewska R, Kozak A, Rosińska J, Gołdyn R. Water quality and phytoplankton structure changes under the influence of effective microorganisms (EM) and barley straw — lake restoration case study. Sci Total Environ. 2019;660:1355–1366. doi: 10.1016/J.SCITOTENV.2019.01.071. [DOI] [PubMed] [Google Scholar]
- Dunalska JA, Sieńska J, Szymański D. The use of biopreparations in lake restoration: experimental research. Oceanol Hydrobiol Stud. 2015;44(4):500–507. doi: 10.1515/ohs-2015-0047. [DOI] [Google Scholar]
- Ekpeghere KI, Kim BH, Son HS, Whang HS, Kim HS, Koh SC. Functions of effective microorganisms in bioremediation of the contaminated harbor sediments. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47(1):44–53. doi: 10.1080/10934529.2012.629578. [DOI] [PubMed] [Google Scholar]
- Escudero-López HJ, Serrano-Carrillo KA, Jácome-Pilco CR, Sanaguano-Salguero HDR, Bayas-Morejón IF (2022) Bacterial selection of the Pseudomonas genus with the capacity to treat water and contaminated soils. J Water Land Dev. 238–241
- Fan YV, Lee CT, Klemeš JJ, Chua LS, Sarmidi MR, Leow CW. Evaluation of effective microorganisms on home scale organic waste composting. J Environ Manage. 2018;216:41–48. doi: 10.1016/j.jenvman.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Gao J, Shao N, Sun Y, Nie Z, Yang X, Dai F, Xu G, Xu P. Impact of effective microorganisms and Chlorella vulgaris on Eriocheir sinensis and water microbiota in ponds experiencing cyanobacterial blooms. Sustainability. 2023;15(9):7362. doi: 10.3390/su15097362. [DOI] [Google Scholar]
- Grabas M, Tomaszek J, Czerwieniec E, Masłoń A, Łuczyszyn J. Application of a biopreparation of effective microorganisms to the processing of wastewater sludge on semi-industrial scale. Environ Prot Eng. 2016;42(1):33–44. doi: 10.5277/epe160103. [DOI] [Google Scholar]
- Gruss Ł, Wiatkowski M, Pulikowski K, Kłos A. Determination of changes in the quality of surface water in the river–reservoir system. Sustainability. 2021;13(6):3457. doi: 10.3390/SU13063457. [DOI] [Google Scholar]
- Hapich H, Andrieiev V, Kovalenko V, Makarova T (2022) The analysis of spatial distribution of artificial reservoirs as anthropogenic fragmentation elements of rivers in the Dnipropetrovsk Region Ukraine. J Water Land Dev 80–85
- International Standardization Organization (ISO) (1999), ISO 6222:1999; Water quality—enumeration of culturable micro-organisms—colony count by inoculation in a nutrient agar culture medium, International Organization for Standardization: Geneva Switzerland
- International Standardization Organization (ISO) (2000) ISO 7899–1 (1998) (cor 1: (2000) Water quality-detection and enumeration of intestinal enterococci—Part 1: Miniaturized method (most probable number) for surface and waste water, International Organization for Standardization Geneva,
- International Standardization Organization (ISO) (2010) ISO 19250:2010 Water quality—detection of Salmonella spp., International Organization for Standardization: Geneva Switzerland
- Ji B, Liang J, Chen R. Bacterial eutrophic index for potential water quality evaluation of a freshwater ecosystem. Environ Sci Pollut Res. 2020;27(26):32449–32455. doi: 10.1007/S11356-020-09585-4. [DOI] [PubMed] [Google Scholar]
- Jilbert T, Couture RM, Huser BJ, Salonen K. Preface: Restoration of eutrophic lakes: current practices and future challenges. Hydrobiologia. 2020;847(21):4343–4357. doi: 10.1007/S10750-020-04457-X. [DOI] [Google Scholar]
- Jóźwiakowski K, Czernaś K, Szczurowska A. Preliminary results of studies on the purification of water in a pond using the SCD Probiotics technology. Ecohydrol Hydrobiol. 2009;9(2–4):307–312. doi: 10.2478/v10104-010-0009-9. [DOI] [Google Scholar]
- Kajak Z. Hydrobiology—limnology. Inland water ecosystems: PWN Publisher; 2001. [Google Scholar]
- Kamath G (2007) Bioremediation of lakes: myths and realities, The Proceedings of Taal, The 12th World Lake Conference: 44–49.
- Kanzari F, Syakti AD, Asia L, Malleret L, Mille G, Jamoussi B, Abderrabba M, Doumenq P. Aliphatic hydrocarbons polycyclic aromatic hydrocarbons polychlorinated biphenyls organochlorine and organophosphorous pesticides in surface sediments from the Arc river and the Berre lagoon France. Environ Sci Pollut Res. 2012;19(2):559–576. doi: 10.1007/S11356-011-0582-5. [DOI] [PubMed] [Google Scholar]
- Khan MN, Mohammad F. Eutrophication: challenges and solutions. Eutrophication Causes Consequences Control. 2014;2:1–15. doi: 10.1007/978-94-007-7814-6_1. [DOI] [Google Scholar]
- Khatri N, Tyagi S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci. 2015;8(1):23–39. doi: 10.1080/21553769.2014.933716. [DOI] [Google Scholar]
- Koundouri P, Ker Rault P, Pergamalis V, Skianis V, Souliotis I. Development of an integrated methodology for the sustainable environmental and socio-economic management of river ecosystems. Sci Total Environ. 2016;540:90–100. doi: 10.1016/J.SCITOTENV.2015.07.082. [DOI] [PubMed] [Google Scholar]
- Kour D, Kaur T, Devi R, et al. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: present status and future challenges. Environ Sci Pollut Res. 2021;28:24917–24939. doi: 10.1007/s11356-021-13252-7. [DOI] [PubMed] [Google Scholar]
- Li X, Guo Q, Wang Y, Xu J, Wei Q, Chen L, Liao L. Enhancing nitrogen and phosphorus removal by applying effective microorganisms to constructed wetlands. Water. 2020;12:2443. doi: 10.3390/w12092443. [DOI] [Google Scholar]
- Lin S-S, Shen S-L, Zhou A, Lyu H-M. Assessment and management of lake eutrophication: a case study in Lake Erhai China. Sci Total Environ. 2020;751:141618. doi: 10.1016/j.scitotenv.2020.141618. [DOI] [PubMed] [Google Scholar]
- Lu C, Tian H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: shifted hot spots and nutrient imbalance. Earth Syst Sci Data. 2017;9:181–192. doi: 10.5194/essd-9-181-2017. [DOI] [Google Scholar]
- Lürling M, Mucci M. Mitigating eutrophication nuisance: in-lake measures are becoming inevitable in eutrophic waters in the Netherlands. Hydrobiologia. 2020;847(21):4447–4467. doi: 10.1007/S10750-020-04297-9. [DOI] [Google Scholar]
- Lürling M, Waajen G, de Senerpont Domis LN. Evaluation of several end-of-pipe measures proposed to control cyanobacteria. Aquat Ecol. 2016;50:499–519. doi: 10.1007/s10452-015-9563-y. [DOI] [Google Scholar]
- Lv K, Guo X, Wang C, Su Q, Liu D, Xiao S, Yang Z. Sediment nitrogen contents controlled by microbial community in a eutrophic tributary in Three Gorges Reservoir. China Environ Pollut. 2022;314:120312. doi: 10.1016/j.envpol.2022.120312. [DOI] [PubMed] [Google Scholar]
- Mazur R. The application of microbiological biopreparations in the process of water remediation of the dam reservoir in Głuchów. Acta Sci Pol Formatio Circumiectus. 2020;19(1):81–95. doi: 10.15576/ASP.FC/2020.19.1.81. [DOI] [Google Scholar]
- Mazurkiewicz J, Mazur A, Mazur R, Chmielowski K, Czekała W, Janczak D. The process of microbiological remediation of the polluted Słoneczko reservoir in Poland: for reduction of water pollution and nutrients management. Water. 2020;12(11):3002. doi: 10.3390/w12113002. [DOI] [Google Scholar]
- Minister of Health in Poland (MHP) (2019) Regulation of the Minister of Health of 11th February 2019 on the supervision of the quality of bathing water and places occasionally used for bathing, J Laws 2019 item 255; Prime Minister of Poland: Warsaw, Poland (in Polish),
- Minister of Infrastructure in Poland (MIP) (2021) Regulation of the Minister of Infrastructure of 25th June 2021 on the classification of ecological status ecological potential and chemical status and the method of classifying the status of surface water bodies as well as environmental quality standards for priority substances; J Laws (2021 item 1475; Prime Minister of Poland: Warsaw, Poland (in Polish),
- Mrozińska N, Glińska-Lewczuk K, Burandt P, Kobus S, Gotkiewicz W, Szymańska M, Bąkowska M, Obolewski K. Water quality as an indicator of stream restoration effects—a case study of the Kwacza River Restoration Project. Water. 2018;10:1249. doi: 10.3390/w10091249. [DOI] [Google Scholar]
- Myronidis D, Ioannou K, Fotakis D, Dörflinger G. Streamflow and hydrological drought trend analysis and forecasting in Cyprus. Water Resour Manage. 2018;32(5):1759–1776. doi: 10.1007/S11269-018-1902-Z. [DOI] [Google Scholar]
- Nugroho FL, Rusmaya D, Yustiani YM, Hafiz FI, Putri RBT. Effect of temperature on removal of COD and TSS from artificial river water by mudballs made from EM rice bran and clay. Int J Geomate. 2017;12(33):91–95. doi: 10.21660/2017.33.2626. [DOI] [Google Scholar]
- O’Hare MT, Aguiar FC, Asaeda T, et al. Plants in aquatic ecosystems: current trends and future directions. Hydrobiologia. 2018;812:1–11. doi: 10.1007/s10750-017-3190-7. [DOI] [Google Scholar]
- Oki T, Kanae S. Global hydrological cycles and world water resources. Science. 2006;313(5790):1068–1072. doi: 10.1126/SCIENCE.1128845. [DOI] [PubMed] [Google Scholar]
- Olle M, Williams IH. Effective microorganisms and their influence on vegetable production — a review. J Hortic Sci Biotechnol. 2015;88(4):380–386. doi: 10.1080/14620316.2013.11512979. [DOI] [Google Scholar]
- Páll E, Niculae M, Kiss T, Şandru CD, Spînu M. Human impact on the microbiological water quality of the rivers. J Med Microbiol. 2013;62(11):1635. doi: 10.1099/JMM.0.055749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GS, Khan AR, Kwak Y, Hong SJ, Jung BK, Ullah I, Kim JG, Shin JH. An improved effective microorganism (EM) soil ball-making method for water quality restoration. Environ Sci Pollut Res Int. 2016;23(2):1100–1107. doi: 10.1007/S11356-015-5617-X. [DOI] [PubMed] [Google Scholar]
- Paruch L, Paruch AM, Eiken HG, Sørheim R. Faecal pollution affects abundance and diversity of aquatic microbial community in anthropo-zoogenically influenced lotic ecosystems. Sci Rep. 2019;9:19469. doi: 10.1038/s41598-019-56058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B, Bhattacharya SS, Gogoi N. Primacy of ecological engineering tools for combating eutrophication: an ecohydrological assessment pathway. Sci Total Environ. 2021;762:143171. doi: 10.1016/J.SCITOTENV.2020.143171. [DOI] [PubMed] [Google Scholar]
- Price JI, Heberling MT. The effects of source water quality on drinking water treatment costs: a review and synthesis of empirical literature. Ecol Econ. 2018;151:195–209. doi: 10.1016/J.ECOLECON.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchlik M, Piekutin J, Dyczewska K. Analysis of the impact of climate change on surface water quality in north-eastern Poland. Energies. 2022;15(1):164. doi: 10.3390/en15010164. [DOI] [Google Scholar]
- Rajfur M, Klos A, Waclawek M. Algae utilization in assessment of the large Turawa Lake (Poland) pollution with heavy metals. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2011;46(12):1401–1408. doi: 10.1080/10934529.2011.606717. [DOI] [PubMed] [Google Scholar]
- Rivoira L, Castiglioni M, Nurra N, Battuello M, Sartor RM, Favaro L, Bruzzoniti MC. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in seawater sediment and biota of neritic ecosystems: occurrence and partition study in Southern Ligurian Sea. Appl Sci. 2022;12:2564. doi: 10.3390/app12052564. [DOI] [Google Scholar]
- Safwat SM. Performance of moving bed biofilm reactor using effective microorganisms. J Clean Prod. 2018;185:723–731. doi: 10.1016/j.jclepro.2018.03.041. [DOI] [Google Scholar]
- Safwat SM, Matta ME. Environmental applications of effective microorganisms: a review of current knowledge and recommendations for future directions. J Eng Appl Sci. 2021;68(1):1–12. doi: 10.1186/S44147-021-00049-1. [DOI] [Google Scholar]
- Schoumans OF, Chardon WJ, Bechmann ME, Gascuel-Odoux C, Hofman G, Kronvang B, Rubæk GH, Ulén B, Dorioz JM. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: a review. Sci Total Environ. 2014;468–469:1255–1266. doi: 10.1016/J.SCITOTENV.2013.08.061. [DOI] [PubMed] [Google Scholar]
- Sharip Z, Razak SBA, Noordin N, Yusoff FM. Application of an effective microorganism product as a cyanobacterial control and water quality improvement measure in Putrajaya Lake, Malaysia. Earth Syst Environ. 2020;4:213–223. doi: 10.1007/s41748-019-00139-4. [DOI] [Google Scholar]
- Sitarek M, Napiórkowska-Trzebietke A, Mazur R, Czarnecki B, Pyka JP, Stawecki K, Olech M, Sołtysiak S, Kapusta A. Application of effective microorganisms technology as a lake restoration tool — a case study of Muchawka Reservoir. J Elem. 2017;22(2):529–543. doi: 10.5601/jelem.2016.21.2.1196. [DOI] [Google Scholar]
- Smith VH, Tilman GD, Nekola JC. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut. 1999;100(1–3):179–196. doi: 10.1016/S0269-7491(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Smolders AJP, Lamers LPM, Lucassen ECHET, van der Velde G, Roelofs JGM. Internal eutrophication: how it works and what to do about it—a review. Chem Ecol. 2007;22(2):93–111. doi: 10.1080/02757540600579730. [DOI] [Google Scholar]
- Sun C, Wang S, Wang H, Hu X, Yang F, Tang M, Zhong J. Internal nitrogen and phosphorus loading in a seasonally stratified reservoir: implications for eutrophication management of deep-water ecosystems. J Environ Manag. 2022;319:115681. doi: 10.1016/j.jenvman.2022.115681. [DOI] [PubMed] [Google Scholar]
- Suwal N, Huang X, Kuriqi A, Chen Y, Pandey KP, Bhattarai KP. Optimisation of cascade reservoir operation considering environmental flows for different environmental management classes. Renewable Energy. 2020;158:453–464. doi: 10.1016/J.RENENE.2020.05.161. [DOI] [Google Scholar]
- Szymańska-Pulikowska A, Wdowczyk A, Wiercik P (2023) Analysis of changes on substrates filling constructed wetland systems after landfill leachate treatment - FTIR study. J Clean Prod 411:137336. 10.1016/j.jclepro.2023.137336
- Talaat NB, Ghoniem AE, Abdelhamid MT, Shawky BT. Effective microorganisms improve growth performance alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress. Plant Growth Regul. 2015;75(1):281–295. doi: 10.1007/S10725-014-9952-6. [DOI] [Google Scholar]
- Tarczyńska M, Romanowska-Duda Z, Jurczak T, Zalewski M. Toxic cyanobacterial blooms in a drinking water reservoir — causes, consequences and management strategy. Water Supply. 2001;1(2):237–246. doi: 10.2166/ws.2001.0043. [DOI] [Google Scholar]
- Ting ASY, Rahman NHA, Isa MIHM, Tan WS. Investigating metal removal potential by Effective Microorganisms (EM) in alginate-immobilized and free-cell forms. Bioresour Technol. 2013;147:636–639. doi: 10.1016/j.biortech.2013.08.064. [DOI] [PubMed] [Google Scholar]
- Ting WHT, Tan IAW, Salleh SF, Wahab NA. Application of water hyacinth (Eichhornia crassipes) for phytoremediation of ammoniacal nitrogen: a review. J Water Process Eng. 2018;22:239–249. doi: 10.1016/J.JWPE.2018.02.011. [DOI] [Google Scholar]
- Tomczyk P, Wiatkowski M. Impact of a small hydropower plant on water quality dynamics in a diversion and natural river channel. J Environ Qual. 2021;50(5):1156–1170. doi: 10.1002/JEQ2.20274. [DOI] [PubMed] [Google Scholar]
- Tomczyk P, Gałka B, Wiatkowski M, Wdowczyk A, Gruss Ł. Toxicity studies on sediments near hydropower plants on the Ślęza and Bystrzyca rivers Poland to establish their potential for use for soil enrichment. Land Degrad Dev. 2022;33(5):756–770. doi: 10.1002/LDR.4210. [DOI] [Google Scholar]
- Tommonaro G, Abbamondi GR, Nicolaus B, Poli A, D’Angelo C, Iodice C, De Prisco R. Productivity and nutritional trait improvements of different tomatoes cultivated with effective microorganisms technology. Agriculture. 2021;11:112. doi: 10.3390/agriculture11020112. [DOI] [Google Scholar]
- Tsuji K, Asakawa M, Anzai Y, Sumino T, Harada K. Degradation of microcystins using immobilized microorganism isolated in an eutrophic lake. Chemosphere. 2006;65(1):117–124. doi: 10.1016/j.chemosphere.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution toxicity and remedial measures: current trends and future perspectives. J Mol Liq. 2019;290:111197. doi: 10.1016/J.MOLLIQ.2019.111197. [DOI] [Google Scholar]
- Vollenweider RA. Material and ideas for a hydrochemistry of water. Mem Dell Istit Ital Idrobiol. 1965;19:213–286. [Google Scholar]
- Wang J, Chen J, Chen Q, Yang H, Zeng Y, Yu P, Jin Z. Assessment on the effects of aluminum-modified clay in inactivating internal phosphorus in deep eutrophic reservoirs. Chemosphere. 2019;215:657–667. doi: 10.1016/J.CHEMOSPHERE.2018.10.095. [DOI] [PubMed] [Google Scholar]
- Wdowczyk A, Szymańska-Pulikowska A (2023) Effect of substrates on the potential of Phragmites australis to accumulate and translocate selected contaminants from landfill leachate. Water Resour Ind 29:100203. 10.1016/j.wri.2023.100203
- Wiatkowski M. Problems of water management in the reservoir Młyny located on the Julianpolka river. Acta Sci Pol Formatio Circumiectus. 2015;14:3:191–203. doi: 10.15576/ASP.FC/2015.14.3.191. [DOI] [Google Scholar]
- Wiatkowski M, Wiatkowska B. Changes in the flow and quality of water in the dam reservoir of the Mała Panew catchment (South Poland) characterized by multidimensional data analysis. Arch Environ Prot. 2019;45:26–41. doi: 10.24425/aep.2019.126339. [DOI] [Google Scholar]
- Wiatkowski M, Rosik-Dulewska C, Tymiński T. Analysis of water management of the Michalice reservoir in relation to its functions. Ecol Chem Eng A. 2010;17(11):1505–1516. [Google Scholar]
- Wiatkowski M, Wiatkowska B, Gruss Ł, Rosik-Dulewska C, Tomczyk P, Chłopek D. Assessment of the possibility of implementing small retention reservoirs in terms of the need to increase water resources. Arch Environ Prot. 2021;47(1):80–100. doi: 10.24425/aep.2021.136451. [DOI] [Google Scholar]
- Yin H, Wang J, Zhang R, Tang W. Performance of physical and chemical methods in the co-reduction of internal phosphorus and nitrogen loading from the sediment of a black odorous river. Sci Total Environ. 2019;663:68–77. doi: 10.1016/J.SCITOTENV.2019.01.3260. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhu M, Ying Zeng G, Ming Yu Z, Gang Cui F, Yang ZZ, Shen LQ. Active capping technology: a new environmental remediation of contaminated sediment. Environ Sci Pollut Res. 2016;23(5):4370–4386. doi: 10.1007/S11356-016-6076-8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li H, Gao D, Yu H. Source identification of surface water pollution using multivariate statistics combined with physicochemical and socioeconomic parameters. Sci Total Environ. 2022;806(3):151274. doi: 10.1016/j.scitotenv.2021.151274. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wei J, Massey IY, Peng T, Yang F. Immobilization of microbes for biodegradation of microcystins: a mini review. Toxins. 2022;14(8):573. doi: 10.3390/toxins14080573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang P, Cui R, Yang H, Li G, Chen A, Wang H. Electrical conductivity and dissolved oxygen as predictors of nitrate concentrations in shallow groundwater in Erhai Lake region. Sci Total Environ. 2022;802:149879. doi: 10.1016/j.scitotenv.2021.149879. [DOI] [PubMed] [Google Scholar]
- Zhao WY, Yang GW, Chen XX, Yu JY, Ma BK. Study on integrated restoration technique for eutrophic artificial lakes. Adv Mat Res. 2013;807–809:1304–1310. doi: 10.4028/WWW.SCIENTIFIC.NET/AMR.807-809.1304. [DOI] [Google Scholar]
- Zhi S, Stothard P, Banting G, Scott C, Huntley K, Ryu K, Neumann NF. Characterization of water treatment-resistant and multidrug-resistant urinary pathogenic Escherichia coli in treated wastewater. Water Res. 2020;182:115827. doi: 10.1016/j.watres.2020.115827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.