Abstract
Two recombinant fragments of diphtheria toxin (DT) were fused to an engineered tandem repeat of the immunoglobulin (Ig) binding domain of protein A, called ZZ. These fragments are (i) the receptor binding domain (DTR), which comprises amino acids 382 to 535 of DT, and (ii) a linear peptide (DT168–220) which comprises residues 168 to 220 of the loop between fragment A and fragment B of DT. The fusion proteins were produced in Escherichia coli and purified by affinity chromatography. In vitro experiments showed that the DTR domain is responsible for the capacity of ZZ-DTR to bind to Vero cells and is capable of inhibiting the cytotoxicity of DT for these cells. These findings suggest that DTR binds to the cell surface receptors of DT and hence adopts a conformation that is similar to that of the receptor binding domain of DT. We compared the capacities of ZZ-DTR, ZZ-DT168–220, and a chemically detoxified form of DT currently used for vaccination to elicit antibodies in rabbits. The toxoid was more immunogenic than ZZ-DT168–220, which in turn was more immunogenic than ZZ-DTR. However, ZZ-DT168–220 antiserum was poorly efficient at neutralizing DT cytotoxicity on Vero cells, whereas ZZ-DTR antiserum was only 15-fold less potent than anti-DT antisera.
With the advent of genetic manipulations, a number of recombinant proteins have been successfully designed as appropriate vaccines (21, 27). Various improvements, including the production of proteins which cannot be readily purified from natural sources and the development of a small and selected number of amino acid substitutions to detoxify the original proteins, have been thus introduced (4, 5, 12). There are, however, additional advantages which may contribute to increase the number and quality of recombinant vaccines. In principle, recombinant technology offers the possibility to generate fragments of proteins which may be structurally organized as domains and which may lack any toxicity. Another advantage offered by genetic manipulations consists of the possibility of fusing an immunogen with a protein capable of increasing the immunogenicity of the fused partner. The present work aims at investigating the consequences of combining these two potential advantages in the case of diphtheria toxin (DT).
DT is an Mr-58,342 exotoxin synthesized as a single polypeptide. After proteolytic cleavage, the amino-terminal fragment A (Mr, 21,150) and the carboxy-terminal fragment B (Mr, 37,200) remain linked via a single disulfide bond (26). The crystallographic structure of DT (6, 7) reveals the presence of three functional domains. The catalytic domain (C domain) is located in fragment A, the translocation domain (T domain) is located in the N-terminal part of fragment B, and the receptor binding domain (R domain) is located in the carboxy-terminal region of fragment B. The toxin is known to target cell surface receptors via its R domain; then the toxin is internalized by receptor-mediated endocytosis and fragment A is translocated into the cytosol. Once in the cytosol, fragment A blocks protein synthesis by causing an ADP-ribosylation of elongation factor 2, thus provoking cell death.
A number of DT regions have been shown to elicit neutralizing anti-DT antibodies. These include the whole R domain (amino acids [aa] 382 to 535) (8, 14, 28, 29, 37–39) and the smaller loop connecting the A and B fragments (aa 188 to 201) (2). We therefore tentatively expressed the DT receptor binding domain, called DTR, and the fragment from aa 168 to 220 (DT168–220), which overlaps the loop region, in Escherichia coli. These fragments were fused individually with the tandem repeat dimer of a modified immunoglobulin (Ig) binding domain of protein A from Staphylococcus aureus, called ZZ (24). This particular fusion moiety was chosen due to the previous observations that (i) the presence of an Ig binding domain associated with an expressed protein offers a simple and efficient means to extract and purify the protein from bacterial media, using IgG affinity chromatography (22, 23), and (ii) ZZ often increases immunogenicity of the fused partner (11, 17–19, 22, 35), presumably by enhancing its presentation to specific T cells (16).
The aim of this work was to investigate the immunogenic properties of the ZZ-DTR and ZZ-DT168–220 fusion proteins in rabbits and to examine the capacity of the elicited antibodies to neutralize DT, using an in vitro assay (39). The two hybrid proteins were cloned, produced in the cytoplasm of E. coli, and purified. Furthermore, the biological activity of ZZ-DTR was examined by determining its ability to (i) protect Vero cells from cytotoxic effects of DT and (ii) bind to DT receptors on Vero cells by its DTR moiety.
MATERIALS AND METHODS
Genetic construction of ZZ fusion proteins.
Plasmid pK5DT, coding for DT, was kindly provided by Patrice Boquet (Sophia Antipolis, France). All DNA manipulations were performed as described by Sambrook et al. (31). Using PCR, a SalI site and a BamHI site were created at the 5′ end and the 3′ end, respectively, of the nucleotide sequence of the DTR domain. Plasmid pK5DT was used as the template, and the following single-stranded oligonucleotides were used as primers: SalI site, 5′GGGACTGCAGGTACCGTCGACGCCGGGTCACAAAACGCAA3′; and BamHI site, 5′GGGACTGCAGGATCCTTATAAGCTTCCGCTTTTGATTTCAAAAAATAG3′. Restriction sites are indicated in boldface; the stop codon is underlined. Oligonucleotides used in PCR were synthesized in an Applied Biosystems DNA synthesizer.
A BamHI site was created after the stop codon of the open reading frame, and a SalI site was introduced at the 5′ end of the codon corresponding to residue 382 of DT. The oligonucleotides were designed to ligate the frame of DT and the ZZ protein. The SalI and BamHI site were used to subclone the amplified fragment in M13mp18 (36), where it was sequenced. Then the DTR fragment was excised from M13 by using SacI and BamHI sites and ligated in a SacI/BamHI-opened pCP vector (10), a pET3a-derived plasmid (33). The SalI site will be used for subcloning in a vector described in a subsequent work; it was unusable in pCP.
The same procedure was used to subclone the DT168–220 fragment in pCP but with the following oligonucleotides as PCR primers: SalI site, GGGACTGCAGGTACCGTCGACGGAAACCCGTGGAAAACGT; and BamHI site, GGGACTGCAGGATCCTTATAAGCTTCCCAAAGACTCTATCTTTGT. (Boldface and underlining are as defined above.)
Production and purification.
E. coli BL21(DE3)LysS was used as the host for the expression of ZZ-DTR and ZZ-DT168–220. Freshly transformed cells were grown in 100 ml of tryptic soy broth (Difco, Detroit, Mich.) supplemented with glucose (5 g/liter), ampicillin (200 μg/ml), and chloramphenicol (30 μg/ml). The cells from a 60-ml overnight culture at 37°C were used to inoculate a 3-liter fermentor (Chemap; B. Braun Sciencetec, Les Ulis, France) containing the same medium as in the preculture. Cells were incubated at 37°C under aeration until the optical density at 600 nm reached 0.5 to 1. Then isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 0.5 mM (final concentration); after 3 h of induction, the cells were harvested by centrifugation (5,000 × g for 15 min), resuspended in lysis buffer (30 mM Tris, 5 mM EDTA, 20% sucrose [pH 8]), and disrupted with an Eaton press. The supernatant containing the fusion protein was purified on an IgG-Sepharose 4B column (Pharmacia Biotech Inc., Uppsala, Sweden); 10 ml of the crude extract was incubated overnight at 4°C with 10 ml of IgG-Sepharose equilibrated in 50 mM Tris-HCl buffer (pH 7.6)–150 mM NaCl–0.05% Tween 20. After a wash with 10 bed volumes of the equilibration buffer, 2 bed volumes of 5 mM ammonium acetate (NH4Ac; pH 5.0) was passed through the column. The bound protein was then eluted with 0.5 M hydrogen acetate (pH 3.4) and immediately neutralized with 1 M Tris-HCl buffer (pH 8). The ZZ-DTR-containing fraction was concentrated at 4 mg/ml by ultrafiltration on Microsep 30 (Filtron, Northborough, Mass.) and used for experiments without further purification. ZZ-DT168–220 eluted from the IgG column was further purified with a Mono S ion-exchange column (Pharmacia Biotech) equilibrated with 10 mM NH4Ac (pH 5.1). The hybrid was eluted with a linear gradient ranging from 10 mM to 1.5 M NH4Ac (pH 5.1). Protein concentration was determined spectrophotometrically, based on the calculated extinction coefficient at 275 nm (ɛM = 12,940 for ZZ-DTR and ɛM = 11,460 for ZZ-DT168–220).
PAGE and Western blot analysis.
The samples were analyzed by polyacrylamide gel electrophoresis (PAGE) in a sodium dodecyl sulfate (SDS)–20% polyacrylamide gel, using the PHAST system (Pharmacia Biotech), and their purity was estimated after Coomassie blue staining of the gel.
For Western blot experiments, ZZ-DTR and ZZ-DT168–220 fractions were subjected to SDS-PAGE on a 15% polyacrylamide gel (8 by 5 cm; Minirad) and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). Membranes were then incubated with a 1/2,000 dilution of horse anti-DT antisera (Pasteur Vaccin, Ville d’Avray, France) for 1 h at room temperature. Binding of the antibodies to the DT moiety was revealed by using F(ab′)2 rabbit anti-horse IgG conjugated to horseradish peroxidase (Jackson Immunoresearch, West Grove, Pa.) and diaminobenzidine (Sigma, St. Quentin Fallavier, France) as substrate.
ELISA.
Microtiter enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at 4°C with either rabbit IgG, DT (Calbiochem, La Jolla, Calif.), or the fusion proteins (each at 1 μg/well) in a 50 mM Tris-HCl buffer (pH 7.4). Plates were subsequently saturated with 0.1 M Tris-HCl (pH 7.4) containing 0.3% casein. Before use, plates were washed five times with 0.01 M Tris-HCl (pH 7.4)–0.05% Tween 20. The fusion proteins and the antibodies were diluted in 0.1 M Tris-HCl (pH 7.4) containing 0.1% casein.
(i) Antigenicity of ZZ-DTR and ZZ-DT168–220.
Rabbit IgG-coated wells were incubated overnight at 4°C with 100 μl of either ZZ-DTR, ZZ-DT168–220, or ZZ at a concentration of 2.3 μg/ml and washed as described above; 100 μl of a horse anti-DT antiserum diluted 1/1,000 (Pasteur Vaccin) was added, and the mixture was incubated for 3 h at room temperature (RT). After a washing step, 100 μl of a goat F(ab′)2 anti-horse antibody conjugated to alkaline phosphatase (Jackson Immunoresearch) diluted 1/5,000 was added, and the mixture was incubated for 30 min at RT. Finally, 200 μl of substrate-containing buffer (0.1 M Tris-HCl [pH 8.2], 2 M NaCl, 1 mM MgCl2, 0.1 mM ZnCl2, 3 mM triethylamine, 10 mM p-nitrophenylphosphate [Amresco, Solon, Ohio]) was added, and the plates were read after 4 h at RT in a Titertek Multiskan MCC/340 spectrophotometer at 414 nm.
(ii) Determination of anti-DT titer.
DT-coated plates were washed, and serial dilutions of the different rabbit antisera were added and incubated overnight at 4°C. The plates were then washed, and goat F(ab′)2 anti-rabbit IgG antibody conjugated to alkaline phosphatase was added in a dilution of 1/5,000 for 30 min at RT. After incubation and washing, p-nitrophenylphosphate was added to each well, and the absorbance at 414 nm was read after 60 min. The titer was defined as the highest serum dilution giving an absorbance value of 0.6 at 414 nm after 60 min, above the negative control. As a control, we used nonimmune rabbit sera.
Assay of binding of ZZ-DTR to Vero cells.
Vero cells (kindly provided by Patrice Boquet) were grown in 250-ml culture flasks (Falcon) at 37°C in Dulbecco modified Eagle medium (DMEM; Biological Industries, Rehovot, Israel) supplemented with 10% fetal calf serum (without β-mercaptoethanol). Cells were passaged at confluency. Vero cells were detached from the flasks for experimental seeding by incubation in a 0.02% trypsin–0.05% EDTA solution (Biological Industries).
For assessment of the binding of ZZ-DTR to Vero cells, ZZ and ZZ-DTR were preincubated overnight at 4°C with a mouse IgG2a monoclonal antibody (MAb) labeled with fluorescein isothiocyanate (FITC) to form complexes. Single-cell suspensions (5 × 105 cells/50 μl) of Vero cells were subsequently incubated with 50 μl of either the FITC-labeled MAb (MAb-FITC), ZZ/MAb-FITC, or ZZDTR/MAb-FITC for 30 min at 4°C. Cells were then washed three times with phosphate-buffered saline (PBS)–0.5% bovine serum albumin, and 5,000 viable cells were analyzed on a FACScan (Becton Dickinson).
For assessment of the inhibition of DT cytotoxicity by ZZ-DTR, 96-well MADV N65 filter plates (Millipore) were seeded with 3 × 104 cells in each well. DT was serially diluted in DMEM and added to the wells in the presence of either CRM197 (Sigma, St. Louis, Mo.), ZZ-DTR, or ZZ (20 nM [final concentration] for each protein). After 3.5 h at 37°C, the medium was removed by filtration using the Millipore multiscreen assay system, and the cells were washed and filtered with cold Hanks balanced salt solution (Biological Industries). Cells were further incubated in Leu-deficient minimal essential medium (MEM; Sigma) for 1 h at 37°C. The medium was then removed by filtration, and MEM containing [14C]Leu (0.2 μCi/well; CEA, Saclay, France) was added. After 2.5 h at 37°C, the medium was removed and cells were washed twice with cold Hanks balanced salt solution. Cells were then solubilized with 0.4 M KOH for 10 min. Proteins were subsequently precipitated with 10% trichloroacetic acid (TCA) and collected on filters by using a TOMTEC apparatus (Wallac, Turku, Finland). Filters were dried, and [14C]Leu incorporation was measured by liquid scintillation using a 1450 Microbeta counter (Wallac).
Immunization.
The immunogenicity of ZZ-DTR, ZZ-DT168–220, and the diphtheria toxoid (Dtx; Pasteur Mérieux, Marcy l’Etoile, France) was tested in Blanc du Bouscat rabbits (2 kg, male) in two independent immunization schemes. In one case, 1.5 ml of PBS (Biological Industries) containing 4.65 × 10−5 M ZZ-DTR, ZZ-DT168–220, or Dtx was mixed with 1.5 ml of complete Freund’s adjuvant (Difco) for the first injection. Incomplete Freund’s adjuvant was used for the subsequent immunizations. In the second immunization scheme, 6.26 × 10−5 M ZZ-DTR or Dtx in 1.25 ml of PBS was mixed with 1.25 ml of aluminum hydroxide (Pierce, Rockford, Ill.). For all three immunizations, 1 ml with the indicated concentration per rabbit was injected subcutaneously.
Immunization with Freund’s adjuvant was made at days 0, 22, and 58 for ZZ-DT168–220 and at days 0, 20, and 56 for ZZ-DTR. Injections using aluminum hydroxide as adjuvant were given at days 0, 14, and 36.
Blood samples were taken 18 days (immunization with Freund’s adjuvant) or 21 days (immunization with aluminum hydroxide) after the third injection. Antisera were subsequently tested for the abilities to (i) recognize DT in an ELISA and (ii) neutralize the cytotoxic effect of DT in the in vitro Vero cell neutralization assay.
Vero cell neutralization assay.
Sera from rabbits immunized with ZZ-DTR, ZZ-DT168–220, or Dtx were serially diluted in DMEM containing 10−10 M DT and incubated overnight at 4°C. Samples were then added to 96-microfilter-well plates (MADV N65; Millipore) at 50 μl/well in the presence of Vero cells (3 × 104/well). After 3.5 h at 37°C, the cells were washed to remove unbound protein by using the Millipore multiscreen assay system, and the incorporation of [14C]Leu was measured as described for the assessment of the inhibition of DT cytotoxicity by ZZ-DTR. The neutralizing titer is expressed as the dilution of antiserum giving 50% inhibition of the cytotoxicity of 10−10 M DT.
RESULTS
Preparation of recombinant ZZ-DTR and ZZ-DT168–220.
Two nonoverlapping regions of DT were selected for this study. One of them, called DTR, corresponds to the region (aa 382 to 535), by which DT binds to cell surface receptors. The recent resolution of the three-dimensional structure of DT indicates that this region is structurally organized as a domain (6, 7) which therefore was anticipated to fold independently in a native-like structure. Several studies showed that numerous neutralizing MAbs raised against DT are directed against the R domain (8, 14, 28, 29, 37–39). Furthermore, a recent work showed that the R domain of DT can be a potential immunogen (13). The second region (aa 168 to 220) overlaps the loop that connects fragments A and B. Audibert et al. (2) have demonstrated that immunization of guinea pigs with peptide 188–201 elicits antibodies that bind to the toxin and neutralize its dermonecrotic effect. In addition, Zucker and Murphy (38) have shown that some of the anti-DT antibodies which recognize the C terminus of fragment A are protective. However, the loop region is described as being poorly antigenic (3). Therefore, we selected a longer sequence (aa 168 to 220) in order to include helix 7 of the C domain and helix 1 of the T domain, which may favor the structural stabilization of the segment.
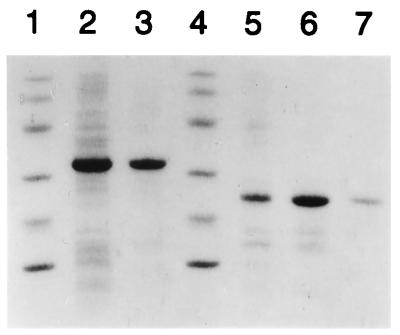
Using PCR, we cloned the sequences encoding DTR and DT168–220 in the pCP vector (10); the recombinant peptides were thus fused to the C terminus of ZZ, a synthetic repeat of the Ig binding domain of protein A from S. aureus (24). Since ZZ was not preceded by a signal sequence, the induction of the system led to the synthesis of ZZ-DTR or ZZ-DT168–220 fusion proteins in cytoplasm of E. coli. Two major proteins migrating at 32 and 22 kDa were detected by SDS-PAGE in the soluble fractions following inductions (Fig. 1, lanes 2 and 5). They have the expected molecular weights for ZZ-DTR and ZZ- DT168–220, respectively. The fractions were centrifuged and applied to an IgG-Sepharose 4B column. ZZ-DT168–220 was further purified on a Mono S column to eliminate the low-molecular-weight components which can be seen in the IgG-Sepharose-purified fraction. SDS-PAGE of the purified fractions is shown in Fig. 1. Approximately 60 mg of ZZ-DTR and 12 mg of ZZ-DT168–220 were obtained per liter of culture.
FIG. 1.
SDS-PAGE of ZZ-DTR and ZZ-DT168–220 at different purification steps and stained with Coomassie blue R-250. Lanes 1 and 4, molecular mass markers (97.4, 66, 45, 31, 21.5, and 14.5 kDa); lane 2, crude extract of ZZ-DTR after induction with IPTG; lane 3, ZZ-DTR eluates from an IgG-Sepharose 4B column; lane 5, crude extract of ZZ-DT168–220 after induction with IPTG; lane 6, ZZ-DT168–220 after passage through an IgG-Sepharose 4B column; lane 7, ZZ-DT168–220 after passage through a Mono S column.
Immunological characterization of ZZ-DTR and ZZ- DT168–220.
We first estimated the antigenicity of the recombinant hybrid proteins by Western blotting using a horse anti-DT antiserum (data not shown). Equine Igs were used since they are 100-fold less efficient than rabbit Igs at binding to protein A (15) and hence to the ZZ moiety of the hybrids. Thus, only the DT part of the hybrid could be revealed with this antiserum. A major protein band located at 32 kDa, which corresponds to the calculated molecular mass of ZZ-DTR, was observed in Western blots. In contrast, in the same conditions, only a faint band corresponding to the calculated molecular weight of ZZ-DT168–220 could be observed.
To further evaluate the antigenicity of the recombinant hybrids, we submitted them to an ELISA in which microtiter plates were coated with rabbit IgG (data not shown). Similar concentrations of ZZ-DTR and ZZ-DT168–220 were added to the wells, and after incubation for 3 h, the plates were washed. The DTR and DT168–220 domains of the two hybrids bound by their ZZ domain to coated rabbit IgGs were detected by using a horse anti-DT serum and a goat anti-horse antibody conjugated to a colorimetric enzyme. A clear coloration was found for ZZ-DTR (743 ± 6 mU of DO), whereas the coloration observed for ZZ-DT168–220 was quite weak and similar to that of ZZ (241 ± 4 and 236 ± 21 mU of DO, respectively). Therefore, only ZZ-DTR cross-reacted with DT antisera.
Binding of ZZ-DTR to Vero cells.
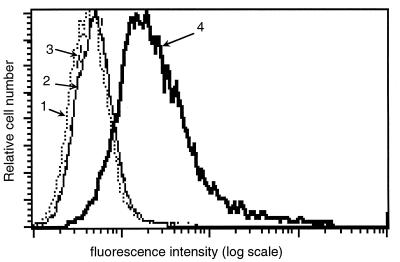
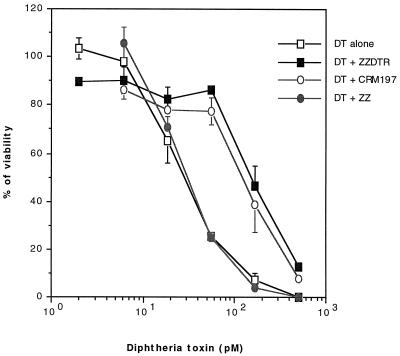
Previous results have shown that the binding of DT to its cell surface receptor is mediated via the R domain (30). To assess whether ZZ-DTR is also able to bind to the DT receptor, we performed a fluorescence-activated cell sorting analysis. ZZ and ZZ-DTR were complexed with MAb-FITC, and binding of the two complexes to Vero cells was investigated after a 30-min incubation at 4°C. As shown in Fig. 2, a shift in the fluorescence intensity of Vero cells was observed with ZZ-DTR/MAb-FITC, while no change was observed with either MAb-FITC alone or ZZ/MAb-FITC. Though this experiment does not tell us if DTR binds to DT receptors, it demonstrates that binding of ZZ-DTR to Vero cells is uniquely due to the DTR moiety. However, further data (Fig. 3) demonstrate that ZZ-DTR is efficient at inhibiting the cytotoxic capacity of DT. Thus, approximately 10 times more DT is required to kill 50% of the Vero cells when 20 nM ZZ-DTR is present in the wells. A control experiment revealed that the presence of an excess of free ZZ does not affect DT cytotoxicity, indicating that the inhibitory effect of the hybrid is specifically associated with the DTR domain. Furthermore, the inhibitory potency of ZZ-DTR is similar to that obtained with CRM197, a nontoxic mutant of DT which is devoid of any enzymatic activity but retains its binding property (20). In aggregate, therefore, our data suggest that the DTR moiety of ZZ-DTR contains sufficient structural information to specifically and efficiently recognize the DT receptor on Vero cells. As a consequence, the DTR domain is likely to be folded similarly in both the ZZ-DTR hybrid and native DT.
FIG. 2.
Binding of ZZ-DTR to Vero cells. ZZ and ZZ-DTR were incubated overnight at 4°C with MAb-FITC. The complexes were subsequently incubated for 30 min at 4°C, and binding to Vero cells was assessed by using a FACScan system. Curves: 1, Vero cells alone; 2, ZZ/MAb-FITC; 3, MAb-FITC; 4, ZZ-DTR/MAb-FITC.
FIG. 3.
ZZ-DTR protects Vero cells from DT-mediated toxicity. Vero cells were incubated with serial dilutions of DT and a fixed amount (20 nM [final concentration]) of either ZZ-DTR, ZZ, or CRM197. After 3.5 h, cells were washed and incubated for 1 h in Leu-deficient MEM and for 2.5 h at 37°C with [14C]Leu. The incorporation of [14C]Leu in cells was subsequently measured. Results are expressed as percent viability, where 1.5 nM DT was set as 0% and the absence of toxin was considered 100%.
Immunogenicity of fused DT fragment.
We investigated the ability of ZZ-DTR, ZZ-DT168–220 and Dtx (a toxoid of DT that is currently used in anti-DT vaccination) emulsified in Freund’s adjuvant, or ZZ-DTR and Dtx emulsified in aluminum hydroxide, to elicit a humoral immune response in rabbits. The rabbits were immunized three times, and blood samples were collected after the last immunization. The antisera were subsequently tested for the capacity to bind to DT coated on microtiter ELISA plates. ZZ-DTR elicited anti-DT titers which varied between either 1/300 and 1/1,500 or 1/400 and 1/3,000, depending on the immunization scheme (Fig. 4). The immunogenicity of ZZ-DT168–220 was substantially higher since in the presence of Freund’s adjuvant, titers of 1/4,900 and 1/38,000 were obtained with the two rabbits. Clearly, Dtx displayed the highest titers, which, however, were nearly 30-fold lower when immunization was performed with aluminum hydroxide rather than Freund’s adjuvant.
FIG. 4.
Antibody response to ZZ-DTR, ZZ-DT168–220, and Dtx in Blanc de Bouscat rabbits. The rabbits were immunized with either Freund’s adjuvant or aluminum hydroxide (Alum). Blood samples were collected, and sera were serially diluted in 0.1 M Tris buffer–0.1% caseine (pH 7.4) and incubated overnight at 4°C on DT-coated microtiter ELISA plates. Antibody binding was revealed with a goat anti-rabbit–PhoA conjugate. Titers are defined as the highest serum dilution that led to an absorbance of 0.6 at 414 nm in 1 h. Each dot represents the titer obtained for one rabbit.
Neutralization of cytotoxicity.
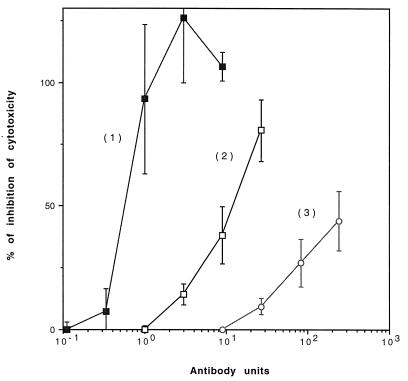
The neutralizing potencies of antisera raised in rabbits against ZZ-DTR, ZZ-DT168–220, and Dtx were tested in vitro, using toxin-sensitive Vero cells. DT was incubated at a concentration of 10−10 M in the presence of various dilutions of the different sera, and the mixtures were added to the Vero cells. The cells were assayed for [14C]Leu incorporation into TCA-precipitable material. Neutralization by immune sera is given as a function of antibody units, which compensates for the differences between the anti-DT titers for each antiserum. As shown in Fig. 5, the antibodies raised against ZZ-DT168–220 are poorly neutralizing whereas the antibodies raised against ZZ-DTR with aluminum hydroxide as adjuvant exerted a substantial neutralizing activity. A higher neutralizing potency was observed with antisera raised against Dtx injected with aluminum hydroxide; the ratio of neutralization was approximately 15-fold higher than that obtained with anti-ZZ-DTR.
FIG. 5.
Neutralization of DT on Vero cells with serum anti-Dtx (curve 1), anti-ZZ-DTR obtained with aluminum hydroxide as adjuvant (curve 2), and anti-ZZ-DT168–220 obtained with Freund adjuvant (curve 3). The different sera were serially diluted and coincubated with DT overnight at 4°C in DMEM; the Vero cells were incubated with this mixture for 3.5 h at 37°C, and [14C]Leu incorporation was detected after 2.5 h in the TCA-precipitable fraction. The data are presented as percent inhibition of cytotoxicity of 10−10 M DT as a function of antibody units. The antibody unit is defined as the highest serum dilution that lead to an absorbance of 0.6 at 414 nm in 1 h.
DISCUSSION
The production of efficient vaccines corresponding to a fragment of a protein still remains a challenge. Part of the difficulty resides in the necessity of producing the selected protein fragment in a structure that resembles the conformation adopted by the same fragment in the cognate protein (25). In this respect, the genetic approach makes it possible to produce large protein fragments which may be structurally organized as domains. In this work, we described the production of two recombinant fragments of DT fused with a synthetic Ig binding domain of protein A, called ZZ. These fragments show distinct immunological properties.
DT168–220 was chosen since peptide 188–201 was previously described to constitute a potentially interesting synthetic vaccine (2). We found that ZZ-DT168–220 has a poor capacity to bind to horse anti-DT antibodies, in agreement with a previous report concerning the antigenicity of this region (3). Several reasons can explain this behavior, including the possibility that (i) DT168–220 has a few reactive antigenic sites, (ii) this region is poorly immunogenic when DT is used as an immunogen, and/or (iii) the conformation of this region differs in the hybrid and in the native protein. At present, we cannot determine which of these possibilities prevails. However, a rather high immunogenic response was obtained when ZZ-DT168–220 was injected in rabbits, since anti-ZZ-DT168–220 antisera displayed a substantial capacity to bind to coated DT. However, despite their high antibody titer, the antisera poorly neutralize the capacity of DT to kill Vero cells. These results suggest that ZZ-DT168–220 does not constitute a potential vaccine.
The biological and immunological results obtained with ZZ-DTR are more promising. DTR is the domain by which DT recognizes its cell surface receptors (6, 7) and to which numerous neutralizing anti-DT antibodies are directed (38). We have seen that purified ZZ-DTR is well recognized by horse anti-DT antibodies and can bind to Vero cells. Furthermore, competition experiments performed with Vero cells revealed that ZZ-DTR inhibits competitively DT cytotoxicity, and this inhibition is as efficient as that observed with CRM197, a nontoxic mutant of DT which retains its full binding property (20). These observations suggest that the R domain of ZZ-DTR is likely to bind to DT receptors as efficiently as DT and hence to adopt a folding that is similar, if not identical, in the hybrid and native toxins. This hypothesis is further supported by the observation that anti-ZZ-DTR antisera bind to coated DT and neutralize in vitro DT cytotoxicity to Vero cells. Interestingly, a higher titer of anti-ZZ-DTR antiserum was obtained upon immunization in rabbits by using aluminum hydroxide, an adjuvant that is commonly used in human vaccination. Altogether, these results suggest that fusion of an Ig binding region of protein A to the amino-terminal end of the R domain of DT hampers neither its receptor binding activity nor its ability to elicit neutralizing antibodies.
However, one cannot yet conclude that the ZZ-DTR hybrid constitutes an appropriate vaccine against diphtheria. First, it remains to be demonstrated that rabbits immunized with ZZ-DTR are protected against DT. Second, our comparative analysis of antisera revealed that the commercial DTx elicits antisera with higher anti-DT titers and higher neutralizing potency compared to ZZ-DTR. Third, the ZZ moiety can bind to rabbit IgGs, and the immunization with such a fusion protein may potentially elicit an anti-IgG immune response. These issues raised the question of how to control DTR immunogenicity. One way might be to use other fusion proteins (34) or to introduce additional T-cell epitopes to the hybrid (19). A number of hybrid proteins have been recently described in the field of vaccine development. Barbieri et al. (4) have reported a fusion protein consisting of fragment A of DT and the S1 subunit of pertussis toxin. Antisera raised against this hybrid were neutralizing for both toxins. Boucher et al. (9) have added protective epitopes to a fusion protein (p75) comprising the pertussis toxin S1 subunit and the protective fragment C of tetanus toxin. The recombinant protein retained the biological activities of both toxin parts and elicited neutralizing antibody. Another approach may consist of using other adjuvants, like muramyl dipeptide (1). Finally, one may also envision the possibility of producing bacteria displaying heterologous proteins on their surface, such as the gram-positive bacterium Staphylococcus carnosus (32).
ACKNOWLEDGMENTS
We gratefully acknowledge Jérome Galon for assistance with FACS analysis. We thank P. Boquet for his gift of plasmid pK5DT.
REFERENCES
- 1.Ada G L. Vaccines. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press Ltd.; 1993. pp. 1309–1352. [Google Scholar]
- 2.Audibert F, Jolivet M, Chedid L, Alouf J E, Boquet P, Rivaille P, Siffert O. Active antitoxic immunization by a diphtheria toxin synthetic oligopeptide. Nature. 1981;289:593–594. doi: 10.1038/289593a0. [DOI] [PubMed] [Google Scholar]
- 3.Autran B, Triebel F, Viguier M, Jolivet M, Falmagne P, Debre P. Monoclonal B-cell response to diphtheria toxoid: evidence for cross-reactive epitopes. Immunology. 1987;60:531–538. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbieri J T, Armellini D, Molkentin J, Rappuoli R. Construction of a diphtheria toxin A fragment-C180 peptide fusion protein which elicits a neutralizing antibody response against diphtheria toxin and pertussis toxin. Infect Immun. 1992;60:5071–5077. doi: 10.1128/iai.60.12.5071-5077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beachey E H, Seyer J M, Dale J B, Simpson W A, Kang A. Type specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature. 1981;292:457. doi: 10.1038/292457a0. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M J, Choe S, Eisenberg D. Refined structure of dimeric diphtheria toxin at 2.3 Å resolution. Protein Sci. 1994;3:1444–1463. doi: 10.1002/pro.5560030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett M J, Eisenberg D. Refined structure of monomeric diphtheria toxin at 2.3 Å resolution. Protein Sci. 1994;3:1464–1475. doi: 10.1002/pro.5560030912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigio M, Rossi R, Nucci D, Antoni G, Rappuoli R, Ratti G. Conformational changes in diphtheria toxoids. Analysis with monoclonal antibodies. FEBS Lett. 1987;218:271–276. doi: 10.1016/0014-5793(87)81060-8. [DOI] [PubMed] [Google Scholar]
- 9.Boucher P, Sato H, Sato Y, Locht C. Neutralizing antibodies and immunoprotection against pertussis and tetanus obtained by use of a recombinant pertussis toxin-tetanus toxin fusion protein. Infect Immun. 1994;62:449–456. doi: 10.1128/iai.62.2.449-456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevet P, Lemaire C, Gasparini S, Zinn-Justin S, Lajeunesse E, Ducancel F, Pinkasfeld S, Courçon M, Boulain J C, Ménez A. High level production and isotope labelling of snake neurotoxins, disulphide rich proteins. Protein Expression Purif. 1997;10:293–300. doi: 10.1006/prep.1997.0740. [DOI] [PubMed] [Google Scholar]
- 11.Ducancel F, Boulain J C, Tremeau O, Ménez A. Direct expression in Escherichia coli of a functionally active protein A-snake toxin fusion protein. Protein Eng. 1989;3:139–143. doi: 10.1093/protein/3.2.139. [DOI] [PubMed] [Google Scholar]
- 12.Fromen-Romano C, Maillère B, Drevet P, Lajeunesse E, Ducancel F, Boulain J C, Ménez A. Transformation of a non-enzymatic toxin into a toxoid by genetic engineering. Protein Eng. 1997;10:101–108. doi: 10.1093/protein/10.10.1213. [DOI] [PubMed] [Google Scholar]
- 13.Fu H, Shen W H, Collier R J. Receptor-binding domain of diphtheria toxin as a potential immunogen. Vaccines. 1993;93:379–383. [Google Scholar]
- 14.Hayakawa S, Uchida T, Mekada E, Moynihan M R, Okada Y. Monoclonal antibody against diphtheria toxin. Effect on toxin binding and entry into cells. J Biol Chem. 1983;258:4311–4317. [PubMed] [Google Scholar]
- 15.Langone J J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- 16.Léonetti, M., R. Thaï, J. Cotton, S. Leroy, P. Drevet, F. Ducancel, J.-C. Boulain, and A. Ménez. Increasing immunogenicity of fused antigens by cell surface targeting. Submitted for publication. [PubMed]
- 17.Löwenadler B, Jansson B, Paleus S, Holmgren E, Nilsson B, Moks T, Palm G, Josephson S, Philipson L, Uhlén M. A gene fusion system for generating antibodies against short peptides. Gene. 1987;58:87–97. doi: 10.1016/0378-1119(87)90032-1. [DOI] [PubMed] [Google Scholar]
- 18.Löwenadler B, Nilsson B, Abrahmsén L, Moks T, Ljungquist L, Holmgren E, Paleus S, Josephson S, Philipson L, Uhlén M. Production of specific antibodies against protein A fusion proteins. EMBO J. 1986;5:2393–2398. doi: 10.1002/j.1460-2075.1986.tb04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowenadler B, Lycke N, Svanholm C, Svennerholm A-M, Krook K, Gidlund M. T and B cell responses to chimeric proteins containing heterologous T helper epitopes inserted at different positions. Mol Immunol. 1992;29:1185–1190. doi: 10.1016/0161-5890(92)90054-2. [DOI] [PubMed] [Google Scholar]
- 20.Mekada E, Uchida T. Binding properties of diphtheria toxin to cells are altered by mutation in the fragment A domain. J Biol Chem. 1985;260:12148–12153. [PubMed] [Google Scholar]
- 21.Michel M-L, Pontisso P, Sobczak E, Malpièce Y, Streeck R E, Tiollais P. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerised human serum albumin. Proc Natl Acad Sci USA. 1984;81:7708. doi: 10.1073/pnas.81.24.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson B, Abrahmsén L. Fusions to staphyloccocal protein A. Methods Enzymol. 1990;185:144–161. doi: 10.1016/0076-6879(90)85015-g. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson B, Forsberg G, Hartmanis M. Expression and purification of recombinant insulin-like growth factor, from Escherichia coli. Methods Enzymol. 1991;198:3–16. doi: 10.1016/0076-6879(91)98003-o. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson B, Moks T, Jansson B, Abrahmsén L, Elmblad A, Holmgren E, Henrichson C, Jones T A, Uhlén M. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 25.Palmenberg A. A vaccine for the common cold? Nature. 1987;329:668. doi: 10.1038/329668a0. [DOI] [PubMed] [Google Scholar]
- 26.Pappenheimer A M. Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- 27.Peppoloni S, Pizza M, DeMagistris M T, Bartoloni A, Rappuoli R. Acellular pertussis vaccine composed of genetically inactivated pertussis toxin. Physiol Chem Phys Med NMR. 1995;27:355–361. [PubMed] [Google Scholar]
- 28.Rolf J M, Eidels L. Structure-function analyses of diphtheria toxin by use of monoclonal antibodies. Infect Immun. 1993;61:994–1003. doi: 10.1128/iai.61.3.994-1003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolf J M, Eidels L. Characterization of the diphtheria toxin receptor-binding domain. Mol Microbiol. 1993;7:585–591. doi: 10.1111/j.1365-2958.1993.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 30.Rolf J M, Gaudin H M, Eidels L. Localization of the diphtheria toxin receptor-binding domain to the carboxyl-terminal Mr approximately 6000 region of the toxin. J Biol Chem. 1990;265:7331–7337. [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Samuelson P, Hanson M, Ahlborg N, Andreoni C, Götz F, Bachi T, Nguyen T N, Binz H, Uhlén M, Stahl S. Cell surface display of recombinant proteins on Staphylococcus carnosus. J Bacteriol. 1995;177:1470–1476. doi: 10.1128/jb.177.6.1470-1476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 34.Uhlén M, Forsberg G, Moks T, Hartmanis M, Nilsson B. Fusion proteins in biotechnology. Curr Opin Biotechnol. 1992;3:363–369. doi: 10.1016/0958-1669(92)90164-e. [DOI] [PubMed] [Google Scholar]
- 35.Valerie K, Fronko G, Long W, Henderson E E, Nilsson B, Uhlén M, de Riel J K. Production and detection of coliphage T4 endonuclease V polyclonal and monoclonal antibodies using staphylococcal protein A hybrid proteins. Gene. 1987;58:99–107. doi: 10.1016/0378-1119(87)90033-3. [DOI] [PubMed] [Google Scholar]
- 36.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimori T, Yamada M, Sugawa H, Mekada E, Uchida T, Okada Y. Monoclonal antibodies against diphtheria toxin fragment A. Characterization and introduction into living cells. Exp Cell Res. 1984;151:344–353. doi: 10.1016/0014-4827(84)90385-9. [DOI] [PubMed] [Google Scholar]
- 38.Zucker D R, Murphy J R. Monoclonal antibody analysis of diphtheria toxin. I. Localization of epitopes and neutralization of cytotoxicity. Mol Immunol. 1984;21:785–793. doi: 10.1016/0161-5890(84)90165-2. [DOI] [PubMed] [Google Scholar]
- 39.Zucker D R, Murphy J R, Pappenheimer A M. Monoclonal antibody analysis of diphtheria toxin. II. Inhibition of ADP-ribosyl-transferase activity. Mol Immunol. 1984;21:795–800. doi: 10.1016/0161-5890(84)90166-4. [DOI] [PubMed] [Google Scholar]