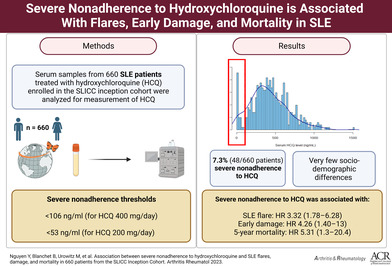
Abstract
Objective
The goals of this study were to assess the associations of severe nonadherence to hydroxychloroquine (HCQ), objectively assessed by HCQ serum levels, and risks of systemic lupus erythematosus (SLE) flares, damage, and mortality rates over five years of follow‐up.
Methods
The Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort is an international multicenter initiative (33 centers throughout 11 countries). The serum of patients prescribed HCQ for at least three months at enrollment were analyzed. Severe nonadherence was defined by a serum HCQ level <106 ng/mL or <53 ng/mL for HCQ doses of 400 or 200 mg/day, respectively. Associations with the risk of a flare (defined as a Systemic Lupus Erythematosus Disease Activity Index 2000 increase ≥4 points, initiation of prednisone or immunosuppressive drugs, or new renal involvement) were studied with logistic regression, and associations with damage (first SLICC/American College of Rheumatology Damage Index [SDI] increase ≥1 point) and mortality with separate Cox proportional hazard models.
Results
Of the 1,849 cohort participants, 660 patients (88% women) were included. Median (interquartile range) serum HCQ was 388 ng/mL (244–566); 48 patients (7.3%) had severe HCQ nonadherence. No covariates were clearly associated with severe nonadherence, which was, however, independently associated with both flare (odds ratio 3.38; 95% confidence interval [CI] 1.80–6.42) and an increase in the SDI within each of the first three years (hazard ratio [HR] 1.92 at three years; 95% CI 1.05–3.50). Eleven patients died within five years, including 3 with severe nonadherence (crude HR 5.41; 95% CI 1.43–20.39).
Conclusion
Severe nonadherence was independently associated with the risks of an SLE flare in the following year, early damage, and five‐year mortality.

INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease in which preventing adverse long‐term outcomes remains a major challenge. The efficacy of antimalarials, especially hydroxychloroquine (HCQ), is well established. 1 , 2 Besides reducing the risk of SLE flares, HCQ is beneficial against SLE‐related comorbidities, including diabetes, thrombotic events, and dyslipidemia, 3 , 4 , 5 , 6 , 7 and against long‐term damage 8 , 9 , 10 and death. 11 It is widely recommended that patients with SLE receive this treatment. 12 , 13 , 14
Like all self‐administered medications, HCQ's effectiveness is impaired by nonadherence, reported to range between 3% and 85% in SLE. 8 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Low whole blood HCQ levels are a marker of SLE exacerbation because of their pharmacokinetic/pharmacodynamic relations. 16 Because its blood half‐life is at least 5 days, and its terminal half‐life 43 days, 31 a very low blood HCQ level is an objective indicator of severe nonadherence, identifying patients who have not taken HCQ for a significant period of time and not those who have just missed a few tablets. 16 , 17 , 25 , 26 , 27 , 28 , 30 , 32 , 33 , 34 In a first study, published in 2007, we retrospectively validated an HCQ cutoff <200 ng/ml in whole blood to identify severely nonadherent patients. 16 Other cutoffs have been proposed since then: 500 ng/ml, 35 100 ng/ml, 15 ng/ml, 17 or undetectable whole‐blood HCQ levels. Most studies have measured HCQ in whole blood. Large longitudinal cohorts, however, most often collect serum samples, with whole blood samples being relatively rare. Recently, we compared whole blood and serum levels 36 and found a mean serum/whole blood HCQ ratio of 0.53 ± 0.15. We concluded that when whole blood is unavailable, serum HCQ levels can be used to assess nonadherence.
In this study, we aimed to assess whether patients prescribed HCQ for at least three months but with objective severe nonadherence, defined by very low HCQ serum levels, were at higher risk of SLE flares in the subsequent year, and of damage and death up to five years later.
What is already known on this topic
In addition to reducing the risk of systemic lupus erythematosus (SLE) flares, hydroxychloroquine has multiple benefits against SLE‐related comorbidities and the risks of long‐term damage and of mortality.
Hydroxychloroquine's effectiveness is impaired by nonadherence, reported to range from 3% to 85% in patients with SLE.
What this study adds
In the large, international, multicenter, longitudinal Systemic Lupus International Collaborating Clinics cohort, using serum hydroxychloroquine levels, we found a 7.3% rate of severe nonadherence.
Severe nonadherence to hydroxychloroquine was independently associated with the risk of an SLE flare, early damage, and mortality.
How this study might affect research, practice, or policy
Our results suggest the benefits of testing for detecting severe nonadherence and of dedicating more resources and more time to these patients to improve their long‐term prognosis.
PATIENTS AND METHODS
The SLICC Inception Cohort
The Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort was recruited between 1999 and 2011 from 33 centers in 11 countries within North America, Europe, and Asia. 8 , 37 Patients were enrolled within 15 months of fulfilling at least four of the 1997 American College of Rheumatology (ACR) revised classification criteria for SLE. 38 After the enrollment visit, patients were seen annually at their study center by a clinician, who completed a detailed case report form. Data were submitted to the coordinating center at the University of Toronto for storage in a centralized database. Annual serum samples have been collected from most patients.
Study participants
We analyzed serum samples of patients who were prescribed HCQ for at least three months at cohort enrollment. The current HCQ course, including its start date and its average dose, were collected at enrollment and at each subsequent visit. We used sera sampled at enrollment in the cohort, or, if unavailable, during the first‐year follow‐up visit after enrollment. The date of the serum sample corresponded to time zero (T0). Patients not treated with HCQ (ie, those for whom the drug was contraindicated), treated for less than three months at enrollment, or who had no follow‐up visit after T0 were excluded. The Institutional Research Ethics Boards of participating centers approved the SLICC Inception Cohort Study in accordance with the Declaration of Helsinki guidelines for research in humans. All patients provided written informed consent.
Serum hydroxychloroquine measurement and definition of severe nonadherence
All serum HCQ levels were assayed at Cochin Hospital by a previously published method. 41 In this study, we compared whole blood and serum levels 36 and found mean ± SD HCQ concentrations of 469 ± 223 ng/mL in serum and 916 ± 449 ng/mL in whole blood, for a mean serum:whole blood HCQ ratio of 0.53 ± 0.15. Two independent groups subsequently confirmed this result, reporting ratios of 0.51 37 and 0.54 41 and high reproducibility. To determine if serum HCQ level cutoffs could be established to identify severely nonadherent patients, we calculated the following thresholds for nonadherence by extrapolation: prescribed HCQ dose of 400 mg/day: <106 ng/mL (corresponding to 200 ng/mL in whole blood); prescribed HCQ dose of 200 mg/day: <53 ng/ml (100 ng/mL in whole blood); other prescribed HCQ daily doses were rounded to the nearest of 200 or 400 mg/day. The 300 mg/day dose was rounded up to 400 mg/day.
In our previous studies, relatively few patients took 200 mg/day (7%–15%), 16 , 30 and we used the same cutoff for patients treated with 200 and 400 mg/day. The relation between HCQ daily dose and HCQ blood level is nonetheless linear, as shown in 2016, 25 and patients treated with 200 mg/day are expected to have blood or serum HCQ levels half those of patients treated with 400 mg/day. We thus chose to adapt our thresholds with different thresholds based on the daily HCQ dose. We also, however, conducted two sensitivity analyses that defined severe nonadherence in all patients by the thresholds of 106 ng/mL and 53 ng/mL, regardless of daily HCQ dose. Patients were considered to have nonquantifiable serum HCQ levels at <20 ng/mL (lower limit of quantification).
Clinical variables
Data from T0 included the following: demographic features including age, sex, Black race (yes/no), educational level (high school education or less vs postsecondary education), dyslipidemia, diabetes mellitus, and body mass index (BMI; <18; 18–25; 25–30, >30 kg/m2). We collected current prescriptions of prednisone and other immunosuppressive medications (methotrexate, azathioprine, mycophenolate mofetil, rituximab, and oral or intravenous [IV] cyclophosphamide) at T0, again including their start dates.
Follow‐up data collected over the subsequent five years included SLE activity, defined by the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI‐2K), 42 damage defined by the SLICC/American College of Rheumatology Damage Index (SDI), 43 , 44 new (since the previous visit) course of oral or IV prednisone or other immunosuppressive agent with its start date, any new renal involvement since the previous visit, and deaths. Causes of death were collected and analyzed.
Outcome definition
An SLE flare was defined by a composite outcome involving at least one of the following events in the first year after T0: (a) increase of at least 4 points in the SLEDAI‐2K; (b) new start of prednisone (oral or IV) or other immunosuppressive agent (azathioprine, methotrexate, mycophenolate mofetil, rituximab, oral or IV cyclophosphamide); (c) new renal involvement since the last visit, including new active nephritis, defined by hematuria (>5 red blood cells/high power field [HPF]) and/or pyuria (>5 white blood cells/HPF), both after exclusion of other causes, a new or recent increase of >500 mg 24‐hour protein, or heme granular or red blood cell casts; or new nephrotic syndrome. Increased damage was defined by an SDI increase of ≥1 point in the five years after T0.
Statistical analyses
Descriptive statistics were used to summarize enrollment data: counts (percentages) for categorical variables and medians (interquartile ranges) or mean ± SD for continuous variables. Characteristics of patients at T0 with and without severe HCQ nonadherence were compared by Student's t‐tests for continuous variables and chi‐squared tests for categorical variables.
To assess the association between severe nonadherence and an SLE flare, we used logistic regression models, with and without adjustment for potentially relevant variables assessed at T0. Sensitivity analyses studied each individual outcome of the primary endpoint separately and assessed the association between nonquantifiable serum HCQ levels and the primary composite outcome. We also performed two other sensitivity analyses, one applying a severe nonadherence threshold of 106 ng/mL and the other 53 ng/mL, for all patients, regardless of their prescribed daily dose.
The association between severe nonadherence and the risk of increased damage was assessed by survival analysis. For five years after T0, patients contributed person‐time from T0 until the first worsening score, loss to follow‐up, or death, whichever occurred first. To assess the risk of early damage, we computed sensitivity survival analyses censoring patients at one, two, three, and four years. Patients with no available SDI score recorded at T0 (because they had been diagnosed for less than six months) had it imputed by the SDI value at the first follow‐up visit. Associations were assessed with Cox proportional hazard models, adjusted for sex, educational level, and relevant covariables associated with SDI worsening in univariate Cox models. Among patients with an SDI increase of ≥1 point in the five years after HCQ measurement, damage included in the SDI was compared between nonadherent patients and the others. We also separately compared damage that was more likely to be related to steroid/cyclophosphamide treatment (including cataracts, retinal change, or optic atrophy, muscle atrophy or weakness, osteoporosis, premature gonadal failure, and diabetes mellitus) versus other damage considered related to SLE itself.
Finally, we assessed the association between severe nonadherence and deaths (all causes) in the five years after T0 with Cox proportional hazard models. All analyses were performed with R version 3.6.1 (R Foundation for Statistical Computing). Data are available upon reasonable request.
RESULTS
Study population
By February 2021, of the 1,849 patients enrolled in the SLICC Inception Cohort, 824 had been treated with HCQ for three months or longer at enrollment (Supplementary Figure 1). Among these 824 patients, 663 had an available serum sample from the enrollment visit or during the first follow‐up visit and met the inclusion criteria. Serum HCQ levels were measured for 660 (99.5%) patients; the other three had technical issues (insufficient serum quantity). Compared with the excluded patients, our study population was slightly older (34 vs 31 years, P = 0.002) and more likely to have a postsecondary education level (63% vs 55%, P < 0.001), but had a lower frequency of renal (20% vs 32%, P < 0.001) and neurologic involvement (2.7% vs 6%, P = 0.002) (Supplementary Table 1). The interval between their diagnosis and inclusion was longer (6.8 vs 4.0 months, P < 0.001), because patients taking HCQ for less than three months at enrollment were excluded. The populations did not differ for sex, cigarette smoking, or Black race.
The HCQ samples were taken at T0, which was either cohort enrollment for 634 (96%), or the first follow‐up visit for 26 (3.9%). Table 1 presents patients’ characteristics at T0. Median follow‐up was 6.1 years (interquartile range [IQR] 3.0–9.7 years) after T0, and 401 (61%) patients were followed up for at least five years.
Table 1.
Characteristics of the study population and outcomes, according to severe nonadherence to HCQ at T0*
| Patients’ characteristics | Overall (n = 660) | Severe nonadherence to HCQ | P value | |
|---|---|---|---|---|
| No (n = 612) | Yes (n = 48) | |||
| Female sex, n (%) | 580 (87.9) | 536 (87.6) | 44 (91.7) | 0.545 |
| Pregnancy, n (%) | 7 (1.1) | 6 (1.0) | 1 (2.1) | 1.00 |
| Black race, n (%) | 107 (16.2) | 96 (15.7) | 11 (22.9) | 0.269 |
| Age at serum sample, mean ± SD, y | 36.2 ± 13.5 | 36.4 ± 13.7 | 33.4 ± 10.7 | 0.132 |
| Months since SLE diagnosis, mean ± SD | 7.2 ± 4.6 | 7.1 ± 4.6 | 8.4 ± 4.9 | 0.062 |
| Education level, n (%) | 0.886 | |||
| Postsecondary | 413 (62.6) | 382 (62.4) | 31 (64.6) | |
| High school or less | 247 (37.4) | 230 (37.6) | 17 (35.4) | |
| Cigarette smoking, n (%) | 0.642 | |||
| Nonsmoker | 437 (66.2) | 408 (66.7) | 29 (60.4) | |
| Current or past smoker | 222 (33.6) | 203 (33.2) | 19 (39.6) | |
| Not available | 1 (0.2) | 1 (0.2) | 0 (0.0) | |
| Main clinical manifestations, n (%) | ||||
| Renal disease | 131 (19.8) | 120 (19.6) | 11 (22.9) | 0.715 |
| Neurologic disorder | 18 (2.7) | 16 (2.6) | 2 (4.2) | 0.861 |
| SLEDAI‐2K at T0, mean ± SD | 4.8 ± 4.9 | 4.8 ± 4.8 | 6.0 ± 5.8 | 0.091 |
| Other comorbidities | ||||
| BMI, mean ± SD, kg/m2 | 25.8 ± 6.1 | 25.7 ± 6.0 | 27.3 ± 7.2 | 0.074 |
| Dyslipidemia, n (%) | 55 (8.3) | 50 (8.2) | 5 (10.4) | 0.786 |
| Diabetes mellitus, n (%) | 17 (2.6) | 16 (2.6) | 1 (2.1) | 0.658 |
| Treatment at T0, n (%) | ||||
| HCQ, daily dose | ||||
| 200 mg/day | 155 (23.5) | 143 (23.4) | 12 (25.0) | 0.936 |
| 400 mg/day | 505 (76.5) | 469 (76.6) | 36 (75.0) | |
| Corticosteroids | 438 (66.4) | 404 (66.0) | 34 (70.8) | 0.602 |
| Other immunosuppressive drugs | 242 (36.7) | 219 (35.8) | 23 (47.9) | 0.127 |
| Azathioprine | 91 (13.8) | 78 (12.7) | 13 (27.1) | 0.011 |
| Cyclophosphamide | 25 (3.8) | 22 (3.6) | 3 (6.2) | 0.592 |
| Methotrexate | 65 (9.8) | 62 (10.1) | 3 (6.2) | 0.537 |
| Mycophenolate mofetil | 58 (8.8) | 53 (8.7) | 5 (10.4) | 0.881 |
| Other immunosuppressant a | 7 (1.1) | 6 (1.0) | 1 (2.1) | 1 |
| Outcomes, n (%) | ||||
| SLE flare within one year | 191 (28.9) | 163 (26.6) | 28 (58.3) | <0.001 |
| ≥4‐point increase in SLEDAI‐2K | 68 (10.3) | 57 (9.3) | 11 (22.9) | 0.006 |
| New steroid and/or IS | 94 (14.2) | 78 (12.7) | 16 (33.3) | <0.001 |
| New renal involvement | 71 (10.8) | 62 (10.1) | 9 (18.8) | 0.107 |
| ≥1‐point increase SDI within 5 y | 167 (25.3) | 152 (24.8) | 15 (31.2) | 0.417 |
| Death within 5 y | 11 (1.7) | 8 (1.3) | 3 (6.2) | 0.047 |
HCQ, hydroxychloroquine; IS, immunosuppressive drug; kg/m2, kilograms per square meter; SD, standard deviation; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLE, systemic lupus erythematosus; SLEDAI‐2K, Systemic Lupus Erythematosus Disease Activity Index 2000; T0, time zero.
Other immunosuppressant users included four patients treated with cyclosporine, one with sulfasalazine, and one with intravenous immunoglobulins (in the “not severely nonadherent” group), and one patient with rituximab (in the “nonadherent” group).
HCQ levels and nonadherence at T0
HCQ had been prescribed for a mean ± SD of 8.7 ± 10.4 months before T0: 7.4 ± 5.5 months for nonadherent patients versus 8.8 ± 10.7 months for the others (P = 0.373). The daily HCQ dose was 400 mg for 428 patients and 200 mg for 141 patients, and the other 91 doses were rounded to the closest daily prescription; doses of 300 mg (n = 62) were rounded up to 400 mg/day. Overall, the median serum HCQ level was 388 ng/mL (IQR 244–566 ng/mL).
For the 155 patients with prescribed HCQ doses of or rounded to 200 mg/day, the median HCQ level was 250 ng/mL (IQR 158–365 ng/mL). Twelve (7.7%) had an HCQ level <53 ng/mL and were thus considered severely nonadherent. For the 505 patients with an HCQ prescription of or rounded to 400 mg/day, the median HCQ level was 427 ng/mL (IQR 287–602 ng/mL); 36 (7.1%) had an HCQ level <106 ng/mL and were thus severely nonadherent.
Accordingly, the overall population contained 48 (7.3%) severely nonadherent patients, 28 (4.2% of the overall cohort) of whom had nonquantifiable serum HCQ levels. Figure 1 presents the distributions of HCQ levels in the overall population and by prescribed HCQ dose.
Figure 1.
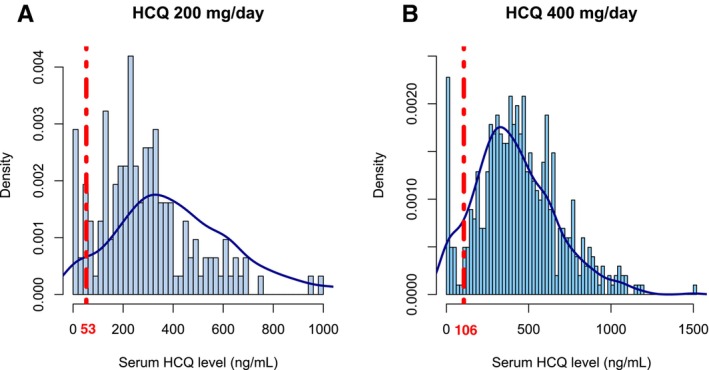
Distribution of serum hydroxychloroquine (HCQ) levels according to daily prescribed dose: (A) 200 mg/day and (B) 400 mg/day. The red dotted lines represent thresholds for severe nonadherence to HCQ: (A) 53 ng/mL and (B) 106 ng/mL.
Of note, among the 62 patients with an HCQ prescription of 300 mg/day, 3 (4.8%) had an HCQ level <106 ng/mL. Moreover, among the seven pregnant patients at T0, only one was considered nonadherent, with an undetectable level of HCQ. None had flares or increased their SDI during follow‐up.
Factors associated with severe nonadherence to HCQ at T0
No sociodemographic, clinical, or laboratory factors were clearly associated with severe nonadherence (Table 1). In the univariate analyses, although the current prescription of immunosuppressive treatment at T0 did not differ significantly between the groups, azathioprine treatment was more frequently currently prescribed in severely nonadherent patients at T0 (27.1% vs 12.7%; P = 0.011). Among the nonadherent patients, azathioprine was prescribed before or concomitantly with HCQ in 72.7% (8 of 11 patients with an available start date), and was prescribed 0.5, 0.8, and 1.2 months after HCQ for the other three patients (Supplementary Figure 2). Several other variables also showed nonsignificant trends, including higher SLEDAI‐2K at T0 (6.0 vs 4.8), higher BMI at T0, (27.3 vs 25.7 kg/m2), and Black race (22.9% vs 16.2%) among nonadherent patients.
Severe nonadherence to HCQ at T0 and risk of SLE flares at 1 year
An SLE flare occurred in the year after T0 in 163 patients without and 28 patients (58.3%) with severe nonadherence. An increase of at least 4 points in the SLEDAI‐2K was observed in 57 patients(9.3%) without and 11 patients (22.9%) with severe nonadherence. New prednisone and/or another immunosuppressive drug was prescribed for 78 patients (12.7%) without and 16 patients (33.3%) with severe nonadherence, and new renal involvement was identified in 62 (10.1%) and 8 patients (18.8%), respectively (Table 1).
In the univariate analyses, age, race, SLEDAI‐2K at T0, current course of immunosuppressive treatments at T0, and severe HCQ nonadherence were all associated with the SLE flare risk (Table 2). In the multivariate analysis, severe nonadherence constituted the most important independent risk factor for flare (adjusted OR [aOR] 3.32; 95% confidence interval [CI] 1.78–6.28) (Table 2).
Table 2.
Odds ratios (95% confidence intervals) for the risk of an SLE flare in the year after the HCQ measurement at T0*
| Demographic data and comorbidities | Overall (n = 660) | SLE flare within one year a | ||
|---|---|---|---|---|
| Patients (n = 191) | Univariate OR (95% CI) | Multivariate OR (95% CI) b | ||
| Age at serum sample, mean ± SD, y | 36.2 ± 13.5 | 33.3 ± 12.2 | 0.98 (0.96–0.99) | 0.98 (0.97–0.99) |
| Male, n (%) | 580 (87.9) | 167 (87.4) | 1.06 (0.63–1.75) | – |
| Black race, n (%) | 107 (16.2) | 47 (24.6) | 2.22 (1.45–3.40) | 2.09 (1.33–3.26) |
| Education level, n (%) | ||||
| Postsecondary | 413 (62.6) | 116 (60.7) | Reference | Reference |
| High school or less | 247 (37.4) | 75 (39.3) | 1.12 (0.79–1.58) | 1.10 (0.76–1.58) |
| Cigarette smoking, n (%) | ||||
| Nonsmoker | 437 (66.2) | 127 (66.8) | Reference | |
| Current or past smoker | 222 (33.6) | 63 (33.2) | 0.97 (0.67–1.38) | |
| BMI, mean ± SD, kg/m2 | 25.8 ± 6.1 | 25.9 ± 6.0 | 1.00 (0.98–1.03) | |
| SLEDAI‐2K at T0, mean ± SD | 4.8 ± 4.9 | 5.9 ± 5.7 | 1.06 (1.02–1.10) | 1.05 (1.01–1.09) |
| Corticosteroids, n (%) | 438 (66.4) | 137 (71.7) | 1.42 (0.99–2.06) | 1.00 (0.67–1.51) |
| Azathioprine, n (%) | 91 (13.8) | 38 (19.9) | 1.95 (1.23–3.07) | 1.63 (0.99–1.51) |
| HCQ adherence, n (%) | ||||
| Severe HCQ nonadherence | 48 (7.3) | 28 (14.7) | 3.86 (2.12–7.12) | 3.32 (1.78–6.28) |
| Nonquantifiable | 28 (4.2) | 16 (8.4) | 3.48 (1.62–7.67) | |
BMI, body mass index; CI, confidence interval; HCQ, hydroxychloroquine; IV, intravenous; OR, odds ratio; SLEDAI‐2K, Systemic Lupus Erythematosus Disease Activity Index 2000; T0, time zero.
An SLE flare was defined by occurrence of one of the following events in the first year after the T0 visit: (a) ≥4 points in the SLEDAI‐2K; (b) new start in prednisone (oral or pulsed) or other immunosuppressive agent (azathioprine, methotrexate, mycophenolate mofetil, rituximab, oral or IV cyclophosphamide); and/or (c) new renal involvement including active nephritis, new nephrotic syndrome, new dialysis, or kidney transplantation.
The following variables were included in the multivariate model: age, Black race, education level (postsecondary; high school or less), SLEDAI‐2000, corticosteroids, azathioprine, and severe HCQ nonadherence. No interaction was found between nonadherence to HCQ and age (P = 0.51), education (P = 0.47), Black race (P = 0.26), SLEDAI‐2K (P = 0.59), azathioprine (P = 0.23), or with corticosteroids (P = 0.49).
When we considered each component of the primary endpoint separately, severe HCQ nonadherence was associated with a SLEDAI‐2K increase of 4 points or more (aOR 3.19; 95% CI 1.42–6.81) (Supplementary Table 2) and with the risk of a new prednisone and/or other immunosuppressive prescription (aOR 3.16; 95% CI 1.59–6.07) (Supplementary Table 3), but not with new renal involvement (aOR 1.41; 95% CI 0.56–3.25) (Supplementary Table 4). Nonquantifiable serum HCQ levels also predicted flare risk defined by the primary composite endpoint (aOR 2.82; 95% CI 1.24–6.54, data not shown).
Finally, applying each of the two thresholds for the definition of severe nonadherence (106 ng/mL and 53 ng/mL) to all patients, regardless of the prescribed HCQ dosage, yielded similar results: these definitions were again associated with the flare risk (aOR 2.38; 95% CI 1.34–4.22 and aOR 3.01; 95% CI 1.55–5.94, respectively) (Supplementary Table 5).
Severe nonadherence to HCQ at T0 and risk of damage at five years
In the five years after T0, the SDI of 167 patients (25.3%) had increased by at least 1 point: 152 patients (24.8%) without and 15 patients (31.2%) with severe nonadherence. In the univariate analyses, age, Black race, education, cigarette smoking, BMI, immunosuppressive treatment, SLEDAI‐2K, and nonquantifiable serum levels of HCQ were associated with risk of damage (Table 3). There was no statistically significant trend toward a higher risk of damage with severe nonadherence (hazard ratio [HR] 1.30; 95% CI 0.74–2.29; Figure 2). In the multivariate analyses, nonquantifiable serum levels of HCQ were independently associated with risk of damage (adjusted HR [aHR] 1.93, 95% CI 1.04–3.59, Table 3, Figure 2), along with age (aHR 1.02 per 1 year; 95% CI 1.01–1.03) and lower educational level (HR 1.94; 95% CI 1.43–2.64 for high school or less education, compared with postsecondary education) (data not shown).
Table 3.
Hazard ratios (95% confidence intervals) for the risk of damage (≥1‐point increase of the SLICC damage index) in the five years after measurement of serum hydroxychloroquine level at time zero (T0) (n = 660)*
| Demographic data and comorbidities | Overall, n = 660 | ≥1‐point increase in SLICC damage index within 5 years | ||
|---|---|---|---|---|
| n events (%) or mean ± SD, n = 167 | Univariate OR (95% CI) | Multivariate OR (95% CI) | ||
| Age at serum sample, mean ± SD, y | 36.2 ± 13.5 | 39.0 ± 15.3 | 1.02 (1.01–1.03) | 1.02 (1.01–1.03) |
| Male, n (%) | 80 (12.7) | 20 ± 12.0 | 1.04 (0.65–1.65) | |
| Black race, n (%) | 107 (16.2) | 36 ± 21.6 | 1.59 (1.10–2.30) | 1.64 (1.11–2.42) |
| Education level, n (%) | ||||
| Post‐secondary | 413 (62.6) | 80 ± 47.9 | Reference | Reference |
| High school or less | 247 (37.4) | 87 ± 52.1 | 2.02 (1.49–2.73) | 1.92 (1.40–2.63) |
| Cigarette smoking, n (%) | ||||
| Non‐smoker | 437 (66.2) | 97 ± 58.1 | Reference | |
| Current or past smoker | 222 (33.6) | 69 ± 41.3 | 1.52 (1.12–2.07) | |
| BMI, kg/m2, mean ± SD | 25.8 ± 6.1 | 26.8 ± 6.5 | 1.03 (1.01–1.06) | |
| SLEDAI‐2K at T0, mean ± SD | 4.8 ± 4.9 | 5.4 ± 5.5 | 1.03 (1.00–1.06) | 1.03 (1.00–1.06) |
| Treatment at T0, n (%) | ||||
| Corticosteroids | 438 (66.4) | 118 ± 70.7 | 1.28 (0.92–1.79) | |
| Azathioprine | 91 (13.8) | 36 ± 21.6 | 2.01 (1.39–2.90) | 1.85 (1.26–2.73) |
| Hydroxychloroquine | ||||
| Severe HCQ nonadherence | 48 (7.3) | 15 ± 9.0 | 1.47 (0.86–2.49) | 1.30 (0.74–2.29) |
| Nonquantifiable serum levels | 28 (4.2) | 11 ± 6.6 | 1.99 (1.08–3.66) | |
Results are expressed as n (%) for categorical variables and mean ± SD for continuous variables. The following variables were included in the multivariate model: age, Black race, education level (post‐secondary; ≤ high school), SLEDAI‐2000, azathioprine, and severe HCQ nonadherence.
BMI, body mass index; CI, 95% confidence interval; HCQ, hydroxychloroquine; kg/m2, kilograms per square meter; OR, odds ratio; SD, standard deviation; SLICC, Systemic Lupus International Collaborating Clinics; SLEDAI‐2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
Figure 2.
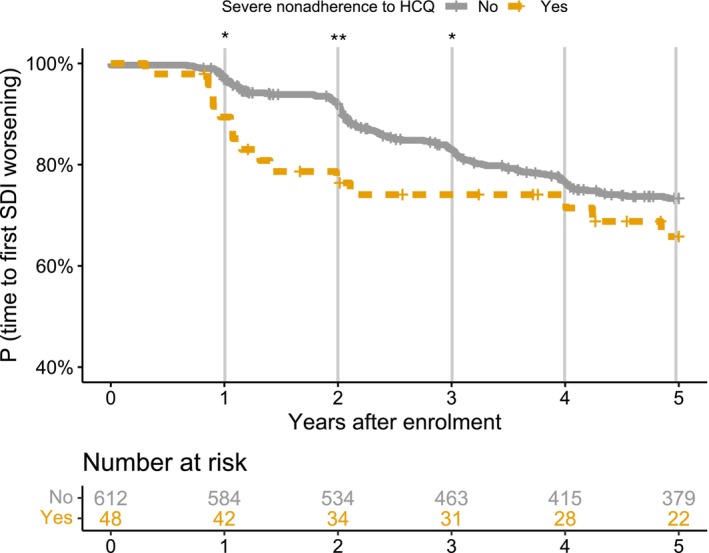
Kaplan‐Meier curves for the risk of damage, defined by a ≥1‐point increase of SDI, according to severe nonadherence. Severe nonadherence was associated with the risk of SDI worsening at one (adjusted HR 4.26; 95% CI 1.40–13), two (adjusted HR 3.54; 95% CI 1.83–6.86), and three years after the HCQ measurement (adjusted HR 1.92; 95% CI 1.05–3.50), with a nonsignificant trend at five years (adjusted HR 1.47; 95% CI 0.86–2.49). The following variables were included in the multivariate models: age, Black race, education level (postsecondary; high school or less), SLEDAI‐2K, azathioprine, and severe HCQ nonadherence. CI, 95% confidence interval; HCQ, hydroxychloroquine; HR, hazard ratio; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SLEDAI‐2K, Systemic Lupus Erythematosus Disease Activity Index 2000. *P < 0.05; **P < 0.01; NS, nonsignificant.
We observed that the trajectories of damage accrual diverged at one, two, and three years and then tended to converge by five years (Figure 2), although precision was limited at that point. The risk of worsening damage was higher during the first year (aHR 4.26; 95% CI 1.40–13), between T0 and year 2 (aHR 3.54; 95% CI 1.83–6.86), and between T0 and year 3 (aHR 1.92; 95% CI 1.05–3.50). We also explored whether effects on damage differed according to whether it was likely related to uncontrolled disease activity or to treatment side effects.
Five years after T0, among patients with an SDI increase ≥1 point (n = 167), patients with severe nonadherence had treatment‐related damage (13.3% vs 33.6%) less frequently and disease‐related damage (100% vs 80.9%) more frequently, although these differences were not statistically significant (Supplementary Table 6).
Severe nonadherence to HCQ at T0 and mortality at five years
In the five years after T0, 11 patients died, including the 3 of 48 patients with severe nonadherence. In the univariate analyses, the HR for the risk of death during this five‐year period was 5.41 (95% CI 1.43–20.39) for patients with severe nonadherence (Table 4). The reported causes of death for the three severely nonadherent patients were multiorgan failure due to SLE and cardiac tamponade, probable septic shock with end‐stage renal disease, and cardiorespiratory arrest with respiratory failure; the adherent patients died from cardiorespiratory failure (n = 3), sepsis (n = 2), pulmonary vasculitis (n = 1), or from unknown causes (n = 2).
Table 4.
Hazard ratios (95% confidence intervals) for the risk of death in the five years after measurement of the serum hydroxychloroquine level at time zero (T0) (N = 660)*
| Demographic data and comorbidities | Overall, n = 660 | Death within 5 years | |
|---|---|---|---|
| n events (%) or mean ± SD, n = 11 | Univariate OR (95% CI) | ||
| Age at serum sample, years, mean ± SD | 36.2 ± 13.5 | 44.8 ± 19.3 | 1.05 (1.01–1.09) |
| Male, n (%) | 80 (12.7) | 0 (0.0) | – |
| Black race, n (%) | 107 (16.2) | 2 (18.2) | 1.31 (0.28–6.06) |
| Education level, n (%) | |||
| Post‐secondary | 413 (62.6) | 5 (45.5) | Reference |
| High school or less | 247 (37.4) | 6 (54.5) | 2.25 (0.69–7.37) |
| Cigarette smoking, n (%) | |||
| Non‐smoker | 437 (66.2) | 5 (45.5) | Reference |
| Current or past smoker | 222 (33.6) | 6 (54.5) | 2.63 (0.80–8.61) |
| BMI, kg/m2, mean ± SD | 25.79 ± 6.1 | 26.3 ± 6.8 | 1.03 (0.93–1.14) |
| SLEDAI‐2K at T0, mean ± SD | 4.8 ± 4.9 | 6.6 ± 6.1 | 1.07 (0.97–1.18) |
| Corticosteroids | 438 ± 66.4 | 11 ± 100.0 | 1.42 (0.99–2.06) |
| Azathioprine | 91 ± 13.8 | 2 ± 18.2 | 1.66 (0.36–7.69) |
| Hydroxychloroquine | |||
| Severe HCQ nonadherence | 48 ± 7.3 | 3 ± 27.3 | 5.41 (1.43–20.39) |
| Nonquantifiable serum levels | 28 ± 4.2 | 1 ± 9.1 | 2.87 (0.37–22.38) |
Results are expressed as n (%) for categorical variables and mean ± SD for continuous variables. BMI, body mass index; CI, 95% confidence interval; HCQ, hydroxychloroquine; HR, hazard ratio; kg/m2, kilograms per square meter; OR, odds ratio; SD, standard deviation; SLEDAI‐2000, Systemic Lupus Erythematosus Disease Activity Index 2000.
DISCUSSION
Our study showed a 7.3% rate of severe nonadherence based on HCQ levels at enrollment in an inception cohort of patients with lupus. In this large, international, multicenter, longitudinal cohort, severe nonadherence to HCQ was independently associated with the risk of an SLE flare in the following year, with damage at one, two, and three years and with five‐year mortality. Nonquantifiable serum HCQ levels were also associated with the risk of damage within five years.
Our results are concordant with the 7% nonadherence rate reported in a previous French series 16 and are lower than other similar published studies of HCQ blood or serum levels, 27 , 28 , 45 which found nonadherence rates as high as 29%. This may be explained by differences (in age distribution, SLE duration, etc) in study populations, because adherence is known to vary by age and decrease over time. 46 Different threshold HCQ concentrations defining nonadherence might also contribute to these discrepancies. Other studies, mainly based on self‐administered questionnaires, found higher nonadherence rates, but questionnaires probably measure different nonadherence patterns (tablets missed relatively infrequently), as reflected by the moderate correlation between these methods. 30
The only characteristic associated with severe nonadherence was azathioprine use at T0, with nonsignificant trends toward higher SLEDAI‐2K, higher BMI, and higher proportions of female and Black patients among those nonadherent. The fact that azathioprine was prescribed before or concomitantly with HCQ for most nonadherent patients makes the likelihood of azathioprine prescription as a consequence of nonadherence very unlikely. A previous French series found a higher SLEDAI‐2K score was the main factor that differentiated adherent from nonadherent patients. 16 An international longitudinal study found that younger age, absence of steroid treatment, higher BMI, and unemployment independently predicted nonadherence defined by blood drug measurements. 30 Some studies assessing nonadherence with self‐reported questionnaires have reported race/ethnicity, disease duration, low education, and/or younger age to be associated with nonadherence, 20 , 21 , 44 but as stated above, self‐reported nonadherence might represent a different pattern, distinguishable from severe nonadherence defined by blood/serum levels. The absence of any predictive marker of severe nonadherence was unsurprising, given the poor correlation between nonadherence by drug level and by physician assessment that some of our group demonstrated in an international study. 30 This suggests the importance of HCQ measurements for unmasking severe nonadherence.
HCQ has long been known to decrease SLE activity and prevent flares. 48 Recently, a study from the SLICC group demonstrated higher SLE flare risk after HCQ discontinuation or taper versus maintenance. 49 However, few studies have assessed the risk of SLE flares associated with HCQ nonadherence, although low blood and serum levels of HCQ are associated with increased SLE activity or subsequent systemic and renal flares during follow‐up. 16 , 17 , 25 , 26 , 33 , 36 , 51 , 52 , 53 We found severe nonadherence was clearly associated with flare risk, defined by a SLEDAI–2K increase of 4 points or more, a new prednisone or other immunosuppressive prescription, or new renal involvement. The remarkable stability of the associations with each component of our composite outcome examined separately strengthens our findings.
The risk of damage (SDI increase) within five years did not differ significantly according to severe nonadherence, but the significance of the association at one, two, and three years suggests an association with early damage. Strikingly, two different kinetics of damage accrual (Figure 2) were observed: early damage in severely nonadherent patients and the convergence of these curves due to later damage accrual in adherent patients. Damage captured by SDI can be related to the disease itself but also to its treatment; corticosteroid use is associated with the transition from no damage to damage and with greater pre‐existing damage. 8 Treatment‐related damage includes diabetes, muscle atrophy, osteoporosis, avascular necrosis, or cataract (possibly due to glucocorticoids), retinal damage (potentially due to HCQ), or premature infertility (potentially due to other immunosuppressive drugs). Such damage usually takes years to occur, whereas damage directly linked to SLE may occur earlier. To explain our findings, particularly the different curves of Figure 2, we hypothesized that treatment‐related damage occurred mainly in adherent patients and appeared after a few years, whereas other damage (often due to SLE activity) occurred more frequently and earlier in the disease in severely nonadherent patients. We indeed found such a tendency, albeit not statistically significant, possibly due to inadequate power. As our group and others have shown that damage in SLE predicts future damage accrual and mortality and that severe nonadherence appears to be a major and potentially modifiable risk factor for damage accrual, these findings strongly argue that severe nonadherence should be actively sought by assessing HCQ levels as a first step toward improving adherence. 17 , 53 , 54
Finally, severe nonadherence to HCQ was associated with the risk of death, even though the small number of events prevented any multivariate analyses. Although HCQ's role in reducing mortality in patients with SLE is known, 11 to our knowledge, this is the first time that a link between severe nonadherence and mortality has been demonstrated in patients with SLE. Admittedly, the inability to use multivariate models limits the strength of our conclusion.
Some limitations must be acknowledged: First, including only patients prescribed HCQ for at least three months at inclusion and with an available sample excluded more than 60% of the cohort (including 40% initially included less than three months after their SLE diagnosis). Nonetheless, our study population differed only slightly from the excluded population (see Supplemental Materials).
Second, to define severe nonadherence, we used prespecified thresholds, based on the prescribed HCQ daily dosage, as our rate of patients taking 200 mg/day was higher than in previous studies. Thus, if the daily dose was not 200 or 400 mg, we had to round to the nearest category, which could have introduced some bias. However, among the patients with 300 mg/day (n = 62), fewer than 5% were considered nonadherent, below the 7.3% rate for the overall population. This finding suggests that this rounding did not artificially increase the number of nonadherent patients. Furthermore, choosing each of the two thresholds we used to define nonadherence regardless of HCQ dose led to very similar results, thus confirming the robustness of our findings.
Third, the validation of the bioanalytical method for HCQ assessment in serum samples according to the European Medicines Agency recommendations ensures the measurement's robustness. 55 The literature include no data about the stability of HCQ in serum for a prolonged interval between sampling and assay. Nonetheless, the median serum HCQ levels we found were very close to reports from other studies and suggests that any potential degradation of HCQ would have had a limited impact on our results.
Fourth, seven patients were pregnant at T0. Although pregnancy can impact adherence and dosing, only one patient was considered severely nonadherent. The patient's undetectable serum level makes it unlikely that the conclusion of nonadherence is wrong. Furthermore, no pregnant patient had a flare or an increase in SDI during follow‐up. It is therefore unlikely that these pregnancies affected our results.
Fifth, we defined SLE flares by a composite endpoint: increased SLEDAI‐2K, new renal involvement, or a new prescription for prednisone and/or other immunosuppressive agent. Follow‐up visits and SLE activity assessment occurred yearly. Using only an increase in SLEDAI‐2K might not have captured an SLE flare occurring between two follow‐up visits, whereas new treatment or new renal involvement since the last visit might well reflect such a flare. Moreover, a similar composite endpoint has previously been used in SLICC cohort studies, 49 and our findings remained significant when the SLEDAI‐2K increase and new treatments were assessed separately.
We acknowledge that we only had one serum HCQ measurement at T0—not repeated measurements. Thus, we could not consider patients with severe nonadherence to HCQ at T0 who became adherent during follow‐up visits or those in the inverse situation. To limit this bias, SLE flares were assessed in the year after T0. We assessed the risk of damage and mortality at five years and showed that severe nonadherence shown by a single measurement was associated with these risks, thus strengthening the demonstration of this measurement's utility, even when performed only once. Finally, two‐thirds of the patients took concomitant steroids, and one‐third had other concomitant immunosuppressive treatments. It is likely that at least some patients with severe nonadherence to HCQ were also nonadherent to other treatments, as previously shown. 16 Unfortunately, it was not possible to measure adherence to other treatments; although the levels of some can be measured in serum samples, they reflect only very recent drug intake, in contrast to HCQ (and azathioprine metabolites), which have a long half‐life. In any case, regardless of adherence to the other drugs, HCQ nonadherence is easy to assess with blood or serum HCQ measurement and may well reflect global treatment adherence. Thus, interventions on HCQ nonadherence might also apply to other drugs if relevant.
Our study has several strengths, including the large cohort size and the multicenter design. Data were collected longitudinally, and very few were missing. The high number of events (disease flares or damage) provided sufficient statistical power to show associations. We also measured HCQ serum levels centrally and demonstrated that serum bank samples can be used when whole blood is not available, as previously suggested. 35 , 36
In conclusion, we demonstrated that severe nonadherence to HCQ is associated with unfavorable outcomes among patients with SLE, including flares, SLE damage, and death. As severe nonadherence is often unknown by the physician and because no predictive clinical or biological factors have been identified, our results underline the benefits of systematically testing to detect severe nonadherence and identify the patients at risk. Once uncovered, dedicating more resources and more time to these patients, and implementing specific strategies for them may help prevent SLE flares and damage and thus improve their long‐term prognosis.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Pr. Costedoat‐Chalumeau had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Nguyen, Blanchet, Urowitz, Hanly, Gordon, Bae, Romero‐Diaz, Clarke, Bernatsky, Wallace, Isenberg, Rahman, Merrill, Fortin, Gladman, Bruce, Petri, Ginzler, Dooley, Ramsey‐Goldman, Manzi, Jönsen, Alarcón, van Vollenhoven, Aranow, Le Guern, Mackay, Ruiz‐Irastorza, Lim, Inanc, Kalunian, Jacobsen, Peschken, Kamen, Askanase, Buyon, Costedoat‐Chalumeau.
Acquisition of data
Nguyen, Blanchet, Costedoat‐Chalumeau.
Analysis and interpretation of data
Nguyen, Blanchet, Clarke, Bernatsky, Gladman, Aranow, Buyon, Costedoat‐Chalumeau.
Supporting information
Disclosure Form
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The authors are indebted to all participants of the SLICC cohort, to all physicians and their study coordinators who included patients in the cohort, and the supporting staff who made it possible to follow up these patients over time. The authors thank Jo Ann Cahn for editorial assistance.
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
The Hopkins Lupus Cohort is supported by the NIH (grants AR43727 and 69572). The Montreal General Hospital Lupus Clinic is partially supported by the Singer Family Fund for Lupus Research. Dr. Hanly's work was supported by Canadian Institutes of Health Research grant MOP‐88526. Dr. Gordon's work was supported by Lupus UK, Sandwell and West Birmingham NHS Trust, and the Birmingham National Institute for Health and Care Research/Wellcome Trust Clinical Research Facility at University Hospital Birmingham NHS Foundation Trust. Dr. Bae's work was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF‐2021R1A6A1A03038899). Dr. Clarke holds The Arthritis Society Chair in Rheumatic Diseases at the University of Calgary. The work of Drs. Isenberg and Rahman was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. Dr. Fortin presently holds a tier 1 Canada Research Chair on Systemic Autoimmune Rheumatic Diseases at Université Laval, and part of this work was done while he was still holding a Distinguished Senior Investigator of The Arthritis Society. Dr. Bruce's work was supported by Arthritis Research UK, the National Institute for Health Research Biomedical Research Unit Funding Scheme, and the NIHR Manchester Biomedical Research Centre and the NIHR/Wellcome Trust Clinical Research Facility at Central Manchester Foundation Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. Dr. Jacobsen's work was supported by the Danish Rheumatism Association (A1028) and the Novo Nordisk Foundation (A05990). Dr. Dooley's work was supported by NIH grant RR00046. Dr. Ramsey‐Goldman's work was supported by the NIH (grants 5UL‐1TR‐001422‐02, formerly 8UL‐1TR‐000150 and UL‐1RR‐025741, K24‐AR‐02318, and P30‐AR‐072579, formerly P60‐AR‐064464 and P60‐AR‐48098). Dr. Ruiz‐Irastorza's work was supported by the Department of Education of the Basque Government, research grant IT 1512‐22.
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/art.42645).
Author disclosures and graphical abstract are available at https://onlinelibrary.wiley.com/doi/10.1002/art.42645.
REFERENCES
- 1. Canadian Hydroxychloroquine Study Group . A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991;324(3):150–154. [DOI] [PubMed] [Google Scholar]
- 2. Costedoat‐Chalumeau N, Dunogué B, Morel N, et al. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med 2014;43(6 Pt 2):e167–e180. [DOI] [PubMed] [Google Scholar]
- 3. Petri M, Konig MF, Li J, et al. Association of higher hydroxychloroquine blood levels with reduced thrombosis risk in systemic lupus erythematosus. Arthritis Rheumatol 2021;73(6):997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babary H, Liu X, Ayatollahi Y, et al. Favorable effects of hydroxychloroquine on serum low density lipid in patients with systemic lupus erythematosus: a systematic review and meta‐analysis. Int J Rheum Dis 2018;21(1):84–92. [DOI] [PubMed] [Google Scholar]
- 5. Stojan G, Petri M. Atherosclerosis in systemic lupus erythematosus. J Cardiovasc Pharmacol 2013;62(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kavanaugh A, Adams‐Huet B, Jain R, et al. Hydroxychloroquine effects on lipoprotein profiles (the HELP trial): a double‐blind, randomized, placebo‐controlled, pilot study in patients with systemic lupus erythematosus. J Clin Rheumatol 1997;3(1):3–8. [PubMed] [Google Scholar]
- 7. Chen YM, Lin CH, Lan TH, et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose‐dependent manner: a population‐based cohort study. Rheumatology (Oxford) 2015;54(7):1244–1249. [DOI] [PubMed] [Google Scholar]
- 8. Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74(9):1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akhavan PS, Su J, Lou W, et al. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol 2013;40(6):831–841. [DOI] [PubMed] [Google Scholar]
- 10. Pakchotanon R, Gladman DD, Su J, et al. More consistent antimalarial intake in first 5 years of disease is associated with better prognosis in patients with systemic lupus erythematosus. J Rheumatol 2018;45(1):90–94. [DOI] [PubMed] [Google Scholar]
- 11. Jin Z, Wang F, Pan W, et al. Association of antimalarial drugs with decreased overall and cause specific mortality in systemic lupus erythematosus. Rheumatology (Oxford) 2021;60(4):1774–1783. [DOI] [PubMed] [Google Scholar]
- 12. Costedoat‐Chalumeau N, Leroux G, Piette JC, et al. Why all systemic lupus erythematosus patients should be given hydroxychloroquine treatment? Joint Bone Spine 2010;77(1):4–5. [DOI] [PubMed] [Google Scholar]
- 13. Gordon C, Amissah‐Arthur MB, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford) 2018;57(1):e1–e45. [DOI] [PubMed] [Google Scholar]
- 14. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78(6):736–745. [DOI] [PubMed] [Google Scholar]
- 15. Costedoat‐Chalumeau N, Pouchot J, Guettrot‐Imbert G, et al. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol 2013;27(3):329–340. [DOI] [PubMed] [Google Scholar]
- 16. Costedoat‐Chalumeau N, Amoura Z, Hulot JS, et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann Rheum Dis 2007;66(6):821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durcan L, Clarke WA, Magder LS, et al. Hydroxychloroquine blood levels in SLE: clarifying dosing controversies and improving adherence. J Rheumatol 2015;42(11):2092–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chambers SA, Raine R, Rahman A, et al. Why do patients with systemic lupus erythematosus take or fail to take their prescribed medications? A qualitative study in a UK cohort. Rheumatology (Oxford) 2009;48(3):266–271. [DOI] [PubMed] [Google Scholar]
- 19. Koneru S, Shishov M, Ware A, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum 2007;57(6):1000–1006. [DOI] [PubMed] [Google Scholar]
- 20. Garcia‐Gonzalez A, Richardson M, Garcia Popa‐Lisseanu M, et al. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol 2008;27(7):883–889. [DOI] [PubMed] [Google Scholar]
- 21. Julian LJ, Yelin E, Yazdany J, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum 2009;61(2):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daleboudt GM, Broadbent E, McQueen F, et al. Intentional and unintentional treatment nonadherence in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63(3):342–350. [DOI] [PubMed] [Google Scholar]
- 23. Oliveira‐Santos M, Verani JFS, Klumb EM, et al. Evaluation of adherence to drug treatment in patients with systemic lupus erythematosus in Brazil. Lupus 2011;20(3):320–329. [DOI] [PubMed] [Google Scholar]
- 24. Marengo MF, Waimann CA, de Achaval S, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus 2012;21(11):1158–1165. [DOI] [PubMed] [Google Scholar]
- 25. Mok CC, Penn HJ, Chan KL, et al. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: A longitudinal cohort analysis. Arthritis Care Res (Hoboken) 2016;68(9):1295–1302. [DOI] [PubMed] [Google Scholar]
- 26. Cunha C, Alexander S, Ashby D, et al. Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome? Nephrol Dial Transplant 2018;33(9):1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iudici M, Pantano I, Fasano S, et al. Health status and concomitant prescription of immunosuppressants are risk factors for hydroxychloroquine nonadherence in systemic lupus patients with prolonged inactive disease. Lupus 2018;27(2):265–272. [DOI] [PubMed] [Google Scholar]
- 28. Geraldino‐Pardilla L, Perel‐Winkler A, Miceli J, et al. Association between hydroxychloroquine levels and disease activity in a predominantly Hispanic systemic lupus erythematosus cohort. Lupus 2019;28(7):862–867. [DOI] [PubMed] [Google Scholar]
- 29. Petri M, Perez‐Gutthann S, Longenecker JC, et al. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med 1991;91(4):345–353. [DOI] [PubMed] [Google Scholar]
- 30. Costedoat‐Chalumeau N, Houssiau F, Izmirly P, et al. A prospective international study on adherence to treatment in 305 patients with flaring SLE: assessment by drug levels and self‐administered questionnaires. Clin Pharmacol Ther 2018;103(6):1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carmichael SJ, Charles B, Tett SE. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther Drug Monit 2003;25(6):671–681. [DOI] [PubMed] [Google Scholar]
- 32. Costedoat‐Chalumeau N, Isenberg D, Petri M. Letter in response to the 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus by Fanouriakis et al. Ann Rheum Dis 2019. [DOI] [PubMed] [Google Scholar]
- 33. Yeon Lee J, Lee J, Ki Kwok S, et al. Factors related to blood hydroxychloroquine concentration in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017;69(4):536–542. [DOI] [PubMed] [Google Scholar]
- 34. Balevic SJ, Green TP, Clowse MEB, et al. Pharmacokinetics of hydroxychloroquine in pregnancies with rheumatic diseases. Clin Pharmacokinet 2019;58(4):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shinjo SK, Bonfá E, Wojdyla D, et al. Antimalarial treatment may have a time‐dependent effect on lupus survival: Data from a multinational Latin American inception cohort. Arthritis Rheum 2010;62(3):855–862. [DOI] [PubMed] [Google Scholar]
- 36. Blanchet B, Jallouli M, Allard M, et al. Hydroxychloroquine levels in patients with systemic lupus erythematosus: whole blood is preferable but serum levels also detect nonadherence. Arthritis Res Ther 2020;22(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Urowitz MB, Gladman D, Ibañez D, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus 2007;16(9):731–735. [DOI] [PubMed] [Google Scholar]
- 38. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40(9):1725–1725. [DOI] [PubMed] [Google Scholar]
- 39. Qu Y, Noe G, Breaud AR, et al. Development and validation of a clinical HPLC method for the quantification of hydroxychloroquine and its metabolites in whole blood. Future Sci OA 2015;1(3):FSO26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baert CA, Nieuwland S, Sokolova T, et al. Comparison of hydroxychloroquine titers measured in frozen/thawed serum and whole blood obtained from lupus patients. Lupus 2022;31(6):767–768. [DOI] [PubMed] [Google Scholar]
- 41. Carlsson H, Hjorton K, Abujrais S, et al. Measurement of hydroxychloroquine in blood from SLE patients using LC‐HRMS—evaluation of whole blood, plasma, and serum as sample matrices. Arthritis Res Ther 2020;22(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29(2):288–291. [PubMed] [Google Scholar]
- 43. Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39(3):363–369. [DOI] [PubMed] [Google Scholar]
- 44. Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997;40(5):809–813. [DOI] [PubMed] [Google Scholar]
- 45. Ting TV, Kudalkar D, Nelson S, et al. Usefulness of cellular text messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. J Rheum 2012;39(1):174–179. [DOI] [PubMed] [Google Scholar]
- 46. Feldman CH, Collins J, Zhang Z, et al. Dynamic patterns and predictors of hydroxychloroquine nonadherence among Medicaid beneficiaries with systemic lupus erythematosus. Semin Arthritis Rheum 2018;48(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosley‐Williams A, Lumley MA, Gillis M, et al. Barriers to treatment adherence among African American and White women with systemic lupus erythematosus. Arthritis Rheum 2002;47(6):630–638. [DOI] [PubMed] [Google Scholar]
- 48. Dima A, Jurcut C, Chasset F, et al. Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge. Ther Adv Musculoskelet Dis 2022;14:1759720X211073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Almeida‐Brasil CC, Hanly JG, Urowitz M, et al. Flares after hydroxychloroquine reduction or discontinuation: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis 2022;81(3):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Costedoat‐Chalumeau N, Amoura Z, Hulot J‐S, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54(10):3284–3290. [DOI] [PubMed] [Google Scholar]
- 51. Costedoat‐Chalumeau N, Galicier L, Aumaître O, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013;72(11):1786–1792. [DOI] [PubMed] [Google Scholar]
- 52. Costedoat‐Chalumeau N, Guern VL, Piette JC. Routine hydroxychloroquine blood concentration measurement in systemic lupus erythematosus reaches adulthood. J Rheumatol 2015;42(11):1997–1999. [DOI] [PubMed] [Google Scholar]
- 53. Costedoat‐Chalumeau N, Houssiau FA. Improving medication adherence in patients with lupus nephritis. Kidney Int 2021;99(2):285–287. [DOI] [PubMed] [Google Scholar]
- 54. Alarcón GS, Roseman JM, McGwin G, et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004;43(2):202–205. [DOI] [PubMed] [Google Scholar]
- 55. EMA . Bioanalytical method validation ‐ Scientific guideline. European Medicines Agency 2018. Available at: https://www.ema.europa.eu/en/bioanalytical-method-validation-scientific-guideline. Accessed February 13, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1: Supporting Information


