Abstract
In this study, we investigated the properties of ascorbic acid (vitamin C), which is a naturally occurring water-soluble vitamin. Our goal is to evaluate its pro-oxidative and/or antioxidant capabilities. To do this, we initially used a confocal laser scanning microscope (CLSM) to visualize the differentiation pattern in U-937 cells under the treatment of variable concentrations of ascorbic acid. Prior to induction, U-937 cells showed a spherical morphology. After treatment, significant morphological changes were observed in the form of prominent pseudopodia and amoeboid structures. Interestingly, pseudopodia incidences increased with an increase in ascorbic acid concentrations. In addition, our analysis of protein modification using anti-malondialdehyde antibodies showed changes in more than one protein. The findings reveal the link between the differentiation of U-937 cells into macrophages and the protein modifications triggered by the production of reactive oxygen species when U-937 cells are exposed to ascorbic acid. Furthermore, the transformation of ascorbic acid from a pro-oxidative to an antioxidant property is also demonstrated.
Keywords: Vitamin C, Reactive oxygen species, Human cells, Antioxidants, Pro-oxidant
Highlights
-
•
Ascorbic acid as a pro-oxidant and antioxidant.
-
•
Cell differentiation and reactive oxygen species generation.
-
•
Malondialdehyde formation during cell differentiation.
1. Introduction
Ascorbic acid (vitamin C) is a natural water-soluble vitamin and a potent reducing and antioxidant agent [[1], [2], [3]]. It functions in fighting bacterial infections, in detoxification, and, in the formation of collagen in fibrous tissue, teeth, bones, connective tissue, skin, and capillaries, besides absorption of iron [4,5]. Since it is obtained from food and available through a wide range of supplements, severe deficiency caused by ascorbic acid is rather rare. In unusual cases, it can lead to conditions such as weakness, fatigue, joint and muscle aches, and bleeding gums, which are also symptoms associated with scurvy [6].
Ascorbic acid is a powerful antioxidant and helps to protect the body from the harmful effects of free radicals [7] Besides this, ascorbic acid is essential for the synthesis of collagen, helps in stimulating the production of white blood cells, iron absorption, synthesis of neurotransmitters. It also acts as natural antihistamines and promotes the formation of connective tissues [[8], [9], [10], [11], [12]]. Free radicals or reactive oxygen species (ROS) are unstable molecules that can damage cells and are associated with the development of chronic diseases such as cancer, heart diseases, and Alzheimer's disease [[13], [14], [15], [16], [17]]. Ascorbic acid, as an antioxidant, neutralizes free radicals by providing electrons to stabilize them, thus reducing their ability to cause cell damage [1,2]. It also helps regenerate other antioxidants, such as vitamin E, and further improves antioxidant properties [18,19]. It is also known for its anti-inflammatory effect; it is important to note that the body can absorb only limited amount of ascorbic acid at a time and that excessive amounts are excreted in the urine. Ascorbic acid supplements are usually safe, but excessive intake can cause side effects such as diarrhea, nausea, and stomach cramps.
Ascorbate (/ascorbate anion) can induce the transformation of Fe3+ into Fe2+, which can lead to the formation of the hydroxyl radical (HO•) in the cytoplasmic pool. Due to this property, ascorbate (/ascorbate anion) can also act as a pro-oxidant. Additionally, the oxidation of ascorbate results in the generation of the ascorbyl radical (AH•) via monodehydroascorbate radical (A•-) [20,21]. Hence, it is essential to approach hypothesis formulation with utmost care to ascertain whether the experimentally obtained results stem directly from ascorbate or ROS during cell supplementation.
In the current study, we aimed to evaluate the pro-oxidant and antioxidant capabilities of ascorbic acid. We have used the U-937 cell line, which is a pro-monocytic myeloid leukemia cell line of human origin [22]. Under in vitro conditions, an unlimited number of uniform cells can be prepared from U-937 cells [23]. These leukemia cells bear the t(10; 11)(p13; q14) translocation, which results in a fusion between the MLLT10 (myeloid/lymphoid or mixed-lineage leukemia) gene and the Ap-3-like clathrin assembly protein PICALM (clathrin assembly lymphoid myeloid leukemia), which is likely important for the tumorous nature of the cell line [24]. We investigated the relationship of ROS production under the effects of variable concentrations/the length of incubation of ascorbic acid. When the immune reactions are activated, monocytes migrate to different tissues and organs within the body. After reaching the target, depending on the specific signals from local micro-environments, they can be differentiated into diverse types of cells. One of the principal cell types which can be differentiated from monocytes are the macrophages. The monocyte-to-macrophage differentiation process involves several phases: Upon accessing the target tissue, monocytes are exposed to cytokines/chemokines which are secreted by the neighbouring cells. Following this, signalling pathways within the monocytes are initiated leading to changes in gene expression/cellular morphology, which is also a typical characteristic of macrophages. Macrophages acquire enlarged morphology with increased capacity for phagocytosis. Additionally, it has been known that the gene expression profile shifts to support macrophage-specific functions such as the generation of inflammatory mediators and the potential for antigen presentation.
Visualization of differentiation under induction was accomplished utilizing confocal laser scanning microscopy (CLSM). Interestingly, it has been shown that ascorbic acid has the potential as a differentiation agent, i.e., unspecialized cells become specialized in different types of cells with specific functions. Research shows that ascorbic acid can induce differentiation in various types of cells, including stem cells, cancerous cells, and immune cells [25,26]. In immune cells, ascorbic acid has been shown to improve the differentiation and function of certain types of immune cells, including T cells and dendritic cells. Overall, the ability of ascorbic acid to induce differentiation in different types of cells is an interesting field of research for potential therapeutic applications. Thus, instead of inducing differentiation using another chemical stimulant, we evaluated the dependence and relation of three parameters: differentiation induction of monocytes to macrophages in U-937, pro-oxidant concentration, and antioxidant concentration of ascorbic acid. Furthermore, protein immunoblotting was used to understand protein modification. Our results show a correlation between the differentiation of U-937 cells into macrophages and protein modification under the effect of generated ROS.
2. Materials and methods
2.1. Chemicals and antibodies
For blotting, a polyclonal anti-malondialdehyde (anti-MDA) antibody suitable for the measurement of MDA was used. The primary antibody was purchased from Abcam (Cambridge, CB2 0AX, UK) [Anti-Malondialdehyde antibody (ab27642)]; CD11b Monoclonal Antibody-FITC from eBioscience™ (ThermoFischer Scientific, USA) and secondary antibody was purchased from Bio-Rad (Hercules, CA, USA) [Goat Anti-Rabbit IgG-HRP Conjugate (1706515)]. Cell culture medium, antibiotics, and inhibitors used were from Biosera (Nuaillé, France) and Roche (Mannheim, Germany).
2.2. Cell line and growing condition
The U-937 cell line is a human pro-monocytic myeloid leukemia cell line [22] obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). The TC20 automated cell counter (Bio-Rad Laboratories, Hercules, CA, United States) was used to determine cell density and was monitored using 0.25 % trypan blue dye. Experiments were performed when viability was close to or above 70 %. To growth medium (RPMI-1640) pre-supplemented with 0.05 mM l-glutamine were added 10 % fetal bovine serum (FBS) and 1 % of the antibiotics (penicillin and streptomycin) in v/v ratio.
2.3. Induction of differentiation
The differentiation of U-937 cells was studied under ascorbic acid concentrations ranging from 0.1 to 10 μM. Culture medium with 1x105 cell suspension/mL was supplemented with ascorbic acid at the final concentration of either 0.1 μM, 1 μM, 5 μM or 10 μM for 72h, and followed by a resting phase of 24 h to achieve cell adhesion and express macrophage characteristic cytokine expression. For validation, we also employed a widely recognized differentiation inducer, phorbol 12-myristate 13-acetate (PMA) (Sigma Aldrich, St. Louis, Missouri, United States) at final concentrations of 150 and 250 nM. The timing of the induction protocol was deduced from our pilot experiments focusing on U-937 cell differentiation stages in relation to ascorbic acid treatment.
2.4. Cell viability assay
The TC20 automated cell counter was used to determine the cell viability (expressed as total cell counts mL−1) after incubation of U-937 cells with 0.25 % trypan blue in a ratio of 4:1 for approximately 2 min. Trypan blue is used to determine the ratio of living and dead cells in a cell suspension, and it is because the dye enters only dead cells and stains them blue, while live cells have intact cell membranes and remain bright and unstained [27]. In our experimental condition, cell density and viability were measured after treatment of U-937 cells with ascorbic acid for 72 h and following the 24 h resting period. The data obtained are presented in Fig. 1.
Fig. 1.
Cell viability of U-937 cells. U-937 cells at different concentrations of ascorbic acid (0.1 μM, 1 μM, 5 μM and 10 μM). The data are presented as the mean value (±SE) of biological replicates (n = 2).
2.5. Confocal laser scanning microscopy
A Fluorview 1000 confocal unit attached to the IX80 microscope was used to image U-937 cells on glass slides (Olympus Czech Group, Prague, Czech Republic). Staining with FM4-64 (15 μM, RT), which is a lipophilic dye was used to monitor cell membrane integrity, and Hoechst 33342 (2 μM, RT) was used to visualize the nucleus under the experimental conditions mentioned. FM4-64 excitation was done using a 543 nm He–Ne laser and its emission was recorded in the range of 655–755 nm. The cells were co-stained with FM4-64 and Hoechst 33342 [Sigma Aldrich GmbH (Germany)]. Following an incubation of 5 min, the cells were transferred to a glass slide for visualization. For immunohistochemistry, after treatments with 5 μM and 10 μM ascorbic acid for 48 h, the culture medium was removed from the culture plates, and 3.5 % paraformaldehyde was added for cell fixation for 30 min. The cells were then washed three times with phosphate buffered saline containing 0.1 % Tween-20 (PBST) for 5 min each.
Afterward, the fixed cells were blocked with 0.5 % BSA in phosphate buffered saline (PBS) for 30 min at room temperature (RT). Subsequently, the blocked samples were probed with CD11b Monoclonal Antibody-FITC (dilution 1:2500) for 60 min at RT, followed by three washes with PBS (5 min each) and Immunofluorescence was measured using excitation achieved by a 488 nm line of an argon laser, and the signal was detected by a 505–550 nm emission filter.
2.6. Protein immunoblotting
Ascorbic acid (72 h, 0.1–10 μM) or PMA (72 h, 150 and 250 nM) pre-treated U-937 cells were incubated in serum-free medium for 24 h (resting time), cells were then collected by centrifugation and washed with phosphate buffer saline (PBS) (pH 7.4) twice. Following this step, cells were sonicated in RIPA lysis buffer [150 mM NaCl, 50 mM Tris (pH 8.0), 0.5 % sodium deoxycholate, 0.1 % SDS, 1 % NP-40] containing 1 % (v/v) protease and phosphatase inhibitor. The processed homogenate was centrifuged at 14,000 rpm (30 min, 4 °C) and the collected supernatant fraction was quantified using a Pierce BCA protein estimation kit (Thermo Fisher Scientific, Paisley, UK).
For anti-MDA blotting, samples were prepared with 5× Laemmli sample buffer along with 100 mM Dithiothreitol (DTT); and a protein concentration of 10 μg/lane was used for electrophoresis. The protein samples were then boiled for 10 min at 70 °C. The proteins were seperated on 10% SDS gels and then transferred to nitrocellulose membranes using the Trans-Blot Turbo transfer system (Bio-Rad, California, USA). The nitrocellulose membranes were then blocked for 90 min at room temperature (RT) with 5 % BSA in tris-buffered saline (TBS) (pH 7.4) and 0.1 % Tween 20 (referred to as TBST). The blocked membranes were probed for 90 min at RT with anti-MDA antibody (dilution 1: 5000) followed by 3X washing (10 min each) with TBST; incubated for another 90 min at RT with HRP-conjugated goat anti-rabbit secondary antibody (dilution 1:10000). Following 3 steps of TBST (10 min each), immunocomplexes were imaged using the Amersham imager 600 and Immobilon Western Chemiluminescent HRP Substrate (Sigma Aldrich, GmbH, Germany) (GE Healthcare, UK).
3. Results
3.1. Cell viability using the trypan blue exclusion test
Quantitative estimation of viable cells exposed to varying concentrations of ascorbic acid in U-937 cells was carried out using trypan blue. U-937 cells were treated for 72 h followed by a 24 h of resting period. In Fig. 1A, the gray bar indicates the total cell count, while the green bar indicates the number of live cells within the population (SE, n = 2), while Fig. 1B indicates the cell viability after the resting period. From Fig. 1, it is evident that cell viability under different concentrations used in the experiments is more than 80 % with ascorbic acid treatment. There was a minor/negligible effect noticed in comparison to the control at different concentrations. Therefore, it is considered that the cells are metabolically active in response to the treatments.
3.2. Cell differentiation induced by ascorbic acid and various inducers
Visual changes were monitored 72 h after the addition of ascorbic acid using a confocal laser scanning microscope. Cell morphology was altered after 72 h of incubation with doses of ascorbic acid. When exposed to various differentiation inducers such as PMA, dimethyl sulfoxide (DMSO), retinoic acid, Zn2+, 12-O-tetradecanoylphorbol-13-acetate (TPA), and low concentrations of glutamine, pro-monocytic cells tend to undergo maturation into monocytes or macrophages. To confirm whether the cellular integrity of the U-937 cells under the experimental conditions does not lead to damage of cells, FM4-64 which is a lipophilic styryl compound, and Hoechst 33342 were used. It can be observed that in U-937 cells treated with variable concentration of ascorbic acid (Fig. 2), no obvious cell damage occurred, and cellular integrity (red fluorescence) and nuclear integrity (blue fluorescence) were maintained under all conditions. Differentiated U-937 cells have distinct extensions bearing amoeboid morphology. Before the treatment, the cell morphology exhibited a clear, spherical structure, whereas, after treatment, significant morphological alterations can be observed in the form of prominent pseudopodia, the incidence of which can be seen to be higher with increasing concentration of ascorbic acid from 0.1 μM until 5 μM. A reduction in these morphological structures was observed at higher concentrations of ascorbic acid (10 μM) (Fig. 2).
Fig. 2.
Double staining using Hoechst 33342 and FM4-64 in 72 h differentiated U-937 cells. Differentiation was induced using variable concentrations of ascorbic acid (0.1–10 μM). Images were captured in various channels at a magnification of 1000 × after staining for 5 min (from left to right are Nomarski DIC, FM4-64 and Hoechst 33342). For each variant, the presented images represent several scans conducted on both biological and technical replicates.
3.3. Expression of cell surface marker CD11b
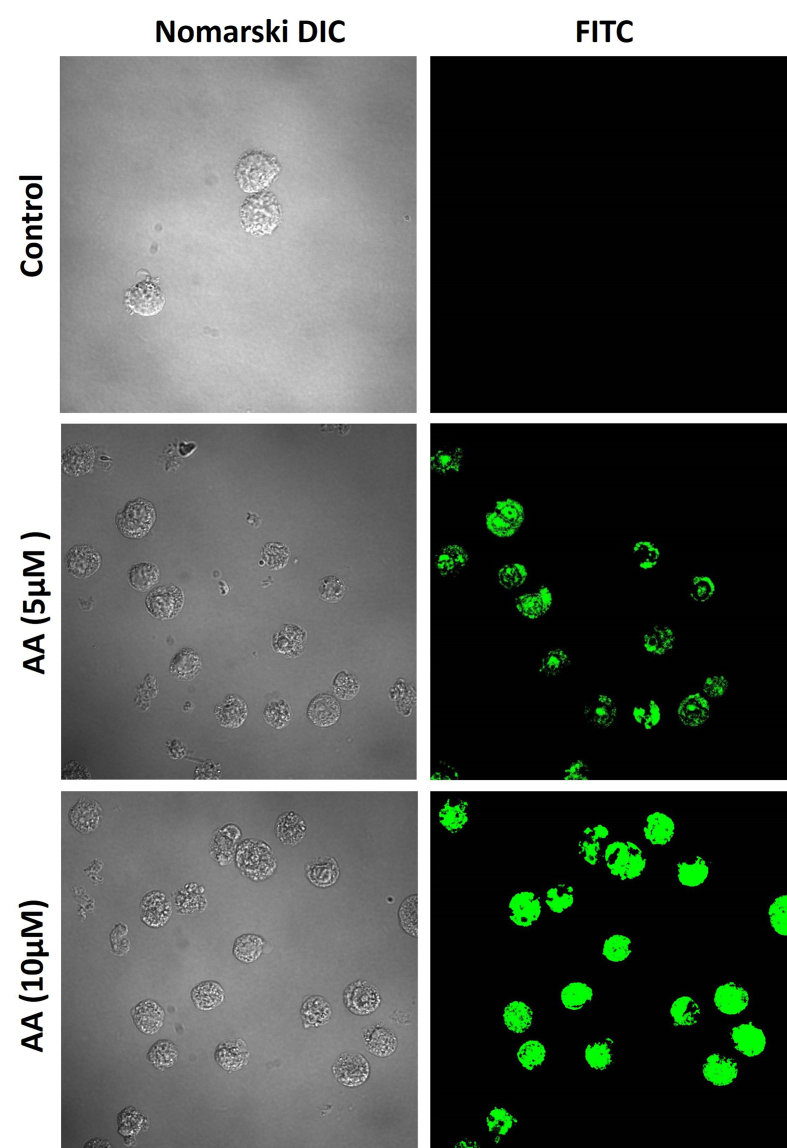
To confirm the differentiation of pro-monocytic cells into macrophages, we tracked the expression of the CD11b surface marker. As shown in the results (Fig. 3), it is evident that in untreated U-937 cells, the surface marker expression is minimal. However, in cells treated with ascorbic acid (at 5 and 10 μM concentrations), FITC fluorescence is visibly present under both treatment conditions.
Fig. 3.
Expression of CD11b surface marker in cell treated without (upper panel) and in the presence of 5 μM (middle panel) and 10 μM (lower panel) of ascorbic acid for 48 h. The images from left to right represent Nomarski DIC and FITC fluorescence.
3.4. Effect of ascorbic acid in U-937 cells and associated protein modification
Protein modification occurs when MDA, a reactive aldehyde compound, reacts with amino acid residues of proteins, resulting in the formation of MDA-protein adducts. This is often used as a marker of oxidative stress and lipid peroxidation in cells and tissues. Whole-cell homogenate from U-937 cells treated with PMA and ascorbic acid separated using SDS-PAGE followed by immunoblotting using anti-malondialdehyde (anti-MDA) antibody showed modification of more than one protein. In PMA-treated U-937 cells, the formation of MDA is most pronounced as displayed by protein bands at approximately 40 kDa (Fig. 4A) which is also evident from the densitogram presented in Fig. 4B. In ascorbic acid treated U-937 cells, the formation of MDA is most pronounced in the protein bands at approximately 20 kDa and 40 kDa, and this effect becomes more prominent with increasing concentrations of ascorbic acid (Fig. 5A and Supplementary data 1). A consistent and linear increase in band intensity at this molecular weight range is observed in treated cells, especially with 1 μM and 5 μM ascorbic acid, as indicated in the densitogram shown in Fig. 5B. Notably, in cells differentiated with 10 μM ascorbic acid, a sudden decline in band intensity is evident. We confirmed our findings by employing reverse-phase HPLC to measure the concentration of MDA in cells treated with 1 and 5 μM ascorbic acid, comparing them to the control (indicated as -). A significant increase was observed in case of ascorbic acid differentiated cell (supplementary data 2). These findings can be associated with the differentiation pattern illustrated in Fig. 2. Unexpectedly, the band intensity at 40 kDa exhibits a slight reduction in the 0.1 μM ascorbic acid-treated sample when compared to the control. We attribute this phenomenon to potential non-specific interactions.
Fig. 4.
A. Protein MDA adducts formed in U-937 cells treated with 250 nM PMA. This illustrates protein modification in U-937 cells that were differentiated for 72 h, visualized on blot with anti-MDA antibody. B. Quantification of protein bands from an anti-MDA blot by densitogram analysis.
Fig. 5.
A. Protein MDA adducts formed in U-937 cells treated with 0.1–10 μM ascorbic acid. This illustrates protein modification in U-937 cells that were differentiated for 72 h, visualized on blot with anti-MDA antibody. B. Quantification of protein bands from an anti-MDA blot by densitogram analysis.
4. Discussion
Monocytes are circulatory precursors that originate from a myeloid lineage which can further differentiate into macrophages or dendritic cells after recruitment in tissues and blood stream [28]. Our immune system gains the advantage of phagocytosis, antigen presentation, and cytokine production from these cells. U-937, a pro-monocytic cell lines differentiate into macrophages or into dendritic cells in vitro in presence of different inducers [29]. The basic feature of this cell line is the synthesis and secretion of lysozyme with the absence of immunoglobulin production.
When cells such as U-937 and THP-1 cells are exposed to differentiation inducers such as PMA, retinoic acid etc., their proliferation is slowed while the differentiation process is triggered. The monocytes under these treatments are known as “macrophage-like” because of their structure. However, the properties of the transformed cell line are not yet well known. It depends on the dose and time of treatment with inducers. Cells treated with different inducers have been shown to express elevated levels of CD11b and CD14. It also starts to induce adherence accompanied by cell cycle arrest [30]. Cell differentiation is also known to activate the calcium and phospholipid-dependent isoforms of protein kinase C (PKC) thereby inducing AMP metabolism, which leads to its maturation into a macrophage [31,32]. It is now well accepted that the treatment with differentiation inducers exogenously activates the NADPH oxidase complex, which can lead to the formation of O2•− [33]. In the presence of superoxide dismutase (SOD), it can form H2O2 and subsequently HO• in the presence of transition metal ions [34]. During the last decades, differentiation studies were done utilizing PMA and retinoic acid as inducers whereas, more recently, ascorbic acid has been known to relate to cell differentiation [35], the molecular mechanism of which is still unclear. Differentiation studies on dental stem cells have been performed using ascorbic acid in the time range of 24–72 h; however, the stability of ascorbic acid over the period of several days in solution should be taken in account considering the instability of the compound [36].
With the production of ROS in cells, biomolecules such as lipids, proteins, and nucleic acids can be damaged. Reactive oxygen species can directly oxidize proteins, leading to structural and functional impairments, as well as the formation of protein aggregates and cross-linking. Altogether, it can lead to change in normal protein folding and conformation resulting which the protein-protein interaction and enzymatic activity can be hampered. Amino acids, including but not limited to cysteine, methionine, and histidine are known to be most affected by ROS generation. Lipid particularly due to the presence of polyunsaturated fatty acids (PUFA's) are prone to peroxidation leading to the formation of lipid hydroperoxide and subsequently other reactive lipid species. In our study, MDA which is formed as a by-product of lipid peroxidation known to be generated through a series of reaction involving cleavage of the peroxide bonds and rearrangement of the resulting radicals. In addition to most damage to lipids and proteins, ROS is known to react with DNA/RNA, oxidizing its bases, leading to the formation of DNA adducts, chains, and DNA-cross links. If not repaired, this can eventually lead to mutations and genomic instability that can be responsible for disease development.
Our study shows the differentiation behavior of U-937 cells under exogenous supplementation of ascorbic acid. During the process of cell differentiation, it has been observed that there is a higher expression of NADPH oxidase complex, which eventually can be hypothesized with the increase in the production of ROS. Based on our previous study, we observed a higher expression of NOX-4 in differentiated U-937 cells [16]. There might be a direct/indirect role of ROS in the activation of NADPH expression and eventually self-oxidation.
5. Conclusions
Prooxidative vs. antioxidative action of ascorbic acid (vitamin C) was evaluated in relation to differentiation of U-937 cell line into macrophages. Analysis of protein modification using anti-malondialdehyde antibodies showed changes in more than one protein. The findings demonstrate the relationship between the differentiation of U-937 cells into macrophages and the protein modification caused by the production of ROS under the influence of ascorbic acid together with a switch from pro-oxidative to the antioxidant property of the compound under investigation. Findings from this study indicate ascorbic acid promotes differentiation of monocytes into macrophages underlying its role in immune response besides its antioxidant activity. Studies focusing on ascorbic acid role in differentiation and development have to be considered.
Funding
This work was funded by the European Regional Development Fund project “Plants as a tool for sustainable global development” (CZ.02.1.01/0.0/0.0/16_019/0000827) and grant no. IGA_PrF_2023_023 entitled “Current studies and research directions in general and molecular biophysics” of Palacký University.
CRediT authorship contribution statement
Ankush Prasad: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Deepak Rathi: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Michaela Sedlářová: Data curation, Investigation, Methodology, Writing – review & editing. Renuka Ramalingam Manoharan: Investigation, Methodology, Writing – review & editing. Eliška Průdková: Data curation, Investigation, Writing – review & editing. Pavel Pospíšil: Data curation, Formal analysis, Investigation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2023.101622.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure S1: Protein MDA adducts formed in U-937 cells treated with 0.1–10 μM ascorbic acid. This illustrates protein modification in U-937 cells that were differentiated for 72 h, visualized on blot with anti-MDA antibody.
Figure S2: Quantification of MDA in non-differentiated (control) and differentiated U-937 cells (72 h, 1 and 5 μM AA) monitored by the reverse-phase HPLC. The retention time of MDA-DNPH derivative was at 5.8 min. Experimental protocol used is briefly mentioned below: The total amount of MDA was assessed through reverse-phase HPLC analysis, following the procedure outlined by Pilz et al. (2000). In summary, HPLC-grade water (LC-MS grade) was added to the fraction samples to reach a total volume of 200 μl. To release MDA from proteins via alkaline hydrolysis, 40 μl of 6 M aqueous sodium hydroxide was added and incubated at 60 °C for 30 minutes. Proteins were precipitated by acidifying the sample using 100 μl of 35% (v/v) perchloric acid, followed by centrifugation at 16,000 g for 10 minutes. The supernatant (125 μl) was transferred to a new Eppendorf tube, and MDA was derivatized by adding 1 μl of 50 mM 2,4-dinitrophenyl hydrazine (DNPH) prepared in 2 M sulphuric acid and incubated at RT for 30 minutes. A 50 μl volume of the derivatized MDA was injected into the HPLC (Alliance e 2695 HPLC System, Waters, Milford, MA, U.S.A.) equipped with a 2998 Photodiode Array (PDA) detector. The isocratic separation was carried out using an Arion ASTRA® C18-HE HPLC column (3.0 μm 150 mm × 4.6 mm) (Chromes s. r.o., Prague, Czech Republic) with acetonitrile: water (50:50 v/v) as the solvent system and a flow rate of 1 ml min−1. The MDA-DNPH derivative was detected in the samples at 310 nm with the PDA detector. Operation and data processing were performed using Empower software.
Data availability
Data will be made available on request.
References
- 1.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Canc. 2012;1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5(35):27986–28006. [Google Scholar]
- 3.Macan A.M., Kraljevic T.G., Raic-Malic S. Therapeutic perspective of vitamin C and its derivatives. Antioxidants. 2019;8(8):36. doi: 10.3390/antiox8080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane D.J.R., Richardson D.R. The active role of vitamin C in mammalian iron metabolism: much more than just enhanced iron absorption. Free Radic. Biol. Med. 2014;75:69–83. doi: 10.1016/j.freeradbiomed.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11) doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricaurte F.R., Kewan T., Daw H. Scurvy: a rare cause of anemia. Cureus. 2019;11(9) doi: 10.7759/cureus.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njus D., Kelley P.M., Tu Y.J., Schlegel H.B. Ascorbic acid: the chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020;159:37–43. doi: 10.1016/j.freeradbiomed.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 8.DePhillipo N.N., Aman Z.S., Kennedy M.I., Begley J.P., Moatshe G., LaPrade R.F. Efficacy of vitamin C supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries A systematic review. Orthopaedic J. Sports Med. 2018;6(10) doi: 10.1177/2325967118804544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Gorkom G.N.Y., Wolterink R., Van Elssen C., Wieten L., Germeraad W.T.V., Bos G.M.J. Influence of vitamin C on lymphocytes: an overview. Antioxidants. 2018;7(3) doi: 10.3390/antiox7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N.Y., Zhao G.J., Wu W.L., Zhang M.X., Liu W.Y., Chen Q.F., Wang X.Q. The efficacy and safety of vitamin C for iron supplementation in adult patients with iron deficiency anemia A randomized clinical trial. JAMA Netw. Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pullar J.M., Carr A.C., Bozonet S.M., Vissers M.C.M. High vitamin C status is associated with elevated mood in male tertiary students. Antioxidants. 2018;7(7) doi: 10.3390/antiox7070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarisch R., Weyer D., Ehlert E., Koch C., Pinkowski E., Jung P., Kähler W., Girgensohn R., Hemmer W., Koch A. Influence of orally taken vitamin C on histamine levels and motion sickness. J. Allergy Clin. Immunol. 2011;127(2):AB261. AB261. [Google Scholar]
- 13.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Meo S., Reed T.T., Venditti P., Victor V.M. 2016. Role of ROS and RNS Sources in Physiological and Pathological Conditions, Oxidative Medicine and Cellular Longevity 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U.S.A. 2018;115(23):5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad A., Manoharan R.R., Sedlářová M., Pospíšil P. Free radical-mediated protein radical formation in differentiating monocytes. Int. J. Mol. Sci. 2021;22(18):17. doi: 10.3390/ijms22189963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pospíšil P., Prasad A., Rac M. Mechanism of the formation of electronically excited species by oxidative metabolic processes: role of reactive oxygen species. Biomolecules. 2019;9(7) doi: 10.3390/biom9070258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan A.C., Tran K., Raynor T., Ganz P.R., Chow C.K. Regeneration of vitamin E in human platelets. J. Biol. Chem. 1991;266(26):17290–17295. [PubMed] [Google Scholar]
- 19.Chan A.C. Partners in defense, vitamin-E and vitamin-C. Can. J. Physiol. Pharmacol. 1993;71(9):725–731. doi: 10.1139/y93-109. [DOI] [PubMed] [Google Scholar]
- 20.Poljsak B., Gazdag Z., Jenko-Brinovec S., Fujs S., Pesti M., Belagyi J., Plesnicar S., Raspor P. Pro-oxidative vs antioxidative properties of ascorbic acid in chromium(VI)-induced damage: an in vivo and in vitro approach. J. Appl. Toxicol. 2005;25(6):535–548. doi: 10.1002/jat.1093. [DOI] [PubMed] [Google Scholar]
- 21.Gegotek A., Skrzydlewska E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants. 2022;11(10) doi: 10.3390/antiox11101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundstrom C., Nilsson K. Establishment and characterization of A human histiocytic lymphoma cell line (U-937) Int. J. Cancer. 1976;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento C.R., Fernandes N.A.R., Maldonado L.A.G., Rossa C. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem. Biophy. Rep. 2022;32 doi: 10.1016/j.bbrep.2022.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreyling M.H., MartinezCliment J.A., Zheng M., Mao J., Rowley J.D., Bohlander S.K. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc. Natl. Acad. Sci. U.S.A. 1996;93(10):4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu J.W., Wu Z.Y., Liu J.F., Wu T.F. Vitamin C: a stem cell promoter in cancer metastasis and immunotherapy. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110588. [DOI] [PubMed] [Google Scholar]
- 26.Ang A., Pullar J.M., Currie M.J., Vissers M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018;46:1147–1159. doi: 10.1042/BST20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strober W. Trypan blue exclusion test of cell viability. Curr. Protoc. Im. 2015;111 doi: 10.1002/0471142735.ima03bs111. A3.B.1-A3.B.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chanput W., Mes J.J., Wichers H.J. THP-1 cell line: an in vitro cell model for immune modulation approach. Int. Immunopharm. 2014;23(1):37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Prasad A., Sedlářová M., Balukova A., Ovsii A., Rac M., Krupka M., Kasai S., Pospíšil P. Reactive oxygen species imaging in U937 cells. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.552569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starr T., Bauler T.J., Malik-Kale P., Steele-Mortimer O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim P.S., Sutton C.R., Rao S. Protein kinase C in the immune system: from signalling to chromatin regulation. Immunology. 2015;146(4):508–522. doi: 10.1111/imm.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musashi M., Ota S., Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int. J. Hematol. 2000;72(1):12–19. [PubMed] [Google Scholar]
- 33.Karlsson A., Nixon J.B., McPhail L.C. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. J. Leukoc. Biol. 2000;67(3):396–404. doi: 10.1002/jlb.67.3.396. [DOI] [PubMed] [Google Scholar]
- 34.Prousek J. Fenton chemistry in biology and medicine. Pure Appl. Chem. 2007;79(12):2325–2338. [Google Scholar]
- 35.Qiao H., May J.M. Macrophage differentiation increases expression of the ascorbate transporter (SVCT2) Free Radic. Biol. Med. 2009;46(8):1221–1232. doi: 10.1016/j.freeradbiomed.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diederich A., Fründ H.J., Trojanowicz B., Navarrete Santos A., Nguyen A.D., Hoang-Vu C., Gernhardt C.R. Influence of ascorbic acid as a growth and differentiation factor on dental stem cells used in regenerative endodontic therapies. J. Clin. Med. 2023;12:1196. doi: 10.3390/jcm12031196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.