Abstract
Background
This retrospective study aimed to determine the risk of venous thromboembolism (VTE) in patients with PsA after surgery for lumbar degenerative disease (LDD).
Methods
The study data of adults aged ≥20 years admitted to U.S. hospitals with diagnoses of LDD and undergoing spinal decompression or fusion between 2005 and 2018 were extracted from the National Inpatient Sample (NIS) database. Patients were further divided into two groups based on a diagnosis of PsA or not via codes ICD-9: 696.0 and ICD-10: L40.50. Patients with missing information were excluded. Propensity score matching (PSM) was employed to enhance comparability between groups. Logistic regression was used to determine associations between PsA and various outcomes, including complications, unfavorable discharge, and prolonged length of stay (LOS).
Results
Data on 471,283 patients with LDD was extracted from the NIS database.
from 2005 to 2018. Before propensity score matching, patients with PsA had higher proportions of overall morbidity (8.8 % vs. 6.9 %), VTE (1.4 % vs. 0.7 %), and unfavorable discharge (20.8 % vs. 16.9 %). After matching, patients with PsA still had higher VTE incidence and unfavorable discharge proportions. After adjustments, multivariable regression analysis indicated that patients with PsA had a higher risk of unfavorable discharge (aOR: 1.26, 95 % CI: 1.03–1.55) and VTE (aOR: 1.99, 95 % CI: 1.05–3.75).
Conclusions
Among patients undergoing surgery for LDD, pre-existing PsA may be associated with increased risks of unfavorable discharge and VTE occurrence. The findings may benefit preoperative risk stratifications before LDD surgeries.
Keywords: Psoriatic arthritis (PsA), Lumbar degenerative disease (LDD), Complication, Fusion, Nationwide inpatient sample (NIS)
1. Introduction
Psoriasis is a chronic skin condition associated with several comorbidities and co-manifestations that reduce patient quality of life [1]. Psoriatic arthritis (PsA) is a rare autoimmune disorder first described by Moll et al., in 1973 [2]. It is a type of inflammatory arthritis included among the spondyloarthritis, a group of rheumatic diseases characterized by different clinical manifestations and associated comorbidities; prevalence of PsA is between 0.1 % and 1 % in the general population and about 20 % in individuals with cutaneous or nail psoriasis [3,4]. PsA may begin with several clinical features such as peripheral arthritis, dactylitis, enthesitis, axial disease, dactylitis, and skin and nail lesions [[5], [6], [7]]. The disease is associated with a substantial psychosocial and functional burden and may lead to irreversible joint damage if left untreated [8].
Lumbar degenerative disease (LDD) is one of the most common musculoskeletal disorders, affecting approximately 31 million people in the United States alone [9]. LDD includes conditions involving disc degeneration, lumbar spinal stenosis, and spondylolisthesis, and manifests symptoms ranging from low back pain to lower extremity radicular pain and weakness [10]. If patients have neurologic compromise or limitations of the activities of daily living, surgical intervention may be required to decompress and stabilize the affected segments [11].
Individuals affected by psoriatic diseases are susceptible to facing significant medical and surgical challenges. Extensive evidence supports the association between psoriatic disease and elevated arterial thrombotic events, such as myocardial infarction (MI) and stroke. These events are a result of accelerated atherosclerosis triggered by an excess of traditional cardiovascular risk factors and chronic, systemic inflammation [12]. In addition, recent research has also indicated a heightened risk of venous thromboembolism (VTE) in individuals with psoriatic disease [13,14]. Moreover, in a population-based study focusing on individuals undergoing total knee arthroplasty (TKA), psoriasis patients exhibit an elevated risk of deep surgical site infections (SSIs) [15]. Due to the inflammatory nature of psoriasis, that study suggested the implementation of enhanced pre-operative counseling, shared decision-making, and strict infectious precautions within the psoriasis population undergoing TKA [15].
At the time of indication for surgery, it is essential to assess the patient's risk of developing complications. Although a substantial number of patients undergoing lumbar surgery for LDD may have concomitant PsA, however, to the best of our knowledge, there have been no comprehensive investigation on the potential impact of PsA in patients undergoing surgery for LDD. Understanding the role of PsA in the context of LDD surgery may help improve preoperative risk stratification and patient care. Therefore, the present study aimed to assess whether or not patients with pre-existing PsA have an increased risk for postoperative complications following LDD surgery in comparison to those without PsA.
2. Material and methods
2.1. Study design and data source
This population-based, retrospective observational study extracted all data from the US Nationwide Inpatient Sample (NIS) database, the largest all-payer, continuous, annually updated inpatient care database in the United States, including about 8 million hospital stays each year. The database is administered by the Healthcare Cost and Utilization Project (HCUP) of the US National Institutes of Health (NIH). Data are collected from about 1050 hospitals in 44 states in the US, representing a 20 % stratified sample of US community hospitals. Patient data include primary and secondary diagnoses, primary and secondary procedures, admission and discharge status, patient demographics, expected payment source, duration of hospital stay, and hospital characteristics (i.e., hospital bed size/location/teaching status/hospital region).
2.2. Ethics statement
All data were obtained through a request to the Online Healthcare Cost and Utilization Project (HCUP) Central Distributor (available at: https://www.distributor.hcup-us.ahrq.gov/), the agency that administers the database (certificate # HCUP-4Q18H34EX). This study conforms to the NIS data-use agreement with HCUP. The data contained in the NIS are de-identified, and the Institutional Review Board of Johns Hopkins Medical Institutions deemed that the study using NIS data is exempt from ethical review.
2.3. Study population
Adults aged ≥20 years admitted to U.S. hospitals between 2005 and 2018 in the NIS database with diagnoses of LDD and related surgeries were identified by the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth (ICD-10) diagnostic codes as follows:ICD-9: 721.3, 721.4721.42, 722.32, 722.5, 722.51, 722.52, 738.4, 756.11, 756.12; ICD-10: M51.05, M51.06, M51.15, M51.16, M51.17, M51.25, M51.26, M51.27, M51.35, M51.36, M51.37, M51.85, M51.86, M51.87, M51.9, M54.30-M54.32, and M54.40-M54.42. Codes for undergoing spinal decompression (ICD-9-PCS: 03.01, 03.09, 03.4, 03.53; ICD-10-PCS: 005Y, 00CY, 0SB0, 0SB2, 0SB3, 0SB4, 0SB5, 0SB6, 00BY, 00NY, 0QS0, 0QS1) or fusion (ICD-9-PCS: 81.04–81.08, 81.34–81.38, 81.61; ICD-10-PCS: 0SG0, 0SG1, 0SG3, 0SG5, 0SG6, 0RGA) were also used. The included patients were further divided into two groups based on whether or not they had PsA identified through ICD-9: 696.0 and ICD-10: L40.50. Patients with no information on mortality status, discharge disposition, or hospital length of stay (LOS) were excluded.
2.4. Study variables and outcome measures
Study outcomes were: incidence of 1) any major complications occurring during admission; 2) prolonged LOS, defined as LOS >75th percentile; and 3) unfavorable discharge, defined as transfer to nursing homes or long-term care facilities. Major complications included acute myocardial infarction (AMI), cerebrovascular accident (CVA), venous thromboembolism (VTE), pneumonia, sepsis, infection, respiratory failure, mechanical ventilation, and acute kidney injury (AKI).
2.5. Covariates
Patients’ baseline characteristics included age, sex, race, household income, and insurance status (primary payer). Significant comorbidities (i.e., ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease (COPD), obesity, other rheumatic diseases, chronic kidney disease (CKD), and coagulopathy) were identified using ICD-9 and ICD-10 diagnostic codes. Weekend admissions and hospital-related characteristics (hospital bed size, location/teaching status, hospital region) were also extracted from the database as part of the comprehensive NIS data available for all patients.
2.6. Statistical analysis
The National Inpatient Sample (NIS) represents nationwide analyses by using weighted models (TRENDWT and DISCWT), strata (NIS_STRATUM), and clusters (HOSPID) (details can be found at: https://hcup-us.ahrq.gov/nisoverview.jsp). Descriptive statistics are expressed as unweighted counts (n) and weighted percentages (%) or mean ± standard error (SE) with the SAS procedures of PROC SURVEYMEANS or PROC SURVEYFREQ for continuous and categorical data, respectively. Comparisons between groups (p-value) were conducted using the SAS procedures of PROC SURVEYMEANS or PROC SURVEYFREQ. Logistic regression models were implemented with the PROC SURVEYLOGISTIC to evaluate the odds ratios (ORs) and associations between psoriatic arthritis and various outcomes, including complications, unfavorable discharge, and prolonged LOS. In addition, propensity score matching (PSM) was conducted in which patients were age- and sex-matched with the propensity score (PS) based on the case: control ratio 1:10. Variables reaching statistical significance in univariable analysis were adjusted for in the multivariable models. A two-sided P-value of <0.05 was regarded as statistically significant. All statistical analyses were performed using the SAS version 9.4 software (SAS Institute, Inc., Cary, North Carolina, USA).
3. Results
3.1. Study population
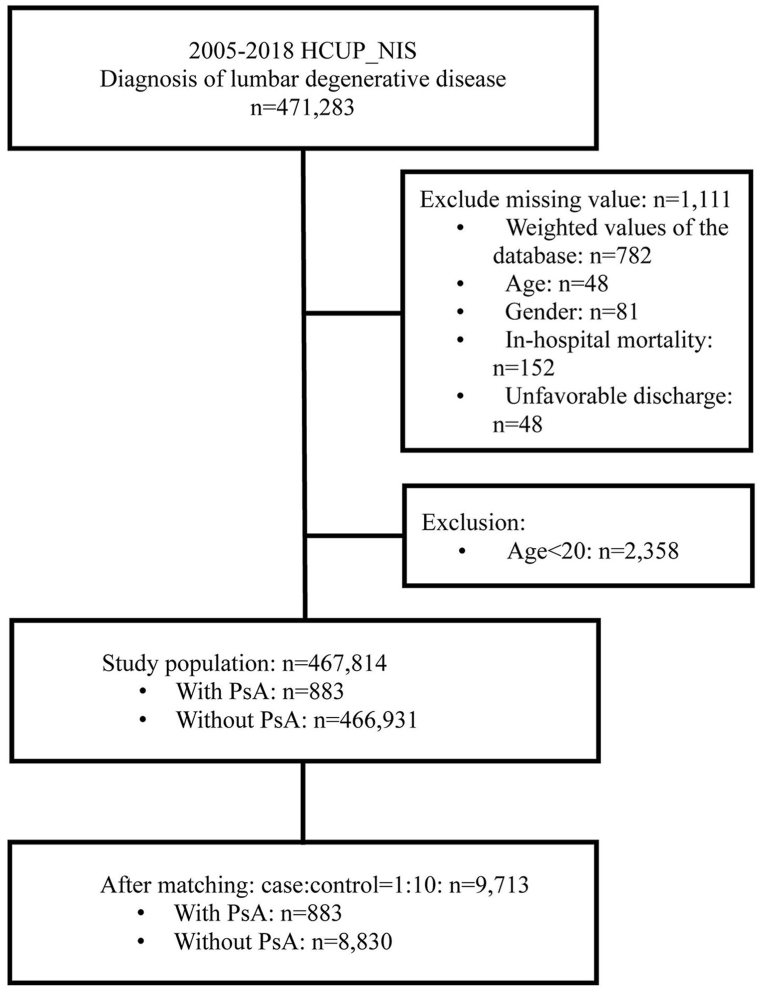
The study subject selection process is depicted in Fig. 1. A total of 471,283 patients with LDD were identified in the NIS database during 2005–2018. Patients with missing information of weighted values, age, sex, in-hospital mortality, and discharge dispositions (n = 1111) or who were aged <20 years were excluded. Among the final study population (n = 467,814), approximately 0.2 % (n = 883) patients had pre-existing PsA diagnosed previously. After age and sex-matching, 883 patients with PsA and 8830 without were included for analysis (Fig. 1). The full model of the associations between study variables and outcomes is displayed in Supplementary Table S1.
Fig. 1.
Flow diagram of patient selection.
3.2. Characteristics of patients undergoing LDD surgery with or without PsA
Patients' baseline characteristics before and after matching are documented in Table 1. Patient’ age (mean ± standard deviation) was 58.1 ± 0.06 years old, and more than half of patients were females (56.2 %). The age range for the majority of the study cohort was 60–69 years (26.3 %), largest racial/ethnic group was White (83.3 %), with similar household incomes (22.0 %–27.0 %), and the highest proportion of primary payers was Medicare/Medicaid (45.5 %) and private insurance such as Health Maintenance Organization (HMO) (44.2 %). About 30.6 % of patients smoked either before or after matching. Major comorbidities included hypertension (51.3 %), dyslipidemia (30.5 %), and diabetes (17.9 %). Spinal fusion had been performed in 95.3 % of the study cohort. Most patients were not admitted to hospitals emergently or on weekends. The majority of hospitals were urban-teaching hospitals (57.1 %), located in the Southern US (40.6 %), and were larger hospitals with a higher bed number (57.7 %). After PS matching, no significant differences were found in most covariates between the two groups (with or without PsA) except for race, household income, primary payer, comorbidities (hypertension, dyslipidemia, obesity, other rheumatic disease, and CKD), type of surgery, and hospital location/teaching status/hospital region (all p-value<0.05).
Table 1.
Characteristics of patients with or without PsA undergoing surgery for LDD.
| Characteristics | Before matching |
After matching |
||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 467,814) | PsA |
P-value | Total (n = 9713) | PsA |
P-value | |||
| Yes (n = 883) | No (n = 466,931) | Yes (n = 883) | No (n = 8830) | |||||
| Demography | ||||||||
| Age, years | 58.1 ± 0.06 | 60.0 ± 0.29 | 58.1 ± 0.06 | <0.001 | 60.1 ± 0.11 | 60.0 ± 0.29 | 60.1 ± 0.12 | 0.857 |
| Age, category | <0.001 | 1.000 | ||||||
| 20–39 | 51363 (10.9) | 34 (3.9) | 51329 (11.0) | 374 (3.9) | 34 (3.9) | 340 (3.8) | ||
| 40–49 | 76055 (16.2) | 106 (12.2) | 75949 (16.2) | 1166 (12.0) | 106 (12.2) | 1060 (12.0) | ||
| 50–59 | 110713 (23.7) | 258 (29.3) | 110455 (23.6) | 2838 (29.2) | 258 (29.3) | 2580 (29.1) | ||
| 60–69 | 122814 (26.3) | 318 (35.9) | 122496 (26.3) | 3498 (36.1) | 318 (35.9) | 3180 (36.1) | ||
| 70–79 | 86228 (18.5) | 147 (16.6) | 86081 (18.5) | 1617 (16.7) | 147 (16.6) | 1470 (16.7) | ||
| 80+ | 20641 (4.4) | 20 (2.2) | 20621 (4.4) | 220 (2.3) | 20 (2.2) | 200 (2.3) | ||
| Sex | 0.012 | 0.912 | ||||||
| Male | 204825 (43.8) | 348 (39.6) | 204477 (43.8) | 3828 (39.5) | 348 (39.6) | 3480 (39.4) | ||
| Female | 262989 (56.2) | 535 (60.4) | 262454 (56.2) | 5885 (60.5) | 535 (60.4) | 5350 (60.6) | ||
| Race | <0.001 | <0.001 | ||||||
| White | 343213 (83.3) | 748 (93.2) | 342465 (83.3) | 7163 (83.9) | 748 (93.2) | 6415 (83.0) | ||
| Black | 28726 (7.0) | 9 (1.1) | 28717 (7.0) | 594 (7.0) | 9 (1.1) | 585 (7.6) | ||
| Hispanic | 23833 (5.8) | 25 (3.1) | 23808 (5.8) | 449 (5.3) | 25 (3.1) | 424 (5.5) | ||
| Others | 16168 (3.9) | 21 (2.6) | 16147 (3.9) | 329 (3.9) | 21 (2.6) | 308 (4.0) | ||
| Missing | 55874 | 80 | 55794 | 1178 | 80 | 1098 | ||
| Household income | <0.001 | <0.001 | ||||||
| Quartile1 | 101144 (22.0) | 142 (16.3) | 101002 (22.0) | 2057 (21.5) | 142 (16.3) | 1915 (22.0) | ||
| Quartile2 | 122694 (26.7) | 220 (25.2) | 122474 (26.7) | 2477 (26.0) | 220 (25.2) | 2257 (26.0) | ||
| Quartile3 | 123881 (27.0) | 234 (26.9) | 123647 (27.0) | 2551 (26.7) | 234 (26.9) | 2317 (26.7) | ||
| Quartile4 | 111367 (24.3) | 278 (31.5) | 111089 (24.3) | 2462 (25.9) | 278 (31.5) | 2184 (25.3) | ||
| Missing | 8728 | 9 | 8719 | 166 | 9 | 157 | ||
| Primary Payer | <0.001 | <0.001 | ||||||
| Medicare/Medicaid | 211789 (45.5) | 403 (45.6) | 211386 (45.5) | 4454 (46.0) | 403 (45.6) | 4051 (46.1) | ||
| Private including HMO | 206811 (44.2) | 443 (50.5) | 206368 (44.2) | 4429 (45.7) | 443 (50.5) | 3986 (45.2) | ||
| Self-pay/no-charge/other | 48244 (10.3) | 35 (4.0) | 48209 (10.3) | 810 (8.3) | 35 (4.0) | 775 (8.8) | ||
| Missing | 970 | 2 | 968 | 20 | 2 | 18 | ||
| Smoker | 142731 (30.6) | 291 (33.2) | 142440 (30.6) | 0.105 | 2964 (30.6) | 291 (33.2) | 2673 (30.4) | 0.084 |
| Comorbidities | ||||||||
| Ischemic heart disease | 49704 (10.6) | 89 (10.1) | 49615 (10.6) | 0.59 | 1058 (10.9) | 89 (10.1) | 969 (11.0) | 0.403 |
| Congestive heart failure | 9219 (2.0) | 19 (2.1) | 9200 (2.0) | 0.765 | 191 (2.0) | 19 (2.1) | 172 (2.0) | 0.739 |
| Atrial fibrillation | 15664 (3.4) | 37 (4.3) | 15627 (3.4) | 0.143 | 322 (3.3) | 37 (4.3) | 285 (3.2) | 0.093 |
| Diabetes | 83689 (17.9) | 191 (21.7) | 83498 (17.9) | 0.003 | 1930 (19.9) | 191 (21.7) | 1739 (19.8) | 0.163 |
| Hypertension | 239933 (51.3) | 535 (60.4) | 239398 (51.3) | <0.001 | 5338 (54.9) | 535 (60.4) | 4803 (54.4) | <0.001 |
| Dyslipidemia | 142075 (30.5) | 342 (38.7) | 141733 (30.5) | <0.001 | 3286 (34.0) | 342 (38.7) | 2944 (33.5) | 0.002 |
| COPD | 66028 (14.1) | 143 (16.2) | 65885 (14.1) | 0.072 | 1464 (15.0) | 143 (16.2) | 1321 (14.9) | 0.285 |
| Obesity | 77486 (16.7) | 240 (27.3) | 77246 (16.6) | <0.001 | 1713 (17.8) | 240 (27.3) | 1473 (16.8) | <0.001 |
| Other rheumatic disease | 16038 (3.4) | 135 (15.3) | 15903 (3.4) | <0.001 | 463 (4.8) | 135 (15.3) | 328 (3.7) | <0.001 |
| CKD | 15201 (3.3) | 43 (4.9) | 15158 (3.3) | 0.009 | 348 (3.6) | 43 (4.9) | 305 (3.5) | 0.04 |
| Coagulopathy | 8897 (1.9) | 24 (2.8) | 8873 (1.9) | 0.059 | 207 (2.1) | 24 (2.8) | 183 (2.1) | 0.172 |
| Type of surgery | 0.012 | <0.001 | ||||||
| Decompression alone | 21482 (4.7) | 57 (6.5) | 21425 (4.7) | 421 (4.4) | 57 (6.5) | 364 (4.2) | ||
| Fusion | 446332 (95.3) | 826 (93.5) | 445506 (95.3) | 9292 (95.6) | 826 (93.5) | 8466 (95.8) | ||
| Emergent admission | 0.97 | 0.803 | ||||||
| No | 425209 (91.1) | 800 (91.0) | 424409 (91.1) | 8845 (91.3) | 800 (91.0) | 8045 (91.3) | ||
| Yes | 41567 (8.9) | 79 (9.0) | 41488 (8.9) | 843 (8.7) | 79 (9.0) | 764 (8.7) | ||
| Missing | 1038 | 4 | 1034 | 25 | 4 | 21 | ||
| Weekend admission | 0.777 | 0.385 | ||||||
| No | 460967 (98.5) | 869 (98.4) | 460098 (98.5) | 9590 (98.7) | 869 (98.4) | 8721 (98.8) | ||
| Yes | 6847 (1.5) | 14 (1.6) | 6833 (1.5) | 123 (1.3) | 14 (1.6) | 109 (1.2) | ||
| Hospital characteristics | ||||||||
| Hospital bed size | 0.172 | 0.283 | ||||||
| Small | 84885 (17.9) | 176 (19.8) | 84709 (17.9) | 1820 (18.5) | 176 (19.8) | 1644 (18.3) | ||
| Medium | 112860 (24.4) | 193 (22.2) | 112667 (24.4) | 2309 (24.1) | 193 (22.2) | 2116 (24.3) | ||
| Large | 268299 (57.7) | 511 (58.0) | 267788 (57.7) | 5546 (57.5) | 511 (58.0) | 5035 (57.4) | ||
| Missing | 1770 | 3 | 1767 | 38 | 3 | 35 | ||
| Hospital location/teaching status | <0.001 | <0.001 | ||||||
| Rural | 21244 (4.5) | 37 (4.1) | 21207 (4.5) | 444 (4.6) | 37 (4.1) | 407 (4.6) | ||
| Urban nonteaching | 179696 (38.4) | 266 (30.1) | 179430 (38.4) | 3611 (37.2) | 266 (30.1) | 3345 (37.9) | ||
| Urban teaching | 265104 (57.1) | 577 (65.7) | 264527 (57.1) | 5620 (58.3) | 577 (65.7) | 5043 (57.5) | ||
| Missing | 1770 | 3 | 1767 | 38 | 3 | 35 | ||
| Hospital region | <0.001 | <0.001 | ||||||
| Northeast | 67092 (14.5) | 173 (19.8) | 66919 (14.4) | 1392 (14.5) | 173 (19.8) | 1219 (13.9) | ||
| Midwest | 111692 (24.0) | 220 (24.8) | 111472 (24.0) | 2350 (24.3) | 220 (24.8) | 2130 (24.2) | ||
| South | 190351 (40.6) | 316 (35.7) | 190035 (40.6) | 3871 (39.8) | 316 (35.7) | 3555 (40.2) | ||
| West | 98679 (21.0) | 174 (19.7) | 98505 (21.0) | 2100 (21.5) | 174 (19.7) | 1926 (21.7) | ||
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HMO: Health Maintenance Organization; PsA, psoriatic arthritis; LDD, lumbar degenerative disease; SD, standard deviation; SE, standard error.
Continuous variables are presented as mean ± SE and categorical variables are presented as unweighted counts (weighted percentage).
3.3. Clinical outcomes of patients undergoing LDD surgery
Table 2 shows the clinical results of hospitalized patients who underwent LDD surgery. Before PS matching, patients with PsA had higher proportions of overall morbidity (8.8 % vs. 6.9 %), VTE (1.4 % vs. 0.7 %), and unfavorable discharge (20.8 % vs. 16.9 %) than non-PsA patients. PsA patients still had higher proportions of VTE and unfavorable discharge after matching.
Table 2.
Clinical outcomes of patients with or without PsA undergoing surgery for LDD before and after PSM.
| Outcomes | Before matching |
After matching |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 467,814) | PsA |
P-value | Total (n = 9713) | PsA |
P-value | |||||
| Yes (n = 883) | No (n = 466,931) | Yes (n = 883) | No (n = 8830) | |||||||
| Any major complication | 32081 (6.9) | 78 (8.8) | 32003 (6.9) | 0.02 | 713 (7.4) | 78 (8.8) | 635 (7.2) | 0.079 | ||
| AMI & CVA | 3385 (0.7) | 7 (0.7) | 3378 (0.7) | 0.96 | 79 (0.8) | 7 (0.7) | 72 (0.8) | 0.823 | ||
| VTE | 3242 (0.7) | 12 (1.4) | 3230 (0.7) | 0.015 | 77 (0.8) | 12 (1.4) | 65 (0.7) | 0.035 | ||
| Pneumonia | 3768 (0.8) | 11 (1.3) | 3757 (0.8) | 0.137 | 89 (0.9) | 11 (1.3) | 78 (0.9) | 0.29 | ||
| Sepsis & Infection | 12699 (2.7) | 28 (3.2) | 12671 (2.7) | 0.399 | 277 (2.9) | 28 (3.2) | 249 (2.8) | 0.563 | ||
| Respiratory failure & Mechanical ventilation | 6895 (1.5) | 14 (1.6) | 6881 (1.5) | 0.751 | 143 (1.5) | 14 (1.6) | 129 (1.5) | 0.773 | ||
| Acute kidney injury | 8237 (1.8) | 17 (1.9) | 8220 (1.8) | 0.701 | 190 (2.0) | 17 (1.9) | 173 (2.0) | 0.932 | ||
| In-hospital mortality | 448 (0.1) | 448 (0.1) | 13 (0.1) | 13 (0.1) | ||||||
| Unfavorable dischargea | 78990 (16.9) | 186 (20.8) | 78804 (16.9) | 0.002 | 1693 (17.5) | 186 (20.8) | 1507 (17.1) | 0.006 | ||
| LOS > 75th percentile (4 days)a | 111525 (23.8) | 232 (26.1) | 111293 (23.8) | 0.101 | 2417 (24.9) | 232 (26.1) | 2185 (24.7) | 0.349 | ||
AMI, acute myocardial infarction; CVA, cerebrovascular accident; PSM, propensity score matching; VTE, venous thromboembolism; PsA, psoriatic arthritis; LDD, lumbar degenerative disease; LOS, length of stay.
Categorical variables are presented as unweighted counts (weighted percentage).
Significant values are shown in bold.
Excluded patients died in hospital.
3.4. Associations between pre-existing PsA and outcomes after LDD surgery
Table 3 shows the associations between PsA and postoperative outcomes of LDD surgery. After adjustments, multivariable regression analysis showed that patients with PsA had a higher risk for unfavorable discharge (aOR: 1.26, 95 % CI: 1.03–1.55) but not for any major complication (aOR: 1.14, 95 % CI: 0.89, 1.47) or prolonged LOS (aOR: 0.96, 95 % CI: 0.81, 1.15). However, significant risk for VTE was shown for patients with PsA (aOR: 1.99, 95 % CI: 1.05–3.75).
Table 3.
Associations between PsA and outcomes of patients undergoing surgery for LDD.
| Outcomes | PsA | Adjusted |
|---|---|---|
| OR (95 % CI)l | ||
| Any major complicationc | Yes vs. No | 1.14 (0.89, 1.47) |
| Unfavorable discharged | Yes vs. No | 1.26 (1.03, 1.55) |
| Prolonged LOSa,b,e | Yes vs. No | 0.96 (0.81, 1.15) |
| AMI & CVAf | Yes vs. No | 0.81 (0.35, 1.88) |
| VTEg | Yes vs. No | 1.99 (1.05, 3.75) |
| Pneumoniah | Yes vs. No | 1.36 (0.72, 2.55) |
| Sepsis & infectioni | Yes vs. No | 1.03 (0.69, 1.54) |
| Respiratory failure & mechanical ventilationj | Yes vs. No | 0.93 (0.53, 1.64) |
| Acute kidney injuryk | Yes vs. No | 0.79 (0.46, 1.36) |
AMI, acute myocardial infarction; CVA, cerebrovascular accident; PSM, propensity score matching; VTE, venous thromboembolism; PsA, psoriatic arthritis; LDD, lumbar degenerative disease; LOS, length of stay.
Significant values were shown in bold.
Excluded In-hospital mortality patients.
Length of hospital stay >75th percentile: 4 days.
Adjusted for age, insurance status, ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, COPD, obesity, other rheumatic disease, CKD, coagulopathy, emergent admission, weekend admission, and hospital bed size.
Adjusted for age, sex, race, household income, insurance status, smoking status, ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, COPD, obesity, other rheumatic disease, CKD, coagulopathy, emergent admission, weekend admission, hospital bed size, hospital location, and hospital region.
Adjusted for age, sex, race, insurance status, ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, COPD, obesity, other rheumatic disease, CKD, coagulopathy, emergent admission, weekend admission, hospital bed size, hospital location, and hospital region.
Adjusted for age, sex, insurance status, ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, COPD, CKD, emergent admission, and hospital location.
Adjusted for age, race, smoking status, atrial fibrillation, COPD, obesity, CKD, coagulopathy, emergent admission, and hospital bed size.
Adjusted for age, ischemic heart disease, congestive heart failure, atrial fibrillation, COPD, and CKD.
Adjusted for insurance status, atrial fibrillation, diabetes, COPD, other rheumatic disease, CKD, coagulopathy, emergent admission, weekend admission, and hospital bed size.
Adjusted for age, insurance status, ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, COPD, obesity, CKD, coagulopathy, and hospital bed size.
Adjusted for age, sex, insurance status, ischemic heart disease, congestive heart failure, atrial fibrillation, diabetes, hypertension, dyslipidemia, COPD, obesity, other rheumatic disease, CKD, coagulopathy, emergent admission, weekend admission, and hospital region.
Covariates with p-value <0.05 were enrolled in multivariate models as adjusted variables.
4. Discussion
The present study is the first to evaluate the effects of PsA on clinical outcomes of LDD surgery, particularly VTE. Among LDD patients in a nationwide US-based sample, PsA did not pose an increased risk for worse inpatient outcomes following decompression and fusion for LDD, including for most major complications or LOS. However, we found that pre-existing PsA was independently associated with a 26 % increase in the risk of unfavorable discharge and twice the risk of VTE compared to not having PsA diagnosed preoperatively.
The present study found that PsA doubles the risk of VTE after LDD surgery. However, no universally accepted guidelines are available for VTE prophylaxis in spine surgery. A previous study aimed to identify VTE risk factors in patients undergoing degenerative spine surgery, reporting that the incidence of readmission with VTE diagnoses after spine surgery was low (0.42 % within 30 days and 0.62 % within 90 days postoperative) [16]. Nevertheless, the devastating consequences of VTE underscore the need to identify patients at highest risk.
Another recent study assessed the incidence and risk factors for VTE in patients with psoriatic disease, finding that older patients with psoriasis, along with those with diabetes and those using corticosteroids, were at higher risk of developing VTE [17]. Another population-based cohort study assessed the association between PsA and VTE events [18]. The authors suggested that the increased risk of VTE in PsA patients appears to be related to their underlying comorbidities rather than being independently associated with PsA diagnosed previously. Age and previous history of VTE were the only risk factors associated with increased risk of VTE in patients with a PsA diagnosis. Results of the above studies support the finding of the present study that patients with PsA double the risk of VTE after LDD surgery, and also support the association shown between age and PsA in LDD patients. Together these findings suggest that anticoagulation therapy should be considered after LDD surgery for older adults with PsA diagnosed preoperatively.
Another previous study has also indicated that the reoperation rate for LDD is associated with patient comorbidities [19]. That study used claims data from the national health insurance database to identify a cohort of patients who underwent spinal surgery for LDD in 2009 and were followed until 2016. Multivariate analysis of comorbidities showed that peripheral vascular disease, pulmonary disease, peptic ulcer, diabetes, and diabetes complications were associated with a significant increase in the reoperation rate, and that these patients had a poorer prognosis overall. Another retrospective case-control study investigated risk factors for 30-day readmission of discharged patients who had undergone lumbar spinal surgery for LDD, reviewing 3933 patients discharged from a university hospital from 2005 to 2012 [20]. In that study, intractable pain was the most common reason for readmission, and independent risk factors for readmission were longer operation times, previous spinal surgery, ICU admission, extended hospital stays, and higher medical expenses. The authors suggested that more attentive management of these risk factors may help to reduce the readmission rate, ultimately improving the quality of care.
Since PsA causes various symptoms that are similar to those reported for rheumatoid arthritis (RA), differentiation between these two entities can be a diagnostic challenge [21]. A few prior studies have focused on the effects of RA, multiple sclerosis (MS), and other autoimmune diseases on outcomes of spine surgery. Significant increases in the risks of sepsis, urinary tract infection, deep vein thrombosis, 90-day emergency room visits, and 90-day readmissions were found in MS patients who had undergone primary posterior lumbar fusion compared with lower risks in patients without MS [22]. Another study compared postoperative outcomes of lumbar spine surgery between RA and non-RA patients through retrospective propensity score-matched analysis, finding that RA had poorer postoperative outcomes for LDD surgery compared with PS-matched non-RA patients [23]. Similarly, the present study demonstrated an association between pre-existing PsA and poorer postoperative complications after LDD surgery.
4.1. Strengths and limitations
The strength of the present study is the use of a massive sample representing a nationwide population. The study groups were carefully matched and adjusted to minimize confounding of measured variables. Nevertheless, the study is inherently limited by its retrospective and observational nature. Coding errors as in other studies that used ICD code systems cannot be ruled out. The severity of comorbidities and disease activity of PsA was not apparent using the NIS data. The database did not record medication usage, especially biologics in treating PsA, thus the effects of medications could not be further investigated. Follow-up data after discharge were also not available, precluding the analyses of late functional outcomes of LDD surgery.
5. Conclusions
In patients undergoing surgery for LDD, pre-existing PsA may be associated with increased likelihood of unfavorable discharge and the occurrence of VTE. The findings may benefit preoperative risk stratification in patients undergoing LDD surgery. Further prospective studies are still warranted to gain more solid evidence.
Funding source
None.
Data availability statement
The data used to support the findings of this study are included within the article.
CRediT authorship contribution statement
Mao-Yu Chen: Conceptualization, Data curation, Formal analysis, Supervision, Writing – original draft. Pin-Yuan Chen: Data curation, Formal analysis, Investigation. Chen-Nen Chang: Formal analysis, Investigation, Methodology. Bo-An Chen: Data curation, Formal analysis, Investigation. Wen-Chun Deng: Data curation, Formal analysis, Methodology. Jiun-Lin Yan: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23613.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baum E.W., Schwartzman S. ALIGN PsA: advancing a multidisciplinary approach in PsA. Semin. Cutan. Med. Surg. 2018;37(6S):S125–S134. doi: 10.12788/j.sder.2018.057. [DOI] [PubMed] [Google Scholar]
- 2.Moll J.M., Wright V. Psoriatic arthritis. Semin. Arthritis Rheum. 1973;3(1):55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Ferrer A., Laiz A., Puig L. Psoriatic arthritis. Med. Clin. 2022;159(1):40–46. doi: 10.1016/j.medcli.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Van den Bosch F., Coates L. Clinical management of psoriatic arthritis. Lancet. 2018;391(10136):2285–2294. doi: 10.1016/S0140-6736(18)30949-8. [DOI] [PubMed] [Google Scholar]
- 5.Veale D.J., Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 6.Gladman D.D. Axial psoriatic arthritis. Curr. Rheumatol. Rep. 2021;23(6):35. doi: 10.1007/s11926-021-00999-8. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb A.B., Merola J.F. Axial psoriatic arthritis: an update for dermatologists. J. Am. Acad. Dermatol. 2021;84(1):92–101. doi: 10.1016/j.jaad.2020.05.089. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb A., Merola J.F. Psoriatic arthritis for dermatologists. J. Dermatol. Treat. 2020;31(7):662–679. doi: 10.1080/09546634.2019.1605142. [DOI] [PubMed] [Google Scholar]
- 9.Yavin D., Casha S., Wiebe S., Feasby T.E., Clark C., Isaacs A., et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80(5):701–715. doi: 10.1093/neuros/nyw162. [DOI] [PubMed] [Google Scholar]
- 10.Bydon M., Alvi M.A., Goyal A. Degenerative lumbar spondylolisthesis: definition, natural history, conservative management, and surgical treatment. Neurosurg. Clin. 2019;30(3):299–304. doi: 10.1016/j.nec.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Donnally I.C., Hanna A., Varacallo M. Lumbar Degenerative Disk Disease. StatPearls. edn; Treasure Island (FL): 2022. [PubMed] [Google Scholar]
- 12.Polachek A., Touma Z., Anderson M., Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res. 2017;69:67–74. doi: 10.1002/acr.22926. [DOI] [PubMed] [Google Scholar]
- 13.Ogdie A., Kay McGill N., Shin D.B., Takeshita J., Jon Love T., Noe M.H., et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population-based cohort study. Eur. Heart J. 2018;39:3608–3614. doi: 10.1093/eurheartj/ehx145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung W.S., Lin C.L. Increased risks of venous thromboembolism in patients with psoriasis: a nationwide cohort study. Thromb. Haemostasis. 2017;117:1637–1643. doi: 10.1160/TH17-01-0039. [DOI] [PubMed] [Google Scholar]
- 15.Gold P.A., Garbarino L.J., Ramkumar P.N., et al. Psoriasis and post-surgical infections in primary total knee arthroplasty: an analysis of 10,727 patients. J. Arthroplasty. 2022;37(8):1575–1578. doi: 10.1016/j.arth.2022.03.055. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan I.A., Lin M., Donoho D.A., Ding L., Giannotta S.L., Attenello F., et al. Venous thromboembolism after degenerative spine surgery: a nationwide readmissions database analysis. World Neurosurg. 2019;125:e165–e174. doi: 10.1016/j.wneu.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damian A.C., Colaco K., Rohekar S., Boyd T., Chandran V., Gladman D.D., et al. The incidence and risk factors for venous thromboembolic events in patients with psoriasis and psoriatic arthritis. Semin. Arthritis Rheum. 2021;51(3):547–552. doi: 10.1016/j.semarthrit.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Gazitt T., Pesachov J., Lavi I., Elias M., Haddad A., Feldhamer I., et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res. Ther. 2022;24(1):16. doi: 10.1186/s13075-021-02703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park H.K., Park S.Y., Lee P.H., Park H.R., Park S.Q., Cho S.J., et al. The influence of comorbidities on reoperations following primary surgery of lumbar degenerative diseases : a nationwide population-based retrospective cohort study from 2009-2016. J. Korean Neurosurg. Soc. 2020;63(6):730–737. doi: 10.3340/jkns.2020.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho P.G., Kim T.H., Lee H., Ji G.Y., Park S.H., Shin D.A. Incidence, reasons, and risk factors for 30-day readmission after lumbar spine surgery for degenerative spinal disease. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-69732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogdie A., Coates L.C., Gladman D.D. Treatment guidelines in psoriatic arthritis. Rheumatology. 2020;59(Suppl 1):i37–i46. doi: 10.1093/rheumatology/kez383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamalapathy P.N., Bell J., Puvanesarajah V., Hassanzadeh H. Postoperative outcomes following posterior lumbar fusion in patients with multiple sclerosis. Clin. Spine Surg. 2022;35(1):E211–E215. doi: 10.1097/BSD.0000000000001212. [DOI] [PubMed] [Google Scholar]
- 23.Kato S., Nakamoto H., Matsubayashi Y., Taniguchi Y., Doi T., Yoshida Y., et al. Postoperative outcomes after degenerative lumbar spine surgery in rheumatoid arthritis patients -a propensity score-matched analysis. BMC Musculoskelet Disord. 2022;23(1):380. doi: 10.1186/s12891-022-05326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article.