Abstract
Next-generation sequencing (NGS) and liquid biopsy (LB) showed positive results in the fight against different cancer types. This paper aims to assess the uptake of advanced molecular diagnostics/NGS for quick and efficient genetic profiles of tumour cells. For that purpose, the European Alliance for Personalised Medicine conducted a series of expert interviews to ascertain the current status across member states. One stakeholder meeting was additionally conducted to prioritize relevant factors by stakeholders. Seven common pillars were identified, and twenty-five measures were defined based on these pillars. Results showed that a multi-faceted approach is necessary for successful NGS implementation and that regional differences may be influenced by healthcare policies, resources, and infrastructure. It is important to consider different correlations when interpreting the results and to use them as a starting point for further discussion.
Keywords: Molecular diagnostics, Next-generation sequencing, Reimbursement, Policy, Infrastructure, Personalised medicine
introduction.
1.1. Background
One of the key elements of the EU's 2021 European Beating Cancer Plan is the 'Cancer Diagnostic and Treatment for All’ initiative [1]. The attainment of this ambitious goal is all the more challenging given persisting variations across countries in rates of incidence, mortality and survival - variations paralleled in risk factors and the intensity and accuracy of diagnostic methods, including screening, The improvements that have been made in cancer survival are not matched by success in cancer control. Improved diagnostic techniques could allow earlier detection, including of indolent tumours [2]. But there is also wide variation in access to the cancer centres that are critical to the development of a cancer control strategy, as well as in their services in prevention, diagnosis, multidisciplinary treatment, supportive care, research and education (Fig. 1). The Organization of European Cancer Institutes (OECI) promotes collaboration and mutual learning to improve the quality of services and to develop comprehensive cancer care through a process that includes accreditation and designation [3].
Fig. 1.
List of factors that need to be taken into account in cancer canters [3].
National and international peer-reviewed publications linked to the centre (first, second or last author employed by the centre)
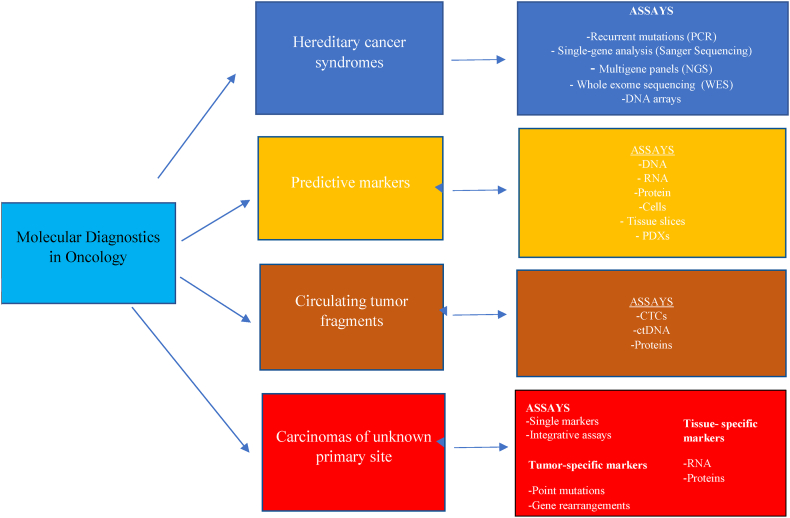
Advances in molecular genetics permit molecular diagnostics to deliver precise characterization of malignant tumours, through sequencing of DNA or RNA, PCR (polymerase chain reaction) based techniques, immunohistochemistry, and in-situ hybridization (Fig. 2). The benefits of molecularly stratified tumour treatment have been demonstrated in non–small-cell lung cancer (NSCLC), colorectal cancer (CRC), breast cancer (BC) and other tumour entities; testing for hereditary cancer syndromes can identify at-risk individuals and personalize systemic treatment; predictive tests can identify specific tumour phenotypes; and molecularly guided detection of residual tumour fragments can monitor malignant disease. Liquid biopsy (LB) has great potential in early cancer diagnosis and screening, and mutation testing and RNA analysis offer new tools for the diagnosis of unknown primary sites (CUPs), distinguishing tumours of different histological origins [4]. NGS enables the sequencing of numerous relevant gene sequences or even whole genes in parallel, dispensing with complex staged diagnostics and reducing the use of biomaterials, although sequencing of large gene panels or whole genomes or exomes needs to be precisely validated before widespread routine clinical application. NGS can also detect with relative ease mutations, insertions, deletions, structural reorganizations, copy number changes, gene fusions and alternatively spliced isoforms [5].
Fig. 2.
Molecular diagnostics in oncology [4].
NGS methods have emerged as versatile tools for genomics and clinical activities involving DNA analysis. NGS is a high-throughput technology that aids in integrating molecular tumour profiles into precision oncology decision-making. Diagnostic centres increasingly utilize NGS tests to assess genomic alterations and select patients for precision oncology [6]. A range of NGS tests is available, including ctDNA MRD tests, ctDNA treatment response monitoring, gene fusion analysis, homologous recombination deficiency (HRD) testing, and tumour mutational burden (TMB) testing [[7], [8], [9]].
In real-world outcomes, the utility of NGS testing becomes evident when dealing with patients who have advanced cancers requiring multiple genetic markers, those with rare cancers, or individuals being considered for molecular clinical trials [10]. NGS technology is playing a pivotal role in characterizing the genomics of various neoplastic diseases, exerting a significant and far-reaching influence in this field [11]. A real-world study conducted on the clinical use of NGS in advanced breast cancer revealed that NGS testing facilitated molecular-guided therapy in merely 4.7 % of the patients [12]. However, NGS testing demonstrated a connection with enhanced outcomes in another study when utilized for making therapeutic decisions in initially advanced cancers, particularly in cases like lung cancer, as well as for clinical trial screening and rare cancers in the early stages [11]. The US Food and Drug Administration has approved NGS-based multigene panel tests for cancer-related genes, and routine clinical use of multigene NGS panels has been recommended by the European Society for Medical Oncology (ESMO) for advanced NSCLC, prostate cancer (PC), ovarian cancer and cholangiocarcinoma [13].
At present, NGS faces significant challenges, including the lack of standardized procedures for quality management, sequencing workflows, data handling, and analysis. Affordability and accessibility issues persist, and sequencing costs in lower-income countries are higher due to taxes, analysis expenses, shipment, and infrastructure costs [14,15]. Although recent technologies have improved NGS speed, read length, and data analysis while reducing costs, drug manufacturers have little incentive to develop tests for specific populations since that could negatively impact existing sales [16].
Additionally, there is limited data on the availability, funding, and adoption of NGS globally, especially outside North America and Western Europe. The promotion of NGS adoption in healthcare systems, with its implications for making personalised medicine accessible worldwide, depends on healthcare professionals and decision-makers assessing the maturity of NGS practices in their respective countries [9].
The future of NGS presents immense potential for advancements across fields as diverse as bioinformatics and liquid handling. Continuing technological advancements will usher in faster and more accurate sequencing methods. Future sequencing platforms will require less input DNA and reagents (zeptoliters down to a few molecules), and become more portable for diagnostic use in medical, agricultural, ecological and other field-based settings. With advancing technology, NGS is poised to play a crucial role in single-cell genomics, long-read sequencing, epigenomics, and the integration of multiple omics datasets. This will enable a deeper comprehension of cellular processes and disease mechanisms, and the development of personalised treatment approaches [17,18].
The paper focuses on lung cancer (LC), breast cancer (BC), gastric cancer (GC), prostate cancer (PC), colorectal cancer (CRC) and sarcoma.
Progress in the diagnosis and treatment of BC is partly due to examination programs and advances in imaging techniques, with NGS an increasingly powerful tool. Treatment of metastases in distant organs in metastatic breast cancer (mBC) is no longer automatically chemotherapy, since commercially available, high-throughput genomic sequencing technologies with NGS now permit new therapeutic possibilities. However, molecular profiling in combination with precision medicine is hampered in daily clinical practice largely by incomplete standardization in interpreting complex genomic data and by delays in timely molecular testing in patients with progressive disease [19].
For sarcomas, where effective treatments are lacking for advanced cases, global genomic signatures detectable by NGS, including tumour mutational load and microsatellite instability, have the potential as biomarkers for response to immunotherapy. NGS could improve the assessment of cancer predisposition, and expert interpretation of NGS data will complement tools in diagnosis and decision-making. Data from Germany and France show benefits to survival from guideline adherence, and further improvements in outcomes from treatment in certified sarcoma centres with specialized Molecular Tumor Boards (MTB) [20,21].
PC is highly treatable in the early stages, but NGS is performed infrequently. More genomically-based studies are urgently needed. A study by Griffin et al. showed only 2.3 % of patients with a history of PC had undergone NGS testing during the study period, and NGS testing was not routine in initial evaluation for metastatic prostate cancer (mPC). The approval of poly (ADP-ribose) polymerase (PARP) inhibitors for later-line treatment of metastatic PC increased the actionability of NGS findings but has not led to routine earlier NGS testing – although greater utilization of NGS testing is anticipated [22].
The asymptomatic nature of early-stage GC impedes timely diagnosis using conventional pathological examinations and imaging tests, but NGS is increasingly adopted to identify tumour genomic alterations for precision medicine. Recent studies using NGS suggest potential cancer-driver genes and a mutational landscape. Molecular profiling using NGS offers hope of efficient prognostic, diagnostic, and therapeutic strategies [23].
Tackling CRC would benefit from targeted NGS in routine practice. Bayle et al. showed how it permitted patients to access a targeted therapy not currently registered for CRC, but targeted NGS panels need to be regularly updated and analysis of prognostic factors needs to be improved. NGS allows the detection of rare RAS/BRAF mutations or molecular alterations that could impede the anti-EGFR response, the identification of potential drug targets (HER2, PIK3CA.), and the possibility of testing the clinical value of co-occurring alterations [24].
In LC, Pop-Bica et al. assessed the genetic profile of cancer genes using The Cancer Genome Atlas (TCGA) datasets for mutations, and validated the results in a separate cohort of 32 patients using tumor tissue and whole blood samples for NGS experiments. TCGA analysis identified the most commonly mutated genes in each LC dataset, with differences among the three histotypes analyzed. NGS analysis revealed tp53, csf1r, pik3ca, flt3, erbb4, and kdr as being the genes most frequently mutated [25].
Narrowing the existing disparities in incorporating NGS and advanced molecular diagnostics into healthcare systems will require a series of initiatives. These encompass the improvement of education and training, the establishment of standardized procedures and quality assurance measures, the resolution of cost-related and reimbursement concerns, the allocation of resources and infrastructure investment, and the promotion of collaboration and engagement among distinct groups of stakeholders. The projected trajectory exhibits significant potential, driven by continuous technological progress, the broadening of clinical utilization, the integration of artificial intelligence and machine learning, pioneering research, and the formulation of regulatory frameworks. This paper aims to marshal input about the uptake of advanced molecular diagnostics/NGS for quick and efficient genetic profiles of tumour cells, allowing Cancer Centres to share cancer profiles and apply the same or similar diagnostic and therapeutic approaches to patients with comparable cancer profiles. It aims to investigate the factors influencing the implementation of NGS in healthcare across European Union countries and centres, revealing correlations between various factors and NGS adoption. If ‘Access and Diagnostics for all’ as well as Public Health Genomics are to be respected in line with the EU principle of universal and equal access to high-quality healthcare, it must be made available to many more citizens than now. What is recommended is a long-term approach to innovation to ensure the translation of new therapies from laboratories to patients.
2. Methods
To comprehensively assess the current uptake of NGS and advanced molecular diagnostics across EU member states, a systematic approach was employed. The European Alliance for Personalised Medicine (EAPM) organized semi-structured interviews, engaging experts in oncology, molecular biology, pathology, and bioinformatics. Expert selection was based on their substantial contributions to peer-reviewed literature on NGS applications in clinical diagnostics, as detailed in the Appendix.
2.1. Interview structure
Semi-structured interviews were conducted over 2 months using the Zoom platform, ensuring flexibility for in-depth discussions. A total of 28 interviews, involving 2 to 4 experts each, were conducted and recorded for thorough analysis.
2.2. Defined pillars and open-ended survey
Experts engaged in open-ended surveys organized around defined pillars. An open-ended survey with predefined questions, consistent across all experts, was employed to maintain standardization. The questions were tailored to cover distinct issues related to genomic testing and diagnostics.
2.3. Transcription and reading through/paraphrasing
The interviews underwent meticulous transcription, capturing the richness of the discussions. The subsequent step involved thorough reading through and paraphrasing, ensuring the accurate representation of expert insights.
2.4. Identifying common pillars
Common pillars were derived from the open-ended survey responses, creating a structured foundation for subsequent analysis. The systematic identification of common themes facilitated a uniform interpretation of the data.
2.5. Scoring system based on defined questions
Measures were identified based on the defined questions in the open-ended survey. A standardized scoring system was applied to quantify expert opinions, adding a quantitative dimension to the qualitative insights.
2.6. Quality assurance
Throughout the data collection process, measures were in place to ensure the quality and reliability of the gathered information. The recorded Zoom interviews served as a verification tool, and the transparency in expert selection added credibility to the study.
2.7. Documentation of scoring procedures
To enhance the transparency and replicability of the study, detailed documentation of the procedures used in scoring, specifically based on the predefined questions, was undertaken. This documentation provides clarity on the scoring criteria and ensures uniformity in their application.
In conclusion, the methodology leveraged open-ended surveys with defined questions to capture expert insights on specific pillars. This structured approach, coupled with the systematic analysis through transcription, paraphrasing, and scoring, reflects a standardized method for evaluating the uptake of NGS and advanced molecular diagnostics across EU member states. The inclusion of a systematic scoring system, based on the defined questions, enhances the rigour and replicability of the research methodology.
Statistical analysis used Spearman rank correlation, the non-parametric test measuring the degree of association between two variables without any assumptions about the distribution of the data. Cohen's standard evaluated the correlation coefficient to determine the strength of the relationship, or the effect size, and one-way analysis of variance (ANOVA) identified any statistically significant differences between the means of three or more independent (unrelated) groups. To determine whether any of the differences between the means are statistically significant, the p-value is compared to our significance level to assess the null hypothesis that the population means are all equal. Usually, a significance level (denoted as α or alpha) of 0.05 works well. A significance level of 0.05 indicates a 5 % risk of concluding that a difference exists when there is no actual difference.
To ensure trustworthiness, an explicit description of the methods undertaken was used, along with participant profile, extensive use of interview transcripts by way of representative quotations, peer review of the data analysis process and invited feedback from interviewees on the overall findings.
A subsequent face-to-face meeting in Rome explored a possible sustainability plan to tackle disparities in and between countries on the uptake of molecular diagnostics, especially NGS (Table 1), where stakeholders from different member states (Table 2) reviewed 28 out of 40 reports presented at the meeting via a SWOT (strengths-weaknesses-opportunities--threats) analysis. The expert panel interviews identified major strengths, weaknesses, opportunities and threats affecting the access and utilization of NGS throughout the EU, and suggested major commonalities.
Table 1.
Addressed topics and participants in the stakeholder meeting.
| Expert panel | Topic(s) addressed | Participants |
|---|---|---|
| High-level Stakeholder Conference Reducing Disparities Across the European Union - stakeholder meeting in Rome |
● Assess and address obstacles to the integration of ‘Access & Diagnostics for All’ & 'Public Health Genomics' into Europe's healthcare systems ● Identify best practices and their added value |
Number of experts: 40 ● Healthcare professionals, patient representatives, researchers, policy makers ● European Parliament representatives |
Table 2.
Number of experts involved in the face-to-face stakeholder meetings organised by EAPM.
| Participants | Number of participants | Percentage (%) |
|---|---|---|
| Healthcare professionals (oncologists, radiologists, haematologists, pathologists, surgeons etc.) | 14 | 35 |
| Patients' representatives | 2 | 5 |
| European Parliament representatives | 6 | 15 |
| European Commission representatives | 3 | 7.5 |
| Pharma industry representatives | 3 | 7.5 |
| Other Agencies and Organisations | 7 | 17.5 |
| Researchers | 5 | 12.5 |
| Total | 40 | 100 |
3. Findings
3.1. Expert interviews
Semi-structured interviews were transcribed, read through and paraphrased, and seven common pillars were identified.
-
1.
Infrastructure and tools needed for conducting NGS and advanced diagnostics
-
2.
Molecular tumour boards and expertise required to appropriately offer genomic testing to patients
-
3.
Reimbursement for NGS and LB
-
4.
Governance in terms of guidelines, recommendations, certifications and accreditation needed to conduct genomic testing
-
5.
Education/training/awareness
-
6.
Healthcare Workforce
-
7.
Data sharing and linking, and security guidelines to ensure the latest technological, regulatory, and ethical considerations are being addressed
On this basis twenty-five measures were defined, scored from the transcript of interviews. In the table describing measures and units of expression (Table 3), correlation coefficients between 0.10 and 0.29 represent a small association, between 0.30 and 0.49 a medium association, and 0.50 and above a large association or relationship. The “*” sign indicates the correlation coefficient value is statistically significant at 0.05 significance level, which implies that there is a significant association or correlation between dependent and independent variables. Through correlation, this data analysis makes possible the attribution of specific factors to performance in specific areas for the implementation of NGS.
Table 3.
Description of scored measures from the expert panel interviews.
| Measure ID | Pillars | Measure Name | Measure Description | Unit of expression |
|---|---|---|---|---|
| 1 | Infrastructure and tools needed for conducting NGS and advanced diagnostics | NGS centre | Availability of NGS centre | Binary |
| 2 | Equipment/infrastructure | Sufficient tools/infrastructure available for NGS testing | Binary | |
| 3 | Funding | Sufficient funding for the development of proper infrastructure | Binary | |
| 4 | Routine utilization | Utilization of NGS on regular basis | Binary | |
| 5 | Molecular tumour boards and expertise required to appropriately offer genomic testing to patients | MTB panel | Availability of MTB | Binary |
| 6 | Consultation | Recurring board meetings for consultation | Weekly-5 Monthly-4 Quarterly-3 Half-yearly-2 Yearly-1 |
|
| 7 | Testing/Discussion | Number of the patients being tested/discussed | High = 3 Medium = 2 Low = 1 |
|
| 8 | Reimbursement for NGS and LB | Reimbursement for NGS | Binary | |
| 9 | Reimbursement for LB | Binary | ||
| 10 | Funding | Enough funding for reimbursement process | Binary | |
| 11 | Governance in terms of guidelines, recommendations, certifications and accreditation needed to conduct genomic testing | ISO accreditation/certification | Labs/institutions are ISO accredited or certified | Binary |
| 12 | Clinical Guidelines | Clinical guidelines are regularly updated | Binary | |
| 13 | Internal Guidelines | Use of internal guidelines | Binary | |
| 14 | External Guidelines | Use of external guidelines | Binary | |
| 15 | External Quality Assessment | Use of external quality assessment | Binary | |
| 16 | Education/training/awareness | Trained Personnel | Availability of sufficient trained workforce/personnel | Binary |
| 17 | Awareness/Understanding | Proper understanding/Awareness regarding NGS testing/application | Scale 1-5 | |
| 18 | Educational programme/workshops to increase awareness | Availability of educational programme/workshops to increase awareness | Binary | |
| 19 | Educational programme for proper training | Availability of educational programme for proper training | Binary | |
| 20 | Healthcare workforce | Sufficient workforce | Availability of sufficient healthcare workforce/personnel | Binary |
| 21 | Data sharing and linking, and security guidelines to ensure the latest technological, regulatory, and ethical considerations are being addressed | Collaborations | Cross-border/cross-disciplinary collaborations | Binary |
| 22 | Data sharing | Routine sharing of data | Binary | |
| 23 | Security guidelines | Availability of security guidelines external/internal | Binary | |
| 24 | Data linking | Data linking to Electronic Health Record | Binary | |
| 25 | Controlling body | Controlling body for data sharing | Binary |
The correlation analysis conducted across participating EU countries, as presented in Table 4, offers valuable insights into the factors influencing the implementation of NGS and advanced molecular diagnostics within healthcare systems. Notably, a robust positive correlation of 0.6831 between Infrastructure and Funding suggests that countries with well-established infrastructure tend to receive greater financial support for their healthcare systems. Further examining the relationship between NGS centres and funding-related variables reveals significant associations. A strong positive correlation of 0.7868 between NGS centres and Equipment/Infrastructure highlights the pivotal role of funding in establishing and enhancing NGS facilities. Similarly, a moderate positive correlation of 0.5606 between NGS centres and Routine Utilization indicates that increased funding is linked to routine application of NGS technologies. Within the domain of Molecular Tumor Boards (MTBs), a compelling positive correlation of 0.8490 with Consultation and 0.7537 with Testing/Discussion underscores the integral connection between these components, suggesting that countries actively engaged in consultation services also demonstrate substantial involvement in testing and discussion aspects of MTBs. Delving into reimbursement dynamics, a moderate positive correlation of 0.5071 between Reimbursement for NGS and Funding implies that countries with higher financial allocations are more likely to have tailored reimbursement mechanisms for NGS procedures. Governance pillars exhibit varying degrees of correlation, with weak associations observed in ISO Accreditation/Certification and Internal Guidelines (0.0348), External Guidelines and Clinical Guidelines (0.2 and 0.1741, respectively). In the realm of education, training, and awareness, positive correlations between Trained Personnel and Awareness/Understanding (0.1816 and 0.4144, respectively) suggest that well-trained personnel contribute to heightened awareness and understanding of advanced diagnostics. The Data Sharing and Linking domain reveals a moderate correlation of 0.3779 between Collaborations and Data Sharing, indicating that collaborative efforts are aligned with data-sharing practices across countries. However, the weaker correlation of 0.0476 between Security Guidelines and Data Sharing raises questions about potential gaps in ensuring the security of shared data. Finally, the establishment of a controlling body shows a weak correlation of 0.3333, suggesting a nuanced relationship with other pillars. These correlation coefficients collectively provide a nuanced understanding of the multifaceted landscape influencing the adoption of NGS technologies in European healthcare systems.
Table 4.
Correlation table of participating countries across EU.
| Pillar | Dependent Variable | Independent Variable | CORRELATIONS COEFFICIENT (ROH) |
|---|---|---|---|
| Infrastructure | Funding | NGS centre | 0.683130051* |
| Equipment/infrastructure | 0.786795792* | ||
| Routine utilization | 0.560611911* | ||
| Molecular Tumour Board | MTB panel | Consultation | 0.849033391* |
| Testing/Discussion | 0.753707581* | ||
| Reimbursement | Funding | Reimbursement for NGS | 0.507092553* |
| Reimbursement for LB | 0.225374468* | ||
| Governance | ISO accreditation/certification | Internal Guidelines | 0.034815531* |
| External Guidelines | 0.2* | ||
| Clinical Guidelines | Internal Guidelines | 0.174077656* | |
| Education/Training/Awareness | Educational programme for proper training | Trained Personnel | 0.18156826* |
| Awareness/Understanding | 0.414363971* | ||
| Educational programme/workshops to increase awareness | 0.480384461* | ||
| Data Sharing and Linking | Collaborations | Data sharing | 0.377964473* |
| Security guidelines | Data sharing | 0.047619048* | |
| Controlling body | 0.333333333* |
Table 5 provides a comprehensive correlation analysis of participating centres across five major European countries—Belgium, Croatia, France, Germany, and Italy. The correlation coefficients (roh) shed light on the relationships between various pillars influencing the adoption of NGS and advanced molecular diagnostics in these healthcare systems.
Table 5.
Correlation table of participating centres across 5 major European countries (France, Germany, Italy, Spain, United Kingdom).
| Pillar | Dependent Variable | Independent Variable | Belgium (Roh) | Croatia (Roh) | France (Roh) | Germany (Roh) | Italy (Roh) |
|---|---|---|---|---|---|---|---|
| Infrastructure | Funding | NGS centre | 0.471404521* | 0.292770022* | 0.666666667* | 0.679366221* | 0.540061725* |
| Equipment/infrastructure | 0.730296743* | 0.774596669* | 0.666666667* | 0.679366221* | 0.788810638* | ||
| Routine utilization | 0.730296743* | 0.745355992* | 0.375* | 0.679366221* | 0.83452296* | ||
| Molecular Tumour Board | MTB panel | Consultation | 0.353553391* | 0.76834982* | 0.333333333* | 0.372104204* | 0.265165043* |
| Testing/Discussion | 0.222222222* | 0.707106781* | 0.333333333* | 0.366088266 | 0.218125718* | ||
| Reimbursement | Funding | Reimbursement for NGS | 1 | 0.466666667* | 0.509175077* | 0.440385506* | 0.658280589* |
| Reimbursement for LB | 1 | 0.774596669* | 0.509175077* | 0.645497224* | 0.859337849* | ||
| Governance | ISO accreditation/certification | Internal Guidelines | 1 | 0 | 0.327326835* | 0.416666667* | 0.490990253* |
| External Guidelines | 1 | 0 | 0.428571429* | 0.416666667* | 0.190476191* | ||
| External Quality Assessment | 0 | 0 | 0.534522484* | 0.408248291* | 0.436435781* | ||
| Clinical Guidelines | Internal Guidelines | 1 | 0.292770022* | 0.666666667* | 0.78173596* | 0.265165043* | |
| External Guidelines | 1 | 0.774596669* | 0.509175077* | 0.284267622* | 0.540061725* | ||
| External Quality Assessment | 0 | 0.6* | 0.408248291* | 0.174077656* | 0.23570226* | ||
| Education/Training/Awareness | Educational programme for proper training | Trained Personnel | 1 | 1 | 0.763762616* | 0.547722558* | 0.536739439* |
| Awareness/Understanding | 0.416666667* | 0.421075961* | 0.607142857* | 0.353553391* | 0.50104431* | ||
| Educational programme/workshops to increase awareness | 0.75* | 0.745355992* | 0.763762616* | 0.679366221* | 0.783349452* | ||
| Data Sharing and Linking | Collaborations | Data sharing | 1 | 0.654653671* | 0.816496581* | 0.825722824* | 0.88273483* |
| Security guidelines | Data sharing | 0.645497224* | 0.333333333* | 0.5* | 0.575757576* | 0.298807152* | |
| Data linking | 0.645497224* | 0.654653671* | 0.21821789* | 0.440385506* | 0.684653197* | ||
| Controlling body | 0.166666667* | 0.333333333* | 0.25* | 0.174077656* | 0.209165007* |
3.2. Belgium
In Belgium, the correlation analysis reveals significant associations between various pillars influencing the implementation of NGS and advanced molecular diagnostics. The positive correlation (0.4714) between Infrastructure and Funding for NGS centres suggests that as infrastructure improves, there is an associated increase in financial support. Notably, strong correlations between Equipment/Infrastructure and Routine Utilization within NGS centres highlight the interconnectedness of having the necessary equipment and the routine application of NGS technologies. The strong correlation (1.0) between Reimbursement for Liquid Biopsy and Funding emphasizes a substantial financial influence on reimbursement mechanisms for different diagnostic methods. In the governance domain, positive correlations between Internal Guidelines and ISO accreditation/certification (1.0) underscore the importance of internal guidelines in achieving accreditation. Moreover, the strong correlations in Educational initiatives, such as Trained Personnel and Awareness/Understanding (1.0), indicate that well-trained personnel significantly contribute to heightened awareness and understanding of advanced diagnostics. Collaborative efforts in data-sharing practices are evident with strong correlations in Collaborations and Data Sharing (1.0). However, the weak correlation with Security Guidelines (0.6455) suggests the need for further examination of security measures in shared data.
3.3. Croatia
Croatia's correlation analysis illuminates the nuanced relationships among the pillars influencing the adoption of NGS and advanced molecular diagnostics. The weak positive correlation (0.2928) between Infrastructure and Funding for NGS centres suggests a less pronounced link compared to other countries. Positive correlations between Equipment/Infrastructure and Routine Utilization underscore the importance of funding in acquiring necessary equipment and the routine application of NGS technologies. The strong correlation for Consultation within MTBs (0.7683) indicates a significant association between consultation services and other MTB components. Notably, the strong correlations (1.0) between Reimbursement for NGS and Funding, as well as Reimbursement for Liquid Biopsy and Funding, emphasize a substantial financial influence on reimbursement mechanisms. In governance, the weak correlation between Internal Guidelines and ISO accreditation/certification (0.0) suggests a nuanced relationship. Strong correlations between Trained Personnel and Awareness/Understanding (1.0) highlight the pivotal role of well-trained personnel in increasing awareness. The strong correlation (0.6547) between Collaborations and Data Sharing emphasizes collaborative efforts in data-sharing practices, while the weak correlation with Security Guidelines (0.3333) suggests a need for further exploration of security measures.
3.4. France
In France, the correlation analysis provides insights into the interconnected nature of distinct pillars influencing the adoption of NGS and advanced molecular diagnostics. A strong positive correlation (0.6667) between Infrastructure and Funding for NGS centres indicates a robust connection between well-established infrastructure and financial support. Positive correlations between Equipment/Infrastructure and Routine Utilization within NGS centres highlight the importance of funding in acquiring necessary equipment and the routine application of NGS technologies. In MTBs, a moderate correlation for Consultation (0.3333) suggests a link between consultation services and other components within MTBs. The correlations in Reimbursement dynamics indicate that increased funding is associated with tailored reimbursement strategies for NGS procedures. In governance, positive correlations between Internal Guidelines and ISO accreditation/certification (0.3273) underscore the importance of internal guidelines in achieving accreditation. The moderate correlation (0.4286) between External Guidelines and ISO accreditation/certification indicates a significant link. Strong correlations between Trained Personnel and Awareness/Understanding (0.5477) highlight the contribution of well-trained personnel to increased awareness. Collaborative efforts in data-sharing practices are evident with strong correlations in Collaborations and Data Sharing (0.8165). However, the weak correlation with Security Guidelines (0.5) suggests a need for further examination of security measures in shared data.
3.5. Germany
Germany's correlation analysis reveals significant associations among the pillars influencing the adoption of NGS and advanced molecular diagnostics. A strong positive correlation (0.6794) between Infrastructure and Funding for NGS centres indicates a significant relationship between well-established infrastructure and financial support. Positive correlations between Equipment/Infrastructure and Routine Utilization within NGS centres highlight the importance of funding in acquiring necessary equipment and the routine application of NGS technologies. In MTBs, a moderate correlation for Consultation (0.3721) suggests a link between consultation services and other components within MTBs. Positive correlations in Reimbursement dynamics indicate that increased funding is associated with tailored reimbursement strategies for NGS procedures. In governance, positive correlations between Internal Guidelines and ISO accreditation/certification (0.4167) underscore the importance of internal guidelines in achieving accreditation. The moderate correlation (0.4167) between External Guidelines and ISO accreditation/certification indicates a significant link. Strong correlations between Trained Personnel and Awareness/Understanding (0.5477) highlight the contribution of well-trained personnel to increased awareness. Collaborative efforts in data-sharing practices are evident with strong correlations in Collaborations and Data Sharing (0.8257). However, the weak correlation with Security Guidelines (0.5758) suggests a need for further examination of security measures in shared data.
3.6. Italy
Italy's correlation analysis sheds light on the nuanced relationships among the pillars influencing the adoption of NGS and advanced molecular diagnostics. A moderate positive correlation (0.5401) between Infrastructure and Funding for NGS centres suggests a moderate link between well-established infrastructure and financial support. Strong correlations between Equipment/Infrastructure and Routine Utilization within NGS centres highlight the importance of funding in acquiring necessary equipment and the routine application of NGS technologies. In MTBs, weak to moderate correlations for Consultation (0.2652) and Testing/Discussion suggest a less pronounced connection within MTBs. The correlations in Reimbursement dynamics indicate that increased funding is associated with tailored reimbursement strategies for NGS procedures. In governance, a moderate correlation (0.7817) between Internal Guidelines and ISO accreditation/certification underscores the importance of internal guidelines in achieving accreditation. The weak correlation (0.2843) between External Guidelines and ISO accreditation.
ANOVA (Analysis of Variance)
Analysis of Variance (ANOVA) was used to compare variances across the means (or average) of different groups. Table 6 shows ANOVA-Single Factor among the common pillars of participating countries, while Table 8 shows results among common pillars of five major countries’ centres.
-
•
P-value ≤ α: The differences between some of the means are statistically significant.
Table 6.
ANOVA-Single Factor among the pillars of participating countries.
| Groups | Count | Sum | Average | Variance |
|---|---|---|---|---|
| Infrastructure | 16 | 53 | 3.3125 | 1.5625 |
| Molecular Tumour Board | 16 | 97 | 6.0625 | 10.4625 |
| Reimbursement | 16 | 16 | 1 | 1.333333 |
| Governance | 16 | 46 | 2.875 | 0.65 |
| Education/Training/Awareness | 16 | 65 | 4.0625 | 3.929167 |
| Healthcare workforce | 16 | 2 | 0.125 | 0.116667 |
| Data Sharing and Linking | 16 | 51 | 3.1875 | 1.629167 |
Table 8.
ANOVA-Single Factor Results among pillars of five major countries’ centres.
| Groups | Count | Sum | Average | Variance |
|---|---|---|---|---|
| Infrastructure | 56 | 179 | 3.196429 | 1.651623 |
| Molecular Tumour Board | 56 | 254 | 4.535714 | 14.58052 |
| Reimbursement | 56 | 109 | 1.946429 | 1.61526 |
| Governance | 56 | 189 | 3.375 | 2.311364 |
| Education/Training/Awareness | 56 | 231 | 4.125 | 5.747727 |
| Healthcare workforce | 56 | 12 | 0.214286 | 0.171429 |
| Data Sharing and Linking | 56 | 191 | 3.410714 | 2.646429 |
If the p-value is less than or equal to the significance level, we reject the null hypothesis and conclude that not all population means are equal.
-
•
P-value > α: The differences between the means are not statistically significant
If the p-value is greater than the significance level, we do not have enough evidence to reject the null hypothesis that the population means are all equal.
In Table 7, the p-value for the ANOVA test is less than 0.001, indicating that there is strong evidence to reject the null hypothesis of no significant difference among the groups. The F-statistic is 21.71888, and the critical F-value at the 0.05 significance level with 6 and 105 degrees of freedom is 2.186134. Therefore, we can conclude that at least one group has a significantly different mean from the others.
Table 7.
Calculation of p-value.
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Groups | 366.4286 | 6 | 61.07143 | 21.71888 | <0.001 | 2.186134 |
| Within Groups | 295.25 | 105 | 2.811905 | |||
| Total | 661.6786 | 111 |
In Table 9, the p-value for the ANOVA test is also less than 0.001, indicating strong evidence to reject the null hypothesis of no significant difference among the groups. The F-statistic is 29.19686, and the critical F-value at the 0.05 significance level with 6 and 385 degrees of freedom is 2.122137. Thus, we can conclude that at least one group has a significantly different mean from the others.
Table 9.
Calculation of p-value.
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Groups | 718.852 | 6 | 119.8087 | 29.19686 | <0.001 | 2.122137 |
| Within Groups | 1579.839 | 385 | 4.103479 | |||
| Total | 2298.691 | 391 |
Overall, both tables suggest that there are significant differences among the groups in terms of their means, indicating that some pillars are more developed in some countries or centres than others. This information can be useful for identifying areas where improvement is needed and for guiding policy decisions.
3.7. SWOT analysis for Expert panel in Rome
SWOT analysis was applied for the analysis of stakeholder's reports presented at the expert panel in Rome organised by EAPM. The aim was to define common measures between the perspectives of different presentations (box1). The measures defined are as follows.
Box 1. SWOT analysis based on opinions/perspectives of different speakers at stakeholder meetings.
4. Scoring of measures
The measures discussed in the previous section were scored based on the extent of agreement among different stakeholders regarding their importance. The first measure, Networking/Stakeholder Involvement, received a total score of 12 out of 28, indicating that 42.85 % of the presenters agreed that networking is a major strength in the EU. The second measure, Data Infrastructure, received a score of 10, indicating that 35.71 % of the experts agreed that data infrastructure is a significant strength in Europe. The majority of presenters (64.28 %) agreed that increased interpretation is a significant strength (Table 10). In terms of weaknesses, the Alliance Complexity measure received a total score of 19 (67.8571428 %). Most of the experts (25 out of 28) identified Complex Hospital Procedures as a significant weakness, while 27 of them identified High Dependency on National Will and National Priorities as a major weakness. External Dependency on Databases was a significant weakness for 15 out of 28 experts (Table 11). The Increased Awareness for the Benefits of Personalised Medicine measure received a total score of 22, indicating that 78.5 % of experts believed it to be a major opportunity. A total of 21 out of 28 experts identified Bottom-Up Initiatives at the National and EU levels as the major opportunities (Table 12). For threats, the External Competition from New and Emerging Research Networks or Technologies measure received a total score of 27, indicating that 27 out of 28 experts identified it as a significant threat. On the other hand, 13 out of 28 experts identified Making Full Use of Artificial Intelligence as a major threat (Table 13).
Table 10.
Scoring of measures for ‘strengths’ category.
| Sr.No. | Measures/Commonalities | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap | Total | Percentage |
|---|---|---|---|---|---|---|---|---|
| 1 | Networking/Stakeholders involvement | 5 | 2 | 1 | 3 | 1 | 12 | 42.85 |
| 2 | Data Infrastructure | 5 | 3 | 2 | 0 | 0 | 10 | 35.71 |
| 3 | Increased interpretation | 4 | 4 | 5 | 4 | 1 | 18 | 64.28 |
| 4 | Collaborations | 6 | 4 | 5 | 3 | 0 | 18 | 64.28 |
| 5 | Cost-effective solution | 4 | 3 | 2 | 4 | 2 | 15 | 53.57 |
| 6 | Increase of patient satisfaction | 3 | 2 | 3 | 5 | 0 | 13 | 46.42 |
| 7 | Scientific advances with a proven impact | 6 | 5 | 5 | 4 | 1 | 21 | 75 |
| 8 | Evidence-based | 4 | 3 | 5 | 5 | 1 | 18 | 64.28 |
Table 11.
Scoring of measures for ‘weaknesses’ category.
| Sr. No. | Measures/Commonalities | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap | Total | Percentage |
|---|---|---|---|---|---|---|---|---|
| 1 | Alliance complexity | 6 | 5 | 3 | 5 | 0 | 19 | 67.85714286 |
| 2 | Sustainability | 7 | 6 | 4 | 4 | 1 | 22 | 78.57142857 |
| 3 | Dependency on external databases | 4 | 4 | 2 | 5 | 0 | 15 | 53.57142857 |
| 4 | Legal basis | 5 | 6 | 5 | 4 | 1 | 21 | 75 |
| 5 | Difficulties for interdepartmental coordination | 5 | 6 | 5 | 6 | 2 | 24 | 85.71428571 |
| 6 | Complex hospital procedures | 7 | 5 | 6 | 6 | 1 | 25 | 89.28571429 |
| 7 | Managing sensitive patient data while maintaining privacy and security can be a challenge, especially considering the GDPR regulations | 7 | 5 | 4 | 5 | 1 | 22 | 78.57142857 |
| 8 | Insufficient grant budget | 6 | 6 | 6 | 4 | 0 | 22 | 78.57142857 |
| 9 | Lack of knowledge in evidence-based policy | 5 | 4 | 5 | 5 | 0 | 19 | 67.85714286 |
| 10 | Training the healthcare workforce | 6 | 5 | 6 | 4 | 0 | 21 | 75 |
| 11 | Inequalities in access | 5 | 6 | 5 | 6 | 1 | 23 | 82.14285714 |
| 12 | Fragmentation | 4 | 6 | 3 | 5 | 2 | 20 | 71.42857143 |
| 13 | Highly dependent on national will and national priorities | 7 | 7 | 6 | 5 | 2 | 27 | 96.42857143 |
Table 12.
Scoring of measures for ‘opportunities’ category.
| Sr. No. | Measures/Commonalities | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap | Total | Percentage |
|---|---|---|---|---|---|---|---|---|
| 1 | Advances in NGS and reduction of costs associated | 7 | 7 | 5 | 4 | 2 | 25 | 89.28571429 |
| 2 | Increased awareness for the benefits of personalised medicine | 7 | 6 | 4 | 4 | 1 | 22 | 78.57142857 |
| 3 | Many new stakeholders | 6 | 6 | 6 | 5 | 2 | 25 | 89.28571429 |
| 4 | Interest by pharma companies | 6 | 5 | 5 | 6 | 1 | 23 | 82.14285714 |
| 5 | Bottom-up initiatives at national and EU level | 5 | 4 | 5 | 5 | 2 | 21 | 75 |
Table 13.
Scoring of measures for ‘threats’ category.
| Sr. No. | Measures/Commonalities | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap | Total | Percentage |
|---|---|---|---|---|---|---|---|---|
| 1 | Barriers in data-sharing | 7 | 6 | 5 | 4 | 1 | 23 | 82.14285714 |
| 2 | Variability across the EU (in data, clinical workflows) | 6 | 4 | 4 | 5 | 1 | 20 | 71.42857143 |
| 3 | External competition from new and emerging research networks or technologies that may challenge our funding, resources, and partnerships | 7 | 7 | 5 | 6 | 2 | 27 | 96.42857143 |
| 4 | Increased competition | 5 | 6 | 6 | 5 | 2 | 24 | 85.71428571 |
| 5 | Data protection limits data sharing | 5 | 5 | 6 | 5 | 2 | 23 | 82.14285714 |
| 6 | High resistant to chemotherapy and non-respondent to immunotherapy | 4 | 3 | 3 | 5 | 0 | 15 | 53.57142857 |
| 7 | Making full use of Artificial Intelligence | 2 | 4 | 3 | 4 | 0 | 13 | 46.42857143 |
| 8 | Different economic contexts | 6 | 6 | 5 | 4 | 1 | 22 | 78.57142857 |
| 9 | Changes of European and national priorities | 7 | 7 | 4 | 5 | 2 | 25 | 89.28571429 |
5. Statistical analysis
The correlation for the ‘strength’ category between the similarities in opinions of various groups of experts who presented at the EU level is shown in Table 14. For e.g. the relationship between the speakers of Tackling the Implementation Gap: SWOT and Public Private partnership & keeping the person in personalised medicine is 0.61 which means that their ideas are highly correlated with each other in a positive direction whereas, if we consider the relationship between Tackling the Implementation Gap: SWOT and Disease Use Cases, SWOT is −0.46, which means that their opinions are inversely correlated with each other or simply we can say that they have opposite opinions regarding the strength at EU level. Furthermore, the relationship between Disease Use Cases: SWOT and Public Private partnership & keeping the person in personalised medicine i.e., 0, means that their opinions are not correlated at all. There are no negative correlations between any category of speakers regarding the threats on the EU level. Overall, the correlations suggest that experts' opinions are generally more similar within each category of the SWOT analysis, with some categories having higher correlations than others (Table 14, Table 15, Table 16, Table 17). This could be due to differences in the level of expertise among the experts, the specific focus of each category, or other factors. It is important to consider these correlations when interpreting the results of the SWOT analysis and to use them as a starting point for further discussion and analysis.
Table 14.
Correlation between the similarities in opinions among different experts for the ‘strength’ category.
| STRENGTH | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap |
|---|---|---|---|---|---|
| (7) Tackling the Implementation Gap: SWOT | 1 | 0.61 | 0.19 | −0.46 | −0.14 |
| (7) Public Private partnership & keeping the person in personalised medicine | 0.61 | 1 | 0.73 | 0 | 0.09 |
| (6) Stakeholder Perspectives: SWOT | 0.19 | 0.73 | 1 | 0.42 | −0.11 |
| (6) Disease Use Cases: SWOT | −0.46 | 0 | 0.42 | 1 | 0.37 |
| (2) Developing Priorities to support a policy roadmap | −0.14 | 0.09 | −0.11 | 0.37 | 1 |
Table 15.
Correlation between the similarities in opinions among different experts for the ‘weakness’ category.
| WEAKNESS | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap |
|---|---|---|---|---|---|
| (7) Tackling the Implementation Gap: SWOT | 1 | 0.24 | 0.47 | −0.12 | 0.03 |
| (7) Public Private partnership & keeping the person in personalised medicine | 0.24 | 1 | 0.38 | −0.06 | 0.7 |
| (6) Stakeholder Perspectives: SWOT | 0.47 | 0.38 | 1 | −0.03 | 0.09 |
| (6) Disease Use Cases: SWOT | −0.12 | −0.06 | −0.03 | 1 | 0.39 |
| (2) Developing Priorities to support a policy roadmap | 0.03 | 0.7 | 0.09 | 0.39 | 1 |
Table 16.
Correlation between the similarities in opinions among different experts for the ‘opportunity’ category.
| OPPORTUNITY | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap |
|---|---|---|---|---|---|
| (7) Tackling the Implementation Gap: SWOT | 1 | 0.89 | −0.42 | −0.64 | −0.32 |
| (7) Public Private partnership & keeping the person in personalised medicine | 0.89 | 1 | 0 | −0.62 | 0.08 |
| (6) Stakeholder Perspectives: SWOT | −0.42 | 0 | 1 | 0.42 | 0.64 |
| (6) Disease Use Cases: SWOT | −0.64 | −0.62 | 0.42 | 1 | −0.21 |
| (2) Developing Priorities to support a policy roadmap | −0.32 | 0.08 | 0.64 | −0.21 | 1 |
Table 17.
Correlation between the similarities in opinions among different experts for the ‘threat’ category.
| THREAT | (7) Tackling the Implementation Gap: SWOT | (7) Public Private partnership & keeping the person in personalised medicine | (6) Stakeholder Perspectives: SWOT | (6) Disease Use Cases: SWOT | (2) Developing Priorities to support a policy roadmap |
|---|---|---|---|---|---|
| (7) Tackling the Implementation Gap: SWOT | 1 | 0.7247844507 | 0.4496979663 | 0.325 | 0.64 |
| (7) Public private partnership & keeping the person in personalised medicine | 0.7247844507 | 1 | 0.5734146387 | 0.2209708691 | 0.7778174593 |
| (6) Stakeholder Perspectives: SWOT | 0.4496979663 | 0.5734146387 | 1 | 0.1843024452 | 0.7814423676 |
| (6) Disease Use Cases: SWOT | 0.325 | 0.2209708691 | 0.1843024452 | 1 | 0.55 |
| (2) Developing Priorities to support a policy roadmap | 0.64 | 0.7778174593 | 0.7814423676 | 0.55 | 1 |
6. Interpretation
The correlation tables provide insights into the factors that affect the implementation of NGS in the participating countries and centres across the European Union. The results suggest that infrastructure, equipment/infrastructure, routine utilization, consultation, and educational programs are positively correlated with NGS implementation, while reimbursement of LB and external guidelines are negatively correlated with NGS implementation. In terms of country-specific results, Germany, Italy, and France have the highest correlations with NGS implementation, while Belgium and Croatia have the lowest correlations. These findings are consistent with previous studies that have investigated the factors affecting NGS implementation. For example, a study by Liu et al. [26] found that lack of infrastructure and expertise, high costs, and inadequate reimbursement policies are major barriers to NGS implementation. Another study by Kim et al. [27] reported that insufficient education and training programs, lack of standardized guidelines, and concerns about data privacy and security are significant obstacles to NGS implementation. These studies, along with the results of our analysis, highlight the importance of addressing the barriers and promoting the factors that positively affect NGS implementation. Furthermore, studies have also highlighted the importance of infrastructure, funding, and education/training/awareness in the successful implementation of NGS. A study by Yates et al. [28] found that infrastructure, funding, and training were critical factors in the successful implementation of NGS in cancer care. Another study [29] identified infrastructure, education, and collaboration as important factors in the implementation of genomic medicine.
Furthermore, the positive correlation between educational programs and NGS implementation underscores the importance of investing in education and training programs to increase awareness and improve the skills of healthcare providers and researchers. This finding is in line with the recommendations of the European Society for Medical Oncology (ESMO), which emphasizes the need for continuous education and training programs to ensure the effective use of NGS in clinical practice [30].
In conclusion, the results of our analysis suggest that infrastructure, equipment/infrastructure, routine utilization, consultation, and educational programs are key factors that positively affect NGS implementation in the European Union. Addressing the barriers and promoting these factors can enhance the adoption and effective use of NGS in clinical practice and research.
The results of the ANOVA test show that there is a significant difference in the mean values among the pillars of participating countries and five major countries' centres (p < 0.001). The Molecular Tumour Board pillar has the highest mean value, indicating that it is the most significant pillar in the implementation of NGS. This result is consistent with previous studies that have shown the importance of MTBs in guiding the use of NGS in cancer treatment [[31], [32], [33], [34]].
The Education/Training/Awareness pillar also has a high mean value, indicating that it is a critical pillar for successful NGS implementation. Previous studies have emphasized the need for education and training programs for healthcare professionals to improve their knowledge and skills in NGS technology [[35], [36], [37]].
In contrast, the Healthcare Workforce pillar had the lowest mean value, indicating a lack of emphasis on workforce development and training in NGS technology. This is consistent with previous studies that have identified a shortage of trained personnel as a significant barrier to the implementation of NGS in clinical practice [38,39].
The variance values indicate that there is variability in the implementation of NGS among the participating countries and five major countries' centres. This variability may be due to differences in resources, infrastructure, and healthcare systems, which can impact the availability and accessibility of NGS technology.
In terms of the other pillars, Infrastructure and Data Sharing and Linking have relatively low mean values in both tables, indicating that they are less critical in the successful implementation of NGS. However, these pillars are still essential for ensuring the efficient and effective use of NGS technology [40].
Overall, the results of the ANOVA tests suggest that a multi-faceted approach is necessary for successful NGS implementation and the importance of considering various factors, such as workforce development, education, and awareness, as well as MTB implementation, when planning and implementing NGS technology in clinical practice. The differences observed between participating countries and major countries' centres suggest that there may be regional differences in the approaches to NGS implementation, which may be influenced by factors such as healthcare policies, resources, and infrastructure.
Common pillars that we identified reflect common challenges in the literature reviews. Infrastructure and tools needed for conducting NGS and advanced diagnostics vary across member states. In Bulgaria, according to the literature, data infrastructure to monitor the burden of cancer and outcomes of care is not fully operational. Infrastructure and access to care vary widely across Europe.
Many different projects exist on the EU level which promote cancer research and care, especially in the context of NGS technology and genomics. Many of them aim to identify people at high risk of cancer by using different technologies, especially NGS (Table 18). Increasing emphasis is put on developing and testing next-generation tools for disease prevention, diagnosis, and personalised treatment, which should be the basis of sustainable healthcare systems.
Table 18.
Projects overview in the field of NGS technology and molecular diagnostics.
| Project name | Description of a project |
|---|---|
| Genomic diagnostics beyond the sequence | This proposal aims to bridge the gap by analyzing long individual DNA molecules without PCR amplification via the utilization of emerging optical DNA mapping technologies. |
| Breast Cancer Risk after Diagnostic Gene Sequencing (BRIDGE) | This project aims to build a knowledge base allowing the identification of women at high risk of BC, in particular through a comprehensive evaluation of DNA variants in known and suspected BC genes. |
| Collaborative Oncological Gene-environment Study | The overarching goal of COGS was to identify individuals with an increased risk of breast, ovary and prostate cancer, evaluating the effect of inherited genetic variation on tumour characteristics and clinical outcome. |
| Genetic testing in Europe - Network for test development harmonization, validation and standardization of services | With the active participation of stakeholders, the proposed EUROGENTEST NOE intends to structure, harmonize and improve the overall quality of genetic services across Europe. |
| Female cancer prediction using cervical omics to individualise screening and prevention | The FORECEE project was aligned with the novel concept of “P4 Medicine” (predictive, preventive, personalised, and participatory): it aimed to translate the risk prediction tool output into personalised recommendations for screening and prevention of female cancers. |
| Comprehensive characterization and effective combinatorial targeting of high-grade serous ovarian cancer via single-cell analysis | The goal of this multidisciplinary project was to comprehensively characterise high-grade serous ovarian cancer (HGS-OvCa) at the single-cell level, identify the best combination of drugs combination to kill HGS-OvCa populations and commercialise a predictive biomarker kit for finding the right therapeutic regimen to the right patient. |
| Next Generation Health Technology Assessment to support patient-centred, societally oriented, real-time decision-making on access and reimbursement for health technologies throughout Europe | The objective of the HTx project is to develop methods using real-world data to estimate relative effectiveness and cost-effectiveness of personalised treatment and to develop prediction models for personalised treatment, based on diagnostic and genetic profiling. |
| Integrated and Standardized NGS Workflows for Personalised Therapy | Driven by patient and clinical needs, innovative NGS workflows from sample-pre-analytics to medical decision-making will be developed. The modular design of the workflow will particularly enable SMEs to contribute, and provide flexibility to adopt emerging user needs and technologies. |
| International consortium for integrative genomics prediction | INTERVENE aims to develop and test next-generation tools for disease prevention, diagnosis, and personalised treatment utilizing the first US-European pool of genomic and health data and integrating longitudinal and disease-relevant -omics data into genetic risk scores. |
| Personalised Engine For Cancer Integrative Study and Evaluation | The aim was to develop predictive computational technology that can exploit molecular and clinical data to improve their understanding of disease mechanisms and to inform clinicians about optimized strategies for therapeutic intervention. |
Funding
This research was funded by Reducing Disparities Across the European Union (BEACON) Project Number: 101080005 and by the European Commission EU4Health Programme 2021–2027 under Grant N° 101080009.
Data availability statement
Data associated with our study have not been deposited into a publicly available repository because data are included in the article/supp. material/referenced in the article.
CRediT authorship contribution statement
Denis Horgan: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Marc Van den Bulcke: Validation, Investigation, Formal analysis, Data curation. Umberto Malapelle: Validation, Investigation, Formal analysis, Data curation. Giancarlo Troncone: Validation, Investigation, Formal analysis, Data curation. Nicola Normanno: Validation, Investigation, Formal analysis, Data curation. Ettore D. Capoluongo: Validation, Investigation, Formal analysis, Data curation. Arsela Prelaj: Validation, Investigation, Formal analysis, Data curation. Carmelo Rizzari: Validation, Investigation, Formal analysis, Data curation. Dario Trapani: Validation, Investigation, Formal analysis, Data curation. Jaya Singh: Validation, Investigation, Formal analysis, Data curation. Marta Kozaric: Validation, Investigation, Formal analysis, Data curation. John Longshore: Validation, Investigation, Formal analysis, Data curation. Manuel Ottaviano: Validation, Investigation, Formal analysis, Data curation. Stefania Boccia: Validation, Investigation, Formal analysis, Data curation. Gabriella Pravettoni: Validation, Investigation, Formal analysis, Data curation. Ivana Cattaneo: Validation, Investigation, Formal analysis, Data curation. Núria Malats: Validation, Investigation, Formal analysis, Data curation. Reinhard Buettner: Validation, Investigation, Formal analysis, Data curation. Karim Lekadir: Validation, Investigation, Formal analysis, Data curation. Francesco de Lorenzo: Validation, Investigation, Formal analysis, Data curation. Paul Hofman: Validation, Investigation, Formal analysis, Data curation. Ruggero De Maria: Validation, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgements
We would like to thank the members of the European Alliance for Personalised Medicine (EAPM), and the representatives of the European Commission and Member States for their kind input. All co-authors of this manuscript participated on the roundtable organized by EAPM. In addition, we want to thank all the experts who participated on expert interviews and surveys organised by EAPM for providing us with their valuable insights. A special thanks to Branka Bernard and Maria Bošković for their contributions at the early stage of the project.
Footnotes
Denis Horgan reports a relationship with European Alliance for Personalised Medicine Brussels that includes: employment. Jaya Singh reports a relationship with European Alliance for Personalised Medicine Brussels that includes: employment. Marta Kozaric reports a relationship with European Alliance for Personalised Medicine Brussels that includes: employment. France Dube reports a relationship with AstraZeneca that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix.
Table 1.
Cancer centres per country involved in the expert interviews organised by EAPM.
| Country | Cancer centre |
|---|---|
| Austria | ● Medical University of Graz |
| Belgium | ● Centre for Human Genetics, University Hospitals Leuven ● Institut Jules Bordet ● Cliniques Universitaires Saint Luc ● University Hospitals Leuven, Leuven, Belgium; Nuclear Medicine & Molecular Imaging, KU Leuven, Leuven ● King Albert II Cancer Institute |
| Bulgaria | ● Military Medical Academy |
| Croatia | ● Department for Respiratory Diseases ‘’Jordanovac’’, UMC Zagreb ● Department for Respiratory Diseases, University Hospital Centre ● Faculty of Science, University of Zagreb ● University Hospital Centre ‘’Sestre Milosrdnice’’ ● Institute Ruđer Bošković, Zagreb ● Department of Oncology, University Hospital Centre |
| France | ● Laboratory of Clinical and Experimental Pathology, FHU OncoAge, IRCAN, Nice Hospital Centre, The University Côte d'Azur, Nice ● Hôpital Tenon, HUEP, Sorbonne université, Paris ● Institut du Thorax Curie Montsouris, Paris ● Institut Curie, Paris ● UFR Simone Veil, Paris Saclay University, Université de Versailles Saint-Quentin-en-Yvelines (UVSQ), Versailles ● Gustave Roussy-Cancer Campus, Villejuif ● Hôpital Cochin, AP-HP, Paris ● Université de Paris, Descartes-Paris ● University Hospital, St. Etienne ● Medical Oncology, Centre Léon Bérard ● AP-HP, Hôpital Saint Louis, Oncology Unit, Vellefaux ● Hospices Civils de Lyon ● Sorbonne Université, Hôpital Saint-Antoine |
| Germany | ● Institute of Medical Genetics and Applied Genomics, University of Tuebingen ● Institute of Clinical Cancer Research, UCT University Cancer Centre, Krankenhaus Nordwest ● Otto-von-Guericke University Hospital ● Sarcoma Center Berlin-Brandenburg, Helios Hospital Bad Saarow/Department of Internal Medicine C, University Hospital Greifswald ● Department of Surgery, Charité – Universitätsmedizin Berlin ● University Hospital Düsseldorf, Heinrich-Heine University Medical Faculty ● University Cancer Centre, Leipzig University Medical Centre ● Klinikum Emil von Behring, Berlin ● Institute for Laboratory Medicine, Marienhospital Stuttgart, Stuttgart ● University Hospital Cologne ● Universitätsklinikum Essen, Westdeutsches Tumorzentrum ● Hannover Medical School (MHH) ● National Center for Tumor Diseases - NCT ● University Hospital Heidelberg ● University of Frankfurt |
| Ireland | ● Oncology Molecular Medicine, Royal College of Surgeons in Ireland, Beaumont Hospital |
| Italy | ● University of Torino, Torino ● Fondazione IRCCS Istituto Nazionale dei Tumori di Milano ● University Vita e Salute-San Raffaele ● IEO European Institute of Oncology ● University "la Sapienza", Rome ● Azienda Ospedaliera per l'Emergenza Cannizzaro, Catania ● University of Naples Federico II, 80138 Naples ● University of Udine Medical School, Udine ● Institute of Clinical Pathology, Santa Maria della Misericordia, University Hospital, Udine ● Istituto Nazionale Tumori Fondazione G. Pascale-Istituto Di Ricovero e Cura a Carattere Scientifico |
| Netherlands | ● Josephine Nefkens Institute, Erasmus Medical Center, Rotterdam |
| Poland | ● Oncology and Immunology Clinic, Warmian-Masurian Cancer Center of the Ministry of the Interior and Administration's Hospital ● National Institute of Tuberculosis and Lung Diseases, Warsaw ● Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw ● Medical University of Gdańsk |
| Portugal | ● Instituto Português de Oncologia de Coimbra Francisco Gentil, Department of Medical Oncology |
| Romania | ● University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca |
| Slovenia | ● Univerzitetni klinični centre Maribor ● Institute of Oncology Ljubljana |
| Spain | ● Institut Hospital del Mar d'Investigacions Mèdiques ● Hospital Universitario Fundación Jiménez Díaz, Universidad Autónoma de Madrid ● eVIDA Research Group, University of Deusto, Bilbao ● IOB Institute of Oncology, Quironsalud Group, Madrid & Barcelona ● Vall d'Hebron Institute of Oncology, Barcelona ● University Hospital A Coruña ● Hospital Universitario Miguel Servet, Medical Oncology Department, Zaragoza |
| Sweden | ● Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg ● Karolinska Institute, Stockholm |
| United Kingdom | ● London Sarcoma Service, Royal National Orthopaedic Hospital ● Cancer Research UK Cambridge Institute ● Cancer Research UK & UCL Cancer Trials Centre, University College London, London W1T 4 TJ ● Institute of Applied Health Sciences, University of Aberdeen ● Translational and Oncology Research, Faculty of Life Sciences and Medicine, King's College London ● Royal Liverpool University Hospital |
References
- 1.European Commission Europe's Beating Cancer Plan: a new EU approach to prevention, treatment and care. 2021. https://ec.europa.eu/commission/presscorner/detail/en/ip_21_342 Available online:
- 2.Kalager M., Adami H.O., Lagergren P., Steindorf K., Dickman P.W. Cancer outcomes research-a European challenge: measures of the cancer burden. Mol. Oncol. 2021 Dec;15(12):3225–3241. doi: 10.1002/1878-0261.13012. Epub 2021 Jun 22. PMID: 34003576; PMCID: PMC8637567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saghatchian M., Thonon F., Boomsma F., Hummel H., Koot B., Harrison C., et al. Pioneering quality assessment in European cancer centers: a data analysis of the organization for European cancer Institutes accreditation and designation program. J Oncol Pract. 2014;10(5) doi: 10.1200/JOP.2013.001331. [DOI] [PubMed] [Google Scholar]
- 4.Sokolenko A.P., Imyanitov E.N. Molecular diagnostics in clinical oncology. Front. Mol. Biosci. 2018 Aug 27;5:76. doi: 10.3389/fmolb.2018.00076. PMID: 30211169; PMCID: PMC6119963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel C., Herold S., Wermke M., Aust D.E., Baretton G.B. Routine molecular pathology diagnostics in precision oncology. Dtsch Arztebl Int. 2021 Apr 16;118:255–261. doi: 10.3238/arztebl.m2021.0025. Forthcoming. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pervez M.T., Hasnain M.J.U., Abbas S.H., Moustafa M.F., Aslam N., Shah S.S.M. A comprehensive review of performance of next-generation sequencing platforms. BioMed Res. Int. 2022:2022. doi: 10.1155/2022/3457806. 3457806. PMCID: PMC9537002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Heydt C., Wölwer C.B., Camacho O.V., Wagener-Ryczek S., Pappesch R., Siemanowski J., Rehker J., Haller F., Agaimy A., Worm K., et al. Detection of gene fusions using targeted next-generation sequencing: a comparative evaluation. BMC Med. Genom. 2021;14:62. doi: 10.1186/s12920-021-00909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Luca X.M., Newell F., Kazakoff S.H., Hartel G., Reed A.E.M., Holmes O., Xu Q., Wood S., Leonard C., Pearson J.V., et al. Using whole-genome sequencing data to derive the homologous recombination deficiency scores. npj Breast Cancer. 2020;6:33. doi: 10.1038/s41523-020-0172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horgan D., Hamdi Y., Lal J.A., Nyawira T., Meyer S., Kondji D., Francisco N.M., De Guzman R., Paul A., Bernard B., et al. Framework for adoption of next-generation sequencing (NGS) globally in the oncology area. Healthcare. 2023;11:431. doi: 10.3390/healthcare11030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti A., Barbareschi M., Barberis M., Buglioni S., Buttitta F., Fassan M., Fontanini G., Marchiò C., Papotti M., Pruneri G., Scarpa A., Stanta G., Tallini G., Troncone G., Veronese S.M., Truini M., Sapino A. Real-world data on NGS diagnostics: a survey from the Italian society of pathology (SIAPeC) NGS network. Pathologica. 2021 Aug;113(4):262–271. doi: 10.32074/1591-951X-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colomer R., Miranda J., Romero-Laorden N., Hornedo J., González-Cortijo L., Mouron S., et al. Usefulness and real-world outcomes of next generation sequencing testing in patients with cancer: an observational study on the impact of selection based on clinical judgement. eClinicalMedicine. 2023:60. doi: 10.1016/j.eclinm.2023.102029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh K.J., Kim S.H., Kim Y.J., Shin H., Kang E., Kim E.K., Lee S., Woo J.W., Na H.Y., Ahn S., Jang B.S., Kim I.A., Park S.Y., Kim J.H. Clinical application of next-generation sequencing in patients with breast cancer: real-world data. J Breast Cancer. 2022 Oct;25(5):366–378. doi: 10.4048/jbc.2022.25.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosele F., Remon J., Mateo J., Westphalen C.B., Barlesi F., Lolkema M.P., et al. vol. 31. Annals of Oncology; 2020. (Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: a Report from the ESMO Precision Medicine Working Group). [DOI] [PubMed] [Google Scholar]
- 14.Morganti S., Tarantino P., Ferraro E., D'Amico P., Viale G., Trapani D., Duso B.A., Curigliano G. Complexity of genome sequencing and reporting: next generation sequencing (NGS) technologies and implementation of precision medicine in real life. Crit. Rev. Oncol. Hematol. 2019;133:171–182. doi: 10.1016/j.critrevonc.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Helmy M., Awad M., Mosa K.A. Limited resources of genome sequencing in developing countries: challenges and solutions. Appl. Transl. Genom. 2016;9:15–19. doi: 10.1016/j.atg.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xuan J., Yu Y., Qing T., Guo L., Shi L. Next-generation sequencing in the clinic: promises and challenges. Cancer Lett. 2013;340:284–295. doi: 10.1016/j.canlet.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satam H., Joshi K., Mangrolia U., Waghoo S., Zaidi G., Rawool S., Thakare R.P., Banday S., Mishra A.K., Das G., et al. Next-generation sequencing technology: current trends and advancements. Biology. 2023;12:997. doi: 10.3390/biology12070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan D., Mills D. Past, present, and future of DNA typing for analyzing human and non-human forensic samples. Front. Ecol. Evol. 2021;9 [Google Scholar]
- 19.Bruzas S., Kuemmel S., Harrach H., Breit E., Ataseven B., Traut A., Rüland A., Kostara A., Chiari O., Dittmer-Grabowski C., Reinisch M. Next-generation sequencing-directed therapy in patients with metastatic breast cancer in routine clinical practice. Cancers. 2021 Sep 11;13(18):4564. doi: 10.3390/cancers13184564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyse Simona, Thway Khinb, Huang Paul H.a, Jones Robin L.b. Next-generation sequencing for the management of sarcomas with no known driver mutations. Curr. Opin. Oncol. July 2021;33(4):315–322. doi: 10.1097/CCO.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 21.Eichler M., Richter S., Hohenberger P., Kasper B., Andreou Di, Heidt V., et al. Current state of sarcoma care in Germany: results of an online survey of physicians. Oncol. Res. Treat. 2019;42(11) doi: 10.1159/000502758. [DOI] [PubMed] [Google Scholar]
- 22.Griffin J., Tsao C.-K., Patel V., Liaw B.C., Guin S., Joshi H., Rossi M., Hantash F., Zhou X., Tewari A., Galsky M.D., Oh W.K., Chen R., Jun T. Clinical actionability and utilization of next-generation sequencing for prostate cancer in a changing treatment landscape. Front. Urol. 2022;2 doi: 10.3389/fruro.2022.997396. [DOI] [Google Scholar]
- 23.Moradi N., Moghadam S.O., Heidarzadeh S. Application of next-generation sequencing in the diagnosis of gastric cancer. Scand. J. Gastroenterol. 2022;57(7):842–855. doi: 10.1080/00365521.2022.2041717. [DOI] [PubMed] [Google Scholar]
- 24.Bayle A., Basile D., Garinet S., Rance B., Laurent-Puig P., Blons H., Taieb J., Perkins G. Next-generation sequencing targeted panel in routine care for metastatic colon cancers. Cancers. 2021;13:5750. doi: 10.3390/cancers13225750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pop-Bica C., Ciocan C.A., Braicu C., Haranguș A., Simon M., Nutu A., Pop L.A., Slaby O., Atanasov A.G., Pirlog R., Al Hajjar N., Berindan-Neagoe I. Next-generation sequencing in lung cancer patients: a comparative approach in NSCLC and sclc mutational landscapes. J. Personalized Med. 2022 Mar 13;12(3):453. doi: 10.3390/jpm12030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Zhou R., Baumbusch L.O., et al. Next-generation sequencing for clinical diagnostics. Nucleic Acid Therapeut. 2020;30(5):219–224. doi: 10.1089/nat.2020.0889. [DOI] [Google Scholar]
- 27.Kim J., Kim S.T., Lee S.J., et al. The process of developing a next-generation sequencing panel for clinical diagnosis of colorectal cancer. Expert Rev. Mol. Diagn. 2019;19(7):599–609. doi: 10.1080/14737159.2019.1634312. [DOI] [Google Scholar]
- 28.Yates L.R., Seoane J., Le Tourneau C., Siu L.L., Marais R., Michiels S., Soria J.C., Campbell P., Normanno N., Scarpa A., Reis-Filho J.S., Rodon J., Swanton C., Andre F. The European society for medical oncology (ESMO) precision medicine glossary. Ann. Oncol. 2018 Jan 1;29(1):30–35. doi: 10.1093/annonc/mdx707. [DOI] [PubMed] [Google Scholar]
- 29.Manolio T.A., Abramowicz M., Al-Mulla F., Anderson W., Balling R., Berger A.C., Bleyl S., Chakravarti A., Chantratita W., Chisholm R.L., Dissanayake V.H., Dunn M., Dzau V.J., Han B.G., Hubbard T., Kolbe A., Korf B., Kubo M., Lasko P., Leego E., Mahasirimongkol S., Majumdar P.P., Matthijs G., McLeod H.L., Metspalu A., Meulien P., Miyano S., Naparstek Y., O'Rourke P.P., Patrinos G.P., Rehm H.L., Relling M.V., Rennert G., Rodriguez L.L., Roden D.M., Shuldiner A.R., Sinha S., Tan P., Ulfendahl M., Ward R., Williams M.S., Wong J.E., Green E.D., Ginsburg G.S. Global implementation of genomic medicine: we are not alone. Sci. Transl. Med. 2015 Jun 3;7(290):290ps13. doi: 10.1126/scitranslmed.aab0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark Z., et al. Clinical impact of genomic sequencing in familial adenomatous polyposis. Genet. Med. 2019;21(3):672–677. [Google Scholar]
- 31.Tafe L.J., et al. The molecular tumor board: using next-generation sequencing to improve cancer care. Clin. Chem. 2018;64(2):218–225. [Google Scholar]
- 32.Gagan J., Van Allen E.M., Lorusso P.M. Role of next-generation sequencing in enabling personalized oncology therapy. Expert Review of Precision Medicine and Drug Development. 2015;1(1):1–12. [Google Scholar]
- 33.Stadler Z.K., Schrader K.A., Vijai J., Robson M.E., Offit K., Zhang L. Cancer genomics and inherited risk. J. Clin. Oncol. 2018;36(20):2011–2018. doi: 10.1200/JCO.2013.49.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amendola L.M., et al. Implementing rapid, robust, cost-effective, patient-centered, routine genetic testing in a diverse population. Genet. Med. 2016;18(10):1022–1031. [Google Scholar]
- 35.Musa A., et al. Next-generation sequencing in clinical oncology: next steps towards clinical validation. Cancers. 2020;12(8):2058. [Google Scholar]
- 36.Sadedin S.P., Pope B., Oshlack A., Bickerstaff P. Best practices for preparing metadata for next-generation sequencing of human immunoglobulin repertoires. Front. Immunol. 2017;8:1172. [Google Scholar]
- 37.Nagarajan R., Scutari M., Shankar R.D., Huan T., Yang Y. Next-generation sequencing workflow for diagnosis of lower limb peripheral arterial disease. J. Med. Syst. 2017;41(10):165. [Google Scholar]
- 38.Song Y., Li S., Ray A. Next-generation sequencing for the diagnosis and management of prostate cancer. Clin. Lab. Med. 2017;37(3):537–549. [Google Scholar]
- 39.Dinh T.A., et al. Implementation of next generation sequencing in clinical molecular diagnostic laboratories: advantages, challenges and potential. Expert Rev. Mol. Diagn. 2017;17(2):225–237. [Google Scholar]
- 40.Mersch J., et al. Preparing for incidental findings in the era of next-generation sequencing. Genet. Test. Mol. Biomarkers. 2015;19(12):697–701. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with our study have not been deposited into a publicly available repository because data are included in the article/supp. material/referenced in the article.