Highlights
-
•
Chemical attributes were identified using UPLC and GC–MS in grape and wine.
-
•
Sensory evaluation of wine was conducted using quantitative descriptive analysis.
-
•
Differences in chemical and sensory attributes of wine were reveled by chemometrics.
-
•
Beibinghong wine obtained the highest sensory scores.
Keywords: Grape, Wine, Chemical attributes, Sensory analysis, Chemometrics
Abstract
Chemical and sensory attributes play a vital role in evaluating the quality of grapes and wines. This study compared basic physicochemical parameters, organic acids, phenolic compounds, and aroma profiles of grapes and wines of six cultivars using chemometrics. The results showed that the reducing sugar contents of Beibinghong, Gongniang, and Granoir grapes were significantly higher than those of others cultivars, whereas their juice yields were significantly lower. The phenolic compound contents in Moldova, Beibinghong, and Gongniang grape skins and wines were higher than those in others cultivars. The organic acid contents in Beibinghong grape and Dunkelfelder wine were highest. Beibinghong and Gongniang grapes and wines showed richer aldehyde and ester concentrations. Beibinghong wine obtained the highest sensory scores. Ethyl decanoate, coumaric acid, and methyl dodecanoate were characteristic variables distinguishing wine cultivars, exhibiting important contributions to their sensory characteristics. These findings were useful for viticulturists and winemakers to select grape varieties.
1. Introduction
Wine is one of the most popular alcoholic drinks around the world, and its quality depends on the quality of grape berries. Grape quality is subject to several factors, such as vineyards’ geographic location, climate, soil, viniculture practices, and grape varieties (Cadot et al., 2010, Granato et al., 2016, Gao et al., 2021). In recent years, consumers’ demand for high-quality, diverse, and distinctive wines has gradually increased. However, only a few varieties are widely planted in major areas in China, such as Cabernet Sauvignon, Merlot, and Cabernet Franc, which results in a single type of wine production for each region. Most varieties that grow well are cultivated only as germplasm resources in the vineyards of each region. The winemaking potential of these varietal grapes will be overlooked because winemakers do not understand their chemical characteristics and flavour profiles.
Aroma is one of the most important sensory characteristics of wine and plays a key role in influencing wine quality (Cadot et al., 2010). The aroma compounds are usually present in the skin and flesh of grape berries as free or bound forms (Gao et al., 2021). Free aroma compounds directly influence grape flavour, while bound components can be hydrolysed into free aroma compounds during fermentation and ageing and serve as an additional flavor reserve (Hjelmeland & Ebeler, 2015). Due to the metabolism of yeast during fermentation, wine aromas after fermentation are mainly composed of higher alcohols, carbonyl compounds, volatile fatty acids, esters, and sulfur compounds (Garde-Cerdán & Ancín-Azpilicueta, 2008). Phenolic compounds in wine originating principally from grape skins and seeds, are also secondary metabolites of grape berries. Anthocyanins in wine are derived from grape skins, whereas proanthocyanidins are derived from both skins and seeds (Ferrero-del-Teso, Arias, Escudero, Ferreira, Fernández-Zurbano & Sáenz-Navajas, 2020). Although the concentration is very low, these compositions are of crucial importance for red wine colour, mouthfeel attributes, and antioxidant activity (Aleixandre-Tudo, Buica, Nieuwoudt, Aleixandre & du Toi, 2017). It was reported that the combination of tannins and anthocyanins can impact wine bitterness, astringency, and colour stabilization (Llaudy, Canals, Canals, Rozéz, Arola & Zamora, 2004). The antioxidant capacity of polyphenols in wines has been demonstrated because they serve as reactive oxygen species scavengers and metal chelators (Li, Wang, Li, Li & Wang, 2009). Organic acids play an important role in the characteristics of taste, stability, and ageing for wine, including acetic acid (AA), citric acid (CA), lactic acid (LA), malic acid (MA), succinic acid (SA), and tartaric acid (TA) (Huang, Jiang, Tan & Li, 2017). CA, MA, and TA essentially derive from grape berries, while AA, LA, and SA are related to microbes principally rooted in alcoholic and malolactic fermentation. Moreover, organic acids have many physiological functions, such as antibacterial, antiviral, increasing coronary flow, and inhibiting lipid peroxide production in brain tissue (Robles, Fabjanowicz, Chmiel & Płotka-Wasylka, 2019). Holistically, the characteristics of these chemical compounds affect the sensory and quality of grapes and wine.
Grape cultivars play a vital role in influencing the chemical profiles of grape berries and wine. Dordevic et al. (2017) reported that Merlot and Cabernet Sauvignon varieties had a significantly higher content of total phenolic compounds compared to the Vranac variety. Moreno-Olivares, Paladines-Quezada, Fernandez-Fernandez, Bleda-Sanchez, Martinez-Moreno & Gil-Munoz (2020) verified that crossing Monastrell and Cabernet Sauvignon varieties showed a predominance of aromas belonging to esters, whereas parent varieties showed a high concentration of alcohols, acids, and some terpenes. In addition, phenolic compounds, volatile compounds, and organic acids are commonly regarded as fingerprints for discriminating the authentication of wine cultivars (Milovanovic et al., 2019, Merkytė et al., 2020). Therefore, the analysis of these chemical components is important for comparing the quality differences of grapes and wines among different varieties.
Current studies on the chemical and sensory characteristics of wine universally focus on grape varieties with large cultivation areas in Chinese regions (Zhao et al., 2019, Yao et al., 2021, Yao et al., 2021, Gao et al., 2021, Li et al., 2021), whereas some rare cultivars have received less attention. In this study, to evaluate the winemaking potential of wine grapes, we determined basic chemical parameters, phenols, organic acids, and aroma compounds for grapes and wines of six cultivars from the Weibei Plateau region in China. Subsequently, chemometrics was employed to further compare the differences in chemical characteristics and find important chemical variables among different cultivars. Finally, we determined the relationship between these variables and sensory characteristics for wine. Overall, this study provides a theoretical basis at the microscale for viticulturists and winemakers to select grape cultivars.
2. Materials and methods
2.1. Grape sampling and winemaking
The grapes used in this study included Granoir (JNH), Dunkelfelder (ZDF), Meili (ML), Moldova (ME), Gongniang (GN), and Beibinghong (BBH). Among them, JNH, ZDF, and ML belong to V. vinifera grapevine and ME is a cross between V. vinifera and V. labrusca, while GN and BBH are the hybrid varieties cross between V. vinifera and V. amurensis. JNH, ZDF, ML, and ME were planted in the vineyard of Shengtang chateau, Yangling, Shaanxi Province, China (E108.04°, N34.31°). The vineyard had an average annual temperature of 15.47 °C and an average annual precipitation of 676.66 mm (concentrated from July to September). GN and BBH were cultivated in the grape experiment demonstration station of Northwest Agriculture and Forestry University in Heyang County, Shaanxi Province, China (E110.15°, N35.24°). The vineyard possessed 15.02 °C of the average annual temperature and 660.65 mm of the average annual precipitation. In addition, all grapevines were cultivated with 1.0 m × 2.0 m of spaces within and between the vine rows.
In this study, grape berries of six cultivars were harvested when they reached the optimal technological maturity, as judged by the ratio of sugar and acid concentrations. Approximately 30 kg of grape berries for each variety were obtained in August 2020 and transported quickly to the laboratory for further winemaking. Each variety included 3 duplicate samples, and each sample (approximately 10 kg) was randomly collected from 15 grapevines (3 grapevines per sampling point) according to the five-point sampling method. To determine the chemical composition of grape berries, three samples for each variety were collected by the same method and each sample included 500 grape berries. The winemaking process was performed by the modified laboratory-based microvinification method reported by Peng, Wen, Tao, and Lan (2013). In short, 30 kg of grape berries for each variety were destemmed, crushed, and transferred to three 10-L glass fermenters as three replicate samples. Then, the musts were treated with 50 mg/L sulfur dioxide. Activated commercial yeast (200 mg/L, Saccharomyces cerevisiae CECA, Angel Yeast Co., Ltd., China) was introduced to start alcohol fermentation after 24 h of maceration. The fermentation was performed at 25–28 °C. To ensure effective maceration and prevent grape juice overflow, cap punching was carried out three times a day during fermentation. Subsequently, wine was separated from pomace when the residual sugar reached a level of less than 2 g/L, transferred to sterile tanks, treated with 50 mg/L sulfur dioxide, clarified, stored at 4 °C for 3 months, and analysed further.
2.2. Chemical reagents and instrumentation
Chemical reagents (Analytical reagent, ≥99.7 %) include sodium hydroxide, copper sulfate, methyl cellulose, potassium biphthalate, sodium carbonate, aluminum chloride, ammonium sulfate, sodium nitrite, and sodium chloride, being purchased from Kemiou Chemical Reagent Co. Ltd. (Tianjin, China). Standards (Chromatographical grade, ≥98 %) were purchased from Sigma-Aldrich corporation (Shanghai, China), including 2-octanol, isopropanol, acetonitrile, ethyl acetate, benzoic acid, caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, gallic acid, resveratrol, syringic acid, salicylic acid, vanillic acid, catechin, coumarin, l-epicatechin, morin hydrate, myricetin, quercetin, rutin, tangeretin, oxalic acid, citric acid, tartaric acid, malic acid, succinic acid, and lactic acid.
Rotary evaporator (RE52AA), pH meter (PHS-3C), freeze dryer (FD-1C-50), electronic balance (FA2014N), centrifuge (5424R) and ultraviolet spectrophotometer (Cary60UV–Vis) were purchased from Yarong Biochemical Instrument Factory (Shanghai, China), Precision Scientific Instruments Co. (Shanghai, China), Bomikang Laboratory Instrument Co. (Beijing, China), Jinghai Instrument Co. (Shanghai, China), Eppendorf corporation (Hamburg, Germany) and Shimadzu corporation (kyoto, Japan), respectively. Ultra-performance liquid chromatography (UPLC, ACQUITY UPLC I-Class) equipped with Empower chromatography workstation, diode array detector, and ACQUITY BEH C18 column (1.7 µm 2.1 × 50 mm) was supplied by Waters corporation in America. High-performance liquid chromatography (HPLC, LC-2010) equipped with CLASS-VP workstation, SPDM20A detector and Mars MOA 10u chromatography column (300 × 7.8 mm, Phenomenex, America) was provided by Shimadzu corporation. Gas chromatography-mass spectrometer (GC–MS, QP2010 Ultra) equipped with a DB-wax chromatographic column (30 m × 250 μm × 0.25 μm, GL Sciences Inc., Tokyo, Japan) was offered by Shimadzu corporation.
2.3. Determination of the physicochemical parameters of grapes and wines
Basic physicochemical parameters of grape berries, including cluster weight, hundred-seeds weight, hundred-berries weight, juice yield, pH, reducing sugar, soluble solids, sugar acid rate and titratable acidity, were determined and repeated for three times according to the official analysis methods (OIV, 2017). Enological parameters, including alcohol, sugar-free extract, pH, reducing sugar, titratable acidity, total dry extract, total sugar, and volatile acidity, were determined and repeated for three times using the methodologies described by the OIV (OIV, 2017). In brief, the alcohol content was determined by the distillation/densimetry method. The reducing sugar was detected by titration with Fehling’s reagent. Grape juice and wine pH was determined using a pH meter. Titratable acidity was titrated with 0.05 mol/L NaOH to an endpoint of pH 8.2 and was expressed as tartaric acid equivalents.
2.4. Phenolic composition analysis
Fifty grape berries for each sample were removed from the −80 °C refrigerator, peeled, and deseeded. Then the skins and seeds were ground into powder in a mortar with liquid nitrogen and placed in a freeze dryer for 24 h to remove moisture. Total phenols in grape skins, seeds, and wines were determined by the Folin-Ciocalteu method (Meng, Fang, Qin, Zhuang & Zhang, 2012), and the contents were expressed as g/kg gallic acid. Total anthocyanins were analysed by the pH differential method (Meng et al., 2012), and the concentrations were expressed as g/kg cyanidin-3-glucoside. Total flavonoids were detected using the NaNO3-AlCl3 method reported by Peinado, de Lerma, Moreno & Peinado (2009), and the contents were expressed as g/kg rutin. Total flavan-3-ols were determined via the method of p-DMACA-hydrochloric acid (Peinado et al., 2009), and the contents were expressed as g/kg catechin. Tannin contents were assessed by the method of methyl cellulose precipitation (Mercurio, Dambergs, Herderich & Smith, 2007), and the results are shown as g/kg catechin.
The monomeric polyphenols of wines were extracted according to the method of Zhang, Guo, Han & Zhang (2016) with minor modifications. Five-millilitre wines were mixed with isovolumetric ethyl acetate. Then, the mixture was oscillated for 30 s by a vortex oscillator and centrifuged at 4 °C and 3500 r/min for 3 min to obtain the supernatant. Then, the above steps were repeated 3 times, and the supernatant was collected. The supernatant was dried at 35 °C by rotary evaporators, and the residue was dissolved in 2 mL of methanol and stored in a refrigerator at −20 °C until further testing. In addition, UPLC was employed to determine the profile of monomeric polyphenols in wine. The testing procedure was as follows: 1 % acetic acid for phase A, acetonitrile for phase B, a flow rate of 0.2 m L/min, a column temperature of 30 °C, and a detection wavelength of 210–400 nm. Each sample was tested three times and averaged to avoid systematic errors.
2.5. Determination of organic acid compounds
To determine the contents of organic acids in grapes and wines, sample pretreatment must be carried out. Grape juice was obtained after 20 grapes were peeled, deseeded, and pressed. A total of 1.00 g of grape juice was mixed with 10 mL of mobile phase solution in a 15-mL centrifuge tube. Next, the mixtures were sonicated and oscillated at 25 °C for 20 min and centrifuged at 4 °C, and 8000 r/min for 20 min. Then, the supernatant was filtered through a 0.22 μm filter membrane for further testing. Regarding wine, 100 μL wine was evenly mixed with 4.9 mL of mobile phase solution, and the mixtures were filtered through a 0.22 μm filter membrane for further testing. Organic acids were detected using HPLC. The processes were performed under the following conditions: 8 mmol/L H2SO4 as the mobile phase solution, 0.5 mL/min flow rate, 210 nm detection wavelength, 50 °C column temperature, and 10 μL injection volume. Each sample was tested three times and averaged to avoid systematic errors.
2.6. Determination of aroma compounds
Aroma compounds in grapes and wines were extracted by headspace solid-phase microextraction (HS-SPME) according to a previous method with minor modifications (Ge et al., 2021). In detail, 100 g of grapes after removing seeds were put into a mortar, frozen with liquid nitrogen, and quickly ground into powder. Then, the powder was placed in a 50 mL centrifuge tube and centrifuged at 4 °C and 10 000 r/min for 5 min to extract the supernatant. NaCl (1.5 g), 5 mL of supernatant, and 10 μL of 0.230 g/L 2-octanol internal standard solutions were added to 20-mL headspace bottles. Considering wines, 5 mL samples, 1.5 g of NaCl, and 10 μL of 0.492 g/L 2-octanol internal standard solutions were added to 20-mL headspace bottles. Then, SPME fibre (50/30 m, DVB/CAR/PDMS, Supelco, Inc., Bellefonte, PA, USA) was inserted into the headspace bottles containing the sample solution, equilibrated in a 40 °C water bath with stirring for 15 min, extracted for 35 min, and desorbed in the GC injector at 250 °C for 3 min.
Aroma compounds were analysed by GC–MS. The procedures were implemented as follows: start at 40 °C, hold for 4 min, then increase at 4 °C/min to 120 °C, increase at 6 °C/min to 240 °C, hold for 11 min, helium as the carrier gas, 70 eV ionization voltage, 230 °C ion source temperature, 230 °C interface temperature, and 35–350 amu as the range of mass spectrometry scan. Subsequently, aroma compounds were identified via the comparison of retention times and mass spectra with those of pure standards in the NIST2017 library (Ge et al., 2021). To quantitate aroma compound content, the relative areas versus the area of the internal standard (2-octanol) were interpolated by calibration graphs established for pure standards. Each sample was tested three times and averaged to avoid systematic errors.
2.7. Sensory analysis
The sensory profiles of all wines were assessed via quantitative descriptive analysis (QDA) according to a previous method with minor modifications (Li et al., 2021). The tasting panel consisted of sixteen well-trained master’s students (8 males and 8 females aged from 25 to 30 years old) from the College of Enology, Northwest A&F University, China, who possess professional sensory capacities for identifying and describing wine flavour. Approximately 30 mL of wine (15 °C) for each sample was analysed by panelists in a tasting room at 23 °C. The interval between two samples was 1–2 min. Panelists scored each wine sample by a 100-point scoring system including the following four sections: appearance (15 %), aroma (30 %), taste (45 %), and integrity (10 %) (Supplementary Table S1).
2.8. Statistical analysis and data visualization
One-way analysis of variance (ANOVA) with Duncan’s multiple comparisons was performed for each parameter or chemical characteristic to determine the significant differences between different varieties of grapes and wines, utilizing SPSS 25.0 software (IBM Inc., USA). Clustering analysis (CA) and principal component analysis (PCA) were used to investigate intuitively the differences of polyphenol monomers and aroma compounds among grapes and wines of six varieties. Random forest (RF) is a supervised machine learning algorithm integrating multiple classification trees. Due to its excellent performance in classification, prediction, and feature extraction, it has been widely used in the fields of food component analysis in recent years (Phan & Tomasino, 2021). In this work, the RF model was introduced to determine important chemical variables among different cultivars of grapes and wines. Moreover, the performance of the RF model was evaluated utilizing the confusion matrix, leave-one-out cross-validation, and the area under the curve (AUC) of the receiver operating characteristic (ROC). Partial least squares regression (PLSR) was employed to determine the correlation between these variables and sensory characteristics. CA, PCA, RF, and PLSR were performed using pheatmap, ade4, randomForest, and mixOmics packages of R programming language (4.03), respectively.
3. Results
3.1. Physicochemical parameters of grapes and wines
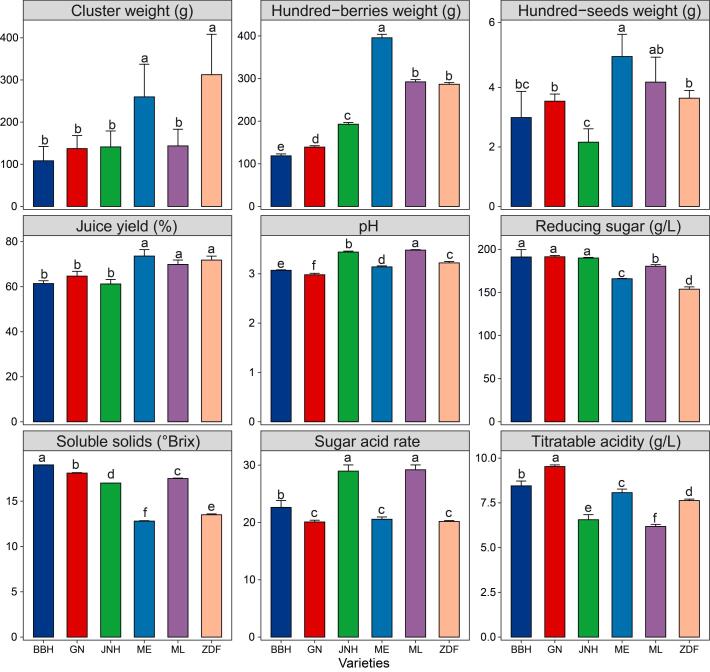
Fig. 1 shows the physicochemical parameters of grapes of six varieties. One-way ANOVA indicated that the cluster weights of the ZDF and ME varieties were significantly higher than those of the JNH, ML, GN, and BBH varieties (p < 0.05). The hundred-seed weight in the ME was highest, followed by that in the ML, ZDF, GN, BBH, and JNH. The hundred-berry weight of the ME variety was significantly higher than that of the ML, ZDF, JNH, GN, and BBH varieties (p < 0.05). The juice yields of the ME, ZDF, and ML grapes were prominently greater than those of the GN, BBH, and JNH grapes (p < 0.05), which are relative to the hundred-berry weight of grape berries. Soluble solids, titratable acidity, and pH showed significant differences among the six varieties (p < 0.05). The contents of reducing sugar in the JNH, GN, and BBH grapes were notably higher than those in the ML, ME, and ZDF grapes (p < 0.05). The sugar acid rates of the JNH and ML grapes were significantly higher than those of the BBH, GN, ME, and ZDF grapes (p < 0.05). In short, the differences in these parameters between varieties were mainly caused by the properties of the grapevine.
Fig. 1.
Basic physical and chemical parameters of grape berries of different varieties. Different letters represent significant differences between the varieties according to ANOVA with Duncan’s test (p < 0.05).
The chemical parameters of wines are related to grape berry attributes and brewing processes. In this work, the chemical parameters of wines of six cultivars are shown in Supplementary Fig. S1. One-way ANOVA revealed that the alcohol content in wines showed significant differences between the six varieties (p < 0.05), which is consistent with the pattern of soluble solids content in grape juice. The alcohol content of the BBH wine was the highest followed by the JNH, GN, ML, ME, and ZDF wines. The sugar-free extract of the BBH and GN wine was significantly higher than that of the other varieties. The pH values of all wines ranged from 3.02 to 3.48, and the JNH and ML wines showed a significantly higher level (p < 0.05). The range of reducing sugar was 0.68 to 1.67 g/L, and the BBH wine showed the highest level, followed by the GN, ML, ZDF, ME, and JNH wines. The contents of the titratable acidity of wines were in the range of 6.15 to 9.03 g/L, and the GN wine had a significantly higher content than the other varieties (p < 0.05). The total dry extract of the BBH wine showed the highest concentration, followed by the GN, ML, JNH, ZDF, and ME wines. The concentrations of total sugar in wines ranged from 2.83 to 3.9 g/L, whereas the difference between varieties was not significant.
3.2. Comparison of phenolic components of grapes and wines among different cultivars
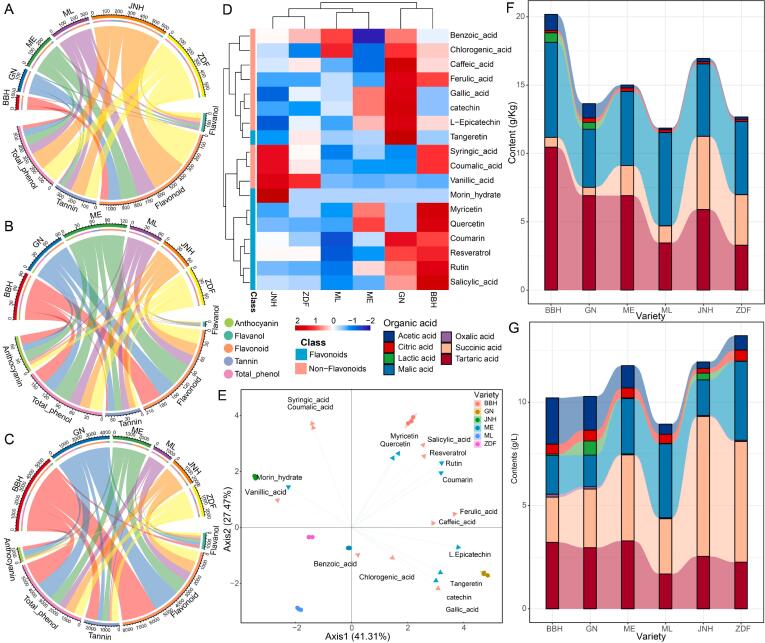
Phenolic compound contents in grape seeds and skins are particularly important for their distribution in wine. The flavonoid in grape seeds was the richest phenolic compound in each variety, with values of 69 to 313 mg/g, and the differences among the JNH, ML, GN, and BBH varieties were significant (p < 0.05) (Fig. 2A). The mean contents of tannin ranged from 10.48 to 128 mg/g, and the differences among different cultivars except between the GN and BBH varieties were significant (p < 0.05) (Supplementary Table S2). The flavanol concentration ranged from 7.44 to 43 mg/g and showed significant differences among the different varieties (p < 0.05). The content of total phenol presented significant differences among the JNH, ML, GN, and BBH varieties with values of 35 to 153 mg/g. The JNH grape seeds had the highest levels of flavonoid, tannin, and total phenol compared with the other varieties. Flavanol in the ZDF grape seeds presented the highest content at 43 mg/g, followed by the JNH, ML, ME, GN, and BBH seeds.
Fig. 2.
The profiles of phenolics compounds and organic acids in the grapes and wines of six varieties. (A) Grape seeds (dry weight); (B) Grape skins (dry weight); (C) Wines; (D) Clustering heatmap for polyphenol monomers in wines; (E) PCA for polyphenol monomers in wines; Organic acids in Grape juices (F) and Wines (G).
Considering grape skins, the ME variety had the highest concentration of phenolic compounds followed by the BBH, GN, ZDF, JNH, and ML varieties (Fig. 2B). Flavonoid was the most abundant phenolic compound in each variety, with values of 23 to 52 mg/g, and the differences among different cultivars were significant (p < 0.05) (Supplementary Table S2). The mean contents of tannin ranged from 4.02 to 17.41 mg/g, and the mean contents of tannin ranged from 4.02 to 17.41 mg/g and showed significant differences among different cultivars except between the ML and GN varieties (p < 0.05). In addition, the contents of flavanol, total phenol, and anthocyanin also presented significant differences among the six varieties (p < 0.05).
Phenolic composition is a key factor influencing wine flavour and quality. Among phenolic compounds, flavonoid in each variety showed the highest level with a range of 744 to 2690 mg/L, ranked in order of BBH > GN > ME > JNH > ZDF > ML (Fig. 2C). Flavonoid concentrations showed significant differences among the JNH, ML, ME, GN, and BBH varieties (p < 0.05) (Supplementary Table S2). The contents of tannin in wine ranged from 105 to 915 mg/L and showed significant differences among the JNH, ZDF, and BBH varieties (p < 0.05). Flavanols concentrations ranged from 114 to 236 mg/L and showed prominent differences among GN, ME, JNH, and ML varieties (p < 0.05). The concentration of total phenol ranged from 555 to 1782 mg/L and presented significant differences among BBH, ME, ML, and GN (p < 0.05). The contents of anthocyanin ranged from 13.37 to 410 mg/L and showed significant differences among different cultivars except between GN and BBH (p < 0.05).
Moreover, a total of eighteen polyphenol monomers in wines of six varieties were identified including ten nonflavonoids and eight flavonoids, using UPLC, and their contents are shown in Supplementary Table S3. One-way ANOVA indicated that the concentrations of benzoic acid, caffeic acid, coumaric acid, gallic acid, syringic acid, and catechin showed significant differences among the six varieties (p < 0.05). The accumulated contents of all polyphenol monomers in the GN wine (1053 mg/L) were the richest, followed by the BBH (985 mg/L), ME (643 mg/L), JNH (567 mg/L), ZDF (560 mg/L), and ML (420 mg/L) wines, showing significant differences among the six varieties. Subsequently, the cluster heatmap intuitively showed that most polyphenol monomers of the BBH and GN wines had significantly higher concentrations than those of the JNH, ZDF, ME, and ML wines (Fig. 2D). PCA was employed to further describe the differences in polyphenol monomers among the six varieties. As shown in Fig. 2E, wines of the same variety were clustered together and separated from other varieties, indicating that the profiles of polyphenol monomers in wines were driven by grape varieties. The first two principal components cumulatively explained 68.78 % of the total variation (PC1, 41.31 %; PC2, 27.47 %). According to the loadings plot of PCA, higher loadings correspond to resveratrol, rutin, coumarin, ferulic acid, caffeic acid, l-epicatechin, tangeretin, catechin, gallic acid, morin hydrate, and vanillic acid, which showed higher loadings in the PC1 direction. Salicylic acid, myricetin, quercetin, syringic acid, and coumaric acid showed higher loadings in the PC2 direction. Among these variables, resveratrol, rutin, coumarin, ferulic acid, caffeic acid, salicylic acid, myricetin, and quercetin were distributed in the first quadrant together with the BBH samples, indicating a higher contribution to BBH. Similarly, l-epicatechin, tangeretin, catechin, and gallic acid showed higher variation contributions to the GN wine, while syringic acid, coumaric acid, morin hydrate, and vanillic acid presented higher variation contributions to the JNH wine. These results were consistent with the higher concentration of these polyphenol monomers shown in the heatmap.
3.3. Determination of organic acids in grapes and wines from different cultivars
Organic acids of grape berries and wines of six varieties were detected by HPLC, and the results are shown in Fig. 2. Oxalic acid was not detected in the grape juice of the ME, GN, and BBH varieties (Fig. 2F). Lactic acid was not detected in the ME, ML, JNH, and ZDF varieties. Tartaric acid, malic acid, and succinic acid were the dominant organic acids in grape juice. The concentrations of tartaric acid and malic acid ranged from 3.28 to 10.46 and 4.25 to 6.95 g/kg, respectively, and BBH was the most abundant variety. Succinic acid showed a range of levels from 0.61 to 5.35 g/kg, and the contents of succinic acid in JNH, ZDF, and ME grapes were significantly higher than those in ML, BBH, and GN grapes (p < 0.05). In addition, the cumulative concentration of organic acids in BBH grape juice reached the highest level, at 19.00 g/kg, followed by the JNH, ME, GN, ZDF, and ML cultivars.
Similarly, tartaric acid, malic acid, and succinic acid were also the dominant organic acids in wines (Fig. 2G). The contents of tartaric acid, malic acid, and succinic acid exhibited significant differences among the six cultivars (p < 0.05). Tartaric acid of the ME wine showed the highest level, with a mean value of 3.29 g/L, followed by BBH, GN, JNH, ZDF, and ML. The concentrations of malic acid in the ZDF wine were significantly higher, at 3.82 g/L, than those of ML, ME, BBH, JNH, and GN. Succinic acids of the JNH, ZDF, and ME wines were significantly higher than those of GN, BBH, and ML. Oxalic acid, citric acid, and lactic acid presented a range of levels from 0.05 to 0.14, 0.23 to 0.53, and 0.01 to 0.68 g/L, respectively. Furthermore, the cumulative concentration of organic acids in ZDF reached the highest level, at 11.69 g/L, followed by JNH, ME, GN, ML, and BBH.
3.4. Aroma profiles of grapes and wines of different cultivars
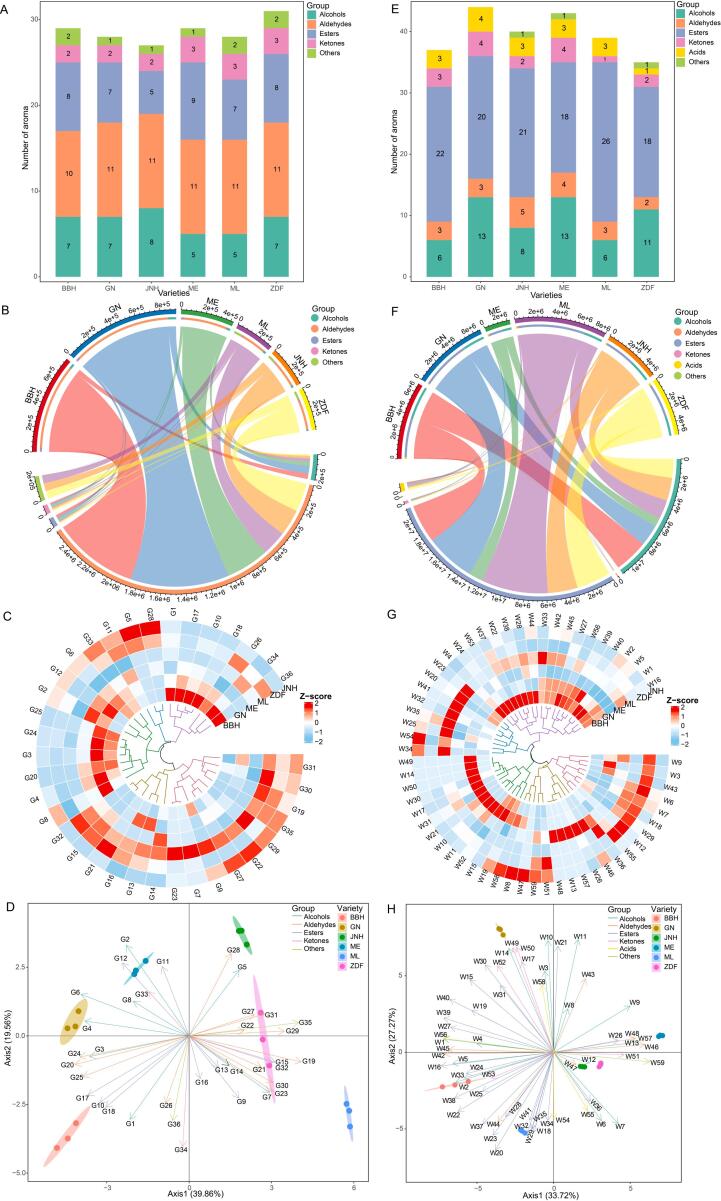
Aroma compounds of grape berries of six varieties were detected by HS-SPME-GC–MS, and a total of thirty-six compounds were identified (Supplementary Table S4). Aroma compounds in the ZDF variety were the most diverse with 31 types, followed by BBH (29), ME (29), GN (28), ML (28), and JNH (27) (Fig. 3A). Aldehydes were the most diverse aroma compounds in grape berries, followed by esters, alcohols, and ketones. A total of 11 aldehydes were detected in six varieties of grape berries (BBH, 10; GN, JNH, ME, ML, ZDF, 11). Nine esters were identified in grape berries, and ME was the richest variety with 9 esters followed by BBH (8), ZDF (8), GN (7), ML (7), and JNH (n = 5). 8, 7, 7, 7, 5, and 5 alcohols were determined in the JNH, BBH, GN, ZDF, ME, and ML varieties, respectively. In addition, aldehydes were the richest compounds in the six varieties of grape berries, followed by alcohols, esters, and ketones (Fig. 3B). The cumulative abundance of aroma components was the highest in the GN variety, followed by BBH, ME, ZDF, JNH, and ML. To deeply compare the differences in the aroma compounds among the six varieties, clustering analyses were performed after the Z score standardization data. In Fig. 3C, thirty-six aroma compounds were clustered into five groups, and the first group (3-methyl-1-butanol, ethyl caprylate, ethyl decanoate, ethyl laurate, benzaldehyde, acetophenone, and d-limonene) showed higher concentrations in BBH. The second group (2-heptanol, ethyl caproate, and (E)-2-octenal) in JNH and ZDF was more abundant. BBH, GN, and ME were distinguished from ML, ZDF, and JNH by the third cluster. Most substances in the fourth and fifth clusters enjoyed more contents in the ML grapes, and they were obviously separated from the BBH, GN, and ME grapes. Furthermore, PCA was applied to visually illustrate the differences in aroma profiles between grape berries of six varieties. The first and second PCs (PC1 and PC2) explained 39.86 % and 19.56 % of the variance, respectively, accounting for 59.42 % of the total variance (Fig. 3D).
Fig. 3.
The profiles of aroma compounds in six varieties of grape berries and wines. (A/E) The varieties of aroma compounds; (B/F) The richness of aroma compounds; (C/G) The clustering heatmap of aroma compounds; (D/H) PCA for aroma compounds. (A, B, C, and D) Grape; (E, F, G, and H) Wine.
Regarding wines of six varieties, a total of fifty-nine aroma compounds were identified (Table 1). GN had the most abundant aromas with forty-four followed by ME (43), JNH (40), ML (39), BBH (37), and ZDF (35) (Fig. 3E). Esters and alcohols were the dominant aroma compounds in wines based on the numbers and cumulative abundances of aroma compounds. A total of twenty-six esters were identified in ML, followed by BBH (22), JNH (21), GN (20), ME (18), and ZDF (18). Thirteen, thirteen, eleven, eight, six, and six alcohol compounds were determined in the GN, ME, ZDF, JNH, BBH, and ML wines, respectively. Furthermore, esters were the most abundant compounds in wines of six varieties followed by alcohols, acids, ketones, and aldehydes (Fig. 3F). The cumulative aroma abundance in the ML wine was the highest followed by the BBH, GN, ZDF, JNH, and ME varieties. Moreover, a clustering heatmap was used to display the distinction of aroma profiles in wines. As shown in Fig. 3G, fifty-nine aroma compounds were clustered into five clusters. Aroma compounds of the first three clusters possessed a richer level in the BBH, ME, and GN wines. For the fourth cluster, one part including (E)-hex-3-en-1-ol, 3-methylpentanoic acid, 3-methyl-butyraldehyde, 2,4-ditert-butylphenol, and 2-octanone manifested richer concentrations in the JNH wine, whereas the other part (1-octen-3-ol, hexyl acetate, ethyl undecanoate, 2-heptanone, nonanoic acid, and 2,4-dimethylbenzaldehyde) showed higher contents in the ME wine. The fifth cluster differentiated the ZDF wine from others with higher concentrations. Afterwards, PCA further demonstrated the differences in wine aroma profiles between the six varieties. PC1 and PC2 contributed 33.72 % and 27.27 % of the variance, respectively, capturing 60.99 % of the total variance (Fig. 3H).
Table 1.
Aromatic compounds contents in wines of six varieties.
| No. | Compounds | JNH/(µg/L) | ZDF/(µg/L) | ML/(µg/L) | ME/(µg/L) | GN/(µg/L) | BBH/(µg/L) | Descriptor (Ge et al., 2021, Li et al., 2021, Zhao et al., 2019) | Odor Threshold/(µg/L) (Ge et al., 2021, Li et al., 2021, Zhao et al., 2019) |
|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||
| W1 | 1-Butanol | 3562 ± 169c | 4694 ± 157b | 5008 ± 2b | 1914 ± 23d | 6216 ± 118a | 6653 ± 694a | Medicinal, alcohol | 150,000 |
| W2 | 3-Methyl-1-butanol | 1877466 ± 1475b | 1448709 ± 8085c | 1577902 ± 127260c | 568136 ± 21821d | 1559501 ± 103349c | 2833155 ± 11159a | Green, floral | 400 |
| W3 | 2-Heptanol | ND | 1001 ± 3b | ND | ND | 1188 ± 54a | ND | Fruity, moldy, musty | 70 |
| W4 | 1-Heptanol | 4073 ± 50e | 4753 ± 474d | 4857 ± 30 cd | 5375 ± 180bc | 5921 ± 306b | 8378 ± 162a | Grape, sweet | 1000 |
| W5 | Phenethyl alcohol | 100851 ± 477b | 93552 ± 1067b | 86471 ± 3548b | 33215 ± 233c | 105325 ± 4877b | 158864 ± 26359a | Sweet rose | 14,000 |
| W6 | 1-Octanol | 20275 ± 413c | 31549 ± 2787a | 25674 ± 183b | 16760 ± 907d | 10507 ± 787e | 12139 ± 1239e | Intense citrus, roses | 120 |
| W7 | 1-Decanol | 8375 ± 112b | 13220 ± 1074a | 13283 ± 18a | 7302 ± 77c | ND | ND | Orange flowery, special fatty |
400 |
| W8 | (E)-hex-3-en-1-ol | 10125 ± 199a | ND | ND | 1560 ± 36c | 5184 ± 349b | ND | Green, floral | 400 |
| W9 | (Z)-hex-3-en-1-ol | ND | 13073 ± 17.2a | ND | 10905 ± 428b | 6308 ± 557c | ND | Green | 400 |
| W10 | (Z)-hex-2-en-1-ol | ND | 1203 ± 3c | ND | 1540 ± 74b | 4821 ± 98a | ND | Green | 400 |
| W11 | 1-Hexanol | 60404 ± 381d | 113185 ± 6781c | ND | 177270 ± 7254b | 266766 ± 20312a | 41695 ± 3414d | Green, grass | 8000 |
| W12 | 1-Dodecanol | ND | 2664 ± 160a | ND | ND | ND | ND | Citrus, orange, lemon, floral, waxy |
15 |
| W13 | 1-Octen-3-ol | ND | ND | ND | 2458 ± 83a | ND | ND | Mushroom, earthy, vegetative |
1 |
| W14 | Citronellol | ND | ND | ND | ND | 4301 ± 205a | ND | Floral, rose, sweet, green, fruity |
100 |
| Esters | |||||||||
| W15 | Methyl acetate | ND | ND | ND | ND | 1945 ± 536a | 1506 ± 183a | nf | nf |
| W16 | Ethyl acetate | 270769 ± 1648d | 258788 ± 4534d | 416486 ± 5803b | 78923 ± 3186e | 391503 ± 20796c | 442160 ± 15424a | Ethereal fruity | 7500 |
| W17 | Ethyl propionate | ND | ND | 3296 ± 46b | 2297 ± 33c | 10395 ± 439a | ND | Fruity, sweet, winey | 550 |
| W18 | Propyl acetate | 1057 ± 22 d | 6652 ± 162a | 5726 ± 67b | ND | ND | 3542 ± 282c | Fruity, banana, honey | 4740 |
| W19 | Ethyl isobutyrate | 12867 ± 870ab | 8901 ± 590c | 7793 ± 57c | 4408 ± 152d | 13903 ± 561a | 12793 ± 491b | Strawberry | 15 |
| W20 | Isobutyl acetate | 24206 ± 16d | 32039 ± 1619c | 85913 ± 1535a | ND | ND | 58685 ± 7569b | Flowery | 1600 |
| W21 | Butyl acetate | ND | 1413 ± 56c | 1601 ± 17c | 10403 ± 565b | 19312 ± 326a | ND | Sweet, ripe, banana | 1800 |
| W22 | Ethyl butyrate | 20354 ± 752c | 14572 ± 490d | 30240 ± 385b | 7352 ± 386e | 15053 ± 382d | 46409 ± 4044a | Strawberry, apple |
20 |
| W23 | Isoamyl acetate | 201144 ± 7879d | 408824 ± 12428c | 1737031 ± 16291a | 207414 ± 10031d | 221665 ± 10669d | 1023924 ± 77839b | Intense banana | 30 |
| W24 | Methyl caproate | 1599 ± 39bc | 2007 ± 40b | 1767 ± 20b | 1100 ± 37c | 2134 ± 92b | 6974 ± 705a | nf | nf |
| W25 | Ethyl caproate | 420436 ± 12146b | 337855 ± 9866d | 553226 ± 2513a | 247663 ± 9286e | 419878 ± 26297b | 385151 ± 5729c | Sweet, pineapple, fruity, waxy |
8 |
| W26 | Hexyl acetate | 4687 ± 212e | 22562 ± 670d | 44981 ± 1115b | 141093 ± 4724a | 34784 ± 2419c | 32074 ± 444c | Pleasant fruity, pear, floral | 670 |
| W27 | Ethyl heptanoate | 2472 ± 30d | 3435 ± 105c | 2854 ± 43d | 1653 ± 35e | 5078 ± 352b | 6317 ± 526a | Pineapple, fruity | 220 |
| W28 | Heptyl acetate | ND | ND | 3109 ± 187b | 2237 ± 51c | ND | 4106 ± 440a | Cherry, pear | 670 |
| W29 | Methyl octanoate | 27389 ± 352d | 51069 ± 3011a | 39643 ± 1871b | 11354 ± 304e | 14199 ± 557e | 34263 ± 2615c | Intense citrus | 200 |
| W30 | Methyl salicylate | 1557 ± 25c | ND | 3351 ± 4b | ND | 15200 ± 1360a | 3458 ± 747b | Mint | 40 |
| W31 | Ethyl caprylate | 1046561 ± 9273d | 1135719 ± 58694c | 1685631 ± 18759b | 364623 ± 8439f | 2857681 ± 47201a | 697189 ± 1518e | Sweet, waxy, fruity, pineapple |
5 |
| W32 | Octyl acetate | 3708 ± 49d | ND | 31361 ± 649a | 6491 ± 28c | ND | 7791 ± 765b | nf | nf |
| W33 | Phenylethyl acetate | 1407 ± 284c | ND | 22913 ± 739a | ND | 12967 ± 41b | 13421 ± 968b | Pleasant, floral | 650 |
| W34 | Ethyl pelargonate | 6012 ± 425b | ND | 7778 ± 318a | ND | ND | ND | Banana, grape | 1300 |
| W35 | Ethyl decanoate | 616205 ± 2065c | 770295 ± 52942b | 1242736 ± 16584a | 157771 ± 1183e | 356338 ± 11887d | 396504 ± 1234d | Fruity, fatty, pleasant | 200 |
| W36 | Ethyl undecanoate | ND | ND | 2136 ± 65a | 1717 ± 24b | ND | ND | Coconut | 100 |
| W37 | Methyl dodecanoate | 3085 ± 123d | 6443 ± 125c | 6902 ± 85b | 2450 ± 41e | 2155 ± 151e | 11511 ± 370a | nf | nf |
| W38 | Ethyl laurate | 62022 ± 1844d | 84656 ± 6834c | 157385 ± 3687b | 16448 ± 329e | 86801 ± 3527c | 311231 ± 17838a | Oily, fatty, fruity | 1500 |
| W39 | Ethyl tetradecanoate | 4213 ± 175e | 7969 ± 192d | 9944 ± 319c | ND | 20299 ± 333b | 21092 ± 1174a | Mild waxy, soapy | 2000 |
| W40 | Ethyl palmitate | 2447 ± 87e | 4234 ± 680d | 7374 ± 508c | ND | 18531 ± 26a | 15073 ± 1504b | Fruity, sweet, fatty | 1500 |
| W41 | Pentyl acetate | ND | ND | 1463 ± 14a | ND | ND | ND | nf | nf |
| Aldehydes | |||||||||
| W42 | Ethanal | 5168 ± 284b | 2543 ± 21c | 7068 ± 20a | 2074 ± 22d | 7004 ± 78a | 7183 ± 6a | Fruity, pungent, green, grassy, apple |
500 |
| W43 | Benzaldehyde | ND | 5940 ± 237a | ND | 2377 ± 39c | 4913 ± 53b | ND | Roasted, almond | 2000 |
| W44 | 4-Ethylbenzaldehyde | 4474 ± 91c | ND | 5368 ± 151b | 3510 ± 74d | ND | 10236 ± 532a | nf | nf |
| W45 | Capraldehyde | 2294 ± 86b | ND | 3436 ± 614a | ND | 3561 ± 131a | 3375 ± 364a | nf | 10 |
| W46 | 2,4-Dimethylbenzaldehyde | 3655 ± 752b | ND | ND | 7132 ± 85a | ND | ND | nf | nf |
| W47 | 3-Methyl-butyraldehyde | 1723 ± 182a | ND | ND | ND | ND | ND | nf | nf |
| Ketones | |||||||||
| W48 | 2-Heptanone | ND | ND | ND | 1009 ± 202a | ND | ND | nf | nf |
| W49 | Acetoin | ND | ND | ND | ND | 6014 ± 522a | ND | Fatty, cream | 150,000 |
| W50 | 2-Undecanone | ND | ND | ND | ND | 2693 ± 32a | ND | nf | nf |
| W51 | 2-Octanone | 45672 ± 409a | 51124 ± 3378a | 27903 ± 7169c | 35649 ± 123b | 30691 ± 493bc | 27090 ± 689c | Lactic, mushroom | 150 |
| W52 | Acetophenone | 2241 ± 0c | 2087 ± 153c | ND | 1460 ± 33c | 4860 ± 446a | 3261 ± 842b | nf | nf |
| W53 | 2,3-Pentanedione | ND | ND | ND | 778 ± 8b | ND | 4122 ± 104a | nf | nf |
| Acids | |||||||||
| W54 | Acetic acid | 13717 ± 140a | ND | 10186 ± 50b | 4946 ± 93d | 833 ± 17e | 5405 ± 704c | Acid, fatty, spicy |
200,000 |
| W55 | Octanoic acid | 82049 ± 1469c | 200156 ± 1141a | 114724 ± 1432b | 81453 ± 1140c | 43831 ± 3109d | 80679 ± 12406c | Cheese, fatty acid |
500 |
| W56 | Hexanoic acid | ND | ND | 13157 ± 316c | ND | 18556 ± 939b | 24948 ± 4686a | Cheese, unpleasant copra, oil odor | 3000 |
| W57 | Nonanoic acid | ND | ND | ND | 9872 ± 2a | ND | ND | Cheese, waxy flavor | 500–800 |
| W58 | 3-Methylpentanoic acid | 8289 ± 85a | ND | ND | ND | 7392 ± 122b | ND | nf | 33 |
| Others | |||||||||
| W59 | 2,4-Di-tert-butylphenol | 4022 ± 37a | 3358 ± 152b | ND | 2882 ± 355c | ND | ND | nf | nf |
Note: Data are expressed as mean values of three independent experiments ± standard deviation (n = 3). nf, not found. The different letters within each row show that mean values are significantly different at the 95 % confidence level according to the analysis of variance with Duncan’s multiple comparison test. ND, not detected. IUPAC name and CAS No. of compounds are summarized in the Supplementary Table S5.
3.5. Important chemical variables among different varieties of grapes and wines
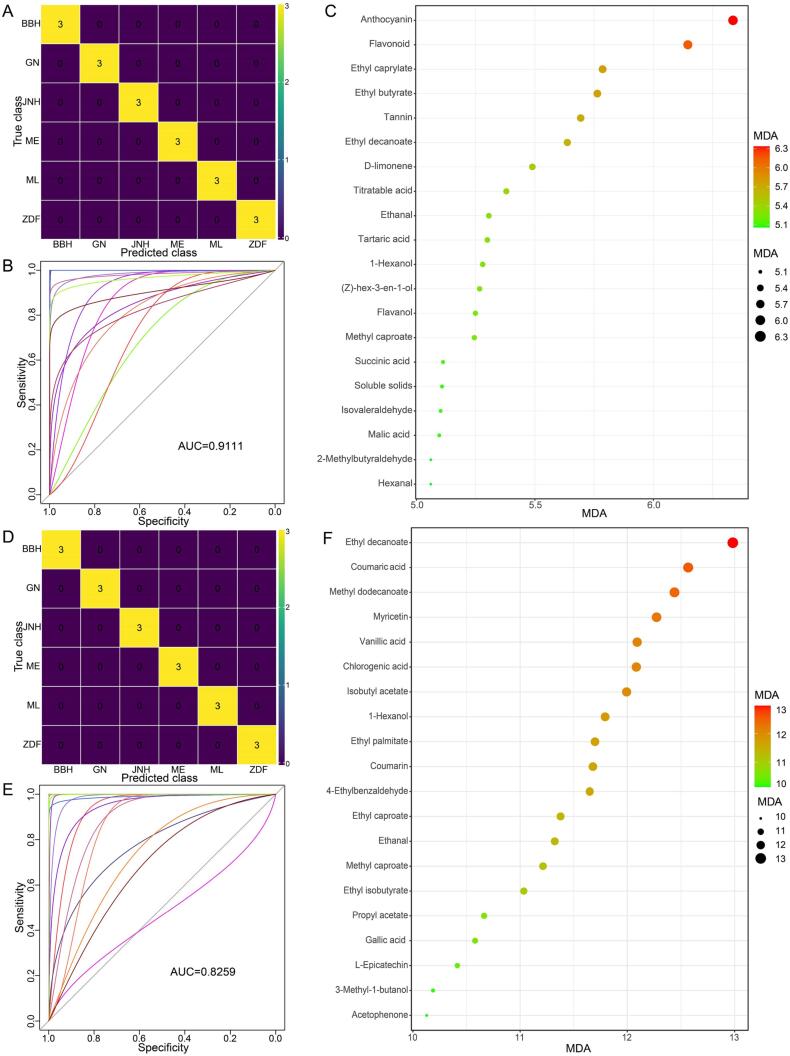
To determine important chemical variables among different varieties of grapes and wines, a random forest model was performed with 7 variables tried at each split (mtry) and 500 trees (ntree). Integrating basic parameters, phenolics, organic acids, and aroma compounds for grape berries, a data matrix consisting of 53 variables and 18 samples was used to build a classification model. The confusion matrix showed 100 % accuracy for predicting the varieties of grape berries (Fig. 4A). Subsequently, leave-one-out cross-validation was introduced to verify the classifier performance, obtaining 0 % out of bag (OOB, used for calculating the class error). The performance was further evaluated via the ROC and AUC. As shown in Fig. 4B, all ROCs based on multiple classifications were located in the upper left, which demonstrated that the AUC values of all curves were greater than 0.5, with an average of 0.9111. Thus, the RF model combined with multiple chemical variables is an excellent classifier for distinguishing grape cultivars. Eventually, the importance of each variable was assessed by the mean decrease accuracy (MDA). The top 20 variables were successively ranked according to MDA values from largest to smallest as anthocyanin, flavonoid, ethyl caprylate, ethyl butyrate, tannin, ethyl decanoate, d-limonene, titratable acid, ethanal, tartaric acid, 1-hexanol, (z)-hex-3-en-1-ol, flavanol, methyl caproate, succinic acid, soluble solids, isovaleraldehyde, malic acid, 2-methylbutyraldehyde, and hexanal (Fig. 4C).
Fig. 4.
A random forest model finding important chemical variables by distinguishing grapes and wines according to cultivars. Confusion matrixes of RF classifier for grapes (A) and wines (D); the ROC curves and the AUC of RF classifier for grapes (B) and wines (E); the top twenty of chemical markers according to MDA values for grapes (C) and wines (F).
Analogously, the RF classifier was employed to distinguish wines based on a data matrix composed of 97 variables and 18 samples. The RF classifier obtained a high accuracy of 100 % for predicting wine varieties according to a confusion matrix (Fig. 4D). Furthermore, the values of OOB (0 %) and AUC (0.8259) further verified that the RF classifier combined with chemical parameters had a preeminent performance in distinguishing wine varieties (Fig. 4E). Ethyl decanoate was the most important variable differentiating wines followed by coumaric acid, methyl dodecanoate, myricetin, vanillic acid, chlorogenic acid, isobutyl acetate, 1-hexanol, ethyl palmitate, coumarin, 4-ethylbenzaldehyde, ethyl caproate, ethanal, methyl caproate, ethyl isobutyrate, propyl acetate, gallic acid, l-epicatechin, 3-methyl-1-butanol, and acetophenone according to MDA values (Fig. 4F).
3.6. The relationship between chemical variables and sensory characteristics of wine
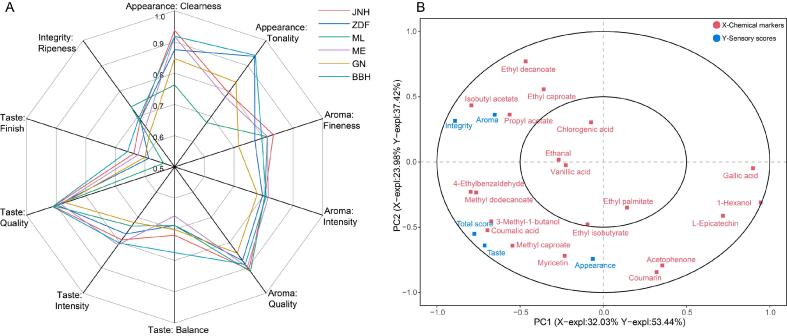
The scores of the sensory analysis for wines of six varieties are summarized in Supplementary Table S6. The mean score of the BBH wine was highest at 86.78 followed by JNH (84.92), ZDF (84.65), ME (83.11), ML (82.24), and GN (80.82). Compared among the six wines, the taste (quality and finish) and appearance (tonality) of the BBH wine were the best, whereas the appearance clearness and aroma fineness of the JNH wine were the best (Fig. 5A). The taste and aroma intensity of the ME wine were the best, whereas the ripe-ness and aroma quality of the ML wine were the best.
Fig. 5.
Sensory quantitative description and PLSR analysis for wines of six varieties. (A) Radar plot of six varieties of wine based on ten key sensory characterizations. (B) Correlation loadings of PLSR between chemical variables (X variables) and sensory scores (Y variables). The outer and inner ellipses on the correlation loadings plot indicate 100% and 50% of explained variance, respectively.
PLSR is a useful tool to evaluate chemical and sensory datasets. In this study, the method was employed to explore the underlying relationships between chemical variables and sensory scores for wines. As shown in Fig. 5B, chlorogenic acid, vanillic acid, ethanal, ethyl palmitate, and ethyl isobutyrate are distributed in the inner circle, indicating a lower interpretive degree of the first two principal components to these variables. In contrast, ethyl decanoate, coumaric acid, methyl dodecanoate, myricetin, isobutyl acetate, 1-hexanol, coumarin, 4-ethylbenzaldehyde, ethyl caproate, methyl caproate, propyl acetate, gallic acid, l-epicatechin, 3-methyl-1-butanol, and acetophenone, manifested a higher interpretive degree of the first two principal components to these variables. The first two principal components explained 56.01 % of the variation in chemical variables and 90.86 % of the variation in sensory scores. Furthermore, ethyl caproate, ethyl decanoate, propyl acetate, and isobutyl acetate presented a large contribution to wine aroma and integrity. Myricetin, methyl caproate, 4-ethylbenzaldehyde, 3-methyl-1-butanol, methyl dodecanoate and coumaric acid were positively related to wine taste and total score. Myricetin, methyl caproate, acetophenone, and coumarin were also well related to wine appearance. Overall, these findings demonstrated that most chemical variables exhibited positive correlations with wine sensory characteristics.
4. Discussion
Chemical and sensory characteristics are key parameters to evaluate the quality of grapes and wine. Alcohol in the BBH, GN, and JNH wines showed significantly higher levels than that in the ME, ML, and ZDF varieties, which is consistent with the pattern of reducing sugar content in grape juices. This finding indicates that the BBH, GN, and JNH grapes are a good fit for brewing wines with high alcohol content. The volatile acidity of the JNH and ME wines was significantly higher than that of ZDF, BBH, GN, and ML under 1.2 g/L according to the OIV limit (OIV, 2017), increasing the risk of wine acidification because it confers an unpleasant vinegary aroma along with an acrid taste (Vilela, 2018). The content of reducing sugar in Meili grape juice was 180 g/L, which is in the range of 156–210 g/L reported by Yang, Jin, Wang, Kong, Liu & Tao (2019) Compared with the ME, ML, and ZDF varieties, the BBH, GN, and JNH grapes and wines exhibited higher phenolic compound contents, which may strengthen their antioxidation, bitterness astringency, colour stability, and shelf life (Ferrer-Gallego, Hernández-Hierro, Rivas-Gonzalo & Escribano-Bailón, 2014). Ristic et al. (2019) reported that the hedonic and emotional responses of wine consumers were related to wine aroma characterization. Thus, the BBH and ML wines may be more popular with consumers due to their more abundant floral and fruity aroma. In addition, wine flavour was indicated by consumers to be the most important factor driving purchase decisions followed by the balance of flavour and wine body (Niimi, Danner, Li, Bossan & Bastian, 2017). That is why the ML and ZDF wines had the richest aroma and organic acid respectively, but their sensory scores were lower than those of the BBH wines.
The differences in chemical and sensory profiles for grapes between different cultivars of the same origin were caused by grapevine attributes. The ME grape berry exhibited lower levels of soluble solids, reducing sugar, and pH than the JNH and ML varieties because the hybrid varieties of V. labrusca and V. vinifera inherit the characteristics of American parents (Wang et al., 2021). Compared with the ‘Gold Finger’ cultivar reported previously in the Yangling region (Feng et al., 2021), the BBH and GN grape berries had similar soluble solids, higher titratable acidities, and richer aroma compounds. The six varieties of grape berries showed higher levels of reducing sugars, titratable acidities, and aldehyde compounds than ‘Hutai-8′ grapes (Yao et al., 2021, Yao et al., 2021). The GN and BBH grapes had lower cluster and hundred-berry weights and higher titratable acid levels than other variables, which are related to parental traits (V. amurensis) with small clusters, small grains, and high acidity (Wang et al., 2021). In this work, the ME grape skins had significantly higher phenolic compounds than the JNH, ZDF, and ML varieties, which may be explained by the varietal differences between V. labrusca and V. vinifera (Santos, Morais, Souza, Cottica, Boroski & Visentainer, 2011).
The variation in chemical characteristics from grape to wine was influenced by the winemaking process. The BBH and GN grapes had higher aroma contents than the ML grapes, but the pattern was reversed in the wine. Some nonvolatile aroma precursors in grapes are released in wine during alcohol fermentation by the action of microorganisms and enzymes (Carpena et al., 2021). Fermented aromas, such as 1-dodecanol, 1-octen-3-ol, and citronellol were detected in the ZDF, ME, and GN wines, respectively, contributing to citrus, mushroom, and floral flavour (Zhang et al., 2016). The three aroma compounds were detected in Moscatel and Gewürztraminer wines, but the concentrations were lower than our values (Soares et al., 2015, Lukić et al., 2016). C6 compound such as (E)-hex-3-en-1-ol, (Z)-hex-3-en-1-ol, (Z)-hex-2-en-1-ol were detected in the JNH, ZDF, ME, and GN wines, giving these varieties of wines a green flavour (Zhao et al., 2019). Esters are the secondary aroma produced during wine fermentation, caused by the combination of alcohols and acids (Soares et al., 2015). A high concentration of ethyl acetate showed significant differences among the six varieties of wines, which is most certainly related to higher contents of its precursor acetic acid since the contents of these two compounds are interdependent (Lukić et al., 2016). Among the six cultivars, the BBH grapes had the highest organic acid contents, but the BBH wines did not have the highest organic acid contents. This finding may be related to the ability of microorganisms to reduce organic acid contents during the fermentation process (Englezos, Torchio, Vagnoli, Krieger-Weber, Rantsiou & Cocolin, 2020). Some microorganisms, such as filamentous fungi or bacteria, can produce acetic acid (Vilela, 2018), leading to an increase in the volatile acidity of wine during fermentation, which is usually controlled by the addition of sulfur dioxide. Moreover, yeasts can cause grape phenolic compounds to undergo various types of transformations during winemaking, further impacting sensory attributes and thus wine quality (Zhang, Ma, Meng, Zhang, Jin & Fang, 2021).
Chemical composition plays a critical role in shaping the sensory characteristics of wines of different varieties. Herein, ester compounds exhibited an important contribution to sensory scores, which is related to the fact that esters are the main aroma compounds in wine. Several volatile compounds showed higher contribution to aroma scores, such as propyl acetate, isobutyl acetate, ethyl caproate, and ethyl decanoate. Isobutyl acetate was detected in JNH, ZDF, ML, and BBH, contributing a flowery flavour for different levels of these wines (Zhao et al., 2019). The concentration of ethyl decanoate giving fruity flavour exhibited significant differences among six varieties of wines, which is richer than that of Cabernet Sauvignon wine (Zhao et al., 2019). Some phenols including coumarin, myricetin, and coumaric acid were related to taste and appearance characteristics. It was reported that coumaric acid is an oxidation substrate and precursor of browning of wine, giving a bitter flavour (Merkytė et al., 2020). In addition, the chemical composition of grapes has an excellent effect on determining wine flavour. In this study, anthocyanin, flavonoid, and tannin showed significant differences among the six cultivars of grape skins and were important variables for distinguishing grapes according to cultivar. It was reported that tannins combined with anthocyanin influence wine taste (bitterness), astringency and colour stabilization (Merkytė et al., 2020). Overall, grape cultivar is a key factor influencing the chemical characterization and sensory profile of grapes and wine.
5. Conclusion
This study preliminarily characterized the chemical and sensory profiles of grapes and wines of six cultivars from the Weibei Plateau region in China. Phenolic contents were higher in the JNH, ZDF, and ML grape seeds, but better in the BBH, GN, and ME grape skins and wines. The contents of organic acids were the highest in BBH grape and ZDF wine. The BBH and GN grapes had the most abundant aroma contents, whereas the aroma content of the ML wine was higher than that of the BBH and GN wines. Chemometrics further proved the differences in chemical characteristics among different cultivars. Furthermore, RF models demonstrated that anthocyanin, flavonoid, ethyl caprylate, ethyl butyrate, tannin and coumaric acid, methyl dodecanoate, myricetin, vanillic acid, and chlorogenic acid were important chemical variables to distinguish grapes and wines according to cultivar. The chemical variables of wine exhibited an important contribution to its sensory characteristics according to PLSR. The sensory quality of wines was ranked by BBH > JNH > ZDF > ME > ML > GN. In summary, these microscale findings may be helpful for viticulturists and winemakers in selecting grape varieties. However, the variations caused by the scale-up effects should be considered when these grape cultivars are used for winemaking on an industrial scale.
CRediT authorship contribution statement
Feifei Gao: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Lingxiao Guan: Data curation, Formal analysis, Methodology. Guihua Zeng: Investigation, Methodology, Writing – review & editing. Xiaoyun Hao: Methodology, Resources. Hua Li: Project administration, Resources, Supervision, Validation. Hua Wang: Project administration, Resources, Supervision, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the high-level talent research project of Shihezi University in China (No. RCZK202347) and Shaanxi Provincial Key Research and Development Projects in China (No. 2020ZDLNY07-08).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101091.
Contributor Information
Feifei Gao, Email: gaofeifei@shzu.edu.cn.
Hua Wang, Email: wanghua@nwafu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Aleixandre-Tudo J.L., Buica A., Nieuwoudt H., Aleixandre J.L., du Toit W. Spectrophotometric analysis of phenolic compounds in grapes and wines. Journal of Agricultural and Food Chemistry. 2017;65:4009–4026. doi: 10.1021/acs.jafc.7b01724. [DOI] [PubMed] [Google Scholar]
- Cadot Y., Caillé S., Samson A., Barbeau G., Cheynier V. Sensory dimension of wine typicality related to a terroir by quantitative descriptive analysis, just about right analysis and typicality assessment. Analytica Chimica Acta. 2010;660:53–62. doi: 10.1016/j.aca.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Carpena M., Fraga-Corral M., Otero P., Nogueira R.A., Garcia-Oliveira P., Prieto M.A., Simal-Gandara J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods. 2021;10:51. doi: 10.3390/foods10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordevic N.O., Novakovic M.M., Pejin B., Mutic J.J., Vajs V.E., Pajovic S.B., Tesevic V.V. Comparative analytical study of the selected wine varieties grown in Montenegro. Natural Product Research. 2017;31:1825–1830. doi: 10.1080/14786419.2017.1289209. [DOI] [PubMed] [Google Scholar]
- Englezos V., Torchio F., Vagnoli P., Krieger-Weber S., Rantsiou K., Cocolin L. Impact of Saccharomyces cerevisiae strain selection on malolactic fermentation by Lactobacillus plantarum and Oenococcus oeni. American Journal of Enology and Viticulture. 2020;71:157–165. doi: 10.5344/ajev.2019.19061. [DOI] [Google Scholar]
- Feng M., Jin X., Yao H., Zhu T., Guo S., Li S., Lei Y., Xing Z., Zhao X., Xu T., Meng J. Evolution of volatile profile and aroma potential of 'Gold Finger' table grapes during berry ripening. Journal of the Science of Food and Agriculture. 2022;102:291–298. doi: 10.1002/jsfa.11357. [DOI] [PubMed] [Google Scholar]
- Ferrer-Gallego R., Hernández-Hierro J.M., Rivas-Gonzalo J.C., Escribano-Bailón M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Research International. 2014;62:1100–1107. doi: 10.1016/j.foodres.2014.05.049. [DOI] [Google Scholar]
- Ferrero-del-Teso S., Arias I., Escudero A., Ferreira V., Fernández-Zurbano P., Sáenz-Navajas M. Effect of grape maturity on wine sensory and chemical features: The case of Moristel wines. LWT-Food Science and Technology. 2020;118 doi: 10.1016/j.lwt.2019.108848. [DOI] [Google Scholar]
- Gao X., Sun D., Wu M., Li H., Liu F., He F., Pan Q., Wang J. Influence of cluster positions in the canopy and row orientation on the flavonoid and volatile compound profiles in Vitis vinifera L. Cabernet franc and Chardonnay berries. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110306. [DOI] [PubMed] [Google Scholar]
- Garde-Cerdán T., Ancín-Azpilicueta C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT-Food Science and Technology. 2008;41:501–510. doi: 10.1016/j.lwt.2007.03.018. [DOI] [Google Scholar]
- Ge Q., Guo C., Zhang J., Yan Y., Zhao D., Li C., Sun X., Ma T., Yue T., Yuan Y. Effects of simultaneous co-fermentation of five indigenous non-Saccharomyces strains with S. cerevisiae on Vidal icewine aroma quality. Foods. 2021;10:1452. doi: 10.3390/foods10071452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D., Carrapeiro M.D., Fogliano V., van Ruth S.M. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: A review. Trends in Food Science & Technology. 2016;52:31–48. doi: 10.1016/j.tifs.2016.03.013. [DOI] [Google Scholar]
- Hjelmeland A.K., Ebeler S.E. Glycosidically bound volatile aroma compounds in grapes and wine: A review. American Journal of Enology and Viticulture. 2015;66:1–11. doi: 10.5344/ajev.2014.14104. [DOI] [Google Scholar]
- Huang X., Jiang Z., Tan J., Li R. Geographical origin traceability of red wines based on chemometric classification via organic acid profiles. Journal of Food Quality. 2017;2038073 doi: 10.1155/2017/2038073. [DOI] [Google Scholar]
- Li H., Wang X., Li Y., Li P., Wang H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chemistry. 2009;112:454–460. doi: 10.1016/j.foodchem.2008.05.111. [DOI] [Google Scholar]
- Li W., Yao H., Chen K., Ju Y., Min Z., Sun X., Cheng Z., Liao Z., Zhang K., Fang Y. Effect of foliar application of fulvic acid antitranspirant on sugar accumulation, phenolic profiles and aroma qualities of Cabernet Sauvignon and Riesling grapes and wines. Food Chemistrry. 2021;351 doi: 10.1016/j.foodchem.2021.129308. [DOI] [PubMed] [Google Scholar]
- Llaudy M., Canals R., Canals J.M., Rozéz N., Arola L., Zamora F. New method for evaluating astringency in red wine. Journal of Agricultural and Food Chemistry. 2004;52:742–746. doi: 10.1021/jf034795f. [DOI] [PubMed] [Google Scholar]
- Lukić I., Radeka S., Grozaj N., Staver M., Peršurić Đ. Changes in physico-chemical and volatile aroma compound composition of Gewürztraminer wine as a result of late and ice harvest. Food Chemistry. 2016;196:1048–1057. doi: 10.1016/j.foodchem.2015.10.061. [DOI] [PubMed] [Google Scholar]
- Meng J.F., Fang Y.L., Qin M.Y., Zhuang X.F., Zhang Z.W. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi county (China) Food Chemistry. 2012;134:2049–2056. doi: 10.1016/j.foodchem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Mercurio M., Dambergs R.G., Herderich M.J., Smith P.A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. Journal of Agricultural and Food Chemistry. 2007;55:4651. doi: 10.1021/jf063674n. [DOI] [PubMed] [Google Scholar]
- Merkytė V., Longo E., Windisch G., Boselli E. Phenolic compounds as markers of wine quality and authenticity. Foods. 2020;9:1785. doi: 10.3390/foods9121785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic M., Žeravík J., Obořil M., Pelcová M., Lacina K., Cakar U., Petrovic A., Glatz Z., Skládal P. A novel method for classification of wine based on organic acids. Food Chemistry. 2019;284:296–302. doi: 10.1016/j.foodchem.2019.01.113. [DOI] [PubMed] [Google Scholar]
- Moreno-Olivares J.D., Paladines-Quezada D., Fernandez-Fernandez J.I., Bleda-Sanchez J.A., Martinez-Moreno A., Gil-Munoz R. Study of aromatic profile of different crosses of Monastrell white wines. Journal of the Science of Food and Agriculture. 2020;100:38–49. doi: 10.1002/jsfa.9991. [DOI] [PubMed] [Google Scholar]
- Niimi J., Danner L., Li L., Bossan H., Bastian S.E.P. Wine consumers' subjective responses to wine mouthfeel and understanding of wine body. Food Research International. 2017;99:115–122. doi: 10.1016/j.foodres.2017.05.015. [DOI] [PubMed] [Google Scholar]
- OIV (International Organisation of Vine and Wine) Paris, France; OIV: 2017. Compendium of International Methods of Wine and Must Analysis OIV-18. https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis. [Google Scholar]
- Peinado J., de Lerma N.L., Moreno J., Peinado R.A. Antioxidant activity of different phenolics fractions isolated in must from Pedro Ximenez grapes at different stages of the off-vine drying process. Food Chemistry. 2009;114:1050–1055. doi: 10.1016/j.foodchem.2008.10.068. [DOI] [Google Scholar]
- Peng C., Wen Y., Tao Y., Lan Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. Journal of Agricultural and Food Chemistry. 2013;61:1542–1553. doi: 10.1021/jf3043874. [DOI] [PubMed] [Google Scholar]
- Phan Q., Tomasino E. Untargeted lipidomic approach in studying pinot noir wine lipids and predicting wine origin. Food Chemistry. 2021;355 doi: 10.1016/j.foodchem.2021.129409. [DOI] [PubMed] [Google Scholar]
- Ristic R., Danner L., Johnson T.E., Meiselman H.L., Hoek A.C., Jiranek V., Bastian S.E.P. Wine related aromas for different seasons and occasions: Hedonic and emotional responses of wine consumers from Australia, UK and USA. Food Quality and Preference. 2019;71:250–260. doi: 10.1016/j.foodqual.2018.07.011. [DOI] [Google Scholar]
- Robles A., Fabjanowicz M., Chmiel T., Płotka-Wasylka J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC-Trends. Analytical Chemistry. 2019;120 doi: 10.1016/j.trac.2019.115630. [DOI] [Google Scholar]
- Santos L.P., Morais D.R., Souza N.E., Cottica S.M., Boroski M., Visentainer J.V. Phenolic compounds and fatty acids in different parts of Vitis labrusca and V. vinifera grapes. Food Research International. 2011;44:1414–1418. doi: 10.1016/j.foodres.2011.02.022. [DOI] [Google Scholar]
- Soares R.D., Welke J.E., Nicolli K.P., Zanus M., Caramão E.B., Manfroi V., Zini C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chemistry. 2015;183:291–304. doi: 10.1016/j.foodchem.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Vilela A. Lachancea thermotolerans, the Non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation. 2018;4:56. doi: 10.3390/fermentation4030056. [DOI] [Google Scholar]
- Wang Z., Xue T., Gao F., Zhang L., Han X., Wang Y., Hui M., Wu D., Li H., Wang H. Intraspecific recurrent selection in V. vinifera: An effective method for breeding of high quality, disease-, cold-, and drought -resistant grapes. Euphytica. 2021;217:111. doi: 10.1007/s10681-021-02851-7. [DOI] [Google Scholar]
- Yang Y., Jin G., Wang X., Kong C., Liu J., Tao Y. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chemistry. 2019;284:155–161. doi: 10.1016/j.foodchem.2019.01.106. [DOI] [PubMed] [Google Scholar]
- Yao H., Jin X., Feng M., Xu G., Zhang P., Fang Y., Xu T., Meng J. Evolution of volatile profile and aroma potential of table grape Hutai-8 during berry ripening. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110330. [DOI] [PubMed] [Google Scholar]
- Yao Y., Chen K., Yang X., Li J., Li X. Comparative study of the key aromatic compounds of Cabernet Sauvignon wine from the Xinjiang region of China. International Journal of Food Science and Technology. 2021;58:2109–2120. doi: 10.1007/s13197-020-04720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Ma W., Meng Y., Zhang Y., Jin G., Fang Z. Wine phenolic profile altered by yeast: Mechanisms and influences. Comprehensive Reviews in Food Science and Food Safety. 2021;20:3579–3619. doi: 10.1111/1541-4337.12788. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Guo, A., Han, F., & Zhang, Y. (2016). Fast determination of phenolics and polyphenolics in wine by ultra performance liquid chromatography. Food Science, 37, 128–133. (in Chinese with English abstract) https://doi.org/10.7506/spkx1002-6630-201610022.
- Zhao T., Wu J., Meng J., Shi P., Fang Y., Zhang Z., Sun X. Harvesting at the right time: Maturity and its effects on the aromatic characteristics of Cabernet Sauvignon wine. Molecules. 2019;24:2777. doi: 10.3390/molecules24152777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.