Abstract
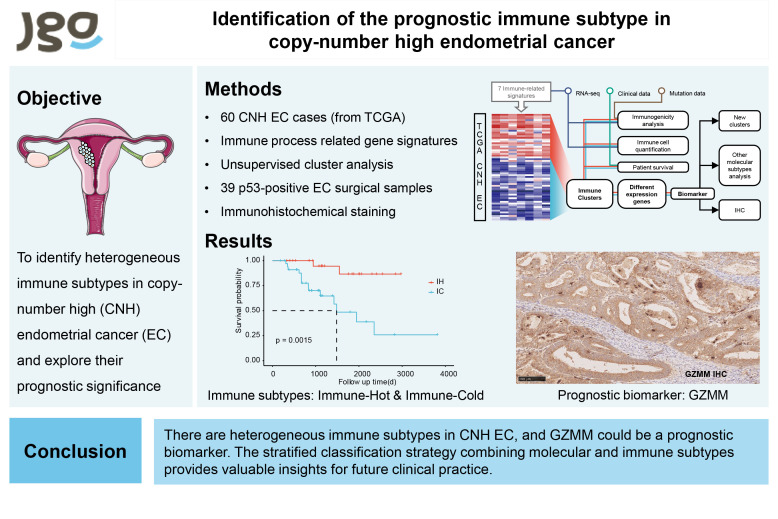
Objective
The TCGA molecular subtype of endometrial cancer (EC) is widely applied, among which the copy-number high (CNH) subtype has the poorest prognosis. However, the heterogeneity of this subtype remains elusive. In this study, we aimed to identify heterogeneous immune subtypes in CNH EC and explore their prognostic significance.
Methods
We collected 60 CNH EC cases in the TCGA database and performed unsupervised cluster analysis based on the enrichment scores of immune-related gene signatures to identify immune subtypes. We described their immune characteristics and prognoses and conducted differential gene analysis and lasso regression to identify a prognostic biomarker, GZMM. For experimental validation, we performed immunohistochemical staining of GZMM in 39 p53-positive EC surgical samples.
Results
We defined two immune subtypes, immune-hot (IH) and immune-cold (IC), which differed in immune cell infiltration, cytokine and chemokine expression and prognosis. The IH subtype has significantly stronger immune activation than the IC subtype, showing a significant infiltration of immune effector cells and high expression of relevant chemokines, with better prognosis. Moreover, the immunohistochemical staining of GZMM in a cohort of 39 p53-positive EC surgical samples confirmed GZMM as a unique prognostic biomarker, with high expression in both tumor cells and lymphocytes predicting a better prognosis.
Conclusion
Our study revealed heterogeneous immune subtypes in CNH EC and identified GZMM as a prognostic biomarker. The stratified classification strategy combining molecular and immune subtypes provides valuable insights for future clinical practice.
Keywords: Endometrial Cancer, Genetic Heterogeneity, Prognostic Factor, Granzyme M
Synopsis
Copy-number high endometrial cancer is heterogeneous and has the worst prognosis. We identified two immune subtypes which are predictive of prognosis. The stratified classification combining molecular and immune subtypes can be a promising strategy and provide a reference for future prognosis assessment and treatment selection.
Graphical Abstract
INTRODUCTION
Endometrial cancer (EC) is a prevalent gynecologic malignancy [1], and primary treatment includes surgery along with comprehensive treatment. However, not all patients benefit from this treatment, highlighting the importance of identifying different types of EC to improve the prognosis.
Over the years, several classification strategies have been proposed for EC. In 1983, Bokhman [2] classified EC into hormone-related and non-hormone-related types based on clinicopathological features and prognosis. In 2014, the World Health Organization proposed a histopathological classification based on the morphology of tumor cells combined with immunohistochemical results. In 2013, The Cancer Genome Atlas (TCGA) proposed a molecular classification strategy, including POLE ultramutated (POLE), microsatellite instability hypermutated (MSI), copy-number low (CNL), and copy-number high (CNH) [3]. Among the four subtypes, the CNH subtype is the most heterogeneous and has the worst prognosis due to extensive somatic copy number variations, low gene mutation rate. However, to our knowledge, there is a lack of research on its heterogeneity, and in-depth exploration will help improve the prognosis of these patients.
Immune cell infiltration has emerged as a potential prognostic factor in cancer treatment [4,5,6,7] and several studies have investigated the immune classification of tumors [8,9,10,11,12]. Bagaev et al. [8] classified pan-cancer into subtypes based on immune-related genes in the tumor microenvironment. Li and Wan [10] defined EC immune subtypes which were different in immune cells, immune regulation, and patient survival. However, the biological behavior of tumor interacts with the immune cells in the tumor microenvironment and the combination of molecular and immune subtypes can achieve precise individualized diagnosis and treatment [13,14,15].
In this study, we aimed to explore the heterogeneity of the CNH subtype of EC by defining two immune subtypes of CNH EC, immune-hot (IH) and immune-cold (IC) subtypes, through unsupervised clustering analysis combining immune-related signatures. The identification of these immune subtypes and their distinct characteristics may contribute to a better understanding of CNH EC’s prognosis and potential implications for personalized treatment strategies.
MATERIALS AND METHODS
1. Data collection and immune-related gene signatures
The study design is displayed in Fig. 1. We obtained the data from the TCGA datasets. The format of fragments per kilobase million (FPKM) gene expression data and clinical information of EC samples were downloaded from GDC Data Portal. Corresponding molecular subtype information were collected using TCGAquery_subtype in TCGABiolinks package. We applied the following steps to preprocess the RNA-seq data to obtain 60 samples of CNH EC: 1) normal tissue sample data were removed; 2) samples without molecular subtype and clinical information were removed; 3) log transformation log2(FPKM+1) was performed.
Fig. 1. Schematic overview of the workflow. RNA-seq data of CNH endometrial cancer from the TCGA database was divided into 2 subtypes based on 7 immune-related signatures. Differences between the subtypes were compared using various methods. Then different expression gene analysis was used to identify prognostic biomarkers.
CNH, copy-number high; EC, endometrial cancer.
We selected 7 immune process related gene signatures for the subsequent analysis, including immune activation [16,17], immune effector cells [18], immune cell infiltration (lymphocyte infiltration, stromal cell infiltration, immune cell infiltration) [19], immune exhaustion (exhausted tumor CD8+ T cells [20], checkpoint molecules [16]) with a total of 420 genes (Table S1).
2. Gene signature enrichment analysis and consensus clustering
The enrichment scores of immune-related gene signatures in each sample were calculated by the GSVA (Gene Set Variation Analysis) package in R. GSVA is an unsupervised nonparametric gene set enrichment method that estimates variation of pathway activity over a sample population [21]. After obtaining the enrichment scores for each sample, we applied the hierarchical clustering algorithm and performed 100 bootstraps each encompassing 80% patients in 60 samples. The final number of clusters was determined based on the cumulative distribution function and the consensus matrix in the consensus clustering (package ConsensusClusterPlus).
3. Assessing cellular and molecular features of immune subtypes
We analyzed the relationship between immune subtypes and immune-related cellular and molecular features. Immunome was used to assess the tumor infiltration immune cells [22]. Immunome contains 28 types of immune cells and is a non-overlapping set of genes representing specific immune cell subpopulations (Table S2), including innate immune cells and adaptive immune cells. Single-sample Gene Set Enrichment Analysis (ssGSEA) was used to calculate the score of different immune cells (package GSVA). Signatures of immune and stromal cell infiltration were derived from ESTIMATE algorithm (package ESTIMATE). The analysis of molecular features mainly included the expression of protumor and antitumor cytokines (Table S3) [8]. We also used ssGSEA to calculate the enrichment scores of molecular features to represent their expression.
4. Tumor mutation analysis
We downloaded the simple nucleotide variation data of 58 patients using GDCquery (package TCGAbiolinks). Tumor mutation load and gene mutations of each sample were obtained using Maftools package in R. We also analyzed and compared the common gene mutations of immune subtypes.
5. Analysis of differential expression genes
Differential gene expression analysis between subtypes was performed using the DESeq2 package, and genes with |Log2FC|>0.25 and p.adj<0.05 were considered as differential expression genes. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses of differential expression genes were performed using the clusterProfiler package to detect the enriched signaling pathways. Subsequently, we performed a single-factor Cox regression analysis for differential expression genes with |Log2FC|>0.5 to screen for genes significantly associated with prognosis.
6. Immunohistochemical staining
We collected 39 EC patients whose postoperative pathology showed TP53 positive in Peking Union Medical College Hospital from January 2015 to December 2020 (Table S4). The follow-up time ended in November 2022. Sections of 4-μm thickness were placed on amino-propyl-tri-ethoxy-silane-coated slides for subsequent IHC with anti-human GZMM (MK43142, Ab-mart). The secondary antibodies were HRP-linked caprine anti-mouse IgG monoclonal antibodies. To evaluate GZMM expression from IHC, the semi-quantitative immunoreactive score was used. The staining intensity determined the score (loss=0, weak=1, moderate=2, strong=3). Two pathologists were blinded to clinical data and independently determined the semi-quantitative immunoreactive score to evaluate the immunostaining.
7. Statistical analysis
The chi-square test was used to check associations between two categorical variables with more than two categories. The Mann-Whitney U test was used for statistical analysis comparing non-categorical variables. Kaplan-Meier was used for survival analysis, and survival differences between clusters were compared using the Log-rank test. In the box plots, the upper whisker indicates the maximum value or 75th percentile +1.5 interquartile range (IQR); the lower whisker indicates the minimum value or 25th percentile 1.5 IQR. Unless otherwise stated, two-sided p-values less than 0.05 were considered statistically significant (ns p≥0.05, *p<0.05, **p<0.01, ***p<0.001). All the statistical analysis and visualization were performed using R (R Foundation, Vienna, Austria).
RESULTS
1. Immune subtypes in CNH EC
We obtained the RNA-seq data and clinical information of 60 CNH EC samples from the TCGA database. In these samples, the median age was 68, most of which were stage 1, G3, and serous carcinoma. A consensus cluster of the 60 samples was performed based on the GSVA score of the 7 immune-related gene signatures. The most stable number of clusters was observed to occur at k=2 (Fig. S1A). Therefore, we divided the samples into two immune subtypes, named IH and IC subtypes (Fig. 2A and B), which contained 41.7% and 58.3% of all samples. The two subtypes differed significantly in the expression of immune-related gene signatures. IH showed a reactive immunogenic pattern characterized by elevated expression of most immune-related gene signatures, especially checkpoint molecules, immune cell infiltration, immune activation genes, and lymphocyte infiltration and effector cells. IC showed an immune dessert pattern characterized by low expression of immune-related signatures. We compared the two subtypes regarding clinical features, including age, stage, and grading. The IH subtype patients were younger, had a low proportion of advanced patients, and consisted mainly of low-grade (age: p=0.200; stage: p=0.300; grade: p=0.040, Fig. S1B). In addition, we analyzed the prognostic value of the immune subtype and found that the prognosis of the IH subtype was significantly better than that of the IC subtype (p=0.002, Fig. 2C). We then examined the prognostic value of the immune subtype using a multi-factor Cox regression analysis, taking into account age and tumor stage. We found that the survival difference between the two subtypes was independent of these clinical factors, indicating that the immune subtype was a significant prognostic factor (hazard ratio [HR]=11.4; 95% confidence interval [CI] 2.3–56.7; p=0.003; Fig. 2D).
Fig. 2. Immune subtypes defined by immune-related signatures. (A) Unsupervised clustering analysis defined two immune subtypes based on GSVA scores. (B) Violin plot showing the difference between the GSVA scores of immune-related signatures. (C) Kaplan–Meier curves for overall survival. (D) Forest plot illustrating estimates of multivariable logistic regression analysis and the 95% CI.
CI, confidence interval; GSVA, Gene Set Variation Analysis; HR, hazard ratio; IC, immune-cold; IH, immune-hot.
2. The immune cells and molecular features of immune subtypes
We evaluated the infiltration abundance of 28 immune cells in each sample to systematically characterize the tumor microenvironment of the immune subtype (Fig. 3A). The immune cell infiltration was significantly higher in IH. IH was not only enriched in anti-tumor immune cells, but also in pro-tumor immune cells, indicating an immune active status. In contrast, IC was only slightly higher than IH on some pro-tumor immune cells, and there was no significant difference. We used ESTIMATE to estimate the overall level of tumor stroma cells and immune cells and found that the overall level of tumor immune infiltrating cells was significantly higher in IH than in IC (Fig. 3B). We then compared the expression of antitumor and protumor cytokines in the two subtypes to assess whether it was consistent with immune cell infiltration (Fig. 3C and D). We found that IH had higher expression of antitumor cytokines, representing immunoreactive and higher cytotoxic T cell infiltration. Protumor cytokine expression in IH was also slightly higher than in IC, consistent with its enrichment in pro-tumor immune cells, but there was no significant difference (p=0.085).
Fig. 3. Immune cells and molecular features associated with immune subtypes. (A) Violin plot comparing immune cell infiltration in two immune subtypes by ssGSEA scores. (B) Immune scores and stromal scores calculated by ESTIMATE. (C) Heatmap showing expression of each antitumor and protumor cytokine. (D) Enrichment scores of antitumor and protumor cytokines calculated by ssGSEA algorithm.
IC, immune-cold; IH, immune-hot; IL, interleukin; ssGSEA, Single-sample Gene Set Enrichment Analysis; TGF, transforming growth factor; TNF, tumor necrosis factor.
3. Tumor immunogenicity in different immune subtypes
The immunogenicity of tumors is the ability to induce adaptive immune responses [23] and can be represented by the cytolytic activity, which can be estimated using the expression of GZMA and PRF1, as this is the ultimate effector mechanism in the cancer immunity [24]. We found that the immunogenicity of IH was much higher than that of IC (Fig. 4A). The immunogenicity is mainly influenced by tumor-intrinsic factors and tumor-extrinsic factors [17]. Tumor-intrinsic factors include the tumor mutational burden (TMB) and the expression levels of MHC, co-inhibitory, and co-stimulatory molecules. Tumor-extrinsic factors include tumor immune cell infiltration, immunomodulatory cytokines, and chemokines that regulate T cell traffic.
Fig. 4. Tumor immunogenicity comparison. (A) Violin plot showing GZMA and PRF1 expression. (B, C) Tumor mutation burden and common mutations in two immune subtypes. (D) Expression of immunomodulatory molecules and MHC for each subtype. (E) Enrichment scores of chemokines that regulate cell traffic calculated by ssGSEA algorithm.
IC, immune-cold; IH, immune-hot; ssGSEA, Single-sample Gene Set Enrichment Analysis; TMB, tumor mutational burden.
First, we evaluated tumor-intrinsic factors. We compared the TMB of the two subtypes, and there was no significant difference in TMB (p=0.160; Fig. 4B). We also showed common mutations in the CNH samples, and the most common mutations included TP53 (93%), PIK3CA (48%), FBXW7 (22%), and PPP2R1A (21%) (Fig. 4C). Genomic mutations frequently found were not consistently associated with the specific immune subtypes, as there was no significant difference between the two immune subtypes. Subsequent analysis of the expression levels of MHC, co-inhibitory and co-stimulatory molecules also showed a similarity between IH and IC (Fig. 4D). These results suggest that there was no significant difference in tumor-intrinsic factors of the immune subtype.
The above results illustrated that the infiltration abundance of tumor-infiltrating immune cells and the expression of tumor-associated cytokines differed between the two subtypes, each with its own unique pattern. We then evaluated chemokines that regulate cell traffic (Fig. 4E). We found that IH showed high expression of relevant chemokines, consistent with the immune cell enrichment of IH. These results indicate that tumor-extrinsic factors of the immune subtype are the main determinants of immunogenicity.
4. Identification of subtype-related differentially expressed genes
We analyzed the differentially expressed genes of the two subtypes and identified 202 differentially expressed genes, which were mostly up-regulated in IH (Fig. S2A). The differentially expressed genes included many immune-related genes, such as NKG7, CCL5, GZMA, which further illustrated the differences in the immune microenvironment of the two subtypes. Subsequently, we performed KEGG and GO enrichment analysis to elucidate the functional roles of differentially expressed genes. The results of KEGG enrichment analysis showed that genes were enriched in immune-related pathways, such as cytokine-cytokine receptor interaction, natural killer cell mediated cytotoxicity (Fig. S2B). The results of GO enrichment analysis also confirmed the enrichment in immune-related pathways. Biological process is enriched in leukocyte mediated immunity, regulation of T cell activation. Cellular component is concentrated in secretory granule membrane. Molecular function is enriched on immune receptor activity, cytokine receptor activity (Fig. S2C).
5. Key genes differed in immune subtypes
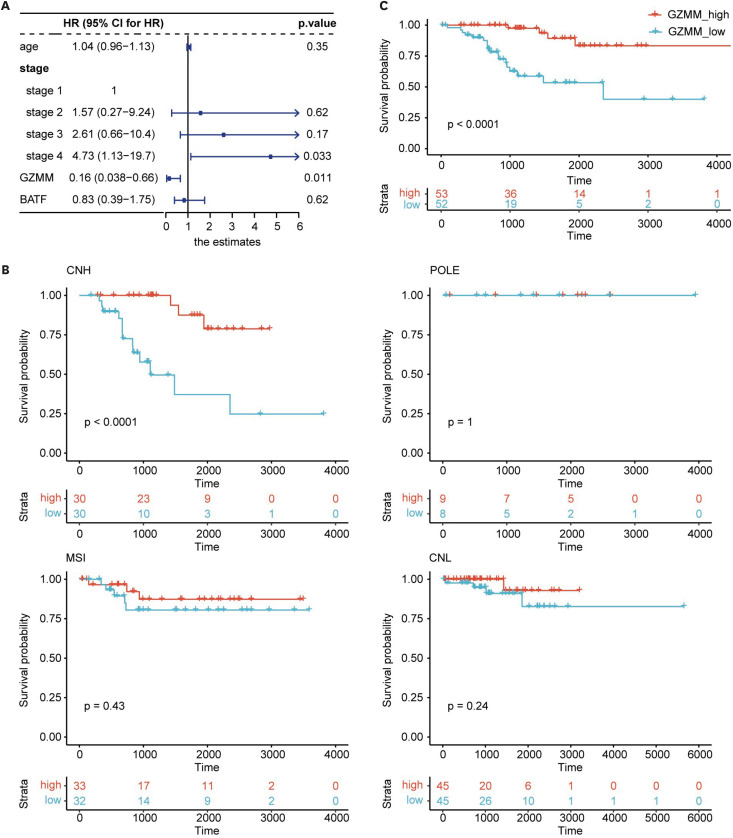
We employed lasso regression analysis to screen for key differentially expressed genes to simplify the classification process and discover prognosis-related biomarkers. Following this analysis, we obtained two genes with prognostic predictive value, namely BATF and GZMM. Next, we constructed a multi-factor Cox regression analysis model using BATF and GZMM combined with other factors. The p-value of GZMM in the model was found to be less than 0.05 (HR=0.160; 95% CI=0.038–0.66; p=0.011; Fig. 5A), indicating that GZMM was an independent predictor of prognosis. To validate the classification effect of GZMM as a single factor, we used the median GZMM expression to divide the samples into GZMM-high and GZMM-low groups. Most samples from the IH group were classified in the GZMM-high group. We performed a survival analysis and found that the level of GZMM expression could serve as an indicator of the prognosis (p<0.001; Fig. 5B).
Fig. 5. Prognostic value of GZMM. (A) Forest plot illustrating estimates of multivariable logistic regression analysis containing GZMM and BATF, and the 95% CI. (B) Kaplan–Meier curves for OS stratified by GZMM expression in different molecular subtypes. (C) Kaplan–Meier curves for OS stratified by GZMM expression in TP53mut samples.
CI, confidence interval; CNH, copy-number high; CNL, copy-number low; HR, hazard ratio; OS, overall survival.
We further attempted to group other molecular subtype samples using GZMM. The preprocessing steps for other samples were consistent with those for CNH samples. We obtained 17 POLE samples, 65 MSI-H samples, and 90 CNL samples. We found that the prognosis of other molecular subtype samples could not be determined using GZMM (Fig. 5B), highlighting the unique role of GZMM in CNH classification.
As the molecular subtype evolves, IHC of p53 protein is increasingly being used in clinical practice instead of copy number variation analysis. P53 protein IHC is an alternative indicator for TP53 mutation analysis, so we analyzed samples with TP53 mutations in TCGA-UCEC. After removing samples without TP53 mutations and those with TP53 nonsense mutations, we obtained 105 samples with TP53 mutations. The prognosis of groups with high and low GZMM expression was significantly different (p<0.001, Fig. 5C), providing a foundation for the clinical application of GZMM in classifying CNH EC.
6. Immunohistochemical staining of GZMM in p53-positive EC
We next analyzed the expression of GZMM by IHC in 39 p53-positive surgical samples of EC, and matched it to its adjacent tumor stroma. The median follow-up was 24 months, and 7 of the 39 samples died. The results showed that GZMM was expressed in both tumor cells and infiltration lymphocytes, while the expression of GZMM in the tumor cells and lymphocytes of dead samples was low (Fig. 6A). To evaluate the prognostic value of GZMM, we grouped samples according to the IHC score in tumor cells and lymphocytes. Scores of 0 and 1 were grouped as low expressions, and scores of 2 and 3 were grouped as high expressions. We found that the results were consistent with the analysis of the TCGA database. Higher expression of GZMM in both tumor cells and lymphocytes predicted a better prognosis, but the difference between the two groups was not significant in tumor cells (p=0.170), whereas it was significantly different in lymphocytes (p=0.040) (Fig. 6B).
Fig. 6. IHC of GZMM in TP53mut endometrial cancer. (A). Representative IHC images of GZMM in samples with different prognoses. (B). Kaplan–Meier curves for overall survival stratified by GZMM expression in tumor cells and in lymphocytes.
DISCUSSION
CNH EC is considered the most aggressive and heterogeneous subtype. The current classification of EC neglects immune-related components in the tumor microenvironment, which plays an essential role in tumor progression [25]. To address this, we proposed a new immune subtype classification for CNH EC patients. To our knowledge, this is the first immune subtype study of CNH EC.
Through unsupervised clustering analysis, we identified two immune subtypes, IH and IC, with IH exhibiting a reactive immunogenic pattern and demonstrating significantly better prognosis than IC. Our findings align with previous studies validating the prognostic value of the immune subtype in different tumors [9,19,26,27]. Interestingly, our analysis revealed the main determinants of immunogenicity were tumor-extrinsic factors, contributing to the heterogeneity in CNH EC immunogenicity. IH demonstrated evident anti-tumor immune cell infiltration, along with consistent expression of related cytokines and chemokines. This may explain why IH had a better prognosis than IC. IH also showed the enrichment of pro-tumor immune cells and high expression of pro-tumor cytokines. Previous studies have reported varying phenotypes across immune subtypes. Some studies have demonstrated that higher pro-tumor immune factors are often accompanied by lower anti-tumor immune factors and are associated with poor prognosis [17,28]. Other studies, like ours, have shown high expression levels of anti-tumor and pro-tumor immune factors and demonstrated better prognosis [29,30]. This contradictory immune phenotype may be associated with the different immune activation status of these subtypes. The above data indicate that different EC molecular subtypes have significant differences in tumor-intrinsic factors, while the heterogeneity of the internal immunogenicity of CNH EC mainly comes from tumor-extrinsic factors. This highlights the potential for stratified classification strategies, combining molecular subtyping with immunological features for improved prognosis prediction.
Moreover, our study offers valuable insights into the future of immunotherapy in EC by revealing distinctive characteristics of the IH and IC subtypes. Previous studies have demonstrated that only a subset of patients can benefit from the immunotherapy [31]. Pembrolizumab as a monotherapy has been adopted to second-line treatment for advanced dMMR or MSI-H patients. Pembrolizumab plus lenvatinib has shown exhibited favorable therapeutic outcomes in advanced EC [32], and it has been approved for advanced pMMR EC patients. Recent research by Eskander et al. [33] and Mirza et al. [34] has shown promising therapeutic efficacy with the combination of chemotherapy and immunotherapy in the first-line treatment of advanced cancer, particularly in specific subgroups like dMMR or MSI-H tumors. These findings underscore the potential benefits of immunotherapy, but the challenge of selecting the most appropriate treatment regimen for individual patients remains unresolved. Although our study did not directly incorporate these contemporary treatments, identifying the IH-subtype holds significant clinical implications. IH exhibited IH features. Immunotherapy may enhance its anti-tumor immune response. The monotherapy with immunotherapy may potentially yield favorable outcomes, while further escalation with additional chemotherapy may lead to exacerbated adverse effects. In contrast, IC displayed IC features, indicating that immunotherapy alone may not be sufficient to stimulate an immune response. Combination therapy, such as targeted therapy or chemotherapy, may be needed to achieve better therapeutic outcomes.
In our study, we identified GZMM as a prognostic biomarker. Our GZMM subgroup analysis in TP53 mutated patients further indicated its potential clinical application. The IHC results showed that GZMM expression in tumor-infiltrating lymphocytes might serve as a potential indicator of good prognosis for patients. We hypothesized that this might be associated with an enhanced killing function of lymphocytes, leading to a better prognosis. GZMM is a unique class of granzymes and may play a role in innate immunity [35]. Some investigators believe that GZMM may induce a specific type of perforin-dependent cell death [36], while others have shown its potential to promote inflammation [37]. Since its controversial cytotoxic potential and mechanism of action, limited research has been conducted on its role in tumor immunity, and its specific function in tumor immunity is still not fully understood and has not yet reached a consensus [38,39]. It is necessary to confirm our findings through further research.
There are several limitations of this study. First, given the rarity of the CNH subtype, our sample size was small, including only 60 patients from the TCGA database and 39 samples for IHC. Second, as this was a retrospective study, we only analyzed RNA-seq data from the TCGA database, without independent RNA-seq data to validate the immune subtype. Thirdly, we did not explore the underlying mechanism of GZMM.
In summary, our study used transcriptomes to provide a detailed characterization of the heterogeneity of CNH EC. We proposed a stratified classification strategy that revealed two immune subtypes with distinct immune characteristics and prognosis. Furthermore, we identified the prognostic biomarker GZMM and validated it in independent clinical samples. Our research provides theoretical support for the future development of clinical diagnosis and treatment strategies.
ACKNOWLEDGEMENTS
We are thankful to the TCGA for providing the data analyzed in this study.
Footnotes
Funding: The funding for this research, including experimental expenses and publication fees, was supported by grants from Beijing Natural Science Foundation (No. 7222126), National Natural Science Foundation of China (No. 82071760), and the Capital's Funds for Health Improvement and Research (No. 2022-1-4011).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: M.M., J.F.
- Investigation: H.R.
- Funding acquisition: X.Y.
- Methodology: M.M., J.F.
- Resources: H.R.
- Software: M.M.
- Supervision: X.Y., J.F.
- Validation: M.M.
- Writing - original draft: M.M.
- Writing - review & editing: X.Y., J.F.
SUPPLEMENTARY MATERIALS
7 immune process related gene signatures
Immunome contains 28 types of immune cells
Protumour and antitumour cytokines
Characteristics of the patients enrolled for IHC
Immune subtypes defined by immune-related signatures. (A) The CDF curve and CDF delta area curve of TCGA-UCEC cohort samples. (B) The proportion of age, grade and stage in different immune subtypes.
Differentially expressed gene analysis. (A) Volcano plot showing DEGs. (B) Gene Ontology enrichment analysis of DEGs. (C) Kyoto Encyclopedia of Genes and Genomes enrichment analysis of DEGs.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 5.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Čermáková P, Melichar B, Tomšová M, Zoul Z, Kalábová H, Spaček J, et al. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in patients with endometrial carcinoma. Anticancer Res. 2014;34:5555–5561. [PubMed] [Google Scholar]
- 7.Guo F, Dong Y, Tan Q, Kong J, Yu B. Tissue infiltrating immune cells as prognostic biomarkers in endometrial cancer: a meta-analysis. Dis Markers. 2020;2020:1805764. doi: 10.1155/2020/1805764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39:845–865.e7. doi: 10.1016/j.ccell.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Liang L, Zhu Y, Li J, Zeng J, Yuan G, Wu L. Immune subtypes and immune landscape analysis of endometrial carcinoma. J Immunol. 2022;209:1606–1614. doi: 10.4049/jimmunol.2200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li BL, Wan XP. Prognostic significance of immune landscape in tumour microenvironment of endometrial cancer. J Cell Mol Med. 2020;24:7767–7777. doi: 10.1111/jcmm.15408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H, Feng PH, Yu SN, Lu ZH, Yu Q, Chen J. Identification and validation of TNFRSF4 as a high-profile biomarker for prognosis and immunomodulation in endometrial carcinoma. BMC Cancer. 2022;22:543. doi: 10.1186/s12885-022-09654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Sun L, Zhang J, Song W, Li M, Wang H. The landscape and prognostic value of immune characteristics in uterine corpus endometrial cancer. Biosci Rep. 2021;41:1–9. doi: 10.1042/BSR20202321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talhouk A, Derocher H, Schmidt P, Leung S, Milne K, Gilks CB, et al. Molecular subtype not immune response drives outcomes in endometrial carcinoma. Clin Cancer Res. 2019;25:2537–2548. doi: 10.1158/1078-0432.CCR-18-3241. [DOI] [PubMed] [Google Scholar]
- 14.Mullen MM, Mutch DG. Endometrial tumor immune response: predictive biomarker of response to immunotherapy. Clin Cancer Res. 2019;25:2366–2368. doi: 10.1158/1078-0432.CCR-18-4122. [DOI] [PubMed] [Google Scholar]
- 15.Horeweg N, de Bruyn M, Nout RA, Stelloo E, Kedziersza K, León-Castillo A, et al. Prognostic integrated image-based immune and molecular profiling in early-stage endometrial cancer. Cancer Immunol Res. 2020;8:1508–1519. doi: 10.1158/2326-6066.CIR-20-0149. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Wang D, Sun M, Liu X, Yang Q. Identification of prognostic and immune-related gene signatures in the tumor microenvironment of endometrial cancer. Int Immunopharmacol. 2020;88:106931. doi: 10.1016/j.intimp.2020.106931. [DOI] [PubMed] [Google Scholar]
- 17.Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Reports. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Milner JJ, Toma C, He Z, Kurd NS, Nguyen QP, McDonald B, et al. Heterogenous populations of tissue-resident CD8+ T cells are generated in response to infection and malignancy. Immunity. 2020;52:808–824.e7. doi: 10.1016/j.immuni.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie P, An R, Yu S, He J, Zhang H. A novel immune subtype classification of ER-positive, PR-negative and HER2-negative breast cancer based on the genomic and transcriptomic landscape. J Transl Med. 2021;19:398. doi: 10.1186/s12967-021-03076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 21.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12:307–313. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahoo SS, Zhang XD, Hondermarck H, Tanwar PS. The emerging role of the microenvironment in endometrial cancer. Cancers (Basel) 2018;10:408. doi: 10.3390/cancers10110408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye S, Li Q, Wu Y, Jiang W, Zhou S, Zhou X, et al. Integrative genomic and transcriptomic analysis reveals immune subtypes and prognostic markers in ovarian clear cell carcinoma. Br J Cancer. 2022;126:1215–1223. doi: 10.1038/s41416-022-01705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekpli X, Lien T, Røssevold AH, Nebdal D, Borgen E, Ohnstad HO, et al. OSBREAC. An independent poor-prognosis subtype of breast cancer defined by a distinct tumor immune microenvironment. Nat Commun. 2019;10:5499. doi: 10.1038/s41467-019-13329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Chen X, Jiang Y, Cheng W. Development of an immune gene prognostic classifier for survival prediction and respond to immunocheckpoint inhibitor therapy/chemotherapy in endometrial cancer. Int Immunopharmacol. 2020;86:106735. doi: 10.1016/j.intimp.2020.106735. [DOI] [PubMed] [Google Scholar]
- 29.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng M. Immune landscape of distinct subtypes in urothelial carcinoma based on immune gene profile. Front Immunol. 2022;13:970885. doi: 10.3389/fimmu.2022.970885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 32.Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial Cancer. N Engl J Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388:2159–2170. doi: 10.1056/NEJMoa2302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novák Z, Black D, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388:2145–2158. doi: 10.1056/NEJMoa2216334. [DOI] [PubMed] [Google Scholar]
- 35.Joeckel LT, Bird PI. Are all granzymes cytotoxic in vivo? Biol Chem. 2014;395:181–202. doi: 10.1515/hsz-2013-0238. [DOI] [PubMed] [Google Scholar]
- 36.Kelly JM, Waterhouse NJ, Cretney E, Browne KA, Ellis S, Trapani JA, et al. Granzyme M mediates a novel form of perforin-dependent cell death. J Biol Chem. 2004;279:22236–22242. doi: 10.1074/jbc.M401670200. [DOI] [PubMed] [Google Scholar]
- 37.Anthony DA, Andrews DM, Chow M, Watt SV, House C, Akira S, et al. A role for granzyme M in TLR4-driven inflammation and endotoxicosis. J Immunol. 2010;185:1794–1803. doi: 10.4049/jimmunol.1000430. [DOI] [PubMed] [Google Scholar]
- 38.Ye Q, Han X, Wu Z. Bioinformatics analysis to screen key prognostic genes in the breast cancer tumor microenvironment. Bioengineered. 2020;11:1280–1300. doi: 10.1080/21655979.2020.1840731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Sun Q, Wu Y, Wang L, Zhou C, Ma W, et al. Granzyme M expressed by tumor cells promotes chemoresistance and EMT in vitro and metastasis in vivo associated with STAT3 activation. Oncotarget. 2015;6:5818–5831. doi: 10.18632/oncotarget.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
7 immune process related gene signatures
Immunome contains 28 types of immune cells
Protumour and antitumour cytokines
Characteristics of the patients enrolled for IHC
Immune subtypes defined by immune-related signatures. (A) The CDF curve and CDF delta area curve of TCGA-UCEC cohort samples. (B) The proportion of age, grade and stage in different immune subtypes.
Differentially expressed gene analysis. (A) Volcano plot showing DEGs. (B) Gene Ontology enrichment analysis of DEGs. (C) Kyoto Encyclopedia of Genes and Genomes enrichment analysis of DEGs.