Abstract
Based on phylogenetic analyses of a multi-gene matrix of nuITS-LSU rDNA, RPB2 and TUB2 sequences and morphology, xylariaceous species with uni- to pauciperitheciate stromata and ascospores having a spirally coiling (helicoid) germ slit are revised and reclassified, including detailed descriptions and illustrations. The genus Helicogermslita is redefined and restricted to seven species with massive, erumpent, clypeus-like carbonaceous stromata, and Rosellinia somala is combined in Helicogermslita. Within the core Xylariaceae, the poorly known Leptomassaria simplex is shown to be closely related to Anthostoma insidiosum, for which the new genus Oligostoma is established, and Anthostoma rhenanum is demonstrated to be synonymous with O. insidiosum. The new genus Albicollum, characterised by immersed ascomata and a collar of white pseudostromatic tissues surrounding the ostioles, is established for Amphisphaeria canicollis, Anthostoma chionostomum, Sordaria (= Helicogermslita) fleischhakii and Anthostoma vincensii. Anthostoma ostropoides is synomymised with Albicollum canicolle, and Al. berberidicola, Al. longisporum and Al. novomexicanum are described as new species. Rosellinia (= Helicogermslita) gaudefroyi is transferred to the new genus Spiririma. Anthostoma amoenum and Euepixylon udum, both with a poroid germ locus, are shown to be only distantly related, and An. amoenum is reclassified within the asexual genus Digitodochium. Based on phylogeny, the genus Euepixylon is treated as a synonym of Nemania. A new species, Nemania ethancrensonii, which is closely related to the two formerly accepted Euepixylon species (E. sphaeriostomum, E. udum) but strongly deviates from the morphological concept of Euepixylon and Nemania, is described from the eastern USA. The genera Anthostomelloides, Clypeosphaeria, Digitodochium, Emarcaea, Induratia, Linosporopsis, Magnostiolata, Occultitheca and Spiririma are revealed to form a morphologically heterogeneous lineage in a basal position of Xylariaceae. Anthostoma vincensii, Quaternaria simplex and Rosellinia gaudefroyi are lectotypified, and Amphisphaeria canicollis, Anthostoma amoenum, An. rhenanum, An. vincensii, Quaternaria simplex, Rosellinia gaudefroyi and Valsa insidiosa are epitypified. Keys to uni- to pauciperitheciate xylariaceous genera with sigmoid to helicoid germ slits and to species of Albicollum are provided.
Citation: Voglmayr H, Tello S, Jaklitsch WM, et al. 2022. About spirals and pores: Xylariaceae with remarkable germ loci. Persoonia 49: 58–98. https://doi.org/10.3767/persoonia.2022.49.02.
Keywords: Anthostoma, Ascomycota, Helicogermslita, molecular phylogeny, new taxa, systematics, Xylariales
INTRODUCTION
Xylariaceae are well-known for their large, multiperitheciate stromata, but there are also numerous, little-known species with uni- to pauciperitheciate stromata. Recently, the classical concept of the family has been shown to be phylogenetically heterogeneous, and the Hypoxylaceae were segregated and re-established as a distinct family for Hypoxylon, Daldinia and their allies (Wendt et al. 2018). In recent years, substantial progress has been achieved in classification and documentation of generic and species diversity of Hypoxylaceae by a combination of multigene phylogenies, morphology, pure culture studies, and secondary metabolite chemistry (e.g., Kuhnert et al. 2014, 2015, 2017, Stadler et al. 2014, Sir et al. 2016a, 2016b, 2019, Wendt et al. 2018, Lambert et al. 2019, 2021, Wongkanoun et al. 2019, 2020, Cedeño-Sanchez et al. 2020, Pourmoghaddam et al. 2020).
The Xylariaceae s.str. have remained much less studied, although containing a substantial number of described as well as undescribed species occupying various ecological niches ranging from saprotrophs, coprophiles, pyrophiles, pathogens, endophytes to insect symbionts. Molecular phylogenetic studies mainly focussed on the large and prominent genus Xylaria, which in its classical morphological circumscription was revealed as polyphyletic (Hsieh et al. 2010, Konta et al. 2020, Samarakoon et al. 2022). Comparatively few species from other genera with large stromata have been included in multi-gene phylogenies, and the diversity of Xylariaceae s.str. remains incompletely sampled. As an additional complication, morphological characters used for traditional generic circumscriptions like stroma morphology commonly do not reflect phylogenetic relationships. This is particularly evident in the large genera Rosellinia and Xylaria which are revealed as para- or polyphyletic in phylogenetic analyses (e.g., Wendt et al. 2018, Wittstein et al. 2020). Thus, although numerous new species and genera have been described in recent years within Xylariaceae s.str. (e.g., Tibpromma et al. 2017, Samarakoon et al. 2020, 2022, Pi et al. 2021) and phylogentic resolution has much improved in the course of multi-gene phylogenies, much still needs to be done towards a comprehensive phylogenetic generic classification, in particular by inclusion of type species of genera that have not yet been sequenced.
Many taxonomists in literature focused on conspicuous large stromata and less so on Xylariaceae with small, reduced and commonly immersed uni- to pauciperitheciate stromata, which, however, contain a substantial species and genus diversity (Daranagama et al. 2016). One of these little-investigated genera is Helicogermslita, which currently contains 10 species (Index Fungorum http://www.indexfungorum.org/Names/Names.asp, accessed 4 Apr. 2022) characterised by brown ascospores with a helicoid germ slit, which, however, is morphologically heterogeneous. Several fresh collections resembling Helicogermslita have been recently collected by us, which led us to initiate a study on Xylariaceae with spirally coiling (helicoid) germ slits, including the generic type of the genus Leptomassaria. During these investigations, several little-known species described and placed in the diatrypaceous genus Anthostoma turned out to be of particular interest. While Anthostoma has been reduced to the generic type species, An. decipiens (Rappaz 1992, Jaklitsch et al. 2014), numerous Anthostoma species await an appropriate generic re-classification, amongst which are An. amoenum, An. insidiosum, An. ostropoides, An. rhenanum and An. vincensii. Therefore, the aim of our study was to provide an improved classification of these species and genera, based on molecular phylogenetic, pure culture and morphological studies of recent collections as well as type studies.
MATERIALS AND METHODS
Sample sources
All isolates included in this study originated from ascospores of freshly collected specimens. Details of the strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the Westerdijk Fungal Biodiversity Centre (CBS-KNAW), Utrecht, The Netherlands. Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. In addition, the following collection of Euepixylon udum (syn. Nemania uda), the generic type species of Euepixylon, was isolated in pure culture and sequenced: Austria, Oberösterreich, St. Willibald, Große Sallet S of B129, N48°21'14.2" E13°42'30.5", on dead twig of Quercus robur, 22 Feb. 2020, H. Voglmayr (WU-MYC 0040046, culture EUU = CBS 148422). Fungarium acronyms are according to Thiers (2021), and citation of exsiccata follows Triebel & Scholz (2021). Specimens have been deposited in the Fungarium of the Department of Botany and Biodiversity Research, University of Vienna (WU).
Table 1.
Isolates and accession numbers used in the phylogenetic analyses. Isolates/sequences in bold were isolated/sequenced in the present study.
| Species | Specimen or strain number1 | Origin | Status2 | GenBank accession numbers3 | References | |||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | TUB2 | |||||
| Albicollum berberidicola | HEV4C | Spain | ON869276 | ON869276 | ON808456 | ON808499 | This study | |
| HEV19 | Spain | ON869277 | ON869277 | N/A | ON808500 | This study | ||
| HG = CBS 147392 | Greece | HT | ON869278 | ON869278 | ON808457 | ON808501 | This study | |
| Albicollum canicolle | HEF = CBS 147281 | Spain | ET | ON869279 | ON869279 | ON808458 | ON808502 | This study |
| HEV | Spain | ON869280 | ON869280 | ON808459 | ON808503 | This study | ||
| HEV8 | Spain | ON869281 | ON869281 | ON808460 | ON808504 | This study | ||
| HEV9 | Spain | ON869282 | ON869282 | ON808461 | ON808505 | This study | ||
| HEV11 | Spain | ON869283 | ON869283 | ON808462 | ON808506 | This study | ||
| HEV13 | Spain | ON869284 | ON869284 | ON808463 | ON808507 | This study | ||
| HEV18 | Spain | ON869285 | ON869285 | ON808464 | ON808508 | This study | ||
| Albicollum longisporum | HEV3 = CBS 147283 | Spain | HT | ON869286 | ON869286 | ON808465 | ON808509 | This study |
| HEV4 | Spain | ON869287 | ON869287 | ON808466 | ON808510 | This study | ||
| HEV5 | Spain | ON869288 | ON869288 | ON808467 | ON808511 | This study | ||
| HEV6 | Spain | ON869289 | ON869289 | ON808468 | ON808512 | This study | ||
| HEV7 | Spain | ON869290 | ON869290 | ON808469 | ON808513 | This study | ||
| HEV10 | Spain | ON869291 | ON869291 | ON808470 | ON808514 | This study | ||
| HEV14 | Spain | ON869292 | ON869292 | ON808471 | ON808515 | This study | ||
| HEV15 | Spain | ON869293 | ON869293 | ON808472 | ON808516 | This study | ||
| HEV16 | Spain | ON869294 | ON869294 | ON808473 | ON808517 | This study | ||
| HEV17 | Spain | ON869295 | ON869295 | ON808474 | ON808518 | This study | ||
| Albicollum novomexicanum | HB6019b | USA | HT | ON869296 | ON869296 | N/A | N/A | This study |
| Albicollum vincensii | ARQ = CBS 147286 | Austria | ET | ON869297 | ON869297 | ON808475 | ON808519 | This study |
| HEV1 | Spain | ON869298 | ON869298 | ON808476 | ON808520 | This study | ||
| HEV2 | Spain | ON869299 | ON869299 | ON808477 | ON808521 | This study | ||
| HEV12 | Spain | ON869300 | ON869300 | ON808478 | ON808522 | This study | ||
| RQ1 | Italy | ON869301 | ON869301 | ON808479 | ON808523 | This study | ||
| Amphirosellinia fushanensis | HAST 91111209 | Taiwan | HT | GU339496 | N/A | GQ848339 | GQ495950 | Hsieh et al. (2010) |
| Amphirosellinia nigrospora | HAST 91092308 | Taiwan | HT | GU322457 | N/A | GQ848340 | GQ495951 | Hsieh et al. (2010) |
| Annulohypoxylon truncatum | CBS 140778 | Texas | ET | KY610419 | KY610419 | KY624277 | KX376352 | Kuhnert et al. (2017), Wendt et al. (2018) |
| Anthostomelloides krabiensis | MFLUCC 15–0678 | Thailand | HT | KX305927 | KX305928 | KX305929 | N/A | Tibpromma et al. (2017) |
| Astrocystis concavispora | MFLUCC 14–0174 | Italy | KP297404 | KP340545 | KP340532 | KP406615 | Daranagama et al. (2015) | |
| Barrmaelia rhamnicola | CBS 142772 | France | ET | MF488990 | MF488990 | MF488999 | MF489018 | Voglmayr et al. (2018) |
| Biscogniauxia marginata | MFLUCC 12–0740 | France | KJ958407 | KJ958408 | KJ958409 | KJ958406 | Daranagama et al. (2015) | |
| Camillea obularia | ATCC 28093 | Puerto Rico | KY610384 | KY610429 | KY624238 | KX271243 | Wendt et al. (2018) | |
| Clypeosphaeria mamillana | CBS 140735 | France | ET | KT949897 | KT949897 | MF489001 | MH704637 | Jaklitsch et al. (2016), Voglmayr et al. (2018), Liu et al. (2019) |
| Collodiscula bambusae | GZUH 0102 | China | KP054279 | KP054280 | KP276675 | KP276674 | Li et al. (2015) | |
| Collodiscula fangjingshanensis | GZUH 0109 | China | HT | KR002590 | KR002591 | KR002592 | KR002589 | Li et al. (2015) |
| Collodiscula japonica | CBS 124266 | China | JF440974 | JF440974 | KY624273 | KY624316 | Jaklitsch & Voglmayr (2012), Wendt et al. (2018) | |
| Creosphaeria sassafras | ST.MA. 14087 | Argentina | KY610411 | KY610468 | KY624265 | KX271258 | Wendt et al. (2018) | |
| Daldinia concentrica | CBS 113277 | Germany | AY616683 | KY610434 | KY624243 | KC977274 | Triebel et al. (2005), Kuhnert et al. (2014), Wendt et al. (2018) | |
| Dematophora buxi | 99 J.D.R. | France | GU300070 | N/A | GQ844780 | GQ470228 | Hsieh et al. (2010) | |
| Dematophora necatrix | CBS 349.36 | Argentina | AY909001 | KF719204 | KY624275 | KY624310 | Pelaez et al. (2008), Wendt et al. (2018) | |
| Diatrype disciformis | CBS 197.49 | Netherlands | N/A | DQ470964 | DQ470915 | N/A | Zhang et al. (2006) | |
| Digitodochium amoenum | AAM | Germany | ON869302 | ON869302 | ON808480 | ON808524 | This study | |
| AAM1 = CBS 147285 | Austria | ET | ON869303 | ON869303 | ON808481 | ON808525 | This study | |
| DIG | Norway | ON869304 | ON869304 | ON808482 | ON808526 | This study | ||
| Digitodochium rhodoleucum | NBRC 32296 | Japan | LC146732 | LC146732 | N/A | N/A | Ban et al. (unpubl.) | |
| Emarcea castanopsidicola | CBS 117105 | Thailand | HT | AY603496 | MK762717 | MK791285 | MK776962 | Duong et al. (2004), Samarakoon et al. (2020) |
| Emarcea eucalyptigena | CBS 139908 | Malaysia | HT | KR476733 | MK762718 | MK791286 | MK776963 | Crous et al. (2015), Samarakoon et al. (2020) |
| Entalbostroma erumpens | ICMP 21152 | New Zealand | HT | KX258206 | N/A | KX258204 | KX258205 | Johnston et al. (2016) |
| Entoleuca mammata | 100 J.D.R. | France | GU300072 | N/A | GQ844782 | GQ470230 | Hsieh et al. (2010) | |
| Entonaema liquescens | ATCC 46302 | USA | KY610389 | KY610443 | KY624253 | KX271248 | Wendt et al. (2018) | |
| Entosordaria perfidiosa | CBS 142773 | Austria | ET | MF488993 | MF488993 | MF489003 | MF489021 | Voglmayr et al. (2018) |
| Eutypa lata | UCR-EL1 | USA | JGI | JGI | JGI | JGI | ||
| Graphostroma platystomum | CBS 270.87 | France | JX658535 | DQ836906 | KY624296 | HG934108 | Zhang et al. (2006), Stadler et al. (2014), Koukol et al. (2015), | |
| Wendt et al. (2018) | ||||||||
| Helicogermslita clypeata | MFLU 18-0852 | HT | MW240666 | MW240596 | MW658647 | MW775614 | Samarakoon et al. (2022) | |
| Helicogermslita somala | PAD S00034 | ILT | MW626901, | N/A | N/A | N/A | Forin et al. (2021) | |
| MW6269095 | ||||||||
| Hypocreodendron sanguineum | J.D.R. 169 | Mexico | GU322433 | N/A | GQ844819 | GQ487710 | Hsieh et al. (2010) | |
| Hypomontagnella monticulosa | MUCL 54604 | French Guiana | ET | KY610404 | KY610487 | KY624305 | KX271273 | Wendt et al. (2018) |
| Hypoxylon fragiforme | MUCL 51264 | Germany | ET | KC477229 | KM186295 | KM186296 | KX271282 | Stadler et al. (2013), Daranagama et al. (2015), Wendt et al. |
| (2018) | ||||||||
| Induratia alba | 9-6 | N/A | HM034857 | HM034865 | N/A | HM034844 | Zhang et al. (2010) | |
| Induratia coffeana | COAD 1842 | Brazil | HT | KM514680 | N/A | KP862881 | N/A | Hongsanan et al. (2015) |
| Induratia fengyangensis | CGMCC 2862 | China | HT | HM034856 | HM034859 | HM034849 | HM034843 | Zhang et al. (2010) |
| Induratia thailandica | MFLUCC 17-2669 | Thailand | HT | MK762707 | MK762714 | MK791283 | MK776960 | Samarakoon et al. (2020) |
| Induratia yunnanensis | CGMCC 3.18908 | China | HT | MG866046 | MG866038 | MG866059 | MG866066 | Chen et al. (2019) |
| Induratia ziziphi | MFLUCC 17-2662 | Thailand | HT | MK762705 | MK762712 | MK791281 | MK776958 | Samarakoon et al. (2020) |
| Jackrogersella multiformis | CBS 119016 | Germany | ET | KC477234 | KY610473 | KY624290 | KX271262 | Kuhnert et al. (2014, 2017), Wendt et al. (2018) |
| Kretzschmaria deusta | CBS 163.93 | Germany | KC477237 | KY610458 | KY624227 | KX271251 | Stadler et al. (2013), Wendt et al. (2018) | |
| Leptomassaria simplex | LSI = CBS 147282 | Austria | ET | ON869305 | ON869305 | ON808483 | ON808527 | This study |
| LSI1 | Austria | ON869306 | ON869306 | ON808484 | ON808528 | This study | ||
| LSI2 | Austria | ON869307 | ON869307 | ON808485 | ON808529 | This study | ||
| LSI3 | Austria | ON869308 | ON869308 | N/A | N/A | This study | ||
| LSI4 | Austria | ON869309 | ON869309 | ON808486 | ON808530 | This study | ||
| LSI5 | Canada | ON869310 | ON869310 | ON808487 | ON808531 | This study | ||
| Linosporopsis ischnotheca | CBS 145761 | Switzerland | ET | MN818952 | MN818952 | MN820708 | MN820715 | Voglmayr & Beenken (2020) |
| Linosporopsis ochracea | CBS 145999 | Germany | ET | MN818958 | MN818958 | MN820714 | MN820721 | This study |
| Lopadostoma turgidum | CBS 133207 | Austria | ET | KC774618 | KC774618 | KC774563 | MF489024 | Jaklitsch et al. (2014), Voglmayr et al. (2018) |
| Magnostiolata mucida | MFLU 19-2133 | Thailand | HT | MW240673 | MW240603 | MW658652 | MW775618 | Samarakoon et al. (2020) |
| Nemania abortiva | BISH 467 | USA | HT | GU292816 | N/A | GQ844768 | GQ470219 | Hsieh et al. (2010) |
| Nemania beaumontii | HAST 405 | Martinique | GU292819 | N/A | GQ844772 | GQ470222 | Hsieh et al. (2010) | |
| Nemania bipapillata | HAST 90080610 | Taiwan | GU292818 | N/A | GQ844771 | GQ470221 | Hsieh et al. (2010) | |
| Nemania ethancrensonii | AEC = CBS 148337 | USA | HT | ON869311 | ON869311 | ON808489 | ON808533 | This study |
| Nemania primolutea | HAST 91102001 | Taiwan | HT | EF026121 | N/A | GQ844767 | EF025607 | Hsieh et al. (2010) |
| Nemania serpens | HAST 235 | Canada | GU292820 | N/A | GQ844773 | GQ470223 | Hsieh et al. (2010) | |
| Nemania sphaeriostoma | 261 J.D.R. | USA | GU292821 | N/A | GQ844774 | GQ470224 | Hsieh et al. (2010) | |
| Nemania uda | EUU = CBS 148422 | Austria | ON869312 | ON869312 | ON808488 | ON808532 | This study | |
| Obolarina dryophila | MUCL 49882 | France | GQ428316 | GQ428316 | KY624284 | GQ428322 | Pažoutová et al. (2010), Wendt et al. (2018) | |
| Occultitheca rosae | HKAS 102393 | China | HT | MW240672 | MW240602 | MW658651 | MW775617 | Samarakoon et al. (2020) |
| Oligostoma insidiosum | ANI = CBS 147280 | Austria | ON869313 | ON869313 | ON808490 | ON808534 | This study | |
| ANI1 = CBS 147288 | Switzerland | ET | ON869314 | ON869314 | ON808491 | ON808535 | This study | |
| ANR = CBS 147287 | Austria | ET4 | ON869315 | ON869315 | ON808492 | ON808536 | This study | |
| ANR1 | Austria | ON869316 | ON869316 | ON808493 | ON808537 | This study | ||
| ANR2 | Austria | ON869317 | ON869317 | ON808494 | ON808538 | This study | ||
| ANR3 | Austria | ON869318 | ON869318 | ON808495 | ON808539 | This study | ||
| OTI | Austria | ON869319 | ON869319 | ON808496 | ON808540 | This study | ||
| Podosordaria mexicana | WSP 176 | Mexico | GU324762 | N/A | GQ853039 | GQ844840 | Hsieh et al. (2010) | |
| Podosordaria muli | WSP 167 | Mexico | HT | GU324761 | N/A | GQ853038 | GQ844839 | Hsieh et al. (2010) |
| Poronia pileiformis | WSP 88113001 | Taiwan | ET | GU324760 | N/A | GQ853037 | GQ502720 | Hsieh et al. (2010) |
| Poronia punctata | CBS 656.78 | Australia | HT | KT281904 | KY610496 | KY624278 | KX271281 | Senanayake et al. (2015), Wendt et al. (2018) |
| Pyrenopolyporus hunteri | MUCL 52673 | Ivory Coast | ET | KY610421 | KY610472 | KY624309 | KU159530 | Kuhnert et al. (2017), Wendt et al. (2018) |
| Rhopalostroma angolense | CBS 126414 | Ivory Coast | KY610420 | KY610459 | KY624228 | KX271277 | Wendt et al. (2018) | |
| Rosellinia aquila | MUCL 51703 | France | KY610392 | KY610460 | KY624285 | KX271253 | Wendt et al. (2018) | |
| Rosellinia corticium | MUCL 51693 | France | KY610393 | KY610461 | KY624229 | KX271254 | Wendt et al. (2018) | |
| Rostrohypoxylon terebratum | CBS 119137 | Thailand | HT | DQ631943 | DQ840069 | DQ631954 | DQ840097 | Tang et al. (2007), Fournier et al. (2011) |
| Ruwenzoria pseudoannulata | MUCL 51394 | D. R. Congo | HT | KY610406 | KY610494 | KY624286 | KX271278 | Wendt et al. (2018) |
| Sarcoxylon compunctum | CBS 359.61 | South Africa | KT281903 | KY610462 | KY624230 | KX271255 | Senanayake et al. (2015), Wendt et al. (2018) | |
| Spiririma gaudefroyi | HGA = CBS 147284 | Spain | ET | ON869320 | ON869320 | ON808497 | ON808541 | This study |
| HGA1 | Spain | ON869321 | ON869321 | ON808498 | ON808542 | This study | ||
| Stilbohypoxylon elaeicola | Y.M.J. 173 | French Guiana | EF026148 | N/A | GQ844826 | EF025616 | Hsieh et al. (2010) | |
| Stilbohypoxylon quisquiliarum | Y.M.J. 172 | French Guiana | EF026119 | N/A | GQ853020 | EF025605 | Hsieh et al. (2010) | |
| Stromatoneurospora phoenix | BCC 82040 | Thailand | MT735133 | MT735133 | MT742605 | MT700438 | Becker et al. (2020) | |
| Thamnomyces dendroidea | CBS 123578 | French Guiana | HT | FN428831 | KY610467 | KY624232 | KY624313 | Stadler et al. (2010), Wendt et al. (2018) |
| Xylaria acuminatilongissima | HAST 95060506 | Taiwan | HT | EU178738 | N/A | GQ853028 | GQ502711 | Hsieh et al. (2010) |
| Xylaria adscendens | 865 J.D.R. | Thailand | GU322432 | N/A | GQ844818 | GQ487709 | Hsieh et al. (2010) | |
| Xylaria aethiopica | Y.M.J. 1136 | Ethiopia | HT | MH790445 | N/A | MH785222 | MH785221 | Fournier et al. (2018b) |
| Xylaria apoda | HAST 90080804 | Taiwan | GU322437 | N/A | GQ844823 | GQ495930 | Hsieh et al. (2010) | |
| Xylaria arbuscula | CBS 126415 | Germany | KY610394 | KY610463 | KY624287 | KX271257 | Fournier et al. (2011), Wendt et al. (2018) | |
| Xylaria atrosphaerica | HAST91111214 | Taiwan | GU322459 | N/A | GQ848342 | GQ495953 | Hsieh et al. (2010) | |
| Xylaria badia | HAST 95070101 | Taiwan | GU322446 | N/A | GQ844833 | GQ495939 | Hsieh et al. (2010) | |
| Xylaria bambusicola | WSP 205 | Taiwan | HT | EF026123 | N/A | GQ844802 | AY951762 | Hsieh et al. (2010) |
| Xylaria brunneovinosa | HAST 720 | Martinique | HT | EU179862 | N/A | GQ853023 | GQ502706 | Hsieh et al. (2010) |
| Xylaria castorea | 600 PDD | New Zealand | GU324751 | N/A | GQ853018 | GQ502703 | Hsieh et al. (2010) | |
| Xylaria cf. castorea | HAST 91092303 | Taiwan | GU324752 | N/A | GQ853019 | GQ502704 | Hsieh et al. (2010) | |
| Xylaria crozonensis | HAST 398 | France | GU324748 | N/A | GQ848361 | GQ502697 | Hsieh et al. (2010) | |
| Xylaria culleniae | 189 J.D.R. | Thailand | GU322442 | N/A | GQ844829 | GQ495935 | Hsieh et al. (2010) | |
| Xylaria curta | HAST 494 | Martinique | GU322444 | N/A | GQ844831 | GQ495937 | Hsieh et al. (2010) | |
| Xylaria digitata | HAST 919 | Ukraine | GU322456 | N/A | GQ848338 | GQ495949 | Hsieh et al. (2010) | |
| Xylaria discolor | HAST 131023 | USA | ET | JQ087405 | N/A | JQ087411 | JQ087414 | Ju et al. (2012) |
| Xylaria enterogena | HAST 785 | French Guiana | GU324736 | N/A | GQ848349 | GQ502685 | Hsieh et al. (2010) | |
| Xylaria frustulosa | HAST 92092010 | Taiwan | GU322451 | N/A | GQ844838 | GQ495944 | Hsieh et al. (2010) | |
| Xylaria cf. glebulosa | HAST 431 | French West Indies | GU322462 | N/A | GQ848345 | GQ495956 | Hsieh et al. (2010) | |
| Xylaria globosa | HAST 775 | French West Indies | GU324735 | N/A | GQ848348 | GQ502684 | Hsieh et al. (2010) | |
| Xylaria grammica | HAST 479 | Taiwan | GU300097 | N/A | GQ844813 | GQ487704 | Hsieh et al. (2010) | |
| Xylaria haemorrhoidalis | HAST 89041207 | Taiwan | GU322464 | N/A | GQ848347 | GQ502683 | Hsieh et al. (2010) | |
| Xylaria cf. heliscus | HAST 88113010 | Taiwan | GU324742 | N/A | GQ848355 | GQ502691 | Hsieh et al. (2010) | |
| Xylaria hypoxylon | CBS 122620 | Sweden | ET | KY610407 | KY610495 | KY624231 | KX271279 | Sir et al. (2016b), Wendt et al. (2018) |
| Xylaria ianthinovelutina | HAST 553 | French West Indies | GU322441 | N/A | GQ844828 | GQ495934 | Hsieh et al. (2010) | |
| Xylaria intracolorata | HAST 90080402 | Taiwan | GU324741 | N/A | GQ848354 | GQ502690 | Hsieh et al. (2010) | |
| Xylaria laevis | HAST 419 | French West Indies | GU324746 | N/A | GQ848359 | GQ502695 | Hsieh et al. (2010) | |
| Xylaria longipes | CBS 148.73 | Germany | MH860649 | MH872351 | KU684280 | KU684204 | Vu et al. (2019), U‘Ren et al. (2016) | |
| Xylaria luteostromata | HAST 508 | French West Indies | GU324739 | N/A | GQ848352 | GQ502688 | Hsieh et al. (2010) | |
| Xylaria multiplex | HAST 580 | Martinique | GU300098 | N/A | GQ844814 | GQ487705 | Hsieh et al. (2010) | |
| Xylaria ophiopoda | HAST 93082805 | Taiwan | GU322461 | N/A | GQ848344 | GQ495955 | Hsieh et al. (2010) | |
| Xylaria oxyacanthae | J.D.R. 859 | USA | GU322434 | N/A | GQ844820 | GQ495927 | Hsieh et al. (2010) | |
| Xylaria palmicola | 604 PDD | New Zealand | GU322436 | N/A | GQ844822 | GQ495929 | Hsieh et al. (2010) | |
| Xylaria phyllocharis | HAST 528 | French West Indies | GU322445 | N/A | GQ844832 | GQ495938 | Hsieh et al. (2010) | |
| Xylaria plebeja | HAST 91122401 | Taiwan | GU324740 | N/A | GQ848353 | GQ502689 | Hsieh et al. (2010) | |
| Xylaria polymorpha | MUCL 49884 | France | KY610408 | KY610464 | KY624288 | KX271280 | Wendt et al. (2018) | |
| Xylaria reevesiae | H.M.H. 2010g | Taiwan | HT | GU322435 | N/A | GQ844821 | GQ495928 | Hsieh et al. (2010) |
| Xylaria regalis | HAST 92072001 | Taiwan | GU324744 | N/A | GQ848357 | GQ502693 | Hsieh et al. (2010) | |
| Xylaria schweinitzii | HAST 92092023 | Taiwan | GU322463 | N/A | GQ848346 | GQ495957 | Hsieh et al. (2010) | |
| Xylaria scruposa | HAST 497 | French West Indies | GU322458 | N/A | GQ848341 | GQ495952 | Hsieh et al. (2010) | |
| Xylaria telfairii | HAST 421 | French West Indies | GU324737 | N/A | GQ848350 | GQ502686 | Hsieh et al. (2010) | |
| Xylaria vivantii | H.M.H. 2010h | French West Indies | HT | GU322438 | N/A | GQ844824 | GQ495931 | Hsieh et al. (2010) |
1 Abbreviations: ATCC: American Type Culture Collection, Manassas, USA; BISH: Bishop Museum, Honolulu, USA; BCC: BIOTEC Culture Collection, National Center for Genetic Engineering and Biotechnology, Khlong Luang, Thailand; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CGMCC: China General Microbiological Culture Collection Center, Beijing, China; COAD: Otávio de Almeida Drumond culture collection, Universidade Federal de Viçosa, Brazil; GZUH: Guizhou University, Guiyang, China; HAST: Academia Sinica, Taipei, Taiwan; HKAS: Herbarium of Cryptogams Kunming Institute of Botany Academia Sinica, China; H.M.H.: Academia Sinica, Taipei, Taiwan; Huei-Mei Hsieh ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; J.D.R.: Jack D. Rogers, Washington State University, Pullman, USA; J.F., Jacques Fournier, Rimont, France; MFLU, MFLUCC: Mae FahLuang University, Chiang Rai, Thailand; MUCL: Università Catholique de Louvain, Louvain-la-Neuve, Belgium; NBRC: Biological Resource Center, National Institute of Technology and Evaluation, Tokyo, Japan; PAD: Botanical Museum-Herbarium, University degli Studi di Padova, Italy; ST.MA.: Marc Stadler, Helmholtz-Zentrum für Infektionsforschung, Braunschweig, Germany; UCR: University of California, Riverside, USA; Y.M.J.: Yu-Ming Ju, Academia Sinica, Taipei, Taiwan; WSP, Washington State University, Pullman, USA.
2 ET epitype; HT holotype; ILT isolectotype.
3 N/A not available; JGI sequences retrieved from JGI-DOE (http://genome.jgi.doe.gov/).
4 Ex-epitype strain of Anthostoma rhenanum.
5 ITS1 and ITS2 sequences combined for analyses.
Morphology
Stereomicroscopy photographs were captured with a Nikon SMZ 1500 dissecting microscope equipped with a Nikon DS-U2 digital camera, a Keyence VHX-6000 Digital Microscope or a Olympus SZ60. For certain images of ascomata the stacking software Zerene Stacker v. 1.04 (Zerene Systems LLC, Richland, WA, USA) was used.
For light microscopy, hand sections of ascomata were made using a razor blade and the following reagents were used as mounting media: tap water, 3 % KOH, 1 % Sodium dodecyl sulfate (SDS), 10 % aqueous NaCl solution, aqueous Congo red solution, Congo red in 1 % SDS, Lugol’s solution (IKI, with 3 % KI and 1 % iodine), Melzer’s reagent, aqueous chlorazol black, Indian ink and black or blue Pelikan ink. PVA-lactophenol was used after 48 h incubation for the observation of ascospore wall ornamentation and inconspicuous germ slits, and aqueous nigrosin was used to stain ascospore appendages. Semi-permanent slides were made with chloral-lactophenol. Slides were examined and photographed using a Zeiss Axio Imager.A1 (Zeiss, Jena, Germany) compound microscope equipped with a Zeiss Axiocam 506 colour digital camera or a Leitz Ortholux equipped with Nikon 995 colour digital camera. Measurements were done with the NIS-Elements D v. 3.0, Piximetre 5.10, or Zeiss ZEN Blue Edition software packages. Measurements are reported as maxima and minima in parentheses and the range representing the mean plus and minus the standard deviation of a number of measurements N given in parentheses; in addition, means of measurements (Me) and of l/w ratios (Qe) are given for ascospores and conidia.
Culture preparation, DNA extraction, PCR, and sequencing
Isolates were prepared from ascospores as described in Jaklitsch (2009) and grown on 2 % malt extract agar (MEA) or on 2 % corn meal agar plus 2 % w/v dextrose (CMD). Growth of liquid culture and extraction of genomic DNA was performed as reported previously (Voglmayr & Jaklitsch 2011, Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) or the Thermo Scientific GeneJET Plant Genomic DNA Purification Kit (Thermo Fisher Scientific Inc., Waltham, Mass., USA). As for A. novomexicanum no cultures were available and in order to save material of the type collection, no DNA extraction was done, but thin sections of ascomatal contents were prepared with a sterile razor blade, which were directly placed in the PCR solution.
The following loci were amplified and sequenced: the complete internal transcribed spacer region (ITS1-5.8S-ITS2) and ac. 0.9–1.3-kb fragment of the large subunit nuclear ribosomal DNA (nuLSU rDNA), a c. 1.2-kb fragment of the RNA polymerase II subunit 2 (RPB2) and a c. 1.6-kb fragment of the beta-tubulin (TUB2) gene. Primers and annealing temperatures for PCR and sequencing are given in Table 2. In the direct PCR approach of A. novomexicanum, the ITS and LSU were amplified and sequenced in short, overlapping fragments according to Voglmayr et al. (2012), but partly using newly designed specific forward primers for the ITS1, and the PCR solution containing ascomatal contents was incubated for 10 min at 80 °C followed by 7 min at 95 °C prior to the PCR. PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK). Sequencing was performed on an automated DNA sequencer (ABI 3730xl Genetic Analyzer, Applied Biosystems).
Table 2.
Primers used to amplify and sequence the nuclear internal transcribed spacer - large subunit (ITS-LSU) rDNA region, the RNA polymerase II second largest subunit (RPB2) and the beta-tubulin (TUB2) genes.
| Gene | Primer | Sequence (5‘–3‘) | Direction | Annealing t (°C) | amplicon length | References |
|---|---|---|---|---|---|---|
| ITS-LSU | V9G | TTAAGTCCCTGCCCTTTGTA | forward | 55 | 1.6–2.1 kb | De Hoog & Gerrits van den Ende (1998) |
| LR5 | TACTTGAAGGAACCCTTACC | reverse | Hopple & Vilgalys (1994) | |||
| LR2R-A1 | CAGAGACCGATAGCGCAC | forward | Voglmayr et al. (2012) | |||
| LR31 | CCGTGTTTCAAGACGGG | reverse | Hopple & Vilgalys (1994) | |||
| ITS41 | TCCTCCGCTTATTGATATGC | reverse | White et al. (1990) | |||
| ITS12 | ITSxyF3 | CTTCCGGACTGGCCCAGAGGA | forward | 60 | This study | |
| ITSxyF13 | AGGAGTCGGCAACGACACCT | forward | 60 | This study | ||
| F5.8Sr | TGCGTTCAAARATTCGATG | reverse | Jaklitsch & Voglmayr (2011) | |||
| ITS22 | F5.8Sf | CAACAACGGATCTCTTGGYTC | forward | 52 | Jaklitsch & Voglmayr (2011) | |
| ITS4 | TCCTCCGCTTATTGATATGC | reverse | White et al. (1990) | |||
| LSU2 | LROR | ACCCGCTGAACTTAAGC | forward | 52 | Moncalvo et al. (1995) | |
| LR2-A | TGCTTTTCATCTTTCGATCAC | reverse | Voglmayr et al. (2012) | |||
| LR2R-A | CAGAGACCGATAGCGCAC | forward | 52 | Voglmayr et al. (2012) | ||
| LR3 | CCGTGTTTCAAGACGGG | reverse | Hopple & Vilgalys (1994) | |||
| LR3R | GTCTTGAAACACGGACC | forward | 52 | Hopple & Vilgalys (1994) | ||
| LR5 | TACTTGAAGGAACCCTTACC | reverse | Hopple & Vilgalys (1994) | |||
| RPB2 | dRPB2-5f | GAYACNGAYGAYCGWGAYCAYTTYGG | forward | 52 | 1.2 kb | Voglmayr et al. (2016a) |
| dRPB2-7r | AANCCCATDGCYTGYTTDCCCAT | reverse | Voglmayr et al. (2016a) | |||
| fRPB2-5F | GAYGAYMGWGATCAYTTYGG | forward | 55 | Liu et al. (1999) | ||
| fRPB2-7cR | CCCATRGCTTGYTTRCCCAT | reverse | Liu et al. (1999) | |||
| TUB2 | T1D | CAANATGCGTGAGATTGTRAGT | forward | 55–58 | 1.5–1.6 kb | Carbone & Kohn (1999) |
| T22D | CTGSACGTTGTTGGGRATCCA | reverse | Carbone & Kohn (1999) | |||
| BtHVf1 | AACTGGGCMAAGGGYCAYTACAC | forward | Voglmayr & Mehrabi (2018) | |||
| BtHV2r1 | CATCATRCGRTCNGGGAACTC | reverse | Voglmayr et al. (2016b, 2017) |
1 internal primers used only for sequencing.
2 for direct PCR of ascomatal contents of A. novomexicanum.
2 newly designed primers near the 3’ end of the nuSSU specific for Xylariaceae.
Data analysis
The newly generated sequences were aligned to a representative matrix of Xylariales, selecting Diatrypaceae and Lopadostomataceae as outgroups according to Voglmayr & Beenken (2020). The GenBank accession numbers of sequences used in the phylogenetic analyses are given in Table 1.
Sequence alignments were produced with the server version of MAFFT v. 7.490 (http://mafft.cbrc.jp/alignment/server/; Katoh et al. 2019), checked and refined using BioEdit v. 7.2.6 (Hall 1999). The ITS-LSU rDNA, RPB2 and TUB2 matrices were combined for subsequent phylogenetic analyses. After exclusion of ambiguously aligned regions and long gaps, the final combined data matrix contained 4694 characters (537 nucleotides of ITS, 1344 nucleotides of LSU, 1282 nucleotides of RPB2 and 1531 nucleotides of TUB2). Familial classification of Xylariaceae and phylogenetically related families follows Voglmayr & Beenken (2020), Voglmayr et al. (2018) and Wendt et al. (2018).
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI v. 1.3 (Silvestro & Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMA substitution model with 1000 bootstrap replicates. The matrix was partitioned for the different gene regions. For evaluation and discussion of bootstrap support, values below 70 % were considered low, between 70 and 90 % medium/moderate, above 90 % high and 100 % maximum.
Maximum parsimony (MP) bootstrap analyses were performed with PAUP v. 4.0a169 (Swofford 2002), with 1000 bootstrap replicates using five rounds of heuristic search replicates with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect, COLLAPSE command set to MINBRLEN, each replicate limited to 100000 rearrangements) during each bootstrap replicate. All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data.
RESULTS
Direct PCR of ascomatal contents in A. novomexicanum
The direct PCR approach of ITS and LSU with specific forward primers resulted in clean sequences attributable to the genus Albicollum, and the sequences differed significantly from the other sequenced species of the genus, confirming the status of A. novomexicanum as a distinct species. Despite the lack of RPB2 and TUB2 sequence data, A. novomexicanum was included in the multigene analyses.
Molecular phylogeny
The combined multilocus matrix used for phylogenetic analyses comprised 4694 characters, of which 2030 were parsimony informative (292 from ITS, 188 from LSU, 699 from RPB2 and 851 from TUB2). The best ML tree (-lnL = 127092.746) obtained by RAxML is shown in Fig. 1. Overall topologies were similar to previous analyses (Voglmayr et al. 2018, Wendt et al. 2018, Voglmayr & Beenken 2020) and are therefore not described in detail here unless of relevance for the taxa of the present study. The Xylariaceae (including Clypeosphaeriaceae and Induratiaceae) received maximum support (Fig. 1). Within Xylariaceae, the genera Digitodochium and Spiririma were contained within a basal clade receiving medium (70 % MP) to high (90 % ML) bootstrap support. While Spiririma was contained within the Emarcea-Induratia clade (‘Induratiaceae’ in Fig. 1) with maximum support, Digitodochium was closest relative of Occultitheca (‘Clypeosphaeriaceae’ in Fig. 1) with medium (73 % ML, 87 % MP) support. The genera Albicollum, Leptomassaria and Oligostoma were placed within the highly supported core Xylariaceae clade. Leptomassaria simplex and Oligostoma insidiosum are closest relatives placed in a highly supported (99 % ML, 95 % MP) subclade together with Xylaria digitata (Fig. 1). The new genus Albicollum formed a distinct subclade with maximum support, but its closest relatives remained uncertain. The two Nemania species formerly classified in Euepixylon (N. uda and N. sphaeriostoma) were revealed as close relatives, but did not form a sister group relationship; while N. uda was sister species with the generic type of Nemania, N. serpens, with high (99 % MP) or maximum (ML) support, N. sphaeriostoma was revealed as closest relative of the newly described N. ethancrensonii. Like in previous analyses, the genus Xylaria was revealed as polyphyletic.
Fig. 1.
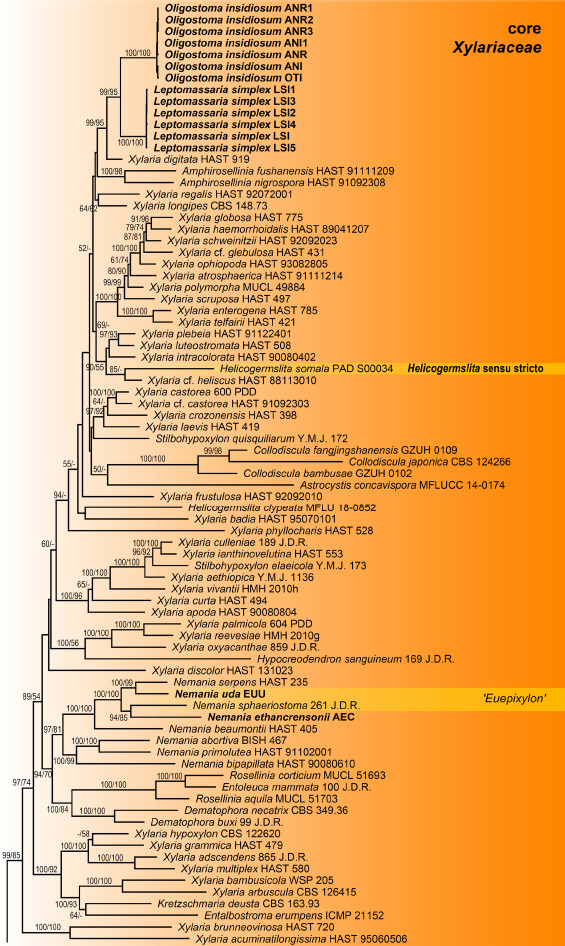
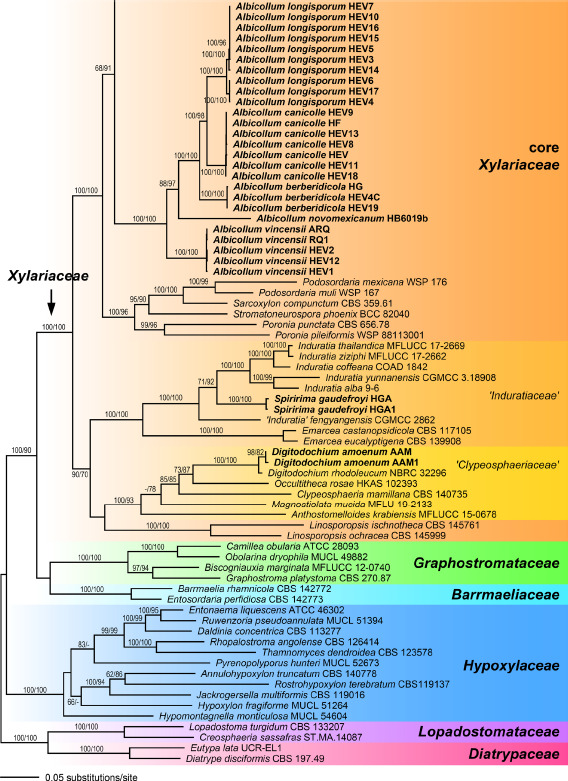
Phylogram of the ML tree (-lnL = 127092.746) revealed by RAxML from an analysis of the combined SSU-ITS-LSU-RPB2-TUB2 matrix of selected Xylariales. Strain/culture numbers or GenBank accession numbers are given following the taxon names. ML and MP bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches. Accessions in bold were sequenced in the present study.
TAXONOMY
Albicollum Voglmayr, J. Fourn., S. Tello & Jaklitsch, gen. nov. — MycoBank MB 844597
Etymology. Albi- = white, collum = collar; referring to the distinct white collar of pseudostromatic tissues surrounding the black ostiolar neck.
Type species. Albicollum vincensii (G. Arnaud) Voglmayr, J. Fourn., S. Tello & Jaklitsch
Genus of Xylariaceae. Pseudostromata immersed in the substrate and erumpent through the bark or wood, reduced mostly to the region around and below the ostioles, forming a whitish to yellowish collar or discoid area. Ascomata perithecial, immersed to barely erumpent, solitary, scattered, or clustered, subglobose to globose, with a central ostiole; ostiolar neck straight to oblique, black. Stromatic tissue around the perithecial venters inconspicuous; upper stromatic layer composed of clusters of white crystals mixed with necrotic wood or bark cells, forming prominent white discoid areas. Peridium pseudoparenchymatous, brown to dark brown; ostiolar canal densely periphysate. Asci cylindrical to fusiform, short-stipitate, with a short-cylindrical to slightly trapezoid, hemi- or euamyloid apical apparatus. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, embedded in a mucilaginous matrix. Ascospores aseptate, variable in shape from subglobose, broadly to narrowly ellipsoid, subcitriform to ellipsoid-fusiform, equilateral to slightly inequilateral, dark brown with a conspicuous helicoid germ slit coiling at least once around the ascospore, sometimes with a hyaline gelatinous secondary appendage; epispore medium to dark brown, smooth or verruculose; without a gelatinous sheath visible in Indian ink. Asexual morph not seen on the natural substrate or in pure culture.
Notes — Within Xylariaceae, Albicollum is well characterised by its distinct white collar of pseudostromatic tissues surrounding the erumpent to projecting black ostiolar necks emerging from the immersed, solitary to aggregated perithecia.
Albicollum berberidicola Voglmayr, J. Fourn., S. Tello & Jaklitsch, sp. nov. — MycoBank MB 844598; Fig. 2
Fig. 2.
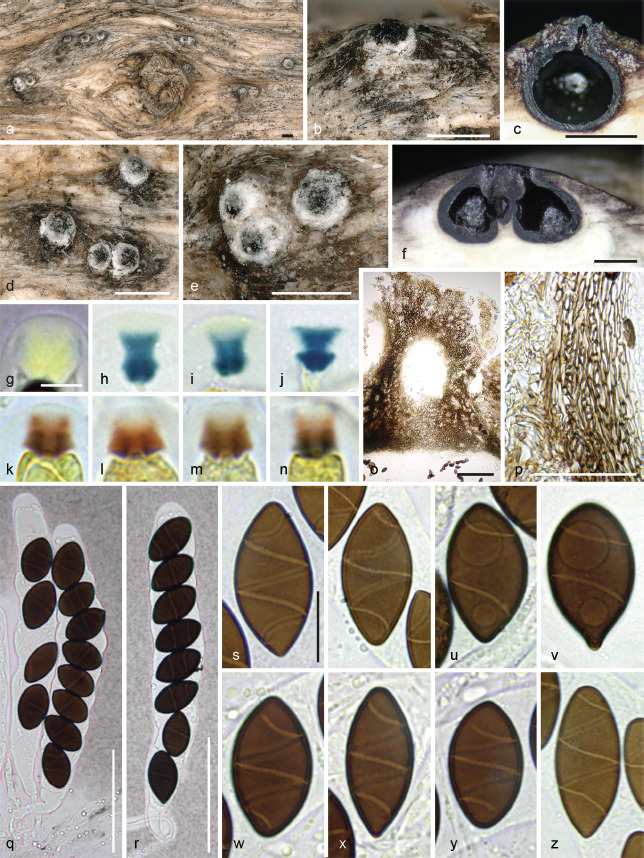
Albicollum berberidicola. a–b, d–e. Habit of ostiolar necks on the host surface; c. ascoma in vertical section showing a blackened and granular pseudostroma; f. two adjacent ascomata in vertical section showing an extensive black apical pseudostroma and bleached wood; g–n. apical apparatuses in black Pelikan ink (g), Lugol’s solution after 3 % KOH pretreatment (h–j), and Lugol’s solution (k–n); o. ostiolar neck in oblique section, in chloral-lactophenol; p. peridium in vertical section, in chloral-lactophenol; q–r. asci in diluted Indian ink; s–y. mature ascospores in 1 % SDS; z. barely mature ascospore in 1 % SDS (a–e, g–i, k–t, w–z. WU-MYC 0043994 - holotype; f, j, u–v. WU-MYC 0043995 - paratype). — Scale bars: a–f = 0.5 mm; g–n = 5 μm; o = 100 μm; p–r = 50 μm; s–z = 10 μm.
Etymology. Referring to its host genus Berberis.
Typus. Greece, Crete, Psiloritis, Ekklisia Analipsi, 1400 m a.s.l., N35°12'20.8" E24°49'53.5", on decorticated weathered twigs of Berberis cretica, soc. Albicollum longisporum, 16 Oct. 2014, W. Jaklitsch (holotype WU-MYC 0043994, ex-holotype culture HG = CBS 147392).
Diagnosis. Differs from A. longisporum by shorter and more broadly ellipsoid ascospores 22.6 × 10.8 μm vs 40.8 × 12.5 μm on average (Qe = 2.1 vs 3.3).
Pseudostromata immersed in the woody substrate to slightly erumpent, raising the host surface up to 0.6 mm high, blackening the wood surface around the ostioles and forming a whitish ring or collar around them, occasionally extending downward and forming a lateral black line. Ascomata perithecial, immersed to partly erumpent, scattered, solitary or in clusters, subglobose, 600–750 μm diam to occasionally depressed-subglobose 650 μm high × 900 μm diam, with a central to slightly eccentric ostiole when in contact; ostiolar neck straight to slightly curved, black, apically flattened, 40–300 μm high, 250–450 μm diam, overlain by a thick, white to off-white coarsely granular tissue forming a basal ring or a continuous sleeve; ostioles minutely porate, at the centre of a black discoid area. Stromatic tissue around the venters inconspicuous, reduced to sparse, loosely interwoven, moderately thick-walled hyaline hyphae 3–4 μm diam, mixed with necrotic wood cells; upper stromatic layer at the base of the ostiolar neck brown, 120–280 μm thick, prosenchymatous, composed of light to dark brown, thin-walled to moderately thick-walled hyphae 2–4 μm diam, originating from the upper part of the peridium and the ostiolar neck wall and extending downward in places, slightly blackening the wood, encasing conspicuous to discrete clusters of white crystals readily dissolving in 5 % HCl, occasionally absent, similar to those present around the ostiolar neck. Peridium 30–60 μm thick at sides, pale brown, turning subhyaline inwardly, a textura prismatica mixed with textura angularis, composed of elongate to polygonal, thin-to thick-walled cells 4–18 μm in greatest dimension with wall 1–1.8 μm thick; darker brown at the apex, 60–80 μm thick, a textura angularis of small, thick-walled, subopaque cells, with rare to abundant white crystals, intergrading into the c. 80 μm thick ostiolar neck wall, of similar texture interspersed with abundant white crystals. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, with small scattered refractive guttules, longer than the asci, 3–4 μm wide at the base, tapering to 1–2 μm wide, embedded in mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate, with (4–)8 obliquely uniseriate overlapping, occasionally irregularly biseriate ascospores, 138–157 × 17–22.5 μm (Me = 147 × 19.5 μm, N = 20) including the 13–26 μm long stipes; with a hemiamyloid apical apparatus not blueing in Melzer’s reagent, barely refractive in water or in chlorazol black; 7–8 × 7–8 μm in black Pelikan ink, stained yellow and appearing roughly hexagonal and apically convex; 3.2–4.4 μm high × 4.7–6.1 μm diam (Me = 3.9 × 5.5 μm, N = 20) in Lugol’s solution, reddish brown to dirty red, short-cylindrical to nearly trapezoid with a sharp lower rim, occasionally appearing obscurely annellate or unevenly stained; 4.6–5.6 μm high × 3.5–4.4 μm diam (Me = 5.2 × 4.1 μm, N = 22) in Lugol’s solution or in Melzer’s reagent after 30 s pretreatment in 3 % KOH, blue, inverted bell-shaped with more or less acute upper and lateral angles. Ascospores (18–)20–26.2(–30.5) × (9–)9.4–12.4(–14.3) μm, Q = (1.5–)1.8–2.4(–2.8) (Me = 22.6 × 10.8 μm, Qe = 2.1; N = 180), ellipsoid to broadly ellipsoid or subcitriform, aseptate, equilateral, dark brown, with narrowly rounded, rarely subacute or apiculate ends; germ slit 0.5–0.6 μm wide, conspicuous, helicoid, obliquely coiled 2.5 times around the ascospore, appearing as broken down into five segments in optical section, curving toward one or both ends; epispore medium to dark brown, smooth; no gelatinous sheath visible in Indian ink, including immature hyaline ascospores. Asexual morph on the natural substrate not seen.
Habitat & Host range — Only known from dead branches of Berberis spp.
Known Distribution — Greece (Crete) and southern Spain (Andalucía).
Other specimens examined (paratypes). Spain, Andalucía, Jaén, Valdepeñas de Jaén, La Pandera, N37°37'47.30" W3°46'21.06", 1786 m a.s.l., on decorticated twig of Berberis hispanica c. 10 mm diam, soc. Albicollum longisporum, 14 Sept. 2017, S. Tello S.T.14091701 (WU-MYC 0043995, culture HEV4C); ibid., 30SVG 31735 65067, N37°37'48.69" W3°46'25.14", 1820 m a.s.l., on decorticated twigs of Berberis hispanica, 9 Feb. 2020, S. Tello S.T. 09022001 (WU-MYC 0043996, culture HEV19).
Notes—Ascomatal morphology of A. berberidicola does not provide sound differential characters when compared with other species of Albicollum. Ascospores resemble those of A. longisporum in having a narrow germ slit coiling 2–2.5 times and differ from those of A. canicolle in size, shape and germ slit morphology. Ascospore shape varying from ellipsoid to subcitriform, 20–26 μm long, appears to be the diagnostic character setting A. berberidicola apart from A. longisporum. Unlike in A. longisporum, the germ slit is distinctive in consistently curving towards one or most often both ends.
Albicollum canicolle (P. Karst.) Voglmayr, J. Fourn., S. Tello & Jaklitsch, comb. nov. — MycoBank MB 844599; Fig. 3, 4
Fig. 3.
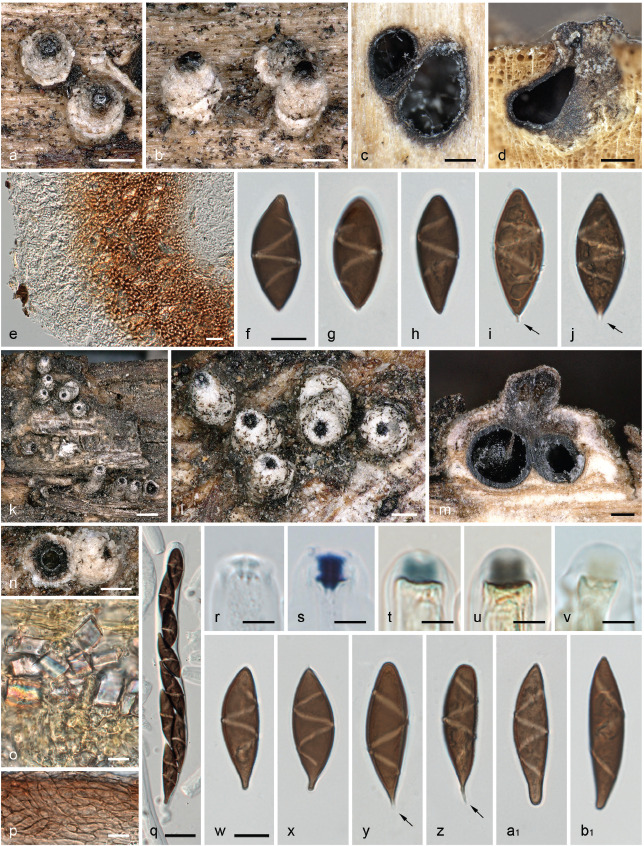
Albicollum canicolle. a–b, k–l, n. Habit of ostiolar necks erumpent from wood (left broken in n); c–d, m. ascomata in transverse (c) and vertical (d, m) section; e. ostiolar neck in transverse section; f–j, w–b1. ascospores, arrows denoting apiculi; o. pseudostroma with large crystals in section, in Melzer’s reagent; p. squash mount of peridium in Lugol’s solution after 3 % KOH pretreatment; q. ascus; r–v. apical apparatuses in 3 % KOH (r), Lugol’s solution after 3 % KOH pretreatment (s), Lugol’s solution (t–u) and Melzer’s reagent (v). All in 3 % KOH, except where noted (a–j. holotype of Amphisphaeria canicollis - H s.n.; k–b1. lectotype of Anthostoma ostropoides - M-0307883). — Scale bars: a–d, l–n = 200 μm; e, q = 20 μm; f–j, o–p, w–b1 = 10 μm; k = 500 μm; r–v = 5 μm.
Fig. 4.
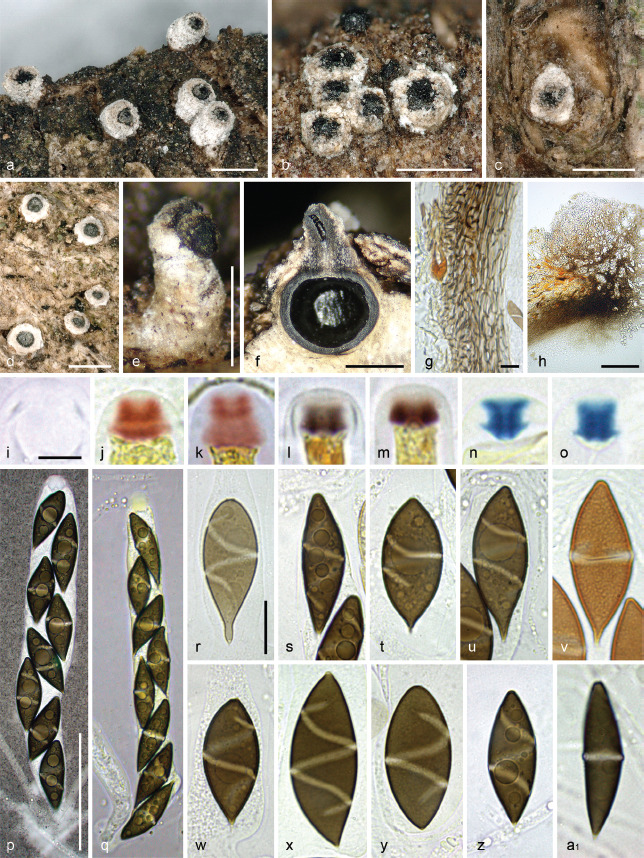
Albicollum canicolle. a–e. Habit of ostiolar necks erumpent from bark; f. ascoma in vertical section; g. peridium in vertical section, in chloral-lactophenol; h. ostiolar neck wall in vertical section, in chloral-lactophenol; i–o. apical apparatuses in water (i), Lugol’s solution (j–m) and Lugol’s solution after 3 % KOH pretreatment (n–o); p–q. asci in diluted Indian ink and black Pelikan ink respectively; r–u, w–z. ascospores in 1 % SDS; v. ascospore in PVA-lactophenol showing an ornamented wall and an equatorial germ slit; a1. ascospore in 3 % KOH showing a slightly prominent equatorial germ slit (a–b, e–h, q, s–v, a1. WU-MYC 0043997 - epitype; c. WU-MYC 0043999; d, i–k, n–p. WU-MYC 0044000; l–m. WU-MYC 0040045; r, w, z. WU-MYC 0044003; x–y. WU-MYC 0044013). — Scale bars: a–f = 0.5 mm; g, r–a1 = 10 μm; h = 100 μm; i–o = 5 μm; p–q = 50 μm.
Basionym. Amphisphaeria canicollis P. Karst., Not. Sällsk. Fauna Fl. Fenn. Förh. 13: 245. 1873 ‘1871–1874’.
Synonym. Anthostoma ostropoides Rehm, Ascomyc. no. 520. 1879.
Typification. Finland, near Åbo (Turku), on decorticated branch of Populus tremula, May 1861, leg. P. Karsten 1248 (H s.n., holotype). – Spain, Andalucía, Fuensanta de Martos, banks of river Víboras, N37°35'20.49" W3°52'56.08", 710 m a.s.l., on dead corticated twigs 5–7 mm diam of Pistacia lentiscus, 30 May 2017, S. Tello S.T.30041701 (WU-MYC 0043997, epitype here designated, MBT 10007728; ex-epitype culture HEF = CBS 147281).
Pseudostromata immersed in the woody substrate and erumpent through bark or wood, reduced mostly to the region around and below the ostioles, forming a whitish collar. Ascomata perithecial, immersed to partly erumpent and raising the host surface, scattered or in clusters, subglobose, 0.8–1 mm diam, with a central ostiole; ostiolar neck straight to slightly curved, black, apically flattened, 100–500(–800) μm high, 160–450 μm diam, overlain by a thick, white to off-white coarsely granular layer forming a continuous sleeve; ostiole minutely porate, at the centre of a black discoid area. Stromatic tissue around the venters inconspicuous, reduced to a loose prosenchymatous tissue composed of moderately thick-walled hyaline hyphae 3–4 μm diam, mixed with necrotic wood cells, encasing scattered clusters of white crystals; upper stromatic layer at the base of ostiolar neck brown, 160–200 μm thick, prosenchymatous, composed of light to dark brown, thin-walled to moderately thick-walled hyphae 3.5–5 μm diam, originating from the upper part of the peridium and the ostiolar neck wall, encasing conspicuous clusters of white crystals readily dissolving in 5 % HCl. Peridium 30–60 μm thick at sides, pale brown, a textura angularis grading inwardly into a subhyaline textura prismatica, composed of moderately thick-walled cells with wall 1.2–1.8 μm thick; darker brown at the apex, up to 125 μm thick, of small, thick-walled, subopaque cells, with few white crystals; ostiolar neck wall 170–180 μm thick at the base, c. 100 μm thick above, dark brown, a textura angularis of small, thick-walled cells, interspersed with abundant white crystals. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, with scattered small refractive guttules, much longer than the asci, 3–4 μm wide at the base, gradually tapering to 1.2–1.8 μm above the asci, embedded in a mucilaginous matrix. Asci cylindrical to subfusiform, short-stipitate to subsessile, with (4–)8 obliquely uniseriate overlapping, frequently irregularly biseriate ascospores, 155–178 × 19–25.5 μm (Me = 166 × 21.5 μm, N = 28) including the 7–15(–23) μm long stipes; with a hemiamyloid apical apparatus not blueing in Melzer’s reagent, barely refractive in water or in chlorazol black; 8–11 μm high × 8–10 μm diam in black Pelikan ink, stained yellow and appearing roughly hexagonal and apically convex; 4.2–6.2 μm high × 4.7–7.6 μm diam (Me = 4.9 × 5.9 μm, N = 60) in Lugol’s solution, reddish brown to dirty red, short-cylindrical to nearly trapezoid, with an apical depression and a convex base occasionally with sharp lateral rims, frequently appearing obscurely annellate or unevenly stained; 4.5–5.3 μm high × 5.2–6.2 μm diam (Me = 5 × 5.7 μm, N = 25) in Lugol’s solution or in Melzer’s reagent after 30 s pretreatment in 3 % KOH, bluish grey to dark blue, short-cylindrical to nearly trapezoid and apically flared, with basal lateral rims. Ascospores (21.8–)24.6–36.7(–40.8) × (7.1–)8.2–13.2(–14.4) μm, Q = (1.5–)2–3.8(–4.7) (Me = 30.0 × 11.2 μm, Qe = 2.7; N = 540), aseptate, ellipsoid-fusiform, equilateral to subequilateral, dark brown, frequently heteropolar, with one end obtusely rounded to slightly truncate, the other end narrowly rounded to frequently apiculate, apiculus spike-like, 0.8–2.5 μm long, colourless to light brown, most often oriented downward in the ascus; germ slit conspicuous, 1.8–2.7 μm wide, helicoid, obliquely coiled 1–1.5 times around the ascospore to equatorial when perpendicular to the ascospore main axis, appearing as broken down into three to five segments when seen in optical section, slightly prominent when seen in optical section, not reaching the ends; epispore medium to dark brown, appearing obscurely ornamented in water and in 3 % KOH, more conspicuously verrucose or pitted when observed after 48 h incubation in PVA-lactophenol; no gelatinous sheath visible in Indian ink, even around immature hyaline ascospores. Asexual morph on the natural substrate not seen.
Habitat & Host range — On dead branches of various deciduous shrubs and trees; confirmed from Crataegus, Pistacia, Populus, Prunus, Rhamnus, Rosa.
Known Distribution — Europe; confirmed from Finland, Germany, Spain.
Other specimens examined. Germany, Bayern, Franken, mountain above Hammelburg, on dead branches of Rosa canina, without date, H. Rehm, in Rehm, Ascomyc. 520 (M-0307883, lectotype of Anthostoma ostropoides here designated, MBT 10007729). – Spain, Jaén, Valdepeñas de Jaén, El Parrizoso, 30SVG 35060 63682, N37°37'4.63" W3°44'9.05", 1075 m a.s.l., on dead corticated twigs 7–10 mm diam of Pistacia terebinthus still attached to the tree, 25 Feb. 2017, S. Tello S.T.25021701 (WU-MYC 0044000, culture HEV); ibid., 30SVG 34397 63415, N37°36'55.81" W3°44'36.02", 1125 m a.s.l., on dead corticated twigs of Pistacia terebinthus, associated with Eutypa sp., 9 Apr. 2019, S. Tello S.T.09041905 (WU-MYC 0044001, culture HEV11); Jaén, Valdepeñas de Jaén, La Pandera, N37°37'54.31" W3°46'12.74", 1790 m a.s.l., on a dead decorticated branch of Rhamnus saxatilis, soc. A. longisporum, 15 Oct. 2017, S. Tello S.T.20101704 (WU-MYC 0040045); ibid., N37°37'54.30" W3°46'12.73", 1790 m a.s.l., on a decorticated branch of Prunus prostrata, soc. A. longisporum, 15 Oct. 2017, S. Tello S. T.20101703 (WU-MYC 0043998, culture HEV8); ibid., La Pandera, N37°37'52.71" W3°46'11.39", 1787 m a.s.l., on a decorticated branch of Prunus mahaleb, soc. A. longisporum, 20 Oct. 2017, S. Tello S.T.20101704 (WU-MYC 0043999, culture HEV9); Jaén, Valdepeñas de Jaén, Los cotos, 30SVG 27389 59333, N37°34'41.45" W3°49'20.41", 990 m a.s.l., on dead wood of Prunus dulcis, 18 June 2019, S. Tello S.T. 18061902 (WU-MYC 0044002, culture HEV18); Jaén, Valdepeñas de Jaén, Puerto de Ranera, N37°37'34.62" W3°49'22.03", 1215 m a.s.l., on a dead branch of Crataegus monogyna, 8 May 2019, S. Tello S.T.08051901 (WU-MYC 0044003, culture HEV13); ibid., on a dead branch of Crataegus monogyna, soc. A. longisporum, 8 May 2019, S. Tello S.T.08051902 (WU-MYC 0044013).
Notes — Albicollum canicolle differs from other known species of the genus by ascospores measuring 24.5–37 × 8–13 μm with a frequently apiculate end, with a roughened epispore and a thick, conspicuous, oblique to equatorial helicoid germ slit coiling 1–1.5 times not reaching the ends. Stromata and ostiolar necks are externally indistinguishable from those of A. longisporum and A. berberidicola which show the same wide range of morphological variations and occasionally occur intermingled on the same host.
Ascospore morphology is likewise variable, including the absence or presence of an apiculus, but average dimensions of 30 × 11 μm clearly set it apart from A. longisporum (41 × 13 μm) and A. berberidicola (23 × 11 μm). The most distinctive feature is the wide germ slit far from reaching the ascospore ends, usually Z-shaped in optical section, occasionally equatorial or nearly so. The finely roughened epispore is a further diagnostic feature.
Albicollum canicolle has been described from decorticated twigs of Populus tremula in Finland (Karsten 1873). The type specimen is overmature and moulded, no asci could be seen and most of the ascospores were collapsed. However, the ascospore characters (size, shape, apiculus, epispore ornamentation and germ slit) fully match the Spanish collections, and we therefore consider them to be conspecific. To ensure nomenclatural stability, a recent Spanish collection (WU-MYC 0043997), for which a culture and DNA data are available, is here selected as an epitype.
Investigation of an isotype specimen of Anthostoma ostropoides showed it matched A. canicolle in most morphological characters, in particular the distinctive wide, Z-shaped germ slit. However, its ascospores measured (24.5–)31.5–40.5(–46) × (8.2–)9.4–11(–12.2) μm, Q = (2.1–)3.0–4.2(–5.6) (Me = 36 × 10.2 μm, Qe = 3.6; N = 100), and were therefore in average distinctly longer, resulting in a higher Qe, than the other specimens examined. Remarkably, the means of our measurements perfectly matched those given in the original description (36 × 10 μm; Rehm 1881). Considering the high variability of ascospore sizes observed in different conspecific Albicollum accessions, we regard this collection to represent a large-spored variant of Albicollum canicolle, and it bridges the distribution gap between the Finnish holotype and the Spanish collections. As the type collection of A. ostropoides has been distributed to several herbaria as part of an exsiccatum series, we here select the specimen M-0307883 as lectotype.
Albicollum chionostomum (Speg.) Voglmayr, comb. nov. — MycoBank MB 844600; Fig. 5
Fig. 5.
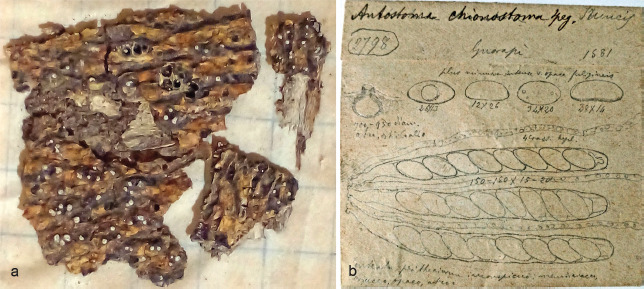
Albicollum chionostomum (holotype LPS 6804). a. Habit of ostiolar necks on host surface; b. line drawings of the type by Spegazzini (courtesy of Jorge Alberto Chayle, LPS).
Basionym. Anthostoma chionostomum Speg. (as ‘chionostoma’), Anales Soc. Ci. Argent. 18(6): 267. 1884.
Typification. Paraguay (‘Brazil’), Guarapí, on bark of dead trunks, 1881, B. Balansa no. 2798 (LPS 6804, holotype).
Notes — Although it has not been possible to microscopically re-investigate the type (Spegazzini specimens from LPS are no longer sent out on loan), the detailed original description of the short whitish ostioles scarcely erumpent through the bark, in combination with the helicoid germ slit (Schrantz 1960, Hladki & Romero 2003), indicate that this species also belongs to Albicollum, in which we combine it here. In his notes to the species, Spegazzini (1884) described it as a “most beautiful species, piercing the bark with white ostioles, then to be recognised!” (translated from Latin), and photographs of the type specimen kindly provided by Jorge Alberto Chayle (LPS) confirm the presence of distinct white ostiolar collars typical for Albicollum (Fig. 5a). Line drawings of the type by Spegazzini show a section of an immersed perithecium with an ostiolar neck piercing the substrate, asci with guttulate paraphyses and ascospores (Fig. 5b; also available at http://www.cybertruffle.org.uk/spegazzini/eng/006804a_.htm (accessed 24 Aug. 2021)). Schrantz (1960: pl. XVII, 2a–d) provided line drawings of an ascomatal section, perithecial wall and ascus apices with ascospores, and Hladki & Romero (2003: f. 4A–E) also published line drawings of the type showing the whitish ostioles piercing the bark, asci, amyloid apical ascus ring and ascospores. All these data match the genus Albicollum.
Albicollum chionostomum shares ellipsoid ascospores with A. fleischhakii, A. novomexicanum and A. vincensii, but has larger ascospores measuring 26–36 × 12–20 μm with a one time helicoid coiling germ slit (Spegazzini 1884, Hladki & Romero 2003). Based on similar ascospore sizes and the helicoid germ slit, Hladki & Romero (2003) synonymised A. chionostomum with Leptomassaria simplex, which, however, is not tenable considering the different hosts, geographical distribution and the prominent white ostioles.
Albicollum fleischhakii (Auersw.) Voglmayr, comb. nov. — MycoBank MB 844601; Fig. 6
Fig. 6.
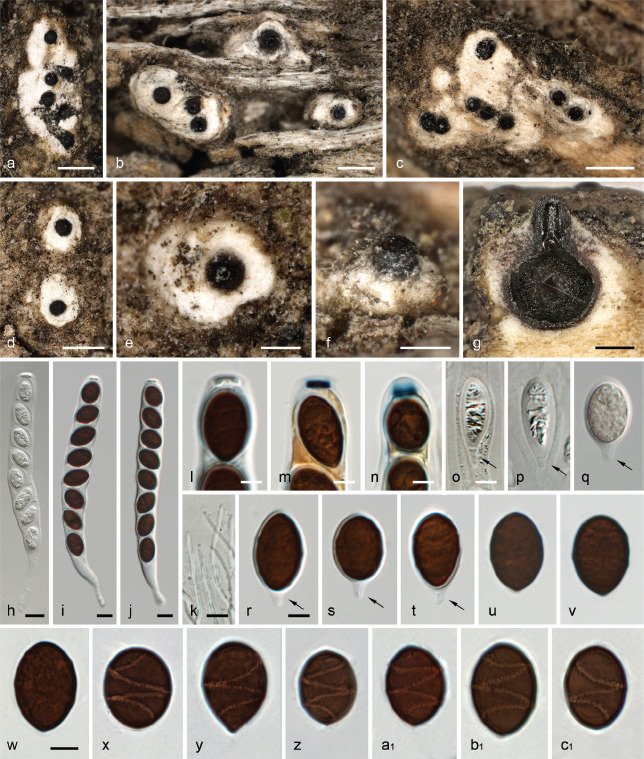
Albicollum fleischhakii. a–f. Habit of ostiolar necks on host surface; g. ascoma in vertical section; h–j. asci (h immature); k. tips of paraphyses; l–n. apical apparatuses in KOH (l), Lugol’s solution after 3 % KOH pretreatment (m) and Lugol’s solution (n); o–c1. immature (o–q) and mature (r–c1) ascospores, arrows denoting basal hyaline gelatinous secondary appendage. All in 3 % KOH, except where noted (a, g. WU 0121796; b, f. WU 0121795; c–e, i–n, u–c1. WU 01217954; h, o–t. WU 0121797 (isotypes)). — Scale bars: a–d = 500 μm; e–g = 200 μm; h–k = 10 μm; l–c1 = 5 μm.
Basionym. Sordaria fleischhakii Auersw. in Rabenh. Fungi Europaei: 1133. 1867.
Synonyms. Anthostoma fleischhakii (Auersw.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 127(8–9): 560. 1918.
Helicogermslita fleischhakii (Auersw.) Læssøe & Spooner, Kew Bull. 49(1): 48. 1994 ‘1993’.
Isotypes. Germany, Thüringen, Arnstadt, on strongly decayed boards of Picea abies, without date, Fleischhak, in Rabenhorst, Fungi Eur. Exs. 1133 (WU 0121791, WU 0121792, WU 0121793, WU 0121794, WU 0121795, WU 0121796, WU 0121797).
Pseudostromata immersed in the woody substrate and erumpent through the wood, prominently developed in the region around and below the ostioles, forming a whitish to yellowish collar or discoid area. Ascomata perithecial, immersed to barely erumpent, scattered, solitary or in small clusters up to 10, subglobose to depressed-spherical, 0.4–0.5 mm diam, with a central ostiole; ostiolar neck straight to slightly oblique, black, apically flattened, opening flush with the surface to slightly projecting; ostiolar canal 60–70 μm wide, ostiole minutely porate, at the centre of a black discoid area 150–230 μm wide. Stromatic tissue around the venters conspicuous, white, of a loose prosenchymatous tissue composed of moderately thick-walled hyaline hyphae 1.5–4 μm diam, mixed with necrotic wood cells; upper stromatic layer composed of clusters of white crystals readily dissolving in 5 % HCl mixed with necrotic wood or bark cells, frequently spreading over the host surface and forming discoid white areas 0.3–1.2 mm wide, up to 2.7 mm wide when confluent from clustered ascomata. Peridium 30–60 μm thick at sides, pseudoparenchymatous, brown to dark brown, a textura angularis grading inwardly into a subhyaline textura prismatica, composed of unevenly pigmented cells with wall 0.5–1 μm thick; ostiolar neck wall 50 μm thick at the base, dark brown, a textura angularis of small, thick-walled, subopaque cells, ostiolar canal densely periphysate. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, much longer than the asci, 3–5 μm wide at the base, gradually tapering to 1.3–1.7 μm above the asci, embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate, with (4–)8 obliquely uniseriate overlapping, frequently irregularly biseriate ascospores, (120–)135–160(–170) × (12–)13.5–17(–19.5) μm (Me = 147 × 15.2 μm, N = 46) including the 10–50(–80) μm long stipes; with an euamyloid apical apparatus strongly refractive in 3 % KOH; 1.3–3.2 μm high × 5.1–7.3 μm diam in Lugol’s solution and Melzer’s reagent, blueing, the colour fading toward the apex, short cylindrical to nearly trapezoid with a sharp lower rim. Ascospores (13.8–)16–18.3(–20) × (8.5–)10–11.8(–13) μm, Q = (1.3–)1.4–1.7(–2.2) (Me = 17.1 × 10.9 μm, Qe = 1.6; N = 162), unicellular, with a hyaline gelatinous secondary appendage commonly disappearing at full maturity, broadly ellipsoid to subglobose, slightly inequilateral, dark brown, with broadly rounded to subacute ends, with a narrow but conspicuous helicoid germ slit 0.5–0.6 μm wide coiling 1.5–2 times around the ascospore, appearing as broken down into four to six segments when seen in optical section, almost reaching the ends; epispore medium to dark brown, smooth in 3 % KOH. Asexual morph on the natural substrate not seen.
Habitat & Host range — On decayed boards of coniferous wood (Picea abies).
Known Distribution — Europe; apparently very rare; known only from France and Germany (fide Rappaz 1995); all known collections are from the 19th century.
Notes — Primarily based on the helicoid slit, the species was combined in Helicogermslita by Læssoe & Spooner (1993). This was accepted by Rappaz (1995), who described a cellular appendage of the ascospores and the extensive apical pseudostroma heavily incrusted with white crystals that surrounds the ostioles, a character typical of Albicollum. He also noted that the first valid description of the species is not by Auerswald (1868), but in the measurements and illustrations on the printed labels of Rabenhorst, Fungi Europaei 1133, and he lectotypified the species with a collection preserved in BRSL. We have not seen this lectotype specimen, but seven rich isotype specimens preserved in WU, upon which our description and illustrations are based.
No recent collections and sequence data are available for A. fleischhakii. However, pseudostroma, ascoma and ascospore morphology match the genus Albicollum. Albicollum fleischhakii differs from all other species of the genus by broadly ellipsoid to almost subglobose ascospores. In addition, the apical ascus apparatus observed in our study is comparatively flat, which, however, may be due to preservation of the old material examined, as Rappaz (1995) illustrated a much higher apical ring in line with the other species of Albicollum. After critical reinvestigation, we do not interpret the ascospore appendages as cellular, but rather as secondary appendages, i.e., extensions of a gelatinous sheath surrounding the developing spores in early stages (Fig. 6o–q), which mostly disappear in mature spores and only rarely remain as a small hyaline appendage. Similar secondary appendages were also seen in A. vincensii, which shares euamyloid apical apparatuses, non-fusiform ascospores and less prominent to fully immersed ostiolar necks. Albicollum fleischhakii is so far the only known species of the genus occurring on coniferous substrates.
Albicollum longisporum Voglmayr, J. Fourn., S. Tello & Jaklitsch, sp. nov. — MycoBank MB 844602; Fig. 7
Fig. 7.
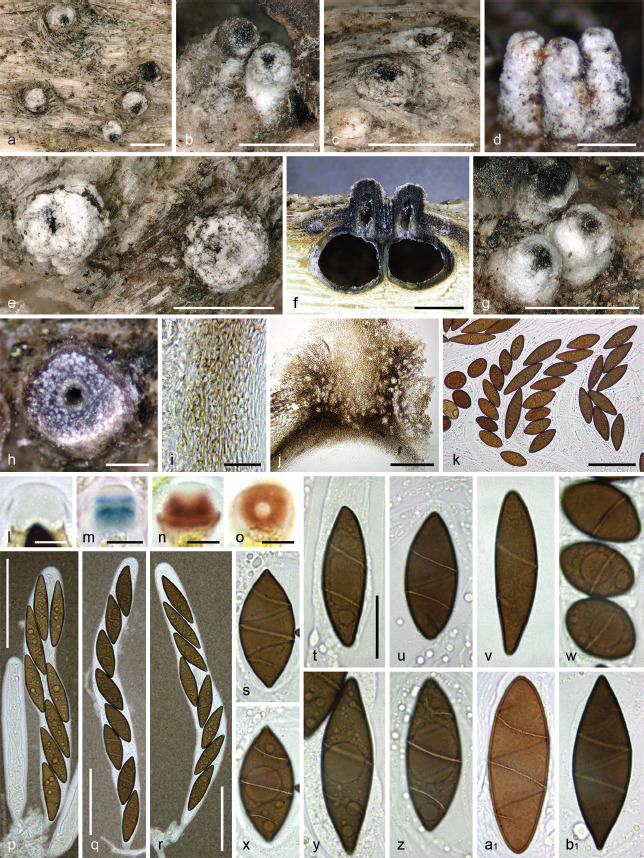
Albicollum longisporum. a–e, g. Habit of ostiolar necks on host surface; f. two adjacent ascomata in vertical section; h. base of ostiolar neck in horizontal section showing white crystals inside and around; i. peridium in vertical section, in chloral-lactophenol; j. ostiolar neck in vertical section, in chloral-lactophenol; k. variously shaped ascospores from the same hymenium, in 1 % SDS; l–o. apical apparatuses in Melzer’s reagent (l), Lugol’s solution after 3 % KOH pretreatment (m) and Lugol’s solution (n–o); p–r. asci in diluted Indian ink; s–b1. ascospores in 1 % SDS (s–z, b1) and in chloral-lactophenol (a1) (a–c, e–g, k–r, t–w, z, a1. WU-MYC 0044004 (holotype); d, h, s, x–y. WU-MYC 0044007; i–j. WU-MYC 0044009; b1. WU-MYC 0044011). — Scale bars: a–g = 0.5 mm; h, j = 100 μm; i, s–b1 = 20 μm; k, p–r = 50 μm; l–o = 5 μm.
Etymology. Referring to its long ascospores.
Holotype. Spain, Andalucía, Jaén, Valdepeñas de Jaén, La Pandera, N37°38'3.63" W3°46'53.22", 1814 m a.s.l., on a dead branch of Berberis hispanica, 15 Sept. 2017, S. Tello S.T. 15091701 (WU-MYC 0044004, culture HEV3 = CBS 147283).
Diagnosis. Differs from A. berberidicola by significantly longer ascospores 40.8 × 12.5 μm vs 22.6 × 10.8 μm on average (Qe = 3.3 vs 2.1).
Pseudostromata immersed in the woody substrate and erumpent through the bark or wood, reduced mostly to the region around and below the ostioles, forming a whitish collar. Ascomata perithecial, immersed to partly erumpent and raising the host surface, scattered, solitary or in small clusters, subglobose, 0.75–1 mm diam, with a central ostiole; ostiolar neck straight to slightly curved, black, apically flattened, (150–)200–500(–1000) μm high, 200–450 μm diam, overlain by a thick, white to off-white coarsely granular layer forming a continuous sleeve; ostioles minutely porate, at the centre of a black discoid area. Stromatic tissue around the venters inconspicuous, reduced to loosely interwoven, moderately thick-walled hyaline hyphae 3–4 μm diam, mixed with necrotic wood cells, occasionally encasing scattered clusters of white crystals; upper stromatic layer at the base of ostiolar neck brown, 180–260 μm thick, prosenchymatous, composed of light to dark brown, thin-walled to moderately thick-walled hyphae 2–4 μm diam, originating from the upper part of the peridium and the ostiolar neck wall, slightly blackening the host tissues, encasing conspicuous clusters of white crystals readily dissolving in 5 % HCl. Peridium 50–80 μm thick at sides, pale brown, turning subhyaline inwardly, a textura prismatica composed of elongate, thin-walled cells 2.5–5 μm wide to textura angularis in places, composed of thick-walled polygonal cells with wall 1.5–1.8 μm thick; darker brown at the apex, 60–100 μm thick, a textura angularis of small, thick-walled, subopaque cells, with rare white crystals; ostiolar neck wall 100–120 μm thick at the base, c. 80 μm thick above, dark brown, a textura angularis of small, thick-walled, subopaque cells, interspersed with abundant white crystals. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, with small scattered refractive guttules, much longer than the asci, 3–4 μm wide at the base, gradually tapering to 1.2–1.8 μm, embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate to subsessile, with (4–)8 obliquely uniseriate overlapping ascospores, frequently irregularly biseriate, 180–220(–230) × (18–)22–25(–26.5) μm (Me = 205 × 23.5, N = 20) including the 12–25 μm long stipes; with a hemiamyloid apical apparatus not blueing in Melzer’s reagent, barely refractive in water or in chlorazol black; 8–10 μm high × 7–10 μm diam in black Pelikan ink, stained yellow and appearing roughly hexagonal and apically convex; 3.3–6.1 μm high × 6–8.9 μm diam (Me = 4.6 × 7.7 μm, N = 50) in Lugol’s solution, reddish brown to dirty red, short-cylindrical to slightly trapezoid, with an apical depression and a convex base with sharp lateral rims; 2.5–5.8 μm high × 5.8–9.2 μm diam (Me = 4.6 × 7.5 μm, N = 50) in Lugol’s solution or in Melzer’s reagent after 30 s pretreatment in 3 % KOH, bluish grey to dark blue, discoid to short-cylindrical to nearly trapezoid and apically flared, occasionally with basal rims curving upward, frequently appearing obscurely annellate or unevenly stained. Ascospores (21.4–)28.5–59.4(–67.5) × (8.5–)9.6–16(–19) μm, Q = (1.2–)2–5.1(–5.8) (Me = 40.8 × 12.5 μm, Qe = 3.3; N = 960), aseptate, highly variable in shape and dimensions even within a single ascoma, ellipsoid-fusiform, occasionally broadly ellipsoid, equilateral to subequilateral, dark brown, with obtusely rounded to acute, occasionally subapiculate ends; germ slit narrow but conspicuous, 0.6–0.8 μm wide, helicoid, obliquely 2–2.5 times coiled around the ascospore, appearing as broken down into four to six segments when seen in optical section, straight to slightly curved toward the ends, not reaching the ends; epispore medium to dark brown, smooth; no gelatinous sheath visible in Indian ink, even around immature hyaline ascospores. Asexual morph on the natural substrate not seen.
Habitat & Host range — On dead branches of various deciduous shrubs and trees; confirmed from Acer, Berberis, Crataegus, Lonicera, Pistacia, Prunus, Rhamnus, Rosa, Stahaelina.
Known Distribution — Europe and North Africa; confirmed from Finland, Germany, Greece, Morocco and Spain.
Other specimens examined (paratypes). Greece, Crete, Analipsis, 1400 m a.s.l., on decorticated weathered twigs of Berberis cretica, soc. Albicollum berberidicola, 16 Oct. 2014, W. Jaklitsch (WU-MYC 0043994). – Morocco, Moyen Atlas, Col de Tafliloute, on branch of Acer monspessulanum, 27 May 1962, R. Bertaut II.095 172/P 62 (MPU B00047). – Spain, Andalucía, Jaén, Fuensanta de Martos, Sierra de la grana, N37°36'18.63" W3°54'57.78", 765 m a.s.l., on a dead twig of Staehelina dubia, 6 May 2019, S. Tello S.T.06051902 (WU-MYC 0044005, culture HEV15); Jaén, Valdepeñas de Jaén, El Parri-zoso, 30SVG 34397 63415, N37°36'55.81" W3°44'36.02", 1125 m a.s.l., on dead twigs of Pistacia terebinthus, 9 Apr. 2019, S. Tello S.T.09041902 (WU-MYC 0044006, culture HEV10); Jaén, Valdepeñas de Jaén, La Pandera, N37°37'47.30" W3°46'21.06", 1786 m a.s.l., on a decorticated twig of Berberis hispanica c. 1 cm diam, soc. Albicollum berberidicola, 14 Sept. 2017, S. Tello S.T.14091701 (WU-MYC 0044009, culture HEV4); ibid., N37°37'52.91" W3°46'31.91", 1818 m a.s.l., on a dead twig of Rosa sicula, 1 Oct. 2017, S. Tello S.T.01101701 (WU-MYC 0044008, culture HEV5); ibid., N37°37'52.46" W3°46'10.55", 1784 m a.s.l., on a decorticated twig of Rhamnus myrtifolia c. 0.5 cm diam, 20 Oct. 2017, S. Tello S. T.20101702 (WU-MYC 0044010, culture HEV6); ibid., N37°37'54.31" W3°46'12.74", 1790 m a.s.l., on a dead decorticated branch of Rhamnus saxatilis, soc. Albicollum canicolle, 15 Oct. 2017, S. Tello S.T.15101704 (WU-MYC 0044011, culture HEV7); ibid., N37°37'54.30" W3°46'12.73", 1790 m a.s.l., on a decorticated branch of Prunus prostrata, soc. Albicollum canicolle, 15 Oct. 2017, S. Tello S.T.15101701 (WU-MYC 0040043); ibid., N37°37'52.71" W3°46'11.39", 1787 m a.s.l., on a decorticated branch of Prunus mahaleb, 20 Oct. 2017, S. Tello S. T.20101705 (WU-MYC 0040044); ibid., 30SVG 32480 65039, N37°37'48.00" W3°45'54.76", 1750 m a.s.l., on dead wood of Prunus mahaleb, 10 June 2019, S. Tello S.T.10061901 (WU-MYC 0044007, culture HEV17); Jaén, Valdepeñas de Jaén, Puerto de las Coberteras, 30SVG 32576 61710, N37°36'49.75" W3°45'38.22", 1340 m a.s.l., on a dead twig of Lonicera arborea still attached to the plant, 7 May 2019, S. Tello S.T.27051901 (WU-MYC 0044012, culture HEV16); Jaén, Valdepeñas de Jaén, Puerto de Ranera, N37°37'34.62" W3°49'22.03", 1215 m a.s.l., on a dead branch of Crataegus monogyna, soc. Albicollum canicolle, 8 May 2019, S. Tello S.T.08051902 (WU-MYC 0044013, culture HEV14).
Notes — Albicollum longisporum is distinguished from A. canicolle, with which it may co-occur on the same substrate, by on average significantly longer ascospores (40.8 × 12.5 μm, Qe = 3.2 vs 30.0 × 11.2 μm, Qe = 2.7). However, owing to considerable variations in ascospore shape and dimensions, even within a single ascoma, the spore sizes of both species overlap. Therefore, ascospore dimensions must be assessed on mean values of a significant number of measurements. The main differential character is the germ slit morphology, in A. longisporum less than 1 μm wide and coiling usually more than two times around the ascospore, vs wider (1.8–2.7 μm wide) and rarely coiling over 1.5 time in A. canicolle. Additional differential characters are the smooth-walled and only occasionally apiculate ascospores of A. longisporum.
Ascospores of A. berberidicola share a narrow and long germ slit with those of A. longisporum but they differ in being significantly smaller and more broadly ellipsoid (22.6 × 10.8 vs 40.8 × 12.5 μm on average, Qe = 2.1 vs 3.3). Both species were encountered intermingled in two collections (WU-MYC 0043994 and WU-MYC 0044009).
Beautiful illustrations of a recent Spanish collection matching A. longisporum are available at http://www.centrodeestudiosmicologicosasturianos.org/?p=303 (accessed 6 Apr. 2022), in which ascospores more typical of A. canicolle can also be observed, suggesting the occurrence of both species closely intermingled on the substrate. A Moroccan collection preserved as MPU B00047 filed under the unpublished name Anthostoma monspessulana Bertault undoubtedly represents A. longisporum, according to the detailed description and illustrations attached to the specimen (see https://science.mnhn.fr/institution/um/collection/mpu/item/mpub00047?listIndex=3&listCount=3, accessed 6 Apr. 2022).
Albicollum novomexicanum Voglmayr, J. Fourn., Baral & Jaklitsch, sp. nov. — MycoBank MB 844603; Fig. 8
Fig. 8.

Albicollum novomexicanum. a–d. Habit of ostiolar necks on host surface (a rehydrated, b–d dry); e–f. ascomata in vertical section (e. rehydrated, f. dry); g. peridium in vertical section, in 1 % SDS; h. ostiolar neck tissue in vertical section in 3 % KOH; i. periphyses in 3 % KOH; j–m. apical apparatuses in Lugol’s solution (j. low concentration, k. high concentration), Lugol’s solution after 3 % KOH pretreatment (l) and Melzer’s reagent (m); n–p. asci in 3 % KOH; q–x. ascospores (q–r. in Melzer’s reagent; s–t in 1 % SDS (flattened by pressure); u–x in Lugols solution followed by 1 % SDS) (holotype WU-MYC 0040048). — Scale bars: a, e = 500 μm; b–d =100 μm; f = 200 μm; g–i =10 μm; j–m, q–x = 5 μm; n–p = 20 μm.
Etymology. Referring to its occurrence in New Mexico, USA.
Holotype. USA, New Mexico, Chihuahuan Desert, 25.5 km SE of Carlsbad, Malaga, N32°14'06" W104°04'35", 910 m a.s.l., on wood of decoricated weathered branch of Koeberlinia spinosa, soc. Orbilia pleiomesaverdiana, 17 June 1996, G. Marson (ex H.B. 6019b, WU-MYC 0040048).
Diagnosis. Differs from A. vincensii by hemiamyloid ascal apical rings and germ slits coiled 1.5–2 times.
Pseudostromata immersed in the woody substrate and erumpent from it, reduced mostly to the region around and below the ostioles, forming a whitish collar or discoid area. Ascomata perithecial, immersed, scattered, solitary or in small clusters, subglobose to depressed-spherical, 330–400 μm high, 480–550 μm diam, with a central ostiole 200–250 μm diam; ostiolar neck straight, black, apically flattened, opening flush with the surface to slightly prominent up to 250 μm high (total height of ostiole 220–350 μm); ostiole minutely porate, at the centre of a black discoid area 90–120 μm wide. Stromatic tissue around the venters inconspicuous, reduced to a loose prosenchymatous tissue composed of moderately thick-walled hyaline hyphae 3–4 μm diam, mixed with necrotic wood cells; upper stromatic layer composed of clusters of hyaline crystals mixed with necrotic wood cells, frequently spreading over the host surface and forming discoid white areas 100–300 μm wide. Peridium 25–35 μm thick at sides, pseudoparenchymatous, brown to dark brown, a textura angularis grading inwardly into a subhyaline textura prismatica, composed of unevenly pigmented cells; ostiolar neck wall 60–80 μm thick at the base, dark brown, a textura angularis of thick-walled, brown cells; ostiolar canal 40–50 μm wide, densely periphysate. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, with scattered small refractive guttules, much longer than the asci, 3–4 μm wide at the base, gradually tapering to 1.2–1.7 μm above the asci, embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate, with (4–)8 obliquely uniseriate overlapping, sometimes irregularly biseriate ascospores, (131–) 142–161(–171) × (12–)14–19(–23) μm (Me = 151 × 16.5 μm, N = 36) including the 10–15 μm long stipes; with a hemiamyloid apical apparatus, barely refractive in water, highly refractive in KOH, faintly greenish blue in Melzer’s reagent, reddish brown to dirty red in highly concentrated Lugol’s solution, blue in Lugol’s solution with low concentration (RB type, Baral 2009), short-cylindrical to slightly trapezoid, with an apical depression and a small ocular chamber, occasionally with sharp lateral rims, (3–)4–6(–7.5) μm high × 4.7–6.5 μm diam (Me = 5 × 5.7 μm, N = 18), frequently appearing obscurely annellate or unevenly stained; 3–4 μm high × 3.8–5.1 μm diam (Me = 3.1 × 4.5 μm, N = 13) in Lugol’s solution after pretreatment in 3 % KOH, dark blue, short-cylindrical to slightly trapezoid, with basal lateral rims. Ascospores (19–)21.5–24.5(–27) × (9–)9.7– 10.8(–11.5) μm, Q = (1.7–)2–2.5(–2.8) (Me = 23 × 10.3 μm, Qe = 2.2; N = 100), aseptate, ellipsoid to narrowly ellipsoid, equilateral, dark brown, with broadly rounded or sometimes obtuse ends, with a narrow but conspicuous helicoid germ slit 0.4–0.6 μm wide coiling 1.5–2 times around the ascospore, almost reaching the ends; epispore medium to dark brown, smooth; no gelatinous sheath visible; containing high amounts of lipid. Asexual morph on the natural substrate not seen.
Habitat & Host range — On wood of dead branches of Koeberlinia spinosa.
Known Distribution — Only known from the type collection in New Mexico (USA).
Notes — Morphologically, the immersed, solitary to aggregated perithecia with black, erumpent ostiolar necks surrounded by a white collar of pseudostromatic tissues place the species in the genus Albicollum. As no living culture of A. novomexicanum was available, no multi-gene data could be generated for the species. However, the ITS-LSU rDNA was successfully sequenced directly from ascomatal contents, and the multi-gene analyses placed it within the Albicollum clade with maximum support (Fig. 1).
Albicollum novomexicanum is most similar to A. vincensii with which it shares ascospores of similar size and shape and ostioles not to only scarcely protruding above the substrate. However, the ascospores of A. novomexicanum are equilateral, have more often coiled germ slits (1.5–2 times vs once in A. vincensii) and more broadly rounded ascospore ends. In addition, A. novomexicanum was collected in a semiarid desert habitat in western North America, while A. vincensii has so far been only confirmed from Europe where it occurs on various woody substrates in (sub)mediterranean habitats. Albicollum novomexicanum was provisionally identified as Helicogermslita celastri, under which name it is mentioned as associated fungus of Orbilia pleiomesaverdiana in Baral et al. (2020: 1150).
Albicollum vincensii (G. Arnaud) Voglmayr, J. Fourn., S. Tello & Jaklitsch, comb. nov. — MycoBank MB 844604; Fig. 9, 10
Fig. 9.
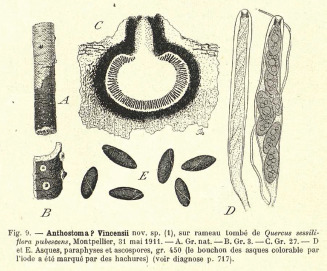
Albicollum vincensii (iconotype; lectotype of Anthostoma vincensii; Arnaud 1925: 662).
Fig. 10.

Albicollum vincensii. a–c, f–g. Habit of ostiolar necks on host surface; d. ascoma in vertical section, in chloral-lactophenol; e. peridium in vertical section, in chloral-lactophenol; h–j. apical apparatuses in chlorazol black (h), Lugol’s solution (i) and Melzer’s reagent (j); k–m. ascomata in vertical section showing a variously developed pseudostroma; n. immature and mature asci, in black Pelikan ink; o. ascus in 1 % SDS; p. ascospore with hyaline gelatinous secondary appendage in Lugol’s solution; q–w. ascospores in 1 % SDS (a, c, i, p. WU-MYC 0044014 - epitype; b, d–e, m–n. WU-MYC 0044017; f–g, k–l, q–w. WU-MYC 0044018; h, j, o. WU-MYC 0044019). — Scale bars: a–c, f–g, k–m = 500 μm; d = 200 μm; e, p–w = 10 μm; h–j = 5 μm; n–o = 50 μm.
Basionym. Anthostoma vincensii G. Arnaud, Les Astérinées: 717. 1925.
Typification. G. Arnaud, Les Astérinées. IV.e partie (Études sur la systématique des champignons pyrénomycétes). Ann. Sci. Nat. Hist. Bot., Ser. 10, Vol. 7, 1925, p. 662, f. 9A–E (iconotype; lectotype here designated, MBT 10007730). – Austria, Burgenland, Siegendorf, Siegendorfer Puszta, N47°46'46" E16°34'53", 170 m a.s.l., on a dead corticated twig of Quercus pubescens, 28 Mar. 2015, G. Friebes (WU-MYC 0044014, epitype here designated, MBT 10007722; ex epitype culture ARQ = CBS 147286).
Pseudostromata immersed in the woody substrate and erumpent through bark or wood, reduced mostly to the region around and below the ostioles, forming a whitish to yellowish collar or discoid area. Ascomata perithecial, immersed to barely erumpent, scattered, solitary or in small clusters, subglobose to depressed-spherical, 0.5–1 mm diam, with a central ostiole; ostiolar neck straight to slightly oblique, black, apically flattened, opening flush with the surface to slightly prominent up to 350 μm high; ostiole minutely porate, at the centre of a black discoid area. Stromatic tissue around the venters inconspicuous, reduced to a loose prosenchymatous tissue composed of moderately thick-walled hyaline hyphae 3–4 μm diam, mixed with necrotic wood cells; upper stromatic layer composed of clusters of white crystals readily dissolving in 5 % HCl mixed with necrotic wood or bark cells, frequently spreading over the host surface and forming discoid white areas 250–500 μm wide. Peridium 35–60 μm thick at sides, up to 100–110 μm thick at the apex, pseudoparenchymatous, brown to dark brown, a textura angularis grading inwardly into a subhyaline textura prismatica, composed of unevenly pigmented cells with wall 0.8–1.2 μm thick; ostiolar neck wall 80–120 μm thick at the base, dark brown, a textura angularis of small, thick-walled, subopaque cells, interspersed with abundant white crystals; ostiolar canal densely periphysate, containing free white crystals. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, with scattered small refractive guttules, much longer than the asci, 3–4 μm wide at the base, gradually tapering to 1.2–1.8 μm above the asci, embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate, with (4–)8 obliquely uniseriate overlapping, frequently irregularly biseriate ascospores, 130–160 × 16–20 μm (Me = 142 × 17.7 μm, N = 20) including the 15–25 μm long stipes; with an euamyloid apical apparatus barely refractive in water or in chlorazol black; 8.5–9 μm high × 7–8 μm diam in black Pelikan ink, stained yellow and appearing roughly hexagonal and apically convex; 4–6.5 μm high × 3–5.3 μm diam (Me = 4.9 × 4 μm, N = 70) in Melzer’s reagent and Lugol’s solution, blueing in Melzer’s reagent, darker blue in Lugol’s solution, the colour fading toward the apex, short-cylindrical to slightly inverted-trapezoid, apically flared. Ascospores (18.8–)20.5–27(–28.5) × (7–)8–11.4(–12.2) μm, Q = (1.8–)2–2.9(–3.2) (Me = 24.5 × 10.1 μm, Qe = 2.4; N = 360), aseptate, ellipsoid to narrowly ellipsoid, slightly inequilateral, dark brown, with narrowly to broadly rounded ends, when young with a secondary appendage, with a narrow but conspicuous helicoid germ slit 0.5–0.6 μm wide coiling once around the ascospore, mostly located on the less convex side of inequilateral ascospores, almost reaching the ends; epispore medium to dark brown, smooth when observed after 48 h incubation in PVA-lactophenol; no gelatinous sheath or appendages visible in Indian ink, even around immature hyaline ascospores. Asexual morph on the natural substrate not seen.
Habitat & Host range — On dead branches of various deciduous shrubs and trees; confirmed from Acer, Berberis, Crataegus, Lonicera, Pistacia, Quercus, Retama, Rubus.
Known Distribution — Infrequent but widely distributed in Central and Southern Europe; known from Austria, France, Italy and Spain. A record from Taiwan (Hsieh et al. 1995) needs confirmation.
Other specimens examined. France, Vendée, La Tranche-sur-Mer, La Terrière-Plage, coastal Quercus ilex forest, N46°21'23.2" W1°28'21.9", 5–10 m a.s.l., on a dead corticated twig of Quercus ilex, 3 June 2003, F. Candoussau 745 (J.F. 03081 (overmature) = WU-MYC 0044015, WSP 74225). – Italy, south of Florence, north of Tavarnelle, Romita, N43°34'46.7" E11°11'28", 318 m a.s.l., Quercus pole in vineyard, 28 Oct. 2015, W. Jaklitsch (WU-MYC 0044016, culture RQ1). – Spain, Andalucía, Jaén, Valdepeñas de Jaén, El Parrizoso, carretera del Quiebrajano, N37°38'13.77" W3°4359.48", 735 m a.s.l., on dead corticated twig 10 mm diam of Pistacia lentiscus, soc. Thyronectria pistaciae, 17 Apr. 2017, S. Tello S.T.17041701 (WU-MYC 0044017, culture HEV1); Jaén, Valdepeñas de Jaén, El Parrizoso, near the dam of Quiebrajano, N37°37'59.93" W3°43'47.46", 710 m a.s.l., on dead weathered twigs 10–15 mm diam of Rubus ulmifolius, 29 Apr. 2016, S. Tello S.T.29041601 (WU-MYC 0044018, culture HEV2); Jaén, Valdepeñas de Jaén, El Parrizoso, N37°38'2.83" W3°44'3.70", 881 m a.s.l., on a dead decorticated branch of Retama sphaerocarpa, 8 Mar. 2018, S. Tello S.T.08031803 (WU-MYC 0044019, culture HEV12).
Notes — The six collections studied match in all respects the protologue of Anthostoma vincensii and the excellent illustrations provided by Arnaud (1925). Despite an extensive search, no type collection could be located in various French herbaria, nor in the herbarium database of French herbaria (https://science.mnhn.fr/all/search/form, accessed 26 July 2021). Likewise, also Rappaz (1995) was unable to locate a type specimen. Therefore, the original illustration in Arnaud (1925: 662, f. 9) is here selected as lectotype (Fig. 9). To ensure nomenclatural stability, a recent Austrian collection (WU-MYC 0044014) from the same host as the type, Q. pubescens, for which a culture and DNA data are available, is here selected as an epitype.
Albicollum vincensii shares the characteristic individual, apical pseudostroma heavily incrusted with white crystals, asci with a massive apical apparatus and large ascospores with a helicoid slit with other known species. Its ascospore size and shape are similar to A. novomexicanum, which differs by hemiamyloid apical apparatuses and by germ slits coiling 1.5–2 times. From the residual Albicollum species it mainly differs by ellipsoid, non-fusiform ascospores with a less strongly coiling germ slit and the reduced to absent brown prosenchymatous tissue in the pseudostroma. Further differences, which are shared with A. fleischhakii, are the euamyloid apical apparatuses, secondary appendages in young ascospores, the less prominent to fully immersed ostiolar necks, and the white granular tissue spreading over the host surface around the ostioles.
Based on similar ascospores, Læssøe & Spooner (1993) synonymised Anthostoma vincensii with Rosellinia (= Helicogermslita) gaudefroyi which also occurs on Quercus; however, our investigations show that the latter represents a distinct, unrelated taxon which is here reclassified as Spiririma gaudefroyi (see below). This synonymy was already questioned by Hsieh et al. (1995) who noted that the illustrations of A. vincensii in Arnaud (1925) showed perithecia with distinctly beaked ostioles, while no beak was mentioned in the original description of Rosellinia gaudefroyi.
The identity of the Taiwanese record of A. vincensii described and illustrated by Hsieh et al. (1995) remains to be confirmed. While the perithecial, ascus and ascospore characters fit the species well, there is no mention of the distinct whitish pseudostromatic tissues around the ostiolar necks that are highly distinctive for the species.
KEY TO SPECIES OF ALBICOLLUM
1. Ascospores mostly with rounded ends, subglobose to narrowly ellipsoid; apical ascusring eu- or (in A. novomexicanum) hemiamyloid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. Ascospores mostly with subacute or apiculate ends, fusiform to ellipsoid; apical ascus ring hemiamyloid . . . . . . . . . . . . 5
2. Ascospores on average shorter than 20 μm, broadly ellipsoid to subglobose . . . . . . . . . . . . . . . . . . . . . . . . A. fleischhakii
2. Ascospores on average longer than 20 μm, ellipsoid to narrowly ellipsoid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Ascospores 26–36 × 12–20 μm; only known from South America (Argentina) . . . . . . . . . . . . . . . . A. chionostomum
3. Ascospores 19–28 μm long, narrower than 12 μm . . . . . 4
4. Apical ascus ring hemiamyloid; ascospore germ slit coiled 1.5–2 times; only known from the western USA (New Mexico) . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. novomexicanum
4. Apical ascus ring euamyloid; ascospore germ slit coiled once; only known from Europe . . . . . . . . . . . . . . . . . A. vincensii
5. Ascospore germ slit coiled 1–1.5 times, Z-shaped in optical section and far from reaching the ascospore ends, 1.8–2.7 μm wide; ascospores 25–37 × 8–13 μm . . . . . . . . A. canicolle
5. Ascospore germ slit coiled more than twice, less than 0.8 μm wide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6. Ascospores 20–26 × 9.5–12.5 μm, Q = 1.8–2.4; germ slit (almost) reaching ascospore ends . . . . . . A. berberidicola
6. Ascospores 28–60 × 9.5–16 μm, Q = 2–5.1; germ slit not reaching ascospore ends . . . . . . . . . . . . . . A. longisporum
Digitodochium Tubaki & Kubono, Sydowia 41: 344. 1989
Type species. Digitodochium rhodoleucum Tubaki & Kubono
Emended description: Genus of Xylariaceae. Pseudostromata erumpent from bark, scattered, pustulate to pulvinate, consisting of a white ectostromatic disc and perithecia embedded in necrotic bark cells mixed with inconspicuous entostromatic hyphae. Ostiolar necks few per disc, barely protruding, black, convergent; ostiolar canal periphysate. Entostroma inconspicuous, reduced. Perithecia arranged in valsoid configuration with ostiolar necks central to eccentric, subglobose. Peridium pseudoparenchymatous, brown. Paraphyses hyphal, hyaline, thin-walled, septate, branched, much longer than the asci, embedded in a mucilaginous matrix. Asci cylindrical to fusiform, short-stipitate, with (4–)8 ascospores and a massive, tubular to barrel-shaped, bipartite, euamyloid apical apparatus. Ascospores aseptate, ellipsoid to subcitriform, equilateral, brown; germination site discoid to ellipsoid, conspicuous, usually central but occasionally eccentric; epispore medium to dark brown, smooth; with conspicuous mucilaginous secondary appendages in Indian ink, not stained by aqueous nigrosin. Conidiomata on natural substrate erumpent from bark, sporodochial, cushionshaped, hemispherical to irregular, pinkish, yellow to orange. Conidiophores micronematous, aggregated, hyaline, branched, terminally bearing conidia. Conidiogenesis holoblastic. Conidia subhyaline, staurosporous, consisting of a cylindrical main axis with whorls of lateral branches arising from the basal cells of the main axis, the basal cells of these lateral branches bearing side branches again, dry in mass.
Notes — The genus Digitodochium is based on D. rhodoleucum, an asexual morph which was described from corticated twigs of Fagus crenata in Japan (Tubaki & Kubono 1989). Until now, no connection with a sexual morph was known. Morphology and sequence data leave no doubt that Anthostoma amoenum is closely related to Digitodochium rhodoleucum, and Anthostoma amoenum is therefore combined in Digitodochium.
The genus Digitochium is remarkable in several respects. Phylogenetically, it is a member of the most basal clade of Xylariaceae s.str., and is closely related to Clypeosphaeria mamillana (Fig. 1). The staurosporous asexual morph is unique and unusual within Xylariaceae. Besides its distinctive asexual morph, Digitodochium can be diagnosed by Lopadostoma-like pseudostromata overlain by a white disc and ascospores with a lateral poroid germination site. Within the Xylariaceae, this distinctive feature is only known from the unrelated Nemania species formerly classified within Euepixylon (see below) and Xylaria rickii (Dennis 1956, Fournier et al. 2020).
Digitodochium amoenum (Nitschke ex Sacc.) Voglmayr, J. Fourn. & Jaklitsch, comb. nov. — MycoBank MB 844605; Fig. 11, 12
Fig. 11.

Digitodochium amoenum, sexual morph. a–e. Habit of ectostromatic discs on host surface in face view; f. pseudostroma and perithecia in vertical section; g. perithecium in vertical section showing strongly melanized ostiolar neck; h. lateral peridium in vertical section; i. entostromatic hyphae surrounding the base of a perithecium; j. structure of the ostiolar neck at the base, in vertical section; k–n. apical apparatuses in Lugol’s solution (k), Melzer’s reagent (l–m) and aqueous Congo red (n); o–q. asci in diluted Indian ink (o–p) and 1 % SDS (q); r. ascospore showing bipolar thickenings (arrows) and conspicuous mucilaginous secondary appendages, in diluted Indian ink; s–u. ascospores showing a discoid germination pore and apical thickenings (arrows); v. immature ascospore; w–y. giant, inequilateral abnormal ascospores showing an ellipsoid germination pore on the convex side. (g, l–n in chloral-lactophenol; s–y in 1 % SDS) (a, d, i, k, p–y. WU-MYC 0044023; b–c, e–h, j, l–o. J.F. 08072). — Scale bars: a, c–f = 500 μm; b, n = 200 μm; g, l–m, r–y = 10 μm; h–k = 5 μm; o–q = 50 μm.
Fig. 12.
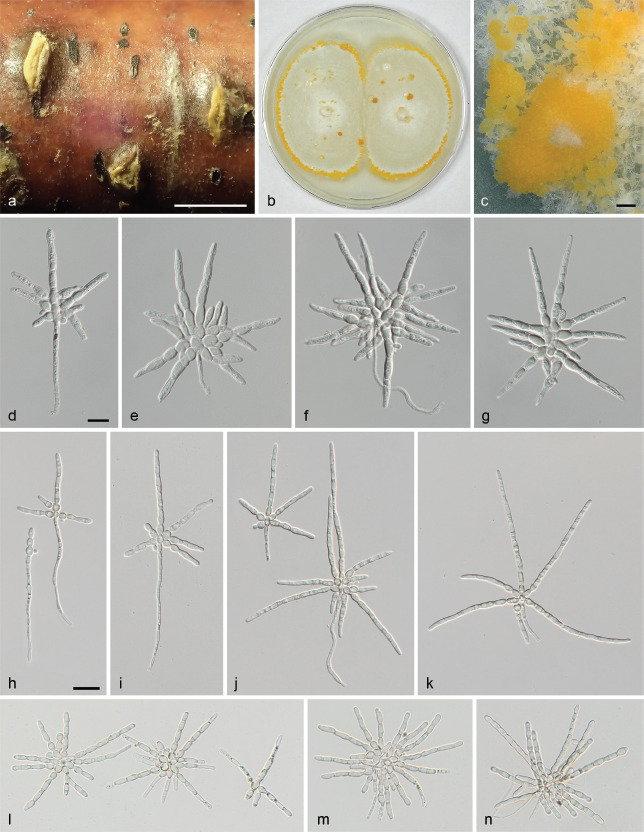
Digitodochium amoenum, asexual morph. a. Habit of sporodochial conidiomata on natural substrate; b. culture with yellow sporodochia (CMD, 20 °C, 16 wk); c. sporodochium on CMD; d–g. conidia from natural substrate; h–n. conidia in pure culture (CMD). All in water (a, d–g. WU-MYC 0044024; c, h–k. DIG; b, l–n. AAM). — Scale bars: a = 1 mm; c = 500 μm; d–g = 10 μm; h–n = 20 μm.
Basionym. Anthostoma amoenum Nitschke ex Sacc., Syll. Fung. (Abellini) 1: 307. 1882.
Synonyms. Fuckelia amoena Nitschke, in Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 224. 1870, nom. inval., Art. 35.1 (Melbourne).
Lopadostoma amoenum (Nitschke ex Sacc.) Shear, Mycologia 30(5): 593. 1938.
Typification. Germany, Hessen, Oestrich im Rheingau, on corticated branches of Fagus sylvatica, without date, L. Fuckel, in Fuckel, Fungi Rhen. Exs. 2052 (B 700009215, lectotype of Anthostoma amoenum here designated, MBT 10007731; WU 0121802, isotype (depauperate). – Austria, Niederösterreich, Rastenfeld, Dobra-Urwald, N48°35'18.8" E15°23'51.1", 500 m a.s.l., on corticated thin branches of Fagus sylvatica, 21 May 2020, H. Voglmayr & I. Krisai-Greilhuber(WU-MYC 0044020, epitype of Anthostoma amoenum here designated, MBT 10007723; ex-epitype culture AAM1 = CBS 147285).
Pseudostromata erumpent from bark, rupturing the periderm, scattered, pustulate to pulvinate, consisting of a white ectostromatic disc and perithecia embedded in necrotic bark cells mixed with inconspicuous entostromatic hyphae. Ectostromatic discs 0.25–1 mm diam, occasionally confluent up to 1.4 mm diam, rounded to polygonal in outline, overlain by a white, fibrous to crumbly tissue composed of tightly intertwined hyaline hyphae 1.5–2.5 μm diam mixed with amorphous colourless substance, gradually wearing off to expose a greyish brown surface, projecting up to 0.5 mm. Ostiolar necks 1–4 per disc, barely protruding, conical with truncate or slightly convex apices, shiny black, smooth, convergent, 120–170 μm diam at the apex; ostiolar canal periphysate. Entostroma inconspicuous, reduced to necrotic bark cells and loosely intertwined, diverticulate hyaline hyphae 2–4 μm diam, mostly present around the base of the perithecia. Perithecia 330–420 μm diam, arranged in valsoid configuration with central to frequently eccentric ostiolar necks, subglobose with usually distinctly flattened base. Peridium 22–35 μm thick, pseudoparenchymatous, brown, a textura angularis of small isodiametric cells with unevenly pigmented wall 1–1.2 μm thick, grading inwardly into larger and slightly prismatic cells; innermost layer inconspicuous, composed of thin-walled, hyaline prismatic cells. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, rarely branched, with scattered small, globose to large and oblong refractive guttules, much longer than the asci, 3–4.5 μm wide at the base, gradually tapering to 1.5–2 μm above the asci, discretely embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate, with (4–)8 obliquely uniseriate overlapping, rarely biseriate ascospores, 170–197 × 12–14.5 μm (Me = 182 × 12.9 μm, N = 20) including the 18–30 μm long fragile stipes; with a massive, tubular to barrel-shaped, bipartite apical apparatus 5.9–7.8 × 4.3–6.6 μm (Me = 6.9 × 5.5 μm, N = 20), not stained in aqueous Congo red, featuring an euamyloid discoid to crown-like basal part with sharp lateral rims, 2.2–4.5 μm high × 4.7–6.7 μm wide (Me = 3.5 × 5.6 μm, N = 40), apically convex, blueing in Melzer’s reagent, darker blue in Lugol’s solution, light yellow in black Pelikan ink; upper part ill-defined, apically convex, hyaline in water, inamyloid in Melzer’s reagent or faintly blueing in the transition zone to the lower half, barely stained blue in diluted blue Pelikan ink. Ascospores (20–)21–29.6(–33.5) × (8.5–)9.2–13(–14) μm, Q = (1.6–)1.7–2.9(–3.2) (Me = 25.2 × 11 μm, Qe = 2.3; N = 230), aseptate, ellipsoid to narrowly ellipsoid or subcitriform, equilateral, dark brown, with narrowly rounded, frequently slightly pinched ends, with a low, inconspicuous bipolar blister-like thickening; germination site circular to slightly elliptic, 3–4 μm diam, conspicuous, usually central but occasionally eccentric, on the convex side of abnormal inequilateral ascospores; epispore medium to dark brown, smooth when observed after 48 h incubation in PVA-lactophenol; conspicuous pad-like mucilaginous secondary appendages can be detected in Indian ink, that are easily overlooked in water and are not stained by aqueous nigrosin. Conidiomata on natural substrate erumpent from bark, sporodochial, cushion-shaped, hemispherical to irregular, pale yellow to bright orange, 0.5–1 mm diam, 150–300 μm high. Conidiophores micronematous, aggregated, hyaline, 1.5–2.5 μm wide, branched, bearing conidiogenous cells and conidia terminally. Conidiogenous cells integrated; conidiogenesis holoblastic. Conidia on natural substrate (55–)75–100(–110) μm diam, N = 25 (Me = 85 μm), subhyaline, staurosporous, consisting of a cylindrical main axis with whorls of lateral branches arising at wide angles from the basal 1–3 cells of the main axis, the basal cells of these lateral branches bearing side branches, too; main axis 43–80 μm long, side branches (12–)20–50 μm long; conidial cells constricted at the septa, at the base of the branches subglobose-ellipsoid, 4.3–6.5 μm wide, with strong constrictions at the septa, becoming elongated, 2.2–4.8 μm wide and less constricted at the septa towards the gradually tapering, narrowly rounded tips. Culture on CMD at 22 °C reaching 35 mm after 1 mo, white, without aerial mycelium, producing bright yellow sporodochia in the centre, scattered over the culture, or in concentric rings at the margins. Conidia similar to those on natural substrate, but more variable and often with distinctly longer branches.
Habitat & Host range — In bark of Fagus sylvatica.
Known Distribution — Europe; confirmed for Austria, Denmark, France, Germany, Norway, Slovenia, Spain, Sweden, Switzerland and Ukraine (Læssoe & Spooner 1993, Hayova 2012, Eriksson 2014, Brackel & Zehm 2020).
Additional specimens examined (all on dead corticated twigs of Fagus sylvatica). Austria, Niederösterreich, N of A21 exit Heiligenkreuz, Füllenberg, N48°03'34" E16°08'40", 400 m a.s.l., 14 Nov. 2020, H. Voglmayr & I. Krisai-Greilhuber (WU-MYC 0044021); Gmünd, Sankt Martin, Joachimstal, Riesenkopf, Luxensteinwand, N48°38'31" E14°44'28", 820 m a.s.l., 31 Oct. 2021, H. Voglmayr & I. Krisai-Greilhuber (WU-MYC 0045129); Oberösterreich, Bez. Grieskirchen, Natternbach, Hochstrass SW Landersberg, N48°23'16" E13°42'10", 540 m a.s.l., 24 Oct. 2021, H. Voglmayr (WU-MYC 0045130); Wien, Hernals, Schottenwald, Moosgraben, N48°13'30" E16°15'34", 300 m a.s.l., 6 Dec. 2021, M. Ploderer (WU-MYC 0045128). – France, Ardennes, Damouzy, Sorel, Bois de la Havetière, N49°48'28" E4°42'06", 300 m a.s.l., 18 Oct. 2007, R. Jouan & R. Collot, comm. C. Lechat CLL 7145 (WU-MYC 0044022, depauperate); Deux Sèvres, Villiers-en-Bois, Forêt de Chizé, 80 m a.s.l., 14 Apr. 2008, P. Leroy (J.F. 08072) (WU-MYC 0033533, culture MUCL 51842). – Germany, Baden-Württemberg, Gerstetten, Kleine Birkle, Schafhaus, N48°37'09" E9°59'23", 670 m a.s.l., 30 Mar. 2017, B. Fellmann (WU-MYC 0044023, culture AAM). – Norway, Vestfold, Faerder, Brattás, N59°11'52" E10°23'16", 10 m a.s.l., 4 Dec. 2018, P. Marstad 20773187 (WU- MYC 0044024, culture DIG). – Slovenia, Gorenjska, Radovljica, Pokljuka, Kranjska dolina, N46°21'55" E13°58'33", 1280 m a.s.l., 14 Oct. 2021, H. Voglmayr (WU-MYC 0045131); Gorenjska, Radovljica, Pokljuka, Na mlakah, N46°19'47" E13°57'02", 1350 m a.s.l., 15 Oct. 2021, H. Voglmayr (WU-MYC 0045132); Gorenjska, Radovljica, Triglav, Krma, Krma dolina, N46°24'05" E13°55'20", 880 m a.s.l., 16 Oct. 2021, H. Voglmayr(WU-MYC 0045133).
Notes — Digitodochium amoenum is closely related to the generic type, D. rhodoleucum, which raises the question whether both are conspecific. For the latter, only an ITS-LSU sequence (LC146732; 1148 bp) from the ex-holotype culture NBRC 32296 is available, which differs by 11 bp (1 gap, 10 substitutions, i.e., 2 % sequence difference) in the ITS from the European accessions, indicating that they represent distinct species. Furthermore, the sporodochial colour of D. rhodoleucum is reported as pink (Tubaki & Kubono 1989), which has never been observed in the European collections which range from pale yellow to bright orange. Given these differences and the vast geographical distribution gap, we here treat D. amoenum and D. rhodoleucum as distinct yet closely related species. However, if both species turn out to be conspecific, D. amoenum will take priority over D. rhodoleucum. A collection of the asexual morph of D. amoenum matching our collections was recently described and illustrated from Germany (Brackel & Zehm 2020, as D. rhodoleucum), and illustrations of a Spanish and a Danish collection can be retrieved at http://www.centrodeestudiosmicologicosasturianos.org/?p=618 and https://svampe.databasen.org/observations/10011806, respectively (accessed 6 Apr. 2022).
Our observations indicate that the pseudostromata develop beneath previously formed sporodochia which display at their periphery a white hyphal mat similar to that on the white ecto-stromatic disc. The minute blister-like bipolar appendages of the ascospores are difficult to interpret. As ascospores are aseptate from their very early stages, they cannot be regarded as cellular appendages. They are also very different from the conspicuous, mucilaginous bipolar secondary appendages that can be observed in Indian ink. They are difficult to elucidate with only a bright-field microscope; the observations suggest a swelling within the epispore involving this blister-like appearence. A similar case can be observed in ascospores of Albicollum canicolle when the spiny apiculus is highly reduced.
The type collection has been distributed as an exsiccatum, and we here lectotypify with a copy in B originating from the herbarium Nitschke (Gerhardt & Hein 1979). Like the copy in WU, it is in very poor condition, with only a single stroma left which is heavily moulded. Therefore, a recent abundant Austrian collection (WU-MYC 0044020) for which a culture and DNA data are available, is here designated as an epitype to ensure nomenclatural stability.
The ITS-LSU sequence KC774569 originating from the culture MUCL 51842 (Jaklitsch et al. 2014) does not represent D. amoenum, but Biscogniauxia nummularia (99.82 % sequence identity).
Helicogermslita Lodha & D. Hawksw., Trans. Brit. Mycol. Soc. 81(1): 91. 1983
Type species. Helicogermslita celastri (S.B. Kale & S.V.S. Kale) Lodha & D. Hawksw.
Genus of Xylariaceae. Pseudostroma erumpent through the bark or wood, massive, conical, semi- to (sub)globose, clypeus-like, sometimes overlain by whitish remnants of wood fibers and host tissues devoid of crystals, uni- to pauciperitheciate, with a more or less prominent apical ostiolar papilla; ectostroma hard, black, carbonaceous, splintering, at the base continuous with host tissues; entostroma reduced to the base or absent. Ascomata perithecial, entirely covered by the clypeus-like pseudostroma, single or in small groups, globose to subglobose, detached and collapsed with age, apically with a central, papillate ostiole; ostiolar canal periphysate. Peridium pseudoparenchymatous, brown to dark brown; innermost layer composed of thin-walled, hyaline cells. Paraphyses hyphal, hyaline, thin-walled, septate, rarely branched, longer than the asci. Asci cylindrical to fusiform, short-stipitate, with 8 ascospores, with a short-cylindrical to trapezoid, amyloid or inamyloid apical apparatus. Ascospores two-celled when young with a basal cell, unicellular when mature and occasionally with a minute hyaline appendage, ellipsoid, equilateral, with a helicoid, rarely sigmoid germ slit; epispore medium to dark brown; without a gelatinous sheath. Asexual morph not known.
Notes — The genus Helicogermslita was established by Hawksworth & Lodha (1983) and was characterised by uni- to pauciperitheciate, clypeus-like, carbonaceous stromata in combination with inamyloid ascus apices and ascospores having a helicoid, spore-length germ slit. However, Dargan et al. (1984) reported on a collection with amyloid ascus apices that in all other respects fully matched the generic type, H. celastri, and they therefore considered this feature to be not of diagnostic value on the generic and species level. They also questioned the status of Helicogermslita as a distinct genus, and suggested that it may be synonymous with Rosellinia, which has similar uni- to pauciperitheciate stromata and a cellular appendage disintegrating upon ascospores maturity. However, they refrained from formally combining H. celastri into the large and heterogenous genus Rosellinia. Also Petrini et al. (1987) did not recognise the genus Helicogermslita but considered H. celastri to belong to Anthostomella.
Læssøe & Spooner (1993) accepted Helicogermslita to be distinct from Rosellinia, stressing that the flat apical apparatus of the former is very distinct from that observed in typical species of Rosellinia. They also widened the generic concept of Helicogermslita by combining three additional species in Helicogermslita. However, their concept made the genus heterogeneous, as it included species with prominent, black, clypeus-like (H. celastri, H. valdiviensis, H. gaudefroyi) as well as poorly developed (H. fleischhakii) stromata. Besides spores with a helicoid germ slit, they considered an extensive white ectostroma to be an important diagnostic feature for the genus. However, this ‘white ectostroma’ is quite different in these species: In H. celastri (and other similar species subsequently added by Petrini 2003), the white ectostroma actually represents remnants of wood fibres attached to the stroma surface (Petrini 2003). In H. gaudefroyi, it represents a thin, pure white pellicle composed of dead bleached periderm cells. Conversely, in H. fleischhakii it represents an ectostroma forming a prominent white collar surrounding the ostioles, consisting of clusters of abundant white crystals mixed with prosenchymatous to intervowen hyphae and necrotic host cells. Based on morphology and phylogenetic data, H. fleischhakii and H. gaudefroyi are here removed from Helicogermslita and recognised within the new genera Albicollum and Spiririma, respectively.
After the additions of Petrini (2003), Lee & Crous (2003) and Samarakoon et al. (2022), there are currently 10 Helicogermslita species according to Index Fungorum http://www.indexfungorum.org/Names/Names.asp (accessed 4 Apr. 2022). Our re-defined, morphology-based generic concept of Helicogermslita basically follows Petrini (2003). We here restrict the genus to species having massive, erumpent, conical to (sub) globose, uni- to pauciperitheciate stromata consisting of a hard, black, splintering, clypeus-like carbonaceous ectostroma and a reduced to absent entostroma, perithecia detached from the stromata and collapsing with age, and unicellular, dark brown ascospores with sigmoid to helicoid germ slits and mostly (possibly always) a cellular appendage. Accordingly, we here recognise seven species within Helicogermslita: H. aucklandica, H. celastri, H. gisbornia, H. johnstonii, H. mackenziei, H. somala and H. valdiviensis. Two species are here formally transferred to other genera (H. fleischhakii, H. gaudefroyi; see above), and two (H. diversa and H. clypeata) likely do not belong to Helicogermslita and currently have an uncertain generic position. Helicogermslita diversa deviates by completely immersed uniperitheciate pseudostromata that are not clypeus-like (Lee & Crous 2003); as no sequence data are available for this species, no alternative classification can currently be proposed. The recently described H. clypeata has a rather reduced clypeus, immersed ascomata with ostioles piercing the substrate and ascospores without a cellular appendage (Samarakoon et al. 2022); in addition, it is phylogenetically remote from H. somala (Fig. 1), a species here newly combined in Helicogermslita that is morphologically highly similar to the generic type (see notes below) and therefore considered representative for the genus. Additional species belonging to Helicogermslita may have been described within the large genus Rosellinia.
The only sequence data available for a species of Helicogermslita s.str. are the recently published ITS1 and ITS2 sequences of the isolectotype specimen of H. somala (as Rosellinia somala; Forin et al. 2021), which is considered to be closely related or even conspecific with the generic type, H. celastri (see notes of H. somala below). Another recently described species for which sequence data are available, H. clypeata, may not be congeneric based on morphology and phylogenetic placement (see above).
Helicogermslita celastri (S.B. Kale & S.V.S. Kale) Lodha & D. Hawksw., Trans. Brit. Mycol. Soc. 81(1): 91. 1983 — Fig. 13
Fig. 13.

Helicogermslita celastri. a–g, j–k. Habit of erumpent (a–g) and immersed (j—k) stromata on host surface in face (a–f, j) and side (g, k) view; h–i. uniperitheciate stromata in vertical (h) and transverse (i) section, arrows denoting peridium; l. clypeate ectostroma in section; m–n. peridium in face view (m) and transverse section (n); o–q. asci; r–u. ascus apices in 3 % KOH (r–s) and Lugol’s solution after 3 % KOH pretreatment (t–u); v–x. apical apparatuses in Lugol’s solution (v–w) and in Lugol’s solution after 3 % KOH pretreatment (x); y–z. young ascospores with cellular appendage in Lugol’s solution after 3 % KOH pretreatment; a1-i1. ascospores (a1, bl in unknown mounting medium; hi, i1 aberrant). All in 3 % KOH, except where noted (a, c, e–i, l, n–p, r, t, c1-d1. IMI 321924; b, d, m, z, ei. IMI 309809; j–k, q, s, u–y, fl–il. IMI 315985; al–bl. IMI 190412 (isotype slide)). — Scale bars: a = 1 mm; b–c = 500 μm; d–k = 200 μm; l–n, r–u, a1 -i1 = 10 μm; o–q = 20 μm; v–z = 5 μm.
Basionym. Amphisphaerella celastri S.B. Kale & S.V.S. Kale, Sydowia 24(1–6): 334. 1971 ‘1970’.
Synonyms. Anthostomella spirilla Panwar & S.J. Kaur, Kavaka 4: 77. 1977 ‘1976’.
?Rosellinia punicae Anahosur [as ‘Rosselinia’], Sydowia 23(1–6): 60. 1970 ‘1969’.
Pseudostromata initially immersed, then erumpent through the bark or wood to almost superficial, massive, subglobose, obpyriform to mammiform, 0.6–1.1 mm diam, 0.4–0.6 mm high, clypeus-like, commonly overlain by whitish remnants of wood fibers and host tissues devoid of crystals, uni- to pauciperitheciate, with a short apical ostiolar papilla 80–160 μm wide; ectostroma clypeus-like, 50–100 μm thick, hard, black, carbonaceous, splintering, at the base continuous with host tissues, composed of a textura angularis of isodiametric to prosenchymatous, thick-walled cells 6–9.5 × 3–5.5 μm with dark brown walls 1.3–4 μm thick. Ascomata perithecial, entirely covered by the tightly appressed clypeus-like pseudostroma, 1–2(–4) per stroma, depressed subglobose with a more or less flattened base, 0.5–1 mm diam, with a central, not protruding ostiole; ostiolar canal c. 40–60 μm wide, lined with periphyses 1–1.5 μm wide. Peridium 25–40 μm thick, pseudoparenchymatous, in squash mounts composed of more or less parallel, tightly appressed hyphae, in transverse section a textura angularis of brown to dark brown cells 2.5–17 × 1–5 μm; innermost layer composed of thin-walled, hyaline cells. Paraphyses copious, hyphal, thin-walled, septate, guttulate, persistent, 4–9 μm wide at the base, gradually tapering to 1.3–2.3 μm towards the apex. Asci cylindrical to fusiform, short-stipitate, with eight obliquely uniseriate ascospores, (135–)145–160(–170) × (10.7–)13.5–17.7(–20.5) μm (Me = 151 × 15.5 μm, N = 40) including stipes; with an inamyloid or euamyloid apical apparatus, in 3 % KOH shallow, indistinct, not or distinctly blueing in Lugol’s solution and then cylindrical to inverted-trapezoid, 1.4–2.4 μm high × 2–3 μm diam (Me = 1.9 × 2.4 μm, N = 50), not stained or dark blue in Lugol’s solution after 3 % KOH pretreatment and then flat trapezoid, 0.6–1.2 μm high × 2.5–4.1 μm diam (Me = 0.9 × 3.4, N = 37). Ascospores (18.8–)20.6–24.1(–27.6) × (8.5–) 9.2–11.2(–12.2) μm, Q = (1.8–)2.0–2.4(–2.8) (Me = 22.4 × 10.2 μm, Qe = 2.2; N = 197), aseptate, ellipsoid, equilateral, dark brown, with narrowly to broadly rounded ends, with a primary appendage cell when young, with a narrow but conspicuous helicoid germ slit 0.3–0.5 μm wide coiling 2–4 times around the ascospore, almost reaching the ends; without gelatinous sheath or appendages. Asexual morph unknown.
Habitat & Host range — In dead bark or wood of various broadleaf shrubs and trees, e.g., Anogeissus latifolia, Celastrus paniculata, Gymnosporia montana, Lantana camara, Punica granatum, Terminalia arjuna (Hawksworth & Lodha 1983; Herb. IMI).
Distribution — Only known from India.
Specimens examined. India, Ramling, on Celastrus paniculata, Jan. 1968, S. T. Tilak & S. B. Kale (IMI 190412, isotype slide of Amphisphaerella celastri); Jabalpur, on dead wood of Punica granatum, 10 Dec. 1987, S. Gupta (IMI 321924); Jabalpur, on dead wood of Anogeissus latifolia, Sept. 1986, R.C. Rajak (IMI 309809); Panchgani, on corticated branch of Terminalia arjuna, 10 Sept. 1986, L.N. Nail (IMI 315985).
Notes — The current morphological species concept of Helicogermslita celastri includes accessions with inamyloid (those cited in Hawksworth & Lodha 1983, including the type collection) as well as euamyloid apical ascus apparatuses (Dargan et al. 1984), which is remarkable. Also the material examined in the present study contained collections with inamyloid (even after KOH pretreatment; IMI 309809, IMI 321924: Fig. 13t) as well as amyloid (IMI 315985: Fig. 13u–x) ascus apices, which matched well in all other characters. Unfortunately, attempts to obtain sequence data from these herbarium specimens failed. In lack of sequence data, it is currently impossible to evaluate whether these are conspecific or represent distinct species.
This uncertainty in the species concept also makes it impossible to decide about the species epithet to be used, as there are several competing older names which potentially take precedence over celastri. One of these is Rosellinia somala (Petrini 2013), which, however, differs in some morphological characters from H. celastri, and we therefore treat them as distinct species and combine R. somala in Helicogermslita below.
According to Petrini (2013), also Rosellinia punicae is conspecific with Helicogermslita celastri. Although in the protologue the germ slit of R. punicae was described and illustrated as longitudinal (Anahosur 1969), Petrini (2013) observed a helicoid germ slit in the type material examined by her. Remarkably, ascospore measurements reported for R. punicae in the protologue (16–18 × 6–9 μm) fit those of H. somala, but amyloidity of the apical ring is unknown.
Helicogermslita somala (Bacc.) Voglmayr, J. Fourn. & Jaklitsch, comb. nov. — MycoBank MB 844606
Basionym. Rosellinia somala Bacc., Risultati scientifici della Missione Stefanini Paoli nella Somalia meridionale. Le Collezioni botani (Firenze): 195. 1916.
Typification. Somalia, Gololonle, su arbusto indeterminato, 29 July 1913, G. Paoli no. 1384 (FT 009431, lectotype of Rosellinia somala designated by Petrini (2013); PAD S00034, isolectotype here designated, MBT 10007772).
Notes — For species description and illustrations, see Forin et al. (2021). Without giving further details, Petrini (2013) considered the older Rosellinia somala to be conspecific with Helicogermslita celastri, which was also followed by Forin et al. (2021). Petrini (2013) selected a specimen from FH as lectotype of R. somala, which could not be traced there despite thorough search (G. Tocci, pers. comm.). However, further research showed that FH was cited in error, and the type specimen investigated by L. Petrini is actually located at FT (Centro Studi Erbario Tropicale, Florence, Italy), where specimen FT 009431 bears her respective annotation labels as type. As according to ICN 9.2 typification errors like incorrect fungarium codes are to be corrected, FT 009431 is here given as the correct lectotype selected by Petrini (2013), and PAD S00034 is cited as isolectotype.
Forin et al. (2021) provided a description and illustrations of a part of the type collection of R. somala deposited in PAD. Their illustrations leave no doubt that R. somala is morphologically close to H. celastri. Forin et al. (2021) also generated ITS1 and ITS2 sequences from the type, which confirm a placement of R. somala within Xylariaceae s.str., with an unsupported (MP) to moderately (85 % ML) supported sister group relationship to Xylaria cf. heliscus (Fig. 1). This result is arguable, since X. heliscus features small kretzschmarioid, gregarious and stipitate stromata and small ascospores c. 10 μm long with a straight germ slit. Moreover, ITS sequences place X. heliscus within a well-defined group of Xylaria called polymorpha aggregate by Hsieh et al. (2010), which is morphologically consistent and not rosellinia-like. However, this placement may change upon availability of additional markers with higher and better resolution than the ITS. While the ascomatal and ascospore characters of R. somala support a close relationship with H. celastri, ascospores of R. somala are somewhat smaller ((14.3–)15.8–19(–21.7) × (6–)7–8(–8.7) μm, Me = 17.4 × 7.5 μm) than those of H. celastri ((18.8–)20.6–24.1(–27.6) × (8.5–)9.2–11.2(–12.2) μm, Me = 22.4 × 10.2 μm). In addition, Forin et al. (2021) reported an amyloid apical ascus ring, which does not match the type collection of H. celastri. Considering the uncertainties of the species concept of H. celastri and the lack of confirmed sequence data of collections matching its type (see notes of H. celastri), the conspecificity of R. somala and H. celastri suggested by Petrini (2013) remains equivocal. However, as in any case the epithet somala takes precedence over celastri (and all other known potentially competing epithets) based on priority, R. somala is here combined in Helicogermslita. Additional studies of fresh collections and DNA sequence data are necessary to finally evaluate whether H. celastri becomes a synonym of H. somala; at present we retain them as distinct species.
Leptomassaria Petr., Ann. Mycol. 12(5): 474. 1914
Type species. Leptomassaria simplex (Nitschke ex G.H. Otth) Petr.
Genus of Xylariaceae. Pseudostroma inconspicuous, immersed in bark, becoming visible as a small clypeus, sometimes slightly erumpent around the ostiole; entostroma prosenchymatous, whitish, well developed near the peridium, mixed with substrate cells, without a melanized zone line. Perithecia solitary, globose, peridium prosenchymatous. Paraphyses copious, hyphal, thin-walled, septate, evanescent. Asci cylindrical, short-stipitate, with a well-developed, discoid, cylindrical to nearly trapezoid euamyloid apical ring. Ascospores two-celled when young with a minute basal cell, unicellular when mature and occasionally with a minute hyaline appendage, symmetrical, ellipsoid with rounded ends, brown, with a narrow helicoid germ slit; with a gelatinous sheath.
Notes — Established by Petrak (1914), the genus Leptomassaria was mostly ignored until Rappaz (1995) recognised the genus as distinct and provided a detailed description. He also was the first to notice the helicoid germ slit. Based on Petrak’s material of L. simplex, Daranagama et al. (2018) provided short descriptions of the genus and the type species, suggesting potential synonymy with the genera Anthostoma, Anthostomella or Helicogermslita. We do not concur with their conclusions and here recognise Leptomassaria as a distinct genus within Xylariaceae s.str. Leptomassaria is phylogenetically clearly distinct from Anthostoma which belongs to Diatrypaceae (see e.g., Jaklitsch et al. 2014, 2016). Helicogermslita and Anthostomella differ in their ascomatal and ascospore characters; in addition, no sequence data are yet available for their generic types.
All previous studies missed the minute basal dwarf cells in the type species, L. simplex, which are here reported for the first time. These are particularly evident in young spores but are occasionally also seen in mature spores as a minute cellular appendage. The fact that these are only well seen in well-developed fresh material may be a reason why they have not been noticed in previous studies that were based on old herbarium material.
Leptomassaria simplex (Nitschke ex G.H. Otth) Petr., Ann. Mycol. 12(5): 474. 1914 — Fig. 14
Fig. 14.

Leptomassaria simplex. a–b. Habit of young ostioles on host surface surrounded by white stromatic tissues; c–d. clypeus with ostiole; e. ostiole of mature ascoma surrounded by ejected ascospores; f–h. ascoma in vertical (f) and transverse (g–h) section, showing reduced, lighter brown entostroma surrounding the perithecia; i. peridium in transverse section (in 3 % KOH); j. loosely intertwined entostromatic hyphae with a crystal (in 3 % KOH); k–l. immature (k) and mature (l) ascus (aqueous NaCl solution); m. paraphyses in water; n–r, c1. apical apparatuses in water (n), 3 % KOH (o), Lugol’s solution (p–q) and Lugol’s solution after 3 % KOH pretreatment (r, c1); s–b1, d1–o1. immature (s, o1) and mature (t–b1, d1–n1) ascospores; arrows denoting basal cellular appendages (s–b1, h1–l1 in water; d1–g1, m1–o1 in 3 % KOH) (a–b, f. WU-MYC 0044028; c. WU-MYC 0044029; d, g, i–j, c1–g1, m1–o1. B 700017008 (lectotype); e, h. WU-MYC 0044027; k–b1, h1–l1. WU-MYC 0044025 (epitype)). — Scale bars: a–h = 200 μm; i–j, m–o1 = 10 μm; k–l = 20 μm.
Basionym. Quaternaria simplex Nitschke ex G.H. Otth, Mitth. Naturf. Ges. Bern 711–744: 95. 1871 ‘1870’.
Synonym. Anthostoma simplex (Nitschke ex G.H. Otth) Sacc., Hedwigia 35(7): XXV. 1896.
Typification. Switzerland, Bern, on dead branches of Tilia sp., late autumn, without date, G.H. Otth no. 10 (B 700017008, lectotype of Quaternaria simplex here designated, MBT 10008059; B 700017007, isotype). – Austria, Burgenland, Hornstein, Lindenallee, N47°52’40.5" E16°26'59.5", 400 m a.s.l., on dead branches of Tilia sp., 4 Nov. 2017, H. Voglmayr & I. Krisai-Greilhuber (WU-MYC 0044025, epitype here designated, MBT 10008060; ex-epitype culture LSI = CBS 147282).
Pseudostromata almost entirely immersed in bark below the periderm, scarcely erumpent, reduced to an apical clypeus-like structure and a poorly developed whitish entostroma surrounding the ascomata. Clypeus highly variable, from virtually absent, much-reduced around the ostiolar region, small-pulvinate and erumpent forming a 0.3–0.7 mm wide whitish to greyish disc, to immersed and forming a black translucent disc 0.5–1.3 mm wide, at its margin sometimes extending downwards in the substrate, then forming a black zone surrounding the upper half of the ascomata; mostly only the ostiole piercing the periderm. Ascomata perithecial, immersed, scattered, solitary or rarely in groups of two, globose, 0.7–1.3 mm diam, with a central ostiole; ostiolar neck straight, black, not projecting above the substrate surface, 150–200 μm wide; ostiole minutely porate, pore c. 50 μm wide. Stromatic tissue around the perithecia inconspicuous, whitish, reduced to a loose hyphal tissue composed of moderately thick-walled hyaline hyphae 2–5 μm diam, mixed with necrotic bark cells and clusters of white crystals; upper layer of the stromatal disc around ostioles overlain with white crystals readily dissolving in 5 % HCl. Peridium 20–50 μm thick, prosenchymatous, of two layers, an outer brown layer of a textura angularis composed of unevenly pigmented cells 4–18 × 1.5–4.5 μm, with wall 0.7–1 μm thick, grading into an inner layer of subhyaline textura prismatica; ostiolar neck wall 60–75 μm thick at the base, dark brown, in transverse section a textura angularis of small, isodiametric, thick-walled, dark brown cells with wall 0.6–1.2 μm thick; ostiolar canal densely periphysate. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, guttulate, much longer than the asci, evanescent, 6–9 μm wide at the base, gradually tapering to 2.3–3.5 μm towards the apex, embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, short-stipitate, with 8 obliquely uniseriate to uniseriate overlapping ascospores, (240–)255–290(–300) × (22–)24–30.5(–35) μm (Me = 272 × 27 μm, N = 27) including stipes, spore-bearing part 165–280 μm long, very unstable and immediately breaking and dissolving in water and 3 % KOH when fresh; with an euamyloid apical apparatus barely refractive in water, in 3 % KOH inverted hat-shaped with a flattened apex and a sharp subapical rim; 5.1–7.3 μm high × 6.3–8.8 μm diam (Me = 6.4 × 7.3 μm, N = 25) in Lugol’s solution, blueing, the colour fading toward the apex, cylindrical to inverted trapezoid, apically flared; 3.7–5.5 μm high × 5.4–6.4 μm diam (Me = 4.5 × 5.8 μm, N = 6) in Lugol’s solution after 3 % KOH pretreatment, dark blue, inverted-hat shaped with a flattened, flared apex and a sharp subapical rim. Ascospores (22.7–)28.8–35.5(–41) × (12–)15–19.5(–21.8) μm, Q = (1.4–)1.7–2.0(–2.3) (Me = 32.2 × 17.3 μm, Qe = 1.9; N = 342), two-celled when young with a minute basal cell, unicellular when mature and occasionally with a minute hyaline appendage, symmetrical, ellipsoid with rounded ends, dark to blackish brown, with a faint, narrow helicoid germ slit, obliquely coiled 1–1.5 times around the ascospore, appearing as broken down into three to five segments when seen in optical section, almost reaching the ends, occasionally with two parallel germ slits; when fresh with a prominent rectangular gelatinous sheath widely expanding after spore release, gel sheath less prominent and rather thin in old herbarium material. Asexual morph on the natural substrate not seen.
Habitat & Host range — In dead bark of Tilia spp.
Distribution — Europe and North America; known from Austria, Canada, Czech Republic, France, Sweden, Switzerland and the USA.
Other specimens examined (all on shed dead corticated branches of Tilia spp.). Austria, Burgenland, Edelstal, northern slope of Spitzerberg, N48°05'42" E16°58'55", 250 m a.s.l., 22 Feb. 2022, H. Voglmayr & I. Greilhuber (WU-MYC 0045985); Niederösterreich, Ebenfurth, Stadtpark, 17 Feb. 2013, Z. Jorgovanovic (specimen lost); Steiermark, Graz, St. Peter, Park of the ORF-Landesstudio Steiermark, N47°03'19" E15°27'58", 360 m a.s.l., 17 Nov. 2017, G. Friebes (WU-MYC 0044026, culture LSI1); Graz-Umgebung, alley at the road to LKH Hörgas-Enzenbach, Standort Hörgas; N47°08'11" E15°18'29", 410 m a.s.l., 1 Dec. 2017, G. Friebes (WU-MYC 0044027, culture LSI2); Graz (Stadt), left bank of the river Mur, Radweg R2, alley between Radetzkybrücke and Augartenbrücke, map grid 8958/2, N47°04'03" E15°26'09", 350 m a.s.l., 15 June 2015, I. Wendelin (GJO 0076055, WU-MYC 0044028; culture LSI3); Südoststeiermark, Edelsbach bei Feldbach, Rohr an der Raab, N46°58'55" E15°48'55", 360 m a.s.l., 19 Jan. 2018, A. Gallé (WU-MYC 0044029, culture LSI4). – Canada, Québec, Montreal, Isle de Souers, Domain St. Paul, N45°27'21" W73°33'09", 40 m a.s.l., 26 Mar. 2018, A. Carter (WU-MYC 0044030, culture LSI5); ibid., 30 Jan. 2018, A. Carter (WU-MYC 0044031).
Notes — Rappaz (1995) could not locate original material of Otth and neotypified the species with a specimen collected and investigated by Petrak (1914). However, two original collections of Otth are extant in the herbarium of Nitschke preserved in B (Hein 1984), which are in rather poor condition as most ascomata are old and heavily moulded. Based on preservation and abundance, we here designate the specimen B 700017008 as lectotype, and epitypify with a recent Austrian collection (WU-MYC 0044025), for which a culture and DNA data are available, to ensure nomenclatural stability.
Nemania Gray, Nat. Arr. Brit. Pl. (London) 1: 516. 1821
Synonym. Euepixylon Füisting, Bot. Zeitung 25(no. 39): 309. 1867.
Notes — The genus Nemania is characterised by dark brown to black, one-layered, more or less carbonaceous pulvinate to effuse stromata, a whitish soft tissue between and/or beneath the perithecia, papillate ostioles, an apical apparatus usually higher than broad, pale brown ascospores usually with a cellular appendage when immature and with a mostly inconspicuous germ slit located on the less convex side, and by geniculosporium-like asexual morphs (Granmo et al. 1999, Læssøe et al. 2000, Ju & Rogers 2002).
The genus Euepixylon was resurrected by Læssøe & Spooner (1993) for a single species, Euepixylon udum. In stromatal characters, Euepixylon udum is similar to Nemania confluens and Anthostomella by perithecia more or less immersed under a black effused superficial layer (clypeus). In addition, like in most members of Nemania and Anthostomella, ascospores of E. udum bear a cellular appendage when immature. However, it morphologically differs from both genera by ascospores having a poroid germ locus, from Nemania in having asci with short stipes and a shallow (i.e., broader than high) apical apparatus, and from Anthostomella in a clypeoid stromatal layer extending deeply into the substrate. Like Nemania, Euepixylon udum has a geniculosporium-like asexual morph (Whalley 1976) while in Anthostomella anamorphs are nodulisporium- or virgariella-like (Martin 1967, Francis et al. 1980).
Currently, two similar species, the European E. udum and the North American E. sphaeriostomum, are accepted within the genus Euepixylon, constituting a morphologically homogeneous genus. A third species, E. quercinum, rather represents a typical Nemania species, as in the protologue a long straight germ slit ismentioned (“fissure germinalis recta et longa”; Vasilyeva 1998: 210). However, in multi-gene analyses E. sphaeriostomum was consistently placed within Nemania (e.g., Voglmayr et al. 2018, Wendt et al. 2018, Voglmayr & Beenken 2020, Wittstein et al. 2020, Pi et al. 2021), while no sequence data were available for the generic type, E. udum. Our newly generated sequences for E. udum confirm a placement within Nemania (Fig. 1) as well as a close relationship with E. sphaeriostomum, but both Euepixylon species are not revealed as closest relatives. In our analyses, a new species, N. ethancrensonii, which morphologically deviates from the current generic concept of Euepixylon and Nemania in several respects (see notes below), is placed as sister species to E. sphaeriostomum, while E. udum is revealed as sister species of the generic type of Nemania, N. serpens, with maximum support (Fig. 1). This supports that the genus Euepixylon should be synonymised with Nemania; a conclusion which is also supported by the recently published study on Nemania by Pi et al. (2021), who showed that E. sphaeriostomum (as N. sphaeriostoma) is embedded within their Nemania clade 6, which is characterised by variable (slit-like, poroid or apparently absent) germ loci.
Nemania ethancrensonii Voglmayr, J. Fourn. & Jaklitsch, sp. nov. — MycoBank MB 844607; Fig. 15, 16
Fig. 15.

Nemania ethancrensonii, sexual morph. a. Top view of white pseudostromata on dead twig after periderm disintegration; b–c. white ectostromatic discs with black ostiolar necks, rupturing the bark; d–h. habit of white pseudostromata with black ostiolar necks in top (d–f) and side (g–h) view after periderm disintegration (in f–g with residual bark flaps); i–j. pseudostromata and perithecia in horizontal (i) and vertical (j) section; k. lateral peridium in vertical section in 3 % KOH; l. polyhedral crystals in pseudostromatic tissue in 3 % KOH; m–n. asci with paraphyses (m) in water; o–r. apical apparatuses in water (o), 3 % KOH (p), Lugol’s solution (q) and Lugol’s solution after 3 % KOH pretreatment (r); s–z. ascospores in water (s–v), 3 % KOH (w) and Melzer’s reagent (x–z) (holotype WU-MYC 0040047). — Scale bars: a = 5 mm; b–c = 200 μm; d–j = 500 μm; k–l, n = 10 μm; m = 50 μm; o–z = 5 μm.
Fig. 16.
Nemania ethancrensonii, culture and asexual morph on CMD. a. Culture after 23 d at 22 °C; b–j. aerial conidiophores with conidia; k. conidia. g–k in water (holotype WU-MYC 0040047). — Scale bars: b = 1 mm; c–d = 200 μm; e–f = 100 μm; g–h = 20 μm; i = 10 μm; j–k = 5 μm.
Etymology. In honor of the collector of the holotype, Ethan Crenson.
Holotype. USA, New York, Brooklyn, Green-Wood cemetery, N40°39'27" W73°59'23", 50 m a.s.l., on corticated twig of an unidentified tree, 1 May 2021, E. Crenson (WU-MYC 0040047; ex-holotype culture AEC = CBS 148337).
Diagnosis. Differs from other Nemania (including Euepixylon) species by the lack of a black clypeus and a prominent whitish entostroma embedded in host tissue.
Pseudostromata immersed, rupturing the bark periderm by 0.5–1.3 mm long elongate or irregular cracks, eventually becoming fully exposed after weathering of the bark and then conspicuous, orbicular to elongate, 1.5–3(–5) mm diam, sometimes confluent, consisting of a white ectostromatic disc and perithecia embedded in a white entostroma. Ectostromatic discs 0.5–1.3 mm diam, fusoid to polygonal in outline, inconspicuous, not protruding above the bark surface, white, pierced by the distinct black apical ostiolar papillae. Ostiolar papillae 1–3(–6) per disc, distinctly protruding 100–500 μm above ectostromatic disc, conical with truncate, rounded or acute apices, black, at the base commonly covered by whitish hyphae, towards the tips smooth and shiny black, 140–450 μm diam towards their base. Entostroma conspicuous, dull white to brownish, consisting of necrotic bark cells intermingled with loosely intertwined hyaline hyphae and massive clusters of white crystals. Perithecia (without ostiolar necks) 1.3–1.5 mm high, 0.9–1 mm diam, oval, with central ostiolar apical papilla. Peridium 53–76 μm thick, pseudoparenchymatous, a textura angularis of small isodiametric to prosenchymatous brown cells, consisting of two layers: a 37–56 μm thick outer layer of dark brown, thick-walled cells and a 13–21 μm thick inner layer of lighter brown, thin-walled cells. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, rarely branched, with numerous refractive guttules, much longer than the asci, 1.7–8 μm wide, discretely embedded in a mucilaginous matrix. Asci cylindrical to slightly fusiform, long-stipitate, with 8 obliquely uniseriate overlapping, rarely irregularly biseriate ascospores, (111–)132–162(–188) × (7–)9–10(–11.5) μm (Me = 147 × 9 μm, N = 40) including the (35–)47–75(–100) μm long stipes, spore-bearing part (62–)74–99(–113) μm long; with an euamyloid cylindrical apical apparatus barely refractive in water, in 3 % KOH inverted trapezoid; 2.7–4.6 μm high × 2.2–4 μm diam (Me = 3.7 × 3.1 μm, N = 40) in Melzer’s reagent and Lugol’s solution, blueing, short-cylindrical to inverted trapezoid; 2–3 μm high × 2.5–3 μm diam (Me = 2.4 × 2.6 μm, N = 13) in Lugol’s solution after pretreatment in 3 % KOH, dark blue, inverted trapezoid to inverted hat-shaped. Ascospores (11.7–)13–14.8(–16) × (5–)5.5–6.4(–7) μm, Q = (1.8–)2.2–2.5(–2.8) (Me = 14 × 6 μm, Qe = 2.4; N = 60), aseptate, ellipsoid, equilateral, pale to medium fuligineous brown, smooth, with rounded ends; with a 3.6–6.6 μm long, indistinct, straight germ slit of about one third of spore-length. Asexual morph on the natural substrate not seen, on CMD geniculosporium-like. Conidiophores forming superficially on sparse aerial mycelium after 2 wk in culture, effuse, decumbent to erect, sympodially branched, not synnematous, mostly with a well-defined main axis, pale brown, 23 μm wide. Fertile regions terminal, proliferating sympodially, geniculate with conspicuous conidial scars. Conidia produced in acropetal succession, (2.8–)3.0–3.6(–4.2) μm diam (Me = 3.3 μm, N = 60), globose with an indistinctly truncate base, hyaline to subhyaline, aseptate, smooth, containing several small guttules when fresh, without a gelatinous sheath or appendages.
Notes — Within Xylariaceae, N. ethancrensonii has a unique character combination, viz. the lack of a clypeus or strongly melanized carbonaceous stromatal tissues, a prominent whitish entrostroma immersed in bark containing few black perithecia with prominent conical apical papillae piercing a small white ectostromatic disc, and equilateral ascospores with an indistinct, short, straight germ slit. The white entostroma and the straight germ slit are reminiscent of the genus Entoleuca, which is phylogenetically distinct (Fig. 1). However, N. ethancrensonii was placed within the Nemania clade with maximum support, where it was revealed as sister species to the North American Nemania (Euepixylon) sphaeriostoma. Morphologically, the cylindrical apical apparatuses, the indistinct germ slits of the ascospores and the geniculosporium-like asexual morph support a classification within Nemania.
It is remarkable that N. ethancrensonii has apparently not been previously found and described. However, it should be considered that the distinct white entostroma becomes only apparent after disintegration of the outer tissues of the host bark, which was the case in most parts of the holotype specimen, while in the small part with intact host periderm the ectostromatic discs rupturing the bark are indistinct.
Oligostoma Voglmayr, J. Fourn. & Jaklitsch, gen. nov. — MycoBank MB 844608
Etymology. Greek oligo = few, stoma = mouth, opening; referring to the few ostioles per ectostromatic disc.
Type species. Oligostoma insidiosum (P. Crouan & H. Crouan) Voglmayr, J. Fourn. & Jaklitsch
Genus of Xylariaceae. Stromata erumpent from bark, pustulate, containing 2–5 perithecia; ectostromatic discs flattened to slightly convex, with few black ostiolar dots. Paraphyses copious, hyphal, thin-walled. Asci cylindrical to obclavate, short-stipitate, with a massive, plug-like, euamyloid apical ring with a flattened apex and a sharp subapical rim. Ascospores unicellular, inequilaterally-ellipsoid to nearly citriform, brown to dark brown with a conspicuous sigmoid germ slit on the ventral side; with a thin slimy sheath and a minute, often inconspicuous cellular appendage on one end; epispore smooth.
Oligostoma insidiosum (P. Crouan & H. Crouan) Voglmayr, J. Fourn. & Jaklitsch, comb. nov. — MycoBank MB 844609; Fig. 17
Fig. 17.

Oligostoma insidiosum. a, c, e–g, j–k. Habit of stromatal discs on host surface in top view; b. biperitheciate stroma in horizontal section; d, h–i. stromata in vertical section; l–m. asci in 3 % KOH; n–o; apical apparatuses in 3 % KOH (n) and Lugol’s solution (o); p. paraphyses at their bases, in water; q–r. immature ascospores with basal appendage (arrows); s, a1–c1. ascospores in side view showing a wide ventral sheath, in water or Indian ink (c1); t–x. ascospores showing a ventral sigmoid germ slit, in water; y–z. mature ascospores showing a basal cellular appendage (arrows) in KOH (y) and water (z) (a–b, f–g, k–v, y–b1. WU-MYC 0044034; c–e, h–j, w–x, c1; J.F. 08073). — Scale bars: a–b, e–g, j–k = 200 μm; c–d, h–i = 500 μm; l–m = 50 μm; n–o = 5 μm; p–c1 = 10 μm.
Basionym. Valsa insidiosa P. Crouan & H. Crouan, Fl. Finistère (Paris): 32. 1867.
Synonyms. Fuckelia rhenana Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 224. 1870 ‘1869–70’, nom. inval., Art. 35.1 (Melbourne).
Diatrype adusta Cooke & Peck, Ann. Rep. N.Y. State Mus. Nat. Hist. 29: 58. 1878 ‘1876’.
Anthostoma insidiosum (P. Crouan & H. Crouan) Sacc., Syll. Fung. (Abellini) 1: 306. 1882.
Anthostoma adustum (Cooke & Peck) Sacc., Syll. Fung. (Abellini) 1: 307. 1882.
Anthostoma rhenanum Fuckel ex Sacc., Syll. Fung. (Abellini) 1: 307. 1882.
Anthostomella mammoides Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 45: 139. 1893.
Anthostomella adusta (Cooke & Peck) M.E. Barr, in Barr, Rogerson, Smith & Haines 1986.
Typification. France, Finistère, on bark of Fagus sylvatica, 6 June 1868, Crouan (CO s.n., holotype). – Switzerland, Bern, Langnau im Emmental, Bachwald, N46°56'35.1" E7°50'48.7", 1020 m a.s.l., on dead corticated fallen twigs of Fagus sylvatica, 11 Sept. 2020, S. Blaser (WU-MYC 0044032, epitype of Valsa insidiosa here designated, MBT 10007725; ex epitype culture ANI1 = CBS 147288). – Germany, Hessen, Oestrich, Mappen, on dead branches of Acer pseudoplatanus, spring, without date, L. Fuckel, in Fuckel, Fungi Rhen. Exs. 2052 (WU 0121799, lectotype of Anthostoma rhenanum here designated, MBT 10007732). – Austria, Oberösterreich, St. Willibald, Große Sallet, 430 m a.s.l., N48°21'56" E13°42'26", on dead corticated fallen branches of Acer pseudoplatanus, 28 Aug. 2020, H. Voglmayr (WU-MYC 0044033, epitype of Anthostoma rhenanum here designated, MBT 10007733; ex epitype culture ANR = CBS 147287).
Stromata erumpent from bark, pustulate, bluntly conical, 0.3–0.7 mm high, comprising a soft, whitish to brownish hyphal entostroma mixed with necrotic bark cells within a black line widely effused between bark and wood, rising above stromata and forming a black leathery clypeus 50–120 μm thick of small, thick-walled, opaque angular cells; ectostromatic discs (0.3–)0.6–1(–1.2) mm in their greatest dimension, dark brown to black, flattened to slightly convex, circular, oblong or often angular or irregular due to surrounding bark flaps, with few black, shiny, flat or convex ostiolar dots 50–130(–200) μm diam, weakly prominent, more rarely opening flush with the surface and appearing as minute perforations occasionally plugged with white substance. Perithecia circinate with ostiolar openings central to slightly eccentric, 1–5 per stroma, depressed subglobose with a more or less strongly flattened base, 0.4–0.5 mm high, 0.45–0.8 mm diam; peridium light brown, 22–35 μm thick, pseudoparenchymatous, of moderately thick-walled unevenly pigmented angular to prismatic cells with wall 1–1.5 μm thick. Paraphyses copious, hyphal, thin-walled, minutely guttulate, 4–6.5 μm diam at the base, apically tapering to 2–2.5 μm diam. Asci cylindrical, occasionally slightly obclavate in KOH, short-stipitate, vital 170–210 × 20.5–27.5 μm (Me = 187 × 23.9 μm, N = 25), in KOH 170–200 μm in total length × 17–21 μm wide (N = 30), with 8 uniseriate, obliquely overlapping ascospores and a massive, plug-like, euamyloid apical ring with a flattened apex and a sharp subapical rim, refractive in 3 % KOH, blue in Melzer’s reagent, dark blue to blackish blue in Lugol’s solution, 6–7.5 μm wide, 4–7 μm high. Ascospores (20.4–)23.8–29.2(–33.4) × (8.7–)10.8–13.2(–17) μm, Q = (1.6–)1.9–2.4(–3.3) (Me = 26.5 × 12 μm, Qe = 2.1; N = 500), inequilaterally ellipsoid to nearly citriform, with broadly to narrowly rounded ends, olivaceous brown to dark brown with a conspicuous sigmoid germ slit on the ventral side, usually not reaching the ends; with a thin slimy sheath swollen to 2–6 μm on the ventral side and a small basal cellular appendage visible on immature ascospores, often collapsed or inconspicuous at maturity; epispore smooth. Asexual morph on the natural substrate not seen.
Habitat & Host range — In dead bark of deciduous trees; confirmed from Acer pseudoplatanus, Fagus sylvatica (type host), Ostrya spp., Tilia sp.
Distribution — Europe, North America; known from Austria, France, Germany, Slovenia, Switzerland and the USA.
Additional specimens examined. Austria, Kärnten, St. Margareten im Rosental, N46°32'50" E14°25'16", 600 m a.s.l., on corticated fallen branches of Ostrya carpinifolia, 5 Feb. 2018, W. Jaklitsch (WU-MYC 0044034, culture ANI = CBS 147280); Velden am Wörthersee, Drauauen between St. Egyden und Dieschitz, N46°34'23" E14°03'11", 470 m a.s.l., on corticated fallen branches of Tilia sp., 12 Mar. 2020, M. Koncilja (WU-MYC 0044035, culture OTI); Niederösterreich, Klausen-Leopoldsdorf, Untergrödl W Schöpflgitter, at the bridge crossing the Lammeraubach, N48°06'00.5" E15°56'26.2", 500 m a.s.l., on corticated fallen branches of Acer pseudoplatanus, 5 Sept. 2020, H. Voglmayr (WU-MYC 0044036, culture ANR3); Oberösterreich, Raab, Rotes Kreuz, Rothmayrberg, N48°22'15.8" E13°40'13.6", 460 m a.s.l., on corticated fallen branches of Acer pseudoplatanus, 23 Aug. 2020, H. Voglmayr (WU-MYC 0044037, culture ANR1); ibid., on corticated fallen branches of Fagus sylvatica, 5 July 2021, H. Voglmayr (WU-MYC 0040048); Enzenkirchen, N Oberantlang, above Siegl, N48°23'05.4" E13°41'56", 470 m a.s.l., on corticated fallen branches of Acer pseudoplatanus, 26 Aug. 2020, H. Voglmayr (WU-MYC 0044038, culture ANR2). – France, Deux-Sèvres, Villiers-en-Bois, Forêt de Chizé, N46°08'24" W0°23'28", 80 m a.s.l., on corticated twig of Fagus sylvatica, 14 Aug. 2008, A. Rossman J.F. 08073 (WU-MYC 0044039). – Germany, Bayern, Gem. Anger, Aufham, N47°46'38" E12°51'57", 520 m a.s.l., near Reitbauernhof, on dead branch of Acer pseudoplatanus, 1 Apr. 2020, I. Rössl (WU-MYC 0044040). – Slovenia, Gorenjska, Radovljica, Triglav, Krma, N margin of Krma dolina, N46°24'05" E13°55'20", 880 m a.s.l., on corticated twig of Fagussylvatica, 16 Oct. 2021, H. Voglmayr (WU-MYC 0045127). – Switzerland, near Zürich, on dead branches of Fagus sylvatica, Sept. 1882, G. Winter, in Rabenhorst-Winter, Fungi Europaei 2870 (WU 0121798).
Notes — The description is based on Jaklitsch et al. (2014) and supplemented with additional characters from fresh material. As pointed out by Jaklitsch et al. (2014), the older (1867) Valsa insidiosa morphologically agrees with the younger (1878) North American Anthostomella (Diatrype) adusta (see Barr et al. 1986: 6, Læssøe & Spooner 1993: 44 and Rappaz 1995: 148 for descriptions of A. adusta), and the latter name is therefore considered to be a synonym. Based on stroma morphology, Lu & Hyde (2000) suggested a placement in Lopadostoma which was refuted by Jaklitsch et al. (2014) who pointed out that the strongly sigmoid slit in broadly ellipsoid ascospores, the asymmetrical slimy sheath and the massive apical ascal plug exclude this fungus from Lopadostoma. Læssøe & Spooner (1993) noted that this fungus may require a new genus, which is confirmed by the molecular data (Fig. 1). Phylogenetically, O. insidiosum is placed as sister species to Leptomassaria simplex with high support (99 % ML and 95 % MP; Fig. 1), with which it also shares brown ascospores with a helicoid germ slit, a cellular appendage and a distinct gel sheath surrounding the ascospores. However, considering the differences in stromatal morphology and ascospore shape, we here place it in the new genus Oligostoma.
Spore size and shape is highly variable between the various collections investigated. However, the molecular data leave no doubt about the conspecificity of the collections from different hosts. To ensure nomenclatural stability, we here epitypify the species with a recent collection from the type host, Fagus sylvatica, for which a living culture and sequence data are available.
There is some confusion in the literature about the identity of Fuckelia rhenana nom. inval. (= Anthostoma rhenanum), which was described by Fuckel (1870) from Acer pseudoplatanus. Höhnel (1918) considered it to be synonymous with F. amoena (= Digitodochium amoenum). However, in two isotype specimens (K(M) 255520, K(M) 255521), Læssoe & Spooner (1993) observed oblong, dark brown ascospores of 26–41 × 11–17 μm without a visible germ locus, which they could not attribute to any described species. However, the isotype specimen of F. rhenana we investigated (WU 0121799) matches O. insidiosum in its stromata and ascospores, and, conversely to the isotypes in K, is also fully in line with the description of ascospores in Fuckel (1870), which were described as oblongovate, curved, on both sides obliquely apiculate, blackish brown, 30 × 12 μm. We therefore conclude that the WU specimen corresponds with Fuckel’s concept of the species, and therefore lectotypify Anthostoma rhenanum with this specimen, and a recent Austrian specimen (WU-MYC 0044033) for which a culture and sequence data are available is selected as an epitype. Sequence data of four recent collections from Acer pseudoplatanus support conspecificity of A. rhenanum with Oligostoma insidiosum (Fig. 1).
Spiririma Voglmayr, J. Fourn., S. Tello & Jaklitsch, gen. nov. — MycoBank MB 844610
Etymology. spiri = spiral, rima = furrow; referring to the helicoid slit of the ascospores.
Type species. Spiririma gaudefroyi (Fabre) Voglmayr, J. Fourn., S. Tello & Jaklitsch
Genus of Xylariaceae. Pseudostromata consisting of a black clypeus, irregularly spreading around the ostioles of single to few ascomata, overlain by a white pellicle composed of dead bleached periderm cells, devoid of crystals. Clypeus leathery to carbonaceous, prosenchymatous, composed of periderm host cells and dark brown hyphae, continuous with the periderm. Ascomata perithecial, corticolous, immersed to erumpent, scattered, solitary or in small clusters, globose to subglobose, apically with a central, papillate black ostiole; ostiolar canal peri-physate. Peridium pseudoparenchymatous, brown to dark brown; innermost layer composed of thin-walled, hyaline prismatic cells. Paraphyses hyphal, hyaline, thin-walled, septate, rarely branched, much longer than the asci, discretely embedded in a mucilaginous matrix. Asci cylindrical to fusiform, short-stipitate, with (4–)8 ascospores, with a massive bipartite amyloid apical apparatus. Ascospores aseptate, ellipsoid, equilateral, with a helicoid germ slit; epispore medium to dark brown; with a gelatinous sheath surrounding the whole ascospore and slightly swollen at ends forming bipolar secondary appendages.
Notes — This phylogenetically isolated genus is characterised by ascomata with a coarsely papillate ostiole beneath a black clypeus, which is overlain by a superficial, expanded white pellicle on the bark, combined with brown ellipsoid equilateral ascospores with a spirally coiling germ slit and small bipolar secondary appendages. According to the description of Rappaz (1995), Leptomassaria unedonis may also belong to Spiririma. nBLAST searches of the ITS of S. gaudefroyi revealed accessions referable to the morphologically quite distinct genus Induratia (syn. Muscodor) as closest matches (92–95 % sequence identity), confirming the results of the phylogenetic analyses (Fig. 1).
Spiririma gaudefroyi (Fabre) Voglmayr, J. Fourn., S. Tello & Jaklitsch, comb. nov. — MycoBank MB 844611; Fig. 18
Fig. 18.

Spiririma gaudefroyi. a–b, d. Habit of ascomata on host surface in top view; c, g. erumpent ascomatal apices in side view; e–f. ascomata in vertical section; h–m. apical apparatuses in black Pelikan ink (h), diluted blue Pelikan ink (i–j), Melzer’s reagent (k–l) and Lugol’s solution (m); n. clypeus and upper peridium in vertical section, in chloral-lactophenol; o. lateral peridium in vertical section, in chloral-lactophenol; p. eight-spored ascus with paraphyses; q–r. mature and immature few-spored asci; s–b1. ascospores showing a helicoid germ slit (s–w, b1) and bipolar secondary appendages (arrows) (p–r, bl in diluted Indian ink; s–a1 in 1 % SDS) (a, c–e, s–w. WU-MYC 0044041 (epitype); b, f–r, x–b1. WU-MYC 0044043). — Scale bars: a–b, d = 500 μm; c, e–g = 200 μm; h–m = 5 μm; n, p–r = 50 μm; o = 20 μm; s–b1 = 10 μm.
Basionym. Rosellinia gaudefroyi Fabre, Ann. Sci. Nat., Bot., sér. 6 9: 79. 1879 ‘1878’.
Synonym. Helicogermslita gaudefroyi (Fabre) Læssøe & Spooner, Kew Bull. 49(1): 50. 1994 ‘1993’.
Typification. France, Provence-Alpes-Côtes d’Azur, Dépt. Vaucluse, Sérignan(-du-Comtat), on bark of trunkbase of Quercus pubescens, soc. Hysterium pulicare and Navicella pileata, June 1879, J.H. Fabre (MNHN-FABR-FABR11249, lectotype of Rosellinia gaudefroyi here designated, MBT 10007726; MNHN-FABR-FABR11250, MNHN-FABR-FABR13089 isotypes). – Spain, Andalucía, Jaén, Valdepeñas de Jaén, El Parrizoso, N37°36'56.56" W3°44'0.05", 1157 m a.s.l., on bark of living trunk of Quercus ilex, 10 Dec. 2017, S. Tello S.T.10121701 (WU-MYC 0044041, epitype of Rosellinia gaudefroyi here designated, MBT 10007727; ex-epitype culture HGA = CBS 147284)
Pseudostroma consisting of a black clypeus 400–1000 μm diam, irregularly spreading around the ostiole of an individual ascoma or those of a small group of adjacent ascomata, overlain by a thin, pure white, widespreading pellicle composed of dead bleached periderm cells, devoid of crystals. Clypeus 50–80(–100) μm thick, gradually thinning at the periphery, leathery to faintly carbonaceous, prosenchymatous, composed of host periderm cells occluded by tightly aggregated dark brown hyphae 3–4 μm diam, with wall 1–1.2 μm thick; seated on a basal layer of necrotic bark cells invaded by a loose textura intricata of pale brown hyphae 1.5–3 μm diam with wall 0.5–0.8 μm thick, continuous with the periderm. Ascomata perithecial, corticolous, immersed to erumpent, scattered, solitary or in small clusters, globose to subglobose, (300–)500–850 μm diam, apically with a central, broadly conical, coarsely papillate ostiole, black, smooth, projecting (100–)200–250 μm above the ascomal venter. Peridium 45–55 μm thick at sides, up to 60 μm thick at the apex, pseudoparenchymatous, brown to dark brown, a textura angularis grading inwardly into textura prismatica, composed of unevenly pigmented cells with wall 1.8–2.5 μm thick, with scattered pale brown hyphae similar to those lying under the clypeus originating from the outermost peridial cells and spreading into the bark tissue; innermost layer inconspicuous, composed of thin-walled, hyaline prismatic cells; ostiolar canal densely periphysate. Paraphyses copious, hyphal, hyaline, thin-walled, remotely septate, rarely branched, with scattered small refractive guttules, much longer than the asci, 4–5 μm wide at the base, gradually tapering to 1.5–2 μm above the asci, discretely embedded in a mucilaginous matrix. Asci cylindrical to subfusiform, short-stipitate, with (4–)8 obliquely uniseriate overlapping, occasionally irregularly biseriate ascospores, 175–210 × 20.3–26.7 μm (Me = 191 × 23.4 μm, N = 20) including the 15–25 μm long fragile stipes; with a massive bipartite apical apparatus 8.7–10 μm high × 5.8–7 μm wide (Me = 9.5 × 6.4 μm, N = 20) featuring an euamyloid short-cylindrical to nearly trapezoid basal part 3.6–4.6 μm high × 2.9–4.0 μm wide (Me = 4.2 × 3.5 μm, N = 30), frequently apically flared with lateral rims, blueing in Melzer’s reagent, darker blue in Lugol’s solution, yellow in black Pelikan ink; upper part 3.3–4.8 μm high × 6–6.9 μm wide (Me = 4.1 × 6.4 μm, N = 12), nearly turbinate, apically flared and convex, inamyloid, hyaline in water or Melzer’s reagent, whitish in black Pelikan ink, stained light blue in diluted blue Pelikan ink. Ascospores (22–)24.3–36.4(–41.8) × (9–)9.6–12(–13.2) μm, Q = (2–)2.3–3.3(–3.8) (Me = 28.7 × 10.6 μm, Qe = 2.7; N = 180), aseptate, varying from ellipsoid to narrowly ellipsoid, occasionally nearly oblong, equilateral, with mostly broadly rounded ends, with a narrow but conspicuous helicoid germ slit c. 0.5 μm wide coiling once around the ascospore, almost reaching the ends; epispore medium to dark brown, smooth when observed after 48 h incubation in PVA-lactophenol; a narrow gelatinous sheath surrounding the whole ascospore and slightly swollen at ends forming bipolar secondary appendages detectable in Indian ink; short, hyaline, flattened appendages can be seen in water on fresh material but frequently becoming inconspicuous with time. Asexual morph on the natural substrate not seen.
Habitat & Host range — In dead bark of living trunks of Quercus spp.
Distribution — Southern France, Spain.
Additional specimens examined. France, Provence-Alpes-Côte d’Azur, Dépt. Alpes-de-Haute-Provence, Savoy Alps, 15 km N of Digne-les-Bains, 3.7 km SSW of Barles, Clues de Barles, N44°13'52" E6°15'30", 910 m a.s.l., in bark of a trunk of Quercus pubescens, soc. Decaisnella mesascium, Hyalorbilia fusispora, Leptogium sp., Proliferodiscus tricolor, 14 Aug. 2009, H.O. Baral H.B. 9156d (WU-MYC 0044042). – Spain, Jaén, Valdepeñas de Jaén, Cerro de Chircales, N37°35'42.79" W3°51'15.18", 988 m a.s.l., in bark of Quercus faginea, 24 Feb. 2018, S. Tello S.T.24021801 (WU-MYC 0044043; culture HGA1).
Notes — Spiririma gaudefroyi is a little-known and -studied species lacking detailed descriptions and illustrations of its ascomatal characters, and it has been variously treated taxonomically. After studying the type, Petrini (1992) considered Rosellinia gaudefroyi to be conspecific with Anthostomella calligoni, a species described from dead stems of Calligonum (Polygonaceae) collected in Turkmenistan, but no morphological description of the type of R. gaudefroyi was given. While according to the protologue the ascospore characters of A. calligoni and R. gaudefroyi are similar, the sparse description and the line drawing in Frolov (1970) are insufficient to evaluate the generic affiliation of A. calligoni. Conspecificity of A. calligoni and R. gaudefroyi was subsequently not accepted by Læssøe & Spooner (1993), who transferred R. gaudefroyi to Helicogermslita. However, they did not investigate the type but only material referrable to a different, yet unrelated, species also growing on Quercus, Anthostoma vincensii, which they considered to be synonymous with R. gaudefroyi. Therefore, their brief description of ascomatal characters represents a different species, Albicollum vincensii (see above).
Leptomassaria simplex is another unrelated species that has been confused with S. gaudefroyi based on similar ascospore size and shape (e.g., Wergen 2018: 133). However, L. simplex differs by its host (Tilia spp.) and by wider blackish ascospores (22–41 × 12–22 μm) with a minute basal appendage and a prominent, widely expanding gel sheath.
Morphologically, Leptomassaria unedonis as described by Rappaz (1995) is very similar to Spiririma gaudefroyi, except that no white tissue on bark surface was mentioned. Unfortunately, the De Notaris specimens maintained in RO are not sent out on loan, but photographic images of the type specimens were kindly provided by Agnese Tilia (RO), showing black clypeus- like structures which, however, were taken at too low magnification to properly evaluate the ascomata in detail.
As the specimens of FABR are not sent out on loan, we could not investigate the original material of R. gaudefroyi. However, L. Petrini kindly provided her notes and documentation on the Fabre collections she investigated in 1989, based on which we here designate MNHN-FABR-FABR11249 as lectotype. To stabilise the species concept, we here propose our recent collection WU-MYC 0044041, for which a culture and sequences are available, as epitype. Although the collection date is given as July in the protologue (Fabre 1879), the specimens bear the collection date June.
KEY TO UNI- TO PAUCIPERITHECIATE XYLARIACEOUS GENERA WITH SIGMOID TO HELICOID GERM SLITS
Notes — This key does not include Anthostomella species with sigmoid to helicoid germ slits, as the genus is in need of a critical revision and reclassification before they can be incorporated in a key. Amphirosellinia species with multiperitheciate stromata and Stilbohypoxylon species lacking sigmoid to helicoid germ slits are not covered by the key.
1. Stromata massive, rosellinia-like, conical, semi- to subglobose, immersed, erumpent or superficial . . . . . . . . . . . . . 2
1. Pseudostromata reduced, not rosellinia-like, immersed to erumpent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2. Stromata superficial, perithecioid; surface brown to black, with cracks, squamules or protuberances; ectostroma carbonaceous or leathery; ascospores inequilateral, with sigmoid germ slits; geniculosporium-like asexual morph associated with or on stromata, either synnematous or conidiophores formed on squamules of the stromata Stilbohypoxylon p.p.
2. Stromata immersed to erumpent; surface black, smooth to verruculose; ectostroma strongly carbonaceous; ascospores with helicoid or sigmoid germ slits; asexual morph not formed on stromata . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Ascospores equilateral; stromata usually erumpent and often covered by substrate remnants; ectostroma clypeus-like, hard, splintering Helicogermslita
3. Ascospores inequilateral; stromata in most species immersed, only occasionally (A. evansii) erumpent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Amphirosellinia p.p.
4. Pseudostromata pustulate, lopadostoma-like, containing 2–5 perithecia, with a blackish ectostromatic disc and few black, ostiolar dots; whitish to brownish hyphal entostroma surrounded by a black line . . . . . . . . . . . . . . . . . . . Oligostoma
4. Pseudostromata immersed to erumpent, without an ectostromatic disc and a black line . . . . . . . . . . . . . . . . . . . . . 5
5. Ostiolar necks black, erumpent to distinctly projecting and surrounded by a striking persistent white collar of pseudo-stromatic tissues; without a . . . . . . . . . . clypeus Albicollum
5. Ostioles not surrounded by a persistent white collar, with a more or less distinct grey to blackish clypeus . . . . . . . . . . 6
6. Clypeus black, leathery, distinct; ostioles central, broadly conical, papillate; asci persistent; ascospores with a narrow gelatinous sheath and bipolar secondary appendages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Spiririma
6. Clypeus light to dark grey, often indistinct; ostioles not to slightly protruding, never conical; asci very unstable and quickly dissolving in water; ascospores with a prominent gelatinous sheath widely expanding in water when fresh and a minute basal cellular appendage (dwarf cell) seen at least when immature . . . . . . . . . . . . . . . . . . Leptomassaria
DISCUSSION
Phylogenetic placement of the genera with helicoid and poroid germ loci
The genera with helicoid (Albicollum, Amphirosellinia, Leptomassaria, Oligostoma, Spiririma) or poroid (Digitodochium, Nemania species formerly classified in Euepixylon) germ loci included in the present analyses were contained within Xylariaceae in the restricted sense of Wendt et al. (2018) and subsequent publications (Voglmayr et al. 2018, Voglmayr & Beenken 2020). In addition to the genera treated here in detail, also Amphirosellinia spp. (Ju et al. 2004, Fournier et al. 2018a), Anthostomella (Francis 1975, Lu & Hyde 2000) as well as several Kretzschmaria (Rogers & Ju 1998, Hladki & Romero 2001), Rosellinia (Petrini 2013), Stilbohypoxylon (Rogers & Ju 1997, Petrini & Ju 2021) and Xylaria species (Læssøe 1999, Rogers & Ju 2004, Ju et al. 2012, Fournier 2014, Fournier et al. 2018c) have sigmoid to helicoid germ slits. Neither the genera with sigmoid to helicoid (Cannon 1987) nor those with poroid germ slits were revealed as closely related but dispersed throughout Xylariaceae. Apart from Xylariaceae s.str., sigmoid to helicoid germ slits are occasionally also found in other related families, e.g., Graphostromataceae (Obolarina spp., Pažoutová et al. 2010). For other xylariaceous genera with helicoid germ slits, like Xylotumulus (Rogers et al. 2006), no molecular data are yet available, and familial affiliation is unclear (Wendt et al. 2018). Therefore, germ slit morphology is not indicative for phylogenetic relationship above the generic level, but usually highly informative at species level.
In most xylariaceous species the germ slit is straight and oriented longitudinally but may be more or less oblique, diagonal to exceptionally circumferential (equatorial). When it is not straight it is termed sigmoid, as long as it is restricted to one face of the ascospore, typically dorsal in Hypoxylaceae and ventral in Xylariaceae, but with some exceptions. The term helicoid applies to germ slits that extend on lateral faces and are more or less coiled around the entire ascospore, occasionally several times, as in Albicollum and Helicogermslita species illustrated herein. Albicollum vincensii (Fig. 10) is a borderline case with a germ slit often barely long enough to make a full coil. Conversely, O. insidiosum (Fig. 17) features a shorter but strongly sigmoid germ slit slightly extending laterally sides and such a configuration is occasionally referred to as spiral or helicoid in literature, making these terms sometimes somewhat ambiguous and requiring accurate observations.
There are some discrepancies about the circumscription of Xylariaceae. Recently, Samarakoon et al. (2020) synonymised Muscodor with Induratia, and established a segregate family Induratiaceae for Emarcea and Induratia, which was adopted by Hyde et al. (2020) who also accepted Clypeosphaeriaceae for Clypeosphaeria and Anthostomelloides. In their phylogenies, Induratiaceae formed an unsupported sister group relationship with Clypeosphaeriaceae (plus Fasciatispora arengae), which were placed as sister group to Xylariaceae. Samarakoon et al. (2022) accepted Induratiaceae and Clypeosphaeriaceae as well; however, they restricted Clypeosphaeriaceae to Clypeosphaeria mamillana, while several genera related to Clypeosphaeria were not assigned to families.
Based on our phylogenies and on morphological considerations, we do not accept Induratiaceae and Clypeosphaeriaceae as distinct families but argue to include them within Xylariaceae. With the addition of Spiririma having dark brown unicellular ascospores with a helicoid germ slit, the Induratiaceae have become heterogeneous. The same applies for Clypeosphaeriaceae, which, if applied to the sister group of Induratiaceae, is morphologically likewise highly heterogeneous without characters setting it apart from the core Xylariaceae. On the other hand, it is certainly not desirable to restrict Clypeopshaeriaceae to Clypeosphaeria and to establish several additional monotypic families for the unclassified genera of Samarakoon et al. (2022), as well as for Digitodochium. In addition, when accepting Induratiaceae and Clypeopshaeriaceae as distinct families, the genus Linosporopsis needs to be classified within a distinct family as well. We do not see much benefit from such a familial splitting into numerous smaller entities that are either again morphologically heterogeneous or monotypic, if the core Xylariaceae contain a morphologically similar heterogeneity.
Acknowledgements
We thank the herbarium curators of B, H, K and M for loans of herbarium specimens and of K for permission for DNA extraction of Helicogermslita specimens, Walter Till (WU) for handling the herbarium loans, Irmgard Greilhuber for incorporating the specimens in the herbarium, Agnese Tilia (RO) for providing photographic illustrations of the type specimens of Sphaeria unedonis and S. umbrinella, Jorge Alberto Chayle (LPS) for sending photos of the type specimen of Anthostoma chionostoma and for permission to use them in our publication, Genevieve E. Tocci (FH) and Lia Pignotti (FT) for providing information on the type specimen of Rosellinia somala, Liliane Petrini for providing her documentation of the Fabre specimens of Rosellinia gaudefroyi from FABR, Stefan Blaser, Adrian Carter, Ethan Crenson, Annemarie Gallé, Bernd Fellmann, Matevž Koncilja, Paul Leroy, Per Marstad, Inge Rössl and Ilse Wendelin for communicating fresh collections, and Yu-Ming Ju for sending cultures.
Declaration on conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Anahosur KH. 1969. Ascomycetes of Coorg (India) Vll. Sydowia 23: 57–62. [Google Scholar]
- Auerswald B. 1868. Sphaeria cubicularis Fr. Hedwigia 7: 17–19. [Google Scholar]
- Arnaud G. 1925. Les Astérinées. IV.e partie (Études sur la systématique des champignons Pyrénomycétes). Annales des Sciences Naturelles. Botanique, ser. 10, 7: 643–722. [Google Scholar]
- Baral HO. 2009. Iodine reaction in Ascomycetes: why is Lugol’s solution superior to Melzer’s reagent? https://in-vivo-veritas.de/articles/iodine-reaction-in-ascomycetes-why-is-lugols-solution-superior-to-melzers-reagent/.
- Baral HO, Weber E, Marson G. 2020. Monograph of Orbiliomycetes (Ascomycota) based on vital taxonomy. Part I + II. National Museum of Natural History Luxembourg. https://www.mnhn.lu/pub/mono_orb. [Google Scholar]
- Barr ME, Rogerson CT, Smith SJ, et al. 1986. An annotated catalog of the Pyrenomycetes described by Charles H. Peck. Bulletin of the New York State Museum 459: 1–74. [Google Scholar]
- Becker K, Wongkanoun S, Wessel AC, et al. 2020. Phylogenetic and chemotaxonomic studies confirm the affinities of Stromatoneurospora phoenix to the coprophilous Xylariaceae. Journal of Fungi 6: E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackel W, Zehm A. 2020. Digitodochium rhodoleucum Tubaki & Kubono: ein für Bayern und Europa neuer Hyphomyzet. Hoppea 80: 150–152. [Google Scholar]
- Cannon PF. 1987. The identity of the genus Spirogramma. Systema Ascomycetum 6: 171–178. [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Cedeño-Sanchez M, Wendt L, Stadler M, et al. 2020. Three new species of Hypoxylon and new records of Xylariales from Panama. Mycosphere 11: 1457–1476. [Google Scholar]
- Chen JJ, Feng XX, Xia CY, et al. 2019. The phylogenetic position of the genus Muscodor and the description of a new Muscodor species. Mycosphere 10: 187–201. [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015. Fungal Planet description sheets 320-370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daranagama DA, Camporesi E, Tian Q, et al. 2015. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Diversity 73: 203–238. [Google Scholar]
- Daranagama DA, Hyde KD, Sir EB, et al. 2018. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Diversity 88: 1–165. [Google Scholar]
- Daranagama DA, Jones EBG, Liu XZ, et al. 2016. Mycosphere essays 13. Do xylariaceous macromycetes make up most of the Xylariomycetidae? Mycosphere 7: 582–601. [Google Scholar]
- Dargan JS, Singh M, Rogers JD. 1984. A note on Helicogermslita celastri. Mycologia 76: 1113–1115. [Google Scholar]
- De Hoog GS, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Dennis RWG. 1956. Some Xylarias of tropical America. Kew Bulletin 11: 401–444. [Google Scholar]
- Duong LM, Lumyong S, Hyde KD, et al. 2004. Emarcea castanopsidicola gen. et sp. nov. from Thailand, a new xylariaceous taxon based on morphology and DNA sequences. Studies in Mycology 50: 253–260. [Google Scholar]
- Eriksson OE. 2014. Checklist of the non-lichenized ascomycetes of Sweden. Symbolae Botanicae Upsalienses 36: 1–499. [Google Scholar]
- Fabre JH. 1879. ‘1878’. Essai sur les Sphaeriacees du Departement de Vaucluse. Annales des Sciences Naturelles, ser. 6, 9: 66–118. [Google Scholar]
- Forin N, Vizzini A, Fainelli F, et al. 2021. Taxonomic re-examination of nine Rosellinia types (Ascomycota, Xylariales) stored in the Saccardo mycological collection. Microorganims 9: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. 2014. Update on European species of Xylaria. http://www.ascofrance.fr/uploads/xylaria/201406.pdf.
- Fournier J, Castro Marcote JM, Rubio Dominguez E, et al. 2018a. Amphirosellinia gallaeciana sp. nov. (Xylariaceae), a new species from Spain. Ascomycete.org 10: 200–204. [Google Scholar]
- Fournier J, Flessa F, Peršoh D, et al. 2011. Three new Xylaria species from southwestern Europe. Mycological Progress 10: 33–52. [Google Scholar]
- Fournier J, Ju YM, Hsieh HM, et al. 2018b. Xylaria aethiopica sp. nov. – a new pod-inhabiting species of Xylaria (Xylariaceae) from Ethiopia. Ascomycete.org 10: 209–215. [Google Scholar]
- Fournier J, Lechat C, Courtecuisse R. 2018c. The genus Xylaria sensu lato (Xylariaceae) in Guadeloupe and Martinique (French West Indies). I. Taxa with penzigioid stromata. Ascomycete.org 10: 131–176. [Google Scholar]
- Fournier J, Lechat C, Courtecuisse R. 2020. The genus Xylaria sensu lato (Xylariaceae) in Guadeloupe and Martinique (French West Indies) III. Taxa with slender upright stromata. Ascomycete.org 12: 81–164. [Google Scholar]
- Francis SM. 1975. Anthostomella Sacc. (Part 1). Mycological Papers 139: 1–97. [Google Scholar]
- Francis SM, Minter DW, Caine TS. 1980. Three new species of Anthostomella. Transactions of the British Mycological Society 75: 201–206. [Google Scholar]
- Frolov IP. 1970. Ascomycetes novi e Turkomania in Calligono sp. inventi. Novosti Sistematiki Nizshikh Rastenii 7: 189–197. [Google Scholar]
- Fuckel L. 1870. Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde 23-24: 1–459. [Google Scholar]
- Gerhardt E, Hein B. 1979. Die nomenklatorischen Typen der von Th. Nitschke beschriebenen Arten im Pilzherbar des Botanischen Museums Berlin-Dahlem. Willdenowia 9: 313–329. [Google Scholar]
- Granmo A, Læssøe T, Schumacher T. 1999. The genus Nemania s.l. (Xylariaceae) in Norden. Sommerfeltia 27: 1–96. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hawksworth DL, Lodha BC. 1983. Helicogermslita, a new stromatic xylariaceous genus with a spiral germ slit from India. Transactions of the British Mycological Society 81: 91–96. [Google Scholar]
- Hayova VP. 2012. Ascomycota of the Gorgany Nature Reserve. Ukrainian Botanical Journal 69: 255–264. [Google Scholar]
- Hein B. 1984. Originalmaterial und Hinweise zu den von G. Otth beschriebenen Fungi-Arten und subspezifischen Taxa im Herbar des Botanischen Museums Berlin-Dahlem. Willdenowia 14: 413–416. [Google Scholar]
- Hladki AI, Romero AI. 2001. The genus Kretzschmaria from Tucuman, Argentina. Mycotaxon 79: 481–496. [Google Scholar]
- Hladki AI, Romero AI. 2003. Two new species of Stilbohypoxylon and the taxonomic positions of Hypoxylon cyclopicum, H. chionostomum and Anthostoma chionostoma. Sydowia 55: 65–76. [Google Scholar]
- Höhnel F. 1918. Mycologische Fragmente. Annales Mycologici 16: 35–174. [Google Scholar]
- Hongsanan S, Hyde KD, Bahkali AH, et al. 2015. Fungal biodiversity profiles 11–20. Cryptogamie Mycologie 36: 355–380. [Google Scholar]
- Hopple JS, Vilgalys R. 1994. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86: 96–107. [Google Scholar]
- Hsieh HM, Lin CR, Fang MJ, et al. 2010. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Molecular Phylogenetics and Evolution 54: 957–969. [DOI] [PubMed] [Google Scholar]
- Hsieh WH, Chen CY, Sivanesan A. 1995. Taiwan fungi: new species and new records of ascomycetes. Mycological Research 99: 917–931. [Google Scholar]
- Hyde KD, Norphanphoun C, Maharachchikumbura SSN, et al. 2020. Refined families of Sordariomycetes. Mycosphere 11: 305–1059. [Google Scholar]
- Jaklitsch WM. 2009. European species of Hypocrea Part I. The green-spored species. Studies in Mycology 63: 1–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Fournier J, Rogers JD, et al. 2014. Phylogenetic and taxonomic revision of Lopadostoma. Persoonia 32: 52–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Gardiennet A, Voglmayr H. 2016. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 37: 82–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. 2012. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia 104: 925–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2011. Nectria eustromatica sp. nov., an exceptional species with a hypocreaceous stroma. Mycologia 103: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2012. Phylogenetic relationships of five genera of Xylariales and Rosasphaeria gen. nov. (Hypocreales). Fungal Diversity 52: 75–98. [Google Scholar]
- Johnston PR, Rogers JD, Park D, et al. 2016. Entalbostroma erumpens gen. et sp. nov. (Xylariaceae) from Phormium in New Zealand. Mycotaxon 131: 766–772 . [Google Scholar]
- Ju YM, Hsieh HM, Rogers JD, et al. 2012. New and interesting penzigioid Xylaria species with small, soft stromata. Mycologia 104: 766–776. [DOI] [PubMed] [Google Scholar]
- Ju YM, Rogers JD. 2002. The genus Nemania (Xylariaceae). Nova Hedwigia 74: 75–120. [Google Scholar]
- Ju YM, Rogers JD, Hsieh HM, et al. 2004. Amphirosellinia gen. nov. and a new species of Entoleuca. Mycologia 96: 1393–1402. [PubMed] [Google Scholar]
- Karsten PA. 1873. Pyrenomycetes novi, in Fennia et Lapponia rossica lecti. Notiser ur Sällskapets pro Fauna et Flora Fennica förhandlingar, ny ser. 13: 245–248. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konta S, Hyde KD, Phookamsak R, et al. 2020. Polyphyletic genera in Xylariaceae (Xylariales): Neoxylaria gen. nov. and Stilbohypoxylon. Mycosphere 11: 2629–2651. [Google Scholar]
- Koukol O, Kelnarová I, Černý K, et al. 2015. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. Forest Pathology 45: 21–27. [Google Scholar]
- Kuhnert E, Fournier J, Peršoh D, et al. 2014. New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Diversity 64: 181–203. [Google Scholar]
- Kuhnert E, Sir EB, Lambert C, et al. 2017. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Diversity 85: 1–43. [Google Scholar]
- Kuhnert E, Surup F, Sir EB, et al. 2015. Lenormandins A–G, new azaphilones from Hypoxylon lenormandii and Hypoxylon jaklitschii sp. nov., recognised by chemotaxonomic data. Fungal Diversity 71: 165–184. [Google Scholar]
- Læssøe T. 1999. The Xylaria comosa complex. Kew Bulletin 54: 605–619. [Google Scholar]
- Læssøe T, Heilmann-Clausen J, Christensen M. 2000. Slægterne Nemania, Euepixylon og Kretzschmaria i Danmark. Svampe 42: 17–29. [Google Scholar]
- Læssøe T, Spooner BM. 1993. Rosellinia & Astrocystis (Xylariaceae): new species and generic concepts. Kew Bulletin 49: 1–70. [Google Scholar]
- Lambert C, Pourmoghaddam MJ, Cedeño-Sanchez M, et al. 2021. Resolution of the Hypoxylon fuscum complex (Hypoxylaceae, Xylariales) and discovery and biological characterization of two of its prominent secondary metabolites. Journal of Fungi 7: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Wendt L, Hladki AI, et al. 2019. Hypomontagnella (Hypoxylaceae): a new genus segregated from Hypoxylon by a polyphasic taxonomic approach. Mycological Progress 18: 187–201. [Google Scholar]
- Lee S, Crous PW. 2003. A new species of Helicogermslita from South Africa. Sydowia 55: 109–114. [Google Scholar]
- Li QR, Kang JC, Hyde KD. 2015. Two new species of the genus Collodiscula (Xylariaceae) from China. Mycological Progress 14: 52. [Google Scholar]
- Liu F, Bonthond G, Groenewald JZ, et al. 2019. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Studies in Mycology 92: 287–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lu BS, Hyde KD. 2000. A world monograph of Anthostomella. Fungal Diversity Research Series 4: 1–376. Fungal Diversity Press, Hong Kong. [Google Scholar]
- Martin P. 1967. Studies in the Xylariaceae: I. New and old concepts. Journal of South African Botamy 33: 205–240. [Google Scholar]
- Moncalvo JM, Wang HH, Hseu RS. 1995. Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87: 223–238. [Google Scholar]
- Pažoutová S, Srutka P, Holuša J, et al. 2010. The phylogenetic position of Obolarina dryophila (Xylariales). Mycological Progress 9: 501–507. [Google Scholar]
- Pelaez F, Gonzalez V, Platas G, et al. 2008. Molecular phylogenetic studies within the family Xylariaceae based on ribosomal DNA sequences. Fungal Diversity 31: 111–134. [Google Scholar]
- Petrak F. 1914. Beiträge zur Pilzflora von Mähren und Österr.-Schlesien. Annales Mycologici 12: 471–479. [Google Scholar]
- Petrini LE. 1992. Rosellinia species of the temperate zones. Sydowia 44: 169–281. [Google Scholar]
- Petrini LE. 2003. Rosellinia and related genera in New Zealand. New Zealand Journal of Botany 41: 71–138. [Google Scholar]
- Petrini LE. 2013. Rosellinia – a world monograph. Bibliotheca Mycologica 205. Cramer, Stuttgart. [Google Scholar]
- Petrini LE, Ju YM. 2021. Notes on Stilbohypoxylon (Sordariomycetes, Xylariales). Nova Hedwigia 113: 471–490. [Google Scholar]
- Petrini LE, Petrini O, Fisher PJ. 1987. Anthostomella calligoni, an endophyte of Suaeda fruticosa in Dorset. Transactions of the British Mycological Society 89: 387–389. [Google Scholar]
- Pi YH, Long SH, Wu YP. et al. 2021. A taxonomic study of Nemania from China, with six new species. MycoKeys 83: 39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmoghaddam MJ, Lambert C, Surup F, et al. 2020. Discovery of a new species of the Hypoxylon rubiginosum complex from Iran and antagonistic activities of Hypoxylon spp. against the Ash Dieback pathogen, Hymenoscyphus fraxineus, in dual culture. MycoKeys 66: 105–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaz F. 1992. Anthostoma decipiens et sa position systématique. Mycologia Helvetica 5: 21–32. [Google Scholar]
- Rappaz F. 1995. Anthostomella and related xylariaceous fungi on hard wood from Europe and North America. Mycologia Helvetica 7: 99–168. [Google Scholar]
- Rehm H. 1881. Ascomyceten. In getrockneten Exemplaren herausgegeben. Berichte des naturwissenschaftlichen Vereins für Schwaben, Augsburg 26: 2–132. [Google Scholar]
- Rogers JD, Ju YM. 1997. The genus Stilbohypoxylon. Mycological Research 101: 135–138. [Google Scholar]
- Rogers JD, Ju YM. 1998. The genus Kretzschmaria. Mycotaxon 68: 345–393. [Google Scholar]
- Rogers JD, Ju YM. 2004. Kretzschmaria varians sp. nov., Xylaria coremiifera sp. nov. and Xylaria umbonata sp. nov. from Costa Rica. Mycological Progress 3: 37–40. [Google Scholar]
- Rogers JD, Ju YM, Hemmes DE. 2006. Hypoxylon subdisciforme sp. nov., Nemania abortiva sp. nov. and Xylotumulus gibbisporus gen. et sp. nov. from Hawaii, Hawaiian Islands. Sydowia 58: 290–299. [Google Scholar]
- Samarakoon MC, Hyde KD, Maharachchikumbura SSN, et al. 2022. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Diversity 112: 1–88. [Google Scholar]
- Samarakoon MC, Thongbai B, Hyde KD, et al. 2020. Elucidation of the life cycle of the endophytic genus Muscodor and its transfer to Induratia in Induratiaceae fam. nov., based on a polyphasic taxonomic approach. Fungal Diversity 101: 177–210. [Google Scholar]
- Schrantz JP. 1960. Recherches sur les pyrenomycetes de I’ordre des Diatrypales, sensu Chadefaud, 1957. Bulletin de la Société Mycologique de France 76: 305–407. [Google Scholar]
- Senanayake IC, Maharachchikumbura SSN, Hyde KD, et al. 2015. Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Diversity 73: 73–144. [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337. [Google Scholar]
- Sir EB, Becker K, Lambert C, et al. 2019. Observations on Texas hypoxylons, including two new Hypoxylon species and widespread environmental isolates of the H. croceum complex identified by a polyphasic approach. Mycologia 111: 832–856. [DOI] [PubMed] [Google Scholar]
- Sir EB, Kuhnert E, Lambert C, et al. 2016a. New species and reports of Hypoxylon from Argentina recognized by a polyphasic approach. Mycological Progress 15: 1–42. [Google Scholar]
- Sir EB, Lambert C, Wendt L, et al. 2016b. A new species of Daldinia (Xylariaceae) from the Argentine subtropical montane forest. Mycosphere 7: 1378–1388. [Google Scholar]
- Speggazini C. 1884. Fungi Guaranitici. Pugillus 1. Anales de la Sociedad Cientifica Argentina 18: 263–286 (nos 178–228). [Google Scholar]
- Stadler M, Fournier J, Læssøe T, et al. 2010. Chemotaxonomic and phylogenetic studies of Thamnomyces (Xylariaceae). Mycoscience 51: 189–207. [Google Scholar]
- Stadler M, Kuhnert E, Peršoh D, et al. 2013. The Xylariaceae as model example for a unified nomenclature following the ‘one fungus-one name’ (1F1N) concept. Mycology 4: 5–21. [Google Scholar]
- Stadler M, Læssøe T, Fournier J, et al. 2014. A polyphasic taxonomy of Daldinia (Xylariaceae). Studies in Mycology 77: 1–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland. [Google Scholar]
- Tang AM, Jeewon R, Hyde KD. 2007. Phylogenetic relationships of Nemania plumbea sp. nov. and related taxa based on ribosomal ITS and RPB2 sequences. Mycological Research 111: 392–402. [DOI] [PubMed] [Google Scholar]
- Thiers B. 2021. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ [accessed 8 July 2021].
- Tibpromma S, Daranagama DA, Boonmee S, et al. 2017. Anthostomelloides krabiensis gen. et sp. nov. (Xylariaceae) from Pandanus odorifer (Pandanaceae). Turkish Journal of Botany 40: 107–116. [Google Scholar]
- Triebel D, Peršoh D, Wollweber H, et al. 2005. Phylogenetic relationships among Daldinia, Entonaema and Hypoxylon as inferred from ITS nrDNA sequences. Nova Hedwigia 80: 25–43. [Google Scholar]
- Triebel D, Scholz P. 2021. IndExs – Index of Exsiccatae. Botanische Staatssammlung München. http://indexs.botanischestaatssammlung.de/ [accessed 8 Oct. 2020]. [Google Scholar]
- Tubaki K, Kubono T. 1989. Digitodochium, a new staurosporous anamorph genus. Sydowia 41: 344–348. [Google Scholar]
- U’Ren JM, Miadlikowska J, Zimmerman NB, et al. 2016. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Molecular Phylogenetics and Evolution 98: 210–232. [DOI] [PubMed] [Google Scholar]
- Vasilyeva LN. 1998. Pyrenomycetes and Loculoascomycetes. In: Azbukina ZM. (ed.), Nizshie Rasteniya, Griby i Mokhoobraznye Dalnego Vostoka Rossii, Griby, Tom 4: Pirenomitsety i Lokuloaskomitsety, Nauka, St. Petersburg. [In Russian.] [Google Scholar]
- Voglmayr H, Akulov OY, Jaklitsch WM. 2016a. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycological Progress 15: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Beenken L. 2020. Linosporopsis, a new leaf-inhabiting scolecosporous genus in Xylariaceae. Mycological Progress 19: 205–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Castlebury LA, Jaklitsch WM. 2017. Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales). Persoonia 38: 136–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Friebes G, Gardiennet A, et al. 2018. Barrmaelia and Entosordaria in Barrmaeliaceae (fam. nov., Xylariales), and critical notes on Anthostomella-like genera based on multi-gene phylogenies. Mycological Progress 17: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Gardiennet A, Jaklitsch WM. 2016b. Asterodiscus and Stigmatodiscus, two new apothecial dothideomycete genera and the new order Stigmatodiscales. Fungal Diversity 80: 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2008. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research 112: 885–905. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2011. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae). Fungal Diversity 46: 133–170. [Google Scholar]
- Voglmayr H, Mehrabi M. 2018. Molecular phylogeny and a new Iranian species of Caudospora (Sydowiellaceae, Diaporthales). Sydowia 70: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt L, Sir EB, Kuhnert E, et al. 2018. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycological Progress 17: 115–154. [Google Scholar]
- Wergen B. 2018. Handbook of Ascomycota Ia. Pyrenomycetes s.l. mit 0–1fach septierten Sporen. Published by the author. [Google Scholar]
- Werle E, Schneider C, Renner M, et al. 1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22: 4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley AJS. 1976. Notes on the conidial state of Hypoxylon udum. Transactions of the British Mycological Society 67: 515–517. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego. [Google Scholar]
- Wittstein K, Cordsmeier A, Lambert C, et al. 2020. Identification of Rosellinia species as producers of cyclodepsipeptide PF1022 A and resurrection of the genus Dematophora as inferred from polythetic taxonomy. Studies in Mycology 96: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongkanoun S, Becker K, Boonmee K, et al. 2020. Three novel species and a new record of Daldinia (Hypoxylaceae) from Thailand. Mycological Progress 19: 1113–1132. [Google Scholar]
- Wongkanoun S, Wendt L, Stadler M, et al. 2019. A novel species and a new combination of Daldinia from Ban Hua Thung community forest in the northern part of Thailand. Mycological Progress 18: 553–564. [Google Scholar]
- Zhang CL, Wang GP, Mao LJ, et al. 2010. Muscodor fengyangensis sp. nov. from southeast China: morphology, physiology and production of volatile compounds. Fungal Biology 114: 797–808. [DOI] [PubMed] [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, et al. 2006. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076–1087. [DOI] [PubMed] [Google Scholar]


