Graphical abstract
Keywords: As removal, Ozone, Ultrasonic, ZnSO4 solution, Zinc roasting dust
Highlights
-
•
A novel one-pot method for efficient arsenic removal from ZnSO4 solution.
-
•
Ultrasonic field can enhance the oxidation effect of ozone and destroy the wrapper.
-
•
Compared to the lime method, the amount of precipitate can be reduced by 54.5%.
-
•
The reaction time is reduced by 60% compared to traditional methods.
Abstract
Currently, removing arsenic (As) from ZnSO4 solution using lime presents several drawbacks, including high wet precipitate content, long reaction time, and the introduction of new impurities. In this study, we propose a novel ultrasonic (US) ozone one-pot method for effectively removing As from a high-arsenic ZnSO4 solution. In this method, as in ZnSO4 solution was removed by ultrasound enhanced ozone oxidation combined with zinc roasting dust (ZRD). No secondary pollution will occur with the addition of ZRD and ozone, as neither introduces new impurities. The experimental results show that under the conditions of initial As and Fe concentrations of 1640 mg/L and 2963 mg/L, US power of 480 W, frequency of 20 kHz, reaction temperature of 60 °C, reaction time of 1 h, ZRD dose of 12 g/L and gas flow rate of 900 mL/min, the removal rate of As can reach 99.4%. The introduction of US can further enhance the oxidation effect of ozone on As(III) and Fe2+ by increasing the solubility of ozone and promoting the production of •OH radicals. Additionally, US cavitation and mechanical action increase the probability of contact between various reactants in the solution, facilitating the occurrence of reactions. US also reduces the aggregation of arsenic-containing precipitates and the encapsulation of ZRD by arsenic containing precipitates, thereby decreasing the amount of arsenic-containing precipitates. In comparison to the traditional lime method, this approach results in a significant reduction in the amount of arsenic-containing precipitate by 54.5% and a 60% decrease in the total reaction time. The As removal mechanism of our method encompasses ZRD neutralization, US-enhanced ozone mass transfer and decomposition, oxidation of As(III) and Fe2+, and adsorption and coprecipitation. Consequently, the proposed method provides a cost-effective, fast, safe and environmentally friendly alternative for treating arsenic-contaminated ZnSO4 solutions.
1. Introduction
With the rapid development of the economy, the consumption of zinc in China’s nonferrous metal industry has reached a significant level, ranking second only to aluminum and copper. Moreover, there is a consistent upward trend in the demand for zinc. According to the statistics of the China Nonferrous Metals Association, the cumulative zinc production in China in 2021 was 6.561 million tons, a year-on-year increase of 1.7% [1]. The production methods of zinc can be divided into two types: pyrometallurgy and hydrometallurgy. However, due to the high environmental pollution and low comprehensive metal recovery rate of pyrometallurgy, 80% of the zinc in the world is produced through hydrometallurgy [2]. The primary process of hydrometallurgical production is to leach the raw materials through two stages to obtain a zinc sulfate solution, which is then purified and sent to an electrolytic cell for zinc electrowinning. The obtained cathode zinc is then melted and cast to obtain coarse zinc ingots or zinc plates.
The primary raw material for hydrometallurgical zinc production is zinc calcine, which is obtained from the oxidized roasting of zinc sulfide concentrate. After two stages of acid leaching, a significant amount of zinc still remains in the residue of the zinc calcine. To recover this zinc, the acid leaching residue is typically reduced and roasted, and the zinc oxide dust is collected through a dust collection bag [3]. Due to the enrichment of over half of the As in the entire Zn leaching process in the acid leaching residue, this arsenic will enter the zinc oxide dust along with the flue gas during the reduction roasting process [4]. Therefore, when zinc oxide dust is leached, these arsenics will also be leached, which makes the As content in the zinc oxide dust leaching solution (500–2000 mg/L) higher than that in the zinc calcine leaching solution (<500 mg/L). The As content in the ZnSO4 solution must be lower than 0.3 mg/L to meet the electrolysis requirements, and excessive concentrations of arsenic can even cause “plate-burning” in the zinc electrowinning process [5], [6]. Therefore, the high concentration of As in the leaching solution of zinc oxide dust must be effectively removed to meet subsequent requirements.
There are many methods available for removing As from solutions [7]. The most commonly used methods for removing As from solutions with high As concentrations include sulfide precipitation, iron salt precipitation, calcium salt precipitation, and adsorption. The stability of arsenic-containing precipitates obtained by the sulfurization precipitation method is poor, and the process of arsenic removal inevitably produces the toxic gas H2S, causing secondary damage to the environment [8]. Although calcium salt precipitation has good effects, low raw material cost, and good slag stability, this method will achieve a large amount of low-grade arsenic containing gypsum slag, and subsequent treatment will also bring many difficulties [9]. The adsorbents used for As removal by the adsorption method generally include molecular sieves, activated carbon, zero valent iron, etc. However, only zero valent iron units have the best As removal effect and are suitable for a wide range of pH values. However, due to the high price of zero valent iron, it is not suitable for large-scale industrial use [10], [11], [12]. As removal by ferric salt mainly includes coagulation coprecipitation and adsorption reactions, ferric salt hydrolysis is mainly used to generate positively charged ferric hydroxide colloids. Coagulation coprecipitation mainly uses Fe3+ to react with arsenate or arsenite in water to generate FeAsO3 or FeAsO4 [13]. Some studies have also found that iron salts form binuclear bridging complexes with arsenic, such as FeO-AsO(OH)O-Fe and FeO-As(OH)O-Fe [14]. Overall, iron salt has a good As removal effect, strong stability of arsenic-containing precipitates, and low price, making it a widely used arsenic removal technology.
Arsenic in nature has difficulty existing in the form of single ions. Arsenic in solution usually exists in various oxygen containing acid radicals, such as trivalent (HAsO32-, H2AsO3-, H3AsO3 and AsO33-) and pentavalent (HAsO42-, H2AsO4-, H3AsO4 and AsO43-). According to the difference in pH value and oxidation reduction potential in solution, it will exist in different ionic forms [15], [16]. As is well known, almost all substances containing arsenic have high toxicity, among which the toxicity of trivalent arsenic is approximately 60 times that of pentavalent arsenic. Moreover, trivalent arsenic compounds have poor stability and strong mobility, making it easy for arsenic-containing precipitate to dissolve and cause secondary pollution during storage [17], [18], [19]. Many studies have also shown that under the same conditions, the removal effect of pentavalent arsenic is significantly better than that of trivalent arsenic [20], [21]. To stably and effectively remove As from a solution, enterprises typically subject it to oxidation treatment prior to As removal.
There are many common oxidants, such as KMnO4, H2O2, air, O2 and O3 [22], [23], [24]. Among these oxidants, KMnO4, air and O2 are commonly used in industrial production. KMnO4 has a good oxidation effect and quick speed, but it introduces impurities into the electrolyte during the oxidation process, increasing the cost of subsequent impurity removal. In addition, potassium permanganate is relatively expensive, which is not conducive to cost control. Air and O2 have long oxidation times, and there are challenges associated with the transportation and storage of H2O2. These factors limit the effectiveness of these oxidants in the production process. However, O3 has a very high oxidation reduction potential of 2.07 V, which is second only to F2 and hydroxyl radical (•OH). Additionally, the ozone oxidation process does not introduce new impurities and features rapid oxidation speeds, leading to its great development prospects [25], [26], [27]. Otgon’s research indicates that the combination of ozone oxidation and iron precipitation technology can achieve excellent oxidation effects in low pH solutions, with arsenic removal rates surpassing 90% at a solution temperature of 90 °C [28]. Cao et al. utilized ozone oxidation to synthesize scorodite for As removal. The As removal rate reached 89.64% after 7 h of reaction at 95 °C and an initial pH of 2 in the solution. The resulting scorodite particles were larger with good crystallinity [29]. In general, the oxidation effect of ozone is positively correlated with ozone dosage. However, its low solubility in water presents a significant disadvantage that hinders the widespread application of ozone [30].
Ultrasound (US) can improve the mass transfer and decomposition process of ozone in solution while also promoting the generation of free radicals. Therefore, it can significantly enhance the oxidation effect of ozone [31]. US refers to sound waves with a frequency greater than 20,000 Hz, which possess strong penetration abilities and propagate efficiently within liquids. US is known to generate various effects within liquids, such as mechanical effects, cavitation effects, chemical effects, and thermal effects [32]. Therefore, US has good applications in many fields, such as material synthesis [33], biotechnology [34], medical testing [35], and mineral leaching [36]. Due to its unique ability to accelerate chemical reactions and improve reaction efficiency, US finds widespread application in hydrometallurgy. According to Yan et al., US can increase the dissolution rate of valuable metals (Li, Ni, Co, and Mn) in waste lithium batteries by over 95% under optimal conditions [37]. The experimental results of Zhang et al. showed that US can increase the leaching concentration of gold in refractory gold ore by 18 kg/t from 6 kg/t with NaCN [38]. Masoum et al. used ultrasonic and peroxide assisted sulfur leaching to make the recovery of vanadium and yttrium in fly ash reach 100% and 97% respectively. The research results also showed that the high-frequency vibration and turbulence formed by ultrasonication in the solution can accelerate the diffusion of leachant to the surface diffusion of fly ash [39]. Chen et al. utilized ultrasound-assisted sulfuric acid leaching to extract vanadium from vanadium shale, achieving an improved leaching rate from 87.5% to 92.9% along with a reduction in leaching time by 87.5% [40]. Gui et al. demonstrated that the combination of US and ozone can significantly enhance the leaching rate of gold in refractory gold ores, increasing it from 49.1% to 93.5%. This is mainly attributed to US significantly suppressing the secondary inclusion layer caused by surface pyrite, refining the ore particle size, and reducing the reaction resistance [31].
The conventional method of using lime and Fe salt to remove As from ZnSO4 solutions produces substantial amounts of gypsum residue, which not only increases the costs but also poses an environmental threat owing to the potential leaching of arsenic from the waste residue. To address these issues, we propose a new strategy of replacing lime with ZRD as a neutralizer, utilizing ozone as an oxidant, and combining it with US to enhance the oxidation effect of ozone to improve the removal rate of arsenic and reduce the amount of residue. The use of ZRD to neutralize H2SO4 in solution has a series of advantages, such as low raw material cost, easy access to raw materials, and no introduction of impurity ions. Meanwhile, employing ozone as an oxidant helps prevent secondary pollution while avoiding the introduction of new impurities. Additionally, incorporating US can further improve the efficiency of arsenic removal. The arsenic-containing precipitates were analyzed by XRD, FE-SEM–EDS, FT-IR and XPS to probe the As removal mechanism. This study is of great importance because it not only efficiently removes As from ZnSO4 solution but also provides a safe, effective, low-cost and environmentally friendly treatment plan.
2. Materials and methods
2.1. Material and reagents
Sulfuric acid (H2SO4, XiLong Chemical Co., Ltd, Guangdong, China;), potassium permanganate (KMnO4, Tianjin Shentai Chemical Reagents Ltd., TSianjin, China; >98%), and calcium oxide (CaO, Tianjin Zhiyuan Chemical Co. Ltd., Tianjin, China; >98%) used in the experiment were of analytical grade. For our experiment, we obtained the arsenic-containing ZRD and ZnSO4 solution after germanium precipitation from a zinc production plant located in Yunnan Province, China. The ZRD was dried in a drying oven at 50 °C and then screened with a 200 mesh sieve to obtain a homogeneous particle size. The chemical composition of the ZRD was determined through a chemical analysis, and the results are presented in Table S1. The ZnSO4 solution containing arsenic is obtained by two-stage leaching of zinc oxide dust with sulfuric acid, followed by precipitation of germanium with tannic acid. Inductively coupled plasma atomic emission spectrometry (ICP–AES) was used to analyze the concentration of elements present in the ZnSO4 solution, with a dilution factor of 200–500 times. The dilution medium used is 5–10% hydrochloric acid solution. The concentration of Fe(II) was determined using the colorimetric O-phenanthroline method [41], and the concentration of Fe(III) was calculated by the following equation: CFe(III) = C(total Fe) - CFe(II), where C(total Fe) was measured using ICP–AES. The analytical results for the concentrations are presented in Table 1. The pH value of zinc sulfate solution is 1.47.
Table 1.
The elemental composition of the ZnSO4 solution from a zinc production plant.
| Element | As(Tol) | Fe2+ | Fe3+ | Zn | Cd | Mg | Pb | Ge |
|---|---|---|---|---|---|---|---|---|
| Concentration(mg/L) | 1640 | 2680 | 283 | 122,560 | 368 | 16.69 | 29 | 1.68 |
2.2. Experimental procedures
In this experiment, As in the ZnSO4 solution was removed by the ZRD neutralization ─ iron salt method. Due to the direct removal of As by the one-pot method, this method can greatly shorten the total reaction time compared with the traditional lime method. For our experiment, we replaced lime with ZRD as a neutralizer, utilized ozone oxidation as an oxidant, and employed a US field to enhance ozone oxidation and the As removal process. A schematic diagram of the experimental process is shown in Fig. 1, which mainly includes an ozone generator, a constant temperature water bath, an ultrasonic generator (VOSHIN-1500C), and a tail gas treatment. The ultrasonic equipment used in this experiment was probe type. The ultrasonic mode of action was continuous, and the adjustable parameters were as follows: ultrasonic power range from 0 to 1200 W, frequency was 20 kHz, and operating temperature range was 0 ∼ 90 °C. The ozone generator is capable of catalyzing the generation of ozone from oxygen, resulting in an ozone concentration of 10–30 mL/L (O3/O2).
Fig. 1.
Schematic diagram of experimental apparatus.
Batch experiments were conducted to explore the effects of different ZRD addition amounts, gas flow rates, US powers, reaction temperatures, and reaction times on the arsenic removal rate, arsenic removal precipitate stability, and precipitate amount. 500 mL of ZnSO4 solution was added to a 1000 mL round bottom flask and placed in a constant-temperature water bath for heating. The solution was stirred at 250 r/min under standard atmospheric pressure, and ZRD (4–16 g/L) was added to the solution when the temperature reaches the set value. At the same time, ozone (100–1000 mL/min) and ultrasound (60–480 W) are activated. Upon completion of the reaction, a vacuum pump was used to filter the solid–liquid mixture. The wet precipitate on the filter paper was washed with distilled water at least three times, followed by drying in a 60℃ oven for 12 h. The conventional method has the same parameters as the above reaction conditions, except for the absence of US. The pH meter is used to determine the final pH of the filtered solution.
The traditional lime method for arsenic removal uses one-stage lime neutralization─KMnO4 oxidation─two-stage lime neutralization. One-stage lime neutralization: 500 mL of zinc sulfate solution was added to a 1000 mL flask, the temperature was raised to the set temperature, lime milk was added to adjust the pH value of the solution to 4.5–4.8, the rotor speed was rotated at 250 r/min, and the mixture was reacted for 1 h. After the reaction was completed, a vacuum pump was used for filtration. KMnO4 oxidation: a filtered solution was put back into the flask, and when the temperature increased to the set temperature, KMnO4 was added. The rotor speed was 250 r/min, and the reaction lasted for 30 min. For two-stage lime neutralization, lime milk was added to the solution after KMnO4 oxidation was completed, the pH value of the solution was adjusted to 5.0–5.4, and the other conditions were the same as those for one-stage neutralization.
2.3. Characterizations
XRD analysis of the ZRD and arsenic-containing precipitate was conducted using a Netherlands Philips X’pert3 diffractometer. The analysis utilized Cu Ka radiation (λ = 1.54 Å) at a scanning rate of 8°/min within the scanning angle (2θ) range of 10-90°. The equipment used a generator voltage of 40 kV and a generator current of 40 mA.
FT-IR (Thermo Fisher Scientific, USA) spectra were collected to detect the surface chemical composition and elemental valence states of the arsenic-containing precipitate using the KBr pellet method at the wavelength scope of 400–4000 cm−1.
XPS (K-Alpha, Thermo Fisher Scientific, USA) measurements were carried out to analyze the chemical valence states transformation of Fe, As and O on the surface of the arsenic-containing precipitate sample using K-Alphaþ equipment equipped with an Al X-ray source (1486.6 eV), and using the C1s peak as an internal standard calibration peak at 284.8 eV.
The pH value all of solutions involved was measured using a Mettler pH meter from Shanghai Mettler Toledo. The particle size of the sample was determined using Mastersizer 3000 particle size analyzer by Malvern, UK.
FE-SEM (Philips XL30ESEM-TMP, Netherlands) was employed to observe the morphology and element mapping (EDS) of arsenic-containing precipitate and ZRD at an accelerated voltage of 15 kV. Due to the poor conductivity of the sample, platinum spraying treatment was carried out before the test.
2.4. Toxicity characteristic leaching test
To evaluate the stability of the arsenic-containing precipitate, the Toxicity Characteristic Leaching Procedure (TCLP) tests were performed following the guidelines outlined in the United States Environmental Protection Agency’s Test Methods [42]. The experimental procedure comprised two distinct stages: the preparation of the leaching solution and the arsenic toxicity leaching experiment.
Preparation of leaching solution:
At that stage, 5.7 mL of glacial acetic acid and 500 mL of distilled water were combined and added to a 1000 mL volumetric flask. Then the mixture was diluted with distilled water to the mark on the volumetric flask. Subsequently, the pH value was adjusted to 2.88 ± 0.05. The resulting solution was utilized as the leaching solution.
Arsenic toxicity leaching experiment:
During that stage, 10 g of arsenic-containing precipitate, ground to a particle size between 100 and 200 mesh, was placed into a conical flask with 200 mL of leaching solution. The liquid–solid ratio was maintained at 20:1 mL/g. The bottle cap was securely tightened, and the flask was positioned vertically on a horizontal shaker. The shaking frequency was adjusted to 180 r/min, and the mixture was continuously agitated for 18 ± 2 h at a temperature of 23 °C. Upon the completion of the leaching experiment, a vacuum pump was employed to separate the solid–liquid mixture. Subsequently, the concentration of As in the supernatant was determined using ICP-AES.
3. Results and discussion
3.1. Removal of arsenic from zinc sulfate solution
3.1.1. Effect of the dosage of ZRD
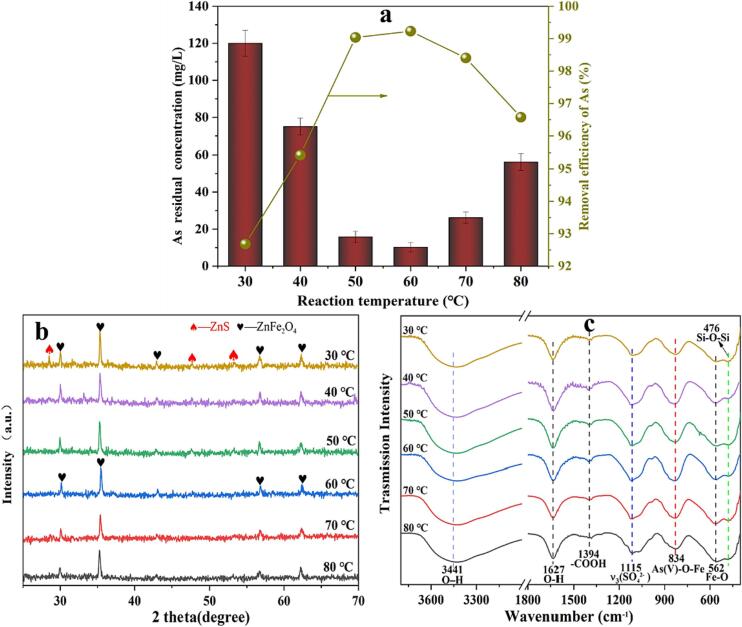
From Table 1, it appears that the ZnSO4 solution contains a significant amount of As(III) and Fe2+, while As(V) and Fe3+ are relatively scarce. Therefore, oxidation is necessary to remove iron and arsenic from the solution. To achieve better hydrolysis of Fe3+ in ZnSO4 solution and higher arsenic removal efficiency, it is necessary to adjust the pH value using ZRD to regulate the oxidation rate of As(III) and Fe2+ [43], [44]. Fig. 2b demonstrates that ZRD effectively regulates the pH of the ZnSO4 solution. With increasing ZRD dosage, the pH value shows an upward trend. This effect can be attributed to ZnO, which accounts for the majority of the composition in ZRD (as shown in Fig. S1). ZnO undergoes a neutralization reaction with H2SO4 present in the ZnSO4 solution. As depicted in Fig. 2a, increasing the ZRD dosage from 4 g/L to 12 g/L results in a decrease in arsenic concentration in the treated ZnSO4 solution from 1640 mg/L to 10.2 mg/L. Consequently, this leads to a remarkable As removal rate of 99.4%. However, when the dosage of ZRD further increased to 16 g/L, the As removal amount showed a declining trend. This could be attributed to the rapid hydrolysis and sedimentation of iron induced by the high pH level. Fig. 2c displays the XRD results of the arsenic-containing precipitate, and it can be observed that as the amount of ZRD gradually increases, the unreacted compounds such as ZnO, ZnS and Zn2SiO4 in the arsenic-containing precipitate also increase. In Fig. 2d, the FT-IR spectrum of the arsenic-containing precipitate is presented. The band observed at 834 cm−1 is associated with the tensile vibration of As(V)-O-Fe in amorphous iron arsenate [45]. Additionally, the band at 562 cm−1 corresponds to the vibration induced by Fe-O coordination, such as Fe-O-As(V) [46]. An interesting finding is that the intensity of the peak at 834 cm−1 decreases with increasing ZRD dosage, while the peak at 562 cm−1 shows the opposite trend. The reason for this result can be explained by the fact that when a small amount of ZRD is added, more arsenic is removed per unit mass of ZRD, resulting in a higher arsenic content in the precipitate. However, as the amount of ZRD added increases, more Fe3+ hydrolysis precipitation enters the residue due to the increase in the final pH value of the solution.
Fig. 2.
The residual As concentration and the corresponding As removal rate with the increase of ZRD dosage from 4 to 16 g/L (a); the final pH (b); XRD patterns of arsenic-containing precipitate at various ZRD dosage (c); and FT-IR spectra (d). All reactions of As removal experiments were conducted at atmospheric pressure temperature of 60 °C, reaction time of 1 h, gas flow rate of 900 mL/min, and US power of 480 W.
3.1.2. Effect of the ozone flow rate
To effectively oxidize both As(III) and Fe2+ in a ZnSO4 solution, the use of ozone, which possesses stronger oxidizing properties than oxygen, is an effective approach. Gui et al.'s study demonstrated that ozone oxidation can significantly enhance the leaching rates of gold and silver [31]. Fig. 3a shows that the size of the gas flow rate affects the removal rates of As and Fe. Increasing the gas flow rate from 100 mL/min to 900 mL/min significantly increased the removal rates of As and Fe from 56.3% and 60.9% to 99.4% and 98.8%, respectively. Fig. 3b shows that the gas flow rate directly affects the stability of the arsenic-containing precipitate. This is because a low gas flow rate leads to a lower amount of ozone being generated, which results in incomplete oxidation of As(Ⅲ) in the solution. As(III) is carried into the precipitate by the Fe(OH)3 colloid and forms unstable FeAsO3. Additionally, the gas flow rate affects the neutralization process of ZRD. As shown in Fig. 3c, a low gas flow rate results in more ZnO and ZnS in the arsenic-containing precipitate. This is mainly due to a lower amount of Fe3+ oxidation, which leads to a reduced production of sulfuric acid during the hydrolysis and arsenic precipitation of Fe3+(Eqs. (1)-(3)):
| Fe2(SO4)3 + 6H2O = 2Fe(OH)3↓ + 3H2SO4. | (1) |
| Fe2(SO4)3 + 2H3AsO3 = 2FeAsO3 + 3H2SO4. | (2) |
| Fe2(SO4)3 + 2H3AsO4 = 2FeAsO4↓ + 3H2SO4. | (3) |
Fig. 3.
The residual As concentration and the corresponding As removal rate with the increase of ozone flow rate from 100 mL/L to 1000 mL/L (a); and leached As concentrations in TCLP with solution final pH (b); XRD patterns (c); and FT-IR spectra (d). All reactions of As removal experiments were conducted at atmospheric pressure temperature of 60 °C, reaction time of 1 h, dosage of ZRD 12 g/L, and US power of 480 W.
Furthermore, Fig. 3b also indicates that higher gas flow rates result in lower final pH values of the solution. Additionally, Fig. 3d shows that increasing the gas flow rate leads to a stronger peak at 834 cm−1, indicating that more As(Ⅲ) in the solution is oxidized to As(V) and enters the precipitate.
3.1.3. Effect of the ultrasound power
To determine the effect of US power on ozone oxidation, the influence of US power from 60 to 480 W on arsenic removal efficiency was explored (Fig. 4). As shown in Fig. 4a, the residual arsenic content in the solution decreases with increasing US power after the reaction is completed. When the ultrasound power was increased from 60 to 480 W, the residual arsenic content in the solution was reduced from 96.5 to 10.2 mg/L. It should be noted that the rationale behind not utilizing higher US power is rooted in the fact that optimal synergy between US and ozone can only be achieved within a specific power range. Simultaneously, experiments have revealed that increased US power will make arsenic removal and precipitation filtration very difficult. This higher US power level also results in relatively turbid filtrate, which proves disadvantageous for the subsequent treatment of the ZnSO4 solution. If other parameters remained constant, the As removal rate was 91.6% without US, while the removal rate of arsenic was 99.4% when the US power was 480 W. This is because higher US power generates a higher energy density in the liquid, and the microjet generated has a stronger ability to peel off the wrapper that reacts and precipitates on the surface of the ZRD [40]. This exposure of new reaction surfaces makes the reaction of the ZRD more comprehensive, as can be verified from Fig. 4b. The increase in US power not only leads to a more thorough ZnO reaction in the ZRD, but also promotes more participation of ZnS and ZnFe2O4 in the reaction. Fig. 4c shows that the higher the US power, the stronger the stretching vibration peak of As(V)-O-Fe at 834 cm−1. There are reports in the literature indicating that US can significantly impact the mass transfer and decomposition of ozone in liquids, while also enhancing the liquid phase volume mass transfer coefficient, and improving the diffusion of ozone in solutions [47]. The collapse of bubbles generated by ultrasonic cavitation can facilitate the decomposition of ozone into O2 and O(3P), and then water vapor reacts with O(3P) to form hydroxyl radicals (•OH):
| (4) |
| (5) |
Fig. 4.
The residual As concentration and the corresponding As removal rate with the increase of US power from 60 W to 480 W (a); XRD patterns (b); and FT-IR spectra (c). All reactions of As removal experiments were conducted at atmospheric pressure temperature of 60 °C, reaction time of 1 h, dosage of ZRD 12 g/L, gas flow rate of 900 mL/min.
Hydroxyl radicals have strong oxidizability, and can participate in the oxidation reaction of Fe2+ and As(Ⅲ) through the liquid–liquid phase, which has a higher reaction efficiency than the gas–liquid phase reaction of ozone oxidation.
3.1.4. Effect of the reaction temperature
The reaction temperature affects various aspects of the solution. It has a certain influence on the viscosity of the solution, the precipitation of the adsorbent and the diffusion of the flocculant during the reaction [22]. Additionally, it impacts the solubility of ozone in the solution [25], [48]. According to Fig. 5a, increasing the temperature from 30 °C to 50 °C has a positive effect on the removal of arsenic. This resulted in an increase in the removal rate of As in the solution from 92.7% to 99.4%. The reason behind this improvement is that a higher temperature reduces the viscosity of the ZnSO4 solution, which in turn lowers the intermolecular diffusion resistance. Consequently, the probability of contact between arsenic and iron hydroxide colloids increases. Moreover, since the hydrolysis of Fe3+ to Fe(OH)3 is an endothermic process, raising the temperature promotes the formation and precipitation of iron hydroxide colloids. However, as the temperature continues to rise, the effectiveness of As removal starts to decline. This is mainly attributed to the decrease in the solubility of ozone, which will result in a lower amount of oxidation of As(III) and Fe2+ in the solution. Additionally, higher temperatures can lead to an increased desorption of arsenic adsorbed by iron hydroxide colloids. Fig. 5b shows that the effect of temperatures above 60 °C on the XRD pattern of the arsenic-containing precipitate remains almost unchanged. Fig. 5c shows that the peak at 834 cm−1 strengthens and then weakens with an increase in temperature, which is consistent with the XRD results.
Fig. 5.
The residual As concentration and the corresponding As removal rate with the increase of reaction temperature from 30 °C to 80 °C (a); XRD patterns (b); and FT-IR spectra (c). All reactions of As removal experiments were conducted at US power of 480 W, reaction time of 1 h, dosage of ZRD 12 g/L, gas flow rate of 900 mL/min.
3.1.5. Effect of the reaction time
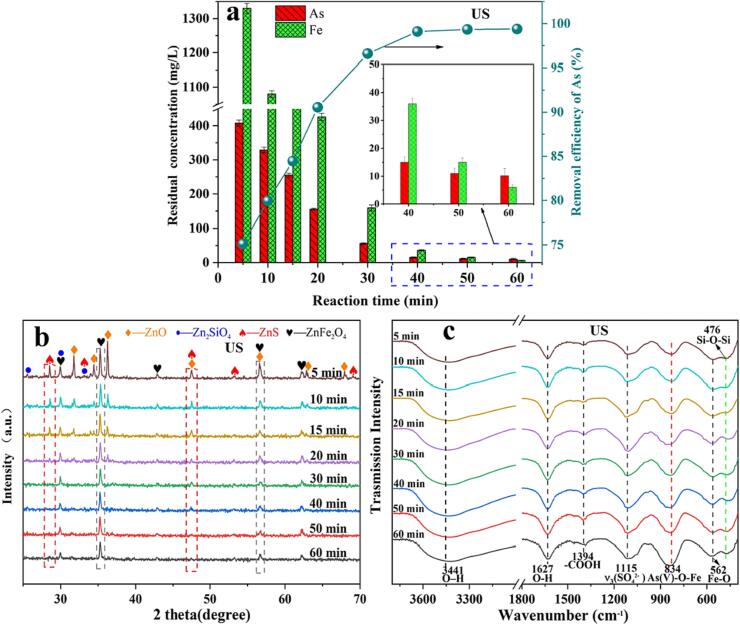
To investigate the effect of reaction time on As removal in ZnSO4 solution, the reaction time ranged from 5 min to 60 min. A comparison between Fig. 6a and Fig. S2(a) reveals that under conventional conditions (without US) and US conditions after 1 h of reaction, the As removal rates are 91.6% and 99.4%, respectively. Interestingly, the removal rates of As under conventional and US conditions were 91.3% and 96.5%, respectively, during the first 30 min of the reaction. This suggests that the removal of arsenic through Fe(OH)3 colloidal flocculation precipitation is a fast process. Fig. S2(a) and Fig. S2(c) show that there is a positive correlation between As and Fe precipitation in zinc sulfate solution. However, the precipitation rate of Fe under US is lower than that under conventional conditions. Comparing Fig. 6b and Fig. S2(b), it is evident that the reaction rate of ZRD under conventional conditions is notably lower than that under US conditions. The conventional arsenic-containing precipitate still contains unreacted ZnO, and the intensity of the peaks corresponding to ZnS and ZnFe2O4 is greater than that of the US arsenic-containing precipitate. This discrepancy can be attributed to the microjets generated by US, which effectively stripped off the deposition on the surface of the ZRD. The newly exposed ZRD under US will continue to participate in the neutralization reaction [49]. Simultaneously, US expedites the transfer of mass between liquid and solid phases in the system, enhancing the kinetics process of the reaction. This is advantageous for facilitating the removal of As. FT-IR spectroscopy of the arsenic-containing precipitate after 1 h of reaction (Fig. 6c and Fig. S2(c)) shows that under US conditions, the peak intensity of the arsenic-containing precipitate is higher at 834 cm−1, indicating a higher arsenic content in the precipitate. This is because the US enhances the oxidation efficiency of ozone on Fe2+ and As(III) in the solution, prompting the presence of more As(V) in the solution to be more easily adsorbed by Fe(OH)3 colloids. This may be the main reason why the arsenic removal rate under US conditions is superior to that under conventional conditions.
Fig. 6.
Under ultrasonic conditions, the concentrations of residual As and Fe in the solution, as well as the corresponding removal rates of As, change as the reaction time increases from 5 min to 60 min (a); XRD patterns (b); and FT-IR spectra (c). All reactions of As removal experiments were conducted at atmospheric pressure temperature of 60 °C, US power of 480 W, dosage of ZRD 12 g/L, gas flow rate of 900 mL/min.
3.2. Arsenic removal mechanism
3.2.1. The influence of different conditions on particle size
The particle sizes under different reaction conditions are presented in Fig. 7. In Fig. 7a, the particle size of ZRD before the reaction is normally distributed, which is D10 = 1.25198 µm, D50 = 4.53779 µm and D90 = 14.62511 µm. Under conventional conditions (Fig. 7b), the particle size of the arsenic-containing precipitate is smaller than that of ZRD, with D10 = 0.73817 µm, D50 = 2.02358 µm and D90 = 6.44017 µm. However, Fig. 7 reveals additional information. It displays two peaks that appear after ultrasonic (US) treatment, resulting in significantly smaller particle sizes, with D10 = 0.54315 µm, D50 = 1.56008 µm and D90 = 4.81956 µm. This is because the microjet generated by US breaks up the agglomerated large particles generated by flocculation precipitation, and at the same time, the US peeling of the ZRD wrapper also exposes a new reaction surface to allow ZRD to react more fully, which also helps reduce the production of arsenic-containing precipitates. As shown in Fig. 7d, the amount of US arsenic-containing precipitate decreased by 54.5% compared to the amount of the arsenic-containing precipitate in lime two-stage neutralization. This is because during the neutralization process of lime milk, in addition to the residue generated by iron hydrolysis, Ca in the lime milk also reacts with SO42- to produce a large amount of gypsum (Fig. S3), which adsorbs a large amount of bound water to form CaSO4·2H2O. Using ZRD as a neutralizing agent can avoid the generation of gypsum and greatly reduce the amount of residue. Additionally, the total reaction time was shortened from 2.5 h to 1 h. Furthermore, when compared to conventional arsenic-containing precipitate, the reduction in arsenic-containing precipitate amount was 16.5%.
Fig. 7.
The particle size distribution of the zinc roasting dust (a), Conventional arsenic-containing precipitate (b) and Ultrasonic arsenic-containing precipitate (c). The wet weight comparison of the arsenic-containing precipitate is conducted with lime and ZRD as a neutralizer, under conventional and Ultrasonic conditions (d).
3.2.2. FE-SEM analysis
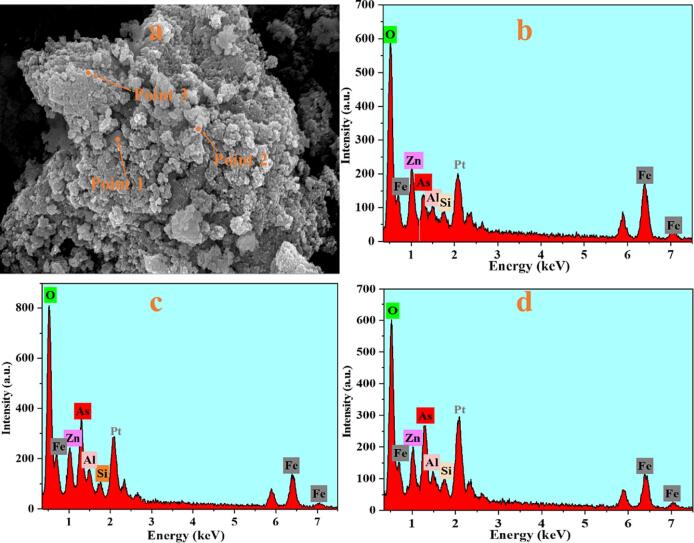
To investigate the morphology and structure of ZRD, conventional precipitate, ultrasonic precipitate, and lime precipitate, we employed FE-SEM for observation. As shown in Fig. 8a, there are two main morphologies of ZRD: layered and granular. Moreover, in Fig. 9a, it is evident that the surface particles of the precipitate under conventional conditions are larger and display noticeable agglomeration. Conversely, Fig. 10a shows that the surface particles of the precipitate after undergoing ultrasonic treatment are finer and more uniformly distributed. As shown in Fig. 11a, there are two main morphologies of lime precipitate: rod and granular. To further analyze the possible components of ZRD, conventional precipitate, ultrasonic precipitate, and lime precipitate, we conducted EDS-point scan analysis, and the results are shown in Tables S2-S5. By analyzing the atomic percentage, we can determine the potential phase composition of the sample.
Fig. 8.
(a) FE-SEM of the zinc roasting dust (ZRD), EDS-point analysis of (b) location 1; (c) location 2; (d) location 3.
Fig. 9.
(a) FE-SEM of the conventional arsenic-containing precipitate, EDS-point analysis of (b) location 1; (c) location 2; (d) location 3.
Fig. 10.
(a) FE-SEM of the US arsenic-containing precipitate, EDS-point analysis of (b) location 1; (c) location 2; (d) location 3.
Fig. 11.
(a) FE-SEM of the lime arsenic-containing precipitate, EDS-point analysis of (b) location 1; (c) location 2; (d) location 3.
In addition, we also conducted EDS-mapping on conventional arsenic-containing precipitates (Fig. S4), ultrasonic arsenic-containing precipitates (Fig. S5), and lime precipitates (Fig. S6). The elements in the two types of residues produced by using ZRD as a neutralizing agent are relatively uniform, but the distribution of elements in the residues produced by using lime as a neutralizing agent is significantly different. The distribution of Fe, As, and O in the arsenic-containing residue of ZRD is consistent, indicating that the precipitated As mainly exists in the form of iron arsenic compounds. The distribution of elements in the lime residue is aggregated in different morphologies, namely rod-shaped (Ca, S, O and As) and granular (Fe, As and O). According to the XRD analysis of the lime residue in Fig. S3, the main rod-shaped component is CaSO4·2H2O, which is one of the reasons for the large amount of arsenic removal residue using the lime method. By comparison, the amount of residue will also affect the As content in the residue. The arsenic content in lime residue is 15.2%, conventional residue As content is 19.5%, and ultrasonic residue As content is 21.9%. In addition, the content of Fe and O in conventional arsenic-containing precipitate is higher than that in US arsenic-containing precipitate, while the content of Zn is lower. This is because more water from crystallization is brought into the arsenic-containing precipitate during the hydrolysis of conventional reaction Fe(OH)3, which is one of the reasons why conventional arsenic-containing precipitate is more abundant than US arsenic-containing precipitate.
3.2.3. XPS analysis
To further explain the mechanism of As removal in ZnSO4 solution by US, XPS examination was conducted to compare the surface chemical valence transitions of ZRD, conventional and US arsenic-containing precipitates, as presented in Fig. 12. Fig. 12a shows that only a single As(V) peak was present in the ZRD. This can be attributed to the fact that ZRD is a dust obtained from the oxidation and roasting of zinc sulfide concentrate. Under oxidation conditions, low-valent arsenic is converted to a high valence state, resulting in the presence of only As(V). In contrast, Fig. 12d and Fig. 12g exhibit the deconvolution of the As 3d spectrum into three overlapping peaks corresponding to As(V) and As(III). Previous literature reports indicate that the As 3d5/2 peak for As(III) can be assigned to a binding energy ranging from 43.40 to 45.31 eV, whereas the As(V) energy ranges from 45.20 to 46.80 eV [50]. Significantly, the percentage of As(V) in the arsenic-containing precipitate increased from 81.4% to 93.3% after the introduction of US. This indicates that US treatment facilitated the oxidation of more As(III), causing it to form amorphous FeAsO4 or be adsorbed by Fe(OH)3 colloids into the precipitate.
Fig. 12.
XPS profiles of As 3d(a, d and g), Fe 2p(b, e and h) and O 1 s(c, f and i) spectra of ZRD and arsenic-containing precipitate.
According to the nonlinear method [51], the Fe 2p XPS spectra of US and conventional arsenic-containing precipitate were fitted, as shown in Fig. 12f and Fig. 12i. The Fe 2p3/2 spectrum was deconvoluted into two main peaks and one satellite peak. The main peaks correspond to Fe(II)(708.46–710.56, 724.30–725.26 eV) and Fe(III)(710.36–713.36, 726.13–726.41 eV), while the Fe(III) satellite peaks appear at 719.41–719.65 eV. After adding US treatment to the solution, the percentage content of Fe(III) in the arsenic-containing precipitate increased from 79.1% to 81.1%. This finding further supports the idea that US can enhance the oxidation effect of ozone and provides a source of Fe3+ for arsenic precipitation and adsorption.
Fig. 12c, Fig. 12f and Fig. 12i display the O1s of the XPS spectra and the fitting results of ZRD, conventional and US arsenic-containing precipitates. These spectra exhibit three overlapping peaks that can be divided into H2O (529.5–530.0 eV), M−OH (531.1–531.9 eV) and M = O (531.9–532.2 eV) [52], [53], [54]. A comparison with ZRD revealed that the percentage content of H2O in conventional and US arsenic-containing precipitates increased from 21.9% to 51.0% and 49.4%, respectively. This increase indicates that arsenic-containing precipitates contain more crystal water. Interestingly, the proportion of water of crystallization in arsenic-containing precipitates after the introduction of US decreases, which is consistent with the FT-IR analysis results. Furthermore, the M−OH content in the conventional arsenic-containing precipitate decreased from 40.2% to 36.7% compared to that in the US arsenic-containing precipitate. This decrease can be attributed to the formation of more As(V)-O, as As binds to the O atom under the influence of US. These findings demonstrate that US treatment leads to more As(V) and Fe3+ in the precipitate, providing further evidence of the superior As removal effect under US conditions. Additionally, the lower crystal water content of the US arsenic-containing precipitate is one of the reasons for its lower quantity.
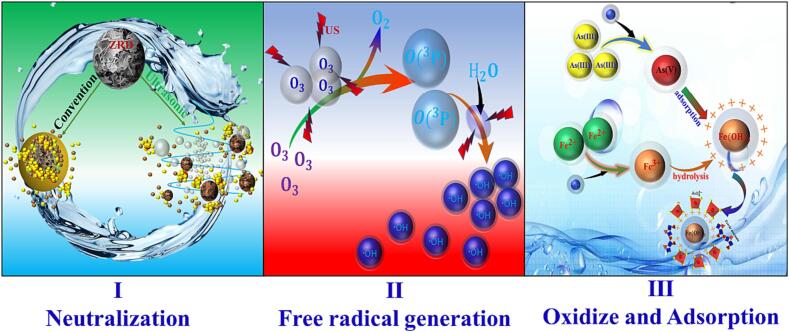
Based on the above characterization results, we propose the reaction mechanism of removing As from ZnSO4 solution through a combination of US enhanced ozonation and ZRD neutralization, as illustrated in Fig. 13. This mechanism involves several reaction pathways, including ZRD neutralization, US enhanced ozone mass transfer and decomposition, oxidation of As(III) and Fe2+, and adsorption and coprecipitation. (i) ZRD reacts with zinc sulfate solution, neutralizing excess sulfuric acid and adjusting the pH. This provides favorable conditions for the subsequent oxidation of As(Ⅲ) and Fe2+. The introduction of US effectively disrupts the wrapper surrounding the ZRD particles, reduces agglomeration, and exposes fresh reaction surfaces to continue the reaction. Consequently, the formation of arsenic-containing precipitates is minimized. (ii) US enhances the solubility of ozone in the zinc sulfate solution. Simultaneously, cavitation caused by US rapidly decomposes ozone, generating O2 and O(3P). The O(3P) species reacts with water vapor, yielding highly reactive hydroxyl radicals (•OH). These •OH radicals oxidize As(III) and Fe2+ in the solution, converting them to As(V) and Fe3+, respectively. The increased precipitation of arsenic occurs predominantly in the form of As(V), which enhances the stability and safety of the resulting arsenic-containing precipitate. (iii) Fe3+ undergoes hydrolysis, forming colloidal Fe(OH)3, which then adsorbs As(V) present in the solution. Additionally, a portion of the adsorbed As(V) transforms into poorly crystalline FeAsO4. This overall process involves coprecipitation and adsorption, facilitating the removal of As from the ZnSO4 solution. In summary, the proposed mechanism underscores the synergistic effects of US enhanced ozonation and ZRD neutralization in achieving efficient and sustainable As removal from ZnSO4 solution.
Fig. 13.
Schematic diagram of US enhanced ozone oxidation combined with ZRD neutralization to remove As from ZnSO4 solution.
4. Conclusions
In this work, we propose an economical and effective method for As removal from ZnSO4 solution. Ozone is used to replacement for KMnO4 as an oxidizing agent, ZRD as a neutralizing agent, and the US field to enhance the As removal process. This method presents several advantages, including a high As removal rate, a short reaction time, no introduction of impurities, and low generation of arsenic-containing precipitates. The optimal conditions for As removal were determined as follows: a ZRD dosage of 12 g/L, gas flow rate of 900 mL/min, US power of 480 W, reaction temperature of 60 °C and time of 1 h. Under these conditions, the As removal rate reached 99.4%. Experimental results indicate that the US field enhance the oxidation efficiency of As and Fe in ZnSO4 solution by ozone and improve the As removal effect during the ZRD neutralization process. In comparison to conventional conditions, US treatment can enhance the oxidation efficiency of As(III) and Fe2+ in ZnSO4 solution. This enhancement occurs through an increase in the solubility of ozone and promoting •OH radicals production. As a result, the stability of arsenic containing precipitates is improved, concurrently reducing the aggregation of arsenic-containing precipitates and the encapsulation of ZRD by arsenic-containing precipitates. When compared to lime and conventional methods, this method reduces the amount of arsenic-containing precipitate by 54.5% and 16.5%, respectively, and significantly shortens the total reaction time from 2.5 h to 1 h. To explore the mechanism of US enhanced ozone arsenic removal process, we analyzed the changes in ion concentration, surface structure of ZRD, and product characteristics of arsenic-containing precipitates.
CRediT authorship contribution statement
Qi Zhang: Writing – original draft. Junchang Liu: Validation. Hongying Xia: Supervision, Project administration. Yingjie Xu: Investigation, Data curation. Libo Zhang: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Financial aid from the following programs is gratefully acknowledged: Supported by the National Key R&D program of China (2021YFC290281), Yunnan Province basic research special key project (202001AS070009), Yunnan Xingdian Talent Support Project-Industrial innovation talents (2019-1096), Yunnan Xingdian Talent Project-Young talents (2018-73).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2023.106748.
Contributor Information
Hongying Xia, Email: hyxiakust@163.com.
Libo Zhang, Email: zhanglibopaper@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.M.o.I.a.I. Technology, Ministry of Industry and Information Technology: Operation of the Lead Zinc Industry in 2021, 91jinshu.com, 2022.4.29. https://news.91jinshu.com/qian/202202/37468.html.
- 2.Dongqing D., Xiu Z., Haimin L. Study on sources and control of Fluorine and Chlorine in Zinc Hydrometallurgy. Jiangsu Sci. Technol. Inform. 2018 https://kns.cnki.net/kns8/defaultresult/index [Google Scholar]
- 3.Yang J., Zhang H., Su X., Ma S. Study on leaching zinc calcine with high iron. Internat. Forum Powder Technol. Appl. 2013 https://doi.rog/10.4028/www.scientific.net/AMR.826.118 [Google Scholar]
- 4.Li Y., Liu Z., Li Q., Zhao Z., Liu Z., Li Z., Li L. Removal of arsenic from arsenate complex contained in secondary zinc oxide. Hydrometall. 2011;109:237–244. https://doi.rog/10.1016/j.hydromet.2011.07.007 [Google Scholar]
- 5.Morrison R., MacKinnon D., Uceda D., Warren P., Mouland J. The effect of some trace metal impurities on the electrowinning of zinc from Kidd Creek electrolyte. Hydrometall. 1992;29:413–430. doi: 10.1016/0304-386X(92)90025-U. [DOI] [Google Scholar]
- 6.Dang X.E., Jun L., Wen-Shuai K.E., Zhou X., Tang C. Analysis of arsenic hazard in zinc hydrometallurgy process and arsenic removal with ferric salt flocculence process. Nonferrous Metals(extractive Metallurgy) 2013 https://kns.cnki.net/kns8/defaultresult/index [Google Scholar]
- 7.Bao S., Xin C., Zhang Y., Chen B., Ding W., Luo Y. Application of capacitive deionization in water treatment and energy recovery: A review. Energies. 2023;16:1136. doi: 10.3390/en16031136. [DOI] [Google Scholar]
- 8.Cai C., Yang Y., Chen Q., Yang Q., Shen Q. Arsenic removal from waste acid by two-stage sulfurization. Nonferr. Metals Sci. Eng. 2019 doi: 10.13264/j.cnki.ysjskx.2019.04.004. [DOI] [Google Scholar]
- 9.H. Sugita, T. Oguma, J. Hara, M. Zhang, Y. Kawabe, M.S. Li, Removal of arsenate from contaminated water via combined addition of magnesium-based and calcium-based adsorbents 2023. https://doi.org/10.3390/su15054689.
- 10.Chen H., Xu J., Lin H., Zhao X., Shang J., Liu Z. Arsenic removal via a novel hydrochar from livestock waste co-activated with thiourea and γ-Fe2O3 nanoparticles. J. Hazard. Mater. 2021;419:126457-. doi: 10.1016/j.jhazmat.2021.126457. [DOI] [PubMed] [Google Scholar]
- 11.Roy M., Genuchten C., Rietveld L., Halem D.V. Integrating biological As(III) oxidation with Fe(0) electrocoagulation for arsenic removal from groundwater. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116531. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Liu L., Yang X., Suib S.L., Qiu G. Removal of As(V) from wastewaters using magnetic iron oxides formed by zero-valent iron electrocoagulation. J. Environ. Manage. 2022;307:114519-. doi: 10.1016/j.jenvman.2022.114519. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z.B., Jin-Xiang L.I., Bian X.Y. Research progress of arsenic removal technology by ferric salt. J. Shandong Jianzhu Univ. 2012 https://kns.cnki.net/kns8/defaultresult/index [Google Scholar]
- 14.Sun D. HE, Adsorption and oxidation of arsenite on goethite. Soil Sci. 1998 https://journals.lww.com/soilsci/ [Google Scholar]
- 15.Eljamal O., Sasaki K., Hirajima T. Sorption Kinetic of Arsenate as Water Contaminant on Zero Valent Iron. J. Water Resour. Prot. 2013 doi: 10.4236/jwarp.2013.56057. [DOI] [Google Scholar]
- 16.Weizhen L., Can W.u., Jingwei T.u. The double influence mechanism of pH on arsenic removal by nano zero valent iron: electrostatic interactions and the corrosion of Fe∼0. Environ. Sci. Nano. 2017;4:1544–1552. doi: 10.1039/C7EN00240H. [DOI] [Google Scholar]
- 17.Yan B., Liang T., Yang X., Gadgil A.J. Superior removal of As(III) and As(V) from water with Mn-doped β-FeOOH nanospindles on carbon foam. J. Hazard. Mater. 2021;126347 doi: 10.1016/j.jhazmat.2021.126347. [DOI] [PubMed] [Google Scholar]
- 18.Shan C., Tong M. Efficient removal of trace arsenite through oxidation and adsorption by magnetic nanoparticles modified with Fe-Mn binary oxide. Water Res. 2013;47:3411–3421. doi: 10.1016/j.watres.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Feng L., Cao M., Ma X., Zhu Y., Hu C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012;217–218:439–446. doi: 10.1016/j.jhazmat.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Zeng T., Deng Z., Zhang F., Fan G., Liu H. Removal of arsenic from “Dirty acid” wastewater via Waelz slag and the recovery of valuable metals. Hydrometall. 2021;200 doi: 10.1016/j.hydromet.2021.105562. [DOI] [Google Scholar]
- 21.Pervez M.N., Chen C., Li Z., Naddeo V., Zhao Y. Tuning the structure of cerium-based metal-organic frameworks for efficient removal of arsenic species: The role of organic ligands. Chemosphere. 2022;303 doi: 10.1016/j.chemosphere.2022.134934. [DOI] [PubMed] [Google Scholar]
- 22.Guiyuan C. Xing, Zhu, Kongzhai, Li, Xianjin, Qi, Yonggang, Self-enhanced and efficient removal of arsenic from waste acid using magnetite as an in situ iron donator. Water Res. 2019 doi: 10.1016/j.watres.2019.03.067. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Qi X., Li G., Wang H. Efficient removal of arsenic from copper smelting wastewater via a synergy of steel-making slag and KMnO4. J. Clean. Prod. 2020;287 doi: 10.1016/j.jclepro.2020.125578. [DOI] [Google Scholar]
- 24.Lahav M.O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution – basic principles and a simple heuristic description. Chemosphere. 2007 doi: 10.1016/j.chemosphere.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Lv X., Zhao H., Zhang Y., Yan Z., Qiu G. Active destruction of pyrite passivation by ozone oxidation of a biotic leaching system. Chemosphere. 2021;130335 doi: 10.1016/j.chemosphere.2021.130335. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Wang S., Liu C., Zhang L., Kong D. Mechanism and kinetics of synergistic decopperization from copper anode slime by ultrasound and ozone. J. Clean. Prod. 2021;322 doi: 10.1016/j.jclepro.2021.129058. [DOI] [Google Scholar]
- 27.Elorza-Rodríguez E., Nava-Alonso F., Jara J., Lara-Valenzuela C. Treatment of pyritic matrix gold–silver refractory ores by ozonization–cyanidation. Miner. Eng. 2006;19:56–61. doi: 10.1016/j.mineng.2005.06.003. [DOI] [Google Scholar]
- 28.Otgon N., Zhang G., Yang C. Arsenic removal from waste water by ozone oxidation combined with ferric precipitation. Mong. J. Chem. 2017;17:18. doi: 10.5564/mjc.v17i43.741. [DOI] [Google Scholar]
- 29.Cao J., Zhang K., Yuanyuan L.I., Zhang G. Arsenic removal by Scorodite synthesis using ozone oxidation. Guocheng Gongcheng Xuebao/Chin. J. Process. Eng. 2018 https://kns.cnki.net/kns8/defaultresult/index [Google Scholar]
- 30.Eriksson M. Ozone chemistry in aqueous solution : ozone decomposition and stabilisation. Inorg. Chem. 2005;1 [Google Scholar]
- 31.Gui Q., Hu Y., Wang S., Zhang L. Mechanism of synergistic pretreatment with ultrasound and ozone to improve gold and silver leaching percentage. Appl. Surface Sci. 2022;576 doi: 10.1016/j.apsusc.2021.151726. [DOI] [Google Scholar]
- 32.Xu X., Cao D., Wang Z., Liu J., Wang Z. Study on ultrasonic treatment for municipal sludge. Ultrason. Sonochem. 2019;57 doi: 10.1016/j.ultsonch.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 33.K. Wannakan, K. Khansamrit, T. Senasu, S. Nanan, Ultrasound-Assisted Synthesis of a ZnO/BiVO 4 S-Scheme Heterojunction Photocatalyst for Degradation of the Reactive Red 141 Dye and Oxytetracycline Antibiotic 2023. https://doi.org/10.1021/acsomega.2c07020. [DOI] [PMC free article] [PubMed]
- 34.Zhu T., Shen Q., Xu Y., Li C. Ionic liquid and ultrasound-assisted extraction of chestnut shell pigment with good hair dyeing capability. J. Clean. Prod. 2022;335:130195-. doi: 10.1016/j.jclepro.2021.130195. [DOI] [Google Scholar]
- 35.Yifei L., Shen Y., Zhang M., Wang J. Real-time 3D ultrasound imaging system based on a hybrid reconstruction algorithm. Chin. J. Electron. 2023;33:1–12. doi: 10.23919/cje.2023.00.002. [DOI] [Google Scholar]
- 36.Ding W., Bao S., Zhang Y., Xiao J. Mechanism and kinetics study on ultrasound assisted leaching of gallium and zinc from corundum flue dust. Miner. Eng. 2022;183 doi: 10.1016/j.mineng.2022.107624. [DOI] [Google Scholar]
- 37.Yan S., Sun C., Zhou T., Gao R., Xie H. Ultrasonic-assisted leaching of valuable metals from spent lithium-ion batteries using organic additives. Sep. Purif. Technol. 2021;257 doi: 10.1016/j.seppur.2020.117930. [DOI] [Google Scholar]
- 38.Zhang G., Wang S., Zhang L., Peng J. Ultrasound-intensified Leaching of Gold from a Refractory Ore. ISIJ Int. 2016;56:714–718. doi: 10.2355/isijinternational.ISIJINT-2015-476. [DOI] [Google Scholar]
- 39.Masoum H.G., Rastegar S.O., Khamforoush M. Ultrasound-assisted leaching of vanadium and yttrium from coal ash: Optimization, kinetic and thermodynamic study. Chem. Eng. Technol. 2021 doi: 10.1002/ceat.202100297. [DOI] [Google Scholar]
- 40.Chen Y. A high-efficiency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound. Sep. Purif. Technol. 2020;240 doi: 10.1016/j.seppur.2020.116624. [DOI] [Google Scholar]
- 41.Saywell L., Cunningham B. Determination of iron: colorimetric o-phenanthroline method. Ind. Eng. Chem. Anal. Ed. 1937;9:67–69. doi: 10.1021/ac50106a005. [DOI] [Google Scholar]
- 42.U.S. Epa, Toxicity Characteristics Leaching Procedure, Method 1311, Test Methods for the Evaluation of Solid Waste, (1992). https://cir.nii.ac.jp/crid/1571135649548764032.
- 43.Xbma B., Ypl A., Lyca B., Zhya B., Shan X.C., Lin L.A., Qzla B. Removal and stabilization of arsenic from anode slime by forming crystal scorodite. Trans. Nonferrous Met. Soc. Chin. 2015;25:1298–1306. doi: 10.1016/S1003-6326(15)63728-1. [DOI] [Google Scholar]
- 44.Yl A., Xing Z.A., Xq A., Bo S.B., Xin Z.B., Kl A., Yw A., Fh A., Hua W.A. Efficient removal of arsenic from copper smelting wastewater in form of scorodite using copper slag. J. Clean. Prod. 2020;270 doi: 10.1016/j.jclepro.2020.122428. [DOI] [Google Scholar]
- 45.Li Y., Qi X., Li G., Duan X., Yang N. Removal of arsenic in acidic wastewater using Lead-Zinc smelting slag: From waste solid to As-stabilized mineral. Chemosphere. 2022;301 doi: 10.1016/j.chemosphere.2022.134736. [DOI] [PubMed] [Google Scholar]
- 46.Jia Y., Xu L., Xin W., Demopoulos G.P. Infrared spectroscopic and X-ray diffraction characterization of the nature of adsorbed arsenate on ferrihydrite. Geochim. Cosmochim. Acta. 2007;71:1643–1654. doi: 10.1016/j.gca.2006.12.021. [DOI] [Google Scholar]
- 47.Zeiger B.W., Suslick K.S. Sonofragmentation of molecular crystals. J. Am. Chem. Soc. 2011;133:14530. doi: 10.1021/ja205867f. [DOI] [PubMed] [Google Scholar]
- 48.Torres R., Lapidus T.G. Platinum, palladium and gold leaching from magnetite ore, with concentrated chloride solutions and ozone. Hydrometall. 2016 doi: 10.1016/j.hydromet.2016.06.009. [DOI] [Google Scholar]
- 49.Luque-Garc J.L., Castro A.M. Ultrasound: a powerful tool for leaching. Trends Anal. Chem. 2003;22:41–47. doi: 10.1016/S0165-9936(03)00102-X. [DOI] [Google Scholar]
- 50.Chowdhury S.R., Yanful E.K., Pratt A.R. Arsenic removal from aqueous solutions by mixed magnetite–maghemite nanoparticles. Environ. Earth Sci. 2011;64:411–423. doi: 10.1007/s12665-010-0865-z. [DOI] [Google Scholar]
- 51.Toru, Peter, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. https://doi.org/10.1016/j.apsusc.2007.09.063.
- 52.Msa B., Mi C., Nh C., Cbt D., Tf A., Ip C., Sj C., Ti C., Ts C., In E. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method - ScienceDirect, Journal of Environmental. Chem. Eng. 2020;8:4. doi: 10.1016/j.jece.2020.104197. [DOI] [Google Scholar]
- 53.Park I., Higuchi K., Tabelin C.B., Jeon S., Hiroyoshi N. Suppression of arsenopyrite oxidation by microencapsulation using ferric-catecholate complexes and phosphate. Chemosphere. 2020;269 doi: 10.1016/j.chemosphere.2020.129413. [DOI] [PubMed] [Google Scholar]
- 54.Zhang T., Zhao Y., Kang S., Li Y., Zhang Q. Formation of active Fe(OH)3 in situ for enhancing arsenic removal from water by the oxidation of Fe(II) in air with the presence of CaCO3. J. Clean. Prod. 2019;227:1–9. doi: 10.1016/j.jclepro.2019.04.199. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.