Abstract
Twelve new species of Inocybe (I. adorabilis, I. comis, I. demetris, I. filiana, I. galactica, I. morganae, I. othini, I. ovilla, I. proteica, I. somae, I. suryana and I. venerabilis) are described from Europe on the basis of detailed morphological and molecular investigation. A portrait of the recently described I. ianthinopes is given. All species are smooth-spored and some pruinose only in the apical part of the stipe, and some on entire length. The new species are compared to 24 type specimens (17 characterized by at least partial ITS sequence data), all of which are described and revised here. Epitypes were selected for two species, I. hirtella and I. sindonia. Based on our studies, we confirm that I. kuehneri and I. sindonia on one hand, and I. subalbidodisca and I. ochroalba on the other, are synonyms and furthermore suggest that I. abietis is synonymous with I. catalaunica, I. exilis with I. rufobrunnea, I. hirtellarum with I. mycenoides, I. lapidicola with I. deianae, I. ochraceolutea with I. sindonia, I. stangliana with I. pelargonium, I. subrubens with I. subhirtella and I. sulfovirescens with I. langei. All of the new species are supported by phylogenetic analyses. Among the 16 previously described species accepted here, 10 are represented by types in the phylogenetic analyses and five by own collections corresponding to the type. Two species, I. eutheloides (remaining doubtful) and I. pallidolutea are only treated morphologically. In summary, we describe as new or verify the taxonomic status and provide or corroborate morphological concepts for 37 smooth-spored species of Inocybe.
Citation: Bandini D, Oertel B, Eberhardt U. 2022. More smooth-spored species of Inocybe (Agaricales, Basidiomycota): type studies and 12 new species from Europe. Persoonia 48: 91–149. https://doi.org/10.3767/persoonia.2022.48.03.
Keywords: Agaricales, alpha taxonomy, epitypification, Inocybaceae, Inocybe, ITS, LSU, molecular systematics, RPB2
INTRODUCTION
All species described or discussed in this article belong to the genus Inocybe, which is one of seven recently constituted genera (Matheny et al. 2020) within the monophyletic family Inocybaceae. This genus is characterized by the presence of cheilocystidia and generally also pleurocystidia with more or less thick walls, whereas this is not the case with the other six genera. With estimated worldwide 850 species (Matheny et al. 2020), Inocybe is by far the largest of the seven genera. The number of species will certainly exceed this estimate by far, since in Germany and adjacent areas alone we have encountered about 470 species and ‘morphospecies’. In many parts of the world a thorough investigation has only just begun, so, e.g., in Africa (Aignon et al. 2021) or China (Fan & Bau 2010, 2013, 2020, Fan et al. 2018). Furthermore, in the last five years, more than 200 new species of the genus have been described considering molecular data from all over the world (e.g., Vauras & Larsson 2016a, b, Crous et al. 2017, Matheny & Bougher 2017, Esteve-Raventós et al. 2016, 2018, Larsson et al. 2018a, b, Cripps et al. 2019b, Bandini et al. 2017, 2019a, b, c, 2020a, b, c, 2021a, b, c, Dovana et al. 2021, Mešić et al. 2021, Muñoz et al. 2022).
In keys (Kuyper 1986, Stangl 1989, Bon 1997a, b, 1998), the genus Inocybe is divided into smooth-spored and nodulose-spored groups, the former appears to be the more species-rich in Europe. Among the about 470 species and ‘morphospecies’ of this genus we have, c. 310 are smooth-spored and 140 are nodulose-spored. In approximately 20 of these species appears to be no fundamental morphological distinction between nodulose and smooth-spored species (see also Kuyper 1986), e.g., I. ambigua, I. helobia, I. pluppiana or I. diabolica – the spores of which can neither be called smooth nor nodulose. Phylogenetically, there is also not always a clear distinction between smooth and nodulose-spored species (e.g., Matheny 2009, Ryberg et al. 2010). Matheny et al. (2020) indicated a ‘smooth-spored temperate boreal clade’ (STBC) that could also be retrieved in other studies (e.g., Bandini et al. 2021b), but it will not become clear how many and which species will be included in this clade, unless further loci are considered for more species.
In his milestone paper, Matheny et al. (2020) employed RPB1, RPB2, TEF1a and coding nuclear ribosomal genes (18s, 28s and 5.8s) of 63 taxa of the Inocybaceae, of which 24 were Inocybe species. For the majority of Inocybe (Inocybaceae) species, only ITS and possibly LSU data exist; combined with the species richness, the comparatively high molecular diversity of the genus and family (even in Europe) and the observation that morphology is not always a good predictor of phylogeny, this implies that the current view of the Inocybe and Inocybaceae (Matheny et al. 2020) may well be challenged as more data become available. Even if we cannot exclude artefactual branches in our phylogeny owing to insufficient data and yet unrecognized key taxa, we consider the validation of existing and description of new taxa as a way forward to a satisfactory comprehensive modern classification of the genus.
An important character used to classify and identify smooth-spored Inocybe species is the pruinosity, i.e., the presence of metuloid caulocystidia and distribution on the stipe (Kuyper 1986, Stangl 1989, Bon 1997a, b, 1998). In the present article only smooth-spored species are treated, some of which are pruinose only near or at the apex, some clearly on the entire length of the stipe, while some are mainly pruinose in the upper half and only so sparely in the lower half, that this can only be observed under the microscope. Some well-established species, such as I. furfurea (see Bandini et al. 2019b), as well as several recently described ones, such as I. perchtana (Bandini et al. 2020a) or I. beatifica (Bandini et al. 2021b), belong to this group – and the same is the case for I. morganae, and I. adorabilis, two species described below.
A portrait of the recently described I. ianthinopes is given. We have not had the opportunity to examine the type and the sequences from this article are not published yet, but the inclusion of sequences published earlier makes it possible to know what I. ianthinopes is. Based on a large number of collections from Germany, we can add detail to the original description and add our interpretation of some observations that differs slightly from the original description (Muñoz et al. 2022).
We furthermore treat I. hirtella var. bispora, which is, at least in Germany and surrounding countries, quite well known and thus included even in several popular mushroom books (e.g., Breitenbach & Kränzlin 2000, Gerhardt 2001, Enderle 2004, Arnolds et al. 2015). Typical for I. hirtella var. bispora is the smell of bitter almonds, which can most easily be observed in fresh basidiomata, and the usually two-spored basidia in combination with rather large spores, i.e., 10–12 µm length. At the end of the 19th century, G. Bresadola described such a species under the name I. hirtella (Bresadola 1881–1887), however, Kuyper (1986) was of the opinion that this species was not the same as the variety he had created. In the present article we discuss this matter with the result, that in our opinion Kuyper’s I. hirtella var. bispora is synonymous with I. hirtella described by Bresadola. Additionally, we describe three further species often smelling of bitter almonds as new, I. morganae, I. somae and I. suryana.
We also investigate I. ochroalba a species which has been synonymized by Kuyper (1986) with I. subalbidodisca and I. subhirtella. We have morphologically examined and sequenced all three holotypes, and found out that I. subhirtella is a good species in its own right.
Unless they belong to a section with very distinctive characteristics, such as the intensive sweetish or disagreeable odour and tendency to redden or become greenish in parts of the species of sect. Lactiferae, European smooth-spored species of genus Inocybe are often not well-known and, lacking obvious special features, are often difficult to distinguish from one another. With the delimitation of 12 new species, we aim to shed some light into the darkness of this group of Inocybe and advance the revision of the genus Inocybe and in particular of the smooth-spored species.
Even in its reduced limits (Matheny et al. 2020), Inocybe is a genus that includes species that are rather variable in their ribosomal genes. A satisfactory comprehensive modern infrageneric classification based on phylogeny has not been achieved yet. Thus, we continue to refer to Bon’s (1997a, b, 1998) classification as a guide through this large genus.
The selection of taxa considered in the analysis was largely driven by the species mentioned in the context of the species treated here, emended with taxa used by Matheny and co-workers (2020) to delimit the genus. Although the molecular infrageneric classification of the genus remains a challenge, the great majority of described Inocybe species can still be identified by ITS data alone. In three species groups (I. adorabilis, I. pseudoscabelliformis and I. urceolicystis, I. mycenoides and I. somae, as well as I. ochroalba and I. subhirtella) in which morphology seemed to suggest the existence of separate species and ITS was not as clear as one would wish, RPB2 data (RNA polymerase II second largest subunit, between the conserved domains 6 and 7) were used in addition to the standard loci ITS and LSU for recent material and (partial) ITS for older collections.
Species delimitation followed the same principles used in an earlier publication (Bandini et al. 2021b), namely that species are described as new if they differ from existing species by the combination of at least three independent characters that are constant among representatives of the new species, and the representatives of the new species are monophyletic in phylogenetic ITS (ITS + LSU (+ RPB2)) analyses. Constant ecological differences between new and existing species are considered as meaningful, but neither ecological nor ITS differences were used unless backed by morphological differences. Species were considered as synonymous, if the overall impression of the species was very similar, if no constant characters could be found that separated the two species, and if neither pronounced ecological preferences nor molecular data (if available) indicated that the two species might be cryptic (Bandini et al. 2021b).
The sequence dataset assembled for this study is made up from sequences that are quite diverse and include different numbers of loci. The analysis was done by Maximum Likelihood, using ultrafast bootstrap (ufb) and SH-like approximate likelihood ratio tests as support. If the phylogenetic signal is strong, normally ultrafast bootstrap and SH-like approximate likelihood ratio tests both support a branch (Guindon et al. 2010); if the support of the two methods widely disagrees, the respective clade has to be treated with caution. It should be taken into account that SH-like approximate likelihood ratio test (SH-aLRT) values tend to behave like traditional bootstrap values and ultrafast bootstrap more like posterior probability values (Minh et al. 2021), thus different cut-off values, ≥ 95 % for ufb and ≥ 80 % for SH-aLRT were selected for reporting test results. SH-aLRT are considered superior to bootstrap where branches are short (Guindon et al. 2010).
MATERIAL AND METHODS
Morphological study
Fresh material was obtaine on forays in Austria, Finland, Germany, the Netherlands and Switzerland between 2011 and 2020. Type material was borrowed from various herbaria. For fresh collections, the relevant macroscopic details, i.e., habit, size and shape of the basidiomata, colour and surface of the pileus, number and colour of lamellae, size, colour, surface and base of the stipe, smell and colour of flesh, colour of exsiccata, habitat and surrounding trees, were noted.
For all collections – if possible, in the fresh, otherwise in the dried state – e.g., basidia, spores, hymenial cystidia, caulocystidia were examined by D. Bandini with a Leica DM-750 microscope in water and 3 % KOH solution, at 400 and 1 000 magnifications. Photographs of microdetails have been taken with a Zeiss AxioCam ERc5s. The measurements of spores and cystidia were determined using Zeiss Axiovision v. 4.8. Cystidia were measured without crystals and basidia without sterigmata. The size of all elements measured is given as length × width. The Q value equals the ratio of spore length to spore width (calculated for each spore). The number of spores or cystidia measured is included in the description.
Pictures of fresh collections, thus all but one photographs of basidiomata on the plates, were taken by D. Bandini with a Panasonic Lumix GH2 with a Leica DG Macro-Elmarit 1 : 2.8/45 mm lens. Figure 16b was taken by B. Oertel also with a Panasonic Lumix GH2 with a Leica DG Macro-Elmarit 1 : 2.8/45 mm lens. For the determination of the colour temperature, a calibration card was photographed together with the fresh collections at the collection site. The RAW files were developed with Silkypix Developer Studio 4.0.
Fig. 16.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Colour codes are taken from Munsell (2009, as ‘Mu’), terminology follows Vellinga (1988) and Kuyper (1986). Herbarium acronyms are according to Holmgren et al. (1990), the acronym D.B. refers to the private herbarium of Ditte Bandini.
Molecular study
DNA extraction, PCR and sequencing of nuclear ribosomal loci (ITS, LSU D1–D3) follows Cripps et al. 2019a and Bandini et al. 2021b. For recent collections of selected taxa, RPB2 was amplified using the primers bRPB2-6F and bRPB2-7.1R and the amplification protocol recommended by Matheny (2005), in 25 µl reaction volumes using TAKARA Taq DNA Polymerase Hotstart version (TAKARA Bio Inc., Otsu, Shiga) according to the instructions of the manufacturers’ and primer concentrations of 0.4 µM.
Bidirectional Sanger sequencing was carried out by LGC Ge-nomics (Berlin, Germany). Sequences were assembled and edited using Sequencher v. 4.9 (Genecodes). Newly generated sequences were submitted to GenBank with acc. no. MN319692, MN319693, MN319699, MZ048356, MZ664390, MZ667615–MZ667617, MZ700324, MZ824395, OK057130–OK057208 and OK078899–OK078918. Raw data for new GenBank accessions OK057115–OK057129 were generated by Alvalab (Oviedo, Spain).
For obtaining additional information on the habitat and distribution of new species, type sequences were BLASTed against GenBank (Johnson et al. 2008) and UNITE (Kõljalg et al. 2005); sequence similarity or identity values were directly copied from BLAST results. Percent values given in the text refer to ITS1-5.8S-ITS2 or fewer positions, if the complete sequence is unavailable for one of the sequences. Conspecificity between public sequences and sequences of types was assumed if sequence variation was 0.5 % or less. Sequence variation attributed to long indels, stretches of N (i.e., in cases in which the ITS was obtained in two amplicons, not allowing to read the entire ITS) or obviously wrong sequence reads in the beginnings or ends of published sequences were ignored. BLAST searches were done 6 May 2021.
For species described here as new, several collections were sequenced for ITS and LSU. Representatives of published sequences putatively belonging to these taxa or very close relative of these taxa (i.e., > 97 % similar in BLAST results) were also included. For species that were synonymized here, we included sequence data from types (if available) or from material that had been morphologically analysed and considered to represent the same species. Based on the phylogeny presented by Matheny et al. (2020), Nothocybe and Pseudosperma spp. were added for rooting.
Multiple alignments were done online in Mafft using the E-INS-i option (Katoh et al. 2005, 2019). Alignments were viewed and reformatted using AliView 1.26 (Larsson 2014).
In provisional analyses (result not shown) all current Inocybe spp. ITS and LSU sequences considered by Matheny et al. (2020) when splitting what used to be Inocybe were included in the alignment, pruning taxa that could not be resolved adequately, i.e., as in the analysis of Matheny et al. (2020) using additional loci. The final ITS + partial LSU alignment consisted of 266 sequences and 1 576 positions of ITS and LSU. For 96 sequences less than 1 280 positions were available, i.e., the LSU was largely missing; for some sequences, mainly types only part of the ITS could be obtained.
RPB2 data were obtained for two pairs and one triplet of species to assist species delimitation. These included I. adorabilis, I. pseudoscabelliformis and I. urceolicystis, I. mycenoides and I. somae, as well as I. ochroalba and I. subhirtella. Obtaining RPB2 data for large numbers of taxa was outside the scope of this study and published data do not exist for many of the species discussed here. Thus, for each of the groups of taxa for which RPB2 data were obtained, a partition was created that included the RPB2 alignment positions for which members of the respective group differed. These partitions were concatenated to the main ITS + partial LSU alignment. For each of the partitions, the alignment for all other taxa (including the members of the other groups) was filled with gaps. The I. adorabilis RPB2 partition included 18 positions, the I. mycenoides RPB2 partition seven positions and the I. ochroalba RPB2 partition 28 positions. The entire alignment included 2 070 positions.
Modeltesting and selection of partitioning schemes under the Bayesian information criterion (BIC) (Kalyaanamoorthy et al. 2017) and ML analyses were run in IQ-TREE v. 1.6 (Nguyen et al. 2015) online (Trifinopoulos et al. 2016). Branch support was obtained through 5 000 replicates of ufb (Minh et al. 2013, Hoang et al. 2018) and SH-aLRT (Guindon et al. 2010). Support values are noted as SH-aLRT support [%] / ufb support [%]. For SH-aLRT support ≥ 80 % and ultrafast bootstrap support ≥ 95 % are given. The tree was visualized using FigTree v. 1.4.4 (Rambaut 2006–2018) and submitted to TreeBASE (accession no. TB2:S28706).
RESULTS
Sequences considered in Fig. 1 are listed in Table 1. Following the model test and partition finder results, the ML tree was calculated under three partitions, one for the ribosomal loci and the RPB2 partition of I. adorabilis, I. urceolicystis and I. pseudoscabelliformis (GTR + F +R5), JC for the RPB2 partition of I. mycenoides and I. somae, and K2P for the RPB2 partition of I. ochroalba and I. subhirtella. Ultrafast bootstrap and H-like approximate likelihood ratio tests were run in 5 000 replicates.
Fig. 1.
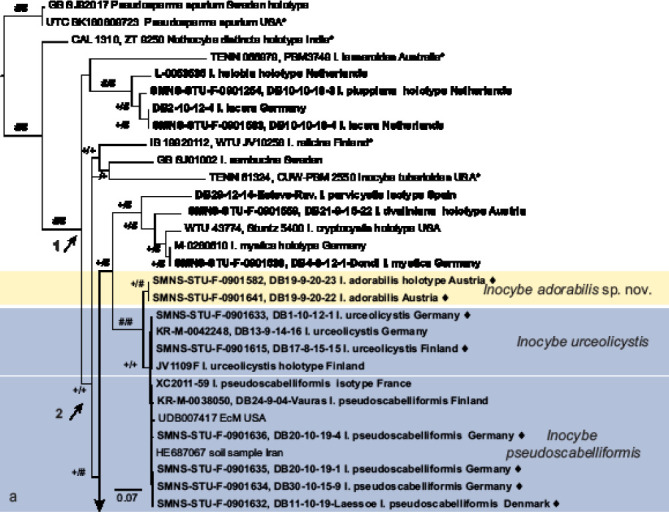



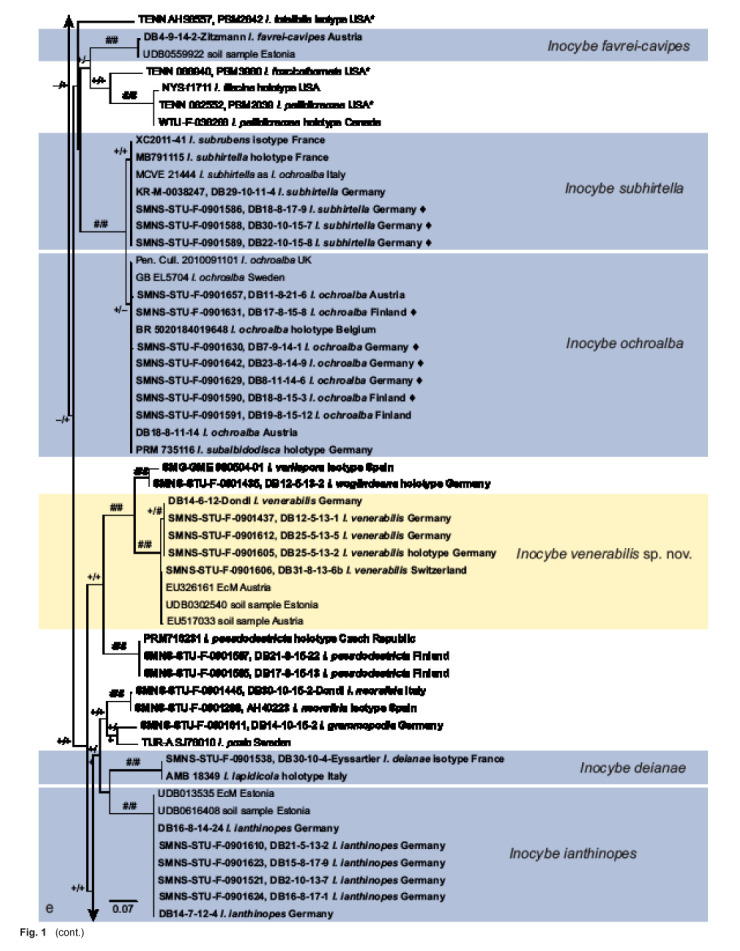

a–f. ML topology of ITS and partial LSU sequences from the species of Inocybe treated or discussed here. Pseudosperma spurium is used for rooting. Clades of species that are described here as new are highlighted in yellow; species clades for which type material is described here are highlighted in blue. Sequences indicated by ♦ also include RPB2 data. Material studied is in bold font. Sequences taken from Matheny et al. (2020) are indicated by *. Arrow 1: Inocybe (genus); arrow 2: smooth-spored temperate boreal clade (STBC, see Matheny et al. 2020) as interpreted here; and arrow 3: STBC as in Matheny et al. (2020). Support values from 5 000 replicates of SH-like approximate likelihood ratio test (SH-aLRT) support / 5 000 replicates of ultrafast bootstrap (ufb) indicated at the branches, # indicating 100 % support, + indicating values ≥ 80 % SH-aLRT or ≥ 0.95 ufb. The SH-aLRT value is given first. The clade of I. urceolicystis (too short to be visible as distinct in the figure) received – / + support
RPB2 data added support to the morphological delimitation for all taxa and collections for which this locus was obtained. In all cases, several, at least two (I. pseudoscabelliformis and I. urceolicystis) alignment positions unambiguously supported the respective species limits. In combination with incomplete data for other sequences in the analysis, the addition of the RPB2 data had little influence on the tree at large and, compared to provisional results without RPB2, limited effect on the species groups under investigation. Within the investigated species pairs and triplet, the monophyly of I. adorabilis (100 % / 100 %) and I. urceolicystis (97 % / 95 %) were supported, but I. pseudoscabelliformis remained paraphyletic in relation to I. urceolicystis. Inocybe ochroalba and I. subhirella were monophyletic in relation to each other, but only I. subhirtella received support (86 % / 99 %). Inocybe mycenoides and I. somae were both monophyletic and at least bootstrap supported (– / 96 % vs 89 % / 98 %).
Figure 1 shows the result of the ML analysis. Based on this alignment, the genus Inocybe receives support (89 % / 96 %). All smooth spored species discussed here apart from I. sambucina are resolved in a single clade. Inocybe sambucina is here included in the clade that corresponds to the clade termed Inocybe sect. Inocybe by Matheny et al. (2020). Inocybe helobia and I. lacera, which have smooth laceroid to (I. he-lobia) almost subangular spores, form an unsupported clade with I. lasseroides (unclassified by Matheny et al. 2020). The clade of I. adorabilis, I. cryptocystis, I. mystica, I. parvicystis, I. pseudoscabellifomis and I. urceolicystis is outside the clade corresponding to Matheny’s et al. (2020) ‘smooth-spored temperate boreal clade’, but as the above listed taxa fit the morpho-ecological circumscription of the clade, we consider them as part of the clade. The joined clade (Fig. 1a) receives 91 % / 98 % support.
All species described here as new received support from ultrafast bootstrap ≥ 95 % and SH-like approximate likelihood ratio test ≥ 80 %. Sequence variation within species, among morphologically analysed collections was normally 0.5 % percent or less, unless, e.g., long indels, stretches N or lacking ITS1 or ITS2 data compromised the result.
For almost all synonymizations with sequence data available for the types of all of the taxa concerned (i.e., I. abietis and I. catalaunica, I. exilis and I. rufobrunnea, I. deianae and I. lapi-dicola, I. subhirtella and I. subrubens), the joined clades received support, in many cases 100 % from both criteria. Exceptions are I. subhirtella and I. ochroalba. Two of the species pairs that are treated below as separate species (I. pseudoscabelliformis and I. urceolicystis, I. heterosemen and I. iseranensis) are not reciprocally monophyletic in relation to each other. In some cases, e.g., I. pelargonium and I. sindonia we did not have sequence data from all types available.
The 26 types (including newly assigned epitypes) that are discussed below correspond to ten species. For 17 of these types, sequence data were available and for 15 of these, data were newly obtained here. Four species are represented by own collections in the phylogenetic analysis. For two species, I. eutheloides and I. pallidolutea, we did not have molecular data available. Inocybe eutheloides is the only species considered doubtful, i.e., for which no morphological concept could be obtained. Species that are treated in the context of synonymizations are highlighted in blue in Fig. 1. New species are indicated in yellow in Fig. 1. One type (I. eutheloides) we were not able to interpret. Species are discussed in detail below.
TAXONOMY
Inocybe abietis (Fig. 17a), accepted name: Inocybe catalaunica
Fig. 17.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Inocybe adorabilis Bandini, B. Oertel & U. Eberh., sp. nov. — MycoBank MB 841145; Fig. 2
Fig. 2.

Inocybe adorabilis sp. nov. a. Holotype, in situ; b. collection DB19-9-20-22, in situ; c. pleurocystidia (coll. DB19-9-20-22); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘adorabilis’ (Latin), because it is a very pretty and thus adorable species.
Typus. AUSTRIA, Salzburg, Tamsweg, ÖK25V 3230-Ost, alt. 1490 m, moist acidulous terrain with Picea abies, 19 Sept. 2020, D. Bandini (holotype STU SMNS-STU-F-0901582; isotype priv. herb. D.B. DB19-9-20-23). GenBank ITS + LSU (partial) OK057159, RPB2 (partial) OK078903.
Diagnosis — Inocybe adorabilis has a bright yellow-ochraceous glabrous to minutely tomentose-lanose pileus, a stipe that is mainly in the upper but sparely also in the lower half pruinose, on average rather narrow smooth spores, measuring 8.0–9.9 µm (av. 8.9 µm) × 4.6–5.6 µm (av. 5.1 µm) and mostly (sub)fusiform to (sub)lageniform hymenial cystidia, often with rounded base, pleurocystidia measuring 37–69 µm (av. 54 µm) × 11–22 µm (av. 15 µm). It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as the closely related I. pseudoscabelliformis.
Basidiomata gregarious or solitary. Pileus 10–30 mm wide, at first (sub)conical, later broadly convex to expanded, with rather low to rather prominent large umbo, margin slightly decurved to straight; young basidiomata with very faint remnants of a pale velipellis; colour bright and warm yellow-ochraceous with or without brownish hue (Mu 10YR 6/6–6/8, 5/6–5/8), at the centre with faint orange hue or somewhat paler; one basidiome appearing somewhat speckled; surface at first glabrous, then minutely tomentose-lanose; no remnants of a cortina observed. Lamellae moderately crowded (c. 45–60, l = 1–3), emarginate adnate, subventricose, at first whitish, later with greyish brownish hue; edge fimbriate, whitish. Stipe 30–45 × 2–3 mm, cylindrical or curved, slightly widening towards the base, base even to slightly thickened, when young covered with whitish tomentum, later longitudinally striate to glabrous, at first whitish, later getting brownish below the apex, while remaining whitish at the apex; pruinose mainly near the apex of the stipe, but some metuloid cystidia also below the middle of the stipe. Context whitish in the pileus and the stipe. Smell spermatic when cut. Colour of exsiccata pileus ochraceous brownish to hazel-brown (Mu 10YR 6/8, 5/6–5/8), lamellae somewhat paler in colour, stipe concolorous or darker brownish towards the base, no darkening or blackening on drying.
Spores 8.0–9.9 µm (av. 8.9 µm, SD 0.4 µm) × 4.6–5.6 µm (av. 5.1 µm, SD 0.2 µm); Q = 1.5–2.1 (av. 1.7, SD 0.1) (n = 80 of 2 coll.), smooth, (sub)amygdaloid, without or with only faint suprahilar depression, apex subacute, often with indistinct pseudoporus. Basidia 23–28 × 7–9 µm, generally 4-spored. Lamellae edges composed of cheilocystidia and numerous colourless, (sub)clavate, thin-walled paracystidia. Pleurocystidia 37–69 µm (av. 54 µm, SD 7 µm) × 11–22 µm (av. 15 µm, SD 3 µm); Q = 2.5–5.1 (av. 3.6, SD 0.6) (n = 30 of 2 coll.); mostly (sub)fusiform, also (sub)lageniform, some almost ovoid, without neck or with short or longer neck, with short pedicel or with rounded base, apex usually crystalliferous, walls up to 3.5(–4.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 3–8(–10) µm wide, with not or only faintly encrusting and parietal yellowish pigment, subcutis with wider and paler to colourless elements. Caulocystidia mainly near the apex of the stipe, but sparely also below the middle, 30–55 × 8–15 µm, (sub)fusiform to (sub)utriform, with short neck and short pedicel, apex usually crystalliferous, walls up to 1.0(–1.5) µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe adorabilis is known from two finds in Austria. The collections were found at a wayside with Picea abies nearby. Apart from those two no other collections or sequences of collections are known to us. There is just one EcM-sequence in GenBank from Canada (MT088375), that may belong to this species.
Additional collection examined. Austria, Salzburg, Tamsweg, ÖK25V 3230-Ost, alt. 1450 m, Picea abies, 19 Sept. 2020, D. Bandini (STU SMNS-STU-F-0901641, DB19-9-20-22).
Notes — Inocybe adorabilis is characterized by bright yellow-ochraceous glabrous to minutely tomentose-lanose pilei, stipes that are mainly in the upper but sparely also in the lower half pruinose, smooth rather narrow spores and hymenial cystidia with often rounded base. Because of its bright colour, the species might on first view be confused with I. leochroma, which differs, e.g., by a rougher pruina on the stipe and shorter spores (Bandini et al. 2019b). Inocybe pseudoscabelliformis can be distinguished from I. adorabilis by generally more ochraceous brownish to nut-brown pileus colours, on average longer spores and hymenial cystidia usually without rounded base (Carteret & Reumaux 2017, pers. observ. and see below) and I. urceolicystis by, e.g., less bright, i.e., duller pileus colours, hymenial cystidia often with rounded or urceoliform base and on average somewhat larger spores. Inocybe pelargonium can be distinguished, e.g., by a more glabrous, sometimes sticky pileus surface, clearly entirely pruinose stipe, smaller spores and shorter hymenial cystidia (Kühner 1955, Kuyper 1986, Stangl 1989, Bandini et al. 2019b, and see also below). Inocybe langei differs, e.g., by much smaller spores and shorter hymenial cystidia (Heim 1931, Kuyper 1986, Stangl 1989, Ludwig 2017), I. iseranensis, an alpine or (sub)boreal species, differs, e.g., by much smaller spores and shorter hymenial cystidia (Ferrari 2010, and see below), while I. demetris (see below) has an abundant velipellis, more brownish pileus colour and on average longer hymenial cystidia. Inocybe suryana (see below) differs, e.g., by more pruina in the lower half of the stipe, on average wider spores and shorter hymenial cystidia as well as growth on humid or moist terrain generally with Salix and Alnus (see below), while I. hirtella usually has two-spored basidia with spores of much larger size (see below). The monophyly of the two sequences we have for I. adorabilis (including RPB2 data) is supported by 92 % / 100 %. It is sister species to I. pseudoscabelliformis and I. urceolicystis, and differs from these only by 2 % in the ITS. RPB2 data support the independence of I. adorabilis from these two taxa, but sequence similarity is also more than 98 %. It can be assumed that I. adorabilis is a fairly rare species, even though it may have been mistaken for one of the above listed species.
Inocybe catalaunica Singer, Collectanea Bot., Barcinone Bot. Inst. 1: 245. 1947 — Fig. 17a, b
Heterotypic synonym. Inocybe abietis Kühner, Bull. Soc. Nat. Oyonnax 9 (Suppl.(Mém. hors sér. 1)): 3. 1955.
Selected descriptions & Iconography — Singer 1947, Esteve-Raventós 1997 (evaluation and drawing of the microdetails of the holotype), Larsson et al. 2014 (evaluation and drawing of the microdetails of the holotype).
Studied material. Lectotype of I. abietis, Fig. 17a, designated by J. Poirier (2016): France, Savoie, alt. 1100–1200 m, at the wayside on needles with Picea, Abies, 15 Aug. 1941, R. Kühner (lectotype G00058749, other number 388325). Spores 8.2–10.4 µm (av. 9.1 µm, SD 0.4 µm) × 4.5–6.0 µm (av. 5.1 µm, SD 0.2 µm); Q = 1.7–2.1 (av. 1.8, SD 0.1) (n = 40), smooth, oblong (sub)amygdaloid, mostly without suprahilar depression, with subacute to subobtuse apex. Basidia 4-spored. Pleurocystidia 55–81 µm (av. 69 µm, SD 8 µm) × 12–19 µm (av. 15 µm, SD 2 µm); Q = 3.1–5.7 (av. 4.7, SD 0.8) (n = 15), mostly (sub)cylindrical or (sub)fusiform, with short or longer pedicel, walls up to 1.0 (1.5) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but more variable in shape. Paracystidia subclavate. Caulocystidia not studied (to preserve the material).
Selected additional collections examined. Germany, Baden-Württemberg, Neckar-Odenwald-Kreis, Schwarzach, TK25 6619/2, alt. 390 m, Picea abies, 5 Sept. 2014, D. Bandini (STU SMNS-STU-F-0901596, DB5-9-14-3); Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 390 m, Picea abies, Abies alba, 5 Sept. 2014, D. Bandini (DB5-9-14-9); Baden-Württemberg, Ortenau-Kreis, Gutach, Hornberg, TK25 7715/3, alt. 630 m, roadside with Picea abies, 14 July 2016, D. Bandini (DB14-7-16-4); Baden-Württemberg, Rhein-Neckar-Kreis, Reichartshausen, TK25 6619/2, alt. 390 m, Picea abies, 27 Oct. 2019, D. Bandini (STU SMNS-STU-F-0901597, DB27-10-19-12); Bayern, Miesbach, Gmund, Marienstein, TK25 8236/3, alt. 880 m, Abies alba, Fagus sylvatica, 7 Sept. 2013, M. Dondl, (DB7-9-13-1-Dondl); Bayern, Ostallgäu, Füssen, Hopfen-am-See, Hopfener Wald, TK25 8330/3, alt. 870 m, wayside with Picea abies, Fraxinus excelsior, Corylus betulus, 14 Oct. 2016, D. Bandini, B. Oertel & J. Christan (DB14-10-16-4).
Notes — Inocybe catalaunica has a brown pileus colour with often a more or less intense foxy tinge. The surface is at first finely to thickly tomentose, later often radially minutely cracked or minutely subsquamulose. The stipe is entirely pruinose, but because it is covered with whitish tomentum, it is, with the bare eye, sometimes difficult to decide whether the stipe is entirely pruinose or not (pers. observ., see also Kühner 1955, Esteve-Raventós 1997). The hymenial cystidia are rather long with short or longer pedicel. The protologue and the drawing of the hymenial cystidia of I. abietis Kühner (1955) fit very well with our several collections of I. catalaunica. The type sequence of I. catalaunica is publicly available. Since the ITS of our collections matches the ITS of the holotype (see Fig. 1), and since not only Esteve-Raventós (1997) but also Larsson et al. (2014) have examined the holotype and published drawings of the hymenial cystidia, we saw no reason to examine the type again ourselves. However, we here include a drawing of the microdetails of one of our own collections (Fig. 17b), demonstrating the similarity between the holotype of I. abietis and I. catalaunica. The spores of the type of I. abietis are on average a bit shorter than those measured by Larsson et al. (2014), with on average 9.7 × 5.3 µm. But the average values of the spores of three own collections (120 spores) are with 9.1 × 5.1 µm exactly the same as those of the type of I. abietis. Also, the measurements of the hymenial cystidia are with 70 × 15 µm (45 spores of 3 coll.) on average almost identical. The ITS sequence obtained from the type of I. abietis is included in the same clade (support 100 % / 100 %) as the type and own collections of I. catalaunica. We therefore consider both species as conspecific. Inocybe catalaunica is a fairly common species, growing in woods preferably in association with Picea abies.
Inocybe comis Bandini & B. Oertel, sp. nov. — MycoBank MB 841146; Fig. 3
Fig. 3.

Inocybe comis sp. nov. a. Holotype, in situ; b. collection DB13-8-13-27, in situ; c. cheilocystidia (coll. DB13-8-13-27); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (coll. DB13-8-13-27). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘comis’ (Latin ‘gracious’), because the species was so gracious to present itself as a new species.
Typus. Austria, Salzburg, Zederhaus, Riedingtal, ÖK25V 3229-Ost, alt. c. 1500 m, Picea abies, Larix decidua, 13 Aug. 2013, D. Bandini & B. Oertel (holotype STU SMNS-STU-F-0901599; isotype priv. herb. D.B. DB13-8-13-3). GenBank ITS + LSU (partial) OK057190.
Diagnosis — The pileus of I. comis is brown with foxy or reddish tinges and with age (sub)lanose. The stipe is pruinose only near the apex of the stipe and reddish near the apex. The smooth spores on average are rather small, measuring 6.8–9.8 µm (av. 8.2 µm) × 4.2–5.8 µm (av. 4.9 µm), and the hymenial cystidia are mainly (sub)fusiform to (sub)utriform, pleurocystidia measuring 46–77 µm (av. 58 µm) × 9–20 µm (av. 13 µm), and often have undate walls. The caulocystidia are long and narrow. The species can be recognized by the combination of the above characters and differs in its ITS sequence from other smooth-spored species, such as I. minimispora.
Basidiomata gregarious or solitary. Pileus 10–25 mm wide, at first (sub)campanulate to subconical, later broadly convex or expanded, with low large umbo, margin at first decurved, later straight or even uplifted, and then pileus depressed around the umbo; young basidiomata with greyish remnants of a velipellis, to be observed as radially scattered garland outside the umbo; colour brown with foxy to reddish hue (Mu 5YR 5/6–5/8; 7.5YR 5/6–5/8); surface at first finely tomentose, later minutely to strongly lanose, sometimes with somewhat paler fibre bundles on darker ones and therefore with mottled aspect; young basidiomata with remnants of a cortina. Lamellae subdistant (c. 30–40, l = 1–3), adnate, (sub)ventricose, at first whitish, later greyish whitish, with age brownish; edge fimbriate, whitish. Stipe 20–35 × 1–2 mm, cylindrical or curved, when young covered with fine whitish tomentum, later longitudinally striate or glabrous, pale flesh-coloured, reddish towards the apex; pruinose only near the apex of the stipe. Context whitish in the pileus and the stipe below the apex, where it is faintly reddish. Smell spermatic, at least when cut. Colour of exsiccata pileus brown with reddish or greyish hue (Mu 7/5YR 4/4–4/6; 10YR 4/4–4/6), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 6.8–9.8 µm (av. 8.2 µm, SD 0.6 µm) × 4.2–5.8 µm (av. 4.9 µm, SD 0.1 µm); Q = 1.4–2.0 (av. 1.7, SD 0.1) (n = 80 of 2 coll.), smooth, (sub)amygdaloid, often with explicit suprahilar depression, apex subacute to subobtuse, with distinct pseudoporus. Basidia 24–28 × 7–9 µm, generally 4-spored, rarely also 2-spored and then spores up to 11.2 µm. Lamellae edges composed of cheilocystidia and numerous colourless, (sub)clavate, thin-walled paracystidia. Pleurocystidia 46–77 µm (av. 58 µm, SD 6 µm) × 9–20 µm (av. 13 µm, SD 3 µm); Q = 3.0–7.0 (av. 4.5, SD 0.9) (n = 30 of 2 coll.); (sub)fusiform to (sub)utriform, sometimes sublageniform or subcylindrical, often with undate walls, usually with short neck, generally with only short pedicel, sometimes without pedicel, at apex generally wide, apex usually crystalliferous, walls up to 2.5(–3.0) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4–11 µm wide, with encrusting and parietal dark brown pigment, subcutis with wider and paler to colourless elements. Caulocystidia only near the apex of the stipe, 50–90 × 7–11 µm, long and narrow subcylindrical to (sub)utriform, with undate walls and often subcapitate apex, apex usually crystalliferous, walls up to 1.0 µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate to subglobose cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Up to now the species is known only from two collections found by us in Austria. It appears to occur in Italy, judging from a sequence in GenBank (as Inocybe sp., JF908230; see also Osmundson et al. 2013: t. S1). Furthermore, an EcM-sequence, which might belong to this species is in GenBank from Mexico (KF041426, with Abies religiosa). Inocybe comis was found on the gravelly banks of the small river Riedingbach, on calcareous soil, associated with conifers. More collections are needed to investigate the ecology of the species.
Additional collection examined. Austria, Salzburg, Zederhaus, Riedingtal, ÖK25V 3229-Ost, at some distance to type collection, alt. c. 1500 m, Picea abies, Larix decidua, 13 Aug. 2013, D. Bandini & B. Oertel (STU SMNS-STU-F-0901598, DB13-8-13-27).
Notes — The pilei of I. comis are sometimes somewhat mottled brown with foxy or reddish hue with sublanose surface. The species is further characterized by rather small spores on average and generally (sub)fusiform to (sub)utriform hymenial cystidia with often undate walls. It was found on calcareous soil. Macroscopically, on first sight it could perhaps be mistaken for I. nitidiuscula or I. involuta. Both species normally have reddish tinges at least in the pileus colour, and the apex of the stipe is reddish as well. However, these species differ from I. comis, e.g., by smoother pileus surface and the spores are much larger (Britzelmayr 1891, Stangl 1983, 1989, Kuyper 1986, 1989, Marchetti et al. 2014, Bandini et al. 2020a, c). The pileus aspect of I. rivierana can be somewhat similar to I. comis. The former species was also found on the border of a river on calcareous soil, even though in a somewhat lower region. However, I. rivierana has much larger spores and longer, mostly (sub)cylindrical hymenial cystidia. Furthermore, the stipe is mostly pruinose also in the lower half (Bandini et al. 2021b). In some collections the pilei of I. tenuicystidiata may be similar in aspect, too, but the surface of pileus is (sub)-hygrophanous, the hymenial cystidia are longer and narrow, and the spores are on average longer and a little wider (Horak & Stangl 1980, Bandini et al. 2021b). Inocybe perchtana, a species with reddish tinged pileus and growing in the mountains of Austria like I. comis, differs from the latter, e.g., by the often reddening context of the stipe, an entirely pruinose stipe and on average larger spores (Bandini et al. 2020a), while I. pipilikae, which was found in the same location as I. comis, has a much more glabrous pileus surface, an entirely pruinose stipe and larger spores (Bandini et al. 2021a). The pileus surface of I. alberichiana, another species of the mountainous regions and associated with conifers, is more glabrous and the spores are larger (Bandini et al. 2021b). Inocybe pseudodestricta and I. nemorosa, both with reddish tinges in the pileus colour, have a more glabrous pileus surface and larger spores, too (Heim 1931, Grund & Stuntz 1968, Stangl & Veselský 1973, Bandini et al. 2019b). Inocybe minimispora is the species with the most similar ITS (94 % similarity) in relation to I. comis and it is also its sister species in Fig. 1. Inocybe minimispora is quite common in the mountainous regions of Austria. It differs from I. comis, e.g., by its smooth to at most finely fibrillose pileus surface, much smaller spores and hymenial cystidia (Reumaux 1986, Bandini et al. 2021b). Inocybe demetris (92 % similar in the ITS to I. comis) is discussed below. Inocybe comis forms a fully supported (100 % / 100 %) clade in Fig. 1.
Inocybe deianae Eyssart., Bull. Mycol. Bot. Dauphiné-Savoie 47(no. 186): 36. 2007 — Fig. 17c
Heterotypic synonym. Inocybe lapidicola Brugaletta, Consiglio & M. Marchetti, in Brugaletta, Consiglio & Marchetti, Riv. Micol. 62(2): 104. 2019.
Selected descriptions & Iconography — Eyssartier 2007, Brugaletta et al. 2019 (as ‘I. lapidicola’).
Studied material. Isotype of I. deianae: France, Port-de-Bouc, Forêt de Castillon, Pinus halepensis, 30 Oct. 2004, G. Eyssartier & A. Bidaud (isotype STU SMNS-STU-F-0901538; DB30-10-4-Eyssartier). Spores 9.6–12.8 µm (av. 10.6 µm, SD 0.7 µm) × 5.7–7.1 µm (av. 6.4 µm, SD 0.3 µm); Q = 1.4–1.9 (av. 1.7, SD 0.1) (n = 40), smooth (broadly) elliptical or (sub)amygdaloid, with (sub)obtuse to (sub)acute apex. Basidia 4-spored. Pleurocystidia 47–62 µm (av. 56 µm, SD 4 µm) × 15–21 µm (av. 18 µm, SD 2 µm); Q = 2.6–3.8 (av. 3.1, SD 0.4) (n = 15), (sub)cylindrical, (sub)fusiform or subutriform, sublageniform, sometimes subclavate, apex usually crystalliferous, walls up to 1.0(–1.5) µm thick, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate. Caulocystidia only at the apex of the stipe, (sub)clavate, (sub)cylindrical, (sub)fusiform or subutriform, intermixed with hyphoid elements.
Notes — The isotype of I. deianae was successfully sequenced. As can be seen in Fig. 1, it matches the sequence of the holotype of I. lapidicola, a recently described species (Brugaletta et al. 2019). We have not examined the holotype of this latter species ourselves, but the detailed protologues of the two species (see also Eyssartier 2007) appear to describe similar species. The microscopic details, i.e., size of spores and shape and thickness of the walls of hymenial cystidia of I. deianae correspond to those of I. lapidicola (Brugaletta et al. 2019). Furthermore, the habitat, sandy soil with Pinus halepensis, is similar. The clade including the isotype of I. lapidicola and the holotype of I. deianae receives full support (100 % / 100 %). Thus, we treat the two species as conspecific.
Inocybe demetris Bandini & U. Eberh., sp. nov. — MycoBank MB 841147; Fig. 4
Fig. 4.

Inocybe demetris sp. nov. a. Holotype, in situ; b. collection DB22-9-20-13, in situ; c. cheilocystidia (coll. DB12-8-14-7); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (coll. DB21-10-17-10). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘demetris’ after the Greek goddess Demeter, goddess of the harvest and the agriculture, because of its colour resembling soil.
Typus. Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Neckarbischofsheim, castle park, TK25 6719/2, alt. 175 m, Abies alba, 27 Oct. 2019, D. Bandini (holotype STU SMNS-STU-F-0901593; isotypes priv. herb. D.B. DB27-10-19-6, TUR-A 209506). GenBank ITS + LSU (partial) OK057184.
Diagnosis — Inocybe demetris has an ochraceous brownish to reddish hued brown pileus with an abundant greyish velipellis, smooth to rimose pileus surface, smooth spores, measuring 7.8–10.3 µm (av. 8.8 µm) × 4.3–5.9 µm (av. 5.1 µm), mostly (sub)utriform to (sub)lageniform hymenial cystidia, with short ones intermixed with long and often undate necks, pleurocystidia measuring 46–76 µm (av. 61 µm) × 9–17 µm (av. 13 µm), and very long caulocystidia, often with long and undate necks on entire length of the stipe, but sometimes rather sparely in the lower half. It can be recognized by the combination of the above characters and differs in its ITS sequence from other smooth-spored species, such as the distantly genetically related I. comis.
Basidiomata mostly gregarious, often in large numbers. Pileus 20–40 mm wide, at first (sub)conical or (sub)campanulate, later broadly convex or expanded, with age with more or less pronounced large umbo, margin at first either slightly incurved or decurved, later clearly decurved to almost straight, sometimes even uplifted, and then pileus depressed around the umbo; young basidiomata with abundant greyish velipellis, remaining often at the centre of older specimens; colour ochraceous brownish, hazelbrown to brownish or brown with more or less intense reddish hue (Mu 5YR 5/6–5/8; 7.5YR 5/6–5/8, 4/4–4/6), at the centre mostly somewhat greyish because of the velipellis, sometimes even darker greyish; surface at first smooth or almost satiny glabrous, later finely rimulose to strongly rimose towards the margin, with fibres more or less diverging, so that the paler trama below is visible, while the centre remains glabrous and unbroken; young basidiomata sometimes with remnants of a whitish cortina. Lamellae rather crowded (c. 60–80, l = 1–3), adnate, subventricose, at first strikingly whitish, in some collections remaining whitish for a long time, in others becoming ochraceous brownish to coffee brown; edge fimbriate, whitish. Stipe 20–50 × 2–4 mm, cylindrical or curved, base equal, when young entirely covered with fine whitish tomentum, later longitudinally striate or glabrous, at first whitish, later pale flesh coloured to pale brownish; pruinose generally on the entire length of the stipe, but sometimes only sparely in the lower half. Context whitish in the pileus and the stipe. Smell spermatic, at least when cut. Colour of exsiccata pileus dark brown, at the centre sometimes almost blackish brown with reddish tinge (Mu 7.5YR 4/4–4/6; 5YR 3/2–3/4), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 7.8–10.3 µm (av. 8.8 µm, SD 0.5 µm) × 4.3–5.9 µm (av. 5.1 µm, SD 0.5 µm); Q = 1.5–2.2 (av. 1.7, SD 0.1) (n = 120 of 3 coll.); smooth, mostly oblong, (sub)amygdaloid, in some collections generally without or with suprahilar depression, apex subacute to subobtuse, with indistinct pseudoporus. Basidia 25–28 × 7–9 µm, generally 4-spored. Lamellae edges composed of cheilocystidia and numerous colourless, (sub)clavate or subcylindrical, thin-walled paracystidia. Pleurocystidia 46–76 µm (av. 61 µm, SD 8.2 µm) × 9–17 µm (av. 13 µm, SD 2.2 µm); Q = 3.1–6.7 (av. 4.7, SD 0.9) (n = 45 of 3 coll.), mostly (sub)utriform to (sub)lageniform, also (sub)fusiform, neck very variable even in the same collection, some cystidia only short, others long and with undate walls, usually with short pedicel or with truncate or rounded base, apex usually crystalliferous, walls up to 1.5(–2.5) µm thick near the apex, often unequally thick in one and the same collection, almost colourless to pale yellowish greenish with 3 % KOH. Cheilocystidia somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4–10 µm wide, with partially encrusting and parietal ochraceous brownish to brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia on entire length of the stipe, but sometimes only sparely in the lower half, 45–110 × 10–15 µm, long and narrow (sub)lageniform, (sub)utriform, subcylindrical, usually with very long, sometimes undate neck, walls up to 1.0(–1.5) µm thick at the apex, almost colourless to pale yellowish greenish with 3 % KOH. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe demetris was found by us always next to conifers, either with Picea abies or Abies alba on calcareous soils, along pebbled waysides or in parks or on cemeteries. Judging from our own experience and numerous collections, it is a quite common species. In spite of this, no sequence data of this taxon appear to be in GenBank or UNITE, only some EcM sequences from France (EU711175, with Epipogium aphyllum), Spain (JQ975963, with Pinus pinaster) and from some soil samples from Estonia (e.g., UDB0613420), that may belong to this species.
Additional collections examined. Austria, Tirol, Imst, Mötz, Locherboden, ÖK25V 2221-Ost, alt. 780 m, Pinus sylvestris, Corylus avellana, 14 Sept. 2017, D. Bandini (DB14-9-17-6). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Wiesenbach TK25 6618/2, alt. 170 m, Picea abies, Fagus sylvatica, 20 Oct. 2012, D. Bandini & B. Oertel (DB 20-10-12-2); Baden-Württemberg, Rhein-Neckar-Kreis, Wiesenbach, TK25 6618/1, alt. 180 m, Fagus sylvatica, Picea abies, 28 Sept. 2013, D. & G. Bandini (DB28-9-13-1); Baden-Württemberg, Rhein-Neckar-Kreis, Neckarbischofsheim, TK25 6719/2, alt. 180 m, Abies alba, 21 Oct. 2017, X. Hielscher, D. Bandini & R. Bandini (DB21-10-17-10); Baden-Württemberg, Rhein-Neckar-Kreis, Neckarbischofsheim, castle park, TK25 6719/2, alt. 175 m, at some distance from type collection, Pseudotsuga menziesii, 27 Oct. 2019, D. Bandini (STU SMNS-STU-F-0901594, DB27-10-19-10); Bayern, Lenggries, Arzbach, Lehen, TK25 8235/3, 820 m, Abies alba, 12 June 2011, M. Dondl (DB12-6-11-Dondl); Bayern, Berchtesgadener Land, Berchtesgaden, TK25 8344/3, alt. 1400 m, Picea abies, Abies alba, 16 Aug. 2011, D. Bandini & B. Oertel (DB16-8-11-6); Bayern, Starnberg, Gilching, TK25 7833/4, alt. c. 550 m, Picea abies, 1 Oct. 2013, H. Grünert (DB1-10-13-13-Grünert); Bayern, Ostallgäu, Füssen, TK25 8430/1, alt. 795 m, Abies alba, 12 Aug. 2014, D. Bandini (DB12-8-14-7); Ibidem, at some distance from former location, Picea abies, Abies alba, Larix decidua, 12 Oct. 2016, D. Bandini (DB12-10-16-5); Ibidem, at some distance from former location, Picea abies, Abies alba, Larix decidua, 12 Oct. 2016, D. Bandini (DB12-10-16-10); Bayern, Oberallgäu, Bad Hindelang, Untergschwend, TK25 8428/2, alt. 1100 m, Picea abies, 15 Sept. 2018, D. Bandini (DB15-9-18-9); Bayern, Schwaben, Ostallgäu, Füssen, TK25 8430/1, alt. 798 m, Picea abies, Abies alba, Larix decidua, 15 Sept. 2019, D. Bandini (DB15-9-19-1); Bayern, Rottal-Inn, Julbach, near Simbach, TK25 7643/4, alt. 400 m, Picea abies, Fagus sylvatica, 22 Sept. 2020, D. Bandini (STU SMNS-STU-F-0901595, DB22-9-20-13); Ibidem, at some distance from former location, Picea abies, Fagus sylvatica, 23 Sept. 2020, D. Bandini (STU SMNS-STU-F-0901581, DB23-9-20-5).
Notes — Inocybe demetris is characterized by an ochraceous brownish, nut-brown to reddish hued brown pileus with glabrous to rimose surface, abundant greyish velipellis and when young striking whitish lamellae. The stipe is entirely pruinose but sometimes sparely in the lower half, the spores are on average rather small and narrow, and the hymenial cystidia are (sub)utriform to (sub)lageniform with short or long necks. The caulocystidia are very long and often have undate necks. At first sight, it may be mistaken for another brown species with entirely pruinose stipe and rather small spores such as I. glabrescens (Velenovský 1920–1922) or I. metrodii, two species equally with greyish velipellis and rather small and narrow spores (see Bandini et al. 2021b). However, the pileus surface of I. glabrescens is more tomentose to sublanose (Stangl 1989, and pers. observ.), and that of I. metrodii finely tomentose to minutely fibrillose and the hymenial cystidia and caulocystidia of both species are clearly shorter (Stangl & Veselský 1979, and pers. observ.). Besides, the basidiomata of I. metrodii often are larger and quite stout (pers. observ.). Inocybe beatifica differs, e.g., by lacking velipellis, clearly longer spores and shorter caulocystidia (Bandini et al. 2021b). Inocybe catalaunica often has a more foxy tinged pileus colour and a tomentose to sublanose or minutely subsquamulose pileus surface (pers. observ., see also above). Furthermore, the hymenial cystidia are generally neckless (see drawing of Kühner 1955 (description of I. abietis), Esteve-Raventós 1997, Larsson et al. 2014, and Fig. 17b), and the caulocystidia are shorter. More ochraceous tinged basidiomata of I. demetris might on first sight be mistaken for I. hirtella. However, the spores of this latter species are much larger and the basidia usually are 2-spored (Bresadola 1881–1887, and see below). Inocybe suryana (see below) has an only faint whitish velipellis, on average wider spores and much shorter hymenial cystidia, and I. somae (see below) differs, e.g., by a pale pileus colour, larger spores and shorter hymenial cystidia. Species like I. leochroma or I. pelargonium can be distinguished, e.g., by much shorter spores and shorter caulocystidia (Kühner 1955, Bandini et al. 2019b). The described species with the most similar ITS (92 %) is I. comis (see above). This species has a sometimes somewhat mottled brown foxy or reddish hued pileus with sublanose surface, the stipe is only near the apex pruinose and the spores are shorter on average. However, there is sequence evidence from Italy (herbarium collection) and Morocco (EcM sequence) that a more closely related, apparently unnamed taxon occurs in the Mediterranean (ITS sequence similarity 97.2–97.6 %; see also Fig. 1). It seems probable that I. demetris has often been mistaken for I. glabrescens, I. metrodii or I. abietis, i.e., I. catalaunica.
Inocybe eutheloides Peck, Bull. New York State Mus. Nat. Hist. 1(no. 2): 13. 1887 — Fig. 17d
Description & Iconography — Peck 1887.
Studied material. Holotype of I. eutheloides: USA, Brewerton, New York, woods, Sept. [C.H. Peck] (holotype NYSf-1117). Spores 8.2–9.4 µm (av. 8.8 µm, SD 0.3 µm) × 4.2–5.6 µm (av. 5.1 µm, SD 0.3 µm); Q = 1.6–2.0 (av. 1.7, SD 0.1) (n = 40), smooth, (sub)amygdaloid, apex subacute. Basidia 4-spored. Pleurocystidia 39–62 µm (av. 54 µm, SD 6 µm) × 16–24 µm (av. 19 µm, SD 3 µm); Q = 2.0–3.9 (av. 2.9, SD 0.5) (n = 15), mostly (sub)utriform, sometimes (sub)-fusiform, with clearly demarcated pedicel, apex usually crystalliferous, walls up to 3.0(–4.0) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia not studied (to preserve the material).
Notes — Inocybe eutheloides was described by Peck (1887). According to his protologue the pileus is umbonate, silky-fibrillose, more or less rimose and varying in colour from greyish cervine to chestnut-brown, the disk is sometimes squamulose. We were not allowed to sequence the type. The spores are with 8.8 µm length on average rather short, and also the hymenial cystidia are with 54 ×19 µm relatively short but wide. The pedicel is clearly demarcated. It can be excluded that I. eutheloides is the same as I. sindonia, which has a paler pileus colour, narrow fusiform hymenial cystidia and on average narrower spores (see below). We are not able to interpret the name I. eutheloides and cannot match it with any material we know.
Inocybe exilis (Fig. 17e), accepted name: Inocybe rufobrunnea
Inocybe favrei-cavipes Bon, Bull. Mycol. Bot. Dauphiné-Savoie 37(no. 144): 93. 1997 — Fig. 17f
Homotypic synonym. Inocybe cavipes J. Favre, Ergebnisse der Wissenschaftlichen Untersuchungen des Schweizerischen Nationalparks 5: 200. 1955, nom. illegit., Art. 53.1 [non I. cavipes (Britzelm.) Sacc. & Traverso, Sylloge Fungorum 19: 968. 1910].
Selected descriptions & Iconography — Favre 1955, Kuyper 1986: f. 89, Bon 1997b.
Studied material. Holotype of I. cavipes: Switzerland, Le Laiets, Val Sesvenna, Salix herbacea, alt. 2573 m, 20 Aug. 1944, J. Favre (holotype G00126210). Spores 9.3–11.7 µm (av. 10.5 µm, SD 0.6 µm) × 5.0–6.7 µm (av. 5.9 µm, SD 0.4 µm); Q = 1.5–2.0 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)acute apex. Basidia 4-spored and 2-spored. Pleurocystidia 57–77 µm (av. 64 µm, SD 5 µm) × 10–19 µm (av. 15 µm, SD 3 µm); Q = 3.3–6.3 (av. 4.5, SD 0.9) (n = 15), mostly subfusiform to (sub)-cylindrical, without or with only short neck, sometimes subcapitate, without or with only short pedicel, apex usually crystalliferous, walls up to 2.5(–3.0) µm thick at the apex, almost colourless to pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia not studied (to preserve the material).
Additional collection examined. Austria, Salzburg, Maria Alm, Steinernes Meer, Riemannhaus, TK25 8543/2, alt. 2100 m, Salix sp., Dryas octopetala, 4 Sept. 2014, H. Zitzmann (DB4-9-14-2-Zitzmann).
Notes — Sequencing of the type was not permitted. We are reasonably sure, though, that the collection listed above is the species in question. The ITS sequence matches with a number of soil sample sequences in the UNITE database (for one example see Fig. 1 and Table 1). Kuyper (1986) synonymized I. favrei-cavipes with I. abjecta. We do not follow this synonymization. We have studied the holotype of I. abjecta, too, and found several differences regarding shape and size of spores and hymenial cystidia. The size of the spores of I. abjecta is larger, the spores typically have a (sub)papillate apex, the hymenial cystidia are on average shorter and their shape is more fusiform with narrowing necks towards the apex, which is not the case in I. favrei-cavipes. Furthermore, the macroscopic descriptions of both species differ distinctly (see Karsten 1879, Favre 1955). We therefore consider I. favrei-cavipes a good species, as did M. Bon (1997b).
Inocybe filiana Bandini, B. Oertel & U. Eberh., sp. nov. — MycoBank MB 841148; Fig. 5
Fig. 5.

Inocybe filiana sp. nov. a. Holotype, in situ; b. collection DB2-10-12-2, in situ; c. pleurocystidia (coll. DB5-5-16-9); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (coll. DB5-5-16-9). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘filiana’ after Fili, a dwarf in the nordic Edda, because the pilei of the species mostly are very small.
Typus. Germany, Nordrhein-Westfalen, Viersen, Brüggen, Nature Reserve Brachter Wald, TK25 4702/2, alt. 40 m, sandy soil with Pinus sylvestris, 4 May 2016, D. Bandini & K. Wehr (holotype STU SMNS-STU-F-0901602; isotypes priv. herb. D.B. DB4-5-16-1, TUR-A 209507). GenBank ITS + LSU (partial) OK057192.
Diagnosis — Inocybe filiana usually has a small reddish tinged or redbrown smooth to subsquamulose-sublanose pileus, at first whitish lamellae contrasting with the reddish brown stipe, smooth spores with rather distinct pseudoporus, measuring 7.7–11.6 µm (av. 9.6 µm) × 5.0–6.4 µm (av. 5.6 µm), and mostly (sub)fusiform neckless and on average rather short hymenial cystidia, pleurocystidia measuring 28–66 µm (av. 47 µm) × 10–18 µm (av. 14 µm). Its preferred habitat is sandy soil with Pinus sylvestris. It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. furfurea.
Basidiomata mostly gregarious, seldom solitary. Pileus 5–20(–30) mm wide, (sub)campanulate or (sub)conical when young, later broadly convex or expanded, with or without low large umbo, margin from strongly incurved or slightly inflexed to decurved or straight; young basidiomata with whitish remnants of velipellis at least in the centre of the pileus; colour nut-brown with faint reddish tinge, often redbrown, seldom dark brown without reddish hue (Mu 7.5YR 5/4–5/6, 4/3–4/6; 10YR 3/4–3/6), sometimes somewhat darker at the centre or paler, when still covered by velipellis; surface from glabrous or finely felty when young to tomentose, minutely innate fibrillose or (sub)squamulose-sublanose with age; young basidiomata with remnants of a whitish cortina. Lamellae rather distant (c. 30–40(–45), l = 1–3), adnexed to adnate, sometimes with subdecurrent tooth, (sub)ventricose, whitish when young, soon pale coffee-brown or ochraceous-brownish to brown; edge fimbriate, whitish. Stipe 10–40(–45) × 2–5 mm, cylindrical to slightly widening towards the base, when young covered entirely with whitish tomentum, later longitudinally striate or glabrous, reddish brownish to intensely redbrown, sometimes also partially dark brown, base remaining whitish; pruinose only near the apex of the stipe. Context whitish in the pileus, redbrown in the stipe, especially in the cortex of the stipe. Smell (sub)spermatic, at least when cut. Colour of exsiccata pileus dark greyish brown or dark brown (Mu 10YR 3/2–3/4; 7.5YR 3/2), lamellae paler in colour, stipe often reddish dark brown, no darkening or blackening with drying.
Spores 7.7–11.6 µm (av. 9.6 µm, SD 0.7 µm) × 5.0–6.4 µm (av. 5.6 µm, SD 0.3 µm); Q = 1.5–2.0 (av. 1.7, SD 0.1) (n = 120 of 3 coll.); smooth, (sub)amygdaloid, sometimes with faint suprahilar depression, apex (sub)obtuse, with rather distinct pseudoporus. Basidia 25–31 × 7–9(–10) µm, generally 4-spored, but in some collections also 2-spored, and then spores up to 14 µm. Lamella edge composed of cheilocystidia and numerous colourless, mostly (sub)clavate or (sub)cylindrical, thin-walled paracystidia. Pleurocystidia 28–66 µm (av. 47 µm, SD 9.7 µm) × 10–18 µm (av. 14 µm, SD 2.0 µm); Q = 1.9–5.3 (av. 3.5, SD 0.8) (n = 45 of 3 coll.), mostly (sub)fusiform, sometimes subutriform, rarely (sub)cylindrical or (sub)clavate, generally without neck and with short pedicel or truncate base, apex usually crystalliferous, walls up to 1.5(–2.0) µm thick near the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, sometimes more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4–11 µm wide, often but not always with finely encrusting and parietal brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia only near the apex of the stipe (30–)35–55 × 10–15(–17) µm, ventricose (sub)fusiform to (sub)clavate, apex usually crystalliferous, walls up to 1.5 µm thick near the apex, yellowish greenish with 3 % KOH. Clamp-connections abundant in all tissues.
Habitat & Distribution — Our own collections of I. filiana are from Austria, Finland and Germany. In all collections but one Pinus sylvestris was present in the vicinity, sometimes as the only tree nearby. The only collection with Salix instead of Pinus in the neighbourhood was one from the subalpine zone in Austria. It cannot be excluded that small Pinus mugo bushes were present at some distance. Several collections were found on sandy soil. Apart from our own collections a sequence exists of a collection from Finland (as Inocybe sp., UDB022424) and some EcM-sequences, which may belong to this species. They are from Canada (KC840640 with Picea glauca; JX630538 and JX630539 with Dryas integrifolia) or the US (JX198534 with Alnus rhombifolia or Betula occidentalis).
Additional collections examined. Austria, Tirol, Großglockner, Zoderer Kaser, ÖK25V 3227-West, alt. c. 1900 m, Salix sp., 18 Aug. 2011, D. Bandini & B. Oertel (DB18-8-11-12). – Finland, Koillismaa, Kuusamo municipality, Oulanka National Park, near Research Station, alt. c. 170 m, Pinus sylvestris, Picea abies, Betula sp., 18 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB18-8-15-2); Koillismaa, Kuusamo municipality, Oulanka National Park, Ampumavaara, Pinus sylvestris, Picea abies, Betula sp., 18 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB18-8-15-11); Ibidem, at some distance from former location, Pinus sylvestris, Salix sp., Alnus incana, Betula sp., Picea abies, 19 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB19-8-15-10); Ibidem, at some distance from former location, Pinus sylvestris, Betula sp., Picea abies, 20 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB20-8-15-5); Ibidem, at some distance from former location, Pinus sylvestris, Betula sp., Picea abies, 22 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB22-8-15-2). – Germany, Hessen, Bergstrasse, Viernheim, Viernheimer Heide, near Glockenbuckel, TK25 6417/1, alt. 97 m, sandy soil with Pinus sylvestris, 28 Oct. 2016, D. Bandini & B. Oertel (STU SMNS-STU-F-0901604, DB28-10-16-10); Ibidem, at some distance from former location, alt. 100 m, sandy soil with Pinus sylvestris, 31 Oct. 2016, D. Bandini & B. Oertel (DB31-10-16-5); Nordrhein-Westfalen, Viersen, Brüggen, near Brachter Wald, TK25 4702/2, alt. 42 m, sandy soil with Pinus sylvestris, 5 May 2016, D. Bandini (STU SMNS-STU-F-0901603, DB5-5-16-8); Ibidem, at some distance from former location, alt. 42 m, sandy soil with Pinus sylvestris, 5 May 2016, D. Bandini (DB5-5-16-9); Sachsen-Anhalt, Harz, Oberharz am Brocken, Kramershai, TK25 4229/4, alt. c. 680 m, wayside with Picea abies, 2 Oct. 2012, D. Bandini & B. Oertel (STU SMNS-STU-F-0901614, DB2-10-12-2).
Notes — Inocybe filiana is a very small species with an at least reddish tinged, but often redbrown smooth to (sub)squamulose-sublanose pileus surface, covered at first by a whitish velipellis. The stipe is often redbrown, too, and therefore often contrasting in colour with the at first whitish lamellae. The stipe is only pruinose near the apex and the hymenial cystidia are rather short on average and mostly neckless, while the spores often have a rather distinct pseudoporus. It could be mistaken for I. furfurea, the pilei of which may look similar to those of I. filiana, and the hymenial cystidia also may be rather short and neckless. The spores are much smaller than those of I. filiana and it usually grows in the vicinity of frondose trees, preferably with Quercus (Kühner 1955, Bandini et al. 2019b). Inocybe laurina can be found in the same habitat. It differs, however, e.g., by an only seldomly reddish tinged pileus, more abundant velipellis, paler stipe, on average narrower spores and long and narrow caulocystidia (Bandini et al. 2020a). Inocybe involuta, which often shows a similar colour contrast between stipe and lamellae, differs from I. filiana, as does I. nitidiuscula, e.g., by larger pilei and larger spores and hymenial cystidia (Britzelmayr 1891, Stangl 1983, 1989, Kuyper 1986, 1989, Marchetti et al. 2014, Bandini et al. 2020a, c). Inocybe astraiana and I. clandestina, both of which have the same habitat preferences as I. filiana, can be distinguished, e.g., by strongly paling pileus surface towards the margin with age, smaller spores on average and differently shaped caulocystidia (Bandini et al. 2020a, 2021b). Inocybe tarda has a darker, sometimes almost blackish brown pileus colour and clearly larger spores (Kühner 1955, and pers. observ.), while I. rufobrunnea (= I. exilis, see below), I. rufuloides (Bon 1984, Bandini et al. 2020c) and I. neorufula (Esteve-Raventós et al. 2012, Bandini et al. 2020c) may look rather similar to I. filiana, but the spores are much larger. This holds true also for the recently described I. distantifolia. The colour of I. robiginosa is more rusty brown and the spores are larger (Ludwig 2017, Eberhardt et al. in prep.). Because of the often rather distinct pseudoporus, in the past, I. filiana was probably often mistaken for I. subporospora, which name was recently shown to be synonymous with I. tjallingiorum (Bandini et al. 2021b). Inocybe tjallingiorum may be similar in aspect, but the stipe is entirely pruinose, though sometimes sparely in the lower half. Furthermore, the spores are smaller on average. We are not aware of any described species that is similar in ITS to I. filiana. Sequences from British Columbia (e.g., HQ604211and HQ604213), published as I. abjecta or I. auricoma, respectively, are 97–97.7 % similar to I. filiana. These sequences are likely from conspecifics, but we do not know the species. Sequences identified as or ascribed to I. filiana form a well-supported (96 % / 99 %) monophyletic group in Fig. 1.
Inocybe galactica Bandini & B. Oertel, sp. nov. — MycoBank MB 841149; Fig. 6
Fig. 6.

Inocybe galactica sp. nov. a. Holotype, in situ; b. collection DB14-9-19-14, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm
Etymology. Named ‘galactica’ because of its appearance reminding of a galaxy.
Typus. Germany, Bayern, Oberallgäu, Bad Hindelang, near Schattwald, TK25 8428/4, alt. 1200 m, Picea abies, 19 Sept. 2018, D. Bandini (holotype STU SMNS-STU-F-0901613; isotype priv. herb. D.B. DB19-9-18-11). GenBank ITS + LSU (partial) OK057196.
Diagnosis — The pilei of young basidiomata of I. galactica are covered by an arachnoid thick pale velipellis, and the hollow stipe is covered by a thick layer of whitish tomentum and is pruinose only near the extreme apex, the spores are smooth, measuring 8.2–11.6 µm (av. 9.9 µm) × 4.9–6.5 µm (av. 5.7 µm), and the hymenial cystidia are mostly rather long (sub)cylindrical to subfusiform, pleurocystidia measuring 50–80 µm (av. 65 µm) × 10–19 µm (av. 14 µm). It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. flocculosa.
Basidiomata mostly gregarious, rarely solitary. Pileus 10–25 mm wide, (sub)campanulate, later broadly convex or expanded, with low large or explicit (sub)papillate umbo, margin at first slightly inflexed, later decurved to straight; young basidiomata at first covered by a thick layer of arachnoid whitish velipellis; colour brownish to nut-brown (Mu 10YR 5/6–5/8; 7.5YR 5/4–5/8), at the centre sometimes darker; surface glabrous to innately fibrillose; young basidiomata with a whitish cortina. Lamellae subdistant (c. 30–40, l = 1–3), thickish, broadly adnate, with or without subdecurrent tooth, even to subventricose, at first dingy whitish with greyish hue, later pale greyish brownish to milkcoffee-brownish; edge sometimes uneven, fimbriate, whitish. Stipe 30–60 × 1–3 mm, cylindrical or curved, when young covered with abundant whitish arachnoid tomentum, later longitudinally striate or reticulate, flesh-coloured to pale brownish beneath the tomentum; pruinose near the apex of the stipe. Context whitish in pileus and stipe, stipe hollow. Smell indistinct. Colour of exsiccata pileus at the centre almost dark brown to sometimes almost blackish brown, outwards nut-brown to greyish brown or brown with reddish hue (Mu 7.5YR 5/4–5/6, 4/4–4/6), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 8.2–11.6 µm (av. 9.9 µm, SD 0.6 µm) × 4.9–6.5 µm (av. 5.7 µm, SD 0.3 µm); Q = 1.4–2.1 (av. 1.7, SD 0.1) (n = 120 of 3 coll.); smooth, (sub)amygdaloid, but sometimes shaped almost like turnips, often with more or less explicit suprahilar depression, apex subacute to (sub)obtuse. Basidia 25–31 × 7–10 µm, generally 4-spored, sometimes 2-spored. Lamella edge composed of cheilocystidia and numerous colourless, (sub)clavate, (sub)cylindrical thin-walled paracystidia. Pleurocystidia 50–80 µm (av. 65 µm, SD 7 µm) × 10–19 µm (av. 14 µm, SD 2 µm); Q = 3.6–5.8 (av. 4.7, SD 0.6) (n = 45 of 3 coll.), mostly (sub)cylindrical to (sub)fusiform, also (sub)utriform, seldom (sub)clavate, generally neckless and with short pedicel, sometimes with truncate base, at apex generally wide, sometimes appearing subcapitate because walls ending before the apex, apex usually crystalliferous, walls up to 2.0(–2.5) µm thick near the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in size, somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 5–10 µm wide, often but not always with finely encrusting and parietal brownish to ochraceous brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia only near the extreme apex of the stipe, 60–100 × 9–13 µm, mostly somewhat misshaped, long and narrow and often with undate walls, apex without crystals, walls up to 0.5(–1.0) µm thick near the apex. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe galactica seems to be a rare species. Besides our own few collections from Austria and Germany no other collections or sequences in databases are known to us. It was found in mountainous regions on cow pastures next to solitary trees of Picea abies on calcareous soil.
Additional collections examined. Austria, Tirol, Reutte, Tannheimer Tal, near Grän, ÖK25V 2214-Ost, alt. c. 1200 m, moist slope with Picea abies, 17 Sept. 2016, D. Bandini (DB17-9-16-13); Tirol, Imst, near Fernpass, ÖK25V 2221-West, c. 1250 m, Picea abies, 14 Sept. 2019, D. Bandini (STU SMNS-STU-F-0901620, DB14-9-19-14). – Germany, Bayern, Oberallgäu, Bad Hindelang, near Schattwald, at some distance from type collection, TK25 8428/4, alt. 1200 m, Picea abies, 19 Sept. 2018, D. Bandini (STU SMNS-STU-F-0901620, DB19-9-18-14).
Notes — Inocybe galactica is characterized by the thick layer of arachnoid velipellis covering the pileus of young basidiomata as well as the thick layer of tomentum which covers the stipe, which is hollow with age. The spores sometimes have the shape of turnips and the hymenial cystidia are rather long and often (sub)cylindrical in shape. The walls are sometimes ending before the apex, so the cystidia appear to be subcapitate. Young basidiomata of I. castorina may on first sight appear similar, however, the species occurs on moist terrain with Alnus and/or Salix, the hymenial cystidia on average are shorter and generally narrow (sub)utriform, and the spores are somewhat narrower on average (Bandini et al. 2020b). Inocybe flocculosa differs, e.g., by less smooth, up to (sub)squamulose pileus surface and much less velipellis as well as much smaller spores (Kuyper 1986, Stangl 1989, Ludwig 2017, and pers. observ.), while the velipellis of I. semifulva is not arachnoid, its stipe is often reddish near the apex, and its hymenial cystidia are generally not (sub)cylindrical (Grund & Stuntz 1981, Bandini 2014, and pers. observ.). Inocybe galactica shares the hollow stipe with I. favrei-cavipes, which is described with ‘fibrillum aranéeux’ (Favre 1955, description of I. cavipes). The spores of this species are larger on average and the hymenial cystidia usually are subfusiform (see above). Besides, I. favrei-cavipes was originally found with Salix herbacea in the alpine region. Inocybe griseovelata has a similar shape of hymenial cystidia as I. galactica, but the velipellis is less abundant, the stipe is not hollow, and not covered by arachnoid tomentum, and the spores on average are larger (Kühner 1955, Kuyper 1986, Stangl 1989, Bandini et al. 2021b, and pers. observ.), and the basidiomata of I. lechiana are smaller, the pilei are not covered by an arachnoid velipellis and the hymenial cystidia are much shorter (Bandini et al. 2020b). Inocybe costinitii which, according to Bizio et al. (2016) has an abundant velipellis, differs, e.g., by (sub)fusiform hymenial cystidia and caulocystidia without undate walls as well as the mediterranean habitat with Pinus halepensis (Bizio et al. 2016). In Fig. 1, I. galactica forms a well-supported (100 % / 100 %) clade. The phylogenetically closest, but by no means close, relative appears to be I. hotsoniana, which differs distinctly by spores with a length up to 17(–20.4) µm (Stuntz 1947).
Inocybe heterosemen Carteret & Reumaux, Bull. Soc. Mycol. France 127(1-2): 48. 2012 ‘2011’ — Fig. 17g
Description & Iconography — Carteret & Reumaux 2012.
Studied material. Isotype of I. heterosemen: France, Yvelines, forêt de Rambouillet, border of Étang d’ Or, Salix sp., 12 Sept. 1998, X. Carteret (isotype XC98.09.12.09). Spores 6.5–8.1 µm (av. 7.6 µm, SD 0.4 µm) × 3.5–4.8 µm (av. 4.2 µm, SD 0.4 µm); Q = 1.5–2.2 (av. 1.8 SD 0.2) (n = 40), smooth, narrow, mixed (sub)amygdaloid with oblong sublaceroid ones, with (sub)acute apex. Basidia 4-spored, but also 2-spored, and therefore many long, very narrow and sublaceroid spores up to 13.4 µm length are present. Pleurocystidia 29–49 µm (av. 38 µm, SD 5 µm) × 12–20 µm (av. 16 µm, SD 2 µm); Q = 1.9–3.0 (av. 2.4, SD 0.3) (n = 15), mostly (sub)clavate, (sub)fusiform to (sub)ellipsoid, with rounded or truncate base, apex usually crystalliferous, walls up to 2.5(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)cylindrical to (sub)clavate. Caulocystidia not studied, because stipe not present.
Additional collections examined. Estonia, Ida-Viru, Illuka commune, Puhatu Nature Reserve, Poruni primeval forest with Betula, Picea abies, Populus tremula, Alnus glutinosa, Salix, Quercus robur, 1 Oct. 2006, J. Vauras (DB1-10-6-Vauras-JV24488F). – Finland, Varsinais-Suomi, Houtskär commune, Hyppeis, Jungfruskär, Storlandet, Southwestern Archipelago National Park, open pastured, fairly moist forest with Betula pubescens, Populus tremula, Alnus glutinosa, 31 Aug. 1998, J. Vauras (14253) (GenBank JX258834, as I. cf. langei).
Notes — The type of I. heterosemen is quite extraordinary microscopically: very short and (sub)clavate to (sub)ellipsoid shaped hymenial cystidia, and small and subamygdaloid spores mixed with long, very narrow and sublaceroid ones, which are due to the many 2-spored basidia. We got a sequence of the isotype. A Finish collection we examined, with only small spores, of a size similar to the ones measured of the isotype, has a published sequence (JX258834, I. cf. langei). The spores of a collection of this species from Estonia, examined by us, were all small, too.
Inocybe langei is a species with very small spores, too, but the pileus colour is more yellow and the hymenial cystidia are narrower (Heim 1931, Kuyper 1986, Stangl 1989, Ferrari 2006). It is with 90 % similarity in the ITS only distantly genetically related to I. heterosemen (see Fig. 1). Inocybe ochroalba and I. subhirtella have a similar type of hymenial cystidia, but they are longer and both species have larger spores (Bruylants 1970, Stangl & Veselský 1975, Bon 1984, and pers. observ.).
Molecularly, the ITS of I. heterosemen differs by less than 2 % (counting gaps, which contribute more than half of the bp differences) from I. iseranensis. Inocybe iseranensis is in Fig. 1 paraphyletic in relation to I. heterosemen, the clade of the latter receiving very good support (99 % / 100 %). The only published sequence presumably belonging to I. iseranensis is from Sweden (FN550905, as I. cf. langei, Ryberg et al. 2010), indicating that I. heterosemen and I. iseranensis may have overlapping, if not the same, distribution.
Inocybe hirtella Bres., Fungi Trident. 1(4–5): 52. 1884 — Fig. 7, 17h
Fig. 7.

Inocybe hirtella. a. Epitype, in situ; b. collection DB12-10-11-2, in situ; c. cheilocystidia (coll. DB28-9-14-5); d. microscopic characters (epitype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (coll. DB3-10-14-3). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Heterotypic synonym. I. hirtella var. bispora Kuyper, Persoonia Supplement 3: 198. 1986.
Selected descriptions & Iconography — Bresadola 1881–1887, Kuyper 1986 (as I. hirtella var. bispora), Stangl 1989 (as I. hirtella var. bispora), Bizio & Marchetti 1998, Carteret & Reumaux 2017 (as I. hirtella var. bispora).
Typus. Italy, northern Italy, Bres., Fungi Trident., plate 58 1 f. 1884 (holotype).
Epitype (MBT 10002967): Germany, Niedersachsen, Emsland, Haselünne, TK25 3310/4, alt. 20 m, Corylus avellana, Quercus robur, Pinus sylvestris, 4 Oct. 2020, D. Bandini (epitype designated here STU SMNS-STU-F-0901607, double priv. herb. D.B. DB4-10-20-19). GenBank ITS + LSU (partial) OK057200.
Basidiomata mostly gregarious, seldom solitary. Pileus 10–50 mm wide, at first (sub)conical or (sub)campanulate, later broadly convex or expanded, without or with rather low large umbo, margin slightly incurved or decurved when young, later straight or even uplifted, and then pileus depressed around the umbo; young basidiomata with faint and often fugitive whitish remnants of a velipellis; colour yellowish, yellow, yellow-ochraceous, ochraceous to pale ochraceous brownish (Mu 10YR 8/4–8/6, 7/4–7/8, 6/8; 7.5YR 8/6, 7/2–7/8); surface at first glabrous to minutely tomentose, later generally thickly tomentose to innately fibrillose, with age excoriate to subsquamulose, sometimes also sublanose; no remnants of a cortina observed. Lamellae subdistant to moderately crowded (c. 25–45, l = 1–3), almost free to adnate, (sub)ventricose, at first whitish, then ivory, pale straw, to pale ochraceous greyish or yellowish brownish; edge fimbriate, whitish. Stipe 20–65 × 2–5 mm, cylindrical or curved, sometimes with thickened or subbulbous base, when young covered with whitish tomentum, later glabrous, at first whitish, later pale straw-coloured, yellowish, near the apex often slightly pinkish; pruinose on the entire length of the stipe. Context whitish to pale straw-coloured in the pileus, whitish to yellowish in the stipe, sometimes faintly pinkish near the apex of the stipe. Smell lamellae or stipe in different intensity like bitter almonds, and sometimes subspermatic when cut. Colour of exsiccata pileus ochraceous brownish to brown(ish) (Mu 10YR 5/6–5/8, 4/6), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 9.8–12.6 µm (av. 11.1 µm, SD 0.6 µm) × 5.0–6.5 µm (av. 6.0 µm, SD 0.2 µm); Q = 1.6–2.1 (av. 1.9, SD 0.1) (n = 120 of 3 coll.), smooth, (sub)amygdaloid, often with more or less explicit suprahilar depression, apex subacute to often (sub)-papillate, mostly with rather distinct pseudoporus. Basidia 24–35 × 7–11 µm, usually 2-spored. Lamellae edges composed of cheilocystidia and numerous colourless, (sub)clavate, thin-walled paracystidia. Pleurocystidia 40–77 µm (av. 54 µm, SD 8 µm) × 10–20 µm (av. 14 µm, SD 2 µm); Q = 2.7–6.3 (av. 3.9, SD 0.7) (n = 45 of 3 coll.); mostly slenderly (sub)fusiform, also (sub)utriform or (sub)clavate, without or with only short neck, with short or longer pedicel, at apex generally wide, apex usually crystalliferous, walls up to 3.0(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 6–10 µm wide, with encrusting and parietal ochraceous to brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia on entire length of the stipe, 35–65 × 10–15 µm, mostly (sub)-fusiform, sometimes (sub)utriform, apex usually crystalliferous, walls up to 1.5 µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate to subglobose cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe hirtella is a very frequent species that can be found with frondose trees, often in association with Corylus avellana, but also with Quercus, Populus, Fagus and others in planar regions, and with conifers, especially in mountainous habitats. Our own collections are from Austria, Germany and the Netherlands. Sequences have been published from collections from Estonia (as I. hirtella, UDB015672), Finland (as I. hirtella var. bispora, UDB022366), Sweden (as I. hirtella var. bispora, AM882932.2), Switzerland (as I. cf. hirtella, MK028433) as well as several EcM-sequences, which likely belong to this species, such as for instance from Belgium (KR082189), Iran (UDB005503), Latvia (UDB026929, with Salix pentandra) or Poland (AY748868, with Salix caprea).
Selected additional collections examined. Austria, Tirol, Imst, near Fernpass, ÖK25V 2221-West, alt. c. 1250 m, Picea abies, 14 Sept. 2019, D. Bandini (STU SMNS-STU-F-0901645, DB14-9-19-12). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 370 m, Fagus sylvatica, Picea abies, Abies alba, 22 Aug. 2011, D. Bandini (DB22-8-11-1); Baden-Württemberg, Rhein-Neckar-Kreis, Wiesenbach, TK25 6619/1, alt. 170 m, Fagus sylvatica, 12 Oct. 2011, D. Bandini (KR-M-0038920, DB12-10-11-2); Ibidem, in some distance from former location, alt. 200 m, Corylus avellana, Fagus sylvatica, Betula pendula, 28 Sept. 2012, D. Bandini (DB28-9-12-3); Baden-Württemberg, Karlsruhe, TK25 6916/3, alt. 117 m, Carpinus betulus, Picea abies, 14 Sept. 2014, D. Bandini (DB14-9-14-16); Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 380 m, Picea abies, 28 Sept. 2014, D. Bandini (DB28-9-14-5); Baden-Württemberg, Rhein-Neckar-Kreis, Wiesenbach, TK25 6619/1, alt. 140 m, Quercus robur, 3 Oct. 2014, D. Bandini (DB3-10-14-3); Baden-Württemberg, Neckar-Odenwald-Kreis, Schwarzach, near Neunkirchen, TK25 6619/2, alt. 350 m, Fagus sylvatica, Tilia sp., 30 Sept. 2017, D. Bandini (DB30-9-17-2); Bayern, Dingolfing-Landau, Mamming, TK25 7341/2, alt. 350 m, Salix sp., Populus sp., 1 Oct. 2013, D. Bandini & B. Oertel (DB1-10-13-9); Bad Tölz-Wolfratshausen, Schlehdorf, TK25 8333/4, alt. c. 700 m, Corylus avellana, Fraxinus excelsior, Fagus sylvatica etc., 9 Sept. 2016, D. Bandini & J. Christan (DB9-9-16-9); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Pinus sylvestris, Corylus avellana, Betula pendula, 22 Oct. 2011, D. Bandini & B. Oertel (KR-M-0038022, DB22-10-11-12). – Netherlands, Drenthe, Ruinen, alt. 10 m, Quercus robur, 8 Oct. 2018, D. Bandini (DB8-10-18-23); Drenthe, Assen, alt. 10 m, Corylus avellana, Quercus robur, 3 Oct. 2019, D. Bandini (STU SMNS-STU-F-0901646, DB3-10-19-8).
Epitype of I. hirtella, Fig. 7c–e: Germany, Niedersachsen, Emsland, Haselünne, TK25 3310/4, alt. 20 m, Corylus avellana, Quercus robur, Pinus sylvestris, 4 Oct. 2020, D. Bandini (STU SMNS-STU-F-0901607, DB4-10-20-19): Spores 9.8–12.0 µm (av. 10.9 µm, SD 0.6 µm) × 5.1–6.4 µm (av. 6.0 µm, SD 0.2 µm); Q = 1.6–2.1 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)-amygdaloid, with suprahilar depression, apex subacute to (sub)papillate. Basidia mostly 2-spored. Pleurocystidia 40–63 µm (av. 49 µm, SD 6 µm) × 11–15 µm (av. 13 µm, SD 1 µm); Q = 3.1–4.6 (av. 3.9, SD 0.5) (n = 15), mostly (sub)fusiform, also (sub)utriform, apex usually crystalliferous, walls up to 2.5(–3.0) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate. Caulocystidia on entire length of the stipe, in size and shape similar to hymenial cystidia, but walls only up to 1.5 µm thick at the apex.
Holotype of I. hirtella var. bispora, Fig. 17h: Netherlands, Leiden, Botanical Garden, on black soil among ferns, 11 Sept. 1961, C. Bas (holotype L-0053535). Spores 9.9–12.8 µm (av. 10.9 µm, SD 0.7 µm) × 5.3–7.0 µm (av. 6.0 µm, SD 0.3 µm); Q = 1.6–2.0 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)-amygdaloid, with more or less explicit suprahilar depression, apex subacute to (sub)papillate. Basidia mostly 2-spored. Pleurocystidia 43–69 µm (av. 54 µm, SD 6 µm) × 12–17 µm (av. 14 µm, SD 2 µm); Q = 3.1–4.6 (av. 3.8, SD 0.5) (n = 15), mostly (sub)fusiform, apex usually crystalliferous, walls up to 3.0(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate to subcylindrical. Caulocystidia not studied (to preserve the material).
Notes — Inocybe hirtella is characterized by a yellowish to ochraceous brownish pileus colour, smooth to (sub)squamulose pileus surface, an evanescent whitish velipellis, an entirely pruinose stipe, a more or less intense smell of bitter almonds and 2-spored basidia with spores that on average are about 11 µm long. The hymenial cystidia are mostly (sub)fusiform. The species is thus quite easy to recognize, and if all characters listed above are taken into account, not to be mistaken for any other species with entirely pruinose stipe.
Inocybe hirtella was first described by Bresadola (1881–1887), and the reproduction of a good watercolour drawing is referred to in the description (tab. LVIII, f. 1; for a detailed and comprehensive ‘history’ of I. hirtella see Bizio & Marchetti 1998). The pileus is yellowish to yellow-ochraceous, the centre of the pileus is glabrous and towards the margin minutely subsquamulose. The stipe is yellowish, the base even to minutely thickened, the cystide which is depicted, is fusiform and the basidium is 2-spored. The smell is described as ‘levi amygdalino’, while the spore size is indicated as 10–12 × 6 µm. Furthermore, Bresadola declared that the species grew in pastures with Corylus. According to M. Floriani and C. Bonomi of the Museo delle Scienze in Trento (e-mail 12 Oct. 2020), no type material of I. hirtella or any other collections of I. hirtella determined by Bresadola are extant (see also Kuyper 1986), and in the Swedish Herbarium of Natural History (S) only two collections of Bresadola of later years exist. Every detail of the original description including the watercolour fits very well with the description given above for the epitype, including the fact that the species often is associated with Corylus. We are confident that we recognize the species and thus decided to choose this epitype.
Kuyper (1986) distinguished two varieties of I. hirtella, one with 2-spored basidia, as I. hirtella var. bispora, and one with 4-spored basidia, as I. hirtella var. hirtella. Kuyper wrote (see also Bizio & Marchetti 1998) that the species meant by Bresadola was 4-spored, arguing that it was “known from Italy and is generally somewhat more bright yellow than the two-spored variety”. The fact that Bresadola’s drawing unmistakably shows a 2-spored basidium, Kuyper explained with the statement “Bresadola was rather inaccurate in this respect” citing as proof two examples in the book of Bresadola. This, however, is not always the case, e.g., the plate of I. grata (Bresadola 1930: pl. 732, 1) shows both a 2-spored basidium and a 4-spored basidium. The 2-spored one has correctly larger sterigmata than the 4-spored basidium, which shows, that Bresadola took notice of this specific detail. A further point in which we do not agree with Kuyper, and which was not explained by him, is the spore size. While the size in Bresadola’s description is indicated as 10–12 µm, the species, which according to Kuyper (1986) is the hirtella sensu Bresadola, has a spore size of only 7.5–10.5 µm. In addition, we have several ‘yellow’ collections of the 2-spored I. hirtella (see Fig. 7b), and since the species occurs in Austria and Switzerland, it is quite possible that it occurs also in the mountainous regions of northern Italy. Therefore, we see no reason not to take Bresadolas’s description literally and assume that the so-called ‘2-spored variety’ is what Bresadola defines as I. hirtella.
Kühner (1955) distinguished two forms of I. hirtella, I. hirtella f. hirtella and I. hirtella f. bispora, and at the end of the description of the 2-spored form he explicitly referred to Bresadola’s plate (Kühner 1955). Other authors did not differentiate between forms or varieties of I. hirtella, but continued to regard I. hirtella as a single taxon (e.g., Dähnke 2001, Gminder 2010, Læssøe & Petersen 2019). References to I. hirtella var. hirtella in literature may refer to any of the four species which in fresh state may smell like almonds, i.e., I. morganae, I. mycenoides, I. somae or I. suryana.
The holotype of I. hirtella var. bispora formed a clade together with other collections of I. hirtella, including the collection we assign here as epitype of I. hirtella (clade support 100 % / 100 %). The ITS of the epitype of I. hirtella matches the sequence of the holotype of I. hirtella var. bispora. Sister clade of I. hirtella is the clade of I. suryana (see Fig. 1 and below). Inocybe hirtella is with 92 % similarity in the ITS only distantly related to I. suryana and I. morganae. No more closely related species are, to the best of our knowledge, described yet.
Inocybe hirtellarum (Fig. 18a), accepted name: Inocybe mycenoides
Fig. 18.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Inocybe ianthinopes Pancorbo, G. Muñoz & Esteve-Rav. Fungi Iberici 2: 15. 2022 — Fig. 8
Fig. 8.

Inocybe ianthinopes. a. Coll. STU SMNS-STU-F-0901623, in situ; b. collection DB14-7-12-4, in situ; c. cheilocystide (coll. DB14-7-12-4); d. microscopic characters (coll. STU SMNS-STU-F-0901623), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (coll. DB14-7-12-4). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Description & Iconography — Muñoz et al. 2022.
Basidiomata mostly gregarious, seldom solitary. Pileus 15–40 mm wide, at first conical, later subconical, broadly convex or expanded, usually with rather pronounced large umbo, but in some collections also with low large umbo, margin at first mostly decurved, sometimes slightly incurved, later straight or even uplifted, and then pileus depressed around the umbo; young basidiomata with sometimes abundant greyish often rather fugitive remnants of a velipellis giving the surface at first often an (almost) lanose aspect; colour brown to dark brown with or without reddish hue, also greyish brownish because of the velipellis (Mu 10YR 4/3–4/6, 3/3–3/6; 7.5YR 4/2–4/6, 3/2–3/4), with age paler towards the margin because of rubbed-off fibres; surface at first sometimes almost lanose because of the velipellis, but mostly soon and sometimes even when young finely tomentose to smooth or finely rim(ul)ose, rarely getting cracked or breaking up and then subsquamulose, with age typically fibres rubbed off at the border; young basidiomata with remnants of a (sometimes dingy) whitish cortina. Lamellae subdistant (c. (30–)35–45, l = 1–3), somewhat thickish, emarginate adnate, (sub)ventricose, at first whitish or beige, later pale greyish brownish to brownish; edge fimbriate, whitish to concolorous. Stipe 25–50 × 2–6 mm, often rather stout, often widening towards the base, when young thickly covered with whitish tomentum, later longitudinally striate, somewhat floccose or glabrous, near the apex when young more or less intense and brightly purplish blueish, later fading in colour, and then entirely pale brown to flesh-coloured or with pinkish violet tinge near the apex; pruinose only near the apex of the stipe. Context whitish in the pileus, purplish blueish in the stipe near the apex and in the cortex of the stipe at least in young basidiomata, otherwise dingy whitish. Smell spermatic, at least when cut. Colour of exsiccata pileus dark brown with reddish hue (Mu 5YR 3/3–3/4; 7.5YR 3/2–3/4), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 8.3–11.1 µm (av. 9.5 µm, SD 0.6 µm) × 4.4–6.3 µm (av. 5.3 µm, SD 0.3 µm); Q = 1.4–2.2 (av. 1.8, SD 0.1) (n = 120 of 3 coll.), smooth, (sub)amygdaloid, mostly oblong and with explicit suprahilar depression, apex subacute, subobtuse, sometimes (sub)papillate. Basidia 25–32 × 7–10(–11) µm, generally 4-spored. Lamellae edges composed of cheilocystidia and abundant colourless, (sub)clavate, thin-walled paracystidia. Pleurocystidia 39–68 µm (av. 56 µm, SD 7 µm) × 10–19 µm (av. 13 µm, SD 2 µm); Q = 2.6–6.3 (av. 4.3, SD 0.8) (n = 45 of 3 coll.), mostly slender (sub)utriform or (sub)-fusiform, sometimes sublageniform, often with rather long and undate neck, occasionally sometimes somewhat misshaped with very long necks, but in some collections also with rather short neck or even without neck, transition from bulge to neck sometimes clearly demarcated, mostly with short pedicel, at the apex generally wide, apex usually crystalliferous, walls up to 1.0(–1.5) µm thick at the apex, but usually rather thin, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4–11 µm wide, with encrusting and parietal dark to almost blackish brown pigment, subcutis with wider and paler to colourless elements. Caulocystidia only near the apex, 45–85(–90) × 10–15 µm, among (sub)lageniform, (sub)utriform or (sub)cylindrical cystidia always present very long and narrow cystidia with undate walls, usually with short pedicel, apex often crystalliferous, walls up to 0.5 (1.0) µm thick at the apex, pale yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate to subglobose cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe ianthinopes was found by us almost exclusively next to frondose trees, often including Carpinus or Salix on lawn in parklike terrain or in cemeteries, but also on rather humid ground next to rivers or lakes. It appears to prefer calcareous soil. All our collections are from Germany and none from mountainous regions; all collections are from low elevations, maximum 400.
Additional collections examined. Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 395 m, Carpinus betulus, 14 July 2012, D. Bandini (DB14-7-12-4); Baden-Württemberg, Rhein-Neckar-Kreis, Bammental, Hollmuth, TK25 6618/2, alt. 180 m, Salix sp., Fraxinus excelsior, Carpinus betulus, 29 Sept. 2012, D. Bandini (DB29-9-12-2); Baden-Württemberg, Rhein-Neckar-Kreis, St. Leon-Rot, TK25 6717/2, alt. 120 m, Salix sp., Pinus sylvestris, Corylus avellana, 21 May 2013, D. Bandini (STU SMNS-STU-F-0901610, DB21-5-13-2); Baden-Württemberg, Karlsruhe, Erzbergerstrasse, TK25 6916/3, alt. 118 m, Quercus petraea, Fagus sylvatica, Carpinus betulus, 1 June 2013, D. Bandini & B. Oertel (KR-M-0038134, DB1-6-13-7); Ibidem, at some distance from former location, 1 June 2013, D. Bandini & B. Oertel (KR-M-0038135, DB1-6-13-8); Baden-Württemberg, Heidelberg, Ehrenfriedhof, TK25 6618/1, alt. 290 m, Quercus robur, Fagus sylvatica, 1 Aug. 2014, D. Bandini (DB1-8-14-5); Ibidem, in some distance from former location, alt. 290 m, Quercus robur, 4 July 2021, X. Hielscher, D. Bandini & R. Bandini (DB4-7-21-4); Baden-Württemberg, Rhein-Neckar-Kreis, Reichartshausen, TK25 6619/2, alt. 285 m, Carpinus betulus, Fagus sylvatica, 2 July 2016, D. Bandini (DB2-7-16-2); Baden-Württemberg, Heidelberg, Johanneskirche, TK25 6518/3, alt. 117 m, Betula pendula, Picea omorica, 16 Aug. 2017, D. Bandini & B. Oertel (STU SMNS-STU-F-0901624, DB16-8-17-1); Baden-Württemberg, Rhein-Neckar-Kreis, Bammental, TK6618/4, alt. 215 m, Tilia sp., 16 Aug. 2017, D. Bandini (DB16-8-17-5); Bayern, Landshut, Wörth an der Isar, TK25 7340/3, alt. 365 m, Betula pendula, Populus sp., 2 Oct. 2013, D. Bandini, B. Oertel & L. Quecke (STU SMNS-STU-F-0901521, DB2-10-13-7); Bayern, Passau, near Vilshofen, TK25 7445/1, alt. 400 m, Fagus sylvatica, 16 Aug. 2014, A. Schwarz (DB16-8-14-24).
Notes — Inocybe ianthinopes is characterized by brown to dark brown pileus colours, often abundant greyish velipellis on pilei of young basidiomata, with age finely tomentose to glabrous or rim(ul)ose pileus surface, only near the apex pruinose stipe, which is at first purplish blueish below the lamellae. The smooth spores are mostly oblong with an explicit suprahilar depression, and the hymenial cystidia often have a long neck often with rather thin undate walls. The species grows with frondose trees and can be found in parks, cemeteries and on the banks of rivers and lakes etc. We used to mistake this species for I. pusio (e.g., Bandini et al. 2021b), but after having studied the type (Bandini et al. 2021b), we became aware of the error.
Muñoz et al. (2022) consider the presence of caulocystidia along different lengths of the stipe as a good character for differentiating species, i.e., caulocystidia only at the top of the stipe or on a quarter or third of its length. Based on our own experience, we hesitate to agree. There are species in which metuloid caulocystidia are always only located directly below the lamellae, so for instance I. lacera, but in many others this can vary greatly, even within one and the same collection. This is, in our experience, also the case in the group of the lilac-stiped species of which we have recently described quite a number (Bandini et al. 2021b).
Somewhat similar to I. ianthinopes in macroscopic and microscopic aspect is I. grammopodia (sensu Bizio & Castellan 2018), which forms fruit bodies next to Quercus in the same sort of habitat. However, the pileus colour usually is paler, the velipellis is less abundant and thus the surface of young basidiomata is not sublanose, the stipe is only pinkish near the apex when young and the spores are not as oblong, on average somewhat shorter and thus with a different Q-value (Malençon & Bertault 1970, Kuyper 1986, and pers. observ.). It should be noted that different interpretations exist of I. grammopodia. Muñoz et al. (2022) propose another species as I. grammopodia, without giving a detailed description or citing any sequence. Bizio & Castellan (2018) published a detailed type study; for the time being we continue to follow their interpretation of I. grammopodia.
Inocybe pusio has wider hymenial cystidia, the shape of which is mostly fusiform without neck (Karsten 1889, Bandini et al. 2021b). Species of the ‘cincinnata-group’, such as I. cincinnata, I. gaiana, I. obscuroides or I. tiburtina differ, e.g., by rougher pileus surface, and especially the typical narrow lanceolate thicker walled hymenial cystidia (to all of them see Bandini et al. 2021b). Inocybe lampetiana grows on acid boggy ground with Alnus. The hymenial cystidia often are (sub)cylindrical (Bandini et al. 2021b). Inocybe amethystina differs, e.g., by thicker walled hymenial cystidia, the necks of which are shorter and not undate (Kuyper 1986, Bandini et al. 2021b). Furthermore, the pileus is minutely appressed scaly at the centre and outwards fibrillose according to the original description (Kuyper 1986). Inocybe knautiana has an abundant whitish velipellis, the spores are on average longer and the hymenial cystidia, too, on average are longer and short-necked (Bandini et al. 2021b), while I. sitibunda has more lanceolate shaped hymenial cystidia and on average smaller spores as well as wider caulocystidia without undate walls (Bandini et al. 2021b). Inocybe griseolilacina and I. dryadiana differ, e.g., by a rougher pileus surface and much paler pileus colours, and I. griseolilacina also by subcapitate hymenial cystidia and smaller spores on average (Lange 1917, Kuyper 1986, Stangl 1989, Ferrari 2006, Bandini et al. 2021b).
In Fig. 1, the clade of I. ianthinopes is well supported (100 % / 100 %) and separated by long branches from other clades. We are not aware of any ITS molecularly similar taxa to I. ianthinopes. Inocybe neorufula is one of the closer relatives, but still only distantly related with 90 % identity in the ITS. The species has much larger spores and differs, e.g., also in not having a purplish blueish stipe near the apex (Esteve-Raventós et al. 2012, Bandini et al. 2020c).
Inocybe iseranensis E. Ferrari, Fungi Non Delineati, Raro vel Haud Perspecte et Explorate Descripti aut Definite Picti 54–55: 79. 2010 — Fig. 18b
Description & Iconography — Ferrari 2010.
Studied material. Holotype of I. iseranensis: France, Parc National de La Vanoise, zone of Col de l’ Iseran, alt. 2600 m, Salix herbacea, 1 Sept. 2007, E. Ferrari (holotype BOT-050501-TRgmb 00981). Spores 7.5–9.4 µm (av. 8.3 µm, SD 0.5 µm) × 4.7–5.7 µm (av. 5.0 µm, SD 0.2 µm); Q = 1.5–1.9 (av. 1.7, SD 0.1) (n = 40), smooth, (sub)amygdaloid to (sub)ellipsoid, with subacute to (sub)obtuse apex. Basidia 4-spored. Pleurocystidia 37–58 µm (av. 46 µm, SD 5 µm) × 14–18 µm (av. 16 µm, SD 1 µm); Q = 2.3–3.9 (av. 2.9, SD 0.4) (n = 15), (sub)cylindrical, subutriform, (sub)fusiform or subclavate, sometimes with rounded base, apex usually crystalliferous, walls up to 1.5(–2.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia only near the apex of the stipe, in shape and size similar to hymenial cystidia, intermixed with subclavate cauloparacystidia.
Additional collections examined. Finland, Enontekiön Lappi, Enontekiö municipality, Kilpisjärvi, at the main road close to the Biological Station, alt. 490 m, Betula pubescens subsp. czerepanovii, Salix sp., 12 Aug. 1990, J. Vauras (DB12-8-90-Vauras-4733F). – Norway, Troms, Storfjord commune, Skibotndaln, alt. 530 m, Betula nana, Betula pubescens subsp. czerepanovii, Salix sp., Pinguicula vulgaris, 22 Aug. 1995, J. Vauras (DB22-8-95-Vauras-10603F).
Notes — The holotype of I. iseranensis has been sequenced (see Fig. 1). The sequence matches with a sequence in GenBank from Sweden (as I. cf. langei, FN550905). The two nordic collections examined by us match the type sequence as well. We consider I. iseranensis a good, probably quite rare species, which is very similar to I. langei in microscopic respect, but differs, e.g., by larger spores on average. Judging from the little data that we have, I. iserensis is different from I. heterosemen, but the two species may well turn out to be hard to separate by ITS data (see under I. heterosemen and Fig. 1). For differences between the two species, see under I. heterosemen.
Inocybe langei R. Heim, Encyclop. Mycol., 1 Le Genre Inocybe (Paris): 335. 1931 — Fig. 19f
Fig. 19.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Heterotypic synonym. Inocybe sulfovirescens Poirier, Doc. Mycol. 31(no. 124): 3. 2002.
Selected descriptions & Iconography — Heim 1931, Kuyper 1986, Stangl 1989, Poirier 2002 (as I. sulfovirescens), Ferrari 2006, Ludwig 2017.
Studied material. Holotype of I. sulfovirescens: France, Forêt domaniale d’Orléans, massif de Lorris, près les Bordes, Loiret, G. Delandre et al. ex Association mycologique de Sully-sur-Loire, 22 Oct. 2000 (holotype G00127003). Spores 6.4–8.0 µm (av. 7.0 µm, SD 0.4 µm) × 3.8–5.0 µm (av. 4.4 µm, SD 0.3 µm); Q = 1.4–1.8 (av. 1.6, SD 0.1) (n = 40), smooth, (sub)-ellipsoid to (sub)amygdaloid, mostly without suprahilar depression, with (sub)-obtuse, seldom also subacute apex. Basidia 4-spored. Pleurocystidia 35–57 µm (av. 47 µm, SD 7 µm) × 9–15 µm (av. 12 µm, SD 2 µm); Q = 3.0–5.2 (av. 4.0, SD 0.6) (n = 15), (sub)utriform, (sub)fusiform, also (sub)cylindrical, mostly with rather short neck, usually with short, often with ‘loop-shaped’ rounded base, walls up to 3.0(–3.5) µm thick at the apex, (pale) yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia not studied (to preserve the material).
Selected additional collections examined. Austria, Oberösterreich, Braunau am Inn, Schalchen, Kobernausser Wald, ÖK25V 3328-Ost, alt. 550 m, Picea abies, Abies alba, Larix decidua, 18 Aug. 2014, D. Bandini (DB18-8-14-13). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 370 m, Picea abies, Betula pendula, Alnus glutinosa, 10 Aug. 2011, D. Bandini (DB10-8-11-1); Baden-Württemberg, Rhein-Neckar-Kreis, Sankt Leon-Rot, TK25 6717/2, alt. 110 m, Salix sp., Betula pendula, Picea abies, 31 Aug. 2014, D. Bandini (STU SMNS-STU-F-0900983, DB31-8-14-7); Baden-Württemberg, Karlsruhe, Kanalweg, TK25 6916/3, alt. 115 m, Quercus robur, 31 May 2016, D. Bandini (DB31-5-16-20); Baden-Württemberg, Neckar-Odenwald-Kreis, Waldbrunn, Katzenbuckel, TK25 6520/1, alt. 550 m, Fraxinus excelsior, Populus sp., Salix sp., 28 July 2017, D. Bandini (DB28-7-17-4); Baden-Württemberg, Rhein-Neckar-Kreis, Mauer, TK25 6618/4, alt. 155 m, Salix sp., Quercus robur, Populus sp., 29 July 2017, D. Bandini (DB29-7-17-3); Baden-Württemberg, Rhein-Neckar-Kreis, Leimen, Gauangelloch, TK25 6518/4, alt. 200 m, Tilia sp., 12 Aug. 2017, D. Bandini (DB12-8-17-3); Baden-Württemberg, Neckar-Odenwald-Kreis, Aglasterhausen, TK25 6619/2, alt. 340 m, Fagus sylvatica, Quercus robur, 29 Sept. 2017, D. Bandini (DB29-9-17-2); Brandenburg, Berlin, TK25 3445/4, Pinus sylvestris, 5 July 2012, P. & W. Eimann (KR-M-0038101, DB5-7-12-E1-Eimann); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Quercus robur, Populus sp., Corylus avellana, 26 July 2014, D. Bandini (DB26-7-14-4).
Notes — Inocybe langei is a species described by Heim (1931) without surviving original material; only an iconotype exists. The depiction of Heim and the holotype of I. langei (pl. XVI, 4) shows a somewhat yellow-orange pileus. This is the usual colour of I. langei, often even more striking, and the microdetails given by Heim are distinctive, too, especially the very small spores, the length of which on average does not exceed 7 µm and the small and slender (‘peu ventrue’) hymenial cystidia. The pileus surface often gets minutely squamulose, the lamellae often have a greyish hue and the stipe is pruinose near the apex, and sometimes also lower down (Heim 1931, Kuyper 1986, Stangl 1989, Ferrari 2006, and pers. observ.). It usually occurs with frondose trees (so also in Heim 1931). Even though no epitype has been chosen so far for I. langei, the species is well known and several sequences, matching our own sequences of the species, have been published under that name, e.g., from a collection from Estonia (UDB016069) and one from Finland (UDB022371) (Fig. 1).
One of the characteristics of I. langei, which we have observed in all of our own collections, but which, quite astonishingly, was not described or depicted in any description we know of, is that the base of at least some, often many hymenial cystidia is rounded to ‘loop-shaped’, something that is not commonly the case with fibre caps. Such cystidia we also found when examining the holotype of I. sulfovirescens, which we were not allowed to sequence. This type of cystidia in combination with the very small spores and the statements in the protologue concerning the colour and surface of the pileus as well as the habitat mostly with frondose trees (Poirier 2002), left no doubt that this species is conspecific with I. langei.
The clade of I. langei receives full support (100 % / 100 %) in Fig. 1; fully supported is also a sister relationship with the joint clade of I. heterosemen and I. iseranensis which is supported by 95 % /100 %. Here, morphological similarity and ML result agree.
Inocybe morganae Bandini, B. Oertel & U. Eberh., sp. nov. — MycoBank MB 841150; Fig. 9
Fig. 9.

Inocybe morganae sp. nov. a. Holotype, in situ; b. collection DB9-9-19-11, in situ; c. cheilocystide (coll. DB25-7-18-16); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘morganae’ after the mythical fairy Morgan Le Fay, who in all her transformations still remains the same, as is the case with the very variable I. morganae.
Typus. Austria, Tirol, Reutte, Biberwier, ÖK25V 2221-Ost, alt. 1120 m, Picea abies, 9 Sept. 2019, D. Bandini (holotype STU SMNS-STU-F-0901459; isotypes priv. herb. D.B. DB9-9-19-7, TUR-A 209508). GenBank ITS + LSU (partial) OK057143.
Diagnosis — Inocybe morganae has a yellow-ochraceous to ochraceous brownish pileus colour, often with an orange reddish hue and a smooth to minutely furfureous-squamulose pileus surface. The stipe is covered by a rough pruina mainly near the apex, and (very) sparely also down to and below the middle, the spores are smooth, measuring 8.6–11.2 µm (av. 9.7 µm) × 4.9–6.1 µm (av. 5.6 µm), and the hymenial cystidia are generally (sub)utriform or (sub)fusiform and on average rather short, pleurocystidia measuring 35–66 µm (av. 52 µm) × 10–27 µm (av. 16 µm). It smells somewhat sweetish or as bitter almonds and occurs on calcareous soil. It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. leochroma.
Basidiomata gregarious. Pileus 15–30 mm wide, at first (sub)-campanulate, later broadly convex to expanded, without or with only low large umbo, margin at first slightly incurved, later decurved, straight or even uplifted, and then pileus depressed around the umbo; young basidiomata with faint whitish remnants of a velipellis; colour yellow-ochraceous with orange-reddish hue, ochraceous brownish with or without faint reddish hue (Mu 7.5YR 7/6–7/8, 6/6–6/8; 10YR 6/8, 5/6–5/8); surface at first smooth and glabrous, then remaining glabrous or becoming minutely furfureous to minutely squamulose with very small squamules and sometimes at the centre getting cracked to areloate diffracted; young basidiomata with remnants of a whitish cortina. Lamellae moderately crowded (c. 40–50, l = 1–3), adnate to broadly adnate, even to subventricose, at first whitish, greyish whitish, then greyish pale ochraceous to ochraceous with greyish hue; edge fimbriate, whitish. Stipe 20–40 × 2–4 mm, cylindrical or curved, sometimes base slightly thickened, when young covered with whitish tomentum, later glabrous, at first dingy whitish, later pale ochraceous brownish to brownish, in parts sometimes also darker brown, and sometimes faintly reddish near the apex; roughly pruinose (like salt-grains) mainly near the apex, and (very) sparely down to and below the middle. Context whitish in the pileus and the stipe. Smell weakly sweetish, as bitter almonds, at least when cut. Colour of exsiccata pileus brown to dark brown with reddish hue (Mu 7/5YR 5/4–5/6, 4/4–4/6), lamellae and stipe concolorous or a little lighter in colour, stipes sometimes getting brownish to brown with age or on drying.
Spores 8.6–11.2 µm (av. 9.7 µm, SD 0.5 µm) × 4.9–6.1 µm (av. 5.6 µm, SD 0.2 µm); Q = 1.5–2.1 (av. 1.7, SD 0.1) (n = 120 of 3 coll.), smooth, (sub)amygdaloid, sometimes with faint suprahilar depression, apex subacute, occasionally (sub)papillate, in some collections with indistinct pseudoporus. Basidia 24–28 × 7–10 µm, generally 4-spored. Lamellae edges composed of cheilocystidia and numerous colourless, (sub)clavate, thin-walled paracystidia. Pleurocystidia 35–66 µm (av. 52 µm, SD 7 µm) × 10–27 µm (av. 16 µm, SD 4 µm); Q = 2.4–5.1 (av. 3.5, SD 0.7) (n = 45 of 3 coll.); generally (sub)utriform or (sub)-fusiform, seldom subclavate, often transition between bulge and neck clearly demarcated, at apex generally wide, usually with short neck and short pedicel, sometimes with truncate base, apex usually crystalliferous, walls up to 1.5(–2.0) µm thick at the apex, sometimes cap-like thickened at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 3–10 µm wide, with encrusting and parietal ochraceous brownish to brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia mainly near the apex, but (very) sparely also down to and below the middle of the stipe, 35–60 × 8–12 µm, (sub)utriform or (sub)cylindrical, without or with only short neck and often with truncate base, apex usually crystalliferous, walls up to 1.0 µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate, sometimes catenate cauloparacystidia.
Habitat & Distribution — Our collections of I. morganae are from lower to higher mountainous regions of Austria and Germany. In all four locations Picea abies was nearby. The collection from Germany was found next to a small rivulet in rather moist terrain, while the other collections were from sun exposed pastures with interspersed old Picea abies trees. No other collections or sequences of collections are known to us. In GenBank are some EcM-sequences that may belong to this species from China (e.g., JQ318613, with Quercus liaotungensis; LC203905, with Picea crassifolia) as well as some soil sequences from Estonia.
Additional collections examined. Austria, Tirol, Reutte, Biberwier, ÖK25V 2221-Ost, alt. 1120 m, Picea abies, 9 Sept. 2019, D. Bandini (DB9-9-19-7); Ibidem, at some distance from former location, alt. 1150 m, Picea abies, 9 Sept. 2019, D. Bandini (STU SMNS-STU-F-0901639, DB9-9-19-11); Tirol, Imst, near Fernpass, ÖK25V 2221-West, alt. c. 1250 m, Picea abies, 14 Sept. 2019, D. Bandini (STU SMNS-STU-F-0901640, DB14-9-19-11). – Germany, Bayern, Rottal-Inn, Aich near Simbach, TK25 7643/4, alt. 420 m, Picea abies, Fagus sylvatica, 25 July 2018, D. Bandini (STU SMNS-STU-F-0901608, DB25-7-18-16).
Notes — Inocybe morganae is rather variable in appearance, in one collection with smooth, in another with furfureous to minutely squamulose ochraceous, yellow-ochraceous to ochraceous brownish pileus surface. The stipe is covered mainly near the apex with rather rough pruina and only very sparely also below the middle. The (sub)utriform or (sub)-fusiform hymenial cystidia are with 52 µm on average rather short. The smell is weakly sweetish, like bitter almonds. This typical odour reminds of I. hirtella (see above). However, this species has usually 2-spored basidia and larger spores. Inocybe suryana (see below) also may smell like bitter almonds, but the pileus colour is paler without orange-reddish tinge, the spores are smaller and the hymenial cystidia are on average shorter. Inocybe somae (see below), having the same smell, is smaller and more fragile in stature, the pileus colour is paler, and the pileus surface is not becoming furfuraceous to minutely squamulose. Furthermore, the spores are somewhat longer and narrower on average, and the hymenial cystidia are somewhat narrower. Inocybe woglindeana may have a somewhat similar appearance, but this species has only near the apex pruinose stipes, entirely differently, often sac-shaped, hymenial cystidia and larger spores. It can be found on sandy or gravelly soil with Salix nearby (Bandini et al. 2020c). Inocybe leochroma also may look somewhat similar, and the pruina of the stipe is as well rather rough like salt grains and sometimes also very sparely below the middle of the stipe, the spores however are much smaller and the hymenial cystidia often are (sub)cylindrical (Bandini et al. 2019b). Inocybe pelargonium differs by an often sticky surface of pileus and also much smaller spores (Kuyper 1986, Stangl 1989, Bandini et al. 2019b). The latter is also true for I. langei, the hymenial cystidia of which are often rounded or ‘loop-shaped’ at the base (see above). Inocybe heterosemen also has smaller spores and shorter hymenial cystidia (see above). Inocybe ochroalba and I. subhirtella differ from I. morganae by mostly paler pileus colour and often (sub)clavate hymenial cystidia (see below). Inocybe hirtelloides differs, e.g., by smaller spores and hymenial cystidia as well as very long and narrow caulocystidia (Stangl & Veselský 1974, Bandini et al. 2019b). Inocybe catalaunica has abundant greyish velipellis, smaller spores and much longer hymenial cystidia. The sequences we have of I. morganae form a well-supported clade (96 % / 100 %). Trusting the identification of PBM 245 (Fig. 1; material not examined), the closest relative of I. morganae may be I. microteroxantha with 96 % similarity in the ITS. The latter named species differs, e.g., by smaller size of basidiomata, bicolorous aspect of pileus, faintly spermatic odour, and, to judge from the drawing of the microdetails given by Grund & Stuntz (1981), wider and thicker walled hymenial cystidia.
Inocybe mycenoides Kuyper, Persoonia, Suppl. 3: 210. 1986 — Fig. 18a, d
Heterotypic synonym. Inocybe hirtellarum Carteret & Reumaux, Bull. Soc. Mycol. France 131(1-2): 56. 2017 ‘2015’.
Selected descriptions & Iconography — Kuyper 1986, Carteret & Reumaux 2017 (as I. hirtellarum).
Studied material. Holotype of I. mycenoides, Fig. 18d: Netherlands, Utrecht Breukelen, Gunterstein, Tilia, 19 Oct. 1983, Th.W. Kuyper (L-0053538). Spores 8.4–10.9 µm (av. 9.4 µm, SD 0.5 µm) × 5.0–6.2 µm (av. 5.5 µm, SD 0.2 µm); Q = 1.5–1.9 (av. 1.7, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)conical apex. Basidia 4-spored. Pleurocystidia 33–56 µm (av. 43 µm, SD 7 µm) × 9–11 µm (av. 10 µm, SD 1 µm); Q = 3.1–5.6 (av. 4.2, SD 0.8) (n = 15), narrow (sub)fusiform to (sub)clavate, apex usually crystalliferous, walls up to 2.0(–2.5) µm thick at the apex, almost colourless to pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia not studied (to preserve the material).
Isotype of I. hirtellarum, Fig. 18a: France, Oise, Forêt de Coye, with frondose trees, 25 Sept. 1983, Michel (priv. herb. P. Reumaux PR-592). Spores 8.6–10.9 µm (av. 9.6 µm, SD 0.5 µm) × 5.1–6.1 µm (av. 5.5 µm, SD 0.2 µm); Q = 1.5–1.9 (av. 1.7, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)-conical apex. Basidia 4-spored. Pleurocystidia 35–56 µm (av. 45 µm, SD 5 µm) × 8–12 µm (av. 10 µm, SD 1 µm); Q = 3.8–5.6 (av. 4.5, SD 0.6) (n = 15), narrow (sub)fusiform, subcylindrical to (sub)clavate, apex usually crystalliferous, walls up to 1.5(–2.0) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)cylindrical to (sub)clavate. Caulocystidia not studied, because stipe not present.
Selected additional collections examined. Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Lobbach, Haag, TK25 6619/1, alt. 330 m, Fagus sylvatica, Picea abies, 11 Oct. 2015, D. Bandini (SMNS-STU-F-0900981; DB11-10 -15-4); Bayern, Oberallgäu, Bad Hindelang, Untergschwend, TK25 8428/2, alt. 1100 m, Picea abies, 15 Sept. 2018, D. Bandini (STU SMNS-STU-F-0901648, DB15-9-18-5); Bayern, Ostallgäu, Halblech, near Kenzenhütte, TK25 8431/1/3, alt. 1200 m, Picea abies, 17 Sept. 2018, D. Bandini (STU SMNS-STU-F-0901647, DB17-9-18-2).
Notes — Inocybe mycenoides is characterized, e.g., by yellow to ochraceous pileus colours, entirely pruinose stipes and especially by short and very narrow mainly (sub)fusiform to (sub)clavate hymenial cystidia. The smell of fresh basidiomata is sometimes more or less intense like bitter almonds (pers. observ.). The protologue of I. hirtellarum by Carteret & Reumaux (2017) fits very well with the one of I. mycenoides by Kuyper (1986), and the examination of the types showed that the measurements are almost identical. We were able to sequence the holotype of I. mycenoides but not the isotype of I. hirtellarum. Because of the striking macroscopic and microscopic similarities, we are of the opinion, that I. hirtellarum and I. mycenoides are conspecific. In Fig. 1, the clade of I. mycenoides, including a number of environmental sequences, receives only bootstrap support, no support through SH-like approximate likelihood ratio tests in relation to the clade of I. somae. RPB2 data from two sequences of each species support the distinction of the two taxa (5 positions cleanly separate the two species in RPB2), although the overall similarity of the RPB2 sequences is 99.5 %. The lack of support for I. mycenoides could still be related to the number of sequences in this clade with lacking (LSU and) RPB2 data.
Inocybe ochroalba Bruyl., Bull. Trimestriel Soc. Mycol. France 85(3): 345. 1970 ‘1969’ — Fig. 18f, 19c
Heterotypic synonym. Inocybe subalbidodisca Stangl & J. Veselský, Česká Mykol. 29(2): 66. 1975.
Selected descriptions & Iconography — Bruylants 1970, Stangl & Veselský 1975 (as I. subalbidodisca), Stangl 1989, Ferrari 2010, Ludwig 2017.
Studied material. Holotype of I. ochroalba, Fig. 18f: Belgium, Anvers, Deurne, Rivierenhof, Kalmthoutsche Steenweg towards Kappellenboch, Fagus sylvatica, 7 May 1944 [in the protologue is given the wrong date 23 Sept. 1967], M. Morren (BR5020184019648). Spores 7.8–9.3 µm (av. 8.4 µm, SD 0.5 µm) × 4.7–5.5 µm (av. 5.1 µm, SD 0.2 µm); Q = 1.6–1.9 (av. 1.6, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)acute apex. Basidia 4-spored, seldom also 2-spored, and therefore with some larger spores. Pleurocystidia 38–56 µm (av. 47 µm, SD 5 µm) × 13–19 µm (av. 16 µm, SD 2 µm); Q = 2.6–3.4 (av. 3.0, SD 0.2) (n = 15), mostly (sub)clavate to (sub)fusi-form, apex usually crystalliferous, walls up to 3.0(–3.5) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia subclavate. Caulocystidia not studied (to preserve the material).
Holotype of I. subalbidodisca, Fig. 19c:Germany, Bayern, Augsburg, Oberschönfeld, pathside with Picea abies, 3 Sept. 1970, J. Stangl (PRM-735116). Spores 7.5–9.1 µm (av. 8.2 µm, SD 0.4 µm) × 4.6–5.4 µm (av. 5.0 µm, SD 0.2 µm); Q = 1.5–1.9 (av. 1.7, SD 0.1) (n = 40), smooth, (sub)-amygdaloid, with (sub)acute to subobtuse apex. Basidia 4-spored. Pleurocystidia 35–66 µm (av. 47 µm, SD 8 µm) × 14–25 µm (av. 19 µm, SD 3 µm); Q = 1.9–3.7 (av. 2.5, SD 0.4) (n = 15), mostly (sub)clavate or broadly fusiform, apex usually crystalliferous, walls up to 3.0(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia clavate and sometimes slightly thick-walled. Caulocystidia not studied (to preserve the material).
Selected additional collections examined. Austria, Osttirol, Großglockner, Hexenküche, ÖK25V 3227-West, alt. c. 1900 m, Pinus mugo, Alnus viridis, Salix sp., 18 Aug. 2011, D. Bandini & B. Oertel (DB18-8-11-14). – Finland, Koillismaa, Kuusamo municipality, Oulanka National Park, near Research Station, Pinus sylvestris, Betula sp., Picea abies 17 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (STU SMNS-STU-F-0901631, DB17-8-15-8); Ibidem, in some distance from former location, Pinus sylvestris, Betula sp., 18 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (STU SMNS-STU-F-0901590, DB18-8-15-3); Koillismaa, Kuusamo municipality, Oulanka National Park, Ampumavaara, Pinus sylvestris, Betula sp., Picea abies, 19 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (SMNS-STU-F-0901591, DB19-8-15-12); Vorarlberg, Lech am Arlberg, Rüfikopf, ÖK25V 2225-Ost, alt. 2350 m, Salix sp., Dryas octopetala, Bistorta vivipara, 11 Aug 2021, D. Bandini (SMNS-STU-F-0901657, DB11-8-21-6). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Waldwimmersbach, TK25 6619/1, alt. 350 m, Fagus sylvatica, Picea abies, 7 Sept. 2014, D. Bandini (STU SMNS-STU-F-0901630, DB7-9-14-1); Baden-Württemberg, Freudenstadt, Baiersbronn, Wilder See, Hornisgrinde, TK25 7415/1, alt. 919 m, Picea omorika, 12 Sept. 2014, D. Bandini (DB12-9-14-14); Bayern, Neumarkt i.d. Oberpfalz, Hörmannsdorf, TK25 6736/3, alt. 560 m, Pinus sylvestris, Fagus sylvatica, Picea abies, 23 Aug. 2014, D. Bandini (STU SMNS-STU-F-0901642, DB23-8-14-9); Bayern, Ostallgäu, Füssen, Hopfen am See, Hopfener Wald, TK25 8330/3, alt. 870 m, Picea abies, Fagus sylvatica, Corylus avellana, 14 Oct. 2016, D. Bandini, B. Oertel & J. Christan (DB14-10-16-14); Hessen, Bergstraße, Viernheim, near Viernheimer Heide, TK25 6417/1, alt. 100 m, Pinus sylvestris, 8 Nov. 2014, D. Bandini (STU SMNS-STU-F-0901629, DB8-11-14-6); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Pinus sylvestris, Fagus sylvatica, 20 Nov. 2011, D. Bandini & B. Oertel (DB20-11-11-2). – Switzerland, Graubünden, Albulapass near La Punt, TK25 1237/3, alt. 2320 m, Dryas octopetala, Salix reticulata, 1 Sept. 2016, B. Oertel (DB1-9-16-3b, BAN1831).
Notes — The macroscopic and microscopic details in the protologues of I. ochroalba (Bruylants 1970) and I. subalbidodisca (Stangl & Veselský 1975) do not differ greatly from each other, nor do the drawings of spores and cystidia. Therefore, Kuyper (1986) had already synonymized the two species, having examined the holotype of I. subalbidodisca, but not the holotype of I. ochroalba. We here confirm this synonymization not only based on the morphological examination but also on sequences of both holotypes. The measurements of the spores and the hymenial cystidia are very similar and the ITS of both types is alike. However, we do not agree with the synonymization of I. subhirtella with I. ochroalba done by Kuyper (1986, see below, notes to I. subhirtella) in the same publication. In Fig. 1, the clades of I. subhirtella and I. ochroalba are reciprocally monophyletic sisters in Fig. 1 with 89 % / – support for I. ochroalba and 86 % / 99 % support for I. subhirtella. For three collections of I. subhirtella and five collections of I. ochroalba RPB2 data was available and used for the ML analysis (Fig. 1), unambiguously supporting the distinction of the two species in 21 positions (= 2.7 %) of which six positions include gaps in I. ochroalba and one position in I. subhirtella. As above, the number of sequences with lacking data is likely to be responsible for the weak support.
Inocybe othini Bandini & B. Oertel, sp. nov. — MycoBank MB 841152; Fig. 10
Fig. 10.

Inocybe othini sp. nov. a. Holotype, in situ; b. collection DB19-9-20-12, in situ; c. cheilocystide (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘othini’ after the Latin name for the norse god Odin, who is known as Magician since the species was found in a magical location.
Typus. Austria, Salzburg, Tamsweg, ÖK25V 3230-Ost, alt. 1500 m, moist terrain with Picea abies, 19 Sept. 2020, D. Bandini (holotype STU SMNS-STU-F-0901622; isotype priv. herb. D.B. DB19-9-20-28). GenBank ITS + LSU (partial) OK057180.
Diagnosis — Inocybe othini is a rather small species with dun coloured smooth to minutely innately fibrillose pileus surface, smooth spores near the apex of the stipe, measuring 8.1–10.5 µm (av. 9.2 µm) × 4.3–5.5 µm (av. 4.8 µm) and long mostly narrow (sub)lageniform or subcylindrical hymenial cystidia with rather thin walls, pleurocystidia measuring 51–82 µm (av. 68 µm) × 9–17 µm (av. 13 µm). It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. semifulva.
Basidiomata gregarious. Pileus 10–25 mm wide, at first sub-conical, later broadly convex or expanded, with rather pronounced large umbo, margin at first slightly decurved, later straight or even uplifted, and then pileus strongly depressed around the umbo; young basidiomata with faint remnants of a greyish velipellis; colour pale milkcoffee-brown, dun (Mu 10YR 6/4–6/6, 5/3–5/4); surface glabrous to tomentose or minutely fibrillose; young basidiomata with remnants of a pale cortina. Lamellae subdistant (c. 25–35, l = 1–3), broadly adnate with decurrent tooth, ventricose, at first dingy whitish, later pale ochraceous with or without faintly greyish hue; edge uneven fimbriate, whitish. Stipe 20–40 × 1–2 mm, cylindrical or curved, when young thickly covered with whitish tomentum, later longitudinally striate, pale flesh-coloured to pale wood-coloured; pruinose only near the apex of the stipe. Context beige in the pileus, whitish in the stipe, reddish in the cortex of the stipe. Smell spermatic, at least when cut. Colour of exsiccata pileus brown with reddish hue (Mu 10YR 4/3–4/4, 4/4), lamellae and stipe somewhat lighter in colour, no darkening or blackening on drying.
Spores 8.1–10.5 µm (av. 9.2 µm, SD 0.5 µm) × 4.3–5.5 µm (av. 4.8 µm, SD 0.3 µm); Q = 1.7–2.2 (av. 1.9, SD 0.1) (n = 80 of 2 coll.), smooth, (sub)amygdaloid, oblong, without or with only faint suprahilar depression, apex acute to subpapillate, with indistinct pseudoporus. Basidia 24–28 × 7–9 µm, generally 4-spored, seldom also 2-spored, and then spores up to 11.5 µm. Lamellaeedgescomposed of cheilocystidia and numerous colourless, catenate (sub)clavate, thin-walled paracystidia. Pleurocystidia 51–82 µm (av. 68 µm, SD 7 µm) × 9–17 µm (av. 13 µm, SD 2 µm); Q = 3.9–7.1 (av. 5.5, SD 0.1) (n = 30 of 2 coll.); mostly narrow (sub)lageniform or subcylindrical, also subfusiform, without explicit neck or with short or rather long, sometimes somewhat undate wide neck, at apex generally wide, with short pedicel or with truncate base, apex usually crystalliferous, walls up to 1.0(–1.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4–10 µm wide, with encrusting and parietal brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia only near the apex of the stipe, 50–80 × 10–13 µm, (sub)fusiform, (sub)-cylindrical, usually without neck, with short pedicel, with only small crystals, walls up to 1.0 µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with catenate cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Up to now I. othini is known only from two own collections from Austria, found in some distance from each other next to Picea abies in very moist location, next to a springlet. Apart from these, no other collections or sequences of collections are known to us from the databases. In GenBank and UNITE are sequences from soil samples from Germany (HF675425), and Estonia (e.g., UDB0190916) which may belong to this species. Inocybe othini seems to be quite rare.
Additional collection examined. Austria, Salzburg, Tamsweg, ÖK25V 3230-Ost, alt. 1490 m, moist terrain with Picea abies, 19 Sept. 2020, D. Bandini (DB19-9-20-12).
Notes— Inocybe othini is characterized by rather small size, pale coffee-brown pileus colour, smooth to minutely innately fibrillose pileus surface, only near the apex pruinose stipe, narrow spores and rather long and narrow (sub)lageniform or (sub)-cylindrical hymenial cystidia with wide necks. Macroscopically superficial similar is I. semifulva, the stipes of which species, however, often are reddish near the apex, the hymenial cystidia often are subcapitate and the species generally grows with Salix or Quercus on calcareous soil (Grund & Stuntz 1981, Bandini 2014, and pers. observ.). Inocybe flocculosa also may have sometimes such a pale appearance, but the pileus surface mostly is more lanose to (sub)squamulose, the spores are on average shorter, and the necks of the hymenial cystidia are narrower and more undate (Kuyper 1986, Stangl 1989, and pers. observ.). Inocybe griseovelata differs, e.g., by abundant velipellis, larger spores and longer and narrower caulocystidia (Kühner 1955, Bandini et al. 2021b). Inocybe castorina, too, has an abundant velipellis and on average larger spores. Besides it grows with Salix and/or Alnus on calcareous soil (Bandini et al. 2020a). Inocybe oetziana has coppery-reddish tinges in the pileus colour, a subhygrophanous pileus surface, on average larger spores and shorter hymenial cystidia (Bandini et al. 2021a). Inocybe gaiana has a dull clayish brown pileus colour which might remind of I. othini, but the surface is often more lanose to subsquamulose and the stipe is violaceous tinged at least near the apex of young basidiomata (Bandini et al. 2021b). Inocybe parvipileus differs from I. othini by on average longer spores, long and narrow caulocystidia and habitat on calcareous soil (Ludwig 2017, Eberhardt et al. in prep., and pers. observ.), while I. costinitii has an abundant whitish velipellis according to the original description and larger spores and grows in mediterranean region with Pinus (Bizio et al. 2016, Bandini et al. 2021b). In Fig. 1, the clade of I. othini, including two environmental sequences, receives full support (100 % / 100 %). We are not aware of any described species that is phylogenetically closely related to I. othinii.
Inocybe ovillaBandini & B. Oertel, sp. nov. — MycoBank MB 841153; Fig. 11
Fig. 11.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘ovilla’ (Latin ‘related to a sheep’) because of the colour of the pilei.
Typus. Germany, Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Quercus robur, Alnus glutinosa, Corylus avellana, Pinus sylvestris, 6 Sept. 2014, D. Bandini (STU SMNS-STU-F-0901600; isotype priv. herb. D.B. DB6-9-14-7). GenBank ITS + LSU (partial) OK057191.
Diagnosis — Inocybe ovilla is a small species with glabrous to finely tomentose beige to dingy straw-coloured pileus, entirely pruinose stipe, rather narrow spores, measuring 8.2–10.7 µm (av. 9.0 µm) × 4.1–5.4 µm (av. 4.7 µm) and often somewhat subcapitate, mostly (sub)utriform hymenial cystidia, pleurocystidia measuring 44–67 µm (av. 57 µm) × 11–18 µm (av. 14 µm). It can be recognized by the combination of the above characters and differs in its ITS sequence from the genetically and morphologically related I. sindonia.
Basidiomata gregarious or solitary. Pileus 10–20 mm wide, at first (sub)conical, later broadly subconical, with pronounced large umbo, margin at first slightly decurved or straight; young and older basidiomata with abundant remnants of a whitish velipellis; colour dingy whitish, beige, pale dingy straw-coloured with brownish hue (Mu 10YR 7/3–7/6, 5/3–5/6; 7.5YR 7/4, 6/4–6/6) surface glabrous to finely tomentose; young basidiomata with remnants of a whitish cortina. Lamellae moderately crowded (c. 45–60, l = 1–3), adnate, (sub)ventricose, at first whitish to whitish with greyish hue, later pale greyish brownish; edge fimbriate, whitish. Stipe 15–25 × 2 mm, cylindrical or curved, when young covered with fine whitish tomentum, later longitudinally striate or glabrous, at first whitish, later pale flesh coloured; at first densely pruinose on the entire length of the stipe, older basidiomata sometimes less densely pruinose in the lower half. Context whitish in the pileus and the stipe. Smell (sub)spermatic, at least when cut. Colour of exsiccata pileus greyish brownish (Mu 10YR 5/3–5/6), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 8.2–10.7 µm (av. 9.0 µm, SD 0.4 µm) × 4.1–5.4 µm (av. 4.7 µm, SD 0.2 µm); Q = 1.7–2.2 (av. 1.9, SD 0.1) (n = 80 of 2 coll.), smooth, oblong (sub)amygdaloid, often with explicit suprahilar depression, apex subacute to subpapillate, with indistinct pseudoporus. Basidia 24–28 × 7–9 µm, generally 4-spored. Lamellae edges composed of heilocystidia and numerous colourless, (sub)clavate or (sub)cylindrical, sometimes catenate thin-walled paracystidia. Pleurocystidia 44–67 µm (av. 57 µm, SD 7 µm) × 11–18 µm (av. 14 µm, SD 2 µm); Q = 3.1–5.6 (av. 4.2, SD 0.6) (n = 30 of 2 coll.); mostly (sub)utriform, also (sub)fusiform, without neck or with short neck, often more or less distinct subcapitate and sometimes with gelatinous cap, often roundish at the apex, with short pedicel, apex usually crystalliferous, walls up to 2.0(–3.0) µm thick at the apex, mostly quite uniformly wide near bulge and apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 5–10 µm wide, with faintly encrusting and parietal ochraceous pigment, subcutis with wider and paler to colourless elements. Caulocystidia on entire length, 40–70 × 10–15 µm, mostly (sub)fusiform or (sub)lageniform, sometimes subcapitate, with only short pedicel or with rounded base, apex usually crystalliferous, walls up to 1.5 µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)-clavate to subglobose cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution— Inocybe ovilla was found only twice by us in Germany, both times in rather humid terrain, one collection (the holotype) in a ditch, the other in a moist thicket with Salix bushes on calcareous ground. Apart of those, just one additional collection is known to us from the UNITE database from Estonia (as I. sindonia, UDB024767). There are some soil sample sequences from Estonia, too (for instance UDB086808), which may belong to this species. It seems to be rather rare.
Additional collection examined. Germany, Bayern, Weilheim-Schongau, Pähl, near Hartschimmelhof, TK25 8033/3, alt. c. 700 m, Salix sp., 14 Sept. 2016, D. Bandini, J. Christan & B. Oertel (STU SMNS-STU-F-0901601, DB14-9-16-5).
Notes — Inocybe ovilla is a rather small species characterized by beige to dingy straw-coloured smooth to finely tomentose pileus, entirely pruinose stipe, rather narrow spores and often subcapitate hymenial cystidia. It could be mistaken for I. sindonia, which species is also the most closely related species (Fig. 1). Inocybe sindonia differs, however, e.g., by larger basidiomata, a less glabrous, often sublanose to even subsquamulose or strongly fibrillose pileus surface, a near the apex very often more or less intense pinkish tinged stipe and generally narrow fusiform hymenial cystidia, which are not subcapitate (Kuyper 1986, Stangl 1989, Ludwig 2017, and see below). Furthermore, the smell of I. sindonia is very characteristic cheesy or farinaceous or like cucumber or radish, which is not the case with I. ovilla. Inocybe kuehneri and I. ochraceolutea are both conspecific with I. sindonia (see below). Inocybe sambucina also has narrow spores and a pale pileus colour. However, this species has much larger basidiomata, and entirely differently shaped, i.e., subclavate hymenial cystidia (Quélet 1872, Kuyper 1986, Stangl 1989, Ludwig 2017, and pers. observ.). Subclavate to clavate hymenial cystidia are also characteristic for I. ochroalba and I. subhirtella. The ITS of Inocybe sindonia is 97 % similar to that of I. ovilla (see Fig. 1) and the only phylogenetically closely related species of I. ovilla we know of (Fig. 1).
Inocybe pallidolutea Carteret & Reumaux,Bull. Soc. Mycol. France 131(1-2): 52. 2017 ‘2015’ — Fig. 18g
Description & Iconography — Carteret & Reumaux 2017.
Studied material. Isotype of I. pallidolutea: France, Ardennnes, Bois de Sommauthe, frondose trees, 28 Aug. 1983, P. Reumaux (XC2000-52). Spores 7.5–10.4 µm (av. 8.8 µm, SD 0.6 µm) × 4.3–6.0 µm (av. 5.0 µm, SD 0.3 µm); Q = 1.5–2.0 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)-acute apex. Basidia 4-spored. Pleurocystidia 49–79 µm (av. 58 µm, SD 8 µm) × 13–17 µm (av. 15 µm, SD 1 µm); Q = 3.1–4.7 (av. 3.9, SD 0.5) (n = 15), mostly (sub)fusiform, apex usually crystalliferous, walls up to 2.0(–2.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate. Caulocystidia not studied, because stipe not present.
Notes — Inocybe pallidolutea is a recently described species with pale ochraceous to yellowish (sub)squamulose pileus and entirely pruinose stipe, which is pinkish to reddish in the upper part according to the protologue. We could not obtain a sequence for the isotype. For the time being we consider it a good species of which apparently we have not found a collection of our own so far.
Inocybe pelargonium Kühner, Bull. Soc. Nat. Oyonnax 9 (Suppl. (Mém. hors sér. 1)): 5. 1955 — Fig. 19b
Heterotopic synonym. I. stangliana Kuyper, Persoonia, Suppl. 3: 213. 1986.
Selected descriptions & Iconography — Kuyper 1986 (as I. pelargonium and as I. stangliana), Stangl 1989, Breitenbach & Kränzlin 2000, Ferrari 2010, Ludwig 2017, Bandini et al. 2019b.
Studied material. Holotype of I. stangliana: Germany, Bayern, Augsburg, Siebentischwald, TK25 7631, Fagus, Fraxinus, 17 Aug. 1984, J. Stangl (L-0054130). Spores 6.0–9.2 µm (av. 7.3 µm, SD 0.7 µm) × 4.2–5.5 µm (av. 4.8 µm, SD 0.3 µm); Q = 1.2–1.8 (av. 1.5, SD 0.1) (n = 40), smooth, (sub)amygdaloid or (sub)ellipsoid, with (sub)obtuse to subconical apex. Basidia 4-spored. Pleurocystidia 39–52 µm (av. 45 µm, SD 4 µm) × 10–19 µm (av. 14 µm, SD 3 µm); Q = 2.7–4.7 (av. 3.3, SD 0.7) (n = 15), (sub)fusiform or subutriform, apex usually crystalliferous, walls up to 2.0(–3.0) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate. Caulocystidia not studied (to preserve the material).
Selected additional collections examined. France, Savoie, St. Bon - Tarentaise, Forêt du Praz, on earth strewn with needles, alt. 1600–1800 m, 5 Sept. 1927, R. Kühner [lectotype of I. pelargonium, designated by Poirier (2016), G00118409]. – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 380 m, Picea abies, Abies alba, Fagus sylvatica, 29 June 2012, D. Bandini (KR-M-0042334, DB29-6-12-7); Bayern, Oberallgäu, Bad Hindelang, Breitenberg, TK25 8429/3, alt. c. 1750 m, Picea abies, Pinus mugo, 11 Aug. 2014, D. Bandini (KR-M-0042363, DB11-8-14-21); Bayern, Weilheim-Schongau, Wessobrunn, Nature Reserve ‘Paterzeller Eibenwald’, TK25 8132/1, alt c. 700 m, Fagus sylvatica, Picea abies, Alnus incana, 12 Sept. 2016, D. Bandini & B. Oertel (KR-M-0042364, DB12-9-16-1); Bayern, Ostallgäu, Füssen, Hopfen am See, Hopfener Wald, TK25 8330/3, alt. 870 m, Picea abies, Fraxinus excelsior, Corylus avellana, 14 Oct. 2016, D. Bandini, B. Oertel & J. Christan (KR-M-0042365, DB14-10-16-5).
Notes — Kuyper (1986) described I. stangliana based on a single specimen only, given to him by Johann Stangl, who collected it in Germany, so Kuyper obviously did not see the species in fresh condition. The pileus colour was described as hazel brown, mixed with faint reddish tinges, the stipe as entirely pruinose and the smell as ‘faint, reminiscent of fruit’. The spores are very small and also the hymenial cystidia are rather short. Those ‘distinctive’ macro- and microscopic characters attributed to the impression of a new species. Upon re-examination of the type of I. stangliana, the same kind – in size and shape – of hymenial cystidia were observed in I. pelargonium, the lectotype of which we had examined (see Bandini et al. 2019b). The spores of the type of I. pelargonium and of our own collections of the species are on average somewhat longer (see Bandini et al. 2019b) than those of I. stangliana,as measured by Kuyper (1986) and confirmed by us. However, our impression is that the majority of the spores of the examined basidiome were not mature which often leads to unbalanced spore sizes and often untypically small spores. Inocybe pelargonium may have an ochraceous-brownish pileus colour, and the odour is ‘fruity’. So apart from the somewhat smaller spores, the description fits well to the one of I. pelargonium given in Bandini et al. (2019b). In the absence of sequence data from the lectotype of I. pelargonium, the ITS-sequence of the holotype of I. stangliana was compared to morphologically confirmed collections of I. pelargonium. The ITS of the holotype of I. stangliana matches that of our collections of I. pelargonium, confirming the morphological results. The respective clade (Fig. 1) received 99 % / 100 % support. We consider the two species as conspecific.
Inocybe proteica Bandini, B. Oertel & U. Eberh., sp. nov. — MycoBank MB 841154; Fig. 12
Fig. 12.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘proteica’ from the Greek water-god Proteus, known for his versatility, because of the highly variable appearance of the species.
Typus. Austria, Salzburg, Tamsweg, ÖK25V 3230-Ost, alt. 1460 m, very moist terrain with springlets, Picea abies, Petasites hybridus, 16 Sept. 2020, D. Bandini (holotype STU SMNS-STU-F-0901658; isotypes priv. herb. D.B. DB16-9-20-17; TUF135805). GenBank ITS + LSU (partial) OK057140.
Diagnosis — Inocybe proteica is characterized by small basidiomata, rather smooth to finely rim(ul)ose ochraceous brownish to reddish brown or even dark brown pileus, in the upper half pruinose stipe, smooth spores, measuring 7.8–10.7 µm (av. 9.5 µm) × 5.1–6.6 µm (av. 5.8 µm), and generally (sub)fusiform to (sub)utriform hymenial cystidia, pleurocystidia measuring 39–77 µm (av. 54 µm) × 10–22 µm (av. 15 µm) and (sub)utriform to (sub)-lageniform caulocystidia. It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. involuta or I. nitidiuscula.
Basidiomata gregarious or solitary. Pileus 10–20 mm wide, (sub)campanulate or subconical when young to broadly convex or expanded, without or with only low large umbo, seldom also with rather pronounced umbo, margin at first sometimes strongly involute or only decurved, later straight or even uplifted, and then depressed at the centre, sometimes deeply torn at the margin; young basidiomata with remnants of a dingy whitish velipellis; colour ochraceous brownish, reddish brown to dark brown with reddish hue (Mu 10YR 5/3–5/6, 4/4–4/6; 7.5YR 5/4–5/6, 4/4–4/6); surface glabrous when young, then finely rim(ul)ose towards the margin; young basidiomata with faint remnants of cortina. Lamellae moderately crowded (c. 40–60, l = 1–3), adnate, partially with subdecurrent tooth, even to subventricose, at first whitish to whitish greyish, later pale brown; edge fimbriate, whitish. Stipe 20–50 × 1–3 mm, cylindrical, base even, when young covered with whitish tomentum, later longitudinally striate or glabrous, at first whitish to ivory-coloured, later pale flesh-coloured; pruinose in the upper half of the stipe. Context whitish in the pileus, faintly flesh-coloured in the upper half or, though sparely, on entire length of the stipe. Smell subnull, subspermatic when cut. Colour of exsiccata pileus brown to dark brown without or with faint reddish tinge (Mu 10YR 4/3–4/4; 7.5YR 4/3–4/6), lamellae somewhat paler, stipe sometimes reddish brown, no blackening on drying.
Spores 7.8–10.7 µm (av. 9.5 µm, SD 0.3 µm) × 5.1–6.6 µm (av. 5.8 µm, SD 0.3 µm); Q = 1.4–2.1 (av. 1.7, SD 0.1) (n = 120 of 3 coll.); smooth, (sub)amygdaloid, sometimes broadly subellipsoid, sometimes with slight suprahilar depression, apex subacute, subobtuse to obtuse, with more or less distinct pseudo-porus. Basidia 25–30 × 7–9 µm, generally 4-spored, sometimes also 2-spored, and then spores up to 12.5 µm. Lamellaedgecomposed of cheilocystidia and numerous colourless, (sub)-clavate, thin-walled paracystidia. Pleurocystidia 39–77 µm (av. 54 µm, SD 9 µm) × 10–22 µm (av. 15 µm, SD 3 µm); Q = 2.2–7.0 (av. 3.7, SD 2.6) (n = 45 of 3 coll.), mostly (sub)fusiform, to (sub)utriform, also (sub)lageniform, generally without or with only short neck, at apex generally wide, usually with short pedicel, sometimes without pedicel and with truncate base, apex usually crystalliferous, walls up to 2.0(–3.0) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4.5–13 µm wide, with coarsely encrusting and parietal brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia in the upper half of the stipe, (35–)40–70 × 10–15 µm, (sub)-utriform or sublageniform, with short or longer neck, with short pedicel, apex crystalliferous or not, walls up to 2.5(–3.0) µm thick at the apex, pale yellowish greenish with 3 % KOH; intermixed with (sub)clavate cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution— Inocybe proteica was collected by us in Austria and Germany, always in very moist or somewhat moist terrain, with Picea abies nearby and on base rich soil. All collections were from elevations above 500 m, some from c. 1800 m. Apart from our own collections, we are not aware of any other collections or sequences. In GenBank and UNITE there is a (very short) EcM-sequence from China (LC204279, with Picea crassifolia) and one from Europe, without indication of the country (LS955034), which may belong to this species as well as numerous soil sample sequences from Estonia (e.g., UDB0310982, UDB0655937).
Additional collections examined. Austria, Tirol, Reutte, Tannheimer Tal, near Grän, ÖK25V 2214-Ost, alt. 1200 m, Picea abies, 16 Sept. 2016, D. Bandini (DB16-9-16-21); Tirol, Reutte, Rieden, ÖK25V 2215-West, alt. 860 m, moist terrain with Picea abies, Betula pendula, Salix sp., 12 Sept. 2018, D. Bandini (DB12-9-18-1). – Germany, Bayern, Berchtesgadener Land, Ramsau, Hintersee, TK25 8343/3, alt. 795 m, on moist border of a rivulet, Picea abies, Salix sp., 22 Aug. 2014, D. Bandini (DB22-8-14-11); Bayern, Weilheim-Schongau, Wessobrunn, Nature Reserve ‘Paterzeller Eibenwald’, TK25 8132/1, alt. c. 700 m, in a ditch with Picea abies, Fagus sylvatica, Alnus incana, 12 Sept. 2016, D. Bandini & B. Oertel (DB12-9-16-14); Bayern, Oberallgäu, Bad Hindelang, Breitenberg, TK25 8429/3, alt. c. 1800 m, very moist terrain near or almost in small spring-rivulet with Picea abies, Pinus mugo, 23 Sept. 2016, D. Bandini (DB23-9-16-18); Ibidem, in several hundred metres distance from former location, alt. c. 1670 m, with Picea abies, Pinus mugo, 23 Sept. 2016, D. Bandini (STU SMNS-STU-F-0901625, DB23-9-16-22); Bayern, Oberallgäu, Bad Hindelang, near Schattwald, TK25 8428/4, alt. 1200 m, Picea abies, 19 Sept. 2018, D. Bandini (STU SMNS-STU-F-0901617, DB19-9-18-12).
Notes — Inocybe proteica is a small species, which grows on humid ground in mountainous regions. The colour may vary from ochraceous brownish to dark brown with reddish hue. In some collections the spores are rather broadly subellipsoid, while in others subamygdaloid. Thus, it may on first sight be mistaken for several other species, such as I. nitidiuscula or I. involuta. Both, however, differ, e.g., by larger size of basidiomata, generally more reddish tinged pileus colours and by larger spores. Inocybe involuta furthermore shows a strong colour contrast between whitish lamellae and reddish stipe, and the hymenial cystidia of I. nitidiuscula are typically (sub)fusiform with long towards the apex narrowing necks (Britzelmayr 1891, Stangl 1983, 1989, Kuyper 1986, 1989, Marchetti et al. 2014, Bandini et al. 2020a, c, and pers. observ.). Inocybe perchtana differs, e.g., by abundant velipellis and reddening context, when bruised, as well as on average smaller spores (Bandini et al. 2020a). Inocybe alberichiana has a greyish velipellis, with age intensely ochraceous brownish lamellae and hymenial cystidia often with rather long and sometimes undate neck (Bandini et al. 2021b). Inocybe laurina differs, e.g., by its abundant whitish velipellis, on average longer hymenial cystidia, narrower caulocystidia and growth on dryer terrain with Pinus (Bandini et al. 2020a), while I. filiana (see above) has a paler velipellis and shorter hymenial cystidia and the stipe is pruinose only near the apex. Furthermore, the species occurs on dryer terrain, often with Pinus. Inocybe nemorosa shows no velipellis, the stipe is pruinose only near the apex and the hymenial cystidia on average are shorter than those of I. proteica (Heim 1931, Grund & Stuntz 1968, Bandini et al. 2019b). In terms of ITS, I. involuta and I. nitidiuscula are with 94 % similarity the closest to I. proteica. In Fig. 1, the clade of I. proteica receives 100 % / 100 % support.
Inocybe pseudoscabelliformis Carteret & Reumaux,Bull. Soc. Mycol. France 131(1-2): 70. 2017 ‘2015’ — Fig. 18h
Description & Iconography — Carteret & Reumaux 2017.
Studied material. Isotype of I. pseudoscabelliformis: France, Ardennes, Bois de Voncques, Picea abies, 28 Oct. 1978, P. Reumaux (XC2001-59). Spores 8.0–10.5 µm (av. 9.2 µm, SD 0.6 µm) × 4.5–5.6 µm (av. 5.1 µm, SD 0.2 µm); Q = 1.6–2.1 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)obtuse or subacute apex. Basidia 4-spored. Pleurocystidia 48–61 µm (av. 55 µm, SD 4 µm) × 11–19 µm (av. 14 µm, SD 2 µm); Q = 2.9–4.7 (av. 3.9, SD 0.6) (n = 15), mostly (sub)fusiform, also (sub)utriform or (sub)-lageniform, apex usually crystalliferous, walls up to 3.5(–4.5) µm thick at the apex, yellow(ish)-green(ish) with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate. Caulocystidia not studied, because stipe not present.
Additional collections examined. Denmark, Vegebjerg, Abies alba, 11 Oct. 2019, T. Læssøe (SMNS-STU-F-0901632, DB11-10-19-Læssøe). – Finland, Koillismaa, Kuusamo municipality, Oulanka National Park, near Research Station, Pinus sylvestris, Betula sp., Picea abies, 17 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB17-8-15-12). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Lobbach, Haag, TK25 6519/4, alt. 420 m, Picea abies, 30 Oct. 2015, D. Bandini (STU SMNS-STU-F-0901634, DB30-10-15-9); Baden-Württemberg, Rhein-Neckar-Kreis, Schwanheim, TK25 6519/4, alt. 400 m, Abies alba, Picea abies, 21 Oct. 2016, D. Bandini (DB21-10-16-1); Baden-Württemberg, Neckar-Odenwald-Kreis, Waldbrunn, Katzenbuckel, TK25 6520/1, alt. 560 m, Pseudotsuga menziesii, Salix sp., Carpinus betulus, 20 Oct. 2019, D. Bandini (STU SMNS-STU-F-0901635, DB20-10-19-1); Ibidem, at some distance from former location, alt. 560 m, Pseudotsuga menziesii, Salix sp., Carpinus betulus, 20 Oct. 2019, D. Bandini (STU SMNS-STU-F-0901636, DB20-10-19-4).
Notes — Inocybe pseudoscabelliformis was only recently described by Carteret & Reumaux (2017). The species is mor-phologically quite close and often difficult to distinguish from I. urceolicystis. However, it differs from the latter named species, e.g., by the less ventricose mostly (sub)fusiform hymenial cystidia with narrowing necks (Stangl & Vauras 1987, Oertel et al. 2014) and from I. adorabilis, e.g., by less bright pileus colours and less smooth pileus surface, by larger spores on average, and not urceoliform hymenial cystidia. Inocybe pseudoscabelliformis and I. urceolicystis are also challenging to differentiate molecularly. The only constant difference in the ITS of both species is an indel of 8 bp in the ITS1, insertion in I. pseudoscabelliformis and deletion in I. urceolicystis. Sequences of RPB2 (I. pseudoscabelliformis four, I. urceolicystis two) display constant differences between the two species in two positions (= 0.3 % difference). Unless these results were challenged by further results, the two species can be differentiated molecularly, too. Both species are sisters and monophyletic each, albeit sitting on very short branches, not resolved in Fig. 1.
Inocybe rufobrunnea J. Favre, Ergebn. Wiss. Untersuch.
Schweiz. Nationalparkes 5(33): 201. 1955 — Fig. 17e, 19a
Heterotypic synonym. I. exilis (Kuyper) Jacobsson & E. Larss., in Knudsen & Vesterholt, Funga Nordica, Agaricoid, Boletoid and Cyphelloid Genera (Gylling): 913 (2008), basionym I. rufuloides var. exilis Kuyper, Persoonia Supplement 3: 110. 1986.
Selected descriptions & Iconography — Favre 1955, Kuyper 1986 (as I. rufuloides var. exilis).
Studied material. Holotype of I. rufobrunnea, Fig. 19a: Switzerland, Parc National, Val Nüglia, near Fuorn, Grisons, alt. 2400 m, Dryas, 15 Aug. 1950, J. Favre (G00126153). Spores 11.0–14.8 µm (av. 12.6 µm, SD 1.0 µm) × 6.6–8.5 µm (av. 7.1 µm, SD 0.1 µm); Q = 1.5–2.0 (av. 1.8, SD 0.1) (n = 40), smooth, oblong (sub)amygdaloid, with (sub)obtuse to (sub)papillate apex. Basidia 4-spored and 2-spored. Pleurocystidia 44–68 µm (av. 57 µm, SD 7 µm) × 14–22 µm (av. 17 µm, SD 3 µm); Q = 2.2–4.0 (av. 3.3, SD 0.6) (n = 15), mostly (sub)fusiform or (sub)utriform, intermixed with rather short broadly fusiform to subclavate cystidia, apex usually crystalliferous, walls up to 2.0 µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)clavate. Caulocystidia not studied (to preserve the material).
Holotype of I. exilis,Fig. 17e:Netherlands, Friesland, Lauwersmeer, Marnewaard, with frondose trees and Salix repens in calcareous dune-sand, 24 Oct. 1984, Th.W. Kuyper (L-0053539). Spores 11.0–15.1 µm (av. 12.5 µm, SD 1.1 µm) × 6.1–8.0 µm (av. 7.0 µm, SD 0.3 µm); Q = 1.6–2.2 (av. 1.8, SD 0.1) (n = 40), smooth, oblong (sub)amygdaloid, with (sub)obtuse to (sub)papillate apex. Basidia 4-spored, but sometimes also 2-spored. Pleurocystidia 37–69 µm (av. 56 µm, SD 9 µm) × 12–21 µm (av. 17 µm, SD 3 µm); Q = 1.8–4.6 (av. 3.3, SD 0.8) (n = 15), (sub)fusiform or (sub)utriform, intermixed with short broadly fusiform to subclavate cystidia, apex usually crystalliferous, walls up to 1.5(–2.0) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia subclavate. Caulocystidia present only near the apex of the stipe, (sub)-utriform, subcylindrical or subfusiform, in size similar to hymenial cystidia.
Additional collections examined. Austria, Tirol, Reutte, Rieden, ÖK25V 2215-West, alt. 890 m, Salix sp., Pinus sylvestris, 21 Sept. 2018, D. Bandini (DB21-9-18-15); Tirol, Reutte, Weißenbach am Lech, border of river Lech, ÖK25V 2215-West, alt. 870 m, Salix sp., Alnus sp., 28 Sept. 2015, D. Bandini (STU SMNS-STU-F-0901441, DB28-9-15-16). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Sandhausen, TK25 6617/4, alt. 100 m, Pinus sylvestris, Quercus robur, Helianthemum, 13 Oct. 2012, D. Bandini, B. Oertel & W. Winterhoff (SMNS-STU-F-0901442, DB13-10-12-4); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Rheinauen, Altrip, TK 6515/4, alt. 95 m, Populus sp., Betula pendula, Pinus sylvestris, Salix sp., 25 May 2013, D. Bandini & B. Oertel (DB25-5-13-11).
Notes — Inocybe exilis shows the same characteristic micro-scopic features as I. rufobrunnea,i.e., same size, large oblong spores often with subpapillate apex and (sub)utriform to (sub)-fusiform hymenial cystidia with wide neck, which are intermixed with short and broadly fusiform to subclavate cystidia with walls up to 2.0 µm thick (see also Favre 1955: f. 92 and Kuyper 1986: f. 74). The protologues of both species state the small size of basidiomata, the dark brown colour of pileus and the presence of a velipellis, as well as the reddish brown context of the stipe. We were able to obtain a sequence of the type of I. exilis, but we were not allowed to sequence the type of I. rufobrunnea. However, because of the above listed similarities we consider the two species as conspecific, whereby I. rufobrunnea is the older name. It is not unusual that an alpine species also occurs in lowland habitats, as is for instance the case with Boletus edulis (see Treindl & Leuchtmann 2019) and with several species of Inocybe, e.g., with I. johannae or I. aurea (pers. observ. of B.O. and D.B.). In Fig. 1, I. rufobrunnea receives full support (100 % / 100 %).
Inocybe sindonia (Fr.) P. Karst., Bidrag Kannedom Finlands Natur Folk 32: 465. 1879 — Fig. 13, 18c, e
Fig. 13.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Heterotypic synonyms. Inocybe clarkii (Berk. & Broome) Sacc., Syll. Fung. (Abellini) 5: 784. 1887. Inocybe kuehneri Stangl & J. Veselský, Česká Mykol. 28(4): 199. 1974. Inocybe ochraceolutea Carteret & Reumaux, Bull. Soc. Mycol. France 131(1-2): 51. 2017 ‘2015’.
Selected descriptions & Iconography — Stangl & Veselský 1974 (as I. kuehneri), Kuyper 1986, Stangl 1989, Ferrari 2006, Carteret & Reumaux 2017 (as I. ochraceolutea), Ludwig 2017, Matheny & Bougher 2017.
Basionym. Agaricus sindonius Fr., Epicrisis Systematis Mycologici: 176. 1838.
Typus. Lectotype selected here (MBT 10002971): lectotype location unknown, Battarra, Fungorum Agri Ariminensis Historia, plate 18 B. 1755.
Epitype designated here (MBT 10002972): Germany, Rheinland-Pfalz, Bad Kreuznach, Hennweiler, TK25 6110/4, alt. 150 m, Fagus sylvatica, 3 Nov. 2013, D. Bandini & J. Walter (epitype STU SMNS-STU-F-0901627, isoepitype priv. herb. D.B. DB3-11-13-1). GenBank ITS + LSU (partial) OK057164.
Basidiomata mostly gregarious. Pileus 15–60(–70) mm wide, at first (sub)conical, also subcampanulate, later broadly convex or expanded, without or with more or less pronounced large umbo, margin at first slightly incurved to decurved, later straight or even uplifted, and then pileus depressed around the umbo; young basidiomata with whitish remnants of a velipellis; colour from whitish, cream, ivory, pale straw, pale yellowish to ochraceous brownish (Mu 10YR 8/2–8/6, 7/4–7/8, 6/4–6/8); surface at first almost glabrous to minutely but densely felty, with age innately fibrillose to excoriate with appressed, often somewhat darker fibre bundles, sometimes also subsquarrose; young basidiomata with abundant remnants of a cortina, often pending from the margin. Lamellae moderately crowded to crowded (c. 40–60, l = 1–3), almost free to (emarginate) adnate, even to subventricose, at first whitish, then cream- to ivory- or straw-coloured, yellowish brownish to brownish or greyish brownish with age; edge fimbriate, whitish. Stipe 23–80(–100) × 2–5(–7) mm, cylindrical or curved, sometimes widening towards the base, base sometimes thickened to subbulbous, glabrous, at first whitish, later cream- or pale straw-coloured or pale yellowish to pale ochraceous, near the apex often slightly pinkish to pinkish; pruinose only in the upper part or on the entire length of the stipe. Context whitish in the pileus and mostly in the stipe, sometimes somewhat yellowish in the stipe. Smell usually rather intensely cheesy or farinaceous or like cucumber or radish. Colour of exsiccata pileus from silvery pale greyish to brownish (Mu 10YR 7/4–7/8, 6/4–6/8), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 7.5–10.1 µm (av. 8.6 µm, SD 0.5 µm) × 4.0–5.4 µm (av. 4.7 µm, SD 0.3 µm); Q = 1.6–2.1 (av. 1.8, SD 0.1) (n = 120 of 3 coll.), smooth, often slender oblong (sub)amygdaloid, without or with only faint suprahilar depression, apex (sub)acute. Basidia 24–36 × 7–10 µm, generally 4-spored. Lamellaeedgescomposed of cheilocystidia and numerous colourless, (sub)clavate or (sub)cylindrical, thin-walled paracystidia. Pleurocystidia 47–78 µm (av. 59 µm, SD 6 µm) × 9–16 µm (av. 13 µm, SD 2 µm); Q = 2.9–7.8 (av. 4.8, SD 1.0) (n = 45 of 3 coll.); mostly slender (sub)fusiform, also (sub)utriform, (sub)cylindrical or sublageniform, narrowing towards the apex, rarely with undate walls, usually with short pedicel, sometimes also without pedicel, apex usually crystalliferous, walls up to 3.0(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size and shape. Pileipellis constituted by an epicutis made up of parallel hyphae 6–12 µm wide, with encrusting and parietal ochraceous brownish to brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia only in the upper part or on entire length of the stipe, 40–85 × 8–20 µm, (sub)utriform or (sub)fusiform, apex usually crystalliferous, walls up to 1.5(–2.0) µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate to subglobose cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution— Inocybe sindonia is a very frequent species and can be found often with Picea abies, but also with Pinus sylvestris, Fagus sylvatica or other frondose trees, from summer until the end of the year, often occurring rather late in the year, from temperate regions at low elevations to subalpine or boreal regions. We gathered about 100 collections from Austria, Finland, Germany, the Netherlands, Spain and Switzerland. In GenBank and UNITE are many sequences of collections from other European countries, such as Estonia, Great Britain (as Inocybe sp., UDB001582), Italy (as I. sindonia, JF908199; see also Osmundson et al. 2013: t. S1), Scotland (as I. sindonia, JQ888173), Sweden (as I. sindonia, MH310763), as well as from Tunesia (as I. sindonia, KU973853), and as (presumably) introduced species from Argentina (as I. sindonia, MH930386, with Salix sp.), Australia (FN550901) and New Zealand (as I. sindonia, GQ267474, with Pinus radiata) (see Matheny & Bougher 2017). Furthermore, there are a number of EcM sequences and soil sample sequences, which may belong to this species.
Selected additional collections examined. Austria, Tirol, Reutte, Tannheimer Tal, near Grän, ÖK25V 2214-West, alt. 1200 m, Picea abies, 22 Sept. 2016, D. Bandini (DB22-9-16-3); Tirol, Reutte, Tannheimer Tal, near Tannheim, ÖK25V 2214-Ost, alt. c. 1250 m, Picea abies, Salix sp., 13 Oct. 2016, D. Bandini (DB13-10-16-31). – Finland, Koillismaa, Kuusamo municipality, Oulanka National Park, Ampumavaara, Pinus sylvestris, Betula, Picea abies, 25 Aug. 2015, D. Bandini, J. Vauras & B. Oertel (DB25-8-15-19). – Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Schönbrunn, TK25 6519/4, alt. 390 m, Picea abies, 21 Sept. 2014, D. Bandini (STU SMNS-STU-F-0901626, DB21-9-14-1); Baden-Württemberg, Heidelberg, Königstuhl, Posseltslust, TK25 6618/1, alt. 460 m, Picea abies, 25 Oct. 2014, D. Bandini (DB25-10-14-13); Bayern, Oberallgäu, Wertach, Jungholz, TK25 8428/2, alt. 1015 m, Fagus sylvatica, Picea abies, 14 Sept. 2018, D. Bandini (STU SMNS-STU-F-0901584, DB14-9-18-23); Bayern, Ostallgäu, Pfronten, Breitenberg, TK25 8429/3, alt. c. 1650 m, Picea abies, 16 Sept. 2018, D. Bandini (DB16-9-18-24); Nordrhein-Westfalen, Märkischer Kreis, Plettenberg, TK25 4813/1, alt. 270 m, Picea abies, Salix sp., Alnus glutinosa, Carpinus betulus, 6 Oct. 2018, D. Bandini (DB6-10-18-21); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Pinus sylvestris, Betula pendula, Quercus robur, 29 Oct. 2011, D. Bandini & B. Oertel (KR-M-0038268, DB29-10-11-6); Ibidem, at some distance from former location, alt. 110 m, Pinus sylvestris, Fagus sylvatica, 29 Oct. 2011, D. Bandini & B. Oertel (DB29-10-11-9). – Netherlands, Friesland, Ameland, Hollum, alt. 4 m, Pinus sylvestris, 4 Dec. 2012, D. Bandini (DB4-12-12-1).
Epitype of I. sindonia, Fig. 13a, d: Germany, Rheinland-Pfalz, Bad Kreuznach, Hennweiler, TK25 6110/4, alt. 1500 m, Fagus sylvatica,3 Nov. 2013, D. Bandini & J. Walter (STU SMNS-STU-F-0901627, DB3-11-13-1). Spores 7.6–9.7 µm (av. 8.7 µm, SD 0.5 µm) × 4.0–5.1 µm (av. 4.7 µm, SD 0.3 µm); Q 1.7–2.1 (av. 1.8, SD 0.1) (n = 40), smooth, slender oblong (sub)amygdaloid, with (sub)acute apex. Basidia 4-spored. Pleurocystidia 53–78 µm (av. 61 µm, SD 7 µm) × 9–12 µm (av. 11 µm, SD 1 µm); Q = 4.5–7.8 (av. 5.7, SD 0.8) (n = 15), mostly slender (sub)fusiform, also (sub)utriform, apex usually crystalliferous, walls up to 2.5(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)cylindrical to (sub)clavate. Caulocystidia on the entire length of the stipe, but sparely in the lower half, similar to hymenial cystidia, intermixed with (sub)clavate cauloparacystidia.
Holotype of I. kuehneri,Fig. 18c: Czech Republic, Moravia, Ostrava, Halda Hrabuvka, with frondose trees, Populus sp., Cornus sanguinea, 19 Sept. 1971, J. Veselský (PRM-710368). Spores 7.7–9.6 µm (av. 8.5 µm, SD 0.4 µm) × 4.3–5.1 µm (av. 4.6 µm, SD 0.2 µm); Q = 1.6–2.1 (av. 1.8, SD 0.1) (n = 40), smooth, slender oblong (sub)amygdaloid, with (sub)acute apex. Basidia 4-spored. Pleurocystidia 47–68 µm (av. 60 µm, SD 6 µm) × 11–14 µm (av. 13 µm, SD 1 µm); Q = 3.9–6.2 (av. 4.8, SD 0.6) (n = 15), mostly slender (sub)fusiform, apex usually crystalliferous, walls up to 3.0(–3.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)cylindrical to (sub)clavate. Caulocystidia examined only near the apex of the stipe, (sub)fusiform to (sub)-utriform, in size similar to hymenial cystidia, intermixed with (sub)clavate cauloparacystidia.
Isotype of I. ochraceolutea,Fig. 18e:France, Ardennes, Bois de Toges, with frondose trees, 15 Oct. 2000, P. Reumaux (XC2001-12). Spores 7.7–10.1 µm (av. 8.8 µm, SD 0.5 µm) × 4.1–5.1 µm (av. 4.7 µm, SD 0.2 µm); Q = 1.6–2.1 (av. 1.8, SD 0.1) (n = 40), smooth, slender oblong (sub)amygdaloid, with (sub)-acute apex. Basidia 4-spored. Pleurocystidia 50–70 µm (av. 59 µm, SD 5 µm) × 12–18 µm (av. 14 µm, SD 2 µm); Q = 3.4–5.8 (av. 4.3, SD 0.6) (n = 15), mostly (sub)fusiform, apex usually crystalliferous, walls up to 2.0(–2.5) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia (sub)cylindrical to (sub)clavate. Caulocystidia not studied, because stipe not present.
Notes — The curators of C and UPS confirmed by e-mail (1 Sept. 2021 and 6 Sept. 2021, respectively) that material selectable as lectotype of I. sindonia does not appear to exist in C or UPS; see also Kuyper (1986, notes on I. sindonia). In 1838, Fries’ concept of A. sindonius was possibly exclusively based on the literature, i.e., on the illustrations he cited and not on own collections. Of the two plates mentioned in the original description, see above and Sowerby (1803) pl. 365, the plate by Battarra (1755) is the more suitable lectotype; it is also the only plate mentioned by Streintz (1862) for the name A. sindonius. A water colour of I. sindonia, commissioned by Fries (1856, https://herbarium.nrm.se/specimens/S0674), cannot be used as type for I. sindonia,because it was carried out after the original description had been published. But it clearly shows that at least in later years Fries’ concept of the species coincided with the concept we have of the taxon today. The species is here represented by some of our collections (see also Bandini et al. 2021b).
Inocybe sindonia is characterized by rather small oblong and narrow spores and slender (sub)fusiform to subutriform hymenial cystidia (see, e.g., Kuyper 1986, Stangl 1989, Ferrari 2006, Ludwig 2017, and pers. observ.). The synonymy of I. clarkei with I. sindonia was discussed in Bandini et al. (2021b). Inocybe kuehneri was already synonymized with I. sindonia by Kuyper (1986). We sequenced and examined the holotype of I. kuehneri and confirm the conspecificity (see Fig. 1). The isotype of I. ochraceolutea, recently described by Carteret & Reumaux (2017), was examined and sequenced by us, too, and the protologue as well as the microscopic details fit very well to I. sindonia. Its ITS sequence matches sequences of I. sindonia (Fig. 1). Thus, we consider I. ochraceolutea and I. sindonia as conspecific. In Fig. 1, the types of the synonymized taxa are included in a well-supported (97 % / 100 %) species clade of I. sindonia collections.
Inocybe somae Bandini, B. Oertel & U. Eberh., sp. nov. — Myco-Bank MB 841155; Fig. 14
Fig. 14.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘somae’ after the Indian moon god Soma, because of the pale yellowish colour.
Typus. Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Mauer, NR ‘Sandgrube Grafenrain’, TK25 6618/4, alt. 66 m, Populus sp., Salix sp., 25 Oct. 2019, D. Bandini (holotype STU SMNS-STU-F-0901652; isotype priv. herb. D.B. DB25-10-19-4). GenBank ITS + LSU (partial) OK057148, RPB2 (partial) OK078901.
Diagnosis — Inocybe somae is a rather small and fragile species with pale yellowish to straw-coloured glabrous to innately fibrillose pileus, entirely pruinose stipe, smooth spores measuring 8.6–11.3 µm (av. 10.0 µm) × 4.9–6.1 µm (av. 5.4 µm), and mostly (sub)fusiform on average rather short hymenial cystidia, pleurocystidia measuring 40–69 µm (av. 52 µm) × 8–18 µm (av. 13 µm). It smells like bitter almonds. It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. suryana.
Basidiomata gregarious. Pileus 5–15 mm wide, at first (sub)-conical, later broadly convex or expanded, without or with only very low large umbo, margin at first decurved, later decurved to straight or even uplifted, and then pileus slightly depressed around the umbo; young basidiomata with faint remnants of a whitish velipellis, visible especially at the umbo of young basidiomata, but vanishing soon; colour pale and dingy yellowish to dull straw-coloured (Mu 10YR 8/4–8/6, 7/4–7/6, 6/4–6/6), at the centre often more intense in colour up to brownish and often with orange or orange reddish hue (Mu 10YR 6/6, 5/6–5/8; 7.5YR 6/6–6/8, 5/6–5/8), therefore often appearing bicoloured with age; surface at first smooth and glabrous, later minutely innately fibrillose towards the margin; no remnants of a cortina observed. Lamellae subdistant (c. 20–35, l = 1–3), almost free to rather broadly adnate with (sub)decurrent tooth, ventricose, at first creamy whitish or whitish greyish, later pale ochraceous with rusty tinge; edge fimbriate, whitish. Stipe 30–50 × 1–2 mm, cylindrical or curved, glabrous, at first whitish, later dingy whitish, pale straw-coloured to pale dingy yellowish; pruinose on the entire length of the stipe. Context whitish to watery yellowish in the pileus, at first whitish, later yellowish in different intensity near the apex of the stipe, dingy whitish below. Smell more or less intense as bitter almonds. Colour of exsiccata sometimes pileus brown at the centre and outwards paler up to straw-coloured, or entirely straw-coloured (Mu 10YR 4/4–4/6, 6/3–6/6), lamellae and stipe lighter in colour, no darkening or blackening on drying.
Spores 8.6–11.3 µm (av. 10.0 µm, SD 0.6 µm) × 4.9–6.1 µm (av. 5.4 µm, SD 0.3 µm); Q = 1.7–2.1 (av. 1.8, SD 0.1) (n = 120 of 3 coll.), smooth, oblong (sub)amygdaloid, (sub)ellipsoid, mostly without, sometimes with faint explicit suprahilar depression, apex subacute to (sub)obtuse. Basidia 23–31 × 7–10 µm, generally 4-spored. Lamellaeedgescomposed of cheilocystidia and numerous colourless, (sub)clavate, thin-walled paracystidia. Pleurocystidia 40–69 µm (av. 52 µm, SD 6 µm) × 8–18 µm (av. 13 µm, SD 2 µm); Q = 2.9–6.1 (av. 3.9, SD 0.7) (n = 45 of 3 coll.); mostly (sub)fusiform, also (sub)utriform, without neck, with short or longer neck and then often with somewhat undate walls, at the apex narrowing, with short pedicel, apex usually crystalliferous, walls up to 1.5(–2.0) µm thick at the apex, yellow-green with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 3–11 µm wide, with faintly encrusting and parietal yellowish pigment, subcutis with wider and paler to colourless elements. Caulocystidia on entire length of the stipe, 35–75 × 8–15 µm, mostly slender (sub)fusiform, sometimes (sub)utriform, usually without or with only short neck, sometimes with longer neck, and with short pedicel or truncate base, apex usually crystalliferous, walls up to 1.0 µm thick at the apex, yellow-green with 3 % KOH; intermixed with numerous (sub)clavate cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe somae was found in Germany with Populus and Salix in shady and somewhat moist sandpits on calcareous ground, where the jawbone of the Homo heidelbergensis was detected. No other collections or sequences of collections are known so far. In GenBank and UNITE are two EcM-sequences from Germany (HF675223, HF675227) and one from Iran (UDB005410), which appears to belong to this species.
Additional collection examined. Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Mauer, NR ‘Sandgrube Grafenrain’, TK25 6618/4, alt. 150 m, Salix sp., Populus sp., 23 Oct. 2020, D. Bandini (STU SMNS-STU-F-0901580, DB23-10-20-2); Ibidem, in some distance from former location, alt. 153 m, Salix sp., Populus sp. 14 Oct. 2021, D. & G. Bandini (DB14-10-21-1); Ibidem, in some distance from former location, alt. 150 m, Salix sp., Populus sp. 18 Oct. 2021, D. & G. Bandini (DB18-10-21-1).
Notes — Inocybe somae is characterized by a small and fragile habit, a rather pale yellowish to dingy straw-coloured glabrous to innately fibrillose pileus, often with an orange tinge at the centre, an entirely pruinose stipe and on average rather short, mostly (sub)fusiform hymenial cystidia. It smells like bitter almonds. Mainly because of the smell it might at first be mistaken for I. hirtella (see above) which differs, e.g., by larger size, more intense pileus colours, usually 2-spored basidia and larger spores. Inocybe suryana (see below), which can also smell like bitter almonds, differs, e.g., by generally less fragile habit, more intense pileus colours, reddish hue near the apex of the stipe, on average shorter spores and usually shorter caulocystidia. Inocybe morganae (see above) differs, e.g., by more intense or more ochraceous brownish, orange reddish tinged pileus colour, with age furfuraceous-(sub)squamulose pileus surface, and rough pruina (like salt grains) on the stipe. It furthermore grows with conifers. Inocybe leochroma and I. pelargonium have more intense pileus colours and much smaller spores (Kuyper 1986, Stangl 1989, Bandini et al. 2019b), and the smell of the basidiomata is different. Inocybe woglindeana, which can be found in a somewhat similar habitat with Salix and Populus, differs, e.g., by an only near the apex pruinose stipe, larger spores and entirely different, often sac-shaped hymenial cystidia (Bandini et al. 2020c). Inocybe ochroalba (see above) and I. subhirtella (see below) have a more velvety, tomentose to sublanose pileus surface, the colour is mostly more dingy beige or brownish, and the hymenial cystidia mostly are (sub)clavate. The ITS of I. somae is almost 99 % similar to that of I. mycenoides (but see above notes to I. mycenoides). This species, however, differs, e.g., by on average shorter spores, shorter and narrower hymenial cystidia and shorter caulocystidia. Furthermore, the smell like bitter almonds is missing (Kuyper 1986, and pers. observ.). It can be assumed that I. somae is a rather rare species, may be due to the special, in central Europe rare habitat in which it was found. In Fig. 1 and there based on three loci, the sequences of the German collections and the Iranian EcM sequence form a clade with 89 % / 98 % support which is sister to the clade of I. mycenoides (see also there).
Inocybe subalbidodisca (Fig. 19c), accepted name: Inocybe ochroalba
Inocybe subhirtellaBon,Doc. Mycol. 14(no. 53): 33. 1984 — Fig. 19d, e
Heterotypic synonym. Inocybe subrubens Carteret & Reumaux, Bull. Soc. Mycol. France 127(1-2): 50. 2012 ‘2011’.
Selected descriptions & Iconography — Bon 1984, Carteret & Reumaux 2012 (as I. subrubens).
Studied material. Holotype of I. subhirtella, Fig. 19d: France, Cayeux-sur-Mer, Somme, Bois de Brighton, Pinus, sandy ground, 15 Nov. 1979, M. Bon (LIP Bon-791115). Spores 8.1–10.1 µm (av. 9.0 µm, SD 0.5 µm) × 4.4–5.7 µm (av. 5.1 µm, SD 0.3 µm); Q = 1.5–2.0 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)acute apex. Basidia 4-spored, but also 2-spored, and then spores somewhat differently shaped, and up to 12.1 µm. Pleurocystidia 40–53 µm (av. 44 µm, SD 4 µm) × 9–18 µm (av. 14 µm, SD 2 µm); Q = 2.4–5.0 (av. 3.3, SD 0.7) (n = 15), mostly (sub)clavate to (sub)-fusiform, apex usually crystalliferous, walls up to 2.0(–3.5) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia down to the base of the stipe, (sub)clavate to subfusiform, somewhat longer than hymenial cystidia.
Isotype of I. subrubens, Fig. 19e: France, Ardennes, Bois de Guignicourt, 1 Nov. 1977, P. Reumaux (XC2000-41). Spores 7.7–9.7 µm (av. 8.8 µm, SD 0.4 µm) × 4.6–5.6 µm (av. 5.0 µm, SD 0.2 µm); Q = 1.6–1.9 (av. 1.8, SD 0.1) (n = 40), smooth, (sub)amygdaloid, with (sub)acute apex. Basidia 4-spored. Pleurocystidia 31–53 µm (av. 43 µm, SD 7 µm) × 11–21 µm (av. 15 µm, SD 3 µm); Q = 2.1–4.3 (av. 2.9, SD 0.6) (n = 15), mostly (sub)clavate to (sub)fusiform, apex usually crystalliferous, walls up to 2.0(–3.5) µm thick at the apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Paracystidia not observed. Caulocystidia not studied, because stipe not present.
Selected additional collections examined. Germany, Baden-Württemberg, Rhein-Neckar-Kreis, Lobbach, Haag, TK25 6519/4, Picea abies, 30 Oct. 2015, D. Bandini (STU SMNS-STU-F-0901588, DB30-10-15-7); Baden-Württemberg, Neckar-Odenwald-Kreis, Waldbrunn, Katzenbuckel, TK25 6520/1, alt. c. 495 m, calcareous soil with Salix sp., Populus sp., Pseudotsuga menziesii, 18 Aug. 2017, D. Bandini (SMNS-STU-F-0901586, DB18-8-17-9); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Pinus sylvestris, Fagus sylvatica, 29 Oct. 2011, D. Bandini & B. Oertel (KR-M-0038247, DB29-10-11-4); Ibidem, at some distance from former location, Tilia sp., Pinus sylvestris, Quercus robur, 22 Oct. 2015, D. Bandini (STU SMNS-STU-F-0901589, DB22-10-15-8). – Netherlands, Drenthe, near Stuifzand, alt. 20 m, Pinus sylvestris, Quercus robur, Betula sp., 30 Sept. 2019, D. Bandini (DB30-9-19-8).
Notes — Inocybe subhirtella is in several respects rather similar to I. ochroalba, in first line by the combination of the entirely pruinose stipe with the peculiar shape of the hymenial cystidia. However, we do not follow Kuyper (1986) in synonymizing the two species. Inocybe subhirtella differs in a number of morphological details and also molecularly from I. ochroalba: The pileus of young basidiomata is covered by a thick layer of velipellis, the pileus colour of all our own collections is more dull, often somewhat pale brownish, in contradiction to what Bon (1984) wrote in the protologue, where he described the colour as ‘ocre jaune assez vif’. The pileus surface of I. subhirtella is generally finer textured, glabrous to velvety (‘veloutée’, Bon 1984) or minutely but densely tomentose, and the spores are on average somewhat longer, and thus have a higher Q-value, whereas the hymenial cystidia often are more variable in shape and somewhat narrower than those of I. ochroalba. Besides the two species deviate genetically with 1.6 % in the ITS from each other.
There is no explicit description in the protologue of I. subrubens, since the intention of Carteret & Reumaux (2012) was apparently to elevate I. obscura var. rubens (Heim 1931) to species rank, but formally the Heim variety is not the basionym of I. subrubens. However, the drawing of the hymenial cystidia by Heim (1931: f. 169) shows a different kind of shape, and the spores are with a length of 9–13 µm much longer than those of I. subrubens (see above). We examined the isotype of I. subrubens morphologically and sequenced the ITS which matches with the sequence of I. subhirtella, as do the microscopic characters. We thus consider the two species as conspecific. The clade of I. subhirtella received 86 % / 99 % support; RPB2 data were included to improve the distinction from I. ochroalba (see also notes of I. ochroalba). Inocybe subhirtella seems to be less common than I. ochroalba and it seems to prefer calcareous soil.
Inocybe subrubens (Fig. 19e), accepted name: Inocybe subhirtella
Inocybe suryana Bandini & B. Oertel, sp. nov. — MycoBank MB 841156; Fig. 15
Fig. 15.

Inocybe ovilla sp. nov. a. Holotype, in situ; b. collection DB14-9-16-5, in situ; c. cheilocystidia (holotype); d. microscopic characters (holotype), Ca = Caulocystidia, Cpa = Cauloparacystidia, Ch = Cheilocystidia, Pa = Paracystidia, Pl = Pleurocystidia, Sp = Spores; e. spores (holotype). — Scale bars: a–b = 1 cm; c, e = 10 μm; d (Ca, Ch, Cpa, Pa, Pl) = 50 μm, d (Sp) = 10 μm.
Etymology. Named ‘suryana’ after the Indian sungod Surya, because of the yellow colour of the pileus.
Typus. Germany, Bayern, Dingolfing-Landau, Mamming, TK25 7341/2, alt. 345 m, Salix sp., Betula pendula, Populus sp., 30 Sept. 2013, D. Bandini & B. Oertel (holotype STU SMNS-STU-F-0901649; isotypes priv. herb. D.B. DB30-9-13-4, TUR-A 209510). GenBank ITS + LSU (partial) OK057149.
Diagnosis — Inocybe suryana has a yellowish to ochraceous pileus with generally smooth to innately fibrillose surface with faint whitish velipellis, entirely pruinose stipe, smooth spores, measuring 7.4–10.0 µm (av. 8.9 µm) × 4.5–6.2 µm (av. 5.5 µm), and mostly (sub)fusiform to (sub)utriform on average rather short hymenial cystidia, pleurocystidia measuring 33–63 µm (av. 48 µm) × 12–20 µm (av. 16 µm). It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. somae.
Basidiomata mostly gregarious. Pileus 10–25 mm wide, at first subcampanulate, soon broadly convex and then expanded, without or with only low umbo, margin at first decurved, later straight or even uplifted, and then pileus depressed around the umbo; very young basidiomata with a thin and fugitive layer of whitish remnants of a velipellis; colour generally pale yellowish, straw to ochraceous (Mu 10YR 7/6–7/8, 6/6–6/8), but sometimes with reddish tinge; surface at first glabrous to minutely tomentose, later tomentose to minutely innately fibrillose, sometimes also subrimose, rarely minutely subsquamulose around the disc, while the disc remains smooth or becomes areolate diffracted; no remnants of a cortina observed. Lamellae moderately crowded (c. 35–50, l = 1–3), adnate, (sub)ventri-cose, at first whitish or very pale yellowish, then pale greyish with yellowish hue to more intensely yellowish or pale yellow-ochraceous; edge fimbriate, whitish. Stipe 30–60 × 1–3 mm, cylindrical or curved, base sometimes subbulbous, when young covered with whitish tomentum, at first whitish to dingy whitish, later with pale ochraceous tinge, near the apex mostly with faintly reddish hue; pruinose on the entire length of the stipe. Context whitish in the pileus and the stipe. Smell either more or less intense like bitter almonds or subspermatic, at least when cut. Colour of exsiccata pileus brown with reddish or coppery hue (Mu 7/5YR 4/4–4/6; 5YR 4/4–4/6), lamellae and stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 7.4–10.0 µm (av. 8.9 µm, SD 0.5 µm) × 4.5–6.2 µm (av. 5.5 µm, SD 0.3 µm); Q = 1.4–1.8 (av. 1.6, SD 0.1) (n = 120 of 3 coll.), smooth, (sub)amygdaloid, without or with only rather faint suprahilar depression, apex (sub)acute. Basidia 24–28 × 7–10 µm, generally 4-spored. Lamellaeedgescomposed of cheilocystidia and numerous colourless, (sub)clavate, cylindrical or subglobose, thin-walled paracystidia. Pleurocystidia 33–63 µm (av. 48 µm, SD 7 µm) × 12–20 µm (av. 16 µm, SD 2 µm); Q = 2.2–4.7 (av. 3.1, SD 0.5) (n = 45 of 3 coll.); mostly (sub)fusiform to (sub)utriform, sometimes also (sub)lageniform, rarely (sub)clavate, without or with only short neck, usually with short or longer pedicel, but sometimes also without pedicel, at apex generally wide, apex usually crystalliferous, walls up to 2.0(–3.0) µm thick at the apex, yellowish greenish with 3 % KOH. Cheilocystidia similar in size, but somewhat more variable in shape. Pileipellis constituted by an epicutis made up of parallel hyphae 4–20 µm wide, with encrusting and parietal brownish pigment, subcutis with wider and paler to colourless elements. Caulocystidia on entire length of the stipe, 30–60 × 10–15 µm, (sub)fusiform to (sub)cylindrical, apex usually crystalliferous, walls up to 1.0 µm thick at the apex, yellowish greenish with 3 % KOH; intermixed with numerous (sub)clavate to subglobose cauloparacystidia. Clamp-connections abundant in all tissues.
Habitat & Distribution — Our own collections of I. suryana are from Austria and Germany. In most cases, only frondose trees were present, most often Corylus avellana was noted. Several collections were found next to a river on rather moist ground. Apart from these, no other collections or sequences of collections are known to us. In the UNITE database only one EcM-sequence from Iran was found, which may belong to this species (UDB005693, with Betula).
Additional collections examined. Austria, Oberösterreich, Braunau am Inn, Oberrothenbuch, ‘Riviera’, ÖK25V3321-Ost, alt. 355 m, Corylus avellana, Salix sp., 27 July 2018, D. Bandini (STU SMNS-STU-F-0901650, DB27-7-18-16). – Germany, Bayern, Dingolfing-Landau, Mamming, TK25 7341/2, alt. 345 m, Salix sp., Betula pendula, Populus sp., 30 Sept. 2013, D. Bandini & B. Oertel (DB30-9-13-1); Ibidem, at some distance from former location, alt. 348 m, Salix sp., Betula pendula, Populus sp., 30 Sept. 2013, D. Bandini & B. Oertel (DB30-9-13-2); Ibidem, at some distance from former location, alt. 345 m, Salix sp., Betula pendula, Populus sp., 30 Sept. 2013, D. Bandini & B. Oertel (DB30-9-13-5); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Böhl-Iggelheim, TK25 6615/4, alt. 110 m, Quercus robur, Alnus glutinosa, Corylus avellana, Pinus sylvestris, 27 Sept. 2014, D. Bandini (STU SMNS-STU-F-0901651, DB27-9-14-2); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Dudenhofen, TK25 6616/3, alt. 125 m, Quercus robur, Corylus avellana, Pinus sylvestris, 25 Oct. 2020, D. Bandini (STU SMNS-STU-F-0901653, DB25-10-20-6); Ibidem, at some distance from former location, alt. 125 m, Quercus robur, Corylus avellana, 25 Oct. 2020, D. Bandini (DB25-10-20-7).
Notes — Inocybe suryana has a yellowish to ochraceous pileus with traces of a whitish velipellis when young. The surface generally is at first glabrous later up to innately fibrillose to minutely subsquamulose around the centre, the stipe is entirely pruinose and the mostly (sub)fusiform to (sub)utriform hymenial cystidia are on average rather short (48 µm). Since it sometimes smells like bitter almonds, it could on first sight be mistaken for I. hirtella, which differs in usually having 2-spored basidia and larger spores (see above). Inocybe somae (see above) is somewhat more fragile and the pileus colours usually are paler. Furthermore, the spores are on average longer and the caulocystidia generally are longer, too. Inocybe catalaunica is more foxy or dull brownish in colour, the pileus surface is usually less glabrous, the spores are smaller and the hymenial cystidia clearly longer. Besides, it is associated with conifers (see above). Inocybe mycenoides has a yellow pileus and entirely pruinose stipe, but the pileus colour is paling with age towards the margin, and the hymenial cystidia generally are much shorter, and the spores on average are shorter, too (see above). The pileus colour of I. morganae (see above) is more intense, with orange-reddish tinge, the spores are larger on average and the hymenial cystidia are on average somewhat longer. Inocybe woglindeana may look somewhat similar, but this species has an only near the apex pruinose stipe, entirely different, often sac-shaped hymenial cystidia and larger spores (Bandini et al. 2020c). Inocybe leochroma and I. pelargonium both have a more intense pileus colour and much smaller spores (Kuyper 1986, Stangl 1989, Bandini et al. 2019b), and the same can be said of I. langei, the hymenial cystidia of which often have a rounded to ‘loop-shaped’ base (see above). Inocybe pallidolutea differs, e.g., by less smooth pileus surface, smaller spores and longer hymenial cystidia (Carteret & Reumaux 2017, and see above), while I. sindonia has at most an only pale yellowish pileus colour, on average narrower spores and longer hymenial cystidia (see above). Inocybe ochroalba and I. subhirtella generally have a paler or more brownish pileus colour, (sub)clavate hymenial cystidia and smaller spores (see above). The species that are most similar in terms of ITS are I. hirtella and I. morganae,but neither is more similar than 92 %. In Fig. 1, I. suryana is well supported (99 % / 100 %).
Inocybe venerabilisBandini, B. Oertel & U. Eberh., sp. nov. — MycoBank MB 841157; Fig. 16
Etymology. Named ‘venerabilis’ (Latin, ‘venerable’) because of its ‘grey hair’, i.e., abundant greyish velipellis.
Typus. Germany, Rheinland-Pfalz, Rhein-Pfalz-Kreis, Neuhofen, near Kistnerweiher, TK25 6516/4, alt. 90 m, Populus sp., Betula pendula, Pinus sylvestris, Salix sp., 25 May 2013, D. Bandini & B. Oertel (holotype STU SMNS-STU-F-0901605; isotypes priv. herb. D.B. DB25-5-13-2, AH). GenBank ITS + LSU (partial) OK057198.
Diagnosis — Inocybe venerabilis is a rather small species with brown to dark brown, smooth to minutely fibrillose cap, covered at first with abundant greyish velipellis, only near the apex pruinose stipe, smooth spores, measuring 8.5–12.7 µm (av. 10.2 µm) × 5.0–6.8 µm (av. 5.9 µm) and usually neckless ventricose hymenial cystidia with rather thin walls, pleurocystidia measuring 34–77 µm (av. 54 µm) × 12–22 µm (av. 16 µm). It can be recognized by the combination of the above characters and differs in its ITS sequence from other superficially similar species, such as I. rufobrunnea.
Basidiomata mostly gregarious or solitary. Pileus 10–20 mm wide, at first (sub)glandular, (sub)conical or (sub)campanulate, sometimes also somewhat deformed, later conico-convex or broadly convex, but not or very rarely expanded, mostly without, seldom with low large umbo, margin incurved when young, later decurved; with abundant remnants of a pale greyish to greyish velipellis, at first as a thick layer on the entire pileus, later only at the centre or radially scattered or only visible near the margin as thin coating; colour brown to dark or umber brown, often with more or less intense greyish tinge because of the velipellis or even silvery greyish (Mu 10YR 4/4–4/6, 3/4–3/6), with age sometimes darker, up to almost dark greyish at the centre; surface at first sublanose because of the velipellis, later finely tomentose to glabrous, sometimes entirely areolate diffracted, or minutely fibrillose towards the margin; young basidiomata with abundant remnants of whitish greyish cortina. Lamellae distant (c. 20–35, l = 1–3), thickish, adnate to broadly adnate or emarginate adnate, ventricose, at first whitish or whitish greyish, later sometimes with ochraceous hue, then coffee-brown to dark rusty or chocolate brown; edge fimbriate, whitish to concolorous. Stipe 20–40 × 1–2 mm, mostly somewhat curved, at first covered with fine whitish tomentum, later longitudinally striate or glabrous, at first beige to pale flesh-coloured, later often brown to dark brown; only sparely pruinose near the apex of the stipe. Context whitish or watery-whitish in the pileus and at first also in the stipe, later brownish especially in the cortex of the stipe. Smell (sub)spermatic, at least when cut. Colour of exsiccata pileus dark brown or dark greyish brown (Mu 7/5YR 4/4, 3/2; 10YR 3/3), lamellae concolorous or, when old, darker brown and with reddish hue, stipe concolorous or a little lighter in colour, no darkening or blackening on drying.
Spores 8.5–12.7 µm (av. 10.2 µm, SD 1.0) × 5.0–6.8 µm (av. 5.9 µm, SD 0.4); Q = 1.4–2.3 (av. 1.7, SD 0.2) (n = 120 of 3 coll.), smooth, (sub)amygdaloid, often with more or less explicit suprahilar depression, apex mostly subacute, sometimes subpapillate, often with more or less distinct pseudoporus. Basidia 25–31 × 7–11 µm, generally 4-spored, seldom also 2-spored and then spores up to 15 µm. Lamellaedgecomposed of cheilocystidia and numerous colourless, (sub)clavate or (sub)cylindrical, sometimes catenate, thin-walled paracystidia. Pleurocystidia 34–77 µm (av. 54 µm, SD 8.2) × 12–22 µm (av. 16 µm, SD 2.1); Q = 2.3–4.8 (av. 3.4, SD 0.6) (n = 45 of 3 coll.), mostly ventricose (sub)fusiform, also (sub)cylindrical or plump sac-shaped to subclavate, generally without neck, at apex often rounded, without or with only short pedicel, base often rounded or truncate, apex usually crystalliferous, walls rather thin, up to 1.0(–1.5) µm thick at the apex, mostly quite uniformly wide near bulge and near apex, pale yellowish greenish with 3 % KOH. Cheilocystidia similar in appearance and size. Pileipellis constituted by an epicutis made up of parallel hyphae 3–10 µm wide, with encrusting and parietal brown pigment, subcutis with wider and paler to colourless elements. Caulocystidia only near the apex of the stipe, 30–70 × 10–15 µm, mostly somewhat deformed subutriform, subfusiform or subcylindrical, also subclavate, often with tapering walls, apex usually crystalliferous, walls up to 1 µm thick near the apex, pale yellowish greenish with 3 % KOH. Clamp-connections abundant in all tissues.
Habitat & Distribution — Inocybe venerabilis was collected by us in Germany and Switzerland, at low elevations as well as at almost 2300 m. We have found it on sun exposed sandy or gravelly terrain in Germany with Salix nearby. Salix was also present in the alpine terrain in Switzerland. Apart from the collections listed below, no other specimens or sequences of collections are known to us. In GenBank are two soil respectively EcM sequences from Austria from the alpine zone (EU517033 and EU326161), which may belong to this species. So, it seems that the species is rather rare. It appears to prefer basic to calcareous soil.
Additional collections examined. Germany, Rheinland-Pfalz, Rhein-Pfalz-Kreis, Rheinauen, Altrip, TK25 6516/4, alt. 90 m, Populus sp., Salix sp., 12 May 2013, D. Bandini & B. Oertel (STU SMNS-STU-F-0901437, DB12-5-13-1); Rheinland-Pfalz, Rhein-Pfalz-Kreis, Neuhofen, Kistnerweiher, TK25 6516/4, alt. 90 m, Populus sp., Betula pendula, Pinus sylvestris, Salix sp., 25 May 2013, D. Bandini & B. Oertel (SMNS-STU-F-0901612, DB25-5-13-5); Bayern, München, Garching, Fröttmaninger Heide, TK25 7735/4, alt. 490 m, Salix caprea, Pinus sylvestris, 14 June 2012, M. Dondl (DB14-6-12-Dondl). – Switzerland, Kanton Obwalden, Engelberg, Jochpass, TK25 1211, alt. 2270 m, Salix herbacea, 31 Aug. 2013, B. Oertel (STU SMNS-STU-F-0901606, DB31-8-13-6b); Ibidem, at some distance from former location, alt. 2270 m, Salix retusa, 31 Aug. 2013, B. Oertel (DB31-8-13-10b).
Notes— Inocybe venerabilis is characterized by rather small size of basidiomata, brown to dark brown pileus colours, smooth to minutely fibrillose surface of pileus and abundant greyish velipellis. The stipe is pruinose only near the apex, the spores are on average > 10 µm long and the hymenial cystidia are rather thin-walled, mostly neckless, ventricose and often rather plump. Most similar in appearance is I. rufobrunnea (often referred to as I. exilis, see above), which can also be found in the same habitats. The pileus colour of I. rufobrunnea is usually more reddish hued than in I. venerabilis and the pileus surface is less glabrous. Furthermore, the spores are larger and the hymenial cystidia on average are longer and thicker walled. The same can be said about I. caulis-aurorae (Ludwig 2017, Eberhardt et al. in prep.). Inocybe carneicaulis differs, e.g., by lacking velipellis and paler pileus colours and larger spores (Ludwig 2017, Eberhardt et al. in prep.). Inocybe rufuloides also can on first view be mistaken for I. venerabilis, but the pileus colour often is more reddish tinged and somewhat paler with less smooth surface, the spores are larger and the hymenial cystidia are shorter and thicker walled than those of I. venerabilis (Bon 1984, Bandini et al. 2020c). Inocybe tarda differs by larger size of basidiomata, less velipellis, larger spores and longer hymenial cystidia which often are (sub)cylindrical (Kühner 1955, Bandini et al. 2021b, and pers. observ.), and I. neorufula differs, e.g., by more reddish pileus colour, whitish velipellis, longer spores on average and less plump and thicker walled hymenial cystidia (Esteve-Raventós et al. 2012, Bandini et al. 2020c). Inocybe deianae is a more robust and larger species with lanose to (sub)squamulose pileus and larger spores (see above). In the same habitat species of the ‘lacera-group’ may occur, which can be similar in aspect. However, the spores of I. lacera are much longer and narrower (Kuyper 1986, Stangl 1989, Ferrari 2006), while the spores of I. helobia and I. pluppiana are often somewhat undulate and also longer (Kuyper 1986 as I. lacera var. helobia, Bandini 2020a). Inocybe woglindeana, incidentally also one of the closest relatives,was collected next to I. venerabilis. The hymenial cystidia are also plump and often sac-shaped with rounded base and the spores are of almost the same size. It can nevertheless easily be distinguished by yellowish pileus colour and abundant whitish velipellis as well as less glabrous pileus surface (Bandini et al. 2020c). Inocybe variispora also has a less smooth pileus, the abundant greyish velipellis is missing and the species seems to grow with conifers in a different habitat (Fernández Sasia 2002, Bandini et al. 2020c). We are not aware of any species that is similar in the ITS to I. venerabilis. The clade of I. venerabilis received full support (100 % / 100 %).
DISCUSSION
This study was made, as others cited above, in the context of a long-term study of the Inocybaceae mainly in Germany and adjacent countries. By combining morphological, molecular and ecological data – and relying on years of experience – we are confident that we can recognize and delimit species. Species recognition is helped by the fact that species pairs or groups that are very similar in the ITS (and possibly other loci), such as I. heterosemen and I. iseranensis or I. pseudoscabelliformis and I. urceolicystis, are relatively few in the genus. Thus, we mostly rely on ITS for molecular species recognition. The numbers of sequences we have available from studied collections may not be sufficient to represent the intraspecific variation; the inclusion of sequences from root or soil, samples and collections not examined here increases the sample size and helps to better represent the natural variation that exists, even if some of the apparent sequence variation may be artefactual (i.e., due to technical difficulties or differences in interpretation of raw data).
In the cases, in which RPB2 was used as additional locus, ITS and RPB2 supported the same conclusions. The amount of information in RPB2 was slightly but not dramatically superior to ITS. Bandini et al. (2020c) used the V6 variable region of the mitSSU to assist the delimitation of I. variispora and I. woglindeana. The advantage of that locus is that amplification from older material such as types, is feasible. Although we were successful sequencing this locus also from older material (results not shown), in the species pairs and triplets investigated here (I. adorabilis, I. pseudoscabelliformis, I. urceolicystis; I. ochroalba and I. subhirtella, and I. mycenoides and I. somae), the data showed slight intraspecific but no consistent interspecific variation.
Earlier works (e.g., Matheny 2005, Ryberg et al. 2010) have shown that none of the traditional morphology based on infrageneric classifications is entirely supported by molecular results. With the data we have available, we are not in a position, either, to resolve the infrageneric phylogeny of higher infrageneric ranks or infrageneric ranks above stirps/subsection. Clade support depends on the loci used, the species considered, and the alignment. As the selection of sequences and taxa included in the analysis presented here was largely dictated by morphological similarity, the closer a supported branch is to the backbone and the longer the internal branches are, the more likely it is that taxa exist or existed and are extinct that would break the relationship as depicted in the tree figure, sometimes irrespective of clade support. Results like the inclusion of I. sambucina in the clade of I. sect. Inocybe or I. helobia and I. lacera forming a clade with I. lasseroides (though unsupported) are unexpected and might be artefacts due to the taxon sampling or the loci used. In some clades, however, morphology and ITS-LSU or multigene based results agree, e.g., in the clades or groups as for instance the I. lanuginosa-group (Matheny & Bougher 2017), the I. mixtilis-group (Esteve-Raventós et al. 2018), the Napipedinae-group (Bon 1998, Bandini et al. 2017), the I. phaeocystidiosa-group (Bandini et al. 2020b) among the nodulose-spored species, or the I. flocculosa-group (Oertel et al. 2014), I. geophylla-group (Matheny & Swenie 2018), the I. melanopus-group (Oertel et al. 2014) or I. sect. Hysterices (see Matheny & Kudzma 2019), respectively, among the smooth-spored species. Additional examples from the present analysis are the clades around I. subbrunnea (Fig. 1b), I. hirtella (Fig. 1d) or I. cincinnata (Fig. 1f). Well-supported clades closer to the backbone, e.g., the clade including both I. catalaunica and I. subnudipes ought to be interpreted with caution, also considering that I. catalaunica has an entirely pruinose stipe (Singer 1947, Esteve-Raventós 1997, Larsson et al. 2014, and see below), while I. subnudipes is pruinose only at the extreme apex of the stipe (Kühner 1955).
The twelve species of Inocybe newly described in this article are all smooth-spored. Of these, I. comis, I. filiana, I. galactica, I. othini, I. proteica and I. venerabilis have caulocystidia only more or less near the apex or in the upper part of the stipe. It is often not so easy to classify these species according to the classification principles of Bon (1997a). Section Inocybe in the sense of Bon is defined by a stipe without caulocystidia or only directly below the lamellae, and sect. Tardae by a stipe with caulocystidia down to a 1/4 or 1/3 of the stipe. This question cannot be answered reliably in several cases, since this was found to vary from collection to collection within the same species. However, since the classification system of Bon is still widely used, especially in France and in Italy (see, e.g., Brugaletta et al. 2019, Cervini 2021), and since it is still the best we have, we will try to assign our new species to sections of Bon’s key as well as possible. Inocybe comis, as related to I. minimispora, we would assign to section Inocybe in the sense of Bon, and likewise I. galactica with caulocystidia only near the extreme apex. Inocybe othini belongs to the ‘I. flocculosa-group’ and thus would have to be assigned to I. sect. Tardae and there to the I. subsect. Gausapatinae.
The stipe of I. adorabilis is mainly pruinose near the apex, but there are also some caulocystidia below the middle, so this species could be assigned both to I. sect. Tardae or to I. sect. Splendentes, which contains species with entirely pruinose stipe.
Inocybe morganae, I. somae and I. suryana often have an odour like bitter almonds that is characteristic for I. hirtella, and they are all included in a fully supported clade (100 % / 100 %)including I. hirtella (Fig. 1),which in our opinion is the I. hirtella var. bispora of Kuyper (see above). Inocybe morganae, I. somae and I. suryana can easily be distinguished from I. hirtella, e.g., by 4-spored basidia with much smaller spores. Inocybe somae is somewhat more fragile in habit than I. suryana and the pileus colours usually are paler. Furthermore, the spores are on average longer, and the caulocystidia generally are longer, too. The pileus surface of I. morganae is minutely furfuraceous to minutely squamulose with very small squamules with age and sometimes getting cracked to areolate diffracted at the centre, furthermore the pruina of the stipe is quite rough.
As has been discussed above (see notes to I. hirtella), we are of the opinion that I. hirtella var. bispora (see Kuyper 1986) is in fact the I. hirtella of Bresadola (1881–1887), because both the details of the original description and the coloured illustration provided with it coincide very well. We thus created an epitype of I. hirtella and added a detailed description. To this ‘hirtella-group’ also belongs I. mycenoides, the basidiomata of which species also may smell like bitter almonds in fresh state (pers. observ. from several own collections). According to Bon’s (1997a) key, all mentioned species would be grouped in I. sect. Splendentes, because the stipes are pruinose on the entire length of the stipe. However, I. morganae could also be assigned to I. sect. Tardae, because its caulocystidia are only sparely to be found below the middle of the stipe.
Bon included I. hirtella in I. subsect. Subbrunneinae. We do not agree with this, since this group (including, e.g., I. subbrunnea, I. leiocephala, I. lindrothii and I. fuscescentipes), have very characteristic cystidia, often (sub)lageniform with ‘sandy’, i.e., finely granulose apex (see Larsson et al. 2014), which thus differ greatly from those of the ‘I. hirtella-group’. Inocybe ochroalba and I. subhirtella have distinctive short mostly subclavate to broadly fusiform hymenial cystidia. These species fit morphologically into I. sect. Splendentes, but not into I. subsect. Subbrunneinae where they are keyed out by Bon (1997a). The members of the core group around I. subbrunnea form a highly supported clade (Fig. 1b), but its position in relation to the species discussed here is unresolved.
Inocybe ovilla, too, has an entirely pruinose stipe and thus would be included in I. sect. Splendentes. The species is, morphologically and genetically, closely related to I. sindonia, and since no type material exists of this latter specieswe created an epitype. The stipe of I. sindonia may be pruinose only in the upper part or on entire length, and thus the species could be assigned to I. sect. Tardae as well as to I. sect. Splendentes.
None of the species discussed here in detail was clearly resolved as member of the I. lilacina clade, possibly with the exception of I. favrei-cavipes (Fig. 1e). Inocybe tarda was included in the analysis (Fig. 1c) and its position is reasonably well resolved. Among the species discussed here, I. rufobrunnea, I. proteica and I. filiana might be considered as members of a I. tarda clade or I. sect. Tardae;the relationship between I. tarda and the other target taxa of this study is unresolved. Inocybe splendens was not included in the analysis; none of the target taxa of this study would be a member of an I. splendens clade or I. sect. Splendentinae. The molecularly and morphologically supported groups or clades are still too few and too insular to construct classical dichotomous keys or device a system of infrageneric taxa with subsections and sections.
Inocybe abietis, a species described in detail by Kühner (1955), is according to our evaluation synonymous with I. catalaunica, a species which had been described not long before by Singer (1947). Inocybe catalaunica was an almost unknown species until its holotype specimen was sequenced by Larsson et al. (2014), but it is quite common at least in Germany.
In 1986 Kuyper described a variety of I. rufuloides with the name of exilis. This variety was raised to species level by Jacobsson & Larsson (in Knudsen & Vesterholt 2008). However, the morphological comparison of the types of I. exilis and of I. rufobrunnea,a species described by Favre (1955),gave very similar results, and also the macroscopic descriptions are very close. Since it is well known by now that alpine species may also be found at low elevations, we concluded that the two names refer to the same species.
Backed by genetic analysis in combination with morphological examination of the respective types and protologues, we furthermore conclude that I. lapidicola is conspecific with I. deianae, I. stangliana with I. pelargonium, I. ochraceolutea with I. sindonia and I. subrubens with I. subhirtella,which is a good species in its own right and not conspecific with I. ochroalba as supposed by Kuyper(1986). We were not allowed to sequence the type of I. sulfovirescens, but the morphological examination – showing very small spores in combination with hymenial cystidia often with rounded or ‘loop-shaped’ base – left no doubt that this species is conspecific with I. langei. Microscopically and macroscopically I. hirtellarum is so similar to I. mycenoides that we consider them synonyms. We could confirm a previous study (Kuyper 1986) by genetic and morphological examination of the types that I. kuehneri is conspecific with I. sindonia and I. subalbidodisca with I. ochroalba.
Of the new species described above, I. demetris, which we found rather often, seems to be quite common at least in Germany, and this is also the case with I. filiana of which we have several own collections from Finland, too. Also the recently described I. ianthinopes appears to be fairly common in Germany. Inocybe proteica is so variable both in macroscopic and microscopic respect that it may have been mistaken for a number of species in the past. It is likely to have been overlooked. Inocybe suryana seems not to be very rare, either, and can be found on waysides on base rich or calcareous ground. Judging by the few entries in GenBank and UNITE and the small proportion (0.35 %) they collectively represent of our own collections (over 8 000), the other species described in this article, i.e., I. adorabilis, I. comis, I. galactica, I. morganae, I. othini, I. ovilla, I. somae and I. venerabilis seem to be rather rare.
Table 1.
title.
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
|---|---|---|---|---|---|---|---|
| Inocybe abietis | G | G00058749 (lectotype) | France | MZ664390 | This study | ||
| I. adorabilis | STU | SMNS-STU-F-0901582 (holotype) | DB19-9-20-23 | Austria | OK057159 | OK057159; OK078903* | This study |
| I. adorabilis | STU | SMNS-STU-F-0901641 | DB19-9-20-22 | Austria | OK057161 | OK057161; OK078904* | This study |
| I. ahmadii | LAH | LAH14215 (holotype) | Pakistan | KX254462 | Farooqi et al. 2017 | ||
| I. alberichiana | STU | SMNS-STU-F-0901514 (holotype) | DB12-9-19-16 | Austria | MW845855 | MW845855 | Bandini et al. 2021b |
| I. amethystina | L | L-0053531 (holotype) | Netherlands | MW845932 | Bandini et al. 2021b | ||
| I. astraiana | STU | SMNS-STU-F-0901240 (holotype) | DB26-10-14-7 | Germany | MN512321 | MN512321 | Bandini et al. 2020a |
| I. beatifica | STU | SMNS-STU-F-0901261 (holotype) | DB12-10-13-3 | Germany | MW845857 | Bandini et al. 2021b | |
| I. beatifica | STU | SMNS-STU-F-0901472 | DB22-10-13-1 | Germany | MW845859 | MW845859 | Bandini et al. 2021b |
| I. brijunica | CNF | CNF 1/7345a (isotype) | D. Haelew. F-1610a | Croatia | MN749370 | MN749492 | Mešić et al. 2021 |
| I. castorina | STU | SMNS-STU-F-0901250 (holotype) | DB21-10-15-2 | Germany | MN512319 | MN512319 | Bandini et al. 2020a |
| I. catalaunica | LE | LE 12646 (holotype) | Spain | KJ399954 | Larsson et al. 2014 | ||
| I. catalaunica | STU | SMNS-STU-F-0901596 | DB5-9-14-3 | Germany | OK057187 | OK057187 | This study |
| I. catalaunica | STU | SMNS-STU-F-0901597 | DB27-10-19-12 | Germany | OK057188 | OK057188 | This study |
| I. chondroderma | WTU | Stuntz 4771 (holotype) | USA | GU949572 | Matheny et al. 2013 | ||
| I. chondroderma | UBC | UBC-F-18986 | Canada | HQ604103 | HQ604103 | Berbee et al. (direct submission 2010, unpublished) | |
| I. cincinnata | STU | SMNS-STU-F-0901571 | DB19-9-20-14 | Austria | MW845946 | MW845946 | Bandini et al. 2021b |
| I. clandestina | STU | SMNS-STU-F-0901267 (holotype) | DB11-10-17-16 | Germany | MW845865 | MW845865 | Bandini et al. 2021b |
| I. comis | STU | SMNS-STU-F-0901599 (holotype) | DB2013-8-13-3 | Austria | OK057190 | OK057190 | This study |
| I. comis | STU | SMNS-STU-F-0901598 | DB13-8-13-27 | Austria | OK057189 | OK057189 | This study |
| I. comis as Inocybe sp. | MCVE | MCVE 21573 | Italy | JF908230 | Osmundson et al. 2013 | ||
| I. corydalina | EIU, WTU | EIU AM10687, WTU 6488 | Russian Federation | MH216083 | AY038314 | Matheny et al. 2002, Matheny & Kudzma 2019 | |
| I. costinitii | MCVE | MCVE 28974 (holotype) | Croatia | KX686581 | Bizio et al. 2016 | ||
| I. cryptocystis | WTU | WTU 43774 (holotype) | Stuntz 5400 | USA | KY923017 | Matheny et al. 2020 | |
| I. deianae | STU | SMNS-STU-F-0901538 (isotype) | DB30-10-4-Eyssartier; double of G. Eyssartier pers. coll. 04-095 | France | OK057117 | This study | |
| I. demetris | STU | SMNS-STU-F-0901593 (holotype) | DB27-10-19-6 | Germany | OK057184 | OK057184 | This study |
| I. demetris | DB | DB20-10-12-2 | Germany | OK057118 | OK057118 | This study | |
| I. demetris | STU | SMNS-STU-F-0901581 | DB23-9-20-5 | Germany | OK057158 | OK057158 | This study |
| I. demetris | STU | SMNS-STU-F-0901594 | DB27-10-19-10 | Germany | OK057185 | OK057185 | This study |
| I. demetris | STU | SMNS-STU-F-0901595 | DB22-9-20-13 | Germany | OK057186 | OK057186 | This study |
| I. dryadiana | STU | SMNS-STU-F-0901259 (holotype) | DB31-8-14-1 | Germany | MW845873 | MW845873 | Bandini et al. 2021b |
| I. dryadiana | STU | SMNS-STU-F-0901481 | DB2-8-14-11 | Germany | MW845875 | MW845875 | Bandini et al. 2021b |
| I. dvaliniana | STU | SMNS-STU-F-0901559 (holotype) | DB21-9-16-22 | Austria | MW647624 | MW647624 | Bandini et al. 2021b |
| I. exilis | L | L-0053539 (holotype) | Kuyper 2657 | Netherlands | MZ667616 | This study | |
| I. favrei-cavipes | DB | DB4-9-14-2-Zitzmann | Austria | UDBxxxxx | This study | ||
| I. filiana | STU | SMNS-STU-F-0901602 (holotype) | DB4-5-16-1 | Germany | OK057192 | OK057192 | This study |
| I. filiana | STU | SMNS-STU-F-0901603 | DB5-5-16-8 | Germany | OK057194 | OK057194 | This study |
| I. filiana | STU | SMNS-STU-F-0901604 | DB28-10-16-10 | Germany | OK057195 | OK057195 | This study |
| I. filiana originally as I. subporospora | STU | SMNS-STU-F-0901614 | DB2-10-12-2 | Germany | MT101895 | OK057116 | Bandini et al. 2020c |
| I. flavoalbida | TENN | TENN 067000 (isotype) | PBM3768 | Australia | KJ729873, KJ729901 | Matheny et al. 2020 | |
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
| I. flocculosa | DB | DB2-10-12-8 | Germany | MW856450 | Bandini et al. 2021b | ||
| I. flocculosa | WTU | PBM2392 | Norway | AY380375 | Matheny 2005 | ||
| I. flocculosa | STU | SMNS-STU-F-0901628 | DB2-10-19-9 | Netherlands | OK057165 | OK057165 | This study |
| I. furfurea | G | G00053152 (lectotype) | France | MG012472 | Bandini et al. 2019b | ||
| I. furfurea | STU | SMNS-STU-F-0901592 | DB29-5-21-1 | Germany | OK057169 | OK057169 | This study |
| I. fuscescentipes | G | G00052202 (holotype) | Kühner 69-328 | France | KJ399947 | Larsson et al. 2014 | |
| I. fuscescentipes | TUR-A | JV5955F | Finland | KJ399948 | KJ399948 | Larsson et al. 2014 | |
| I. fuscicothurnata | TENN | TENN 068940 | PBM3980 | USA | MF487844 | KY990485 | Matheny & Swenie 2018 |
| I. gaiana | STU | SMNS-STU-F-0901482 (holotype) | DB10-10-18-22 | Germany | MW845876 | MW845876 | Bandini et al. 2021b |
| I. galactica | STU | SMNS-STU-F-0901613 (holotype) | DB19-9-18-11 | Germany | OK057196 | OK057196 | This study |
| I. galactica | STU | SMNS-STU-F-0901620 | DB19-9-18-14 | Germany | OK057178 | OK057178 | This study |
| I. galactica | STU | SMNS-STU-F-0901621 | DB14-9-19-14 | Austria | OK057179 | OK057179 | This study |
| I. glabrescens | STU | SMNS-STU-F-0901570 | DB17-10-17-6 | Germany | MW845941 | MW845941 | Bandini et al. 2021b |
| I. glabripes | STU | SMNS-STU-F-0900979 (neotype) | DB2-6-13-12 | Germany | MW845881 | MW845881 | Bandini et al. 2021b |
| I. grammopodia | STU | SMNS-STU-F-0901611 | DB14-10-15-2 | Germany | OK057203 | OK057203 | This study |
| I. griseolilacina | KR | KR-M-0038014 | DB16-8-11-12 | Germany | MW856429 | MW856429 | Bandini et al. 2021b |
| I. griseovelata | GB | EL20906 | France | FN550931 | FN550931 | Ryberg et al. 2010 | |
| I. helobia | L | L-0053536 (holotype) | Kuyper 2124 | Netherlands | MN319699 | This study | |
| I. heterosemen | X. Carteret pers. coll. | XC98091209 (isotype) | France | OK057119 | This study | ||
| I. heterosemen as I. cf. langei | TUR-A | TUR-A 8532 | JV14253 | Finland | JX258834 | Matheny (direct submission 2012, unpublished) | |
| I. hirtella | STU | SMNS-STU-F-0901607 (epitype) | DB4-10-20-19 | Germany | OK057200 | OK057200 | This study |
| I. hirtella | GB | EL13505 | Sweden | AM882933 | Ryberg et al. 2008 | ||
| I. hirtella | KR | KR-M-0038022 | DB22-10-11-12 | Germany | OK057128 | OK057128 | This study |
| I. hirtella | STU | SMNS-STU-F-0901645 | DB14-9-19-12 | Austria | OK057150 | OK057150 | This study |
| I. hirtella | STU | SMNS-STU-F-0901646 | DB3-10-19-8 | Netherlands | OK057152 | OK057152 | This study |
| I. hirtella as I. hirtella var. bispora | GB | EL12505 | Sweden | AM882932.2 | AM882932.2 | Ryberg et al. 2008 | |
| I. hirtella var. bispora | L | L-0053535 (holotype) | Netherlands | MZ700324 | This study | ||
| I. hirtelloides | PRM | PRM727125 (holotype) | Germany | MG012471 | Bandini et al. 2019b | ||
| I. hirtelloides | KR | KR-M-0042354 | DB1-8-14-4 | Germany | MH366618.2 | Bandini et al. 2019b | |
| I. hotsoniana | WTU | WTU 43770 (holotype) | Stuntz 525 | USA | MT239043 | Matheny & Swenie (direct submission 2020, unpublished) | |
| I. hotsoniana as I. flocculosa | UBC | UBC-F-19241 | Canada | HQ604174 | HQ604174 | Berbee et al. (direct submission 2010, unpublished) | |
| I. ianthinopes | STU | SMNS-STU-F-0901623 | DB15-8-17-9 | Germany | OK057181 | OK057181 | This study |
| I. ianthinopes originally as I. pusio | DB | DB14-7-12-4 | Germany | MH366589.2 | OK057115 | Bandini et al. 2019b; this study | |
| I. ianthinopes originally as I. pusio | DB | DB16-8-14-24 | Germany | MH366588.2 | Bandini et al. 2019b | ||
| I. ianthinopes | STU | SMNS-STU-F-0901521 | DB2-10-13-7 | Germany | OK057144 | OK057144 | This study |
| I. ianthinopes | STU | SMNS-STU-F-0901610 | DB21-5-13-2 | Germany | OK057202 | OK057202 | This study |
| I. ianthinopes | STU | SMNS-STU-F-0901624 | DB16-8-17-1 | Germany | OK057182 | OK057182 | This study |
| I. involuta | L | L-0017086 (holotype) | Netherlands | MN319696 | Bandini et al. 2020a | ||
| I. involuta | STU | SMNS-STU-F-0901270 | DB13-10-16-19 | Austria | MN512329 | MN512329 | Bandini et al. 2020a |
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
| I. involuta | STU | SMNS-STU-F-0901618 | DB14-8-13-7 | Austria | OK057177 | OK057177 | This study |
| I. involuta | STU | SMNS-STU-F-0901643 | DB16-9-20-25 | Austria | OK057162 | OK057162 | This study |
| I. ionochlora | M | M-0276187 | Germany | MF804316 | Bandini et al. 2013 | ||
| I. iseranensis | TR | TR gmb 00981 (holotype) | France | OK057141 | OK057141 | This study | |
| I. iseranensis as I. cf. langei | GB | EL8306 | Sweden | FN550905 | FN550905 | Ryberg et al. 2010 | |
| I. knautiana | STU | SMNS-STU-F-0901491 (holotype) | DB22-8-16-1 | Germany | MW845887 | MW845887 | Bandini et al. 2021b |
| I. kuehneri | PRM | PRM 710368 (holotype) | Czech Republic | MN319693 | This study | ||
| I. lacera | DB | DB2-10-12-4 | Germany | OK057120 | OK057120 | This study | |
| I. lacera | STU | SMNS-STU-F-0901583 | DB10-10-18-4 | Netherlands | OK057130 | OK057130 | This study |
| I. lampetiana | STU | SMNS-STU-F-0901494 (holotype) | DB6-7-14-3 | Germany | MW845891 | MW845891 | Bandini et al. 2021b |
| I. lanatopurpurea | AH | AH 40445 (holotype) | Spain | KJ432290 | Esteve-Raventós et al. in rous et al. 2014 | ||
| I. langei | GB | EL7705 | Sweden | AM882919 | Ryberg et al. 2008 | ||
| I. langei | KR | KR-M-0038101 | DB5-7-12-Eimann | Germany | OK057121 | OK057121 | This study |
| I. langei | MCVE | MCVE 9778 | Finland | JF908138 | Osmundson et al. 2013 | ||
| I. langei | STU | SMNS-STU-F-0900983 | DB31-8-14-7 | Germany | OK057205 | OK057205 | This study |
| I. langei | TUF | TUF101799 | Estonia | UDB016069 | UDB016069 | Saar (direct submission 2012, unpublished) | |
| I. lapidicola | AMB | AMB 18349 (holotype) | Italy | MN449987 | MN449989 | Brugaletta et al. 2019 | |
| I. lasseroides | TENN | TENN 066979 | PBM3749 | Australia | KP171145 | KP170924 | Matheny et al. 2020 |
| I. laurina | STU | SMNS-STU-F-0901247 (holotype) | DB23-10-16-6 | Germany | MN512325 | MN512325 | Bandini et al. 2020a |
| I. lechiana | STU | SMNS-STU-F-0901268 (holotype) | DB18-9-18-26 | Austria | MN512330 | MN512330 | Bandini et al. 2020a |
| I. leiocephala | WTU | Stuntz 4739 (holotype) | USA | KJ399884 | KJ399884 | Larsson et al. 2014 | |
| I. leochroma | KR | KR-M-0042372 (holotype) | DB25-9-15-21 | Austria | MH366611.2 | Bandini et al. 2019b | |
| I. lilacina | NYS | NYS-f1711 (holotype) | USA | MH024860 | Matheny & Swenie 2018 | ||
| I. lindrothii | TUR-A | TUR-A 144764 (epitype) | Vauras 2390F | Finland | KJ399915 | KJ399915 | Larsson et al. 2014 |
| I. luteifolia | TENN, CUW | TENN AHS6557 (isotype) | PBM2642 | USA | FJ436331 | EU307814 | Matheny et al. 2020 |
| I. melanopus | GB | JV4986 | Finland | AM882727.2 | AM882727.2 | Ryberg et al. 2008 | |
| I. melleiconica | WTU | WTU-F-043792 (isotype) | DG1906 | Canada | KY923034, KY923047 | KY923037 | Matheny et al. 2020 |
| I. metrodii | PRM | PRM756354 (holotype) | Germany | MN319692 | This study | ||
| I. microteroxantha | TENN | TENN 075005 | PBM4245 | USA | MT197007 | Matheny & Swenie (direct submission 2020, unpublished) | |
| I. microteroxantha as I. cf. hirtella | PBM 2624 | USA | EU523572 | Hughes et al. (direct submission 2008, unpublished) | |||
| I. minima | ACAD | ACAD 13144 | Canada | MH578003 | Matheny & Hobbs (direct submission 2018, unpublished) | ||
| I. minimispora | STU | SMNS-STU-F-0901264 | DB12-7-17-9 | Austria | MW845934 | MW845934 | Bandini et al. 2021b |
| I. minimispora as I. glabripes | GB | EL8103 | Sweden | AM882971.2 | AM882971.2 | Ryberg et al. 2008 | |
| I. minutissima | X. Carteret pers. coll. | XC2001-77 (isotype) | France | OK057122 | This study | ||
| I. minutissima as Inocybe sp. | MCVE | MCVE 21586 | Italy | JF908235 | Osmundson et al. 2013 | ||
| I. morganae | STU | SMNS-STU-F-0901459 (holotype) | DB9-9-19-7 | Austria | OK057143 | OK057143 | This study |
| I. morganae | STU | SMNS-STU-F-0901608 | DB25-7-18-16 | Germany | OK057201 | OK057201 | This study |
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
| I. morganae | STU | SMNS-STU-F-0901639 | DB9-9-19-11 | Austria | OK057146 | OK057146 | This study |
| I. morganae | STU | SMNS-STU-F-0901640 | DB14-9-19-11 | Austria | OK057147 | OK057147 | This study |
| I. muricellata | DB | DB16-10-11-2 | Germany | MW856432 | Bandini et al. 2021b | ||
| I. muricellata | STU | SMNS-STU-F-0900985 | DB23-9-14-2 | Germany | MW845931 | MW845931 | Bandini et al. 2021b |
| I. muricellatoides | HMJAU | HMJAU 26201 (holotype) | Gansu 2012130 | China | KY402220 | KY402216 | Fan & Bau 2020 |
| I. mycenoides | L | L-0053538 (holotype) | Kuyper 2486 | Netherlands | MZ667617 | This study | |
| I. mycenoides | STU | SMNS-STU-F-0900981 | DB11-10-15-4 | Germany | OK057204 | OK057204 | This study |
| I. mycenoides | STU | SMNS-STU-F-0901647 | DB17-9-18-2 | Germany | OK057156 | OK057156; OK078899* | This study |
| I. mycenoides | STU | SMNS-STU-F-0901648 | DB15-9-18-5 | Germany | OK057151 | OK057151; OK078900* | This study |
| I. mycenoides as I. hirtella | GB | MR00025 | Sweden | AM882934.2 | AM882934.2 | Ryberg et al. 2008 | |
| I. mystica | M | M-0280610 (holotype) | M-1266 | Germany | KY349131 | Rodriguez Campo & Esteve-Raventós (direct submission 2016, unpublished) | |
| I. mystica | STU | SMNS-STU-F-0901638 | DB4-8-12-1-Dondl | Germany | OK057145 | OK057145 | This study |
| I. nemorosa | ACAD | ACAD 19518 | DG1887 | Canada | MH586780, MH586817 | Matheny & Hobbs (direct submission 2018, unpublished) | |
| I. neorufula | STU | SMNS-STU-F-0901288 (isotype) | AH40223 | Spain | MT101890 | Bandini et al. 2020a | |
| I. neorufula | STU | SMNS-STU-F-0901445 | DB30-10-15-2-Dondl | Italy | MT101876 | MT101876 | Bandini et al. 2020a |
| I. nitidiuscula | M | M-0229745 (epitype) | Germany | KM873364 | Marchetti et al. 2014 | ||
| I. nitidiuscula | DB | DB16-8-11-15 | Germany | MT101891 | Bandini et al. 2020c | ||
| I. ochroalba | BR | BR5020184019648 (holotype) | Belgium | MZ824395 | This study | ||
| I. ochroalba | DB | DB18-8-11-14 | Austria | OK057123 | This study | ||
| I. ochroalba | GB | EL5704 | Sweden | AM882882.2 | AM882882.2 | Ryberg et al. 2008 | |
| I. ochroalba | P. Cullington pers. coll. | PC2010091101 | UK | UDB0754138 | UDB0754138 | Saar (submission 2019, unpublished) | |
| I. ochroalba | STU | SMNS-STU-F-0901590 | DB18-8-15-3 | Finland | OK057137 | OK057137; OK078918* | This study |
| I. ochroalba | STU | SMNS-STU-F-0901591 | DB19-8-15-12 | Finland | OK057138 | OK057138 | This study |
| I. ochroalba | STU | SMNS-STU-F-0901629 | DB8-11-14-6 | Germany | OK057166 | OK057166; OK078911* | This study |
| I. ochroalba | STU | SMNS-STU-F-0901630 | DB7-9-14-1 | Germany | OK057167 | OK057167; OK078912* | This study |
| I. ochroalba | STU | SMNS-STU-F-0901631 | DB17-8-15-8 | Finland | OK057168 | OK057168; OK078913* | This study |
| I. ochroalba | STU | SMNS-STU-F-0901642 | DB23-8-14-9 | Germany | OK057160 | OK057160; OK078905* | This study |
| I. ochroalba | STU | SMNS-STU-F-0901657 | DB11-8-21-6 | Austria | OK057139 | This study | |
| I. oetziana | STU | SMNS-STU-F-0901526 (holotype) | DB13-9-17-23 | Austria | MW845897 | MW845897 | Bandini et al. 2021b |
| I. othini | STU | SMNS-STU-F-0901622 (holotype) | DB19-9-20-28 | Austria | OK057180 | OK057180 | This study |
| I. othini | DB | DB19-9-20-12 | Austria | UDB07670889 | This study | ||
| I. ovilla | STU | SMNS-STU-F-0901600 (holotype) | DB6-9-14-7 | Germany | OK057191 | OK057191 | This study |
| I. ovilla | STU | SMNS-STU-F-0901601 | DB14-9-16-5 | Germany | OK057193 | OK057193 | This study |
| I. ovilla as I. sindonia | TUF | TUF120387 | Estonia | UDB024767 | UDB024767 | Liiv (direct submission 2015, unpublished) | |
| I. pallidicremea | WTU | WTU-F-038286 (holotype) | Canada | KY923033 | Matheny et al. 2020 | ||
| I. pallidicremea | TENN, WTU | TENN 062552 | PBM2039 | USA | KY990553 | AY380385 | Matheny et al. 2020 |
| I. parvicystis | AH | AH 46600 (holotype) | DB29-12-14-Esteve-Rav. (isotype); | Spain | KY349121 | Crous et al. 2017 | |
| PRC 141229-02 | |||||||
| I. pelargonium | KR | KR-M-0042334 | DB29-6-12-7 | Germany | MH366623.2 | Bandini et al. 2019b | |
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
| I. pelargonium | MCVE | MCVE 21637 | Italy | JF908252 | Osmundson et al. 2013 | ||
| I. perchtana | STU | SMNS-STU-F-0901245 (holotype) | DB21-9-16-18 | Austria | MN512326 | MN512326 | Bandini et al. 2020a |
| I. pholiotinoides | DB | DB28-9-12-14 | Germany | OK057125 | OK057125 | This study | |
| I. picetorum | TUF | TUF124448 | Italy | UDB028399 | UDB028399 | Saitta (direct submission, unpublished) | |
| I. pipilikae | STU | SMNS-STU-F-0901539 (holotype) | DB18-9-20-7 | Austria | MW647629 | MW647629 | Bandini et al. 2021a |
| I. pluppiana | STU | SMNS-STU-F-0901254 (holotype) | DB10-10-18-3 | Netherlands | MN512327 | MN512327 | Bandini et al. 2020a |
| I. proteica | STU | SMNS-STU-F-0901658 (holotype) | DB16-9-20-17 | Austria | OK057140 | OK057140 | This study |
| I. proteica | STU | SMNS-STU-F-0901617 | DB19-9-18-12 | Germany | OK057176 | OK057176 | This study |
| I. proteica | STU | SMNS-STU-F-0901625 | DB23-9-16-22 | Germany | OK057183 | OK057183 | This study |
| I. pseudodestricta | PRM | PRM716231 (holotype) | Czech Republic | MG012468 | Bandini et al. 2019b | ||
| I. pseudodestricta | STU | SMNS-STU-F-0901585 | DB17-8-15-13 | Finland | OK057132 | OK057132 | This study |
| I. pseudodestricta | STU | SMNS-STU-F-0901587 | DB21-8-15-22 | Finland | OK057134 | OK057134 | This study |
| I. pseudoscabelliformis | X. Carteret pers. coll. | XC2011-59 (isotype) | France | OK057208 | This study | ||
| I. pseudoscabelliformis | KR | KR-M-0038050 | DB24-9-04-Vauras | Finland | OK057126 | OK057126 | This study |
| I. pseudoscabelliformis | STU | SMNS-STU-F-0901634 | DB30-10-15-9 | Germany | OK057172 | OK057172; OK078908* | This study |
| I. pseudoscabelliformis | STU | SMNS-STU-F-0901635 | DB20-10-19-1 | Germany | OK057173 | OK057173; OK078909* | This study |
| I. pseudoscabelliformis | STU | SMNS-STU-F-0901632 | DB11-10-19-Laessoe | Denmark | OK057170 | OK057170; OK078906* | This study |
| I. pseudoscabelliformis | STU | SMNS-STU-F-0901636 | DB20-10-19-4 | Germany | OK057174 | OK057174; OK078910* | This study |
| I. pusio | TUR-A | SJ78010 | Sweden | KJ432286 | Esteve-Raventós et al. in Crous et al. 2014 | ||
| I. relicina | IB, WTU | IB 19920112, JV10258 | Finland | AF325664 | AY038324 | Peintner et al. 2001; Matheny et al. 2002 | |
| I. rivierana | STU | SMNS-STU-F-0901249 (holotype) | DB24-7-18-16 | Austria | MW845910 | MW845910 | Bandini et al. 2021b |
| I. rivierana | STU | SMNS-STU-F-0901503 | DB24-7-18-19 | Austria | MW845912 | MW845912 | Bandini et al. 2021b |
| I. roseifolia | CSU | CO5576 | USA | MH578026 | MK421968 | Matheny et al. 2020 | |
| I. rufobrunnea | DB | DB25-5-13-11 | Germany | MT101888 | Bandini et al. 2020c | ||
| I. rufobrunnea | STU | SMNS-STU-F-0901441 | DB28-9-15-16 | Austria | MT101873 | MT101873 | Bandini et al. 2020c |
| I. rufobrunnea as I. exilis | GB | JVe6575 | Denmark | FN550919 | FN550919 | Ryberg et al. 2008 | |
| I. rufuloides | PERTH | PERTH 08305978 | NLB00618 | Australia | JN035291 | Bougher & Matheny 2011 | |
| I. rufuloides | STU | SMNS-STU-F-0901442 | DB13-10-12-4 | Germany | MT101878 | Bandini et al. 2020c | |
| I. sambucina | GB | SJ01002 | Sweden | AM882757.2 | AM882757.2 | Ryberg et al. 2008 | |
| I. semifulva | STU | SMNS-STU-F-0901000 | DB28-8-14-4 | Germany | MW845916 | MW845916 | Bandini et al. 2021b |
| I. semifulva | WTU | WTU-ACAD11651 (isotype) | F 043791 | Canada | HQ222006 | HQ222007 | Matheny & Wolfenbarger (direct submission 2010, unpublished) |
| I. sindonia | STU | SMNS-STU-F-0901627 (epitype) | DB3-11-13-1 | Germany | OK057164 | OK057164 | This study |
| I. sindonia | DB | DB29-10-11-9 | Germany | MW856445 | Bandini et al. 2021b | ||
| I. sindonia | AM | AM-AR17-019 | Argentina | MH930386 | Mujic & Smith (direct submission 2018, unpublished) | ||
| I. sindonia | DB | DB4-12-12-1 | Netherlands | MW856446 | Bandini et al. 2021b | ||
| I. sindonia | GB | EL364-16 | Sweden | MH310763 | Wurzbacher et al. 2019 | ||
| I. sindonia | KR | KR-M-0038268 | DB29-10-11-6 | Germany | MW856447 | Bandini et al. 2021b | |
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
| I. sindonia | KR | KR-M-0042635 | Germany | MT005949 | Scholler (direct submission 2020, unpublished) | ||
| I. sindonia | STU | SMNS-STU-F-0901584 | DB14-9-18-23 | Germany | OK057131 | OK057131 | This study |
| I. sindonia | STU | SMNS-STU-F-0901626 | DB21-9-14-1 | Germany | OK057163 | OK057163 | This study |
| I. sitibunda | STU | SMNS-STU-F-0901504 (holotype) | DB10-10-15-9 | Germany | MW845918 | MW845918 | Bandini et al. 2021b |
| I. somae | STU | SMNS-STU-F-0901652 (holotype) | DB25-10-19-4 | Germany | OK057148 | OK057148; OK078901* | This study |
| I. somae | STU | SMNS-STU-F-0901580 | DB23-10-20-2 | Germany | OK057157 | OK057157; OK078902* | This study |
| I. stangliana | L | L-0054130 (holotype) | Germany | MZ667615 | This study | ||
| I. subalbidodisca | PRM | PRM 735116 (holotype) | Germany | MZ048356 | This study | ||
| I. subbrunnea | G | G00126460 (lectotype) | G388231 | France | KJ399934 | Larsson et al. 2014 | |
| I. subhirtella | LIP | MB791115 (holotype) | France | OK057206 | This study | ||
| I. subhirtella | KR | KR-M-0038247 | DB29-10-11-4 | Germany | OK057127 | OK057127 | This study |
| I. subhirtella | STU | SMNS-STU-F-0901586 | DB18-8-17-9 | Germany | OK057133 | OK057133; OK078915* | This study |
| I. subhirtella | STU | SMNS-STU-F-0901588 | DB30-10-15-7 | Germany | OK057135 | OK057135; OK078916* | This study |
| I. subhirtella | STU | SMNS-STU-F-0901589 | DB22-10-15-8 | Germany | OK057136 | OK057136; OK078917* | This study |
| I. subhirtella as I. ochroalba | MCVE | MCVE 21444 | Italy | JF908169 | Osmundson et al. 2013 | ||
| I. subnudipes | KR | KR-M-0042701 | DB13-9-14-3 | Germany | MH732915 | Scholler et al. (direct submission 2018, unpublished) | |
| I. subrubens | X. Carteret pers. coll. | XC2011-41 (isotype) | France | OK057207 | This study | ||
| I. suryana | STU | SMNS-STU-F-0901649 (holotype) | DB30-9-13-4 | Germany | OK057149 | OK057149 | This study |
| I. suryana | STU | SMNS-STU-F-0901650 | DB27-7-18-16 | Austria | OK057153 | OK057153 | This study |
| I. suryana | STU | SMNS-STU-F-0901651 | DB27-9-14-2 | Germany | OK057155 | OK057155 | This study |
| I. suryana | STU | SMNS-STU-F-0901653 | DB25-10-20-6 | Germany | OK057154 | OK057154 | This study |
| I. tarda | STU | SMNS-STU-F-0901443 | DB21-10-12-28 | Germany | MW845922 | MW845922 | Bandini et al. 2021b |
| I. tenuicystidiata | M | M-0281792 (holotype) | M-1039 | Germany | MW856454, MW856453 | Bandini et al. 2021b | |
| I. tiburtina | STU | SMNS-STU-F-0901565 (holotype) | DB25-10-19-15 | Germany | MW845939 | MW845939 | Bandini et al. 2021b |
| I. tigrina | STU | SMNS-STU-F-0901532 (epitype) | DB24-10-15-3 | Germany | MW845933 | MW845933 | Bandini et al. 2021b |
| I. tjallingiorum | L | L-0053540 (holotype) | Kuyper 1902 | Netherlands | MW845929 | Bandini et al. 2021b | |
| I. tubarioides | TENN, CUW | TENN 61324 | PBM 2550 | USA | EU439453 | AY732211 | Matheny & Moreau 2009 |
| I. urceolicystis | KUO | JV1109F (holotype) | Finland | MF804315 | Oertel et al. 2014 | ||
| I. urceolicystis | KR | KR-M-0042248 | DB13-9-14-16 | Germany | MH734512 | Scholler et al. (direct submission 2018, unpublished) | |
| I. urceolicystis | STU | SMNS-STU-F-0901615 | DB17-8-15-15 | Finland | OK057175 | OK057175; OK078914* | This study |
| I. urceolicystis | STU | SMNS-STU-F-0901633 | DB1-10-12-1 | Germany | OK057171 | OK057171; OK078907* | This study |
| I. variispora | SMG-GME | SMG-GME 980504-01 (isotype) | Spain | MT101872 | Bandini et al. 2020c | ||
| I. venerabilis | STU | SMNS-STU-F-0901605 (holotype) | DB25-5-13-2 | Germany | OK057198 | OK057198 | This study |
| I. venerabilis | DB | DB14-6-12-Dondl | Germany | OK057129 | This study | ||
| I. venerabilis | STU | SMNS-STU-F-0901612 | DB25-5-13-5 | Germany | OK057197 | OK057197 | This study |
| I. venerabilis | STU | SMNS-STU-F-0901437 | DB12-5-13-1 | Germany | OK057142 | OK057142 | This study |
| I. venerabilis | STU | SMNS-STU-F-0901606 | DB31-8-13-6b | Switzerland | OK057199 | OK057199 | This study |
| I. woglindeana | STU | SMNS-STU-F-0901435 (holotype) | DB12-5-13-2 | Germany | MT101882 | MT101882 | Bandini et al. 2020c |
| Inocybe sp. | MCVE | MCVE 21486 | Italy | JF908193 | Osmundson et al. 2013 | ||
| Inocybe sp. as I. abjecta | UBC | UBC-F-19255 | Canada | HQ604211 | HQ604211 | Berbee et al. (direct submission 2010) unpublished | |
| Taxon/sample | Herbarium | Voucher no. | Other voucher numbers | Country | GenBank/UNITE acc. no. ITS | GenBank acc. no. LSU; RPB2 | References |
| Inocybe sp. as I. auricoma | UBC | UBC-F-19651 | Canada | HQ604213 | HQ604213 | Berbee et al. (direct submission 2010) unpublished | |
| Inocybe sp. as I. cf. hirtelloides | GB | EL9506 | Sweden | FN550903 | FN550903 | Ryberg et al. 2010 | |
| Inocybe sp. as I. subporospora | MCVE | MCVE 21472 | Italy | JF908186 | Osmundson et al. 2013 | ||
| Nothocybe distincta | CAL | CAL 1310 (holotype) | ZT 9250 | India | KX171343 | KX171344 | Latha et al. 2016 |
| Pseudosperma spurium | GB | SJ92017 (holotype) | Sweden | AM882784.2 | AM882784.2 | Ryberg et al. 2008 | |
| P. spurium | UTC | BK180809723 | USA | JQ408794 | EU600868 | Kropp et al. 2013, Matheny et al. 2009 | |
| EcM | Italy | GU256192 | Lancellotti & Franceschini 2013 | ||||
| EcM | Canada | JX630539 | Timling et al. 2012 | ||||
| EcM | Austria | EU326161 | Mühlmann & Peintner 2008 | ||||
| EcM | France | JQ975963 | JQ975963 | Rincón et al. 2014 | |||
| EcM | France | EU711175 | Roy et al. 2009 | ||||
| EcM | 2-1F_03 | USA | UDB007417 | Smith et al. 2004 | |||
| EcM | B588_Ino_Nowshr | Iran | UDB005410 | Bahram (direct submission 2008, unpublished) | |||
| EcM | G4030 | Morocco | UDB026613 | Tedersoo (direct submission 2016, unpublished) | |||
| EcM | TS1206 | Estonia | UDB013535 | Pärnad (direct submission 2006) | |||
| environmental sample | Iran | FR852221 | Bahram et al. 2012 | ||||
| environmental sample | Iran | FR852249 | Bahram et al. 2012 | ||||
| environmental sample | Iran | FR852239 | Bahram et al. 2012 | ||||
| soil sample | Iran | HE687067 | Bahram et al. 2013 | ||||
| soil sample | PAC2C5a-09 | France | JF506770 | Shahin (direct submission 2011, unpublished) | |||
| soil sample | Canada | KF297178 | KF297178 | Timling et al. 2014 | |||
| soil sample | Austria | EU517033 | EU517033 | Kühnert et al. 2012 | |||
| soil sample | G3564 | Estonia | UDB0356885 | Tedersoo et al. 2020 | |||
| soil sample | G4207 | Estonia | UDB0559922 | Tedersoo et al. 2020 | |||
| soil sample | G4215 | Estonia | UDB0232116 | Tedersoo et al. 2020 | |||
| soil sample | G4231 | Estonia | UDB0302540 | Tedersoo et al. 2020 | |||
| soil sample | G4233 | Estonia | UDB0320504 | Tedersoo et al. 2020 | |||
| soil sample | G4379 | Latvia | UDB0310982 | Tedersoo et al. 2020 | |||
| soil sample | G4419 | Estonia | UDB0613420 | Tedersoo et al. 2020 | |||
| soil sample | G4561 | Estonia | UDB0616408 | Tedersoo et al. 2020 | |||
| soil sample | G4703 | Estonia | UDB0655937 | Tedersoo et al. 2020 | |||
| soil sample | G4809 | Estonia | UDB0201710 | Tedersoo et al. 2020 | |||
| soil sample | G4812 | Estonia | UDB0190916 | Tedersoo et al. 2020 | |||
| soil sample | H06IK44 | Germany | HF675425 | Kapturska et al. (direct submission 2013, unpublished) | |||
| soil sample | IH.M07 | Estonia | UDB061504 | Tedersoo et al. 2020 |
Acknowledgments
We are grateful to the curators Petra Ballings and Ann Bogaerts (BR), Philippe Clerc and Michelle Price (G), Markus Scholler (KR), Nicolien Sol (L), Régis Courtecuisse (LIP), Lorinda Leonardi and Diana Hurlbut (NYS), Jan Holec (PRM) and Marco Floriani (TR) for the loan of specimens in their keeping. We are particularly thankful to Holger Thüs (STU) for handling the numerous loans for us. The Citizen Scientist project of the University of Tartu, i.e., Urmas Kõljalg and Irja Saar, are thanked for providing us with sequences for some of our collections. We greatly appreciate the help of Konstanze Bensch, Shaun R. Pennycook and Volker Braun with all kinds of questions around types and taxa. We would furthermore like to express our gratitude to Jukka Vauras and the herbarium TUR-A for the gift of several specimens, to Xavier Carteret and Patrick Reumaux for the gift of small pieces of several isotypes, and to Guillaume Eyssartier for the gift of an isotype of I. deianae. We would furthermore like to express our gratitude to Matthias Dondl, Petra & Werner Eimann, Stefan Ekman, Åsa Kruys, Thomas Læssøe, Jukka Vauras, Karl Wehr and Helmut Zitzmann for providing us with fresh collections or specimens or for other help. We thank Martin Bemmann for bibliographical, technical and other help. Giovanni Bandini is thanked for logistic and other support. Reviewing long manuscripts can be a burden; we are grateful to the reviewers for having accepted the task and for their valuable input. Thank you also very much to the editorial team of Persoonia.
Declaration on conflict of interest The authors declare that there is no conflict of interest.
REFERENCES
- Aignon HL, Naseer A, Boukary AA. et al. 2021. Inocybaceae and affiliated taxa from West Africa. Moroccan Journal of Agricultural Sciences 2(1): 9–13. [Google Scholar]
- Arnolds E, Chrispijn R, Enzlin R. 2015. Ecologische atlas van paddenstoelen in Drenthe. 3 vols. Stichting Paddestoelen Werkgroep Drenthe, Beilen. [Google Scholar]
- Bahram M, Kõljalg U, Kohout P, et al. 2013. Ectomycorrhizal fungi of exotic pine plantations in relation to native host trees in Iran: evidence of host range expansion by local symbionts to distantly related host taxa. Mycorrhiza 23(1): 11–19. [DOI] [PubMed] [Google Scholar]
- Bahram M, Põlme S, Kõljalg U, et al. 2012. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytologist 193(2): 465–473. [DOI] [PubMed] [Google Scholar]
- Bandini D. 2014. Zusammenfassung der Inocybe-Funde während der siebten Bayerischen Mykologischen Tagung. Mycologia Bavarica 15: 33–36. [Google Scholar]
- Bandini D, Christan J, Eberhardt U. et al. 2017. Inocybe sphagnophila sp. nov., eine neue Art der höckersporigen Untersektion Napipedinae der Gattung Inocybe (Agaricales), Mycologia Bavarica 18: 11–34. [Google Scholar]
- Bandini D, Hampe F, Oertel B. 2013. Eine kleine seltene Inocybe:Inocybe ionochlora Romagnesi. Zeitschrift für Mykologie 79(1): 79–98 . [Google Scholar]
- Bandini D, Huijser H. 2017. Vezelkoppen (Inocybe) van Ameland, Inocybe griseotarda Poirier. Coolia 60(4): 243–247. [Google Scholar]
- Bandini D, Oertel B, Eberhardt U. 2021a. Noch mehr Risspilze (2): Dreizehn neue Arten der Familie Inocybaceae, Mycologia Bavarica 21: 27–98. [Google Scholar]
- Bandini D, Oertel B, Eberhardt U. 2021b. A fresh outlook on the smooth-spored species of Inocybe: type studies and 18 new species, Mycological Progress 20: 1019–1114. [Google Scholar]
- Bandini D, Oertel B, Eberhardt U. 2021c. Inocybe blandula, eine neue höcker-sporige Art der Gattung Inocybe, Sektion Marginatae. Zeitschrift für Mykologie 87(2): 211–228. [Google Scholar]
- Bandini D, Oertel B, Moreau P-A. et al. 2019a. Three new hygrophilous species of Inocybe, subgenus Inocybe. Mycological Progress 18: 1101–1119. 10.1007/s11557-021-01712-w. [DOI] [Google Scholar]
- Bandini D, Oertel B, Ploch S. et al. 2019b. Revision of some central European species of Inocybe (Fr. : Fr.) Fr. subgenus Inocybe, with the description of five new species. Mycological Progress 18: 247–294. 10.1007/s11557-018-1439-9. [DOI] [Google Scholar]
- Bandini D, Oertel B, Ploch S. et al. 2019c. Inocybe heidelbergensis, eine neue Risspilz-Art der Untergattung Inocybe. Zeitschrift für Mykologie 85(2): 195–213. [Google Scholar]
- Bandini D, Oertel B, Schüßler C, et al. 2020a. Noch mehr Risspilze: Fünfzehn neue und zwei wenig bekannte Arten der Gattung Inocybe. Mycologia Bavarica 20: 13–101. [Google Scholar]
- Bandini D, Sesli E, Oertel B. et al. 2020b. Inocybe antoniniana, a new species of Inocybe section Marginatae with nodulose spores. Sydowia 72: 95–106. https://doi.org/ 10.12905/0380.sydowia72-2020-0095. [DOI] [Google Scholar]
- Bandini D, Vauras J, Weholt Ø, et al. 2020c. Inocybe woglindeana, a new species of the genus Inocybe, thriving in exposed habitats with calcareous sandy soil. Karstenia 58: 41–56. [Google Scholar]
- Battarra GA. 1755. Fungorum agri ariminensis historia. Typis Ballantianis, Faenza, Italy. [Google Scholar]
- Bizio E, Castellan A. 2018. Inocybe acutofulva e Inocybe grammopodia var. paleoveneta, due nuovi taxa dall’Alta Marca Trevigiana (Treviso, Veneto, Italia). Micologia e Vegetazione Mediterranea. 32(2): 103–124. [Google Scholar]
- Bizio E, Ferisin G, Dovana F. 2016. Inocybe costinitii. A new species from the Istrian coast. Micologia e Vegetazione Mediterranea 31(2): 95–102. [Google Scholar]
- Bizio E, Marchetti M. 1998 ‘1997’. Le Inocybe dell’Abate Bresadola (attraverso gli autori successivi e revisione di materiale d’erbario), prima parte, Bollettino del Gruppo Micologico G. Bresadola 40(2/3): 91–112. [Google Scholar]
- Bon M. 1984. Macromycètes de la zone maritime picarde (8ème supplément. Les inocybes sabulicoles). Documents Mycologiques 14: 9–40. [Google Scholar]
- Bon M. 1997a. Clé monographique du genre Inocybe (Fr.) Fr. (2ème partie). Documents Mycologiques 27(108): 1–77. [Google Scholar]
- Bon M. 1997b. Clé monographique des inocybes alpins. Bulletin Trimestriel de la Fédération Mycologique Dauphiné-Savoie 37(144): 71–109. [Google Scholar]
- Bon M. 1998. Clé monographique du genre Inocybe (Fr.) Fr. (3ème partie). Documents Mycologiques 28(111): 1–45. [Google Scholar]
- Bougher NL, Matheny PB. 2011. Two species of Inocybe (fungi). Nuytsia 21: 139–148. [Google Scholar]
- Breitenbach J, Kränzlin F. 2000. Pilze der Schweiz, vol. 5. Mykologia, Luzern. [Google Scholar]
- Bresadola G. 1881–1887. Fungi tridentini novi, vel nondum delineati, descripti et iconibus illustrati, vol. I. Zippel, Trento. [Google Scholar]
- Bresadola G. 1930. Iconographia Mycologica, vol.15/16,Società Botanica Italiana, Milano. [Google Scholar]
- Britzelmayr M. 1891. Hymenomyceten aus Südbayern 10/VII. Friedländer & Sohn, Berlin. [Google Scholar]
- Brugaletta E, Consiglio G, Marchetti M. 2019. Inocybe lapidicola, una nuova specie delle Sicilia. Rivista di Micologia 62(2): 99–117. [Google Scholar]
- Bruylants J. 1970 ‘1969’. Inocybe ochroalba sp. nov.. Bulletin de la Société Mycologique de France 85(3): 345–349. [Google Scholar]
- Carteret X, Reumaux P. 2012 ‘2011’. Miettes sur les Inocybes (6ème série), études de quelques nains des feuillus de la plaine, accompagnée d’une clé de détermination des taxons de la section Lilacinae R. Heim. Bulletin de la Société Mycologique de France 127(1-2): 1–53. [Google Scholar]
- Carteret X, Reumaux P. 2017 ‘2015’. Miettes sur les Inocybes (8e série). Inocybes jaunes ou jaunâtres. Bulletin de la Société Mycologique de France 131(1-2): 1–96. [Google Scholar]
- Cervini M. 2021. Inocybe messapica (Inocybaceae, Agaricales, Basidiomycota), a new species in section Splendentes, from Mediterranean oak woods. Phytotaxa 480(2): 174–184. [Google Scholar]
- Cripps CL, Eberhardt U, Schütz N, et al. 2019a. The genus Hebeloma in the Rocky Mountain alpine zone. MycoKeys 46: 1–54. 10.3897/mycokeys.46.32823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps CL, Larsson E, Vauras J. 2019b. Nodulose-spored Inocybe from the Rocky Mountain alpine zone molecularly linked to European and type specimens. Mycologia 112: 133–153. 10.1080/00275514.2019.1677419. [DOI] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W. et al. 2014. Fungal Planet description sheets: 213–280. Persoonia 32: 184–306 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI. et al. 2017. Fungal Planet description sheets: 558–624. Persoonia 38: 240–384. https://doi.org/ 10.3767/003158517X698941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dähnke RM. 2001. 1200 Pilze in Farbfotos. Bechtermünz und Weltbild Verlag, Augsburg. [Google Scholar]
- Dovana F, Bizio E, Garbelotto M. et al. 2021. Inocybe cervenianensis (Agaricales, Inocybaceae), a new species in the I. flavoalbida clade from Italy. Phytotaxa 484(2): 227–236. [Google Scholar]
- Enderle M. 2004. Die Pilzflora des Ulmer Raumes, Süddeutsche Verlagsgesellschaft Ulm, Ulm. [Google Scholar]
- Esteve-Raventós F. 1997. Studies on the genus Inocybe in the Iberian Peninsula and Balearic Islands III. nocybe catalaunica. Mycotaxon 62: 421–425. [Google Scholar]
- Esteve-Raventós F, Bandini D, Oertel B, et al. 2018. Advances in the knowledge of the Inocybe mixtilis group (Inocybaceae, Agaricomycetes), through molecular and morphological studies. Persoonia 41: 213–236. 10.3767/persoonia.2018.41.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve-Raventós F, Macau N, Ferville A. 2012 ‘2011’. Inocybe neorufula sp. nov., un nouveau nom pour I. rufula au sens de Malençon. Bulletin de la Société Mycologique de France 127: 189–200. [Google Scholar]
- Esteve-Raventós F, Moreno G, Alvarado P, et al. 2016. Unraveling the Inocybe praetervisa group through type studies and ITS data: Inocybe praetervisoides sp. nov. from the Mediterranean region. Mycologia 108: 123–134. [DOI] [PubMed] [Google Scholar]
- Eyssartier G. 2007. Inocybe deianae sp. nov., un taxon proche d’Inocybe tenuicystidiata. Bulletin Mycologique et Botanique Dauphiné-Savoie 186: 35–42. [Google Scholar]
- Fan Y-G, Bau T. 2010. A revised checklist of the genus Inocybe (Fr.) Fr. in China. Journal of Fungal Research 8(4): 189–193. [Google Scholar]
- Fan Y-G, Bau T. 2013. Two striking Inocybe species from Yunnan Province, China. Mycotaxon 123(1): 169–181. [Google Scholar]
- Fan Y-G, Bau T. 2020. Two new smooth-spored species of Inocybe (Inocybaceae, Agaricales) from Gansu Province, northwestern China. Mycosystema 39(9): 1694–1705. [Google Scholar]
- Fan Y-G, Wu RH, Bau T. 2018. Two new species and eight newly recorded species of Inocybe subg. Inocybe from China. Journal of Fungal Research 16(2): 70–83. [Google Scholar]
- Farooqi A, Aqdus F, Niazi AR. et al. 2017. Inocybe ahmadii sp. nov. and a new record of I. leptocystis from Pakistan. Mycotaxon 132: 257–269. [Google Scholar]
- Favre J. 1955. Les champignons supérieurs de la zone alpine du parc national Suisse. Ergebnisse der wissenschaftlichen Untersuchungen des schweizerischen Nationalparks NF 5: 1–212. [Google Scholar]
- Fernández Sasia R. 2002. Sur une nouvelle espèce du genre Inocybe (Fr. : Fr.) Fr. identifiée au Pays Basque espagnol: Inocybe variispora. Documents Mycologiques 231(124): 25–31. [Google Scholar]
- Ferrari E. 2006. Inocybe alpine e subalpine. Fungi non Delineati, vol. XXXIV–XXXVI. Edizioni Candusso, Alassio. [Google Scholar]
- Ferrari E. 2010. Inocybe dai litorali alla zona alpina. Fungi non delineati, vol. LIV–LV. Edizioni Candusso, Alassio. [Google Scholar]
- Fries E. 1836–1838. Epicrisis systematis mycologici seu synopsis hymenomycetum. Typographia academica, Upsala. [Google Scholar]
- Gerhardt E. 2001. Der große BLV Pilzführer für unterwegs. Über 1200 Arten, über 1000 Farbfotos. BLV Verlagsgesellschaft mbH, München. [Google Scholar]
- Gminder A. 2010. Ständerpilze: Blätterpilze III. (Die Großpilze BadenWürttembergs, vol. 5), Ulmer, Stuttgart. [Google Scholar]
- Grund DW, Stuntz DE. 1968. Nova Scotian Inocybes. I. Mycologia 60: 406–425. [Google Scholar]
- Grund DW, Stuntz DE. 1981. Nova Scotian Inocybes. VI. Mycologia 73: 655–674. [Google Scholar]
- Guindon S, Dufayard J-F, Lefort V, et al. 2010. New algorithms and methods to estimate Maximum-Likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biololgy 59: 307–321. 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Heim R. 1931. Le genre Inocybe (Encyclopédie Mycologique). Lechevalier, Paris. [Google Scholar]
- Hoang DT, Chernomor O, Von Haeseler A, et al. 2018. UFBoot 2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren PK, Holmgren NH, Barnett LC. 1990. Index Herbariorum, 8th edn. Botanic Garden, New York. [Google Scholar]
- Horak E, Stangl J. 1980. Notizen zur Taxonomie und Verbreitung von Inocybe leptocystis. Sydowia 33: 145–151. [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y. et al. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res 36 (Web Server issue): W5–W9. 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF. et al. 2017. ModelFinder: Fast model selection for accurate phylogenetic estimates, Nature Methods 14: 587–589. 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten PA. 1879. Rysslands, Finlands och den Skandinaviska halföns Hattsvampar. Förra Delen: Skifsvampar. Bidrag till Kännedom av Finlands Natur och Folk 32: 1–571. [Google Scholar]
- Karsten PA. 1889. Kritisk öfverisgt af Finlands Basidsvampar (Basidiomycetes; Gastero- & Hymenomycetes). Bidrag till Kännedom av Finlands Natur och Folk 48: 1–470. [Google Scholar]
- Katoh K, Kuma K, Toh H. et al. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511–518. 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H, Vesterholt J. 2008. Funga Nordica: agaricoid, boletoid and cyphelloid genera. Nordsvamp, Copenhagen. [Google Scholar]
- Kõljalg U, Larsson K-H, Abarenkov K, et al. 2005. UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytologist 166: 1063–1068. [DOI] [PubMed] [Google Scholar]
- Kropp BR, Matheny PB, Hutchison LJ. 2013. Inocybe section Rimosae in Utah: phylogenetic affinities and new species. Mycologia 105: 728–747. 10.3852/12-185. [DOI] [PubMed] [Google Scholar]
- Kühner R. 1955. Compléments a la ‘Flore analytique’ V. Inocybes léiosporés cystidiés. Espèces nouvelles ou critiques. Bulletin de la Société des Naturalistes d’Oyonnax pour l’étude et la diffusion des sciences naturelles dans la région 9: 3–95. [Google Scholar]
- Kühnert R, Oberkofler I, Peintner U. 2012. Fungal growth and biomass development is boosted by plants in snow-covered soil. Microbial Ecology 64: 79–90. [DOI] [PubMed] [Google Scholar]
- Kuyper TW. 1986. A revision of the genus Inocybe in Europe. I. Subgenus Inosperma and the smooth-spored species of subgenus Inocybe. Persoonia Suppl. 3: 1–247. [Google Scholar]
- Kuyper TW. 1989. Studien in Inocybe IV. Zeitschrift für Mykologiex 55: 111–114. [Google Scholar]
- Læssøe T, Petersen JH. 2019. Fungi of temperate Europe. 2 vols. Princeton University Press, Princeton and Oxford. [Google Scholar]
- Lancellotti E, Franceschini A. 2013. Studies on the ectomycorrhizal community in a declining Quercus suber L. stand. Mycorrhiza 23: 533–542. 10.1007/s00572-013-0493-z. [DOI] [PubMed] [Google Scholar]
- Lange JE. 1917. Studies in the Agarics of Denmark. III. Pluteus, Collybia, Inocybe. Dansk botanist Arkiv 2(7): 1–50. [Google Scholar]
- Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 30: 3276–3278. 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E, Cripps CL, Vauras J. 2014. Inocybe leiocephala Stuntz, a species with an intercontinental distribution range mainly in arctic, alpine and subalpine habitats – disentangling the I. leiocephala – subbrunnea – catalaunica morphological species complex. Karstenia 54: 15–39. [Google Scholar]
- Larsson E, Vauras J, Cripps CL. 2018a ‘2017’. Inocybe lemmi, a new species of section Marginatae from the alpine region of Sweden. Karstenia 57: 1–9. [Google Scholar]
- Larsson E, Vauras J, Cripps CL. 2018b. Inocybe praetervisa group – A clade of four closely related species with partly different geographical distribution ranges in Europe. Mycoscience 59(4): 277–287. 10.1016/j.myc.2017.11.002. [DOI] [Google Scholar]
- Latha KPD, Manimohan P, Matheny PB. 2016. A new species of Inocybe representing the Nothocybe lineage. Phytotaxa 267: 40–50. [Google Scholar]
- Ludwig E. 2017. Pilzkompendium, vol. 4 (parts 1 & 2). Fungicon, Berlin. [Google Scholar]
- Malençon G, Bertault R. 1970. Flore des champignons superieurs du Maroc, vol. 1, Faculté de Science, Rabat. [Google Scholar]
- Marchetti M, Franchi P, Consiglio G. 2014. Tipificatione di alcune Inocybe di Britzelmayr. Rivista di Micologia 57(2): 127–178. [Google Scholar]
- Matheny PB. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Molecular Phylogenetics and Evolution 35(1): 1–20. [DOI] [PubMed] [Google Scholar]
- Matheny PB. 2009. A phylogenetic classification of the Inocybaceae. McIlvainea 18: 11–21. [Google Scholar]
- Matheny PB, Aime MC, Bougher NL. et al. 2009. Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. Journal of Biogeography 36: 577–592. 10.1111/j.1365-2699.2008.02055.x. [DOI] [Google Scholar]
- Matheny PB, Bougher NL. 2017. Fungi of Australia, Inocybaceae. ABRS & CSIRO Publishing, Canberra & Melbourne. [Google Scholar]
- Matheny PB, Hobbs AM, Esteve-Raventós F. 2020 ‘2019’. Genera of Inocybaceae: New skin for the old ceremony. Mycologia 112(1): 83–120. 10.1080/00275514.2019.1668906. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Kudzma LV. 2019. New species of Inocybe (Inocybaceae) from eastern North America 1 . The Journal of the Torrey Botanical Society 146(3): 213–235. [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF. et al. 2002. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Moreau PA. 2009. A rare and unusual lignicolous species of Inocybe (Agaricales) from eastern North America. Brittonia 61: 163–171. 10.1007/s12228-008-9066-4. [DOI] [Google Scholar]
- Matheny PB, Norvell LL, Giles EC. 2013. A common new species of Inocybe in the Pacific Northwest with a diagnostic PDAB reaction. Mycologia 105(2): 436–446. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Swenie RA. 2018. The Inocybe geophylla group in North America: a revision of the lilac species surrounding I. lilacina. Mycologiax 110: 618–634. 10.1080/00275514.2018.1469880. [DOI] [PubMed] [Google Scholar]
- Mešić A, Haelewaters D, Tkalčec Z, et al. 2021. Inocybe brijunica sp. nov., a new ectomycorrhizal fungus from Mediterranean Croatia revealed by morphology and multilocus phylogenetic analysis. Journal of Fungi 7: 199. 10.3390/jof7030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Lanfear R, Trifinopoulos J. et al. 2021. IQ-TREE version 2.1.2: Tutorials and ManualPhylogenomic software by maximum likelihood. March 15, 2021. http://www.iqtree.org/doc/iqtree-doc.pdf. [Google Scholar]
- Minh BQ, Nguyen MAT, Von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30: 1188–1195. 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlmann O, Peintner U. 2008. Mycobionts of Salix herbacea on a glacier forefront in the Austrian Alps. Mycorrhiza 18(4): 171–180. [DOI] [PubMed] [Google Scholar]
- Muñoz G, Pancorbo F, Turégano Y, et al. 2022. New species and combinations of Inocybe with lilac or violet colours in Europe. Fungi Iberici 2: 7–26. 10.51436/funiber//02.001. [DOI] [Google Scholar]
- Munsell O. 2009. Soil color charts. X-Rite, Grand Rapids MI. [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel B, Bandini D, Vauras J. 2014. Zwei aus Finnland beschriebene Risspilze in Deutschland nachgewiesen: Inocybe urceolicystis Stangl & Vauras und Inocybe ericetorum Vauras & Kokkonen. Zeitschrift für Mykologie 80(1): 43–79. [Google Scholar]
- Osmundson TW, Robert VA, Schoch CL. et al. 2013. Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLoS One 8(4): e62419. 10.1371/journal.pone.0062419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CH. 1887. Descriptions of new species of New York fungi. Bulletin of the New York State Museum 1(2): 5–24. [Google Scholar]
- Peintner U, Bougher NL, Castellano MA. et al. 2001. Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). American Journal of Botany 88(12): 2168–2179. [PubMed] [Google Scholar]
- Poirier J. 2002. Notes sur le genre Inocybe – 1. Documents Mycologiques 31(124): 3–13. [Google Scholar]
- Poirier J. 2016. Études dans le genre Inocybe – 3. Bulletin Mycologique et Botanique Dauphiné-Savoie 223: 53–63. [Google Scholar]
- Quélet L. 1872. Les Champignons du Jura et des Vosges. Mémoires de la Société d’Émulation de Montbéliard. ser. 2, 5: 43–332. [Google Scholar]
- Rambaut A.2006–2018. FigTree. Tree figure drawing tool version 14.4. Institute of Evolutionary Biology, University of Edinburgh. Available from http://tree.bio.ed.ac.uk/ [accessed 20 Mar. 2019]. [Google Scholar]
- Reumaux P. 1986. Notes sur quatres Inocybes d’allure insignifiante – 1ère partie. Bulletin trimestriel de la Fédération mycologique et botanique Dauphiné-Savoie 25(100): 13–17. [Google Scholar]
- Rincón A, Santamaría BP, Ocaña L, et al. 2014. Structure and phylogenetic diversity of post-fire ectomycorrhizal communities of maritime pine. Mycorrhiza 24: 131–141. [DOI] [PubMed] [Google Scholar]
- Roy M, Yagame T, Yamato M. et al. 2009. Ectomycorrhizal Inocybe species associate with the mycoheterotrophic orchid Epipogium aphyllum but not its asexual propagules. Annals of Botany 104(3): 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg M, Larsson E, Jacobsson S. 2010. An evolutionary perspective on morphological and ecological characters in the mushroom forming family Inocybaceae (Agaricomycotina, Fungi). Molecular Phylogenetics and Evolution 55: 431–442. [DOI] [PubMed] [Google Scholar]
- Ryberg M, Nilsson RH, Kristiansson E. et al. 2008. Mining metadata from unidentified ITS sequences in GenBank, a case study in Inocybe (Basidiomycota). BMC Evolutionary Biology 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. 1947. Champignons de la Catalogne. Espèces observées en 1934. Collectanea Botanica 1(3): 199–246. [Google Scholar]
- Smith JE, McKay D, Niwa CG. et al. 2004. Short-term effects of seasonal prescribed burning on the ectomycorrhizal fungal community and fine root biomass in ponderosa pine stands in the Blue Mountains of Oregon. Canadian Journal of Forest Research 34: 2477–2491. [Google Scholar]
- Sowerby J. 1803. Coloured figures of English fungi or mushrooms. Wilks, London, Great Britain. [Google Scholar]
- Stangl J. 1983. Inocybe nitidiuscula (Britz.) Sacc., gültiger Name für Inocybe friesii Heim. International Journal of Mycology and Lichenology 1(2): 161–168. [Google Scholar]
- Stangl J. 1989. Die Gattung Inocybe in Bayern. Hoppea 46: 5–388. [Google Scholar]
- Stangl J, Vauras J. 1987. Über das Genus Inocybe in Finnland. Die neuen Arten I. mytiliodora und I. urceolicystis. Karstenia 27(1): 15–21. [Google Scholar]
- Stangl J, Veselský J. 1973. Zweiter Beitrag zur Kenntnis der selteneren Inocybe-Arten. Česká Mykologie 27: 11–25, pl. 83. [Google Scholar]
- Stangl J, Veselský J. 1974. Fünfter Beitrag zur Kenntnis der selteneren Inocybe-Arten. Česká Mykologie 28: 195–218, pl. 86. [Google Scholar]
- Stangl J, Veselský J. 1975. Beiträge zur Kenntnis der selteneren Inocyben Nr. 6, Inocybe albidodisca Kühner und etliche ähnliche der gänzlich stielbereiften Glattsporigen. Ceská Mykologie 29(2): 65–78. [Google Scholar]
- Stangl J, Veselský J. 1979. Inocybe metrodii sp. nov.. Beiträge zur Kenntnis seltenerer Inocyben 16. Česká Mykologie 33(4): 220–224. [Google Scholar]
- Streintz WM. 1862. Nomenclator fungorum exhibens ordine alphabetico nomina tam genera quam specifica ac synonyma a scripteribus de scientia botanica fungis imposita. Gerischek, Vienna, Austria. [Google Scholar]
- Stuntz DE. 1947. Studies in the genus Inocybe. I. New and noteworthy species from Washington. Mycologia. 39(1): 21–55. [PubMed] [Google Scholar]
- Tedersoo L, Anslan S, Bahram M. et al. 2020. Regional-scale in-depth analysis of soil fungal diversity reveals strong pH and plant species effects in northern Europe. Frontiers Microbiology x 11: 1953. 10.3389/fmicb.2020.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timling I, Dahlberg A, Walker DA. et al. 2012. Distribution and drivers of ectomycorrhizal fungal communities across the North American Arctic. Ecosphere 3(11): 1–25. [Google Scholar]
- Timling I, Walker DA, Nusbaum C. et al. 2014. Rich and cold: diversity, distribution and drivers of fungal communities in patterned-ground ecosystems of the North American Arctic. Molecular Ecology 23(13): 3258–3272. [DOI] [PubMed] [Google Scholar]
- Treindl AD, Leuchtmann A. 2019. A king amongst dwarfs: Boletus edulis forms ectomycorrhiza with dwarf willow in the Swiss Alps. Alpine Botany 129(2): 185–189. 10.1007/s00035-019-00218-2. [DOI] [Google Scholar]
- Trifinopoulos J, Nguyen L-T, Von Haeseler A, et al. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44 (W1): W232–W 235 . 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauras J, Larsson E. 2016a ‘2015’. Inocybe caprimulgi and I. lacunarum, two new nodulose-spored species from Fennoscandia. Karstenia 55: 1–18. [Google Scholar]
- Vauras J, Larsson E. 2016b. Inocybe baltica and I. suecica, two new smooth-spored species from the Baltic Sea region. Karstenia 56: 13–26. [Google Scholar]
- Velenovský J. 1920–1922. České Houby, Díl 1- 5.České Botanické Společnosti, Praze. [Google Scholar]
- Vellinga EC. 1988. Glossary. In: Bas C, Kuyper TW, Noordeloos ME, et al. (eds), Flora Agaricina Neerlandica vol 1. Balkema, Rotterdam. [Google Scholar]
- Wurzbacher C, Larsson E, Bengtsson-Palme J, et al. 2019. Introducing ribosomal tandem repeat barcoding for fungi. Molecular Ecology Resources 19: 118–127. [DOI] [PubMed] [Google Scholar]


