Abstract
CT scan imaging provides high-resolution images of the lungs in patients with chronic respiratory diseases. Extensive research over the last several decades has focused on developing novel quantitative CT scan airway measurements that reflect abnormal airway structure. Despite many observational studies demonstrating that associations between CT scan airway measurements and clinically important outcomes such as morbidity, mortality, and lung function decline, few quantitative CT scan measurements are applied in clinical practice. This article provides an overview of the relevant methodologic considerations for implementing quantitative CT scan airway analyses and provides a review of the scientific literature involving quantitative CT scan airway measurements used in clinical or randomized trials and observational studies of humans. We also discuss emerging evidence for the clinical usefulness of quantitative CT scan imaging of the airways and discuss what is required to bridge the gap between research and clinical application. CT scan airway measurements continue to improve our understanding of disease pathophysiologic features, diagnosis, and outcomes. However, a literature review revealed a need for studies evaluating clinical benefit when quantitative CT scan imaging is applied in the clinical setting. Technical standards for quantitative CT scan imaging of the airways and high-quality evidence of clinical benefit from management guided by quantitative CT scan imaging of the airways are required.
Key Words: airway disease, COPD, CT scan, imaging, quantitative imaging
Diseases affecting the airways continue to be a major source of morbidity and mortality worldwide.1 Over the last 40 years, numerous studies have shown that CT scan imaging can provide high-resolution images of airway structures. According to the Quantitative Imaging Biomarkers Alliance,2 quantitative imaging is the extraction of quantifiable disease features from medical images that reflect the severity, degree of change, or status of a disease. Quantitative imaging also involves the standardization of acquisition protocols, data analysis, and display methods to enable accurate and reproducible measurements to be obtained.2 Application of quantitative CT scan measures of airway structure in the research setting have provided important insights into disease pathophysiologic characteristics.
Quantitative CT scan airway measurements have been applied extensively in the research setting to advance our understanding of airway disease risk, pathophysiologic features, treatment effect, and prognosis. For example, CT scan airway measurements can identify early structural changes before irreversible airway flow limitation is detected in patients with COPD.3,4 CT scan airway wall measurements in COPD also have been shown to correlate significantly with patient physiologic features,5 symptoms,6 exacerbations,7 morbidity,8,9 mortality,8, 9, 10 and lung function decline.8 More recently, it has been shown that individuals with evidence on CT scan imaging of dysanapsis, a mismatch between airway tree caliber and lung size arising early in life,11 have an increased risk of COPD developing years later,12 even in the absence of traditional risk factors such as smoking. These observations suggest that standardized quantitative CT scan airway measurements have potential to advance understanding of disease pathogenesis, risk prediction and prognosis, and participant selection for clinical trials.
However, high-quality evidence of clinical benefit from quantitative CT scan imaging-guided management of the airway is limited. Translation of such tools to clinical practice can be described best as emergent in select areas. Accordingly, the goal of this article is to provide a review of the relevant literature supporting clinical benefit of quantitative CT scan imaging of the airway and to discuss what evidence is required to bridge the gap between research and clinical application to improve respiratory health outcomes.
Methodological Considerations for CT Scan Airway Analysis
To quantify airway tree structure on CT scan imaging, one must consider the complex branching structure of the airway tree and the methods used both to acquire the CT scan images and to extract the airway trees from those images. Herein, we highlight some of the relevant considerations and innovations that have made quantitative CT scan assessment of airway tree structure feasible.
Quantifying Airway Tree Structure on CT Scan Imaging: Image Acquisition
Contemporary thoracic CT scan images are acquired using multidetector CT scan imaging scanners with the patient supine and coached to achieve a specific lung volume (typically suspended maximal inspiration, although multivolume acquisitions yield additional information). A number of important technical considerations apply when acquiring CT scan images for quantitative airway analysis. Numerous studies have demonstrated factors that impact image quality, including scanner-related factors (eg, CT scan imaging scanner and model, radiation dose, reconstruction kernel, field of view, and voxel size) as well as subject-related factors (eg, BMI, positioning within the CT scan imaging scanner bore, and breath-hold volume).13,14 Specifically, these factors impact the Hounsfield units (HU), the voxel-level CT scan density that reflects the underlying tissue composition that in turn is used to identify airway structures (eg, lumen and wall). Importantly, standardized image acquisition protocols to be used across sites for large, multicenter quantitative CT scan imaging research studies have been developed and implemented successfully for image data collection.15 Although these standardized protocols minimize differences in image quality between sites, either harmonization or analysis methods used after acquisition to overcome heterogeneity across the diversity of scanner and patient factors encountered in clinic practice are still needed. Toward this goal, Quantitative Imaging Biomarkers Alliance Biomarker Committees have worked to harmonize acquisition methods across vendors and to define quantitative CT scan protocol requirements that obtain repeatable CT scan measurements, such as the Lung Density Biomarker Committee for measuring CT scan emphysema.16
Quantifying Airway Structure on CT Scan Imaging: Segmentation
Another technical consideration is how to extract (segment) the airway tree from the surrounding lung parenchyma. A number of techniques have been developed for airway tree segmentation over the last several decades.17 However, airway tree segmentation in CT scan images is a challenging task because of the multiplane (ie, airway branches are directed in various orientations) and multiscale (ie, airway diameters ranging from 10-20 mm [eg, trachea] to submillimeter [eg, terminal bronchioles]) anatomic features of the airway tree, combined with the finite resolution of CT scan images. For example, CT scan images typically have a slice thickness of approximately 1 mm and may contain > 300 slices of the chest. Because of the airway branching structure, not all airways are aligned perpendicular to the imaging plane. For the larger airways, misalignment may lead to partial volume effects, whereby the HU values inside the airway lumen increase because of partial volume averaging of the airway wall. This reduced contrast between the airway lumen and wall can lead to challenges when performing image segmentation. In addition, for the smaller airways that align within the plane of the CT scan image, the airway may fall partially or entirely within the slice thickness, making these airways impossible to segment.
Quantifying Airway Structure on CT Scan Imaging: Measurements
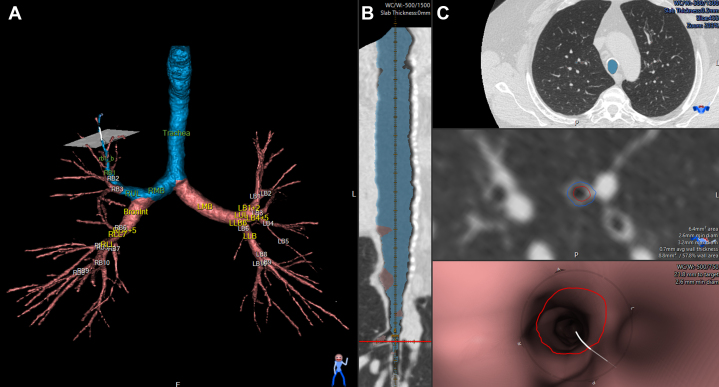
Airway tree segmentation can be used for extraction of numerous morphometric measurements. Figure 1 shows an example of commercial software for generating 3-D CT scan airway tree segmentation (VIDA Diagnostics, Inc.). The segmented and labeled airway tree can be visualized in three dimensions (Fig 1A), as the curved planar reconstruction to a particular airway of interest (Fig 1B), and in an orthogonal view on the CT scan image or a fly-through view (Fig 1C). As shown in Figure 1C, segmentation of the airway lumen and outer wall can generate numerous quantitative measurements, including airway lumen and outer wall area or diameter, wall area or thickness, or percentage of the airway that is airway wall. The square root of wall area of a 10-mm lumen perimeter, is calculated by plotting the square root wall area vs the internal perimeter and is used as a global measurement of airway wall thickness. Importantly, such morphologic measurements have been investigated for quantifying abnormalities in the trachea,18 the large airways (right main bronchi, left main bronchi, right intermediate bronchus, trifurcation of left lower lobe),19 and at the segmental and subsegmental level.6,20 Although VIDA software for airway tree segmentation is detailed herein, we note that a number of vendors, such as the Airway Inspector - Chest Imaging Platform, 3D Slicer, Imbio, Thirona, and Fluidda, provide airway tree segmentation.
Figure 1.
A-C, Example of commercial software for airway tree segmentation. A, CT scan airway tree segmentation generated using VIDA Vision Software (VIDA Diagnostics, Inc.) showing the segmented and labeled airway tree. A path to the subsegment of interest (RB1_b) is highlighted in blue. B, Curved planar reconstructions of the airway path to the subsegment of interest. C, Orthogonal segmentation of the airway lumen and airway outer wall. Segmentation of the lumen and outer wall can produce numerous quantitative measurements, such as the lumen and outer wall area or diameter, as well as the airway wall area or thickness.
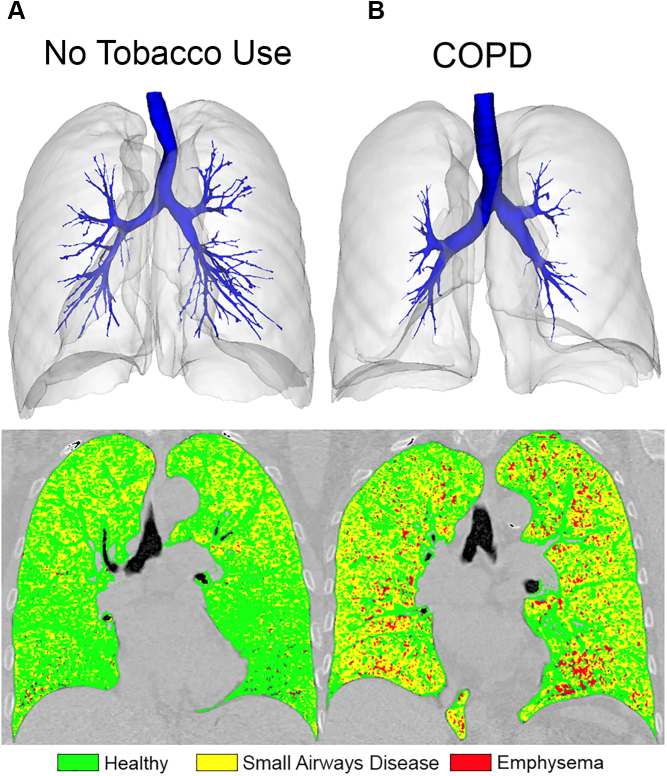
Quantitative approaches also have been introduced to overcome the limitation of quantifying the small airways beyond the resolution of the CT scan system. For example, counting the total number of airways visible on CT scan imaging, known as the total airway count (TAC),21 does not require accurate segmentation of the airway lumen or wall, only airway detection. A reduction in the number of airways detected on CT scan imaging may be caused by narrowing or obstruction of the airway lumen resulting from remodeling or mucus plugging, thinning of the airway wall, or destruction of the airway. Importantly, reduced CT scan imaging TAC is associated with severity of COPD,21 and airway count on CT scan imaging has been shown to be associated with the total number of terminal bronchioles.22 The parametric response map23 is another approach for quantifying gas trapping resulting from small airways obstruction by voxel-wise registration of CT scan images acquired at full inspiration and full expiration. The CT scan parametric response mapping (PRM) small airways disease measurement also has been shown to correlate with terminal bronchiole narrowing, loss, and destruction in COPD.24 Figure 2 shows the 3-D airway tree segmentation overlaid with the whole lung segmentation and PRM map for a 71-year-old woman who had never used tobacco and a 64-year-old woman with COPD. The number of airways that can be quantified on CT scan imaging is reduced in the patient who had never used tobacco with COPD (TAC, 128) compared with the patient who had never used tobacco (TAC, 300), and the extent of small airways disease (indicated by the yellow voxels) is increased in the individual with COPD (patient who had never used tobacco, PRM functional small airway disease = 28.8%; patient with COPD, PRM functional small airway disease = 49.6%).
Figure 2.
CT scan 3-D airway tree and PRM map for a participant who had never used tobacco (A) and a participant with COPD (B). 3-D airway tree (in blue) with the lung segmentation overlaid, as well as the PRM map (green = normal, yellow = small airways disease, red = emphysema) is provided for two representative participants. The participant who had never used tobacco (A) is a 71-year-old woman with FEV1 of 115.3% predicted and FEV1 to FVC ratio of 74.8%. The participant with COPD (B) is a 64-year-old woman with FEV1 of 46.9% predicted and FEV1 to FVC ratio of 52.9%. Compared with the participant who had never used tobacco, the participant with COPD showed a visually and quantitatively reduced number of CT scan airways as measured by total airway count (TAC; patient who had never used tobacco, TAC = 300; patient with COPD, TAC = 128) and increased extent of small airways disease as measured by PRM functional small airway disease (patient who had never used tobacco, CT PRM functional small airway disease = 28.8%; patient with COPD, CT PRM functional small airway disease = 49.6%). PRM = parametric response mapping.
Literature Search: The Gap Between Research and Clinical Application
Quantitative CT scan imaging of the airways has substantially advanced our scientific understanding of normal and abnormal physiologic features of the respiratory system. From population-based studies demonstrating interindividual differences in native airway tree structure12,25 to innovative imaging techniques to detect small airways pathophysiologic features in vivo,22,24 research applications of quantitative CT scan imaging of the airways have provided unique insights into the heterogeneity of airway disease susceptibility, impairment, and prognosis. In the therapeutic space, quantitative CT scan imaging of the airways also has been used as a surrogate end point in clinical studies targeting airway diseases (eg, cystic fibrosis, sleep-disordered breathing, asthma, COPD, bronchiectasis, and chronic rhinosinusitis).26, 27, 28, 29, 30, 31 Despite these important research advances, evidence-based clinical applications of quantitative CT scan imaging of the airways remain nascent. Quantitative CT scan imaging of the airways has the potential to facilitate diagnosis, to increase the yield of diagnostic procedures, to prognosticate, and to guide treatment, but real-world uptake requires high-quality evidence of meaningful patient benefit and value in the intended setting. Herein is a survey of quantitative CT scan imaging of the airways techniques that are approaching clinical application. We searched Medline from 1971 (first patient CT scan) through November 1, 2022, for the terms (“computed tomography” OR “CT”) AND (“airway” OR “bronchus” OR “bronchial”), restricting the search to clinical or randomized trials and observational studies of humans (n = 741 citations).
Evidence Review
Clinical Applications
Tracheobronchomalacia and Excessive Dynamic Airway Collapse
Airway lumen narrowing on expiration is associated with chronic dyspnea, cough, and airflow obstruction.32 Although these clinical features overlap with diseases such as COPD and asthma, the underlying pathophysiologic features32, 33, 34, 35 and potential management strategies36, 37, 38 differ. The prevalence of excessive central airway lumen narrowing among patients with chronic respiratory symptoms has been estimated via meta-analysis to be 27% (95% CI, 11%-46%),39 making it an important consideration when evaluating patients with respiratory symptoms.
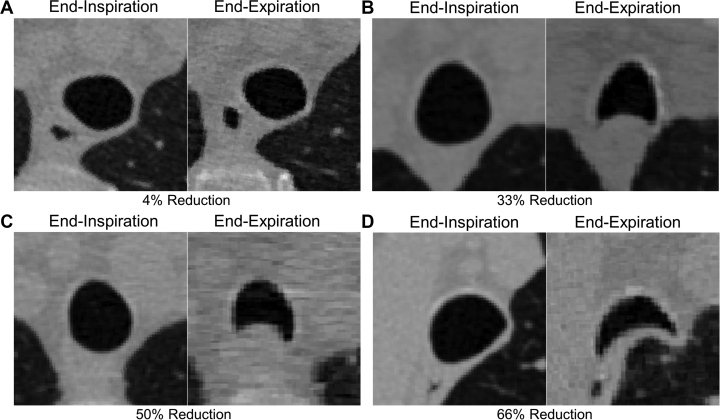
Tracheobronchomalacia and excessive dynamic airway collapse (EDAC) both are airway abnormalities categorized by expiratory central airway collapse (ECAC). Although tracheobronchomalacia and EDAC are used interchangeably in the literature, they are distinct diseases, which leads to confusion and no clear guidelines for diagnosis and treatment. Tracheobronchomalacia is defined strictly as a pathologic weakness of the cartilage that supports the tracheobronchial tree and may occur in the trachea, trachea and main bronchi, or the bronchi alone.32 In contrast, EDAC is used to describe the excessive invagination of the posterior tracheal membrane into the airway lumen during expiration, but with intact cartilage.32 Quantitative CT scan imaging is the commonly reported approach to diagnose ECAC40, 41, 42, 43 using a threshold of ≥ 50% reduction in cross-sectional lumen area from end inspiration to expiration. Figure 3 shows CT scan images acquired at end inspiration and end expiration for four participants with COPD with various degrees of ECAC as quantified by the magnitude of reduction in cross-sectional lumen area from end inspiration to expiration. The top two participants with COPD did not have ECAC by definition, with a reduction in the cross-sectional lumen area of the trachea from end inspiration to end expiration that measured 4% (Fig 3A) and 33% (Fig 3B). In contrast, the bottom two participants with COPD did have ECAC, with a reduction in cross-sectional area from end inspiration to end expiration that measured 50% (Fig 3C) and 66% (Fig 3D). However, EDAC often is defined as a ≥ 80% reduction in cross-sectional lumen area.42,43 Another important methodologic consideration is the breathing protocol used during image acquisition. Studies show that tracheal collapse on CT scan imaging measured during a static end-expiration breathing maneuver differs from tracheal measurements obtained during dynamic or forced expiration and that the magnitude of static end-expiratory tracheal collapse is not predictive of the magnitude of dynamic expiratory collapse.42 Dynamic expiratory imaging should be considered when excessive expiratory tracheal collapse is suspected clinically.
Figure 3.
A-D, CT scan imaging quantifying end-expiratory central airway collapse in four participants with COPD. A, B, Two participants with COPD without central airway collapse (defined as present if ≥ 50% decrement in cross-sectional airway lumen area from inspiration to expiratory scan). The participants showed a 4% (A) and 33% (B) reduction in cross-sectional lumen area within the trachea between end-inspiration and end-expiration CT scan imaging. C, D, Two participants with COPD with central airway collapse. The participant showed a 50% reduction (C) and a 66% reduction (D) in cross-sectional airway lumen area from inspiratory to expiratory scan, respectively.
In the largest study to date, Bhatt et al33 assessed ECAC among tobacco-exposed people using paired noncontrast inspiratory-expiratory CT scans acquired at maximum inspiration and end-tidal expiration. First, CT scan airway segmentation software (VIDA Diagnostics, Inc.) was used to screen inspiratory-expiratory scans, and participants with at least a 50% reduction in cross-sectional lumen area from inspiration to expiration were categorized as having screen-positive results for ECAC. Using image viewing window levels set at −550 to −700 HU and window width of 1,200 to 1,500 HU, ECAC then was confirmed by expert reader manual measurements and was defined as present if a ≥ 50% decrement in cross-sectional airway lumen area from inspiration to expiratory scan was observed at one of three standard anatomic locations (at the level of the aortic arch, at the main carina, or at the bronchus intermedius, just distal to the origin of the right upper lobe bronchus). The 50% threshold is the most commonly used criterion for excessive central airway collapse,39 and Bhatt et al,33 evaluating various thresholds, showed that this threshold is associated with a clinically meaningful difference in respiratory health status. However, it should be noted that Bhatt et al33 used an existing cohort of patients with end-expiratory CT scan images available to identify ECAC. Identifying ECAC on end-expiration CT scan imaging is less sensitive than doing so on dynamic imaging, which is considered by some to be the reference standard technique for detecting EDAC.
Double Lumen Endotracheal Tube Selection
Thoracic surgery routinely requires single-lung ventilation. Successful single-lung ventilation can be achieved using a double-lumen endotracheal tube, but requires appropriate sizing to avoid complications related to oversizing (eg, trauma to the bronchial wall related to insertion) or undersizing (eg, trauma to the bronchial wall related to high endotracheal tube cuff volume). Considerable interindividual variation in central airway lumen size, independent of age, sex, and body height, has been described,33 limiting the usefulness of such factors in selecting an appropriate double-lumen endotracheal tube size.44 Quantitative CT scan imaging of the airways has been shown to improve double-lumen endotracheal tube selection. For example, Liu et al,45 using preoperative axial CT scan images acquired at maximum inspiration, measured the left mainstem airway lumen diameter to guide double-lumen tube selection (selecting an outer tube diameter 1-2 mm smaller than the airway lumen diameter) and measured the distance from vocal cords to main carina to guide double-lumen tube positioning. In a randomized trial, this quantitative CT scan imaging of the airways strategy was associated with a higher proportion of optimal placement and fewer bronchial injuries when compared with blind double-lumen tube insertion.46
Emerging Clinical Applications
Several innovative studies have applied quantitative CT scan imaging of the airways to address clinical problems, but these approaches have not been integrated into routine clinical practice. A survey of these emerging applications is presented herein, followed by a discussion of what is needed to bridge the gap between research and clinical application.
Diagnosis
Bronchiolitis obliterans syndrome is an important complication of hematopoietic stem cell transplantation. Diagnosis relies on a constellation of clinical, functional, and radiographic features, in addition to the exclusion of other disease processes. Observational studies using multivolume quantitative CT scan imaging techniques, such as PRM, to assess functional small airways disease, have shown relatively high discriminative accuracy for bronchiolitis obliterans syndrome.46, 47, 48 Establishing the clinical advantage of this diagnostic strategy, as well as the development of effective treatments for bronchiolitis obliterans syndrome, will facilitate clinical uptake of this diagnostic tool.
Asthma and COPD share several similar clinical features (eg, symptoms, physiologic impairment), but have distinct management strategies.49,50 For some patients, distinguishing between these two disease entities (or establishing coexistence) can be challenging. Studies using quantitative CT scan imaging of both airways and parenchyma have demonstrated statistical differences between groups,19,51,52 suggesting potential for clinical usefulness. However, studies evaluating the discriminative accuracy of such approaches in the intended clinical setting (ie, patient population), as well as randomized trials demonstrating better clinical outcomes when guided by an imaging-based diagnostic strategy, are needed.
Treatment
Several studies have used quantitative CT scan imaging of the airways as a surrogate end point in therapeutic trials (eg, biologics in asthma and chronic rhinosinusitis, hypertonic saline in cystic fibrosis, oral devices in OSA).26, 27, 28, 29, 30, 31 Such research applications are important for advancing the most promising therapies to larger trials evaluating clinical end points, but do not themselves justify clinical uptake. For this reason, the evidence for clinical benefit from treatment guided by quantitative CT scan imaging of the airways is best described as emergent.
Prognosis
Several quantitative measures of CT scan imaging have been shown to predict adverse outcomes in various disease states. For example, airway fractal dimension,9 airway surface area to volume ratio8; and measures of airway wall thickness10 predict mortality in COPD; total airway count on CT scan imaging predicts lung function decline in COPD21; and extent of airway disease on CT scan imaging predicts progressive structural lung disease in children with cystic fibrosis.53 Although application of such knowledge in clinical research is apparent (eg, clinical trial enrichment), studies demonstrating clinical benefit resulting from management strategies guided by quantitative CT scan imaging of the airways are needed.
Bridging the Gap Between Research and Clinical Application
A better understanding of CT scan image standardization is required for quantitative airway measurements to be adopted widely in clinical practice. Although large, multicenter cohort studies (the Genetic Epidemiology of COPD [COPDGene], Severe Asthma Research Program [SARP], the Multi-Ethnic Study of Atherosclerosis [MESA], Evaluation of Chronic Obstructive Pulmonary Disease Longitudinally to Identify Predictive Surrogate End-points [ECLIPSE], Subpopulations and Intermediate Outcomes in COPD [SPIROMICS], and Canadian Cohort Obstructive Lung Disease [CanCOLD]) have advanced our understanding of the sources of variability in image acquisition13 and reconstruction techniques and analysis of CT scan airway measurements,54, 55, 56 no standard for CT scan image acquisition for airway analysis has been accepted. Also an increasing number of commercial vendors as well as academic groups have software for CT scan airway segmentation (eg, VIDA Diagnostics, Imbio, Thirona, Fluidda, and Chest Imaging Platform). However, not all software tools use the same algorithms, and standardization of CT scan airway measurements across platforms is lacking, with no consensus on airway segmentation methodology or which airways should be sampled. Further, few studies have evaluated airway measurement repeatability and reproducibility,56, 57, 58, 59 which is critical for technical validation. Similar to the need for technical standards in pulmonary function measurement and reporting,60,61 technical standards are needed for quantitative CT scan imaging of the airways to facilitate clinical implementation across the diverse set of scanner manufacturers, models, imaging protocols, and software vendors (both commercial and academic). Standardization initiatives for protocol development are required for CT scan airway measurements, such as the initiatives by Quantitative Imaging Biomarkers Alliance for measuring lung densitometry.16
CT scan airway measurements that have achieved real-world uptake are simple and easy to implement. For example, expiratory central airway collapse detection and double-lumen endotracheal tube size selection involve single plane measurements at standard anatomic locations. In contrast, many of the CT scan measurements developed in recent years are more expensive computationally and are conceptually complex. Although it is clear that these latter measurements provide important mechanistic and prognostic information, their usefulness in routine clinical practice is less clear. As such, clinical research directed toward the demonstration of airway measurements as treatable traits and randomized controlled trials demonstrating clinically meaningful benefits from management strategies guided by quantitative CT scan imaging of the airways are needed.
Summary
Quantitative CT scan airway measurements have provided important insights into disease pathophysiologic features, with potential to facilitate diagnosis, to increase the yield of diagnostic procedures, to prognosticate, and to guide treatment. However, only a few applications to date have successfully translated quantitative CT scan imaging into the clinical setting: diagnosing ECAC, guiding double-lumen tube selection, and navigational bronchoscopy. Although other nascent areas of clinical application were identified, a need exists for technical standards for quantitative CT scan imaging of the airways and randomized controlled trials demonstrating better clinical outcomes when guided by a quantitative CT scan imaging-based diagnostic strategy.
Funding/Support
M. K. acknowledges support from the NSERC Discovery Grant, the Early Researchers Award Program, and the Canada Research Chair Program (Tier II). B. M. S. acknowledges support from the National Institutes of Health (R01-HL130506, R01-HL155816), the Canadian Institutes of Health Research, the Canadian Lung Association, and the Quebec Health Research Fund.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
References
- 1.Labaki W.W., Han M.L.K. Chronic respiratory diseases: a global view. Lancet Respir Med. 2020;8(6):531–533. doi: 10.1016/S2213-2600(20)30157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radiological Society of North America Quantitative Imaging Biomarkers Alliance® (QIBA®). 2010. Radiological Society of North America website. Accessed June 23, 2022. http://www.rsna.org/QIBA/
- 3.Regan E.A., Lynch D.A., Curran-Everett D., et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff P.G., Barr R.G., Bleecker E., et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakano Y., Muro S., Sakai H., et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 6.Martinez C.H., Chen Y.-H.H., Westgate P.M., et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han M.K., Kazerooni E.A., Lynch D.A., et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodduluri S., Puliyakote A.K., Nakhmani A., Charbonnier J.P., Reinhardt J.M., Bhatt S.P. Computed tomography-based airway surface area-to-volume ratio for phenotyping airway remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(2):185–191. doi: 10.1164/rccm.202004-0951OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodduluri S., Kizhakke Puliyakote A.S., Gerard S.E., et al. Airway fractal dimension predicts respiratory morbidity and mortality in COPD. J Clin Invest. 2018;128(12):5374–5382. doi: 10.1172/JCI120693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannessen A., Skorge T.D., Bottai M., et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 11.Green M., Mead J., Turner J.M. Variability of maximum expiratory flow volume curves. J Appl Physiol. 1974;37(1):67–74. doi: 10.1152/jappl.1974.37.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Smith B.M., Kirby M., Hoffman E.A., et al. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA. 2020;323(22):2268–2280. doi: 10.1001/jama.2020.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen-Mayer H.H., Fuld M.K., Hoppel B., et al. Standardizing CT lung density measure across scanner manufacturers. Med Phys. 2017;44(3):974–985. doi: 10.1002/mp.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakker M.E., Stolk J., Reiber J.H.C., Stoel B.C. Influence of inspiration level on bronchial lumen measurements with computed tomography. Respir Med. 2012;106(5):677–686. doi: 10.1016/j.rmed.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Sieren J.P., Newell J.D., Jr., Barr R.G., et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quantitative Imaging Biomarkers Alliance Lung Density Committee QIBA profile: computed tomography: lung densitometry. Quantitative Imaging Biomarkers Alliance website. qibawiki.rsna.org/images/c/c9/QIBA_CT_Lung_Density_Profile_062619-appendix-resolved.pdf
- 17.Van Rikxoort E.M., Van Ginneken B. Automated segmentation of pulmonary structures in thoracic computed tomography scans: a review. Phys Med Biol. 2013;58(17):R187. doi: 10.1088/0031-9155/58/17/R187. [DOI] [PubMed] [Google Scholar]
- 18.Gallardo Estrella L., Pompe E., Kuhnigk J.M., et al. Computed tomography quantification of tracheal abnormalities in COPD and their influence on airflow limitation. Med Phys. 2017;44(7):3594–3603. doi: 10.1002/mp.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi S., Haghighi B., Choi J., et al. Differentiation of quantitative CT imaging phenotypes in asthma versus COPD. BMJ Open Respir Res. 2017;4(1) doi: 10.1136/bmjresp-2017-000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschirren J., Hoffman E.A., McLennan G., Sonka M. Segmentation and quantitative analysis of intrathoracic airway trees from computed tomography images. Proc Am Thorac Soc. 2005;2(6):484–487. doi: 10.1513/pats.200507-078DS. 503-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby M., Tanabe N., Tan W.C., et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am J Respir Crit Care Med. 2018;197(1):56–65. doi: 10.1164/rccm.201704-0692OC. [DOI] [PubMed] [Google Scholar]
- 22.Kirby M., Tanabe N., Vasilesc D.M., et al. Computed tomography total airway count is associated with the number of micro-computed tomography terminal bronchioles. Am J Respir Crit Care Med. 2020;201(5):613–615. doi: 10.1164/rccm.201910-1948LE. [DOI] [PubMed] [Google Scholar]
- 23.Galban C.J., Han M.K., Boes J.L., et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasilescu D.M., Martinez F.J., Marchetti N., et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;200(5):575–581. doi: 10.1164/rccm.201811-2083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith B.M., Traboulsi H., Austin J.H.M.M., et al. Human airway branch variation and chronic obstructive pulmonary disease. Proc Natl Acad Sci. 2018;115(5):E974–E981. doi: 10.1073/pnas.1715564115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiddens H.A.W.M., Chen Y., Andrinopoulou E.R., et al. The effect of inhaled hypertonic saline on lung structure in children aged 3-6 years with cystic fibrosis (SHIP-CT): a multicentre, randomised, double-blind, controlled trial. Lancet Respir Med. 2022;10(7):669–678. doi: 10.1016/S2213-2600(21)00546-4. [DOI] [PubMed] [Google Scholar]
- 27.Van Gaver H., Op de Beeck S., Dieltjens M., et al. Functional imaging improves patient selection for mandibular advancement device treatment outcome in sleep-disordered breathing: a prospective study. J Clin Sleep Med. 2022;18(3):739–750. doi: 10.5664/jcsm.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laidlaw T.M., Bachert C., Amin N., et al. Dupilumab improves upper and lower airway disease control in chronic rhinosinusitis with nasal polyps and asthma. Ann Allergy Asthma Immunol. 2021;126(5):584–592.e1. doi: 10.1016/j.anai.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghdoust M., Mirsadraee M., Aligolighasemabadi F., Khakzad M.R., Hashemi Attar A., Naghibi S. Effect of azithromycin on bronchial wall thickness in severe persistent asthma: a double-blind placebo-controlled randomized clinical trial. Respir Med. 2021;185:106494. doi: 10.1016/j.rmed.2021.106494. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino M., Akitsu K., Kubota K. Effect of sublingual immunotherapy on airway inflammation and airway wall thickness in allergic asthma. J Allergy Clin Immunol Pract. 2019;7(8):2804–2811. doi: 10.1016/j.jaip.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 31.De Backer W., De Backer J., Vos W., et al. A randomized study using functional respiratory imaging to characterize bronchodilator effects of glycopyrrolate/formoterol fumarate delivered by a metered dose inhaler using co-suspension delivery technology in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2673–2684. doi: 10.2147/COPD.S171707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslam A., Cardenas J.D.L., Morrison R.J., et al. Tracheobronchomalacia and excessive dynamic airway collapse: current concepts and future directions. Radiographics. 2022;42(4):1012–1027. doi: 10.1148/rg.210155. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt S.P., Terry N.L., Nath H., et al. Association between expiratory central airway collapse and respiratory outcomes among smokers. JAMA. 2016;315(5):498–505. doi: 10.1001/jama.2015.19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudali T.H., Bodduluri S., Dransfield M.T., Bhatt S.P. Association between inhaled corticosteroids and expiratory central airway collapse in smokers. Am J Respir Crit Care Med. 2021;203(4):518–521. doi: 10.1164/rccm.202008-3122LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copeland C.R., Nath H., Terry N.L.J., et al. Paratracheal paraseptal emphysema and expiratory central airway collapse in smokers. Ann Am Thorac Soc. 2018;15(4):479–484. doi: 10.1513/AnnalsATS.201709-713OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zafar MA, Sengupta R, Bates A, et al. Oral positive expiratory pressure device for excessive dynamic airway collapse caused by emphysema. Chest. 2021;160(4):e333–e337. doi: 10.1016/j.chest.2021.04.059. [DOI] [PubMed] [Google Scholar]
- 37.Majid A., Guerrero J., Gangadharan S., et al. Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest. 2008;134(4):801–807. doi: 10.1378/chest.08-0728. [DOI] [PubMed] [Google Scholar]
- 38.Zafar M.A., Sengupta R., Bates A., et al. Oral positive expiratory pressure device for excessive dynamic airway collapse caused by emphysema. Chest. 2021;160(4):e333–e337. doi: 10.1016/j.chest.2021.04.059. [DOI] [PubMed] [Google Scholar]
- 39.Mitropoulos A., Song W.J., Almaghlouth F., Kemp S., Polkey M., Hull J.H. Detection and diagnosis of large airway collapse: a systematic review. ERJ Open Res. 2021;7(3) doi: 10.1183/23120541.00055-2021. 00055-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boiselle P.M., O’Donnell C.R., Loring S.H., Bankier A.A. Reproducibility of forced expiratory tracheal collapse: assessment with MDCT in healthy volunteers. Acad Radiol. 2010;17(9):1186–1189. doi: 10.1016/j.acra.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boiselle P.M., Michaud G., Roberts D.H., et al. Dynamic expiratory tracheal collapse in COPD: correlation with clinical and physiologic parameters. Chest. 2012;142(6):1539–1544. doi: 10.1378/chest.12-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Donnell C.R., Bankier A.A., O’Donnell D.H., Loring S.H., Boiselle P.M. Static end-expiratory and dynamic forced expiratory tracheal collapse in COPD. Clin Radiol. 2014;69(4):357–362. doi: 10.1016/j.crad.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Donnell C.R., Litmanovich D., Loring S.H., Boiselle P.M. Age and sex dependence of forced expiratory central airway collapse in healthy volunteers. Chest. 2012;142(1):168–174. doi: 10.1378/chest.11-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim D., Son J.S., Ko S., Jeong W., Lim H. Measurements of the length and diameter of main bronchi on three-dimensional images in Asian adult patients in comparison with the height of patients. J Cardiothorac Vasc Anesth. 2014;28(4):890–895. doi: 10.1053/j.jvca.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z., Zhao L., Jia Q., Yang X., Liang S.J., He W. Chest computed tomography image for accurately predicting the optimal insertion depth of left-sided double-lumen tube. J Cardiothorac Vasc Anesth. 2018;32(2):855–859. doi: 10.1053/j.jvca.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Sharifi H., Lai Y.K., Guo H., et al. Machine learning algorithms to differentiate among pulmonary complications after hematopoietic cell transplant. Chest. 2020;158(3):1090–1103. doi: 10.1016/j.chest.2020.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng G.S., Selwa K.E., Hatt C., et al. Multicenter evaluation of parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Am J Transplant. 2020;20(8):2198–2205. doi: 10.1111/ajt.15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boes J.L., Bule M., Kitko C.L., et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(10):1592–1598. doi: 10.1016/j.bbmt.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Global Initiative for Asthma Global Initiative for Asthma homepage. Global Initiative for Asthma website. https://ginasthma.org/
- 50.Global Initiative for Chronic Obstructive Lung Disease Global Initiative for Chronic Obstructive Lung Disease homepage. Global Initiative for Chronic Obstructive Lung Disease webiste. https://goldcopd.org/
- 51.Moslemi A., Kontogianni K., Brock J., Wood S., Herth F., Kirby M. Differentiating COPD and asthma using quantitative CT imaging and machine learning. Eur Respir J. 2022;60(3) doi: 10.1183/13993003.03078-2021. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T., Tada Y., Kawata N., et al. Clinical, physiological, and radiological features of asthma-chronic obstructive pulmonary disease overlap syndrome. Int J COPD. 2015;10:947–954. doi: 10.2147/COPD.S80022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wijker N.E., Vidmar S., Grimwood K., et al. Early markers of cystic fibrosis structural lung disease: follow-up of the ACFBAL cohort. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.01694-2019. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez A., Ranallo F.N., Judy P.F., Gierada D.S., Fain S.B. CT reconstruction techniques for improved accuracy of lung CT airway measurement. Med Phys. 2014;41(11) doi: 10.1118/1.4898098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith B.M., Hoffman E.A., Rabinowitz D., et al. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(11):987–996. doi: 10.1136/thoraxjnl-2014-205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown R.H., Henderson R.J., Sugar E.A., Holbrook J.T., Wise R.A., Brown R.H. Reproducibility of airway luminal size in asthma measured by HRCT. J Appl Physiol. 2017;123:876–883. doi: 10.1152/japplphysiol.00307.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montaudon M., Desbarats P., Berger P., de Dietrich G., Marthan R., Laurent F. Assessment of bronchial wall thickness and lumen diameter in human adults using multi-detector computed tomography: comparison with theoretical models. J Anat. 2007;211(5):579–588. doi: 10.1111/j.1469-7580.2007.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little S.A., Sproule M.W., Cowan M.D., et al. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax. 2002;57(3):247–253. doi: 10.1136/thorax.57.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maetani T., Tanabe N., Terada S., et al. Physiological impacts of computed tomography airway dysanapsis, fractal dimension and branch count in asymptomatic never smokers. J Appl Physiol. 2023;134(1):20–27. doi: 10.1152/japplphysiol.00385.2022. [DOI] [PubMed] [Google Scholar]
- 60.Graham B.L., Steenbruggen I., Barjaktarevic I.Z., et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):E70–E88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culver B.H., Graham B.L., Coates A.L., et al. Recommendations for a standardized pulmonary function report. An official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. doi: 10.1164/rccm.201710-1981ST. [DOI] [PubMed] [Google Scholar]