Abstract
Background
This study investigated the outcomes of biventricular repair using right ventricle to pulmonary artery (RV-PA) conduit placement in patients aged <1 year.
Methods
Patients aged <1 year who underwent biventricular repair using an RV-PA conduit between 2011 and 2020 were included in this study. The outcomes of interest were death from any cause, conduit reintervention, and conduit dysfunction (peak velocity of ≥3.5 m/sec or moderate or severe regurgitation).
Results
In total, 141 patients were enrolled. The median age at initial conduit implantation was 6 months. The median conduit diameter z-score was 1.3. The overall 5-year survival rate was 89.6%. In the multivariable analysis, younger age (p=0.006) and longer cardiopulmonary bypass time (p=0.001) were risk factors for overall mortality. During follow-up, 61 patients required conduit reintervention, and conduit dysfunction occurred in 68 patients. The 5-year freedom from conduit reintervention and dysfunction rates were 52.9% and 45.9%, respectively. In the multivariable analysis, a smaller conduit z-score (p<0.001) was a shared risk factor for both conduit reintervention and dysfunction. Analysis of variance demonstrated a nonlinear relationship between the conduit z-score and conduit reintervention or dysfunction. The hazard ratio was lowest in patients with a conduit z-score of 1.3 for reintervention and a conduit z-score of 1.4 for dysfunction.
Conclusion
RV-PA conduit placement can be safely performed in infants. A significant number of patients required conduit reintervention and had conduit dysfunction. A slightly oversized conduit with a z-score of 1.3 may reduce the risk of conduit reintervention or dysfunction.
Keywords: Right ventricle to pulmonary artery conduit, Infancy, Conduit reintervention, Conduit dysfunction, Conduit diameter z-score
Introduction
In patients who require complete repair of various congenital cardiac defects associated with a discontinuous right ventricular outflow tract (RVOT) or the Ross operation for congenital aortic valve diseases, establishing continuity between the right ventricle (RV) and the pulmonary artery (PA) entails the placement of an RV-PA conduit.
Various conduits, such as homografts, porcine-valved Dacron conduits (Hancock; Medtronic Inc., Minneapolis, MN, USA), bovine jugular vein-valved conduits (Contegra; Medtronic Inc.), and polytetrafluoroethylene tubes (PTFE; W.L. Gore & Associates Inc., Newark, DE, USA) with or without a PTFE membrane valve, have been used for establishing RV-PA continuity; however, homografts, which were the traditionally preferred option, are no longer widely available in most countries [1-3]. Although surgery using an RV-PA conduit has excellent survival outcomes, the conduit failure and reintervention rates are still high [3-5]. According to previous studies, younger age is associated with higher rates of reintervention or conduit failure, and most patients who undergo RV-PA connection with a conduit in infancy require conduit reintervention during their lifetime [3,6,7]. This may be attributable to the patient’s outgrowth, conduit degeneration and subsequent calcification, external compression by the sternum, distal anastomosis site obstruction, and the use of an oversized conduit [4,6].
The majority of prior RV-PA conduit studies included patients of varying ages and employed homografts, which are currently difficult to obtain. As a result, we focused on younger patients for whom selecting the conduit type and size is difficult, as well as patients who had RV-PA conduit placement since 2011 (i.e., during a period when homografts were infrequently used). Therefore, this study aimed to investigate the outcomes of RV-PA conduit placement for biventricular repair in patients aged <1 year.
Methods
Patient selection and data collection
This study was approved by the institutional review board of Asan Medical Center (approval number: 2023-1557-0001; approval date: July 21, 2023), and the requirement for informed consent was waived given the retrospective study design.
Patients aged <1 year who underwent biventricular repair using an RV-PA conduit between January 2011 and September 2020 were included in this study. Patients who underwent RV-PA connection using a homograft were excluded. Baseline characteristics, morphological characteristics, operative details, and perioperative outcomes were collected by reviewing electronic medical records. Follow-up data were obtained from outpatient visit records and telephone contact.
Definitions
The outcomes of interest were death from any cause or transplantation, conduit reintervention or reoperation, and conduit dysfunction. The date of death, first reintervention, and first documentation of conduit dysfunction were the study endpoints. Early mortality was defined as any death that occurred either within 30 days after the initial operation or before hospital discharge.
Conduit reintervention was defined as any catheter-based or surgical reintervention performed on the conduit during follow-up. Catheter-based interventions included balloon dilatation or stenting of the conduit.
Serial echocardiographic examinations following RV-PA conduit implantation were reviewed to evaluate conduit function. To evaluate size mismatch, the conduit size was converted to a z-score using a standard formula. Conduit dysfunction was defined as a peak conduit velocity ≥3.5m/sec or moderate or severe regurgitation [8].
Surgical techniques
After a midline incision was made and median sternotomy was performed, cardiopulmonary bypass (CPB) was established by aortobicaval cannulation. Immediately after CPB initiation, systemic-to-PA shunts or persistent arterial ducts were occluded. Under moderate hypothermic CPB and cardioplegic arrest, intracardiac procedures such as closure of ventricular or atrial septal defects and right ventricular incision for the RV-PA connection were performed. During rewarming after the release of the aortic cross-clamp (ACC), the distal end of the RV-PA conduit was first anastomosed to the PA, after which the proximal end was beveled and connected to the longitudinal incision on the RV. The conduit was chosen at the surgeon’s discretion. If necessary, narrow segments of the PA were augmented using various patch materials. Intraoperative transesophageal echocardiography was routinely performed to evaluate procedural success after the patient was weaned from CPB.
Statistical analysis
The normality of the data distribution was tested using the Shapiro-Wilk test. Categorical variables were described as absolute numbers with percentages, and continuous variables were described as medians with interquartile ranges. The probabilities of survival, freedom from conduit reintervention, and freedom from conduit dysfunction were estimated using the Kaplan-Meier method. The intergroup equality of survival curves was assessed with the log-rank test. Factors associated with death or transplantation, conduit reintervention, and conduit dysfunction were identified using a logistic regression model and Cox proportional hazard models. Analysis of variance (ANOVA) was used to examine the significance of nonlinear relationships, and restricted cubic splines were used to analyze variables with a nonlinear relationship with the outcome. The analysis results for these variables were reported using partial effect plots. Variables with a p-value of <0.05 in the univariable analysis were further assessed in the multivariable analysis. Statistical analysis was performed using R software ver. 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
A total of 141 patients between January 2011 and September 2020 were included. Table 1 summarizes the baseline characteristics of the patients. The median age and body weight at initial conduit implantation were 6 months (interquartile range, 1–8 months) and 6.5 kg (interquartile range, 3.8–7.6 kg), respectively. Of these patients, 25 (17.7%) were neonates, and 16 (11.3%) weighed <3 kg. Moreover, 26 infants (18.4%) were born prematurely. Chromosomal abnormalities were found in 25 patients (17.7%). The primary diagnosis was categorized into 5 groups. Pulmonary atresia with ventricular septal defect was the most common category, accounting for 58.1% (n=82) of cases.
Table 1.
Baseline characteristics of the patients
| Characteristic | Value |
|---|---|
| Total no. of patients | 141 |
| Age at operation (mo) | 6 (1–8) |
| Neonate at operation (<28 day) | 25 (17.7) |
| Weight at operation (kg) | 6.5 (3.8–7.6) |
| Low weight at operation (<3.0 kg) | 16 (11.3) |
| Gender | |
| Male | 62 (44.0) |
| Female | 79 (56.0) |
| Premature | 26 (18.4) |
| Chromosomal anomalies | 25 (17.7) |
| DiGeorge syndrome/22q11.2 microdeletion | 9 (6.3) |
| Down syndrome | 3 (2.1) |
| CHARGE syndromea) | 2 (1.4) |
| VACTERL syndromeb) | 1 (0.7) |
| Others | 10 (7.0) |
| Primary cardiac diagnosis | |
| Pulmonary atresia with VSD | 82 (58.1) |
| Truncus arteriosus | 18 (12.7) |
| DORV/ToF | 16 (11.3) |
| ToF/absent pulmonary valve syndrome | 10 (7.0) |
| Others | 15 (10.6) |
Values are presented as number, median (interquartile range), or number (%).
VSD, ventricular septal defect; DORV, double outlet right ventricle; ToF, tetralogy of Fallot.
a)CHARGE is an abbreviation for several of the features common in the disorder: coloboma, heart defects, atresia choanae (also known as choanal atresia), growth retardation, genital abnormalities, and ear abnormalities. b)VACTERL syndrome is based on an acronym for the affected organs and systems: V (vertebral anomalies), A (anal atresia), C (cardiovascular abnormalities), TE (tracheoesophageal fistula), R (renal anomalies), and L (limb defects).
Operative details
Table 2 describes the operative details of the patients. Previous palliative cardiac surgery had been performed in 95 patients, and the most common palliative surgery was a modified Blalock-Taussig shunt (n=65, 46%). The types of conduits for RV-PA connection were Contegra in 82 patients (54.2%), homemade PTFE membrane-valved PTFE conduit in 34 patients (24.1%), valveless PTFE conduit in 19 patients (13.5%), and Hancock in 6 patients (4.3%). The median diameter of the implanted RV-PA conduits was 12 mm (interquartile range, 12–14 mm), and the median z-score of the conduit diameter was 1.3 (interquartile range, 0.8–1.8). Concomitant pulmonary arterioplasty was performed in 92 patients (65.2%) at initial conduit implantation. The differences in characteristics according to conduit type are described in Table 3.
Table 2.
Operative details
| Variable | Value |
|---|---|
| Previous palliative cardiac surgery | |
| Modified Blalock-Taussig shunt | 65 |
| Central shunt | 14 |
| Pulmonary artery banding | 9 |
| Pulmonary artery angioplasty | 8 |
| MAPCA unifocalization | 8 |
| Modified norwood operation | 4 |
| Closure patent ductus arteriosus | 3 |
| Valvuloplasty | 3 |
| Truncal separation | 1 |
| Others | 2 |
| No. of previous cardiac surgery | |
| 0 | 46 (32.6) |
| 1 | 77 (54.6) |
| 2 | 14 (9.9) |
| ≥3 | 4 (2.8) |
| Type of conduit | |
| Bovine jugular vein (Contegra) conduit | 82 (54.2) |
| Valved PTFE conduit | 34 (24.1) |
| Valveless PTFE | 19 (13.5) |
| Porcine-valved Dacron (Hancock) conduit | 6 (4.3) |
| Conduit diameter (mm) | 12 (12–14) |
| 8 | 9 (6.3) |
| 10 | 13 (9.2) |
| 12 | 61 (43.2) |
| 14 | 55 (39.0) |
| 16 | 3 (2.1) |
| Conduit/weight ratio | 1.99 (1.7–2.8) |
| Conduit diameter, z-score | 1.34 (0.8–1.8) |
| Concomitant pulmonary artery angioplasty | 92 (65.2) |
| Cardiopulmonary bypass time (min) | 151 (123–203) |
| Aortic cross-clamp time (min) | 54 (40–88) |
Values are presented as number, median (interquartile range), or number (%).
MAPCA, major aortopulmonary collateral arteries; PTFE, polytetrafluoroethylene.
Table 3.
Preoperative and operative characteristics according to the type of conduits
| Characteristic | All | Contegra | Valved PTFE | Valveless PTFE | Hancock | p-value |
|---|---|---|---|---|---|---|
| Total no. of patients | 141 | 82 | 34 | 19 | 6 | |
| Age at operation (mo) | 6 (1 to 8) | 6 (4 to 8) | 5 (1 to 9) | 1 (0 to 5) | 11 (7 to 12) | <0.001 |
| Weight at operation (kg) | 6.5 (3.8 to 7.6) | 6.7 (5.3 to 7.7) | 6 (3.7 to 7.7) | 3.2 (2.8 to 6.1) | 7.7 (7.0 to 8.6) | <0.001 |
| Male gender | 62 (44.0) | 36 (85.0) | 13 (38.0) | 8 (42.0) | 5 (83.0) | 0.235 |
| Premature | 26 (18.4) | 17 (20.4) | 5 (14.7) | 3 (15.7) | 1 (16.6) | 0.873 |
| Chromosomal anomalies | 25 (17.7) | 10 (12.1) | 11 (32.3) | 2 (10.5) | 2 (33.3) | 0.039 |
| Primary cardiac diagnosis | 0.002 | |||||
| Pulmonary atresia with VSD | 82 (58.1) | 52 (63.4) | 17 (50.0) | 7 (36.8) | 6 (100.0) | |
| Truncus arteriosus | 18 (12.7) | 9 (10.9) | 3 (8.8) | 6 (31.5) | - | |
| DORV/ToF | 16 (11.3) | 11 (13.4) | 4 (11.7) | - | - | |
| ToF/Absent pulmonary valve syndrome | 10 (7.0) | 4 (4.8) | 6 (17.6) | 1 (5.2) | - | |
| Others | 15 (10.6) | 6 (7.3) | 4 (11.7) | 5 (26.3) | - | |
| Conduit diameter (mm) | 12 (12 to 14) | 12 (12 to 14) | 12 (12 to 14) | 10 (8 to 10) | 14 (14 to 14) | <0.001 |
| 8 | 9 (6.3) | - | - | 9 (47.3) | - | |
| 10 | 13 (9.2) | - | 7 (20.5) | 6 (31.5) | - | |
| 12 | 61 (43.2) | 43 (52.5) | 16 (47.0) | 2 (10.5) | - | |
| 14 | 55 (39.0) | 38 (46.3) | 10 (29.4) | 2 (10.5) | 5 (83.3) | |
| 16 | 3 (2.1) | 1 (1.2) | 1 (2.9) | - | 1 (16.6) | |
| Conduit diameter, z-score | 1.34 (0.8 to 1.8) | 1.47 (0.9 to 2.0) | 1.00 (0.8 to 1.7) | 0.08 (-0.2 to 0.8) | 1.27 (1.0 to 1.6) | <0.001 |
| Cardiopulmonary bypass time (min) | 151 (123 to 203) | 143 (119 to 175) | 176 (147 to 209) | 204 (149 to 246) | 153 (141 to 172) | 0.007 |
| Aortic cross-clamp time (min) | 54 (40 to 88) | 48 (37 to 73) | 61 (48 to 90) | 95 (76 to 110) | 45 (42 to 57) | 0.001 |
Values are presented as number, median (interquartile range), or number (%).
PTFE, polytetrafluoroethylene; VSD, ventricular septal defect; DORV, double outlet right ventricle; ToF, tetralogy of Fallot.
Survival
Eight early deaths (5.6%) occurred, and the causes of early death were low cardiac output syndrome in 4 patients, respiratory failure in 2 patients, and sepsis in 2 patients. The median follow-up duration from initial conduit implantation was 4.4 years (interquartile range, 2.4–6.2 years). Six late deaths (4.2%) occurred, and the causes of late death included respiratory failure in 1 patient, sepsis in 1 patient, gastrointestinal disease in 1 patient, and unknown in 3 patients who died outside the hospital. The overall survival rates were 91.5% and 89.6% at 1 and 5 years, respectively (Fig. 1). In the univariable analysis, younger age, lower body weight, no previous palliative surgery, and longer CPB or ACC time were associated with overall mortality. In the multivariable analysis, younger age (p=0.006) and longer CPB time (p=0.001) were identified as risk factors for overall mortality (Table 4).
Fig. 1.
Overall survival after right ventricle to pulmonary artery conduit insertion. The shaded area represents 95% confidence interval.
Table 4.
Factors associated with overall mortality (n=14)
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age at operation (mo) | 0.76 (0.64–0.91) | 0.003 | 0.78 (0.65–0.93) | 0.006 | |
| Weight at operation (kg) | 0.63 (0.46–0.85) | 0.002 | |||
| Prematurity | 2.52 (0.84–7.52) | 0.097 | |||
| Previous palliative cardiac surgery | 0.34 (0.11–0.99) | 0.048 | 0.95 (0.22–4.13) | 0.953 | |
| Chromosomal abnormality | 0.75 (0.16–3.39) | 0.718 | |||
| Conduit diameter, z-score | 1.53 (0.76–3.08) | 0.232 | |||
| Concomitant pulmonary arterioplasty | 0.36 (0.12–1.05) | 0.063 | |||
| Type of conduit | 1.01 (0.60–1.68) | 0.968 | |||
| Primary cardiac diagnosis | 1.36 (0.98–1.88) | 0.063 | |||
| Cardiopulmonary bypass time | 1.01 (1.01–1.01) | 0.001 | 1.01 (1.00–1.01) | 0.001 | |
| Aortic cross-clamp time | 1.01 (1.00–1.03) | 0.001 | |||
HR, hazard ratio; CI, confidence interval.
Conduit reintervention
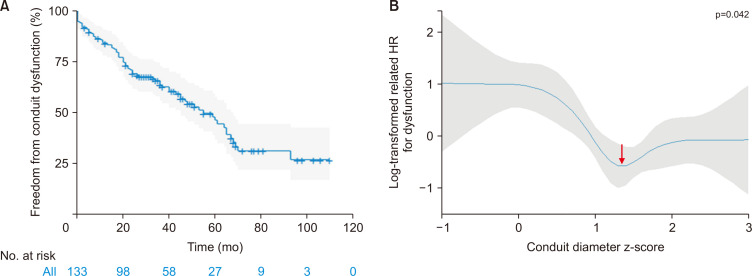
During follow-up, 61 patients required conduit reintervention, including surgical reintervention in 44 patients and catheter-based reintervention in 17 patients, at a median of 29 months after initial conduit implantation. The indications for conduit reintervention included stenosis in 30 patients (49.1%), regurgitation in 8 patients (13.1%), combined stenosis and regurgitation in 15 patients (24.5%), infective endocarditis in 2 patients (3.2%), and others in 6 patients (9.8%). The freedom from conduit reintervention rates were 90% and 52.9% at 1 and 5 years, respectively (Fig. 2A). In the univariable analysis, younger age, lower body weight, no previous cardiac surgery, a smaller conduit diameter z-score, and the use of a valved or valveless PTFE conduit were associated with conduit reintervention. In the multivariable analysis, a smaller conduit diameter z-score (p=0.003) was identified as a risk factor for conduit reintervention (Table 5). ANOVA demonstrated that the conduit diameter z-score had a significant nonlinear relationship with conduit reintervention (p=0.012). To account for this nonlinearity, restricted cubic splines were used to model the relationship, and this showed that the lowest risk was at a conduit diameter z-score of 1.3 (Fig. 2B).
Fig. 2.
Conduit reintervention following right ventricle to pulmonary artery (RV-PA) conduit insertion. (A) Kaplan-Meier curve for freedom from conduit reintervention and (B) a partial effect plot for the RV-PA conduit diameter z-score and the log-transformed related hazard ratio (HR) for conduit reintervention. The shaded area represents the 95% confidence interval. The arrow indicates the conduit diameter z-score (1.3) with the lowest risk of conduit reintervention.
Table 5.
Factors associated with conduit reintervention (n=61)
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age at operation (mo) | 0.87 (0.81–0.93) | <0.001 | 0.92 (0.82–1.03) | 0.175 | |
| Weight at operation (kg) | 0.76 (0.67–0.87) | <0.001 | |||
| Prematurity | 0.63 (0.28–1.38) | 0.252 | |||
| Previous palliative cardiac surgery | 0.30 (0.18–0.52) | 0.001 | 0.44 (0.18–1.06) | 0.068 | |
| Chromosomal abnormality | 0.71 (0.36–1.42) | 0.341 | |||
| Conduit diameter, z-score | 0.59 (0.42–0.84) | 0.003 | 0.57 (0.40–0.83) | 0.003 | |
| Concomitant pulmonary arterioplasty | 0.73 (0.42–1.25) | 0.253 | |||
| Type of conduit | 0.78 (0.61–0.98) | 0.038 | 1.05 (0.82–1.03) | 0.742 | |
| Contegra conduit | Reference | ||||
| Valved PTFE conduit | 2.33 (1.28–4.24) | 0.005 | |||
| Valveless PTFE | 2.43 (1.13–5.19) | 0.021 | |||
| Hancock valved conduit | 1.10 (0.37–3.30) | 0.857 | |||
| Primary cardiac diagnosis | 1.21 (0.93–1.34) | 0.225 | |||
| Cardiopulmonary bypass time | 1.00 (0.99–1.00) | 0.330 | |||
| Aortic cross-clamp time | 1.00 (0.99–1.01) | 0.308 | |||
HR, hazard ratio; CI, confidence interval; PTFE, polytetrafluoroethylene.
Conduit dysfunction
During follow-up, conduit dysfunction occurred in 68 patients at a median interval of 21 months. The causes of conduit dysfunction included stenosis in 29 patients (42.6%), regurgitation in 22 patients (32.3%), combined stenosis and regurgitation in 15 patients (22%), and infective endocarditis in 2 patients (2.9%). The freedom from conduit dysfunction rates were 84% and 45.9% at 1 and 5 years, respectively (Fig. 3A). In the univariable analysis, younger age, lower body weight, no previous cardiac surgery, smaller conduit diameter z-score, type of conduit (valveless conduit), and longer ACC time were associated with conduit dysfunction. In the multivariable analysis, younger age, smaller conduit diameter z-score, and longer ACC time were identified as independent risk factors for conduit dysfunction (Table 6). According to ANOVA, the conduit diameter z-score showed a significant nonlinear relationship with conduit dysfunction (p=0.043). To account for this nonlinearity, restricted cubic splines were used to model the relationship, and this showed that the lowest risk was at a conduit diameter z-score of 1.4 (Fig. 3B).
Fig. 3.
Conduit dysfunction following right ventricle to pulmonary artery (RV-PA) conduit insertion. (A) Kaplan-Meier curve for freedom from conduit dysfunction, and (B) a partial effect plot for RV-PA conduit diameter z-score and the log-transformed related hazard ratio (HR) for conduit dysfunction. The shaded area represents the 95% confidence interval. The arrow indicates the conduit diameter z-score (1.4) with the lowest risk of conduit dysfunction.
Table 6.
Factors associated with conduit dysfunction (n=68)
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age at operation (mo) | 0.89 (0.83–0.95) | 0.001 | 0.86 (0.78–0.95) | 0.003 | |
| Weight at operation (kg) | 0.83 (0.73–0.93) | 0.003 | |||
| Prematurity | 0.49 (0.22–1.07) | 0.076 | |||
| Previous palliative cardiac surgery | 0.49 (0.30–0.81) | 0.005 | 1.15 (0.56–2.36) | 0.699 | |
| Chromosomal abnormality | 0.74 (0.38–1.42) | 0.374 | |||
| Conduit diameter, z-score | 0.58 (0.42–0.81) | <0.001 | 0.67 (0.48–0.95) | 0.024 | |
| Concomitant pulmonary arterioplasty | 0.62 (0.37–1.03) | 0.068 | |||
| Type of conduit | 1.35 (1.07–1.70) | 0.010 | 1.29 (0.95–1.75) | 0.094 | |
| Contegra conduit | Reference | ||||
| Valved PTFE conduit | 1.18 (0.65–2.14) | 0.574 | |||
| Valveless PTFE | 5.98 (3.22–11.10) | <0.001 | |||
| Hancock valved conduit | 0.81 (0.24–2.68) | 0.736 | |||
| Primary cardiac diagnosis | 1.11 (0.93–1.32) | 0.166 | |||
| Cardiopulmonary bypass time | 1.00 (0.99–1.00) | 0.709 | |||
| Aortic cross-clamp time | 1.01 (1.00–1.02) | 0.003 | 1.01 (1.00–1.02) | 0.009 | |
HR, hazard ratio; CI, confidence interval; PTFE, polytetrafluoroethylene.
Discussion
The use of an RV-PA conduit to establish continuity between the ventricle and pulmonary circulation is essential for achieving complete repair of various congenital cardiac defects associated with a discontinuity between the RV and the PA.
Corrective surgery, including RVOT reconstruction using an RV-PA conduit, is a safe procedure with a low risk of death. The findings of this study also support this conclusion, although overall mortality was not negligible. Younger age and longer CPB time were identified as risk factors for death. A possible explanation is that younger patients requiring repair had more severe disease; therefore, poor preoperative conditions might have affected their surgical outcomes. In addition, a longer CPB time might have been associated with mortality because it indicates higher disease complexity and a poorer heart condition before surgery.
Although previous studies have reported significant improvements in the survival of RVOT reconstruction using an RV-PA conduit, the rate of conduit reintervention remains high [2]. Caldarone et al. [7] reported a strong relationship between conduit failure and age at the time of implantation, and Willetts et al. [1] reported that weight was the most significant factor associated with conduit failure. Similarly, younger age was identified as an independent risk factor for conduit dysfunction in our study. Because the available conduit size is limited in small babies regardless of the conduit type, placing an adequate-sized conduit within the limited space is difficult; consequently, external compression, which may cause conduit failure, is more likely to occur [9,10]. Furthermore, age-related immune mechanisms that affect conduits are more prevalent in younger patients, and the likelihood of requiring reintervention increases over time because of rapid degeneration and calcification [4,6,11]. Saxena et al. [3] also reported that most patients required conduit replacement before 5 years of age. Thus, the selection of the conduit type or size in small patients such as neonates and infants is challenging, which is why we exclusively enrolled and evaluated patients aged <1 year in this study.
An interesting finding in our study was that a longer ACC time was a risk factor for conduit dysfunction. Given that most patients who require longer ACC may be younger, the same explanation about the association between younger age and conduit dysfunction may be applicable to that between conduit dysfunction and longer ACC time.
Although an appropriate conduit size is critical for the longevity of the implanted RV-PA conduit in young children [12,13], earlier studies have made inconsistent recommendations regarding the conduit size. Poynter et al. [14] noted that an oversized conduit could be advantageous for durability when considering somatic growth in patients aged <2 years. Conversely, Askovich et al. [15] demonstrated that an oversized conduit with a z-score ≥2.7 may reduce the durability of the conduit. An oversized conduit may lead to external compression of the conduit because of the limited space and subsequent pulmonary valve distortion and distal PA distortion attributable to accelerating pulmonary valve insufficiency and failure [7,15,16].
In the present study, the conduit diameter z-score was identified as a shared risk factor for both conduit reintervention and conduit dysfunction, and the results indicated that a higher conduit diameter z-score was associated with lower risk. Furthermore, the lowest risk was observed at a conduit diameter z-score of 1.3 for conduit reintervention and a conduit diameter z-score of 1.4 for conduit dysfunction, which suggests that a too-large or too-small conduit diameter could be disadvantageous for the patients. Karamlou et al. [12] reported that a conduit diameter z-score of 1–3 was associated with improved outcomes in patients aged <2 years at initial implantation. Recently, Kim et al. [17] suggested that an oversized conduit may have poor longevity, and even without a valve, smaller conduits may be better for small babies.
Allografts have been predominantly used to establish RV-PA connections for over 50 years and have long been believed to be the most durable option. However, many countries and centers, including ours, are experiencing extreme graft shortages. Therefore, since 2011, alternative conduits such as homemade PTFE membrane-valved or non-valved PTFE conduits, Contegra, and Hancock have been exclusively used at our center.
Since its introduction in 1999, Contegra has been predominantly used in many countries because of its advantages, such as being easy to handle and having a wide range of sizes for selection; furthermore, the durability of Contegra was comparable to that of homograft [6,10], and may even be better than that of homografts [16]. However, Mery et al. [13] reported that Contegra, particularly in younger patients, was associated with a higher incidence of distal conduit stenosis and early conduit insufficiency. Homemade PTFE valved conduits began to be used in earnest by Japanese groups and are a good alternative to RV-PA conduits, with a low risk of reoperation or reintervention [11,18-21]. Our center adopted a homemade tricuspid PTFE-valved conduit that was made using the technique introduced by Kim et al. [22] in 2013. Hancock conduits have been used for a long time and have shown comparable outcomes to those of other RV-PA conduits in previous studies [6,23]. In the present study, no conduit was clearly superior to others in terms of longevity. Hopefully, future research will suggest suitable conduits for each patient with different characteristics based on the advantages and disadvantages of each conduit type.
Limitation
This study has inherent limitations because of its retrospective design. Throughout the study period, the predominantly used conduits changed based on their availability and surgeons’ preferences, without definitive criteria. This variation precludes a fair comparison and hinders the ability to draw meaningful conclusions. Although the number of events was sufficient to conduct a multivariable analysis, readers should cautiously interpret the results because of the small number of patients and events.
Conclusion
The results of this study reveal that RV-PA conduit placement in infants can be performed safely. A significant number of patients required conduit reintervention and had conduit dysfunction. A slightly oversized conduit with a z-score of 1.3 might reduce the risk of conduit reintervention or dysfunction.
Funding Statement
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article information
Author contributions
Conceptualization: PCS, JDH. Data curation: PCS, JDH, KDH. Formal analysis: PCS, JDH, KDH. Methodology: PCS, JDH, KDH, CES. Visualization: JDH, KDH. Writing–original draft: PCS, JDH. Writing–review & editing: all authors. Final approval of the manuscript: all authors.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Willetts RG, Stickley J, Drury NE, et al. Four right ventricle to pulmonary artery conduit types. J Thorac Cardiovasc Surg. 2021;162:1324–33. doi: 10.1016/j.jtcvs.2020.12.144. https://doi.org/10.1016/j.jtcvs.2020.12.144. [DOI] [PubMed] [Google Scholar]
- 2.Alsoufi B. Right ventricle-to-pulmonary artery conduits: do we really have an option? J Thorac Cardiovasc Surg. 2016;151:442–3. doi: 10.1016/j.jtcvs.2015.10.100. https://doi.org/10.1016/j.jtcvs.2015.10.100. [DOI] [PubMed] [Google Scholar]
- 3.Saxena A, Salve GG, Betts K, et al. Outcomes following heterotopic placement of right ventricle to pulmonary artery conduits. World J Pediatr Congenit Heart Surg. 2021;12:220–9. doi: 10.1177/2150135120975769. https://doi.org/10.1177/2150135120975769. [DOI] [PubMed] [Google Scholar]
- 4.Ong K, Boone R, Gao M, et al. Right ventricle to pulmonary artery conduit reoperations in patients with tetralogy of Fallot or pulmonary atresia associated with ventricular septal defect. Am J Cardiol. 2013;111:1638–43. doi: 10.1016/j.amjcard.2013.01.337. https://doi.org/10.1016/j.amjcard.2013.01.337. [DOI] [PubMed] [Google Scholar]
- 5.Dearani JA, Danielson GK, Puga FJ, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75:399–411. doi: 10.1016/S0003-4975(02)04547-2. https://doi.org/10.1016/s0003-4975(02)04547-2. [DOI] [PubMed] [Google Scholar]
- 6.Vitanova K, Cleuziou J, Horer J, et al. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age? Eur J Cardiothorac Surg. 2014;46:961–6. doi: 10.1093/ejcts/ezu080. https://doi.org/10.1093/ejcts/ezu080. [DOI] [PubMed] [Google Scholar]
- 7.Caldarone CA, McCrindle BW, Van Arsdell GS, et al. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg. 2000;120:1022–31. doi: 10.1067/mtc.2000.110684. https://doi.org/10.1067/mtc.2000.110684. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. https://doi.org/10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Andersen ND, Turek JW. Commentary: decision-making for right ventricle to pulmonary artery conduit selection: statistical models and clinical practice. J Thorac Cardiovasc Surg. 2021;162:1334–5. doi: 10.1016/j.jtcvs.2021.01.018. https://doi.org/10.1016/j.jtcvs.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Prior N, Alphonso N, Arnold P, et al. Bovine jugular vein valved conduit: up to 10 years follow-up. J Thorac Cardiovasc Surg. 2011;141:983–7. doi: 10.1016/j.jtcvs.2010.08.037. https://doi.org/10.1016/j.jtcvs.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Shinkawa T, Chipman C, Bozzay T, Tang X, Gossett JM, Imamura M. Outcome of right ventricle to pulmonary artery conduit for biventricular repair. Ann Thorac Surg. 2015;99:1357–66. doi: 10.1016/j.athoracsur.2014.07.095. https://doi.org/10.1016/j.athoracsur.2014.07.095. [DOI] [PubMed] [Google Scholar]
- 12.Karamlou T, Blackstone EH, Hawkins JA, et al. Can pulmonary conduit dysfunction and failure be reduced in infants and children less than age 2 years at initial implantation? J Thorac Cardiovasc Surg. 2006;132:829–38. doi: 10.1016/j.jtcvs.2006.06.034. https://doi.org/10.1016/j.jtcvs.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Mery CM, Guzman-Pruneda FA, De Leon LE, et al. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J Thorac Cardiovasc Surg. 2016;151:432–9. 441. doi: 10.1016/j.jtcvs.2015.10.069. https://doi.org/10.1016/j.jtcvs.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 14.Poynter JA, Eghtesady P, McCrindle BW, et al. Association of pulmonary conduit type and size with durability in infants and young children. Ann Thorac Surg. 2013;96:1695–702. doi: 10.1016/j.athoracsur.2013.05.074. https://doi.org/10.1016/j.athoracsur.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 15.Askovich B, Hawkins JA, Sower CT, et al. Right ventricle-to-pulmonary artery conduit longevity: is it related to allograft size? Ann Thorac Surg. 2007;84:907–12. doi: 10.1016/j.athoracsur.2007.04.104. https://doi.org/10.1016/j.athoracsur.2007.04.104. [DOI] [PubMed] [Google Scholar]
- 16.Barron DJ, Guariento A. Oversize it: valuable advice for larger right ventricle-to-pulmonary artery conduits. Ann Thorac Surg. 2021;112:1508. doi: 10.1016/j.athoracsur.2021.02.040. https://doi.org/10.1016/j.athoracsur.2021.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Kwon YK, Choi ES, Kwon BS, Park CS, Yun TJ. Risk factors for early adverse outcomes after bovine jugular vein conduit implantation: influence of oversized conduit on the outcomes. Interact Cardiovasc Thorac Surg. 2022;35:ivac197. doi: 10.1093/icvts/ivac197. https://doi.org/10.1093/icvts/ivac197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki T, Yamagishi M, Maeda Y, et al. Long-term outcomes of expanded polytetrafluoroethylene conduits with bulging sinuses and a fan-shaped valve in right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg. 2018;155:2567–76. doi: 10.1016/j.jtcvs.2017.12.137. https://doi.org/10.1016/j.jtcvs.2017.12.137. [DOI] [PubMed] [Google Scholar]
- 19.Si MS. Expanded polytetrafluoroethylene right ventricle to pulmonary artery conduit: time to adopt? J Thorac Cardiovasc Surg. 2018;156:1637–8. doi: 10.1016/j.jtcvs.2018.05.020. https://doi.org/10.1016/j.jtcvs.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Shinkawa T, Yamagishi M, et al. Right ventricle to pulmonary artery conduit with tricuspid expanded polytetrafluoroethylene valves. Ann Thorac Surg. 2021;112:831–7. doi: 10.1016/j.athoracsur.2020.06.119. https://doi.org/10.1016/j.athoracsur.2020.06.119. [DOI] [PubMed] [Google Scholar]
- 21.Ootaki Y, Welch AS, Walsh MJ, Quartermain MD, Williams DA, Ungerleider RM. Medium-term outcomes after implantation of expanded polytetrafluoroethylene valved conduit. Ann Thorac Surg. 2018;105:843–50. doi: 10.1016/j.athoracsur.2017.07.013. https://doi.org/10.1016/j.athoracsur.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Sung SC, Chang YH, Lee HD, Park JA. A new simplified technique for making tricuspid expanded polytetrafluoroethylene valved conduit for right ventricular outflow reconstruction. Ann Thorac Surg. 2013;95:e131–3. doi: 10.1016/j.athoracsur.2012.12.047. https://doi.org/10.1016/j.athoracsur.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Belli E, Salihoglu E, Leobon B, et al. The performance of Hancock porcine-valved Dacron conduit for right ventricular outflow tract reconstruction. Ann Thorac Surg. 2010;89:152–8. doi: 10.1016/j.athoracsur.2009.09.046. https://doi.org/10.1016/j.athoracsur.2009.09.046. [DOI] [PubMed] [Google Scholar]