Abstract
Background
Prothrombin complex concentrate (PCC) and fresh frozen plasma (FFP) are commonly used to manage bleeding in patients during cardiac surgery. However, the relative efficacy and safety of these 2 strategies remain uncertain.
Methods
MEDLINE, Embase, and Cochrane were searched for studies comparing PCC and FFP in patients who underwent cardiac surgery complicated by bleeding. Review Manager (RevMan) ver. 5.4 (Nordic Cochrane Centre, The Cochrane Collaboration) was used for statistical analysis. Binary and continuous outcomes were compared using pooled risk ratios and mean differences, respectively. The meta-analysis protocol was registered in the International Prospective Register of Systematic Reviews under protocol number CRD42022379144.
Results
We included 8 studies with 1,500 patients, of whom 613 (40.9%) received PCC. The mean follow-up period ranged from 28 to 90 days. The PCC group had significantly lower chest tube drainage at 24 hours (mean difference [MD], -148.50 mL; 95% CI, -253.02 to -43.99 mL; p=0.005; I2=42%). Fewer units of red blood cells (RBCs) were transfused within the first 24 hours (MD, -1.02 units; 95% CI, -1.81 to -0.24 units; p=0.01; I2=56%), and fewer patients required RBC transfusion within the first 24 hours (risk ratio, 0.85; 95% CI, 0.78–0.93; p<0.007; I2=45%) in the PCC group. There were no statistically significant differences in secondary outcomes. Nonetheless, a subgroup analysis of randomized controlled trials failed to corroborate the results obtained from the main analysis.
Conclusion
Our findings suggest that PCC can be effective, without increased adverse events, when compared with FFP in patients undergoing cardiac surgery complicated by bleeding.
Keywords: Prothrombin complex concentrate, Plasma, Thoracic surgery, Hemorrhage, Meta-analysis
Introduction
Cardiopulmonary bypass (CPB) is an essential component of cardiac surgery, as it helps maintain the functions of the circulatory and respiratory systems during the procedure [1]. However, CPB can trigger a complex interaction between inflammatory, fibrinolytic, and coagulation factors that can lead to coagulopathy and bleeding, which can increase morbidity and mortality rates. Consequently, it is essential to minimize the need for transfusions and surgical re-explorations [2].
Traditionally, fresh frozen plasma (FFP) has been the preferred treatment for patients with deficient coagulation factors. However, the possibility of adverse events associated with FFP administration such as fluid overload, transfusion reaction, and the transmission of infectious diseases should be carefully considered [3,4]. As a result, prothrombin complex concentrate (PCC) has emerged as an alternative to FFP. PCC offers a full complement of procoagulant and anticoagulant factors and is available in 3-factor (3F; factors II, IX, X) and 4-factor (4F; factors II, VII, IX, X, with select anticoagulants) formulations [5]. While PCC was initially used for the treatment of patients with hemophilia B, its applications have expanded to include other bleeding disorders, such as urgent reversal of oral anticoagulation (e.g., warfarin) and congenital vitamin K-dependent coagulation deficiencies at the time of surgical procedures [6,7]. Nevertheless, its use has been associated with an increased risk of thrombotic/thromboembolic complications and acute kidney injury (AKI) [8,9].
Therefore, we performed a systematic review and meta-analysis to evaluate the effects of PCC versus FFP on coagulopathy and bleeding in patients who underwent cardiac surgery.
Methods
Search strategy
Our systematic review and meta-analysis was performed according to the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10]. The pre-specified research protocol was registered with the International Prospective Register of Systematic Reviews under the protocol number CRD42022379144. We systematically searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception to August 14, 2023. The search terms were: frozen plasma, plasma, standard care, prothrombin complex concentrate, 4-factor prothrombin, PCC, prothrombin, cardiac surgical patient, cardiopulmonary bypass, heart surgery, cardiac surgery, cardiac surgical procedures, aortic replacement surgery, coronary artery bypass, valve surgery, aortic surgery, and cardiac transplantation. Specific detailed information regarding the search strategy for each database is presented in the Supplementary Material 1. The references from all included studies, previous systematic reviews, and meta-analyses were also searched manually for any additional studies, and any missing data was requested from the authors. Eventual conflicts were resolved by consensus among the authors. Data extraction was conducted independently by 4 authors (J.H., J.P., J.S., and P.V.) who collected the following information from each individual study: (1) study characteristics, including study design, time of follow-up, and sample size per group; (2) patient baseline characteristics, such as age (years), sex, body mass index (BMI, kg/m2), and comorbidities; and (3) outcomes of interest.
Selection criteria
There were no restrictions regarding publication date or language. The eligibility criteria included: (1) randomized controlled trials (RCTs) or observational cohorts; (2) comparisons of PCC and FFP in patients who underwent cardiac surgery complicated by coagulopathy or bleeding; and (3) reports on at least one outcome of interest. We excluded: (1) patients with pre-existing coagulopathy or those using anticoagulants 5 days before surgery; (2) patients who received both PCC and FFP; (3) studies with overlapping populations, (i.e., studies recruiting from the same institution over an overlapping period); (4) studies with only an abstract available; and (5) studies that did not report any outcomes of interest.
Endpoints and subgroup analyses
Our primary endpoint was chest tube drainage, defined as the volume of fluid drained from the chest cavity within the first 24 hours after intervention [3,4,11-13]. Secondary endpoints included: (1) total units of transfused red blood cells (RBCs); (2) number of patients requiring RBC transfusion; (3) length of stay (LOS) in the hospital, and (4) LOS in the intensive care unit (ICU) in days; (5) duration of mechanical ventilation in hours; (6) 30-day incidence of stroke or transient ischemic attack (TIA); (7) other thromboembolic events within 30 days such as deep vein thrombosis, mesenteric ischemia, myocardial infarction or pulmonary embolism [13-15]; (8) AKI defined in accordance with the consensus of Risk, Injury, Failure, Loss of Kidney Function, and End-Stage Kidney Disease criteria or AKI Network recommendations, within 30 days [12,14,15]; (9) renal replacement therapy (RRT) within 30 days; (10) reoperation for bleeding within 48 hours; and (11) 30-day mortality. In addition, we performed a subgroup analysis of data restricted to RCTs.
Quality assessment
We assessed the risk of bias for each study using the Cochrane Risk of Bias tool for randomized trials (RoB 2) and Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) for non-randomized studies, in accordance with the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions [16-18]. Four independent investigators (M.P., P.B., P.V., and V.F.) conducted the risk of bias for each study and recorded their findings in Supplementary Figs. 1 and 2. Any disagreements were resolved through discussion and consensus.
Due to the limited number of included studies, we could not conduct a comprehensive assessment of publication bias. The utility of funnel plots in detecting bias is limited when the sample size is small, and the Egger test is not recommended unless at least 10 studies are included in the analysis [19].
Statistical analysis
We computed risk ratios (RRs) using the Mantel-Haenszel test for dichotomous outcomes and used 95% confidence intervals (CIs) as a measure of effect size. We considered p-values of less than 0.05 to indicate statistical significance. We used mean difference (MD) to report the effect size for continuous outcomes, also with 95% CIs.
To assess heterogeneity, the Cochran Q test and I2 statistics were utilized. We classified I2 values of <25%, 25%–75%, and >75% as low, moderate, and high heterogeneity, respectively. To account for potential disparities in both clinical and methodological aspects across studies, we applied the restricted maximum-likelihood estimator and a DerSimonian and Laird random-effects model to all outcomes. Our meta-analysis was conducted using Review Manager ver. 5.4 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
Study selection and characteristics
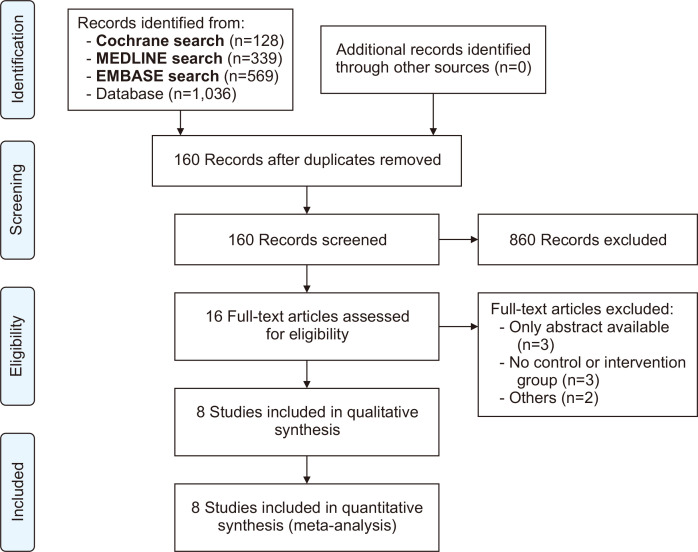
The systematic search yielded 1,036 studies, as illustrated in Fig. 1. After screening for duplicate reports and studies with an exclusion criterion based on title or abstract review, 16 remained and were fully assessed. Finally, 8 articles were included (3 RCTs and 5 observational studies). A total of 1,500 adult patients were included, of whom 613 (40.9%) received PCC, and 234 (38.2%) were female [3,4,11-15,20]. The baseline characteristics of the PCC and FFP groups exhibited a generally homogeneous distribution of gender. The study by Bartoszko et al. [14] was the exception and had a notably higher number of female patients. In addition, there were similarities in mean age and BMI when comparing the groups. Notably, Arnékian et al. [11] and Ortmann et al. [20] reported the longest CPB times, exceeding 300 minutes in duration. While not all studies provided detailed comorbidity data, it was observed that patients who received either PCC or FFP exhibited a similar incidence of diabetes and hypertension across the included studies. Most of the surgeries analyzed were performed on an emergency basis. The study by Karkouti et al. [3] was the only one to offer information on elective procedures. The dose of PCC varied from 15 to 25 IU/kg. Details of the study characteristics are reported in Table 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) screening and selection flow diagram for a study evaluating the effects of prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) in cardiac surgery.
Table 1.
The design and characteristics of 8 studies included in a meta-analysis to compare the use of PCC and FFP in adult cardiac surgery
| Variable | PCC/FFP | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Arnékian et al. [11] (2012) | Bartoszko et al. [14] (2021) | Cappabianca et al. [12] (2016) | Fitzgerald et al. [15] (2018) | Karkouti et al. [3] (2021) | Ortmann et al. [20] (2015) | Green et al. [4] (2021) | Smith et al. [13] (2022) | |
| Type of study | Retrospective | Post-hoc | Retrospective | Retrospective | RCT | Retrospective | RCT | RCT |
| Follow-up (day) | NA | 28 | NA | NA | 28 | 30 | 90 | 30 |
| Patientsa) | 24/26 | 72/343 | 225/225 | 117/117 | 54/47 | 45/55 | 25/25 | 51/49 |
| Mean age (yr) | 64.0/72.0 | 59.4/65.3 | 69.2/69.7 | 59.6/58.9 | 62.8/65.2 | 61.0/62.0 | 68.3/65.6 | 70.0/67.6 |
| Femalea) | 6/8 | 30/99 | 91/91 | 40/45 | 14/14 | 19/18 | 9/9 | 25/14 |
| BMI (kg/m2) | 27±3.6/26±4 | 21±5/23±4.5 | 25±4.7/25±4.9 | NA | 23.6±4.5/23±4.7 | 28.8±6/27±5 | 27±6/29±5 | 28.6±6/29±5 |
| CPB time (min) | 310±50/317±8 | NA | 152±68/150±65 | 142±69/148±65 | 172±71/166±46 | 351±61/344±51 | NA | 164±76/160±63 |
| Diabetesa) | 6/6 | 12/72 | 37/36 | 15/20 | 11/10 | NA | 6/6 | NA |
| Hypertensiona) | 19/18 | NA | 142/143 | 65/67 | 36/31 | NA | 19/13 | NA |
| Emergent surgerya) | NA | 8/60 | 41/45 | 18/16 | 11/15 | NA | NA | NA |
| Elective surgerya) | NA | NA | NA | NA | NA | NA | 19/20 | NA |
| Procedureb) | ||||||||
| CABG | 9/9 | NA | 36/39 | NA | 20/15 | NA | NA | 1/0 |
| Valve surgery and CABG | 2/6 | NA | 53/47 | NA | NA | NA | 6/5 | 13/8 |
| Othersc) | 13/11 | NA | 136/140 | 88/92 | 106/87 | 45/55 | 5/7 | 37/41 |
| eGFR (mL/min/1.73 m2) | NA | NA | 64±26/64±23 | NA | NA | NA | NA | NA |
Values are presented as number or mean±standard deviation, unless otherwise stated.
PCC, prothrombin complex concentrate; FFP, fresh frozen plasma; RCT, randomized controlled trial; NA, not available; BMI, body mass index; CPB, cardiopulmonary bypass; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate.
a)Absolute number of patients. b)Absolute number of procedures. c)Examples of other procedures include: aortic replacement, mitral valve, tricuspid valve, single valve, surgery on aorta, multivalve repair, aortic replacement and valve repair, congenital defect repair, and pericardiectomy.
Pooled analysis of all studies
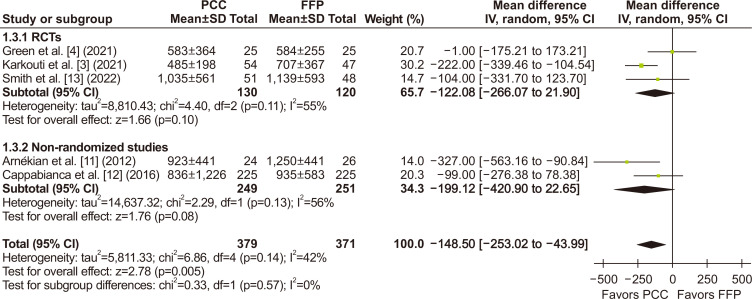
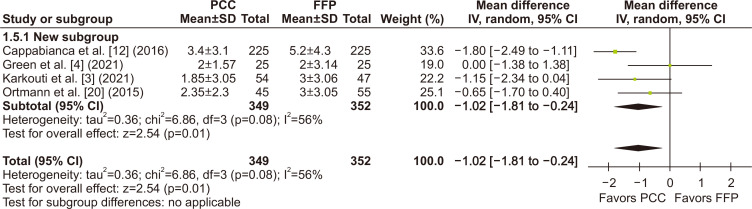
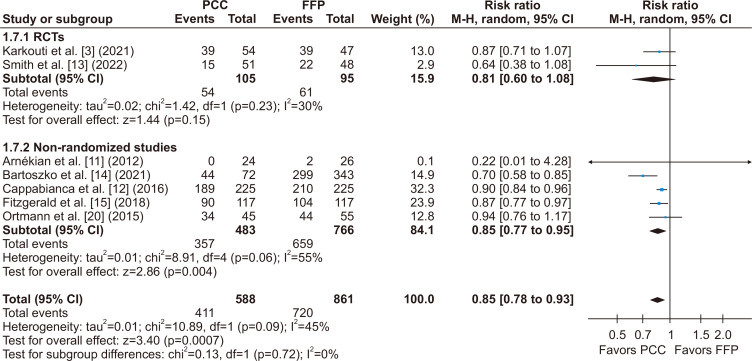
Regarding the main outcome, chest tube drainage output at 24 hours was significantly lower in the PCC group when compared to the FFP group (MD, -148.50 mL; 95% CI, -253.02 to -43.99 mL; p=0.005; I2=42%) (Fig. 2). There was also a significant decrease in the number of units of RBCs transfused within the first 24 hours (MD, -1.02 units; 95% CI, -1.81 to -0.24 units; p=0.01; I2=56%) (Fig. 3). The number of patients requiring RBC transfusion within the first 24 hours was also lower in the PCC group (RR, 0.85; 95% CI, 0.78–0.93; p<0.0007; I2=45%) (Fig. 4).
Fig. 2.
In an evaluation of the effects of prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) in cardiac surgery, there was a significant reduction in chest tube drainage output within 24 hours, favoring the PCC group. SD, standard deviation; IV, inverse variance; CI, confidence interval; RCT, randomized controlled trial; df, degrees of freedom. p-values <0.05 indicate statistical significance.
Fig. 3.
The number of red blood cell (RBC) units transfused within the first 24 hours was significantly lower in the patients receiving prothrombin complex concentrate (PCC) during cardiac surgery when compared with fresh frozen plasma (FFP). SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom. p-values <0.05 indicate statistical significance.
Fig. 4.
There was a significant decrease in the number of patients requiring red blood cell (RBC) transfusions within the first 24 hours after cardiac surgery, favoring patients who received prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) during surgery for coagulopathy or bleeding. M-H, Mantel-Haenszel; CI, confidence interval; RCT, randomized controlled trial; df, degrees of freedom. p-values <0.05 indicate statistical significance.
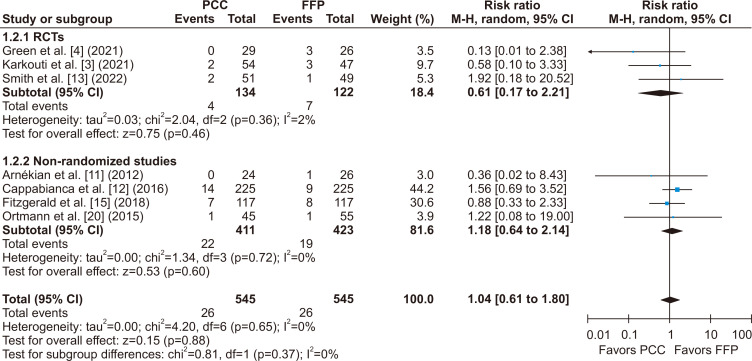
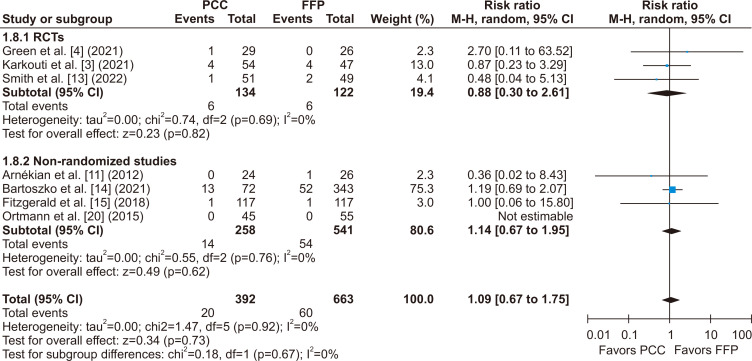
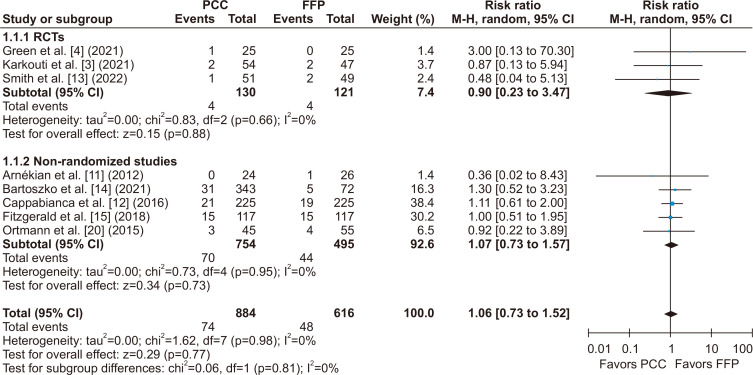
There was no statistically significant difference between groups for the incidence of stroke or TIA (RR, 1.04; 95% CI, 0.61–1.80; p=0.37; I2=0%) (Fig. 5), thromboembolic events (RR, 1.09; 95% CI, 0.67–1.75; p=0.67; I2=0%) (Fig. 6), and all-cause mortality (RR, 1.06; 95% CI, 0.73–1.52; p=0.81; I2=0%) (Fig. 7).
Fig. 5.
There was no significant difference between groups for the incidence of stroke or transient ischemic attack after cardiac surgery when comparing patients who received prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) during surgery for bleeding or coagulopathy. M-H, Mantel-Haenszel; CI, confidence interval; RCT, randomized controlled trial; df, degrees of freedom. p-values <0.05 indicate statistical significance.
Fig. 6.
There was no significant difference in the incidence of thromboembolic events after cardiac surgery between patients who received prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) during surgery for coagulopathy or bleeding. M-H, Mantel-Haenszel; CI, confidence interval; RCT, randomized controlled trial; df, degrees of freedom. p-values <0.05 indicate statistical significance.
Fig. 7.
All-cause mortality within 30 days of cardiac surgery was not significantly different between patients who received prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) during surgery for coagulopathy or bleeding. M-H, Mantel-Haenszel; CI, confidence interval; RCT, randomized controlled trial; df, degrees of freedom. p-values <0.05 indicate statistical significance.
Similarly, the LOS in the hospital (MD, -1.02 days; 95% CI, -2.32 to 0.28 days; p=0.12; I2=67%) (Supplementary Fig. 3), LOS in the ICU (MD, 0.06 days; 95% CI, -0.58 to 0.71 days; p=0.85; I2=56%) (Supplementary Fig. 4), duration of mechanical ventilation (MD, -3.43 hours; 95% CI, -7.88 to 1.02 hours; p=0.13; I2=50%) (Supplementary Fig. 5), reoperation for bleeding (RR, 0.81; 95% CI, 0.61–1.08; p=0.60; I2=0%) (Supplementary Fig. 6), incidence of AKI (RR, 1.07; 95% CI, 0.89–1.29; p=0.48; I2=0%) (Supplementary Fig. 7A), and incidence of RRT (RR, 1.89; 95% CI, 0.92–3.87; p=0.08; I2=45%) (Supplementary Fig. 7B) were not significantly different between the 2 groups.
Subgroup analysis
When considering subgroup analyses of the RCTs, we found no significant difference in the chest tube output within the first 24 hours (MD, - 91.54 mL; 95% CI, -256.38 to 73.31 mL; p=0.28; I2=65%) (Fig. 2), in the number of units of RBCs transfused within 24 hours (MD, -0.63 units; 95% CI, -1.75 to 0.49 units; p=0.27; I2=35%) (Fig. 3), and in the number of patients who required RBC transfusions within 24 hours (RR, 0.81; 95% CI, 0.60–1.08; p=0.72; I2=45%) (Fig. 4) when compared to a pooled analysis of all studies. In this analysis of the RCTs, there was also no significant difference in outcomes for length of hospital stay (MD, -0.41 days; 95% CI, -1.75 to 0.93 days; p=0.55; I2=40%) (Supplementary Fig. 3), stroke incidence (RR, 0.61; 95% CI, 0.17–2.21; p=0.46; I2=2%) (Fig. 5), and all-cause mortality (RR, 0.90; 95% CI, 0.23–3.47; p=0.88; I2=0%) (Fig. 7).
Quality assessment
A summary of the individual evaluations of each RCT included in the meta-analysis using the RoB2 quality assessment tool is presented in Supplementary Fig. 1. Two RCTs were rated at low risk of bias, whereas one presented some concerns (Supplementary Fig. 1) [16]. Using the ROBINS-I tool for observational studies, 1 study was rated low risk of bias, 3 studies were moderate risk, and 1 study was rated a critical risk (Supplementary Fig. 2) [17]. These ratings suggest that the overall risk of bias in the studies included in the meta-analysis was low to moderate.
Discussion
In our systematic review and meta-analysis of 8 studies involving 1,500 patients, we compared the use of PCC to FFP in patients who underwent cardiac surgery and experienced significant bleeding. A summary of our main findings follows. First, PCC was associated with lower chest tube drainage output and a reduced need for RBC transfusions within the first 24 hours after start of surgery. Second, there were no significant differences between the PCC and FFP groups for outcomes including reoperation for bleeding, mortality, LOS in the hospital, ICU duration of mechanical ventilation, AKI, and RRT. Finally, no significant difference was found between patients treated with PCC or FFP for the incidence of stroke or other thromboembolic events.
Higher rates of RBC transfusion have been consistently associated with unfavorable outcomes in cardiovascular surgery, including risk of infection, acute lung injury, mortality, and prolonged length of hospital stay. This may be, at least partially, attributable to fluid overload induced by transfusions [21,22]. In addition to RBC transfusion, intraoperative administration of FFP has also been correlated with fluid overload, increased inpatient mortality, and worse outcomes [23,24]. Indeed, fluid overload events such as pulmonary edema and congestive cardiac failure are more common in patients receiving FFP than those receiving PCC [24]. Our meta-analysis also showed that PCC did not increase the risk of these events when compared to FFP.
Thromboembolic complications are a potential concern with the use of PCC [25]. This may be related to the accumulation of factor II after repeated administration of PCC and to the use of PCC for the reversal of warfarin. When patients with hemophilia are excluded, the overall incidence of thrombotic complications was found to be low [8,26]. In a porcine laboratory study using a dose of 50 IU/kg, PCC was correlated with an increased risk of thromboembolism, but a lower dose of 35 IU/kg safely improved coagulation [27]. Similarly, in a multivariable analysis adjusted for potential confounding variables such as procedure types, comorbidities, and other demographic characteristics, PCC was not associated with an increased risk of thromboembolic events in patients who underwent cardiac surgery [14]. In this meta-analysis, the PCC dose among studies ranged from 15 to 25 IU/kg, and we found no significant difference between PCC and FFP in the incidence of stroke or thromboembolic events.
A previous meta-analysis comparing PCC to FFP in patients who underwent CPB was published in 2019 [28]. The authors found a significant decrease in the number of patients requiring RBC transfusions and in the number of units received. However, that meta-analysis included retrospective studies only and had a smaller sample size of 861 patients. Our study builds on prior work by adding RCT data and nearly doubling the sample size. We also confirmed findings of decreased transfusion need and the safety of PCC in a subgroup analysis of randomized studies. Overall, our results provide more robust evidence supporting the use of PCC as an effective and safe alternative to FFP for the management of coagulopathy and bleeding in patients who undergo cardiac surgery.
Our study had limitations. First, 5 of the 8 studies included in the analysis were observational, 3 of which were assessed as having a moderate risk of bias and one as having a critical risk of bias. To address this limitation, we conducted a subgroup analysis with the RCTs exclusively. Second, we observed substantial heterogeneity in the primary outcomes. This heterogeneity could be attributed to chance alone or to methodological differences between studies such as variations in the administered dose of PCC. Finally, it was not possible to conduct a meta-regression of the influence of PCC dose on the pooled treatment estimates due to limited data availability.
Conclusion
In this meta-analysis with 1,500 patients, the use of PCC was associated with a significant decrease in chest tube drainage output at 24 hours and a reduction in the need for RBC transfusion when compared with the use of FFP in patients undergoing cardiac surgery complicated by bleeding. Treatment with PCC did not increase the risk of thromboembolic events, stroke, AKI, RRT, or mortality. Altogether, these findings suggest that PCC may be considered a safe and effective alternative to FFP.
Supplementary materials
Supplementary materials can be found via https://doi.org/10.5090/jcs.23.081. Supplementary Material 1. Search strategy for each database. Supplementary Fig. 1. Quality assessment according to the Cochrane Collaboration tool (RoB2) of 3 randomized controlled trials in a systematic review and meta-analysis comparing PCC versus FFP in adult cardiac surgery patients. Supplementary Fig. 2. Quality assessment according to the Cochrane Collaboration tool (ROBINS-I) for the observational studies in a systematic review and meta-analysis comparing PCC versus FFP in adult cardiac surgery patients. Supplementary Fig. 3. There was no significant difference in the length of hospital stay after cardiac surgery between patients who received PCC versus FFP during surgery for coagulopathy or bleeding. Supplementary Fig. 4. There was no significant difference in the length of ICU stay after cardiac surgery between patients who received PCC versus FFP during surgery for coagulopathy or bleeding. Supplementary Fig. 5. There was no significant difference in duration of mechanical ventilation (hours) after cardiac surgery between patients who received PCC versus FFP during surgery for bleeding or coagulopathy. Supplementary Fig. 6. There was no significant difference in the rate of reoperation for bleeding within 48 hours of cardiac surgery between patients who received PCC versus FFP during surgery for bleeding or coagulopathy. Supplementary Fig. 7. (A) There was no significant difference in the incidence of acute kidney injury after cardiac surgery between patients who received PCC versus FFP during surgery for bleeding or coagulopathy. (B) There was no significant difference in the risk of renal replacement therapy after cardiac surgery between patients who received PCC versus FFP during surgery for bleeding or coagulopathy.
Acknowledgments
We thank Dr. Rhanderson Cardoso for his assistance with methods, statistics, and paper revision.
Funding Statement
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article information
Authors contributions
Conceptualization and project administration: PV. Investigation: PV, JR, JP, MP, TC, PB, VF. Triage of studies: PV, TC. Data acquisition: PV, JR, JP, JS. Data analysis and interpretation: PV, JP. Risk of bias assessment: MP, PB, PV, VF. Formal analysis: PV, JP, JR. Writing–review and editing: all authors. Final approval of the manuscript: all authors.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Sarkar M, Prabhu V. Basics of cardiopulmonary bypass. Indian J Anaesth. 2017;61:760–7. doi: 10.4103/ija.IJA_379_17. https://doi.org/10.4103/ija.IJA_379_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiele RH, Raphael J. A 2014 update on coagulation management for cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. 2014;18:177–89. doi: 10.1177/1089253214534782. https://doi.org/10.1177/1089253214534782. [DOI] [PubMed] [Google Scholar]
- 3.Karkouti K, Bartoszko J, Grewal D, et al. Comparison of 4-factor prothrombin complex concentrate with frozen plasma for management of hemorrhage during and after cardiac surgery: a randomized pilot trial. JAMA Netw Open. 2021;4:e213936. doi: 10.1001/jamanetworkopen.2021.3936. https://doi.org/10.1001/jamanetworkopen.2021.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green L, Roberts N, Cooper J, et al. Prothrombin complex concentrate vs. fresh frozen plasma in adult patients undergoing heart surgery: a pilot randomised controlled trial (PROPHESY trial) Anaesthesia. 2021;76:892–901. doi: 10.1111/anae.15327. https://doi.org/10.1111/anae.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashmi NK, Ghadimi K, Srinivasan AJ, et al. Three-factor prothrombin complex concentrates for refractory bleeding after cardiovascular surgery within an algorithmic approach to haemostasis. Vox Sang. 2019;114:374–85. doi: 10.1111/vox.12774. https://doi.org/10.1111/vox.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tornkvist M, Smith JG, Labaf A. Current evidence of oral anticoagulant reversal: a systematic review. Thromb Res. 2018;162:22–31. doi: 10.1016/j.thromres.2017.12.003. https://doi.org/10.1016/j.thromres.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8:149–54. doi: 10.2450/2010.0149-09. https://doi.org/10.2450/2010.0149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghadimi K, Levy JH, Welsby IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg. 2016;122:1287–300. doi: 10.1213/ANE.0000000000001188. https://doi.org/10.1213/ANE.0000000000001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Key NS, Negrier C. Coagulation factor concentrates: past, present, and future. Lancet. 2007;370:439–48. doi: 10.1016/S0140-6736(07)61199-4. https://doi.org/10.1016/S0140-6736(07)61199-4. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group, author. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul 21;339:b2535. doi: 10.1136/bmj.b2535. https://doi.org/10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnekian V, Camous J, Fattal S, Rezaiguia-Delclaux S, Nottin R, Stephan F. Use of prothrombin complex concentrate for excessive bleeding after cardiac surgery. Interact Cardiovasc Thorac Surg. 2012;15:382–9. doi: 10.1093/icvts/ivs224. https://doi.org/10.1093/icvts/ivs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappabianca G, Mariscalco G, Biancari F, et al. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Crit Care. 2016;20:5. doi: 10.1186/s13054-015-1172-6. https://doi.org/10.1186/s13054-015-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MM, Schroeder DR, Nelson JA, et al. Prothrombin complex concentrate vs plasma for post-cardiopulmonary bypass coagulopathy and bleeding: a randomized clinical trial. JAMA Surg. 2022;157:757–64. doi: 10.1001/jamasurg.2022.2235. https://doi.org/10.1001/jamasurg.2022.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartoszko J, Callum J, Karkouti K FIBRES study investigators, author. The association of prothrombin complex concentrates with postoperative outcomes in cardiac surgery: an observational substudy of the FIBRES randomized controlled trial. Can J Anaesth. 2021;68:1789–801. doi: 10.1007/s12630-021-02100-4. https://doi.org/10.1007/s12630-021-02100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald J, Lenihan M, Callum J, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth. 2018;120:928–34. doi: 10.1016/j.bja.2018.02.017. https://doi.org/10.1016/j.bja.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. https://doi.org/10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. https://doi.org/10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions: version 6.3. Cochrane; 2022. [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. https://doi.org/10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortmann E, Besser MW, Sharples LD, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesth Analg. 2015;121:26–33. doi: 10.1213/ANE.0000000000000689. https://doi.org/10.1213/ANE.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. https://doi.org/10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 22.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. https://doi.org/10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt HV, Subramaniam K. PRO: prothrombin complex concentrate should be used in preference to fresh frozen plasma for hemostasis in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2018;32:1062–7. doi: 10.1053/j.jvca.2017.05.044. https://doi.org/10.1053/j.jvca.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 24.Refaai MA, Goldstein JN, Lee ML, Durn BL, Milling TJ, Jr, Sarode R. Increased risk of volume overload with plasma compared with four-factor prothrombin complex concentrate for urgent vitamin K antagonist reversal. Transfusion. 2015;55:2722–9. doi: 10.1111/trf.13191. https://doi.org/10.1111/trf.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler M, Hellstern P, Lechler E, Uberfuhr P, Muller-Berghaus G. Thromboembolic complications associated with the use of prothrombin complex and factor IX concentrates. Thromb Haemost. 1998;80:399–402. doi: 10.1055/s-0037-1615219. https://doi.org/10.1055/s-0037-1615219. [DOI] [PubMed] [Google Scholar]
- 26.Majeed A, Eelde A, Agren A, Schulman S, Holmstrom M. Thromboembolic safety and efficacy of prothrombin complex concentrates in the emergency reversal of warfarin coagulopathy. Thromb Res. 2012;129:146–51. doi: 10.1016/j.thromres.2011.07.024. https://doi.org/10.1016/j.thromres.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Grottke O, Braunschweig T, Spronk HM, et al. Increasing concentrations of prothrombin complex concentrate induce disseminated intravascular coagulation in a pig model of coagulopathy with blunt liver injury. Blood. 2011;118:1943–51. doi: 10.1182/blood-2011-03-343046. https://doi.org/10.1182/blood-2011-03-343046. [DOI] [PubMed] [Google Scholar]
- 28.Roman M, Biancari F, Ahmed AB, et al. Prothrombin complex concentrate in cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2019;107:1275–83. doi: 10.1016/j.athoracsur.2018.10.013. https://doi.org/10.1016/j.athoracsur.2018.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.