Abstract
Healthy adults are susceptible to infection with small numbers of Cryptosporidium parvum oocysts, resulting in self-limited infection. We investigated if infection of humans with C. parvum is protective 1 year after primary exposure. At 1 year after a primary challenge with 30 to 106 oocysts, 19 healthy immunocompetent adults were rechallenged with 500 oocysts and monitored for the development of infection and/or illness. Oocyst excretion was quantitated by direct immunofluorescence with a C. parvum-specific monoclonal antibody, and anti-C. parvum antibodies in serum were detected by an enzyme-linked immunosorbent assay. Fewer subjects shed oocysts after the second exposure (3 of 19; 16%) than after the first exposure (12 of 19; 63%) (P < 0.005). Although the rates of diarrhea were comparable after each of the two exposures, the clinical severity as determined by the mean number of unformed stools passed was lower after reexposure (11.25 versus 8.62; P < 0.05). The number of anti-Cryptosporidium immunoglobulin G and A seroconversions increased after secondary exposure. However, the C. parvum serum antibody response did not correlate with the presence or absence of infection.
Resistance to repeated Cryptosporidium parvum infection is the norm for most animal species, including mice and calves (12, 13, 21), but anecdotal evidence suggests that this may not be the case in humans. The mechanisms for the resistance seen in many species are not well understood but are thought to involve both innate and acquired factors. For example, C. parvum challenge of neonatal mice results in a nonfatal infection that resolves in approximately 3 weeks, coincident with the development of a mature gut and the acquisition of normal flora (10). Further, when the primary challenge of mice is delayed until adulthood, the infection is light and even more limited (3 to 5 days) than in neonates (11). An age-related resistance can also be observed in calves (12). The acquired response also plays a significant role and involves CD4 (17) and CD8 (1) lymphocytes, as well as gamma interferon (4). Interestingly, primates are susceptible to reinfection within 2 weeks after primary exposure but are protected from symptomatic disease (18). This resistance has also been observed in broilers after repeated exposure to C. baileyi (6).
While primary exposure frequently results in proven infection and symptomatic disease in healthy adults previously seronegative for C. parvum (7), the resulting susceptibility to reinfection and illness is unknown. Epidemiological data obtained for Brazilian children suggests that primary infection with C. parvum does not completely block reinfection upon subsequent exposure (20) but may protect the host against clinical illness (5). However, recurrent infections have been described in healthy adults (5) and in high-risk populations in areas with high seroprevalence for the disease (20), suggesting that repeated infection may result in diarrheal illness. The proportion of subjects who develop disease and/or infection after reexposure is unknown. In the present study, 19 healthy adult volunteers were challenged with C. parvum on two occasions separated by 1 year to determine the role of primary exposure in the development of protective immunity. A 1-year interval between challenges was selected to resemble the seasonal occurrence of C. parvum infections in nature (19).
MATERIALS AND METHODS
The C. parvum Iowa isolate was prepared for volunteer use as previously described (7). Primary exposure was carried out with 30 to 106 oocysts to determine a 50% infective dose (ID50) for healthy adults (7). The primary challenge was conducted between September 1993 and August 1994. An interim analysis of the primary challenge at the time of the design and initiation of this study revealed an ID100 of 500 oocysts; therefore, this dose was selected for the rechallenge. Once the primary challenge was completed, 500 oocysts represented an ID86. Volunteers were rechallenged within 11 to 13 months after their primary challenge date. Subjects who agreed to participate were asked to provide informed consent and were interviewed for any illness in the intervening months following the initial oocyst challenge. A physical examination and screening laboratory studies for immunodeficiency were performed as previously described (7). The study was approved by the University of Texas Committee for the Protection of Human Subjects.
Evaluation of stools and definition of terms.
Volunteers collected all stools passed for the first 2 weeks of the study and collected stools during three 24-h collection periods per week thereafter for a total of 6 weeks after challenge. All stools collected were kept in a cooler with ice and brought to the University Clinical Research Center at Hermann Hospital the following morning. The stools were weighed, and an aliquot was placed in 10% buffered formalin (1:4, vol/vol). A diary was kept by each volunteer describing the time and characteristics of all stools passed and the symptoms experienced. All stools were examined for the presence of Cryptosporidium by a direct immunofluorescence assay (DFA) (9).
Diarrhea was defined as the passage of three unformed stools in an 8-h period, the passage of four or more unformed stools in a 24-h period, or the passage of unformed stools in excess of 200 g/24 h accompanied by at least two of the following symptoms: nausea, vomiting, abdominal pain and/or cramping, tenesmus, fecal urgency, or gas-associated complaints. Symptomatic subjects included those who experienced two or more symptoms. Subjects were presumed to be infected if oocysts could be identified in their stools or if they experienced diarrhea and gastrointestinal symptoms in the first 30 days after challenge. Volunteers were considered uninfected if oocysts could not be identified in feces and no symptoms were experienced during the duration of the study. The duration of diarrhea was defined as previously described (8). Wellness was defined as a 24-h period with no gastrointestinal symptoms or diarrhea. Relapse was defined as the recurrence of diarrhea after a 24-h period of wellness.
Antibody assays.
Serum samples collected on days 0 and 45 after each challenge were assayed for the presence of anti-C. parvum antibodies (immunoglobulin M [IgM], IgG, and IgA) by a modified anti-C. parvum enzyme-linked immunosorbent assay (ELISA) as previously described (7), except that a biotinylated monoclonal antihuman antibody followed by peroxidase-conjugated streptavidin was used as the detecting antibody. An increase of 0.1 optical density unit from the prechallenge baseline was considered evidence for seroconversion.
Statistical methods.
The proportions of infected or symptomatic volunteers were compared by Fishers’ exact test. The Kruskal-Wallis nonparametric analysis of variance was used to analyze the mean time of onset of infection, and the Mann-Whitney test was used to compare the duration of excretion in the primary and secondary challenges.
RESULTS
No adventitious agents were identified in the oocyst preparations by conventional cultures, cell culture, or electron microscopy. Excystation rates (85.5 to 93.4%), sporozoite yields (38.7 to 43%), time from oocyst production to the date of use, and mouse infectivity were similar throughout the study. The mean number of oocysts ingested by volunteers in the second challenge ranged from 500 to 510 with a coefficient of variation of 0.9 to 3.5%.
Of the 29 volunteers who underwent an initial challenge with C. parvum, 19 (66%) were available for rechallenge 1 year later. Altogether, 15 (83%) of 18 previously infected volunteers and (36%) of 11 who were previously uninfected after the primary challenge were enrolled in the rechallenge. Five of these volunteers received ≥1,000 oocysts during the primary challenge.
After rechallenge, symptoms compatible with cryptosporidiosis occurred in similar proportions (Table 1) to those reported after the initial exposure. Presumed infections were identified in 15 of 19 volunteers after the first exposure and in 16 of 19 after the second. Nine subjects were infected on both occasions. Of the 19 volunteers, 8 had diarrhea after the first and second challenge, 3 only after the first challenge and 3 only after the second challenge, and 5 did not experience diarrhea at any time. For the eight volunteers who experienced diarrhea on both challenges, the mean time to onset (6.6 and 8.18 days) and the duration of diarrhea (67 and 60 h) were similar. However, the intensity of diarrhea as determined by the mean number of unformed stools passed per diarrheal illness was lower after the rechallenge (8.62 after rechallenge and 11.25 after the first challenge [P < 0.05]).
TABLE 1.
Clinical outcome in 19 volunteers exposed to C. parvum oocysts 1 year aparta
| Clinical outcome | No. of volunteers with outcome after
|
|
|---|---|---|
| First challenge (n = 19) | Second challenge (n = 19) | |
| Presumed infection | 15 | 16 |
| Diarrhea | 11 | 11 |
| Oocysts detected by DFA | 9 | 2b |
| Oocysts not detected by DFA | 2 | 9 |
| Enteric symptoms, oocysts detected by DFA | 2 | 1 |
| Enteric symptoms, oocysts not detected by DFA | 0 | 4 |
| Asymptomatic, oocysts detected by DFA | 2 | 0 |
| No infection | 4 | 3 |
| Asymptomatic, oocysts not detected by DFA | 4 | 3 |
The initial challenge was conducted with 30 to 106 oocysts. Volunteers were rechallenged with 500 oocysts. Clinical outcomes are defined in Materials and Methods.
P < 0.01 compared to first challenge.
After primary exposure to C. parvum 13 (68%) of the 19 volunteers had oocysts identified by DFA. In contrast, after secondary challenge, only (16%) of the 19 volunteers shed oocysts in detectable numbers (P < 0.005). A reduced occurrence of shedding after repeated exposure was also apparent when the comparison was restricted to volunteers who had received 500 oocysts on both occasions. In that instance, 3 (100%) of 3 volunteers shed oocysts after the first challenge compared to only 3 (16%) of 19 after the second exposure (P < 0.02). No differences were seen in the mean prepatent period (9.8 and 15 days) or the duration of oocyst excretion (6 and 2.6 days) between those shedding oocysts after primary and secondary challenge, respectively.
Relapses of diarrhea were common. Eleven volunteers enrolled in the rechallenge study had diarrhea after the primary challenge. Of these, 7 (64%) had one relapse and 2 (18%) had two or three relapses. In comparison, 4 (45%) had one relapse after rechallenge and 2 (18%) had three or five relapses. The duration and severity of relapses were similar between groups.
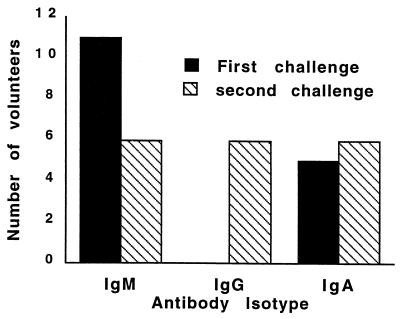
After the primary exposure, increased antibody reactivity (as measured by ELISA) was most noticeable for IgM (58%) and was seen in both infected and uninfected volunteers; no volunteers developed an IgG response (Fig. 1). Of interest, five volunteers demonstrated antibody reactivity prior to rechallenge; three volunteers had reactive IgG (two of them also had reactive IgM and one had reactive IgA), one had IgA and IgM reactivity, and one had IgM reactivity alone. Antibody reactivity after rechallenge was predominantly of the IgG and IgA isotypes. However, no correlation was seen between the development of antibodies after primary challenge and subsequent protection as measured by the various outcome variables (symptoms, diarrhea, and presence of oocysts).
FIG. 1.
IgM, IgG, and IgA serologic responses to C. parvum as measured by ELISA on day 45 after the primary and secondary challenges.
DISCUSSION
This study shows that in healthy adults seronegative for C. parvum, an initial exposure is not sufficient to protect against clinical illness 1 year later. Although the incidence, onset, and duration of clinical illness were similar, there was a decrease in the severity of disease (as determined by a significant decrease in the number of unformed stools passed) and the intensity of infection (as determined by a lesser likelihood of detecting oocysts).
The difference in the proportions of subjects shedding oocysts was not a function of volunteers receiving a higher inoculum in the primary challenge, since a separate analysis of volunteers receiving 500 oocysts on both occasions demonstrated that when adjusted for inoculum dose, the infection rate was lower after rechallenge. Furthermore, the decreased excretion of oocysts is supported by two other observations: (i) according to our data from the primary infectivity study, a dose of 500 oocysts during rechallenge in the absence of protection should have resulted in 86% volunteers excreting oocysts; and (ii) our previous study has shown that volunteers challenged with high doses of oocysts actually excrete fewer oocysts than those who received lower doses (3).
An unexpected finding was the occurrence of diarrhea in the absence of detectable oocysts (as measured by DFA) after the secondary exposure. However, epidemiological data supporting this observation has previously been described in large-scale outbreaks (15). This might be explained by the lack of sensitivity of currently available methods, such as DFA, which is unable to detect fewer than 10,000 oocysts per ml (2). We speculate that individuals with clinical cryptosporidiosis who are DFA negative are excreting few if any oocysts as a result of an active host immune response. Indeed, for stools from four of these volunteers examined by flow cytometry, three were found to be oocyst positive (unpublished data).
Approximately 21% of persons with watery diarrhea during a large-scale outbreak in Milwaukee in 1993 experienced a clinical relapse up to 45 days after resolution of the initial symptoms (16). In our studies, relapses were seen in equal proportions after the first and second challenges. None of our volunteers had experienced diarrhea during the year between challenges.
Previous studies have shown that antibodies to C. parvum can be identified in 19 to 60% of the U.S. population (7, 14). Seroprevalence can be even higher in developing nations and in regions with repeated exposure to hoofed animals. However, the significance and relevance of anti-C. parvum antibodies are unknown. While others have reported a specific antibody response to C. parvum infection in humans (20) in endemic settings, only a modest correlation was seen between infection and/or symptoms and the development of antibodies in serum in our study. IgM conversion was more frequent after primary challenge, while IgG and IgA conversion was more frequent after rechallenge. Of interest, five volunteers demonstrated antibody reactivity immediately prior to rechallenge without any history of diarrheal illness in the time interval between challenges. No protection from clinical illness was seen in this subgroup of subjects when rechallenged. The low antibody response rates seen by ELISA were not completely unexpected given that infection with this parasite is limited to the intestinal epithelium, that the primary protective response to C. parvum is mediated by CD4+ lymphocytes, and that the antigens used are limited to the oocyst/sporozoite stages. Improved methods to study the human humoral and cellular response to C. parvum are needed.
Our data suggests that after repeated exposure to C. parvum, illness is as likely to occur but oocysts are less likely to be detected. Further, the IgM response that predominated after the primary challenge shifted toward an increased IgG reactivity after rechallenge. Thus, we speculate that the IgG-seropositive individuals in the general population have experienced two or more exposures to C. parvum, that symptomatic C. parvum infection may be less intense and associated with lower levels of oocyst excretion after repeated exposure to C. parvum, and that repeated exposure may be necessary for the development of complete immunity.
ACKNOWLEDGMENTS
This study was supported by a Cooperative Agreement with the Environmental Protection Agency (CR-819894) and by a grant from the NIH through the General Clinical Research Centers (MO1-RR-02558).
We thank John J. Mathewson and Melinda Cox for performing the bacteriologic studies. The personnel involved in laboratory studies were Han Dang, Nazario Siytangco-Johnson, Phu-Nguyen, Janice Jackson, and Marilyn M. Marshall. The CRC medical staff caring for the volunteers were Madelene Jewell, Julie Rice, Terry Talbot, Nai-hui Chiu, Paula Officer, and Inge Weiser.
REFERENCES
- 1.Abrahamsen M, Lancto C, Walcheck B, Layton W, Jutila M. Localization of α/β and γ/δ lymphocytes in Cryptosporidium parvum-infected tissues in naive and immune calves. Infect Immun. 1997;65:2428–2433. doi: 10.1128/iai.65.6.2428-2433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrowood M J, Sterling C R. Comparison of conventional staining methods and monoclonal antibody-based methods for Cryptosporidium oocyst detection. J Clin Microbiol. 1989;27:1490–1495. doi: 10.1128/jcm.27.7.1490-1495.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chappell C, Okhuysen P, Sterling C, DuPont H. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J Infect Dis. 1996;173:232–236. doi: 10.1093/infdis/173.1.232. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Harp J. Gamma interferon functions in resistance to Cryptosporidium parvum infection in severe combined immunodeficient mice. Infect Immun. 1993;61:3548–3551. doi: 10.1128/iai.61.8.3548-3551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Current W, Bick P. Immunobiology of Cryptosporidium spp. Pathol Immunopathol Res. 1989;8:141–160. doi: 10.1159/000157146. [DOI] [PubMed] [Google Scholar]
- 6.Current W, Snyder D. Development of and serologic evaluation of acquired immunity to Cryptosporidium baileyi by broiler chickens. Poult Sci. 1988;67:720–729. doi: 10.3382/ps.0670720. [DOI] [PubMed] [Google Scholar]
- 7.DuPont H, Chappell C, Sterling C, Okhuysen P, Rose J, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 8.DuPont, H., M. Cooperstock, M. Corrado, R. Fekety, and D. Murray. 1992. General guidelines for the evaluation of new anti-infective drugs for the treatment of acute infectious diarrhea. Clin. Infect. Dis. 15(Suppl. 1):S228–S235. [DOI] [PubMed]
- 9.Goodgame R W, Genta R M, White A C, Chappell C L. Intensity of infection in AIDS-associated cryptosporidiosis. J Infect Dis. 1993;167:704–709. doi: 10.1093/infdis/167.3.704. [DOI] [PubMed] [Google Scholar]
- 10.Harp J, Chen W, Harmsen A. Resistance of severe combined immunodeficient mice to infection with Cryptosporidium parvum: the importance of intestinal microflora. Infect Immun. 1992;60:3509–3512. doi: 10.1128/iai.60.9.3509-3512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harp J, Wannemuehler M, Woodmansee D, Moon H. Susceptibility of germfree and antibiotic treated adult mice to Cryptosporidium parvum. Infect Immun. 1988;56:2006–2010. doi: 10.1128/iai.56.8.2006-2010.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harp J, Woodmansee D, Moon H. Resistance of calves to Cryptosporidium parvum: effects of age and previous exposure. Infect Immun. 1990;58:2237–2240. doi: 10.1128/iai.58.7.2237-2240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korich D, Mead J, Madore M, Sinclair N, Sterling C. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990;56:1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhls T, Moiser D A, Crawford D, Griffs J. Seroprevalence of cryptosporidial antibodies during infancy, childhood and adolescence. Clin Infect Dis. 1994;5:731–735. doi: 10.1093/clinids/18.5.731. [DOI] [PubMed] [Google Scholar]
- 15.MacKenzie W, Hoxie N, Proctor M, Gradus M, Blair K, Peterson D, Kazmierczak J, Addiss D, Fox K, Rose J, Davis J. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie W, Schell W, Blair K, Addiss D, Peterson D, Hoxie N, Kazmierczak J, Davis J. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of Illness and risk of secondary transmission. Clin Infect Dis. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- 17.McDonald V, Robinson H, Kelly J, Bancroft G. Immunity to Cryptosporidium muris infection in mice is expressed through gut CD4+ intraepithelial lymphocytes. Infect Immun. 1996;64:2556–2562. doi: 10.1128/iai.64.7.2556-2562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller R, Brodsdon M, Morton W. Experimental cryptosporidiosis in a primate model. J Infect Dis. 1990;161:312–315. doi: 10.1093/infdis/161.2.312. [DOI] [PubMed] [Google Scholar]
- 19.Newman R, Wuhib T, Lima A, Guerrant R, Sears C. Environmental sources of Cryptosporidium in an urban slum in northwestern Brazil. Am J Trop Med Hyg. 1993;49:270–275. doi: 10.4269/ajtmh.1993.49.270. [DOI] [PubMed] [Google Scholar]
- 20.Newman R, Zu S, Wuhib T, Lima A, Guerrant R, Sears S. Household epidemiology of Cryptosporidium parvum infection in an urban community in Northeast Brazil. Ann Intern Med. 1994;120:500–505. doi: 10.7326/0003-4819-120-6-199403150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood D, Angus K, Snodgrass D, Tzipori S. Experimental cryptosporidiosis in laboratory mice. Infect Immun. 1982;38:471–475. doi: 10.1128/iai.38.2.471-475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]