Abstract
Resistin and suppressors of cytokine signaling (SOCSs) have been reported to regulate prostate cancer (PCa) cell proliferation and survival, respectively. Whether any of the SOCS molecules mediate the mitogenic effect of resistin on PCa cells is unknown. Using PC-3 human PCa cells, we found that resistin upregulates the expression of SOCS3 and SOCS5 mRNA, but not SOCS7 mRNA, in a dose- and time-dependent manner. The resistin-induced increases in SOCS3 and SOCS5 expression and cell proliferation were prevented by pretreatment with specific inhibitors of the TLR4, ERK, p38 MAPK, JNK, PI3K, and JAK2 proteins. However, pretreatment with a TLR2 inhibitor had no effect on resistin-mediated SOCS3 and SOCS5 expression. In addition, the effects of resistin on SOCS3, SOCS5, and SOCS7 mRNA levels were cell type-specific. Overexpression of either SOCS3 or SOCS5 enhanced further resistin-stimulated growth of PC-3 cells, whereas silencing SOCS3 or SOCS5 antagonized resistin-increased cell growth. Further PCa tissue analysis demonstrated higher levels of RETN, TLR4, SOCS3, and SOCS5 mRNAs in cancer tissues than benign prostate hyperplasia and indicated positive correlations among RETN, TLR4, and SOCS5. These data suggest that SOCS5, TLR4, and, to a lesser extent, SOCS3 can mediate the mitogenic effect of resistin on PC-3 PCa cells.
Keywords: Resistin, suppressor of cytokine signaling (SOCS), human prostate cancer cell
Impact statement
The development of prostate cancer (PCa) is characterized by an increased number of abnormal prostate cells due to mitogenesis and is associated with obesity. Adipocyte resistin has been found to associate with obesity and stimulate PCa cell proliferation. However, the exact mechanism of its action on PCa growth in association with SOCS is still not clear. This study investigated the pathway of SOCS3 and SOCS5 involvement in resistin modulation of PCa cell growth. In PC-3 PCa cells, we found that resistin upregulates the expression of SOCS3 and SOCS5, but not SOCS7, genes and induces cell growth via the TLR4, ERK, p38 MAPK, JNK, PI3K, and JAK2 proteins. In addition, silencing SOCS3 or SOCS5 antagonizes resistin-increased cell proliferation. Therefore, we found that the SOCS3- and SOCS5-regulated pathway mediates the mitogenic effect of resistin on PC-3 cells.
Introduction
Prostate cancer (PCa) is the most common reproductive cancer in elderly men. 1 The American Cancer Society estimates that 164,690 new cases of PCa and 29,430 deaths due to PCa occurred in the United States of America in 2018. 1 The development of PCa is characterized by an increased number of abnormal prostate cells due to mitogenesis and is associated with age, race, and obesity.2–9 The mitogenic process is regulated by endocrine, genetic, environmental, and nutritional factors.4,6–16 Resistin, a cysteine-rich peptide hormone discovered in the adipose tissues of rodents that has a functional link between obesity and type II diabetes,17,18 has been found to stimulate the proliferation of androgen-independent PCa cells via the PI3K/AKT pathway. 19 Although suppressor of cytokine signaling (SOCS) 3, the major resistin signaling protein, has been found to control the liver X receptor-mediated and cAMP-mediated growth of PCa cells,20–22 whether SOCS3 mediates the stimulatory effect of resistin on the growth of PCa cells has not been demonstrated. Notably, a recent study reported that SOCS5 may transduce an oncogenic signal of microRNA (miR)-9 in the human prostate. 23 Unfortunately, the results did not demonstrate whether SOCS5 acts as a resistin signaling molecule to control PCa cell growth. No reports have investigated whether resistin selectively regulates SOCS gene expression in PCa cells or whether any of the SOCSs can mediate the mitogenic effect of resistin on androgen-independent PCa cells. Careful examination of the signaling pathway through which resistin affects the growth of PCa cells, particularly androgen-independent PCa cells, would help in our understanding obesity-related PCa.
The signaling actions of resistin have been described extensively in non-PCa cells, but not much in PCa cells.19,20,24,25 In non-PCa cells, resistin stimulates smooth muscle cell proliferation through the activation of ERK1/2 and PI3K, 24 and SOCS3 acts as a signaling molecule, mediating the insulin-resistant effect of resistin. 20 A recent study reported that Toll-like receptor (TLR) 4, but not TLR2, could serve as a receptor for the actions of resistin in human leukocytes. 25 In PCa cells, the overexpression of resistin increases intracellular phosphorylation of Akt and proliferation of PC-3 PCa cells. 19 SOCS3 and TLR4 have been associated with the development and progression of PCa, respectively.26–34 For example, SOCS3 methylation occurs more frequently in PCa subjects compared to BPH and normal control subjects and inhibits its mRNA and protein expression, 26 and downregulation of SOCS3 causes apoptosis of PC-3, DU145, LNCaP-IL-6 + PCa cells but not parental LNCaP cells. 33 In contrast, overexpression of SOCS3 reduces cell growth and induces cell apoptosis in DU145 and TRAMP-C2 cells, but not in PC-3 cells. 27 These disparate findings may be explained by the presence of various signaling molecules or isoforms of SOCSs in different PCa cell types. This notion may be supported by the downregulation of SOCS5 during miR-9-mediated migration and invasion of M12 human PCa cells. 23 These results, taken together with the fact that SOCS3 is the major resistin signaling protein, 20 have created much controversy surrounding the possible role of the various resistin signaling molecules in regulating SOCS3 and SOCS5 expression in PCa cells and the possible involvement of SOCS3, SOCS5, and TLR4 in the resistin regulation of androgen-independent PCa cell growth. Thus, we hypothesized that resistin may regulate the expression of SOCS3 and SOCS5 genes in PCa cells in a TLR4- and PCa cell type-dependent manner in association with PCa cell growth.
This study was designed to understand the influence of resistin on the expression of SOCS3, SOCS5, and SOCS7 in PC-3 human PCa cells. We show that resistin stimulates the expression of SOCS3 and SOCS5, but not SOCS7, and confirm that this stimulation is mediated through the TLR4, ERK, p38 MAPK, JNK, PI3K, and JAK2 pathways, but not the TLR2 pathway. In addition, we demonstrate the specific effect of resistin on SOCS3, SOCS5, and SOCS7 mRNA expression in different cell types. We also show that SOCS3 and SOCS5 are required for resistin-stimulated PC-3 cell growth. Our results indicate positive correlations among the levels of SOCS5, RETN, and TLR4 mRNAs in human prostate carcinoma tissue.
Materials and methods
Chemical reagents
RPMI1640 medium, fetal bovine serum (FBS), trypsin, the protein marker, and Trizol reagent were purchased from Thermo Fisher Scientific (Waltham, MA). TissueScan Prostate Cancer cDNA Arrays I, II, and III were purchased from OriGene Technologies, Inc. (Rockville, MD). All other materials were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) unless otherwise stated.
Cell culture and treatment
PC-3 cells are the most commonly used cell line and derived from bone metastases. 35 They do not express androgen receptor (AR) and are androgen-independent. 35 To the best of our knowledge, androgen can regulate SOCS3 gene expression 36 and PCa proliferation. 29 To avoid interference by androgen, we used PC-3 cells to evaluate the effect of resistin on SOCS gene expression and PCa cell growth. In some experiments, LNCaP-FGC, DU145, and U937 cells were used for cell type-specific comparisons. All cell lines were maintained at 37°C in a 5% CO2 atmosphere. All human cell lines were purchased from the American Type Culture Collection (Manassas, VA). PC-3, LNCaP-FGC, DU145, and U937 cells were maintained in RPMI 1640 medium supplemented with 10% FBS and 1% antibiotics (100 U/mL penicillin, 100 mg/mL streptomycin). 9 To study the effect of resistin on SOCS gene expression in PC-3 cells, we plated 8 × 105 cells in a 6-cm dish and allowed them to attach. After 48 h, the medium was exchanged for medium lacking FBS. After 12–16 h, serum-starved cells were treated with 50 ng/mL human recombinant resistin (PeproTech, Inc., Rocky Hill, NJ) for 0, 1, 2, 4, 6, 12, or 24 h, followed by 0, 5, 12.5, 25, 50, or 100 ng/mL human recombinant resistin for 2 h. The SOCS mRNA levels were determined relative to GAPDH at each time point as described below for the real-time PCR analysis.
Real-time PCR
We used real-time PCR to determine the mRNA levels of SOCS3, SOCS5, SOCS7, RETN, TLR4, and GAPDH according to the methods of Chang et al. 37 Total RNA was isolated using the Trizol kit and cDNA synthesized from equal amounts (5 μg) of RNA using 100 units of M-MLV reverse transcriptase (Thermo Fisher Scientific) in the presence of 40 units of RNase inhibitor (Thermo Fisher Scientific). The adapter primer was 5′-GGCCACGCGTCGACTAGTAC(T)19-3′. Real-time PCR was performed twice in duplicate using the power SYBR green PCR master mix (Kapa Biosystems, Boston, MA) and ABI 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) under the following conditions: initial denaturing cycle at 95°C for 5 min, followed by 40 amplification cycles comprising denaturation at 95°C for 3 s and annealing at 60°C for 30 s. The forward and reverse primers are given in Table 1. GAPDH mRNA levels were determined in parallel reactions for normalization. The relative expression of SOCS transcripts to GAPDH transcript was calculated as described elsewhere37,38 and then expressed as a fold or percentage of the control. To validate the amplification specificity of the primers for qRT-PCR, a dissociation curve analysis performed from 60°C to 95°C at a rate of 0.1°C/s after the PCR indicated a single peak for RETN, TLR4, SOCS3, SOCS5, SOCS7, or GAPDH (Supplemental Figure S1). The amplification efficiency for each amplicon was close to or greater than 83% (Supplemental Figure S2).
Table 1.
Primers for the detection of SOCS3, SOCS5, SOCS7, RETN, TLR4, and GAPDH genes expression by real-time PCR.
| Genes | Sequence of primers | Accession number (sizes of PCR product) |
|---|---|---|
| SOCS3 | FP: 5′-GGGGAGTACCACCTGAGTCT-3′ | NM_003955.4 |
| RP: 5′-CGAAGTGTCCCCTGTTTGGA-3′ | (128 bp) | |
| SOCS5 | FP: 5′-ATCTGGAGACAGCCATACCCA-3′ | NM_014011.4 |
| RP: 5′-CAAATCAGGCACGAGGCAGT-3′ | (91 bp) | |
| SOCS7 | FP: 5′-CCTTCAGCCTGTGGTGTCAT-3′ | NM_014598.4 |
| RP: 5′-TCGGGACACTGGATAGAGCA-3′ | (170 bp) | |
| RETN | FP: 5′-AGCCATCAATGAGAGGATCCAG-3′ | NM_001193374.2 |
| RP:5′-TCCAGGCCAATGCTGCTTA-3′ | (72 bp) | |
| TLR4 | FP: 5′-AAGCCGAAAGGTGATTGTTG-3′ | NM_003266.4 |
| RP:5′-CTGAGCAGGGTCTTCTCCAC-3′ | (153 bp) | |
| GAPDH | FP: 5′-GGAGCCAAAAGGGTCATCATCTC-3′ | NM_001256799.3 |
| RP: 5′-GAGGGGCCATCCACAGTCTTCT-3′ | (233 bp) |
Western blot analysis
Immunoblot analysis was performed essentially as described by Ku et al. 39 Briefly, cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 20 mM Na2HPO4 (pH 7.4), 100 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 1% Triton X-100) containing aprotinin and leupeptin, and then centrifuged at 14,500 rpm for 10 min at 4°C. Protein samples (100 μg) were separated by 12% SDS-PAGE in 2× gel-loading buffer (100 mM Tris-HCl (pH 6.8), 0.2% bromophenol blue, 4% SDS, 10% β-mercaptoethanol, and 20% glycerol). Antibodies specific for SOCS3, β-actin, GAPDH, and donkey anti-rabbit IgG conjugated with horseradish peroxidase (HRP) were obtained from Cell Signaling Technology (Danvers, MA). All other antibodies (i.e. anti-SOCS5 and anti-SOCS7) were purchased from Abcam (Cambridge, MA). The primary antibodies were used at a dilution of 1:1000 (0.2 μg/mL) and secondary antibody at a dilution of 1:2000 (~0.2 μg/mL). After washing, the immunoblots were visualized using the TOPBIO ECL Substrate Kit (TOP BIO, New Taipei City, Taiwan) for 3 min, and chemiluminescence was detected by the chemiluminescence imaging system MultiGel-21 (TOP BIO, New Taipei City, Taiwan). Using a Gel-Pro Analyzer (Media Cybernetics, Bethesda, MD), each scanned band was quantified, the integrated optical density (IOD) calculated, and the data normalized to β-actin according to the IOD. Protein levels were expressed as a percentage of the control.
Cell proliferation assay
PC-3 cells (5000 cells/well) were plated in triplicate wells of a 96-well plate. 40 To evaluate whether resistin dose-dependently stimulated the growth of PC-3 PCa cells, we treated the cells with different dosages of resistin (0, 5, 12.5, 25, 50, 100, and 200 ng/mL) for 24, 48, and 72 h. In addition, after treatment with different inhibitors for 0.5 h, serum-starved cells were treated with 50 or 100 ng/mL human recombinant resistin for 24 and 48 h. We followed the MTT assay method from our previous report 41 and Mosmann 42 to detect cell proliferation. After incubating cells for the indicated times, we added tetrazolium dye, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazlium bromide), and incubated the cells in the dark at 37°C for 2 h. Next, the medium was removed, and we added 100 μL of 100% dimethyl sulfoxide (DMSO) to each well to stop the reaction. The cells were then incubated for 10 min on a shaker at 300 rpm. The insoluble product (formazan) dissolved in the DMSO, and the absorbance was read at 570 nm. We also used a commercial bromodeoxyuridine (BrdU) ELISA kit (Roche Applied Science, Mannheim, Germany) to measure cellular proliferation. Briefly, 36 hour serum-starved PC-3 cells (5000 cells/well) seeded in RPMI 1640 medium in four wells of a 96-well microplate were incubated with or without resistin for 18 h at 37°C, and then BrdU (10 μM) was added for an additional 16 h. After incubation, cell pellets were washed with 10 mM phosphate buffered saline (PBS) and dried at 55°C for 0.5 h. Cell pellets were fixed with FixDenat solution (200 μL/well) for 30 min at room temperature, probed with mouse anti-BrdU-POD (10 μL) for 1.5 h, and visualized by the addition of 3,3,5,5-tetramethylbenzidine substrate (100 μL/well) for 5 min for color development. To stop the reaction, we added 1 N H2SO4 (25 μL/well) to the culture medium for an additional 5 min on a shaker at 300 rpm. The absorbance was read at a wavelength of 450 nm using the BMG LABTECH’s SPECTROstar® Nano. Blank controls for non-specific binding were culture medium alone and cells incubated with anti-BrdU-POD in the absence of BrdU.
Overexpression of wild-type and short hairpin SOCS
Following our previous method using Lipofectamine 2000 (Thermo Fisher Scientific),41–43 5 × 105 cells/well in a 6-well plate were transiently transfected with empty vector (pCDNA3.1, TRC1, or EG5 vectors), the recombinant wild-type SOCSs plasmid (2 μg), scramble RNA, or shSOCSs for 48 h and incubated with or without 100 ng/mL resistin for 72 h. After transfection, real-time PCR and Western blot analysis were performed to detect differences in the levels of SOCS3 and SOCS5 expression in PC-3 cells. After 72 h incubation with resistin, MTT was added for 1 h, and then 100 μL of DMSO was added to dissolve the remaining MTT formazan crystals. The absorption was read at 570 nm. The cells were co-transfected with pSV-β-galactosidase cDNA to determine the transfection efficiency. Expression of SOCS3 and SOCS5 genes and proteins were validated by qPCR and Western blot analysis, respectively.
Statistical analysis
Data were expressed as mean ± standard error (SE). Unpaired Student’s t-test was used to examine differences between the control and resistin-treated groups. One-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls multiple-range test was used to examine differences among multiple groups. The Kruskal–Wallis test followed by the Mann–Whitney U test was used to examine differences between prostate gland, benign prostatic hyperplasia (BPH), and prostate carcinoma tissue samples. Differences were considered significant if P < 0.05. Pearson correlation analysis was used to examine correlations between different genes. Statistical analyses were performed using SigmaPlot (Jandel Scientific, Palo Alto, CA) and SPSS software (SPSS Inc., Chicago, IL).
Results
Resistin stimulated expression of SOCS3 and SOCS5 in PC-3 cells
Resistin increased the steady-state levels of SOCS3 and SOCS5 mRNA, but not SOCS7 mRNA, in a time-dependent (Figure 1(A)) and dose-dependent (Figure 1(B)) manner. Resistin (50 ng/mL) significantly altered the levels of SOCS3 and SOCS5 mRNA at 0.5 to 2 h, increasing them 21–31% and 80–133%, respectively (Figure 1(A)). After 2 h of treatment, we observed no significant alterations in the levels of SOCS3 and SOCS5 mRNAs. The dose-dependent effect was observed with resistin doses of 5–100 ng/mL (Figure 1(B)).
Figure 1.
Resistin time-dependently and dose-dependently stimulated SOCS3 and SOCS5 mRNA expression in PC-3 cells. (A) Serum-starved PC-3 cells were incubated in the presence (experimental group; •) or absence (control group; ○) of resistin hormone (50 ng/mL) for 0, 1, 2, 4, 8, 12, and 24 h. *P < 0.05 versus the control at same time. (B) Serum-starved PC-3 prostate cancer cells were incubated in the presence or absence (control group) of different doses of resistin hormone (0, 5, 12.5, 25, 50, and 100 ng/mL) for 2 h. Groups with different letters are significantly different (P < 0.05) from each other. (C) The effect of resistin was blocked by actinomycin D (Acti-D, 5 μg/mL) pretreatment for 0.5 h. After 2 h of resistin treatment, SOCS mRNAs were analyzed by real-time PCR. Controls were not treated with resistin or Acti-D. *P < 0.05 versus control; #P < 0.05, resistin versus resistin + Acti-D; §P < 0.05, Acti-D versus resistin + Acti-D. The levels of SOCS mRNAs were determined by real-time PCR. Data are expressed as mean ± SE from replicates of three experiments.
To determine whether the stimulatory effects of resistin on the expression of SOCS3 and SOCS5 require new mRNA synthesis or are attributable to an alteration in mRNA stability, we pretreated PC-3 cells with the transcriptional inhibitor Acti-D for 30 min and analyzed changes in the levels of the SOCS3 and SOCS5 mRNAs (Figure 1(C)). Acti-D alone reduced the SOCS3 and SOCS5 mRNA levels, but not SOCS7 mRNA levels. In the presence of resistin, Acti-D prevented resistin stimulation of SOCS3 and SOCS5 expression.
Next, we examined the possibility that resistin affects SOCS3, SOCS5, and SOCS7 protein levels (Figure 2). At 2 h, resistin was able to increase the protein levels of SOCS3 and SOCS5, but not SOCS7, in a concentration-dependent manner, with significant induction at 100 ng/mL resistin.
Figure 2.

Resistin dose-dependently stimulated SOCS3 and SOCS5 protein expression in PC-3 cells. Serum-starved PC-3 prostate cancer cells were incubated in the presence or absence (control group) of different doses of resistin hormone (0, 5, 12.5, 25, 50, 100, and 200 ng/mL) for 2 h. (A) After 2 h of resistin treatment, SOCS proteins were determined by Western blot. Data are expressed as mean ± SE from replicates of three experiments. (B) Groups with different letters shown in (B) are significantly different (P < 0.05) from each other.
Resistin increased SOCS3 and SOCS5 gene expression via the TLR4 pathway
To examine whether the resistin-induced increase in the expression of SOCS3 and SOCS5 mRNA was mediated by the TLR4 pathway, cells were pretreated with either the TLR4 antagonist TAK-242 (1 μM), 44 or the TLR1/2 antagonist CU-CPT22 (1 μM) 45 for 0.5 h, and then incubated with or without 50 ng/mL resistin for 2 h (Figure 3). Neither TAK-242 nor CU-CPT22 alone altered the steady-state levels of SOCS3, SOCS5, or SOCS7 mRNAs. However, TAK-242 prevented the resistin-induced increases in SOCS3 and SOCS5 mRNAs.
Figure 3.

The toll-like receptor 4 (TLR4) antagonist TAK-242, but not the toll-like receptor 1/2 (TLR1/2) antagonist CU-CPT22, blocked resistin-altered levels of SOCS3 and SOCS5 mRNAs. Serum-starved PC-3 cells were pretreated with or without TAK-242 (1 µM) or CU-CPT22 (1 µM) for 0.5 h and then stimulated with 50 ng/mL resistin. After 2 h of resistin treatment, levels of SOCS3, SOCS5, and SCOS7 mRNAs were analyzed by quantitative real-time PCR. Data are expressed as mean ± SE from replicates of three experiments. *P < 0.05 versus the control; #P < 0.05, resistin versus inhibitor + resistin.
Functional ERK MEK, JNK MAPK, p38 MAPK, PI3K, and JAK2 proteins were necessary for resistin to stimulate SOCS3 and SOCS5 gene expression
We attempted to find the downstream signaling transducers involved in activating the resistin-induced expression of SOCS3 and SOCS5, such as the MAPK pathway (e.g. ERK MEK, JNK MAPK, p38 MAPK), PI3K/AKT, and JAK2 proteins (Figures 4 to 6). PC-3 PCa cells were pretreated with ERK MEK inhibitors U-0126 and PD-98059, JNK inhibitor SP-600125, p38 MAPK inhibitor SB-203580, PI3K inhibitors wortmannin and LY-294002, or JAK2 inhibitor AG-490, followed by 50 ng/mL resistin for 2 h. U-0126, PD-98059, SB-203580, SP-600125, wortmannin, LY-294002, and AG-490 all blocked the resistin-stimulated expression of SOCS3 and SOCS5 mRNAs. In the absence of resistin, SB-203580, SP-600125, wortmannin, LY-294002, and AG-490 alone did not change the SOCS3, SOCS5, and SOCS7 mRNA levels. However, U-0126 and PD-98059 alone decreased the SOCS3 or SOCS5 mRNA level approximately 21%.
Figure 4.

Specific inhibitors of the mitogen-activated protein kinases, such as U-0126, PD-98059, SP-600125, and SB-203580, reduced resistin-induced increases in the levels of SOCS3 and SOCS5 mRNA. (A) Serum-starved PC-3 cells were pretreated with U-0126 (25 μM; in A), (B) PD-98059 (50 μM), (C) SP-600125 (20 μM), or (D) SB-203580 (20 μM) for 0.5 h and then stimulated with resistin (50 ng/mL) for 2 h. Levels of SOCS3, SOCS5, and SOCS7 mRNAs were analyzed by real-time PCR. Data were expressed as mean ± SE from replicates of three experiments. *P < 0.05 versus the control; #P < 0.05, resistin versus resistin + kinase inhibitor.
Figure 6.

The JAK2/STAT3 inhibitor AG-490 reduced resistin-induced increases in levels of SOCS3 and SOCS5 mRNAs. Serum-starved PC-3 cells were pretreated with AG-490 (10 μM) for 0.5 h and then stimulated with resistin (50 ng/mL) for 2 h. Levels of SOCS3, SOCS5, and SOCS7 mRNAs were analyzed by real-time PCR. Data were expressed as mean ± SE from replicates of three experiments. *P < 0.05 versus the control; #P < 0.05, resistin versus resistin + kinase inhibitor.
Figure 5.

The PI3K inhibitors, such as LY-294002 and wortmannin, reduced resistin-induced increases in levels of SOCS3 and SOCS5 mRNAs. (A) Serum-starved PC-3 cells were pretreated with LY-294002 (50 μM; in A) or (B) wortmannin (0.2 μM) for 0.5 h and then stimulated with resistin (50 ng/mL) for 2 h. Levels of SOCS3, SOCS5, and SOCS7 mRNAs were analyzed by real-time PCR. Data were expressed as mean ± SE from replicates of three experiments. *P < 0.05 versus the control; #P < 0.05, resistin versus resistin + kinase inhibitor.
The cell type–specific effect of resistin on SOCS gene expression
To evaluate whether the effect of resistin on the expression of SOCS3 and SOCS5 mRNAs was cell type-specific, we compared the results in PC-3 cells to the same assays performed in LNCaP-FGC, DU145, and U937 (lymphoma) cells (Figure 7). Resistin at 50 and 100 ng/mL for 2 h induced a significant 53–77% increase in SOCS5 mRNA levels in LNCaP-FGC cells, another PCa cell line (Figure 7(A)). Similar to PC-3 cells, resistin did not alter the expression of SOCS7 mRNA levels in LNCaP-FGC cells. Interestingly, in LNCaP-FGC cells, 25, 50, or 100 ng/mL resistin for 2 h did not affect SOCS3 mRNA levels. In DU145 cells, 25, 50, or 100 ng/mL resistin for 2 h did not affect SOCS3, SOCS5, or SOCS7 mRNA levels. In U937 lymphoma cells, 50 and 100 ng/mL resistin alone for 2 h significantly increased the levels of SOCS3 and SOCS7 mRNA by approximately 19–70% (Figure 7(B)), but 25, 50, or 100 ng/mL resistin for 2 h did not affect the SOCS5 mRNA levels. However, resistin induced higher SOCS7 mRNA levels in U937 cells compared to the control.
Figure 7.

Resistin altered SOCS3, SOCS5, and SOCS7 mRNA expression in cancer cells. (A) LNCaP-FGC prostate cancer cells, (B) DU145 prostate cancer cells, and (C) U937 lymphoma cells. Serum-starved cells were incubated with 0 (control group), 25, 50, or 100 ng/mL of resistin for 2 h. Levels of SOCS3, SOCS5, and SOCS7 mRNAs were analyzed by quantitative real-time PCR. Data are expressed as mean ± SE from replicates of three experiments. Groups with different letters for expression of in a given SOCS mRNA significantly differed (P < 0.05) from each other.
Resistin signaling molecules were required for the stimulatory effect of resistin on PC-3 cell growth
MTT analysis showed that resistin dose-dependently (12.5–200 ng/mL resistin) induced the growth of PC-3 cells at 24, 48, and 72 h (Figure 8(A)) by 12–35%, 30–57%, and 33–45%, respectively. BrdU analysis also showed the dose-dependent effect of resistin on PC-3 cells growth (Figure 8(B)). To evaluate which, if any, of the resistin signaling pathways were responsible for modulating PCa cell growth, we pretreated PC-3 cells with different specific inhibitors of the individual protein kinases for 0.5 h, and then treated them with 50 or 100 ng/mL resistin (Figure 8(C) and (D)). CU-CPT22, TAK-242, U-0126, SB-203580, SP-600125, LY-294002, and AG-490 prevented the resistin-stimulated growth of PC-3 cells.
Figure 8.

TAK-242, CU-CPT22, U-0126, SP-600125, SB-203580, LY-294002, and AG-490 reduced resistin-stimulated growth of PC-3 cells. (A and B) Serum-starved PC-3 cells were incubated in the presence or absence (control group) of different doses of resistin hormone for MTT assay and BrdU assay. Group with different letters are significantly different (P < 0.05) from each other. (C and D) Serum-starved PC-3 cells were pretreated with CU-CPT22 (1 μM), TAK-242 (1 μM), U-0126 (25 μM), SP-600125 (20 μM), SB-203580 (20 μM), LY-294002 (50 μM), or AG-490 (10 μM) for 0.5 h and then stimulated with resistin (50 or 100 ng/mL) for MTT assay and BrdU assay. Data are expressed as mean ± SE from replicates of three experiments. *P < 0.05 versus the control; #P < 0.05, resistin versus resistin + kinase inhibitor.
Overexpression or knockdown of SOCS3 or SOCS5 altered the effect of resistin-induced PC-3 cell growth
Overexpression or knockdown of SOCS3 or SOCS5 was achieved to demonstrate whether SOCS3 and SOCS5 are both required for resistin to stimulate the growth of PC-3 cells (Figure 9). After transfection, we validated significant changes in the levels of SOCS3 and SOCS5 expression at both the mRNA and protein level (Figure 9(A) to (D)). Our results indicate that transient overexpression of SOCS3 or SOCS5 enhanced the resistin-stimulated growth of PC-3 cells (Figure 9(E) and (F)) and that knockdown of SOCS3 or SOCS5 suppressed the resistin-increased growth of PC-3 cells (Figure 9(G) and (H)).
Figure 9.
(A, B, E, F) Overexpression of SOCS3 or SOCS5 enhanced the stimulatory effect of resistin on the growth of PC-3 cells. (C, D, G, H) Knockdown of SOCS3 or SOCS5 reduced resistin-stimulated growth of PC-3 cells. PC-3 cells transfected with empty vector, SOCSs or shSOCSs were treated with 100 ng/mL resistin. After 72 h incubation, cell growth was measured by the MTT assay (E-H). To verify the mRNA (bar graphs in A–D) and protein levels (gel graphs in A–D) of SOCS3 and SOCS5, we used real-time PCR and Western blot, respectively. Data are expressed as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control. #P < 0.05, resistin versus resistin + vector.
Expression of SOCS3, SOCS5, SOCS7, RETN, and TLR4 mRNAs in prostate tissues
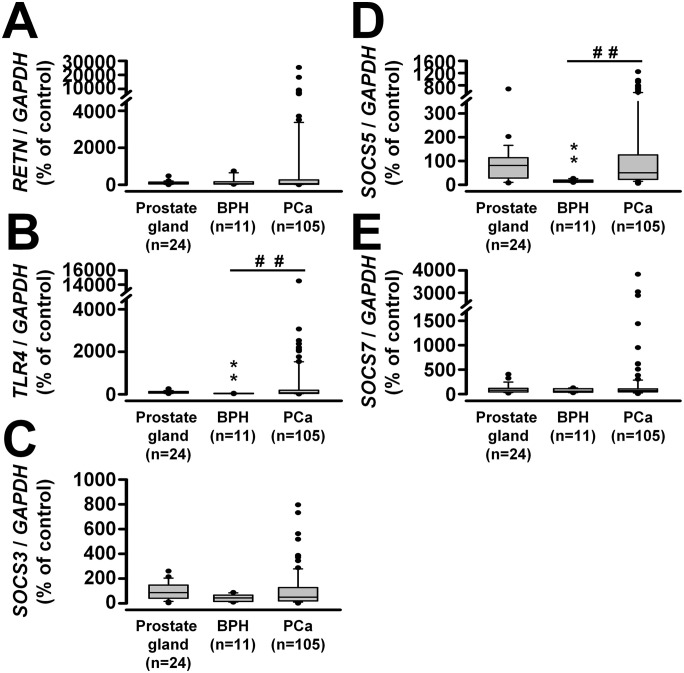
For clinical validation, TissueScan Prostate Cancer cDNA Arrays I, II, and III were used. The mRNA expression profiles of SOCS3, SOCS5, SOCS7, RETN, and TLR4 in the prostate gland (n = 24), BPH (n = 11), and prostate carcinoma (n = 105) are presented in Figure 10. Patients with BPH had lower levels of SOCS5 and TLR4 mRNAs, but no significant differences were found in the levels of SOCS3, SOCS7, and RETN mRNAs compared to the normal prostate gland. Although significant differences in the levels of SOCS3, SOCS5, SOCS7, RETN, and TLR4 mRNAs were not observed between normal prostate gland and prostate carcinoma, significant differences were found in the levels of SOCS5 (P < 0.01) and TLR4 (P < 0.01) mRNA, but not SOCS3, SOCS7, or RETN mRNA, in prostate carcinoma compared to BPH. Interestingly, when we compared changes in RETN mRNA levels between different stages of PCa with normal prostate gland, we observed greater RETN mRNA expression in stages II, III, and IV PCa relative to normal prostate gland (Supplemental Figure S3).
Figure 10.
Differences in mRNA levels of (A) RETN, (B) TLR4, (C) SOCS3, (D) SOCS5, and (E) SOCS7 in prostate gland (n = 24), benign prostate hyperplasia (BPH, n = 11), and prostate carcinoma (n = 105) samples. **P < 0.01 versus prostate gland; ##P < 0.01, BPH versus prostate carcinoma.
The correlations among SOCS3, SOCS5, SOCS7, RETN, and TLR4 in a given group of tissues are summarized in Table 2. In the prostate gland, we found positive correlations between SOCS5 and RETN (P < 0.001) or TLR4 (P < 0.01), and between SOCS7 and TLR4 (P < 0.05). In prostate carcinoma, positive correlations occurred between SOCS5 and SOCS7 (P < 0.001), RETN (P < 0.001), or TLR4 (P < 0.001), and between RETN and TLR4 (P < 0.001). However, we found no significant correlations between SOCS3, SOCS5, SOCS7, RETN, and TLR4 in BPH.
Table 2.
The Pearson correlations (r) among SOCS3, SOCS5, SOCS7, RETN, and TLR4 in the prostate gland, benign prostatic hyperplasia (BPH), and prostate carcinoma tissues.
| Parameters | Prostate gland, BPH, and prostate carcinoma a | |||
|---|---|---|---|---|
| SOCS3 | SOCS5 | SOCS7 | TLR4 | |
| RETN | −0.281 | 0.780*** | 0.036 | 0.237 |
| 0.094 | −0.123 | −0.358 | −0.111 | |
| −0.058 | 0.397*** | −0.083 | 0.639*** | |
| SOCS3 | −0.396 | −0.063 | −0.130 | |
| 0.127 | 0.052 | 0.013 | ||
| −0.018 | 0.174 | −0.075 | ||
| SOCS5 | 0.311 | 0.520** | ||
| −0.111 | −0.083 | |||
| 0.604*** | 0.410*** | |||
| SOCS7 | 0.412* | |||
| −0.255 | ||||
| 0.013 | ||||
“−” means negative correlation.
The first, second, and third numbers represent correlation coefficients between different genes in the prostate gland, BPH, and prostate carcinoma, respectively.
P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
This study demonstrated that resistin selectively affects particular types of SOCS family members in PC-3 PCa cells. Resistin likely upregulates SOCS3 and SOCS5 mRNA levels by stimulating gene transcription. This conclusion is supported by Acti-D treatment preventing the resistin-induced increases in SOCS3 and SOCS5 mRNA levels. However, resistin antagonized the Acti-D suppression of SOCS5, but not SOCS3, mRNA levels compared to Acti-D treatment alone (Figure 1(C)). This suggests that mRNA stability is also required for the effect of resistin on SOCS5 but not SOCS3.
We attempted to determine the signaling proteins required for resistin induction of SOCS3 and SOCS5 gene expression. A recent study demonstrated that TLR4 can act as a resistin receptor in human leukocytes. 25 In support of this notion, we found that pretreatment of PC-3 cells with the TLR4 inhibitor, but not the TLR2 inhibitor, significantly blocked the resistin-stimulated expression of SOCS3 and SOCS5 mRNAs. This suggests that functional TLR4 is necessary for the effect of resistin. This is also consistent with the reported effects of resistin on interleukin-6 release from leukocytes, 25 fractalkine expression in smooth muscle cells, 46 and SOCS3 expression in colon cancer cells. 47 Because the TLR2 inhibitor did not block the resistin-induced increases in SOCS3 and SOCS5 mRNA levels in PC-3 cells, the results suggest a TLR2-independent effect of resistin. Therefore, it is likely that resistin regulates SOCS3 and SOCS5 mRNA expression in PC-3 cells through particular types of TLR family molecules.
In this study, when kinase signaling proteins downstream of resistin were examined, we observed that increases in the level of SOCS3 and SOCS5 mRNAs induced by resistin could be attributed to its activation of the respective MAPK, PI3K/AKT, and JAK2/STAT3 proteins. Any specific inhibitors of these protein kinases significantly prevented resistin-induced increases in the levels of SOCS3 and SOCS5 mRNAs in PC-3 cells. Our findings are consistent with those reported for the ERK-dependent effect of resistin on SOCS3 expression in colon cancer cells, 47 as well as those reported for the p38 and JNK MAPK-dependent effect of resistin on fractalkine expression in human endothelial cells. 48
The functions of SOCS3 and SOCS5 are necessary for resistin to stimulate the growth of PC-3 PCa cells. In particular, knockdown of SOCS3 or SOCS5 suppresses resistin-stimulated growth of PC-3 cells. Furthermore, overexpression of SOCS3 or SOCS5 enhanced the resistin-stimulated growth of PC-3 cells. In support of the SOCS3- and SOCS5-dependent effect of resistin on PC-3 cell growth, treatment with specific inhibitors of the TLR4, ERK, JNK, p38 MAPK, PI3K, and JAK2 proteins decreased the resistin-stimulated growth of PC-3 cells, which accompanied the decrease in SOCS3 and SOCS5 mRNA levels. Taken together, these observations suggest that the effect of resistin on PC-3 cell growth is mediated through the SOCS3 and SOCS5 proteins in a TLR4, ERK, JNK, p38 MAPK, PI3K, and JAK2-dependent pathway.
Resistin also dose- and time-dependently stimulated the growth of DU145 cells (Supplemental Figure S4). In addition, resistin dose-dependently stimulated SOCS5 mRNA expression in LNCaP cells and increased SOCS3 mRNA levels in U937 cells. However, resistin did not alter SOCS7 mRNA levels in DU145 cells or LNCaP cells. Interestingly, the effects of resistin on SOCS3 and SOCS5 mRNA expression in DU145 cells were somewhat different from the effects in PC-3 (Figure 1(A) and (B)) and LNCaP-FGC cells (Figure 7). These observations indicate a PCa cell type-dependent effect of resistin on the regulation of SOCS3 and SOCS5 gene expression. The notion is consistent with the reported cell type-specific regulation of SOCS3 by ET-1 37 and IL-6. 49 Possible explanations for the cell type-dependent effect of resistin on SOCS3 and SOCS5 mRNA expression are that the distinct types of SOCS family members respond to resistin at varying levels, and that signaling pathways required for the actions of resistin vary with the nature of PCa cell types, SOCS members, or kinase signaling cascades. 19 The notion is supported by our findings that high levels of TLR4, MYD88, SOCS3, and SOCS7 proteins were found in LNCaP-FGC cells, and high levels of SOCS5 protein were found in DU145 cells (Supplemental Figure S5). Notably, other reports have indicated that overexpression of SOCS3 decreases phosphorylation of pSTAT3 and influences cell growth and apoptosis in DU145 and TRAMP-C2 cells, but not in PC-3 cells, which do not express pSTAT3. 27 Furthermore, downregulation of SOCS3 causes apoptosis of PC-3 cells, DU145 cells, and LNCaP-IL-6-positive cells, but not parental LNCaP cells. 33 In addition, SOCS5 mRNA levels are not significantly different between P69 and M12 PCa cells after transfection of the miR-9 inhibitor. However, the miR-9 inhibitor reduces SOCS5 protein levels in M12 PCa cells. Moreover, different signaling pathways control cell growth in PC-3, LNCaP, and DU145 cells, which express different levels of the SOCS family members.27–33 We used real-time PCR to detect RETN mRNA in normal (RWPE-1) and cancerous (PC-3, DU145, and LNCaP-FGC) human prostate cells relative to U937 lymphoma cells and found that all RWPE-1, DU-145, PC-3, and LNCaP-FGC cells expressed less RETN mRNA than U937 cells (Supplemental Figure S6(A)). However, changes in the RETN mRNA levels were not altered in PC-3, LNCaP-FGC, and U937 cells after 2 h of resistin (25, 50, and 100 ng/mL) treatment (Supplemental Figure S6(B)). The presence of RETN mRNA expression in RWPE-1, DU145, and PC-3 cells is consistent with the report by Kim et al. 19 Interestingly, our observations indicate that stages II, III, and IV of PCa from tissue array analysis exhibited higher levels of RETN mRNA than normal prostate gland. These findings are also consistent with higher resistin protein levels in PCa tissue than normal prostate tissue and BPH tissue. 19 Parallel to these findings, we found higher levels of resistin mRNA in cancerous (PC-3, DU145, and LNCaP-FGC) human prostate cells than normal (RWPE-1) human prostate cells (Supplemental Figure S6). As LNCaP-FGC, DU145, and PC-3 are metastatic PCa cells, our observations suggest an association of resistin with the development and progression of PCa. Androgen-independent cell lines (PC-3 and DU145) and androgen-dependent cell line (LNCaP-FGC) are isolated from clinical metastatic lesions, such as bone, brain, and lymph node. In addition, based on the invasiveness of PCa cells, LNCaP-FGC cells are an earlier stage than PC-3 and DU145 cells. 50 Our study could not exclude that the differential effects of resistin on SOCS3 and SOCS5 mRNA levels among the three cell lines vary with metastatic ability or the characteristics and microenvironment of PCa cells in the presence or absence of AR. For example, the microenvironment of PCa includes abundant tumor-associated peripheral blood mononuclear cells, such as macrophages and dendritic cells, which are able to express resistin;51,52 thus, the abundance of these myeloid cells may explain greater RETN mRNA and protein levels in late stages of PCa. This explanation may be evident by our findings that CD11c (a marker gene expressed in monocytes, macrophages and dendritic cells) mRNA was expressed at higher levels in PCa than normal prostate gland, and that a positive correlation was observed between RETN and CD11c (Supplemental Figure S7(A) and (B)). Our observations are consistent with those reported in the TCGA data (Supplemental Figure S7(C) and (D)) using the GEPIA data set analysis (http://gepia.cancer-pku.cn/index.html).
Previous clinical studies have indicated an association of SOCS genes with PCa.53,54 In this study, to strengthen the TLR4 signaling of resistin in the regulation of SOCS expression, we looked at whether any correlations of SOCS3, SOCS5, SOCS7, RETN, and TLR4 occurred among normal and cancerous prostate tissues. We used Pearson correlation analysis to find positive correlations among the levels of SOCS5, TLR4, and RETN mRNA in prostate carcinoma (Table 2). Our observations were consistent with those reported in the TCGA data (Supplemental Table S1) using the GEPIA data set analysis (http://gepia.cancer-pku.cn/index.html). Collectively, firm conclusions as to whether any of these clinical associations of RETN with the SOCS5 and TLR4 genes can be explained by its in vitro effects on SOCS5 and TLR4 levels will require more thorough studies. As the significant differences in the levels of TLR4 and SOCS5 mRNAs occurred between BPH and prostate carcinoma, further studies are needed to demonstrate whether the TLR4/SOCS5 pathway can mediate the pro-tumorigenic effect of multiple substances, including resistin. Although no correlation of SOCS3 with the RETN and TLR4 genes were observed among normal and cancerous prostate tissues in our study, SOCS3 tended to be highly expressed in prostate carcinoma, and its overexpression and knockdown were able to alter resistin-mediated cell growth of PC-3. According to the RNA-Seq data, SOCS3 mRNA levels in PCa correlated with RETN, SOCS5, and TLR4 mRNA levels. Thus, our study could not exclude the possibility that SOCS3 signaling may help explain the stimulation of PCa cell growth by resistin.
We concluded that resistin stimulates SOCS3 and SOCS5 expression in androgen-independent PC-3 PCa cells via the TLR4, MAPK, PI3K, and JAK2 pathways. Increased expression of SOCS3 and SOCS5 is necessary for resistin to stimulate the proliferation of PC-3 cells. As resistin and SOCS proteins have been found to regulate obesity in animals, and obesity is associated with PCa progression,17,55 it will be worthwhile to study whether resistin-induced alterations in the expression of SOCS genes can help explain the mechanism by which resistin modulates the processes underlying obesity-associated PCa.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702231191206 for Resistin stimulates PC-3 prostate cancer cell growth through stimulation of SOCS3 and SOCS5 genes by Chi-Wei Liu, Hsuan-Yu Peng, An-Ci Siao, Yi-Wei Tsuei, Yen-Yue Lin, Shine-Gwo Shiah, Li-Jane Shih, Chien-Chih Yeh, Shih-Wei Lee and Yung-Hsi Kao in Experimental Biology and Medicine
Acknowledgments
The authors thank Dr Kuo-Ting Chang at Translational Medicine Center, Taoyuan General Hospital, for excellent technical support. We thank Mr Yi-Qi Chen (Department of Life Sciences at National Central University) for his technical assistance.
Footnotes
Authors’ Contributions: Experiment was conceived and designed by C-WL and Y-HK and performed by C-WL, H-YP, and A-CS. Experiment data were analyzed by C-WL, H-YP, and Y-HK. Reagent/materials/analysis tools were contributed by C-WL, S-WL, Y-WT, Y-YL, L-JS, C-CY, S-GS, and Y-HK. Paper was written by C-WL and Y-HK. The final manuscript has the approval of all authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Landseed Hospital, Taiwan (LSHIRB IRB No./Protocol No. 17-018-C0) and the Ethics Committee of Taoyuan General Hospital, Taiwan (TYGHIRB IRB No./Protocol No. 110-08).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Ministry of Science and Technology, Taiwan (MOST-106-2320-B-008-006, MOST-106-2320-B-008-008-MY3, and MOST-109-2320-B -008-001-MY3); the National Central University and Landseed Hospital Joint Research, Taiwan (NCU-LSH-107-B-006, NCU-LSH-108-B-009, and NCU-LSH-109-B-014); the Taoyuan General Hospital, Taiwan (PTH10419 and PTH111024); the Department of Health and Welfare, Executive Yuan, Taiwan (10706); and the Taoyuan Armed Forces General Hospital, Taiwan (AFTYGH-10510, AFTYGH-10611, AFTYGH-10612, AFTYGH-10717, and AFTYGH-10814; AFTYGH-A-109001 and AFTYGH-A-109002; MAB-108-094).
ORCID iD: Chi-Wei Liu  https://orcid.org/0000-0002-8800-7711
https://orcid.org/0000-0002-8800-7711
Supplemental material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30 [DOI] [PubMed] [Google Scholar]
- 2. Robinson WR, Stevens J, Gammon MD, John EM. Obesity before age 30 years and risk of advanced prostate cancer. Am J Epidemiol 2005;161:1107–14 [DOI] [PubMed] [Google Scholar]
- 3. Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review). Int J Oncol 2006;28:737–45 [PubMed] [Google Scholar]
- 4. Buschemeyer WC, 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol 2007;52:331–43 [DOI] [PubMed] [Google Scholar]
- 5. Freedland SJ, Aronson WJ. Examining the relationship between obesity and prostate cancer. Rev Urol 2004;6:73–81 [PMC free article] [PubMed] [Google Scholar]
- 6. Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol 2007;52:46–53 [DOI] [PubMed] [Google Scholar]
- 7. Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, Higgins B, Lokshin A, Troyer D, Hernandez J, Lynch S, Leach RJ, Thompson IM. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev 2006;15:1331–5 [DOI] [PubMed] [Google Scholar]
- 8. Albanes D, Weinstein SJ, Wright ME, Männistö S, Limburg PJ, Snyder K, Virtamo J. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst 2009;101:1272–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int 2008;101:1317–22 [DOI] [PubMed] [Google Scholar]
- 10. Somasundar P, Frankenberry KA, Skinner H, Vedula G, McFadden DW, Riggs D, Jackson B, Vangilder R, Hileman SM, Vona-Davis LC. Prostate cancer cell proliferation is influenced by leptin. J Surg Res 2004;118:71–82 [DOI] [PubMed] [Google Scholar]
- 11. Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am J Surg 2004;188:560–5 [DOI] [PubMed] [Google Scholar]
- 12. López Fontana CM, Maselli ME, Pérez Elizalde RF, Di Milta Mónaco NA, Uvilla Recupero AL, López Laur JD. Leptin increases prostate cancer aggressiveness. J Physiol Biochem 2011;67:531–8 [DOI] [PubMed] [Google Scholar]
- 13. Samuel-Mendelsohn S, Inbar M, Weiss-Messer E, Niv-Spector L, Gertler A, Barkey RJ. Leptin signaling and apoptotic effects in human prostate cancer cell lines. Prostate 2011;71:929–45 [DOI] [PubMed] [Google Scholar]
- 14. Hsing AW, Chua S, Jr, Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst 2001;93:783–9 [DOI] [PubMed] [Google Scholar]
- 15. Moore SC, Leitzmann MF, Albanes D, Weinstein SJ, Snyder K, Virtamo J, Ahn J, Mayne ST, Yu H, Peters U, Gunter MJ. Adipokine genes and prostate cancer risk. Int J Cancer 2009;124:869–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer 2002;9:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 2001;409:307–12 [DOI] [PubMed] [Google Scholar]
- 18. Banerjee RR, Lazar MA. Resistin: molecular history and prognosis. J Mol Med 2003;81:218–26 [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, Kim MK, Kim W, Myung SC. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int 2011;108:E77–83 [DOI] [PubMed] [Google Scholar]
- 20. Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol 2005;25:1569–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu W, Yao J, Huang Y, Li Q, Li W, Chen Z, He F, Zhou Z, Yan J. LXR agonist regulates the carcinogenesis of PCa via the SOCS3 pathway. Cell Physiol Biochem 2014;33:195–204 [DOI] [PubMed] [Google Scholar]
- 22. Bellezza I, Neuwirt H, Nemes C, Cavarretta IT, Puhr M, Steiner H, Minelli A, Bartsch G, Offner F, Hobisch A, Doppler W, Culig Z. Suppressor of cytokine signaling-3 antagonizes cAMP effects on proliferation and apoptosis and is expressed in human prostate cancer. Am J Pathol 2006;169:2199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seashols-Williams SJ, Budd W, Clark GC, Wu Q, Daniel R, Dragoescu E, Zehner ZE. miR-9 acts as an OncomiR in prostate cancer through multiple pathways that drive tumour progression and metastasis. PLoS ONE 2016;11:e0159601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation 2004;110:3335–40 [DOI] [PubMed] [Google Scholar]
- 25. Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med 2010;14:1419–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pierconti F, Martini M, Pinto F, Cenci T, Capodimonti S, Calarco A, Bassi PF, Larocca LM. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate 2011;71:318–25 [DOI] [PubMed] [Google Scholar]
- 27. Yoneda T, Kunimura N, Kitagawa K, Fukui Y, Saito H, Narikiyo K, Ishiko M, Otsuki N, Nibu KI, Fujisawa M, Serada S, Naka T, Shirakawa T. Overexpression of SOCS3 mediated by adenovirus vector in mouse and human castration-resistant prostate cancer cells increases the sensitivity to NK cells in vitro and in vivo. Cancer Gene Ther 2019;26:388–99 [DOI] [PubMed] [Google Scholar]
- 28. Trengove MC, Ward AC. SOCS proteins in development and disease. Am J Clin Exp Immunol 2013;2:1–29 [PMC free article] [PubMed] [Google Scholar]
- 29. Neuwirt H, Puhr M, Cavarretta IT, Mitterberger M, Hobisch A, Culig Z. Suppressor of cytokine signalling-3 is up-regulated by androgen in prostate cancer cell lines and inhibits androgen-mediated proliferation and secretion. Endocr Relat Cancer 2007;14:1007–19 [DOI] [PubMed] [Google Scholar]
- 30. Fu X, Ren L, Chen J, Liao K, Fu Y, Qian X, Xiao J. Characterization of the roles of suppressor of cytokine signaling-3 in prostate cancer development and progression. Asia Pac J Clin Oncol 2015;11:106–13 [DOI] [PubMed] [Google Scholar]
- 31. Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll–the function and regulation of Toll-like receptors in cancer cells. Oncogene 2008;27:225–33 [DOI] [PubMed] [Google Scholar]
- 32. Rezania S, Amirmozaffari N, Rashidi N, Mirzadegan E, Zarei S, Ghasemi J, Zarei O, Katouzian L, Zarnani AH. The same and not the same: heterogeneous functional activation of prostate tumor cells by TLR ligation. Cancer Cell Int 2014;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puhr M, Santer FR, Neuwirt H, Susani M, Nemeth JA, Hobisch A, Kenner L, Culig Z. Down-regulation of suppressor of cytokine signaling-3 causes prostate cancer cell death through activation of the extrinsic and intrinsic apoptosis pathways. Cancer Res 2009;69:7375–84 [DOI] [PubMed] [Google Scholar]
- 34. Housa D, Vernerova Z, Heracek J, Cechak P, Rosova B, Kuncova J, Haluzik M. Serum resistin levels in benign prostate hyperplasia and non-metastatic prostate cancer: possible role in cancer progression. Neoplasma 2008;55:442–6 [PubMed] [Google Scholar]
- 35. Van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate 2003;57:205–25 [DOI] [PubMed] [Google Scholar]
- 36. Basu S, Tindall DJ. Androgen action in prostate cancer. Horm Cancer 2010;1:223–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang HH, Huang YM, Wu CP, Tang YC, Liu CW, Huang CH, Ho LT, Wu LY, Kuo YC, Kao YH. Endothelin-1 stimulates suppressor of cytokine signaling-3 gene expression in adipocytes. Gen Comp Endocrinol 2012;178:450–8 [DOI] [PubMed] [Google Scholar]
- 38. Chang HH, Tsai PH, Liu CW, Ku HC, Kao CC, Kao YH. Cycloheximide stimulates suppressor of cytokine signaling-3 gene expression in 3T3-L1 adipocytes via the extracellular signal-regulated kinase pathway. Toxicol Lett 2013;217:42–9 [DOI] [PubMed] [Google Scholar]
- 39. Ku HC, Chang HH, Liu HC, Hsiao CH, Lee MJ, Hu YJ, Hung PF, Liu CW, Kao YH. Green tea (-)-epigallocatechin gallate inhibits insulin stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. Am J Physiol Cell Physiol 2009;297:C121–32 [DOI] [PubMed] [Google Scholar]
- 40. Lin CY, Huo C, Kuo LK, Hiipakka RA, Jones RB, Lin HP, Hung Y, Su LC, Tseng JC, Kuo YY, Wang YL, Fukui Y, Kao YH, Kokontis JM, Yeh CC, Chen L, Yang SD, Fu HH, Chen YW, Tsai KK, Chang JY, Chuu CP. Cholestane-3β, 5α, 6β-triol suppresses proliferation, migration, and invasion of human prostate cancer cells. PLoS ONE 2013;8:e65734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huo C, Kao YH, Chuu CP. Androgen receptor inhibits epithelial-mesenchymal transition, migration, and invasion of PC-3 prostate cancer cells. Cancer Lett 2015;369:103–11 [DOI] [PubMed] [Google Scholar]
- 42. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63 [DOI] [PubMed] [Google Scholar]
- 43. Liu CW, Yang SY, Lin CK, Liu HS, Ho LT, Wu LY, Lee MJ, Ku HC, Chang HH, Huang RN, Kao YH. The forkhead transcription factor FOXO1 stimulates the expression of the adipocyte resistin gene. Gen Comp Endocrinol 2014;196:41–51 [DOI] [PubMed] [Google Scholar]
- 44. Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol 2011;79:34–41 [DOI] [PubMed] [Google Scholar]
- 45. Cheng K, Wang X, Zhang S, Yin H. Discovery of small-molecule inhibitors of the TLR1/TLR2 complex. Angew Chem Int Ed Engl 2012;51:12246–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gan AM, Butoi ED, Manea A, Simion V, Stan D, Parvulescu MM, Calin M, Manduteanu I, Simionescu M. Inflammatory effects of resistin on human smooth muscle cells: up-regulation of fractalkine and its receptor, CX3CR1 expression by TLR4 and Gi-protein pathways. Cell Tissue Res 2013;351:161–74 [DOI] [PubMed] [Google Scholar]
- 47. Singh S, Chouhan S, Mohammad N, Bhat MK. Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS3. FEBS Lett 2017;591:1371–82 [DOI] [PubMed] [Google Scholar]
- 48. Manduteanu I, Dragomir E, Calin M, Pirvulescu M, Gan AM, Stan D, Simionescu M. Resistin up-regulates fractalkine expression in human endothelial cells: lack of additive effect with TNF-alpha. Biochem Biophys Res Commun 2009;381:96–101 [DOI] [PubMed] [Google Scholar]
- 49. Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology 2007;132:384–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laniado ME, Lalani EN, Fraser SP, Grimes JA, Bhangal G, Djamgoz MB, Abel PD. Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am J Pathol 1997;150:1213–21 [PMC free article] [PubMed] [Google Scholar]
- 51. Barakat DJ, Suresh R, Barberi T, Pienta KJ, Simons BW, Friedman AD. Absence of myeloid Klf4 reduces prostate cancer growth with pro-atherosclerotic activation of tumor myeloid cells and infiltration of CD8 T cells. PLoS ONE 2018;13:e0191188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilkinson R, Kassianos AJ, Swindle P, Hart DN, Radford KJ. Numerical and functional assessment of blood dendritic cells in prostate cancer patients. Prostate 2006;66:180–92 [DOI] [PubMed] [Google Scholar]
- 53. Zhu JG, Dai QS, Han ZD, He HC, Mo RJ, Chen G, Chen YF, Wu YD, Yang SB, Jiang FN, Chen WH, Sun ZL, Zhong WD. Expression of SOCSs in human prostate cancer and their association in prognosis. Mol Cell Biochem 2013;381:51–9 [DOI] [PubMed] [Google Scholar]
- 54. Zhu JG, Yuan DB, Chen WH, Han ZD, Liang YX, Chen G, Fu X, Liang YK, Chen GX, Sun ZL, Liu ZZ, Chen JH, Jiang FN, Zhong WD. Prognostic value of ZFP36 and SOCS3 expressions in human prostate cancer. Clin Transl Oncol 2016;18:782–91 [DOI] [PubMed] [Google Scholar]
- 55. Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes 2004;53:1671–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702231191206 for Resistin stimulates PC-3 prostate cancer cell growth through stimulation of SOCS3 and SOCS5 genes by Chi-Wei Liu, Hsuan-Yu Peng, An-Ci Siao, Yi-Wei Tsuei, Yen-Yue Lin, Shine-Gwo Shiah, Li-Jane Shih, Chien-Chih Yeh, Shih-Wei Lee and Yung-Hsi Kao in Experimental Biology and Medicine