Abstract
Introduction:
Visually impaired individuals may experience increased frequency of sleep/wake disorders and cognitive decline.
Method:
HCHS/SOL Miami Field Center participants ages 45–74 years (n=665) at Visit-1, who returned for cognitive test 7-years later (SOL-INCA). Participants completed the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ), validated sleep questionnaires and test for sleep apnea at Visit-1. They completed tests for verbal episodic learning and memory (Brief Spanish-English Verbal Learning Test), verbal fluency (Controlled Oral Word Association), processing speed and executive functioning (Digit Symbol Substitution) at Visit-1 and at SOL-INCA. Processing speed/executive functioning were added to SOL-INCA (Trails-A, -B). We examined global cognition and change using a regression-based reliable change index, adjusting for the time lapse between Visit-1 and SOL-INCA. We used regression models to test whether (1) persons with sleep apnea, self-reported sleep duration, insomnia and daytime sleepiness have an increased risk for self-reported visual impairment, (2a) self-reported visual impairment is associated with worse cognitive function and/or decline, and (2b) sleep disorders attenuate any of these associations.
Result:
Sleepiness (β =0.04; p<0.01) and insomnia (β =0.04; p<0.001) were cross-sectionally associated with self-reported visual impairment, adjusting for sociodemographic characteristics, behavioral factors, acculturation, and health conditions. Self-reported visual impairment was associated with lower global cognitive function at Visit 1 (β =−0.16; p<0.001) and on average 7-years later (β =−0.18; p<0.001). Self-reported visual impairment was also associated with a change in verbal fluency (β =−0.17; p<0.01). Sleep apnea, self-reported sleep duration, insomnia and daytime sleepiness did not attenuate any of the associations.
Conclusion:
Self-reported visual impairment was independently associated with worse verbal fluency, memory, and executive functioning and predicted 7-year decline in verbal fluency.
Keywords: visual impairment, sleep disorders, cognitive decline, Hispanic/Latinos, Health disparities
INTRODUCTION
Visual impairment, which affects >10% of U.S. Hispanic/Latino older adults[1] may lead to a shortened photoperiod and worse circadian entrainment, contributing to poor sleep.[2–11] Similar to visual impairment,[12] sleep disorders are associated with cognitive impairment and decline. [13–17] [6, 11] Therefore, examining the interplay between visual impairment and sleep on cognitive health can help identify individuals at risk for cognitive decline and inform treatment studies. The mechanisms by which visual impairment affect cognition are not understood. Prior work suggests that cardiometabolic disorders lead to concurrent vision and cognitive decline through microvascular disease and or atherosclerosis.[18–20] Visual impairment can also lead to mood disorders and social isolation.[18] However, the epidemiologic and clinical evidence that links sleep and cognition provides an impetus to examine sleep as a possible mechanistic link between visual impairment and cognitive decline. Studies on the association between visual impairment and sleep have been conducted in predominantly non-Hispanic/Latino white clinical samples or in older adults.[10, 11, 21] Race-ethnic disparities exist in disorders that may cause visual impairment (i.e. diabetic retinopathy, glaucoma), sleep disorders and cognition.[22] Studies suggest that Hispanics/Latinos have a greater burden of visual impairment as well as high prevalence of sleep disorders, factors that could contribute to increase burden of cognitive decline and dementia risk.[23–25] This study examines associations between self-reported visual impairment and sleep on cognitive function and cognitive decline in participants from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Miami-site. We hypothesize that (1) persons with sleep disorders, specifically obstructive sleep apnea (OSA), insomnia and daytime sleepiness, have an increased self-reported visual impairment, (2a) self-reported visual impairment is associated with worse baseline and average 7-years cognitive function and/or cognitive decline, and (2b) OSA, insomnia and daytime sleepiness attenuate any of these associations.
MATHERIALS AND METHODS
Data:
HCHS/SOL is a multisite, prospective cohort study of community-dwelling Hispanic/Latino adults from diverse backgrounds. Data were collected from field centers in four U.S. metropolitan regions with diverse Hispanic/Latino populations (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). The Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA), an ancillary study of HCHS/SOL, examines neurocognition among a subset of participants from HCHS/SOL. At Visit-2 of HCHS/SOL, n=6,377 eligible HCHS/SOL participants met the inclusion criteria for SOL-INCA (being age 50-years and older at Visit 2 and having completed a Visit-1 neurocognitive module), agreed to participate, and completed the SOL-INCA visit. Detailed HCHS/SOL rationale and sampling methods have been published previously.[26, 27] All participants provided informed consent, and the study was approved by the IRB in all study site and institutions.
Analytic Subpopulation.
The study cohort are 1,235 Miami Field Center participants that completed an interview on self-reported visual impairment and utilization.[28] Participants completed validated sleep questionnaires and objective measures of sleep apnea in Visit-1 (2008–2011), as well as neurocognitive test in Visit-1 and 7-year follow-up during SOL-INCA.[29, 30]
Outcomes: Neurocognitive function and Decline
Neurocognitive tests administered at Visit-1 included the: Brief- Spanish English Verbal Learning Test (B-SEVLT; verbal episodic learning and memory); Word Fluency (WF; phonemic verbal fluency); and Digit Symbol Substitution test (DSS; processing speed) of the Wechsler Adult Intelligence Scale-Revised. All tests were z-scored (X-Mean/SD) to facilitate interpretation of results across a common metric. A global cognitive composite score was generated by averaging the z-scores of the above tests. To evaluate neurocognitive decline, SOL-INCA repeated the above neurocognitive battery at Visit-2. [31, 32] For the repeated cognitive measures and the global measure of cognition, a change score indicator was generated using regression-based techniques [33]. Briefly, these change scores were calculated using survey linear regression to predict cognitive performance at Visit-2 as a function of Visit-1 cognitive performance, adjusting for lapsed time (in days) between cognitive assessments. Test specific standardized measures of change (Δ) were subsequently calculated using (T2 - T2pred)/RMSE. T2 represents a respondent’s score on a cognitive test at Visit-2, T2pred is the predicted value for that respondent on the test derived from the regression model specified above and RMSE is Root Mean Squared Error of the fit model.
Main Exposures: Self-reported Visual Impairment
We used the self-administered National Eye Institute-Visual Functioning Questionnaire (NEI-VFQ) score as a continuous variable. The NEI-VFQ is a composite score generated using 25 items on the 12 different subscales that measure self-reported visual health, which has been validated in Spanish and Latino populations.[34–37] Each item is coded so that the lowest values represent a minimal degree of functional problems whereas the highest values represent worse functional issues. The items are first summed across the measures within the subscales where none of each item in the subscales is missing, and the summed scales are then averaged across the subscales. One item of the mental health subscale was omitted from the survey and was therefore not included in the calculation (How much of the time do you worry about your eyesight), and the mean score of this subscale was determined using the three available items. This modification did not affect the total scoring of NEI-VFQ, and our scores are comparable with those of other studies.[28]
In the analysis, we used the standardized form of these averaged scales.
Sleep measures from Visit-1.
HCHS/SOL’s sleep questionnaire evaluates weekday and weekend bedtime and wake time, napping behaviors, as well as related sleep apnea symptoms. We used this average sleep duration as a continuous variable. We also constructed a trichotomous sleep duration variable to separate ‘short’ (<6 hours), ‘average’ (between 6 and 9 hours), and ‘long’ (>9 hours) sleep. The Epworth Sleepiness Scale (ESS), assesses the likelihood of falling asleep in eight common situations, totaling up to 24 points. Insomnia questions were adapted from the Women’s Health Initiative Insomnia Rating Scale (WHIIRS), which has a total of 20 points derived from five items scored from 0–4 each. The insomnia score was tested both as continuous and a binary measure, whereby respondents were considered to have met criteria for ‘Insomnia’ if their ESS score was ≧10 and ‘no Insomnia’ otherwise. Self-reported sleep quality was defined as ‘Very sound or restful’, ‘Sound or restful’, ‘Average quality’, ‘Restless or very restless’ based on response to the probe “Overall [what is your] …typical night’s sleep during the past 4 weeks?” Similarly, napping was defined as a four-category indicator grouping respondents self-reporting ‘None’, ‘1–2 times’, ‘3–4 times’, ‘5 or more times’ for the number of times they napped for 5 minutes or more during a usual week. All questionaires were administered in either English or Spanish, based on participant’s preference.[15–17, 25]
Obstructive sleep apnea (OSA) data were collected using the ARES Unicorder 5.2; B-Alert (Carlsbad, CA).[38] The ARES is a home sleep apnea testing device used to evaluate for OSA according with the practice parameters of the American Academy of Sleep Medicine.[39] Sleep records were scored at the HCHS/SOL Sleep Reading Center. The apnea-hypopnea index (AHI) was calculated as the number of events divided by estimated sleep time, using methods described previously.[17, 25] We used AHI as a continuous variable and as a categorical variable in secondary analyses. For categorical construction we classified three groups: ‘No Sleep Apnea’ if AHI was <5, ‘Mild Sleep Apnea’ if between 5 and 15, and ‘Moderate to Severe Sleep Apnea’ if ≧15.
Main Covariates.
Sociodemographic factors included age in years at Visit-1, sex, Hispanic/Latino heritage, education (less than high school, high school or equivalent, greater than high school), marital status (single, married/cohabit, separated/divorced/widowed), household income (less than $20,000, between $20,000 and $40,000, more than $40,000, not reported), insurance (uninsured, insured/Medicaid), and employment status (employed, retired, and not currently employed, unemployed). Behavioral risk factors included alcohol use status (never, former, current), smoking status (never, former, current), physical activity (no, yes), and body mass index (BMI). Additionally, we accounted for language preference (Spanish, English, both equally) and an acculturation subscale (language) based on the Short Acculturation Scale for Hispanics. We also included self-reported history of the following chronic conditions (not present, present): hypertension, diabetes, coronary heart disease (CHD), heart failure, and stroke/transient ischemic attack (TIA). Lastly, we included years lived in US and an acculturation subscale (social) in the sensitivity analyses (see discussion below).
Statistical Analyses
Analytic Sample.
A total of 1,235 participants (ages 40–74 years) were enrolled in the Visit 1 vision study at the Miami Field Center. We included n=846 participants (ages 45–74 at Visit 1) that participated in the neurocognitive modules at both Visit-1 (concurrent with the vision study) and recruited and returned for the second neurocognitive visit on average 7-years later. From there, we excluded n=123 individuals who did not participate in the sleep module. Finally, we excluded n=2 individuals who had missing self-reported vision status (defined as ‘Excellent, Good, Fair, Poor, or Very poor’ based on response to a questionnaire “your eyesight using both eyes”) and n=56 individuals who had any missing covariate for a final analytic sample of 665 individuals included in the analyses. Supplemental Table 3 also includes descriptive characteristics comparing the excluded and included subsamples. Overall, we found no evidence for differences between these two groups.
Analytic Approach.
First, we report descriptive statistics for the overall target population at Miami site. The survey weighted estimates are presented in Table 1.
Table 1.
Descriptive characteristics of target population (unweighted n=665)
| (Unweighted n=665) | |
|---|---|
|
| |
| Sex (%) | |
| Female | 52.7 (2.6) |
| Male | 47.3 (2.6) |
| Heritage (%) | |
| Cuban | 69.7 (2.9) |
| Central/South American | 22.9 (2.4) |
| Other | 7.4 (1.3) |
| Education (%) | |
| Less than HS | 26.3 (2.6) |
| HS or equivalent | 21.5 (1.9) |
| Greater than HS | 52.2 (2.7) |
| Marital Status (%) | |
| Single | 17.3 (1.9) |
| Married/cohabit | 52.8 (2.9) |
| Separated/Divorced/Widowed | 29.9 (2.5) |
| Income (%) | |
| <20k | 54.4 (3.5) |
| 20–40k | 25.0 (2.8) |
| 40k+ | 8.6 (1.9) |
| Not reported | 12.0 (1.5) |
| Insurance (%) | |
| Uninsured | 57.9 (2.8) |
| Insured/Medicaid | 42.1 (2.8) |
| Employment (%) | |
| Employed | 44.6 (2.9) |
| Retired and not currently employed | 17.3 (2.9) |
| Unemployed | 38.1 (2.7) |
| Alcohol (%) | |
| Never | 32.5 (2.2) |
| Former | 20.8 (2.2) |
| Current | 46.8 (2.8) |
| Smoking (%) | |
| Never | 50.2 (2.7) |
| Former | 28.3 (2.7) |
| Current | 21.5 (1.9) |
| Physical Activity (%) | |
| No | 45.9 (2.7) |
| Yes | 54.1 (2.7) |
| Language (%) | |
| Spanish preferred | 93.4 (1.2) |
| English preferred | 2.8 (1.0) |
| Both equally | 3.7 (0.9) |
| Hypertension (%) | |
| No | 52.5 (2.3) |
| Yes | 47.5 (2.3) |
| Diabetes (%) | |
| No | 71.5 (2.8) |
| Yes | 28.5 (2.8) |
| CHD (%) | |
| No | 91.8 (1.7) |
| Yes | 8.2 (1.7) |
| Heart Failure (%) | |
| No | 97.1 (1.1) |
| Yes | 2.9 (1.1) |
| Stroke/TIA (%) | |
| No | 96.1 (1.6) |
| Yes | 3.9 (1.6) |
| Age (mean, SD) | 57.4 (8.1) |
| BMI (mean, SD) | 29.7 (5.6) |
| Acculturation - language (mean, SD) | 1.4 (0.6) |
Note 1: Variables are measured at Visit 1
Note 2: HS=High School, CHD=Coronary heart disease, TIA=Transient ischemic attack, BMI=Body mass index, SD=standard deviation
To test our first hypothesis (that individuals in the target population with sleep disorders and disturbances have increased risk for visual impairment), we performed survey generalized linear regressions analysis with our continuous outcome of interest (i.e., standardized NEI-VFQ). The following sleep measures were included as exposures independently: Sleepiness, Sleep Apnea, Insomnia, Sleep Duration, Sleep Quality, and Napping Frequency. For each exposure, we fit four regression models. Model (1) was crude (unadjusted for covariables), (2) adjusted for age, sex, and education, (3) adjusted for additional sociodemographic factors, behavioral risk factors, and acculturation measures, and (4) accounted for the full set of covariates as detailed earlier (Main Covariates section). The estimates for the regression coefficients along with their standard errors are presented in Table 2.
Table 2.
Associations between visual impairment measured through the NEI-VFQ and sleep exposures. Results are derived from survey regression analyses.
| Exposure | M1 β(SE) | M2 β(SE) | M3 β(SE) | M4 β(SE) |
|---|---|---|---|---|
|
| ||||
| Sleepiness | 0.04** (0.01) | 0.04** (0.01) | 0.04** (0.01) | 0.03** (0.01) |
| Unweighted n | 665 | 665 | 665 | 665 |
| Insomnia | 0.04*** (0.01) | 0.04*** (0.01) | 0.04*** (0.01) | 0.04*** (0.01) |
| Unweighted n | 665 | 665 | 665 | 665 |
| Apnea | 0.002 (0.004) | 0.003 (0.004) | 0.004 (0.004) | 0.002 (0.004) |
| Unweighted n | 665 | 665 | 665 | 665 |
| Duration | 0.05 (0.03) | 0.04 (0.03) | 0.01 (0.04) | 0.01 (0.03) |
| Unweighted n | 665 | 665 | 665 | 665 |
| Weekly Nap | ||||
| none | ref | ref | ref | ref |
| 1–2 times | −0.07 (0.15) | −0.05 (0.13) | −0.07 (0.12) | −0.07 (0.12) |
| 3–4 times | −0.04 (0.15) | −0.05 (0.13) | −0.08 (0.15) | −0.05 (0.14) |
| > 5 times | −0.11 (0.13) | −0.09 (0.15) | −0.08 (0.16) | −0.10 (0.15) |
| Unweighted n | 665 | 665 | 665 | 665 |
| Quality | ||||
| very sound/very restful | ref | ref | ref | ref |
| sound/restful | 0.08 (0.20) | 0.07 (0.19) | 0.06 (0.18) | 0.04 (0.17) |
| average | 0.43* (0.20) | 0.42* (0.18) | 0.44* (0.18) | 0.38* (0.17) |
| restless/very restless | 0.43* (0.20) | 0.37* (0.18) | 0.34 (0.18) | 0.33 (0.17) |
| Unweighted n | 665 | 665 | 665 | 665 |
Note 1: NEI VFQ = National Eye Institute Visual Function Questionnaire; standardized measure is used in the estimations
Note 2: Model 1 (M1) is crude, Model 2 (M2) is adjusted for age, sex, and education, Model 3 (M3) is additionally adjusted for all sociodemographic characteristics, behavioral risk factors and acculturation measures, and Model 4 (M4) is full covariate adjusted
Note 3:
p<0.001;
p<0.01;
p<0.05
To test our second set of hypotheses that (1) self-reported visual impairment is inversely linked to Visit-1 and average 7-years cognitive function and/or change in cognition and (2) that sleep can attenuate these associations, we performed survey generalized linear regression for each of the continuous outcomes of interest (i.e., SEVLT-Sum, SEVLT-Recall, Word Fluency, Digit and Symbol Substitution, and Global Cognition). Self-reported visual impairment (i.e., standardized NEI-VFQ) was the primary exposure, and for each outcome we fit 2 different models: (1) crude and (2) fully adjusted for all sleep measures, sociodemographic characteristics, behavioral risk factors, acculturation, and health conditions. Estimates for the regression coefficients and their standard errors are presented in Table 3 for the Visit-1 cognitive performance and on average 7 years later, and in Table 4 for the cognitive change. In post-hoc analyses, we calculated and plotted marginal means derived from the regression models with their 95% confidence intervals to highlight differences across the exposure groups (Figure 1).
Table 3:
Associations between visual impairment (NEI VFQ) and cognitive performance at Visit 1 and at SOLINCA, on average 7-years later. Results are based on survey regression models.
| Visit 1 | SOL-INCA | |||
|---|---|---|---|---|
| M1 | M2 | M1 | M2 | |
|
|
||||
| Outcome | β(SE) | β(SE) | β(SE) | β(SE) |
|
| ||||
| B-SEVLT-Sum | −0.12** (0.04) | −0.09* (0.04) | −0.15* (0.06) | −0.14** (0.04) |
| Unweighted n | 662 | 662 | 663 | 663 |
| B-SEVLT- Recall | −0.14** (0.04) | −0.13** (0.04) | −0.09 (0.05) | −0.10** (0.04) |
| Unweighted n | 665 | 665 | 662 | 662 |
| Word Fluency | −0.12** (0.04) | −0.05 (0.04) | −0.23*** (0.05) | −0.18*** (0.04) |
| Unweighted n | 662 | 662 | 663 | 663 |
| Digit & Symbol | −0.24*** (0.04) | −0.17*** (0.03) | −0.24*** (0.06) | −0.19*** (0.03) |
| Unweighted n | 656 | 656 | 659 | 659 |
| Global Cognition | −0.16*** (0.03) | −0.12*** (0.03) | −0.18*** (0.05) | −0.15*** (0.03) |
| Unweighted n | 665 | 665 | 663 | 663 |
Note 1: NEI VFQ = National Eye Institute Visual Function Questionnaire; standardized measure is used in the estimations
Note 2: Model 1 (M1) is crude, Model 2 (M2) is adjusted for all sleep measures (sleepiness, insomnia, apnea, duration, nap, quality) and full covariates (sociodemographic characteristics, behavioral risk factors, acculturation measures, and health conditions)
Note 3:
p<0.001;
p<0.01;
p<0.05
Table 4:
Associations between visual impairment (NEI VFQ) and cognitive change. Results are based on survey regression models.
| Outcome | M1 β(SE) | M2 β(SE) |
|---|---|---|
|
| ||
| B-SEVLT-Sum | −0.09 (0.07) | −0.09 (0.06) |
| Unweighted n | 660 | 660 |
| B-SEVLT-Recall | −0.02 (0.06) | −0.02 (0.05) |
| Unweighted n | 662 | 662 |
| Word Fluency | −0.17** (0.06) | −0.17** (0.05) |
| Unweighted n | 660 | 660 |
| Digit & Symbol | −0.02 (0.08) | −0.00 (0.07) |
| Unweighted n | 650 | 650 |
| Global Cognition | −0.08 (0.08) | −0.07 (0.07) |
| Unweighted n | 663 | 663 |
Note 1: NEI VFQ = National Eye Institute Visual Function Questionnaire; standardized measure is used in the estimations
Note 2: Model 1 (M1) is crude, Model 2 (M2) is adjusted for all sleep measures (sleepiness, insomnia, apnea, duration, nap, quality) and full covariates (sociodemographic characteristics, behavioral risk factors, acculturation measures, and health conditions)
Note 3:
p<0.001;
p<0.01;
p<0.05
Figure 1.
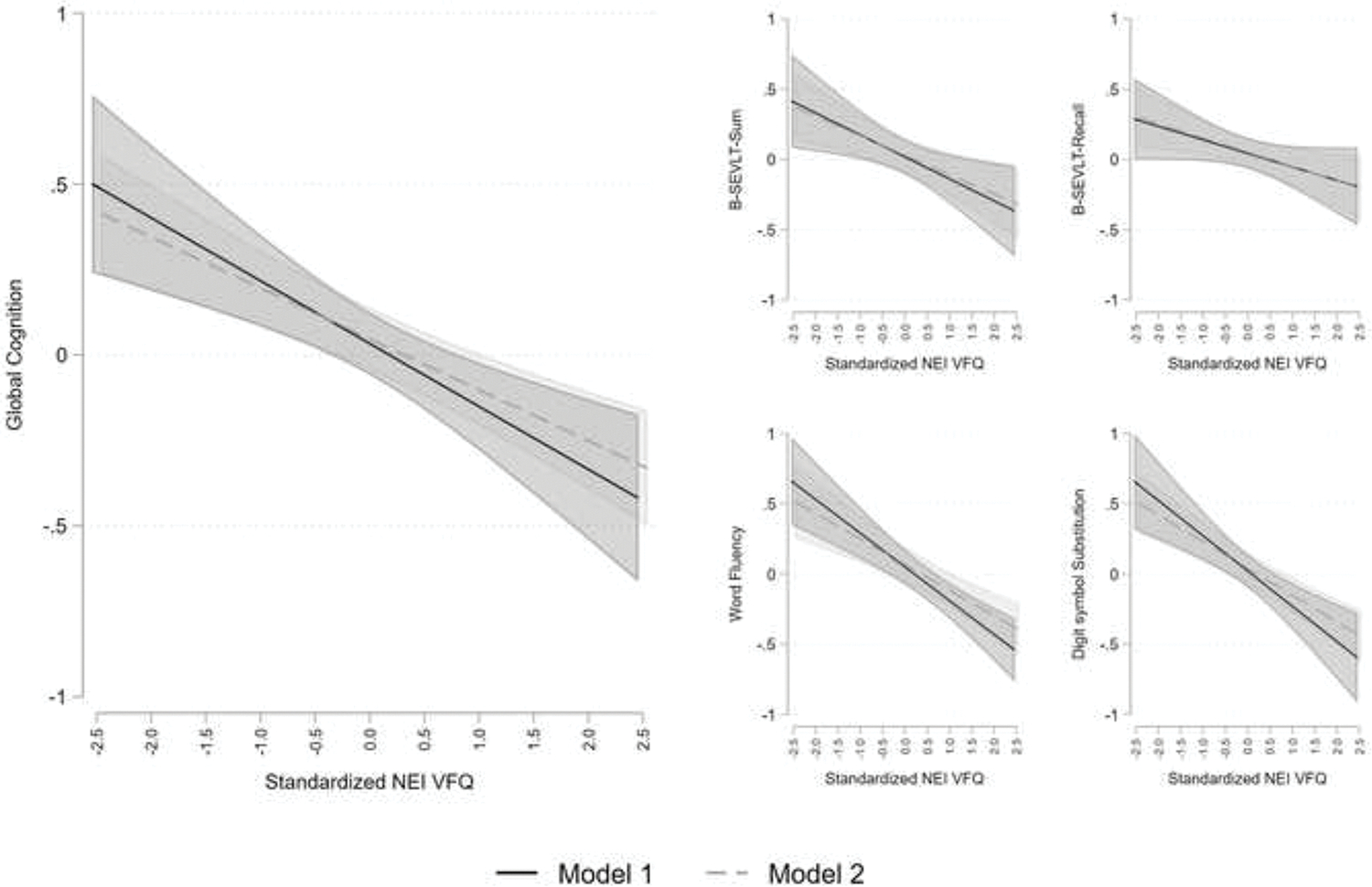
Associations between visual impairment (NEI VFQ) and cognitive performance at SOL-INCA, on average 7-years later. Results are based on survey regression models. Model 1 is crude, Model 2 is adjusted for all sleep measures (sleepiness, insomnia, apnea, duration, nap, quality) and full covariates (sociodemographic characteristics, behavioral risk factors, acculturation measures, and health conditions).
As the supplemental analysis, we fit 2 additional models: (1) adjusted for each of the sleep measures (i.e., Sleepiness, Insomnia, Apnea, Duration, Napping, and Sleep Quality) independently, (2) adjusted for each of the sleep measures, age, sex, and education (Supplemental Table 1–2). The margins are also plotted and presented in Supplemental figures 1–3. To determine the robustness of the models, we conducted sensitivity analyses where we additionally included two covariates (years lived in US and acculturation subscale - social) in the model adjustments (model 3 and model 4 in the first hypothesis and model 4 in the second hypothesis). These results are briefly discussed below (output of results from the sensitivity analyses are available from authors).
Lastly, we replicated the above analyses while using the categorical classifications for Apnea, Insomnia, and Duration (see sleep exposures section above). The additional results from this analysis are also available through authors. All survey regression analyses were performed using the survey functionalities in Stata V16.1 and incorporated the SOL-INCA sampling design.
To facilitate more detailed visual exploration of all the results generated from these analytic steps, we created and published an online dashboard. Interested readers can access and explore all our published results at the following website [https://solincalab.shinyapps.io/Sleep-Vision-Cognition-3/].
RESULTS
Descriptive statistics.
The characteristics for the overall target population are shown in Table 1. Average age was 57.4 years, 52.7% were women, 69.7% were Cuban heritage, and close to a quarter were Central/ South American heritage. 52% of the target population had education of more than high school, more than half was either married or cohabited, and more than 54% had income less than $20,000. Additionally, 42% were insured and 45% were employed at Visit-1. Average BMI of the target population was 29.7, 47% consumed alcohol, slightly more than 1 in 5 smoked at Visit-1, and 54% met the 2008 physical activity level guidelines. 93% preferred Spanish language over English. Finally, 48% had hypertension, 29% had diabetes, 8.2% had CHD, 2.9% self-reported heart failure, and 3.9% had prevalent stroke or TIA.
Associations among OSA, self-reported sleep duration, insomnia and daytime sleepiness with NEI-VFQ (Table 2).
Sleepiness and Insomnia were associated with worse self-reported visual impairment, and the associations were robust to adjustment for sociodemographic characteristics, acculturation, behavioral risk measures, and health conditions. Having average or restless/ very restless quality of sleep was also associated with worse self-reported visual impairment; however, these associations attenuated after adjusting for covariates. Apnea, sleep duration, and napping frequency were not linked with self-reported visual impairment.
Self-reported visual impairment and cognitive function (Table 3, Supplemental Table 1).
At Visit-1, worse self-reported visual impairment was inversely associated with global cognitive function. These associations were consistent across all considered cognitive domains and were not attenuated through adjustment for any of the sleep measures. Slight attenuations were observed for the relationships between self-reported visual impairment with global cognition, learning and memory (B-SEVLT Sum, B-SEVLT recall), and processing speed/executive functioning (DSST) after adjustment for sociodemographic characteristics, behavioral characteristics, acculturation, and health conditions. The association between self-reported visual impairment with word fluency was fully explained by adjustment of covariates.
Worse self-reported visual impairment was associated with lower global cognitive function on average 7-years later. As with Visit-1, the associations were not attenuated by adjustment to sleep, and only slightly so after adjusting for sociodemographic, acculturative, behavioral or health characteristics. Self-reported visual impairment was also inversely associated with learning (B-SEVLT Sum), verbal fluency and processing speed/executive functioning, and these associations were robust to sleep and other covariables adjustment. In the fully adjusted model, worse self-reported visual impairment was associated with lower scores in memory.
Self-reported visual impairment and cognitive change (Table 4, Supplemental Table 2).
Despite consistent inverse links to Visit-1 and Visit-2 cognitive function, self-reported visual impairment was not associated with change in global cognition. We also find no evidence for linking self-reported visual impairment with memory (learning and recall) or processing speed/executive functioning. However, higher NEI-VFQ (implicating worse visual functioning) was associated with more pronounced decline in performance on verbal fluency. The association between the NEI-VFQ and change in verbal fluency was not attenuated by adjustment for the considered sleep measures and remained robust to additional adjustment for sociodemographic, behavioral, acculturation, and health conditions.
Sensitivity Analysis.
After adding two additional covariates (years lived in US and an acculturation subscale - social) and excluding individuals with missing covariates, the sample size was reduced to n=590. While some of the estimated effects lost significance due to the reduced sample size, the results and interpretation from the original results remained stable.
Secondary Analyses.
The results above were consistent in the secondary analyses where insomnia, sleep apnea, and duration were operationalized as categorical variables. These results are available from authors and the online dashboard (see Sensitivity Analysis section above).
DISCUSSION
In this study population of middle-aged and older Hispanic/Latinos from the HCHS/SOL Miami site and SOL-INCA, self-reported visual impairment was associated with worse verbal fluency, memory, and processing speed/executive functioning at baseline and follow-up. In addition, visual disturbances predicted 7-year cognitive decline in the verbal fluency domain. Like our findings, prior studies observed associations between vision loss and cognitive decline. However, most studies used measures of global cognition (e.g., Mini-Mental State examination) or evaluated cohorts of older adults.[19, 40] An analysis from the Baltimore Longitudinal Study on Aging (mean age 71 years; 71% White) described associations between visual acuity with worse cognitive function and six-year cognitive decline in the domains of language and memory, while visual contrast sensitivity was associated with decline in language, memory, attention, and visuospatial ability.[20] Similar to our analysis, self-rated vision (poor compared to normal) was associated with incident dementia in participants 50–69 years of age from the English longitudinal study of ageing.[41] Our study used self-report to measure the main exposure; nonetheless we also observed domain specific cognitive dysfunction in the cross-sectional analysis and 7-year decline in language (i.e., verbal fluency). These findings suggest that impaired visual input may result in errors in perceptual processing, with consequent decline in higher-order cognitive performance.
Our findings also suggest that self-reported visual impairment contributes to cognitive decline and could lead to increase dementia risk in Hispanic/Latino adults, who have up to a fourfold increased risk of dementia and early cognitive decline compared to non-Hispanic white adults.
We also observed associations between self-reported visual impairment, with daytime sleepiness, and insomnia symptoms, but not with OSA or self-reported sleep duration. Other studies observed associations between disorders that cause vision loss (e.g., glaucoma) and OSA. Our study may not be powered to reproduce these associations.[42, 43] Insomnia and daytime sleepiness could be the consequence of circadian misalignment in participants with visual impairment.[44] In addition, cognitive decline can be the consequence of increments in cognitive load associated with visual impairment.[12] Alternatively, visual and cognitive impairment[12] in Hispanic/Latinos, could be in part associated with high prevalence of cardiovascular diseases, as well as sleep disorders affecting cognitive health.[17, 45–47]
Strengths and Limitations
The strengths of our study include our large, diverse Hispanic/Latino population of diverse heritage, the use of a robust self-reported visual functioning tool, validated in Spanish and Hispanic/Latino populations, and finally our extensive, longitudinal, cognitive testing. Our study does have limitations. HCHS/SOL is a community-based population study that may have unmeasured confounders.
Our relatively smaller sample size and evaluation within a single site limited our analysis to confounders considered a priori. However, follow-up studies should include the effects of important confounders such as physical activity, dietary habits, measures of anxiety and depression, medications that could affect sleep and cognition, as well as behaviors, including use of caffeine, tobacco and alcohol. However, the influence of these confounders was minimized through strict regulations, and guidelines to evaluate our participants, as well as systematic controlling for covariates in our statistical models. As with other studies, using subjective data can also introduce classification error. The NEI-VFQ assesses the effect of self-reported visual functioning on emotional well-being and social functioning. Follow-up studies utilizing clinical exams and objective measurements of vision are warranted In addition, there were a limited number of measures available from the home sleep apnea test.[48] HCHS/SOL was designed to address gaps in research associated with U.S. Hispanic/Latino health. However, factors such as low health literacy, low education and the implementation of culturally specific protocols may serve as unknown confounders. Importantly, the chosen questionnaires are available and validated in Spanish and English.
Conclusion
Among Hispanic/Latinos from the HCHS/SOL Miami site target population, self-reported visual impairment was associated with cognitive function and cognitive decline, independent of covariates, including sleep disorders. Our findings suggest that self-reported visual impairment contributes to cognitive decline and could lead to increase dementia risk in Hispanic/Latino adults.
Supplementary Material
Acknowledgements
The investigators would like to thank the participants and staff of the Hispanic Community Health Study/Study of Latinos.
Footnotes
Conflict of Interest
No relevant conflict of interest
REFERENCES:
- [1].Lam BL, Lee DJ, Zheng DD, Davila EP, Christ SL, Arheart KL (2009) Disparity in Prevalence of Self-Reported Visual Impairment in Older Adults Among U.S. Race-Ethnic Subgroups. Ophthalmic Epidemiology 16, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lanzani MF, de Zavalia N, Fontana H, Sarmiento MI, Golombek D, Rosenstein RE (2012) Alterations of locomotor activity rhythm and sleep parameters in patients with advanced glaucoma. Chronobiology international 29, 911–919. [DOI] [PubMed] [Google Scholar]
- [3].Lockley SW, Arendt J, Skene DJ (2007) Visual impairment and circadian rhythm disorders. Dialogues Clin Neurosci 9, 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJ (2018) Longitudinal Associations Between Visual Impairment and Cognitive Functioning: The Salisbury Eye Evaluation Study. JAMA Ophthalmol 136, 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nagarajan N, Assi L, Varadaraj V, Motaghi M, Sun Y, Couser E, Ehrlich JR, Whitson H, Swenor BK (2022) Vision impairment and cognitive decline among older adults: a systematic review. BMJ Open 12, e047929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin SC (2008) Prevalence of glaucoma in patients with obstructive sleep apnoea--a cross-sectional case-series. Eye 22, 1105–1109. [DOI] [PubMed] [Google Scholar]
- [7].Gordo MA, Recio J, Sanchez-Barcelo EJ (2001) Decreased sleep quality in patients suffering from retinitis pigmentosa. Journal of sleep research 10, 159–164. [DOI] [PubMed] [Google Scholar]
- [8].Jean-Louis G, Kripke D, Cohen C, Zizi F, Wolintz A (2005) Associations of ambient illumination with mood: contribution of ophthalmic dysfunctions. Physiol Behav 84, 479–487. [DOI] [PubMed] [Google Scholar]
- [9].Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, Mathis J (1999) High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology 106, 1009–1012. [DOI] [PubMed] [Google Scholar]
- [10].Wee R, Van Gelder RN (2004) Sleep disturbances in young subjects with visual dysfunction. Ophthalmology 111, 297–302; discussion 302–293. [DOI] [PubMed] [Google Scholar]
- [11].Zizi F, Jean-Louis G, Magai C, Greenidge KC, Wolintz AH, Heath-Phillip O (2002) Sleep complaints and visual impairment among older Americans: a community-based study. The journals of gerontology. Series A, Biological sciences and medical sciences 57, M691–694. [DOI] [PubMed] [Google Scholar]
- [12].Nagarajan N, Assi L, Varadaraj V, Motaghi M, Sun Y, Couser E, Ehrlich JR, Whitson H, Swenor BK (2022) Vision impairment and cognitive decline among older adults: a systematic review. 12, e047929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Altman NG, Izci-Balserak B, Schopfer E, Jackson N, Rattanaumpawan P, Gehrman PR, Patel NP, Grandner MA (2012) Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep medicine 13, 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li X, Sotres-Alvarez D, Sofer T, Gallo L, Aviles-Santa L, Perreira K, Isasi C, Ramos A, Zee P, Savin K (2019) Associations of sleep disordered breathing and insomnia with incident hypertension and diabetes: a prospective analysis of data from the Hispanic Community Health Study/Study of Latinos In C99. SRN: CARDIOMETABOLIC CONSEQUENCES OF SLEEP DISORDERED BREATHING AND THE ROLE OF CPAP THERAPY American Thoracic Society, pp. A5605–A5605. [Google Scholar]
- [15].Ramos A, Tarraf W, Daviglus M, Davis S, Gallo L, Mossavar-Rahmani Y, Penedo F, Redline S, Rundek T, Sacco R (2015) Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. [Google Scholar]
- [16].Ramos A, Tarraf W, Wu B, Kaur S, Daviglus M, Shah N, Sostres-Alvarez D, Gallo L, Muñoz E, Wohlgemuth WJSm (2019) Age and sex interactions between sleep disordered breathing and sleep duration with neurocognitive decline in Sol-Inca, an ancillary to the hispanic community health study/study of latinos. 64, S313–S313. [Google Scholar]
- [17].Ramos AR, Tarraf W, Rundek T, Redline S, Wohlgemuth WK, Loredo JS, Sacco RL, Lee DJ, Arens R, Lazalde P, Choca JP, Mosley T Jr., Gonzalez HM (2015) Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology 84, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peltzer K, Phaswana-Mafuya N (2017) Association between Visual Impairment and Low Vision and Sleep Duration and Quality among Older Adults in South Africa. Int J Environ Res Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJJJo (2018) Longitudinal associations between visual impairment and cognitive functioning: the Salisbury Eye Evaluation Study. 136, 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Varadaraj V, Munoz B, Deal JA, An Y, Albert MS, Resnick SM, Ferrucci L, Swenor BKJJNO (2021) Association of vision impairment with cognitive decline across multiple domains in older adults. 4, e2117416–e2117416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Asplund R (2000) Sleep, health and visual impairment in the elderly. Arch Gerontol Geriatr 30, 7–15. [DOI] [PubMed] [Google Scholar]
- [22].Ramos AR, Wallace DM, Williams NJ, Spence DW, Pandi-Perumal SR, Zizi F, Jean-Louis G (2014) Association between visual impairment and sleep duration: analysis of the 2009 National Health Interview Survey (NHIS). BMC Ophthalmol 14, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee DJ, Gomez-Marin O, Lam BL, Ma F, Vilar NF (2000) Prevalence of usual-corrected distance visual acuity impairment in Hispanic and non-Hispanic children and adolescents. Paediatr Perinat Epidemiol 14, 357–362. [DOI] [PubMed] [Google Scholar]
- [24].Lee DJ, Gomez-Marin O, Lam BL (1998) Prevalence of uncorrected binocular distance visual acuity in Hispanic and non-Hispanic adults. Results from the HHANES and the NHANES I. Ophthalmology 105, 552–560. [DOI] [PubMed] [Google Scholar]
- [25].Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, Shah N, Ries A, Arens R, Barnhart J, Youngblood M, Zee P, Daviglus ML (2014) Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 189, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].LaVange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J (2010) Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Annals of epidemiology 20, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M (2010) Design and implementation of the Hispanic community health study/study of Latinos. Annals of epidemiology 20, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McClure LA, Zheng DD, Lam BL, Tannenbaum SL, Joslin CE, Davis S, López-Cevallos D, Youngblood ME Jr., Zhang ZM, Chambers CP, Lee DJ (2016) Factors Associated With Ocular Health Care Utilization Among Hispanics/Latinos: Results From an Ancillary Study to the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). JAMA Ophthalmol 134, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tarraf W, Kaplan R, Daviglus M, Gallo LC, Schneiderman N, Penedo FJ, Perreira KM, Lamar M, Chai A, Vasquez PM, Gonzalez HM (2020) Cardiovascular Risk and Cognitive Function in Middle-Aged and Older Hispanics/Latinos: Results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). J Alzheimers Dis 73, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gonzalez HM, Tarraf W, Fornage M, Gonzalez KA, Chai A, Youngblood M, Abreu MLA, Zeng D, Thomas S, Talavera GA, Gallo LC, Kaplan R, Daviglus ML, Schneiderman N (2019) A research framework for cognitive aging and Alzheimer’s disease among diverse US Latinos: Design and implementation of the Hispanic Community Health Study/Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimers Dement 15, 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Crowe SF (1998) The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J Clin Psychol 54, 585–591. [DOI] [PubMed] [Google Scholar]
- [32].Besha XS, Spencer RJ, Bieliauskas LAJIJoN (2017) PPVT-I administration rules significantly shorten PPVT-III/IV administration. 127, 412–416. [DOI] [PubMed] [Google Scholar]
- [33].Duff K (2012) Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol 27, 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marella M, Pesudovs K, Keeffe JE, O’Connor PM, Rees G, Lamoureux EL (2010) The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci 51, 2878–2884. [DOI] [PubMed] [Google Scholar]
- [35].Baker RS, Bazargan M, Calderón JL, Hays RD (2006) Psychometric performance of the National Eye Institute visual function questionnaire in Latinos and non-Latinos. Ophthalmology 113, 1363–1371. [DOI] [PubMed] [Google Scholar]
- [36].Alvarez-Peregrina C, Sánchez-Tena MA, Caballé-Fontanet D, Thuissard-Vasallo IJ, Gacimartín-García MB, Orduna-Magán C (2018) Crosscultural adaptation and validation into Spanish of the questionnaire National Eye Institute Visual Function Questionnaire 25. Arch Soc Esp Oftalmol (Engl Ed) 93, 586–591. [DOI] [PubMed] [Google Scholar]
- [37].Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, Investigators NEIVFQFT (2001) Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 119, 1050–1058. [DOI] [PubMed] [Google Scholar]
- [38].Westbrook PR, Levendowski DJ, Cvetinovic M, Zavora T, Velimirovic V, Henninger D, Nicholson D (2005) Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest 128, 2166–2175. [DOI] [PubMed] [Google Scholar]
- [39].Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG (2017) Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. 13, 479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BE, Klein R, Tweed TSJJotAGS (2016) Age-related sensory impairments and risk of cognitive impairment. 64, 1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Davies-Kershaw HR, Hackett RA, Cadar D, Herbert A, Orrell M, Steptoe AJJotAGS (2018) Vision impairment and risk of dementia: findings from the English Longitudinal Study of Ageing. 66, 1823–1829. [DOI] [PubMed] [Google Scholar]
- [42].Ramos AR, Wallace DM, Williams NJ, Spence DW, Pandi-Perumal SR, Zizi F, Jean-Louis GJBo (2014) Association between visual impairment and sleep duration: analysis of the 2009 National Health Interview Survey (NHIS). 14, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Santos M, Hofmann RJJJoCSM (2017) Ocular manifestations of obstructive sleep apnea. 13, 1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lockley SW, Arendt J, Skene DJJDicn (2007) Visual impairment and circadiam rhythm disorders. 9, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Agudelo C, Tarraf W, Wu B, Wallace DM, Patel SR, Redline S, Kaur S, Daviglus M, Zee PC, Simonelli G, Mossavar-Rahmani Y, Sotres-Alvarez D, Zeng D, Gallo LC, Gonzalez HM, Ramos AR (2021) Actigraphic sleep patterns and cognitive decline in the Hispanic Community Health Study/Study of Latinos. Alzheimers Dement 17, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ramos AR, Tarraf W, Wu B, Redline S, Cai J, Daviglus ML, Gallo L, Mossavar-Rahmani Y, Perreira KM, Zee P, Zeng D, Gonzalez HM (2020) Sleep and neurocognitive decline in the Hispanic Community Health Study/Study of Latinos. Alzheimers Dement 16, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ramos AR, Tarraf W, Daviglus M, Davis S, Gallo LC, Mossavar-Rahmani Y, Penedo FJ, Redline S, Rundek T, Sacco RL, Sotres-Alvarez D, Wright CB, Zee PC, Gonzalez HM (2016) Sleep Duration and Neurocognitive Function in the Hispanic Community Health Study/Study of Latinos. Sleep 39, 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Patel SR, Blackwell T, Ancoli-Israel S, Stone KL, Osteoporotic Fractures in Men-Mr OSRG (2012) Sleep characteristics of self-reported long sleepers. Sleep 35, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


