Abstract
Pulmonary veno-occlusive disease (PVOD), also known as “pulmonary arterial hypertension (PAH) with overt features of venous/capillary involvement”, is a rare cause of PAH characterised by substantial small pulmonary vein and capillary involvement, leading to increased pulmonary vascular resistance and right ventricular failure. Environmental risk factors have been associated with the development of PVOD, such as occupational exposure to organic solvents and chemotherapy, notably mitomycin. PVOD may also be associated with a mutation in the EIF2AK4 gene in heritable forms of disease. Distinguishing PVOD from PAH is critical for guiding appropriate management. Chest computed tomography typically displays interlobular septal thickening, ground-glass opacities and mediastinal lymphadenopathy. Life-threatening pulmonary oedema is a complication of pulmonary vasodilator therapy that can occur with any class of PAH drugs in PVOD. Early referral to a lung transplant centre is essential due to the poor response to therapy when compared with other forms of PAH. Histopathological analysis of lung explants reveals microvascular remodelling with typical fibrous veno-occlusive lesions. This review covers the main features distinguishing PVOD from PAH and two clinical cases that illustrate the challenges of PVOD management.
Tweetable abstract
PVOD is a rare form of pulmonary arterial hypertension linked to mutations or occupational exposure. Early referral to a lung transplant centre is essential, due to the poor response to therapy when compared with other forms of PAH. https://bit.ly/3sFR8Hk
Introduction
Rare forms of pulmonary arterial hypertension (PAH) characterised by pulmonary venous and capillary remodelling are grouped under the entity PAH with signs of venous and/or capillary involvement, named pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis (PCH) [1–3]. Pre-capillary pulmonary hypertension (PH) is defined by a mean pulmonary arterial pressure (mPAP) >20 mmHg at rest together with pulmonary vascular resistance (PVR) >2 Wood units (WU) with a normal pulmonary artery wedge pressure (PAWP) [4]. According to the latest European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines on the diagnosis and management of PH, the entity “PAH with features of venous/capillary involvement now corresponds to the group 1.1 within the group 1 of the clinical classification of PH, following the Proceedings of the 6th World Symposium on PH, in contrast with the 2015 ESC/ERS Guidelines, where PVOD corresponded to a special subgroup 1′ [4, 5]. Scientific evidence supports that PVOD and PCH are in fact diverse forms of expression of the same disorder, since both conditions share overlapping clinical, histopathological and genetic features [6–8]. In this review, we refer to PVOD as a diagnosis that encompasses both entities. Prevalence and incidence of PVOD remain difficult to estimate since many cases are misclassified until histological diagnosis is available after lung transplantation. Estimated incidence and prevalence rates are respectively 0.1–0.5 and 1–2 cases per million [1]. Most cases are discovered in children and young adults, although PVOD cases have been reported until the seventh decade [9, 10].
Early diagnosis of PVOD is critical because these patients have a poorer response to PAH-specific therapies, with an increased risk of pulmonary oedema and a worse prognosis without lung transplantation when compared with other forms of PAH [11]. This review covers the main features of PVOD, highlighted by the description of two illustrative cases.
Risk factors and conditions associated with PVOD
Associated risk factors for PVOD include exposure to alkylating agents and organic solvents in sporadic forms of disease. Heritable forms of PVOD are caused by biallelic mutations in the eukaryotic translation initiation factor 2 α kinase 4 (EIF2AK4) gene with an autosomal recessive transmission [12].
Genetics
Autosomal recessive biallelic mutations in the EIF2AK4 gene cause heritable PVOD [12]. In the French PH referral centre, genetic counselling and EIF2AK4 mutation screening have been offered to all patients with confirmed or suspected PVOD, with or without a suggestive family history [13, 14]. Biallelic EIF2AK4 mutations were identified in all familial cases of PVOD, but mutations were also found in 9% (7/81) of PVOD cases suspected to be sporadic [7]. The EIF2AK4 gene encodes for GCN2 (general control nonderepressible 2), which belongs to a family of four kinases that phosphorylate the α-subunit of eIF2 (eukaryotic translation initiation factor 2). This protein is involved in the control of general translation in response to various cellular stresses, such as amino acid deprivation [12, 15]. Patients carrying the EIF2AK4 mutation are characterised by a younger age at diagnosis (median 26 years versus 60 years) and a quasi-equal male/female ratio without exposure to organic solvents or previous chemotherapy compared to noncarriers (male predominance, solvent exposure in 42% and chemotherapy in 10% of patients) [16]. However, the two groups have similar disease severity with comparable imaging, including interlobular septal thickening, ground-glass opacities and mediastinal lymphadenopathy on chest high-resolution computed tomography (HRCT) [16].
Drugs associated with PVOD
Numerous case reports identified a temporal association between exposure to several chemotherapeutic agents and PVOD development [10, 16, 17]. After a systematic analysis of chemotherapy-induced PVOD, Ranchoux et al. [18] reported a predominance of alkylating agents associated with chemotherapy-induced PVOD. Alkylating agents are predominantly associated with chemotherapy-induced PH, particularly cyclophosphamide, mitomycin C (MMC) and cisplatin [18]. Although cyclophosphamide has been used successfully in autoimmune diseases associated with PAH, this drug has been shown to be toxic to the pulmonary endothelium in vitro and in vivo. Among the chemotherapeutic agents associated with PVOD, MMC has been frequently described in chemotherapy-induced PVOD, along with demonstrated in vitro direct toxicity to the lung endothelium [19, 20]. Interestingly, amifostine, a cytoprotective agent, prevented the development of MMC-induced PVOD in rats [20]. Finally, chemotherapy-induced PVOD has been reported in paediatric oncology cases, particularly after haematopoietic stem cell and bone marrow transplantation, possibly related to alkylating agent exposure [21, 22].
Occupational exposures: organic solvents and tobacco
Occupational exposure to organic solvents, especially trichloroethylene, a chlorinated solvent, has been significantly associated with PVOD [1, 16, 23]. In a case-control study using an expert consensus approach and an occupational exposure matrix, we demonstrated that PVOD was significantly associated with occupational exposure to organic solvents (adjusted OR 12.8, 95% CI 2.7–60.8). Exposure to trichloroethylene was the most commonly implicated exposure, occurring in 42% of patients with PVOD compared to 3% of patients with PAH (adjusted OR 8.2, 95% CI 1.4–49.4) [23]. Trichloroethylene was demonstrated to increase endothelial permeability in vivo, providing a mechanistic scenario for PVOD associated with solvent exposure [24].
Tobacco smoke exposure has also been associated with alterations of vascular permeability and endothelial barrier dysfunction [25]. Higher cumulative tobacco exposure has been reported in PVOD compared to idiopathic PAH [11]. In this case–control study, all PVOD patients with significant exposure to trichloroethylene had concurrent tobacco exposure, suggesting a cumulative toxic effect of these two exposures [11].
Other conditions with venous and/or capillary involvement (PVOD-like)
There is an increasing recognition of the significant venular involvement in PAH associated with connective tissue disease, particularly systemic sclerosis [26–28]. However, the exact prevalence of significant venous and/or capillary involvement in connective tissue diseases associated with PAH is unknown. The observed PVOD-like features may explain why a poorer clinical response to PAH therapy is occasionally described in systemic sclerosis-associated PAH.
Clinical features of PVOD
PVOD and PAH share some common clinical signs and symptoms, such as progressive dyspnoea, fatigue and exercise intolerance [1]. However, PVOD patients classically have a more severe phenotype, characterised by profound dyspnoea and hypoxaemia [16, 29]. Pulmonary function tests show a reduced diffusion capacity for carbon monoxide with generally preserved lung volumes [16]. The pulmonary haemodynamics in PVOD are indistinguishable from those in other forms of pre-capillary PH diagnosed by right heart catheterisation (RHC), with mPAP ≥20 mmHg, PAWP ≤15 mmHg and elevated PVR >2 WU [4]. Acute vasoreactivity testing is not routinely recommended in PVOD since no reported cases presented a long-term response to calcium channel blockers and pulmonary oedema has been reported during testing [4, 30]. Although PVOD displays significant remodelling of pulmonary capillaries and venules, larger pulmonary veins are relatively spared, likely explaining the normal PAWP during RHC.
Radiology
Imaging can capture haemodynamic derangements resulting from vascular obstruction in the alveolar capillary bed or pulmonary venules and small veins. Echocardiography cannot distinguish PVOD from other causes of PAH but is helpful to exclude left heart disease. The role of chest radiography is limited, with findings such as prominent upper lobe pulmonary vasculature, nodular opacities, septal lines and pleural effusions mimicking signs of heart failure. On ventilation/perfusion scintigraphy, perfusion is normal in most cases, although a small proportion may show mismatched perfusion defects [31]. These defects could arise due to increased downstream resistance caused by narrowing and obliteration of pulmonary veins and venules [32]. Multidetector computed tomography (CT) is the noninvasive imaging modality of choice as it often provides a clue to the underlying diagnosis.
The presence of widespread centrilobular ground-glass opacities, smooth interlobular septal thickening, mediastinal lymphadenopathy and pleuropericardial effusions in the context of PH should raise the suspicion of PVOD, but the parenchymal changes can be present even before development of PH [33]. In addition, the conglomeration of features may not be present in all cases. In a small cohort of 12 patients with PVOD/PCH, 50% were shown to have perfusion abnormalities on dual-energy CT with a small number (3/12) showing lobular and pulmonary embolism-type perfusion defects in the absence of thromboembolic disease [34]. An important caveat to remember is that the HRCT findings can be seen in a variety of other conditions, the commonest being pulmonary oedema, but the presence of a normal-sized left atrium in PVOD is an important discriminator. Other differential diagnoses include interstitial lung disease, infection, lympho-proliferative disorders and pulmonary tumour thrombotic microangiopathy. It is therefore necessary to interpret imaging with correlative clinical information.
Histopathological features
Typical morphological changes in the PVOD (figure 1d) comprise diffuse involvement of the septal veins and pre-septal venules in a vasculopathy that is characterised by fibrous thickening and obliteration of the lumen to various degrees. Involvement of the small pre-septal venules, typically <100 µm in diameter, is characteristic and necessary for the diagnosis. Intimal remodelling may vary from loose fibrous tissue in myxoid stroma to paucicellular fibrosis, possibly reflecting disease evolution. Patchy capillary congestion and proliferation, often referred to in the literature as PCH, is considered a spectrum of the disease and is probably the consequence of diffuse pre-septal venous involvement. Arterial remodelling consisting of arterial intimal fibrosis and medial hypertrophy is common in PVOD, but in most cases is less prominent than venous remodelling.
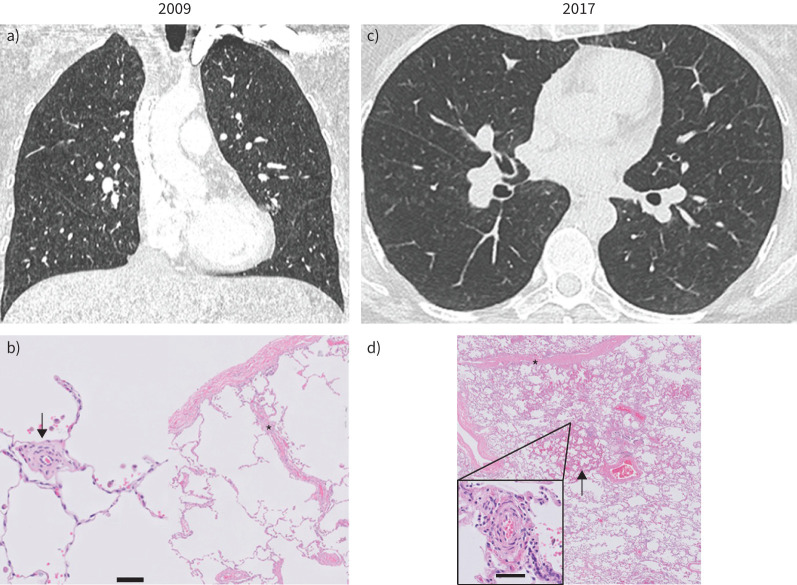
FIGURE 1.
Computed tomography and histopathological images of patient 1 at diagnosis (2009) (a and b) and at the time of lung transplantation (2017) (c and d). a) In 2009, chest computed tomography revealed mild nonspecific ground-glass opacities and interlobular septal thickening, without lymph node enlargement (not shown). c) In 2017, ground-glass opacities and interlobular septal thickening are more pronounced. b) Lung histology in 2009 (surgical biopsy, haematoxylin and eosin staining) and d) in 2017 (lung transplant, haematoxylin and eosin–saffron staining) are shown. In b), pulmonary microcirculation (scale bar = 50 µm) shows extensive thickening of the intima (arrow) with subtle venous remodelling (asterisk). d) Subtotal obliteration of septal veins is confirmed in the explanted lung in 2017 (asterisk) with patchy capillary congestion and proliferation (arrow) and more extensive remodelling of the microcirculation (zoom-in panel; scale bar = 50 µm).
Management and outcomes
Natural history and prognosis
The overall prognosis of PVOD is generally severe, with relentless progression despite medical therapy. 1-year mortality has been reported to be close to 72% [35]. No randomised clinical trials have ever evaluated the prognosis of PVOD patients under PAH therapy. Most patients either die or are evaluated for lung transplantation within 2 years of diagnosis. We reported a mean time from diagnosis to death or lung transplantation of 11.8 months and a mean time from the first reported symptoms to death or lung transplantation of 24.4 months [11]. Early referral to a lung transplant centre after PVOD diagnosis is therefore essential.
General measures, PAH-approved drugs and transplantation
PAH drugs targeting endothelial dysfunction (endothelin receptor antagonists, phosphodiesterase type 5 inhibitors and prostacyclin derivatives) have significantly improved the outcome of patients with PAH over the two last decades [36–38]. In contrast, no medical therapy has demonstrated proven efficacy in randomised trials in PVOD. In the largest published cohort of heritable and sporadic PVOD, 81% of EIF2AK4 mutation carriers and 94% of noncarriers received an approved PAH therapy [16]. Drug-induced pulmonary oedema occurred in more than 20% of patients treated with these drugs, reinforcing the need for cautious use of PAH therapy and for PVOD patients to be handled in expert centres.
Supportive measures comprise oxygen administration in hypoxaemic patients to prevent further exacerbation of PH due to hypoxic pulmonary vasoconstriction and diuretics to reduce right ventricular pre-load and prevent fluid retention. Routine anticoagulation is not recommended in patients with PVOD due to a lack of evidence of its benefit and the possibility of occult pulmonary haemorrhage, but it may be considered on an individual basis [4]. Immunosuppressive drugs are generally not recommended for PAH and PVOD, except in cases of systemic diseases such as systemic lupus erythematosus. In a recent small case series of PVOD, immunosuppressive drugs provided clinical improvement or stability [39]. These findings require further studies.
In conclusion, the 2022 ESC/ERS guidelines on PH have revised some general recommendations for the diagnosis and management of PVOD (table 1). Bilateral lung transplantation remains the definitive treatment for PVOD. Referral to an expert centre should be discussed at the time of diagnosis with eligible patients. Post-transplant survival rates are similar between PAH and PVOD [16].
TABLE 1.
Revised European Society of Cardiology and European Respiratory Society guidelines for PAH with signs of venous/capillary involvement
| Recommendation in 2015 [ 40 ] | Class | Recommendations in 2022 [ 4 ] | Class |
| A combination of clinical findings, physical examination, bronchoscopy and radiological findings is recommended to diagnose PVOD/PCH | I | A combination of clinical and radiological findings is recommended to diagnose PAH with signs of venous and/or capillary involvement (PVOD/PCH) | I |
| In patients with PVOD/PCH, the use of drugs approved for PAH may be considered with careful monitoring of clinical symptoms and gas exchange | IIb | ||
| Lung biopsy is not recommended to confirm a diagnosis of PVOD/PCH | III |
Table adapted from [4]. PAH: pulmonary arterial hypertension; PCH: pulmonary capillary haemangiomatosis; PVOD: pulmonary veno-occlusive disease.
Illustrative case 1
We report the management of a patient displaying familial PVOD, who presented at an early stage in the disease's progression and was followed over 8 years in the French PH referral centre. At the first consultation in 2009, this female patient was 45 years old, reporting a mild dyspnoea on exertion (New York Heart Association (NYHA) functional class II). Family history was highly suggestive of PAH, since two elder sisters had previously undergone lung transplantation for the condition. In both sisters, histopathology of the explanted lungs eventually established a diagnosis of PVOD. In 2009, our patient was in functional class II (table 2), 6-min walk distance (6MWD) was slightly reduced (550 m, with a minimal saturation of 90% on room air). Spirometry and arterial blood gases were normal, diffusing capacity of the lung for carbon monoxide (DLCO) was mildly reduced. Cardiopulmonary exercise testing (CPET) revealed a reduced oxygen uptake and exercise hypoxaemia. RHC at rest was normal, mPAP was 17 mmHg, cardiac output (CO) was 7.2 L·min−1 and PVR was 2.2 WU. During exercise, the mPAP increased to 36 mmHg with a CO of 13.2. The mPAP/CO slope was 3.2 mmHg·min·L−1 between rest and exercise. Considering the normal haemodynamics at rest, a lung biopsy was performed after multidisciplinary team discussion. Histopathological analysis confirmed mild pulmonary vascular disease with focal remodelling of microvessels smaller than 100 μm (figure 1b) with subtle involvement of the pulmonary veins.
TABLE 2.
Evolution of clinical, functional and haemodynamic parameters of patient 1
| Parameter | July 2009 | June 2013 | October 2016 | ||
| NYHA FC | II | II | III/IV | ||
| 6MWD, m | 550 | 506 | NA | ||
| DLCO, %pred | 51 | 35 | 23 | ||
| KCO, %pred | 53 | 39 | 25 | ||
| ABG on room air | |||||
| PaO2, mmHg | 92 | 86 | 46 | ||
| PaCO2, mmHg | 30 | 27 | 27 | ||
| CPET | |||||
| V′O2, mL·min−1·kg−1 (% pred) | 21.4 (69%) | 16 (52%) | Not feasible | ||
| VD/VT | 0.29 | 0.44 | Not feasible | ||
| Peak PaO2, mmHg | 63 | 53 | Not feasible | ||
| PA–aO2, mmHg | 57 | 71 | Not feasible | ||
| BNP, ng·L−1 (<80) | 34 | 34 | – | ||
| NT-proBNP, ng·L−1 (<300) | NA | NA | 160 | ||
| Haemodynamics | Rest | 100 W | Rest | 60 W | Rest |
| RAP, mmHg | 0 | NA | 2 | NA | 3 |
| mPAP, mmHg | 17 | 36 | 25 | 45 | 38 |
| PAWP, mmHg | 1 | 15 | 4 | 14 | 6 |
| CI, L·min−1·m−2 | 3.8 | 7.0 | 3.49 | 6.4 | 2.6 |
| TPR, WU | 2.4 | 2.7 | 3.9 | 3.8 | 7.8 |
| PVR, WU | 2.2 | 1.6 | 3.3 | 2.6 | 6.6 |
6MWD: 6-min walk distance; ABG: arterial blood gases; BNP: brain natriuretic peptide; CI: cardiac index; CPET: cardiopulmonary exercise testing; DLCO: diffusing capacity of the lung for carbon monoxide; FC: functional class; KCO: transfer coefficient of the lung for carbon monoxide; mPAP: mean pulmonary artery pressure; NA: not available; NT-proBNP: N-terminal proBNP; NYHA: New York Heart Association; PA–aO2: alveolar–arterial oxygen tension gradient; PaCO2: arterial carbon dioxide tension; PaO2: arterial oxygen tension; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure; TPR: total pulmonary resistance; VʹO2: oxygen uptake; VD/VT: dead space/tidal volume ratio; WU: Wood unit.
The patient was followed without specific therapy from 2019 to 2016, with a progressive deterioration. Haemodynamic evaluation from 2013 confirmed PH for the first time at rest (table 2). Oxygenation at rest and during exercise also worsened. In 2016, the patient was in functional class III to IV, 6MWD was not evaluable despite oxygen supply. Diffusion capacity was severely reduced to 23%, arterial oxygen tension (PaO2) at rest was 46 mmHg, with a reduced arterial carbon dioxide tension (PaCO2) while breathing room air. Chest HRCT analysis (figure 1c) showed subtle ground-glass opacities and interlobular thickening. Haemodynamics were aggravated with PVR of 6.6 WU. Considering the level of hypoxaemia despite moderate PH and a slightly reduced cardiac index (CI), the patient was not treated with PAH-specific therapies, but rather listed for lung transplantation. Double lung transplantation was performed in 2017. Histology of the explanted lungs showed widespread remodelling of microvessels and marked fibrous veno-occlusive remodelling (figure 1d).
Illustrative case 2
In our second illustrative case, we describe a patient who developed MMC-induced PH.
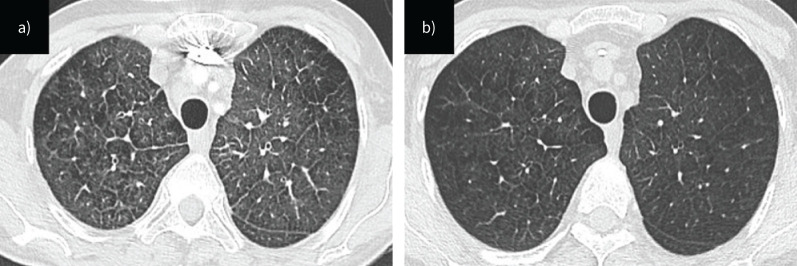
A 54-year-old woman was admitted to the intensive care unit with severe hypoxaemia. She was a former smoker (40 pack-years) and had a history of anal cancer diagnosed 6 months earlier. As part of her treatment, she had received two cycles of chemotherapy with fluorouracil and MMC, along with local radiotherapy. 3 months after completing chemotherapy, she experienced a rapid and progressive dyspnoea which led to her admission to the intensive care unit. At admission, she was in NYHA functional class IV and suffered from severe hypoxaemia and required 6 L·min−1 of oxygen. Her pulmonary auscultation revealed mild lung crackles and brain natriuretic peptide (BNP) was significantly increased to 1083 ng·L−1 (normal value <80 ng·L−1). Doppler echocardiography was performed showing normal left ventricular function, dilatation of right heart chambers with paradoxical septal motion and mild pericardial effusion. The estimated systolic pulmonary arterial pressure was 70 mmHg. In addition, right ventricular function was severely impaired. RHC confirmed severe pre-capillary PH, with an mPAP of 37 mmHg, CI of 1.9 L·min−1·m−2, PAWP of 6 mmHg and PVR of 11.3 WU (table 3). There was no acute response to the nitric oxide vasoreactivity test. CT pulmonary angiogram demonstrated no evidence of thromboembolic disease, but revealed findings suggestive of PVOD, including septal lines, centrilobular ground-glass opacities and enlarged mediastinal lymph nodes (figure 2). Considering the presence of pre-capillary PH, abnormal CT findings highly suggestive of PVOD and exposure to alkylating agents, it is suspected that MMC may have induced PVOD. Screening for EIF2AK4 mutations later proved negative.
TABLE 3.
Evolution of clinical, functional and haemodynamic parameters of patient 2
| Parameter | Baseline | 2 weeks later | 5 months later | 1 year after diagnosis |
| NYHA FC | IV | III | II | IV |
| 6MWD, m | NA | 351 | 462 | NA |
| BNP, ng·L−1 | 1083 | 159 | 63 | 747 |
| RAP, mmHg | 12 | NA | 3 | 16 |
| mPAP, mmHg | 37 | NA | 26 | 52 |
| CO, L·min−1 | 2.7 | NA | 5.8 | 2.5 |
| CI, L·min−1·m−2 | 1.9 | NA | 4.1 | 1.3 |
| PVR, WU | 11.3 | NA | 3.5 | 23 |
| PaO2, mmHg | 69 | 70 | 50 | |
| DLCO, % | NA | 41 | 41 | 25 |
6MWD: 6-min walk distance; BNP: brain natriuretic peptide; CI: cardiac index; CO: cardiac output; DLCO: diffusing capacity of the lung for carbon monoxide; FC: functional class; mPAP: mean pulmonary artery pressure; NA: not available; NYHA: New York Heart Association; PaO2: arterial oxygen tension; PVR: pulmonary vascular resistance; RAP: right atrial pressure; WU: Wood unit.
FIGURE 2.
Computed tomography images of patient 2, a) at diagnosis and b) 2 weeks later.
The initial treatment approach consisted of intravenous diuretics, dobutamine, initial monotherapy with an oral endothelin receptor antagonist and a high-dose of intravenous corticosteroids (administered as three pulses of 250 mg methylprednisolone followed by an oral dose of 1 mg·kg−1·day−1). Corticosteroids are usually not recommended for treating PVOD; however, in this case, their use was chosen based on observations of a significant inflammatory response in an animal model of PVOD induced by MMC [20]. After 2 weeks, our patient improved her functional class to reach NYHA functional class III, was weaned off dobutamine and oxygen, and her BNP level decreased to 159 ng·L−1. The 6MWD was 351 m. Radiological abnormalities also improved. Her pulmonary function test results were normal except for a reduced DLCO of 41% and a PaO2 of 69 mmHg. Over time, corticosteroids were tapered to 20 mg·day−1 while the patient continued to improve. 5 months after discharge, she was in NYHA functional class II, the 6MWD was 462 m and haemodynamics had improved with an mPAP of 26 mmHg, CI of 4 L·min−1·m−2 and PVR of 3.5 WU (table 3). However, DLCO and PaO2 remained stable. The corticosteroid dose was further reduced to 5 mg·day−1.
1 year after the initial diagnosis, the patient deteriorated and was admitted to the hospital due to right heart failure. RHC confirmed the patient's deterioration with an mPAP of 52 mmHg, CI of 1.28 L·min−1·m−2 and PVR of 23 WU (table 3). Chest HRCT showed more pronounced septal lines, ground-glass opacities and enlarged lymph nodes. Emergency treatment included dobutamine, norepinephrine, diuretics, intravenous pulses of high-dose corticosteroids and the initiation of oral phosphodiesterase type 5 inhibitors. She initially improved, but long-term follow-up was marked by recurrent episodes of right heart failure. In addition, pulmonary functional tests revealed decreased DLCO (25%) and PaO2 (50 mmHg).
A few months later (i.e. 2 years after completing treatment for anal cancer), due to refractory right heart failure she was listed for urgent lung transplantation and was successfully transplanted after spending 5 days on the waiting list. Pathologic assessment of explanted lungs confirmed the diagnosis of PVOD, revealing involvement in all three pulmonary vascular compartments, including arterial remodelling without plexiform lesions, fibrous venous occlusion and capillary congestion [19, 20].
In animal models of MMC-induced PVOD, intimal thickening and eosinophilic inflammation of pulmonary veins have been described [20]. The inflammatory toxic effect of MMC is therefore a possible explanation for the response to corticosteroids in MMC-induced PVOD. As we have previously reported, we can hypothesise a temporal evolution of histological abnormalities, namely an initial inflammatory phase which could explain a response to corticosteroids in certain patients, followed by irreversible vascular remodelling that is insensitive to corticosteroids [19].
In our experience, this inflammatory component is limited to drug-induced PVOD, but varies greatly depending on the toxic agent. Corticosteroids appear to be more effective when administered early in the course of the disease.
7 years after lung transplantation, neither PVOD nor anal cancer had recurred in our patient.
Conclusion
PVOD is a rare and fascinating form of pre-capillary PH, characterised by a distinct phenotype and a poor prognosis. Clinical, functional and radiological signs are critical to identify and guide appropriate management. The two cases presented in this review illustrate two underlying risk factors for PVOD, EIF2AK4 mutation in patient 1 and exposure to MMC in patient 2. These two clinical scenarios exemplify the natural history of PVOD and the management challenges. To date, in the absence of proven efficacy, no medical therapies can reverse the fatal nature of the disease, justifying prompt referral for lung transplantation. Further research is urgently needed to understand and potentially reverse the progressive remodelling of the small pulmonary venules and capillaries. Of note, deciphering the mechanisms involved in the EI2AK4 pathway may reveal innovative therapeutic targets.
Footnotes
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Condliffe R, Durrington C, Hameed A, et al. Clinical–radiological–pathological correlation in pulmonary arterial hypertension. Eur Respir Rev 2023; 32: 230138. No. 2: Lichtblau M, Mayer L, Gopalan D, et al. Clinical–radiological–pathological correlation in pulmonary hypertension with unclear and/or multifactorial mechanisms. Eur Respir Rev 2023; 32: 230119. No. 3: Verbelen T, Godinas L, Dorfmüller P, et al. Clinical–radiological–pathological correlation in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2023; 32: 230149.
Number 4 in the Series “Clinical–radiological–pathological correlation in pulmonary hypertension” Edited by Robin Condliffe, Anton Vonk Noordegraaf, Olivier Sitbon, Peter Dorfmüller and Deepa Gopalan
This article has an editorial commentary: https://doi.org/10.1183/16000617.0237-2023
Conflict of interest: B. Lechartier reports travel support from Janssen and advisory board participation with MSD, outside the submitted work. A. Boucly reports grants from Acceleron, Janssen and MSD; lecture honoraria from Janssen, MSD, AOP Orphan and Ferrer; and travel support from Janssen, MSD and Ferrer, outside the submitted work. D. Montani reports grants from Acceleron, Janssen and MSD; consulting fees from Acceleron, Merck MSD, Janssen and Ferrer; and lecture honoraria from Bayer, Janssen, Boehringer, Chiesi, GSK, Ferrer and Merck MSD, outside the submitted work. All other authors have nothing to disclose.
References
- 1.Montani D, Lau EM, Dorfmüller P, et al. Pulmonary veno-occlusive disease. Eur Respir J 2016; 47: 1518–1534. doi: 10.1183/13993003.00026-2016 [DOI] [PubMed] [Google Scholar]
- 2.Mandel J, Mark EJ, Hales CA. Pulmonary veno-occlusive disease. Am J Respir Crit Care Med 2000; 162: 1964–1973. doi: 10.1164/ajrccm.162.5.9912045 [DOI] [PubMed] [Google Scholar]
- 3.Montani D, Price LC, Dorfmüller P, et al. Pulmonary veno-occlusive disease. Eur Respir J 2009; 33: 189–200. doi: 10.1183/09031936.00090608 [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lantuéjoul S, Sheppard MN, Corrin B, et al. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. Am J Surg Pathol 2006; 30: 850–857. doi: 10.1097/01.pas.0000209834.69972.e5 [DOI] [PubMed] [Google Scholar]
- 7.Hadinnapola C, Bleda M, Haimel M, et al. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136: 2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weatherald J, Dorfmüller P, Perros F, et al. Pulmonary capillary haemangiomatosis: a distinct entity? Eur Respir Rev 2020; 29: 190168. doi: 10.1183/16000617.0168-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montani D, Dorfmüller P, Girerd B, et al. Natural history over 8 years of pulmonary vascular disease in a patient carrying biallelic EIF2AK4 mutations. Am J Respir Crit Care Med 2018; 198: 537–541. doi: 10.1164/rccm.201802-0317LE [DOI] [PubMed] [Google Scholar]
- 10.Woerner C, Cutz E, Yoo S-J, et al. Pulmonary venoocclusive disease in childhood. Chest 2014; 146: 167–174. doi: 10.1378/chest.13-0172 [DOI] [PubMed] [Google Scholar]
- 11.Montani D, Achouh L, Dorfmüller P, et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine 2008; 87: 220–233. doi: 10.1097/MD.0b013e31818193bb [DOI] [PubMed] [Google Scholar]
- 12.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46: 65–69. doi: 10.1038/ng.2844 [DOI] [PubMed] [Google Scholar]
- 13.Girerd B, Lau E, Montani D, et al. Genetics of pulmonary hypertension in the clinic. Curr Opin Pulm Med 2017; 23: 386–391. doi: 10.1097/MCP.0000000000000414 [DOI] [PubMed] [Google Scholar]
- 14.Girerd B, Montani D, Jaïs X, et al. Genetic counselling in a national referral centre for pulmonary hypertension. Eur Respir J 2016; 47: 541–552. doi: 10.1183/13993003.00717-2015 [DOI] [PubMed] [Google Scholar]
- 15.Donnelly N, Gorman AM, Gupta S, et al. The eIF2α kinases: their structures and functions. Cell Mol Life Sci 2013; 70: 3493–3511. doi: 10.1007/s00018-012-1252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montani D, Girerd B, Jaïs X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. doi: 10.1016/S2213-2600(16)30438-6 [DOI] [PubMed] [Google Scholar]
- 17.Trobaugh-Lotrario AD, Greffe B, Deterding R, et al. Pulmonary veno-occlusive disease after autologous bone marrow transplant in a child with stage IV neuroblastoma: case report and literature review. J Pediatr Hematol 2003; 25: 405–409. doi: 10.1097/00043426-200305000-00011 [DOI] [PubMed] [Google Scholar]
- 18.Ranchoux B, Günther S, Quarck R, et al. Chemotherapy-induced pulmonary hypertension: role of alkylating agents. Am J Pathol 2015; 185: 356–371. doi: 10.1016/j.ajpath.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 19.Certain M-C, Chaumais M-C, Jaïs X, et al. Characteristics and long-term outcomes of pulmonary venoocclusive disease induced by mitomycin C. Chest 2021; 159: 1197–1207. doi: 10.1016/j.chest.2020.09.238 [DOI] [PubMed] [Google Scholar]
- 20.Perros F, Günther S, Ranchoux B, et al. Mitomycin-induced pulmonary veno-occlusive disease: evidence from human disease and animal models. Circulation 2015; 132: 834–847. doi: 10.1161/CIRCULATIONAHA.115.014207 [DOI] [PubMed] [Google Scholar]
- 21.Hackman RC, Madtes DK, Petersen FB, et al. Pulmonary venoocclusive disease following bone marrow transplantation. Transplantation 1989; 47: 989–992. doi: 10.1097/00007890-198906000-00014 [DOI] [PubMed] [Google Scholar]
- 22.Steward CG, Pellier I, Mahajan A, et al. Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol 2004; 124: 63–71. doi: 10.1046/j.1365-2141.2003.04739.x [DOI] [PubMed] [Google Scholar]
- 23.Montani D, Lau EM, Descatha A, et al. Occupational exposure to organic solvents: a risk factor for pulmonary veno-occlusive disease. Eur Respir J 2015; 46: 1721–1731. doi: 10.1183/13993003.00814-2015 [DOI] [PubMed] [Google Scholar]
- 24.Caliez J, Riou M, Manaud G, et al. Trichloroethylene increases pulmonary endothelial permeability: implication for pulmonary veno-occlusive disease. Pulm Circ 2020; 10: 2045894020907884. doi: 10.1177/2045894020907884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rounds S, Lu Q. Cigarette smoke alters lung vascular permeability and endothelial barrier function (2017 Grover Conference Series). Pulm Circ 2018; 8: 2045894018794000. doi: 10.1177/2045894018794000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorfmüller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007; 38: 893–902. doi: 10.1016/j.humpath.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 27.Johnson SR, Patsios D, Hwang DM, et al. Pulmonary veno-occlusive disease and scleroderma associated pulmonary hypertension. J Rheumatol 2006; 33: 2347–2350. [PubMed] [Google Scholar]
- 28.Dorfmüller P, Montani D, Humbert M. Beyond arterial remodelling: pulmonary venous and cardiac involvement in patients with systemic sclerosis-associated pulmonary arterial hypertension. Eur Respir J 2010; 35: 6–8. doi: 10.1183/09031936.00081009 [DOI] [PubMed] [Google Scholar]
- 29.Laveneziana P, Montani D, Dorfmüller P, et al. Mechanisms of exertional dyspnoea in pulmonary veno-occlusive disease with EIF2AK4 mutations. Eur Respir J 2014; 44: 1069–1072. doi: 10.1183/09031936.00088914 [DOI] [PubMed] [Google Scholar]
- 30.Montani D, Savale L, Natali D, et al. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J 2010; 31: 1898–1907. doi: 10.1093/eurheartj/ehq170 [DOI] [PubMed] [Google Scholar]
- 31.Seferian A, Helal B, Jaïs X, et al. Ventilation/perfusion lung scan in pulmonary veno-occlusive disease. Eur Respir J 2011; 40: 75–83. doi: 10.1183/09031936.00097911 [DOI] [PubMed] [Google Scholar]
- 32.Bailey CL, Channick RN, Auger WR, et al. “High probability” perfusion lung scans in pulmonary venoocclusive disease. Am J Respir Crit Care Med 2000; 162: 1974–1978. doi: 10.1164/ajrccm.162.5.2003045 [DOI] [PubMed] [Google Scholar]
- 33.Resten A, Maitre S, Humbert M, et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. Am J Roentgenol 2004; 183: 65–70. doi: 10.2214/ajr.183.1.1830065 [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre B, Kyheng M, Giordano J, et al. Dual-energy CT lung perfusion characteristics in pulmonary arterial hypertension (PAH) and pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis (PVOD/PCH): preliminary experience in 63 patients. Eur Radiol 2022; 32: 4574–4586. doi: 10.1007/s00330-022-08577-x [DOI] [PubMed] [Google Scholar]
- 35.Holcomb BW, Loyd JE, Ely EW, et al. Pulmonary veno-occlusive disease: a case series and new observations. Chest 2000; 118: 1671–1679. [PubMed] [Google Scholar]
- 36.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. doi: 10.1161/CIRCULATIONAHA.109.911818 [DOI] [PubMed] [Google Scholar]
- 38.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 2012; 142: 448–456. doi: 10.1378/chest.11-1460 [DOI] [PubMed] [Google Scholar]
- 39.Bergbaum C, Samaranayake CB, Pitcher A, et al. A case series on the use of steroids and mycophenolate mofetil in idiopathic and heritable pulmonary veno-occlusive disease: is there a role for immunosuppression? Eur Respir J 2021; 57: 2004354. doi: 10.1183/13993003.04354-2020 [DOI] [PubMed] [Google Scholar]
- 40.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]