Abstract
Background:
The prevalence of tuberculosis (TB)-associated pulmonary hypertension (PH) has not previously been quantified, resulting in an underappreciated burden of disease. We aimed to estimate the prevalence of PH in post-TB and active TB populations.
Methods:
In this systematic review and meta-analysis, we searched PubMed/Medline, Cochrane Library, EBSCOhost, Scopus, African Journals Online and Google Scholar, with no language restriction, for available literature published after 1950. Eligible studies described adult participants (≥16 years), with documented evidence of active or prior TB, diagnosed with PH. Study quality was assessed using a risk of bias tool specifically developed for prevalence studies. Aggregate prevalence estimates with 95% confidence intervals were synthesised using a random-effects meta-analysis model, incorporating the Freeman–Tukey transformation. Subgroup analysis was conducted to ascertain prevalence estimates in specific patient populations.
Results:
We identified 1452 unique records, of which 34 met our inclusion criteria. 23 studies, with an acceptable risk of bias and where PH was diagnosed at right heart catheterisation or echocardiography, were included in the meta-analysis. In post-TB studies (14/23), the prevalence of PH was 67.0% (95% CI 50.8–81.4) in patients with chronic respiratory failure, 42.4% (95% CI 31.3–54.0) in hospitalised or symptomatic patients and 6.3% (95% CI 2.3–11.8) in nonhealthcare-seeking outpatients (I2=96%). There was a lower estimated prevalence of PH in studies of populations with active TB (9.4%, 95% CI 6.3–13.0), I2=84%).
Conclusion:
Our results highlight the significant burden of PH in post-TB and active TB populations. We emphasise the need for increased recognition of TB-associated PH and additional high-quality prevalence data.
Tweetable abstract
This systematic review and meta-analysis highlights the significant burden of pulmonary hypertension in post-tuberculosis and active tuberculosis populations. An increased recognition of tuberculosis-associated pulmonary hypertension is needed. https://bit.ly/47NnTkC
Introduction
Tuberculosis (TB) contributes substantially to the global burden of disease [1]. In 2021, there were an estimated 10.6 million incident TB cases and TB remains one of the leading causes of death from a single infectious agent worldwide [1, 2]. Global TB efforts have traditionally focused on a microbiological cure, resulting in an improved treatment success rate and increasing the number of TB survivors [3]. Despite these advancements, the significant long-term morbidity and mortality of TB-associated sequelae has only recently started to be appreciated [4]. In addition to the permanent lung damage that frequently results from active and previously treated pulmonary TB, pulmonary vascular disease is likely an under-recognised and significant TB-associated complication [5–7].
Pulmonary hypertension (PH), defined by a mean pulmonary arterial pressure (mPAP) greater than 20 mmHg at rest, is estimated to affect 1% of the global population, with more than 80% of people living with PH residing in low- and middle-income countries (LMICs) [8]. PH is classified into five heterogenous clinical groups based on pathophysiology, clinical presentation and haemodynamic profile [9]. Irrespective of the underlying aetiology and clinical group, the development of PH is often associated with poor outcomes and increased mortality [8, 9]. Both active and previously treated pulmonary TB are associated with the development of PH, possibly through distinct pathophysiological mechanisms [10–13]. In high TB burden countries, post-TB lung disease (PTLD) has been found to be one of the most common causes of group III PH (PH associated with lung diseases and/or hypoxia) and, when cohorts of patients with PH from African countries were studied, previous or current TB was identified in 23% of patients [14, 15]. Despite the acknowledged clinical correlation between TB, PTLD and PH, TB is rarely cited as a causative condition for PH and the magnitude of the TB-associated PH burden is ill-defined [7].
TB disproportionately affects LMICs, where more than 90% of incident TB cases occur and the majority of an estimated 155 million global survivors of TB reside [1, 3]. In these resource-constrained and high TB burden countries, the diagnosis of PH presents unique challenges, resulting in the under-detection and under-reporting of PH [16]. Right heart catheterisation (RHC) is the gold standard for the confirmation and classification of PH; however, this invasive and specialised procedure is infrequently available in under-resourced hospitals [14]. In these clinical settings, other imaging and clinical criteria are used as surrogate diagnostic tools for identifying PH and, as a result, TB-associated PH may be underappreciated [16].
The global prevalence of TB-associated PH has not previously been quantified and needs to be determined in order to appreciate the burden of this disease entity. Given that TB-associated PH may develop in young, economically active patients and often has a poor prognosis, the impact of this disease on communities is likely substantial [7, 17, 18]. Knowledge of the prevalence of TB-associated PH will quantify the burden of PH in TB and post-TB populations, assist in the development of targeted screening algorithms and, ultimately, promote the transition towards earlier identification and appropriate intervention. We aimed to estimate, through critical evaluation of the available literature, the prevalence of PH in post-TB and active TB populations.
Methods
Search strategy and eligibility criteria
For this systematic review and meta-analysis, we conducted an extensive search, with no language restriction, of the available literature published after 1950 indexed in electronic databases (Medline accessed via PubMed, Cochrane Library, EBSCOhost, Scopus and African Journals Online). Additionally, we conducted supplementary handsearching of grey literature, including Google Scholar, university literature and the reference lists of other included studies. Our search strategy is available in the supplementary material (appendix S1).
We included studies where participants (≥16 years old) had a history of previous TB and had completed anti-TB treatment at the time of diagnosis of PH (the post-TB group) or were diagnosed with active TB and were on anti-TB treatment at the time of PH diagnosis (the active TB group). These two groups were analysed separately. We excluded studies where participants had other significant predisposing conditions for the development of PH or documented PH pre-dating the diagnosis of pulmonary TB. HIV was not an exclusion criterion in this systematic review; however, this data was extracted and is reported in our results.
Due to the wide variation in the diagnosis of PH, for the purpose of inclusion in this prevalence review, PH was broadly defined to prioritise sensitivity and to include settings where specialised centres with RHC are not available. Therefore, the diagnosis of PH was taken “as defined by the authors”. The strength and reliability of the diagnosis of PH (RHC measurements, echocardiography parameters, ECG, radiography and clinical criteria) was assessed and, for the meta-analysis, only data from studies with RHC or transthoracic echocardiography diagnoses were included.
Studies with no clear numerator or denominator, case series with fewer than 10 cases and commentary or review articles were excluded from this prevalence review. Published abstracts were not excluded, but authors were contacted for further information and, if a more comprehensive text was available, this was used. Similarly, where data were duplicated the more detailed text was used.
Study selection and data extraction
All titles and abstracts retrieved during electronic searches were downloaded and managed on the Rayyan online platform, a collaborative web-based tool [19]. Abstracts were screened by two researchers for eligibility, using pre-determined criteria and researchers were blinded until the initial screening was completed. A third researcher with experience in the field resolved any disagreement regarding the inclusion of studies. Full texts were only considered unavailable after two university librarians had contacted other local and international libraries without success. Included articles were translated prior to data extraction, using both online translation as well as professional translation services when necessary. Study selection is reported using the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]. Our protocol is available in the PROSPERO online database (reference number CRD42023395311).
Data were extracted onto standardised forms and managed using Microsoft Excel software. The data-extraction process was completed by one researcher and independently reviewed by another researcher. The study characteristics, study population and basis of TB and PH diagnosis were collected. A full list of the variables collected is available in the supplementary material (appendix S2).
Quality assessment
An assessment of the risk of bias of the included articles, as well as the overall risk of bias of the evidence, was conducted using a modified version of the risk of bias tool formulated by Hoy et al. [21]. Items and examples from this risk assessment are outlined in the supplementary material (table S1). Studies classified as “low risk” per risk of bias item were allocated zero points and one point if “high risk”, allowing for an overall risk of bias score out of 10. Publication bias was assessed graphically using Doi plots and the LFK index is reported.
Data analysis
Data incorporated into the meta-analysis were combined using a random-effects model to account for inter-study heterogeneity and appropriate packages from STATA software version 17 (STATA Corporation, College Station, TX, USA) and R software version 4.2 (R Foundation for Statistical Computing, Vienna, Austria) were used. The prevalence data from eligible studies were combined using the metaprop_one package in STATA to produce prevalence estimates with 95% confidence intervals (CIs) and displayed using forest plots. The Freeman–Tukey double arcsine transformation of populations was incorporated to stabilise the variance from small studies. The DerSimonian and Laird method was used for weighting and 95% CI values were measured using the binominal exact method.
A subgroup analysis was completed for studies from the post-TB and active TB groups according to patient population and clinical setting. The post-TB group was subdivided into three populations, considering disease severity, presentation to healthcare with symptoms and clinical setting. Studies including participants on home oxygen therapy, or where there was documented chronic respiratory failure, were categorised into the first subgroup. Studies including hospitalised patients or symptomatic patients presenting to outpatient departments comprised the second subgroup. Finally, community-based studies including nonhealthcare-seeking participants were separated into a third subgroup. The active TB population was divided into two groups according to clinical setting.
Heterogeneity between studies and subgroups was tested using Cochran's Q statistics and the Higgins I2 statistic (a value of ≥75% variability was considered as high heterogeneity). Sensitivity analysis was conducted for the post-TB group, sequentially omitting potential factors (HIV, smoking, diagnostic cut-off at echocardiography, risk of bias and publication date) that could alter results. Sensitivity analysis in the active TB group adopted a leave-one-out method, which iteratively omitted individual studies from the meta-analysis to assess change in the overall estimate. In addition to the prevalence data analysed, characteristics were compared between the PH post-TB and non-PH post-TB groups using odds ratios and mean differences. Only articles with separate data in each group were eligible for this analysis. Pooled odds ratios and mean differences (with 95% CI) were synthesised using a random-effects model and the metan command in STATA. For the reporting of continuous data, the metamean package in R software was used, incorporating a random-effects model. This package allowed for minimal loss of data by approximating means from other measures of dispersion where means were not available (by internally using the functions described by Shi et al. [22]) allowing us to report pooled means with 95% CI. For single-value transformations, the formulae described by Wan et al. [23] were used for computation.
Results
Study selection and general characteristics
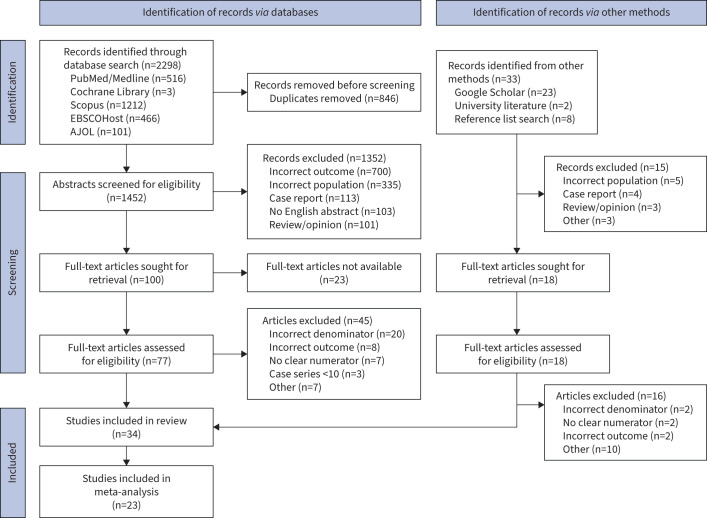
Our search yielded 2298 records, of which 846 duplicate records were removed (figure 1). 1452 abstracts and titles were screened for eligibility, including abstracts from published articles, conference abstracts and published abstracts. Based on our eligibility criteria, we excluded 1352 records which were not relevant to our systematic review. A further 33 records were identified by manual search of the grey literature (Google Scholar and university literature) and from the screening of citations. A total of 95 full-text articles were assessed for eligibility. Articles assessed to have the incorrect denominator or no clear numerator were excluded as these missing elements would have precluded us from finding prevalence estimates [13, 14, 24, 25].
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of studies included in the systematic review and meta-analysis. AJOL: African Journals Online.
The characteristics of included studies and the sample populations are shown in the supplementary material (table S2). Studies included originated from a total of 16 countries, with the largest number of studies originating from India (7/34, 20.6%) and Japan (4/34, 11.8%). When considered according to World Health Organization regions, the Western Pacific region (11/34, 32.4%), South East Asian region (8/34, 23.5%) and African region (7/34, 20.6%) comprised more than two-thirds of the literature included. Included studies’ publication dates ranged from 1961 to 2023; however, more than 70% of studies were published after 2000.
The population sampled in the included studies consisted of hospitalised patients only (13/34, 38.2%) or a combination of hospitalised and outpatients (11/34, 32.4%). 10 out of the 34 (29.4%) studies were conducted in outpatients only, of which 2/34 (5.9%) used random sampling to select and screen participants. Retrospective cohort studies were the most frequent study design, comprising 15/34 (44.1%) studies, followed by cross-sectional studies (10/34, 29.4%).
Age was reported in 29/34 (85.3%) studies and yielded a pooled mean age of 48.1 years (95% CI 43.2–53.1). There was a male predominance in 22/28 (78.6%) studies where sex was reported, with a pooled male prevalence of 58.3% (95% CI 53.6–63.0). Data on the inclusion of participants living with HIV was noted in 11/34 (32.4%) studies and HIV was used as an exclusion criterion in four of these 11 studies. The pooled prevalence of HIV in the population of the remaining seven studies was 13.0% (95% CI 5.1–23.7).
Articles were grouped into the post-TB (n=17) and active TB (n=17) groups as defined in our methods section. The details of the included TB population, diagnostic measure of PH and reported prevalence of TB-associated PH of the two groups are available in table 1. In three studies from the active TB group, the diagnosis of cor pulmonale was made post mortem, limiting their inclusion in the meta-analysis [26–28]. Additionally, one study that met our inclusion criteria was inappropriate for inclusion in the meta-analysis due to the limited population it studied. This study describes 33 cases of TB-associated fibrosing mediastinitis and PH was detected in 20/33 (60.6%) cases [29].
TABLE 1.
Tuberculosis (TB) and post-TB sample populations and prevalence of pulmonary hypertension (PH) for studies in the systematic review (n=34)
| Study | TB population included in study | Investigation used to detect PH | Risk of bias | Sample size |
Prevalence of
TB-associated PH, % (95% CI) # |
| Post-tuberculosis studies (n=17) | |||||
| Ahmed et al. [57] | Microbiologically confirmed previous TB Completed ATT Presented with dyspnoea |
Echocardiography | 3 | 30 | 46.67 (28.34–65.67) |
| Akkara et al. [58] | Completed ATT Presented with symptoms |
Echocardiography | 4 | 264 | 27.27 (21.99–33.07) |
| Jafri et al. [32] | Microbiologically confirmed previous TB Completed ATT Respiratory failure in 91% |
Echocardiography | 4 | 90 | 88.89 (80.51–94.54) |
| Jo et al. [18] | TDL: history of previous TB + Radiological findings of destroyed lung parenchyma Including patients on home oxygen therapy |
Echocardiography | 4 | 195 | 18.97 (55.66–81.66) |
| Kalla et al. [50] | Completed ATT Non-healthcare-seeking Randomly selected from TB registry |
Echocardiography | 1 | 20 | 0.00 (0.00–16.84) |
| Kuriyama et al. [37] | History of previous TB + Post-TB pulmonary sequalae |
Right heart catheterisation | 5 | 86 | 67.44 (56.48–77.16) |
| Lee et al. [59]¶ | TDL: history of previous TB + Radiological findings of destroyed lung parenchyma |
CT chest | 7 | 93 | 46.24 (35.84–56.88) |
| Louw et al. [30] | Completed ATT Non-healthcare-seeking Randomly selected from TB registry |
Echocardiography | 1 | 100 | 9.00 (4.20–16.40) |
| Mkoko et al. [60]¶ | Previous TB + Chronic lung disease |
ECG/clinical examination | 6 | 173 | 21.90 (11.98–31.62) |
| Moneger et al. [36] | Microbiologically confirmed previous TB Respiratory failure in 65% |
Right heart catheterisation | 5 | 58 | 43.10 (30.16–56.77) |
| Nicola et al. [61] | Previous TB + Post-TB pulmonary sequalae |
CXR/clinical examination | 7 | 362 | 17.13 (13.39–21.41) |
| Nishimura et al. [62] | History of previous TB + Post-TB pulmonary sequalae Home oxygen therapy for at least 6 months |
Echocardiography | 4 | 31 | 35.48 (19.23–54.63) |
| Rajeev [31] | History of previous TB + Post-TB pulmonary sequelae on CXR |
Echocardiography | 4 | 100 | 44.00 (34.08–54.28) |
| Sasaki et al. [35] | History of previous TB + Post-TB pulmonary sequalae 86% on home oxygen therapy |
Right heart catheterisation | 4 | 21 | 95.24 (76.18–99.88) |
| Singh et al. [63] | Completed ATT + Respiratory symptoms |
Echocardiography | 3 | 94 | 45.74 (35.42–56.34) |
| Singhal et al. [64] | TDL: history of previous TB + Radiological findings of destroyed lung parenchyma |
Echocardiography | 5 | 44 | 54.55 (38.85–69.61) |
| Tomono [38] | History of previous TB + Post-TB pulmonary sequalae + Chronic respiratory failure |
Right heart catheterisation | 4 | 78 | 67.69 (45.98–68.81) |
| Active tuberculosis studies (n=17) | |||||
| Al Obaidy et al. [65] | Microbiologically confirmed TB | Echocardiography | 3 | 50 | 8.00 (2.22–19.23) |
| Dasti et al. [66] | Microbiologically confirmed TB | Echocardiography | 4 | 100 | 3.00 (0.62–8.52) |
| Desalu et al. [67] | Medical records | Medical records | 5 | 108 | 12.96 (7.27–20.79) |
| Fawibe et al. [68] | Microbiologically confirmed TB Complicated by unilateral lung destruction |
Echocardiography Clinical examination |
5 | 74 | 18.92 (10.75–29.70) |
| Kang et al. [69] | Medical records | Medical records | 2 | 355 929 | 2.59 (2.54–2.65) |
| Liu et al. [29] | Microbiologically confirmed TB, previous TB or TB exposure with suggestive radiographic findings |
Echocardiography | 3 | 33 | 60.61 (42.14–77.09) |
| Marjani et al. [70] | Microbiologically confirmed TB Current ATT |
Echocardiography | 3 | 777 | 9.52 (7.55–11.81) |
| Meressa et al. [71] | Drug-resistant TB | Echocardiography Clinical examination |
5 | 612 | 5.23 (3.60–7.30) |
| Nigam et al. [72] | Microbiologically confirmed TB | CXR/ECG/clinical examination | 4 | 139 | 17.27 (11.39–24.59) |
| Tiwari et al. [17] | Current ATT | Echocardiography | 4 | 728 | 14.00 (11.82–17.04) |
| Todoroff et al. [73] | Clinical findings | CXR/ECG/clinical examination | 6 | 1492 | 17.43 (15.53–19.45) |
| Vasavi et al. [74] | Diagnosis of TB by treating physician | Echocardiography | 2 | 50 | 12.00 (4.53–24.31) |
| Wen-ting et al. [10] | Microbiologically confirmed TB Current ATT |
Echocardiography | 3 | 121 | 14.88 (9.06–22.49) |
| Wibowo et al. [75] | Drug-resistant TB | Echocardiography | 4 | 65 | 4.62 (0.96–12.90) |
| Levinsky [26] | TB at time of death | Autopsy | 6 | 201 | 22.89 (17.27–29.32) |
| Oh et al. [27] | Microbiologically confirmed TB or clinical findings | Cause of death according to medical records | 6 | 55 | 9.09 (3.02–19.95) |
| Salami et al. [28] | Microbiologically confirmed TB | Cause of death according to medical records | 5 | 202 | 10.89 (6.95–16.02) |
ATT: anti-TB treatment; CT: computed tomography; CXR: chest radiography; TDL: TB-destroyed lung. #: 95% confidence interval calculated using the Clopper–Pearson (binomial exact) method. ¶: Lee et al. [58]: prevalence range of 46.2–73.1% according to different CT criteria used; Mkoko et al. [60]: prevalence range of 21.9%–24.7% according to different ECG criteria used.
For the remaining 30 studies (17 post-TB and 13 active TB), we assessed the reliability of the diagnoses of TB and PH prior to inclusion in the meta-analysis (supplementary material, table S3). All studies used one or more criteria to define previous or active TB, with 15/30 (50%) studies specifying the use of established guidelines in the diagnosis of TB. The most frequent primary investigation used to detect PH was echocardiography (19/30 studies, 63.3%), although RHC was used in 4/17 (23.5%) studies including participants with previous TB. The estimated pulmonary artery systolic pressure (PASP) value at echocardiography used as a cut-off point by the authors to diagnose PH differed between studies, including values of 30 mmHg, 35 mmHg and 40 mmHg. However, in 12/19 (63.2%) studies a PASP of greater or equal to 40 mmHg was used to diagnose PH and 12/19 (63.2%) studies referred to using established echocardiography guidelines in their methods section. Three of the four studies which made use of RHC in the diagnosis of PH reported their haemodynamic findings and all three used an mPAP of 20 mmHg or greater at rest to establish a diagnosis of PH.
Quality assessment
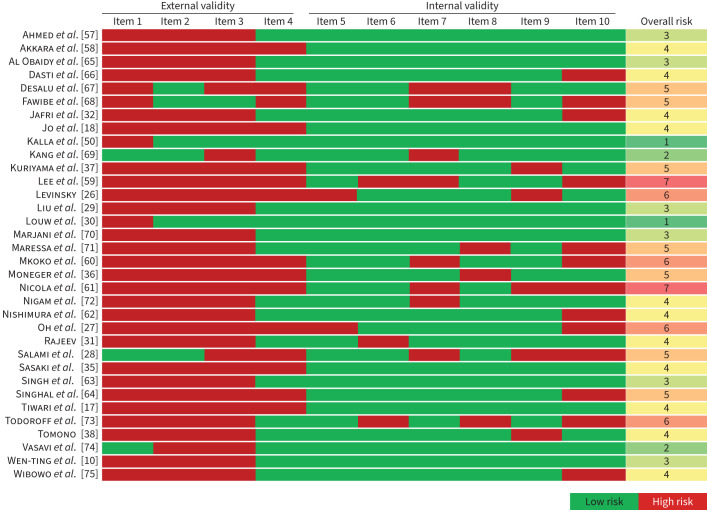
The risk of bias assessment for all included studies is summarised in figure 2. The included articles showed a moderate to high risk of bias, with a median overall risk of bias score of 4 (range 1–7). There was a higher risk of bias when articles were assessed for external validity in comparison to internal validity. Only studies with a risk of bias score ≤5 were included in the meta-analysis. Asymmetry of study effect was more notable in post-TB studies than in active TB studies (supplementary material, figure S1), with corresponding LFK indices of 1.0 and 0.3, respectively.
FIGURE 2.
Risk of bias assessment for all studies included in the systematic review. Based on the tool described by Hoy et al. [21].
Meta-analysis of the prevalence of PH in post-TB studies
14 studies where the diagnosis of PH was made at echocardiography (n=10) or RHC (n=4) were included in the meta-analysis. These 14 studies included a pooled sample of 1069 participants, with a pooled mean age of 52.5 years (95% CI 46.2–58.8) and a male predominance of 62.3% (95% CI 54.2–70.0). Data on smoking status was available in 7/14 (50%) studies, where it was an exclusion criterion in two studies and in the remaining five studies there was a pooled prevalence of smoking (previous or current) of 37.8% (95% CI 15.0–63.7). In 7/14 (50%) studies, data on COPD were available and COPD was an exclusion criterion in 4/7 (57%) of these studies, not listed as a comorbid condition in 1/7 (14%) study and had a prevalence of 11.9% (95% CI 7.6–17.0) in the remaining studies.
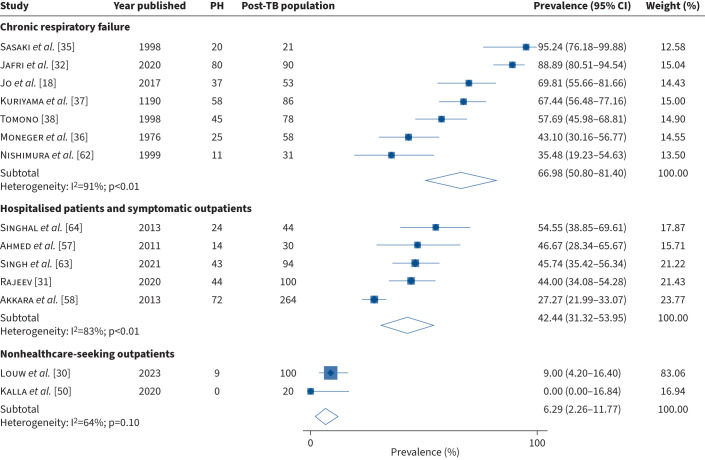
There was an overall pooled prevalence of PH post-TB of 48.1% (95% CI 33.4–63.0); with a high heterogeneity between studies (I2=96%, p<0.01) (supplementary material, figure S2). Subgroup analysis was performed according to the subgroups set out in our methods. In studies including participants with chronic respiratory failure, we found a pooled prevalence of PH post-TB of 67.0% (95% CI 50.8–81.4) with high heterogeneity between studies (I2=91%; p<0.01) and a prevalence estimate of 42.4% (95% CI 31.3–54.0) (I2=83%; p<0.01) in hospitalised patients and symptomatic outpatients (figure 3). Only 2/14 (14.3%) studies reported prevalence data in nonhealthcare-seeking outpatients, with a pooled prevalence of PH post-TB of 6.3% (95% CI 2.3–11.8) (I2=64%, p=0.10).
FIGURE 3.
The prevalence of pulmonary hypertension (PH) in post-tuberculosis (TB) studies: subgroup analysis.
There was a moderate risk of bias amongst the 14 included studies (median risk of bias score 4; range 1–5). Sensitivity analysis showed that removing studies with a higher risk of bias resulted in a lower overall prevalence whereas the removal of studies with smokers or HIV participants had little effect on the overall estimates (supplementary material, table S4). In four studies where PH was diagnosed by echocardiography, the mean PASP was reported, yielding a pooled mean PASP of 58.6 mmHg (95% CI 52.8–64.4) in these studies [18, 30–32].
Data on the duration since the previous episode of TB was available in 8/14 (57.1%) studies, with a pooled mean time from TB to PH diagnosis of 12.4 years (95% CI 2.3–22.6). When participants with PH post-TB were compared to non-PH post-TB participants, there was a shorter duration between the previous episode of TB and the current assessment with a mean difference of −1.3 years (95% CI −2.5 to −0.2; p=0.03) (I2=0%). Additionally, we found a lower mean forced expiratory volume in 1 s/forced vital capacity (FVC) (%) and a lower mean FVC (% predicted) in those with PH post-TB when compared to the non-PH post-TB participants with mean differences of −2.8% (95% CI −7.0–1.3; p=0.18) (I2=0%) and −9.0 (95% CI −20.8–2.6; p=0.127) (I2=87%), respectively (supplementary material, figures S3a–S3c). The pooled odds ratio of being male and a current or previous smoker were 0.9 (0.2–3.7; p=0.91) and 0.9 (95% CI 0.3–3.2; p=0.91), respectively.
Meta-analysis of the prevalence of PH in active TB studies
In the active TB group, nine studies with an echocardiography diagnosis of PH and risk of bias score ≤5 were included in the meta-analysis. This pooled cohort consisted of 2577 participants, with a pooled mean age of 43.2 years (95% CI 36.4–50.0) and 58.8% (95% CI 51.7–65.7) of the cohort were males. The pooled HIV prevalence was 15.3% (95% CI 8.5–23.5) (n=3 studies) and 19% were previous or current smokers (95% CI 12.2–26.7) (n=5 studies).
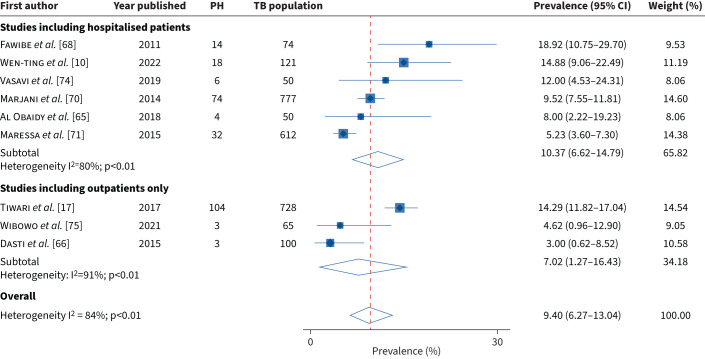
The prevalence of PH active TB ranged from 3.0% (95% CI 0.6–8.5) to 18.9% (95% CI 10.8–29.7) (supplementary material, figure S4) with an overall pooled prevalence of 9.4% (95% CI 6.3–13.0) and high heterogeneity between studies (I2=84%; p<0.01). In a subgroup analysis we found the pooled prevalence of PH active TB to be 10.4% (95% CI 6.6–14.8) (I2=80%) in studies including hospitalised patients and 7.0% (95% CI 1.3–16.4) (I2=91%) in studies including outpatients only (figure 4). No single study was noted to have a significant influence on the overall pooled estimate presented (supplementary material, figure S5).
FIGURE 4.
The prevalence of pulmonary hypertension (PH) in active tuberculosis (TB) studies: subgroup analysis.
Discussion
In this systematic review and meta-analysis, we critically evaluated the available prevalence data pertaining to TB-associated PH, using this data to generate prevalence estimates. We identified that the current body of literature reporting prevalence data on PH post-TB and PH active TB is limited. We found that the prevalence of PH post-TB is high, particularly in those with chronic respiratory failure (67.0%, 95% CI 50.8–81.4) or who are symptomatic or hospitalised (42.4%, 95% CI 31.3–54.0), and lower in nonhealthcare-seeking outpatients (6.3%, 95% CI 2.3–11.8). Overall, the prevalence was lower, yet still substantial, in active TB, where PH is estimated to affect approximately 10% of the TB population. We found that the diagnosis of PH in TB populations is made predominantly at transthoracic echocardiography without RHC confirmation and we observed a lack of uniformity in the diagnostic parameters used.
We found that the quality of data informing the prevalence of TB-associated PH is poor, limiting the accurate quantification of this disease entity. Our decision to exclude articles with a high risk of bias and those without echocardiography or RHC attempted to improve the quality of generated prevalence estimates presented. Nevertheless, in those included in the meta-analysis, we observed studies with small sample sizes, selective bias and a disproportionate representation of studies including hospitalised or symptomatic patients. This symptom-based (as opposed to population-based) sampling has been reported in other prevalence studies of PH in LMICs and is likely driven by resource constraints in these settings [33]. Although the screening of large numbers of patients with active or previous TB would not be feasible in many low-income settings, when accounting for the significant morbidity, mortality and cost implications that may be related to TB-associated PH, appropriate targeted screening may indeed have long-term cost benefits [4, 34].
We observed a high heterogeneity in the screening and diagnosis of TB-associated PH. Given that the majority of the included studies were conducted in LMICs, it is unsurprising that RHC was used in only four studies, of which three originated from Japan and one from France [35–38]. Similarly, a systematic review of the aetiologies of PH in African countries by Bigna et al. [33] found that in only one out of 21 studies the diagnosis of PH was made at RHC. The lack of availability of this gold standard to diagnose PH in the majority of studies may partially explain the shortage of available data on TB-associated PH. Unfortunately, due to inequality of global health resources, it is unlikely that widespread use of RHC will become available in contexts with the highest TB burdens in the foreseeable future. Thus, we emphasise the need for screening and diagnostic algorithms using alternative methods in these resource-limited settings. Transthoracic echocardiography is a relatively reliable noninvasive screening tool (estimated sensitivity of ∼85%; specificity of ∼74%) and was used in 19/23 (82.6%) studies included in this meta-analysis [39]. However, the use of echocardiography as a screening tool in TB and post-TB populations may have several limitations, most notably an acknowledged reduced diagnostic accuracy in patients with chronic lung disease [39]. Echocardiography may frequently overestimate PASP in patients with chronic lung disease and, although accuracy can be improved through the use of other measurements at echocardiography, these were usually not reported in the included studies [40, 41].
It is estimated that there are 155 million survivors of TB globally [3]. Our results show a high prevalence of PH within this population. However, estimates varied according to patient population and clinical setting and were analogous to the severity of PTLD, defined as “evidence of chronic respiratory abnormality attributable at least in part to previous TB” [42]. PTLD may be the cause of 15–30% of PH cases in high TB burden areas [15, 43]. Our prevalence estimates (42–65% in symptomatic patients) are comparable to other significant chronic lung diseases that are associated with group III PH, including COPD (estimated PH prevalence in a recent meta-analysis of 39.2% overall and 62.5% in the severe group), idiopathic pulmonary fibrosis (8–50% and >60% in those awaiting lung transplant) and combined pulmonary fibrosis and emphysema (approximately 30–50%) [44–47]. In light of these findings, PTLD is likely an underappreciated contributor to group III PH globally.
Despite the high prevalence estimates of PH post-TB found in this study, TB or PTLD is rarely cited in the available PH literature. This highlights that there is a clear disparity between the real burden of PH post-TB and the acknowledged burden within the PH research community. This discrepancy has been emphasised by multiple experts in the field and can be explained by global disparities in healthcare, where the lack of specialised centres to screen for PH in low-resource settings results in the under detection of PH in TB-burden countries, as well as the fact that most PH literature stems from high-resource countries where TB is uncommon [5–7]. It is notable that even mild elevations in pulmonary pressures in other chronic lung diseases have been associated with an increased mortality [46]. A 2019 meta-analysis estimated the all-cause mortality to be 2.91 times (95% CI 2.21–3.84) higher amongst the post-TB population compared to age-matched and sex-matched controls [48]. It is possible that PH post-TB contributes significantly to this mortality estimate [49].
Post-TB PH may share several pathophysiological mechanisms with other PH-chronic lung diseases and, additionally, may have several alternative pathophysiological pathways relating to other PH groups. Extensive post-TB lung damage, destruction of the pulmonary bed with reduced cross-sectional area of the pulmonary vasculature and hypoxic-induced vascular remodelling leading to the subsequent development of PH are the most cited mechanisms [11, 12]. Additionally, TB directly affects the pulmonary vasculature, causing a vasculitis and endarteritis obliterans and may be associated with the development of chronic thromboembolic PH. It is noted that the average time from incident TB to assessment of PH was 12 years and time may be an important variable in the pathogenesis of some forms of post-TB PH. Also noteworthy is the high prevalence of post-TB PH in the fibrosing mediastinitis subgroup (not included in the meta-analysis) which is due to proliferation of excess fibrosing tissue in the mediastinum [29]. Thus, in addition to group III PH, post-TB PH may share pathophysiological mechanisms with PH groups I, IV and V, respectively [29, 50–52].
The estimated prevalence of PH active TB ranged from 3.0% to 18.9% which was lower compared to the post-TB group. This proportion is still significant, particularly since PH developing during active TB has been considered a poor prognostic indicator in certain studies [17]. Although both PH active TB and PH post-TB contribute to the overall burden of TB-associated PH, these two disease entities may indeed be distinct with different pathophysiological mechanisms and courses of disease, the former occurring during acute respiratory illness and the latter developing more distally. The pooled cohort with active TB in this systematic review had a relatively young age, were less frequently smokers than the post-TB group and, although numbers are small, had a relatively high HIV prevalence (15% versus 11% in the global TB population) [1]. Data from the Pan African Pulmonary Hypertension Cohort (PAPUCO) showed that 5–7% of PH patients had active TB at the time of PH diagnosis, rising to 13% in the HIV population [13, 14]. Although HIV may cause PH in its own right in approximately 0.5% of cases, the critical interplay between these two chronic infectious diseases and the combined effect on the pulmonary vasculature is unknown and is an important area of research [53, 54].
PH developing in active TB is likely a result of the parenchymal destruction caused by active TB disease; however, multiple other pathophysiological mechanisms may contribute to its development. Such factors may include the release of multiple inflammatory cytokines, local hypoxic vasoconstriction, in addition to the direct destructive effect of TB on the pulmonary vasculature [50, 51]. Additionally, active TB is associated with endothelial activation with the development of a pro-thrombotic state and may well induce in situ thromboses in the pulmonary circulation, thus being causatively related to acute and potentially chronic venous thromboembolic disease [55]. It is notable that in this systematic review we did not search specifically for studies with acute PH developing in the setting of an acute pulmonary embolism and this subset of patients would likely have increased the prevalence of PH in this group.
Strengths and limitations
Our study has several limitations. Most notably, although our results demonstrate a particularly high burden of PH in the post-TB population, several factors should be considered which may have overinflated these estimates. First, although COPD was an exclusion criterion in some studies, the high prevalence of smokers in the included studies means that there may have been an overlap between COPD and PTLD. However, this overlap is not unique to our study, and it should be considered that many people who are diagnosed with COPD in high TB-burden areas may also have PTLD. In the PLATINO study, when COPD patients with and without previous TB were compared, it was found that airway obstruction was observed in 30.7% of patients with a positive history of TB, while the incidence was only 13.9% in those without a TB history [56]. Second, our estimates may have been influenced by reporting bias and it is possible that studies where a low incidence of PH was found were not published. Our inclusion of the negative study by Kalla et al. [50] in the meta-analysis is notable; however, the sample size in this study was small. Third, in the majority of cases, the diagnosis of PH was not confirmed at RHC and an echocardiography diagnosis of PH has several limitations, which have been discussed. Lastly, the high number of studies reporting PH prevalence in chronically ill patients likely contributed to high prevalence estimates and we found that these studies also had a moderately high-risk of bias. Interpretation was improved using subgroup analysis; however, this was somewhat limited by the small number of studies in the subgroup of nonhealthcare-seeking outpatients.
Other limitations of this study are the small sample sizes and limited populations studied, which may limit the generalisability of the prevalence estimates presented. The marked heterogeneity between studies, which was only partially accounted for by the use of a random-effects meta-analysis model and subgroup analysis, may limit the interpretation of results. Additionally, missing data precluded the meaningful comparison of risk factors for the development of TB-associated PH. Our systematic review and meta-analysis also have certain strengths. We used a rigorous approach, employing a wide search strategy, to assess the available literature on this topic and synthesise prevalence estimates. Additionally, by adopting a broad definition of PH in our inclusion criteria, we did not exclude studies where RHC was not available and were able to gauge current diagnostic means available for PH in TB-endemic settings.
Conclusion
There are 155 million survivors of TB globally and 10.6 million new cases each year. Our results present evidence of a high prevalence of PH in both of these populations, which may comprise two disease entities, namely PH post-TB and PH active TB. The contribution of TB-associated PH to morbidity in these populations is not known but is likely substantial. Prevalence estimates of TB-associated PH vary according to patient population, lung disease severity and clinical setting. Our findings highlight the need for a heightened awareness of TB-associated PH, the establishment of universal screening guidelines in low-resource settings, in the absence of availability of RHC, and the integration of these methods to produce high-quality prevalence data.
Questions for future research
Additional high-quality prevalence data on TB-associated PH
Understanding the pathophysiological mechanisms and risk factors for TB-associated PH
Screening algorithms for PH post-TB and PH active TB in low-resource settings
Quantification of the mortality attributable to TB-associated PH
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0154-2023.SUPPLEMENT (2.5MB, pdf)
Acknowledgements
We acknowledge the librarians at the University of Cape Town and Stellenbosch University libraries for their assistance in the retrieval of articles which were not available online. We are grateful to the professional translators at SimplyTranslate and Este van Zyl (independent translator) for the translation of Japanese, Romanian and Korean articles.
Provenance: Submitted article, peer reviewed.
Data availability: The data in this article is available from the respective cited articles. Additionally, synthesised or transformed data as well as analytical code is available on request from the first author.
Author contributions: B.W. Allwood and F. Thienemann conceptualised the project. J.K. van Heerden wrote the protocol and all authors approved the final version of the protocol. J.K. van Heerden and E.H. Louw completed the screening of abstracts, full text reads and data extraction and were supervised by M.E. Engel, B.W. Allwood and F. Thienemann. All disputes over included articles were resolved by B.W. Allwood and discussed with F. Thienemann. Data analysis was done by J.K. van Heerden and M.E. Engel. All authors critically reviewed the manuscript and approved the final version.
Conflicts of interest: None to declare.
References
- 1.World Health Organization . Global tuberculosis report 2022. Date last updated: 21 April 2023. Date last accessed: 5 March 2023. www.who.int/publications/i/item/9789240061729
- 2.Ntoumi F, Nachega JB, Aklillu E, et al. World Tuberculosis Day 2022: aligning COVID-19 and tuberculosis innovations to save lives and to end tuberculosis. Lancet Infect Dis 2022; 22: 442–444. doi: 10.1016/S1473-3099(22)00142-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd PJ, Yuen CM, Jayasooriya SM, et al. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis 2021; 21: 984–992. doi: 10.1016/S1473-3099(20)30919-1 [DOI] [PubMed] [Google Scholar]
- 4.Menzies NA, Quaife M, Allwood BW, et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health 2021; 9: e1679–e1687. doi: 10.1016/S2214-109X(21)00367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allwood BW, Maarman GJ, Kyriakakis CG, et al. Post-pulmonary tuberculosis complications in South Africa and a potential link with pulmonary hypertension: premise for clinical and scientific investigations. S Afr Med J 2018; 108: 12339. doi: 10.7196/samj.2018.v108i7.13359 [DOI] [PubMed] [Google Scholar]
- 6.Raine R. Screening for pulmonary hypertension secondary to pulmonary tuberculosis. Afr J Thorac Crit Care Med 2020; 26: 128. doi:10.7196%2FAJTCCM.2020.v26i4.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh KF, Lui JK. Post-tuberculosis pulmonary hypertension: a case of global disparity in health care. Lancet Glob Health 2022; 10: e476. doi: 10.1016/S2214-109X(22)00042-0 [DOI] [PubMed] [Google Scholar]
- 8.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4: 306–322. doi: 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Kovacs G, Hoeper MM, et al. 2022. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 10.Wen-ting L, Chang-wei W, De-jian B, et al. Arterial partial pressure of oxygen and procalcitonin levels correlate with pulmonary artery systolic pressure in patients with active pulmonary tuberculosis. Int J Infect Dis 2022; 117: 87–92. doi: 10.1016/j.ijid.2022.01.060 [DOI] [PubMed] [Google Scholar]
- 11.Bousseau S, Sobrano Fais R, Gu S, et al. Pathophysiology and new advances in pulmonary hypertension. BMJ Med 2023; 2: e000137. doi: 10.1136/bmjmed-2022-000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maarman G, Lecour S, Butrous G, et al. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm Circ 2013; 3: 739–756. doi: 10.1086/674770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thienemann F, Katoto PDMC, Azibani F, et al. Long-term follow-up of human immunodeficiency virus-associated pulmonary hypertension: clinical features and survival outcomes of the Pan Africa Pulmonary Hypertension Cohort (PAPUCO). Open Forum Infect Dis 2022; 9: ofac604. doi: 10.1093/ofid/ofac604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thienemann F, Dzudie A, Mocumbi AO, et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: insights from the Pan African Pulmonary Hypertension Cohort (PAPUCO) Registry. Int J Cardiol 2016; 221: 205–211. doi: 10.1016/j.ijcard.2016.06.242 [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AR, Irfan M, Ashraf Z, et al. Pulmonary tuberculosis is often over-looked as an etiology of pulmonary hypertension but a high index of suspicion unveils the diagnosis. A retrospective study of 231–cases. Ann Punjab Med Coll 2020; 14: 302–307. doi: 10.29054/APMC/2020 [DOI] [Google Scholar]
- 16.Maarman GJ, Shaw J, Allwood B. Pulmonary hypertension in majority countries: opportunities amidst challenges. Curr Opin Pulm Med 2020; 26: 373–383. doi: 10.1097/MCP.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 17.Tiwari M, Gami SP. Pulmonary hypertension in pulmonary tuberculosis – a prognostic indicator. Eur Respir J 2017; 50: Suppl. 61, PA2432. doi: 10.1183/1393003.congress-2017.PA2432 [DOI] [Google Scholar]
- 18.Jo YS, Park JH, Lee JK, et al. Risk factors for pulmonary arterial hypertension in patients with tuberculosis-destroyed lungs and their clinical characteristics compared with patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2017; 12: 2433–2443. doi: 10.2147/COPD.S136304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65: 934–939. doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods 2020; 11: 641–654. doi: 10.1002/jrsm.1429 [DOI] [PubMed] [Google Scholar]
- 23.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu YJ, Lee JH, Chun EM, et al. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis 2011; 15: 246–250. [PubMed] [Google Scholar]
- 25.Aminde LN, Dzudie A, Kengne AP, et al. Gender disparities in pulmonary hypertension at a tertiary centre in Cameroon. S Afr Med J 2017; 107: 892–899. doi: 10.7196/SAMJ.2017.v107i10.12321 [DOI] [PubMed] [Google Scholar]
- 26.Levinsky L. Tuberculosis and cardiopulmonary failure. Dis Chest 1961; 40: 564–571. doi: 10.1378/chest.40.5.564 [DOI] [PubMed] [Google Scholar]
- 27.Oh SJ, Yoon KH, Yoo JH, et al. A survey of deaths in hospitalized patients for pulmonary tuberculosis. Tuberc Respir Dis 1993; 40: 694–699. doi: 10.4046/trd.1993.40.6.694 [DOI] [Google Scholar]
- 28.Salami A, Oluboyo P. Management outcome of pulmonary tuberculosis: a nine year review in Ilorin. West Afr J Med 2003; 22: 114–118. doi: 10.4314/wajm.v22i2.27928 [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Gao L, Xie S, et al. Clinical and imaging spectrum of tuberculosis-associated fibrosing mediastinitis. Clin Respir J 2018; 12: 1974–1980. doi: 10.1111/crj.12766 [DOI] [PubMed] [Google Scholar]
- 30.Louw E, Baines N, Maarman G, et al. The prevalence of pulmonary hypertension after successful tuberculosis treatment in a community sample of adult patients. Pulm Circ 2023; 13: e12184. doi: 10.1002/pul2.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajeev GS. Prevalence and risk factors for pulmonary artery hypertension and cor pulmonale in post tuberculosis pulmonary sequelae (Masters thesis). Tirunelveli, Tirunelveli Medical College, 2020. [Google Scholar]
- 32.Jafri S, Jawad N, Ahmed N, et al. Post pulmonary tuberculosis: the right heart story. Biol Med 2020; 12: 446. doi: 10.35248/0974-8369.20.12.466 [DOI] [Google Scholar]
- 33.Bigna JJ, Noubiap JJ, Nansseu JR, et al. Prevalence and etiologies of pulmonary hypertension in Africa: a systematic review and meta-analysis. BMC Pulm Med 2017; 17: 183. doi: 10.1186/s12890-017-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quaife M, Houben RMGJ, Allwood B, et al. Post-tuberculosis mortality and morbidity: valuing the hidden epidemic . Lancet Respir Med 2020; 8: 332–333. doi: 10.1016/S2213-2600(20)30039-4 [DOI] [PubMed] [Google Scholar]
- 35.Sasaki Y, Yamagishi F, Kounori S, et al. Survival and pulmonary hemodynamics in patients with sequelae of pulmonary tuberculosis who received antituberculosis chemotherapy and home oxygen therapy. Nihon Kokyuki Gakkai Zasshi 1998; 36: 934–938. [PubMed] [Google Scholar]
- 36.Moneger P, Oury M. Evaluation du retentissement fonctionnel des séquelles de la tuberculose pulmonaire. A propos de 112 observations. [Evaluation of functional repercussion of sequelae of pulmonary tuberculosis. A case report of 112 observations]. Poumon Coeur 1976; 32: 227–232. [PubMed] [Google Scholar]
- 37.Kuriyama T, Yasuda J. Tuberculosis sequelae: pathophysiological aspects (pulmonary circulation). Kakkaku 1990; 65: 81–91. [PubMed] [Google Scholar]
- 38.Tomono K. Tuberculous death. The causes of death of pulmonary tuberculosis: late sequelae of pulmonary tuberculosis. Kekkaku 1998; 73: 751–754. [PubMed] [Google Scholar]
- 39.Ni JR, Yan PJ, Liu SD, et al. Diagnostic accuracy of transthoracic echocardiography for pulmonary hypertension: a systematic review and meta-analysis. BMJ Open 2019; 9: e033084. doi: 10.1136/bmjopen-2019-033084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu T, Levi A, Hasdai D, et al. Preoperative evaluation of pulmonary hypertension in lung transplant candidates: echocardiography versus right heart catheterization. BMC Cardiovasc Disord 2022; 22: 53. doi: 10.1186/s12872-022-02495-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167: 735–740. doi: 10.1164/rccm.200210-1130OC [DOI] [PubMed] [Google Scholar]
- 42.Allwood BW, Van Der Zalm MM, Amaral AFS, et al. Post-tuberculosis lung health: perspectives from the First International Symposium. Int J Tuberc Lung Dis 2020; 24: 820–828. doi: 10.5588/ijtld.20.0067 [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharyya P, Saha D, Bhattacherjee P, et al. Tuberculosis associated pulmonary hypertension: the revelation of a clinical observation. Lung India 2016; 33: 135. doi: 10.4103/0970-2113.177433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shorr AF, Wainright JL, Cors CS, et al. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 2007; 30: 715–721. doi: 10.1183/09031936.00107206 [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Liu Y, Zhao S, et al. The incidence and prevalence of pulmonary hypertension in the COPD population: a systematic review and meta-analysis. Int J COPD 2022; 17: 1365–1379. doi: 10.2147/COPD.S359873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura M, Taniguchi H, Kondoh Y, et al. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration 2013; 85: 456–463. doi: 10.1159/000345221 [DOI] [PubMed] [Google Scholar]
- 48.Romanowski K, Baumann B, Basham CA, et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019; 19: 1129–1137. doi: 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 49.Park SY, Lee CY, Kim C, et al. One-year prognosis and the role of brain natriuretic peptide levels in patients with chronic cor pulmonale. J Korean Med Sci 2015; 30: 442. doi: 10.3346/jkms.2015.30.4.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalla I, Seedat F, Miri A. Occult pulmonary arterial hypertension in patients with previous pulmonary tuberculosis. Afr J Thorac Crit Care Med 2020; 26: 133. doi: 10.7196/AJTCCM.2020.v26i4.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HY, Song KS, Goo JM, et al. Thoracic sequelae and complications of tuberculosis. Radiographics 2001; 21: 839–858. doi: 10.1148/radiographics.21.4.g01jl06839 [DOI] [PubMed] [Google Scholar]
- 52.Park SY, Lee SM, Shin JW, et al. Epidemiology of chronic thromboembolic pulmonary hypertension in Korea: results from the Korean registry. Korean J Int Med 2016; 31: 305–312. doi: 10.3904/kjim.2014.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bigna JJR, Sime PSD, Koulla-Shiro S. HIV related pulmonary arterial hypertension: epidemiology in Africa, physiopathology, and role of antiretroviral treatment. AIDS Res Ther 2015; 12: 36. doi: 10.1186/s12981-015-0078-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basyal B, Jarrett H, Barnett CF. Pulmonary hypertension in HIV. Can J Cardiol 2019; 35: 288–298. doi: 10.1016/j.cjca.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 55.Moodley P, Martinson NA, Joyimbana W, et al. Venous thromboembolic disease in adults admitted to hospital in a setting with a high burden of HIV and TB. Afr J Thorac Crit Care Med 2021; 27: 99. doi: 10.7196/AJTCCM.2021.v27i3.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menezes AMB, Hallal PC, Perez-Padilla R, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J 2007; 30: 1180–1185. doi: 10.1183/09031936.00083507 [DOI] [PubMed] [Google Scholar]
- 57.Ahmed AEH, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: analysis of 14 consecutive cases. Clin Med Insights Circ Respir Pulm Med 2011; 5: 1–5. doi:10.4137%2FCCRPM.S6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akkara SA, Shah AD, Adalja M, et al. Pulmonary tuberculosis: the day after. Int J Tuberc Lung Dis 2013; 17: 810–813. doi: 10.5588/ijtld.12.0317 [DOI] [PubMed] [Google Scholar]
- 59.Lee SW, Shim SS, Ryu YJ, et al. Tuberculous-destroyed lung: cardiovascular CT findings and prognostic imaging factors. Clin Imaging 2013; 37: 1000–1005. doi: 10.1016/j.clinimag.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 60.Mkoko P, Naidoo S, Mbanga LC, et al. Chronic lung disease and a history of tuberculosis (post-tuberculosis lung disease): clinical features and in-hospital outcomes in a resource-limited setting with a high HIV burden. S Afr Med J 2019; 109: 169–173. doi: 10.7196/SAMJ.2019.v109i3.13366 [DOI] [PubMed] [Google Scholar]
- 61.Nicola V, Didilescu C. Risk factors in chronic post-tubercular cor pulmonale in the elderly. Rev Med Chir Soc Med Nat Iasi 1990; 94: 491–493. [PubMed] [Google Scholar]
- 62.Nishimura E, Ikeda S, Naito T, et al. Evaluation of right-ventricular function by Doppler echocardiography in patients with chronic respiratory failure. J Int Med Res 1999; 27: 65–73. doi: 10.1177/030006059902700202 [DOI] [PubMed] [Google Scholar]
- 63.Singh GV, Kumar S, Pratap Pandey B, et al. Pulmonary arterial hypertension in patients of healed pulmonary tuberculosis and its association with quality of life. Med Res Chronicles 2021; 8: 325–333. [Google Scholar]
- 64.Singhal A, Kumar N. Cardiac dysfunction in patients with chronic airflow obstruction due to tuberculous destroyed lung. Lung India 2013; 30: Suppl. 1, S17–S43. [Google Scholar]
- 65.Al Obaidy MW, Al Jubouri AM, Eidan MB. Pulmonary hypertension in active pulmonary tuberculosis patients. Sci Int 2018; 30: 407–416. [Google Scholar]
- 66.Dasti MA, Fasih AHS, Sajid AJM, et al. Pulmonary tuberculosis: cardiac manifestations. Prof Med J 2015; 22: 733–737. doi: 10.29309/TPMJ/2015.22.06.1240 [DOI] [Google Scholar]
- 67.Desalu OO, Ojo OO, Busari OA,et al. Pattern of respiratory diseases seen among adults in an emergency room in a resource-poor nation health facility. Pan Afr Med J 2011; 9: 24. doi:10.4314%2Fpamj.v9i1.71199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fawibe AE, Salami AK, Oluboyo PO, et al. Profile and outcome of unilateral tuberculous lung destruction in Ilorin, Nigera. West Afr J Med 2011; 30: 130–135. [PubMed] [Google Scholar]
- 69.Kang W, Du J, Yang S, et al. The prevalence and risks of major comorbidities among inpatients with pulmonary tuberculosis in China from a gender and age perspective: a large-scale multicentre observational study. Eur J Clin Microbiol 2020; 40: 787–800. doi: 10.1007/s10096-020-04077-2 [DOI] [PubMed] [Google Scholar]
- 70.Marjani M, Baghaei P, Malekmohammad M, et al. Effect of pulmonary hypertension on outcome of pulmonary tuberculosis. Braz J Infect Dis 2014; 18: 487–490. doi: 10.1016/j.bjid.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meressa D, Hurtado RM, Andrews JR, et al. Achieving high treatment success for multidrug-resistant TB in Africa: initiation and scale-up of MDR TB care in Ethiopia – an observational cohort study. Thorax 2015; 70: 1181–1188. doi: 10.1136/thoraxjnl-2015-207374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nigam P, Dubey AL, Goyal BM, et al. Cor pulmonale in pulmonary tuberculosis. Clinician 1982; 46: 406–413. [Google Scholar]
- 73.Todoroff T, Giacomuzzi F. Findings and statistics on the impact of pulmonary heart disease in patients with pulmonary tuberculosis in the pre-antibiotic and current era. Minerva Med 1961; 52: 749–754. [PubMed] [Google Scholar]
- 74.Vasavi B, Reddy BK, Suresh P, et al. Study of prevalence of cor pulmonale in patients with pulmonary tuberculosis with reference to ECG, echocardiographic changes and radiological extent of disease. J Med Dent Sci 2019; 11: 26–29. doi: 10.9790/0853-1808102629 [DOI] [Google Scholar]
- 75.Wibowo A, Burhan E, Putra AC. Proportion of pulmonary hypertension in drug resistant tuberculosis patients. Respirology 2001; 26: 447–447. doi: 10.1111/resp.14150_908 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0154-2023.SUPPLEMENT (2.5MB, pdf)