Abstract
Under-utilized orange peel waste contains natural colorants that might be used for textile dyeing. Research into orange peel waste as natural colorants provides benefits for both the agricultural and fashion industry with a creative and sustainable solution. This research performed the extraction of colorants from the orange peel as plant dyes and investigated their potential dyeing capability of silk fabrics. With full factorial analysis, we determined the optimal extraction conditions by comparing 100 % ethanol, 70 % ethanol, and water, aiming to achieve the highest absorbance for the extracted solution. Conditions obtained with the best performance include an extraction temperature of 60 °C, an extraction time of 120 min, and a material-to-liquor ratio of 1:20 (wt/vol) for both 100 % and 0 % ethanol. To attain the highest K/S values on textiles with orthogonal experimental design, the optimal dyeing profiles of silk fabrics with water solution were found to be 100 °C, 60 min, pH 3, and Liquid Ratio of 1:15. Colorfastness results of crocking, washing, and sunlight are in favor of the usage of orange peel color extracts for textile application.
Keywords: Orange peel colorants, Agriculture wastes, Natural dyeing, Silk fibers
1. Introduction
From both environmental and agricultural perspectives, government, industries, and consumers have become more and more interested in discovering innovative solutions for reducing agricultural byproducts, as they may bring negative impacts on our environments. Based on a report by the Environmental Protection Agency, agriculture/food by-product consists of approximately 22 % of total municipal solid waste generated in the United States, and 95 % of this type of waste is ultimately landfilled [1]. The idea of finding new sustainable solutions to reduce agricultural waste is on the rise so that existing resources can be repurposed in a new approach to a greater extent. The under-utilized agricultural byproducts, e.g., berry, grape, pumpkin, dragon fruit, and orange pomace waste, contain natural colorants such as carotenoid, lutein, anthocyanidin etc. These natural colorants could potentially be utilized in textile coloration to a broader scope, as a substitute to synthetic dyestuffs which are dominantly utilized in the textile coloration. Overall, creative repurposing of the agricultural byproducts as natural colorants will benefit both agricultural and fashion industry.
Oranges are one of the most popular fruits consumed and have an annual yield over 100 million tons globally. According to United States Department of Agriculture (USDA), orange production within California is 147,000 acres in 2018–2019 season, which translated to 49.8 million boxes, being 13 % higher than the previous season [2]. However, orange pomace remains as one of the major challenges in the orange juicing industry for applications. These juice processing wastes consist of peel (flavedo), pith (albedo), pulp, as well as the core and seeds (pip); and these components can take up to 45 %–60 % of the fruit [3]. The orange peel alone represents roughly 20 %–35 % of the total orange [4] and, therefore, is an abundant, cheap, and readily available biomass to expand the horizon of orange industry.
There have been some efforts to utilize orange waste in different ways. In the food industry, the rising consumer demands for “all natural” have driven the broader use of natural colorants. It was studied that orange peel was a potential resource of natural food colorant [5] Further, an investigation suggested that orange peel colors are mainly attributed to phenolic and carotenoid compounds [5]. The redness of orange peel decreases, and the yellowness increases as the concentration of the phenolic compounds becoming more dominant. Ionizing irradiation and storage increased concentration of the total phenols, and therefore the color of orange peel became more yellowish [6]. The paper suggested that scutellarein was one of phenolic compounds in orange peel and was about 0.28 mg/g dried orange peel. In addition, extraction of pigments from orange peels have mainly been carried out mostly by using organic-solvents [[7], [8], [9], [10]], although the extraction methods depend on the solvation properties of the natural extract which are mostly either aqueous extraction or organic solvent extraction.
It is expected that orange peel colorants have potentials to be repurposed for textile materials’ coloration. With a bright orange color, extracts from orange peel could be an attractive dyestuff for various dyeing applications of textiles. Hou [11] investigated UV-protective properties and antimicrobial properties of orange peel extractions. Water extraction orange peel were applied to wool fabric dyeing. In addition, they analyzed the water extracted, with main-colored compounds as phenolic colorants and pectin by UV–visible spectroscopy and FTIR (Fourier transform infrared spectroscopy). Wool fabrics were dyed by orange colorants with pre-, one-bath and post-mordanting method. In another research, Yi identified flavonoids as the main colorant extracted by ethanol [7].
There is a need for safer, more natural, and more environmental-friendly dyes for textile industries due to the overwhelming usage of synthetic dyestuffs. Millions of tons of textile dyeing, printing, and finishing, wastewater have been discharged into the ecosystem annually. It is essential to be aware of the relevant chemical restrictions in textiles (and other materials) that are fundamental to the retail and manufacture of consumer products, as REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulates the substances and articles manufactured and sold in fashion industry. Organizations within the fashion and textiles supply chain should keep up with upcoming restriction within the REACH. More than 33 chemicals for textile application have been added to Annex XVII of REACH under a major new amendment, which will come into force in November 2020 [12]. Because of a continued ban on some toxic synthetic dyes, textile and polymer scientists are in constant search for alternative greener dyestuffs and agents that can reduce pollution from coloration.
Dyeing application using natural colorants is one of promising technologies that could greatly minimize environmental impact. Colorants extracted from natural materials might produce delicate and subdued shades and are to be utilized as novel functional agents in the achievement of highly active textile surfaces with deodorizing, antioxidant, antimicrobial, antifeedant, and UV protection properties [13]. Many current perspectives are intended to outline the functional finishing of different textile substrates and functional agents exploited from natural sources including colorations. In order to find an alternative colorant to the current synthetic dyestuffs, natural dyestuff has been suggested as a candidate with better biodegradability, renewability, and environmental-compatibility features [14]. Up to present, there are a few preliminary studies that have been conducted toward orange peel textile coloration. It is a common practice that natural dyes are commonly used on protein fibers other than cotton textiles [[15], [16], [17]]. Additionally, the dyeing properties and antimicrobial activity of citrus grandis extract was studied [7]. In a recent article, water extracts from orange peel were used to dye wool fabrics with UV-protection properties [11].
This study aimed to expand scope of study builds upon previous publications that have explored the utilization of orange peel extracts in textile dyeing processes. Specifically, we aim to In-depth analysis of the chemical composition of Orange Peel Color Extracts (OPCEs) using analytical chemistry, utilize full factorial method on various factors which affect colorant extraction with both water and ethanol solvents, and experimenting simultaneous extraction/impregnation dyeing of OPCEs with silk fabrics (see Fig. 1).
Fig. 1.
Schematic diagram of natural dyeing with OPECs.
2. Experimental
2.1. Material
Orange peels used in experiments were recycled from waste oranges obtained from Packing house of local farm store in Campus. The commercial name of the orange is Navel under genus Citrus × sinensis.
Silk Chiffon fabrics (42.24 GSM and 112*124 of warp and weft Count) were purchase from Dharma Trading Co.
To characterize the orange peel color extract (OPCEs), α-carotene and β-carotene standards were purchased from Sigma Aldrich (St. Louis, MO) and utilized for qualitative and quantitative analysis. HPLC grade methanol and acetonitrile were purchased through Fisher Scientific (Hampton, NH) and EMD Millipore (Billerica, MA), respectively. Acetic acid (ACS certified plus grade) was also purchased from Fisher Scientific. All chemicals were used as received without any further purification.
2.2. Spectral and HPLC analysis
Standard solutions of both α-carotene and β-carotene with 4 ppm were spectrophotochemically scanned for absorbances in the range of 400 nm–700 nm using Cary 60 UV–vis spectrometer manufactured by Agilent Technologies.
The HPLC analysis of orange peel extract samples was performed by using a 1260 Infinity II LC system manufactured by Agilent Technologies (Santa Clara, CA), equipped with a Poroshell 120 EC-C18 column (Agilent Technologies with dimensions of 4.6 × 100 mm and a particle size of 2.7 μm). Reversed phase HPLC analysis using a diode array detector was performed on all orange peel waste sample as well as α-carotene and β-carotene standards at various concentrations. The mobile phase used was comprised of two solvents: 5 % solvent A and 95 % solvent B. Solvent A was composed of 100 % methanol, and solvent B was 99.9 % acetonitrile with 0.1 % acetic acid, respectively. The collected data were processed by the OpenLab EZChrom software developed by Agilent Technologies.
2.3. Extraction profile of orange peel colorants
The orange powder (500 mg) was first weighed and transferred into a 20-ml glass vial. Subsequently, 10 ml of solvent (water, 70 % ethanol, or 100 % ethanol) was added to the orange powder to achieve a liquid-to-solid ratio of 1:20 (wt/vol). This specific ratio of 1:20 was determined through a preliminary experiment to ensure the maximum amount of solid could be mixed with the solvent without forming a thick paste. The solvent concentration was determined based on previous published literature [11]. The solution was then vigorously shaken to ensure thorough mixing of all the powders with the solvent. The glass vials were then placed in a water bath (General Purpose Water Bath, VWR International, Pennsylvania) set at different temperature conditions (ambient, 40 °C, or 60 °C) for varying time intervals (30 min, 1 h, or 2 h). The highest temperature, 60 °C, was chosen for lab safety considerations, and the time intervals were determined based on previous research [11]. During incubation, the glass vials were inverted every 15 min.
After the extraction process, 1 ml of the solution from the glass vial was transferred into a 1.5 ml microcentrifuge tube and centrifuged using the Eppendorf MiniSpin centrifuge (Eppendorf, Germany) at 14,000×g for 1 min. The supernatant was then diluted five times and transferred into a 1.5 ml quartz cuvette for absorbance measurement. The absorbance of the samples was measured using the GENESYS™ 10 UV–Vis Spectrophotometer (Thermo Fisher Scientific, Massachusetts), which conducted a full scan within the 380 nm −800 nm range with a 1 nm increment. Duplicates of the extraction process were performed, but two independent absorbance measurements were conducted from each glass vial. The statistical significance of each parameter was evaluated using the Tukey-HSD test with SAS (Version 9.4).
2.4. Simultaneous extraction/impregnation dyeing
The dyeing of fabrics with OPCEs was performed using Ahiba™ Infrared dyeing machinery (Datacolor Inc.). To concurrently extract and dye, an orange peel powder dyeing solution was prepared using deionized water. Fabric samples were then immersed in the dyeing solution, which had been formulated based on an orthogonal experimental design incorporating factors such as material-to-liquid ratio, temperature, time, and pH values. The subsequent procedure follows the common processes of dyeing, washing, and drying.
The dyeing experiment was started at room temperature with a heating/cooling rate of 3 °C/min was kept on the dyeing machinery. After the temperature was raised to preset dyeing temperatures, the dyeing bath was kept constant degree for a determined period as defined by the orthogonal experiment. The samples were then tumbling at the dyeing temperature in the dyeing machine. After dyeing, fabric samples were cooled down at the same rate to room temperature. Then, fabric samples were washed with distilled water, soaped, squeezed, and dried. Analytical grades of glacial acetic acid and sodium carbonate were utilized to adjust the pH of the dyeing solution, while no mordants were added.
2.5. Color measurement
The dyeing profiles (parameters) and colorfastness of fabric samples were measured to identify the characteristics of the extracted OP colorants. Transmission data were measured by a UV-VIS-NIR spectrophotometer, while K/S value and color data were measured by a color spectrophotometer. All measurements were conducted in a controlled environment.
K/S value is a function of the color depth of a dyed sample. The higher the value of K/S, the deeper the color, The K/S value can be calculated using equation (1).
| (1) |
- where R refers to the reflectance data (%) of fabric samples.
A color spectrophotometer (Xrite i5 with QC software) was used to measure color reflectance data and K/S values. The colorfastness to washing, wet and dry crocking, and sunlight were measured and evaluated in accordance with the AATCC TM 61–2013: “Colorfastness to Laundering: Accelerated”, AATCC TM 8–2016:” Colorfastness to Crocking: Crockmeter Method”, AATCC TM 16.3–2014: “Colorfastness to Light: Xenon-Arc”, and AATCC TM 15–2023: “Colorfastness to Perspiration”, respectively. The evaluation was based on AATCC Evaluation Procedure 1–2012: “Gray Scale for Color Change” and AATCC Evaluation Procedure 1–2012: Gray Scale for Color Staining.
3. Result and discussion
3.1. Spectral Presentation and HPLC results
This section focuses on identifying and quantifying the various compounds present, such as flavonoids, phenolic compounds, essential oils, and organic acids. Advanced analytical techniques, like HPLC (High-Performance Liquid Chromatography) and UV–vis spectrophotometry, were employed for this purpose. The UV–vis spectrophotometry was first used to identify the maximum wavelength of absorbance of standard carotenes and orange peel extract. Illustrated as Fig. 2, both carotenes have strong absorbance in the wavelength range of 400 nm–500 nm with stronger absorbance around 450 nm The maximum absorbance peaks of standard carotenes are in agreement with literature values [18]; Popescu et al., 2022), On the other hand, no predominant peaks and only minute humps around 450 nm and 480 nm were observed for the orange peel extract. The non-distinguishable peaks are mainly due to the complexity of the orange sample extract, which is normal for biological samples (where numerous compounds are present in the sample matrix) and the low concentration of carotenes in the orange peel extract. As a result, a separation analysis technique, HPLC, was employed to confirm the presence of the carotenes in the orange peel extract, with 450 nm as the detection wavelength based on the UV–vis spectrophotometric results.
Fig. 2.
UV–vis spectra of standard α- and β-carotenes and orange peel extract.
Standard solutions of α-carotene and β-carotene were run first to determine the retention times of each. As indicated in Fig. 3, the retention time of α-carotene was 16.1 min, and that of β-carotene was 17.4 min, respectively. The presence of α-carotene and β-carotene in the orange peel extract was evidenced by the matching retention times between the standard carotenes and the orange peel extract. The small peak around 14.6 min was an unidentified compound that was in the orange peel extract and had an absorbance at 450 nm. The unknown peak also echoes the UV–vis spectral result where the orange peel extract had an absorbance value but no distinguishable peak around 450 nm due to the sample complexity. Additionally, the peak size is proportional to the amount of carotenes in the sample. Shown on Fig. 3, more α-carotene was present in the orange peel waste sample than β-carotene due to the greater size of α-carotene peak.
Fig. 3.
Chromatogram of carotene standards (blue) and orange peel extract (red). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Extraction profile of orange peel colorants
Orange peel powders were prepared and utilized for colorant extraction, described in the Experimental section. The independent parameters of the extraction studied included concentrations of the solvent (0 %, 70 %, and 100 % ethanol), extraction temperature (20 °C, 40 °C, and 60 °C), and extraction time duration (30 min, 60 min, and 120 min). This study employed a full factorial design with three factors, each at three levels, coded as +1, 0, and −1, as illustrated in Table 1.
Table 1.
Extraction conditions with coded and uncoded values and experimental data.
| Test order | Ethanol concentration (%) | Temperature, (°C) | Time (min) | Absorbance | SD |
|---|---|---|---|---|---|
| 1 | 0 (−1) | 20 (−1) | 30 (−1) | 0.2018 | 0.0153 |
| 2 | 0 (−1) | 40 (0) | 30 (−1) | 0.3233 | 0.0238 |
| 3 | 0 (−1) | 60 (1) | 30 (−1) | 0.2620 | 0.0091 |
| 4 | 0 (−1) | 20 (−1) | 60 (0) | 0.3703 | 0.1303 |
| 5 | 0 (−1) | 40 (0) | 60 (0) | 0.3560 | 0.0446 |
| 6 | 0 (−1) | 60 (1) | 60 (0) | 0.3043 | 0.0289 |
| 7 | 0 (−1) | 20 (−1) | 120 (1) | 0.4013 | 0.1702 |
| 8 | 0 (−1) | 40 (0) | 120 (1) | 0.3570 | 0.0454 |
| 9 | 0 (−1) | 60 (1) | 120 (1) | 0.5275 | 0.0826 |
| 10 | 70 (0) | 20 (−1) | 30 (−1) | 0.1693 | 0.0132 |
| 11 | 70 (0) | 40 (0) | 30 (−1) | 0.2310 | 0.0428 |
| 12 | 70 (0) | 60 (1) | 30 (−1) | 0.2333 | 0.0094 |
| 13 | 70 (0) | 20 (−1) | 60 (0) | 0.1873 | 0.0180 |
| 14 | 70 (0) | 40 (0) | 60 (0) | 0.2275 | 0.0353 |
| 15 | 70 (0) | 60 (1) | 60 (0) | 0.2430 | 0.0228 |
| 16 | 70 (0) | 20 (−1) | 120 (1) | 0.1738 | 0.0087 |
| 17 | 70 (0) | 40 (0) | 120 (1) | 0.2478 | 0.0276 |
| 18 | 70 (0) | 60 (1) | 120 (1) | 0.3293 | 0.0336 |
| 19 | 100 (1) | 20 (−1) | 30 (−1) | 0.3145 | 0.0054 |
| 20 | 100 (1) | 40 (0) | 30 (−1) | 0.4128 | 0.0365 |
| 21 | 100 (1) | 60 (1) | 30 (−1) | 0.4155 | 0.0124 |
| 22 | 100 (1) | 20 (−1) | 60 (0) | 0.3463 | 0.0075 |
| 23 | 100 (1) | 40 (0) | 60 (0) | 0.4510 | 0.1005 |
| 24 | 100 (1) | 60 (1) | 60 (0) | 0.4610 | 0.0182 |
| 25 | 100 (1) | 20 (−1) | 120 (1) | 0.3608 | 0.0029 |
| 26 | 100 (1) | 40 (0) | 120 (1) | 0.4800 | 0.0769 |
| 27 | 100 (1) | 60 (1) | 120 (1) | 0.5135 | 0.1078 |
Shown in Table 2, statistical analysis was conducted to assess the impact of extraction solvent concentration, temperature, and time. According to the statistical results provided in the table, all three parameters evaluated demonstrated a significant influence (P < 0.05) on the extraction process. In general, higher ethanol concentration, longer time, and higher temperature led to increased absorbance, indicating a higher concentration of colorants in the extraction liquor.
Table 2.
Statistical analysis of the extraction process on the importance of extraction ethanol concentration, temperature, and time.
| R-Square | Coeff Var | Root MSE | Absorbance Mean |
|---|---|---|---|
| 0.8024 | 17.9232 | 0.0591 | 0.3296 |
| Source | DF | Anova SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Time | 2 | 0.1524 | 0.0762 | 21.83 | <.0001 |
| Temperature | 2 | 0.1393 | 0.0697 | 19.95 | <.0001 |
| Time*Temperature | 4 | 0.0626 | 0.0157 | 4.49 | 0.0025 |
| Solvent | 2 | 0.4109 | 0.2055 | 58.87 | <.0001 |
| Time*Solvent | 4 | 0.0995 | 0.0249 | 7.13 | <.0001 |
| Temperature*Solvent | 4 | 0.1316 | 0.0329 | 9.43 | <.0001 |
| Time*Tempera*Solvent | 8 | 0.1519 | 0.0190 | 5.44 | <.0001 |
3.2.1. Effect of solvent concentration
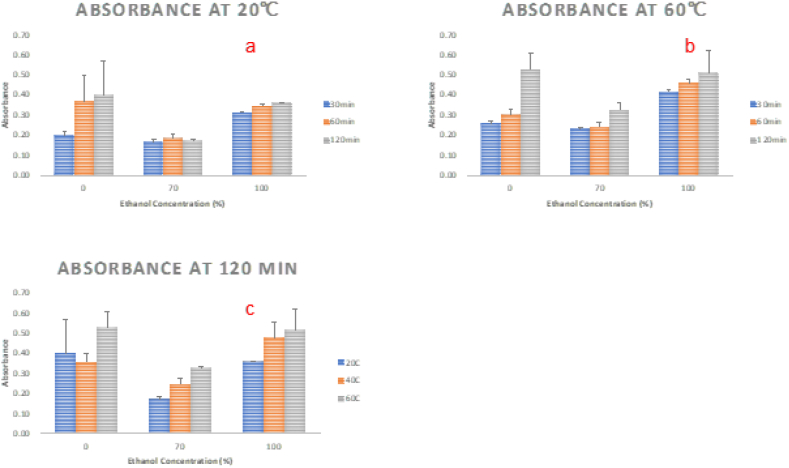
The influence of ethanol concentration on extraction efficiency was assessed across three levels: 0 %, 70 %, and 100 %. As depicted in Fig. 4, the liquor's absorbance when using 0 % ethanol exhibited a similar value to that observed with 100 % ethanol for 60 min and 120 min of extraction. However, the color, as visually observed, appeared lighter (picture not shown). This similarity in absorbance between 0 % and 100 % ethanol mostly resulted from the presence of oil droplet emulsions within the liquor phase, rendering the liquor turbid. Consequently, the absorbance value was deemed invalid due to the lack of transparency in the solution. Clear liquor phases were observed in both the 70 % and 100 % ethanol extraction processes. Therefore, further discussion regarding the impact of ethanol concentration was focused exclusively on these two concentrations.
Fig. 4.
Optimization of extraction profile of orange peel colorants under various conditions. 4a, Absorbance of the extracted colorants at 20 °C. 4b, Absorbance of the extracted colorants at 60 °C. 4c, Absorbance of the extracted colorants for 120 min. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Comparing 70 % with 100 % ethanol extraction processes, it became evident that a higher concentration of ethanol resulted in a greater absorbance, indicating the extraction of more colorants from the orange peel powders. Most of the colorants in orange peels are carotenoids, which are compounds with higher solubility in organic solvent. Consequently, as the ethanol concentration increased, it was foreseeable that the extraction of lipid-soluble carotenoids would increase proportionally.
3.2.2. Effect of extraction time
As shown in Fig. 4b, the impact of extraction time was examined across three distinct durations: 30 min, 60 min, and 120 min respectively. Statistical analysis revealed that the extraction time significantly influenced the extraction of colorants. As illustrated in Fig. 4b, it is evident that with an extended duration, the absorbance of the extracted liquor increased across all three ethanol concentrations at 60 °C. Similar findings were observed at 20 °C and 40 °C as well.
3.2.3. Effect of extraction temperature
Additionally, the study also assessed the impact of extraction temperature (Fig. 4c) at three different levels: 20 °C, 40 °C, and 60 °C, on color extraction. As depicted in Fig. 4c, at ethanol concentrations of 70 % and 100 %, higher temperatures led to increased absorbance values in the extracted liquor. However, no significant difference was observed when using 0 % ethanol (water). As mentioned previously, the elevated absorbance in the water sample was likely to be attributed to the presence of oil droplet emulsions in the extracted liquor, resulting in the turbidity of the liquor solution and thus rendering the influence of temperature on extraction non-discernible. These trends were consistent with the results obtained from 30-min and 60-min extractions.
The colorants in the orange peel powders were extracted and the absorbance at each condition was measured as shown in Table 1. As shown in the table, the maximum absorbance at 0.5275 was found using 0 % ethanol at 60 °C for 120 min. Another condition that achieved high absorbance at 0.5135 was using 100 % ethanol under the same condition (60 °C for 120 min). Since there are no significant discrepancies in the extraction efficiency between pure ethanol and water, the following dyeing experiment will choose a water medium for silk dyeing with an even rigorous extraction condition of 100 °C, which is also a very common textile dyeing temperature for natural fibers.
3.3. Dyeing profile of OPCEs with silk fabrics
To achieve optimal dyeing outcome, an orthogonal design of the experiment was utilized, and the four main factors were selected, which were material ratio, temperature, pH value, and time separately, to examine the parameters’ impact on the dyeing process. A detail of the orthogonal design of the experiment is shown in Table 3, where levels for each of the four factors were defined respectively. After fabric dyeing with OPCEs, results of K/S values of each dyed fabric were collected, followed by an orthogonal range analysis that was shown in Table 4. It is observed that the 6# sample achieved the highest K/S value. Based on a range analysis of the outcomes, we identified the sequence of influence on K/S value to be C > B > A > D. The pH value of the dye solution is the most prominent factor affecting the results, while the impact of dyeing time was found to be the least significant factor. Based upon the K/S value result from the orthogonal design experiment, the condition of orange peel dyeing for silk fabrics has the highest optical density obtained at 410 nm under acidic conditions specifically pH value 3 at 100 °C with a material-to ratio of 1:15 maintained for a duration of 60 min. The same dyeing experiment was repeated three times and the average data were utilized for the orthogonal analysis. Therefore, upon optimizing the K/S values, the optimal outcome was achieved following as A2, B3, C1, D3 which is material to liquid ratio 1:15 (10 g/150 ml water), dyeing at 100 °C with a pH of 3 for 60 min.
Table 3.
Factors and levels in the orthogonal design of the experiment for the dyeing process of orange peel.
| Levels | Factors |
|||
|---|---|---|---|---|
| A. Concentration | B. Temperature (°C) | C. pH value | D. Time (min) | |
| 1 | 1:30 | 80 | 3 | 30 |
| 2 | 1:15 | 90 | 6 | 60 |
| 3 | 1:10 | 100 | 9 | 90 |
Table 4.
The K/S values from the orthogonal experiment and their range analysis.
| No. | A | B | C | D | K/S Value | Range analysis | A | B | C | D | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 1 | 1 | 1 | 1 | 1.798 | K/S Valuea | K1 | 2.026 | 2.178 | 3.991 | 2.360 |
| 2# | 1 | 2 | 2 | 2 | 1.987 | ||||||
| 3# | 1 | 3 | 3 | 3 | 2.293 | K2 | 3.453 | 2.706 | 2.674 | 3.338 | |
| 4# | 2 | 1 | 2 | 3 | 2.607 | ||||||
| 5# | 2 | 2 | 3 | 1 | 1.854 | K3 | 3.279 | 3.874 | 2.092 | 3.059 | |
| 6# | 2 | 3 | 1 | 2 | 5.899 | ||||||
| 7# | 3 | 1 | 3 | 2 | 2.129 | R | 1.427 | 1.696 | 1.899 | 0.978 | |
| 8# | 3 | 2 | 1 | 3 | 4.278 | ||||||
| 9# | 3 | 3 | 2 | 1 | 3.429 | ||||||
OPTIMAL FACTORS: A2, B3, C1, D2.
The K/S values were measured at 410 nm.
In order to verify if the resulting optimal factors have the same performance for different batches of the orange peel of different seasons and years. The same orthogonal experiment was repeated for a new batch of orange peel wastes from the same source in another year. The result is shown in Table 5. Upon further analysis, the optimum dyeing performance factors’ were found to be consistent with the new batch of OPCEs, which was A2:B3:C1:D2, the same as the previous batches. Even though measured K/S values of new OPCEs dyed fabrics may vary compared with data of the previous OPCEs batches, both chemical composition and dyeing performance of OPCEs remain consistent in principle. Thereby, the optimal parameters achieved with this experiment are repeatable for any new batch of orange peel wastes, though there are variations on hue, chroma, and lightness of colors that would be obtained.
Table 5.
The K/S values from new batches of orange peels of orthogonal experiment and their range analysis.
| No. | A | B | C | D | K/S Value | Range analysis | A | B | C | D | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 1 | 1 | 1 | 1 | 1.566 | K/S Valuea | K1 | 1.536 | 1.971 | 3.212 | 2.052 |
| 2# | 1 | 2 | 2 | 2 | 1.611 | ||||||
| 3# | 1 | 3 | 3 | 3 | 1.432 | K2 | 2.927 | 2.322 | 2.224 | 2.736 | |
| 4# | 2 | 1 | 2 | 3 | 2.334 | ||||||
| 5# | 2 | 2 | 3 | 1 | 1.865 | K3 | 2.743 | 2.913 | 1.770 | 2.418 | |
| 6# | 2 | 3 | 1 | 2 | 4.582 | ||||||
| 7# | 3 | 1 | 3 | 2 | 2.014 | R | 1.391 | 0.942 | 1.442 | 0.684 | |
| 8# | 3 | 2 | 1 | 3 | 3.489 | ||||||
| 9# | 3 | 3 | 2 | 1 | 2.726 | ||||||
OPTIMAL FACTORS: A2, B3, C1, D2.
The K/S values were measured at 410 nm.
3.3.1. Colorfastness evaluation
The colorfastness results for optimally dyed silk fabrics are shown in Table 6. Overall, the OPCEs dyed silk fabric showed slightly changed colorfastness with a rate ranging from 4.5 to 5 of the AATCC color staining value. For dyed silk fabrics, colorfastness to laundry (multifiber fabrics staining) was rated as “negligible or no change,” with a grade of 5 except for polyester which indicated "slightly changed", with a grade of 4.5 as well as the colorfastness to laundry indicates minimal color change, with a rating of 4. Also, colorfastness to crocking was indicated as “slightly changed” at a rate of 4.5 in both wet and dry conditions. Issues of colorfastness were found on silk fabrics as “considerably changed” when exposed to sunlight, resulting in grades of 2.5 and 1.5 after 12 h and 24 h of exposure. Overall, the colorfastness testing results were mostly acceptable for textile end uses that are primarily used indoors. For future research, bio-mordants such as Myrobalan and Walnut Husk mixture might be utilized to improve K/S value and colorfastness for fabric from OPCEs dyeing [19].
Table 6.
Colorfastness of OPCEs dyed silk fabrics.
| Versus white clothes | Colorfastness to crocking | |
| CIE DE* | Color staining | |
| Wet | 3.28 | 4.5 |
| Dry |
1.72 |
4.5 |
| Colorfastness to sunlight | ||
| Versus original sample |
CIE DE* |
Color change |
| 12h | 4.34 | 2.5 |
| 24h |
8.37 |
1.5 |
| Colorfastness to laundry | ||
| Versus original sample |
CIE DE* |
Color Change |
| 1.44 |
4 |
|
| Colorfastness to laundry | ||
| AATCC Multifiber Fiber Fabrics (MMF) |
CIE DE* |
Color staining |
| Acetate | 0.26 | 5 |
| Cotton | 0.3 | 5 |
| Nylon | 0.69 | 5 |
| Polyester | 1.97 | 4.5 |
| Acrylic | 0.97 | 5 |
| Wool |
0.72 |
5 |
| Colorfastness to perspiration | ||
| Versus original sample |
CIE DE* |
Color Change |
| 2.12 |
4 |
|
| Colorfastness to perspiration | ||
| AATCC Multifiber Fiber Fabrics (MMF) |
CIE DE* |
Color staining |
| Acetate | 0.5 | 5 |
| Cotton | 0.62 | 5 |
| Nylon | 2.08 | 4.5 |
| Polyester | 1.70 | 4.5 |
| Acrylic | 1.93 | 4.5 |
| Wool | 0.78 | 5 |
Data availability statement
We are glad to deposit data and results into a publicly available repository.
4. Conclusions
This research discusses the extraction of colorants from orange peel, one agriculture waste, as plant dyes and investigates the potential dyeing capability on silk fabrics. To achieve the greatest absorbency rate of extracted solution, the following optimum extraction conditions were obtained after comparing 100 % Ethanol with water as solvent: extraction temperature of 60 °C, extraction time of 120 min, and material-to-liquor ratio of 1: 10. To attain the highest K/S values, the optimal dyeing profiles for both silk fabrics were found to be 100 °C, 60 min, PH 3, and material to liquid ratio of 1:15. Colorfastness results were met the basic requirement of textile application of indoor usage. The overall results provide solid evidence the concepts of repurposing agricultural waste as natural, alternate colorants to synthetic dyestuff for the fashion industry.
This OPECs dyeing initiative delves into the innovative concept of repurposing agricultural byproducts as natural colorants for fabric dyeing. It is evident that various agricultural by-products harbor untapped potential as natural sources of color. The methodology outlined in this study holds promise for extension to various other agricultural wastes, fostering reduced waste generation, sustainable dyeing practices, and a more environmentally friendly approach. However, certain limitations may impact future development. The color derived from orange peels can exhibit variability, and color intensity and shade may be less predictable. Additionally, the extraction and dyeing process may be less efficient, necessitating a larger quantity of raw materials to achieve the desired color. Although the use of mordants can enhance color depth and colorfastness, this aspect was not explored in this research, as we aimed to minimize their usage. Subsequently, we anticipate exploring the potential of bio-mordants derived from plants for natural dyeing using agricultural byproducts. Despite these limitations, the growing interest in sustainable and eco-friendly practices has spurred ongoing research and innovations to address challenges in natural dyeing processes. Advances in technology and increased awareness of environmental issues hold the potential to contribute to the refinement of natural dyeing techniques utilizing agricultural waste.
CRediT authorship contribution statement
Jiangning Che: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Jonathan M. Dannenberg: Data curation, Formal analysis. Myunggyo Yu: Data curation, Formal analysis. Xu Yang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. Yan Liu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jiangning Che reports "Partial funding for this project has been provided by the California State University Agricultural Research Institute (ARI), United States (U.S.)”.
References
- 1.Environmental Protection Agency, U. S . U S Environmental Protection; 2018. National Overview: Facts and Figures on Materials, Wastes and Recycling; pp. 1–13.https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials [Google Scholar]
- 2.Agricultural Statistics Service, N. 2020. United States Department of Agriculture National Agricultural Statistics Service.https://www.nass.usda.gov/Publications/Todays_Reports/reports/vegean20.pdf [Google Scholar]
- 3.O'Shea N., Arendt E.K., Gallagher E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innovative Food Sci. Emerging Technol. 2012;16:1–10. doi: 10.1016/j.ifset.2012.06.002. [DOI] [Google Scholar]
- 4.Sawalha S.M.S., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A. Quantification of main phenolic compounds in sweet and bitter orange peel using CE-MS/MS. Food Chem. 2009;116(2):567–574. doi: 10.1016/j.foodchem.2009.03.003. [DOI] [Google Scholar]
- 5.Vasso Oreopoulou and Constantina Tzia . Utilization of By-Products and Treatment of Waste in the Food Industry; 2006. Utilization of Plant By-Products for the Recovery of Proteins, Dietary Fibers, Antioxidants, and Colorants; pp. 209–232. [Google Scholar]
- 6.Moussaid M., Caillet S., Nketsia-Tabiri J., Boubekri C., Lacroix M. Phenolic compounds and the colour of oranges subjected to a combination treatment of waxing and irradiation. J. Sci. Food Agric. 2004;84(13):1625–1631. doi: 10.1002/jsfa.1849. [DOI] [Google Scholar]
- 7.Yi E., Yoo E.S. A novel bioactive fabric dyed with unripe Citrus grandis Osbeck extract part 1: dyeing properties and antimicrobial activity on cotton knit fabrics. Textil. Res. J. 2010;80(20):2117–2123. doi: 10.1177/0040517510373633. [DOI] [Google Scholar]
- 8.Shukla P.S., Gawande S.S., Thorat A.V. Extraction, Identification and utilization of pigments extracted from citrus wastes. Int. J. Sci. Res. 2015;5(5):840–847. doi: 10.21275/v5i5.nov163451. [DOI] [Google Scholar]
- 9.Susan H. The Swedish of Textile Report; 2014. pp. 1–61. (Extraction of β -carotene from Orange Peel and Carrot Waste for Cotton Dyeing). [Google Scholar]
- 10.Kumar C.S.S., Dhinakaran M. Extraction and application of natural dyes from orange peel and Lemon peel on cotton fabrics. International Research Journal of Engineering and Technology(IRJET) 2017;4(5):2008–2009. https://www.irjet.net/archives/V4/i5/IRJET-V4I541.pdf [Google Scholar]
- 11.Hou X., Chen X., Cheng Y., Xu H., Chen L., Yang Y. Dyeing and UV-protection properties of water extracts from orange peel. J. Clean. Prod. 2013;52:410–419. doi: 10.1016/j.jclepro.2013.03.004. [DOI] [Google Scholar]
- 12.New Chemical Restrictions in Textiles: Are You Ready? 2019. https://www.chem-map.com/chemical_news/new-chemical-restrictions-in-textiles-are-you-ready/ [Google Scholar]
- 13.Shahid-ul-Islam, Wani S.A., Mohammad F. Imparting functionality viz color, antioxidant and antibacterial properties to develop multifunctional wool with Tectona grandis leaves extract using reflectance spectroscopy. Int. J. Biol. Macromol. 2018;109:907–913. doi: 10.1016/j.ijbiomac.2017.11.068. [DOI] [PubMed] [Google Scholar]
- 14.Oktav Bulut M., Akar E. Ecological dyeing with some plant pulps on woolen yarn and cationized cotton fabric. J. Clean. Prod. 2012;32:1–9. doi: 10.1016/j.jclepro.2012.03.010. [DOI] [Google Scholar]
- 15.Tayade P.B., Adivarekar R.V. Colour gamut with easy sources of natural dyes. Int. J. Cloth. Sci. Technol. 2016;28(5):558–569. doi: 10.1108/IJCST-12-2015-0136. [DOI] [Google Scholar]
- 16.Tang A.Y.L., Wang Y., Lee C.H., Kan C. wai. Comparison of computer colour matching of water-based and solvent-based reverse micellar dyeing of cotton fibre. Color. Technol. 2018;134(4):258–265. doi: 10.1111/cote.12333. [DOI] [Google Scholar]
- 17.Yusuf M., Shabbir M., Mohammad F. Natural colorants: Historical, processing and sustainable Prospects. Natural Products and Bioprospecting. 2017;7(1):123–145. doi: 10.1007/s13659-017-0119-9. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen Gupta S., Ghosh M. In vitro antioxidative evaluation of α - and β -carotene, isolated from crude palm oil. Journal of Analytical Methods in Chemistry. 2013;2013 doi: 10.1155/2013/351671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinnezhad M., Gharanjig K., Belbasi S., Saadati S.H.S., Saeb M.R. The Use of Sumac as a natural mordant in green production of Iranian Carpet. Fibers Polym. 2018;19(9):1908–1912. doi: 10.1007/s12221-018-7961-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We are glad to deposit data and results into a publicly available repository.