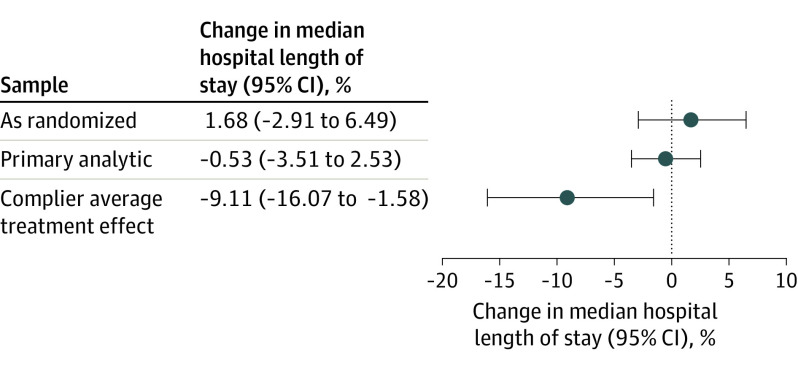Key Points
Question
Does ordering palliative care by default (allowing opt-out) increase consultation and improve clinical outcomes?
Findings
In this pragmatic trial conducted from March 2016 to November 2018 among 24 065 inpatients 65 years or older with advanced chronic obstructive pulmonary disease, dementia, or kidney disease, default orders for palliative care did not significantly reduce length of stay. Default orders significantly increased consultation rate compared with usual care (43.9% vs 16.6%), decreased time to consultation by 1.2 days, and increased odds of hospice discharge and do-not-resuscitate orders at discharge.
Meaning
Default palliative care consult orders did not reduce length of stay for older inpatients with advanced chronic illnesses, but improved the rate and timing of consultation and some end-of-life care processes.
Abstract
Importance
Increasing inpatient palliative care delivery is prioritized, but large-scale, experimental evidence of its effectiveness is lacking.
Objective
To determine whether ordering palliative care consultation by default for seriously ill hospitalized patients without requiring greater palliative care staffing increased consultations and improved outcomes.
Design, Setting, and Participants
A pragmatic, stepped-wedge, cluster randomized trial was conducted among patients 65 years or older with advanced chronic obstructive pulmonary disease, dementia, or kidney failure admitted from March 21, 2016, through November 14, 2018, to 11 US hospitals. Outcome data collection ended on January 31, 2019.
Intervention
Ordering palliative care consultation by default for eligible patients, while allowing clinicians to opt-out, was compared with usual care, in which clinicians could choose to order palliative care.
Main Outcomes and Measures
The primary outcome was hospital length of stay, with deaths coded as the longest length of stay, and secondary end points included palliative care consult rate, discharge to hospice, do-not-resuscitate orders, and in-hospital mortality.
Results
Of 34 239 patients enrolled, 24 065 had lengths of stay of at least 72 hours and were included in the primary analytic sample (10 313 in the default order group and 13 752 in the usual care group; 13 338 [55.4%] women; mean age, 77.9 years). A higher percentage of patients in the default order group received palliative care consultation than in the standard care group (43.9% vs 16.6%; adjusted odds ratio [aOR], 5.17 [95% CI, 4.59-5.81]) and received consultation earlier (mean [SD] of 3.4 [2.6] days after admission vs 4.6 [4.8] days; P < .001). Length of stay did not differ between the default order and usual care groups (percent difference in median length of stay, −0.53% [95% CI, −3.51% to 2.53%]). Patients in the default order group had higher rates of do-not-resuscitate orders at discharge (aOR, 1.40 [95% CI, 1.21-1.63]) and discharge to hospice (aOR, 1.30 [95% CI, 1.07-1.57]) than the usual care group, and similar in-hospital mortality (4.7% vs 4.2%; aOR, 0.86 [95% CI, 0.68-1.08]).
Conclusions and Relevance
Default palliative care consult orders did not reduce length of stay for older, hospitalized patients with advanced chronic illnesses, but did improve the rate and timing of consultation and some end-of-life care processes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02505035
This cluster randomized trial examines whether the effect of ordering palliative care consultation by default for seriously ill hospitalized patients without requiring greater palliative care staffing increased consultations and improved outcomes.
Introduction
Palliative care is widely advocated1,2,3,4,5,6 and increasingly available7 for hospitalized patients with serious illness. Multiple studies suggest that inpatient palliative care reduces hospital length of stay (LOS) and costs8,9,10,11,12,13 and improves other patient-centered outcomes.10,14,15,16,17 Many of these studies were observational, and those that were randomized trials were typically highly controlled, explanatory designs evaluating the efficacy of specific palliative care models. Thus no large-scale, experimental evidence of the effectiveness of inpatient palliative care delivered in routine practice exists.
Moreover, despite rapid growth in inpatient palliative care services, many patients—particularly those with serious illnesses other than cancer—are never referred to palliative care or only receive it near the end of life. Low or late referrals stem, in part, from clinicians serving as gatekeepers: under usual care, clinicians must identify patients likely to benefit from palliative care and choose to order a consult. Because even experienced clinicians struggle to determine who will benefit from palliative care,18,19,20 many health systems have developed referral criteria.21 However, it is unknown whether such “trigger” approaches or other interventions designed to increase palliative care delivery change the frequency or timing of palliative care or affect patient outcomes.
To address these knowledge gaps, this pragmatic randomized trial examined the hypotheses that ordering palliative care by default22 (and enabling clinicians to opt-out) for older, hospitalized patients with common noncancer serious illnesses would lead to increased consultation and improve patient outcomes compared with usual care.
Methods
Study Design
We conducted a stepped-wedge, cluster randomized trial comparing usual care with a default order for palliative care consultation among adults with chronic serious illnesses admitted to 11 Ascension hospitals across 8 US states between March 21, 2016, and November 14, 2018, with follow-up through January 31, 2019. The protocol was approved by the institutional review board at the University of Pennsylvania, Ascension’s ethics department, and each hospital’s medical executive committees with a waiver of informed consent. The trial was registered (NCT02505035) and overseen by a data and safety monitoring board. The protocol was published previously, and the trial protocol and statistical analysis plan can be found in Supplement 1 and Supplement 2.23
Trial Sites and Patients
The trial was conducted in 11 not-for-profit community hospitals that each used the Cerner Corporation electronic health record (EHR) and had an established palliative care program, but differed in hospital bed size and palliative care team staffing (eTable 1 in Supplement 3). Consistent with a pragmatic approach, we neither required nor prohibited hospitals from increasing palliative care staffing during the trial. No hospital employed other triggers for palliative care before or during the trial.
Patients 65 years and older were eligible for inclusion if they had preexisting diagnoses of advanced chronic obstructive pulmonary disease (COPD), kidney failure, or dementia and if 1 or more of these diagnoses met the following consensus criteria for identifying hospitalized patients with high likelihoods of having unmet palliative care needs: at least 2 hospitalizations within the past 12 months or long-term oxygen dependence (COPD), long-term dialysis dependence (kidney failure), and admission from a long-term care facility, presence of a gastrostomy or jejunostomy tube, or at least 2 hospitalizations within the past 12 months (dementia).1 Because the unit of analysis was a hospital encounter, patients could be enrolled more than once.
An EHR algorithm screened all admitted patients 65 years and older to identify eligible diagnostic codes (eTable 2 in Supplement 3) and the secondary criteria. If only an eligible diagnosis was identified, nurses received an EHR alert to complete the secondary criteria screening (eFigure 1 in Supplement 3) because they may not be captured reliably in structured EHR data. Eligible patients were enrolled at 3 pm on the first full hospital day (eFigure 2 in Supplement 3). We defined the primary analytic sample a priori as all patients with a hospital LOS of at least 72 hours because the timing of the intervention made it unlikely to affect patients discharged sooner. Because LOS could not be measured at the time of randomization, we secondarily evaluated the as-randomized (ie, intention-to-treat) sample.
Randomization, Masking, and Procedures
In this 32-month stepped-wedge trial, each hospital began enrolling patients under usual care and then adopted the intervention of default orders over time in a randomly determined sequence (eFigure 3 in Supplement 3). Patients were blinded to the trial, but clinicians were not blinded to preserve their abilities to cancel the default order.
During the intervention phase, a palliative care consult order with a 24-hour delay was generated by default at 3 pm on the first full hospital day (Figure 1). Clinicians received an open-chart alert notifying them of the order, explaining how to cancel it, and instructing them to specify reasons for cancellations (eFigure 4 in Supplement 3). Noncancelled orders became active at 3 pm the following day. Throughout the trial, clinicians could order palliative care consults for any patient at any time. Regardless of how consults were ordered, palliative care teams retained discretion regarding consult prioritization, the types of services provided, and documentation.
Figure 1. Flow of Participants in a Study of Default Palliative Care Consultation for Seriously Ill Hospitalized Patients.
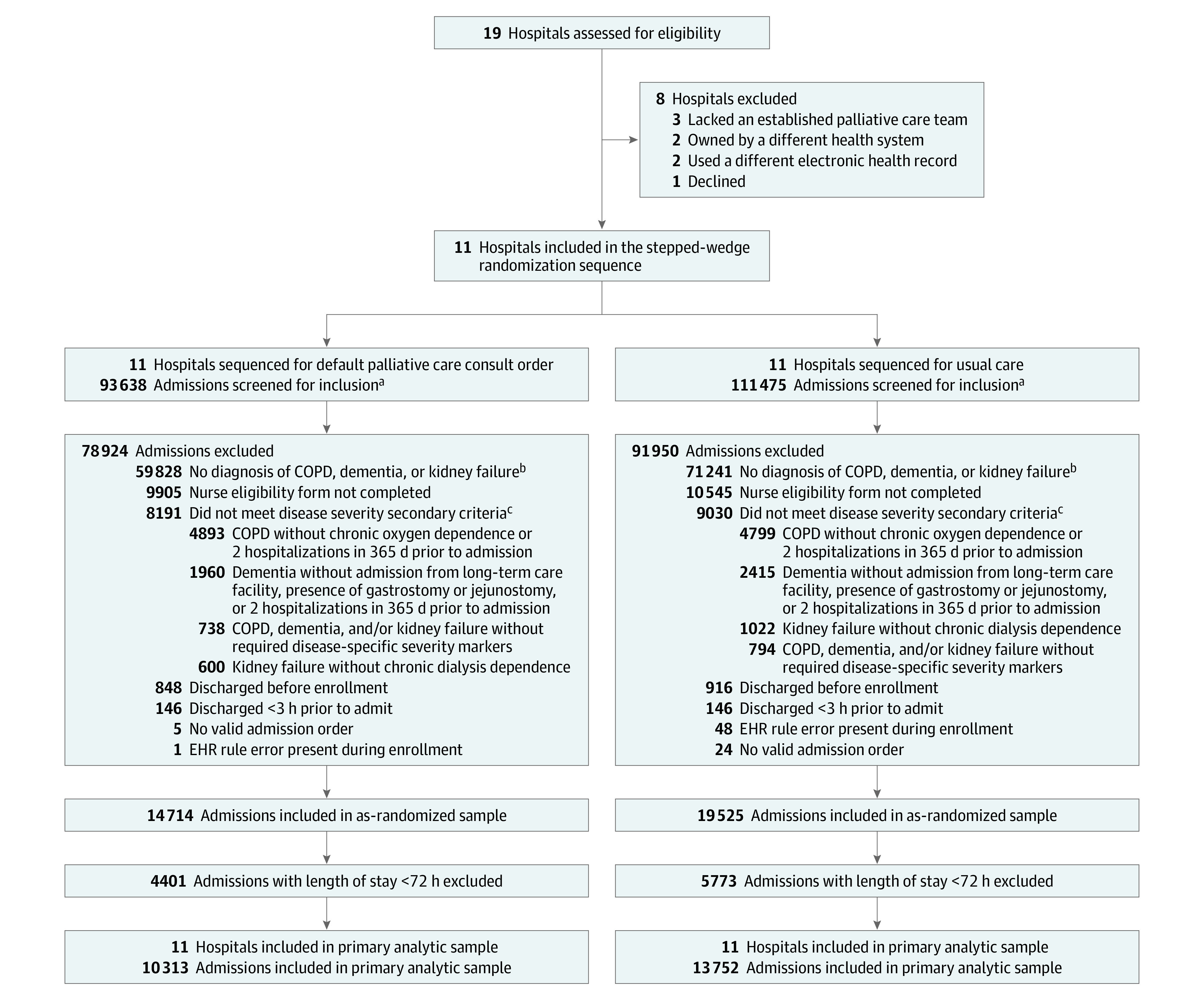
aIncludes admissions with a discharge date prior to January 31, 2019, when trial outcomes data collection ended.
bThe electronic health record (EHR) eligibility algorithm screened International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes for eligible diagnoses (detailed in eTable 2 in Supplement 3) and chronic oxygen dependence (Z99.81) and ≥2 admissions in the past 12 months.
cNurse-reported eligibility criteria included chronic oxygen dependence, ≥2 admissions in the past 12 months, dialysis dependence, admission from a long-term care facility, and presence of a jejunostomy or gastrostomy tube.
COPD indicates chronic obstructive pulmonary disease.
Outcomes
All participant characteristics and outcomes were collected during routine care and extracted from Ascension’s EHR and administrative data warehouses. The primary outcome was hospital LOS using a “placement of death” approach, whereby in-hospital deaths were treated as equivalent to the 99th percentile of the LOS distribution. Incorporating these 2 traditional serious illness care metrics is more patient-centered than either metric alone24,25 and prevents biased estimates of LOS due to informative truncation by death.26
Secondary clinical outcomes included rates of intensive care unit (ICU) mortality, in-hospital mortality, 30-day readmissions, cardiopulmonary resuscitation, invasive mechanical ventilation, do-not-resuscitate (DNR) orders at discharge, discharge to hospice, and ICU transfers after ward admissions. Palliative care process outcomes included the proportion of patients who received palliative care consultation, defined as a signed note from a palliative care clinician; rates at which consult orders were completed, cancelled, or neither cancelled nor completed; and, among those who received a consult, the time from admission to the first signed palliative care clinician note.
Statistical Analysis
A detailed description of the sample size estimate has been previously reported.23 Briefly, we sought to have at least 80% power to detect a difference in the primary outcome of at least 0.5 days at the median of the LOS distribution, assuming a hospital intraclass correlation coefficient of up to 0.20 and rates of palliative care consultation of 10% with usual care and at least 30% less than the intervention. Preliminary data from 2 Ascension hospitals suggested that if we enrolled 15 088 patients, we would have 89% power.
For primary analyses, we used mixed-effects linear regression to compare hospital LOS (with deaths ranked at the 99th percentile) between groups, with adjustment for the following patient characteristics at enrollment: age, sex, race, ethnicity, marital status, admission source, Agency for Healthcare Research and Quality Elixhauser Comorbidity Index,27 eligible diagnosis (kidney failure, COPD, and dementia), number of days between repeated enrollments to account for disease progression, and ICU vs ward location. Analyses also included a fixed effect for time and random effects for cluster and patients to account for repeated measures. LOS data were log-transformed prior to analysis. We tested interaction terms between the intervention and preselected baseline patient characteristics. Race and ethnicity information was collected from the EHR data warehouse because they are associated with health care outcomes and were thus included in our analysis.
A prespecified secondary analysis evaluated the efficacy of completed palliative care consults. Because per-protocol and as-treated analyses are fraught with selection biases, we modeled the randomization group as an instrumental variable28 in analyses of the complier average treatment effect (CATE)29,30 to account for the expected incomplete adherence to the default order. This analysis, described in detail in Supplement 3, uses data on all eligible encounters to estimate the treatment effect among participants who would receive a palliative care consult only if randomized to the intervention group. Because the optimal method for CATE analyses in cluster-randomized trials is uncertain, we used both 2-stage least-squares regression in STATA with time as a fixed effect and cluster as a random effect and the “ivmodel” package in R, which uses cluster robust variance and produces an Anderson-Rubin test and CI.31
Secondary outcomes were analyzed using logistic regression for binary outcomes and negative binomial regression for number of readmissions with an offset term to account for differential follow-up. Time to consult was analyzed using a competing-risks model in which death was the competing event and alive at discharge was the censoring event.
We conducted prespecified sensitivity analyses of the primary outcome (1) without adjustment for patient characteristics, (2) including a time × cluster interaction term to account for potentially heterogeneous effects of time among clusters, (3) recoding the intervention variable as the number of weeks since the intervention launched in each cluster to account for a potential delayed time effect, and (4) ranking in-hospital deaths at the 75th, 85th, and 95th percentiles of the LOS distribution. We also conducted the prespecified analyses of hospital LOS with the following alternate approaches for handling deaths: a clustered competing-risks model in which death was considered a competing event and a mixed-effects Cox proportional model in which death was a censoring event.
Results
Participants
Among 205 113 encounters for patients aged 65 years or older, 34 239 (16.7%) were enrolled in the as-randomized population (eTable 3 in Supplement 3). Of these, 24 065 (70.3%) encounters among 15 275 unique patients had LOS of at least 72 hours and were included in the primary analytic sample (eFigure 4 and eTable 4 in Supplement 3). Hospitals contributed a median (IQR) of 1871 (1143 to 3784) encounters. Baseline characteristics were balanced across the default order intervention (n = 10 313) and usual care (n = 13 752) groups (Table 1). The mean (SD) age of participants was 77.9 (8.3) years; 69.4% of patients were eligible based on a diagnosis of COPD, 30.9% had dementia, and 12.3% had kidney failure.
Table 1. Characteristics of Participants in the Primary Analytic Samplea.
| Characteristic | No. (%) | |
|---|---|---|
| Default order (n = 10 313) | Usual care (n = 13 752) | |
| Age, median (IQR), y | 77.0 (71.0-84.0) | 77.0 (71.0-84.0) |
| Sex | ||
| Female | 5631 (54.6) | 7707 (56.0) |
| Male | 4682 (45.4) | 6045 (44.0) |
| Race | (n = 10 245) | (n = 13 668) |
| American Indian or Alaska Native | 12 (0.1) | 17 (0.1) |
| Asian, Pacific Islander, or Native Hawaiian | 69 (0.7) | 114 (0.8) |
| Black or African American | 1362 (13.3) | 2658 (19.4) |
| Multiple races | 44 (0.4) | 64 (0.5) |
| Other (not specified) | 14 (0.1) | 24 (0.2) |
| White | 8744 (85.3) | 10 791 (79.0) |
| Ethnicity | (n = 10 183) | (n = 13 658) |
| Hispanic or Latino | 327 (3.2) | 491 (3.6) |
| Not Hispanic or Latino | 9856 (96.8) | 13 167 (96.4) |
| Marital status | (n = 10 258) | (n = 13 683) |
| Single | 1280 (12.5) | 1886 (13.8) |
| Married/partnered | 4191 (40.9) | 5046 (36.9) |
| Divorced/separated | 1363 (13.3) | 1968 (14.4) |
| Widowed | 3424 (33.4) | 4783 (35.0) |
| Source of admission | ||
| Home | 8499 (82.4) | 12 090 (87.9) |
| Skilled nursing facility | 544 (5.3) | 682 (5.0) |
| Transfer from another hospital | 1270 (12.3) | 980 (7.1) |
| Eligible diagnosis | ||
| Chronic obstructive pulmonary disease | 7267 (70.5) | 9422 (68.5) |
| Dementia | 3245 (31.5) | 4194 (30.5) |
| Kidney failure | 1124 (10.9) | 1831 (13.3) |
| Elixhauser Comorbidity Index, mean (SD)b | 15.04 (11.69) | 15.04 (11.39) |
| Location at enrollment | ||
| Hospital ward | 7524 (73.0) | 10 435 (75.9) |
| Intensive care unit | 2789 (27.0) | 3317 (24.1) |
The primary analytic sample includes admissions with length of stay ≥72 hours. Characteristics of patients included in the as-randomized sample are presented in eTable 3 in Supplement 3. There were no significant differences between the groups. Percentages may not total to 100 because of rounding or because categories are not mutually exclusive (ie, eligible diagnosis).
The Agency for Healthcare Research and Quality Elixhauser Comorbidity Index was calculated as a weighted sum of each of 29 binary comorbidity variables identified in the electronic health record by International Classification of Diseases, Tenth Revision codes present on admission. Higher scores indicate a greater comorbidity burden and severity of illness.
Palliative Care Consult Processes
More patients received palliative care consults under the default order intervention than usual care (43.9% vs 16.6%; adjusted odds ratio [aOR], 5.17 [95% CI, 4.59-5.81]) (Figure 2). Additionally, among patients who received a consult, the mean time to consultation was 26.7% (95% CI, −33.0% to −20.7%) less with the default order vs usual care (mean [SD] of 3.4 [2.6] days vs 4.6 [4.8] days) (eFigure 5 in Supplement 3). Clinicians cancelled 9.6% of default orders overall (eFigure 6 in Supplement 3), with a range of 1.2% to 16.4% across the 11 hospitals. Cancellations were most common among patients with kidney failure (12.3%), followed by COPD (9.7%) and dementia (7.8%). The most common reasons clinicians provided for cancelling the order was “no palliative care needs at this time” (53.1%) and “primary team is meeting all of the patient’s palliative care needs” (29.7%) (eTable 5 in Supplement 3).
Figure 2. Predicted Probability of a Completed Palliative Care Consult by Hospital.
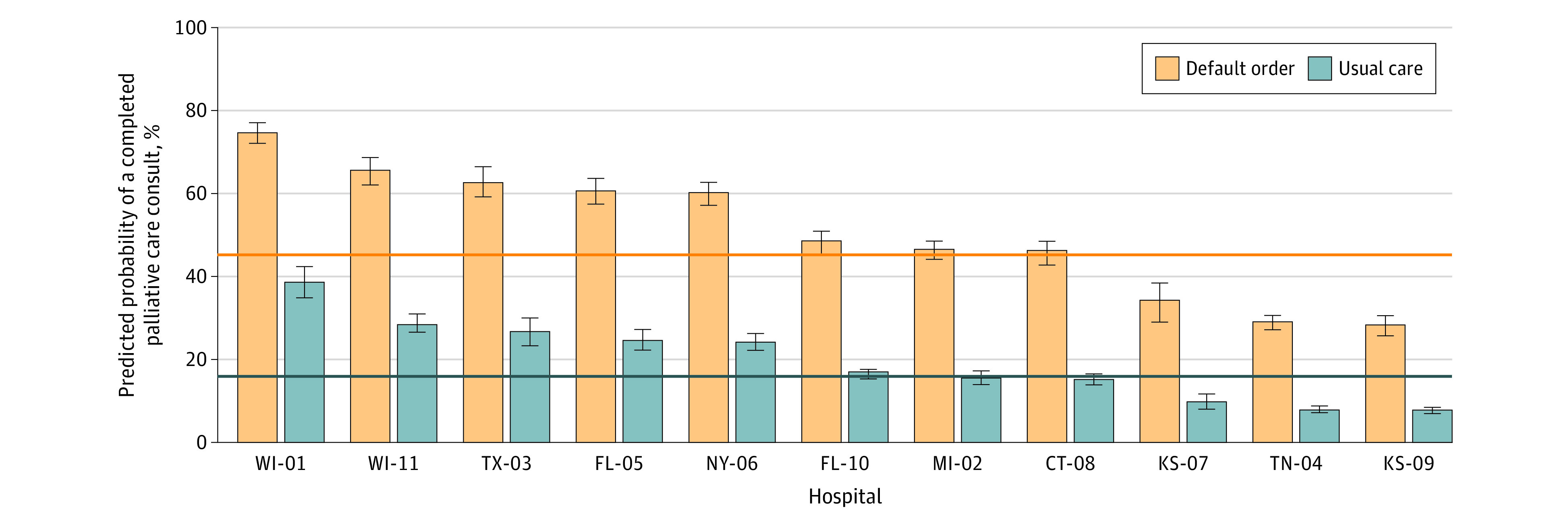
Results are shown for the primary analytic sample. Estimates were adjusted for time and cluster. Hospital number corresponds to randomization sequence; see eFigure 3 in Supplement 3 for more details. Horizonal lines denote the overall mean probability of a completed palliative care consult for usual care (16.6%) and intervention (43.9%) groups. Whiskers indicate 95% CIs. Percentages may not total to 100 due to rounding.
Effectiveness of Intervention
With in-hospital deaths (n = 1065 [4.4%]) ranked at the 99th percentile of the LOS distribution (26.9 days), there was no difference in the median hospital LOS between the default order and usual care groups (4.9 days vs 5.0 days; percent difference, −0.53% [95% CI, −3.51% to 2.53%]). Similar results were found among the as-randomized sample (Figure 3) and in all sensitivity analyses (eTable 6 in Supplement 3). Analyses of LOS that did not rank death also produced nonsignificant results (hazard ratio, 1.06 [95% CI, 0.97-1.16] in competing risk analyses; hazard ratio, 1.02 [95% CI, 0.97-1.07] in the Cox proportional model). The default order led to greater reductions in LOS among older patients (interaction estimate, −0.4% [95% CI, −0.6% to −0.2%]), and the intervention effect was not modified by other patient characteristics (eTable 7 in Supplement 3).
Figure 3. Primary Outcome of Hospital Length of Stay.
All estimates are adjusted for time, hospital, age, gender, race, ethnicity, marital status, source of admission, Agency for Healthcare Research and Quality Elixhauser Comorbidity Index, eligible diagnosis (kidney failure, chronic obstructive pulmonary disease [COPD], and dementia), number of days between repeated enrollment, and location in the intensive care unit at enrollment. The primary analytic sample includes encounters with length of stay ≥72 hours. In-hospital deaths were ranked at the 99th percentile of the distribution of LOS for each sample. Whiskers indicate 95% CIs.
Efficacy of Palliative Care Consultation
The CATE analysis revealed that the median LOS was reduced by 9.6% (95% CI, −17.5% to −1.6%) in the intervention group compared with usual care among the estimated 27.3% of patients who would have received a consult only if assigned to the intervention group (sometimes called “marginal patients”) (Figure 3). The R package produced nearly identical results (9.6% reduction in LOS [95% CI, −17.6% to −1.7%]).
Secondary Outcomes
A total of 487 patients (4.7%) in the default order group and 578 patients (4.2%) in the usual care group died in the hospital (aOR, 0.86 [95% CI, 0.68-1.08]) (Table 2). Patients in the default order group had greater odds of a hospice discharge (aOR, 1.30 [95% CI, 1.07-1.57]) and of having a DNR order at discharge (aOR, 1.40 [95% CI, 1.21-1.63]). Rates of ICU transfer, ICU mortality, cardiopulmonary resuscitation, invasive mechanical ventilation, and 30-day readmission did not differ significantly between groups (Table 2). Secondary outcomes were similar in the as-randomized sample (eTable 8 in Supplement 3).
Table 2. Secondary Outcomes Among the Primary Analytic Sample (n = 24 065)a.
| Outcome | Unadjusted No. (%) | Unadjusted difference (95% CI), % | Adjusted odds ratio (95% CI)b | P value | |
|---|---|---|---|---|---|
| Default order (n = 10 313) | Usual care (n = 13 752) | ||||
| Hospice discharge | 700 (6.8) | 952 (6.9) | −0.13 (−0.78 to 0.51) | 1.30 (1.07 to 1.57) | .008 |
| DNR at dischargec | 1886/5098 (37.0) | 3200/10594 (30.2) | 6.80 (5.20 to 8.37) | 1.40 (1.21 to 1.63) | <.001 |
| ICU mortality | 274 (2.7) | 311 (2.3) | 0.40 (−0.02 to 0.79) | 0.96 (0.71 to 1.29) | .78 |
| In-hospital death | 487 (4.7) | 578 (4.2) | 0.53 (0.02 to 1.06) | 0.86 (0.68 to 1.08) | .19 |
| ICU transferd | 735/9571 (7.7) | 976/13119 (7.4) | 0.24 (−0.45 to 0.94) | 1.13 (0.94 to 1.36) | .19 |
| Readmissions within 30 de | 1869 (18.1) | 2706 (19.7) | −1.55 (−2.55 to −0.55) | 1.01 (0.91 to 1.13) | .82 |
| Mean (SD) | 0.2 (0.5) | 0.2 (0.5) | −0.14 (−0.026 to −0.002) | Rate ratio, 1.02 (0.93 to 1.12) | .67 |
| Palliative care consultation | 4528 (43.9) | 2283 (16.6) | 27.30 (26.16 to 28.45) | 1.63 (1.52 to 1.76) | <.001 |
| Receipt of cardiopulmonary resuscitation | 71 (0.7) | 106 (0.8) | −0.09 (−0.30 to 0.13) | 0.97 (0.56 to 1.68) | .90 |
| Receipt of mechanical ventilation | 321 (3.1) | 412 (3.0) | 0.11 (−0.32 to 0.56) | 0.96 (0.74 to 1.24) | .73 |
| Time to consult, mean (SD), df | 3.4 (2.6) | 4.6 (4.8) | −1.16 (−1.38 to −0.96) | Subdistribution hazard rate, 3.97 (2.29 to 6.86) | <.001 |
The primary analytic sample includes participants with length of stay ≥72 hours.
Estimates adjusted for time, hospital, age, sex, race, ethnicity, marital status, source of hospital admission, Agency for Healthcare Research and Quality Elixhauser Comorbidity Index, eligible diagnosis (kidney failure, COPD, or dementia), number of days between repeated eligible admissions, and intensive care unit (ICU) location at enrollment.
Code status data were not available at 3 hospitals (15 692 missing).
ICU transfer analyses excluded participants who were in an ICU for the entire encounter (1375 excluded).
Readmissions included inpatient admissions to participating hospitals and other Ascension hospitals; observation visits were excluded.
Time-to-consult analyses included participants with a completed palliative care consult (n = 6811), defined as the time from hospital admission to the first signed consult note.
Discussion
In this pragmatic, stepped-wedge cluster randomized trial among older hospitalized patients with advanced noncancer illnesses, ordering palliative care by default (and allowing clinicians to opt-out) without requiring increased staffing did not significantly reduce LOS, but did improve the rate and timing of palliative care consultation and some end-of-life care processes. The World Health Organization32 and professional societies33,34 recommend that hospitalized patients with a broad range of serious illnesses receive palliative care services. Yet health systems have struggled to improve the frequency and timing of palliative care delivery, particularly among patients with noncancer serious illness, and evidence supporting the benefits of inpatient palliative care consultation has been limited. Previous randomized trials of inpatient palliative care have been small, at risk of bias, and conducted in highly controlled settings, primarily among patients with cancer or heart failure in academic medical centers.35,36,37 This pragmatic trial, conducted among diverse hospitals in a large health system, addresses these knowledge gaps by providing 4 key sets of findings.
First, default orders for palliative care consultation nearly tripled the percentage of older adults with advanced COPD, kidney failure, and dementia who were seen by specialist palliative care clinicians. Default orders also reduced the time to consultation by more than 1 day. Delivering palliative care earlier in patients’ hospital courses aligns with guidelines for high-quality palliative care6 and is associated with reduced hospital costs in adults with cancer and noncancer serious illness.38 Furthermore, less than 10% of the default orders were cancelled by clinicians, providing quantitative evidence of high acceptance of default orders for palliative care consultation that aligns with previous qualitive evidence of the acceptability of such nudges for palliative care.39,40 Although default options are understood to be ethical22,41 approaches to change patients’ decisions42 and clinicians’ drug ordering practices,43,44 this study demonstrates that health systems may also use default options to improve the timing and frequency of guideline-recommended clinical consultation.
Second, default orders for palliative care did not affect hospital LOS. Confidence in this result is supported by the consistency of results across multiple sensitivity analyses and different strategies for analyzing LOS. LOS was chosen as the primary outcome based on evidence from smaller palliative care studies and because shorter LOS is valued by all stakeholders.45 However, LOS may be influenced by multiple patient, social, and process-of-care factors that are not modifiable by palliative care. Another potential explanation for this null primary result is that, despite achieving significant separation between groups in consult rates and exceeding the target sample size, a slight majority of patients in the intervention group still did not receive a consult before hospital discharge due to insufficient palliative care team resources. As in other trials attempting to increase recommended care,46 incomplete adherence to the default orders was anticipated because hospitals were neither funded nor instructed to increase palliative care staffing.
Third, although such incomplete adherence reflects the real-world nature of this pragmatic trial, it constrains the potential benefits of the default intervention. Thus, CATE analyses were prespecified to help distinguish between the possibility that palliative care consultation does not impact LOS and the possibility that it does reduce LOS when actually delivered. The CATE analyses showed that when palliative care was delivered, it did reduce LOS among participants who would only have received it if it was ordered by default, although the width of the CI suggests uncertainty about the precise effect. This suggests the efficacy of expanding palliative care delivery and that coupling default orders with increased health system investment in palliative care staffing might yield benefits in future studies.
Fourth, it was found that ordering inpatient palliative care by default increased the odds that patients would have a DNR order at discharge and that they would be discharged to hospice. These changes in end-of-life care among older seriously ill patients occurred without concomitant effects on in-hospital mortality. Although increased mortality would not necessarily have constituted an adverse effect in such a seriously ill population,47 the absence of a change in mortality supports an inference that the observed increases in DNR orders and hospice use may reflect improved goal-concordant care.
Limitations
Results of this analysis must be interpreted in the context of certain limitations. First, although several patient-centered outcomes were measured during and shortly after hospitalization, the trial design did not enable measurement of patient-reported outcomes (eg, quality of life, symptoms) or longer-term outcomes that may be influenced by inpatient palliative care. Similarly, given the multiple influences on LOS, other primary outcomes, such as hospital-free days,48,49 should be considered in future trials of inpatient palliative care interventions. Second, the generalizability of the findings to other seriously ill populations is constrained because patients younger than 65 years and those with other diagnoses commonly referred for palliative care, such as cancer and heart failure, were excluded. These choices were designed to limit burdens on palliative care teams and to generate evidence about palliative care for persons with 3 common serious illnesses that have been underrepresented in prior trials. Third, eligibility screening was incomplete for 20 450 patients due to reliance on busy bedside nurses. Natural language processing methods using existing clinical documentation for screening may overcome the limitations of structured EHR data.
Fourth, although CATE analyses are less susceptible to bias than per-protocol analyses and 2 different CATE analyses produced identical results, these demonstrations of the efficacy of palliative care consultation on LOS require several assumptions that cannot be tested directly.28 Among these, the monotonicity assumption should hold—namely, there should be no patients who would receive palliative care if assigned to usual care but not if assigned to receive it by default. But similar logic cannot be used to verify the stable unit treatment value assumption—that is, the assumption that there are not different versions of palliative care that produce different effects in the 2 groups. Unlike a drug with a reliable biologic effect, palliative care consultation is a human process that might be delivered differently when ordered using an opt-in vs opt-out approach.
Fifth, the study did not measure primary palliative care provided by other clinicians. Health systems are increasingly supportive of training all clinicians to provide palliative care50 to promote sustainable palliative care delivery. If primary palliative care delivery occurred less commonly under the intervention than usual care because clinicians more often deferred to specialists, this could have biased comparisons toward the null. Sixth, what palliative care needs were addressed during consultation was not measured. Although such data may have elucidated mechanisms of inpatient palliative care, it was not stipulated what services specialists should provide because doing so constrains professional autonomy and has proven unsuccessful in other settings.51
Conclusions
These results demonstrate that a default order for palliative care consultation was insufficient to reduce hospital LOS among older patients with advanced COPD, kidney failure, or dementia despite yielding increased and expedited palliative care delivery and improvements in certain processes of end-of-life care. Although secondary analyses provide preliminary evidence that when a palliative care consult is completed for these patients, hospital LOS is reduced, this finding requires replication. Future trials should seek to couple the default intervention with increased palliative care team staffing to enable greater reach in the intervention group or to enrich the target population based on unmet palliative care needs once methods to systematically identify that such patients are ready for use at the point of care.52
Trial protocol
Statistical analysis plan
eMethods, eTables, and eFigures
Data sharing statement
References
- 1.Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi: 10.1089/jpm.2010.0347 [DOI] [PubMed] [Google Scholar]
- 2.Lin RJ, Adelman RD, Diamond RR, Evans AT. The sentinel hospitalization and the role of palliative care. J Hosp Med. 2014;9(5):320-323. doi: 10.1002/jhm.2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fail RE, Meier DE. Improving quality of care for seriously ill patients: opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. doi: 10.12788/jhm.2896 [DOI] [PubMed] [Google Scholar]
- 4.Ferrell BR, Twaddle ML, Melnick A, Meier DE. National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th edition. J Palliat Med. 2018;21(12):1684-1689. doi: 10.1089/jpm.2018.0431 [DOI] [PubMed] [Google Scholar]
- 5.A National Framework and Preferred Practices for Palliative and Hospice Care Quality. National Quality Forum ; 2006. [Google Scholar]
- 6.National Consensus Project for Quality Palliative Care . Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. [Google Scholar]
- 7.America’s Care of Serious Illness: A State-by-State Report Card on Access to Palliative Care in Our Nation’s Hospitals. Center to Advance Palliative Care; 2019. Accessed September 18, 2023. https://reportcard.capc.org/wp-content/uploads/2020/05/CAPC_State-by-State-Report-Card_051120.pdf [DOI] [PMC free article] [PubMed]
- 8.Morrison RS, Penrod JD, Cassel JB, et al. ; Palliative Care Leadership Centers’ Outcomes Group . Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783-1790. doi: 10.1001/archinte.168.16.1783 [DOI] [PubMed] [Google Scholar]
- 9.Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood). 2011;30(3):454-463. doi: 10.1377/hlthaff.2010.0929 [DOI] [PubMed] [Google Scholar]
- 10.Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190. doi: 10.1089/jpm.2007.0055 [DOI] [PubMed] [Google Scholar]
- 11.Smith S, Brick A, O’Hara S, Normand CE. Evidence on the cost and cost-effectiveness of palliative care: A literature review. Palliat Med. 2013;28(2):130-150. doi: 10.1177/0269216313493466 [DOI] [PubMed] [Google Scholar]
- 12.Whitford K, Shah ND, Moriarty J, Branda M, Thorsteinsdottir B. Impact of a palliative care consult service. Am J Hosp Palliat Care. 2014;31(2):175-182. doi: 10.1177/1049909113482746 [DOI] [PubMed] [Google Scholar]
- 13.Starks H, Wang S, Farber S, Owens DA, Curtis JR. Cost savings vary by length of stay for inpatients receiving palliative care consultation services. J Palliat Med. 2013;16(10):1215-1220. doi: 10.1089/jpm.2013.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casarett D, Pickard A, Bailey FA, et al. Do palliative consultations improve patient outcomes? J Am Geriatr Soc. 2008;56(4):593-599. doi: 10.1111/j.1532-5415.2007.01610.x [DOI] [PubMed] [Google Scholar]
- 15.Higginson IJ, Finlay IG, Goodwin DM, et al. Is there evidence that palliative care teams alter end-of-life experiences of patients and their caregivers? J Pain Symptom Manage. 2003;25(2):150-168. doi: 10.1016/S0885-3924(02)00599-7 [DOI] [PubMed] [Google Scholar]
- 16.Casarett D, Johnson M, Smith D, Richardson D. The optimal delivery of palliative care: a national comparison of the outcomes of consultation teams vs inpatient units. Arch Intern Med. 2011;171(7):649-655. doi: 10.1001/archinternmed.2011.87 [DOI] [PubMed] [Google Scholar]
- 17.Elsayem A, Swint K, Fisch MJ, et al. Palliative care inpatient service in a comprehensive cancer center: clinical and financial outcomes. J Clin Oncol. 2004;22(10):2008-2014. doi: 10.1200/JCO.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 18.Liu AY, O’Riordan DL, Marks AK, Bischoff KE, Pantilat SZ. A comparison of hospitalized patients with heart failure and cancer referred to palliative care. JAMA Netw Open. 2020;3(2):e200020. doi: 10.1001/jamanetworkopen.2020.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beernaert K, Cohen J, Deliens L, et al. Referral to palliative care in COPD and other chronic diseases: a population-based study. Respir Med. 2013;107(11):1731-1739. doi: 10.1016/j.rmed.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Steinhauser KE, Arnold RM, Olsen MK, et al. Comparing three life-limiting diseases: does diagnosis matter or is sick, sick? J Pain Symptom Manage. 2011;42(3):331-341. doi: 10.1016/j.jpainsymman.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitner R, Rogers M, Silvers A, Courtright KR, Meier DE. Palliative care team perceptions of standardized palliative care referral criteria implementation in hospital settings. J Palliat Med. 2021;24(5):747-750. doi: 10.1089/jpm.2020.0296 [DOI] [PubMed] [Google Scholar]
- 22.Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi: 10.1056/NEJMsb071595 [DOI] [PubMed] [Google Scholar]
- 23.Courtright KR, Madden V, Gabler NB, et al. Rationale and Design of the Randomized Evaluation of Default Access to Palliative Services (REDAPS) trial. Ann Am Thorac Soc. 2016;13(9):1629-1639. doi: 10.1513/AnnalsATS.201604-308OT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital length of stay. J Palliat Med. 2010;13(6):761-767. doi: 10.1089/jpm.2009.0379 [DOI] [PubMed] [Google Scholar]
- 25.Holloway RG, Quill TE. Mortality as a measure of quality: implications for palliative and end-of-life care. JAMA. 2007;298(7):802-804. doi: 10.1001/jama.298.7.802 [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Halpern SD, Kerlin MP, Small DS. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2017;26(1):292-311. doi: 10.1177/0962280214545121 [DOI] [PubMed] [Google Scholar]
- 27.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. doi: 10.1097/MLR.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 28.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33(13):2297-2340. doi: 10.1002/sim.6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng J, Small DS, Tan Z, Ten Have TR. Efficient nonparametric estimation of causal effects in randomized trials with noncompliance. Biometrika. 2009;96(1):19-36. doi: 10.1093/biomet/asn056 [DOI] [Google Scholar]
- 30.Sussman JB, Hayward RA. An IV for the RCT: using instrumental variables to adjust for treatment contamination in randomised controlled trials. BMJ. 2010;340:c2073. doi: 10.1136/bmj.c2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson TW, Rubin H. Estimation of the parameters of a single equation in a complete system of stochastic equations. Ann Math Stat. 1949;20:46-63. doi: 10.1214/aoms/1177730090 [DOI] [Google Scholar]
- 32.World Health Organization . Strengthening of palliative care as a component of comprehensive care throughout the life course. May 24, 2014. Accessed September 7, 2020. https://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R19-en.pdf
- 33.Halpern SD, Becker D, Curtis JR, et al. ; Choosing Wisely Taskforce; American Thoracic Society; American Association of Critical-Care Nurses; Society of Critical Care Medicine . An official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine policy statement: the Choosing Wisely Top 5 list in Critical Care Medicine. Am J Respir Crit Care Med. 2014;190(7):818-826. doi: 10.1164/rccm.201407-1317ST [DOI] [PubMed] [Google Scholar]
- 34.Fischberg D, Bull J, Casarett D, et al. ; HPM Choosing Wisely Task Force . Five things physicians and patients should question in hospice and palliative medicine. J Pain Symptom Manage. 2013;45(3):595-605. doi: 10.1016/j.jpainsymman.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 35.Quinn KL, Shurrab M, Gitau K, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA. 2020;324(14):1439-1450. doi: 10.1001/jama.2020.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ernecoff NC, Check D, Bannon M, et al. Comparing specialty and primary palliative care interventions: analysis of a systematic review. J Palliat Med. 2020;23(3):389-396. doi: 10.1089/jpm.2019.0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness: a meta-analysis. JAMA Intern Med. 2018;178(6):820-829. doi: 10.1001/jamainternmed.2018.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh RB, Manz CR, Nelson MN, et al. Oncologist perceptions of algorithm-based nudges to prompt early serious illness communication: a qualitative study. J Palliat Med. 2022;25(11):1702-1707. doi: 10.1089/jpm.2022.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders S, Downar J, Subramaniam S, Embuldeniya G, van Walraven C, Wegier P. mHOMR: the acceptability of an automated mortality prediction model for timely identification of patients for palliative care. BMJ Qual Saf. 2021;30(10):837-840. doi: 10.1136/bmjqs-2020-012461 [DOI] [PubMed] [Google Scholar]
- 41.Gorin M, Joffe S, Dickert N, Halpern S. Justifying clinical nudges. Hastings Cent Rep. 2017;47(2):32-38. doi: 10.1002/hast.688 [DOI] [PubMed] [Google Scholar]
- 42.Halpern SD, Small DS, Troxel AB, et al. Effect of default options in advance directives on hospital-free days and care choices among seriously ill patients: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e201742. doi: 10.1001/jamanetworkopen.2020.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel MS, Day SC, Halpern SD, et al. Generic medication prescription rates after health system-wide redesign of default options within the electronic health record. JAMA Intern Med. 2016;176(6):847-848. doi: 10.1001/jamainternmed.2016.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado MK, Shofer FS, Patel MS, et al. Association between electronic medical record implementation of default opioid prescription quantities and prescribing behavior in two emergency departments. J Gen Intern Med. 2018;33(4):409-411. doi: 10.1007/s11606-017-4286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khandelwal N, Brumback LC, Halpern SD, Coe NB, Brumback B, Curtis JR. Evaluating the economic impact of palliative and end-of-life care interventions on intensive care unit utilization and costs from the hospital and healthcare system perspective. J Palliat Med. 2017;20(12):1314-1320. doi: 10.1089/jpm.2016.0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bretthauer M, Løberg M, Wieszczy P, et al. ; NordICC Study Group . Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi: 10.1056/NEJMoa2208375 [DOI] [PubMed] [Google Scholar]
- 47.Halpern SD, Temel JS, Courtright KR. Dealing with death as an outcome in supportive care clinical trials. JAMA Intern Med. 2021;181(7):895-896. doi: 10.1001/jamainternmed.2021.1816 [DOI] [PubMed] [Google Scholar]
- 48.Auriemma CL, Butt MI, Silvestri JA, Halpern SD, Courtright KR. Stakeholder perspectives on minimum clinically important difference and noninferiority margin for hospital-free days to assess interventions. JAMA Intern Med. 2023;183(7):739-742. doi: 10.1001/jamainternmed.2023.0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auriemma CL, Taylor SP, Harhay MO, Courtright KR, Halpern SD. Hospital-free days: a pragmatic and patient-centered outcome for trials among critically and seriously ill patients. Am J Respir Crit Care Med. 2021;204(8):902-909. doi: 10.1164/rccm.202104-1063PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers MM, Chambers B, Esch A, Meier DE, Bowman B. Use of an online palliative care clinical curriculum to train US hospital staff: 2015-2019. J Palliat Med. 2021;24(4):488-495. doi: 10.1089/jpm.2020.0514 [DOI] [PubMed] [Google Scholar]
- 51.Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316(1):51-62. doi: 10.1001/jama.2016.8474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarmet M, Kabani A, Coelho L, Dos Reis SS, Zeredo JL, Mehta AK. The use of natural language processing in palliative care research: a scoping review. Palliat Med. 2023;37(2):275-290. doi: 10.1177/02692163221141969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods, eTables, and eFigures
Data sharing statement