Key Points
Question
Can a nurse and social worker palliative telecare team improve quality of life in outpatients with chronic obstructive pulmonary disease (COPD), heart failure (HF), and interstitial lung disease (ILD)?
Findings
This randomized clinical trial included 306 participants with COPD, HF, or ILD at high risk of hospitalization or death, who reported poor quality of life. Compared with usual care, participants in the intervention reported significantly improved quality of life on the Functional Assessment of Chronic Illness Therapy–General (FACT-G) measure at 6 months (difference in change from baseline, 4.6 points; minimal clinically important difference, ≥4 points).
Meaning
For adults with COPD, HF, or ILD at high risk of hospitalization and death and poor quality of life, a nurse and social worker palliative telecare team demonstrated clinically meaningful improvements in quality of life at 6 months.
Abstract
Importance
Many patients with chronic obstructive pulmonary disease (COPD), heart failure (HF), and interstitial lung disease (ILD) endure poor quality of life despite conventional therapy. Palliative care approaches may benefit this population prior to end of life.
Objective
Determine the effect of a nurse and social worker palliative telecare team on quality of life in outpatients with COPD, HF, or ILD compared with usual care.
Design, Setting, and Participants
Single-blind, 2-group, multisite randomized clinical trial with accrual between October 27, 2016, and April 2, 2020, in 2 Veterans Administration health care systems (Colorado and Washington), and including community-based outpatient clinics. Outpatients with COPD, HF, or ILD at high risk of hospitalization or death who reported poor quality of life participated.
Intervention
The intervention involved 6 phone calls with a nurse to help with symptom management and 6 phone calls with a social worker to provide psychosocial care. The nurse and social worker met weekly with a study primary care and palliative care physician and as needed, a pulmonologist, and cardiologist. Usual care included an educational handout developed for the study that outlined self-care for COPD, ILD, or HF. Patients in both groups received care at the discretion of their clinicians, which could include care from nurses and social workers, and specialists in cardiology, pulmonology, palliative care, and mental health.
Main Outcomes and Measures
The primary outcome was difference in change in quality of life from baseline to 6 months between the intervention and usual care groups (FACT-G score range, 0-100, with higher scores indicating better quality of life, clinically meaningful change ≥4 points). Secondary quality-of-life outcomes at 6 months included disease-specific health status (Clinical COPD Questionnaire; Kansas City Cardiomyopathy Questionnaire-12), depression (Patient Health Questionnaire-8) and anxiety (Generalized Anxiety Disorder-7) symptoms.
Results
Among 306 randomized patients (mean [SD] age, 68.9 [7.7] years; 276 male [90.2%], 30 female [9.8%]; 245 White [80.1%]), 177 (57.8%) had COPD, 67 (21.9%) HF, 49 (16%) both COPD and HF, and 13 (4.2%) ILD. Baseline FACT-G scores were similar (intervention, 52.9; usual care, 52.7). FACT-G completion was 76% (intervention, 117 of 154; usual care, 116 of 152) at 6 months for both groups. Mean (SD) length of intervention was 115.1 (33.4) days and included a mean of 10.4 (3.3) intervention calls per patient. In the intervention group, 112 of 154 (73%) patients received the intervention as randomized. At 6 months, mean FACT-G score improved 6.0 points in the intervention group and 1.4 points in the usual care group (difference, 4.6 points [95% CI, 1.8-7.4]; P = .001; standardized mean difference, 0.41). The intervention also improved COPD health status (standardized mean difference, 0.44; P = .04), HF health status (standardized mean difference, 0.41; P = .01), depression (standardized mean difference, −0.50; P < .001), and anxiety (standardized mean difference, −0.51; P < .001) at 6 months.
Conclusions and Relevance
For adults with COPD, HF, or ILD who were at high risk of death and had poor quality of life, a nurse and social worker palliative telecare team produced clinically meaningful improvements in quality of life at 6 months compared with usual care.
Trial Registration
ClinicalTrials.gov Identifier: NCT02713347
This randomized clinical trial compares differences in quality of life among 306 adults at high risk of death who have COPD, heart failure, or interstitial lung disease and who received nurse and social worker palliative telecare vs usual care.
Introduction
Chronic obstructive pulmonary disease (COPD), chronic heart failure (HF), and interstitial lung disease (ILD) can cause severe morbidity, making them ideal for early palliative care provided alongside disease-specific treatments. Quality of life is reduced in these illnesses because, despite disease-specific treatments, breathlessness, fatigue, pain, and sleep disturbance reduce quality of life and persist over time.1,2,3,4 Between 20% and 30% of patients with HF5 or COPD6 have depressive disorders. Between 50% and 60% have clinically significant depressive symptoms.5,7 Anxiety and depressive symptoms are also common in ILD.8 In addition, HF and COPD commonly co-occur.9
The National Academy of Medicine, the World Health Organization, and the National Quality Forum call for palliative care to be available to patients with serious illnesses in the community (ie, outpatient) setting.10 The 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America joint guideline for the management of heart failure recommends that palliative care be integrated into the care of all patients with heart failure.11 Similar calls are made by the American Thoracic Society12 and the American College of Chest Physicians.13 Yet access to outpatient palliative care, particularly for patients with COPD, HF, or ILD, is largely absent in the United States.10,14 Furthermore, evidence of successful ways to provide outpatient palliative care in COPD, HF, or ILD are lacking. Disease management programs tend to focus on disease-specific therapies rather than nonspecific, palliative (ie, symptom or quality of life–focused) approaches. Given limited numbers of palliative care specialists, providing outpatient palliative care for patients with serious noncancer illnesses such as COPD, HF, and ILD require new care models.15
Accordingly, we developed the Advancing Symptom Alleviation With Palliative Treatment (ADAPT) intervention. The ADAPT intervention built on our prior work with the Collaborative Care to Alleviate Symptoms and Adjust to Illness (CASA) intervention16 and was designed to be integrated into ongoing outpatient care and be scalable. The multisite ADAPT trial evaluated the effect of a nurse and social worker palliative telecare team on the primary outcome of quality of life in adult outpatients with COPD, HF, or ILD at high risk of hospitalization and death who reported poor quality of life.
Methods
Study Design
The ADAPT study was a single-blind, 2-group, patient-level randomized clinical trial that compared a nurse and social worker palliative telecare team to usual care in 2 Veterans Health Administration (VA) health systems (Colorado, Washington). The methods have been previously reported (Supplement 1).17 This article reports the primary quality-of-life outcome, secondary quality-of-life and mood outcomes, and hospitalization and mortality outcomes.
Population
Patients with a hospital discharge diagnosis or 2 or more outpatient visits with COPD, HF, or both diagnoses at either VA health system were identified from the electronic health record.17 Eligibility was expanded to patients with ILD in May 2018 to enhance recruitment. We used the Care Assessment Need score to identify patients in the top 20th percentile of risk for hospitalization or death in the next year.18 The probability of death or hospitalization at 1 year at this cutoff ranged from 16% to 98%. We reviewed medical records to confirm COPD, HF, and ILD diagnoses and evaluate the following exclusion criteria: metastatic cancer, current substance misuse, current incarceration, pregnancy, or participation in the CASA intervention. Potentially eligible patients were contacted to confirm whether they met criteria for poor quality of life (Functional Assessment of Chronic Illness Therapy–General [FACT-G] score ≤70),19 symptoms (bothered by ≥1: pain, fatigue, depression, shortness of breath, or trouble sleeping), presence of a primary care clinician, not living in a nursing home, consistent access to a phone, and not having a prior heart or lung transplant.
Eligible patients who provided informed consent completed baseline measures and were randomized to the ADAPT intervention or to usual care. The randomization sequence was computer generated by the statistician using random block sizes, stratified by study site and disease, and concealed from study personnel. Participants were enrolled by coordinators blinded to the study group, who entered patients’ self-reported outcome data. The study nurse or social worker communicated with participants based on their corresponding randomized group. Accrual occurred between October 2016 and April 2020, ending when the goal sample size was accrued.
This study was approved by the Colorado multiple institutional review board (15–1891), the VA Puget Sound multiple institutional review board (00857). The trial was regularly reviewed by the VA independent data and safety monitoring committee.
ADAPT Intervention
ADAPT was a team intervention based on the effective collaborative care model20 applied to palliative care. Collaborative care was used because this model of care is scalable and effective in improving depression and other symptoms in medically ill patients.21,22,23 A registered nurse addressed symptoms and a social worker provided structured counseling. The nurse and social worker were trained to provide the ADAPT intervention; they were not specialist palliative care clinicians. The intervention was conducted by phone and codified in treatment manuals and structured templates. Six phone calls each (nurse and social worker) were planned twice a month. The number and duration of these calls were allowed to vary depending on the patients’ needs. The ADAPT intervention is a revised version of the CASA intervention16; it was modified based on participant and interventionist feedback and supplemented with goals of care communication.24
During the first call, the patient and nurse selected an initial symptom (from a choice of pain, breathlessness, fatigue, sleep disturbance, or depression) to target for the intervention.2,25 The nurse assessed and managed symptoms using structured guidelines that included disease-specific and palliative approaches.17 Additional symptoms were addressed if needed. The nurse was trained on helping communication (1 hour), motivational interviewing (4 hours), and the symptom guidelines (3 hours).
In subsequent calls, the nurse implemented team recommendations and reassessed symptoms using a structured symptom rating scale; identified an activity goal with the patient and helped with barriers to progress; and provided education about the patient’s disease and navigation through health care system. Symptom data were used by the intervention team for ongoing management of symptoms that were not improving. The nurse applied motivational interviewing to promote changes in health behaviors (eg, medication adherence, diet, physical activities) that could improve patient symptoms.26
The social worker conducted an initial psychosocial assessment and provided 5 counseling calls to improve adjustment to illness and depression symptoms, if present.27 The counseling included the following topics: pacing, deep breathing and relaxation, care goals,24 change in role and asking for/accepting help (optional calls included grief/loss/acceptance and rumination). The social worker received 8 hours of psychosocial intervention training and follow-up supervision.
The nurse and social worker met weekly with a study primary care clinician, palliative care specialist, and a cardiologist and pulmonologist as needed. Based on review of patients’ medical records and discussion with the nurse and social worker, the team ordered tests and medications for patients’ primary care clinician to approve. Each site had its own intervention team, although the same nurse provided the nursing intervention for both health systems during much of the study.
Usual Care
Patients in the usual care group received care at the discretion of their clinicians, which could include care from cardiology, pulmonology, palliative care, and mental health specialties. Patients were given an educational handout developed for the study that outlined self-care for COPD, HF, or ILD. For patients in the usual care group who had significant depressive symptoms, we notified the patient and their primary care clinician. Primary care clinicians assumed responsibility treating their patient’s depression.
Outcomes
The primary outcome was difference in change in quality of life from baseline to 6 months between the ADAPT intervention group and the usual care group in patient-reported quality of life assessed with the FACT-G. The FACT-G is a valid, reliable quality-of-life questionnaire28,29 used to measure quality of life in HF, COPD, and ILD30,31,32,33,34 with validity in noncancer illnesses demonstrated by correlation with disease severity35 and responsiveness. Population norms have been established, allowing it to be used across study populations.28 It is responsive to specialist palliative care interventions.36 It measures 4 subscales that contribute to quality of life: physical, social/family, emotional, and functional well-being. The questionnaire consists of 27 self-report items, each scored on a 5-point Likert scale. Total FACT-G scores range from 0 to 108, with higher scores indicating better quality of life and a minimal clinically important difference of 4. The FACT-G was also assessed at month 12 to explore whether intervention effects persisted. Disease-specific quality of life was measured in participants with HF using the Kansas City Cardiomyopathy Questionnaire (KCCQ-12 [range, 0-100 points]; minimal clinically important difference, 3.6-5 points)37,38 and in those with COPD using the Clinical COPD Questionnaire (CCQ [range, 0-6 points]; minimal clinically important difference, 0.4 points).39 Outcomes for the small number of participants with ILD were not studied separately. Depressive symptoms were assessed using the Patient Health Questionnaire-8 (PHQ-8 [range, 0-24 points]; minimal clinically important difference, 3 points).40,41,42 Anxiety symptoms were measured using the Generalized Anxiety Disorder Questionnaire (GAD-7 [range, 0-21 points]; minimal clinically important difference, 2-4 points).41,43,44 Survey data were self-reported by participants who were compensated $10 for completing the baseline, 4-month, and 12-month surveys and $15 for completing the 6-month survey. Demographics, including race and ethnicity data, were collected by patient report at baseline according to National Institutes of Health categories to assist in understanding representativeness of the sample. Hospitalizations and all-cause mortality were assessed by medical records in each health system supplemented with patient or family self-report.
Sample Size
We planned a sample size of 300 to detect a standardized mean difference of 0.4, which corresponds with a clinically meaningful change in mean (SD) FACT-G score of 4 (10) points.45 We anticipated 25% of participants would have missing primary outcome data due to death, dropping out, or being unreachable. Thus, approximately 113 veterans per study group would provide primary outcome data at 6 months. With this sample size, we would have approximately 85% power to detect a clinically meaningful effect (2-sided test, α = .05).
Statistical Analysis
Data from all participants were analyzed as randomized regardless of intervention adherence (Supplement 2). For descriptive purposes, participants were classified as receiving the intervention as randomized if they received nursing calls that included all nursing topics, all social work topics (including goals of care) and a close-out call. Fidelity was assessed through review of audio recordings of intervention calls and review of interventionist call documentation. Fidelity was measured using 22 items (nurse) and 21 items (social worker) related to specific intervention components. All nurse and social worker intervention calls of a random sample of 10% to 15% of intervention participants were reviewed for fidelity by a nurse or social worker who did not provide the intervention calls being reviewed.
In the primary analysis, we prespecified a plan to exclude surveys that were obtained beyond 31 days before or after the data collection due date. Analysis models were adjusted for the disease stratification variable used in randomization. Analyses of the repeated measures was performed with SAS version 9.4 using maximum likelihood estimation for incomplete data, linear mixed models for continuous outcomes, and generalized linear mixed models with a logit link for binary end points. Time-specific differences between treatment groups were estimated from these models at baseline, 4, 6, and 12 months. To understand the potential impact of missing data, we examined plots of group means over time, stratified by the time of the last completed observation, to determine if biases were evident due to missing data.46 Using this method, data did not appear to have informative missingness. Sensitivity analyses included the following items: (1) including survey data outside of our prespecified exclusion window of 31 days before or after survey due dates, and (2) using medical record review definitions of disease, which differed slightly from the randomization definition.
Heterogeneity of treatment effect for the FACT-G was examined among those with HF only vs COPD only. There were not enough data available to assess for heterogeneity of treatment effect in patients with ILD. A 3-way interaction of time, intervention group, and disease indicator was examined using a similar linear mixed model. We examined whether the number of hospitalizations at 6 months differed between treatment groups (0, 1, 2 or more hospitalizations) using a Pearson χ2 test. Survival was compared between treatment groups through 12 months using a log-rank test.
Results
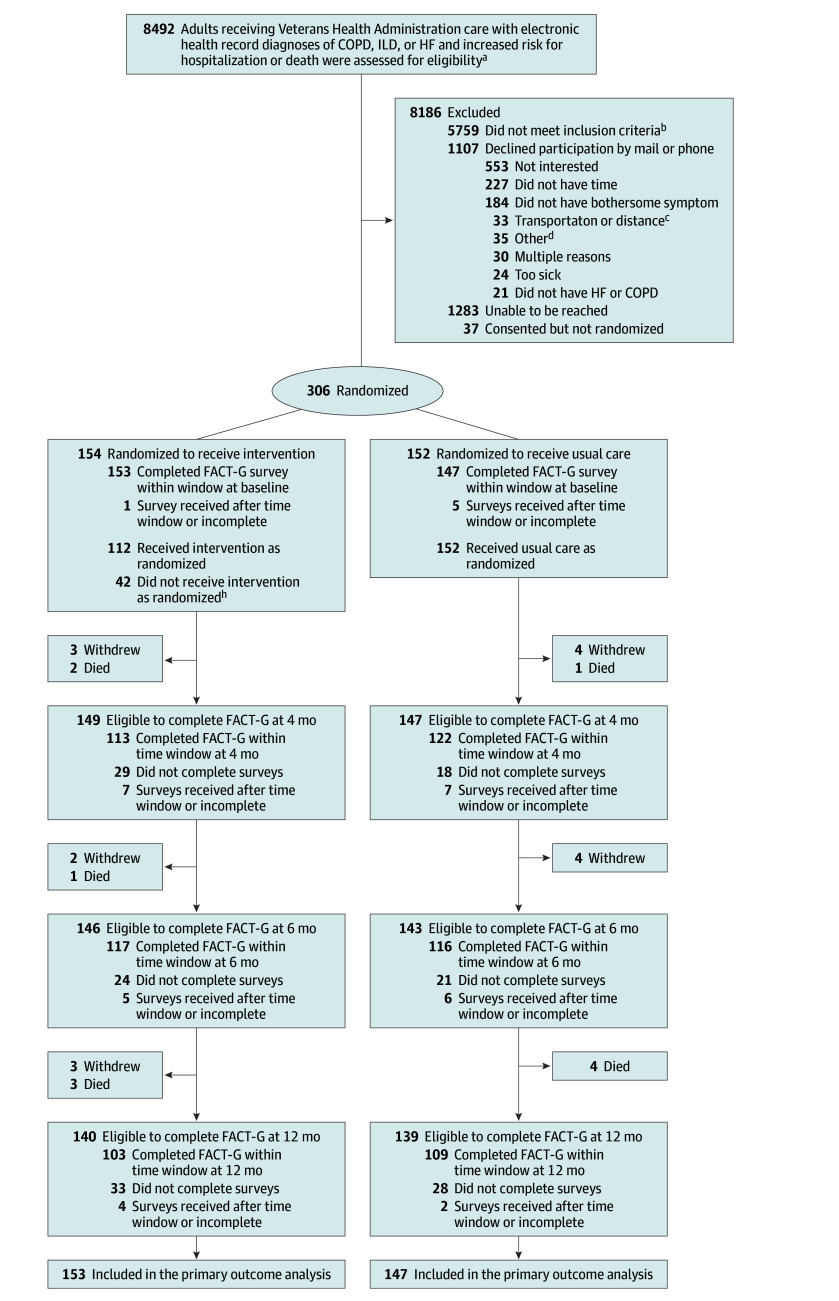
All potentially eligible patients in both health systems with diagnostic codes for COPD, HF, or ILD were evaluated for eligibility (n = 8492). Most (n = 5759) did not meet the disease, severity, poor quality of life, or other inclusion criteria (Figure 1). The recruitment goal of 300 was met, and 306 were randomized (154 to the ADAPT intervention and 152 to usual care). The overall population was predominantly male (90.2%), White (80.1%), and the mean (SD) age was 68.9 (7.7) years (Table; eAppendix, eTable 1, and eTable 2 in Supplement 3). There was a range of education (≤28% completed high school or less) and income (58% total household income ≤$40 000). Among the randomized patients, 177 (58%) had COPD only, 67 (22%) had HF only, 49 (16%) had COPD and HF, and 13 (4%) had ILD. Almost half had been hospitalized in the prior 12 months, and 21% had 2 or more hospitalizations. Many were seeing mental health specialists for medication management (28%) or counseling (32%). Sixty-one percent of patients with COPD and 77% of patients with ILD had seen a pulmonologist, and 75% of patients with HF had seen a cardiologist in the past 6 months. One patient had seen a palliative care specialist.
Figure 1. Screening, Enrollment, Randomization, and Follow-Up in the ADAPT Trial.
aSee Population section for eligibility criteria. bMost did not meet criteria because they did not have a diagnosis of HF or COPD or their FACT-G score was >70 (see Outcomes section for explanation) or they were not bothered by any target symptoms. Specific numbers for reasons not meeting each eligibility criterion are not available because (1) patients were reconsidered during the accrual period to see if they developed eligibility, and the reasons for noneligibility changed with subsequent medical record reviews or screening calls; or (2) some disease-specific criteria changed during the accrual period, including dropping the requirement for spirometry. cIncluded concerns with the ability to get facility-based medical care recommended by the intervention. dPhone problems (16) were most common.
Table. Baseline Sociodemographic and Clinical Characteristics by Randomization Status.
| Characteristic | No. (%)a | |
|---|---|---|
| ADAPT intervention (n = 154) | Usual care (n = 152) | |
| Demographic, No. (%) | ||
| Age, mean (SD), y | 68.87 (8.04) | 68.88 (7.42) |
| Female | 15 (9.7) | 15 (9.9) |
| Male | 139 (90.3) | 137 (90.1) |
| Ethnicity (n = 273)b | ||
| Hispanic | 10 (7.2) | 8 (6.0) |
| Non-Hispanic | 128 (92.8) | 127 (94.1) |
| Race (n = 301)b | ||
| American Indian or Alaska Native | 2 (1.3) | 1 (0.7) |
| Asian or Pacific Islander | 1 (0.7) | 2 (1.3) |
| Black or African American | 13 (8.6) | 7 (4.7) |
| Native Hawaiian or Pacific Islander | 1 (0.7) | 1 (0.7) |
| White | 118 (78.1) | 127 (84.7) |
| Otherc | 16 (10.6) | 12 (8.0) |
| Employment status | ||
| Retired | 73 (47.4) | 90 (59.2) |
| Disabled | 49 (31.8) | 38 (25.0) |
| Employed full time | 8 (5.2) | 4 (2.6) |
| Employed part time | 11 (7.1) | 7 (4.6) |
| Educational level | ||
| <High school completion | 5 (3.2) | 1 (0.7) |
| High school graduate or GED | 38 (24.7) | 41 (27.0) |
| Some college | 73 (47.4) | 66 (43.4) |
| College graduate | 21 (13.6) | 28 (18.4) |
| Any postgraduate education | 15 (9.7) | 16 (10.5) |
| Annual income, $ | ||
| ≤20 000 | 29 (18.8) | 38 (25.0) |
| 20 001-40 000 | 52 (33.8) | 58 (38.1) |
| 40 001-60 000 | 36 (23.4) | 25 (16.5) |
| >60 000 | 31 (20.1) | 23 (15.1) |
| Medical history, collected from medical record review | ||
| COPD | 112 (72.7) | 114 (75.0) |
| Hypertension | 107 (69.5) | 109 (71.7) |
| Obstructive sleep apnea | 80 (51.9) | 71 (46.7) |
| Diabetes | 76 (49.4) | 65 (42.8) |
| Depression | 74 (48.1) | 85 (55.9) |
| Heart failure | 62 (40.3) | 54 (35.5) |
| Atrial fibrillation or atrial flutter | 38 (24.7) | 38 (25.0) |
| Myocardial infarction | 38 (24.7) | 25 (16.4) |
| Percutaneous coronary intervention | 37 (24.0) | 26 (17.1) |
| Coronary artery bypass graft | 17 (11.0) | 15 (9.9) |
| Stroke or transient ischemic attack | 11 (7.1) | 15 (9.9) |
| Interstitial lung disease | 6 (4.0) | 7 (4.6) |
| Clinical characteristicsd | ||
| Comorbidities, mean (SD) | 7.7 (2.4) | 7.5 (2.3) |
| Hospitalized in the prior 12 mo | 74 (49.0) | 68 (45.3) |
| ≥2 Hospitalizations in the prior 12 mo | 30 (19.5) | 34 (22.4) |
| Specialty care in prior 6 mod | ||
| Mental health: medication management | 44 (28.6) | 41 (27.0) |
| Mental health: counseling | 52 (33.8) | 47 (30.9) |
| Pain specialist | 27 (17.5) | 28 (18.4) |
| Palliative care | 0 | 1 (0.7) |
| Opiate prescription | 36 (23.4) | 33 (21.7) |
| Antidepressant prescription | 62 (40.3) | 62 (40.8) |
| Heart failure characteristics | n = 62 | n = 54 |
| Left ventricular ejection fractiond | ||
| ≥50%, Normal | 31 (50.0) | 21 (39.6) |
| 40%-49%, Mildly reduced | 14 (22.6) | 19 (35.8) |
| 30%-39%, Moderately reduced | 10 (16.1) | 7 (13.2) |
| <30%, Severely reduced | 6 (9.7) | 6 (11.3) |
| New York Heart Association classe | ||
| 1, No limitation of physical activity | 3 (6.0) | 5 (10.2) |
| 2, Slight limitation of physical activity; comfortable at rest | 13 (26.0) | 17 (34.7) |
| 3, Marked limitation of physical activity; comfortable at rest | 25 (50.0) | 20 (40.8) |
| 4, Symptoms of heart failure at rest; any physical activity causes further discomfort | 9 (18.0) | 7 (14.3) |
| Cardiologist visit in prior 6 mo | 44 (71) | 43 (76.6) |
| COPD characteristicsf | n = 112 | n = 114 |
| GOLD staged,f | n = 78 | n = 71 |
| 1 or 2, Mild or moderate, | 41 (53) | 37 (52) |
| 3 or 4, Severe or very severe | 37 (47) | 34 (48) |
| Oxygen use | 72 (64.3) | 69 (62.2) |
| At rest | 52 (46.4) | 50 (45.0) |
| With exertion | 54 (48.2) | 53 (47.7) |
| During sleep | 61 (54.5) | 58 (52.2) |
| Pulmonologist visit in prior 6 mo | 45 (40.2) | 42 (36.8) |
| Initial symptom targetedg | n=153 | |
| Shortness of breath | 52 (34.0) | |
| Pain | 23 (15.0) | |
| Sleep disturbance | 23 (15.0) | |
| Depression | 22 (14.4) | |
| Fatigue | 22 (14.4) | |
| Other symptom | 16 (10.5) | |
Abbreviations: COPD, chronic obstructive lung disease; GED, General Educational Development; GOLD, global initiative for COPD; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
Data are No. (%) unless otherwise reported.
Race and ethnicity were collected by self-report that allowed a single selection from a predetermined list.
Indicates self-reported race not included on the list. See eAppendix in Supplement 3 for itemization.
Collected using medical record review.
Determined by participant rating. Additional heart failure–specific data are provided in eTable 1 in Supplement 3.
Determined using definitions at the time of study initiation, which required postbronchodilator spirometry for all stages of COPD (stage 1, mild [ FEV1 > 80% predicted]; 2, moderate [FEV1 50%-79% predicted]; 3, severe [ FEV1 30%-<50% predicted]; 4, very severe [FEV1 < 30% predicted]). Oxygen use was missing in 3 usual care patients. Additional COPD-specific data are provided in eTable 2 in Supplement 3. Prebronchodilator values were used only when postbronchodilator values were unavailable.
One intervention participant did not report an initial target symptom, and 5 chose 2 symptoms.
At baseline, patients reported poor overall quality of life (FACT-G) (Figure 2 and Figure 3) and poor disease-specific health status (Figure 4). Mean depression symptoms were above the screening cutoff for depressive disorder (≥10), indicating moderate depressive symptoms, and mean anxiety symptoms were in the mild to moderate range.
Figure 2. Regression-Estimated Means and Change Scores for Quality-of-Life Outcomes.

Values were obtained from linear mixed models (interaction between treatment status and dummy variables for time) and adjusted for disease status at randomization. All outcome measures are patient-reported. aSee Outcomes section for explanation of the FACT-G. bFor physical and for social/family well-being, the score range is 0 to 28; for emotional and for functional well-being, the score range is 0 to 24. Higher scores indicate better well-being; minimal clinically important difference [MCID], 2-3. cScore range, 0 to 24 (higher scores indicate more depressive symptoms; MCID, 3). Negative mean difference scores indicate improvement in symptoms.dScore range, 0 to 21 (higher scores indicate more anxiety symptoms; MCID, 2-4). Negative mean difference scores indicate improvement in symptoms.
Figure 3. Six-Month Change in FACT-G Score (Primary Outcome) by Participant and Randomization Group.

The FACT-G is patient-reported (score range, 0-108, with higher scores indicating better quality of life; minimal clinically important difference, 4). For box plots, the ends of the boxes are located at the first and third quartiles. The horizontal black line in the middle illustrates the median, and the diamonds indicate the mean. Whiskers extend to the highest and lowest values within 1.5 times the IQR, and markers outside the boxes indicate outlying data. The parallel line plot contains 1 vertical line for each participant, which extends from their baseline value to their 6-month value. Descending lines indicate a reduction in outcome. Baseline values are placed in ascending order for the ADAPT intervention group and descending order for the usual care group.
Figure 4. Regression-Estimated Mean Values and Change Scores for Disease-Specific Health Status Outcomes.
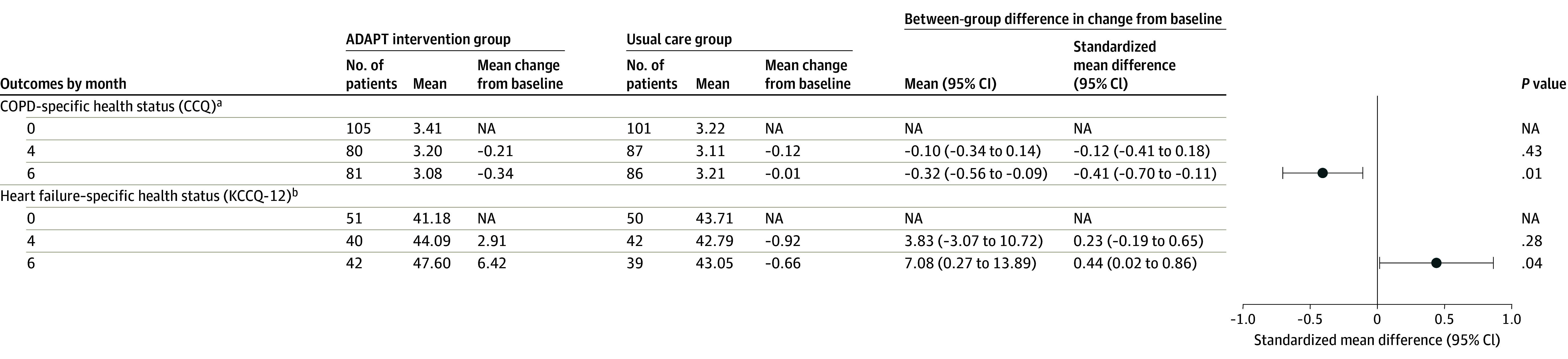
aCOPD-specific health status (CCQ) is patient-reported (score range, 0-6; higher scores indicate worse health status; minimal clinically important difference, 0.4).For the CCQ, negative mean difference scores indicate better status.
bHeart failure–specific health status (KCCQ-12) is patient-reported (score range, 0-100; higher scores indicate better health status; minimal clinically important difference, 3.6-5). See the eFigure in Supplement 1.
Between 67% and 80% of participants completed the FACT-G primary outcome measure at various time points (Figure 1). Among the 154 patients randomized to the ADAPT intervention group, 112 (73%) received the intervention as randomized. There was a mean (SD) of 10.4 (3.3) intervention calls per patient (8.6 [2.9] from the nurse and 7.1 [2.3] from the social worker; many were joint nurse and social worker calls). The intervention duration was a mean of 115.1 (33.4) days. All intervention calls with 21 participants (14% of those randomized to the ADAPT group) were reviewed for intervention fidelity. Fidelity was 99.8% for nurse call components and 98.5% for social worker components.
At 6 months, the primary outcome of mean FACT-G score improved 6.0 points in the intervention group (117/154 [76.0%] reporting) and 1.4 points in the usual care arm (116/152 [76.3%] reporting) (difference, 4.6 [95% CI, 1.8-7.4]; P = .001; standardized mean difference, 0.41) (Figure 2 and Figure 3; eFigure in Supplement 3). The test for heterogeneity comparing effects on those with HF only vs those with COPD only was not statistically significant (P = .64). The nurse and social worker palliative telecare team also improved COPD health status (standardized mean difference, 0.41; P = .01), HF health status (standardized mean difference, 0.44; P = .04), depression (standardized mean difference, −0.50; P < .001), and anxiety (standardized mean difference, −0.51; P < .001) symptoms at 6 months (Figure 2 and Figure 4; eFigure in Supplement 3). In exploratory analyses, the positive intervention effect was observed at all time points (4-month difference, 3.5 [95% CI, 0.6-6.4]; standardized mean difference, 0.30 [P = .02]; 12-month difference, 4.9 [95% CI, 1.4-8.4]; standardized mean difference, 0.36 [P = .007]). Sensitivity analyses showed no significant differences.
There were no adverse events or harms attributed to the intervention. At 6 months, there was no difference between the intervention and usual care groups among patients who had not been hospitalized (109, intervention; 119, control), hospitalized once (24, intervention; 17, control), or hospitalized twice or more (9, intervention; 9, control) (P = .45). At 1 year, 6 of 154 (3.9%) patients in the intervention group and 5 of 152 (3.3%) in the usual care group had died (P = .76).
Discussion
In two large VA health systems, a nurse and social worker palliative telecare team demonstrated early, persistent, and clinically meaningful improvements in quality of life for symptomatic, high-risk patients with COPD, HF, or ILD. Study strengths included the attempt to reach all potentially eligible patients in both health systems; study population education and income diversity; virtual nature of the intervention; intervention integration into primary care and efficient use of nurses, social workers, physicians and specialists; high intervention fidelity; and reasonably high follow-up rates. The ADAPT intervention improved overall quality of life; depression and anxiety symptoms for COPD, HF, and ILD; and disease-specific health status for both COPD and HF. The intervention lasted a mean of 3.8 months, and the improvement in quality of life persisted to 12 months.
Larger intervention effects were seen on the FACT-G emotional well-being subscale as well as on depression and anxiety symptoms. This suggests that the intervention improved quality of life, in part, by helping patients cope with the limitations and symptoms of illness. These data are consistent with the ADAPT conceptual framework, which posited that improving adjustment to illness and addressing symptoms (including psychological symptoms) would improve quality of life. A process evaluation of ADAPT is underway to inform the mechanisms of ADAPT success and intervention cost estimates.17
According to a recent Agency for Healthcare Research and Quality meta-analysis, interventions integrating palliative care into ambulatory care of noncancer serious illness have generally been ineffective in addressing quality of life and depression.15 ADAPT is one of a very limited number of randomized clinical trials of outpatient palliative care in COPD, several of which have had mixed or negative effects.47,48 In HF, the Pal-HF trial found a specialist palliative care team improved multiple quality-of-life outcomes,31 while the ENABLE CHF-PC telecare nursing intervention did not influence quality of life or mood.30 The ADAPT intervention differed in several ways from these interventions, including its use of collaborative care, a nurse and social worker, structured counseling, and integration of information from this intervention into ongoing outpatient care.
Compared with other interventions described in this paragraph, ADAPT was a single intervention that led to improvements for multiple illnesses, not only in overall quality of life but also depression and anxiety symptoms and disease-specific health status. The magnitude of the intervention effects on quality of life, depression, and anxiety are comparable with or greater than other palliative care and disease-specific care delivery interventions, pharmacotherapy, and psychotherapy.49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65 Specifically, palliative care has demonstrated a standardized mean effect of 0.27 (95% CI, 0.15-0.38) on patient quality of life in advanced cancer in a Cochrane review49 and 0.46 (95% CI, 0.08-0.83) in another meta-analysis.50 Some disease-specific virtual care delivery interventions improve quality of life in similarly ill patients.51,52 Pharmacologic treatments alone have generally not improved depression in COPD,53 while cognitive-behavioral therapy demonstrated beneficial effects in meta-analyses (standardized mean difference, 0.19-0.54).54,55 Psychological and pharmacologic therapies for anxiety in COPD show a possible effect of cognitive-behavioral therapy,55 yet there are few studies, heterogeneity, and low-quality evidence.56,57 Behavioral interventions tend to show benefit for depression in HF,58,59,60 yet pharmacotherapy has not demonstrated benefit.61,62 Greater than 8 sessions of cognitive-behavioral therapy is usually required for improvement of depression in COPD.55,63 In ILD, a nurse-led early palliative care intervention improved knowledge and disease preparedness but not quality of life, anxiety, or depression.64 A recent trial of pulmonary rehabilitation in ILD had mixed effects and did not evaluate quality-of-life outcomes.65
This intervention presents opportunities for health systems and payors in context of value-based care. Effective delivery to patients with complex multimorbidity or serious illness often requires teams of individuals with advanced knowledge. Health care systems can develop multidisciplinary teams that are organized around patient needs, reducing the need for patients to coordinate their care services. The virtual and population approach of this intervention lends itself to a hub and spoke model that can improve the reach of palliative care services to areas that may be traditionally underresourced. Payors can support these transformations using bundled payments for services that incentivize quality and outcomes of care while reducing incentives based on volume of care.
Limitations
The VA study population and the high proportion of men may limit the external validity of the study findings. While the study intentionally enrolled high-risk patients, mortality was low, suggesting that some of the sickest patients did not participate. We were unable to shield knowledge of the randomization group from participants due to the nature of the intervention. Using an active comparator group may have helped determine which aspects of the intervention led to the observed effects. However, patients not improving were allowed additional intervention calls, so an attention control would be difficult to design. The test for heterogeneity should be interpreted with caution given the small sample size. The accuracy of the hospitalization data may have been improved by collecting participant-specific claims data. In addition, number of days hospitalized (or number of days spent at home) would be a better measure of resource use and patient benefits vs burdens. The number of participants with ILD was small, so inferences about intervention effect in this specific population should be interpreted with caution.
Conclusions
For adults with COPD, HF, or ILD who had a high risk of death and poor quality of life, a nurse and social worker palliative telecare team demonstrated clinically meaningful improvements in quality of life at 6 months. Future studies should evaluate implementation of this care model.
Trial Protocol
Statistical Analysis Plan
eAppendix. Patients’ Self-Reported Race Categorized as Other
eTable 1. Additional Characteristics for Participants With Heart Failure
eTable 2. Additional Characteristics for Participants With COPD
eFigure. Boxplots of ADAPT Intervention Effect on Primary and Secondary Outcomes at All Time Points
Data Sharing Statement
References
- 1.Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle R, Fried TR. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med. 2007;167(22):2503-2508. doi: 10.1001/archinte.167.22.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi: 10.1016/j.cardfail.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 3.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis. Thorax. 2005;60(7):588-594. doi: 10.1136/thx.2004.035220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coelho AC, Knorst MM, Gazzana MB, Barreto SS. Predictors of physical and mental health-related quality of life in patients with interstitial lung disease. J Bras Pneumol. 2010;36(5):562-570. doi: 10.1590/S1806-37132010000500007 [DOI] [PubMed] [Google Scholar]
- 5.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527-1537. doi: 10.1016/j.jacc.2006.06.055 [DOI] [PubMed] [Google Scholar]
- 6.Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211. doi: 10.1016/S0012-3692(15)34468-8 [DOI] [PubMed] [Google Scholar]
- 7.Ng TP, Niti M, Fones C, Yap KB, Tan WC. Co-morbid association of depression and COPD: a population-based study. Respir Med. 2009;103(6):895-901. doi: 10.1016/j.rmed.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 8.Yohannes AM. Depression and anxiety in patients with interstitial lung disease. Expert Rev Respir Med. 2020;14(9):859-862. doi: 10.1080/17476348.2020.1776118 [DOI] [PubMed] [Google Scholar]
- 9.Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease. Eur J Heart Fail. 2006;8(7):706-711. doi: 10.1016/j.ejheart.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine; Committee on Approaching Death: Addressing Key End-of-Life Issues . Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. The National Academies Press; 2015. [PubMed] [Google Scholar]
- 11.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure. J Am Coll Cardiol. 2022;79(17):e263-e421. doi: 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Lanken PN, Terry PB, Delisser HM, et al. ; ATS End-of-Life Care Task Force . An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177(8):912-927. doi: 10.1164/rccm.200605-587ST [DOI] [PubMed] [Google Scholar]
- 13.Selecky PA, Eliasson CAH, Hall RI, Schneider RF, Varkey B, McCaffree DR; American College of Chest Physicians . Palliative and end-of-life care for patients with cardiopulmonary diseases. Chest. 2005;128(5):3599-3610. doi: 10.1378/chest.128.5.3599 [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Atkins C, Wilson AM. Barriers to specialist palliative care in interstitial lung disease: a systematic review. BMJ Support Palliat Care. 2019;9(2):130-138. doi: 10.1136/bmjspcare-2018-001575 [DOI] [PubMed] [Google Scholar]
- 15.Chyr LC, DeGroot L, Waldfogel JM, et al. Implementation and effectiveness of integrating palliative care into ambulatory care of noncancer serious chronic illness. Ann Fam Med. 2022;20(1):77-83. doi: 10.1370/afm.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekelman DB, Allen LA, McBryde CF, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure. JAMA Intern Med. 2018;178(4):511-519. doi: 10.1001/jamainternmed.2017.8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graney BA, Au DH, Barón AE, et al. Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life. Trials. 2019;20(1):355. doi: 10.1186/s13063-019-3417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi: 10.1097/MLR.0b013e31827da95a [DOI] [PubMed] [Google Scholar]
- 19.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. 2005;28(2):192-211. doi: 10.1177/0163278705275341 [DOI] [PubMed] [Google Scholar]
- 20.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med. 1997;127(12):1097-1102. doi: 10.7326/0003-4819-127-12-199712150-00008 [DOI] [PubMed] [Google Scholar]
- 21.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314-2321. doi: 10.1001/archinte.166.21.2314 [DOI] [PubMed] [Google Scholar]
- 22.Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69(5):506-514. doi: 10.1001/archgenpsychiatry.2011.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K, Krebs EE, Wu J, Yu Z, Chumbler NR, Bair MJ. Telecare collaborative management of chronic pain in primary care. JAMA. 2014;312(3):240-248. doi: 10.1001/jama.2014.7689 [DOI] [PubMed] [Google Scholar]
- 24.Bekelman DB, Johnson-Koenke R, Ahluwalia SC, Walling AM, Peterson J, Sudore RL. Development and feasibility of a structured goals of care communication guide. J Palliat Med. 2017;20(9):1004-1012. doi: 10.1089/jpm.2016.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115-123. doi: 10.1016/j.jpainsymman.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 26.Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing. Br J Gen Pract. 2005;55(513):305-312. [PMC free article] [PubMed] [Google Scholar]
- 27.Turvey CL, Klein DM. Remission from depression comorbid with chronic illness and physical impairment. Am J Psychiatry. 2008;165(5):569-574. doi: 10.1176/appi.ajp.2007.07081224 [DOI] [PubMed] [Google Scholar]
- 28.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system. Health Qual Life Outcomes. 2003;1(1):79. doi: 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172-191. doi: 10.1177/0163278705275340 [DOI] [PubMed] [Google Scholar]
- 30.Bakitas MA, Dionne-Odom JN, Ejem DB, et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure. JAMA Intern Med. 2020;180(9):1203-1213. doi: 10.1001/jamainternmed.2020.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure. J Am Coll Cardiol. 2017;70(3):331-341. doi: 10.1016/j.jacc.2017.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinhauser KE, Alexander S, Olsen MK, et al. Addressing patient emotional and existential needs during serious illness. J Pain Symptom Manage. 2017;54(6):898-908. doi: 10.1016/j.jpainsymman.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 33.Grudzen CR, Shim DJ, Schmucker AM, Cho J, Goldfeld KS; EMPallA Investigators . Emergency Medicine Palliative Care Access (EMPallA): protocol for a multicentre randomised controlled trial comparing the effectiveness of specialty outpatient versus nurse-led telephonic palliative care of older adults with advanced illness. BMJ Open. 2019;9(1):e025692. doi: 10.1136/bmjopen-2018-025692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiss F, Farkas N, Nagy G, et al. Minimal Clinically Important Differences (MCID) for the Functional Assessment of Chronic Illness Therapy Fatigue Scale in patients with systemic sclerosis. Int J Environ Res Public Health. 2022;20(1):771. doi: 10.3390/ijerph20010771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinhauser KE, Arnold RM, Olsen MK, et al. Comparing three life-limiting diseases. J Pain Symptom Manage. 2011;42(3):331-341. doi: 10.1016/j.jpainsymman.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer. Lancet. 2014;383(9930):1721-1730. doi: 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]
- 37.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469-476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler J, Khan MS, Mori C, et al. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22(6):999-1005. doi: 10.1002/ejhf.1810 [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, Zhou A, Zhao Y, Chen P. Evaluating the clinical COPD questionnaire. Respirology. 2017;22(2):251-262. doi: 10.1111/resp.12970 [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163-173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Baye F, Lourens SG. Comparative validity and responsiveness of PHQ-ADS and other composite anxiety-depression measures. J Affect Disord. 2019;246:437-443. doi: 10.1016/j.jad.2018.12.098 [DOI] [PubMed] [Google Scholar]
- 42.Lynch CP, Cha EDK, Jenkins NW, et al. The Minimum Clinically Important Difference for Patient Health Questionnaire-9 in minimally invasive transforaminal interbody fusion. Spine (Phila Pa 1976). 2021;46(9):603-609. doi: 10.1097/BRS.0000000000003853 [DOI] [PubMed] [Google Scholar]
- 43.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 44.Toussaint A, Hüsing P, Gumz A, et al. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J Affect Disord. 2020;265:395-401. doi: 10.1016/j.jad.2020.01.032 [DOI] [PubMed] [Google Scholar]
- 45.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561. doi: 10.1016/S0885-3924(02)00529-8 [DOI] [PubMed] [Google Scholar]
- 46.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials. CRC Press; 2010. doi: 10.1201/9781420061185 [DOI] [Google Scholar]
- 47.Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness. Lancet Respir Med. 2014;2(12):979-987. doi: 10.1016/S2213-2600(14)70226-7 [DOI] [PubMed] [Google Scholar]
- 48.Janssens JP, Weber C, Herrmann FR, et al. Can early introduction of palliative care limit intensive care, emergency and hospital admissions in patients with severe chronic obstructive pulmonary disease? a pilot randomized study. Respiration. 2019;97(5):406-415. doi: 10.1159/000495312 [DOI] [PubMed] [Google Scholar]
- 49.Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6(6):CD011129. doi: 10.1002/14651858.CD011129.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benzo R, Hoult J, McEvoy C, et al. Promoting chronic obstructive pulmonary disease wellness through remote monitoring and health coaching: a clinical trial. Ann Am Thorac Soc. 2022;19(11):1808-1817. doi: 10.1513/AnnalsATS.202203-214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Au DH, Collins MP, Berger DB, et al. Health system approach to improve chronic obstructive pulmonary disease care after hospital discharge. Am J Respir Crit Care Med. 2022;205(11):1281-1289. doi: 10.1164/rccm.202107-1707OC [DOI] [PubMed] [Google Scholar]
- 53.Pollok J, van Agteren JEM, Carson-Chahhoud KV. Pharmacological interventions for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;12(12):CD012346. doi: 10.1002/14651858.CD012346.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollok J, van Agteren JE, Esterman AJ, Carson-Chahhoud KV. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3(3):CD012347. doi: 10.1002/14651858.CD012347.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Yin C, Tian W, Lu D, Yang X. Effects of cognitive behavioral therapy on anxiety and depression in patients with chronic obstructive pulmonary disease. Clin Respir J. 2020;14(10):891-900. doi: 10.1111/crj.13226 [DOI] [PubMed] [Google Scholar]
- 56.Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3(3):CD010673. doi: 10.1002/14651858.CD010673.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Usmani ZA, Carson KV, Cheng JN, Esterman AJ, Smith BJ. Pharmacological interventions for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(11):CD008483. doi: 10.1002/14651858.CD008483.pub2 [DOI] [PubMed] [Google Scholar]
- 58.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients. JAMA Intern Med. 2015;175(11):1773-1782. doi: 10.1001/jamainternmed.2015.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119-131. doi: 10.1016/j.jpsychores.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherwood A, Blumenthal JA, Koch GG, et al. Effects of coping skills training on quality of life, disease biomarkers, and clinical outcomes in patients with heart failure: a randomized clinical trial. Circ Heart Fail. 2017;10(1):e003410. doi: 10.1161/CIRCHEARTFAILURE.116.003410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajeswaran T, Plymen CM, Doherty AM. The effect of antidepressant medications in the management of heart failure on outcomes. Int J Psychiatry Clin Pract. 2018;22(3):164-169. doi: 10.1080/13651501.2017.1401085 [DOI] [PubMed] [Google Scholar]
- 62.Zambrano J, Celano CM, Januzzi JL, et al. Psychiatric and psychological interventions for depression in patients with heart disease: a scoping review. J Am Heart Assoc. 2020;9(22):e018686. doi: 10.1161/JAHA.120.018686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis. 2020;15:903-919. doi: 10.2147/COPD.S178049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindell KO, Klein SJ, Veatch MS, et al. Nurse-led palliative care clinical trial improves knowledge and preparedness in caregivers of patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(11):1811-1821. doi: 10.1513/AnnalsATS.202012-1494OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kataoka K, Nishiyama O, Ogura T, et al. ; FITNESS Study Collaborators . Long-term effect of pulmonary rehabilitation in idiopathic pulmonary fibrosis: a randomised controlled trial. Thorax. 2023;78(8):784-791. doi: 10.1136/thorax-2022-219792 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. Patients’ Self-Reported Race Categorized as Other
eTable 1. Additional Characteristics for Participants With Heart Failure
eTable 2. Additional Characteristics for Participants With COPD
eFigure. Boxplots of ADAPT Intervention Effect on Primary and Secondary Outcomes at All Time Points
Data Sharing Statement