Key Points
Question
Is intravenous allogeneic multipotent adult progenitor cell (MultiStem) therapy safe and effective for patients with acute ischemic stroke?
Findings
In this randomized clinical trial with 206 participants, intravenous administration of MultiStem therapy within 18 to 36 hours of ischemic stroke onset was safe but did not improve short-term outcomes at 90 days compared with placebo. There were no grade 3 or 4 allergic reactions, including in older patients.
Meaning
The results of this study support the safety of MultiStem, but further research is needed to determine whether MultiStem therapy for ischemic stroke has a beneficial effect in patients who meet specific criteria.
This randomized clinical trial examines the efficacy and safety of MultiStem allogeneic stem cell therapy for acute ischemic stroke.
Abstract
Importance
Cell therapy is a promising treatment approach for stroke and other diseases. However, it is unknown whether MultiStem (HLCM051), a bone marrow–derived, allogeneic, multipotent adult progenitor cell product, has the potential to treat ischemic stroke.
Objective
To assess the efficacy and safety of MultiStem when administered within 18 to 36 hours of ischemic stroke onset.
Design, Setting, and Participants
The Treatment Evaluation of Acute Stroke Using Regenerative Cells (TREASURE) multicenter, double-blind, parallel-group, placebo-controlled phase 2/3 randomized clinical trial was conducted at 44 academic and clinical centers in Japan between November 15, 2017, and March 29, 2022. Inclusion criteria were age 20 years or older, presence of acute ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score of 8-20 at baseline), confirmed acute infarction involving the cerebral cortex and measuring more than 2 cm on the major axis (determined with diffusion-weighted magnetic resonance imaging), and a modified Rankin Scale (mRS) score of 0 or 1 before stroke onset. Data analysis was performed between May 9 and August 15, 2022.
Exposure
Patients were randomly assigned to either intravenous MultiStem in 1 single unit of 1.2 billion cells or intravenous placebo within 18 to 36 hours of ischemic stroke onset.
Main Outcomes and Measures
The primary end points were safety and excellent outcome at day 90, measured as a composite of a modified Rankin Scale (mRS) score of 1 or less, a NIHSS score of 1 or less, and a Barthel index score of 95 or greater. The secondary end points were excellent outcome at day 365, mRS score distribution at days 90 and 365, and mRS score of 0 to 1 and 0 to 2 at day 90. Statistical analysis of efficacy was performed using the Cochran-Mantel-Haenszel test.
Results
This study included 206 patients (104 received MultiStem and 102 received placebo). Their mean age was 76.5 (range, 35-95) years, and more than half of patients were men (112 [54.4%]). There were no between-group differences in primary and secondary end points. The proportion of excellent outcomes at day 90 did not differ significantly between the MultiStem and placebo groups (12 [11.5%] vs 10 [9.8%], P = .90; adjusted risk difference, 0.5% [95% CI, −7.3% to 8.3%]). The frequency of adverse events was similar between treatment groups.
Conclusions and Relevance
In this randomized clinical trial, intravenous administration of allogeneic cell therapy within 18 to 36 hours of ischemic stroke onset was safe but did not improve short-term outcomes. Further research is needed to determine whether MultiStem therapy for ischemic stroke has a beneficial effect in patients who meet specific criteria, as indicated by the exploratory analyses in this study.
Trial Registration
ClinicalTrials.gov Identifier: NCT02961504
Introduction
Stroke is the second leading global cause of death and a major cause of disability, with 6.6 million deaths attributed to stroke in 2019.1 Evidence-based reperfusion therapies, including intravenous thrombolysis2,3 and mechanical thrombectomy (MT),4 are widely used for stroke treatment. Despite varied outcomes with these treatments, approximately 50% of patients still experience disability 3 months after stroke,4 underlining the complexity and ongoing debate surrounding this therapeutic approach. This dynamic landscape highlights the need for new treatment options and expansion of the therapeutic window.
Cell therapy shows promise5 in enhancing functional recovery by modulating the immune response, providing neuroprotection,6 and restoring neural circuits in the injured brain.7 Various cell types (mesenchymal stem cells, bone marrow mononuclear cells,8 neural stem cells,9 and induced pluripotent stem cells7,10) and administration routes (intravenous, intra-arterial, and intracerebral)11,12,13 have been explored in different time windows (hours to months).4,14
Previous randomized clinical trials (RCTs) of autologous cell therapies for subacute and chronic stroke suggested improved outcomes pertaining to disability and activities of daily living.15,16,17,18 However, these trials did not focus on acute stroke, had small sample sizes, and used time-consuming procedures for stem cell preparation and administration.
Allogeneic stem cells offer promising advantages over autologous stem cells for therapy because they have off-the-shelf properties and can be used early in the disease course.19 MultiStem is a mass-produced stem cell product that provides benefits through various mechanisms. For example, MultiStem reduces inflammation, modulates immune dysregulation, protects damaged cells, and promotes angiogenesis, tissue repair, and healing.20 Currently, few RCTs have examined the use of allogeneic stem cells, such as MultiStem, for stroke treatment.19,21 The MultiStem in Acute Stroke Treatment to Enhance Recovery (MASTERS) trial used an allogenic, bone marrow–derived, clinical-grade, multipotent adult progenitor cell line and provided valuable insights, albeit within a 24- to 48-hour poststroke window.19
This study reports findings of the phase 2/3 Treatment Evaluation of Acute Stroke Using Regenerative Cells (TREASURE) trial, which explored MultiStem safety and efficacy for patients treated within 18 to 36 hours after ischemic stroke onset.
Methods
This multicenter, double-blind, parallel-group, placebo-controlled RCT (ClinicalTrials.gov: NCT02961504) was conducted in 44 academic and clinical centers across Japan. The TREASURE rationale, design, and methods were reported previously.22 The trial protocol and statistical analysis plan are presented in Supplement 1. The TREASURE trial was approved by relevant local ethics committees and institutional review boards at each participating site. All patients or their representatives provided written informed consent before enrollment. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
An independent data safety monitoring board evaluated the safety data after approximately 30% and 60% of the planned number of participants completed the 7-day follow-up. Bioimaging assessments were independently evaluated by a core assessment board via diffusion-weighted magnetic resonance imaging (DW-MRI) at baseline. All investigators had access to the data, and all authors vouch for the accuracy and completeness of the data, adherence to the trial protocol and statistical analysis plan (Supplement 1), and completeness of adverse event reporting.
Patients
The inclusion criteria were as follows: age 20 years or older, history of ischemic stroke with a persistent neurologic deficit (corresponding to a National Institutes of Health Stroke Scale [NIHSS] score of 8-20 at baseline), confirmed acute infarction involving the cerebral cortex and measuring more than 2 cm on the major axis (as determined with DW-MRI), and a modified Rankin Scale (mRS) score of 0 or 1 before stroke onset. We excluded patients with lacunar or brainstem infarction and a change in NIHSS score of 4 or greater during a minimum period of 6 hours between screening and randomization, and we also excluded those who received combined reperfusion therapy (intravenous thrombolysis plus MT). Patients who received only 1 reperfusion modality were included. The inclusion and exclusion criteria are summarized in eTable 1 in Supplement 2.
Randomization and Masking
A computer-generated randomization process was adopted to randomly assign patients (1:1) to receive MultiStem (HLCM051; Lonza) or placebo treatment. Randomization was stratified according to baseline NIHSS score (≤12 or ≥13), receipt of reperfusion therapy for acute ischemic stroke (yes or no), and age (≤64 or ≥65 years).
After 200 patients were randomized, the inclusion criterion for age was amended to reflect data safety monitoring board recommendations. An upper limit of 84 years was established because the 4 patients who died within 7 days of stroke onset were aged older than 84 years.
At each site, a designated staff member from a pharmacy or equivalent facility who was unblinded to the treatment assignment contacted an interactive web-response system vendor to access the assigned treatment of each patient and prepare the product accordingly. Patients and all trial personnel, including investigators and clinicians, were blinded to the treatment assignment. An opaque cover and sleeve were used over the intravenous infusion bag and tubing.
Procedures
The investigational product (both MultiStem and placebo) was sent to each trial site and stored appropriately by the pharmacy or relevant facility department. MultiStem was provided in a single unit containing 1.2 billion cells (±20%), which was cryopreserved in a medium comprising Plasma-Lyte A (for dilution), dimethylsulfoxide, and human serum albumin. The placebo consisted of only cryopreservation medium. Both MultiStem and placebo were supplied separately in 6-mL vials and sealed securely. The investigational product required storage at temperatures below −140 °C in a designated facility.
When needed for administration, appropriate trial personnel thawed the investigational product and prepared it for infusion. The product was then delivered to the department responsible for administration, following specific procedures.
Patients received a single intravenous dose of either MultiStem or placebo under gravity flow, which lasted 30 to 60 minutes and occurred between 18 and 36 hours after stroke onset.22 Patient visits were scheduled at days 7, 30, 90, and 365 after randomization. Furthermore, patients were contacted via telephone at day 60 and every 2 months after day 90. Brain DW-MRI was performed at baseline. The central institution acquired brain MRI images obtained during the screening process, and researchers evaluated infarct volume and Alberta Stroke Program Early Computed Tomography score independently under blinded status.
Efficacy Assessment
The primary efficacy outcome was the proportion of patients with an excellent outcome at day 90. This was defined as meeting the following composite scoring criteria: mRS score of 1 or less (range, 0-6), NIHSS total score of 1 or less (range, 0-42), and Barthel index (BI) score of 95 or greater (range, 0-100).
The key secondary end points were the proportion of patients with an excellent outcome at day 365, distribution of mRS scores at days 90 and 365, and proportion of patients with mRS scores of 0 to 1 and 0 to 2 at day 90. eTable 2 in Supplement 2 presents a complete list of secondary parameters.
Safety Assessment
The safety assessment comprised several primary safety end points. These were as follows: grade 3 or 4 infusion-related reactions (Common Terminology Criteria for Adverse Events, version 4.0), including cardiovascular and respiratory function abnormalities or allergic reactions occurring within 24 hours after infusion; serious adverse events occurring within 7 days after treatment; worsening of neurological symptoms, defined as an increase of 4 or more points in NIHSS score vs baseline assessed through 7 days after treatment related to the investigational product; death or life-threatening adverse events up to day 90; and secondary infection up to day 90.
Statistical Analysis
Based on the tissue plasminogen activator (t-PA) and MT data from the MASTERS trial,19 we estimated that including 100 patients per group would yield 90% power at a 5% significance level to detect a treatment effect of the primary efficacy outcome. The primary efficacy outcome effect sizes were assumed to be 18.5% and 3.8% in the MultiStem and placebo groups, respectively. We adjusted the number of patients to 110 per group to account for possible exclusions or dropouts. Efficacy analyses were based on the full analysis set, including patients who underwent randomization and mRS, NIHSS, and BI assessments at least once after day 7. Safety outcomes were evaluated in the safety analysis set, which included all randomized and treated participants. The superiority of the MultiStem treatment to placebo was evaluated using the Cochran-Mantel-Haenszel (CMH) test (with a 2-tailed significance level of .05), adjusted for baseline NIHSS score (≤12 or ≥13), concomitant reperfusion therapy (yes or no), and age (≤74 or ≥75 years). The mRS score distribution was summarized with counts and percentages at days 90 and 365 and was compared between groups using the CMH test with modified ridit scores and stratified by baseline adjustment factors. Exploratory subgroup analyses of mRS scores of 2 or less at day 90 with no correction for multiple comparisons were conducted using the CMH test with adjustments for subgroups of age (≤64, ≥65, ≤74, or ≥75 years) and stroke volume (<25, ≥25, <50, ≥50, <70, or ≥70 mL). These subgroup analyses were defined in the statistical analysis plan (version 1.0) finalized before trial unmasking (Supplement 1).
To evaluate the long-term effects of MultiStem, a post hoc analysis was conducted for the proportion of patients with global stroke recovery at day 365. This analysis comprised a mRS score of 2 or less, an NIHSS score improvement of 75% or greater, and a BI score of 95 or greater.
Patients who missed the evaluation at day 90 were assessed using their last postrandomization primary efficacy assessment (day 7 or later) carried forward (LOCF) to impute the missing day 90 assessment. For the secondary end points (ie, excellent outcome at day 365 and distribution of mRS scores at days 90 and 365) and post hoc analysis of global stroke recovery, BI score of 95 or greater, and mRS score of 2 or less at day 365, missing data were imputed in a similar manner. Sensitivity analysis for missing data included evaluation of excellent outcomes at days 90 and 365 for observed patients using the CMH test. Other secondary efficacy data were not imputed. Patients who died during the study were assigned a mRS score of 6, a binary NIHSS score of greater than 1, and a BI score of less than 95 at all subsequent time points.
In all efficacy analyses, we used age 74 years or younger or 75 years or older as an adjusted factor (instead of age ≤64 and ≥65 years as a randomization factor) in the CMH test, with blind review and redefinition of the primary analysis in the revised protocol with Pharmaceuticals and Medical Devices Agency consultation before unmasking.
In this study, statistical multiplicity was not considered for the analysis of secondary end points, subgroup analysis, and post hoc analysis. All data analyses were performed with SAS, version 9.4, and with SAS/STAT, version 14.2 (SAS Institute). Data analysis was performed between May 9 and August 15, 2022.
Results
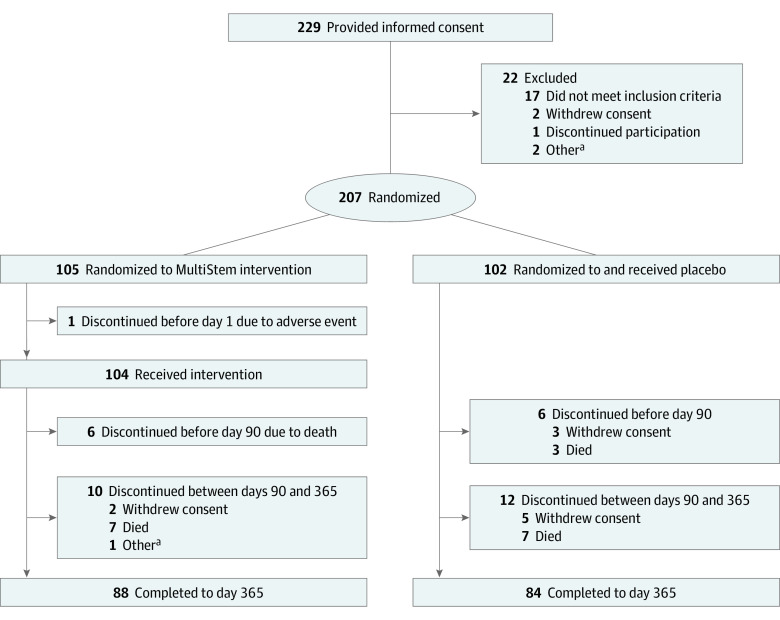
A total of 229 patients were recruited between November 15, 2017, and March 30, 2021, with follow-up to day 365 on March 29, 2022. Among them, 207 patients were randomized to treatment: 105 to MultiStem and 102 to placebo. One patient discontinued participation before day 1. Thus, 206 patients received an intravenous infusion of MultiStem (n = 104) for 33 to 72 minutes or placebo (n = 102) for 30 to 77 minutes and were included in the safety analysis and full analysis sets (Figure 1). More than half of patients were men (112 [54.4%]), and 94 (45.6%) were women; the mean age of patients was 76.5 (range, 35-95) years. Before day 90 after dosing, 6 patients from each group withdrew.
Figure 1. Trial Profile.
aOther indicates that the participant became a welfare recipient during the study and withdrew prematurely because the hospital policy prohibits welfare recipients from participating in clinical trials.
Table 1 presents patient demographics and baseline characteristics. This study included a high proportion of older patients, with a median age of 79 (range, 40-95) years in the MultiStem group and 78 (range, 35-95) years in the placebo group. Proportions of patients who received reperfusion therapy, mean NIHSS scores, and infarct volumes were similar between groups.
Table 1. Patient Demographic and Baseline Characteristics.
| Characteristic | Valuea | |
|---|---|---|
| MultiStem (n = 104) | Placebo (n = 102) | |
| Sex | ||
| Male | 56 (53.8) | 56 (54.9) |
| Female | 48 (46.2) | 46 (45.1) |
| Age, y | ||
| Mean (SD) | 76.7 (10.4) | 76.2 (10.6) |
| Median (range) | 79 (40-95) | 78 (35-95) |
| ≥65 | 94 (90.4) | 90 (88.2) |
| ≥75 | 66 (63.5) | 66 (64.7) |
| Patients with left hemisphere events | 61 (58.7) | 42 (41.2) |
| Patients who received reperfusion therapy | ||
| Yes | 56 (53.8) | 52 (51.0) |
| Tissue plasminogen activator | 24 (23.1) | 12 (11.8) |
| Mechanical thrombectomy | 32 (30.8) | 40 (39.2) |
| No | 48 (46.2) | 50 (49.0) |
| NIHSS score | ||
| Mean (SD) | 13.7 (3.9) | 13.9 (3.9) |
| Median (range) | 14 (8-20) | 14 (8-20) |
| ≤12 | 41 (39.4) | 37 (36.3) |
| Infarct volume, mLb | ||
| Mean (SD) | 42.0 (48.4) | 54.3 (57.0) |
| Median (range) | 26.5 (0.1-308.9) | 39.7 (0.9-423.5) |
Abbreviation: NIHSS, National Institutes of Health Stroke Scale.
Unless indicated otherwise, values are presented as number (percentage) of patients.
The number of patients for the MultiStem and placebo groups was 101 and 97, respectively.
The rate of excellent outcomes at day 90 did not differ significantly between the MultiStem and placebo groups (12 [11.5%] vs 10 [9.8%], P = .90; adjusted risk difference, 0.5% [95% CI, −7.3% to 8.3%]). Furthermore, there were no statistically significant between-group differences for any of the secondary end points (Table 2). The mRS score distribution at days 90 and 365 is presented in Figure 2.
Table 2. Primary and Secondary Efficacy End Points.
| End point | Day 90 | Day 365 | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Adjusted risk difference, % (95% CI) | P value | No. (%) | Adjusted risk difference, % (95% CI) | P value | |||
| MultiStem (n = 104) | Placebo (n = 102) | MultiStem (n = 104) | Placebo (n = 102) | |||||
| Excellent outcome | ||||||||
| Imputationa,b | 12/104 (11.5) | 10/102 (9.8) | 0.5 (−7.3 to 8.3) | .90 | 16/104 (15.4) | 11/102 (10.8) | 3.4 (−5.0 to 11.8) | .43 |
| Observeda,c | 12/103 (11.7) | 10/98 (10.2) | 0.4 (−7.6 to 8.4) | .92 | 14/97 (14.4) | 11/93 (11.8) | 0.4 (−8.1 to 8.9) | .93 |
| mRS score distributionb,d | ||||||||
| 0 | 5 (4.8) | 3 (2.9) | NA | .62 | 9 (8.7) | 1 (1.0) | NA | .42 |
| 1 | 9 (8.7) | 8 (7.8) | NA | 11 (10.6) | 13 (12.7) | NA | ||
| 2 | 20 (19.2) | 12 (11.8) | NA | 18 (17.3) | 13 (12.7) | NA | ||
| 3 | 15 (14.4) | 17 (16.7) | NA | 15 (14.4) | 17 (16.7) | NA | ||
| 4 | 31 (29.8) | 39 (38.2) | NA | 22 (21.2) | 33 (32.4) | NA | ||
| 5 | 18 (17.3) | 20 (19.6) | NA | 16 (15.4) | 15 (14.7) | NA | ||
| 6 | 6 (5.8) | 3 (2.9) | NA | 13 (12.5) | 10 (9.8) | NA | ||
| Global stroke recoveryc | 20/96 (20.8) | 16/94 (17.0) | 0.9 (−9.0 to 10.9) | .86 | NA | NA | NA | NA |
| mRS scorec | ||||||||
| ≤1 | 14/97 (14.4) | 11/95 (11.6) | 1.1 (−7.7 to 9.9) | .81 | NA | NA | NA | NA |
| ≤2 | 34/97 (35.1) | 23/95 (24.2) | 8.3 (−3.6 to 20.2) | .17 | NA | NA | NA | NA |
| NIHSS scorec | ||||||||
| ≤1 | 19/85 (22.4) | 19/85 (22.4) | −2.3 (−13.9 to 9.3) | .71 | NA | NA | NA | NA |
| Improvement ≥75%c | 30/85 (35.3) | 29/85 (34.1) | −1.6 (−15.4 to 12.2) | .82 | NA | NA | NA | NA |
| Barthel index ≥95c | 31/97 (32.0) | 24/93 (25.8) | 3.7 (−8.0 to 15.3) | .54 | NA | NA | NA | NA |
Abbreviations: CMH, Cochran-Mantel-Haenszel; mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale.
Treatments were compared using the CMH test adjusted for baseline NIHSS score (≤12 or ≥13), receipt of concomitant reperfusion therapy (yes or no), and age (20-74 or ≥75 years). Risk differences and corresponding 2-sided 95% CIs between the MultiStem and placebo groups were adjusted for the same factors applied in the CMH test and calculated using the Mantel-Haenszel method by Sato T.
Imputation was performed by last postrandomization efficacy assessment of excellent outcome and mRS score carried forward.
Observed cases. Regarding excellent outcome, included death cases as worst case and available partial missing case (ie, the case in at least available 1 of 3 composite scoring criteria for excellent outcome) of composite scoring criteria for excellent outcome.
P values were calculated using the CMH test adjusted for baseline NIHSS score (≤12 or ≥13), receipt of concomitant reperfusion therapy (yes or no), and age (20-74 or ≥75 years) using modified ridit scores.
Figure 2. Distribution of Modified Rankin Scale Score on Days 90 and 365.
A and B, Modified Rankin Scale scores on days 90 (A) and 365 (B). Scores range from 0 to 6, with 0 indicating no symptoms; 1, symptoms without clinical disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death. Imputation was performed by last postrandomization primary efficacy assessment carried forward.
The proportions of missing assessments, defined as the difference in total patient numbers between LOCF data and observed patient data, for excellent outcomes at day 90 were 1 of 104 (1.0%) and 4 of 102 (3.9%) in the MultiStem and placebo groups, respectively (Table 2). Similarly, at day 365, the proportions of missing assessments for excellent outcomes were 7 of 104 (6.7%) and 9 of 102 (8.8%) in these groups. The proportions of missing data for the primary end point assessment were low in both treatment groups.
Exploratory subgroup analyses of mRS scores of 2 or less at day 90 with no correction for multiple comparisons were conducted for the age and ischemic core volume subgroups. Patients with an ischemic core volume of 50 mL or less had significantly better outcomes in the MultiStem vs placebo groups (8 of 27 [29.6%] vs 3 of 37 [8.1%], P = .04; adjusted risk difference, 20.4% [95% CI, 1.0% to 39.9%]). Patients aged 64 years or younger also tended to have better outcomes in the MultiStem vs placebo groups, although the difference between groups was not statistically significant (8 of 10 [80.0%] vs 5 of 12 [41.7%], P = .08; adjusted risk difference, 37.2% [95% CI, −0.4% to 74.8%]; Figure 3). Although the results of subgroup analyses had potential statistical multiplicity, these beneficial effects were not observed in patients with an ischemic core volume of less than 50 mL for both the MultiStem vs placebo groups (26 of 67 [38.8%] vs 20 of 54 [37.0%], P = .87; adjusted risk difference, −1.4% [95% CI, −16.9% to 14.2%]), nor were they observed for patients with an ischemic core volume of less than 25 mL (17 of 46 [37.0%] vs 14 of 32 [43.8%], P = .70; adjusted risk difference, −4.3% [95% CI, −25.6% to 17.1%]), 25 mL or greater (17 of 48 [35.4%] vs 9 of 59 [15.3%], P = .11; adjusted risk difference, 11.7% [95% CI, −3.2% to 26.6%]), less than 70 mL (29 of 80 [36.3%] vs 22 of 66 [33.3%], P = .85; adjusted risk difference, 1.4% [95% CI, −12.6% to 15.3%]), or 70 mL or greater (5 of 14 [35.7%] vs 1 of 25 [4.0%], P = .05; adjusted risk difference, 25.4% [95% CI, −2.2% to 52.9%]). Beneficial effects also were not observed for patients aged 65 years or older (26 of 87 [29.9%] vs 18 of 83 [21.7%], P = .23; adjusted risk difference, 7.7% [95% CI, −4.8% to 20.3%]), 74 years or younger (18 of 37 [48.6%] vs 12 of 35 [34.3%], P = .42; adjusted risk difference, 8.7% [95% CI, −13.1% to 30.5%]), or 75 years or older (16 of 60 [26.7%] vs 11 of 60 [18.3%], P = .27; adjusted risk difference, 8.0% [95% CI, −5.8% to 21.9%]).
Figure 3. Subgroup Analyses of Modified Rankin Scale Score Scores of 0 to 2 at Day 90 Stratified by Age and Ischemic Core Volume.
Risk differences and corresponding 2-sided 95% CIs in the MultiStem and placebo groups adjusted using the same factors in the Cochran-Mantel-Haenszel (CMH) test were calculated using the Mantel-Haenszel method by Sato T.
aTreatments were compared after categorizing patients by age at baseline using the CMH test adjusted for baseline National Institutes of Health Stroke Scale (NIHSS) score (≤12 or ≥13) and receipt of concomitant reperfusion therapy (yes or no). There were 97 patients in the MultiStem group and 95 in the placebo group.
bTreatments were compared after stratifying patients by diffusion-weighted imaging (DWI) ischemic core volume at baseline using the CMH test adjusted for baseline NIHSS score (≤12 or ≥13), receipt of concomitant reperfusion therapy (yes or no), and age (20-74 or ≥75 years) There were 94 patients in the MultiStem group and 91 in the placebo group.
eTable 3 in Supplement 2 presents the results of exploratory post hoc analyses of proportions of patients in the MultiStem group with global stroke recovery and a BI score of 95 or greater at day 365 with no correction for multiple comparisons, which were better than those in placebo group. For global stroke recovery, 29 patients (27.9%) in the MultiStem group and 16 (15.7%) in the placebo group had improvement (adjusted risk difference, 11.0% [95% CI, 0.8% to 21.3%]; P = .04). For BI scores of 95 or greater, 37 patients (35.6%) in the MultiStem group and 23 (22.5%) in the placebo group had higher scores (adjusted risk difference, 11.3% [95% CI, 0.2% to 22.4%], P = .05).
The primary safety end points including grade 3 or 4 infusion-related allergic reactions did not differ between groups (eTable 4 in Supplement 2). Treatment-emergent adverse events (eTable 5 in Supplement 2) related to the study drug occurred in 31 MultiStem-treated patients (29.8%) and 12 placebo-treated patients (11.8%).
Discussion
To our knowledge, this is the first double-blind phase 2/3 RCT to evaluate cell therapy for acute ischemic stroke. Previous stem cell therapy RCTs used autologous stem cells,15,16,17,18 rendering double-blind studies challenging. Because multicenter RCTs of allogeneic cells in acute stroke are limited,19,21 this study has substantial potential to impact novel treatments for ischemic stroke.
Although there were no significant differences in the primary and secondary end points between the MultiStem and placebo groups in this study, exploratory subgroup analyses with no correction for multiple comparisons conducted with patients with mRS scores of 0 to 2 at day 90 seemed to show better outcomes in the MultiStem group, particularly for patients with ischemic core volumes of 50 mL or greater and those aged 64 years or younger. Exploratory post hoc analyses with no correction for multiple comparisons indicated significantly higher proportions of patients with global stroke recovery and a BI of 95 or greater at day 365 in the MultiStem vs placebo groups. The occurrence of adverse events was comparable between groups.
Contrary to our hypothesis, MultiStem did not improve clinical outcomes as expected. Previous post hoc analysis of early treatment (<36 hours) in phase 2 of the MASTERS trial reported substantially increased rates of excellent outcomes at day 365 in the MultiStem group.19 Additionally, another post hoc analysis of the MASTERS trial showed a higher rate of excellent outcomes in early treatment (<36 hours) excluding patients who received t-PA plus MT19; this exclusion criterion was also used in the TREASURE study.22 The disparity in results between the MASTERS and TREASURE trials may be attributable to the inclusion of older patients, which may have masked the immediate effect of MultiStem treatment. However, a trend toward better outcomes was observed in patients aged younger than 64 years. The median age of TREASURE participants was 78 to 79 years, which was substantially higher than the age in almost all clinical stroke studies, including the previous MASTERS trial on MultiStem,19 by more than 10 to 15 years. One potential reason may be Japan’s aging population, as the median age of stroke in Japan is 74 (IQR, 66-82) years.23 Interestingly, this age distribution concurred with participants in the TREASURE study. Furthermore, based on the safety results of the MASTERS trial, no upper age limit was set at the beginning of the TREASURE trial.19 The influence of the substantial number of older participants on the findings of this study remains uncertain. Exploration of the impact of MultiStem therapy on aging animals in future studies could provide valuable insights. Cell therapy aims to facilitate regeneration, repair, and plasticity of surviving neural tissues, which may require longer evaluation periods. The underlying mechanisms of MultiStem involve modulating the peripheral immune system and promoting a regenerative environment, which may contribute to long-term efficacy.5,24 Results from the MASTERS trial at 1 year support improved outcomes in the MultiStem group compared with the control group, despite intravenously administered MultiStem disappearing from the body shortly after administration.19 Our findings of a better trend in outcomes at 1 year, as determined by the exploratory post hoc analysis, aligns with the exploratory post hoc analysis of the MASTERS trial.19
Autologous stem cell therapy for stroke can improve long-term outcomes and survival.16 Selim25 proposed evaluating outcomes at day 365 for intracerebral hemorrhage trials to detect long-term treatment effects. Other cell therapies for cerebral infarction can restore corticospinal tract function up to 1 year after stem cell administration.26 Applying this principle to interventions promoting neural repair in ischemic stroke seems beneficial.
In our exploratory subgroup analyses with no correction for multiple comparisons, MultiStem seemed to be effective when the cerebral infarction was 50 mL or greater. This is probably because smaller infarct volumes generally respond better to conventional therapy, and it can be challenging to detect the efficacy of cell therapy due to ceiling effects.27 For patients with large infarction volumes, thrombectomy may be less effective, leading to poor outcomes and increased intracranial hemorrhage, even after successful recanalization.28 Although recent studies have demonstrated the efficacy of endovascular therapy for large infarctions, infarct volume remains a substantial factor in poor outcomes. Therefore, our finding that individuals with cerebral infarction of 50 mL or greater benefit from cell therapy holds crucial clinical implications, as these patients may not benefit from conventional treatments like thrombectomy.
In this study, MultiStem also demonstrated an acceptable safety profile. Our study findings align with the observations of Rasmusson et al,29 as we did not encounter grade 3 or 4 allergic reactions associated with MultiStem infusions. Moreover, the MultiStem mechanism of action includes immunomodulation, and the absence of suspected serious adverse reactions within the first 7 days after administration further confirms the safety of this cell therapy approach.
Limitations
Patient heterogeneity is a limitation of this trial. The inclusion of patients with advanced age and posterior circulation involvement in stroke may have diluted the treatment effects. The decision to include such patients remains controversial, as previous RCTs of MT in this subgroup yielded varying results.30,31,32,33,34 Statistical multiplicity was not considered for the subgroup analysis and post hoc analysis in this study. All of these results are exploratory, and most subgroup analyses had small sample sizes. Moreover, patients treated with both t-PA and MT were excluded. The MASTERS-2 clinical trial including patients treated with both t-PA and MT is being conducted.
Conclusions
In the TREASURE RCT, intravenous allogeneic cell therapy within 18 to 36 hours of ischemic stroke onset failed to demonstrate a short-term therapeutic effect. However, efficacy was suggested in patients with large infarcts and possibly in younger patients based on exploratory subgroup analyses with no correction for multiple comparisons. A combined analysis of the TREASURE and ongoing MASTERS-2 trials will be conducted to confirm the efficacy and safety of MultiStem therapy.
Trial Protocol and Statistical Analysis Plan
eTable 1. Inclusion and Exclusion Criteria
eTable 2. List of Secondary Efficacy End Points
eTable 3. Efficacy End Points in the Post Hoc Analysis
eTable 4. Safety End Points
eTable 5. Treatment-Emergent Adverse Events
Nonauthor Collaborators. The TREASURE Study Investigators
Data Sharing Statement
References
- 1.Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18-29. doi: 10.1177/17474930211065917 [DOI] [PubMed] [Google Scholar]
- 2.Jauch EC, Saver JL, Adams HP Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5.Mays RW, Savitz SI. Intravenous cellular therapies for acute ischemic stroke. Stroke. 2018;49(5):1058-1065. doi: 10.1161/STROKEAHA.118.018287 [DOI] [PubMed] [Google Scholar]
- 6.Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20(2):101-107. doi: 10.1159/000086518 [DOI] [PubMed] [Google Scholar]
- 7.Palma-Tortosa S, Tornero D, Grønning Hansen M, et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci U S A. 2020;117(16):9094-9100. doi: 10.1073/pnas.2000690117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahidy FS, Rahbar MH, Zhu H, Rowan PJ, Bambhroliya AB, Savitz SI. Systematic review and meta-analysis of bone marrow-derived mononuclear cells in animal models of ischemic stroke. Stroke. 2016;47(6):1632-1639. doi: 10.1161/STROKEAHA.116.012701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, et al. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J Neurosci. 2016;36(41):10529-10544. doi: 10.1523/JNEUROSCI.1643-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palma-Tortosa S, Coll-San Martin B, Kokaia Z, Tornero D. Neuronal replacement in stem cell therapy for stroke: filling the gap. Front Cell Dev Biol. 2021;9:662636. doi: 10.3389/fcell.2021.662636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59-69. doi: 10.1002/ana.22458 [DOI] [PubMed] [Google Scholar]
- 12.Moniche F, Gonzalez A, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43(8):2242-2244. doi: 10.1161/STROKEAHA.112.659409 [DOI] [PubMed] [Google Scholar]
- 13.Qiao LY, Huang FJ, Zhao M, et al. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014;23(suppl 1):S65-S72. doi: 10.3727/096368914X684961 [DOI] [PubMed] [Google Scholar]
- 14.Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50(10):2835-2841. doi: 10.1161/STROKEAHA.119.026318 [DOI] [PubMed] [Google Scholar]
- 15.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874-882. doi: 10.1002/ana.20501 [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators . A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099-1106. doi: 10.1002/stem.430 [DOI] [PubMed] [Google Scholar]
- 17.Jaillard A, Hommel M, Moisan A, et al. ; for the ISIS-HERMES Study Group . Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. 2020;11(5):910-923. doi: 10.1007/s12975-020-00787-z [DOI] [PubMed] [Google Scholar]
- 18.Chung JW, Chang WH, Bang OY, et al. ; STARTING-2 Collaborators . Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96(7):e1012-e1023. doi: 10.1212/WNL.0000000000011440 [DOI] [PubMed] [Google Scholar]
- 19.Hess DC, Wechsler LR, Clark WM, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360-368. doi: 10.1016/S1474-4422(17)30046-7 [DOI] [PubMed] [Google Scholar]
- 20.Auletta JJ, Cooke KR, Solchaga LA, Deans RJ, van’t Hof W. Regenerative stromal cell therapy in allogeneic hematopoietic stem cell transplantation: current impact and future directions. Biol Blood Marrow Transplant. 2010;16(7):891-906. doi: 10.1016/j.bbmt.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, et al. Final results of allogeneic adipose tissue-derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): a phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplant. 2022;31:9636897221083863. doi: 10.1177/09636897221083863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osanai T, Houkin K, Uchiyama S, Minematsu K, Taguchi A, Terasaka S. Treatment evaluation of acute stroke for using in regenerative cell elements (TREASURE) trial: rationale and design. Int J Stroke. 2018;13(4):444-448. doi: 10.1177/1747493017743057 [DOI] [PubMed] [Google Scholar]
- 23.Toyoda K, Yoshimura S, Nakai M, et al. ; Japan Stroke Data Bank Investigators . Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol. 2022;79(1):61-69. doi: 10.1001/jamaneurol.2021.4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savitz SI, Cox CS Jr. Cell-based therapies for neurological disorders—the bioreactor hypothesis. Nat Rev Neurol. 2023;19(1):9-18. doi: 10.1038/s41582-022-00736-4 [DOI] [PubMed] [Google Scholar]
- 25.Selim MH. Time for a new perspective on intracerebral hemorrhage. JAMA Neurol. 2022;79(9):844-845. doi: 10.1001/jamaneurol.2022.1988 [DOI] [PubMed] [Google Scholar]
- 26.Haque ME, Hasan KM, George S, et al. Longitudinal neuroimaging evaluation of the corticospinal tract in patients with stroke treated with autologous bone marrow cells. Stem Cells Transl Med. 2021;10(7):943-955. doi: 10.1002/sctm.20-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu J, Bakreen A, Juntunen M, et al. Combined adipose tissue-derived mesenchymal stem cell therapy and rehabilitation in experimental stroke. Front Neurol. 2019;10:235. doi: 10.3389/fneur.2019.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303-1313. doi: 10.1056/NEJMoa2118191 [DOI] [PubMed] [Google Scholar]
- 29.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208-1213. doi: 10.1097/01.TP.0000082540.43730.80 [DOI] [PubMed] [Google Scholar]
- 30.Jovin TG, Li C, Wu L, et al. ; BAOCHE Investigators . Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387(15):1373-1384. doi: 10.1056/NEJMoa2207576 [DOI] [PubMed] [Google Scholar]
- 31.Tao C, Nogueira RG, Zhu Y, et al. ; ATTENTION Investigators . Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387(15):1361-1372. doi: 10.1056/NEJMoa2206317 [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Dai Q, Ye R, et al. ; BEST Trial Investigators . Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115-122. doi: 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 33.Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, et al. ; BASICS Study Group . Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384(20):1910-1920. doi: 10.1056/NEJMoa2030297 [DOI] [PubMed] [Google Scholar]
- 34.Berberich A, Finitsis S, Strambo D, et al. Endovascular therapy versus no endovascular therapy in patients receiving best medical management for acute isolated occlusion of the posterior cerebral artery: a systematic review and meta-analysis. Eur J Neurol. 2022;29(9):2664-2673. doi: 10.1111/ene.15410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Inclusion and Exclusion Criteria
eTable 2. List of Secondary Efficacy End Points
eTable 3. Efficacy End Points in the Post Hoc Analysis
eTable 4. Safety End Points
eTable 5. Treatment-Emergent Adverse Events
Nonauthor Collaborators. The TREASURE Study Investigators
Data Sharing Statement