Abstract
The in vitro binding of surface-exposed material and outer membrane proteins of Helicobacter pylori to high-molecular-weight salivary mucin was studied. We identified a 16-kDa surface protein which adhered to high-molecular-weight salivary mucin. This protein binds specifically to sulfated oligosaccharide structures such as sulfo-Lewis a, sulfogalactose and sulfo-N-acetyl-glucosamine on mucin. Sequence analysis of the protein proved that it was identical to the N-terminal amino acid sequence of neutrophil-activating protein. Moreover, this adhesin was able to bind to Lewis x blood group antigen.
Helicobacter pylori is a causative agent in chronic active gastritis, duodenal ulcer, and gastric malignancies (4, 19, 25). This bacterium colonizes the mucus layer as well as the cell surface of the gastric epithelium, especially at intracellular junctions (14, 15). The main component of mucus is a highly glycosylated protein (mucin) that covers and protects the underlying mucosa. It has been reported that H. pylori binds to gastric and nongastric epithelial cells in vivo and in vitro (16, 17, 23) and that the binding involves surface structures, namely, phosphatidylethanolamine (18), GM3 ganglioside and lactosylceramide sulfate (28, 30), N-acetylneuraminyllactose (7, 8), H type 2 antigens, and blood group-related Lewis b (1, 5). We have previously demonstrated that H. pylori binds to sulfated glycans present on high-molecular-weight salivary mucins and that the binding is enhanced at lower pHs (36).
The aim of the present study was to identify the H. pylori adhesin that binds to specific structures on salivary mucin. We identified a 16-kDa surface protein that adhered specifically to high-molecular-weight salivary mucin. This adhesin proved to be H. pylori neutrophil-activating protein (NAP). It mediates adhesion to sulfated carbohydrates on mucin and also binds to Lewis x blood group antigen.
MATERIALS AND METHODS
H. pylori strains.
Two strains of bacteria were used: H. pylori ATCC 43504 and H. pylori 3B3, which was isolated from the subgingival plaque of a patient with a duodenal ulcer at the University Hospital, Vrije Universiteit, Amsterdam, The Netherlands (22). The bacteria were grown for 4 days under microaerophilic conditions (CO2, 10%; O2, 5%; N2, 85%) at 37°C on Dent agar plates (6) supplemented with 40 mg of 2,3,5-triphenyltetrazolium chloride (Sigma Chemical Co., St. Louis, Mo.) per ml. They were stored in brain heart infusion medium (Oxoid-Unipath, Basingstoke, United Kingdom) with 20% glycerol at −80°C.
Protein isolation.
Outer membrane proteins (OMPs) were isolated with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS)-Sarkosyl as described previously (40), and the extracted membrane pellets were suspended in 10 mM Tris-HCl (pH 7.2) and stored at −80°C. To prepare bacterial extracts, the bacterial cells from two confluent agar plates were suspended in 0.15 M NaCl, vortexed for 1 min, and centrifuged for 30 min at 5,000 × g (13). The supernatant containing the bacterial extracts was stored at −80°C. After column chromatography, these materials were solubilized in sodium dodecyl sulfate (SDS) for SDS-polyacrylamide gel electrophoresis (PAGE).
Isolation of high-molecular-weight mucin and coupling to epoxy-activated Sepharose 6B.
Unstimulated human whole saliva from one donor (nonsecretor, blood group A, lacking the secretor gene, encoding α[1-2]fucosyltransferase) was collected in an ice-cooled vessel. Isolation and purification of high-molecular-weight mucins (MG1; molecular mass, >106 kDa) from this saliva were performed, as previously described (37), by ultracentrifugation followed by filtration over Sephacryl HR 500.
Purified mucin was coupled to an epoxy-activated Sepharose 6B (Pharmacia Biotech, Uppsala, Sweden) column (8.5 cm by 1.8 cm) as specified by the manufacturer (26). The coupling efficiency was 80 to 90%; this was calculated by an enzyme-linked immunosorbent assay (ELISA) measurement of the amount of residual mucin left in the supernatant when monoclonal antibody (MAb) F2, raised against high-molecular-weight salivary mucin, was used (38). This MAb recognizes the epitope SO3-3Galβ1-3GlcNAc- moiety of the sulfo-Lewis a antigen. The absence of direct binding of proteins to the Sepharose matrix alone was verified by using the matrix material after incubation with 1 M ethanolamine to hydrolyze activated groups.
Affinity chromatography and SDS-PAGE.
Affinity chromatography was performed by loading 0.5 mg of bacterial extracts diluted in 1 column volume (5 ml) of washing buffer (50 mM sodium acetate, 50 mM NaCl [pH 5.0]) on the Sepharose-mucin column. After 60 min at room temperature, the column was washed extensively with 6 column volumes of washing buffer–0.1% Tween 20 to remove unbound proteins. Proteins bound to mucin were eluted stepwise (flow rate, 1 ml/min) with 1.5 and 3 M guanidine-HCl (Sigma). The fractions obtained after elution were dialyzed against distilled water for 24 h at 4°C. Protein was precipitated by mixing 1 ml of cold acetone (−20°C) with 200 μl of dialysis fractions. After incubation for 10 min at −20°C, the precipitated proteins were collected by centrifugation for 5 min at 19,000 × g. The pellet was air dried and solubilized in 50 μl of sample buffer (0.06 M Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 0.001% bromophenol blue, 5% β-mercaptoethanol). Protein profiles of each solubilized fraction were analyzed by SDS-PAGE in 12.5% discontinuous Tricine-based polyacrylamide gels (29) followed by silver staining.
Binding assays.
The binding of H. pylori components to purified mucin and a panel of biotinylated synthetic oligosaccharides (Table 1) was studied by an ELISA. Microtiter plates (Immulon II; Greiner) were coated with 100 μl of bacterial extract or affinity-purified proteins from the 1.5 and 3 M guanidine-HCl elution fractions (containing 1 to 2 μg of protein/ml). As a negative control, we also coated microtiter plates in the same manner with bovine serum albumin (BSA), two H. pylori heat shock proteins (HSPA and HSPB), and Hpn. Heat shock proteins and BSA, denatured with guanidine-HCl and renatured in distilled water, were also included. HSPA and HSPB were obtained as fusion proteins with mannose binding protein (kindly provided by A. Labigne, Pasteur Institute, Paris, France). Hpn is a metal binding protein of H. pylori (a kind gift from G. Plaut, Gastroentrology Division, New England Medical Center, Boston, Mass.). After the microtiter plates were washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBST), mucin (20 μg/ml) or synthetic oligosaccharide (1 μg/ml) dissolved in 50 mM sodium acetate–150 mM NaCl–0.5% Tween 20 (pH 5.0) was added, and the mixture was incubated for 2 h at 37°C. After being washed, the plates were probed with MAb F2 (1 μg/ml in PBST) for detection of mucin or with streptavidin-conjugated peroxidase for detection of biotinylated oligosaccharides. The plates were washed, and bound antibodies were detected with peroxidase-conjugated goat anti-mouse immunoglobulin (American Qualex, La Mirada, Calif.) with o-phenylenediamine (0.4 mg/ml) and H2O2 (0.012%, vol/vol) as substrates. Binding of biotinylated synthetic oligosaccharides (1 μg/ml) to H. pylori components and other proteins was studied by enzymatic detection with streptavidin conjugated to peroxidase. After the color reaction was stopped with 0.1 M sulfuric acid, the optical density in the wells was read at 492 nm. Neuraminidase and sodium metaperiodate (Sigma) mucin treatments were performed as described previously (34).
TABLE 1.
Synthetic oligosaccharides, multivalently bound to polyacrylamide carriers, used in this studya
| Oligosaccharide | Structure |
|---|---|
| Lewis x | Galβ1-4[Fucα1-3]GlcNAcβ-R |
| Lewis y | Fucα1-2Galβ1-4[Fucα1-3]GlcNAcβ-R |
| Lewis a | Galβ1-3[Fucα1-4]GlcNAcβ-R |
| Lewis b | Fucα1-2Galβ1-3[Fucα1-4]GlcNAcβ-R |
| Sialyl-Lewis a | Neu5Acα2-3Galβ1-3[Fucα1-4]GlcNAcβ-R |
| Sialyl-Lewis x | Neu5Acα2-3Galβ1-4[Fucα1-3]GlcNAcβ-R |
| Sulfo-Lewis a | SO3-3Galβ1-3[Fucα1-4]GlcNAcβ-R |
| Sulfo-Lewis x | SO3-3Galβ1-4[Fucα1-3]GlcNAcβ-R |
| Sulfogalactose | SO3-3Galβ-R |
| Sulfo-N-acetylglucosamine | β-d-GlcNAc-6-SO3 |
| H type 1 | Fucα1-2]Galβ1-3GlcNAcβ-R |
| H type 2 | Fucα1-2Galβ1-4GlcNAcβ-R |
| Mannose-6-phosphate |
Gal, galactose; GlcNAc, N-acetylglucosamine; Fuc, fucose; R, synthetic polyacrylamide carrier.
Amino acid sequence.
The N-terminal sequence of affinity-purified adhesin was determined after SDS-PAGE, transfer to a polyvinylidene difluoride membrane, and staining with Coomassie blue to localize the protein. The band was excised and analyzed with an automated Edman degradation protein sequencer (Applied Biosystems, Foster City, Calif.) for 12 degradation cycles.
RESULTS
Binding of H. pylori proteins to MG1.
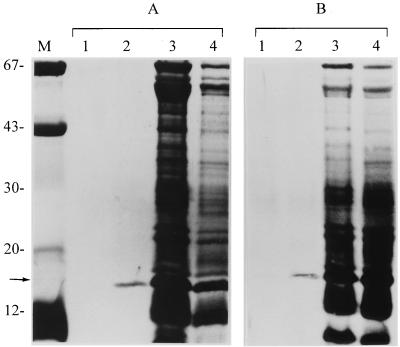
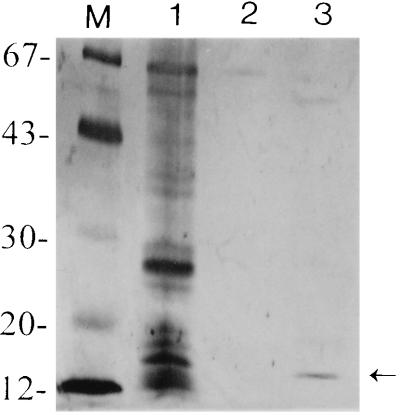
To determine whether the surface-exposed structures and OMPs of H. pylori are involved in binding to MG1, affinity chromatography was used. The bacterial extract of H. pylori ATCC 43504 and 3B3 and OMP of strain ATCC 43504 were subjected to affinity chromatography on a mucin-Sepharose column. In several separate experiments, we consistently observed binding of a protein with molecular mass of approximately 16 kDa to chromatographic media, which could be eluted from the column with 1.5 M guanidine-HCl (Fig. 1, lanes 2). Occasionally, a very small quantity of this protein could be found in the 3 M guanidine-HCl fraction. The same 16-kDa protein was also isolated from the OMP with 1.5 M guanidine-HCl (Fig. 2, lane 3). Sometimes a very small trace of 15- and 67-kDa protein bands was found as the background. None of the H. pylori protein fractions bound to a Sepharose matrix control column. These results showed that a 16-kDa adhesin was present in the bacterial extract and OMP and that it bound specifically to MG1.
FIG. 1.
SDS-PAGE (12.5% polyacrylamide) of protein fractions of H. pylori ATCC 4350 (A) and 3B3 (B) after affinity chromatography. The affinity column was eluted with 3 M guanidine-HCl (lanes 1) and 1.5 M guanidine-HCl (lanes 2). Unbound proteins after washing are shown in lanes 3. The total bacterial extract profiles are shown in lanes 4. The arrow indicates the position of the 16-kDa mucin-binding adhesin. Molecular size markers (lane M) in kilodaltons are shown on the left.
FIG. 2.
SDS-PAGE (12.5% polyacrylamide) of OMPs of H. pylori ATCC 43504 after affinity chromatography. Lanes: 1, total OMP profiles; 2 and 3, fractions eluted with 3 M and 1.5 M guanidine-HCl, respectively. The arrow indicates the 16-kDa mucin binding adhesin eluted with 1.5 M guanine-HCl. Molecular size markers (lane M) in kilodaltons are shown on the left.
Binding of bacterial extract and 16-kDa adhesin to mucin and oligosaccharides.
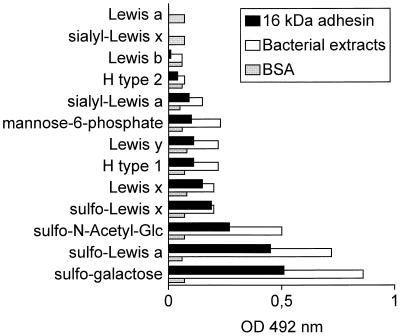
We previously found that whole cells of H. pylori possess a receptor for sulfated oligosaccharides (36). Therefore, the chemical nature of the oligosaccharides involved in the binding of the 16-kDa protein isolated from H. pylori adhesin was further investigated by using ELISA to screen a series of synthetic oligosaccharides (Table 1), multivalently attached to a polysaccharide carrier. The results showed that the bacterial extract and 16-kDa adhesin bound most avidly to polymeric sulfated carbohydrates, including sulfogalactose, sulfo-Lewis a, and sulfo-N-acetylglucosamine. Intermediate binding was observed with Lewis x, sulfo-Lewis x, Lewis y, H type 1, and mannose-6-phosphate. Very little to no binding was observed with H type 2, Lewis b, sialyl-Lewis x, and Lewis a (Fig. 3). For comparison, a number of other H. pylori proteins including native and denatured HSPA, HSPB, mannose binding protein (MBP), and Hpn, as well as BSA, were tested for binding to mucin and synthetic oligosaccharides. Only HSPA bound moderately to Lewis x, sulfo-Lewis a, and mucin, while no binding was observed with the other proteins, again showing the specificity of the 16-kDa adhesin (data not shown).
FIG. 3.
Binding of synthetic biotinylated oligosaccharides to bacterial extracts, 16-kDa adhesin of H. pylori, and BSA by as determined ELISA. Bound oligosaccharides were detected with streptavidin conjugated to horseradish peroxidase. OD, optical density.
Treatment of mucin with sodium metaperiodate and neuraminidase did not affect its binding to the 16-kDa adhesin.
Amino acid sequence of the 16-kDa protein.
The 16-kDa protein contained an N-terminal methionine residue (MKTFEILKHL GADAIVL). This sequence is identical to the N-terminal amino acid sequence of NAP of H. pylori (11).
DISCUSSION
We isolated from the bacterial extract and the OMPs of H. pylori a 16-kDa protein that binds specifically to the MG1 fraction of salivary mucins. Sequence analysis of the protein proved that it was identical to NAP, a previously recognized protein of H. pylori. Mucins are a family of highly glycosylated proteins which cover epithelial tissues throughout the human body. These proteins are involved in host mucosal defense, but they may also function as recognition and binding sites for microorganism, due to the high diversity of carbohydrate structures that they contain (39). Binding by mucin retards the access of microorganisms to the surface of the epithelial cells of the mucosa and favors their removal (21). On the other hand, some motile microorganisms such as H. pylori may use their temporary attachment to mucin as a means of “tracking” toward the epithelium, where they colonize and secrete virulence factors such as cytotoxins (10).
H. pylori binds to human gastric mucin (30, 34). We used salivary mucins because they are easily obtained and provide an interesting and valuable model system to study the structural and functional aspects of mucins in general (31). Salivary mucins, as constituents of mucous pellicles on epithelial and dental tissues, have a number of functions analogous to those of mucins elsewhere in the body (24). Moreover, H. pylori has been detected in various sites of the oral cavity by culture as well as by PCR (22). MG1 has characteristics in common with mucins in other mucous fluids and contains a wide spectrum of structurally different oligosaccharide side chains, some of which carry blood group antigens and function as receptors for bacterial adhesins (24, 35).
In a previous study (35), we reported that H. pylori bound most avidly to sulfated mucins and that the binding was enhanced at lower pHs (6.0 to 5.0). In experiments with synthetic polyacrylamide-coupled oligosaccharides, it was found that SO3-3-Gal and the SO3-3-Lewis a blood group antigen bound to H. pylori. In contrast, the binding of sialylated Lewis a and Lewis b antigens was much weaker. In this study, we have identified a 16-kDa adhesin which bound specifically to sulfated carbohydrate structure such as sulfo-Lewis a on MG1. In an inhibition experiment, the effect of polyanions such as dextran sulfate and DNA, at the same concentration of oligosaccharides as was used in this study, on the binding of NAP to mucin was tested. Dextran sulfate and DNA reduced the binding to 30 and 70% respectively (data not shown), suggesting that the binding has some specificity for chain geometry and distribution of negative charges. Recently, it has been reported that NAP is homologous to the Escherichia coli DNA binding protein Dps (20).
Several candidate molecules on gastric epithelial cells have been proposed as receptors for H. pylori adhesin. In particular, heparan sulfate and heparin bind specifically to H. pylori at low pH (2, 3). A 20-kDa hemagglutinin has been identified as a putative colonization factor on H. pylori (7). This antigen binds to N-acetylneuraminyllactose on mammalian cells in tissue culture. The amino acid sequence and the gene (hpaA) sequence of this adhesin are similar to those of the sialic acid-binding motif of E. coli SfaS, K99, and CFA/I (9) but are essentially different from those of the 16-kDa adhesin found in this study. Fauchere and Blaser (13) described a 15-kDa antigen of H. pylori in bacterial extract that adheres to HeLa cells. Neuraminidase treatment of the HeLa cells had no effect on binding, suggesting that the bacterial extract of H. pylori contains a receptor different from the N-acetylneuraminyllactose binding hemagglutinin identified by Evans et al. (7). This adhesin was not further characterized.
H. pylori is able to bind to human gastric mucin and sialic acid. Carbohydrate structures other than sialic acid are responsible for this interaction (34). However, the mucin-binding adhesins were not identified. Recently, a 20-kDa membrane-associated protein has been isolated from H. pylori (27). Details of this study have not been published yet, but the preliminary results indicate that this protein, like the 16-kDa adhesin isolated in the present study, binds to Lewis x but not to Lewis b antigens.
Analysis of the N-terminal amino acid sequence of the 16-kDa adhesin revealed sequence homology to H. pylori NAP (11). NAP is a bacterioferritin-type protein, and the gene (napA) that encodes it is detected in all strains tested; however, there is considerable strain variation in the level of expression of NAP activity in vitro (12). The N-terminal amino acid sequence of the 16-kDa protein was compared with the translation product of the genomic sequence of H. pylori published recently (33). There were no other genes whose products had similar N-terminal sequences. Furthermore, Yoshida et al. (41) demonstrated that a water extract of H. pylori promotes neutrophil adhesion to endothelial cells via CD11a/CD18- and CD11b/CD18-dependent interaction with ICAM-1. Later, it was shown that this proadhesive activity is associated with NAP (12). Recently, it was reported that NAP binds selectively to four compounds of the acid glycosphingolipid fraction of neutrophils (32). It would be interesting to study whether these glycosphingolipids possess sulfated structures similar to those found in this study. These observations suggest that NAP is able to display different functions: binding to nonglycolipid, sulfated carbohydrate structures such as the sulfo-Lewis a fraction of high-molecular-weight salivary mucin and binding to the glycolipid fraction of neutrophils. Moreover, the binding of the 16-kDa adhesin to Lewis x suggests the possibility that this adhesin regulates neutrophil function through cross-linking of Lewis x on CD11/CD18.
REFERENCES
- 1.Alkout A M, Blackwell C C, Weir D M, Poxton I R, Elton R A, Luman W, Palmer K. Isolation of a cell surface component of Helicobacter pylori that binds H type 2, Lewisa, and Lewisb antigens. Gastroentrology. 1997;112:1179–1187. doi: 10.1016/s0016-5085(97)70129-x. [DOI] [PubMed] [Google Scholar]
- 2.Ascencio F, Franson L A, Wadström T. Affinity of the gastric pathogen Helicobacter pylori for the N-sulphated glycosaminoglycan heparan sulphate. J Med Microbiol. 1993;38:240–244. doi: 10.1099/00222615-38-4-240. [DOI] [PubMed] [Google Scholar]
- 3.Ascencio F, Hansson H-A, Larm O, Wadström T. Helicobacter pylori interaction with heparin and heparin-dependent growth factor. FEMS Immunol Med Microbiol. 1995;12:256–272. doi: 10.1111/j.1574-695X.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaser M J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 5.Borén T, Falk P, Rath K A, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 6.Dent J C, McNulty C A M. Evaluation of a new selective medium for Campylobacter pylori. Eur J Clin Microbiol Infect Dis. 1988;7:555–568. doi: 10.1007/BF01962615. [DOI] [PubMed] [Google Scholar]
- 7.Evans D G, Evans D J, Jr, Moulds J J, Graham D Y. N-Acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans D G, Evans D J, Graham D Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayer. Infect Immun. 1989;57:2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans D G, Karjalainen T K, Evans D J, Jr, Graham D Y, Lee C H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D J, Jr, Evans D G. Colonization factor antigen of human pathogens. Curr Top Microbiol Immun. 1990;151:129–145. doi: 10.1007/978-3-642-74703-8_7. [DOI] [PubMed] [Google Scholar]
- 11.Evans D J, Jr, Evans D G, Lampert H C, Nakano H. Identification of four new prokaryotic bacterioferritins, from Helicobacter pylori, Anabaena variabilis, Bacillus subtilis and Treponema pallidum, by analysis of gene sequences. Gene. 1995;153:123–127. doi: 10.1016/0378-1119(94)00774-m. [DOI] [PubMed] [Google Scholar]
- 12.Evans D J, Jr, Evans D G, Takemura T, Nakano H, Lampert H C, Graham D Y, Granger D N, Kvietys P E. Characterization of a Helicobacter pylori neutophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauchere J L, Blaser M J. Adherence of Helicobacter pylori cells and their surface components to HeLa cell membranes. Microb Pathog. 1990;9:427–439. doi: 10.1016/0882-4010(90)90061-t. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin C S, Armstrong J A, Marshall B J. Campylobacter pyloridis gastritis and peptic ulceration. J Clin Pathol. 1986;39:353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazell S L, Lee A, Brady L, Hennessy W. Campylobacter pylori and gastritis: association with intracellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986;153:658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- 16.Hessey S J, Spencer J, Wyatt J I, Sobala G, Rathbane B J, Axon A T R, Dixon M F. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990;31:134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi Y, Okazaki K I, Murakami K. Adhesion of Helicobacter pylori to gastric epithelial cells in primary cultures obtained from stomach of various animals. Infect Immun. 1993;61:4058–4063. doi: 10.1128/iai.61.10.4058-4063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingwood C A, Huesca M, Kuksis A. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect Immun. 1992;60:2470–2474. doi: 10.1128/iai.60.6.2470-2474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall B J, Warren J R. Unidentified curved bacillus in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;ii:1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 20.McGowan, C. C., A. S. Necheva, T. L. Cover, and M. J. Blaser. 1997. Acid-induced expression of oxidative stress protein homologs in H. pylori. Gut 41(Suppl. 1):A18.
- 21.McSweegan E, Walker R I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namavar F, Roosendaal R, Kuipers E J, de Groot P, van der Bijl M W, Pena A S, de Graaff J. Presence of Helicobacter pylori in the oral cavity, oesophagus, stomach and faeces of patients with gastritis. Eur J Clin Microbiol Infect Dis. 1995;14:234–237. doi: 10.1007/BF02310363. [DOI] [PubMed] [Google Scholar]
- 23.Neman-Simha V, Megraud F. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988;56:3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieuw Amerongen A V, Bolscher J G M, Veerman E C I. Salivary mucins: protective functions in relation to their diversity. Glycobiology. 1995;5:733–740. doi: 10.1093/glycob/5.8.733. [DOI] [PubMed] [Google Scholar]
- 25.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 26.Pharmacia Biotech. Affinity chromatography: principles and methods. Uppsala, Sweden: Pharmacia Biotech; 1993. [Google Scholar]
- 27.Rasko, D. A., C. M. Szymanski, G. D. Armstrong, and D. E. Taylor. 1997. Binding of Helicobacter pylori to Lewis antigens. Ir. J. Med. Sci. 166(Suppl.3):P40.
- 28.Saito T, Natomi N, Zhao W, Okuzumi K, Sugano K, Iwamori M, Nagai Y. Identification of glycolipid receptors for Helicobacter pylori by TLC immunostaining. FEBS Lett. 1991;282:385–387. doi: 10.1016/0014-5793(91)80519-9. [DOI] [PubMed] [Google Scholar]
- 29.Schägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 Kda. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 30.Slomiany B L, Piotrowski J, Samanta A, Vanhorn K, Murty V L N, Slomiany A. Campylobacter pylori colonization factor shows specificity for lactosylceramide sulfate and GM3 ganglioside. Biochem Int. 1989;19:929–936. [PubMed] [Google Scholar]
- 31.Tabak L A. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 32.Teneberg, S., H. Miller-Podraza, H. C. Lampert, D. J. Evans, Jr., D. G. Evans, D. Danielsson, and K. A. Karlsson. 1997. Carbohydrate-binding specificity of the neutrophil-activating protein of Helicobacter pylori. Ir. J. Med. Sci. 166(Suppl. 3):P36. [DOI] [PubMed]
- 33.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 34.Tzouvelekis L S, Mentis A F, Makris A M, Spiliadis C, Blackwell C, Weir D M. In vitro binding of Helicobacter pylori to human gastric mucin. Infect Immun. 1991;59:4252–4254. doi: 10.1128/iai.59.11.4252-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veerman E C I, Ligtenberg A J M, Schenkels L C P M, Walgreen-Weterings E, Nieuw Amerongen A V. Binding of human high-molecular-weight salivary mucins (MG1) to Hemophilus parainfluenzae. J Dent Res. 1995;74:351–357. doi: 10.1177/00220345950740011101. [DOI] [PubMed] [Google Scholar]
- 36.Veerman, E. C. I., C. M. C. Bank, F. Namavar, B. J. Appelmelk, J. G. M. Bolscher, and A. V. Nieuw Amerongen. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology, in press. [DOI] [PubMed]
- 37.Veerman E C I, Valentijn-Benz M, Bank R, Nieuw Amerongen A V. Isolation of high molecular weight mucins from human whole saliva by ulteracentrifugation. J Biol Buccale. 1989;17:302–312. [PubMed] [Google Scholar]
- 38.Veerman E C I, Valentijn-Benz M, van den Keijbus P A M, Rathman W M, Sheehan J K, Nieuw Amerongen A V. Immunochemical analysis of salivary mucins using monoclonal antibodies. Arch Oral Biol. 1991;34:923–932. doi: 10.1016/0003-9969(91)90125-e. [DOI] [PubMed] [Google Scholar]
- 39.Wadolkowski E A, Laux D C, Cohen P S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of adhesion to mucosal receptors. Infect Immun. 1988;56:1036–1043. doi: 10.1128/iai.56.5.1036-1043.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worst D J, Otto B R, de Graaff J. Iron-repressible outer membrane proteins of Helicobacter pylori involved in heme uptake. Infect Immun. 1995;63:4161–4165. doi: 10.1128/iai.63.10.4161-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida N, Granger D N, Evans D J, Jr, Evans D G, Graham D Y, Anderson D C, Wolf R E, Kvietys P R. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]