Summary
Background and Aims:
Whether hepatocellular carcinoma (HCC) increases the familial risk for hepatic fibrosis has not been thoroughly explored, particularly in Mexican Americans who are disproportionately affected by obesity and metabolic syndrome. We evaluated the risk of significant hepatic fibrosis in first-degree relatives of Mexican American adults with HCC.
Methods:
We performed a cross-sectional analysis of a prospective cohort of Mexican American probands with HCC and first-degree relatives enrolled in the Hispanic Liver Cancer Cohort study. We evaluated the prevalence of hepatic fibrosis in first-degree relatives, defined by liver stiffness measurement (LSM)>= 7.0 kPa with transient elastography (TE). Secondary outcomes included the prevalence of definite hepatic steatosis, defined by controlled attenuation parameter >=288 dB/m.
Results:
We identified 70 probands diagnosed with HCC; 47% were female and the mean age was 62 years (±13 years). Among 112 first-degree relatives with a mean age of 43 years (±14 years), 19 (17%) had significant fibrosis and 47 (42%) had definite hepatic steatosis, respectively. The prevalence of significant fibrosis was 20% in first-degree relatives 40 years of age or older. Regression analysis revealed that diabetes (OR 3.2, 95% CI: 1.1–9.2, p = 0.03) and aspartate aminotransferase >=30 units/L (OR 4.0, 95% CI: 1.4–11.7, p = 0.01) were predictors of significant fibrosis in first-degree relatives.
Conclusions:
Using a well-phenotyped familial cohort, we found that the prevalence of significant fibrosis and definite hepatic steatosis are high in first-degree relatives of Mexican Americans with HCC, particularly those with diabetes, suggesting that this population may benefit from screening for liver disease.
1 |. INTRODUCTION
The Hispanic population is the largest ethnic group within the United States with a growing population estimated to be around 110 million by the year 2060,1 necessitating the need to accurately evaluate health disparities that affect this population. Hispanic Americans have a higher incidence rate of nonalcoholic fatty liver disease (NAFLD) and hepatocellular carcinoma (HCC).2–7 More specifically, Mexican Americans living in the southern region of Texas have one of the highest prevalence rates of NAFLD as well as the highest age-adjusted HCC incidence rate of any ethnic group in the United States,8 representing a public health concern.1
Hepatic fibrosis is a primary risk factor for liver-related events and HCC. Previous studies have shown that both hepatic steatosis and hepatic fibrosis are heritable.9 A recent study revealed increased incidences of fibrosis in first-degree relatives of patients with NAFLD cirrhosis, suggesting advanced fibrosis screening could be considered in first-degree relatives of patients with NAFLD cirrhosis.10 However, to our knowledge there are no prospective data examining the prevalence of advanced fibrosis and hepatic steatosis in first-degree relatives of Mexican Americans with HCC. As such, there is currently insufficient insight into whether family members should be screened for NAFLD and hepatic fibrosis. As screening for fibrosis becomes more accessible with non-invasive imaging modalities, this discussion becomes more relevant and essential in this understudied patient population.11–13
To address this knowledge gap, we evaluated the prevalence of hepatic fibrosis and definite hepatic steatosis in first-degree relatives of Mexican Americans with HCC in a population-based cohort in South Texas and examined clinical factors associated with hepatic fibrosis and steatosis.
2 |. MATERIALS AND METHODS
2.1 |. Study population and design
This is a cross-sectional analysis of a prospective cohort study which included 70 adult probands with HCC enrolled in the Hispanic Liver Cancer Cohort (HLCC), a randomly ascertained, community-based cohort study of Mexican Americans, developed as an ancillary cohort to the Cameron County Hispanic Cohort and using the same protocols.14,15 Briefly, individuals were recruited from border communities on the Texas-Mexico border. All probands included were diagnosed with HCC by a board-certified gastroenterologist either based on cross-sectional imaging (using the OPTN/LIRADs staging system) or histology of a targeted biopsy.
Detailed contact information for up to 10 first-degree relatives of each study participant with HCC was obtained. Study team members then attempted to contact the first-degree relatives: first by phone, then by up to five home visits at varying days and times. Each first-degree family member completed the standard Cameron County Hispanic Cohort examination described previously which includes a detailed physical examination, anthropometric measurements, fasting blood draw, urinalysis, personal medical history and vibration-controlled transient elastography (VCTE).14,16 Diabetes (i.e. type 2 diabetes mellitus) was defined using (1) physician diagnosis, (2) HbA1c level (≥6.5%), (3) fasting blood glucose (≥ 126 mg/dl) or (4) anti-diabetic medication status according to American Diabetes Association diagnostic criteria.17
VCTE was performed using FibroScan® 502 Touch model (Echosens) by a trained technician, according to previously described methods.18 VCTE liver stiffness measurement was obtained in the supine position during a 10-s breath hold. Participants were provided standardised instructions to fast for at least 3 hours and avoid alcohol use prior to VCTE. The procedure included a minimum of 10 measurements to determine the median valid liver stiffness measurements in kilopascals (kPa) and the IQR. According to the manufacturer protocol, all patients were first scanned using the M probe (3.5 MHz) and when indicated by the equipment upon initial assessment, patients were re-scanned using the XL probe (2.5 MHz). The controlled attenuation parameter (CAP) value in dB/m was simultaneously measured for the assessment of liver steatosis measurements, co-localised to the valid liver stiffness measurements. Technical failure was defined as inability to obtain ≥10 valid measurements.19 Liver stiffness measurement (LSM) was considered unreliable when the interquartile range (IQR)/median was >0.30 in patients with a median ≥7.1 kPa.20 Patients with a technical failure and/or unreliable exam were excluded from analyses. Patients with chronic viral hepatitis and significant alcohol use (defined by >=7 drinks per week in women or >=14 drinks per week in men) were excluded from our analyses to minimise confounding factors that may influence LSM.
2.2 |. Primary and secondary outcomes
The primary outcome was the prevalence of significant hepatic fibrosis in first-degree relatives, defined by LSM >=7.0 kPa with VCTE.21 Secondary outcomes included definite hepatic steatosis and suspected cirrhosis. Hepatic steatosis was defined by CAP >= 288 dB/m.22 Suspected cirrhosis was defined by either LSM >= 12 kPa or FIB-4>= 2.67.21
We subsequently performed sensitivity analyses to examine the prevalence of significant fibrosis, suspected cirrhosis and steatosis at other validated LSM (>9.7 and >13.6 kPa, respectively) and CAP (>274) cut-off points (Table S1).23
2.3 |. Statistical analysis
Descriptive characteristics of data are presented as mean (SD), median (IQR) or number (%). Categorical data were compared using the chi-square and Fisher’s exact tests. Differences between groups were analysed using a two-independent-sample t test or Wilcoxon–Mann–Whitney test. Univariable and multivariable logistic regression models were used to examine clinical factors associated with significant fibrosis and hepatic steatosis in first-degree relatives. Covariates in adjusted analyses were selected based on biologic plausibility to act as confounders as well as p < 0.20 in unadjusted analyses. Covariates that were not statistically significant (p < 0.05) in the multivariable models were sequentially omitted with backward stepwise regression. Sensitivity analyses were performed in the subset of the cohort with family membership data. Odds ratios were derived from generalised estimation equations (PROC GENMOD) to account for correlation within family members.
All analyses were performed using SAS 9.4 (SAS Institute). A two-tailed p ≤ 0.05 was considered statistically significant for all analyses.
2.4 |. Study approval
All study participants provided informed consent. The study protocol was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston. All authors had access to the study data and reviewed and approved the final manuscript.
3 |. RESULTS
3.1 |. Characteristics of Mexican American probands with hepatocellular carcinoma
Clinical characteristics of the 70 probands with HCC are detailed in Table S2. Probands had a mean age of 63 years (SD 13 years) and 53% were male. The mean body mass index (BMI) was 31.9 kg/m2 (SD 8.9 kg/m2) and 56% had type 2 diabetes mellitus. 90% of the proband cohort reported no alcohol use. Mean serum aspartate aminotransferase (AST) was 49 IU/L (SD 27 IU/L), and total bilirubin was 1.0 mg/dl (SD 0.7 mg/dl).
3.2 |. Prevalence and factors associated with hepatic fibrosis in first-degree relatives
We identified 112 first-degree relatives, among which the mean age was 43 years (SD 14 years) with 58% (65/112) relatives that were age 40 or older (Table 1). 65% of first-degree relatives were female. The mean LSM among the first-degree relative cohort was 5.8 kPa (SD 5.4 kPa). The prevalence of hepatic fibrosis was 17% (19/112) in the overall cohort and 20% when limiting analyses to first-degree relatives aged 40 and older (Table 1 and Figure 1). Among first-degree relatives 40 years or older, 5% met the criteria for suspected cirrhosis.
TABLE 1:
Characteristics of first-degree relatives of probands with hepatocellular carcinoma
| Characteristic | All relatives (n = 112) | Relatives ≥ 40 years old (n = 65) |
|---|---|---|
| Age (years)a | 42.8 (14.4) | 52.9 (9.1) |
|
| ||
| Female, n (%) | 73 (65.2%) | 43 (66.2%) |
|
| ||
| Controlled attenuation parameter (CAP) | ||
| CAP (dB/m)a | 273.5 (62.0) | 274.6 (60.2) |
| CAP ≥ 250 dB/m, n (%) | 74 (66.1%) | 46 (70.8%) |
| CAP ≥ 288 dB/m, n (%) | 47 (42.0%) | 26 (40.0%) |
|
| ||
| Liver stiffness with VCTE | ||
| VCTE (kPa)a | 5.8 (5.4) | 6.3 (6.9) |
| VCTE (kPa) ≥ 7 kPa | 19 (17.0%) | 13 (20.0%) |
| VCTE (kPa) ≥ 12 kPa | 4 (3.6%) | 3 (4.6%) |
|
| ||
| FIB-4 indexa | 0.7 (0.4) | 0.9 (0.4) |
|
| ||
| FIB-4 index ≥ 2.67, n (%) | 1 (0.9%) | 1 (1.7%) |
|
| ||
| VCTE (kPa) ≥ 12 kPa and/or FIB-4 ≥ 2.67 | 4 (3.6%) | 3 (4.6%) |
Abbreviations: CAP, controlled attenuation parameter; HbA1c, glycated haemoglobin; VCTE, vibration-controlled transient elastography.
Mean (SD).
FIGURE 1.
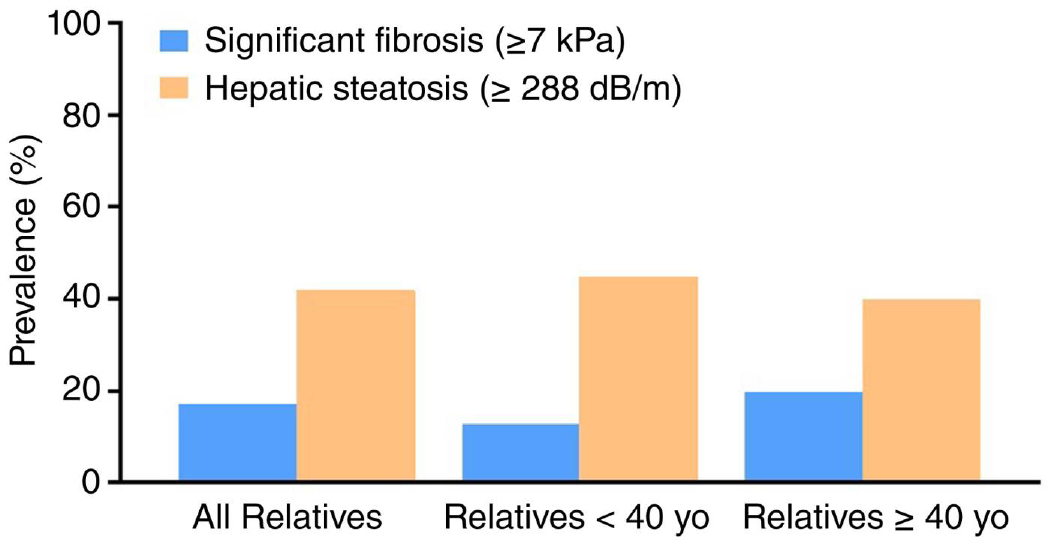
Prevalence of significant fibrosis and steatosis (with or without fibrosis) in first-degree relatives of Mexican Americans with hepatocellular carcinoma.
Comparisons between first-degree relatives with and without fibrosis are shown in Table 3. First-degree relatives with fibrosis had significantly higher BMI (34.1 vs. 30.9 kg/m2, p = 0.05) and were more likely to have type 2 diabetes (42% vs. 19%, p = 0.04). AST was significantly higher in first-degree relatives with significant fibrosis. There were no significant differences in age, sex, hypertension, hypercholesterolemia, alcohol use or tobacco use between first-degree relatives with and without significant fibrosis (Table 2). Among first-degree relatives with hepatic fibrosis, 58% had serum AST < 30 IU/L and 32% were non-obese (BMI < 30 kg/m2).
TABLE 3.
Factors associated with liver fibrosis (liver stiffness measurement ≥ 7 kPa) in first-degree relatives of Mexican American probands with hepatocellular carcinoma
| OR (95% CI)a | p-value | |
|---|---|---|
| Age (1-year increase) | 1.02 (0.98–1.05) | 0.41 |
| Male gender | 0.83 (0.29–2.38) | 0.72 |
| Body mass index ≥ 30kg/m2 | 1.90 (0.67–5.44) | 0.23 |
| Type 2 diabetes | 3.21 (1.12–9.19) | 0.03 |
| Hypercholesterolemia | 1.40 (0.50–3.95) | 0.52 |
| Hypertension | 1.40 (0.50–3.95) | 0.52 |
| Tobacco use | 0.66 (0.14–3.16) | 0.60 |
| Any alcohol use | 0.66 (0.14–3.16) | 0.60 |
| AST ≥ 30 IU/L | 4.00 (1.37–11.71) | 0.01 |
| Albumin < 3.5 g/dl | 5.24 (0.67–39.76) | 0.11 |
Note: Bold values represent the significance of p-value (p < 0.05). Abbreviation: AST, aspartate aminotransferase.
Results from univariable analysis are shown.
TABLE 2.
Characteristics of first-degree relatives of Mexican Americans with hepatocellular carcinoma by the presence of liver fibrosis
| First-degree relatives with fibrosis (N = 19) | First-degree relatives without fibrosis (N = 93) | p-value* | |
|---|---|---|---|
| Age (years)a | 45.3 (13.4) | 42.3 (14.6) | 0.42 |
|
| |||
| BMI (kg/m2), n (%) | 34.1 (7.2) | 30.9 (6.1) | 0.05 |
|
| |||
| Female, n (%) | 13 (68.4%) | 60 (64.5%) | 0.74 |
|
| |||
| Medical co-morbidities, n (%) | |||
| Hypertension | 7 (36.8%) | 28 (30.1%) | 0.56 |
| Hypercholesterolemia | 7 (36.8%) | 27 (29.0%) | 0.50 |
| Type 2 diabetes | 8 (42.1%) | 17 (18.5%) | 0.04 |
|
| |||
| Alcohol use (drinks/week), n (%) | |||
| None | 17 (89.5%) | 79 (85.0%) | 1.00 |
| 1–7 | 1 (5.3%) | 6 (6.4%) | |
| ≥ 7 | 1 (5.3%) | 8 (8.6%) | |
|
| |||
| Tobacco use, n (%) | 2 (10.5%) | 14 (15.1%) | 1.00 |
|
| |||
| Biochemical dataa | |||
| AST (IU/L) | 36.1 (28.2) | 22.3 (12.4) | 0.04 |
| ALT (IU/L) | 46.6 (32.4) | 36.4 (26.9) | 0.085 |
| Bilirubin (mg/dl) | 0.5 (0.2) | 0.5 (0.3) | 0.59 |
| Albumin (g/dl) | 3.8 (0.3) | 4.1 (0.3) | 0.002 |
| Total cholesterol (mg/dl) | 188.4 (32.7) | 177.4 (39.3) | 0.24 |
| HDL (mg/dl) | 49.1 (8.8) | 49.0 (11.4) | 0.86 |
| LDL (mg/dl) | 107.9 (32.6) | 97.5 (34.1) | 0.29 |
| TG (mg/dl) | 156.5 (63.4) | 145.7 (93.6) | 0.16 |
| Platelet count (109/L) | 252.5 (65.1) | 265.0 (61.9) | 0.36 |
| HbA1c (%) | 6.6 (1.5) | 6.1 (1.5) | 0.069 |
|
| |||
| FIB-4a | 1.0 (0.6) | 0.7 (0.4) | 0.02 |
|
| |||
| FIB4 ≥ 2.67, n (%) | 1 (5.3%) | 0 | 0.18 |
Note: Bold values represent the significance of p-value (p < 0.05).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HbA1c, glycated haemoglobin; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides.
Mean (SD).
p-values for biochemical data from Wilcoxon two-sample test, otherwise from the t test, chi-square or Fisher’s as appropriate.
In logistic regression analyses, type 2 diabetes mellitus (OR 3.21, p = 0.03) and serum AST >= 30 IU/L (OR 4.0, p = 0.01) were factors significantly associated with hepatic fibrosis among first-degree relatives (Table 3). Serum AST >= 30 IU/L (OR 4.76, 95% CI: 1.57–14.5, p = 0.006) and serum albumin <3.5 g/dl (OR 8.3, 95% CI: 1.04–66.6, p = 0.05) were factors significantly associated with fibrosis in multivariable analyses. A sensitivity analysis performed in 102 first-degree relatives with family membership data revealed type 2 diabetes mellitus (OR 3.8, p = 0.01), serum AST >= 30 IU/L (OR 4.3, p = 0.01) and BMI >= 30 kg/m2 (OR 2.7, p = 0.02) as factors most associated with fibrosis (Table S3).
3.3 |. Prevalence and factors associated with hepatic steatosis in first-degree relatives
The prevalence of definite hepatic steatosis (defined by CAP ≥ 288 dB/m) in first-degree relatives was 42% with a mean CAP of 273.5 dB/m (SD 62 dB/m; Table 1 and Figure 1). Comparisons between first-degree relatives with and without definite hepatic steatosis are shown in Table 4. Mean age was similar among first-degree relatives with and without definite hepatic steatosis. BMI (35.2 vs. 28.8 kg/m2, p =< 0.001) and serum alanine aminotransferase (ALT) level (46 vs. 33 IU/L, p = 0.001) were significantly higher in first-degree relatives with hepatic steatosis. First-degree relatives with definite hepatic steatosis were more likely to have type 2 diabetes (30% vs. 17%, p = 0.04).
TABLE 4.
Characteristics of first-degree relatives with definite hepatic steatosis
| First-degree relatives with hepatic steatosis (n = 47) | First-degree relatives without hepatic steatosis (N = 65) | p-value* | |
|---|---|---|---|
| Age (years)a | 43.5 (13.1) | 42.4 (15.3) | 0.69 |
|
| |||
| BMI (kg/m2)a | 35.2 (6.1) | 28.8 (5.2) | <0.0001 |
|
| |||
| Female, n (%) | 32 (68.1%) | 41 (63.1%) | 0.58 |
|
| |||
| Medical co-morbidities, n (%) | |||
| Hypertension | 19 (40.4%) | 16 (24.6%) | 0.07 |
| Hypercholesterolemia | 15 (31.9%) | 19 (29.2%) | 0.76 |
| Type 2 diabetes | 14 (29.8%) | 11 (17.2%) | 0.042 |
|
| |||
| Alcohol use (drinks/week) | |||
| None | 41 (87.2%) | 55 (84.6%) | 0.84 |
| 1–7 | 2 (4.3%) | 5 (7.7%) | |
| ≥ 7 | 4 (8.5%) | 5 (7.7%) | |
|
| |||
| Tobacco use, n (%) | 8 (17.0%) | 8 (12.3%) | 0.48 |
|
| |||
| Biochemical dataa | |||
| AST (IU/L) | 27.7 (19.9) | 22.6 (14.2) | 0.06 |
| ALT (IU/L) | 45.5 (30.7) | 32.9 (24.9) | 0.0007 |
| Bilirubin (mg/dl) | 0.5 (0.2) | 0.5 (0.3) | 0.96 |
| Albumin (g/dl) | 4.0 (0.3) | 4.0 (0.3) | 0.25 |
| Total cholesterol (mg/dl) | 186.1 (39.8) | 174.4 (36.7) | 0.17 |
| HDL (mg/dl) | 45.2 (9.6) | 51.8 (11.1) | 0.003 |
| LDL (mg/dl) | 105.2 (36.5) | 95.3 (31.7) | 0.25 |
| TG (mg/dl) | 178.6 (91.2) | 125.3 (80.8) | 0.0003 |
| Platelet count (109/L) | 253.1 (67.0) | 269.6 (58.5) | 0.094 |
| HbA1c (%) | 6.4 (1.5) | 6.1 (1.5) | 0.01 |
|
| |||
| FIB-4 indexa | 0.8 (0.4) | 0.7 (0.4) | 0.24 |
|
| |||
| FIB-4 Ȧ 2.67, n (%) | 0 | 1 (1.6%) | 1.00 |
Note: Bold values represent the significance of p-value (p < 0.05).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HbA1c, glycated haemoglobin; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides.
Mean (SD).
p-values for biochemical data from Wilcoxon two-sample test, otherwise from the t test, chi-square or Fisher’s as appropriate.
Univariable logistic regression analysis revealed that BMI >= 30 kg/m2 (OR 12.7, p < 0.001), serum ALT >= 30 IU/L (OR 3.5, p = 0.002) and serum triglycerides (every 10 unit increase, OR 1.08, p = 0.004) were significantly associated with hepatic steatosis in first-degree relatives (Table 5). BMI >= 30 kg/m2 (OR 13.6, 95% CI: 4.65–39.75, p < 0.001) and serum triglycerides (OR 1.08, 95% CI: 1.02–1.14, p = 0.008) were factors significantly associated with hepatic steatosis in multivariable analysis. A sensitivity analysis that included 102 first-degree relatives with family membership revealed that BMI >= 30 kg/m2 (OR 8.55, p < 0.001), serum ALT >= 30 IU/L (OR 2.94, p = 0.007) and serum triglycerides (OR 1.01, p = 0.05) were the strongest clinical factors associated with hepatic steatosis (Table S4).
TABLE 5.
Factors associated with definite hepatic steatosis in first-degree relatives of Mexican Americans with hepatocellular Carcinoma
| OR (95% CI)a | p-value | |
|---|---|---|
| Age (1-year increase) | 1.01 (0.98–1.03) | 0.66 |
| Male gender | 0.78 (0.35–1.73) | 0.54 |
| Body mass index ≥ 30 kg/m2 | 12.73 (4.68–34.64) | <0.0001 |
| Type 2 diabetes | 2.04 (0.83–5.04) | 0.12 |
| Hypercholesterolemia | 1.11 (0.49–2.51) | 0.80 |
| Any alcohol use | 0.79 (0.27–2.35) | 0.67 |
| ALT >= 30 IU/L | 3.49 (1.58–7.73) | 0.002 |
| AST >= 30 IU/L | 1.91 (0.74–4.89) | 0.18 |
| TG (10 unit increase, mg/dl) | 1.08 (1.02–1.14) | 0.004 |
Note: Bold values represent the significance of p-value (p < 0.05).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TG, triglycerides.
Results from univariable analysis shown.
4 |. DISCUSSION
In this prospective, community-based cohort study, which included 70 Mexican American adults with HCC and 112 of their first-degree relatives, we found that hepatic fibrosis was highly prevalent in first-degree relatives. Specifically, 17% of first-degree relatives had hepatic fibrosis (VCTE >= 7 kPa). The prevalence of fibrosis was 20% in first-degree relatives aged 40 years and older and 5% met the criteria for cirrhosis. Compared with first-degree relatives without fibrosis, those with fibrosis had significantly higher BMI and serum AST and were more likely to have type 2 diabetes. Regression analysis revealed that type 2 diabetes (OR 3.2) and AST >= 30 IU/L (OR 4.0) were independent risk factors for hepatic fibrosis in first-degree relatives. Our finding that diabetes was associated with a threefold increased risk of fibrosis in first-degree relatives corroborates prior findings that elevated HbA1c is associated with liver fibrosis in Mexican Americans.16
We also found a high prevalence of definite hepatic steatosis in first-degree relatives, as 42% had CAP >= 288 dB/m. Notably, we used a high cut-off for CAP to define hepatic steatosis. With a CAP cut-off of >250 dB/m, which has been shown to correlate with grade 1 steatosis in several prior studies,24 the prevalence of hepatic steatosis in first-degree relatives was 66%. Obesity (OR 12.7), ALT >= 30 (OR 3.5) and triglycerides (every 10 unit increase, OR 1.1) were clinical factors most significantly associated with hepatic steatosis in first-degree relatives.
Altogether, these findings highlight the high prevalence of clinically significant liver disease in first-degree relatives of Mexican Americans with HCC and suggest that screening should be considered, to facilitate early identification of those at risk of subsequent liver-related morbidity. Previous studies have shown that hepatocyte ballooning and formation of Mallory bodies develop more rapidly in Hispanics, potentially underscoring the pathophysiology of why hepatic steatosis and fibrosis are increasing at an alarming rate in Mexican Americans living in the United States.25 The majority of first-degree relatives had no evidence of viral hepatitis or history of alcohol consumption, and we suspect that liver disease is predominantly related to nonalcoholic fatty liver. Over the past decade, screening for hepatic fibrosis has become more accessible with non-invasive modalities such as VCTE (e.g. FibroScan), magnetic resonance elastography and risk stratification systems (e.g. FIB-4 index).11–13 Early identification of liver disease in the Mexican American population could yield improved outcomes. Further studies to predict the risk of liver disease in first-degree relatives of Mexican Americans with HCC and to delineate clinical factors associated with liver disease in this population are needed.
Mexican American probands with HCC in our cohort had a high prevalence of obesity, diabetes, hypertension and hypercholesterolemia, which corroborates previous studies characterising Hispanic Americans with HCC.2,4,26–28 Several population-based studies have indicated that there may a hereditary component of hepatic steatosis and fibrosis.29–32 Our group has also previously demonstrated an increased incidence of hepatic fibrosis in the first-degree relatives of patients with cirrhosis secondary to nonalcoholic fatty liver disease (NAFLD).10 Over the past few decades, there has been a significant leap in the discovery of possible pathophysiologic mechanisms that may explain the hereditary predisposition of hepatic fibrosis.33 In genome-wide association studies, a number of genetic polymorphisms have been associated with increased risk for NAFLD and HCC via diverse mechanisms including lipid metabolism, oxidative stress and hepatic lipid compartmentalization.26,33–37 Of these identified variants, the PNPLA3 rs73849 (I148M) variant has the strongest association with hepatic steatosis, fibrosis progression and risk of HCC development.26,38,39 Additional genome-wide studies have shown that the PNPLA3 I148M variant occurs twice as frequently in Hispanic populations than in non-Hispanics.40–43
There is a paucity of data on the prevalence of hepatic fibrosis and hepatic steatosis in first-degree relatives of Mexican American probands with HCC. To our knowledge, our study is the first to evaluate the prevalence of both hepatic steatosis and fibrosis as well as risk factors for these in this patient population. We used a well-characterised cohort of Mexican American adults with HCC and first-degree relatives (n = 112) with extensive data collection on co-morbidities as well as non-invasive assessment for hepatic steatosis and fibrosis with VCTE. Our study does have limitations. The sample size is small which limited multivariable analyses (particularly for factors associated with fibrosis); however, to our knowledge this is the largest study examining the risk of liver disease in first-degree relatives of Mexican Americans with HCC. Non-invasive assessment with VCTE was used to identify hepatic steatosis and fibrosis in first-degree relatives. While liver biopsy remains the gold standard for diagnosing hepatic steatosis and fibrosis, VCTE has been shown to reliably estimate hepatic fibrosis and steatosis,11,24,44 although the interpretation of VCTE must take into account laboratory results and clinical parameters.11,24,44,45 The LSM cut-off used in our study could have resulted in misclassification; therefore, we also performed sensitivity analyses to examine the prevalence at different cut-points with higher specificity for advanced fibrosis.23 Our study comprised Mexican Americans living in Texas, a population previously shown to have one of the highest prevalence of hepatic steatosis and incidences of HCC in the United States, but whether these results are reflective of liver disease incidence in all Mexican Americans in the United States is not known. We excluded patients with significant alcohol use history from our analyses; however, we note the limitations of self-reported alcohol use and that alcohol intake may be underestimated, especially in a cohort of individuals previously shown to be at higher risk for alcohol use and related disorders.46 Finally, while the primary focus of our study was to examine the prevalence of fibrosis and steatosis in first-degree relatives of Mexican Americans with HCC, future studies in the same geographic area with appropriate controls and long-term outcomes are warranted to examine whether the risk of fibrosis is significantly increased in this cohort compared with the general population. Nevertheless, our study has revealed the prevalence and risk factors associated with hepatic steatosis and advanced hepatic fibrosis in first-degree relatives of Mexican Americans with HCC, which provides valuable information for future research and has implications on patient care.
In summary, hepatic fibrosis and steatosis are common in Mexican American adults with a first-degree family history of HCC, particularly in those with type 2 diabetes and obesity, which should prompt consideration of screening for liver disease in this patient population.
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interests: All authors who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take responsibility of the content, including participation in design, analysis, writing and revision of the manuscript. All authors approved the final manuscript. The authors would like to thank the cohort team, particularly Rocío Uribe and Ivana Zavala, who recruited and interviewed the participants. Marcela Morris, BS, and Hugo Soriano and their teams for laboratory and data support respectively; Norma Pérez-Olazarán, BBA, and Christina Villarreal, BA, for administrative support; Valley Baptist Medical Center, Brownsville, Texas, for providing us space for our Center for Clinical and Translational Science Clinical Research Unit is located; and the community of Brownsville and the participants who so willingly participated in this study in their city.
FUNDING INFORMATION
This study was funded in part by the Center for Clinical and Translational Sciences, National Institutes of Health Clinical and Translational Award grant no. UL1 TR000371 from the National Center for Advancing Translational Sciences. SS received funding from the AASLD Foundation. RL received funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), DOD PRCRP (W81XWH-18-2-0026), NIDDK (U01DK061734, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and NIAAA (U01AA029019). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICT OF INTEREST
RL serves as a consultant for Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Inipharm, Intercept, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Sagimet, 89 Bio and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer and Siemens. He is also a co-founder of Liponexus, Inc. All other authors have no conflicts of interest to disclose.
Footnotes
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section.
REFERENCES
- 1.Vespa J, Medina L, Armstrong DM. Demographic turning points for the United States: population projections for 2020 to 2060. Current Population Reports, P25-1144. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2020. [Google Scholar]
- 2.Mcglynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atiemo K, Mazumder NR, Caicedo JC, Ganger D, Gordon E, Montag S, et al. The Hispanic paradox in patients with Liver cirrhosis: current evidence from a large regional retrospective cohort study. Transplantation. 2019;103:2531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asemota J, Oladunjoye O, Babalola A, Nwosu U, Liu PH, Oladunjoye AO, et al. Comparison of hepatocellular carcinoma in Hispanics and non-Hispanics patients. Cureus. 2021;13(5):e14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and ethnic disparities in nonalcoholic fatty Liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:198–210.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez AG, Weiss NS, Holden AEC, Suarez L, Cooper SP, Munoz E, et al. Incidence and risk factors for hepatocellular carcinoma in Texas Latinos: implications for prevention research. PLoS One. 2012;7:e35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 2015;149(7):1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen CH, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest. 2017;127:2697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty Liver disease. Gastroenterology. 2017;152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371–8. [DOI] [PubMed] [Google Scholar]
- 14.Fisher-Hoch SP, Rentfro AR, Salinas JJ, Pérez A, Brown HS, Reininger BM, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004-2007. Prev Chronic Dis. 2010;7(3):A53. [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher-Hoch SP, Vatcheva KP, Rahbar MH, JB MC. Undiagnosed diabetes and pre-diabetes in health disparities. PLoS One. 2015;10(7):e0133135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watt GP, De La Cerda IA-O, Pan JJ, Fallon MB, Beretta L, Loomba R, et al. Elevated glycated hemoglobin is associated with liver fibrosis, as assessed by elastography, in a population-based study of Mexican Americans. Hepatol Commun. 2020;4(12):1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–33. [DOI] [PubMed] [Google Scholar]
- 18.Sporea I Gradinaru-Tascau O, Bota S, Popescu A,Sirli R,Jurchis A, et al. How many measurements are needed for liver stiffness assessment by 2D-shear wave elastography (2D-SWE) and which value should be used: the mean or median? Med Ultrason. 2013;15(4):268–72. [DOI] [PubMed] [Google Scholar]
- 19.Paisant A, Lemoine S, Cassinotto C, de Lédinghen V, Ronot M, Irlès-Depé M, et al. Reliability criteria of two-dimensional shear wave elastography: analysis of 4277 measurements in 788 patients. Clin Gastroenterol Hepatol. 2022;20(2):400–8.e10. [DOI] [PubMed] [Google Scholar]
- 20.Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57(3):1182–91. [DOI] [PubMed] [Google Scholar]
- 21.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62. [DOI] [PubMed] [Google Scholar]
- 22.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67:1348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 24.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. [DOI] [PubMed] [Google Scholar]
- 25.Mohanty SR, Troy TN, Huo D, O’Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797–804. [DOI] [PubMed] [Google Scholar]
- 26.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ajayi F, Jan J, Singal AG, Rich NE. Racial and sex disparities in hepatocellular carcinoma in the USA. Curr Hepatol Rep. 2020;19:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao J, Watt GP, Lee M, Rahbar MH, Vatcheva KP, Pan JJ, et al. Cirrhosis and advanced fibrosis in Hispanics in Texas: the dominant contribution of central obesity. PLoS One. 2016;11(3):e0150978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13. [DOI] [PubMed] [Google Scholar]
- 30.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–61. [DOI] [PubMed] [Google Scholar]
- 31.Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caussy C, Bhargava M, Villesen IF, Gudmann NS, Leeming DJ, Karsdal MA, et al. Collagen formation assessed by N-terminal propeptide of type 3 procollagen is a heritable trait and is associated with liver fibrosis assessed by magnetic resonance elastography. Hepatology. 2019;70(1):127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. [DOI] [PubMed] [Google Scholar]
- 34.Orabi D, Berger NA, Brown JM. Abnormal metabolism in the progression of nonalcoholic fatty Liver disease to hepatocellular carcinoma: mechanistic insights to chemoprevention. Cancers (Basel). 2021;13:3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallwitz E, Tayo BO, Kuniholm MH, Daviglus M, Zeng D, Isasi CR, et al. Association of HSD17B13 rs72613567:TA with nonalcoholic fatty liver disease in Hispanics/Latinos. Liver Int. 2020;40:889–93. [DOI] [PubMed] [Google Scholar]
- 36.Youssefian L, Vahidnezhad H, Saeidian AH, Pajouhanfar S, Sotoudeh S, Mansouri P, et al. Inherited non-alcoholic fatty liver disease and dyslipidemia due to monoallelic ABHD5 mutations. J Hepatol. 2019;71:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taliento AE, Dallio M, Federico A, Prati D, Valenti L. Novel insights into the genetic landscape of nonalcoholic fatty liver disease. Int J Environ Res Public Health. 2019;16:2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stender S, Loomba R. PNPLA3 genotype and risk of liver and all-cause mortality. Hepatology. 2020;71:777–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao J, Kwan SY, Sabotta CM, Tanaka H, Veillon L, Warmoes MO, et al. Circulating fatty acids associated with advanced liver fibrosis and hepatocellular carcinoma in South Texas Hispanics. Cancer Epidemiol Biomarkers Prev. 2021;30(9):1643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker RW, Belbin GM, Sorokin EP, Van Vleck T, Wojcik GL, Moscati A, et al. A common variant in PNPLA3 is associated with age at diagnosis of NAFLD in patients from a multi-ethnic biobank. J Hepatol. 2020;72:1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallwitz ER, Tayo BO, Kuniholm MH, Cai J, Daviglus M, Cooper RS, et al. American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in Hispanic/Latino adults. Clin Gastroenterol Hepatol. 2019;17:2301–9. [DOI] [PubMed] [Google Scholar]
- 43.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Heredia NI, Balakrishnan M, Thrift AP. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: results from NHANES 2017-2018. PLoS One. 2021;16:e0252164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair:; EASL Governing Board Representative:; Panel Members. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. [DOI] [PubMed] [Google Scholar]
- 46.Mills BA, Caetano R. Alcohol use and related problems along the United States-Mexico border. Alcohol Res. 2016;38:79–81. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


