Summary
Background:
Emerging data suggest that statins, aspirin and metformin may protect against hepatocellular carcinoma (HCC) development. However, prior meta-analyses were limited by heterogeneity and inclusion of studies without adequate adjustment for baseline risks.
Aim:
To examine by an updated meta-analysis the association between these medications and HCC risk.
Methods:
Medline and Embase databases were searched from inception to March 2022 for studies that balanced baseline risks between study groups via propensity score matching or inverse probability of treatment weighting, that reported the impact of statins, aspirin or metformin on HCC risk. Multivariable-adjusted hazard ratios (HRs) for HCC were pooled using a random effects model.
Results:
Statin use was associated with reduced HCC risk overall (HR: 0.52; 95% CI: 0.37–0.72) (10 studies, 1,774,476), and in subgroup analyses for cirrhosis, hepatitis B/C, non-alcoholic fatty liver disease, studies accounting for concurrent aspirin and metformin consumption and lipophilic statins. Aspirin use was associated with reduced HCC risk overall (HR: 0.48; 95% CI: 0.27–0.87) (11 studies, 2,190,285 patients) but not in studies accounting for concurrent statin and metformin use. Metformin use was not associated with reduced HCC risk overall (HR: 0.57; 95% CI: 0.31–1.06) (3 studies, 125,458 patients). Most analyses had moderate/substantial heterogeneity, except in follow-up <60 months for aspirin (I2 = 0%).
Conclusion:
Although statin and aspirin use were associated with reduced HCC risk, only statin use was significant in subgroup analyses accounting for concurrent medications. Metformin use was not associated with reduced HCC risk. These data have implications for future clinical trial design.
1 |. INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide.1,2 Patients with HCC have a 5-year survival of less than 20% overall.3–7 The poor prognosis of HCC, in general, has led to increased interest in HCC prevention. Emerging data suggest that the use of statins, aspirin and metformin may be chemoprotective against HCC.8–12 However, prior meta-analyses on this topic had some limitations such as pooling odds ratios that are not time-to-event measurements,11,13–16 or pooling data that had not been adequately adjusted for background differences in treated versus untreated patients. There have also been several recent relevant large studies that were not included in previous meta-analyses.17–20
In light of these considerations, we performed an updated meta-analysis to determine the association between the risk of HCC and the use of statins, aspirin or metformin. We included cohort studies that balanced patient baseline characteristics between groups by propensity score matching (PSM) or inverse probability of treatment weighting (IPTW) to provide robust estimates of the comparative risk of HCC between study groups.21–24 We performed a pooled analysis of co-variate-adjusted hazard ratios (HRs) to account for censoring of events and investigated heterogeneity by performing multiple subgroup analyses for relevant factors such as cirrhosis, sex, liver disease aetiology, method of HCC diagnosis, concurrent medication use, follow-up duration and the use of competing risks of death.
2 |. METHODS
2.1 |. Search strategy
With reference to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines,25 a search was conducted on Medline and Embase databases via Ovid for articles relating to the risk of HCC among patients who consumed statins, aspirin and metformin from inception to 10 March 2022. Key search terms included but were not limited to synonyms of ‘Carcinoma, Hepatocellular’, ‘Metformin’, ‘Hydroxymethylglutaryl CoA Reductase Inhibitors’, ‘Aspirin’ and other related terms in the titles and abstracts. The full search strategy is included in Appendix S1. All references were imported into Endnote X9 for the removal of duplicates. We manually screened the bibliographies of included articles for additional relevant data.
2.2 |. Eligibility and selection criteria
Two pairs of authors (JNY and RWLZ and DJHT and CEF) independently screened abstracts, followed by full-text review to check for study eligibility and inclusion, with discrepancies resolved through consensus from a third independent author (DQH). Only original articles, including prospective and retrospective cohort studies, were considered for inclusion. Cross-sectional studies were excluded from the analysis. Only studies that employed PSM or IPTW to balance patient baseline characteristics between groups were considered for inclusion. The process of matching is designed to minimise selection bias and to achieve balance in baseline characteristics between treatment groups, and existing statistical literature has demonstrated that the resultant effect estimates are empirically equivalent to those of an RCT.21–23 Systematic reviews, meta-analyses, commentaries and editorials were excluded. Studies inferring results from the same databases were also removed to avoid duplication of the same cohort. Additionally, only articles written or translated into the English language were considered for inclusion. Studies were included if they compared HCC incidence between users of statin, aspirin or metformin versus non-users and reported effect estimates in hazard ratios (HRs) or provided sufficient raw data to allow for calculation of incidental HCC. All the included studies excluded patients with prevalent HCC at the start of the study period. Studies on the paediatric population and animal-related studies were excluded from the analysis.
2.3 |. Data extraction
Two pairs of authors (CEF and RZWL and JNY and DJHT) independently extracted relevant data from the included articles in blinded pairs. The extracted data comprised of study characteristics, including but not limited to the author, year, country, geographical region and sample size; patient characteristics including age, gender, race, comorbidities such as hypertension (HTN), type 2 diabetes mellitus (DM), hyperlipidemia (HLD), smoking, cirrhosis and aetiologies of liver disease such as hepatitis B virus (HBV), hepatitis C virus (HCV) and non-alcoholic fatty liver disease (NAFLD). The primary outcome of interest was the comparative risk of HCC with statin, aspirin or metformin use compared with non-users. Co-variate adjusted hazard ratios (HR) and 95% confidence intervals (CIs) for HCC incidence in patients receiving statin, aspirin or metformin versus non-users were recorded. The co-variates included in multivariable models or PSM were also extracted across studies. In studies that only reported median and interquartile ranges, we conducted the transformation of values into mean and standard deviations via the widely adopted formulas by Wan et al.26
2.4 |. Statistical analysis
All analyses were conducted in R Studio (Version 4.1.2) using the meta package, and statistical significance was considered for outcomes with a p ≤ 0.05. Statistical heterogeneity was assessed via I2 and Cochran Q test values, where an I2 value of <50%, 50%–75% and >75% represented low, moderate and a high degree of heterogeneity respectively.27,28 Random effect models were used in all analyses regardless of heterogeneity scores, as it has been shown to provide more robust estimates compared to fixed effects models.29 To compare HCC incidence between patients receiving statin, aspirin or metformin versus non-users, hazard ratios (HRs) were pooled via the inverse variance method using the DerSimonian and Laird random effects model.30 Analysis was stratified based on the drug class the patient received (statin, aspirin or metformin). Where sufficient studies were available, pre-specified subgroup and sensitivity analysis based on the aetiology of liver disease (i.e HBV, HCV, NASH and alcohol-associated liver disease), the presence of cirrhosis, sex, verification of HCC diagnosis by imaging or histology (versus relying on International Classification of Diseases [ICD] codes), whether studies accounted for concurrent hepatoprotective medications (by either stratified analysis or adjusted multivariable Cox regression analysis), by study follow-up duration, and for studies that accounted for competing risks of mortality without HCC. Further subgroup analyses were conducted for the type of statins (lipophilic versus hydrophilic). Lipophilic statins included atorvastatin, simvastatin, fluvastatin and lovastatin, while hydrophilic statins included pravastatin and rosuvastatin.
2.5 |. Quality assessment and publication bias
Quality assessment of included articles was done with the Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence studies, which rates the risk of bias of cohort studies on the premises of appropriateness of sample frame, sampling method, adequacy of sample size, data analysis, methods for identification and measurement of relevant conditions, statistical analysis and response rate adequacy.31 Studies were characterised as having high (JBI checklist score 1–3), moderate (4–6) or low (7–9) risk of bias. Publication bias was assessed for outcomes where sufficient studies were involved in the analysis (n ≥ 10) (Appendix S4), via visual inspection of funnel plots for asymmetry and with Egger’s test.32
3 |. RESULTS
3.1 |. Summary of included articles
A systematic search of the literature using the previously mentioned search strategy yielded 1848 articles after the removal of duplicates. After 1750 articles were excluded based on the study title and abstract, 98 articles were selected for full-text review, of which 21 articles met the inclusion criteria. Two additional articles were retrieved for full-text review after screening references of included articles, and a total of 23 articles were included in the final analysis (Figure 1). In total, nine articles originated from Taiwan,17,19,33–39 five from Korea,18,40–43 three from Hong Kong,44–46 three from the United States,20,47,48 two from Sweden,49,50 and one from the United Kingdom.51 Five studies used IPTW and the remaining 18 studies conducted PSM to adjust for confounding baseline characteristics. The key characteristics and quality assessment for the included articles are summarised in Appendix S2. A total of 4,090,219 individuals were included, comprising 1,774,476 individuals in the analysis for statins, 2,190,285 patients in the analysis for aspirin, and 125,458 individuals in the analysis for metformin. There were a total of 34,422 incident cases of HCC in the included studies. All studies were assessed to have a low (n = 18) to moderate (n = 5) risk of bias based on the JBI appraisal tool.
FIGURE 1.
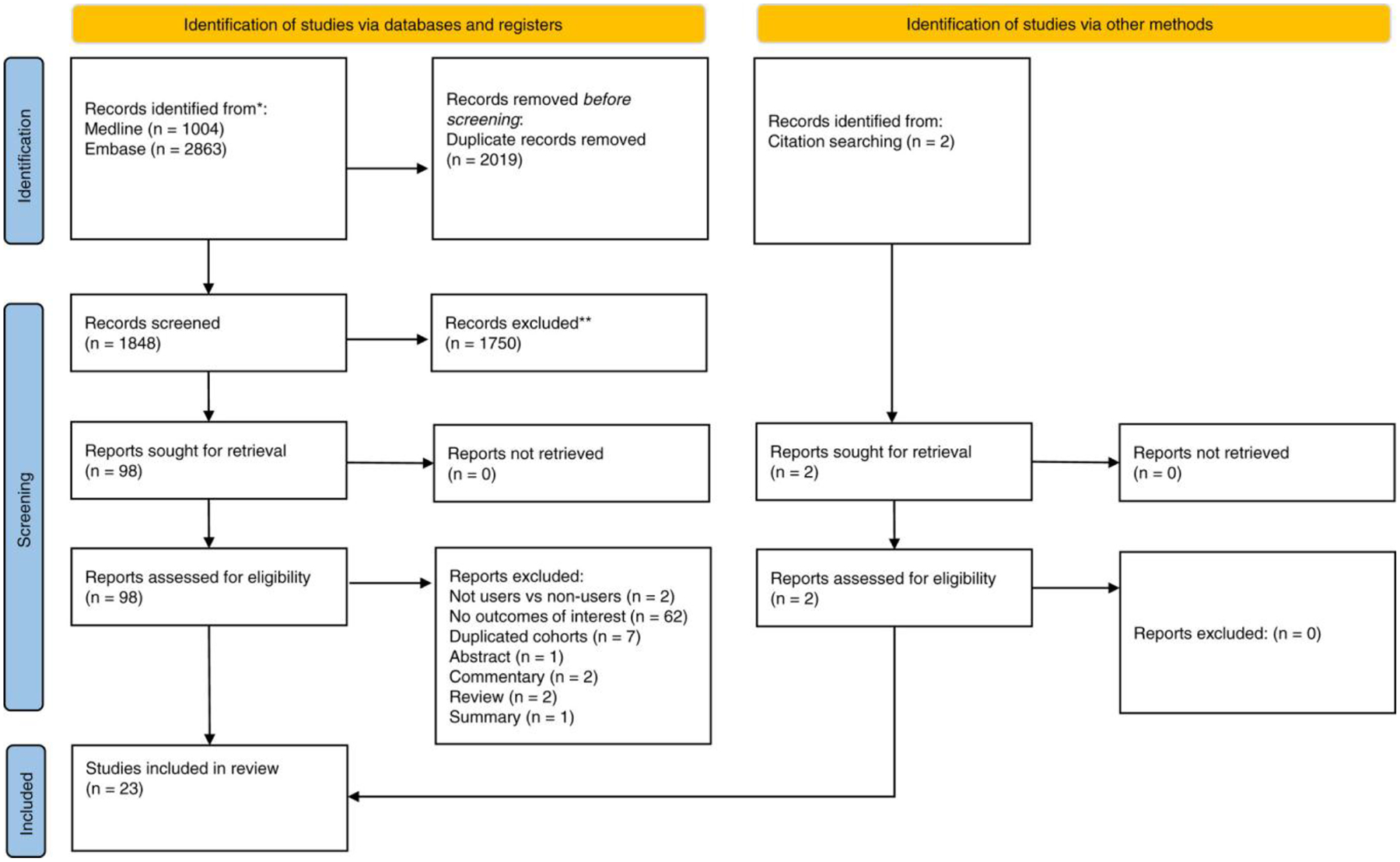
PRISMA flowchart.
3.2 |. Statin use and HCC risk
3.2.1 |. Overall
A pooled analysis of 10 studies and 1,774,476 patients determined that the overall risk of HCC incidence in statin users was lower (HR: 0.52; 95% CI: 0.37–0.72; p < 0.01; I2 = 97.80%) compared to non-users (Table 1, Appendix S3).
TABLE 1.
Hepatocellular carcinoma risk among statin users
| No, of studies | Sample size | HR | 95% confidence interval | p value | I2 (%) | p value for Cochran Q test of heterogeneity | Subgroup difference | |
|---|---|---|---|---|---|---|---|---|
| Statins | ||||||||
| Overall | 10 | 1,774,476 | 0.52 | 0.37–0.72 | <0.01 * | 97.80 | <0.01 | |
| Cirrhosis | 3 | 21,534 | 0.95 | 0.91–0.99 | 0.04 * | 90.20 | <0.01 | |
| Hepatitis B | 5 | 152,716 | 0.53 | 0.32–0.88 | 0.01 * | 96.70 | <0.01 | |
| Hepatitis C | 3 | 16,058 | 0.79 | 0.64–0.99 | 0.04 * | 81.70 | <0.01 | |
| NAFLD | 2 | 242,751 | 0.68 | 0.59–0.77 | <0.01 * | 90.80 | <0.01 | |
| Accounted for competing risk of death without HCC | 5 | 980,486 | 0.51 | 0.32–0.81 | <0.01 * | 97.50 | <0.01 | |
| Sex | ||||||||
| Male | 2 | 115,411 | 0.45 | 0.24–0.85 | 0.01 * | 85.90 | <0.01 | 0.83 |
| Female | 2 | 115,411 | 0.50 | 0.22–1.17 | 0.11 | 75.00 | 0.01 | |
| HCC diagnosis method | ||||||||
| Verified by imaging/histology | 2 | 66,445 | 0.60 | 0.33–1.09 | 0.09 | 95.10 | <0.01 | 0.59 |
| ICD codes | 8 | 1,708,031 | 0.49 | 0.31–078 | <0.01 * | 98.60 | <0.01 | |
| Statin type | ||||||||
| Lipophilic | 3 | 1,083,952 | 0.46 | 0.37–0.57 | <0.01 | 64.00 | 0.06 | 0.93 |
| Hydrophilic | 3 | 1,083,952 | 0.48 | 0.18–1.27 | 0.14 | 99.10 | <0.01 | |
| Accounted for concurrent use of aspirin, NSAIDs and metformin | ||||||||
| Yes | 9 | 1,534,926 | 0.52 | 0.37–0.75 | <0.01 * | 98.10 | <0.01 | |
| Mean follow-up duration | ||||||||
| <60 months | 4 | 814,691 | 0.49 | 0.39–0.62 | <0.01 * | 49.50 | 0.12 | 0.10 |
| ≥60 months | 4 | 965,093 | 0.67 | 0.49–0.92 | 0.01 * | 96.20 | <0.01 | |
Abbreviations: HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, non-alcoholic fatty liver disease; ICD, International Classification of Diseases; HR, hazard ratio; I2, level of heterogeneity.
Bolded p ≤ 0.05 denotes statistical significance.
3.2.2 |. By sex
Statin usage was associated with reduced HCC risk in males (HR: 0.45; 95% CI: 0.24–0.85; p = 0.01) (2 studies, 115,411 patients), but not in females (HR: 0.50; 95% CI: 0.22–1.17; p = 0.11) (2 studies, 115,411 patients). However, there was no significant subgroup difference (p = 0.83).
3.2.3 |. By aetiology of liver disease
Statin use was associated with reduced HCC incidence among patients with HBV, (HR: 0.53; 95% CI: 0.32–0.88; p = 0.01) (5 studies, 152,716 patients), HCV (HR: 0.79; 95% CI: 0.64–0.99; p = 0.04) (3 studies, 16,058 patients) and NAFLD (HR: 0.68; 95% CI: 0.59–0.77; p < 0.01) (2 studies, 242,751 patients).
3.2.4 |. By the presence of cirrhosis
Among patients with cirrhosis, statin use was associated with a reduced risk of HCC (HR: 0.95; 95% CI: 0.91–0.99; p = 0.04) (3 studies, 21,584 patients).
3.2.5 |. Studies that accounted for competing risk of death without HCC
Statin usage was associated with reduced HCC risk (HR: 0.51; 95% CI: 0.32–0.81; p < 0.01) in studies that accounted for competing risk of death without HCC (5 studies, 980,486 patients).
3.2.6 |. Confirmation of HCC diagnosis
Statin usage was associated with reduced HCC incidence in studies that relied on ICD codes for HCC diagnosis (HR: 0.49; 95% CI: 0.31–0.78; p < 0.01) (8 studies, 1,708,031 patients), but not in studies that verified the presence of HCC by imaging or histology (HR: 0.60: 95% CI: 0.33–1.09; p = 0.09) (2 studies, 66,445 patients).
3.2.7 |. Studies that accounted for concurrent aspirin, NSAID and metformin use
In our pooled analysis of the nine studies involving 1,534,926 patients accounted for the use of aspirin, non-steroidal anti-inflammatory drugs (NSAIDs) and metformin, statin use continued to be associated with reduced HCC incidence compared to non-users (HR: 0.52; 95% CI: 0.37–0.75; p < 0.01).
3.2.8 |. Lipophilic versus hydrophilic statins
Lipophilic statin users had a significantly lower risk of HCC incidence compared to non-users (HR: 0.46; 95% CI: 0.37–0.57; p < 0.01) (3 studies, 1,083,952 patients), but not hydrophilic statin users (HR: 0.48; 95% CI: 0.18–1.27; p = 0.14) (3 studies, 1,083,952 patients).
3.2.9 |. Mean follow-up duration
Statin usage was associated with reduced HCC incidence in studies with a mean follow-up duration shorter than 60 months (HR: 0.49; 95% CI: 0.39–0.62; p < 0.01), and in studies with a mean follow-up duration of 60 months or longer (HR: 0.67; 95% CI: 0.49–0.92; p = 0.01), with no significant subgroup difference (p = 0.10).
3.3 |. Aspirin use and HCC risk
3.3.1 |. Overall
Pooled analysis of 11 articles and 2,190,285 patients determined a lower risk of HCC in aspirin users compared to non-users (HR: 0.48; 95% CI: 0.27–0.87; p = 0.01; I2 = 99.50%) (Table 2, Appendix S3).
TABLE 2.
Hepatocellular carcinoma risk among aspirin users
| No. of studies | Sample size | HR | 95% confidence interval | p value | I2 (%) | p value for Cochran Q test of heterogeneity | Subgroup difference | |
|---|---|---|---|---|---|---|---|---|
| Aspirin | ||||||||
| Overall | 11 | 2,190,285 | 0.43 | 0.27–0.87 | 0.01 * | 99.50 | 0.00 | |
| Cirrhosis | 5 | 46,526 | 0.68 | 0.51–0.91 | 0.01 * | 84.90 | <0.01 | |
| Hepatitis B | 5 | 97,643 | 0.45 | 0.12–1.60 | 022 | 99.80 | <0.01 | |
| Hepatitis C | 3 | 43,255 | 0.69 | 0.59–0.80 | <0.01 * | 50.20 | 0.13 | |
| Accounted for competing risk of death without HCC | 6 | 142,281 | 0.50 | 0.18–1.36 | 0.17 | 99.70 | <0.01 | - |
| Sex | ||||||||
| Male | 3 | 365,231 | 0.79 | 0.67–0.90 | <0.01 * | 0.00 | 0.39 | 0.66 |
| Female | 3 | 365,231 | 0.71 | 0.46–1.04 | 0.08 | 67.00 | 0.05 | |
| HCC diagnosis method | ||||||||
| Verified by imaging/histology | 4 | 58,907 | 0.28 | 0.06–1.19 | 0.08 | 99.80 | <0.01 | 0.24 |
| ICD codes | 7 | 2,131,378 | 0.66 | 0.54–0.82 | <0.01 * | 93.40 | <0.01 | |
| Accounted for concurrent use of statins, NSAIDs and metformin | ||||||||
| Yes | 8 | 493,285 | 0.53 | 0.23–1.21 | 0.13 | 99.60 | <0.0 | 0.60 |
| No | 3 | 1,697,000 | 0.42 | 0.27–0.64 | <0.01 * | 93.40 | <0.01 | |
| Mean follow-up duration | ||||||||
| <60 months | 2 | 42,545 | 0.74 | 0.63–0.87 | <0.01 * | 0.00 | 0.35 | 0.93 |
| ≥60 months | 2 | 1,134,408 | 0.69 | 0.63–0.75 | <0.01 * | 0.00 | 0.89 | |
Abbreviations: HCC, hepatocellular carcinoma; LT, liver transplantation; ICD, International Classification of Diseases; HR, hazard ratio; I2, level of heterogeneity.
Bolded p ≤ 0.05 denotes statistical significance
3.3.2 |. By sex
Aspirin usage was associated with reduced HCC risk in males (HR: 0.78; 95% CI: 0.67–0.90; p < 0.01) (3 studies, 365,231 patients), but not in females (HR: 0.71; 95% CI: 0.48–1.04; p = 0.08) (3 studies, 365,231 patients).
3.3.3 |. By aetiology of liver disease
Aspirin use was associated with reduced HCC risk in patients with HCV (HR: 0.69; 95% CI: 0.59–0.80; p < 0.01) (3 studies, 48,255 patients), but not in those with HBV (HR: 0.45; 95% CI: 0.12–1.60; p = 0.18) (5 studies, 97,848 patients).
3.3.4 |. By the presence of cirrhosis
Among patients with cirrhosis, aspirin use was associated with a significant reduction in HCC risk (HR: 0.68; 95% CI: 0.51–0.91; p = 0.01) (5 studies, 46,526 patients).
3.3.5 |. Studies that accounted for competing risk of death without HCC
In a pooled analysis of the six studies involving 142,281 patients which accounted for the competing risk of death without HCC, aspirin use was not associated with a reduced risk of HCC (HR: 0.50; 95% CI: 0.18–1.36; p = 0.17).
3.3.6 |. Confirmation of HCC diagnosis
In studies that relied on ICD codes for HCC diagnosis (7 studies, 2,131,378 patients), aspirin use was associated with significantly reduced HCC risk (HR: 0.66; 95% CI: 0.54–0.82; p < 0.01), but not in studies that verified the diagnosis of HCC by imaging or histology (HR: 0.28; 95% CI: 0.06–1.19; p = 0.08) (4 studies, 58,907 patients).
3.3.7 |. Studies that accounted for a concurrent statin, NSAID and metformin use
In studies that did not account for concurrent use of metformin, NSAIDs and statins, aspirin use was associated with reduced HCC risk (HR: 0.42; 95% CI: 0.27–0.64; p < 0.01) (3 studies, 1,697,000 patients). However, in studies that accounted for concurrent use of metformin, NSAIDs and statins, aspirin use was not associated with reduced HCC risk (HR: 0.53; 95% CI: 0.23–1.21; p = 0.13) (8 studies, 493,285 patients).
3.3.8 |. Mean follow-up duration
Aspirin usage was associated with reduced HCC incidence in studies with a mean follow-up duration shorter than 60 months (HR: 0.74; 95% CI: 0.63–0.87; p < 0.01) and in studies with a mean follow-up duration longer than 60 months (HR: 0.69; 95% CI: 0.63–0.75; p < 0.01), with no significant subgroup difference (p = 0.93).
3.4 |. Metformin use and HCC risk
From a pooled analysis of three articles and 125,458 patients, metformin use was not associated with a lower risk of HCC versus non-users (HR: 0.57; 95% CI: 0.31–1.06; p = 0.08; I2 = 95.90%) (Table 3, Appendix S3).
TABLE 3.
Hepatocellular carcinoma risk in metformin users
| No. of studies | Sample size | HR | 95% confidence interval | p value | I2 (%) | p value for Cochran Q test of heterogeneity | Subgroup difference | |
|---|---|---|---|---|---|---|---|---|
| Metformin | ||||||||
| Overall | 3 | 125,458 | 0.57 | 0.31–1.06 | 0.03 | 95.90 | <0.01 | |
| Confirmation of HCC diagnosis | ||||||||
| ICD codes | 3 | 125,458 | 0.57 | 0.31–1.06 | 0.08 | 95.90 | <0.01 | |
| Accounted for use of statins, aspirin and NSAIDs | ||||||||
| Yes | 2 | 101,611 | 0.97 | 0.60–1.58 | 0.91 | 95.70 | <0.01 | |
| Mean follow-up duration | ||||||||
| <60 months | 2 | 67,647 | 0.23 | 0.02–2.71 | 0.24 | 96.00 | <0.01 | |
| ≥60 months | 1 | - | ||||||
Abbreviations: HCC, hepatocellular carcinoma; LT, liver transplantation; ICD, International Classification of Diseases; HR, hazard ratio; I2, level of heterogeneity.
3.4.1 |. Confirmation of HCC diagnosis
All included studies in the pooled analysis of metformin use and HCC risk used ICD codes to verify HCC diagnosis. In these studies, the risk of HCC was comparable between metformin users and non-users (HR: 0.57; 95% CI: 0.31–1.06; p = 0.08).
3.4.2 |. Studies that accounted for a concurrent statin, NSAID, and aspirin use
In studies that accounted for a concurrent statin, NSAID, and aspirin use, metformin use was not associated with the risk of HCC (HR: 0.97; 95% CI: 0.60–1.58; p = 0.91) (2 studies, 101,611 patients).
3.5 |. Heterogeneity and publication bias
There was a moderate to high degree of heterogeneity in all analyses for the association between statin use and HCC risk, except in subgroup analysis for follow-up time <60 months (I2 = 49.5%, p = 0.12 for heterogeneity). Likewise, there was a moderate to high degree of heterogeneity in all analyses for the association between aspirin use and HCC risk, except in the subgroup analysis for the male sex (I2 = 0%, p = 0.39 for heterogeneity), and analyses for follow-up duration (all I2 = 0%, and p > 0.34 for heterogeneity). There was a high degree of heterogeneity in all analyses for the effect of metformin use on HCC risk. Egger’s test for publication bias was significant for statins (p = 0.026), although no significant publication bias was detected in the overall analysis of aspirin (p = 0.671). However, visual inspection of funnel plots was suggestive of publication bias in the overall analysis for both statins and aspirin due to the asymmetrical distribution of data points across the vertical axis (Appendix S4). There were insufficient studies included in the analysis for metformin for publication bias to be assessed.
4 |. DISCUSSION
4.1 |. Main findings
In this systematic review and meta-analysis of 23 moderate–high-quality matched studies involving 4,090,219 individuals, we determined that statin and aspirin use were all associated with a substantial reduction in HCC risk in the overall analyses, while metformin was not associated with reduced HCC risk. The strongest association with HCC risk reduction appeared to be among statin users, where the reduction of HCC risk persisted in multiple subgroup analyses, including studies that accounted for concurrent use of aspirin and metformin by either stratified analysis or adjusted multivariable analysis. By contrast, data for aspirin and metformin were limited, and the association between aspirin or metformin use and HCC risk persisted in fewer/none of the subgroup analyses and did not reach statistical significance in subgroup analyses that accounted for concurrent statin use. These data suggest that statins may have an important role in the chemoprevention of HCC but require validation in randomised controlled trials before routine use in clinical practice.
Statin use was associated with reduced HCC in risk in most of the subgroup analyses such as those with cirrhosis, HBV, HCV, NAFLD, male sex, follow-up duration, studies that accounted for competing risks of death without HCC and studies that accounted for concurrent use of aspirin, NSAIDs and metformin. Notably, lipophilic statins, but not hydrophilic statins were associated with a reduction in HCC risk. There was no significant reduction in HCC risk among females, which may be related to the lower background risk of HCC in this subgroup. There was moderate to severe heterogeneity in most of the analyses for statins, calling for caution when interpreting these data. However, there was no significant heterogeneity in the subgroup analysis for study follow-up <60 months, suggesting that follow-up duration was a major source of heterogeneity.
Aspirin use was associated with a significant reduction in HCC risk in subgroup analyses for patients with cirrhosis, HCV, males and among studies that did not verify HCC risk with imaging or histology. Notably, aspirin was associated with reduced HCC risk in studies that did not account for concurrent use of statins, NSAIDs and metformin, but not among studies that accounted for concurrent use of these medications by either stratified analysis or adjusted multivariable analysis. This calls for a cautious interpretation of the overall analyses of the association between aspirin and HCC risk. There was moderate to severe heterogeneity in the overall and all the subgroup analyses, except the analyses for male sex and follow-up duration.
Metformin use was not associated with a significant reduction in HCC risk in the overall analysis. There was also no significant association with reduced HCC risk among studies which accounted for concurrent medication use by stratified or adjusted multivariable analyses. Overall, the data for the association between metformin use and HCC risk appeared to be less robust.
4.2 |. In context with current literature
The current study provides a detailed, updated analysis of the existing literature, with multiple additional subgroup analyses that provide confidence for the potential benefits of statins and inform clinical trial design. Some of the previous meta-analyses were limited by the use of odds ratios13,14,16,52 for HCC risk, lack of PSM or IPTW analyses to balance baseline characteristics between study groups,11,16,53 or did not thoroughly investigate sources of heterogeneity through detailed subgroup analyses.11,54,55 A recent meta-analysis focusing on aspirin use and HCC risk pooled HRs after propensity score matching and determined similar findings (HR 0.54), although only 4 studies were included.12 In contrast, we pooled adjusted hazard ratios that account for censoring of events and were adjusted for co-variates, included only moderate to high-quality studies that performed balancing of baseline characteristics for HCC risk, and performed extensive subgroup analyses to identify sources of heterogeneity.
4.3 |. Limitations
The current study is not without limitations. All included studies were observational, and hence subject to confounding and bias, such as immortal time bias and indication bias. Though each study attempted to balance the baseline characteristics between groups, residual confounders, such as family history or fibrosis stage, may not have been accounted for. There was potential publication bias in the overall analyses for aspirin and statin use. In addition, several of the subgroup analyses were limited by a small number of included studies, and there were insufficient studies for subgroup analyses for NAFLD in the analyses of HCC risk in aspirin or metformin.
5 |. CONCLUSION
Statin and aspirin use were all associated with a substantial reduction in HCC risk in the overall analyses, while metformin use was not associated with reduced HCC risk. The strongest association with HCC risk reduction appeared to be among statin users, where the association with reduced HCC risk persisted in multiple subgroup analyses including for studies that accounted for concurrent use of aspirin and metformin. By contrast, subgroup data for metformin were limited, and the association between aspirin or metformin use and HCC risk reduction was not statistically significant in subgroup analyses for studies that accounted for concurrent statin use. These data have implications for future clinical trial design.
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interests: All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures and tables has not been previously published and the manuscript is not under consideration elsewhere.
FUNDING INFORMATION
No external funding was required for this study.
CONFLICT OF INTEREST
MHN: Research support: Pfizer, Enanta, Gilead, Exact Sciences, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, B.K. Kee Foundation. Consulting and/or Advisory Board: Intercept, Exact Science, Gilead, GSK, Eli Lilly, Laboratory of Advanced Medicine, Janssen. R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835) and DOD PRCRP (W81XWH-18-2-0026). RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc. D.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01). In addition, he has served as an advisory board member for Eisai. The other authors declare no conflict of interest.
Footnotes
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34(7):969–977.e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.Reveron-Thornton RF, Teng MLP, Lee EY, Tran A, Vajanaphanich S, Tan EX, et al. Global and regional long-term survival following resection for HCC in the recent decade: a meta-analysis of 110 studies. Hepatol Commun. 2022;6:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan DJH, Lim WH, Yong JN, Ng CH, Muthiah MD, Tan EX, et al. UNOS Down-staging criteria for liver transplantation of hepatocellular carcinoma: systematic review and meta-analysis of 25 studies. Clin Gastroenterol Hepatol. 2022. 10.1016/j.cgh.2022.02.018 [DOI] [PubMed] [Google Scholar]
- 6.Koh JH, Tan DJH, Ong Y, Lim WH, Ng CH, Tay PW, et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr. 2021;11(1):78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang DQ, Tran A, Tan EX, Nerurkar SN, Teh R, Teng MLP, et al. Characteristics and outcomes of hepatocellular carcinoma patients with macrovascular invasion following surgical resection: a meta-analysis of 40 studies and 8,218 patients. Hepatobiliary Surg Nutr. 2022;11:848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facciorusso A, El Aziz MAA, Singh S, Pusceddu S, Milione M, Giacomelli L, et al. Statin use decreases the incidence of hepatocellular carcinoma: an updated meta-analysis. Cancer. 2020;12(4):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memel ZN, Arvind A, Moninuola O, Philpotts L, Chung RT, Corey KE, et al. Aspirin use is associated with a reduced incidence of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Commun. 2021;5(1):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunha V, Cotrim HP, Rocha R, Carvalho K, Lins-Kusterer L. Metformin in the prevention of hepatocellular carcinoma in diabetic patients: a systematic review. Ann Hepatol. 2020;19(3):232–7. [DOI] [PubMed] [Google Scholar]
- 12.Tan JL, Sidhu-Brar S, Woodman R, Chinnaratha MA. Regular aspirin use is associated with a reduced risk of hepatocellular carcinoma (HCC) in chronic liver disease: a systematic review and meta-analysis. J Gastrointest Cancer. 2022. 10.1007/s12029-022-00842-y [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wang W, Wang M, Shi J, Jia X, Dang S. A meta-analysis of statin use and risk of hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2022;2022:5389044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wang M, Liu C, Wang W, Shi J, Dang S. Aspirin use and the risk of hepatocellular carcinoma: a meta-analysis. J Clin Gastroenterol. 2022;56(7):e293–302. [DOI] [PubMed] [Google Scholar]
- 15.Khajeh E, Moghadam AD, Eslami P, Ali-Hasan-al-Saegh S, Ramouz A, Shafiei S, et al. Statin use is associated with the reduction in hepatocellular carcinoma recurrence after liver surgery. BMC Cancer. 2022;22(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Ke Y, Lei X, Wu T, Li Y, Bao T, et al. Meta-analysis: the efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Ann Hepatol. 2020;19(3):320–8. [DOI] [PubMed] [Google Scholar]
- 17.Sung FC, Yeh YT, Muo CH, Hsu CC, Tsai WC, Hsu YH. Statins reduce hepatocellular carcinoma risk in patients with chronic kidney disease and end-stage renal disease: a 17-year longitudinal study. Cancer. 2022;14(3):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang H, Lee YB, Moon H, et al. Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology. 2022;76(2):492–501. [DOI] [PubMed] [Google Scholar]
- 19.Chiu W-C, Shan J-C, Yang Y-H, Chen VC-H, Chen P-C. Statins and the risks of decompensated liver cirrhosis and hepatocellular carcinoma determined in patients with alcohol use disorder. Drug Alcohol Depend. 2021;228:109096. [DOI] [PubMed] [Google Scholar]
- 20.Zou B, Odden MC, Nguyen MH. Statin use and reduced hepatocellular carcinoma risk in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022. 10.1016/j.cgh.2022.01.057 [DOI] [PubMed] [Google Scholar]
- 21.Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109(8):djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grose E, Wilson S, Barkun J, Bertens K, Martel G, Balaa F, et al. Use of propensity score methodology in contemporary high-impact surgical literature. J Am Coll Surg. 2020;230(1):101–112.e102. [DOI] [PubMed] [Google Scholar]
- 23.Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA. 2001;286(7):821–30. [DOI] [PubMed] [Google Scholar]
- 24.Hogan JW, Lancaster T. Instrumental variables and inverse probability weighting for causal inference from longitudinal observational studies. Stat Methods Med Res. 2004;13(1):17–48. [DOI] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher J What is heterogeneity and is it important? BMJ. 2007;334(7584):94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 30.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29(12):1282–97. [DOI] [PubMed] [Google Scholar]
- 31.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CI, Kuan CF, Fang YA, Liu SH, Liu JC, Wu LL, et al. Cancer risk in HBV patients with statin and metformin use a population-based cohort study. Medicine. 2015;94(6):e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018;38(11):2018–27. [DOI] [PubMed] [Google Scholar]
- 36.Lee T-Y, Hsu Y-C, Tseng H-C, Yu SH, Lin JT, Wu MS, et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med. 2019;179(5):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee TY, Hsu YC, Tseng HC, Lin JT, Wu MS, Wu CY. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2020;18(12):2784–2792.e2787. [DOI] [PubMed] [Google Scholar]
- 38.Liao YH, Hsu RJ, Wang TH, Wu CT, Huang SY, Hsu CY, et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 2020;20(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, et al. Statins decrease the risk of decompensation in hepatitis B virus-and hepatitis C virus-related cirrhosis: a population-based study. Hepatology. 2017;66(3):896–907. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66(5):1556–69. [DOI] [PubMed] [Google Scholar]
- 41.Hwang IC, Chang J, Kim K, Park SM. Aspirin use and risk of hepatocellular carcinoma in a National Cohort Study of Korean adults. Sci Rep. 2018;8(1):4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin S, Lee SH, Lee M, Kim JH, Lee W, Lee HW, et al. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine. 2020;99(9):e19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi WM, Kim HJ, Jo AJ, Choi SH, Han S, Ko MJ, et al. Association of aspirin and statin use with the risk of liver cancer in chronic hepatitis B: a nationwide population-based study. Liver Int. 2021;41(11):2777–85. [DOI] [PubMed] [Google Scholar]
- 44.Hsiang JC, Wong GLH, Tse YK, Wong VWS, Yip TCF, Chan HLY. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: a propensity score landmark analysis. J Hepatol. 2015;63(5):1190–7. [DOI] [PubMed] [Google Scholar]
- 45.Hui VWK, Yip TCF, Wong VWS, Tse YK, Chan HLY, Lui GCY, et al. Aspirin reduces the incidence of hepatocellular carcinoma in patients with chronic hepatitis B receiving oral nucleos(t)ide analog. Clin Transl Gastroenterol. 2021;12(3):e00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long-term use of low-dose aspirin for cancer prevention: a 10-year population cohort study in Hong Kong. Int J Cancer. 2019;145(1):267–73. [DOI] [PubMed] [Google Scholar]
- 47.Petrick JL, Sahasrabuddhe VV, Chan AT, Alavanja MC, Beane-Freeman LE, Buring JE, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res. 2015;8(12):1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan DE, Serper MA, Mehta R, Fox R, John B, Aytaman A, et al. Effects of hypercholesterolemia and statin exposure on survival in a large National Cohort of patients with cirrhosis. Gastroenterology. 2019;156(6):1693–1706.e1612. [DOI] [PubMed] [Google Scholar]
- 49.Simon TG, Duberg A-S, Aleman S, Hagstrom H, Nguyen LH, Khalili H, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a Nationwide Swedish population. Ann Intern Med. 2019;171(5):318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020;382(11):1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran KT, McMenamin UC, Coleman HG, Cardwell CR, Murchie P, Iversen L, et al. Statin use and risk of liver cancer: evidence from two population-based studies. Int J Cancer. 2020;146(5):1250–60. [DOI] [PubMed] [Google Scholar]
- 52.Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine. 2017;96(19):e6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Zhang T, Sun Y, Li C, Ding X, Zhu Y, et al. Systematic review and meta-analysis: association of aspirin with Incidence of hepatocellular carcinoma. Front Pharmacol. 2022;13:764854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pradelli D, Soranna D, Scotti L, Zambon A, Catapano A, Mancia G, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22(3):229–34. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y-Y, Zhu G-Q, Liu T, Zheng JN, Cheng Z, Zou TT, et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep. 2016;6:33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


