Abstract
Background
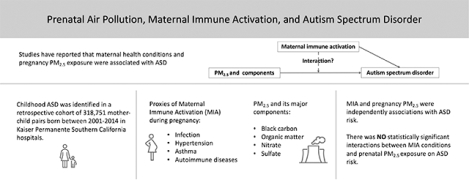
Autism Spectrum Disorder (ASD) risk is highly heritable, with potential additional non-genetic factors, such as prenatal exposure to ambient particulate matter with aerodynamic diameter< 2.5 μm (PM2.5) and maternal immune activation (MIA) conditions. Because these exposures may share common biological effect pathways, we hypothesized synergistic associations of prenatal air pollution and MIA-related conditions would increase ASD risk in children.
Objectives
This study examined interactions between MIA-related conditions and prenatal PM2.5 or major PM2.5 components on ASD risk.
Methods
In a population-based pregnancy cohort of children born between 2001–2014 in Southern California, 318,751 mother-child pairs were followed through electronic medical records (EMR); 4,559 children were diagnosed with ASD before age 5. Four broad categories of MIA-related conditions were classified, including infection, hypertension, maternal asthma, and autoimmune conditions. Average exposures to PM2.5 and four PM2.5 components, black carbon (BC), organic matter (OM), nitrate (NO3−), and sulfate (SO42−), were estimated at maternal residential addresses during pregnancy. We estimated the ASD risk associated with MIA-related conditions, air pollution, and their interactions, using Cox regression models to adjust for covariates.
Results
ASD risk was associated with MIA-related conditions [infection (hazard ratio 1.11; 95% confidence interval 1.05–1.18), hypertension (1.30; 1.19–1.42), maternal asthma (1.22; 1.08–1.38), autoimmune disease (1.19; 1.09–1.30)], with higher pregnancy PM2.5 [1.07;1.03–1.12 per interquartile (3.73 μg/m3) increase] and with all four PM2.5 components. However, there were no interactions of each category of MIA-related conditions with PM2.5 or its components on either multiplicative or additive scales.
Conclusions
MIA-related conditions and pregnancy PM2.5 were independently associations with ASD risk. There were no statistically significant interactions of MIA conditions and prenatal PM2.5 exposure with ASD risk.
Keywords: Interaction, susceptibility, PM2.5, PM2.5 chemical components, autism spectrum disorders
Graphical Abstract
1. Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social communication and interaction, and restricted, repetitive and stereotyped behaviors (American Psychiatric Association 2013). The estimated prevalence of ASD is 1 in 54 among 8-year-old children in the United States (Maenner et al. 2020). Although ASD risk is heritable, only approximately 15–20% of diagnoses are due to spontaneous single gene or chromosomal mutations (Bai et al. 2019; Rylaarsdam and Guemez-Gamboa 2019); other factors that may contribute to increased risk or clinical severity, are not well understood and are likely multifactorial. Diagnoses of ASD can be made starting around age 2, and subtle social and communication impairment may be present earlier (Bacon et al. 2018; Charman and Baird 2002). Therefore, prenatal and early life environment has been the focus of autism environmental epidemiology.
Accumulating evidence indicates that prenatal ambient air pollutants, especially particulate matter (PM) with aerodynamic diameter < 2.5 μm (PM2.5), are modifiable environmental risk factors for ASD (Jo, Eckel, Wang, et al. 2019; Chun et al. 2020; Lam et al. 2016; Rahman et al. 2022). PM2.5 is a complex mixture of solid and liquid particles with varying sizes, chemical composition, and toxicity (Adams et al. 2015; Fong et al. 2019). A previous study from our group found that prenatal exposure to some key components of PM2.5 such as black carbon (BC), organic matter (OM), nitrate (NO3−), and sulfate (SO42-) were associated with increased risk of ASD (Rahman et al. 2023). Understanding the effects of different PM2.5 components can help to better develop source-specific ambient air quality standards and prevention strategies.
Maternal infection (Zerbo et al. 2013; Jiang et al. 2016), hypertension (Maher et al. 2018), asthma (Theoharides et al. 2016), and autoimmune disease (Lyall et al. 2014) during pregnancy have been shown consistently to be associated with ASD and other neuropsychiatric or neurodevelopmental disorders in children (Estes and McAllister 2016; Malkova et al. 2012). These maternal health conditions may disturb the immune function of mothers and trigger systemic inflammation during pregnancy (Simoes et al. 2018; Patterson 2011). Therefore, they have been proposed to be proxies for maternal immune activation (O’Connor and Ciesla 2022; Estes and McAllister 2016).
Maternal immune activation is a potentially useful framework for understanding ASD neurobiology (Meldrum et al. 2013), but most children exposed to health conditions that are proxies for maternal immune activators (MIA) do not develop ASD. It has been hypothesized that subsequent additional exposures are required for ASD symptoms to occur, and co-existing risk factors sharing similar or complementary biological pathways may provide sufficient cause (Estes and McAllister 2016; Bilbo et al. 2018). Several studies have shown separate associations between prenatal environmental exposures including ambient air pollution (Rahman et al. 2022), nutritional intake (Li et al. 2019), social environment (Bhasin and Schendel 2007), and maternal health conditions (Zerbo et al. 2013; Lyall et al. 2014) during pregnancy on ASD risk, but there has been little examination of the interactive effects among these risk factors on ASD (X. Yu, Rahman, Wang, et al. 2022). Animal models have shown that PM and MIA conditions are both associated with impaired social communication in offspring (Carlezon et al., 2019; Jones et al., 2020; Klocke et al., 2018; Klocke et al., 2017; Zhang et al., 2018), and with increased oxidative stress and inflammatory cytokine levels (Leni, Künzi, and Geiser 2020; Smith et al. 2007). Therefore, we hypothesized that the co-exposure of prenatal PM2.5 or PM2.5 components and MIA-related conditions may have interactive effects on the subsequent risk of ASD. Synergies between MIA-related conditions and air pollution-induced ASD would have important implications for a large co-burden of disease from these exposures and for the development of clinical and public health interventions to reduce ASD risk.
We tested this hypothesis using data from a large, population-based Southern California pregnancy cohort, because a large sample size is required to detect interactions between co-exposures. Information on ASD and diagnoses of maternal health conditions, proxies of MIA, were extracted from the electronic medical record (EMR). The large Southern California gradients in air pollution exposure during pregnancy were assessed using high-resolution spatiotemporal hybrid models (Van Donkelaar et al. 2019).
2. Methods
2.1. Study population
This study utilized a population-based retrospective pregnancy cohort that included mothers with singleton deliveries (n=370,723) at Kaiser Permanente Southern California (KPSC) hospitals between January 1, 2001 and December 31, 2014. KPSC is a large integrated healthcare system with over 4.5 million members across Southern California. KPSC membership reflects the diverse socioeconomic demographics in the study region (Koebnick et al. 2012). Information related to the mothers, including maternal address history, and to the children were extracted from high-quality integrated electronic medical records (EMR) maintained by KPSC. Addresses were geocoded using ArcGIS (ArcGIS 2021). Addresses based only on street name, 5-digit postal code, locality, or administrative unit were considered too uncertain to be geolocated into the correct grid used for exposure assignments.
A total of 51,152 births were excluded due to 1) missing gender, maternal race/ethnicity and age at delivery, implausible age of delivery or birth weight (n=666); 2) maternal age at delivery (n=159); 3) incomplete maternal residential address history in pregnancy or geocodes not suitable for exposure assignment (n=51,147). The final data analysis included 318,751 mother-child pairs with complete data on residential estimates of PM2.5 composition exposures (Figure S1 in the supplement). Both KPSC and the University of Southern California Institutional Review Boards approved this study with waiver of individual subject consent.
2.2. ASD ascertainment
The outcome was ASD diagnosis before age 5. Children were followed from birth through EMR until clinical diagnosis of ASD, loss to follow-up, or age 5, whichever came first, as described previously (Coleman et al. 2015; Jo, Eckel, Wang, et al. 2019; Xiang et al. 2018; Xiang et al. 2015). The presence or absence of ASD in children was identified by International Classification of Diseases, Ninth Revision (ICD- 9) codes 299.0, 299.1, 299.8, 299.9 from the EMR records before October 1, 2015 (the date of KPSC implementation of ICD-10 codes) and subsequently ICD-10 codes F84.0, F84.5, F84.9 F84.0, F84.3, F84.5, F84.8, F84.9.
2.3. Air pollution exposure assessment
Monthly estimates of PM2.5 and four major PM2.5 chemical components [BC, OM, NO3−, and SO42-] with a 1 km spatial resolution were estimated by a hybrid model (version V4.NA.02) that integrates chemical transport model outputs, satellite observations, and ground-based measurements as developed by the Atmospheric Composition Analysis Group at Washington University in St. Louis. The exposure model (Van Donkelaar et al. 2019) and its application to this cohort (Rahman et al. 2023), are described elsewhere. Exposures to PM2.5 and these selected components were assigned to maternal address during the entire pregnancy and each of three trimesters. Exposures were time-weighted to account for changes in maternal addresses during pregnancy.
2.4. Conditions triggering maternal immune activation
We considered four broad categories of MIA-related conditions during pregnancy: infections, gestational hypertension, asthma, and autoimmune diseases. Any occurrence (incident or recurring) during pregnancy of each category of conditions was operationalized as a yes/no variable. The pregnancy period was defined as the time between the last menstrual period and the date of delivery. Autoimmune conditions were identified from 1 year before pregnancy and the pregnancy period, considering that most autoimmune diseases are chronic with long-lasting effects.
Since the cohort included births from 2001 to 2014, prior to KPSC adoption of ICD-10 codes (in 2015), only ICD-9 codes were used to identify each of the MIA-related conditions diagnoses during pregnancy (Table S1 in the supplement). The ICD-9 codes for maternal infection during pregnancy were based on Zerbo et al. (2013). The ICD-9 codes for hypertension were from Savitz et al. (2014). Asthma was categorized based on the ICD-9 code 493.xx and the usage of medication for asthma during pregnancy (Martinez et al. 2020). Maternal autoimmune disease classification was based on Croen et al. (2005), to which we added antiphospholipid antibody syndrome, aplastic anemia, dermatitis herpetiformis, giant cell arteritis, hemolytic anemia, Kawasaki’s disease, and Sydenham chorea [from Lyall et al. (2014)].
2.5. Covariates
We included the priori known potential risk factors for ASD including child sex, maternal age at delivery, parity, and maternal history of severe comorbidities [>1 diagnosis of heart, lung, kidney, liver disease, or cancer](Xiang et al. 2015). We also adjusted for birth years as a non-linear term with 4 degrees of freedom to account for the non-linear increasing trend of ASD prevalence. An indicator variable for the season at conception (dry from April-October; wet from November-March) was used to adjust for the potential air pollution seasonality. In a sensitivity analysis adjusting for month of birth (instead of season), associations of air pollutants and of MIA-related conditions with ASD risk were similar, so only the adjustment for season was included in the final analyses. Maternal race/ethnicity, maternal education [≤high school, some college, and ≥> college] and neighborhood disadvantage index (Levy, Owens, and Sampson 2019) at birth have been shown to be associated with ASD in this cohort (Xin Yu, Rahman, Carter, et al. 2022) and were adjusted as socioeconomic covariates in this study. A missing indicator variable was used for missing values in categorical covariates (parity [n = 17,860], education [n = 3,024]). We used the same set of covariates for models assessing ASD associations with MIA-related conditions during pregnancy, air pollution, and interactions to ensure that the estimated associations were comparable under the same modeling strategy.
2.6. Statistical analyses
The main associations of ASD with average air pollution exposures, any MIA (the presence of at least one category of MIA-related conditions) and 4 types of MIA-related conditions during pregnancy and each trimester were estimated as hazard ratios (HRs) using multivariable Cox proportional hazard models, adjusting for covariates described above. Children were followed from birth through the EMR until clinical diagnosis of ASD, loss to follow-up, or age 5, whichever came first. Standard errors were estimated using robust sandwich estimators to control for potential correlation within families. Air pollution exposures were modeled as continuous variables with linear effects, as modeling them using spline functions did not show evidence of non-linearity. Pregnancy air pollution exposures were scaled to the interquartile range (IQR) of each air pollutant to reflect the distribution of air pollutants in this cohort. Each category of MIA-related conditions (e.g., any infection) was represented as a binary indicator (1=present; 0=absent). The proportional hazards assumption of Cox proportional hazard models was assessed using the Schoenfeld residual plot (Schoenfeld 1982). No clear non-random patterns with follow-up time were observed.
We tested the interactions between each indicator of MIA-related conditions (the presence of any MIA during pregnancy or 4 indicators for each category) and PM2.5 and its components during pregnancy on ASD risk. The multiplicative interaction was examined by adding a multiplicative interaction term between the pollutant and MIA-related conditions to the Cox proportional hazard model, adjusting for the main effects and covariates. The associations between air pollutants and ASD for each MIA condition strata (present/absent) were estimated based on the multiplicative interaction models by reparametrizing the indicator of MIA. The additive interaction was estimated by post-hoc relative excess risk due to interaction (RERI) between each indicator MIA-related conditions variable and air pollution (continuous variables per IQR increase) using the method described in VanderWeele and Knol (2014) based on estimated coefficients from interaction models. Sensitivity analysis was conducted including all follow up until December 31, 2019 without censoring at age 5.
Two-sided statistical tests were applied at an alpha level of 0.05 and the uncertainty in estimates was reported by 95% confidence intervals (CIs). Data analyses were performed in R, version 4.2.
3. Results
There were 4,559 children (1.4%) diagnosed with ASD before age 5 (Table 1). The median age at diagnosis was 3.53 years. Children with ASD were 4.3 times more likely to be boys (n=3,703) than girls (n=856). Mothers of children with ASD were slightly older at delivery (31.3 years, IQR=27.5–35.3) than mothers of children without ASD (30.4 years, IQR=26.2–34.3). Mothers of children with ASD were more likely to have more than high school education. The prevalence of gestational infection, hypertension, asthma, autoimmune diseases, and any MIA condition was 48.5%, 9.7%, 7.2%, 11.2%, and 59.4% respectively for the entire cohort. The prevalence of all five MIA indicators was generally higher in mothers of children with ASD than mothers of children without ASD.
Table 1.
Characteristics of children, with and without autism spectrum disorder (ASD)
| Children, No. (%) or median [interquartile range] | |||
|---|---|---|---|
| Characteristics | Overall | With ASD | Without ASD |
| (n =318,751) | (n= 4,559) | (n= 314,192) | |
| Sex = Male (%) | 163,182 (51.2) | 3,703 (81.2) | 159,479 (50.8) |
| Maternal age at delivery, | 30.4 [26.3, 34.3] | 31.3 [27.5, 35.3] | 30.4 [26.2, 34.3] |
| median [IQR*], years | |||
| Maternal immune activation (MIA) conditions; N(%) | |||
| Infection | 154,894 (48.6) | 2,396 (52.6) | 152,498 (48.5) |
| Hypertension | 30,762 (9.7) | 616 (13.5) | 30,146 (9.6) |
| Asthma | 22,860 (7.2) | 429 (9.4) | 22,431 (7.1) |
| Autoimmune diseases | 35,669 (11.2) | 651 (14.3) | 35,018 (11.1) |
| Any MIA | 189,373 (59.4) | 2,977 (65.3) | 186,396 (59.3) |
| Parity; N (%) | |||
| 0 | 111,981 (35.1) | 1,844 (40.4) | 110,137 (35.1) |
| 1 | 104,561 (32.8) | 1,495 (32.8) | 103,066 (32.8) |
| >=2 | 84,176 (26.4) | 903 (19.8) | 83,273 (26.5) |
| Unknown | 18,033 (5.7) | 317 (7.0) | 17,716 (5.6) |
| Maternal Education; N(%) | |||
| High school or lower | 112,096 (35.2) | 1,335 (29.3) | 110,761 (35.3) |
| Some college | 94,525 (29.7) | 1,477 (32.4) | 93,048 (29.6) |
| College graduate or higher | 109,087 (34.2) | 1,713 (37.6) | 107,374 (34.2) |
| Unknown | 3,043 (1.0) | 34 (0.7) | 3,009 (1.0) |
| Neighborhood disadvantage index [IQR]a | 0.07 [−1.22, 1.48] | 0.07 [−1.31, 1.53] | 0.07 [−1.21, 1.48] |
| Race/ethnicity; N (%) | |||
| Non-Hispanic white | 81,050 (25.4) | 956 (21.0) | 80,094 (25.5) |
| Non-Hispanic black | 29,773 (9.3) | 447 (9.8) | 29,326 (9.3) |
| Hispanic | 161,415 (50.6) | 2,300 (50.4) | 159,115 (50.6) |
| Asian/Pacific Islander | 39,974 (12.5) | 744 (16.3) | 39,230 (12.5) |
| Other | 6,539 (2.1) | 112 (2.5) | 6,427 (2.0) |
| Any history of maternal severe comorbiditiesb; N (%) | 46,717 (14.7) | 839 (18.4) | 45,878 (14.6) |
| Year of birth; N (%) | |||
| 2001–2005 | 78,257 (24.6) | 818 (17.9) | 77,439 (24.6) |
| 2006–2010 | 111,174 (34.9) | 1,308 (28.7) | 109,866 (36.0) |
| 2011–2014 | 129,320 (40.6) | 2,433 (53.4) | 126,887 (40.4) |
Abbreviations: IQR, interquartile range.
Census tract level neighborhood disadvantage index. Higher values represent more deprived neighborhoods
>=1 diagnosis of heart, lung, kidney, or liver disease; cancer.
PM2.5 concentrations, NO3−, SO42- and BC during the study period decreased over time, while OM was relatively stable (Figure S2 in the supplement). The median levels of PM2.5, BC, OM, NO3−, and SO42-, during pregnancy were 15.1 micrograms per meter-cubed (μg/m3) (IQR=13.1–16.9), 1.83 μg/m3 (IQR=1.35–2.20), 6.40 μg/m3 (IQR=5.41–7.39), 2.79μg/m3 (IQR=2.33–3.49), and 1.47 μg/m3 (IQR=1.23–1.73), respectively.
Pregnancy average PM2.5 was associated with ASD risk [HR=1.07 (95% CI 1.03–1.12) per IQR=3.73 μg/m3] after adjustment for child sex, maternal race/ethnicity, maternal age at delivery, parity, maternal education, maternal history of pre-pregnancy severe comorbidities, neighborhood disadvantage index, birth year, and season at conception (Table 2), consistent with previous analysis in a subset of this cohort (Jo, Eckel, Wang, et al. 2019). The presence of MIA-related conditions during pregnancy was associated with higher risk of ASD [infection: HR=1.11 (95% CI 1.05–1.18); hypertension: HR=1.30 (95% CI 1.19–1.42); asthma: HR=1.22 (95% CI 1.08–1.38); autoimmune conditions: HR=1.19 (95% CI 1.09–1.30); any MIA: HR=1.17 (95% CI 1.10–1.25), respectively after adjusting for covariates.
Table 2.
Adjusted hazard ratios and 95% confidence intervals for ASD associated with each air pollutant during pregnancy (in single pollutant models) and with each MIA (in single condition models)
| HRa (95% CI) | |
|---|---|
| Pregnancy Air Pollution Exposures b | |
| PM2.5 per 3.73 μg/m3 | 1.07 (1.03, 1.12) |
| BC per 0.84 μg/m3 | 1.06 (1.02, 1.10) |
| OM per 1.98 μg/m3 | 1.09 (1.04, 1.14) |
| NO3− per 1.15 μg/m3 | 1.05 (1.00, 1.10) |
| SO42- per 0.50 μg/m3 | 1.08 (1.03, 1.15) |
| Maternal Immune Activation | |
| Infection | 1.11 (1.05, 1.18) |
| Hypertension | 1.30 (1.19, 1.42) |
| Asthma | 1.22 (1.08, 1.38) |
| Autoimmunec | 1.19 (1.09, 1.30) |
| Any MIAd | 1.17 (1.10, 1.25) |
Abbreviations: CI, confidence interval; HR, hazard ratio
Adjusted for child sex, maternal race/ethnicity, maternal age at delivery, parity, education, maternal history of severe comorbidities, neighborhood disadvantage index, birth year, and season.
Each air pollutant was scaled to its interquartile range.
Any autoimmune disease within 1 year prior or during pregnancy. The other three categories of MIA-related conditions were restricted to pregnancy.
Any MIA represents the presence of at least one of the categories of MIA-related conditions.
No statistically significant interactions were observed between the five indicators of MIA-related conditions (4 categories and any MIA) and pregnancy average PM2.5 on either multiplicative or additive scales (Table 3). The interactions between MIA-related conditions and pregnancy-average exposure of each PM2.5 component exposure were also not statistically significant, except for the interaction between SO42- and autoimmune disease indicator (p-interaction=0.002) (Figure 1). For interactions between trimester specific PM2.5 components exposures and MIA indicators (Figure S3–S5 in the supplement), only the interaction between the second trimester average SO42- and the autoimmune disease indicator was significant (p-interaction=0.001). However, if these interaction p-values were adjusted for false discovery rate for 100 comparisons [for 5 MIA indicators, 4 components and 4 exposure windows (all pregnancy and 3 trimester exposures)] the threshold p-value for significance would be 0.0005 after the Bonferroni correction, so the isolated SO42- interactions cannot be considered statistically significant.
Table 3.
The interaction between MIA-related conditions and pregnancy PM2.5 on risk of ASD in children
| PM2.5-associated risk HRa (95% CI) | multiplicative p-interaction | additive RERI (95% CI) | ||
|---|---|---|---|---|
| Infection | No | 1.07 (1.01, 1.13) | 0.92 | 0.01 (−0.06, 0.08) |
| Yes | 1.07 (1.02, 1.13) | |||
| Hypertension | No | 1.08 (1.04, 1.13) | 0.17 | −0.07 (−0.20, 0.06) |
| Yes | 1.01 (0.92, 1.11) | |||
| Asthma | No | 1.07 (1.02, 1.11) | 0.52 | 0.07 (−0.07, 0.20) |
| Yes | 1.11 (0.99, 1.25) | |||
| Autoimmuneb | No | 1.06 (1.02, 1.11) | 0.20 | 0.09 (−0.03, 0.22) |
| Yes | 1.13 (1.03, 1.25) | |||
| Any MIAc | No | 1.07 (1.01, 1.14) | 0.99 | 0.01 (−0.06, 0.08) |
| Yes | 1.07 (1.02, 1.12) |
Abbreviations: CI, confidence interval; HR, hazard ratio; RERI, relative excess risk due to interaction
Adjusted for child sex, maternal race/ethnicity, maternal age at delivery, parity, education, maternal history of severe comorbidities, neighborhood disadvantage index, birth year, and season. Results were scaled per interquartile (3.73 μg/m3) increase in PM2.5 exposure.
Any autoimmune disease within 1 year prior or during pregnancy. The other three categories of MIA-related conditions were restricted to pregnancy.
Any MIA represents the presence of at least one of the categories of MIA-related conditions.
Figure 1.
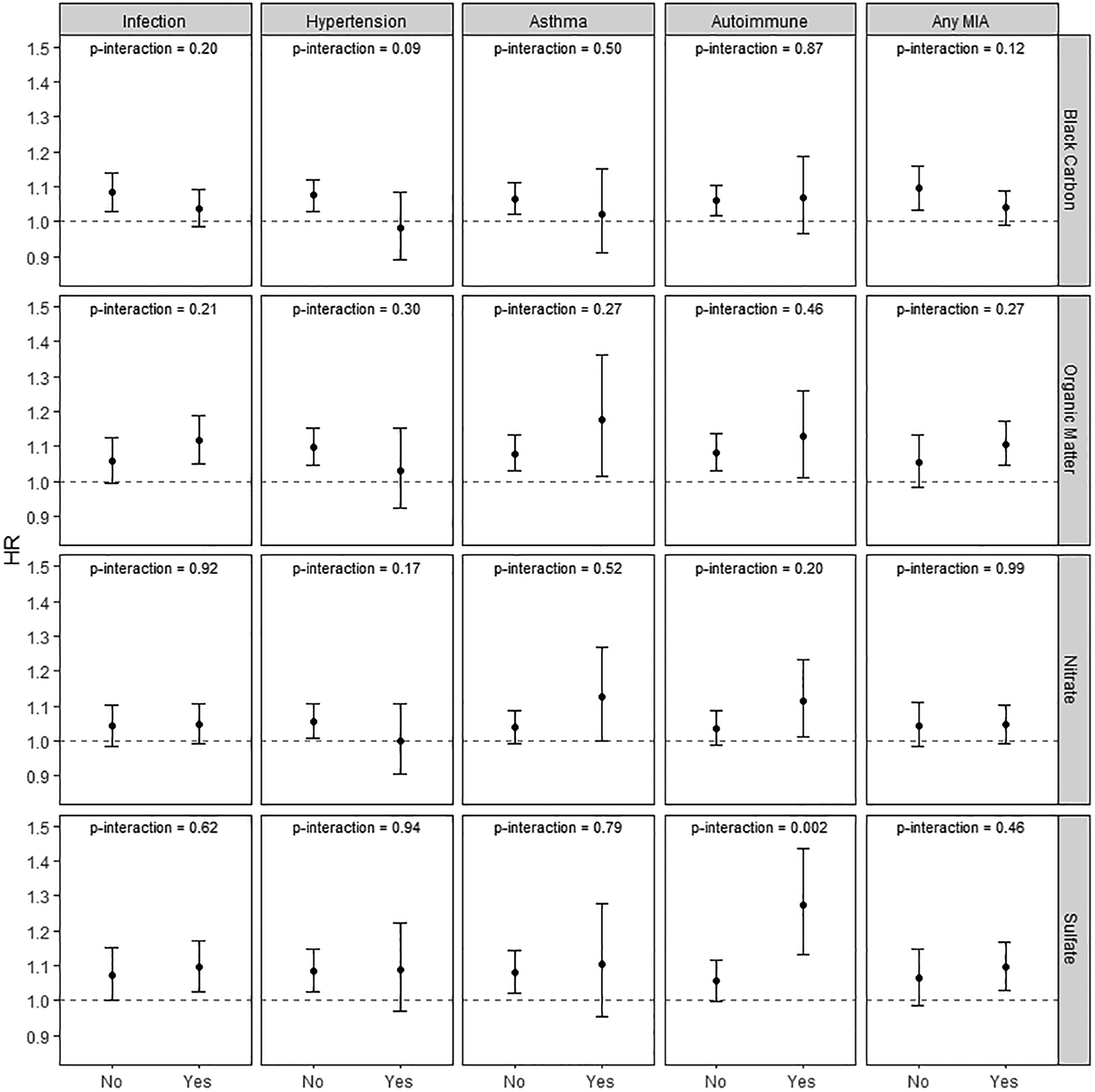
ASD risk associations with interactions between MIA indicators and the concentration of PM2.5 components during pregnancy
Notes: Models were adjusted for child sex, maternal race/ethnicity, maternal age at delivery, parity, education, maternal history of severe comorbidities, neighborhood disadvantage index, birth year, and season.
Air pollutants were scaled to their interquartile range.
We also examined the independent association of ASD risk with pregnancy PM2.5 exposure and all four categories of MIA-related conditions in one co-adjusted model. The associations of ASD risk with pregnancy PM2.5 exposure, each MIA-related conditions indicator and ASD were attenuated but remained statistically significant in the co-adjusted model (Table S2 in the supplement). Results were similar in sensitivity analysis with 6,366 ASD cases during follow up without censoring at age 5 (Table S3–S4 in the supplement).
4. Discussion
Higher levels of average PM2.5 exposures during pregnancy and the presence of each of the four broad categories of conditions that are maternal immune activators were associated with ASD risk. However, we did not observe associations of ASD risk in children with interactions between prenatal PM2.5 or its components and MIA-related conditions either on the multiplicative or additive scales.
The conceptual framework of MIA has been proposed in recent years for studying the effects of co-occurring prenatal environmental exposures with fetal neurodevelopment (O’Connor and Ciesla 2022; Estes and McAllister 2016). However, to our knowledge, this is the first large population-based cohort study explicitly examining the interactions of prenatal particulate air pollution exposure with a broad spectrum of maternal clinical conditions during pregnancy that are proxies of maternal immune activation on the risk of ASD in offspring. A previous cohort study from our group reported interactions between gestational diabetes mellitus and O3 exposure on ASD risk (Jo, Eckel, Chen, et al. 2019). One case-control study showed that maternal immune biomarkers mediated associations between prenatal air pollution exposure and ASD risk (Volk et al. 2020). However, interactions between immune biomarkers or proxies of MIA and air pollution were not examined in that case-control study.
PM2.5 is comprised of a heterogeneous mixture of solid and liquid particles with varying chemical composition that may have different toxicity. We have previously found that prenatal exposure to PM2.5 components BC, OM, NO3−, SO42−were associated with increased ASD risk (Rahman et al. 2023). Studies have shown that the association between prenatal PM2.5 and fetal neurodevelopment may vary by exposure time windows (Pagalan et al. 2019; Raz et al. 2015). In the cohort used for this study, our previous research reported that PM2.5 exposure during the first and second trimesters was associated with higher ASD risk (Rahman et al. 2022). Therefore, we also examined the interactions between trimester specific PM2.5 exposure and MIA-related conditions. Similar patterns of associations were found as with the pregnancy average PM2.5. Overall, no effect modification by MIA-related conditions on the association between PM components and ASD risk was found in our study.
Combinations of different component factors, such as PM2.5 and MIA-related conditions, may be required for disease development (Rothman 1976). Thus, it has been hypothesized that multiple risk factors sharing similar or complementary biological mechanisms are required for ASD to occur (Estes and McAllister 2016; Bilbo et al. 2018). Evidence from animal and in vitro studies has shown that both proxies of MIA and prenatal PM exposure may induce neuroinflammation of glial cells and oxidative stress, leading to impaired neurodevelopment (Baines et al. 2020; Morris et al. 2021; Di Domenico et al. 2020). Children with ASD also exhibit metabolic disruption, with increased oxidative stress (James et al. 2004; James et al. 2006; Gorrindo et al. 2013) and chronic neuroimmune activation in the central nervous system (Onore, Careaga, and Ashwood 2012; Vargas et al. 2005; Voineagu et al. 2011). Thus, it has been hypothesized that co-existing MIA-related conditions and high PM exposure may have synergistic effects on ASD risk. Although our results did not support this hypothesis, this “multiple-exposure” framework may be useful for identifying other multifactorial risk factors for developing ASD.
The present study has several strengths. The large study population provided statistical power to assess even modest associations of ASD risk with interactions between MIA-related conditions and prenatal air pollution, had these effects been present. Mother-child pairs were followed through the EMR in a single integrated healthcare system with standard diagnostic criteria, which helped avoid screening and ascertainment bias. The high-quality EMR also provided relevant confounders in an ethnically diverse sample of children. Current state-of-the-art high spatiotemporal air pollution exposure models were used to assign prenatal ambient exposures accounting for residential mobility.
We also acknowledge some limitations. Beyond clinical diagnoses of maternal immune activators, we lack biomarkers for MIA, which would be expensive to collect for a large cohort. Clinical symptoms and diagnoses of MIA-related conditions can often indicate immune dysregulation in mothers (Mor and Cardenas 2010), which may influence fetal development (Simoes et al. 2018; Patterson 2011). Thus, using the diagnoses of clinical conditions as proxies of MIA in a large population can also provide insights into the potential etiology of ASD. We also acknowledge the limitation of using ICD codes to extract information on maternal health conditions during pregnancy. We lack information on the severity of MIA-related conditions and may misclassify MIA-related conditions if mothers did not come for care if they had mild symptoms. Other epidemiological studies have examined interactions of air pollution with nutritional and genetic risks (Goodrich et al. 2018; Volk et al. 2014; Kim et al. 2017; X. Yu, Rahman, Wang, et al. 2022), but information on these risks was not available in this large cohort. Changes in residential addresses during pregnancy were accounted for in the pollutant exposure assignment, but the exposures away from home at work or elsewhere were unavailable. Since our main hypothesis was about interactions among MIA-related conditions and prenatal air pollution during pregnancy on ASD, we did not assess mediation of air pollution effects by MIA-related conditions. In the co-adjusted model with four categories of clinical conditions and pregnancy PM2.5 (Table S2 in the supplement) the estimated effect sizes were similar to the single exposure models (Table 2), suggesting that these effects of MIA-related conditions and PM2.5 were independent.
In conclusion, our findings do not support the hypothesis that ASD risk in children is associated with multiplicative or additive interaction in analyses of a broad spectrum of MIA-related conditions during pregnancy and prenatal air pollution exposure in this large population-based cohort. However, PM2.5 and four broad categories of conditions related to MIA were independently associated with ASD risk.
Supplementary Material
Highlights.
Prenatal conditions of maternal immune activation (MIA) were associated with ASD.
Exposure to PM2.5 and its major components during pregnancy were associated with ASD.
Prenatal MIA conditions unexpectedly did not augment effects of particles on ASD.
Acknowledgements
The authors thank the patients of Kaiser Permanente for helping us improve care through the use of information collected through our electronic health record systems, and the Kaiser Permanente and the Utility for Care Data Analysis (UCDA) team within Kaiser Permanente for creating the GEMS Datamart with consolidated addresses histories available to facilitate our research.
Funding
This research was supported by National Institutes of Environmental Health Sciences (R01 ES029963 (Xiang, McConnell); R56ES028121 (Xiang); P30ES007048 and P2CES033433 (McConnell); Simms/Mann Chair in Neurogenetics (PL); partly supported by Kaiser Permanente Southern California Direct Community Benefit Funds. Joel Schwartz was supported by EPA grant RD-8358720. Randall Martin acknowledges funding from NASA HAQAST (80NSSC21K0508).
CRediT authorship contribution statement
Xin Yu: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Md Mostafijur Rahman: Conceptualization, Writing – original draft, Writing – review & editing.
Sarah A. Carter: Conceptualization, Writing – review & editing.
Jane C. Lin: Software, Validation, Data curation, Writing – review & editing.
Zimin Zhuang: Software, Data curation, Writing – review & editing.
Ting Chow: Software, Data curation, Writing – review & editing.
Frederick W. Lurmann: Resources, Writing – review & editing.
Michael J. Kleeman: Writing – review & editing.
Mayra P. Martinez: Project administration, Writing – review & editing.
Aaron van Donkelaar: Resources, Writing – review & editing.
Randall V. Martin: Resources, Writing – review & editing.
Sandrah P. Eckel: Methodology, Writing – review & editing.
Zhanghua Chen: Writing – review & editing.
Pat Levitt: Writing – review & editing.
Joel Schwartz: Writing – original draft.
Daniel Hackman: Writing – original draft, Writing – review & editing, Supervision.
Jiu-Chiuan Chen: Conceptualization, Methodology, Writing – review & editing.
Rob McConnell: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Anny H. Xiang: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Footnotes
Conflict of interests
The authors declare they have no actual or potential competing interests. Joel Schwartz declares that he has testified on behalf of the U.S. Department of Justice in a case involving a Clean Air Act violation.
Ethical approval
Both KPSC and the University of Southern California Institutional Review Boards approved this study.
Data statement
KPSC Institutional Review Board approved this study, with waiver of informed consent with the condition that raw data remain confidential and would not be shared. Thus, due to the sensitive nature of these data, the data are not available to be shared.
References:
- Adams K, Greenbaum DS, Shaikh R, van Erp AM, and Russell AG. 2015. “Particulate matter components, sources, and health: Systematic approaches to testing effects.” J Air Waste Manag Assoc 65 (5): 544–58. 10.1080/10962247.2014.1001884. https://www.ncbi.nlm.nih.gov/pubmed/25947313. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- ArcGIS. 2021. “The geocoding process.” Accessed Oct 28. https://desktop.arcgis.com/en/arcmap/latest/manage-data/geocoding/the-geocoding-process.htm.
- Bacon EC, Courchesne E, Barnes CC, Cha D, Pence S, Schreibman L, Stahmer AC, and Pierce K. 2018. “Rethinking the idea of late autism spectrum disorder onset.” Dev Psychopathol 30 (2): 553–569. 10.1017/S0954579417001067. https://www.ncbi.nlm.nih.gov/pubmed/28803559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Yip BHK, Windham GC, Sourander A, Francis R, Yoffe R, Glasson E, Mahjani B, Suominen A, Leonard H, Gissler M, Buxbaum JD, Wong K, Schendel D, Kodesh A, Breshnahan M, Levine SZ, Parner ET, Hansen SN, Hultman C, Reichenberg A, and Sandin S. 2019. “Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort.” JAMA Psychiatry 76 (10): 1035–1043. 10.1001/jamapsychiatry.2019.1411. https://www.ncbi.nlm.nih.gov/pubmed/31314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines Kelly J, Hillier Dendra M, Haddad Faraj L, Rajakumar Nagalingam, Schmid Susanne, and Renaud Stephen J. 2020. “Maternal immune activation alters fetal brain development and enhances proliferation of neural precursor cells in rats.” Frontiers in Immunology 11: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin TK, and Schendel D. 2007. “Sociodemographic risk factors for autism in a US metropolitan area.” J Autism Dev Disord 37 (4): 667–77. 10.1007/s10803-006-0194-y. https://www.ncbi.nlm.nih.gov/pubmed/16951989. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, and Tran PK. 2018. “Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders.” Exp Neurol 299 (Pt A): 241–251. 10.1016/j.expneurol.2017.07.002. https://www.ncbi.nlm.nih.gov/pubmed/28698032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, and Baird G. 2002. “Practitioner review: Diagnosis of autism spectrum disorder in 2- and 3-year-old children.” J Child Psychol Psychiatry 43 (3): 289–305. 10.1111/1469-7610.00022. https://www.ncbi.nlm.nih.gov/pubmed/11944873. [DOI] [PubMed] [Google Scholar]
- Chun H, Leung C, Wen SW, McDonald J, and Shin HH. 2020. “Maternal exposure to air pollution and risk of autism in children: A systematic review and meta-analysis.” Environ Pollut 256: 113307. 10.1016/j.envpol.2019.113307. https://www.ncbi.nlm.nih.gov/pubmed/31733973. [DOI] [PubMed] [Google Scholar]
- Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, Lynch FL, Madden JM, Owen-Smith A, Pearson JA, Pearson KA, Rusinak D, Quinn VP, and Croen LA. 2015. “Validation of Autism Spectrum Disorder Diagnoses in Large Healthcare Systems with Electronic Medical Records.” J Autism Dev Disord 45 (7): 1989–96. 10.1007/s10803-015-2358-0. https://www.ncbi.nlm.nih.gov/pubmed/25641003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, and Van de Water J. 2005. “Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study.” Arch Pediatr Adolesc Med 159 (2): 151–7. 10.1001/archpedi.159.2.151. https://www.ncbi.nlm.nih.gov/pubmed/15699309. [DOI] [PubMed] [Google Scholar]
- Di Domenico M, Benevenuto SGM, Tomasini PP, Yariwake VY, de Oliveira Alves N, Rahmeier FL, da Cruz Fernandes M, Moura DJ, Nascimento Saldiva PH, and Veras MM. 2020. “Concentrated ambient fine particulate matter (PM2.5) exposure induce brain damage in pre and postnatal exposed mice.” Neurotoxicology 79: 127–141. 10.1016/j.neuro.2020.05.004. https://www.ncbi.nlm.nih.gov/pubmed/32450181. [DOI] [PubMed] [Google Scholar]
- Estes ML, and McAllister AK. 2016. “Maternal immune activation: Implications for neuropsychiatric disorders.” Science 353 (6301): 772–7. 10.1126/science.aag3194. https://www.ncbi.nlm.nih.gov/pubmed/27540164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KC, Di Q, Kloog I, Laden F, Coull BA, Koutrakis P, and Schwartz JD. 2019. “Relative toxicities of major particulate matter constituents on birthweight in Massachusetts.” Environ Epidemiol 3 (3): e047. 10.1097/EE9.0000000000000047. https://www.ncbi.nlm.nih.gov/pubmed/31342007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, and Schmidt RJ. 2018. “Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder.” Autism Res 11 (1): 69–80. 10.1002/aur.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo P, Lane CJ, Lee EB, McLaughlin B, and Levitt P. 2013. “Enrichment of elevated plasma F2t-isoprostane levels in individuals with autism who are stratified by presence of gastrointestinal dysfunction.” PLoS One 8 (7): e68444. 10.1371/journal.pone.0068444. https://www.ncbi.nlm.nih.gov/pubmed/23844202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, and Neubrander JA. 2004. “Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism.” Am J Clin Nutr 80 (6): 1611–7. 10.1093/ajcn/80.6.1611. https://www.ncbi.nlm.nih.gov/pubmed/15585776. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, and Gaylor DW. 2006. “Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism.” Am J Med Genet B Neuropsychiatr Genet 141B (8): 947–56. 10.1002/ajmg.b.30366. https://www.ncbi.nlm.nih.gov/pubmed/16917939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, Yang F, Deng M, and Ruan B. 2016. “Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis.” Brain Behav Immun 58: 165–172. 10.1016/j.bbi.2016.06.005. https://www.ncbi.nlm.nih.gov/pubmed/27287966. [DOI] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Chen JC, Cockburn M, Martinez MP, Chow T, Lurmann FW, Funk WE, Xiang AH, and McConnell R. 2019. “Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder.” Environ Int 133 (Pt A): 105110. 10.1016/j.envint.2019.105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Wang X, Chen JC, Cockburn M, Martinez MP, Chow T, Molshatzki N, Lurmann FW, Funk WE, Xiang AH, and McConnell R. 2019. “Sex-specific associations of autism spectrum disorder with residential air pollution exposure in a large Southern California pregnancy cohort.” Environ Pollut 254 (Pt A): 113010. 10.1016/j.envpol.2019.113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Volk H, Girirajan S, Pendergrass S, Hall MA, Verma SS, Schmidt RJ, Hansen RL, Ghosh D, Ludena-Rodriguez Y, Kim K, Ritchie MD, Hertz-Picciotto I, and Selleck SB. 2017. “The joint effect of air pollution exposure and copy number variation on risk for autism.” Autism Res 10 (9): 1470–1480. 10.1002/aur.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, and Jacobsen SJ. 2012. “Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data.” Perm J 16 (3): 37–41. 10.7812/tpp/12-031. https://www.ncbi.nlm.nih.gov/pubmed/23012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, Lawler C, Davidson L, Daniels N, Newschaffer C, and Woodruff T. 2016. “A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder.” PLoS One 11 (9): e0161851. 10.1371/journal.pone.0161851. https://www.ncbi.nlm.nih.gov/pubmed/27653281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leni Zaira, Künzi Lisa, and Geiser Marianne. 2020. “Air pollution causing oxidative stress.” Current opinion in toxicology 20: 1–8. [Google Scholar]
- Levy Brian L, Owens Ann, and Sampson Robert J. 2019. “The varying effects of neighborhood disadvantage on college graduation: Moderating and mediating mechanisms.” Sociology of Education 92 (3): 269–292. [Google Scholar]
- Li M, Francis E, Hinkle SN, Ajjarapu AS, and Zhang C. 2019. “Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis.” Nutrients 11 (7). 10.3390/nu11071628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, and Hertz-Picciotto I. 2014. “Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay.” J Autism Dev Disord 44 (7): 1546–55. 10.1007/s10803-013-2017-2. https://www.ncbi.nlm.nih.gov/pubmed/24337796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee LC, Harrington RA, Huston M, Hewitt A, Esler A, Hall-Lande J, Poynter JN, Hallas-Muchow L, Constantino JN, Fitzgerald RT, Zahorodny W, Shenouda J, Daniels JL, Warren Z, Vehorn A, Salinas A, Durkin MS, and Dietz PM. 2020. “Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016.” MMWR Surveill Summ 69 (4): 1–12. 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher GM, O’Keeffe GW, Kearney PM, Kenny LC, Dinan TG, Mattsson M, and Khashan AS. 2018. “Association of Hypertensive Disorders of Pregnancy With Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis.” JAMA Psychiatry 75 (8): 809–819. 10.1001/jamapsychiatry.2018.0854. https://www.ncbi.nlm.nih.gov/pubmed/29874359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, and Patterson PH. 2012. “Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism.” Brain Behav Immun 26 (4): 607–16. 10.1016/j.bbi.2012.01.011. https://www.ncbi.nlm.nih.gov/pubmed/22310922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MP, Lin J, Chow T, Chung J, Wang X, and Xiang AH. 2020. “Maternal Gestational Diabetes and Type 2 Diabetes During Pregnancy and Risk of Childhood Asthma in Offspring.” J Pediatr 219: 173–179 e1. 10.1016/j.jpeds.2019.12.053. https://www.ncbi.nlm.nih.gov/pubmed/31987655. [DOI] [PubMed] [Google Scholar]
- Meldrum Suzanne Jacqueline, Strunk Tobias, Currie Andrew, Prescott Susan Lynne, Simmer Karen, and Whitehouse Andrew JO. 2013. “Autism spectrum disorder in children born preterm—role of exposure to perinatal inflammation.” Frontiers in neuroscience 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, and Cardenas I. 2010. “The immune system in pregnancy: a unique complexity.” Am J Reprod Immunol 63 (6): 425–33. 10.1111/j.1600-0897.2010.00836.x. https://www.ncbi.nlm.nih.gov/pubmed/20367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RH, Counsell SJ, McGonnell IM, and Thornton C. 2021. “Early life exposure to air pollution impacts neuronal and glial cell function leading to impaired neurodevelopment.” Bioessays: e2000288. 10.1002/bies.202000288. https://www.ncbi.nlm.nih.gov/pubmed/33751627. [DOI] [PubMed] [Google Scholar]
- O’Connor Thomas G, and Ciesla Allison A. 2022. “Maternal immune activation hypotheses for human neurodevelopment: Some outstanding questions.” Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 7 (5): 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, and Ashwood P. 2012. “The role of immune dysfunction in the pathophysiology of autism.” Brain Behav Immun 26 (3): 383–92. 10.1016/j.bbi.2011.08.007. https://www.ncbi.nlm.nih.gov/pubmed/21906670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagalan L, Bickford C, Weikum W, Lanphear B, Brauer M, Lanphear N, Hanley GE, Oberlander TF, and Winters M. 2019. “Association of Prenatal Exposure to Air Pollution With Autism Spectrum Disorder.” JAMA Pediatr 173 (1): 86–92. 10.1001/jamapediatrics.2018.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH 2011. “Maternal infection and immune involvement in autism.” Trends Mol Med 17 (7): 389–94. 10.1016/j.molmed.2011.03.001. https://www.ncbi.nlm.nih.gov/pubmed/21482187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Carter SA, Lin JC, Chow T, Yu X, Martinez MP, Chen Z, Chen JC, Rud D, Lewinger JP, van Donkelaar A, Martin RV, Eckel SP, Schwartz J, Lurmann F, Kleeman MJ, McConnell R, and Xiang AH. 2023. “Associations of Autism Spectrum Disorder with PM(2.5) Components: A Comparative Study Using Two Different Exposure Models.” Environ Sci Technol 57 (1): 405–414. 10.1021/acs.est.2c05197. https://www.ncbi.nlm.nih.gov/pubmed/36548990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Shu YH, Chow T, Lurmann FW, Yu X, Martinez MP, Carter SA, Eckel SP, Chen JC, Chen Z, Levitt P, Schwartz J, McConnell R, and Xiang AH. 2022. “Prenatal Exposure to Air Pollution and Autism Spectrum Disorder: Sensitive Windows of Exposure and Sex Differences.” Environ Health Perspect 130 (1): 17008. 10.1289/EHP9509. https://www.ncbi.nlm.nih.gov/pubmed/35040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, and Weisskopf MG. 2015. “Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort.” Environ Health Perspect 123 (3): 264–70. 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ 1976. “Causes.” Am J Epidemiol 104 (6): 587–92. 10.1093/oxfordjournals.aje.a112335. [DOI] [PubMed] [Google Scholar]
- Rylaarsdam L, and Guemez-Gamboa A. 2019. “Genetic Causes and Modifiers of Autism Spectrum Disorder.” Front Cell Neurosci 13: 385. 10.3389/fncel.2019.00385. https://www.ncbi.nlm.nih.gov/pubmed/31481879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Danilack VA, Engel SM, Elston B, and Lipkind HS. 2014. “Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995–2004.” Matern Child Health J 18 (4): 829–38. 10.1007/s10995-013-1307-9. https://www.ncbi.nlm.nih.gov/pubmed/23793484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld David. 1982. “Partial residuals for the proportional hazards regression model.” Biometrika 69 (1): 239–241. [Google Scholar]
- Simoes LR, Sangiogo G, Tashiro MH, Generoso JS, Faller CJ, Dominguini D, Mastella GA, Scaini G, Giridharan VV, Michels M, Florentino D, Petronilho F, Reus GZ, Dal-Pizzol F, Zugno AI, and Barichello T. 2018. “Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats.” J Psychiatr Res 100: 71–83. 10.1016/j.jpsychires.2018.02.007. https://www.ncbi.nlm.nih.gov/pubmed/29494891. [DOI] [PubMed] [Google Scholar]
- Smith Stephen EP, Li Jennifer, Garbett Krassimira, Mirnics Karoly, and Patterson Paul H. 2007. “Maternal immune activation alters fetal brain development through interleukin-6.” Journal of Neuroscience 27 (40): 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Tsilioni I, Patel AB, and Doyle R. 2016. “Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders.” Transl Psychiatry 6 (6): e844. 10.1038/tp.2016.77. https://www.ncbi.nlm.nih.gov/pubmed/27351598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Donkelaar Aaron, Martin Randall V, Li Chi, and Burnett Richard T. 2019. “Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors.” Environmental science & technology 53 (5): 2595–2611. [DOI] [PubMed] [Google Scholar]
- VanderWeele Tyler J, and Knol Mirjam J. 2014. “A tutorial on interaction.” Epidemiologic methods 3 (1): 33–72. [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, and Pardo CA. 2005. “Neuroglial activation and neuroinflammation in the brain of patients with autism.” Ann Neurol 57 (1): 67–81. 10.1002/ana.20315. https://www.ncbi.nlm.nih.gov/pubmed/15546155. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, and Geschwind DH. 2011. “Transcriptomic analysis of autistic brain reveals convergent molecular pathology.” Nature 474 (7351): 380–4. 10.1038/nature10110. https://www.ncbi.nlm.nih.gov/pubmed/21614001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, and Campbell DB. 2014. “Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene.” Epidemiology 25 (1): 44–7. 10.1097/ede.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Park B, Hollingue C, Jones KL, Ashwood P, Windham GC, Lurman F, Alexeeff SE, Kharrazi M, Pearl M, Van de Water J, and Croen LA. 2020. “Maternal immune response and air pollution exposure during pregnancy: insights from the Early Markers for Autism (EMA) study.” J Neurodev Disord 12 (1): 42. 10.1186/s11689-020-09343-0. https://www.ncbi.nlm.nih.gov/pubmed/33327930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, and Feldman RK. 2018. “Maternal Type 1 Diabetes and Risk of Autism in Offspring.” JAMA 320 (1): 89–91. 10.1001/jama.2018.7614. https://www.ncbi.nlm.nih.gov/pubmed/29936530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Buchanan TA, Coleman KJ, and Getahun D. 2015. “Association of maternal diabetes with autism in offspring.” JAMA 313 (14): 1425–34. 10.1001/jama.2015.2707. https://www.ncbi.nlm.nih.gov/pubmed/25871668. [DOI] [PubMed] [Google Scholar]
- Yu X, Rahman MM, Wang Z, Carter SA, Schwartz J, Chen Z, Eckel SP, Hackman D, Chen JC, Xiang AH, and McConnell R. 2022. “Evidence of susceptibility to autism risks associated with early life ambient air pollution: A systematic review.” Environ Res 208: 112590. 10.1016/j.envres.2021.112590. https://www.ncbi.nlm.nih.gov/pubmed/34929192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Xin, Mostafijur Rahman Md, Carter Sarah A, Lin Jane C, Chow Ting, Lurmann Frederick W, Chen Jiu-Chiuan, Martinez Mayra P, Schwartz Joel, and Eckel Sandrah P. 2022. “Neighborhood Socioeconomic Context and Autism Spectrum Disorder.” ISEE Conference Abstracts. [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, and Croen LA. 2013. “Maternal Infection During Pregnancy and Autism Spectrum Disorders.” J Autism Dev Disord 45 (12): 4015–25. 10.1007/s10803-013-2016-3. https://www.ncbi.nlm.nih.gov/pubmed/24366406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
KPSC Institutional Review Board approved this study, with waiver of informed consent with the condition that raw data remain confidential and would not be shared. Thus, due to the sensitive nature of these data, the data are not available to be shared.


