Abstract
Background:
Dysregulated bile acid (BA) metabolism has been linked to steatosis, inflammation, and fibrosis in nonalcoholic fatty liver disease (NAFLD).
Aim:
To determine whether circulating BA levels accurately stage liver fibrosis in NAFLD.
Methods:
We recruited 550 Chinese adults with biopsy-proven NAFLD and varying levels of fibrosis. Ultra-performance liquid chromatography coupled with tandem mass spectrometry was performed to quantify 38 serum BAs.
Results:
Compared to those without fibrosis, patients with mild fibrosis (stage F1) had significantly higher levels of secondary BAs, and increased diastolic blood pressure (DBP), alanine aminotransferase (ALT), body mass index, and waist circumstance (WC). The combination of serum BAs with WC, DBP, ALT, or Homeostatic Model Assessment for Insulin Resistance performed well in identifying mild fibrosis, in men and women, and in those with/without obesity, with AUROCs 0.80, 0.88, 0.75 and 0.78 in the training set (n = 385), and 0.69, 0.80, 0.61 and 0.69 in the testing set (n = 165), respectively. In comparison, the combination of BAs and clinical/biochemical biomarkers performed less well in identifying significant fibrosis (F2–4). In women and in non-obese subjects, AUROCs were 0.75 and 0.71 in the training set, 0.65 and 0.66 in the validation set, respectively. However, these AUROCs were higher than those observed for the fibrosis-4 index, NAFLD fibrosis score, and Hepamet fibrosis score.
Conclusions:
Secondary BA levels were significantly increased in NAFLD, especially in those with mild fibrosis. The combination of serum BAs and clinical/biochemical biomarkers for identifying mild fibrosis merits further assessment.
1 |. INTRODUCTION
It has been estimated that nonalcoholic fatty liver disease (NAFLD) affects up to a third of the world’s adult population and the global prevalence of NAFLD will increase markedly in the next decade.1,2 NAFLD includes a spectrum of potentially progressive liver conditions, ranging from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) and cirrhosis.3 About 20% of patients with NASH may progress to cirrhosis,4,5 and it has been reported that the severity of liver fibrosis is the strongest histological predictor of liver-related outcomes and mortality in NAFLD.6,7 To date, liver biopsy remains the “gold standard” method for staging fibrosis in NAFLD,8 but this method is invasive, expensive, can cause morbidity and cannot be routinely used for monitoring disease progression or treatment responses in clinical practice.9,10
Dysregulated bile acid (BA) metabolism has been implicated in the pathophysiology of chronic liver diseases, including NAFLD.11,12 Primary BAs are synthesised from cholesterol in the liver. Following their synthesis, BAs are conjugated to an amino acid such as taurine and glycine and secreted into bile, concentrated in the gall bladder, and then released into the intestine after food ingestion.13 BAs carry out their important digestive functions aiding in the absorption of fats and fat-soluble vitamins.14 Besides, primary BAs are transported into the distal small bowel from where they are actively reabsorbed by the gut epithelium and return to the liver via enterohepatic circulation.12 Additionally, BAs pass into the colon and are transformed into secondary BAs by intestinal microbiota through multiple different reactions, including deconjugation, 7α-dehydroxylation, 6α-hydroxylation or epimerization.15 These secondary BAs are also absorbed and diversify the BA pool in the body. During this process, BAs can enter into the systemic circulation, and act as biologically active signalling molecules to regulate glucose and lipid homeostasis,16 mainly through the activation of specific receptors, such as farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5).17 Dysregulated BA homeostasis and impaired BA signalling can lead to liver damage, thereby contributing to the development and progression of NAFLD.18 Hepatic BA accumulation leads to hepatocyte apoptosis, mitochondrial damage and endoplasmic reticulum stress.19 Both conjugated and unconjugated BAs at cholestatic levels lead to a release of multiple proinflammatory cytokines, which activate hepatic stellate cells and induce hepatic fibrogenesis.20 Thus, it is conceivable that modulation of BA synthesis and metabolism could become a valid therapeutic option for NAFLD and its related metabolic diseases.13,21
It is known that the high heterogeneity of NAFLD may result from a complex and multilayered dynamic interaction between different factors, such as sex, obesity, diabetes and other coexisting metabolic disorders,22,23 which are also closely associated with BA synthesis and metabolism. BA synthesis is higher in men than in women with a wider inter-individual variation.24,25 Sex-related differences in BA synthesis and metabolism have been also shown in steatosis, NASH and hepatocellular carcinoma.26 The differential BAs, related gut microbiota and signalling pathways need to be further investigated to better understand their effects on disease heterogeneity.27 Moreover, individuals with lean NAFLD have an obesity-resistant phenotype that could be, at least in part, mediated by higher levels of certain BAs and different gut microbiota composition (with higher amounts of microbes involved in BA metabolism), thus contributing to explain their milder liver disease and more favourable metabolic profiles compared to NAFLD individuals with obesity.28 Distinct signatures of gut microbiome and BAs have been also identified in the stool samples of individuals with lean NAFLD and fibrosis.29 Thus, it is reasonable to assume that a better understanding of BA profiles in different subgroups of NAFLD individuals can also help to better decipher the clinical heterogeneity of NAFLD and to develop more targeted pharmacotherapies for NAFLD and NASH.
Therefore, in a large cohort of Chinese adults with biopsy-confirmed NAFLD and fibrosis, we aimed to examine the differences in a large panel of circulating BA levels in patients with varying levels of liver fibrosis. In addition, we developed and validated prediction models using serum BAs and clinical/biochemical biomarkers, alone or in combination, for the non-invasive identification of mild and significant fibrosis, both in the whole cohort and in different subgroups of patients stratified by sex, and the presence or absence of obesity and metabolic syndrome.
2 |. MATERIALS AND METHODS
2.1 |. Patient recruitment
This is a retrospective analysis of our well-characterised Prospective Epidemic Research Specifically of NASH (PERSONS) cohort. All NAFLD patients in this study were consecutively recruited from 2016 to 2019 at the First Affiliated Hospital of Wenzhou Medical University in Wenzhou (China). The inclusion and exclusion criteria have been described extensively elsewhere.30 Briefly, patients were initially diagnosed with suspected NAFLD based on the presence of imaging-defined hepatic steatosis and/or persistently elevated serum transaminase levels with coexisting metabolic risk factors (such as overweight/obesity, type 2 diabetes, or metabolic syndrome), in the absence of significant alcohol consumption (≥140 g/week in men or ≥70 g/week in women). All these patients underwent a diagnostic liver biopsy. Subsequently, we excluded from the analysis, patients with at least one of the following criteria: (1) patients with chronic liver disease from other etiologies (such as viral hepatitis or autoimmune hepatitis), (2) those chronically treated with drugs potentially inducing steatosis, (3) those with liver cancers or other extrahepatic malignancies, and (4) those with liver fat content <5% on histology. According to these exclusion criteria and the availability of serum samples, 550 Chinese adults with biopsy-proven NAFLD were included in the present study. Overweight/obesity was defined as BMI≥25. Metabolic syndrome was defined as having three or more of the following metabolic risk abnormalities: overweight/obesity, high triglyceride level (≥1.7 mmol/L), reduced HDL cholesterol levels (HDL-c < 1.03 mmol/L for men and <1.29 mmol/L for women), high blood pressure ≥ 130/85 mm Hg, and elevated fasting glucose level (≥5.6 mmol/L) or diagnosed with type 2 diabetes. Written informed consent was obtained from each subject before study participation. The research protocol was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (2016–246, 1 December 2016) and registered in the Chinese Clinical Trial Registry (ChiCTR-EOC-17013562).
2.2 |. Liver histology assessment
An ultrasound-guided liver biopsy was performed using a 16-gauge Hepafix needle (Gallini). All biopsy specimens were analysed by an experienced liver pathologist, who was blinded to participants’ clinical and laboratory data. The histologic features of NAFLD were scored according to the NASH-Clinical Research Network (NASH-CRN) scoring system.31 The stage of fibrosis was quantified according to Brunt’s criteria.32 Mild and significant fibrosis was defined as fibrosis F1 and ≥ F2 on histology, respectively.
2.3 |. Clinical and laboratory parameters
In all participants, demographic characteristics and anthropometric measurements were collected on the day of the liver biopsy examination. Venous blood samples were obtained after overnight fasting for standard laboratory biochemical tests, including serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), bilirubin, albumin, glucose, insulin, lipids, creatinine, uric acid and complete blood count. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to the following formula: fasting insulin (μU/ml) × fasting glucose (mmol/L)/22.5.33 Three commonly used non-invasive fibrosis scores were also calculated using established equations,34–36 including the fibrosis-4 (FIB-4) index, NAFLD fibrosis score (NFS), and Hepamet fibrosis score (HFS).
2.4 |. Serum BA measurements
A 20 μl of serum sample was extracted with 180 μl of acetonitrile/methanol (8:2) containing 10 internal standards in a 96-well plate. The metabolite extraction was centrifuged at 10°C and 3000 rpm for 20 min. After centrifugation, the supernatant was transferred to a 96-well plate tube for lyophilization using a FreeZone freeze dryer equipped with a stopping tray system (Labconco). The supernatant was transferred to a 96-well plate for ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) analysis (ACQUITY UPLC-Xevo TQ-S, Waters Corp.).37 The BA standards were obtained from Steraloids Inc. and TRC Chemicals, and 15 stable isotope-labelled standards were obtained from C/D/N Isotopes Inc. and Steraloids Inc., respectively. Column ACQUITY UPLC Cortecs C18 1.6 μM VanGuard pre-column (2.1 × 5 mm) and ACQUITY UPLC Cortecs C18 1.6 μM analytical column (2.1 × 100 mm) were used. Column temperature and sample manager temperature were 30 and 10°C, respectively. The mobile phases consist of water with formic acid (pH = 3.25) (A) and acetonitrile/methanol (80:20) (B). The flow rate was set as 0.4 ml/min and gradient conditions were as follows: 0–1 min (5% B), 1–3 min (5%–30% B), 3–15 min (30%–100% B), 15–16 min (100%–5% B), 16–17 min (5% B). The source temperature and desolvation temperatures were 150 and 550°C, respectively. Raw data generated by UPLC-MS/MS were processed using the TargetLynx software to perform peak integration, calibration and quantitation for each BA metabolite. Missing values were preprocessed using the quantile regression imputation of left-censored data (QRLIC) method.38 A total of 38 BAs were identified and quantified in serum samples (Table S1). Serum BAs were classified into 8 categories according to their chemical structures, including primary glycine or taurine conjugated BAs, primary unconjugated BAs, secondary glycine or taurine conjugated BAs, secondary unconjugated BAs, sulfated BAs, and glucuronidated BAs, respectively.
2.5 |. Statistical analysis
R software (version 3.6.3, R Foundation for Statistical Computing) was applied for statistical analysis and visualisation. The normal distribution of variables was initially tested using the Shapiro–Wilk test. Then, parametric tests including the Student’s t test and the one-way anova were performed on variables with normal distribution. Meanwhile, non-parametric methods including the Mann–Whitney U test and the Kruskal Wallis test were used for variables that were not normally distributed. The chi-square test was used for categorical variables. Spearman’s rank correlation coefficients were calculated to examine the associations between liver fibrosis, serum BAs, and other clinical and biochemical parameters. Random forest analysis was applied for biomarker selection. Binary logistic regression analysis was then performed to build predictive models for fibrosis. Areas under receiver operating characteristic curves (AUROC) were calculated to evaluate the performance of non-invasive predictive models for identifying mild (F1) or significant fibrosis (F2–4). Two-tailed p < 0.05 value was considered statistically significant. All p values were further adjusted for multiple testing corrections by the Benjamini and Hochberg statistical procedure.
3 |. RESULTS
3.1 |. Clinical and biochemical biomarkers associated with mild and significant fibrosis
The study design is summarised in Figure 1. A total of 550 Chinese adults with biopsy-proven NAFLD were included in the study. They were further divided into six subgroups according to sex, and the presence or absence of obesity (OB) or metabolic syndrome (MetS). They were also divided into patients without fibrosis (stage F0), patients with mild fibrosis (stage F1), and those with significant fibrosis (stages F2–4). Clinical characteristics and BA profiles were then examined at different fibrosis stages for each patient subgroup. Meanwhile, all these 550 patients with NAFLD were randomly subdivided into the training (n = 385) and validation (n = 165) sets, according to a ratio of 7:3,39 to develop and validate predictive models for the non-invasive diagnosis of mild and significant fibrosis.
FIGURE 1.
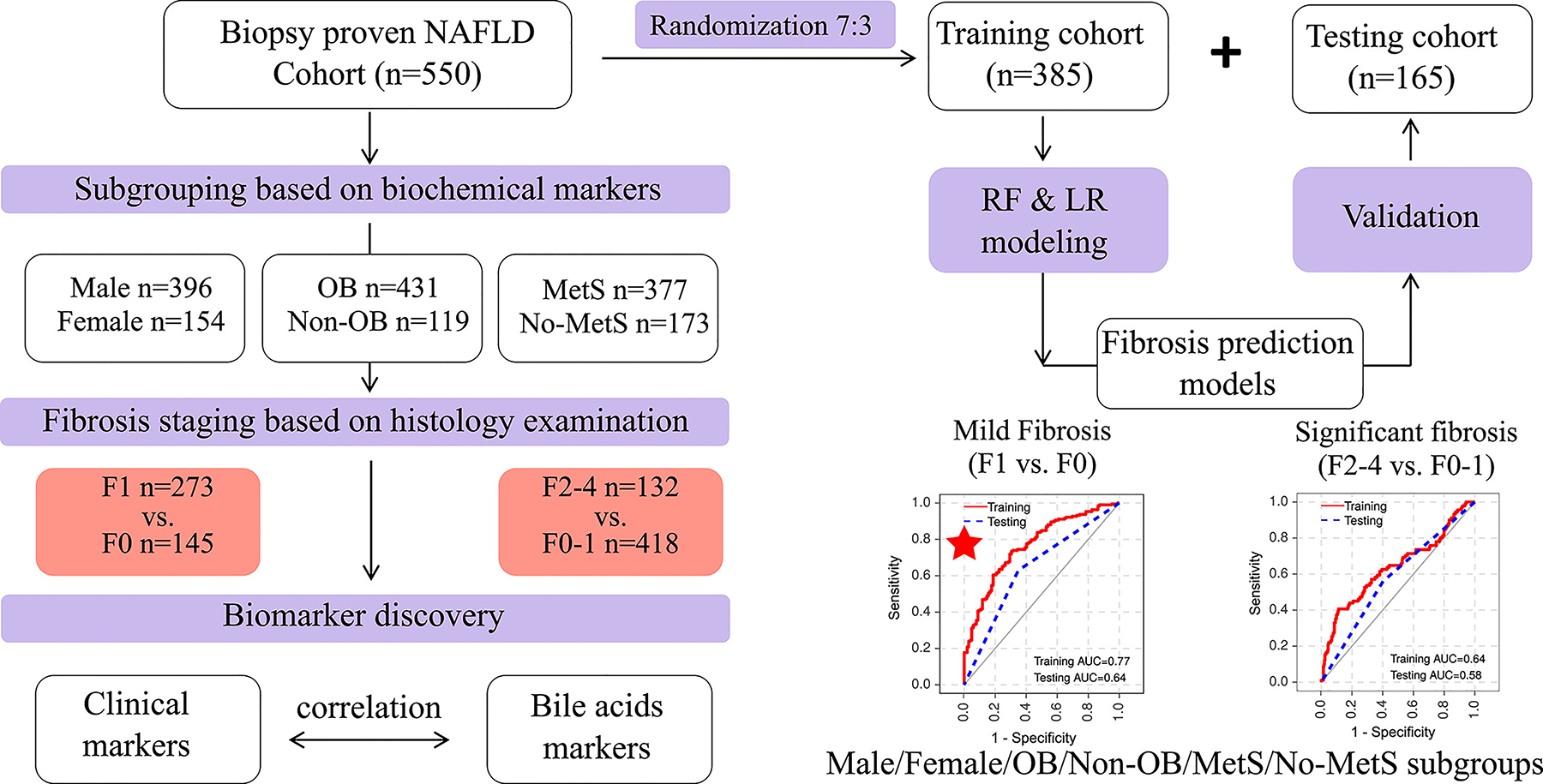
The flowchart of biomarker discovery and validation in patients with biopsy-proven NAFLD.
The main clinical, biochemical and histological characteristics of the whole cohort of NAFLD patients, stratified by increasing fibrosis stages are summarised in Table 1. Adiposity measures, diastolic blood pressure (DBP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), HOMA-IR, haemoglobin A1c (HbA1c), white blood cell count (WBC) and haemoglobin increased significantly across fibrosis stages. Similarly, the histological severity of hepatic steatosis, lobular inflammation, and NAFLD Activity Score (NAS) progressively increased across fibrosis stages. The Hepatic Fibrosis Score (HFS) emerged as the best non-invasive score for staging fibrosis compared to FIB-4 and NFS scores.
TABLE 1.
Demographic, biochemical, and histological characteristics of patients with biopsy-confirmed NAFLD (n = 550), stratified by increasing stages of liver fibrosis
| Characteristics | F0 (n = 145) | F1 (n = 273) | F2–4 (n = 132) | p-value | p-value* |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 42.34 ± 12.26 | 42.82 ± 12.07 | 43.45 ± 13.28 | 0.900 | 0.949 |
| Male sex, n (%) | 104 (72%) | 203 (74%) | 89 (67%) | 0.345 | 0.345 |
| Height (cm) | 167.14 ± 8.44 | 167.63 ± 8.24 | 166.58 ± 9.34 | 0.607 | 0.763 |
| Weight (kg) | 72.11 ± 12.28 | 75.86 ± 13.24 | 76.29 ± 14.89 | 1.66E-02 | 5.00E-02 |
| BMI (kg/m2) | 25.68 ± 2.96 | 26.88 ± 3.61 | 27.4 ± 4.46 | 2.84E-04 | 1.80E-03 |
| WC (cm) | 89.1 ± 7.91 | 92.08 ± 8.42 | 93.75 ± 9.49 | 2.30E-05 | 3.00E-04 |
| SBP (mm Hg) | 125.43 ± 16.16 | 128.83 ± 14.95 | 128.27 ± 16.74 | 0.127 | 0.291 |
| DBP (mm Hg) | 77.68 ± 10.07 | 83.07 ± 9.98 | 81.43 ± 10.82 | 2.37E-06 | 4.60E-05 |
| Obesity, n (%) | 101 (70%) | 219 (80%) | 111 (84%) | 8.25E-03 | 1.40E-02 |
| Type 2 diabetes, n (%) | 51 (35%) | 126 (46%) | 72 (55%) | 4.90E-03 | 1.20E-02 |
| Hypertension, n (%) | 73 (50%) | 178 (65%) | 81 (61%) | 1.22E-02 | 1.50E-02 |
| MetS, n (%) | 80 (55%) | 194 (71%) | 103 (78%) | 1.04E-04 | 5.20E-04 |
| Biochemical parameters | |||||
| ALT (U/L) | 63.9 ± 92.88 | 73.35 ± 61.25 | 73.46 ± 67.85 | 4.11E-03 | 1.80E-02 |
| AST (U/L) | 41.36 ± 44.45 | 45.26 ± 32.7 | 49.6 ± 36.93 | 5.52E-03 | 2.20E-02 |
| ALP (U/L) | 89.06 ± 44.86 | 86.33 ± 27.22 | 85.7 ± 36.78 | 0.806 | 0.916 |
| GGT (U/L) | 71.48 ± 97.04 | 70.55 ± 59.48 | 78.49 ± 100.94 | 0.221 | 0.384 |
| TBIL (μmol/L) | 14.74 ± 12.04 | 14.14 ± 7.09 | 14.04 ± 6.7 | 0.951 | 0.951 |
| DBIL (μmol/L) | 5.83 ± 9.35 | 4.8 ± 2.35 | 4.87 ± 2.2 | 0.332 | 0.498 |
| IBIL (μmol/L) | 8.92 ± 4.44 | 9.34 ± 5.03 | 9.16 ± 4.91 | 0.547 | 0.711 |
| Total protein (g/L) | 76.83 ± 5.42 | 76.85 ± 5.87 | 76.93 ± 5.58 | 0.83 | 0.916 |
| Albumin (g/L) | 45.97 ± 3.85 | 45.79 ± 4.29 | 45.55 ± 4.22 | 0.943 | 0.951 |
| Globulin (g/L) | 31 ± 4.1 | 30.95 ± 3.82 | 31.38 ± 4.15 | 0.473 | 0.659 |
| A/G ratio | 1.51 ± 0.24 | 1.5 ± 0.21 | 1.48 ± 0.26 | 0.469 | 0.659 |
| Glucose (mmol/L) | 5.67 ± 1.86 | 5.82 ± 1.57 | 6.01 ± 1.94 | 0.072 | 0.186 |
| Insulin (pmol/L) | 121.34 ± 125.77 | 125.76 ± 109.18 | 136.71 ± 119.09 | 0.181 | 0.371 |
| HOMA-IR score | 4.94 ± 7.79 | 5.04 ± 6.35 | 5.4 ± 5.5 | 3.62E-02 | 0.101 |
| HbA1c (%) | 6.07 ± 1.75 | 6.25 ± 1.52 | 6.57 ± 1.51 | 6.28E-04 | 3.50E-03 |
| Creatinine (μmol/L) | 67.77 ± 14.53 | 67.73 ± 13.91 | 65.86 ± 15.91 | 0.158 | 0.343 |
| Uric acid (μmol/L) | 384.57 ± 101.22 | 388.24 ± 101.55 | 381.17 ± 111.2 | 0.771 | 0.916 |
| Total cholesterol (mmol/L) | 5.12 ± 1.15 | 5.15 ± 1.17 | 5.15 ± 1.27 | 0.846 | 0.916 |
| Triglycerides (mmol/L) | 2.31 ± 1.69 | 2.21 ± 1.33 | 2.51 ± 2.92 | 0.821 | 0.916 |
| HDL-c (mmol/L) | 1.04 ± 0.24 | 1.01 ± 0.22 | 1 ± 0.24 | 0.296 | 0.482 |
| LDL-c (mmol/L) | 3.1 ± 0.9 | 3.03 ± 0.89 | 2.99 ± 1.02 | 0.499 | 0.671 |
| WBC (×109/L) | 5.92 ± 1.35 | 6.27 ± 1.53 | 6.47 ± 1.76 | 1.40E-02 | 5.00E-02 |
| RBC (×109/L) | 4.96 ± 0.49 | 4.97 ± 0.51 | 4.89 ± 0.56 | 0.226 | 0.384 |
| Hb (g/L) | 146.52 ± 14.63 | 149.53 ± 14.21 | 145.06 ± 16.29 | 1.55E-02 | 5.00E-02 |
| PLT (×109/L) | 248.37 ± 63.88 | 240.21 ± 59.46 | 252.42 ± 66.12 | 0.207 | 0.384 |
| Liver histology features | |||||
| Steatosis, n (%) | |||||
| 0 | 43 (30%) | 42 (15%) | 17 (13%) | 4.26E-05 | 4.10E-04 |
| 1 | 51 (35%) | 85 (31%) | 37 (28%) | ||
| 2 | 19 (13%) | 56 (21%) | 31 (23%) | ||
| 3 | 32 (22%) | 90 (33%) | 47 (36%) | ||
| Ballooning, n (%) | |||||
| 0 | 0:21 (14%) | 64 (23%) | 20 (15%) | 0.203 | 0.384 |
| 1 | 80 (55%) | 134 (49%) | 74 (56%) | ||
| 2 | 44 (30%) | 75 (27%) | 38 (29%) | ||
| Lobular inflammation, n (%) | |||||
| 0 | 19 (13%) | 14 (5%) | 2 (2%) | 2.55E-07 | 1.00E-05 |
| 1 | 112 (77%) | 200 (73%) | 89 (67%) | ||
| 2 | 13 (9%) | 56 (21%) | 37 (28%) | ||
| 3 | 1 (1%) | 3 (1%) | 4 (3%) | ||
| NAS score | 3.41 ± 1.65 | 3.93 ± 1.78 | 4.28 ± 1.59 | 7.72E-05 | 6.00E-04 |
| Non-invasive fibrosis scores | |||||
| FIB-4 | 14.73 ± 6.63 | 14.99 ± 6.86 | 17.33 ± 10.53 | 0.325 | 0.498 |
| NFS | 0.55 ± 1.41 | 0.76 ± 1.38 | 0.95 ± 1.68 | 0.076 | 0.186 |
| HFS | 0.06 ± 0.09 | 0.09 ± 0.12 | 0.12 ± 0.18 | 3.00E-03 | 1.50E-02 |
Note: p-values are assessed by parametric or non-parametric tests among three groups. p < 0.05 is highlighted in bold.
Abbreviations: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; MetS, metabolic syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyltransferase; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; A/G, the ratio of albumin to globulin; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, haemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; WBC, white blood cell count; RBC, red blood cell count; Hb, haemoglobin; PLT, counts of platelet; S, steatosis; B, ballooning; L, lobular inflammation; FIB-4, Fibrosis-4 index; NFS, NAFLD Fibrosis Score; HFS, Hepatic Fibrosis Score.
p-values assessed by the Benjamini–Hochberg procedure after multiple testing corrections.
Baseline clinical and biochemical parameters for discriminating mild and significant fibrosis were further evaluated and compared in the (aforementioned) six patient subgroups. Figure 2A shows the heatmap of their fold-change values by comparing either F1 vs. F0 or F2–4 vs. F0–1, respectively. DBP was increased significantly in most patient subgroups with mild fibrosis (p < 0.001), except for women. Adiposity measures (BMI and WC) were increased in men and OB, MetS patient subgroups with mild and significant fibrosis. Serum liver enzymes (ALT, AST and GGT) were increased in mild fibrosis as compared to F0. HbA1c and WBC were increased in significant fibrosis. The histological severity of steatosis and lobular inflammation increased with increasing fibrosis stages in most patient subgroups (all p < 0.01). Also, NAS was a significant marker for discriminating mild fibrosis in men and women, as well as in OB and MetS patient subgroups. Among the three commonly used non-invasive fibrosis scores, HFS was better than FIB-4 and NFS scores to stage fibrosis. To summarise, twelve differential markers for mild or significant fibrosis that were identified in more than three patient subgroups are reported in Figure 2B, showing their differences among F0, F1, and F2–4 subgroups in the whole cohort.
FIGURE 2.
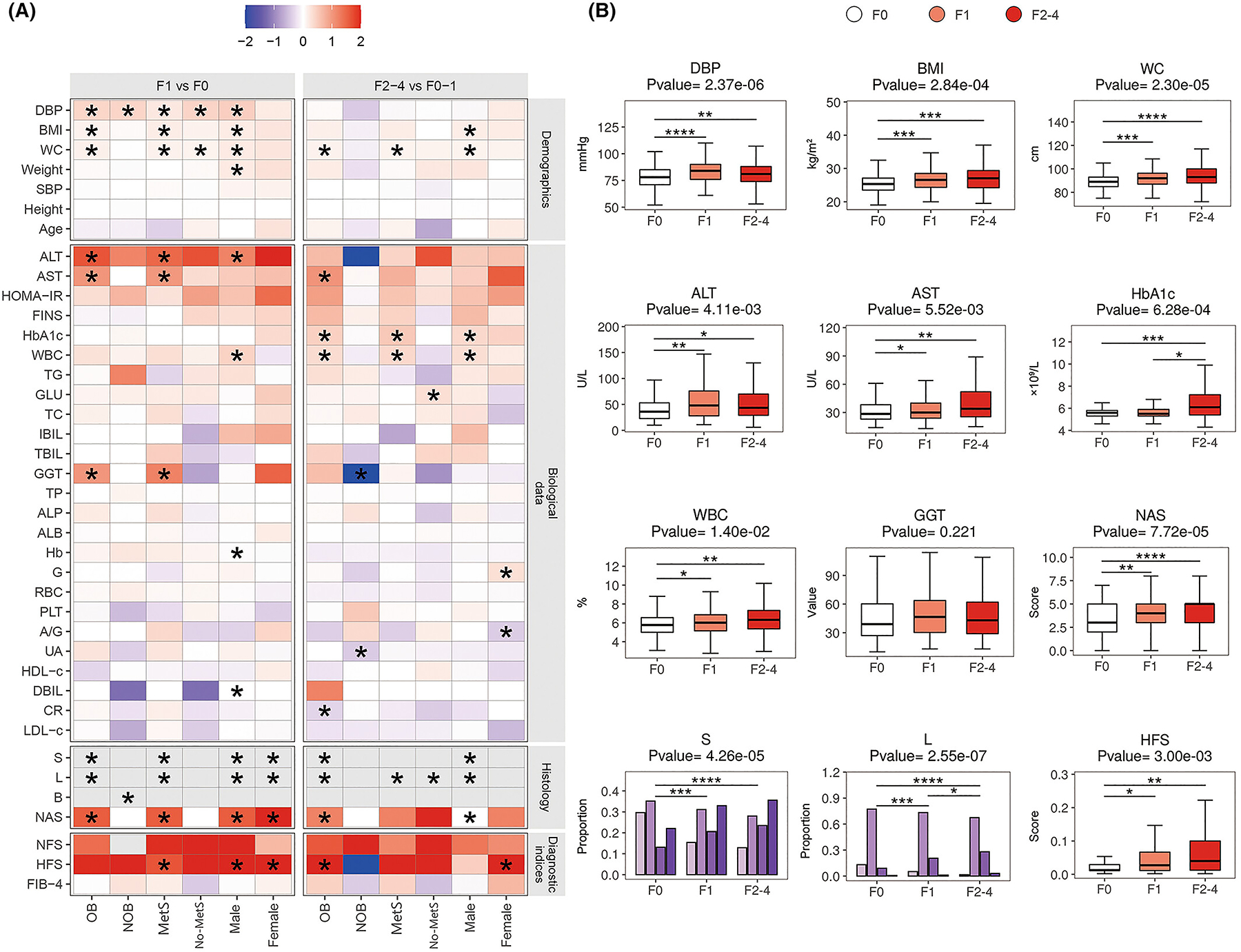
Comparison of clinical parameters in six subgroups of NAFLD patients in the training cohort. (A) The heatmap of fold changes of demographic, biological, and histological variables and non-invasive diagnostic indices between F1 and F0, and between F2–4 and F0–1 in the six patient subgroups of the training cohort. p values were tested by parametric or non-parametric tests (as appropriate). (B) The changes of potential clinical risk factors among NAFLD patients with F0, F1, and F2–4 stages. p < 0.05 is demonstrated as an asterisk (*), p < 0.01 is demonstrated as two asterisks (**), p < 0.001 is demonstrated as three asterisks (***), and p < 0.0001 is demonstrated as four asterisks (****).
3.2 |. Overall BA profiles in NAFLD with mild and significant fibrosis
We found that secondary unconjugated BAs, primary glycine-conjugated BAs, and primary unconjugated BAs covered more than 80% of the BA pool in the serum (Figure 3A). Secondary unconjugated BAs were increased in patients with mild and significant fibrosis. The top eleven abundant BAs were GCDCA, βUDCA, CDCA, CDCA, βCDCA, GCA, DCA, GUDCA, GLCA-3 S, CA and UDCA, respectively (Figure 3B). The chemical names of individual BAs and their classifications are reported in Table S1. The circus plots showed differential BAs (p < 0.05 highlighted in red) and their fold changes by comparing F1 vs. F0 (mild fibrosis, Figure 3C), and F2–4 vs. F0–1 (significant fibrosis, Figure 3D), respectively. TCA and nine secondary BAs were increased in mild fibrosis, while UCA, βUCA, 7-DHCA and NorCA were found to be increased in significant fibrosis.
FIGURE 3.
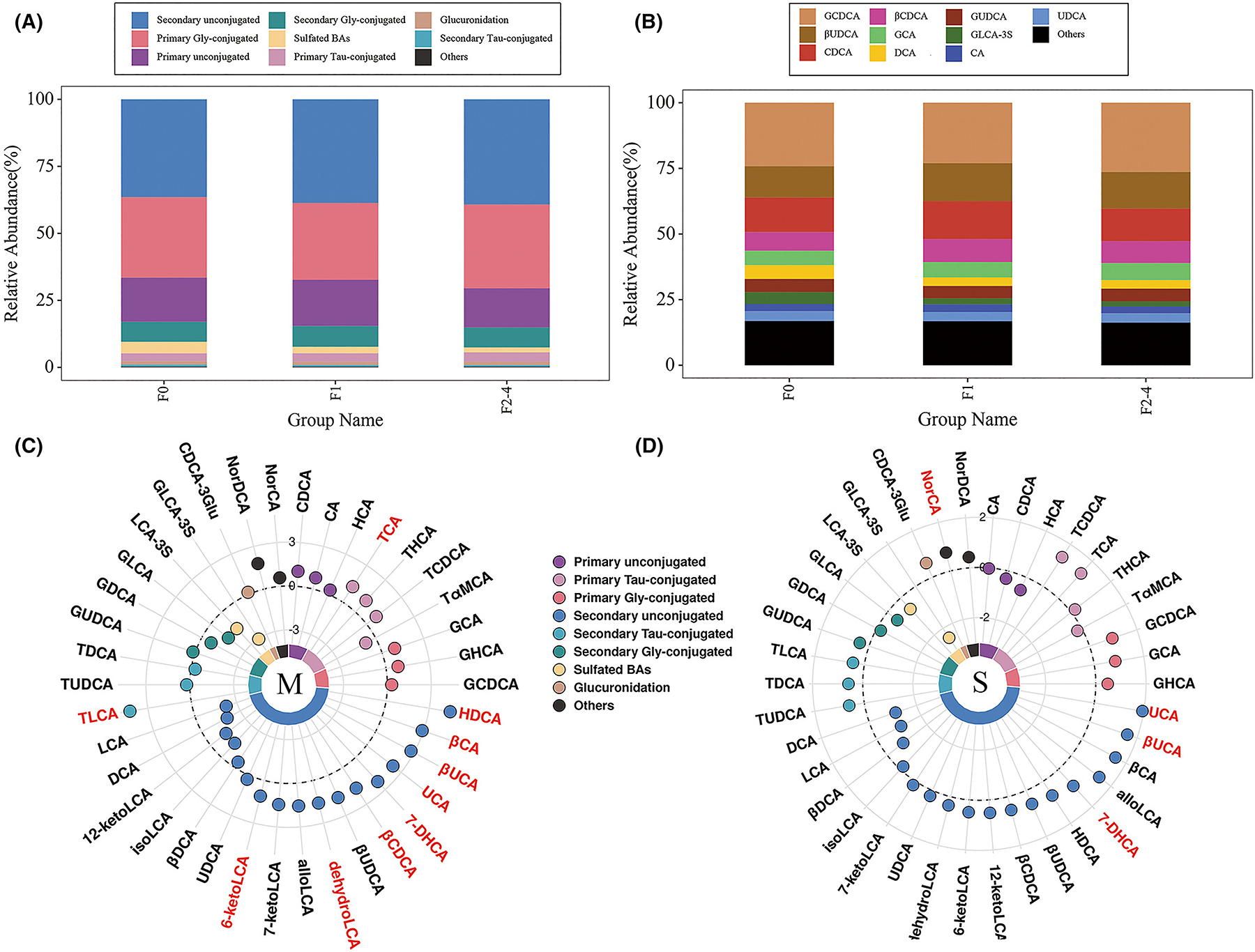
Overall bile acid profiles of patients with NAFLD. (A) The bar plot of relative abundances of different groups of bile acids from F0, F1 to F2–4 stages. (B) The bar plot of relative abundances of top-ten abundant bile acids from F0, F1 to F2–4 stages. (C, D) The circus plot of differential bile acids (fold changes) by comparing F1 vs. F0, and F2–4 vs. F0–1, respectively. The reference circle value is defined as a fold-change value of 1.
3.3 |. BA changes in mild and significant fibrosis in different subgroups
We compared the changes of individual BAs in the presence of mild and significant fibrosis across the six subgroups of NAFLD patients (Figure 4A). In general, BA profiles changed more significantly in those with mild fibrosis than in those with significant fibrosis. Specifically, secondary BAs (HDCA, UCA, βCA, 7-DHCA, βUCA, dehydroLCA, 6-ketoLCA and TLCA) were significantly increased in patients with mild fibrosis (F1), compared to those without fibrosis (F0). However, in the presence of significant fibrosis (F2–4 vs. F0–1), CA and CDCA increased in women, but decreased in both men and the no-MetS subgroup. Three secondary BAs (βCA, 7-DHCA, and βUCA) were increased in women and in the OB and MetS patient subgroups. In the whole cohort of patients, ten serum BAs increased progressively across fibrosis stages (Figure 4B, all p < 0.05).
FIGURE 4.
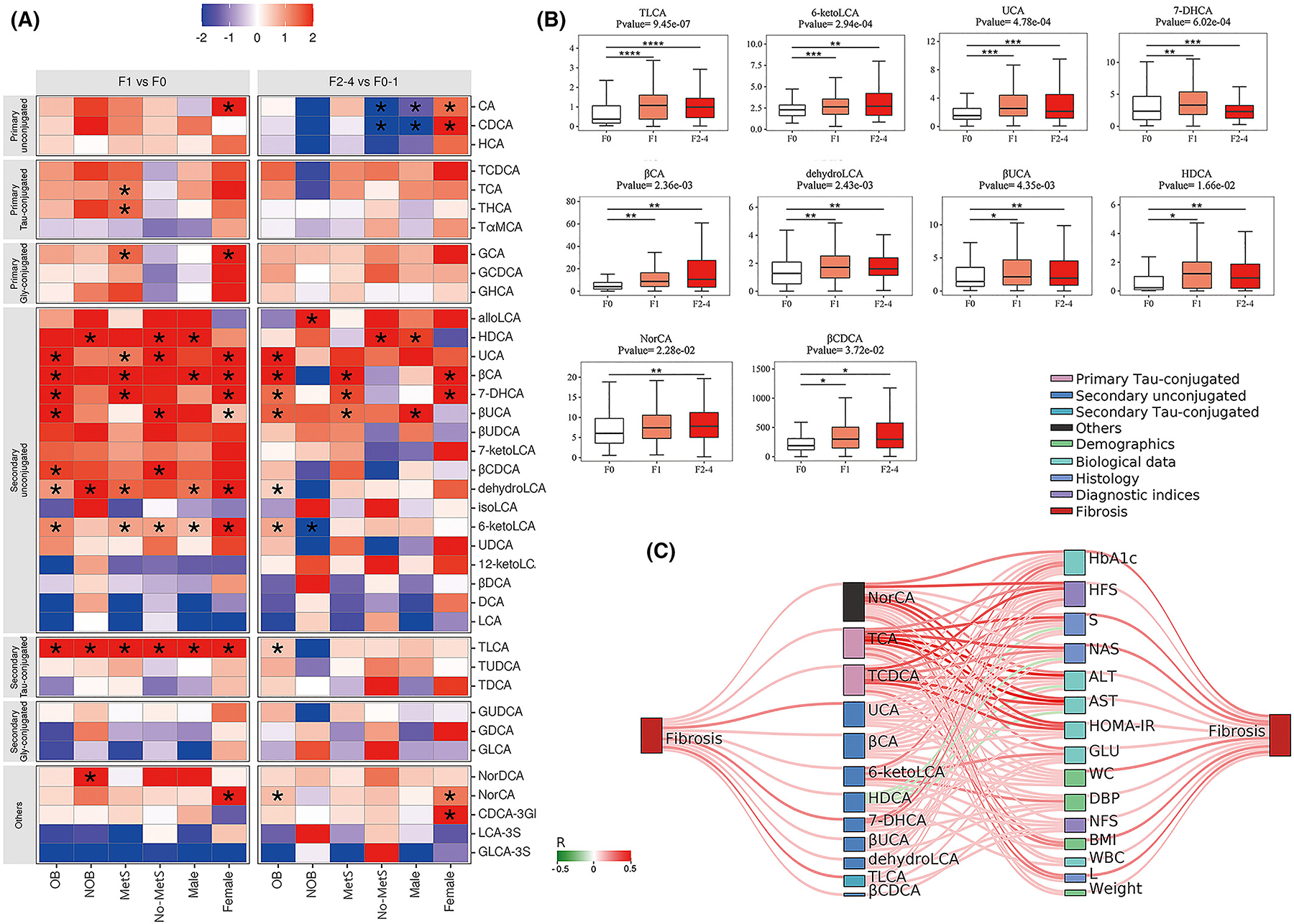
The bile acid changes with the development of fibrosis in six subgroups of NAFLD patients in the training cohort. (A) The heatmap of fold changes of bile acids between F1 and F0, and F2–4 and F0–1 in the six patient subgroups of training cohort. p values were determined by non-parametric tests (as appropriate). (B) The bar plots of differential bile acid biomarkers among patients with F0, F1, and F2–4 fibrosis. p < 0.05 is demonstrated as an asterisk (*), p < 0.01 is demonstrated as two asterisks (**), p < 0.001 is demonstrated as three asterisks (***), and p < 0.0001 is demonstrated as four asterisks (****). (C) The Sankey network of correlations among bile acids, clinical parameters and fibrosis in patients with NAFLD. Bile acids, clinical parameters and liver fibrosis stage are shown as different colours of dots. The connecting lines between dots represent the correlation, with red lines referring to positive correlation, and green lines referring to negative correlation. Also, the width of connecting lines is depending on the correlation coefficients. p values and correlation coefficients were assessed by Spearman’s analysis. Only Spearman’s correlation analyses with a p < 0.05 were depicted.
Spearman’s rank correlation analyses showed that twelve secondary BAs and two primary BAs were significantly associated with liver fibrosis, including NorCA, UCA, βCA, 6-ketoLCA, HDCA, 7-DHCA, βUCA, dehydroLCA, TLCA, βCDCA, TCA and TCDCA (Figure 4C). Meanwhile, six biochemical parameters (HbA1c, fasting glucose, HOMA-IR, ALT, AST and WBC) and four demographic and anthropometric parameters (weight, BMI, WC and DBP) were significantly associated with liver fibrosis, together with HFS, NFS, S and L indices. These aforementioned BAs and clinical/biochemical parameters were ordered by the number of connections between them in the Sankey plot, which indicated that NorCA, TCA and TCDCA were more strongly (darker red colour) associated with clinical/biochemical parameters.
3.4 |. Biomarker discovery and validation for identifying fibrosis severity
Through biomarker selection by random forest analysis and predictive modelling by logistic regression analysis, the combination of serum BAs and clinical/biochemical biomarkers enabled us to obtain optimal non-invasive predictive models for identifying liver fibrosis (Figure 5 and Table 2). Notably, the performance of these non-invasive predictive models differed in the six subgroups of NAFLD patients. In particular, we obtained four predictive models that had good performance in identifying mild fibrosis in men and women, as well as in OB and NOB patient subgroups, with AUROCs of 0.80, 0.88, 0.75, 0.78 in the training set (threshold >0.7), respectively, and 0.69, 0.80, 0.61, 0.69 in the validation set (threshold >0.6), respectively. Among these, the predictive model for identifying mild fibrosis in women was the best one. Accordingly, the predictive model for the whole cohort also achieved a relatively good performance in identifying mild fibrosis with AUROC values of 0.77 in the training set and 0.64 in the validation set, respectively. We also compared three commonly used non-invasive scores of fibrosis, and found that the HFS had the best performance in most of our NAFLD patients, while FIB-4 had the best performance in the no-MetS patient subgroup. However, both of these non-invasive fibrosis scores failed to achieve a good performance for identifying mild fibrosis (AUROC value <0.7 in the training set or <0.6 in the testing set).
FIGURE 5.
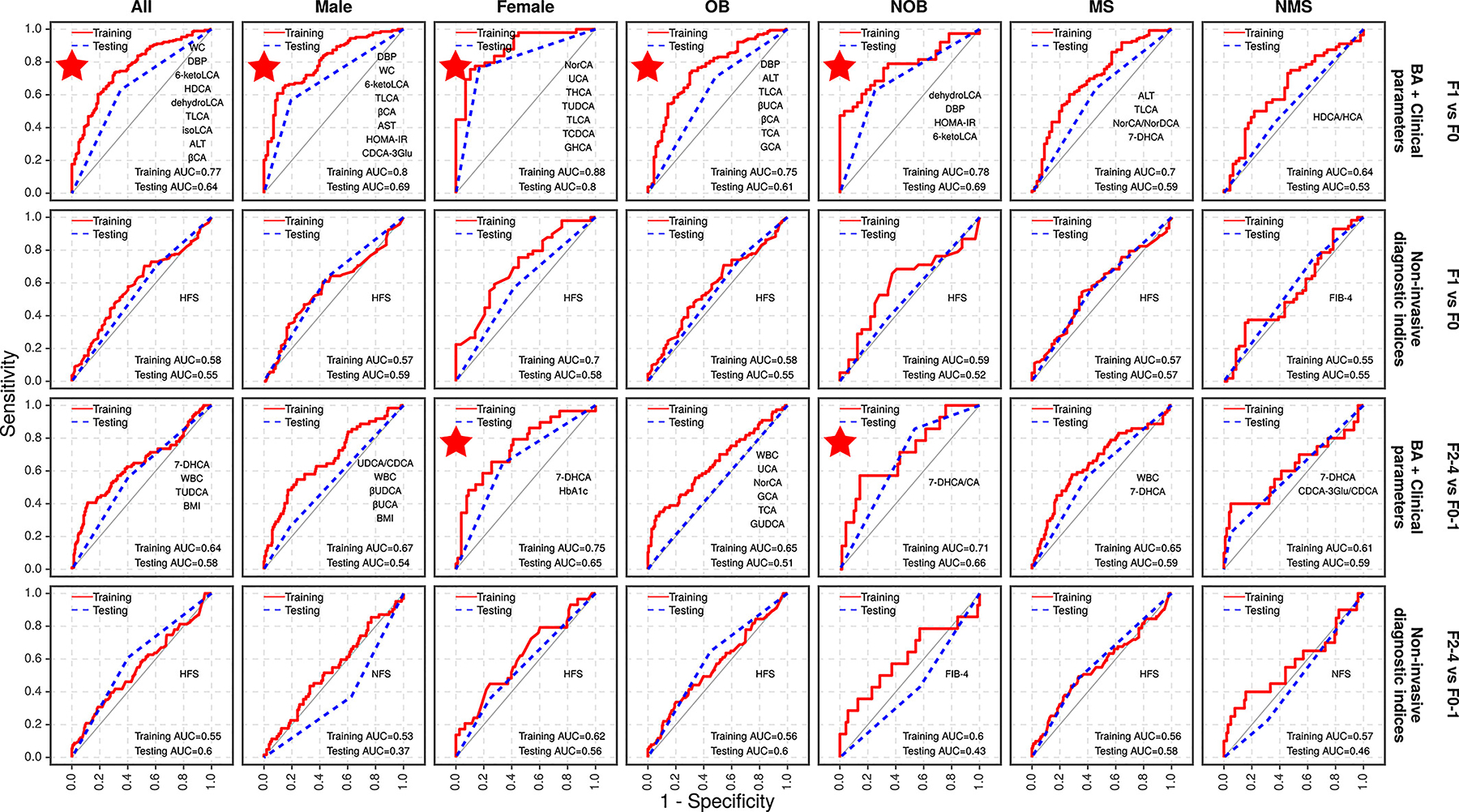
Predictive models for mild and significant liver fibrosis. The figure shows the area under the receiver operating characteristic curves (AUROC) with recommended prediction models with an excellent diagnostic performance marked with a red star.
TABLE 2.
Predictive diagnostic performance of established non-invasive models for different stages of liver fibrosis in the training and testing sets
| Group | Panel | Content | Training AUC | Testing AUC |
|---|---|---|---|---|
| Stage F1 vs. F0 | ||||
| All (n = 550) | BA + Clinical panel | WC + DBP + 6-ketoLCA + HDCA + dehydroLCA + TLCA + isoLCA + ALT + βCA | 0.77 | 0.64 |
| Non-invasive diagnostic index | HFS | 0.58 | 0.55 | |
| Men (n = 396) | BA + Clinical panel | DBP + WC + 6-ketoLCA + TLCA + βCA + AST + HOMA-IR + CDCA-3Glu | 0.80 | 0.69 |
| Non-invasive diagnostic index | HFS | 0.67 | 0.54 | |
| Women (n = 154) | BA panel | NorCA + UCA + THCA + TUDCA + TLCA + TCDCA + GHCA | 0.88 | 0.80 |
| Non-invasive diagnostic index | HFS | 0.75 | 0.65 | |
| Obese (n = 431) | BA + Clinical panel | DBP + ALT + TLCA + βUCA + βCA + TCA + GCA | 0.75 | 0.61 |
| Non-invasive diagnostic index | HFS | 0.65 | 0.51 | |
| Non-obese (n = 119) | BA + Clinical panel | dehydroLCA + DBP + HOMA-IR + 6-ketoLCA | 0.78 | 0.69 |
| Non-invasive diagnostic index | HFS | 0.71 | 0.66 | |
| MetS (n = 377) | BA + Clinical panel | ALT + TLCA + NorCA/NorDCA +7-DHCA | 0.70 | 0.59 |
| Non-invasive diagnostic index | HFS | 0.65 | 0.59 | |
| No-MetS (n = 173) | BA panel | HDCA/HCA | 0.64 | 0.53 |
| Non-invasive diagnostic index | FIB-4 | 0.61 | 0.59 | |
| Stage F2–4 vs. F0–1 | ||||
| All (n = 550) | BA + Clinical panel | 7-DHCA + WBC + TUDCA + BMI | 0.64 | 0.58 |
| Non-invasive diagnostic index | HFS | 0.55 | 0.60 | |
| Men (n = 396) | BA + Clinical panel | UDCA/CDCA + WBC + βUDCA + βUCA + BMI | 0.67 | 0.54 |
| Non-invasive diagnostic index | NFS | 0.53 | 0.37 | |
| Women (n = 154) | BA panel | 7-DHCA + HbA1c | 0.75 | 0.65 |
| Non-invasive diagnostic index | HFS | 0.62 | 0.56 | |
| Obese (n = 431) | BA + Clinical panel | WBC + UCA + NorCA + GCA + TCA + GUDCA | 0.65 | 0.51 |
| Non-invasive diagnostic index | HFS | 0.56 | 0.60 | |
| Non-obese (n = 119) | BA + Clinical panel | 7-DHCA/CA | 0.71 | 0.66 |
| Non-invasive diagnostic index | FIB-4 | 0.60 | 0.43 | |
| MetS (n = 377) | BA + Clinical panel | WBC + 7-DHCA | 0.65 | 0.59 |
| Non-invasive diagnostic index | HFS | 0.56 | 0.58 | |
| No-MetS (n = 173) | BA panel | 7-DHCA + CDCA-3Glu/CDCA | 0.61 | 0.59 |
| Non-invasive diagnostic index | NFS | 0.57 | 0.46 | |
Note: Panel type of predictive models, contents and performances in both the training cohorts and testing cohorts of different prediction models are reported. For the sake of clarity, the recommended prediction models with good performances have been marked in bold.
In comparison, predictive models for identifying significant fibrosis showed overall good performance in women and the NOB subgroup with AUROCs of 0.75 and 0.71 in the training set, and 0.65 and 0.66 in the validation set, respectively (Figure 5 and Table 2). Similar to the above-mentioned predictive models for mild fibrosis, the three commonly used non-invasive scores of fibrosis failed to achieve a good performance for identifying significant fibrosis in both the training and validation sets, with AUROCs <0.60.
4 |. DISCUSSION
The novel findings in this study show that compared to those without fibrosis, patients with NAFLD and mild fibrosis (stage F1) had significantly higher secondary BAs, as well as higher values of DBP, ALT, BMI, and WC. The combination of serum BAs with WC, DBP, ALT or HOMA-estimated insulin resistance performed well in identifying mild fibrosis, especially in men and women, and in those with or without obesity. Conversely, the combination of serum BA and clinical/biochemical biomarkers performed less well in identifying significant fibrosis (F2–4), although predictive models in this fibrosis group performed better in women and non-obese subjects. Importantly, the AUROCs including BAs were significantly higher than those observed for other commonly used non-invasive fibrosis scores, including the fibrosis-4 index, NAFLD fibrosis score, and Hepamet fibrosis score.
4.1 |. BA changes and their associations with liver fibrosis
Increased serum and hepatic BAs have been recognised as important metabolic factors in the pathophysiology of NAFLD and reported to be associated with greater severity of NAFLD and liver fibrosis.29,40–46 However, most previously published studies had a case–control design and compared BA profiles between NAFLD patients and healthy controls or non-NAFLD individuals (Table S2). In the present study, we measured a large panel of circulating BA levels and compared their changes in the presence of mild and significant liver fibrosis among different subgroups of NAFLD patients. We found an altered BA profile in the presence of mild fibrosis that was specifically characterised by increased primary BAs, mainly represented by CA, TCA, and GCA in the female and MetS patient subgroups, and by secondary BAs, mainly HDCA, UCA, CA, 7-DHCA, UCA, dehydroLCA, 6-ketoLCA and TLCA, in the whole patient population (Figure 4A,B). The overall secondary BA profiles were also increased in the presence of significant fibrosis, but their increases as compared to mild fibrosis (F2–4 vs. F0–1) were not as significant and consistent as the increases observed in mild fibrosis (F1 vs. F0). Specifically, CA and CDCA were significantly increased in women but decreased in both men and the no-MetS patient subgroup. Secondary BAs were found to be significantly increased in women and the OB, and MetS patient subgroups. Moreover, those BAs that were closely associated with liver fibrosis were also significantly correlated with serum liver enzymes and glycemic parameters (Figure 4C).
The significant changes in secondary BA metabolism might be causally linked with intestinal dysbiosis and greater severity of liver fibrosis.47–49 In our study, we found that LCA species were significantly increased in patients with mild fibrosis, including TLCA, 6-ketoLCA, and dehydroLCA, and were closely associated with fibrosis severity (Figure 4). LCA species also increased significantly in the OB patient subgroup with significant fibrosis (Figure 4). A previous cross-sectional study of 390 Mexican-American subjects screened with liver elastography also reported that higher serum LCA levels were associated with significant fibrosis.50 Compared to primary BAs, secondary BAs (e.g. DCA and LCA) were more effectively activate TGR5, which is expressed in Kupffer cells and hepatic stellate cells (HSCs).51,52 LCA is considered to be hepatotoxic as the most hydrophobic BA,53 and it has been used to produce a model of cholestatic liver damage.54 Serving as a physiological sensor of LCA, the pregnane X receptor (PXR) could be activated to protect against the LCA-induced liver damage.55 However, experimental data revealed that LCA and its derivatives inhibit HSC activation and have anti-inflammatory and anti-fibrotic effects by inhibiting glycolysis and promoting oxidative phosphorylation, thus leading to macrophage polarisation toward the M2 phenotype.56 To summarize, secondary BAs that are produced and/or modified through gut microbiota, and their enterohepatic circulation and conjugation in the liver, could play a role in the early stage of fibrosis in NAFLD.
4.2 |. Predictive models combining clinical and BA markers for fibrosis severity
Several non-invasive fibrosis scores, such as FIB-4, NFS and HFS scores, have been regarded as sufficiently reliable biomarkers for ruling out advanced fibrosis in NAFLD.34–36 In our study, HFS was better than FIB-4 and NFS to identify mild and significant fibrosis, but in any case, HFS did not achieve a good performance in identifying fibrosis (AUROC < 0.6) in most subgroups of our NAFLD patients. The diagnostic performance of these three commonly used non-invasive fibrosis scores might vary with ethnicity, age, sex, disease severity, comorbidities, and treatment of patients.57,58 Here, the integration of machine learning and logistic regression analyses allowed us to build non-invasive predictive models for identifying mild and significant fibrosis by combining clinical/biochemical and BA biomarkers. DBP, ALT, AST, HOMA-IR, fasting insulin, WBC and HbA1c were closely associated with the severity of liver fibrosis, together with WC and BMI (Table 1, Figures 2 and 4). The predictive model by combining WC, DBP and ALT with 6-ketoLCA, HDCA, dehydroLCA, TLCA, isoLCA, and βCA performed well in identifying mild fibrosis in our whole patient population (Figure 5 and Table 2). WBC and BMI were the two biochemical biomarkers selected for predicting significant fibrosis with 7-DHCA and TUDCA.
4.3 |. Clinical heterogeneity of NAFLD
A spectrum of variables, including sex, obesity and metabolic disorders, may contribute to the high heterogeneity of NAFLD observed in clinical practice.59 Thus, more accurate and refined characterisation and stratification of this common liver disease are needed for precision medicine in NAFLD.60,61 Applying a targeted quantitative metabolomics approach and integrating clinical biomarkers can help us to better define the specific metabolic features and biochemical snapshots among different patient subgroups that might contribute to precision medicine in NAFLD. In our study, increased serum aminotransferase levels and impaired glycemic control were associated with increased liver fibrosis, particularly in men and in the OB and MetS patient subgroups (Figure 2). In contrast, these associations were weaker in women and the NOB and no-MetS patient subgroups. These results are consistent with the existence of sex-related differences in NAFLD, supporting that men are at higher risk of visceral adiposity and MetS.62,63 Meanwhile, we identified that BAs were closely associated with mild and significant fibrosis in women and the OB and MetS patient subgroups (Figure 4A). The non-invasive predictive models combining clinical markers and serum BAs showed good performance in identifying mild fibrosis in both sexes and in the OB/NOB patient subgroups, but not so good in the MetS/no-MetS patient subgroups. For identifying significant fibrosis, we found that the predictive model performed better in women and in the NOB patient subgroup. Thus, these results suggest that the effect of dysregulated BA metabolism on hepatic fibrogenesis in NAFLD might play a differential role in different patient subgroups. It is, therefore, of great clinical importance to consider disease heterogeneity for exploring diagnostic and prognostic biomarkers of liver fibrosis in NAFLD.
Taken together, this is one of the largest cross-sectional cohorts of Chinese adults with biopsy-proven NAFLD focusing on the biomarker discovery for mild and significant liver fibrosis. The changes of secondary BAs observed in mild fibrosis, instead of in significant fibrosis, suggest an important role of these molecules in the early development of liver fibrosis in NAFLD. Different from some previous case–control studies, the exploratory subgroup analyses helped us to identify the specific metabolic features for different NAFLD patient subgroups. However, compared to the total number of participants in this study, the sample size was reduced accordingly due to subgroup stratification, thus future studies are needed to verify our findings from each subgroup. Moreover, more research is needed to effectively evaluate whether BA profiles may significantly differ between NAFLD and chronic liver diseases from other etiologies. It is also valuable to elucidate whether different etiologies of chronic liver disease may differentially affect BA pools for any given stage of fibrosis. Finally, gut microbiota composition in relation to BA metabolism and their complex cross-talk are future priorities in the NAFLD research arena.
5 |. CONCLUSION
This large cross-sectional study show that the circulating levels of secondary BAs (including LCA species) were biomarkers for predicting mild liver fibrosis in Chinese adults with biopsy-proven NAFLD. In addition, the combination of serum BAs and clinical/biochemical biomarkers had good performance in identifying liver fibrosis in NAFLD. Our newly developed predictive models achieved a better diagnostic performance in identifying mild fibrosis than significant fibrosis. We suggest further studies in different ethnic populations are now required to validate our findings.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank all the investigators who contributed to this study.
FUNDING INFORMATION
This work was funded by the National Natural Science Foundation of China (81900510, 82070588, 82170583), and the National Key Research and Development Program of China (2021YFC2701904). GT is supported in part by grants from the School of Medicine, University of Verona, Verona, Italy. CDB is supported in part by the Southampton NIHR (National Institute for Health and Care Research) Biomedical Research Centre (IS-BRC-20004), UK. Vincent Wong is supported in part by a Direct Grant from the Chinese University of Hong Kong (2020.045). RL receives funding support from National Center for Advancing Translational Sciences (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and NIAAA (U01AA029019).
Funding information
National Natural Science Foundation of China, Grant/Award Number: 82170583, 81900510, 82070588 and 82000690; National Key Research and Development Program of China, Grant/Award Number: 2021YFC2701904; National Center for Advancing Translational Sciences, Grant/Award Number: 5UL1TR001442; School of Medicine University of Verona, Verona, Italy; Southampton NIHR (National Institute for Health and Care Research) Biomedical Research Centre, Grant/Award Number: IS-BRC-20004; Chinese University of Hong Kong, Grant/Award Number: 2020.045; NIDDK, Grant/Award Number: P30DK120515, R01DK124318, R01DK121378, R01DK106419, U01DK130190 and U01DK061734; NHLBI, Grant/Award Number: P01HL147835; NIAAA, Grant/Award Number: U01AA029019
CONFLIC T OF INTEREST
Vincent Wai-Sun Wong served as a speaker and/or consultant for Echosens. Mingming Su is employed by Shanghai Keyi Biotech., Shanghai, China. Other authors have no conflicts of interest. RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
Footnotes
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–25. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. [DOI] [PubMed] [Google Scholar]
- 4.Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224–9. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654.e1–9. quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 7.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–40. [DOI] [PubMed] [Google Scholar]
- 9.Rios RS, Zheng KI, Targher G, Byrne CD, Zheng MH. Non-invasive fibrosis assessment in non-alcoholic fatty liver disease. Chin Med J (Engl). 2020;133:2743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: past, present and future. J Hepatol. 2022;76:1362–78. [DOI] [PubMed] [Google Scholar]
- 11.Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694.e3. [DOI] [PubMed] [Google Scholar]
- 12.Farooqui N, Elhence A, Shalimar. A current understanding of bile acids in chronic liver disease. J Clin Exp Hepatol. 2022;12:155–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93. [DOI] [PubMed] [Google Scholar]
- 14.Perino A, Schoonjans K. Metabolic messengers: bile acids. Nat Metab. 2022;4:416–23. [DOI] [PubMed] [Google Scholar]
- 15.Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat Rev Endocrinol. 2019;15:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs CD, Trauner M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol. 2022;19:432–50. [DOI] [PubMed] [Google Scholar]
- 18.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–26. [DOI] [PubMed] [Google Scholar]
- 20.Svegliati-Baroni G, Ridolfi F, Hannivoort R, Saccomanno S, Homan M, De Minicis S, et al. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology. 2005;128:1042–55. [DOI] [PubMed] [Google Scholar]
- 21.Jiao TY, Ma YD, Guo XZ, Ye YF, Xie C. Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease. Acta Pharmacol Sin. 2022;43:1103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrese M, Arab JP, Barrera F, Kaufmann B, Valenti L, Feldstein AE. Insights into nonalcoholic fatty-liver disease heterogeneity. Semin Liver Dis. 2021;41:421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki A, Diehl AM. Nonalcoholic steatohepatitis. Annu Rev Med. 2017;68:85–98. [DOI] [PubMed] [Google Scholar]
- 24.Galman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Intern Med. 2011;270:580–8. [DOI] [PubMed] [Google Scholar]
- 25.Xiang X, Backman JT, Neuvonen PJ, Niemi M. Gender, but not CYP7A1 or SLCO1B1 polymorphism, affects the fasting plasma concentrations of bile acids in human beings. Basic Clin Pharmacol Toxicol. 2012;110:245–52. [DOI] [PubMed] [Google Scholar]
- 26.Xie G, Wang X, Zhao A, Yan J, Chen W, Jiang R, et al. Sex-dependent effects on gut microbiota regulate hepatic carcinogenic outcomes. Sci Rep. 2017;7:45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang JYL. Linking sex differences in non-alcoholic fatty liver disease to bile acid signaling, gut microbiota, and high fat diet. Am J Pathol. 2017;187:1658–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71:1213–27. [DOI] [PubMed] [Google Scholar]
- 29.Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11:4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu WY, Zheng KI, Pan XY, Ma HL, Zhu PW, Wu XX, et al. Effect of PNPLA3 polymorphism on diagnostic performance of various noninvasive markers for diagnosing and staging nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:1057–64. [DOI] [PubMed] [Google Scholar]
- 31.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 32.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 34.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 35.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- 36.Ampuero J, Pais R, Aller R, Gallego-Durán R, Crespo J, García-Monzón C, et al. Development and validation of hepamet fibrosis scoring system-a simple, noninvasive test to identify patients with nonalcoholic fatty liver disease with advanced fibrosis. Clin Gastroenterol Hepatol. 2020;18:216–225.e5. [DOI] [PubMed] [Google Scholar]
- 37.Yin S, Su M, Xie G, Li X, Wei R, Liu C, et al. Factors affecting separation and detection of bile acids by liquid chromatography coupled with mass spectrometry in negative mode. Anal Bioanal Chem. 2017;409:5533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei R, Wang J, Su M, Jia E, Chen S, Chen T, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep. 2018;8:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen QH, Ly HB, Ho LS, Al-Ansari N, Le HV, Tran VQ, et al. Influence of data splitting on performance of machine learning models in prediction of shear strength of soil. Math Probl Eng. 2021;2021:1–5. [Google Scholar]
- 40.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–91. [DOI] [PubMed] [Google Scholar]
- 41.Caussy C, Hsu C, Singh S, Bassirian S, Kolar J, Faulkner C, et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther. 2019;49:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sydor S, Best J, Messerschmidt I, Manka P, Vilchez-Vargas R, Brodesser S, et al. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC. Clin Transl Gastroenterol. 2020;11:e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Zheng M, Liu J, Luo Y, Yang W, Yang J, et al. Ratio of conjugated chenodeoxycholic to muricholic acids is associated with severity of nonalcoholic steatohepatitis. Obesity (Silver Spring). 2019;27:2055–66. [DOI] [PubMed] [Google Scholar]
- 46.Chen T, Zhou K, Sun T, Sang C, Jia W, Xie G. Altered bile acid glycine: taurine ratio in the progression of chronic liver disease. J Gastroenterol Hepatol. 2022;37:208–15. [DOI] [PubMed] [Google Scholar]
- 47.Ji Y, Yin Y, Sun L, Zhang W. The molecular and mechanistic insights based on gut-liver axis: nutritional target for non-alcoholic fatty liver disease (NAFLD) improvement. Int J Mol Sci. 2020;21:3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu H, Lin A, Kong M, Yao X, Yin M, Xia H, et al. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J Gastroenterol. 2020;55:142–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park E, Jeong JJ, Won SM, Sharma SP, Gebru YA, Ganesan R, et al. Gut microbiota-related cellular and molecular mechanisms in the progression of nonalcoholic fatty liver disease. Cell. 2021;10:2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwan SY, Jiao J, Qi J, Wang Y, Wei P, McCormick JB, et al. Bile acid changes associated with liver fibrosis and steatosis in the Mexican-American population of South Texas. Hepatol Commun. 2020;4:555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. [DOI] [PubMed] [Google Scholar]
- 52.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiang JYL, Ferrell JM. Bile acids as metabolic regulators and nutrient sensors. Annu Rev Nutr. 2019;39:175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q, Song GC, Weng FY, Zou B, Jin JY, Yan DM, et al. Hepatoprotective effects of glycyrrhetinic acid on lithocholic acid-induced cholestatic liver injury through choleretic and anti-inflammatory mechanisms. Front Pharmacol. 2022;13:881231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98(6):3369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao J, Ge T, Tang C, Wang G, Pang L, Chen Z. Synergistic anti-inflammatory effect of gut microbiota and lithocholic acid on liver fibrosis. Inflamm Res. 2022;71:1389–401. [DOI] [PubMed] [Google Scholar]
- 57.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lonardo A, Arab JP, Arrese M. Perspectives on precision medicine approaches to NAFLD diagnosis and management. Adv Ther. 2021;38:2130–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lonardo A. Precision medicine in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2022;37:1175–8. [DOI] [PubMed] [Google Scholar]
- 61.Lonardo A, Byrne CD, Targher G. Precision medicine approaches in metabolic disorders and target organ damage: where are we now, and where are we going? Metab Target Organ Damage. 2021;1(3):1–14. [Google Scholar]
- 62.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


