Abstract
BACKGROUND & AIMS:
Alcohol is one of the leading causes of hepatocellular carcinoma (HCC). However, pooled estimates of HCC incidence in alcohol-associated cirrhosis have not been evaluated systematically. We performed a pooled analysis of time-to-event data to provide robust estimates for the incidence of HCC in alcohol-associated cirrhosis.
METHODS:
Medline, Embase, Cochrane Central Register, Scopus, and Web of Science were searched from inception to August 2021. Individual patient data were reconstructed from published Kaplan–Meier curves, and a pooled analysis of cumulative HCC incidence was performed using a random-effects model.
RESULTS:
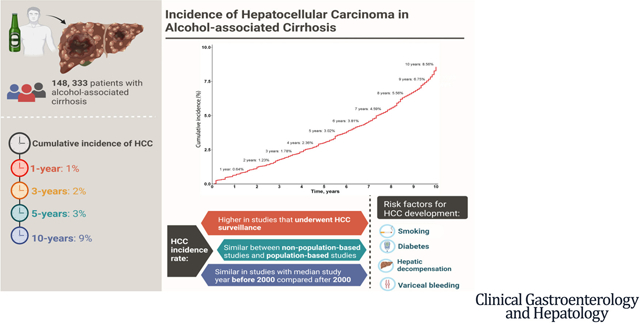
We screened 5022 articles and included 18 studies (148,333 patients). In the pooled analysis, the cumulative incidence of HCC in alcohol-associated cirrhosis at 1, 5, and 10 years among studies that accounted for the competing risk of death without HCC was 1%, 3%, and 9%, respectively. A secondary analysis by traditional meta-analysis determined that the HCC incidence rate was higher in cohorts enrolled in a HCC surveillance program (18.6 vs 4.8 per 1000 person-years; P [ .001) vs those who were not enrolled in a surveillance program. Meta-regression showed that diabetes, smoking, variceal bleeding, and hepatic decompensation were associated with a higher risk of HCC.
CONCLUSIONS:
Our analysis determined that the 5- and 10- year cumulative risk of HCC in alcohol-associated cirrhosis was 3% and 9%, respectively, with a higher incidence in cohorts that were enrolled in a HCC surveillance program. These data should be validated further in large prospective studies, and may have important implications for HCC screening and surveillance among patients with alcohol-associated cirrhosis.
Keywords: Alcohol, Cirrhosis, Hepatocellular Carcinoma, Incidence
Graphical Abstract
Alcohol is one of the leading causes of liver diseases worldwide.1 Alcohol use is associated with a wide spectrum of liver injuries ranging from steatosis, alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC).1–7 HCC is now the third leading cause of cancer deaths worldwide.8 In 2019, an estimated 19% of deaths from HCC were secondary to alcohol.9,10 The age-adjusted death rates resulting from alcohol-associated HCC are increasing in parallel with the increasing global per-capita alcohol consumption, in contrast to hepatitis B virus– and hepatitis C virus (HCV)-associated HCC, which have decreasing death rates.9,11–13
The reported incidence of alcohol-associated HCC varies widely, with estimates of annual incidence ranging from 0.6% to 5.6%.14–16 The wide range in HCC incidence may be owing to varying study settings, time periods, and adherence to HCC surveillance. A systematic review of the incidence of HCC among patients with alcohol-associated cirrhosis has not been reported. Therefore, through a systematic review and meta-analytic approach, we provide estimates for the incidence of HCC among patients with alcohol-associated cirrhosis.
We performed the primary analysis by reconstructing individual patient data from published Kaplan–Meier curves to provide robust estimates of time-to-event data to account for censoring of events. We performed a secondary analysis by traditional meta-analysis methods to evaluate the incidence rate of HCC per 1000 person-years, overall and in subgroup analyses for study setting, enrollment into a HCC surveillance program, and time period.
Methods
Search Strategy and Inclusion Criteria
With reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines,17 a medical librarian (D.G.) searched the literature for articles relating to the incidence of HCC among patients with alcohol-associated cirrhosis. The search strategies were created using a mix of keywords and standardized index terms. Key search terms included “alcohol,” “liver cirrhosis,” “liver cancer,” and “hepatocellular carcinoma.” The full search strategy can be found on page 4 of the Supplementary file. The search was performed on August 25, 2021, in the Cochrane Central Register of Controlled Trials, Embase, Medline, Scopus, and the Web of Science Core Collection. A total of 8545 citations were retrieved. Duplicates were removed in EndNote following the Bramer et al18 method, leaving 5202 citations. Manual screening of the references in the included articles also was conducted for a comprehensive search.
Study Selection
Two authors independently screened titles and abstracts, with subsequent full-text review, for eligibility for inclusion. Discrepancies were resolved by discussion or through review by a third independent author. The primary outcome of interest was the incidence of HCC among patients with alcohol-associated cirrhosis. Retrospective and prospective cohort studies were considered for inclusion, and no date filter was applied. Studies were included if they were performed in adult (age, ≥18 y) patients with alcohol-associated cirrhosis and provided data for the number of incident HCC cases over time.
Studies that were cross-sectional, case–control studies, editorials, systematic reviews, meta-analyses, commentaries, or that included patients with concomitant viral hepatitis were excluded. Studies that included other histologies of liver cancer apart from HCC, such as cholangiocarcinoma, were excluded. When multiple studies provided data for HCC incidence from overlapping cohorts, priority was given to studies that provided time-to-event data for reconstruction of individual patient data, or the most updated/larger cohort.16,19 The majority of the studies from the initial search were excluded at the title and abstract screening stage (Figure 1) because they were not relevant to the aims of the current meta-analysis, or had a study design that did not allow for the evaluation of HCC incidence.
Figure 1.
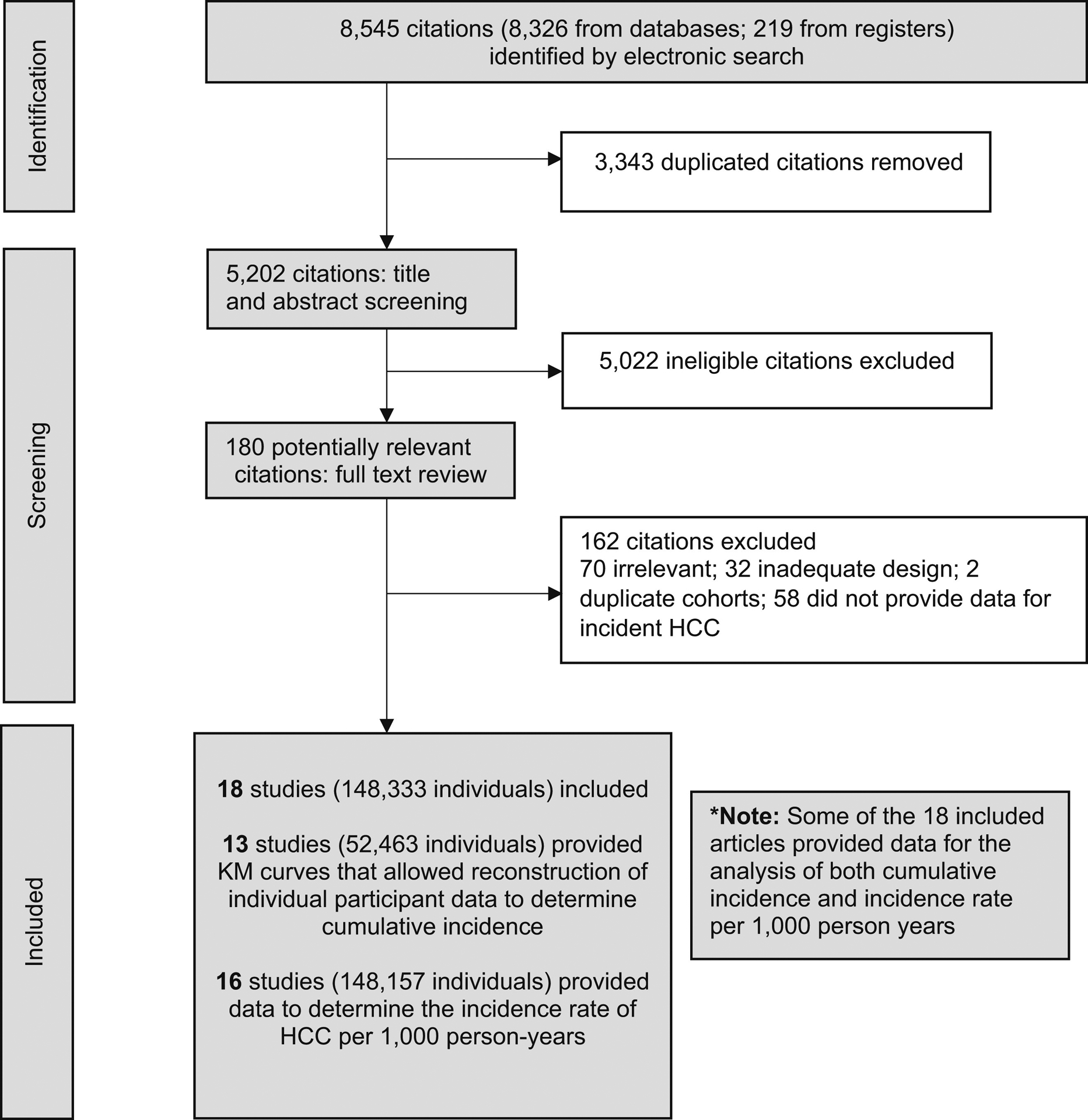
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of included articles. HCC, hepatocellular carcinoma.
Data Extraction
Relevant data from each included article were extracted by a pair of independent authors on a standardized data collection form. Study characteristics, patient demographics, and incident HCC cases were obtained from the included studies.
Quality Assessment
Quality assessment of included studies was performed using scales developed for this review based on the Newcastle–Ottawa scale for retrospective studies.20
Statistical Analysis
The incidence of HCC among patients with alcohol-associated cirrhosis was reported in terms of cumulative incidence and the incidence rate per 1000 person years. The main analysis to determine cumulative incidence was performed among studies that accounted for competing risks of death and liver transplantation, by reconstructing individual patient data from published Kaplan–Meier curves from inverted Kaplan–Meier product-limit equations by Guyot et al.21,22 The method proposed by Guyot et al21 has been widely used in the extraction of individual patient data from Kaplan–Meier curves.23–25 A pooled analysis then was conducted using the MetaSurv package (Divat, Nantes, France), in which arcsine-transformed survival probabilities were aggregated using a random-effects model.26,27 Subgroup/ sensitivity analyses were conducted based on enrollment into a HCC surveillance program (defined as imaging of the liver at least every 6 months with or without α-fetoprotein for the purpose of HCC screening), study setting (population-based vs non–population-based), biopsy-proven cirrhosis, and time period (study median year before the year 2000 vs 2000 and beyond). We could not perform a direct statistical comparison between subgroups using reconstructed individual patient data because data from each subgroup were extracted from separate studies. Therefore, a secondary analysis was performed using a generalized linear mixed model with Clopper–Pearson28,29 intervals to compare between subgroups in terms of incidence rate per 1000 person-years. Statistical heterogeneity was assessed via I2 and the Cochran Q test values, where an I2 value of >40% or a Cochran Q test with a P value ≤.10 was considered heterogeneous.30,31 Currently, there is a lack of an appropriate heterogeneity tool in single-arm meta-analysis32 and I2 can prove an inadequate tool in the assessment of heterogeneity for meta-analysis of proportions, with previous analysis usually exceeding >90%.33,34 In addition, a random-effects meta-regression was conducted to identify baseline characteristics associated with the incidence of HCC. Publication bias was not conducted in the absence of an appropriate tool for the measure of publication bias in a single-arm analysis.35 All statistical analyses were conducted in RStudio (version 4.0.3) and a P value <.05 was considered as the threshold for statistical significance.
Results
Summary of Included Articles
A systematic search of the literature using the previously mentioned search strategy yielded 5202 articles after removal of duplicates. After 5022 articles were excluded after review of the study title and abstract, 180 articles were selected for full-text review, of which 18 articles met the final inclusion criteria (Figure 1). In total, 4 studies were from Japan; 3 studies each were from Spain, Sweden, and the United States; and 1 study each was conducted in Belgium, Denmark, France, and the United Kingdom. In addition, there was 1 multicenter study that included study sites from both France and Belgium. A total of 148,333 patients with alcohol-associated cirrhosis, providing a total of 1,162,744.33 patient-years of follow-up evaluation, were included for analysis. Supplementary Table 1 summarizes the characteristics of included studies, Supplementary Table 2 summarizes patient characteristics, and Supplementary Table 3 provides the quality assessment for the included articles. The mean age of the patients in the included studies ranged from 48.7 to 60.3 years, while the proportion of males ranged from 54% to 100% (Supplementary Table 2). Included studies were assessed to have a low (n = 16) or moderate (n = 2) risk of bias based on the Newcastle–Ottawa scale appraisal tool.
Cumulative Incidence of Hepatocellular Carcinoma in Alcohol-Associated Cirrhosis
Pooled analysis including studies with time-to-event data that accounted for competing risk of death without HCC (4 studies, 4084 patients)36–39 determined that the cumulative incidence of HCC at 1, 3, 5, and 10 years was 0.64% (95% CI, 0.06–1.22), 1.78% (95% CI, 0.29–3.23), 3.02% (95% CI, 0.57–5.35), and 8.56% (95% CI, 4.00–12.66), respectively (Figure 2).
Figure 2.
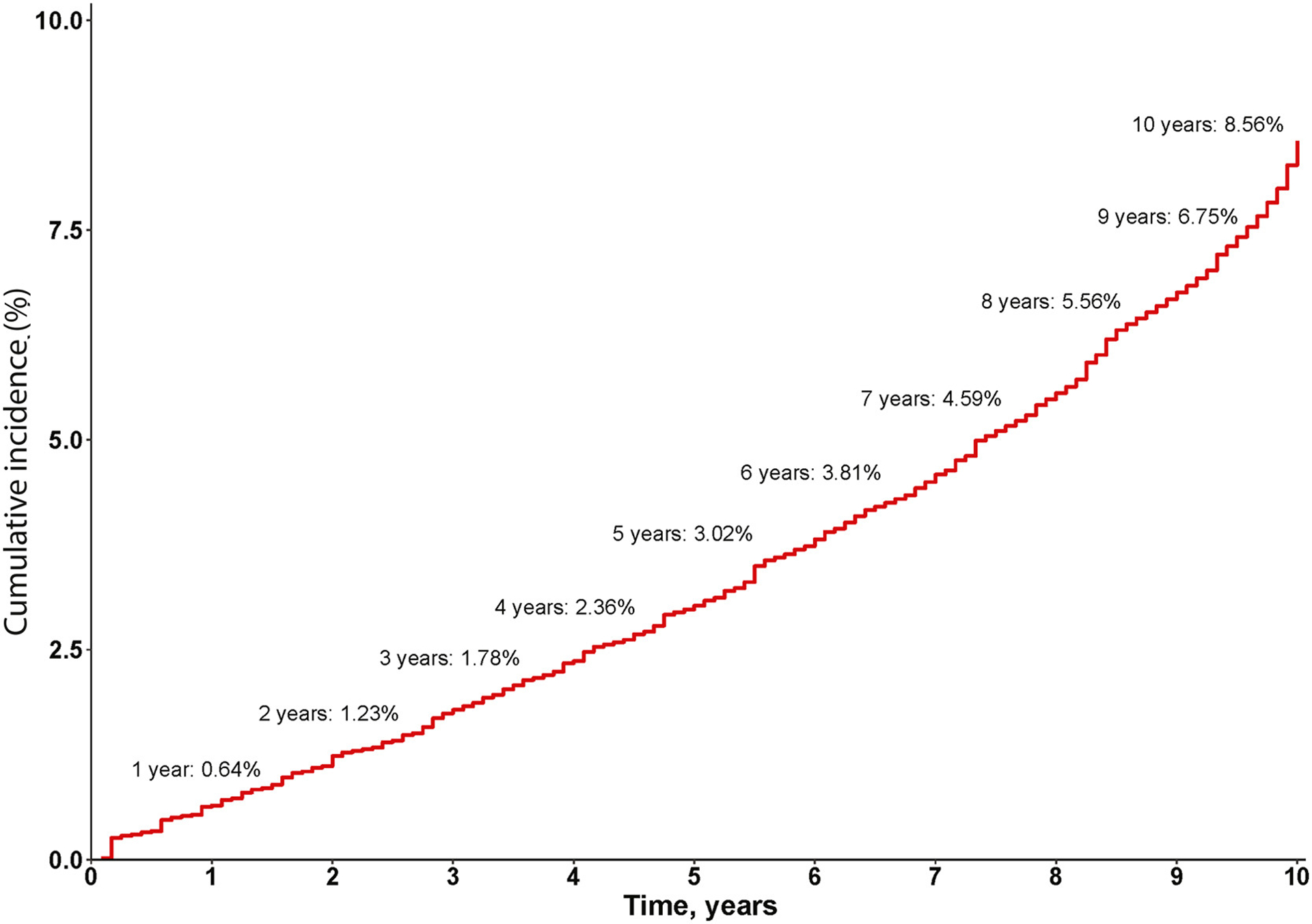
Cumulative risk of hepatocellular carcinoma (HCC) among patients with alcohol-associated cirrhosis among studies that accounted for competing risk of death without HCC, by reconstructed individual patient data meta-analysis.
From pooled analysis of all studies that provided time-to-event data (13 studies, 52,463 patients), the cumulative incidence of HCC in patients with alcohol-associated cirrhosis at 1, 3, 5, and 10 years was 0.88% (95% CI, 0.26–1.50), 3.05% (95% CI, 1.98–4.10), 5.27% (95% CI, 3.12–7.37), and 11.75% (95% CI, 7.35–15.94), respectively.
Subgroup/Sensitivity Analyses
To perform comparisons between subgroups, we then used a secondary analysis for incidence rate using traditional meta-analysis. The pooled incidence rate of HCC in patients with alcohol-associated cirrhosis among studies that accounted for the competing risk of death without HCC and available data for the incidence rate was 9.96 (95% CI, 4.83–20.42) (Table 1 and Supplementary Figure 1A). The pooled incidence rate among all included studies with available data for the incidence rate was 8.29 (95% CI, 4.77–14.39) per 1000 person-years (Supplementary Figure 1B).
Table 1.
Incidence Rate of HCC per 1000 Person-Years in Alcohol-Associated Cirrhosis, Overall and in Subgroups
| Analysis | Studies, n | Patients, n | Incidence rate (per 1000 person-years) | I2, % | Q | P valuea |
|---|---|---|---|---|---|---|
| Studies that accounted for competing risk of death without HCC | 4 | 6542 | 9.96 (4.83–20.42) | 97.00 | <0.01 | |
| Total | 16b | 148,157 | 8.29 (4.77–14.39) | 99.60 | <0.01 | |
| HCC surveillance program | ||||||
| No surveillance program | 9 | 145,212 | 4.82 (2.36–9.81) | 99.70 | <0.01 | .001a |
| Surveillance program | 7 | 2945 | 18.59 (12.08–28.50) | 87.40 | <0.01 | |
| Median study year | ||||||
| Before 2000 | 7 | 101,871 | 4.99 (1.70–14.55) | 99.50 | <0.01 | .100 |
| 2000 and after | 9 | 46,286 | 12.58 (9.18–17.23) | 95.70 | <0.01 | |
| Study setting | ||||||
| Non–population-based | 9 | 134,197 | 10.91 (6.13–19.35) | 100.0 | <0.01 | .08 |
| Population-based | 4 | 13,960 | 3.75 (1.31–10.65) | 99.00 | <0.01 |
HCC, hepatocellular carcinoma.
P value for comparison between subgroups.
Two studies provided time-to-event data for the cumulative incidence of HCC, but not data for incidence rates per 1000 person-years, hence they were not included in this analysis.
By enrollment into a hepatocellular carcinoma surveillance program
The incidence rate was higher among studies with patients who were enrolled in a HCC surveillance program vs studies that did not enroll patients in a HCC surveillance program: 18.59 (95% CI, 12.08–28.50) vs 4.82 (95% CI, 2.36–9.81) per 1000 person-years (P =.001) (Table 1 and Supplementary Figure 2).
By median study year
The pooled incidence rate of HCC between studies with a median year before 2000 vs 2000 and beyond was 4.99 (95% CI, 1.70–14.55) per 1000 person-years vs 12.58 (95% CI, 9.18–12.58) (P = .10) (Table 1 and Supplementary Figure 3).
By study setting
The incidence rate of HCC non–population-based studies vs population-based studies was 10.91% (95% CI, 6.13–19.35) vs 3.75% (95% CI, 1.31–10.65) per 1000 person-years (P = .08) (Table 1 and Supplementary Figure 4).
Biopsy-confirmed cirrhosis
A sensitivity analysis conducted among studies with biopsy-confirmed cirrhosis (3 studies, 1679 patients) determined that the HCC incidence rate was 21.14 (95% CI, 11.45–38.70) per 1000 person-years.
Factors Associated With the Rate of Hepatocellular Carcinoma Development in Alcohol-Associated Cirrhosis
Meta-regression of study-level data showed that smoking (β = 8.92; 95% CI, 5.78–12.06; P < .001), diabetes (β = 9.09; 95% CI, 0.53–17.65; P = .04), and hepatic decompensation (β = 2.45; 95% CI, 0.14–4.77; P=.04) were significant factors associated with an increased rate of HCC (Table 2).
Table 2.
Factors Associated With the Rate of Hepatocellular Carcinoma Development in Alcohol-Associated Cirrhosis
| Risk factor | Articles, n | Coefficient | 95% CI | P value |
|---|---|---|---|---|
| Age | 12 | 0.19 | −0.02 to 0.40 | .08 |
| Male | 12 | 0.61 | −1.90 to 3.13 | .62 |
| BMI | 5 | −0.14 | −0.54 to 0.26 | .48 |
| Smoking | 4 | 8.92 | 5.78–12.06 | <.001 |
| Diabetes | 9 | 9.09 | 0.53–17.65 | .04 |
| Decompensation | 8 | 2.45 | 0.14–4.77 | .04 |
| Ascites | 5 | 0.50 | −2.20 to 3.20 | .72 |
| Mean bilirubin | 7 | 0.001 | −0.02 to 0.02 | .93 |
| Mean albumin | 7 | 0.01 | −0.01 to 0.04 | .31 |
| Mean platelet count | 6 | −0.009 | −0.02 to 0.006 | .23 |
| AST | 5 | −0.009 | −0.02 to 0.0004 | .06 |
| ALT | 5 | −0.02 | −0.04 to 0.005 | .11 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval.
Sensitivity Analysis for Relative Risk of Developing Hepatocellular Carcinoma by Etiology of Cirrhosis
Alcohol-associated cirrhosis was associated with a lower risk of developing HCC compared with nonalcoholic fatty liver disease (NAFLD) cirrhosis (8 studies, 20,602 patients) (relative risk, 0.65; 95% CI, 0.44–0.95; P = .03; I2 = 81.8%) and HCV cirrhosis (6 studies, 1056 patients) (relative risk, 0.29; 95% CI, 0.23–0.37; P < .001; I2 = 0%). There were insufficient studies (n = 2) to allow for comparison with hepatitis B virus cirrhosis.
Discussion
Main Findings
In this systematic review and meta-analysis of 18 studies and 148,333 patients, we provide robust estimates for the incidence of HCC in alcohol-associated cirrhosis. We reconstructed individual patient data from studies that accounted for the competing risk of death without HCC and determined that the cumulative incidence of HCC in patients with alcohol-associated cirrhosis at 1, 3, 5, and 10 years was 1%, 2%, 3%, and 9%, respectively. By conventional meta-analysis, the overall pooled incidence rate of HCC in patients with alcohol-associated cirrhosis, among studies that accounted for the competing risk of death without HCC, was 10.0 per 1000 person-years.
HCC incidence was significantly higher among cohorts that were enrolled in a HCC surveillance program vs cohorts that were not enrolled in a surveillance program (18.6 vs 4.8 per 1000 person-years, respectively). HCC incidence was numerically higher among studies from 2000 onward vs before 2000, and among non–population-based vs population-based studies, but these 2 subgroup analyses did not reach a level of statistical significance. Meta-regression of study-level data showed that diabetes, smoking and hepatic decompensation, were associated were with the rate of development of HCC among patients with alcohol-associated cirrhosis. Among these risk factors, diabetes had the strongest association with HCC incidence.
Our findings have several important implications for clinical practice. First, while the overall incidence of HCC in alcohol-associated cirrhosis appeared to be lower than other etiologies of liver disease, the incidence of HCC was higher among studies that were enrolled in a HCC screening program (18.6 per 1000 person-years). Patients with alcohol-associated cirrhosis are known to have lower HCC surveillance rates, which may be related to poor disease awareness, clinic time constraints caused by other active medical issues, and provider beliefs regarding the likelihood of adherence.40–42 However, we recognize that the lower screening rates in alcohol-associated liver disease often are related to poor disease awareness, and patients often have advanced HCC at the time of liver disease diagnosis. Public health campaigns that use social media and traditional news outlets may help improve disease awareness and adherence to surveillance among patients with alcohol-associated cirrhosis. A recent multicenter trial in patients with cirrhosis (not focused on alcohol) showed that a mailed outreach strategy significantly improved adherence to HCC surveillance.43 Further studies specific to improving surveillance rates in patients with alcohol-associated cirrhosis are required. Second, the overall HCC incidence was only 9% over 10 years (by individual patient data meta-analysis), and 10.0 per 1000 person-years, which is lower than the 1.5%/year threshold proposed by the American Association for the Study of Liver Disease.44 However, there was a higher incidence among cohorts that underwent surveillance (18.6 per 1000 person-years), suggesting that HCC surveillance in alcohol-associated cirrhosis may be cost effective if validated further.44 Finally, diabetes, smoking, and hepatic decompensation were associated with an increased risk of HCC, with the presence of diabetes having the strongest association with HCC incidence. Therefore, patients with alcohol-associated cirrhosis should be screened for diabetes to identify the patients at high risk for HCC development. In addition, patients with alcohol-associated cirrhosis should be advised to stop smoking,45 while patients with hepatic decompensation should be monitored carefully for the development of HCC if clinically appropriate.
In Context With Current Literature
Our data provide similar results to that of a recent nationwide, biopsy-confirmed cohort of alcohol-associated cirrhosis from Sweden (HCC incidence of 8.6 per 1000 person-years),16 even though this study was excluded from analyses because of overlap with another cohort that provided time-to-event data.19 The current study provides important data that are useful for clinical practice and clinical trial design.
Strengths and Limitations
This meta-analysis used reconstructed individual participant data, which is considered to be the gold standard for reporting survival data because it accounts for censoring of events.46,47 However, this study was not without its limitations. Data from the included studies were heterogeneous, which is common among larger meta-analyses, and these findings require cautious interpretation. It is possible that studies that reported a higher risk of HCC may have included patients with a lower risk of death without HCC, however, many of the included studies did not provide data for mortality rates. Data were lacking from Africa, Southeast Asia, and the Eastern Mediterranean region, therefore it is unclear if the estimates provided in the current study may be generalized to those regions, and more studies are required. Most of the included studies were retrospective in nature, several had small sample sizes, and the included studies used a variety of definitions for cirrhosis, which may have introduced bias into the results. Some studies included prevalent cases of HCC while others did not. These likely contributed to the heterogeneity in the secondary analyses using traditional meta-analysis methods that persisted despite performing multiple subgroup analyses. However, the primary analyses performed using individual patient data address between- and within-study heterogeneity to provide more robust estimates for HCC risk. It is unclear if these data are generalizable to females because the majority of patients included in the analysis were male. There were insufficient data to perform subgroup analysis by sex. Meta-regression did not show a significant association between study-level data for sex and HCC incidence, which may have been related to the limitation of using study level data. More studies are required in females, given the increasing burden of alcohol-associated liver disease among females. In the analysis for the relative risk of HCC in alcohol-associated cirrhosis vs NAFLD cirrhosis or HCV cirrhosis, we were unable to adjust for potential confounders such as age and antiviral treatment status owing to a lack of data, therefore these results should be interpreted with caution. Data also were insufficient to provide a comparison of HCC incidence between alcohol-associated cirrhosis vs NAFLD cirrhosis or HCV cirrhosis among cohorts that underwent HCC screening.48,49 In addition, patients with alcohol-associated cirrhosis may have harbored concomitant NAFLD, and there may have been some degree of overlap between etiological groups. Finally, there were insufficient data to evaluate the impact of alcohol cessation or continuation, obesity, as well as the impact of lead-time bias from surveillance on HCC incidence.
Conclusions
This meta-analysis showed that the cumulative risk of HCC in patients with alcohol-associated cirrhosis was 3% after 5 years, and 9% after 10 years. There was a higher annual incidence of HCC in subgroup analyses for cohorts that underwent HCC surveillance. These data should be validated in large prospective studies and may have important implications for HCC screening and surveillance among patients with alcohol-associated cirrhosis.
Supplementary Material
What You Need to Know.
Background
The reported incidence of alcohol-associated hepatocellular carcinoma varies widely. A systematic review of the incidence of hepatocellular carcinoma among patients with alcohol-associated cirrhosis has not been reported.
Findings
The 5- and 10- year cumulative risk of HCC in alcohol-associated cirrhosis was 3% and 9% respectively, with a higher incidence in cohorts that underwent hepatocellular carcinoma surveillance.
Implications for patient care
These data suggest that hepatocellular carcinoma surveillance in alcohol-associated cirrhosis may be cost effective, and should be validated in large prospective studies.
Funding
Supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA), United States grant U01AA029019; National Institute of Environmental Health Sciences (NIEHS), United States grant 5P42ES010337; National Center for Advancing Translational Sciences (NCATS), United States grant 5UL1TR001442; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), United States grants U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, and R01DK124318; National Heart, Lung, and Blood Institute (NHLBI), United States grant P01HL147835; and Department of Defence Peer Reviewed Cancer Research Program (DOD PRCRP), United States grant W81XWH-18-2-0026 (R.L.).
Conflicts of interest
These authors disclose the following: Daniel Q. Huang has received funding support from the Singapore Ministry of Health’s National Medical Research Council under its National Medical Research Council (NMRC) Research Training Fellowship (MOH-000595-01).
Abbreviations used in this paper:
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.06.032.
CRediT Authorship Contributions
Daniel Q Huang, MBBS, MRCP (Conceptualization: Lead; Data curation: Equal; Supervision: Equal; Writing – review & editing: Lead)
Darren J.H. Tan, MBBS (Data curation: Equal; Formal analysis: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Cheng Han Ng, MBBS (Conceptualization: Equal; Data curation: Equal; Investigation: Equal; Writing – review & editing: Equal)
Maral Amangurbanova, MD (Methodology: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Nancy Sutter, MD (Data curation: Supporting; Investigation: Supporting; Writing – review & editing: Supporting)
Phoebe Wen Lin Tay, MBBS (Visualization: Equal)
Wen Hui Lim, MBBS (Formal analysis: Supporting; Investigation: Supporting)
Jie Ning Yong, MBBS (Data curation: Supporting)
Ansel Tang, MBBS (Methodology: Supporting)
Nicholas Syn, MBBS (Conceptualization: Supporting; Data curation: Supporting)
Mark D Muthiah, MBBS, MRCP, MMED (Conceptualization: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Eunice Tan (Data curation: Supporting; Writing – review & editing: Supporting)
Shravan Dave, MD (Writing – review & editing: Supporting)
Benjamin Tay (Data curation: Supporting; Writing – review & editing: Supporting)
Abdul M. Majzoub, MD (Supervision: Supporting; Writing – review & editing: Supporting)
Danielle Gerberi, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Beom Kyung Kim, MD (Methodology: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Rohit Loomba, MD, MHSc (Conceptualization: Equal; Data curation: Equal; Methodology: Equal; Writing – review & editing: Equal)
Data availability statement
Data are publicly available.
References
- 1.Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA 2021;326:165–176. [DOI] [PubMed] [Google Scholar]
- 2.Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2020;71:306–333. [DOI] [PubMed] [Google Scholar]
- 3.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 4.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol 2015;62:S38–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol 2018;69:154–181. [DOI] [PubMed] [Google Scholar]
- 6.Rehm J, Taylor B, Mohapatra S, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev 2010;29:437–445. [DOI] [PubMed] [Google Scholar]
- 7.Mathurin P, Beuzin F, Louvet A, et al. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther 2007;25:1047–1054. [DOI] [PubMed] [Google Scholar]
- 8.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 9.GBD 2019 Diseases; Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969–977.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global status report on alcohol and health 2018. Geneva: World Health Organization, 2018. [Google Scholar]
- 12.El-Serag HB, Kanwal F, Feng Z, et al. Risk factors for cirrhosis in contemporary hepatology practices-findings from the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 2020;159:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reveron-Thornton RF, Teng MLP, Lee EY, et al. Global and regional long-term survival following resection for HCC in the recent decade: a meta-analysis of 110 studies. Hepatol Commun 2022;6:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toshikuni N, Izumi A, Nishino K, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol 2009; 24:1276–1283. [DOI] [PubMed] [Google Scholar]
- 15.N’Kontchou G, Paries J, Htar MT, et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol 2006; 4:1062–1068. [DOI] [PubMed] [Google Scholar]
- 16.Hagström H, Thiele M, Sharma R, et al. Risk of cancer in biopsy-proven alcohol-related liver disease: a population-based cohort study of 3,410 persons. Clin Gastroenterol Hepatol 2022; 20:918–929.e8. [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson E, Anderson H, Sargenti K, et al. Risk and outcome of hepatocellular carcinoma in liver cirrhosis in Southern Sweden: a population-based study. Scand J Gastroenterol 2019; 54:1027–1032. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2022. https://ohri.ca/programs/clinical_epidemiology/oxford.asp; https://web.archive.org/web/20210716121605id_/; http://www3med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf. Accessed February 20, 2022.
- 21.Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology 2015;62:292–302. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB Treatment-2017, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018;392:821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong JN, Lim WH, Ng CH, et al. Outcomes of nonalcoholic steatohepatitis after liver transplantation: An updated meta-analysis and systematic review. Clin Gastroenterol Hepatol 2023;21:45–54.e6. [DOI] [PubMed] [Google Scholar]
- 25.Lim WH, Tan DJH, Ng CH, et al. Laparoscopic versus open resection for rectal cancer: an individual patient data meta analysis of randomized controlled trials. Eur J Surg Oncol 2022; 48:1133–1143. [DOI] [PubMed] [Google Scholar]
- 26.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–1297. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 28.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, et al. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019;10:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–413. [Google Scholar]
- 30.Fletcher J What is heterogeneity and is it important? BMJ 2007; 334:94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 32.Borges Migliavaca C, Stein C, Colpani V, et al. How are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol 2020;20:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:739–752. [DOI] [PubMed] [Google Scholar]
- 34.Huang DQ, Yeo YH, Tan E, et al. ALT levels for Asians with metabolic diseases: a meta-analysis of 86 studies with individual patient data validation. Hepatol Commun 2020;4:1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014;67:897–903. [DOI] [PubMed] [Google Scholar]
- 36.Ganne-Carrie N, Nahon P, Chaffaut C, et al. Impact of cirrhosis aetiology on incidence and prognosis of hepatocellular carcinoma diagnosed during surveillance. JHEP Rep 2021;3:100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marot A, Henrion J, Knebel JF, et al. Alcoholic liver disease confers a worse prognosis than HCV infection and nonalcoholic fatty liver disease among patients with cirrhosis: an observational study. PLoS One 2017;12:e0186715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsson E, Anderson H, Sargenti K, et al. Clinical course and mortality by etiology of liver cirrhosis in Sweden: a population based, long-term follow-up study of 1317 patients. Aliment Pharmacol Ther 2019;49:1421–1430. [DOI] [PubMed] [Google Scholar]
- 39.West J, Card TR, Aithal GP, et al. Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: a population-based cohort study. Aliment Pharmacol Ther 2017; 45:983–990. [DOI] [PubMed] [Google Scholar]
- 40.Bucci L, Garuti F, Camelli V, et al. Comparison between alcohol and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment Pharmacol Ther 2016;43:385–399. [DOI] [PubMed] [Google Scholar]
- 41.Eskesen AN, Bjøro K, Aandahl EM, et al. Low use of surveillance and early diagnosis of hepatocellular carcinoma in Norway–a population-based cohort study. Cancer Epidemiol 2014; 38:741–747. [DOI] [PubMed] [Google Scholar]
- 42.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singal AG, Reddy S, Radadiya Aka Patel H, et al. Multicenter randomized clinicaltrial of a mailed outreach strategy for hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol 2022; 20:2818–2825.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67:358–380. [DOI] [PubMed] [Google Scholar]
- 45.Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br J Cancer 2018;118:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340:c221. [DOI] [PubMed] [Google Scholar]
- 47.Tan DJH, Lim WH, Yong JN, et al. UNOS down-staging criteria for liver transplantation of hepatocellular carcinoma: systematic review and meta-analysis of 25 studies. Clin Gastroenterol Hepatol 2022. 10.1016/j.cgh.2022.02.018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang DQ, Fowler KJ, Liau J, et al. Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: A prospective study. Aliment Pharmacol Ther 2022. Apr;55:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicly available.


