Abstract
Psychedelic drugs, including the serotonin 2a (5-HT2A) receptor partial agonist psilocybin, are receiving renewed attention for their possible efficacy in treating a variety of neuropsychiatric disorders. Psilocybin induces widespread dysregulation of cortical activity, but circuit-level mechanisms underlying this effect are unclear. The claustrum is a subcortical nucleus that highly expresses 5-HT2A receptors and provides glutamatergic inputs to arguably all areas of the cerebral cortex. We therefore tested the hypothesis that psilocybin modulates claustrum function in humans. Fifteen healthy participants (10M, 5F) completed this within-subjects study in which whole-brain resting-state blood-oxygenation level-dependent (BOLD) signal was measured 100 min after blinded oral administration of placebo and 10 mg/70 kg psilocybin. Left and right claustrum signal was isolated using small region confound correction. Psilocybin significantly decreased both the amplitude of low frequency fluctuations as well as the variance of BOLD signal in the left and right claustrum. Psilocybin also significantly decreased functional connectivity of the right claustrum with auditory and default mode networks (DMN), increased right claustrum connectivity with the fronto-parietal task control network (FPTC), and decreased left claustrum connectivity with the FPTC. DMN integrity was associated with right-claustrum connectivity with the DMN, while FPTC integrity and modularity were associated with right claustrum and left claustrum connectivity with the FPTC, respectively. Subjective effects of psilocybin predicted changes in the amplitude of low frequency fluctuations and the variance of BOLD signal in the left and right claustrum. Observed effects were specific to claustrum, compared to flanking regions of interest (the left and right insula and putamen). This study used a pharmacological intervention to provide the first empirical evidence in any species for a significant role of 5-HT2A receptor signaling in claustrum functioning, and supports a possible role of the claustrum in the subjective and therapeutic effects of psilocybin.
Keywords: Psychedelics, fMRI, Resting state, Serotonin 2A receptor
1. Introduction
Administration of sufficient doses of classic psychedelic drugs, including psilocybin, acutely alter sensory perception (Barrett et al., 2018a; Kometer and Vollenweider, 2018) and executive function (Barrett et al., 2018b; Preller and Vollenweider, 2016; Pokorny et al., 2019), and may have long-term therapeutic value in treating a variety of neuropsychiatric disorders without the adverse reactions associated with current medication (Bogenschutz et al., 2015; Carhart-Harris et al., 2016a; Griffiths et al., 2016; Johnson et al., 2014; Ross et al., 2016). Recent findings suggest that psilocybin alters the integrity of and coupling between large-scale brain networks (Petri et al., 2014), including the default mode network (DMN) (Carhart-Harris et al., 2012; Muthukumaraswamy et al., 2013; Smigielski et al., 2019) as well as sensory and executive control networks (including task-positive networks) (Roseman et al., 2014). However, the exact mechanisms through which these effects occur are unclear. Understanding how psilocybin alters large-scale brain networks may reveal mechanisms of therapeutic effects and shed light on new therapeutic targets for many mental disorders.
The effects of psilocybin are largely achieved through its action as a partial agonist of the serotonin 2a (5-HT2A) receptor (Nichols, 2016), which likely modulates glutamatergic signaling across a range of cortical and subcortical afferents (Aghajanian and Marek, 1997; Scruggs et al., 2000, 2003). 5-HT2A receptor protein is highly expressed in a subcortical telencephalic nucleus with widespread bidirectional, glutamatergic connectivity with the cerebral cortex: the claustrum (Nichols, 2016; Mathur, 2014; McKenna and Saavedra, 1987; Pazos et al., 1985). Functional imaging evidence in human subjects shows that claustrum activation occurs at the onset of a demanding cognitive task (Krimmel et al., 2019a). This claustrum activation coincides with the onset of task-positive control networks and the offset of the DMN (Krimmel et al., 2019a). Further, the claustrum is significantly functionally connected both with task-positive networks and the DMN (Krimmel et al., 2019a). These observations are unique to the claustrum, distinct from the role of neighboring structures such as the insula and putamen, and consistent with the idea that the claustrum supports the co-activation of widespread cortical regions participating in the networks necessary for cognitive control of behavior (Krimmel et al., 2019a; Reser et al., 2014; White and Mathur, 2018). Overall, this implicates the claustrum as a target for psilocybin.
We herein test the hypothesis that the 5-HT2A partial agonist psilocybin disrupts claustrum activity and functional brain connectivity in humans. Using functional magnetic resonance imaging (fMRI), we show that psilocybin alters the fluctuation of blood-oxygenation level-dependent (BOLD) signal in the claustrum (a possible proxy for population-level neuronal activity) and disrupts claustrum functional connectivity with widespread cortical networks that support perception and cognition. These effects are associated with subjective effects of psilocybin, and are dissociated from psilocybin effects on the neighboring structures of the insula and putamen.
2. Materials and methods
2.1. Participants
20 healthy volunteers participated in a blinded, placebo-controlled study of the effects of psilocybin on meditation. Five participants completed all study procedures but were excluded from analysis due to excessive head motion and registration failures during one or both of the scans (see Preprocessing below), leaving 15 participants who underwent final analysis and reporting (10 M, 5F; mean age: 51.3 years old [range: 25–68, STD: 12.3]). All participants provided written informed consent and were medically and psychologically healthy as assessed by medical history and physical examination as well as the Structured Clinical Interview for DSM-IV (SCID-IV). All participants had a long-term meditation practice and had been administered a 25 mg/70 kg dose of psilocybin in a previous study, approximately two months before completing the present study. Participants had also completed two to three 90-min fMRI scanning sessions in the previous study, before enrolling in the current study, and reported being comfortable in the scanning environment.
All participants were Caucasian and non-Hispanic. Participants reporting having completed a baccalaureate (n = 5), masters (n = 8), or doctoral or professional (n = 2) degree. Participants were excluded if they presented with clinically relevant cardiac abnormalities, had a first or second degree relative with a history of bipolar disorder, psychosis, or a related disorder, met criteria for substance use disorder, or had used a psychedelic drug after the establishment of their meditation practice. 59% of the participants had previous experience with a hallucinogen before participating in the previous experiment, but none had exposure to a hallucinogen within 5 years of study enrollment in the previous experiment. Of those individuals who had previous hallucinogen exposure, median previous hallucinogen use was 2 separate occasions (range = 1 to 11). All participants were confirmed negative by urinalysis for pregnancy (females) and for recent illicit drug use before enrollment and on the morning of drug administration and scanning procedures.
2.2. Procedures
Participants received psychological support before, during and after drug administration and scanning procedures in this study consistent with guidelines for the safe administration of psilocybin in a research context (Johnson et al., 2008). This included preparation, close supportive care during sessions, and de-briefing. Participants had already built strong rapport with the study team in a previous experiment, and were familiar both with the MRI environment used in the current study and with the subjective effects of a high dose of psilocybin (25 mg/70 kg). In the current study, participants completed 4 h of preparation before their drug administration sessions to reacclimate themselves to expected psilocybin effects and to prepare for experiencing psilocybin effects within the MRI. During sessions, participants were continuously accompanied by at least one staff member and were closely monitored during acute effects of placebo and psilocybin, including having a staff member in the MRI room with a hand placed on the participant’s ankle during scanning. All participants completed a debriefing with study staff after resolution of acute psilocybin effects and before leaving the research unit.
Participants completed two scanning procedures, each beginning 90 min after administration of placebo or 10 mg/70 kg psilocybin. The timing of scanning procedures coincided with peak subjective effects of this dose of psilocybin (Griffiths et al., 2011). Scanning procedures included resting-state EPI scans as well as other imaging procedures that will be reported elsewhere. To minimize expectancy effects, participants were informed both verbally and in the consent form that on each occasion, they may receive placebo, an extremely low dose of psilocybin, or a moderately low but still psychoactive dose of psilocybin (relative to the high dose of 25 mg/70 kg psilocybin they had received previously). Unknown to the participant, the first administration/scan was always after placebo administration at 8am, and the second was after psilocybin administration at 12 noon.
Psilocybin administration occurred in an aesthetic living-room environment in the Behavioral Pharmacology Research Unit (BPRU) at the Johns Hopkins Bayview Medical Center, in Baltimore, MD. After capsule administration, participants reclined on a couch with eye shades and headphones and listened to music. Participants were transported by hired car and accompanied by two session monitors from the psilocybin session room to the MRI facilities 60 min after capsule administration. Resting-state scans began approximately 100 min after capsule administration (±11 min). Participants were instructed to not meditate during resting-state scans, and had previous experience completing resting-state scans following this instruction on at least two previous occasions. All participants confirmed after the MRI that they had not been meditating during the resting-state scan. Participants were accompanied by two study team members and immediately transported back to the BPRU after scanning procedures were completed, and remained at the BPRU until the drug effects subsided.
All procedures were approved by the Johns Hopkins Medicine Institutional Review Board and were carried out in accordance with the Declaration of Helsinki. This study was conducted as part of a clinical trial that was registered at ClinicalTrials.gov (NCT02145091). No serious adverse events were encountered, there were no cases of prolonged effects of drug administration, and no pharmacological, medical, or psychological interventions were necessary in response to study procedures.
2.3. Subjective effects ratings
Immediately after each resting-state scan, participants used a verbal 11 point scale from 0 (none; not at all) to 10 (extreme; strongest imaginable) to rate the degree to which they experienced a series of subjective effects during their resting state scan. Participants first rated the overall strength of psilocybin-like effects. Participants then rated three subjective effects related to mindfulness: now-ness (the feeling of being in the present moment), letting go (the degree to which a person was able to let go of control of the experience), and equanimity (equipose, felt sense of being in balance, emotional balance). Participants then rated a series of subjective effects derived from the mystical experience questionnaire (Maclean et al., 2012; Barrett et al., 2015): pure being and pure awareness, fusion of your personal self into a larger whole, sense of reverence or sacredness, timelessness (lack of a sense of space and time), ineffability (inability to explain the experience with words), feelings of joy, and feelings of peace and tranquility. Finally, participants rated two dimensions of more general emotional experience: positive emotional valence and negative emotional valence. Each rating was verbally prompted by a study team member. Participants had extensive experience with these ratings during fMRI scans in the previous study, and participants confirmed that they understood the meaning and definition of each rating before each round of scanning procedures in the current study.
2.4. MRI acquisition
Data were collected using 3T Philips Achieva MRI scanners (Philips Medical Systems, Best, The Netherlands) at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute in Baltimore, MD. Each resting-state scan was acquired with eyes closed using echo planar imaging (axial acquisition, 3 mm isotropic voxel size, 1 mm slice gap, 37 ascending slices, 210 vol retained for analysis, TR of 2.2 s, TE of 30 ms, sense acceleration factor of 2, and four initial TRs discarded for magnetization equilibrium; total analyzed scan time was 7 min and 42 s). One high-resolution T1-weighted anatomical scan was also collected for each participant (magnetization-prepared rapid acquisition gradient echo, or MPRAGE: 3D acquisition reconstructed in the sagittal plane, 1 mm isotropic voxel size, sense acceleration factor 2, total scan time 5 min and 56 s).
2.5. Preprocessing
Preprocessing steps followed Krimmel and colleagues (Krimmel et al., 2019a), and consisted of slice timing correction, realignment (motion correction), coregistration of the T1-weighted anatomical scan to the first realigned functional image, segmentation of the structural scan, and normalization of the structural and realigned functional images to a standard MNI template using unified segmentation (Ashburner and Friston, 2005). These steps were carried out using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/). Resting-state data were further preprocessed by simultaneous regression (Hallquist et al., 2013; Lindquist et al., 2019) consisting of nuisance variables, detrending, despiking (Patel et al., 2014), and band pass filtering [0.008–0.09 Hz] using the CONN toolbox (Whitfield-Gabrieli et al., 2012). Global signal was not removed, as doing so can introduce structured statistical artifacts into resting-state functional connectivity data (Murphy and Fox, 2017). Nuisance regressors included six motion regressors from realignment, a scrubbing vector generated using outlier detection and intermediate settings (global-signal z-value threshold = 5, subject-motion mm threshold = 0.9) in the ART toolbox (Whitfield-Gabrieli et al., 2012), and the first 5 principal components of twice eroded cerebro-spinal fluid and four-eroded white matter masks, as the signal in these regions controls for non-neural physiological variance (including artifacts introduced by respiration and cardiac pulsation) (Behzadi et al., 2007; Muschelli et al., 2014) and masks at these erosion levels do not contain global signal (Power et al., 2017), nor did the eroded WM mask include the claustrum. Five participants were excluded from analysis due to excessive motion (more than 10% of volumes identified for scrubbing, n = 3) normalization or errors in (n = 2). The remaining 15 participants did not differ in motion between placebo (mean number of scrubbed volumes = 2.7, SD = 4.5; mean framewise displacement = 0.122, SD = 0.071) and extracted psilocybin (mean number of scrubbed volumes = 4.7, SD = 4.9; mean framewise displacement = 0.162, SD = 0.092) scans. We then extracted the 264 ROI time courses from these preprocessed, unsmoothed, whole-brain BOLD data using a sphere with 10 mm diameter around each ROI coordinate defined in the Power Atlas (Power et al., 2011).
We followed our previously reported method of small region confound correction (SRCC) to isolate ROI time courses for the left and right claustrum from the surrounding insula and putamen (Krimmel et al., 2019a, 2019b). Briefly, the average timeseries from each claustrum ROI (left and right) was extracted from slice time-corrected, realigned, coregistered, normalized, unsmoothed whole-brain BOLD data, and was regressed on the timeseries of the ipsilateral ‘flanking’ ROIs, along with previously described artifacts (motion, twice-eroded cerebro-spinal fluid mask, and a 4-eroded white matter mask), and the residuals from this analysis constituted corrected right and left claustrum corrected timeseries (e.g. corrected for signal in the flanking insula and putamen sources) that were then used as the claustrum seeds in all subsequent analyses. Group-averaged template-space ROIs for claustrum and flanking regions were defined previously (Krimmel et al., 2019a), based on the average of claustrum ROIs that were hand-drawn onto high-resolution (0.7 mm isotropic voxels) T1-weighted anatomical images acquired at 7T from 22 individuals. Fit of the claustrum and flanking ROI templates to normalized structural data for each participant in the current dataset was confirmed by visual inspection.
2.6. Analysis
Analysis of subjective ratings.
Subjective effects ratings were compared between drug conditions using a paired T-test. Significant differences between drug conditions were assessed after Bonferroni correction for multiple comparisons.
Analysis of claustrum activity and connectivity.
The amplitude of low-frequency fluctuations (Zang et al., 2007) as well as the variance of each claustrum ROI timecourse was calculated and compared between placebo and psilocybin conditions using a paired Student’s T-test. All pairwise correlations between each claustrum ROI (left and right) and each Power atlas ROI were calculated using CONN, and used to construct a functional connectome for placebo scans and a functional connectome for psilocybin scans for each participant. We then submitted Fisher-transformed correlations to mixed-effects general linear models, with subject and ROI as random effects, to assess differences between psilocybin and placebo scans in the functional connectivity of the claustrum with networks defined in the Power atlas. The following general linear model was fit:
where z is the Fisher-transformed correlation (edge) for a given claustrum-to-ROI connection. Thus, the unit of analysis is the claustrum connectivity strength for every ROI.
Main effects and interactions of drug condition and network label were assessed using the model above. Planned comparisons of the effect of psilocybin on connectivity in each network were assessed using the following mixed-effects general linear model, assessed separately for each network defined in the Power atlas, with subject and ROI as random effects:
We then tested the association of claustrum connectivity with measures of network modularity and network integrity during psilocybin. Network integrity was defined as the average of within-network pairwise Fisher-transformed correlations for each ROI in a given network. A decrease in within-network pair-wise Fisher-transformed correlations across nodes in a given network was interpreted as a decrease in the integrity of that network. Network modularity was assessed using the network theory measure called participation coefficient for each node in a given network (Rubinov and Sporns, 2010). Participation coefficient, when applied to a continuous connectivity measure such as a Fisher-transformed correlation, is a measure of the strength of connections of a node in a given network with nodes in other networks. Decreases in participation coefficient across nodes of a network represent increased modularity of that network (lower inter-network connectivity of network nodes), whereas increases in participation coefficient represent decreased network modularity (greater inter-network connectivity of nodes in that network). We used the inverse of participation coefficient as a measure of network modularity.
To test the association of claustrum connectivity with network modularity and integrity during psilocybin, we fit separate mixed effects models for each network, with subject and ROI as random effects, to node-wise measures calculated from psilocybin connectomes using the following general linear model:
Separate models were fit with DV defined as either the average of all pair-wise within-network Fisher-transformed correlations for nodes (ROIs) of a given network (network integrity), or the inverse of the node-wise participation coefficient for ROIs of a given network (network modularity). Thus, the unit of analysis is a metric for every ROI. ßclaustrum_connectivity represents the Fisher-transformed connectivity of the given ROI with the claustrum. Separate models were also fit for left and right claustrum connectivity, for a total of four mixed effects models per network. Analyses were restricted to networks for which psilocybin significantly altered claustrum connectivity.
For all mixed-effects models that were fit, a compound symmetry covariance structure was specified for random effects. In all mixed-effects models, likelihood ratio tests demonstrated that models including both subject and ROI as random effects produced better fits that models excluding these effects. Mixed-effects models were fit in MATLAB. Statistical significance levels in all cases were corrected for multiple-comparisons and family-wise error rate using the Holm-Bonferroni method (Holm, 1979).
Specificity of claustrum findings.
To determine the specificity of psilocybin effects on claustrum activity and connectivity, we used paired T-tests to analyze the effects of psilocybin (compared to placebo) on ALFF and variance of the BOLD signal in ROIs flanking the claustrum (left and right insula and putamen). We also used mixed effects ANOVAs and planned comparisons (using Tukey’s method) to compare the connectivity of claustrum with each Power atlas network to the connectivity of the insula and putamen with each Power atlas network. Comparisons were limited to networks for which psilocybin significantly altered claustrum connectivity.
Association of psilocybin subjective and neural effects.
We used a series of general linear models to estimate the associations between each subjective effect outcome measure (as independent variables) and the variance of blood-oxygenation level-dependent (BOLD) signal and the amplitude of low-frequency fluctuations (ALFF) in BOLD signal in the left and right claustrum (as dependent variables). We also included the insula and putamen as dependent variables to assess the specificity of any findings in relation to flanking ROIs. The first models for each ROI assessed overall strength of drug effect ratings as the sole independent variable. Subsequent models assessed each additional subjective effect outcome variable separately, with overall strength of drug effect entered as a covariate.
3. Results
3.1. Subjective effects
Consistent with previous reports (Barrett et al., 2018b; Griffiths et al., 2011; Hasler et al., 2004; Carter et al., 2004, 2005; Wittmann et al., 2007; Wackermann et al., 2008; Vollenweider et al., 2007; Studerus et al., 2011; Kometer et al., 2011; Schmidt et al., 2013; Kraehenmann et al., 2015a, 2015b; Pokorny et al., 2016; Carbonaro et al., 2018), administration of 10 mg/70 kg psilocybin resulted in substantial effects on a number of subjective effects measures, including a rating of overall strength of drug effects, and ratings related to both emotion and mystical experience (Fig. 1).
Fig. 1.
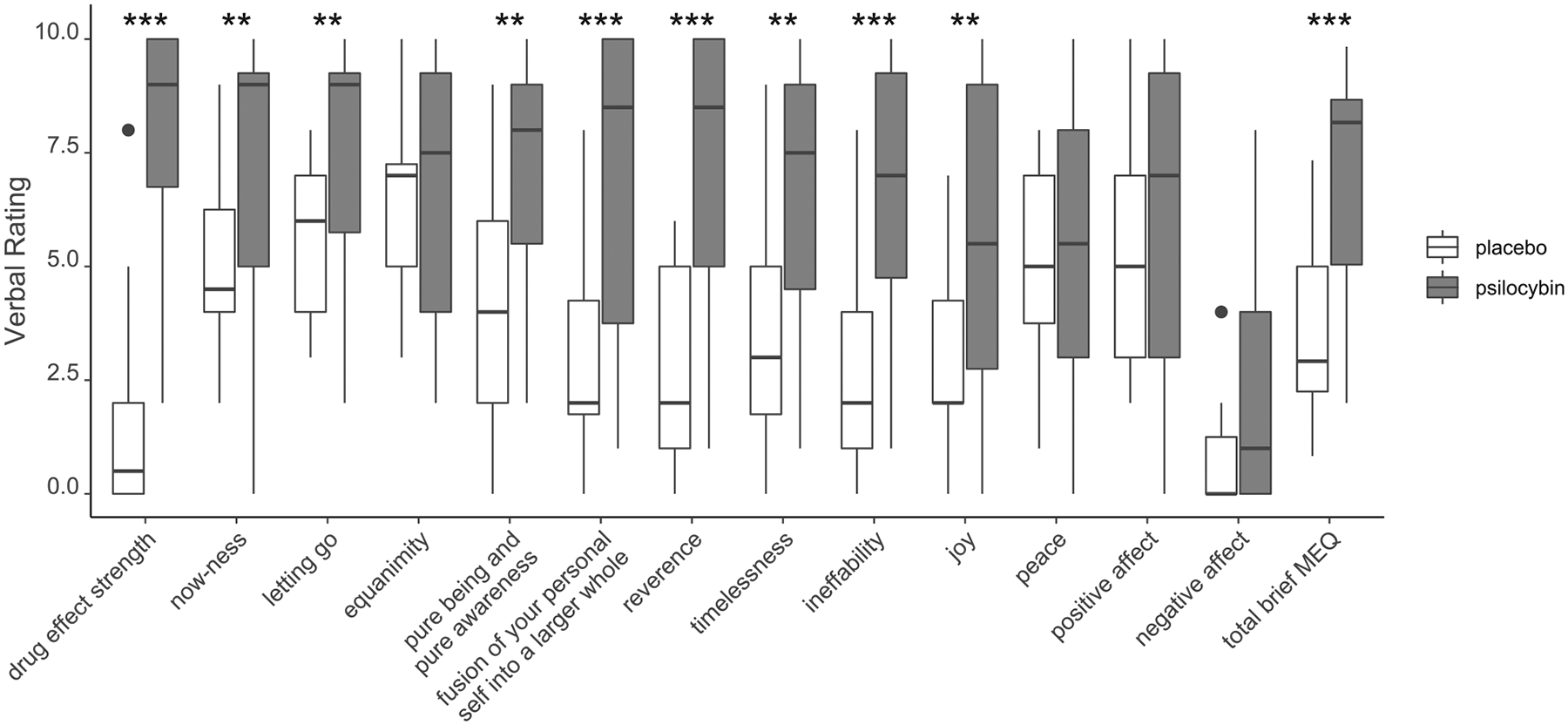
Subjective effects ratings completed immediately after each resting-state scan in 15 participants each of whom received placebo and psilocybin. Items were rated on a scale from 0 = none; not at all to 10 = extreme; strongest imaginable. Box plots denote the median (middle line), interquartile range (box limits), upper and lower whiskers (1.5 * interquartile range), and outliers (dots) for each subjective effect rating. MEQ: mystical experiences questionnaire. ** = p < 0.001, *** = p < 0.0001.
3.2. Integrity of blinding procedures
More than half of the participants (9/15) rated subjective drug strength as greater than 0 for the placebo scan, and these individuals confirmed that they thought they may have received an extremely low or moderately low dose of psilocybin for the placebo scan. However, the average rating of strength of subjective drug effects was significantly greater during psilocybin (M = 8.1, SD = 2.31, range = 2 – 10) than placebo (M = 1.35, SD = 2.08, range = 0 – 5).
3.3. Psilocybin decreases the amplitude of low frequency fluctuations and variance in Bilateral claustrum
Psilocybin reduced variance in BOLD signal in the left claustrum (t[14] = 2.169, p = 0.011), right claustrum (t[14] = 2.269, p = 0.021), and left putamen (t[14] = 1.976, p = 0.008) (Fig. 2A), and the amplitude of low-frequency fluctuations (ALFF) of left claustrum (t[14] = 2.098, p = 0.013), right claustrum (t[14] = 2.292, p = 0.023), and left putamen (t[14] = 2.117, p = 0.004) compared to placebo (Fig. 2B). Psilocybin did not significantly alter variance in the left insula (t[14] = 0.003, p = 0.510), right insula (t[14] = 0.141, p = 0.450), or the right putamen (t[14] = 0.226, p = 0.424), or ALFF in the left insula (t[14] = 0.104, p = 0.462), right insula (t[14] = 0.065, p = 0.475), or the right putamen (t[14] = 0.124, p = 0.448).
Fig. 2.
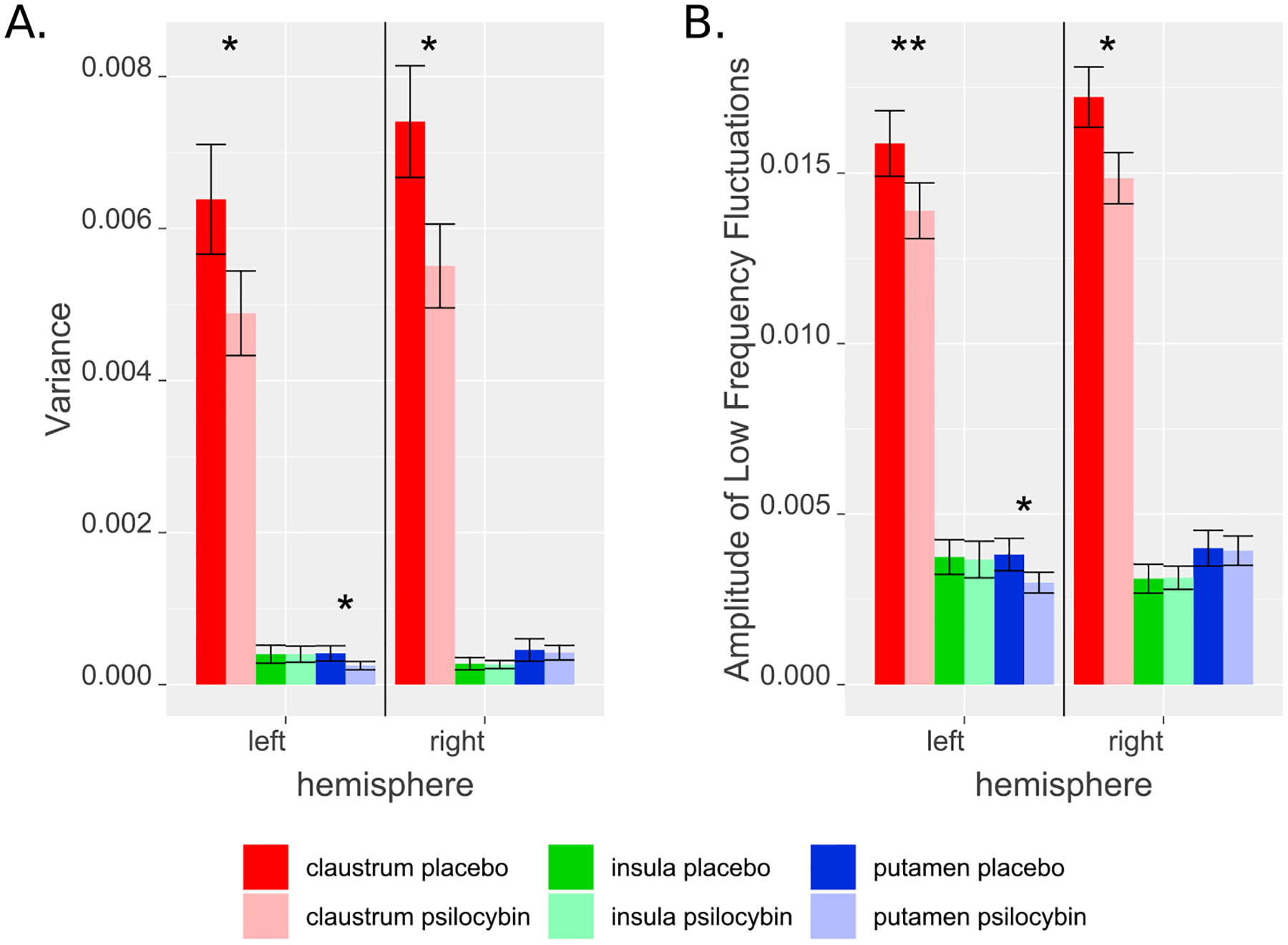
Effect of psilocybin on the average variance (A) and amplitude of low frequency fluctuations (ALFF; B) of resting-state BOLD signal in the left and right claustrum, insula, and putamen. * = p < 0.05, ** = p < 0.01. Error bars show standard error.
3.4. Psilocybin alters claustrum connectivity with brain networks that support perception and cognition
A main effect of network predicting connectivity between the claustrum and cortical ROIs was observed for the left claustrum model (F [12,7053] = 3.285, p < 0.0001) and the right claustrum model (F [12,7053] = 3.623, p < 0.0001). Interactions between drug condition (placebo vs psilocybin) and network were also observed for both left claustrum (F[12,7053] = 2.846, p < 0.001) and right claustrum (F[12,7053] = 4.479, p < 0.0001).
Separate models for each network demonstrated that psilocybin significantly decreased left claustrum connectivity with the fronto-parietal task control network (FPTC; Fig. 3A), decreased right claustrum connectivity with the auditory network and the DMN (Fig. 3B), and increased right claustrum connectivity with the FPTC (Fig. 3B). Post-hoc tests demonstrated that psilocybin had a significantly greater effect on FPTC connectivity with the left claustrum than with the left insula (z = 4.800, p < 0.0001) or left putamen (z = 4.801, p < 0.0001). Psilocybin administration led to a greater change in functional connectivity of the DMN with the right claustrum than with the right insula (z = 4.505, p <0.0001), but not the right putamen (z = 6.954, p < 0.0001), which showed greater decreased connectivity with the DMN following psilocybin. There were no significant differences in the degree to which psilocybin altered FPTC connectivity with the right claustrum compared to the right insula (z = 0.658, p = 0.511) or the right putamen (z = 1.91, p = 0.112), or auditory network connectivity with the right claustrum compared to the right insula (z = 1.265, p = 0.617) or right putamen (z = 0.170, p = 0.865). Individual edges between the claustrum and each ROI for all participants and for each network are plotted as dots overlaid on a bar graph in Supplemental Material (Figure S1). Insula (Figure S2) and putamen (Figure S3) connectivity are also presented in Supplemental Material.
Fig. 3.
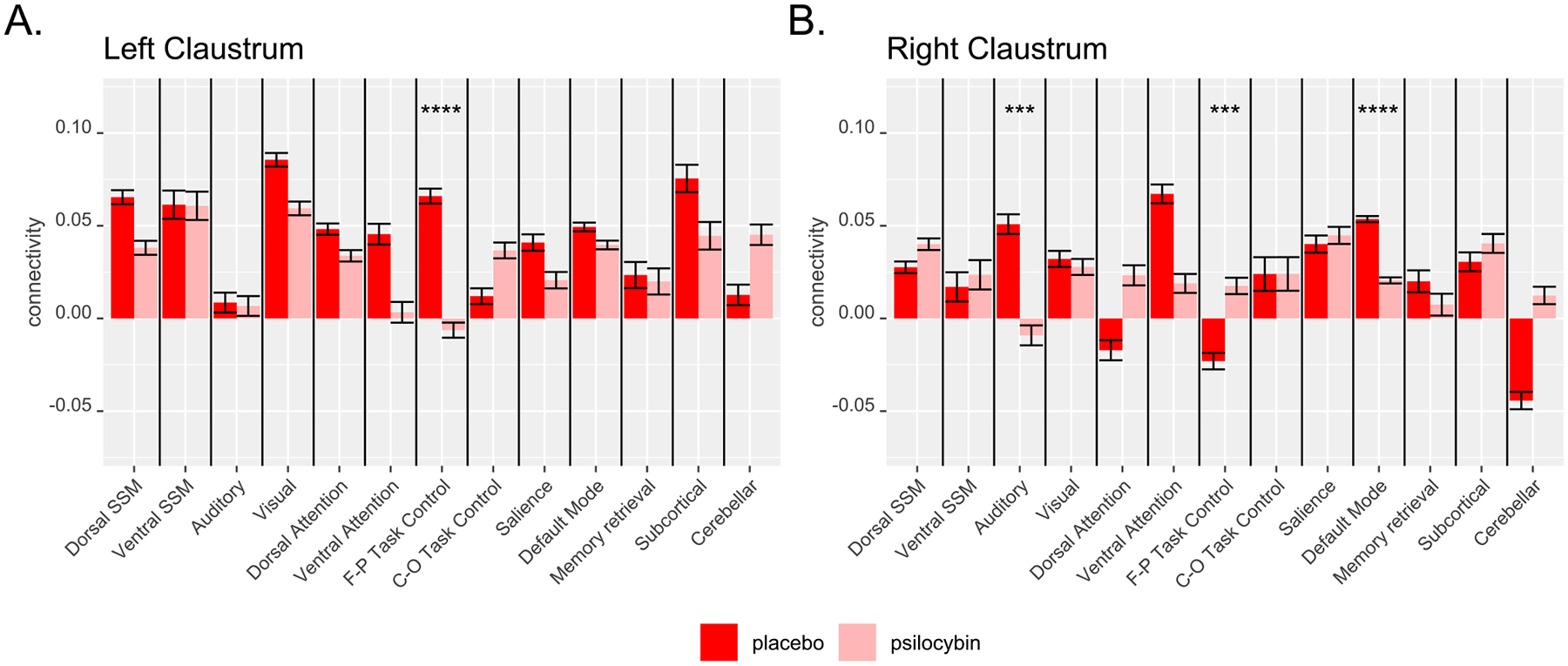
Effects of psilocybin on average left (A) and right (B) claustrum BOLD resting-state connectivity with brain networks defined in the Power atlas. SSM: sensory/somatomotor; C-O: cingulo-opercular; F-P: fronto-parietal. *** = p < 0.001, **** = p < 0.0001. Error bars show standard error.
3.5. Claustrum connectivity is associated with network integrity and modularity
Right claustrum connectivity with the DMN was positively associated with network integrity of the DMN when estimating a model including both psilocybin and placebo scans (b = 0.039, SE = 0.013, t[1738] = 3.063, p = 0.0022) and also when only modeling psilocybin scans (b = 0.04, SE = 0.0193, t[868], p = 0.0367; Fig. 4A). Right claustrum connectivity with the FPTC was negatively associated with network integrity of the FPTC when modeling both psilocybin and placebo scans (b = −0.097, SE = 0.025, t[747] = 3.904, p = 0.0001) but not when modeling only the psilocybin scan (b = −0.011, SE = 0.039, t[373] = 0.289, p = 0.773;Fig. 4B). Left claustrum connectivity with the FPTC was negatively associated with FPTC modularity when including both placebo and psilocybin scans in the model (b −0.023, SE = 0.0053, t[747] = −4.468, p < 0.0001) and also when only modeling the psilocybin scan (b = 0.024, SE = 0.0078, t[373] = 3.097, p = 0.0021; Fig. 4C). No other significant associations between ROI connectivity and network integrity or modularity were observed.
Fig. 4.
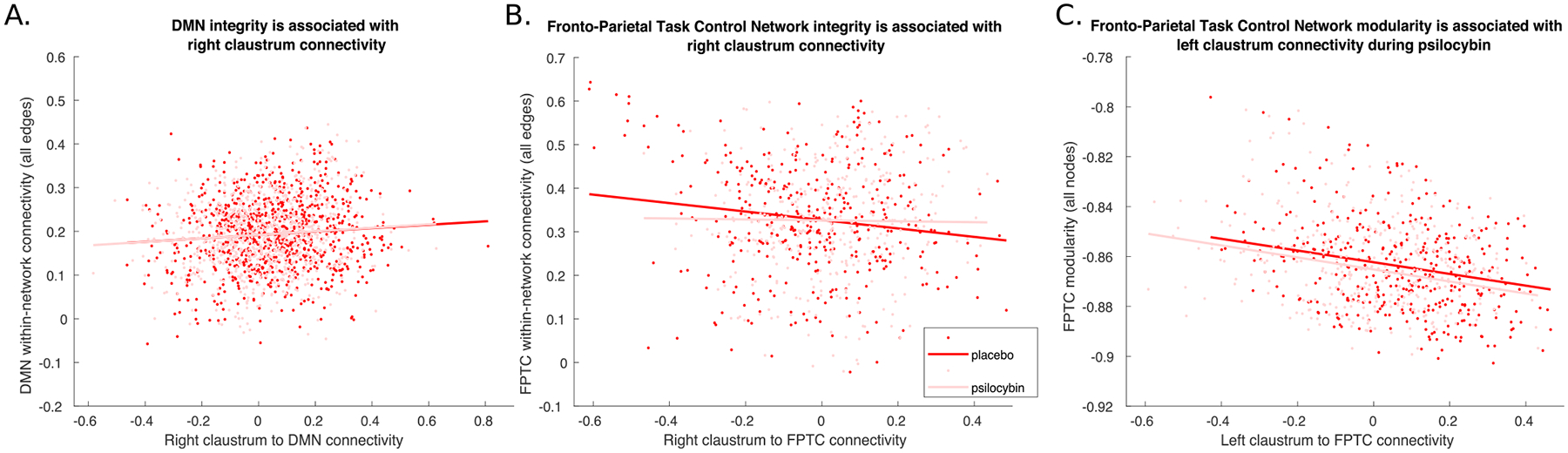
Network integrity and modularity is associated with claustrum connectivity. (A and B) Network integrity (within-network functional connectivity) of the default mode network (DMN) is positively associated with connectivity between the right claustrum and the DMN (A), and network integrity of the fronto-parietal task control network (FPTC) is negatively associated with connectivity between the right claustrum and the FPTC (B). Each dot in the figure represents a single node in the given network for a single individual during a given drug condition (placebo or psilocybin). The average within-network connectivity of a given node within each individual is plotted on the ordinate, and the connectivity of that given node with the right claustrum is plotted on the abscissa. (C) Network modularity (the inverse of the participation coefficient) of the FPTC is negatively associated with connectivity between the left claustrum and the FPTC. Each dot in each panel represents a single node in the given network for a single individual during a given drug condition (placebo or psilocybin). The network modularity for a given node within each individual and within each drug condition is plotted on the ordinate, and the connectivity of that given node with the left claustrum is plotted on the abscissa. Connectivity on each axis is expressed as a Pearson’s “r”. A line of best fit is plotted through each panel, separately for placebo and psilocybin conditions.
3.6. Associations between subjective and neural effects
Analysis of the association between subjective effects during resting state scans and the variance and ALFF of BOLD signal demonstrated a significant negative association between ratings of the overall strength of drug effects and both ALFF (t = 3.269, p[unc] = 0.006, R2adj = 0.409; Table 1) and variance (t = 3.386, p[unc] = 0.005, R2adj = 0.428; Table 2) of the right claustrum, brief total MEQ score and ALFF in the left claustrum (t = 2.239, p[unc] = 0.043, R2adj = 0.223; Table 1), and ineffability and right claustrum ALFF (t = 3.287, p[unc] = 0.006, R2adj = 0.412; Table 1), right claustrum variance (t = 3.47, p[unc] = 0.004, R2adj = 0.441; Table 2), left claustrum ALFF (t = 2.519, p[unc] = 0.026, R2adj = 0.276; Table 1), and left claustrum variance (t = 2.32, p[unc] = 0.037, R2adj = 0.239; Table 2). None of the subjective effects were associated significantly (α = 0.05, uncorrected) with change in the variance or ALFF of the left or right putamen or insula, and no other subjective effects were significantly associated with change in ALFF or variance of claustrum, insula, or putamen regions. While none of the above effects survived correction for multiple comparisons, these relationships yielded moderate coefficients of determination. These findings suggest specificity of the relationship between subjective effects and changes in the activity of the claustrum compared to flanking regions.
Table 1.
Association of subjective effects measures with amplitude of low frequency fluctuations (ALFF) in blood-oxygenation level-dependent (BOLD) signal in left and right claustrum, insula, and putamen regions of interest. Statistics are limited to equations involving a subjective effect that significantly predicted ALFF in at least one region of interest. ß = standardized regression coefficient of the independent variable. Equations where the independent variable is significant (critical α = 0.05, uncorrected) are presented in bold font. MEQ: mystical experience questionnaire.
| Equation (dependent variable ~ independent variable) | ß | t | F | p (unc) | R2adj |
|---|---|---|---|---|---|
| left claustrum ALFF ~ overall strength of drug effect | −0.0008 | 2.069 | 4.28 | 0.059 | 0.189 |
| left claustrum ALFF ~ brief total MEQ | −0.008 | 2.239 | 5.01 | 0.043 | 0.223 |
| left claustrum ALFF ~ ineffability | −0.0006 | 2.519 | 6.35 | 0.026 | 0.276 |
| right claustrum ALFF ~ overall strength of drug effect | −0.001 | 3.269 | 10.7 | 0.006 | 0.409 |
| right claustrum ALFF ~ brief total MEQ | −0.0008 | 1.975 | 3.9 | 0.069 | 0.172 |
| right claustrum ALFF ~ ineffability | −0.0008 | 3.287 | 10.8 | 0.006 | 0.412 |
| left insula ALFF ~ overall strength of drug effect | −2.67e-05 | 0.077 | 0.006 | 0.939 | −0.076 |
| left insula ALFF ~ brief total MEQ | −0.0002 | 0.660 | 0.436 | 0.521 | −0.042 |
| left insula ALFF ~ ineffability | −3.89e-05 | 0.172 | 0.029 | 0.866 | −0.075 |
| right insula ALFF ~ overall strength of drug effect | 7.17e-05 | 0.342 | 0.117 | 0.738 | −0.067 |
| right insula ALFF ~ brief total MEQ | −4.49e-05 | 0.241 | 0.058 | 0.813 | −0.072 |
| right insula ALFF ~ ineffability | 1.15e-06 | 0.008 | 7.03e-05 | 0.993 | −0.077 |
| left putamen ALFF ~ overall strength of drug effect | −9.03e-05 | 0.477 | 0.227 | 0.642 | −0.058 |
| left putamen ALFF ~ brief total MEQ | −1.78e-05 | 0.105 | 0.011 | 0.918 | −0.076 |
| left putamen ALFF ~ ineffability | 3.38e-05 | 0.271 | 0.073 | 0.791 | −0.071 |
| right putamen ALFF ~ overall strength of drug effect | −0.0003 | 1.137 | 1.29 | 0.276 | 0.020 |
| right putamen ALFF ~ brief total MEQ | −0.0002 | 0.829 | 0.687 | 0.422 | −0.023 |
| right putamen ALFF ~ ineffability | −0.0002 | 1.126 | 1.27 | 0.280 | 0.019 |
Table 2.
Association of subjective effects measures with variance in the BOLD signal in left and right claustrum, insula, and putamen regions of interest. Statistics are limited to equations involving a subjective effect that significantly predicted ALFF in at least one region of interest. ß = standardized regression coefficient of the independent variable. Equations where the independent variable is significant (critical α = 0.05, uncorrected) are presented in bold font. MEQ: mystical experience questionnaire.
| Equation (dependent variable ~ independent variable) | ß | t | F | p(unc) | R2adj |
|---|---|---|---|---|---|
| left claustrum variance ~ overall strength of drug effect | −0.0006 | 2.047 | 4.19 | 0.061 | 0.186 |
| left claustrum variance ~ brief total MEQ | −0.0005 | 2.018 | 4.07 | 0.065 | 0.180 |
| left claustrum variance ~ ineffability | −0.0004 | 2.32 | 5.4 | 0.037 | 0.239 |
| right claustrum variance ~ overall strength of drug effect | −0.001 | 3.386 | 11.5 | 0.005 | 0.428 |
| right claustrum variance ~ brief total MEQ | −0.0007 | 2.09 | 4.39 | 0.056 | 0.195 |
| right claustrum variance ~ ineffability | −0.0007 | 3.47 | 12 | 0.004 | 0.441 |
| left insula variance ~ overall strength of drug effect | −9.17e-06 | 0.123 | 0.015 | 0.904 | −0.076 |
| left insula variance ~ brief total MEQ | −5.20e-05 | 0.807 | 0.651 | 0.434 | −0.026 |
| left insula variance ~ ineffability | −1.05e-05 | 0.217 | 0.047 | 0.832 | −0.073 |
| right insula variance ~ overall strength of drug effect | 7.43e-06 | 0.193 | 0.037 | 0.850 | −0.074 |
| right insula variance ~ bM brief total MEQ EQ | −1.62e-05 | 0.478 | 0.229 | 0.641 | −0.058 |
| right insula variance ~ ineffability | −5.34e-06 | 0.212 | 0.045 | 0.836 | −0.073 |
| left putamen variance ~ overall strength of drug effect | −1.98e-05 | 0.498 | 0.248 | 0.627 | −0.057 |
| left putamen variance ~ brief total MEQ | −1.01e-05 | 0.285 | 0.081 | 0.780 | −0.070 |
| left putamen variance ~ ineffability | 4.61e-06 | 0.176 | 0.031 | 0.863 | −0.074 |
| right putamen variance ~ overall strength of drug effect | −9.31e-05 | 1.293 | 1.67 | 0.219 | 0.046 |
| Right putamen variance ~ brief total MEQ | −6.92e-05 | 1.06 | 1.13 | 0.307 | 0.009 |
| right putamen variance ~ ineffability | −6.27e-05 | 1.336 | 1.78 | 0.205 | 0.053 |
4. Discussion
The current report applied a recently developed method of interrogating claustrum function in humans (Krimmel et al., 2019a) to assess the effects of psilocybin on claustrum activity and connectivity. Psilocybin reduced measures of activity (variance and amplitude of low-frequency fluctuations) of both left and right claustrum during the acute effects of psilocybin, and led to alterations in both left and right claustrum connectivity with brain networks that support sensory and cognitive processes. Subjective effects (overall strength of drug effect, as well as mystical experience and/or ineffability scores while controlling for overall strength of drug effects) were associated with decreases in variance and ALFF of the claustrum, but not the flanking brain structures of the insula or putamen.
4.1. Psilocybin decreased claustrum connectivity with the auditory cortex
A reliable effect of a high psychedelic dose is a marked alteration of sensory experience, including the sense of being in a dream-like or imaginal state (Kraehenmann, 2017; Kraehenmann et al., 2017a, 2017b; Sanz and Tagliazucchi, 2018). Sensory alterations during psychedelic drug action include both subjective and neural alterations in both visual (Carter et al., 2004, 2007; Daumann et al., 2010; Roseman et al., 2016) and auditory perception (Barrett et al., 2017, 2018a; Eisner and Cohen, 1958) and auditory to visual synesthesia (Luke and Terhune, 2013; Terhune et al., 2016). Activity and connectivity of both visual (Roseman et al., 2016; Carhart-Harris et al., 2016b; Kaelen et al., 2016) and auditory (Barrett et al., 2017) sensory cortices are also associated with the strength of sensory effects of psychedelic drugs. Given the connectivity of the claustrum with sensory cortices, it is possible that the claustrum may contribute to psilocybin-induced disruptions in sensory experience.
4.2. Psilocybin alters claustrum connectivity with networks that support cognition
Subjective effects of psychedelic drugs are also characterized by shifts in attention and executive function (Barrett et al., 2018b; Preller and Vollenweider, 2016; Daumann et al., 2008). Psilocybin dose-dependent alterations in executive function have been demonstrated, including impaired associative learning, working memory, and episodic recall, as well as overall psychomotor slowing (Barrett et al., 2018b); these effects may reflect difficulties with attention (Barrett et al., 2018b). Such alterations in attention and executive function may manifest in commonly-reported subjective effects of psilocybin and other psychedelic drugs, including the often reported difficulty of putting the experience into words (ineffability), and the potentially challenging subjective effects of dissociation, depersonalization (Cohen, 1960), confusion, and paranoid delusions (Strassman, 1984). A sense of not being in control of the experience may also manifest, which in some cases may be reflected in the temporary feeling of losing one’s mind or going insane (Barrett et al., 2016). For these reasons, serotonergic (classic, 5-HT2A-mediated) psychedelic drugs were studied extensively (Vollenweider and Geyer, 2001; Vollenweider et al., 1998) in the search for pharmacological models of psychosis, though other drug classes have proven more useful in eliciting frank psychosis (Ellinwood et al., 1973; Snyder, 1973; Steeds et al., 2015).
Our findings demonstrate that psilocybin decreases functional connectivity of the right claustrum with DMN and auditory networks (task-negative and sensory networks), and increases right claustrum connectivity with the FPTC (a task-positive network). This is of particular interest given previous reports of increased DMN connectivity with auditory, visual, and task-positive networks during the acute effects of psilocybin (Roseman et al., 2014). Furthermore, in the present study, the subjective effects of psilocybin, and ineffability in particular, were found to be associated with measures of claustrum activity. Given the potential role of the claustrum in the initiation and stabilization of executive and task-based networks (Krimmel et al., 2019a) and top-down control of action (White et al., 2018), the claustrum may play a role in subjectively sensed alterations in executive function through aberrant modulation of frontal cortical regions with which the claustrum heavily connects in rodents (Mathur et al., 2009; White et al., 2017) and monkeys (Reser et al., 2017).
4.3. Psilocybin alters default mode network integrity and fronto-partial task control network modularity by reducing claustrum functional connectivity with these networks
Psilocybin acutely reduces DMN connectivity and increases the connectivity of task-positive networks (Carhart-Harris et al., 2012; Roseman et al., 2014). Psilocybin-evoked reductions in DMN connectivity correlate with ratings of subjective effects and peak (mystical, or ego-dissolutive) experiences (Carhart-Harris et al., 2012; Smigielski et al., 2019; Kometer et al., 2015). How this network disruption occurs is unknown. Our analyses demonstrate decreased connectivity between claustrum and the DMN during the effects of psilocybin, as well as a positive association of right claustrum connectivity with DMN integrity. These analyses also demonstrate decreased connectivity between left claustrum and the FPTC, as well as a negative association between left claustrum connectivity with the FPTC and modularity of this network. Together, the present findings support the notion that the claustrum may be involved at a circuit-level to exert psilocybin-induced disruptions in both the DMN and task-positive networks.
4.4. Association with subjective effects
While we did assess a limited range of subjective effects measures after each resting-state scan, including measures of mystical experience, the small sample renders the study potentially under-powered to detect relationships between brain measures and subjective reports. However, regressing the variance of BOLD signal and ALFF in the right and left corrected claustrum regions of interest revealed modest associations between right and left claustrum variance and amplitude and subjective measures of mystical experience, ineffability, and overall strength of drug effect. Identified associations are specific to the right and left claustrum and are not reflected in the insula or the putamen, providing evidence supporting the hypothesis that disruption of claustrum function may be associated with subjective effects of psilocybin.
4.5. Limitations
The present findings have a number of limitations. First, the order of drug administration was fixed across participants, with placebo always administered first. This design feature reduced potential carry-over effects of exposure, as substantial effects of this dose in healthy individuals may persist for at least 6 h after administration (Griffiths et al., 2011; Carbonaro et al., 2018). Participants were instructed that they may receive placebo, an extremely low dose, or moderately low dose of psilocybin in either scan in the current study. After the first scan (placebo), more than half of the participants provided ratings indicating that they thought they may have received psilocybin, suggesting that blinding was intact and that expectancy due to drug condition order effects was not a major confound. Order effects related to comfort in the MRI environment have been previously noted, but the participants within this study had all recently completed two or three MRI sessions, and were oriented to and reported being comfortable within the MRI environment, which gives us confidence that effects we have observed are not simply due to acclimation to the MRI environment. However, it is still possible that diurnal variations in stress hormones may be a contributing factor to these findings. Second, only a single dose of psilocybin was assessed. Multiple active dose conditions may have allowed for an analysis of dose-dependent effects on brain function. Third, the sample was highly selected, consisting of individuals who had a long-term meditation practice and who had previously been administered a high dose of psilocybin in an experimental setting. The average age of this sample was also rather high (>50 years old) compared to most other studies in the field.
While the dose administered in the current study was moderate (10 mg/70 kg) and lower than those doses (e.g. 25 mg/70 kg) evaluated for clinical efficacy in the context of mood disorders (Carhart-Harris et al., 2016a; Griffiths et al., 2016; Ross et al., 2016) and substance use disorders (Bogenschutz et al., 2015; Johnson et al., 2014), a 10 mg/70 kg dose has been shown to reliably induce a substantially altered state of consciousness in dose effect studies of both subjective (Griffiths et al., 2011; Hasler et al., 2004; Pokorny et al., 2016; Carbonaro et al., 2018) and cognitive (Barrett et al., 2018b; Carter et al., 2004, 2005; Wittmann et al., 2007; Wackermann et al., 2008; Vollenweider et al., 2007) effects of psilocybin.
4.6. Future directions
While resting-state connectivity can be useful for understanding circuit-level brain function, more can be learned by integration of a task-based/behavioral paradigm during scanning. Comparing the effects of psilocybin and other drugs on activity and connectivity of the claustrum in the context of cognitive and behavioral tasks should be explored, and understanding drug-induced changes in dynamic and effective connectivity within this and other imaging modalities during rest and task will greatly expand our understanding of the effects of psychedelic and other drugs on claustrum function. Further investigation of laterality effects in psilocybin function should also be explored. As a future direction that may apply broadly to the psychedelic field, comparison of the effects of psychedelic drugs in participants with and without previous exposure to psychedelics will be important to understanding interactions between the acute and enduring effects of these compounds. While clear effects of an interaction between drug condition and network on claustrum connectivity were observed (see Fig. 1), we also observed a large distribution of claustrum connectivity values (see Figure S1). Though the current sample was not large enough to investigate individual differences in claustrum connectivity or effects of psilocybin on claustrum connectivity, future studies or mega-analyses (e.g. pooled analysis of primary, subject-level data from multiple datasets) may benefit from methods such as clustering, classification, or machine learning approaches to search for unique patterns in the connectivity of the claustrum with canonical brain networks among sub-sets of individuals.
5. Conclusion
Given the network disruptions that underlie neuropsychiatric disorders (Bressler and Menon, 2010; Cao et al., 2006; Gutierrez et al., 2009; Uhlhaas and Singer, 2010), including mood (Leibenluft and Pine, 2013; Zhang et al., 2016) and substance use disorders (Sutherland et al., 2012; Pariyadath et al., 2016), the widespread connectivity of the claustrum suggests this structure may be implicated in those pathologies. Indeed, claustrum volume reductions occur in depression and schizophrenia (Bernstein et al., 2016). The current report utilizes a pharmacological intervention to provide empirical evidence for a significant role of 5-HT2A signaling in claustrum function, provides evidence for the potential effects of psilocybin on claustrum activity and connectivity, and highlights the need for additional efforts to further explore the potential role of the claustrum in both the subjective and therapeutic effects of psilocybin (Nichols, 2016; Nichols et al., 2017).
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Terri Brawner, Ivana Kusevic, and Kathleen Kahl for their contribution to the acquisition of the MR data, as well as Mary Cosimano, M.S.W., Taylor Marcus, Annie Umbricht, M.D., Darrick May, M.D., Theresa Carbonaro, Ph.D., Laura Doyle, and Kishore Bharadwaj for their contributions monitoring participants during the course of acute drug effects.
Funding and disclosures
This work was supported by a grant from the Heffter Research Institute (R.R.G.), National Institute on Alcohol Abuse and Alcoholism grant R01AA024845 (B.N.M.), National Institute on Drug Abuse grant R03DA042336 (F.S.B.), and by the financial support of the Johns Hopkins Center for Psychedelic and Consciousness Research by Tim Ferriss, Matt Mullenweg, Craig Nerenberg, Blake Mycoskie, and the Stephen and Alexandra Cohen Foundation. Dr. Griffiths is a board member of the Heffter Research Institute. No other authors have conflicts of interest.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.116980.
References
- Aghajanian GK, Marek GJ, 1997. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36, 589–599. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K, 2005. J. Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR, 2015. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J. Psychopharmacol 29, 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos J-MS, Johnson MW, Griffiths RR, 2016. The Challenging Experience Questionnaire: characterization of challenging experiences with psilocybin mushrooms. J. Psychopharmacol 30, 1279–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Preller KH, Herdener M, Janata P, Vollenweider FX, 2017. Serotonin 2A receptor signaling underlies LSD-induced alteration of the neural response to dynamic changes in music. Cerebr. Cortex 1–12. 10.1093/cercor/bhx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Preller KH, Kaelen M, 2018. Psychedelics and music: neuroscience and therapeutic implications. Int. Rev. Psychiatr 30, 350–362. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Carbonaro TM, Hurwitz E, Johnson MW, Griffiths RR, 2018. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: effects on cognition. Psychopharmacology (Berlin). 10.1007/s00213-018-4981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H-G, et al. , 2016. Bilaterally reduced claustral volumes in schizophrenia and major depressive disorder: a morphometric postmortem study. Eur. Arch. Psychiatr. Clin. Neurosci 266, 25–33. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, et al. , 2015. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol 29, 289–299. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V, 2010. Large-scale brain networks in cognition: emerging methods and principles. Trends Cognit. Sci 14, 277–290. [DOI] [PubMed] [Google Scholar]
- Cao Q, et al. , 2006. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport 17, 1033–1036. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Johnson MW, Hurwitz E, Griffiths RR, 2018. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology (Berlin) 235, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. , 2012. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. U.S.A 109, 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. , 2016. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3, 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. , 2016. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. U.S.A 113, 4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, et al. , 2004. Psilocybin impairs high-level but not low-level motion perception. Neuroreport 15, 1947–1951. [DOI] [PubMed] [Google Scholar]
- Carter OL, et al. , 2005. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cognit. Neurosci 17, 1497–1508. [DOI] [PubMed] [Google Scholar]
- Carter OL, et al. , 2007. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berlin) 195, 415–424. [DOI] [PubMed] [Google Scholar]
- Cohen S, 1960. Lysergic acid diethylamide: side effects and complications. J. Nerv.Ment. Dis 130, 30–40. [DOI] [PubMed] [Google Scholar]
- Daumann J, et al. , 2008. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berlin) 200 (4), 573–583. [DOI] [PubMed] [Google Scholar]
- Daumann J, et al. , 2010. Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J. Psychopharmacol 24, 1515–1524. [DOI] [PubMed] [Google Scholar]
- Eisner BG, Cohen S, 1958. Psychotherapy with lysergic acid diethylamide. J. Nerv.Ment. Dis 127, 528–539. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Sudilovsky A, Nelson LM, 1973. Evolving behavior in the clinical and experimental amphetamine (model) psychosis. Aust. J. Pharm 130, 1088–1093. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, et al. , 2011. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berlin) 218, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, et al. , 2016. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J. Psychopharmacol 30, 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez RC, et al. , 2009. Altered synchrony and connectivity in neuronal networks expressing an autism-related mutation of neuroligin 3. Neuroscience 162, 208–221. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B, 2013. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82, 208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX, 2004. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berlin) 172, 145–156. [DOI] [PubMed] [Google Scholar]
- Holm SA, 1979. Simple sequentially rejective multiple test procedure. Scand. J. Stat 6, 65–70. [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR, 2008. Human hallucinogen research: guidelines for safety. J. Psychopharmacol 22, 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR, 2014. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol 28, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, et al. , 2016. LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur. Neuropsychopharmacol 26, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Kometer M, Vollenweider FX, 2018. Serotonergic hallucinogen-induced visual perceptual alterations. Curr Top Behav Neurosci 36, 257–282. [DOI] [PubMed] [Google Scholar]
- Kometer M, Cahn BR, Andel D, Carter OL, Vollenweider FX, 2011. The 5-HT2A/1A agonist psilocybin disrupts modal object completion associated with visual hallucinations. Biol. Psychiatr 69, 399–406. [DOI] [PubMed] [Google Scholar]
- Kometer M, Pokorny T, Seifritz E, Volleinweider FX, 2015. Psilocybin-induced spiritual experiences and insightfulness are associated with synchronization of neuronal oscillations. Psychopharmacology (Berlin) 232, 3663–3676. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, 2017. Dreams and psychedelics: neurophenomenological comparison and therapeutic implications. Curr. Neuropharmacol 15, 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, et al. , 2015. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol. Psychiatr 78, 572–581. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, et al. , 2015. The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. Neuroimage Clin 11, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, et al. , 2017. LSD increases primary process thinking via serotonin 2A receptor activation. Front. Pharmacol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, et al. , 2017. Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berlin) 234, 2031–2046. [DOI] [PubMed] [Google Scholar]
- Krimmel SR, et al. , 2019. Resting state functional connectivity and cognitive task-related activation of the human claustrum. Neuroimage 196, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimmel SR, et al. , 2019. Resting state functional connectivity of the rat claustrum. Front. Neuroanat 13, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Pine DS, 2013. Resting state functional connectivity and depression: in search of a bottom line. Biol. Psychiatr 74, 868–869. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Geuter S, Wager TD, Caffo BS, 2019. Modular preprocessing pipelines can reintroduce artifacts into fMRI data. Hum. Brain Mapp 40, 2358–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke DP, Terhune DB, 2013. The induction of synaesthesia with chemical agents: a systematic review. Front. Psychol 4, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean KA, Leoutsakos J-MS, Johnson MW, Griffiths RR, 2012. Factor Analysis of the mystical experience questionnaire: a study of experiences occasioned by the hallucinogen psilocybin. J. Sci. Stud. Relig 51, 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur BN, 2014. The claustrum in review. Front. Syst. Neurosci 8, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur BN, Caprioli RM, Deutch AY, 2009. Proteomic analysis illuminates a novel structural definition of the claustrum and insula. Cerebr. Cortex 19, 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna DJ, Saavedra JM, 1987. Autoradiography of LSD and 2,5-dimethoxyphenylisopropylamine psychotomimetics demonstrates regional, specific cross-displacement in the rat brain. Eur. J. Pharmacol 142, 313–315. [DOI] [PubMed] [Google Scholar]
- Murphy K, Fox MD, 2017. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J, et al. , 2014. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, et al. , 2013. Broadband cortical desynchronization underlies the human psychedelic state. J. Neurosci 33, 15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, 2016. Psychedelics. Pharmacol. Rev 68, 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Johnson MW, Nichols CD, 2017. Psychedelics as medicines: an emerging new paradigm. Clin. Pharmacol. Ther 101, 209–219. [DOI] [PubMed] [Google Scholar]
- Pariyadath V, Gowin JL, Stein EA, 2016. Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog. Brain Res 224, 155–173. [DOI] [PubMed] [Google Scholar]
- Patel AX, et al. , 2014. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage 95, 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Cortés R, Palacios JM, 1985. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 346, 231–249. [DOI] [PubMed] [Google Scholar]
- Petri G, et al. , 2014. Homological scaffolds of brain functional networks. J. R. Soc. Interface 11, 20140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny T, Preller KH, Kraehenmann R, Vollenweider FX, 2016. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol 26, 756–766. [DOI] [PubMed] [Google Scholar]
- Pokorny T, Duerler P, Seifritz E, Vollenweider FX, Preller KH, 2019. LSD acutely impairs working memory, executive functions, and cognitive flexibility, but not risk-based decision-making. Psychol. Med 1–10. 10.1017/S0033291719002393. [DOI] [PubMed] [Google Scholar]
- Power JD, et al. , 2011. Functional network organization of the human brain. Neuron 72, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, Martin A, 2017. Sources and implications of whole-brain fMRI signals in humans. Neuroimage 146, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Vollenweider FX, 2016. Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci. 10.1007/7854_2016_459. [DOI] [PubMed] [Google Scholar]
- Reser DH, et al. , 2014. Claustrum projections to prefrontal cortex in the capuchin monkey (Cebus apella). Front. Syst. Neurosci 8, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser DH, et al. , 2017. Topography of claustrum and insula projections to medial prefrontal and anterior cingulate cortices of the common marmoset (Callithrix jacchus). J. Comp. Neurol 525, 1421–1441. [DOI] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL, 2014. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front. Hum. Neurosci 8, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, et al. , 2016. LSD alters eyes-closed functional connectivity within the early visual cortex in a retinotopic fashion. Hum. Brain Mapp 37, 3031–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, et al. , 2016. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol 30, 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O, 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Sanz C, Tagliazucchi E, 2018. The experience elicited by hallucinogens presents the highest similarity to dreaming within a large database of psychoactive substance reports. Front. Neurosci 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F, 2013. The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacology (Berlin) 225, 227–239. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY, 2000. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J. Neurosci 20, 8846–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY, 2003. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci. Lett 346, 137–140. [DOI] [PubMed] [Google Scholar]
- Smigielski L, Scheidegger M, Kometer M, Vollenweider FX, 2019. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage 196, 207–215. [DOI] [PubMed] [Google Scholar]
- Snyder SH, 1973. Amphetamine psychosis: a ‘model’ schizophrenia mediated by catecholamines. Aust. J. Pharm 130, 61–67. [DOI] [PubMed] [Google Scholar]
- Steeds H, Carhart-Harris RL, Stone JM, 2015. Drug models of schizophrenia. Ther Adv Psychopharmacol 5, 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman RJ, 1984. Adverse reactions to psychedelic drugs. A review of the literature. J. Nerv. Ment. Dis 172, 577–595. [DOI] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX, 2011. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J. Psychopharmacol 25, 1434–1452. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA, 2012. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage 62, 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhune DB, et al. , 2016. A placebo-controlled investigation of synaesthesia-like experiences under LSD. Neuropsychologia 88, 28–34. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci 11, 100–113. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA, 2001. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res. Bull 56, 495–507. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, B€abler A, Vogel H, Hell D, 1998. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB, 2007. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 32, 1876–1887. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, Vollenweider FX, 2008. Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci. Lett 435, 51–55. [DOI] [PubMed] [Google Scholar]
- White MG, Mathur BN, 2018. Claustrum circuit components for top-down input processing and cortical broadcast. Brain Struct. Funct 223, 3945–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, et al. , 2017. Cortical hierarchy governs rat claustrocortical circuit organization. J. Comp. Neurol 525, 1347–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, et al. , 2018. Anterior cingulate cortex input to the claustrum is required for top-down action control. Cell Rep. 22, 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon, Conn A, 2012. A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Wittmann M, et al. , 2007. Effects of psilocybin on time perception and temporal control of behaviour in humans. J. Psychopharmacol 21, 50–64. [DOI] [PubMed] [Google Scholar]
- Zang Y-F, et al. , 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. [DOI] [PubMed] [Google Scholar]
- Zhang K, et al. , 2016. Molecular, functional, and structural imaging of major depressive disorder. Neurosci Bull 32, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


