Abstract
Phellinus spp. have historically been used as traditional medicines to treat various diseases owing to their antioxidant, antitumor, and antidiabetic activities. Polysaccharides exhibit antidiabetic activity. In the present study, the polysaccharide contents of four Phellinus strains were compared. Phellinus igniarius QB72 possessed higher polysaccharide production, stronger 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, and α-amylase inhibitory activity. The three polysaccharides were sequentially extracted and partially purified from the fermentation mycelia using hot water, 1 % (NH4)2C2O4, and 1.25 M NaOH. Hot water extract polysaccharides exhibited higher DPPH radical scavenging and strong inhibitory activity against α-amylase with an IC50 value of 6.84 ± 0.37 mg/mL. The carbohydrate content of A1 (approximately 17457 Da) was approximately 88.28 %. The α-amylase inhibitory activity IC50 was decreased (3.178 ± 0.187 mg/mL) after DEAE water elution. P. igniarius QB72 hot-water extracts of partially purified polysaccharides have great potential as α-amylase inhibitors in food and medication-assisted additives.
Keywords: Phellinus igniarius QB72, Fermentation mycelia, Polysaccharides, α-amylase inhibitory
1. Introduction
Diabetes mellitus (DM) is a group of chronic diseases characterized by high blood glucose levels, leading to systemic disruption of all types of metabolism and damage to the micro- and macrovascular systems of the body [1]. Among all types of diabetes mellitus, type 2 diabetes mellitus (T2DM) is the main pathophysiological reason for DM development. The worldwide incidence of T2DM is expected to increase to 300 million by 2025 [2]. Controlling postprandial blood glucose levels is vital in the treatment of T2DM. α-amylase and α-glucosidase are two key enzymes regulating blood glucose absorption postprandial. Effective carbohydrate hydrolysis by these two enzyme inhibitors is a feasible therapeutic method for ameliorating T2DM by alleviating postprandial hyperglycemia [3]. Medicinal mushrooms have been used for centuries because of their natural bioactive compounds, which can be hypoglycemic and antidiabetic agents. Bioactive ingredients, such as polysaccharides, phytonutrients, terpenoids, and other compounds from fruiting bodies, cultured mycelia, and cultivated broth of mushrooms, have been successfully used as biological antihyperglycemic agents with no harmful side effects in clinical and experimental settings [4,5]. The polysaccharide AAMP-N significantly reduces blood glucose levels, improves lipid metabolism, and quenches superoxide and hydroxyl radicals in vitro [6]. In addition, oxidative stress is a major mediator of diabetes and can influence each other to worsen diabetes [7]. Antioxidants can also be used to treat DM. Therefore, natural products with antioxidant properties can be used to prevent and treat diabetes [8]. Mushrooms contain antioxidants such as polysaccharides, flavonoids, and phenolic compounds, which play important roles in alleviating diseases such as diabetes, inflammation, cancer, and heart disease by fighting reactive oxygen species (ROS) [6]. Phellinus Quél is one of the largest genera in Hymenochaetaceae, with approximately 220 species identified to date [9]. Some Phellinus fungi, such as P. igniarius, Phellinus baumii, Phellinus linteus, and Phellinus vaninii, are recorded as traditional medicines for disease treatment in eastern Asian countries [10]. Polysaccharides are responsible for the therapeutic potential of the active metabolites of Phellinus mushrooms, and possess excellent antioxidant, antidiabetic, triglyceride absorption, obesity, and anticancer activities [11]. Mushroom polysaccharides ameliorate hyperglycemia and hypocholesteremia associated with diabetes, reduce the blood glucose level and free radical damage, reduce liver lipid peroxidation, elevate the hepatic antioxidant system, increase glycogen and insulin levels, and aid in the recovery of injured pancreatic β-cells [2].
Healthy commercial products from medicinal mushrooms are typically obtained from fruiting bodies [12]. Mushroom biomass (mycelia) can be consumed in the form of tablets and powders as functional foods and dietary supplements. Submerged fermentation of mushrooms is a promising alternative for efficient production of valuable products from biomass fermentation [13].
In this study, four Phellinus strains were selected for evaluation of their growth characteristics, fermentation biomass, and antioxidant and α-amylase inhibitory activities. These three solvents were then used to sequentially extract polysaccharides from the fermentation mycelia. The characteristics of these polysaccharides and their antioxidant and α-amylase inhibitory activities were evaluated.
2. Materials and methods
2.1. Strains
P. igniarius QB72 is a mutant obtained by pulsed light irradiation and screened using a laccase screening plate. The wild-type strain used for pulsed-light irradiation was P. igniarius 5.95, which was obtained from the China General Microbiological Culture Collection Center (CGMCC). 2H is a wild-type strain from Jilin Province, Baishan City, and 5.1260 and 5.891 are two strains from CGMCC. All four strains were identified using molecular biology methods (Beijing Tsingke Biotech Co., Ltd.). The plate, liquid, and fermentation media were prepared as described previously [14]. The cultivation time was 13 d for comparison of fermentation biomass.
2.2. Reagents and standards
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were obtained from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Acarbose (purity >95 %) and α-amylase (11 units/mg) were purchased from Porcine Panireas. Uronic acid and glucose were of (HPLC) grade. All other chemicals and reagents were of analytical grade.
2.3. Preparation of the mycelia extracts
2.3.1. Strains for polysaccharides
Before sequential polysaccharide extraction, strains for fermentation from four Phellinus strains were selected for comparison. The growth speed on the plate and the liquid fermentation were considered, and the DPPH radical scavenging ability and α-amylase inhibitory activity were evaluated in this study.
The mycelial growth rates (mm/d) of the four strains were measured using the cross method [15]. Mycelium length was measured every 2 d, and the average growth speed of each strain was calculated. The polysaccharide content was also evaluated to compare the four strains.
2.3.2. Polysaccharide extraction
Freeze-dried mycelial powder was prepared after 8 d of fermentation by P. igniarius QB72. The freeze-dried mycelial powder was ground to a fine powder (80-mesh sieve) using a laboratory mill. A diagram of the extraction of the freeze-dried mycelial powder is shown in Fig. 1.
Fig. 1.
Scheme for extraction from the Phellinus fermentation mycelia by different extraction media.
To obtain water extracts, an extraction procedure was performed according to a previously described method, with some modifications [16]. Ten grams of the freeze-dried mycelia powder was extracted using 200 mL of distilled water for 3 h at 90 °C and centrifuged at 8000 r/min for 10 min (4 °C). The residue was extracted twice, and the supernatant was combined and concentrated to 100 mL under vacuum at 40 °C using a rotary evaporator (A1). The residue from the water extract was extracted again with 200 mL of 1 %(NH4)2C2O4 for 3 h at 90 °C. After centrifugation at 8000 r/min for 10 min (4 °C), extraction was repeated twice using the same method, and the supernatant was combined and concentrated to 100 mL under vacuum at 40 °C using a rotary evaporator (A2). The residue from the 1 %(NH4)2C2O4 extract was extracted with 200 mL of 1.25 M NaOH at 25 °C for 3 h, then extracted twice as previously described. The supernatants were combined, neutralized using 36 % acetic acid, and concentrated to 100 mL under vacuum at 40 °C using a rotary evaporator (A3). The supernatant was concentrated to 100 mL, precipitated with four volumes of ethanol at 4 °C overnight, and centrifuged at 8000 rpm for 10 min. The three precipitates were dissolved in distilled water, deproteinized using Sevage reagent, and dialyzed using an 8000–10000 Da dialysis membrane (RC). All the samples were freeze-dried to obtain partially purified polysaccharides. UV light was measured using a JASCO V-530 UV/VIS spectrophotometer to determine the purity of the polysaccharides [17].
2.4. Principal properties of polysaccharides
The polysaccharide content was measured using the phenol-sulfuric acid colorimetric method, with glucose as a standard [18]. One hundred microliters of crude polysaccharide solution and 100 μL of 6 % phenol were mixed, then 500 μL of concentrated sulfuric acid was added and the mixture was maintained at room temperature(10°C-30 °C) for 30 min. The absorbance was recorded at 490 nm using a microplate reader.
The protein content of the three extracts was determined using the Folin-Ciocalteu method [19], with some modifications using casein as a standard. An appropriately diluted extract (50 μL) was placed in a 2 mL sterilized centrifugal tube, and distilled water was added to a total volume of 0.1 mL. Then, an alkaline copper sulfate reagent (0.5 mL) was added and incubated for 10 min at room temperature. Folin-Ciocalteu's reagent (50 μL) was added, shaken, and incubated for 30 min at room temperature. The absorbance was measured at 750 nm using a microplate reader. Distilled water was used as a blank.
The uronic acid content of the three samples was determined using the sulfuric acid-carbazole method, with glucuronic acid as a standard [20]. With minor modifications, the assay mixture (720 μL) contained 100 μL diluted samples or water as a blank. Then, 600 μL borate sodium sulfuric acid reagent was added. After vortexing and boiling for 20 min, 20 μL of 0.2 % carbazole ethanol was added to the mixtures and boiled for another 10 min. After cooling to room temperature, absorbance was recorded at 520 nm using a microplate reader.
2.5. The polysaccharide characteristics of the extracts
2.5.1. Fourier-transform infrared spectroscopy (FT-IR)
FT-IR spectra were obtained using a Spectrum Two FT-IR spectrometer in the range 4000–400 cm−1 (Thermo Fisher, SCIENTIFIC) [21]. Polysaccharides (1 mg) were ground using potassium bromide (KBr) powder and compressed into slices.
2.5.2. Determination of the molecular weight
High-performance gel permeation chromatography (HPGPC) (Shimadzu, Japan) was used to determine the homogeneity and average molecular weight of the polysaccharide fractions. The HPGPC system was equipped with a TSKgel GMPWXL column (7.8 × 300 mm, column temperature 35 °C) and Refractive Index Detector (RID-20). A 20 μL sample solution was performed in each run at a flow rate of 0.6 mL/min, and 0.1 N NaNO3+0.05 % NaN3 was their mobile phase. The column was calibrated with pullulan (642000, 334000, 49400, 22000, and 6300) as the standard, and the molecular weights of the three fractions were measured using the calibration curve.
2.5.3. Congo red test
The Congo red test was used to assess the chain conformation of the polysaccharides, in which a red shift in maximum absorption indicated that the polysaccharide chains contained triple helices [22]. Sample aqueous solution (1 mg/mL) 50 μL was combined with the 91 μM Congo red 50 μL, then the mixture was treated separately with 4 M NaOH at various concentrations ranging 0–0.5 M for 25 min at room temperature. The visible light absorption spectrum of the solution was obtained over the 400–600 nm range using a microplate reader with deionized water as a control.
2.6. The bioactivity of the extracts
2.6.1. Antioxidant activity
The three antioxidant activities were evaluated to compare the bioactivities of the extracts.
2.6.1.1. Ferric reducing antioxidant power (FRAP)
The FRAP assay of the polysaccharide samples was performed according to the method reported by Benzie and Strain [23]. The FRAP solution, acetate buffer (300 mM, pH 3.6), TPTZ (10 mM, dissolved in 40 mM HCl), and FeCl3 (20 mM) were mixed at a ratio of 10:1:1 in the FRAP assay solution [24], which was freshly prepared.
Twenty microliters of each polysaccharide fraction combined with 180 μL of FRAP solution was placed in a 96-well microplate (Costar, Cambridge, MA). The cells were incubated at 37 °C for 30 min in the dark. Absorbance was measured at 595 nm using a microplate reader.
2.6.1.2. ABTS
The ABTS radical scavenging ability was measured according to a previously described method with some modifications [18]. The resulting ABTS·+ solution (500 μL) was mixed with 25 μL of diluted sample, and after 10 min of reaction, the absorbance was read at 734 nm. The scavenging rate of each sample was calculated as follows:
where Abss is the absorbance of the sample and Absb is the absorbance value of the blank. where Absc is the absorbance of the control sample.
2.6.1.3. DPPH
DPPH radical scavenging ability was evaluated according to a previously described method with some modifications [25]. One hundred microliters of polysaccharide extract was mixed with 100 μL of 0.2 mM DPPH solution. The reaction mixture was incubated in the dark for 30 min at room temperature and the absorbance was read at 517 nm using a microplate reader. The DPPH radical-scavenging rate was calculated using the following equation:
where Abss is the absorbance of the sample, Absb is the absorbance value of the blank, And Absc is the absorbance of the control sample.
2.6.2. α-Amylase inhibitory assay
The α-amylase inhibitory activity was determined according to a previous method with minor modifications [26]. The reaction was incubated in a 200 μL EP tube. Twenty-five microliters of 5 mg/mL mushroom extracts was added to 25 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing porcine pancreatic α-amylase (1.0 U/mL), then the mixture was incubated at 37 °C for 10 min. Then, 50 μL of 1 % starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) was added. Incubated at 37 °C for an additional 3 min and stopped with 100 μL of dinitrosalicylic acid color reagent. After boiling for 8 min and cooling to room temperature, absorbance was measured at 540 nm using a microplate reader. Acarbose was used as a positive control.
2.6.3. Kinetics analysis for the α-amylase inhibition type
The enzyme inhibitory kinetics of acarbose on α-amylase were investigated using the Michaelis-Menten method with Lineweaver-Burk double reciprocal plots [27,28].
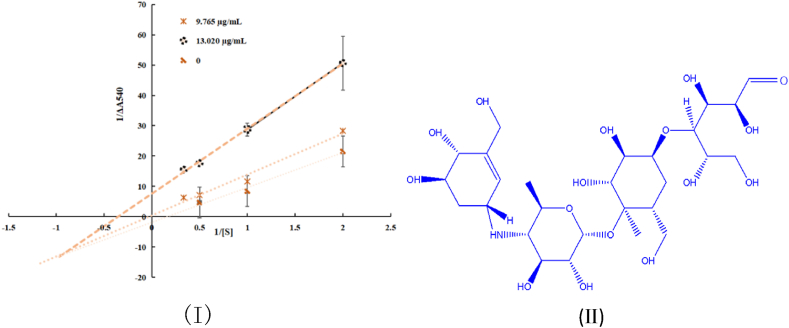
For α-amylase, 25 μL of acarbose with varying concentrations was first preincubated with 25 μL of α-amylase in phosphate-buffered saline (PBS; (0.1 mol/L, pH 6.9) at 37 °C for 10 min. The reaction was initiated by adding 50 μL cooked starch solution (1 %), and the mixtures were incubated at 37 °C for 3 min. After termination of the reaction with 100 μL of dinitrosalicylic acid, the tubes were incubated in a boiling water bath for 8 min followed by cooling using an ice bath. Absorbance was measured at 540 nm to determine the initial velocity (v). The α-amylase concentration versus v at various acarbose concentrations was evaluated to determine the reversibility of acarbose inhibition. The v values at different acarbose concentrations (9.765, 13.020 μg/mL) for different starch concentrations (0.125–2.0 mg/mL) in the presence of α-amylase were determined using the above method to estimate the mode of inhibition on α-amylase.
2.7. DEAE isolation and purification of A1
A1 (100 mg) was dissolved and injected into a DEAE-52 column (26 mm × 30 cm), followed with a stepwise elution with increasing concentrations of NaCl (0, 0.01, 0.05, 0.1, 0.2 M and 0.3 M NaCl) at a rate of 0.5 mL/min. After concentration, lyophilization, the fraction that eluted with water was collected. The principal properties of polysaccharides of the water elution part was determined and the α-amylase inhibitory activity was also evaluated.
2.8. Statistical analysis
The results were analyzed for statistical significance using one-way analysis of variance (ANOVA) in SPSS 17.0. All data are expressed as the mean ± S.D. The test numbers are shown at the corresponding locations in the figure and tables.
3. Results
3.1. Strain selection for mycelia fermentation
Four strains were selected the test strains for mycelial fermentation. The growth characteristics differed according to the different strains (Fig. 2. A), with 5.891 having the highest growth rate among the four strains, 2H and 5.126 having almost the same growth rate, and P. igniarius QB72 having the lowest growth rate (5.057 mm/d).
Fig. 2.
The strains and their characters. (A) the growth characters of the four strains on the plate. (B) the growth speed of the four strains (n = 13), the mycelia fermentation (n = 10) of the biomass fermentation, and the polysaccharide production (n = 4). (C) The DPPH radical scavenging ability of hot water extractions of the four strains(n = 3), Vc 0.025 mg/mL (D) α-amylase inhibitory activity of hot water extracts the four strains (n = 5), acrobose 0.625 mg/mL (n = 3).
The 5.891 and 2H strains had relatively high growth rates, and the hyphal margins of the two strains were sparse. At 5.1260, the growth rate was higher than that of P. igniarius QB72; however, the lawn of P. igniarius QB72 was thicker than that at 5.1260 (Fig. 2. B). Interestingly, the mycelial fermentation of P. igniarius QB72 was the highest among the four strains (29.60 g/L), and strains 5.891 and 5.1260 had nearly the same biomass (18.6 and 18.76 g/L), whereas for strain 2H, the mycelia fermentation was not as high as that of the other strains (Fig. 2. B). The same trend was observed for polysaccharide production: P. igniarius QB72 had the highest polysaccharide production compared to the other three Phellinus strains (Fig. 2. B). For bioactivity, the highest DPPH radical scavenging ability occurred in the 5.891 strain (Fig. 2. C); however, for α-amylase inhibitory activity, the highest inhibitory activity occurred in the P. igniarius QB72 strains (Fig. 2. D). Collectively, P. igniarius QB72 was selected as the strain for polysaccharide purification.
3.2. Preliminary properties and compositions of three partially purified polysaccharides
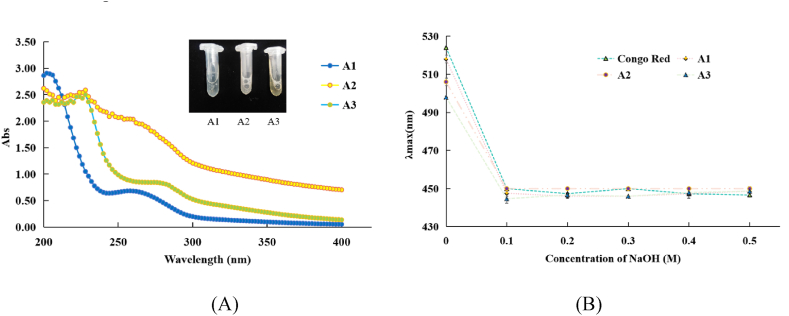
Polysaccharide components were obtained from QB72 fermentation mycelia using sequential extraction from hot water, 1 %(NH4)2C2O4 and NaOH extracts after ethanol precipitation and dialysis. The total polysaccharide, protein, and uronic acid contents of the three polysaccharides and their compositions were determined (Table 1). The hot water extract polysaccharides (A1) yielded 6.2 %, with clear and easily soluble characteristics, and (A2 and A3) yielded 0.795 % and 3.519 %, respectively. However, the two polysaccharides were not as soluble as A1, with milky white and light brown colors, respectively. The carbohydrate contents of the three polysaccharides were 88.28 ± 5.61, 64.14 ± 5.92, and 42.29 ± 5.69 %, respectively. However, the uronic acid (%) from the hot water extracts 12.84 ± 1.48 % was higher than that from the other two extracts (11.32 ± 1.57 and 3.46 ± 0.28 %). The results are shown in the ultraviolet–visible (UV/VIS) spectra of the three polysaccharides (Fig. 3 (A)). A1 from the hot water extracts showed no absorption peak at 280 nm but had a slight absorption peak at 260 nm. A2 and A3 had no notable absorption peaks at 260 and 280 nm, suggesting that the water extracts contained small amounts of protein and nucleic acid, which was in keeping with the protein content 2.098 ± 0.262 %, 2.882 ± 0.119 %, and 4.337 ± 0.316 %, respectively, measured using the Folin-Phenol method.
Table 1.
Preliminary properties and compositions of polysaccharides extracts from the Ph. igniarius QB72 freezed dried mycelia powder.
| Physicochemical properties | Water(A1) | 1 %(NH4)2C2O4(A2) | 1.25 M NaOH(A3) |
|---|---|---|---|
| Color | Clear | Milky white | Light brown |
| Solubility | Easily soluble | Soluble | Soluble |
| Yield(%) | 6.2 % | 0.795 % | 3.519 % |
| Carbohydrate(%) (n = 3) | 88.28 ± 5.61a | 64.14 ± 5.92b | 42.29 ± 5.69c |
| Protein(%) (n = 3) | 2.098 ± 0.262c | 2.882 ± 0.119b | 4.337 ± 0.316a |
| Uronic acid(%) (n = 3) | 12.84 ± 1.48a | 11.32 ± 1.57a | 3.46 ± 0.28b |
| Mw(Da) | 17457 | 12468 1049 |
28334 |
| Mn | 8373 | 6852 905 |
10989 |
| Mw/Mn | 2.08491 | 1.81958 1.16003 |
2.57838 |
Fig. 3.
UV scan of the three partially purified polysaccharides(A). Structural properties of three polysaccharides in aqueous solutions at various NaOH concentrations(n = 3)(B).
The Congo red test was used to detect the chain conformation of polysaccharides, and a red shift in the maximum absorption indicated whether the polysaccharide chains contained a triple helix [29]. The results are shown in Fig. 3 (B), in the range of 0.1–0.5 M NaOH, the maximum absorption wavelength of the three polysaccharides did not change visibly, suggesting that none of them had a triple-helical conformation in solution.
3.3. GPC of the different extracts of the polysaccharides
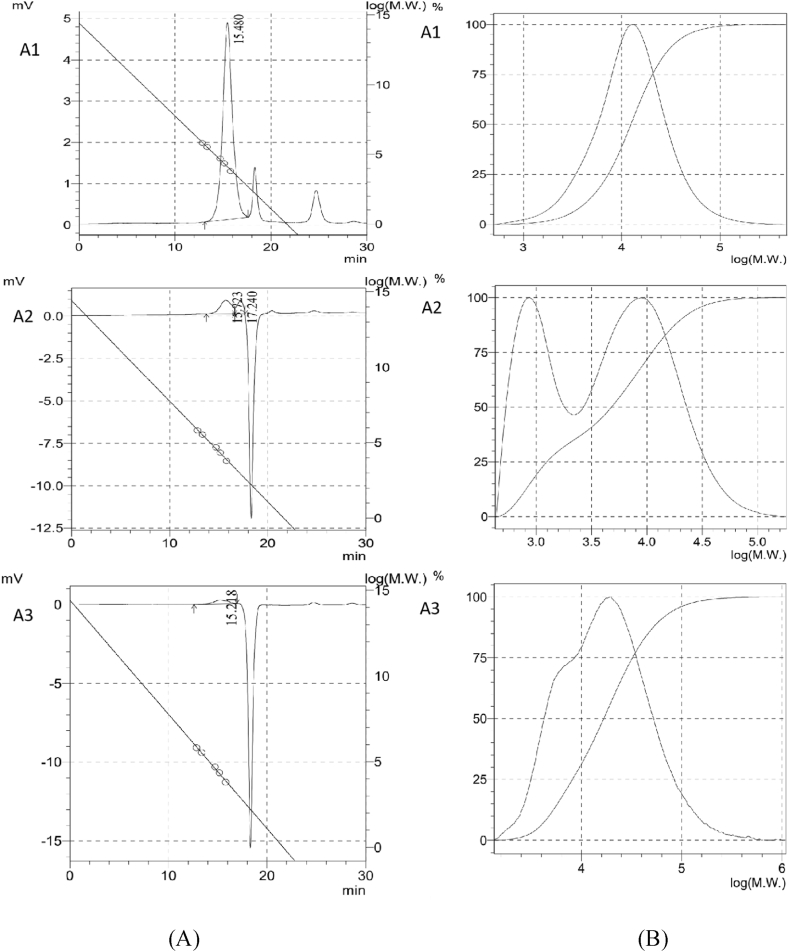
Natural polysaccharides isolated from medicinal mushrooms and fungi have different molar masses (103–108 Da). In this study, the molecular properties and chain conformations of water-soluble polysaccharides, partially purified from P. igniarius QB72, were investigated. The Mw of the three polysaccharides was determined using size-exclusion chromatography, and the results are shown in Fig. 4. The HPGPC results in Fig. 4 (A) show that the water-soluble extracts of A1 had a single symmetrical peak, indicating that they were homogeneous Fig. 4 (B). The molecular weight of A1 is approximately 17457 Da. For the 1 %(NH4)2C2O4 extraction polysaccharides from the water extract residue A2, there were two peaks, and their molecular weights were approximately 12468 and 1049 Da, respectively. For the A3 polysaccharides from the 1.25 M NaOH extracts of the acid extract residues, the molecular weight was approximately 28334 Da.
Fig. 4.
The GPC of the different extracts of the polysaccharides (A), and the molecular weight distribution curve (B).
3.4. The bioactivity of each extract from the P. igniarius QB72 fermentation mycelia
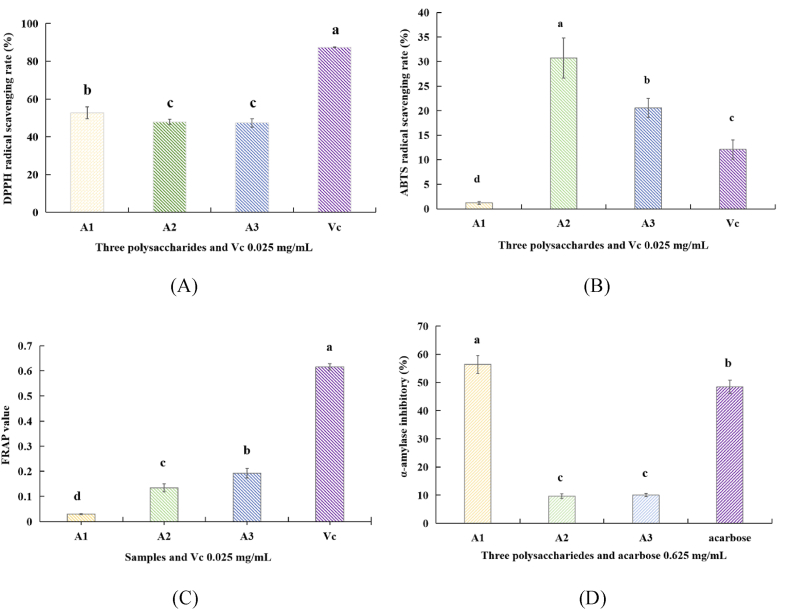
Three antioxidant and α-amylase inhibitory activity assays were used to evaluate the bioactivity of three partially purified polysaccharides from P. igniarius QB72 fermentation mycelia. All three partially purified polysaccharides possessed antioxidant activities; however, different results were obtained using different methods. Fig. 5 (A) shows the DPPH radical-scavenging ability of the three polysaccharide samples. The activities of all three antioxidants were evaluated at a concentration of 2 mg/mL. The DPPH radical scavenging ability of the three polysaccharides was approximately 50 %; however, for ABTS radical scavenging, the polysaccharides from the acid extraction possessed a relatively higher scavenging ability than the other two polysaccharides Fig. 5 (B). A3 from the 1.25 M NaOH extraction with a higher FRAP value Fig. 5 (C). Among the three polysaccharides, the water extraction polysaccharides possess excellent α-amylase inhibitory ability at a concentration of 5 mg/mL Fig. 5 (D).
Fig. 5.
Antioxidant and α-amylase inhibitory activity of the each extracts. (A) DPPH (n = 3) (B) ABTS (C) FRAP (n = 3) activity at the concentration of 2 mg/mL; (D) α-amylase inhibitory activity at the concentration of 5 mg/mL. All the data in the figure are three independent experiments.
3.5. FTIR of the polysaccharide
FTIR was used to determine the type of sugar ring and functional groups in polysaccharide samples. From the FTIR spectra, the polysaccharide samples were analyzed for structural characteristics based on the position, intensity, and shape of the peaks. The results (Fig. 6.) showed that all three polysaccharides had O–H and C–H stretching vibration peaks, which are the main absorption peaks of polysaccharide compounds [30].
Fig. 6.
FTIR of the three polysaccharides.(I) A1 partially purified from water extracts.(Ⅱ)A2 was a partially purified polysaccharide from 1 %(NH4)2C2O4 extraction of the water extract residue. (Ⅲ)A3 was a partially purified polysaccharide from 1.25 M NaOH extraction of the 1 %(NH4)2C2O4 extract residue.
Conventionally, the stretching vibration absorption peak in the 3600–3200 cm−1 region is attributed to intramolecular hydroxyl O–H bonds. The stretching vibration absorption peak of the C–H bond typically appears in the region 3000–2800 cm−1. The infrared spectrum of A1 is shown in Fig. 6 (A). The bands at 3306 and 2927.07 cm−1 were the stretching vibration peaks of O–H and C–H, respectively. A2 has bands at 3291.19 and 2930.54 cm−1 Fig. 6. (Ⅱ), and for A3, the bands of the stretching vibration peaks of O–H and C–H occurred at 3294.38 and 2961.43 cm−1, respectively Fig. 6. (Ⅲ). A similar band appeared at 1640 cm−1, which represented the C O group [30]. The three polysaccharides showed absorption peaks at approximately 1649.26, 1643.53, and 1656.83 cm−1. The weaker absorption peak in the 1400–1200 cm−1 region is the angular vibration absorption peak of the C–H bond. Three polysaccharide vibration absorption peaks of the C–H bond occurred at 1362.92, 1314.94, and 1313.38 cm−1. In addition, the broad peak at 1200–1000 cm−1 verified the existence of the pyran ring, which is primarily caused by the C–O stretching vibration of C–O–H and C–O–C. All the three polysaccharides exhibited three stretching vibrations; however, the strengths of the three absorption peaks were different. The 1000–700 cm−1 region in the infrared spectrum is the fingerprint region, which is typically used to judge the type of polysaccharide and the configuration of the sugar ring. The C–O–H bond on the deformation vibration of the α-pyran ring produces an absorption peak at 800 cm−1. Two weak absorption peaks appeared at 801.76 and 862.98 cm−1 for polysaccharide A2 (Fig. 6. (Ⅱ)). The absorption peaks at approximately 876 and 808 cm−1 are characteristic signals of glucose and mannose, respectively [30].
3.6. Inhibitory effect of A1 on α-amylase
The α-amylase inhibitory activity of A1, A1 DEAE water elution and acarbose showed a dose-dependent inhibitory effect at the tested concentrations. The α-amylase inhibitory activities of A1, A1 DEAE water elution and acarbose are shown in Fig. 7. (A, C). The IC50 values of the α-amylase inhibitory activities of acarbose, A1 and the A1 DEAE water elution were 0.455 ± 0.095, 6.84 ± 0.37 mg/mL and 3.178 ± 0.187 mg/mL, respectively. The carbohydate content of the A1 DEAE water elution is about 90.65 %. The full sacn of the A1 DEAE water elution without obvious protein and the content of the protein is minor. After isolation using the DEAE water elution, the α-amylase inhibitory increased.
Fig. 7.
The impact of the A1 (I)(n = 3) A1 DEAE water elution(Ⅱ) on the α-amylase inhibitory activity and full sacn of the A1 DEAE water elution(Ⅲ). Isolation and purification of A1(B), the acarbose (B)(n = 3) on the α-amylase inhibitory activity(C).
4. Discussion
Phellinus spp. have been known for their bioactivity for centuries and deserve further development. The fruiting bodies of wild Phellinus are rare, and the relatively slow process of traditional cultivation of fruiting bodies significantly affects the availability of this resource. Thus, using biotechnological fermentation techniques to maximize the productivity [31] of fermentation biomass is an alternative method of obtaining this type of resource. Polysaccharides (50–60 %) are the major structural components of mushroom cell walls [32]. In addition to polysaccharides, secondary metabolites from fruiting bodies and fermentation mycelia also possess excellent bioactivities. The biomass and metabolic composition of the fermented mycelia varied depending on the medium used. In addition, the use of natural culture media is a good choice for obtaining biocompounds and utilizing inexpensive raw materials.
In this study, a natural culture medium was applied to four Phellinus strains to evaluate their growth, metabolism, and antioxidant and α-amylase inhibitory activities. Among the media used in this study, QB72 strain achieved the highest biomass fermentation. Polysaccharides are the main biocompounds in the fruiting body and even fermentation mycelia; thus, the media in this study have the potential to obtain P. igniarius polysaccharides in large-scale production. Strain 5.891 was identified as P. vaninii HZNK, a widely cultivated Phellinus strain [33] in China. Biocompounds are typically obtained from fruiting bodies; however, liquid fermentation is also an alternative and fast method for flavonoid production [34] and hispidin polyphenols [35]. Strain 5.1260 was identified as Phellinus tuberculosus isolate fs102, which possesses antioxidant and potent radical-scavenging activities [36]. In recent years, research has focused on identifying sesquiterpenoids in fungal cultures [37,38]. In this study, the DPPH radical scavenging ability of 5.1260 was not higher than that of 5.891 but was higher than that of QB72 and 2H, and the results partially agree with previous research [36]. The 2H strain, identified as the wild-type strain, was Sanghuangporus alpinus voucher SH2022. However, limited research has been conducted on polysaccharides or other biocomponents. The fermentation biomass of this strain was not higher than that of the other three strains, and the growth rate of this strain on the plate was not the lowest. Therefore, this strain may be suitable for cultivation. The small molecule content of the four strains must be compared in future research. Strain improvement was also required for these four strains using a protoplast fusion approach.
The structure-activity relationship and mechanism of action of polysaccharides are important research topics in glycobiology. The bioactivity of polysaccharides is closely related to their structure and conformation [39]. Carbohydrate-hydrolyzing enzymes are essential for the development of drugs to delay glucose absorption for the treatment of T2DM to delay glucose absorption [40]. α-Glucosidase and α-amylase inhibitors are two key enzymes that decrease glucose release from starch and delay carbohydrate absorption by inhibiting the activity of carbohydrate-hydrolyzing enzymes in the small intestine. Cheng et al. evaluated the hypoglycemic effect of a neutral polysaccharide (SSIPS1) in vitro and found that polysaccharides can inhibit the activity of α-amylase and α-glucosidase [41]. Crude heteropolysaccharides from P. linteus are composed of glucose, arabinose, fucose, galactose, xylose, mannose, and arabinose, which increase the levels of short-chain fatty acids (SCFAs) by increasing the abundance of SCFA-producing bacteria. Thus, the lipopolysaccharide content in the blood is reduced, which helps to reduce systemic inflammation and reverse insulin resistance [42]. In one study, P. linteus polysaccharide (PLP) was homogeneous (153 kDa), and neutral sugar composition analysis revealed Ara (7.0 %), Xyl (3.7 %), Glc (21.1 %), Gal (24.1 %), and Man (44.2 %), which significantly decreased FBG levels and improved glucose intolerance by altering the metabolism of hepatic phospholipids and rescuing insulin signal transduction via the FOXO signaling pathway [43]. PLP also suppressed the cytokine expression of inflammatory markers such as macrophages, interleukins, IFN-γ, and TNF-α [44].
In this study, hot water extract polysaccharides exhibited inhibitory activity against α-amylase with an IC50 value of 2.189 ± 0.097 mg/mL, whereas they had no effective inhibition of α-glucosidase (data not shown in the text). Both A1 and acarbose showed mixed competitive inhibition against α-amylase.
Acarbose (Fig. 8.) is an oligosaccharide (with a molecular weight of 645.605) which can slow glucose release from starch and delay carbohydrate absorption. A1 is a polysaccharide obtained from hot water extracts, and its carbohydrate content is approximately 88 %, with an Mw of 17457 Da. However, the structure of A1 was analyzed and identified after purification. Evaluation of the effect of A1 and the purified polysaccharide components on sugar absorption in the cell model requires further efforts, and the cytokine expression of inflammatory markers such as macrophages, ILs, IFN-γ, and TNF-α also should be studied further. In addition, the structure-activity relationship must be discussed. According to previous research, mixed-type inhibition can be explained by the binding of the sample to the enzyme, which causes a conformational change that reduces enzyme activity. In addition, irreversible modifications of the α-amylase structure may occur. This inhibition could arise because the sample interacts with a later intermediate in the reaction but not with the initial enzyme-substrate complex.In this research, the carbohydrate content of the A1 water elution is about 90 %, the A1 DEAE water elution part need more work to make sure purify enough to conducted the α-amylase inhibitory again. After purification, the inhibitory activity is increase, but it may not as effective as medicine acarbose, for the polysaccharides purify partially from the biomass of the fermentation could applied for the food and even medication-assisted additives.
Fig. 8.
Kinetics analysis for the α-amylase inhibition type of acarbose(I) The molecule structure of the acarbose(II).
5. Conclusion
One therapeutic approach for preventing DM is to retard glucose absorption via the inhibition of α-amylase. Therefore, the search for α-amylase inhibitors in natural sources is important. Phellinus spp. as a medicinal mushroom could be treated one of the potential α-amylase inhibitors. Four Phellinus strains were evaluated, among the four Phellinus strains, hot water extracts from P. igniarius QB72 had obvious α-amylase inhibitory activity compared with the other three strains. Partially purification A1 from the hot water polysaccharides (17457 Da) with better α-amylase inhibitory activity (IC50 value of (2.189 ± 0.097) mg/mL) than the 1 % (NH4)2C2O4 extract and 1.25 M NaOH extracts when compared at a concentration of 5 mg/mL with 56.46 %, 9.59 %, and 10.01 % inhibitory rate. Phellinus spp. fermentation mycelia could be used as a source of bioactive polysaccharides. A1 from P. igniarius QB72 may have potential for development as a novel natural α-amylase inhibitor applied in food and medication-assisted additives.
Data availability statement
Important part of the data we share on the figureshare with the doi at the following places.
https://doi.org/10.6084/m9.figshare.22794032.v2
https://doi.org/10.6084/m9.figshare.24227722.v1
https://doi.org/10.6084/m9.figshare.20544084.v1
https://doi.org/10.6084/m9.figshare.24234946.v1
https://doi.org/10.6084/m9.figshare.24235525.v1
CRediT authorship contribution statement
Yating Dong: Writing - review & editing, Writing - original draft, Software, Methodology, Formal analysis. Tao Wang: Project administration, Investigation, Funding acquisition. Bingcheng Gan: Supervision, Project administration, Conceptualization. Solomon P. Wasser: Investigation. Zhiyuan Zhang: Resources, Investigation. Jin Zhao: Data curation. Xinlian Duan: Supervision, Data curation. Luping Cao: Supervision, Data curation. Rencai Feng: Supervision, Investigation. Renyun Miao: Supervision, Resources, Investigation. Junjie Yan: Supervision, Resources, Investigation. Zhi Wu: Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Thanks to the Local Financial Funds of National Agricultural Science and Technology Center, Chengdu (No.NASC2022KR03) and the Agricultural Science and Technology Innovation Program (No. 34-IUA-06) support some related work about the polysaccharides research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23370.
Contributor Information
Yating Dong, Email: dyt0623xiang@126.com.
Bingcheng Gan, Email: ganbingcheng@caas.cn.
Abbreviations
- (ROS)
Reactive oxygen species
- CGMCC
(China General Microbiological Culture Collection Center)
- (HPGPC)
High-performance gel-permeation chromatography
- (FRAP)
ferric reducing antioxidant power
- (ANOVA)
analysis of variance
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Antonceva E., Shamtsyan M. Antidiabetical and hypoglycemic action of mushroom polysaccharides. E3S Web of Conferences. 2020;215 doi: 10.1051/e3sconf/202021505001. [DOI] [Google Scholar]
- 2.Friedman M. Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods. 2016;5(4) doi: 10.3390/foods5040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan H., Yu G., Zheng M.J., Zhu Y.B., Ni H., Oda T., Jiang Z.D. Inhibitory effects of a low-molecular-weight sulfated fucose-containing saccharide on alpha-amylase and alpha-glucosidase prepared from ascophyllan. Food Funct. 2022;13(3):1119–1132. doi: 10.1039/d1fo03331j. [DOI] [PubMed] [Google Scholar]
- 4.De Silva D.D., Rapior S., Hyde K.D., Bahkali A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012;56(1):1–29. doi: 10.1007/s13225-012-0187-4. [DOI] [Google Scholar]
- 5.Arunachalam K., Sreeja P.S., Yang X. The antioxidant properties of mushroom polysaccharides can potentially mitigate oxidative stress, beta-cell dysfunction and insulin resistance. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.874474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khursheed R., Singh S.K., Wadhwa S., Gulati M., Awasthi A. Therapeutic potential of mushrooms in diabetes mellitus: role of polysaccharides. Int. J. Biol. Macromol. 2020;164:1194–1205. doi: 10.1016/j.ijbiomac.2020.07.145. [DOI] [PubMed] [Google Scholar]
- 7.Jo Y.H., Lee S., Yeon S.W., Ryu S.H., Turk A., Hwang B.Y., Lee M.K. Anti-alpha-glucosidase and anti-oxidative isoflavonoids from the immature fruits of Maclura tricuspidata. Phytochemistry. 2022;194 doi: 10.1016/j.phytochem.2021.113016. [DOI] [PubMed] [Google Scholar]
- 8.Umeno A., Horie M., Murotomi K., Nakajima Y., Yoshida Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules. 2016;21(6) doi: 10.3390/molecules21060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez-Armenta F.J., Leyva J.M., Mata-Haro V., Gonzalez-Aguilar G.A., Cruz-Valenzuela M.R., Esqueda M., Ayala-Zavala J.F. Phenolic compounds of Phellinus spp. with antibacterial and antiviral activities. Braz. J. Microbiol. 2022;53(3):1187–1197. doi: 10.1007/s42770-022-00745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He P., Zhang Y., Li N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: a review. Food Funct. 2021;12(5):1856–1881. doi: 10.1039/d0fo02342f. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Tan H., Liu Q., Zheng X., Zhang H., Liu Y., Xu L. A Review: the bioactivities and pharmacological applications of Phellinus linteus. Molecules. 2019;24(10) doi: 10.3390/molecules24101888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y.J., Zhang W., Zhong J.J. Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and Ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum. Bioresour. Technol. 2009;100(5):1852–1859. doi: 10.1016/j.biortech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Papaspyridi L.M., Aligiannis N., Topakas E., Christakopoulos P., Skaltsounis A.L., Fokialakis N. Submerged fermentation of the edible mushroom Pleurotus ostreatus in a batch stirred tank bioreactor as a promising alternative for the effective production of bioactive metabolites. Molecules. 2012;17(3):2714–2724. doi: 10.3390/molecules17032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y.T., Ma H.L., Golly M.K., Qu W.J., Wang B., Zhou C.S., Ye X.F., Zhang H., Gan B.C., Yan J.J., Han X., Zhao J., Feng R.C., Miao R.Y., Zhang Z.Y. Pulsed light mutagenesis of the willow bracket mushroom, Phellinus igniarius (Agaricomycetes), for enhanced production of flavonoids, laccase, and fermentation biomass. Int. J. Med. Mushrooms. 2022;24(10):31–43. doi: 10.1615/IntJMedMushrooms.2022044961. [DOI] [PubMed] [Google Scholar]
- 15.Qi Y., Zhang R., Zhang M., Wen Q., Shen J. Effects of exogenous ascorbic acid on the mycelia growth and primordia formation of Pleurotus ostreatus. J. Basic Microbiol. 2021;61(8):736–744. doi: 10.1002/jobm.202100143. [DOI] [PubMed] [Google Scholar]
- 16.Sengul U., Gokhan Z., Abdurrahman A., Sukru K. Fatty acid composition, total sugar content and anti-diabetic activity of methanol and water extracts of nine different fruit tree leaves collected from mediterranean region of Turkey. Int. J. Food Prop. 2015;18(10):2268–2276. doi: 10.1080/10942912.2014.973964. [DOI] [Google Scholar]
- 17.Zhu M.Y., Wang C.J., Gu Y., He C.S., Teng X., Zhang P., Lin N. Extraction, characterization of polysaccharides from Morinda officinalis and its antioxidant activities. Carbohydr. Polym. 2009;78:497–501. doi: 10.1016/j.carbpol.2009.05.008. [DOI] [Google Scholar]
- 18.Badshah S.L., Riaz A., Muhammad A., Tel Cayan G., Cayan F., Emin Duru M., Jaremko M. Isolation, characterization, and medicinal potential of polysaccharides of Morchella esculenta. Molecules. 2021;26(5) doi: 10.3390/molecules26051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Hsien. A new colorimetric method for the determination of plasma proteins. J. Biol. Chem. 1922;51(1):33–39. doi: 10.1016/s0021-9258(18)85898-7. [DOI] [Google Scholar]
- 20.Bitter T., Muir H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z.B., Pei J.J., Ma H.L., Cai P.F., Yan J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr. Polym. 2014;109:49–55. doi: 10.1016/j.carbpol.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Yan J.K., Wang W.Q., Li L., Wu J.Y. Physiochemical properties and antitumor activities of two α-glucans isolated from hot water and alkaline extracts of Cordyceps (Cs-HK1) fungal mycelia. Carbohydr. Polym. 2011;85(4):753–758. doi: 10.1016/j.carbpol.2011.03.043. [DOI] [Google Scholar]
- 23.Vincenzo F., Veronica V., Giacomino R., Alberto R. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999;47:1035–1040. doi: 10.1021/jf980496s. [DOI] [PubMed] [Google Scholar]
- 24.Prabu M., Kumuthakalavalli R. Antidiabetic potential of the oyster mushroom pleurotus Florida (mont.) singer. Int. J. Curr. Pharmaceut. Res. 2017;9(4):51. doi: 10.22159/ijcpr.2017v9i4.20765. [DOI] [Google Scholar]
- 25.Mărghitaş L.A., Stanciu O., Dezmirean D., Bobiş O., Popescu O., Bogdanov S., Campos M.G.N.C. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009;115(3):878–883. doi: 10.1016/j.foodchem.2009.01.014. [DOI] [Google Scholar]
- 26.Stojkovic D., Smiljkovic M., Ciric A., Glamoclija J., Van Griensven L., Ferreira I.C.F.R., Sokovic M. An insight into antidiabetic properties of six medicinal and edible mushrooms: inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. South Afr. J. Bot. 2019;120:100–103. doi: 10.1016/j.sajb.2018.01.007. [DOI] [Google Scholar]
- 27.Wang Z., Zuo G., Hwang S.H., Kwon S.H., Kang Y.H., Lee J.Y., Lim S.S. Affinity measurement of ligands in Perilla frutescens extract towards alpha-glucosidase using affinity-based ultrafiltration-high-performance liquid chromatography. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2019;1125 doi: 10.1016/j.jchromb.2019.121725. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Wang J., Liu Y., Ji Y., Guo Y., Zhao J. Interaction mechanism of carnosic acid against glycosidase (alpha-amylase and alpha-glucosidase) Int. J. Biol. Macromol. 2019;138:846–853. doi: 10.1016/j.ijbiomac.2019.07.179. [DOI] [PubMed] [Google Scholar]
- 29.Jia Y., Xue Z., Wang Y., Lu Y., Li R., Li N., Chen H. Chemical structure and inhibition on alpha-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohydr. Polym. 2021;252 doi: 10.1016/j.carbpol.2020.117185. [DOI] [PubMed] [Google Scholar]
- 30.Shi X.D., Nie S.P., Yin J.Y., Que Z.Q., Zhang L.J., Huang X.J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocolloids. 2017;73:176–183. doi: 10.1016/j.foodhyd.2017.06.039. [DOI] [Google Scholar]
- 31.Chegwin-Angarita C., Bello-Forero R.A., Serrato-Bermúdez J.C. Optimization of culture conditions in a bioreactor for the production of biomass and metabolites of the Macromycete Lentinula Edodes. Carbohydr Polym. 2021;97:627–634. doi: 10.21203/rs.3.rs-610654/v1. [DOI] [Google Scholar]
- 32.Liu X.F., Luo D.H., Guan J.J., Chen J., Xu X.F. Mushroom polysaccharides with potential in anti-diabetes: biological mechanisms, extraction, and future perspectives: a review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1087826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan X.L., Jin X., Xie M.L., Liu J., Gontcharov A.A., Wang H., Lv R.N., Liu D.Y., Wang Q., Li Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020;163:865–877. doi: 10.1016/j.ijbiomac.2020.06.279. [DOI] [PubMed] [Google Scholar]
- 34.Ma X.K., Ma H.Y., Chen Q., Ma Y., Daugulis A.J., Liang J., Zheng P. The influence of monochromatic lights on flavonoid production by the fungus Sanghuangporus vaninii: modeling of kinetic profiles and expression levels of important genes in flavonoid synthesis. Biochem. Eng. J. 2021;166 doi: 10.1016/j.bej.2020.107876. [DOI] [Google Scholar]
- 35.Yuan W.W., Yuan W.H., Zhou R., Lv G.Y., Sun M.N., Zhao Y.X., Zheng W.F. Production of hispidin polyphenols from medicinal mushroom Sanghuangporus vaninii in submerged cultures. Chin Herb Med. 2023 doi: 10.1016/j.chmed.2022.07.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seephonkai P., Samchai S., Thongsom A., Sunaart S., Kiemsanmuang B., Chakuton K. DPPH radical scavenging activity and total phenolics of Phellinus mushroom extracts collected from northeast of Thailand. Chin. J. Nat. Med. 2011;9:441–445. [Google Scholar]
- 37.He J.B., Feng T., Zhang S., Dong Z.J., Li Z.H., Zhu H.J., Liu J.K. Seven new drimane-type sesquiterpenoids from cultures of fungus Phellinus tuberculosus. Nat Prod Bioprospect. 2014;4:21–25. doi: 10.1007/s13659-014-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He J.B., Tao J., Miao X.S., Feng Y.P., Bu W., Dong Z.J., Li Z.H., Feng T., Liu J.K. Two new illudin type sesquiterpenoids from cultures of Phellinus tuberculosus and Laetiporus sulphureus. J Asian Nat Prod Res. 2015;17:1054–1058. doi: 10.1080/10286020.2015.1040774. [DOI] [PubMed] [Google Scholar]
- 39.Qin D.D., Han S., Liu M.L., Guo T.Y., Hu Z.M., Zhou Y.P., Luo F.J. Polysaccharides from Phellinus linteus: a systematic review of their extractions, purifications, structures and functions. Int. J. Biol. Macromol. 2023;230 doi: 10.1016/j.ijbiomac.2023.123163. [DOI] [PubMed] [Google Scholar]
- 40.Mphahlele M.J., Maluleka M.M., Choong Y.S., Monchusi B.A., Mbazima V.G. An in vitro study of the 5-methyl- and 5-bromo/chloro substituted 2-hydroxy-3-nitrochalcones as α-glucosidase and/or α-amylase inhibitors with potential anti-inflammatory activity. Med. Chem. Res. 2022;31:2243–2259. [Google Scholar]
- 41.Cheng J.W., Song J.L., Wei H.L., Wang Y.B., Huang X.B., Liu Y., Lu N., He L., Lv G.Y., Ding H.M., Yang S.Z., Zhang Z.F. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020:1643305–1643314. doi: 10.1016/j.ijbiomac.2020.08.202. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y.Y., Wang C.R., Li J.S., Li T.T., Zhang Y., Liang Y.X., Mei Y.X. Phellinus linteus polysaccharide extract improves insulin resistance by regulating gut microbiota composition. Faseb. J. 2020;34:1065–1078. doi: 10.1096/fj.201901943RR. [DOI] [PubMed] [Google Scholar]
- 43.Feng H., Zhang S.J., Man-Fan W., Gui L.F., Ruan M.C., Li N., Zhang H.Y., Liu Z.G., Wang H.L. Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice. Carbohydr. Polym. 2018;200:144–153. doi: 10.1016/j.carbpol.2018.07.086. [DOI] [PubMed] [Google Scholar]
- 44.Kim H.M., Kang J.S., Kim J.Y., Park S.K., Kim H.S., Lee Y.J., Yun J.U., Hong J.T., Kim Y., Han S.B. Evaluation of antidiabetic activity of polysaccharide isolated from Phellinus linteus in non-obese diabetic mouse. Int. Immunopharm. 2010;10:72–78. doi: 10.1016/j.intimp.2009.09.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Important part of the data we share on the figureshare with the doi at the following places.
https://doi.org/10.6084/m9.figshare.22794032.v2
https://doi.org/10.6084/m9.figshare.24227722.v1
https://doi.org/10.6084/m9.figshare.20544084.v1
https://doi.org/10.6084/m9.figshare.24234946.v1
https://doi.org/10.6084/m9.figshare.24235525.v1