Abstract
The endocardial vegetation which is formed in the course of bacterial endocarditis (BE) contains tissue factor (TF)-dependent procoagulant activity. Earlier studies showed that monocytes are the main source of TF in the vegetations. The TF activity (TFA) of vegetations isolated from Streptococcus sanguis-infected rabbits depended on the numbers of bacteria as well as monocytes in the vegetation. In this study, we investigated whether for Staphylococcus epidermidis, a frequent pathogen in BE, an effect similar to that found for S. sanguis could be shown. In vitro, S. epidermidis was found to stimulate TFA of fibrin adherent monocytes significantly. This stimulation was maximal at a bacterium-to-monocyte ratio of 7. In vivo, TFA was found to be significantly higher in S. epidermidis-infected than in sterile catheter-induced vegetations. Reduction of vegetational bacterial numbers by teicoplanin treatment lead to a small but significant decrease of TFA. Reduction of monocyte numbers by etoposide did not affect vegetational TFA. Comparison of data for S. epidermidis and S. sanguis revealed that at equivalent bacterial numbers, vegetational TFAs were approximately the same for both microorganisms. Combining the results of the present study with those of a previous study using S. sanguis, we conclude that the main factor determining monocyte-dependent vegetational TFA is the number of vegetation-associated bacteria. The lower TFA found for S. epidermidis-infected than for S. sanguis-infected vegetations can be explained by the significantly lower bacterial numbers in the infected vegetations and consequently a lower stimulation of vegetation-associated monocytes.
Bacterial endocarditis (BE) is an inflammatory process on heart valves, in which activation of the coagulation system plays a major role (7). A fibrin clot containing monocytes, granulocytes, thrombocytes, matrix proteins, and infecting microorganisms, called an endocardial vegetation (11), is formed on the heart valve. Activation of the coagulation system occurs via the extrinsic pathway (7). A key protein in this process is the cell-associated tissue factor (TF). In an in vitro model of BE, we demonstrated that the expression of monocyte TF activity (TFA) depends not only on an interaction with bacteria but also on the adherence of these cells to a fibrin surface (3). In earlier studies in the rabbit model of BE, we have shown that monocytes do account for the TFA of endocardial vegetations (4, 15). After streptococci, staphylococci are the most frequent causative microorganisms in BE. Staphylococcus epidermidis is frequently isolated in prosthetic valve endocarditis (1, 6). In the rabbit model of BE, the effects of warfarin treatment on the induction and course of the infection of catheter-induced vegetations were studied for S. epidermidis and Streptococcus sanguis (12, 13). Results indicated that with S. epidermidis, warfarin-treated rabbits needed larger bacterial inocula to induce infection, with a lower degree of infection of the vegetations (13), whereas with S. sanguis, warfarin treatment had no effect on the induction or course of the infection (12). The results of these two studies suggest that species-specific effects occur in the pathogenesis of BE. Data from recent studies, both in an in vivo and in an in vitro model for BE, suggest that with S. sanguis, the numbers of monocytes as well as of bacteria are positively correlated with the TFA of endocardial vegetations (3, 4). The main objective of the present study was to investigate whether for S. epidermidis a similar effect on monocytes in the activation of the coagulation system could be found, in vitro as well as in vivo. To achieve this goal, we studied in vitro the ability of S. epidermidis to induce TF expression on fibrin-adherent monocytes. In vivo, the effects of monocytopenia and antibiotic treatment were assessed in the rabbit model of BE.
MATERIALS AND METHODS
Microorganism:
S. epidermidis (ATCC 149900) was cultured overnight at 37°C in Todd-Hewitt broth (Oxoid, London, England). Cultures were washed twice with phosphate-buffered saline and diluted to appropriate concentrations. For the in vivo experiments, S. epidermidis suspensions were diluted to approximately 108 CFU/ml.
Adherence of S. epidermidis to a fibrin surface.
The adherence of S. epidermidis to a fibrin surface was assessed as described for S. sanguis (3, 4).
Effect of S. epidermidis on TFA of fibrin-adherent monocytes.
The effect of S. epidermidis on monocyte TFA was also determined as described for S. sanguis (4, 16).
Rabbit model of BE.
BE was induced in male New Zealand White rabbits as described elsewhere (2, 4, 5, 8).
Cytostatic drug.
The cytostatic drug etoposide (Vepesid; kindly donated by Bristol-Meyers Squibb BV, Woerden, The Netherlands) was used as described previously (4) to induce a selective monocytopenia.
Antibiotic treatment.
On 2 consecutive days, one daily dose of teicoplanin (30 mg/kg of body weight; Gist-Brocades, Delft, The Netherlands) was injected subcutaneously. The first dose was given 20 h after injection of staphylococci. Rabbits were sacrificed 24 h after the last injection of teicoplanin (72 h of infection). The blood concentration of teicoplanin was determined with the Innufluor reagent set for the quantitative determination of teicoplanin (International Bioclinical, Inc., Portland, Oreg.). The MIC and MBC of teicoplanin were determined as described previously (4). The MIC of teicoplanin was 8 μg/ml; the MBC was 16 μg/ml.
Quantitation of blood monocytes.
Monocyte numbers in 1-ml blood samples were determined as described before (4).
Blood cultures.
Immediately before rabbits were sacrificed, 1 ml of blood was drawn from a marginal ear vein and collected in vials containing 10 mg of EDTA. Two hundred μl of blood was plated on blood agar plates. After overnight incubation at 37°C, the CFU/milliliter of blood was determined.
Handling of vegetations.
The isolated vegetations were weighed and homogenized in 2 ml of PBS. Part of the homogenate was used to determine the log CFU/gram of vegetation, while another part was used for measurement of the TFA as described elsewhere (4). The procedure for measuring TFA on the surface of fibrin-adherent monocytes in the presence of S. epidermidis was the same as described previously (3) for S. sanguis.
Statistical analysis.
For determination of significance of differences between the vegetational TFAs, weights, and infections of control rabbits, etoposide-treated rabbits, and teicoplanin-treated rabbits, multifactorial analysis of variance was used with Newman-Keuls correction. The significance level α was 5%.
RESULTS
S. epidermidis-induced monocyte TFA in vitro.
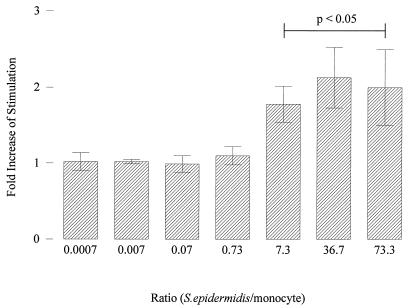
Overnight staphylococcal cultures were washed and layered on the fibrin plates in concentrations ranging from 1.5 × 104 to 1.5 × 1010 CFU/ml. The percentage adherence of S. epidermidis was not affected by dilution of the cultures, being ±7% of the inoculum. Next, TFA of fibrin-adherent monocytes was assessed after a 4-h incubation of the cells at 37°C and 5% CO2 in the absence or presence of staphylococci. As shown before (3), the adherence of the monocytes to the fibrin plate induced a TFA of 55 ± 13 pmol of factor Xa (FXa)/min/106 monocytes. The presence of S. epidermidis led to an inoculum-dependent increase of monocyte TFA (Fig. 1). A bacterium-to-monocyte ratio of 7 was needed to give a maximal TFA (Fig. 1). At this maximum, TFA was increased twofold by the addition of staphylococci. These results were comparable to those with S. sanguis, where monocyte TFA reached a maximum with a twofold increase at a bacterium-to-monocyte ratio of 4.5 (3).
FIG. 1.
Influence of bacterium-to-monocyte ratio on TFA of fibrin-adherent monocytes in the presence of S. epidermidis, expressed as fold increase of stimulation over TFA of fibrin-adherent monocytes in the absence of bacteria. Cells were stimulated for 4 h. Significant increases in TFA are indicated.
Effect of etoposide on the number of peripheral blood monocytes and granulocytes.
On 6 consecutive days, one daily dose of 12.5 mg of etoposide was injected in a marginal ear vein. During this period, the course of the number of peripheral blood leukocytes was monitored in each rabbit. At the time of catheterization (day 4 of etoposide treatment), the numbers of peripheral blood monocytes had dropped to 5 to 10% of initial values, and they remained at this level during the rest of the experiment, even after infection of the vegetation. As shown before, the number of blood granulocytes (±9.9 × 102 cells/mm3) did not significantly change during the experiment (4).
Effects of monocytopenia on vegetations.
After 48 h of infection, all vegetations of S. epidermidis-injected rabbits were infected. The degree of infection was 7.01 ± 1.16 log CFU/g of vegetation. Monocytopenia did not cause a difference in vegetational infection (7.53 ± 0.92). All control rabbits had sterile vegetations. Blood cultures of staphylococcus-challenged monocytopenic rabbits were S. epidermidis positive, while all blood cultures of nonmonocytopenic rabbits were sterile. Also, after 48 h of infection, vegetational weights of S. epidermidis-infected rabbits were higher than those of noninfected rabbits, being 16.11 ± 9 and 11.19 ± 5.07 mg, respectively (P < 0.008). Etoposide treatment slightly increased this difference, the weights of S. epidermidis-infected and noninfected vegetations being 19.54 ± 6.36 and 11.40 ± 4.53 mg, respectively (P < 0.005). There were no differences in the vegetational weights of noninfected control and noninfected etoposide-treated rabbits. However, contrary to expectation, vegetational weights of etoposide-treated S. epidermidis-infected rabbits were slightly but not significantly higher than those of non-etoposide-treated S. epidermidis-infected rabbits, being 19.54 ± 6.36 and 16.11 ± 9.00 mg, respectively. At day 2 of infection, the TFA of infected vegetations was significantly higher than that of sterile vegetations, being 162 ± 8 versus 116 ± 23 pmol of FXa/g of vegetation/min for the nonmonocytopenic rabbits (P < 0.05) and 158 ± 32 versus 99 ± 21 pmol of FXa/g of vegetation/min for the monocytopenic rabbits (P < 0.006). The TFA of sterile vegetations of monocytopenic rabbits was found to be fractionally lower than that of nonmonocytopenic rabbits, but this difference was not significant (116 ± 23 versus 99 ± 21 pmol of FXa/g of vegetation/min). Etoposide treatment did not affect the TFA of staphylococcus-infected vegetations (162 ± 8 versus 158 ± 32 pmol of FXa/g of vegetation/min for infected and etoposide-treated infected vegetations).
Effect of antibiotic treatment on vegetations.
Although serum levels of teicoplanin did not permanently reach the MBC, at 72 h of infection, bacterial numbers of the vegetations dropped below the detection level (<3.8 log CFU/g), whereas in non-teicoplanin-treated rabbits, the infection level was 7.18 ± 1.75 log CFU/g. Vegetational weights did not differ between teicoplanin-treated and control rabbits, being 18.24 ± 11.9 and 18.53 ± 8.69 mg, respectively. At day 3 of infection, all blood cultures were sterile. TFA of vegetations from S. epidermidis-infected rabbits was significantly higher than that of S. epidermidis-infected teicoplanin-treated rabbits (100 ± 19 versus 80 ± 9 pmol of FXa/g of vegetation/min; P < 0.01).
DISCUSSION
From the results of this study, we conclude that in vitro, S. epidermidis can adhere to fibrin and stimulate monocytes to express TFA. In vivo, we found that infection of the vegetation with S. epidermidis resulted in an increase of the vegetational weight and TFA compared to that of noninfected vegetations. Treatment of the rabbits with etoposide led to a 95% reduction of peripheral blood monocytes. Blood cultures from etoposide-treated infected rabbits were positive, whereas those of control infected rabbits remained sterile. Therefore, the slightly though not significantly higher weights of vegetations in the former group might be due to reseeding of the vegetations from the circulation. Further, no significant changes in TFA or infection of the vegetations in etoposide-treated rabbits were observed. Reduction of the vegetational infection with teicoplanin led to a significant decrease of the vegetational infection and also to a small but significant decrease of the TFA of the vegetations but did not affect the vegetational weights. In vitro, S. epidermidis and S. sanguis have the same effect on the TFA of fibrin-adherent monocytes. They induce the same maximal twofold increase in TFA at comparable bacterium-to-monocyte ratios (7 for S. epidermidis and 4.5 for S. sanguis [3]). Also, they adhere to fibrin comparably (7% [S. epidermidis] and 5% [S. sanguis] of the inoculum) (3). The finding that the adherence of the bacteria (staphylococci as well as streptococci [3]) to a fibrin surface invariably proved to be approximately 6% of the original inoculum may be due to the fact that even with high inocula, the saturation of binding sites present on the fibrin surface is not complete. At higher inocula, causing complete saturation of all binding sites, most probably the adherence of the bacteria on this surface will decrease. However, in vivo findings with S. epidermidis appeared to differ from those obtained with S. sanguis (9, 10, 12–14). Infection of the endocardial vegetations leads to a considerably lower number of bacteria as well as a lower TFA for S. epidermidis than for S. sanguis. Since the TFA of monocytes depends on the ratio between these cells and the number of bacteria, the lower TFA of S. epidermidis-infected vegetations can be explained by the lower number of bacteria in the vegetations. For S. sanguis, reduction of vegetation-associated monocytes by etoposide treatment as well as reduction of vegetational bacterial numbers by penicillin treatment resulted in a significantly lower vegetational TFA (Table 1). With S. epidermidis, no effect on TFA was found after etoposide treatment and only a small but significant reduction of TFA by lowering bacterial numbers by teicoplanin treatment. Apparently TFA in the S. epidermidis-infected vegetations is already so low that it is influenced not at all by a reduction of vegetation-associated monocytes and to only a small degree by lowering bacterial numbers in the vegetations. Interestingly, at equivalent bacterial numbers, TFAs of the vegetations are comparable for S. sanguis and S. epidermidis. For instance, the number of streptococci per gram of vegetation after penicillin treatment is approximately 107, which is about the same as in control non-teicoplanin-treated S. epidermidis-infected vegetations. Vegetational TFAs in these groups are comparable (Table 1). Treatment of S. epidermidis-infected rabbits with teicoplanin reduces bacterial numbers below the detection limit, and the TFAs of the vegetations in these rabbits became comparable to that of sterile vegetations of noninfected rabbits (Table 1). Thus, the number of bacteria in the vegetations seems to be the main factor determining their TFAs and consequently their weight, as shown by the considerably higher weight of the S. sanguis-infected than of S. epidermidis-infected vegetations. In conclusion, S. epidermidis can stimulate monocytes to express TFA dose dependently. It can do so as effectively as S. sanguis. However, due to significantly lower bacterial numbers, the TFA of S. epidermidis-infected vegetations was already so low that a reduction of monocytes by etoposide treatment had no effect and reduction of bacterial numbers by teicoplanin had only a very small though significant effect.
TABLE 1.
Comparison of in vivo parameters of S. sanguis-infecteda and S. epidermidis-infected vegetations
| Treatment | Infection (log CFU/g) | Wt (mg) | TFA (pmol of FXa/g) |
|---|---|---|---|
| Sterile | None | 14.0 ± 1 | 392 ± 101 |
| Sterile + etoposide | None | 13.0 ± 6 | 249 ± 93 |
| S. sanguis | 8.7 ± 0.7 | 41.0 ± 23 | 736 ± 156 |
| S. sanguis + etoposide | 8.7 ± 0.7 | 31.7 ± 7 | 278 ± 65 |
| Sterile | None | 11.0 ± 5 | 116 ± 23 |
| Sterile + etoposide | None | 11.0 ± 5 | 99 ± 21 |
| S. epidermidis | 7.0 ± 1.2 | 16.0 ± 9 | 162 ± 8 |
| S. epidermidis + etoposide | 7.5 ± 0.9 | 18.0 ± 12 | 158 ± 32 |
| S. sanguis | 8.7 ± 0.7 | 22.4 ± 4.2 | 602 ± 56 |
| S. sanguis + penicillin G | 7.1 ± 0.1 | 21.0 ± 5.8 | 250 ± 50 |
| S. epidermidis | 7.2 ± 1.8 | 18.5 ± 8.7 | 100 ± 19 |
| S. epidermidis + teicoplanin | <3.8 | 18.2 ± 11.9 | 80 ± 9 |
Data obtained from reference 4.
ACKNOWLEDGMENT
This work was supported by The Netherlands Heart Foundation grant 91.058.
REFERENCES
- 1.Arber N, Militiana A, Ben-Yehuda A, Krivoy N, Pinkhas J, Sidi Y. Native valve Staphylococcus epidermidis endocarditis, response of seven cases and review of the literature. Am J Med. 1991;90:758–762. [PubMed] [Google Scholar]
- 2.Archer G, Fehety F R. Experimental endocarditis due to Pseudomonas aeruginosa. I. Description of a model. J Infect Dis. 1976;134:1–7. doi: 10.1093/infdis/134.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bancsi M J L M F, Thompson J, Bertina R M. Stimulation of monocyte tissue factor expression in an in vitro model of bacterial endocarditis. Infect Immun. 1994;62:5669–5672. doi: 10.1128/iai.62.12.5669-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancsi M J L M F, Veltrop M H A M, Bertina R M, Thompson J. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetations in rabbits infected with Streptococcus sanguis. Infect Immun. 1996;64:448–451. doi: 10.1128/iai.64.2.448-451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buiting A G M, Thompson J, van den Keur D, Schmall-Bauer W C, Bertina R M. Procoagulant activity of endocardial vegetations in rabbits with Streptococcus sanguis endocarditis. Thromb Haemostasis. 1989;62:1029–1033. [PubMed] [Google Scholar]
- 6.Calderwood S B, Swinski L A, Waternaux C M, Karchmer A W, Buckley M J. Risk factors for development of prosthetic valve endocarditis. Circulation. 1985;72:31–37. doi: 10.1161/01.cir.72.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Drake T A, Rodgers G M, Sande M A. Tissue factor is a major stimulus for vegetation formation in enterococcal endocarditis in rabbits. J Clin Invest. 1984;73:1750–1753. doi: 10.1172/JCI111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonisation of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 9.Meddens M J M, Thompson J, Eulderink F, Bauer W C, Mattie H, van Furth R. Role of granulocytes in experimental Streptococcus sanguis endocarditis. Infect Immun. 1982;36:325–332. doi: 10.1128/iai.36.1.325-332.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meddens M J M, Thompson J, Bauer W C, Hermans J, van Furth R. Role of granulocytes and monocytes in experimental Staphylococcus epidermidis endocarditis. Infect Immun. 1983;41:145–153. doi: 10.1128/iai.41.1.145-153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheld W M, Sande M A. Endocarditis and intravascular infections. In: Mandell G L M, Douglas R G, Bennet J E, editors. Principals and practices of infectious diseases. 3rd ed. New York, N.Y: John Wiley; 1990. pp. 670–706. [Google Scholar]
- 12.Thompson J, Eulderink F, Lemkes H, van Furth R. Effect of warfarin on the induction and course of experimental endocarditis. Infect Immun. 1976;14:1284–1289. doi: 10.1128/iai.14.6.1284-1289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thörig L, Thompson J, Eulderink F. Effect of warfarin on the induction and course of experimental Staphylococcus epidermidis endocarditis. Infect Immun. 1977;17:504–509. doi: 10.1128/iai.17.3.504-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thörig L, Thompson J, van Furth R. Effect of immunisation on the induction of experimental Streptococcus sanguis and Staphylococcus epidermidis endocarditis. Infection. 1980;8:267–274. [Google Scholar]
- 15.Thörig L, Thompson J, Eulderink F, Emeiss J J, van Furth R. Effects of monocytopenia and anticoagulation in experimental Streptococcus sanguis endocarditis. Br J Pathol. 1980;61:108–116. [PMC free article] [PubMed] [Google Scholar]
- 16.van der Meer J W M, van de Gevel J S, Elzenga-Claesen I, van Furth R. Suspension cultures of mononuclear phagocytes in the Teflon culture bag. J Immunol Methods. 1988;108:19–26. doi: 10.1016/0008-8749(79)90236-3. [DOI] [PubMed] [Google Scholar]