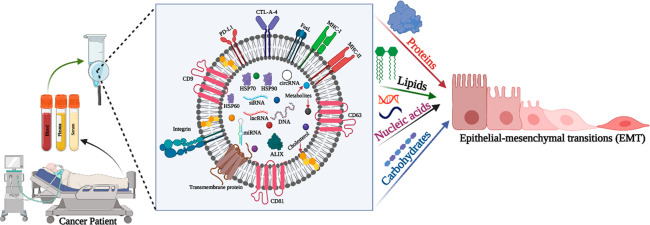
Abstract
Epithelial–mesenchymal transition (EMT) is a fundamental process driving cancer metastasis, transforming non-motile cells into a motile population that migrates to distant organs and forms secondary tumors. In recent years, cancer research has revealed a strong connection between exosomes and the EMT. Exosomes, a subpopulation of extracellular vesicles, facilitate cellular communication and dynamically regulate various aspects of cancer metastasis, including immune cell suppression, extracellular matrix remodeling, metastasis initiation, EMT initiation, and organ-specific metastasis. Tumor-derived exosomes (TEXs) and their molecular cargo, comprising proteins, lipids, nucleic acids, and carbohydrates, are essential components that promote EMT in cancer. TEXs miRNAs play a crucial role in reprogramming the tumor microenvironment, while TEX surface integrins contribute to organ-specific metastasis. Exosome-based cancer metastasis research offers a deeper understanding about cancer and an effective theranostic platform development. Additionally, various therapeutic sources of exosomes are paving the way for innovative cancer treatment development. In this Review, we spotlight the role of exosomes in EMT and their theranostic impact, aiming to inspire cancer researchers worldwide to explore this fascinating field in more innovative ways.
Keywords: Exosome, Cancer, Metastasis, EMT, Biomarkers, Therapeutic
1. Introduction
Cancer, the deadliest noncommunicable disease, arises from uncontrolled cell proliferation and constitutes a significant global health burden. The burden of cancer is expected to grow over the next two decades.1 Risk factors such as tobacco use, alcohol consumption, poor nutrition, physical inactivity, and air pollution contribute to cancer and other noncommunicable diseases. Certain chronic infections can also pose risks, particularly affecting low- and middle-income countries.2 Cancer is projected to claim nearly 10 million lives globally in 2020, making it the leading cause of death.3 Recent cancer research has uncovered intriguing links between cancer and extracellular vehicles (EVs).4 All active cells secrete EVs,5 which are classified into major subpopulations, including macrovesicles, exosomes, large endosomes, and apoptotic bodies.6 Exosomes have emerged as the most prominent EV subpopulation in cancer research, playing a crucial role in cell-to-cell communication. Tumor-derived exosomes (TEXs) and their molecular cargo play a significant part in cancer development and progression.7−11 Cancer metastasis, the most complex event in cancer progression, is driven by the core process of epithelial–mesenchymal transition (EMT). During EMT, cells become motile, enter the circulatory system, and develop the ability to form secondary tumors.12 TEXs molecular cargos, including proteins, lipids, miRNAs, and surface molecules, promote EMT in cancer.13 Circulating exosomes within the human body serve as a valuable source of diagnostic and prognostic markers for cancer. Various exosome sources (mesenchymal stem cell-derived exosomes, immune cell-derived exosomes, plant-derived exosomes,14 etc.) exhibit cancer-fighting properties. As a result, exosome-related cancer research has helped unravel many complex aspects of cancer in greater detail. In this Review, we spotlight exosome regulatory activity in EMT and its theranostic applications in cancer treatment.
2. Biogenesis and Components of Exosomes
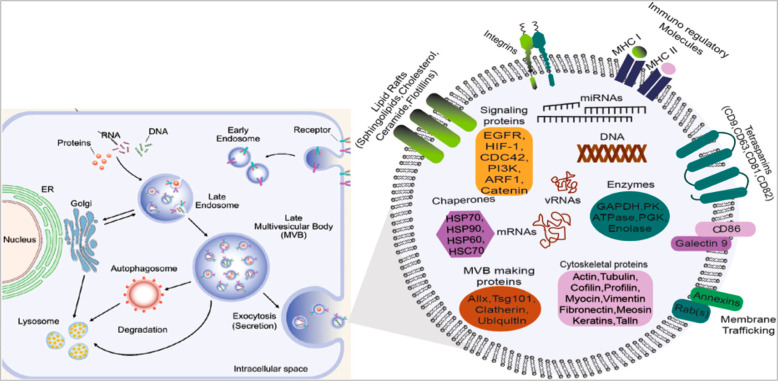
Extracellular vesicles (EVs) are membrane-bound structures that are found in human blood, plasma, serum, etc.15 EV membranes are composed of lipids that resemble the ones present in the plasma membrane of the cell.16 It has been established that a large variety of proteins are also integrated into, bound to, or present in the intraluminal area of EVs.17 Exosomes are known to be a subset of extracellular vesicles.18 It was earlier considered that they carried a cargo of garbage outside the cell,19 but in due course, these nanosized vesicles have grabbed great attention among scientists due to their role in cellular communication and cell signaling.20 The origin of exosomes from the endosomes (Figure 1) and the intermediate stage is the multivesicular body (MVB) maturing into late endosomes. MVBs carry several intraluminal vesicles (ILVs).21 According to recent research, the endosomal sorting complex required for transport (ESCRT) is involved in ILV development. ESCRT has four subsets such as ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. Together, they work in MVB development, cargo selection, and promoting vesicle budding. ESCRT-0 activated ESCRT-I and ESCRT-II. ESCRT-I and ESCRT-II play a role in cargo packaging, and ESCRT-III is involved in vesicle budding.22 ESCRT-independent pathway of exosome biogenesis regulated via surface tetraspanin protein, lipids, and ceramide. The composition of exosomes is a combination of many proteins, including receptors, extracellular matrix proteins, transcription factors, enzymes, lipids, and nucleic acids (DNA, miRNA, mRNA, etc.).13,23
Figure 1.
Exosome biogenesis and its molecular cargo (Adapted from ref (106). Copyright 2021 American Chemical Society.).
3. Exosomes in Immune Cell Reprogramming and Cancer Progression
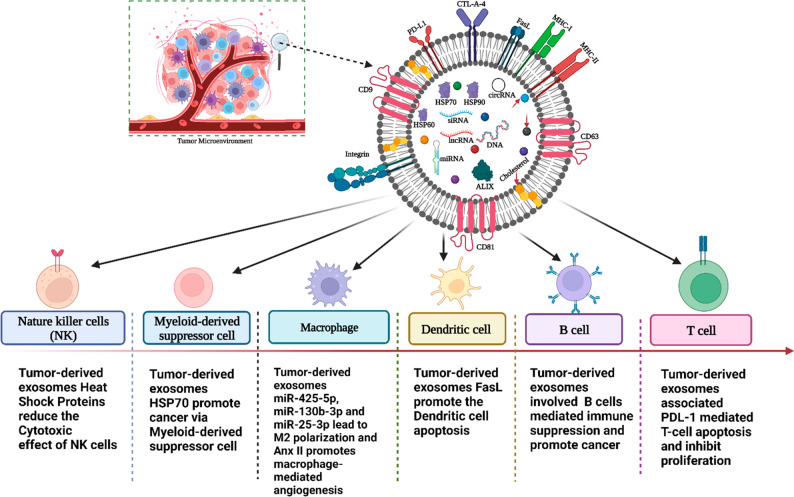
During cancer, immune cell reprogramming is a vital cell event, as a result cancer cells escape the immune surveillance.121 TEXs play a major role in the process. They originate from the plasma membrane and dynamic functional biomolecules that signal the target cell to either suppress or stimulate an immune signal in pathological conditions such as infection and cancer.24 There has been increasing evidence of how these cargos contained within exosomes modulate myeloid as well as lymphoid cell function.25 Myeloid-derived suppressor cells (MDSCs) expand during infection, inflammation, and cancer.26 Scientific research evidence suggests that exosomes released by hypoxia-induced glioma cells delivered miRNA-10a and miRNA-21 which promoted MDSC activation and differentiation.27 Similarly, exosomes carrying miRNA-9 and miRNA-181a shed from breast cancer cells and miRNA-107 from gastric cancer cells caused the expansion of MDSCs.25 Various studies have confirmed that exosomes derived from several cancers promote M1 to tumor-promoting M2 phenotype;28 for example, exosomes derived from HCC harboring miRNA-146-5p induced M2 polarization while inhibiting interferon α, β expression, and high expression of program cell death legend-1 reprogramming of T cells mediated an immune response in cancer.27 Similarly, the exosome-mediated delivery of miRNA-222 in EOC cells, miRNA-301a-3 in pancreatic cancer cells, and miRNA-425-5p, miRNA-25-3p, and miRNA-130b-3p in colon cancer cells led to the formation of M2 phenotype which promoted tumor growth, EMT induction, and angiogenesis that ultimately enhanced metastasis.25 Reprogramming of macrophages through exosome-mediated delivery of miRNA-1246 in p53 mutant cancer cells induced the anti-inflammatory state required for tumor progression.25 In another study, exosomes shed from cells of hepatocellular carcinoma under endoplasmic reticulum stress stimulated macrophages to secrete MCP-1, IL-6, IL-10, and TNF-α, whereas cells of breast cancer under ER stress released exosome miRNA-27a-3p upregulated the PD-L1 expression in macrophages leading to immune evasion.29 Studies have also indicated that tumor-derived exosomes simulate NF-κB signaling in macrophages.30 In gastric carcinoma caused by the Epstein–Barr virus, the exosomes shed by infected epithelial cells inhibited dendritic cell (DC) maturation.27 In another study, it was reported that exosomes bearing miRNA-212-3p released by pancreatic tumors caused immune tolerance by inhibiting RFXAP expression and downregulating expression of MHC-II on dendritic cells.2 Furthermore, exosomes carrying miRNA-203 in pancreatic cancer cells decreased the TLR-4 expression and interleukin-12 tumor necrosis factor-α in DC. Another study showed that cancer cell-derived exosomes from B-cell chronic lymphocytic leukemia and 4T1 breast cancer suppressed differentiation while inducing programmed cell death and enhancing PD-L1 expression in DCs.25 Additionally, T regulatory (Treg) cells have also been shown to modulate DC function via exosome miRNA-142-3p and miRNA-150-5p resulting in tolerogenic DC phenotype.27 Studies show that IL-2 inhibits the activation of Natural Killer (NK) cells through exosomes derived from tumor cells. In blood cancer B cells, T cells, and NK cells, immunosuppressive effects are regulated via exosomes. In yet another study, exosomes harboring TGF-β1 released by pancreatic adenocarcinoma cells dysregulated NK cell function by inhibiting TNF-α, INF-γ, NKG2D, and CD107a expression.27 In contrast, exosomes bearing HSP70 stimulate the production of INF-γ by NK cells in multiple myeloma cells via activating the NF-κB pathway conferring antitumor immunity. Pancreatic and colon cancer cell-derived exosomes carrying HSP70/Bag4 enhanced migratory potential and stimulated cytotoxicity in NK cells.27 TEX’s molecular cargo led to the induction of apoptosis or activation or suppression of T cell function. For instance, exosomes bearing FasL in oral squamous cell carcinoma cells induce apoptosis of T cells via extrinsic and intrinsic pathways.31 Similarly, exosomes carrying FasL in prostatic cancer cells also induced programmed cell death in CD8+ T cells and suppressed their growth. FasL-mediated cell death is caused by melanosomes.32 Exosome-based skin cancer progression led via INF-γ and PDL-1 to higher expression associated cytotoxic T cell downregulation.32 In breast cancer, PDL1-mediated immune suppression takes place in the tumor microenvironment (TME).27 Exosomes harboring 14-3-3(phospho-serine binding proteins) released by hepatocellular carcinoma cells inhibited the antitumor effects of T cells in the TME.27 In colorectal cancer stem cells, exosome-mediated transfer of miRNA-146a-5p enhanced tumor progression along with the decrease in the number of cytotoxic T cells infiltrating the TME. Exosomes also alter B cell function. HCC cell-derived exosomes are involved in B-cell proliferation, expressing interleukin-10 and inhibiting the proliferation of CD8+ T cells, thereby drastically decreasing the antitumor immunity in TME.33 All immune cell alteration development is a favorable condition for cancer progression (Figure 2).
Figure 2.
Tumor-derived exosomes (TEXs) alter immune cell function in cancer (created with BioRender.com).
4. Exosomes in Extracellular Matrix (ECM) Remodeling in Cancer
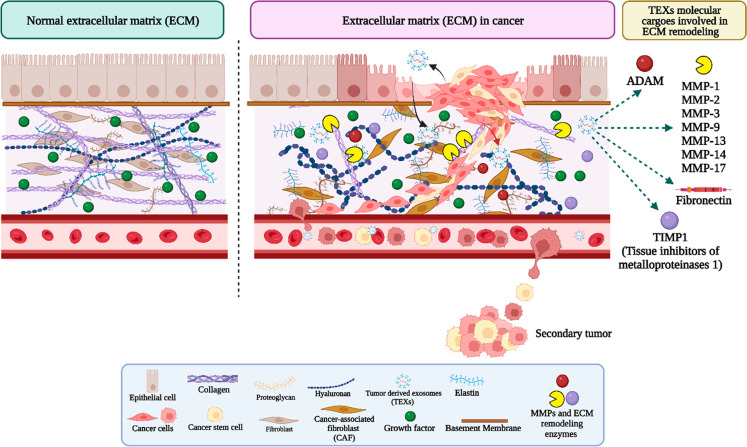
In the tumor microenvironment, cancer cell extracellular matrix remodeling promotes cell motility development. This event leads to metastasis. ECM is composed of protein, glycoprotein, and peptidoglycan. The deep exploration of the exosome and cancer interlink defines tumor-derived exosome (TEX) metalloproteinase (MMPs),107−115 ADAM,116 and fibronectin involved in ECM34 (Figure 3). The protein profiling of ECM events suggests that integrin, annexins, integrin α3, and metalloproteinase participate in cancer cell migration and ECM.35 The tumor microenvironment (TME) associated fibroblast is the most influential cell population in cancer development.36 This has a major contribution to cancer-associated fibroblast (CAF) development and CAF participation in EMT. Tumor growth and development are regulated via CAF cells secreted by inflammatory signaling molecules and growth factors. Adipocytes from cancer patients have higher levels of IL6, IL1, and MMP11 (CAAs). Adipocytes develop fibroblasts involved in extracellular matrix remodeling, and they support metastasis.37 The clinical investigation suggests that in brain cancer the patient’s serum containing the miRNA molecular signature of exosome promotes brain cancer progression. Advanced stage cancer patients carry a huge number of exosomes in their body fluids, and these exosomes develop complex cell signaling toward cancer development.38 Exosome long noncoding RNA SNHG3 acted as a sponge for miRNA-330, favorably regulating pyruvate kinase M1/M2 (PKM), decreasing oxidative phosphorylation, increasing glycolysis, and stimulating the proliferation of breast cancer cells.39 Exosomes formed from breast-cancer-derived miRNA-105 can alter the metabolism of CAFs. CAFs also had altered metabolic profiles, which promote the growth of cancer cells. Exosomes harboring virus-encoded microRNAs were absorbed by neighboring cells, shifting their metabolism toward glycolysis and limiting mitochondrial biogenesis. Exosomes promoted angiogenesis in Kaposi’s sarcoma by altering the metabolism of neighboring cells. Exosomes deliver angiogenic medicines or microRNA to ECs, altering their metabolism and angiogenic action. Exosomes produced from SMAD4-deficient pancreatic ductal adenocarcinoma (PDAC) cells can induce immunosuppression.40 Immunosuppression caused by exosome-mediated metabolic reprogramming may hasten tumor growth. Exosome proteins, microRNAs, noncoding RNAs, and metabolites all have an impact on metabolic reprogramming. In addition to microRNAs, lncRNAs and circRNAs have been studied.41 Exosome-based ECM investigation supports the understanding of several facts about cancer metastasis.10,11,117
Figure 3.
Exosomes in the extracellular matrix remodeling in cancer (created with BioRender.com).
5. Role of Exosomes in Cancer Metastasis
First observed in developmental biology research during the 1970s, EMT plays a vital role in embryo development and organogenesis.42 This process of epithelial cells changing into mesenchymal cells is known as epithelial-to-mesenchymal transition (EMT).152 Many essential developmental processes, including the gastrulation process and healing of wounds, rely on this mechanism.153 The EMT, on the other hand, can play a role in the progression of cancer by enabling cancer cells to detach from tumors and infect adjacent tissues. A complex system of signaling channels regulates the EMT. Several transcription factors, including ZEB1/2, SNAIL, Slug, and Twist, have been identified as crucial regulators of the EMT.154 These factors hinder the expression of epithelial markers like E-cadherin while promoting the production and expression of mesenchymal markers like Vimentin and N-cadherin.155 The transcription factors stimulate the loss of epithelial cell–cell adhesion and the emergence of mesenchymal features, allowing cells to migrate and invade by modifying the expression of these proteins.156 When these so-called transcription factors are activated, epithelial cells undergo alterations such as loss of polarity, enhanced motility, and invasiveness.157 During EMT, epithelial cells lose their unique apical-basal polarity, whereas mesenchymal cells are more invasive and can penetrate the basement membrane. PI3K/Akt, TGF-β, Notch, and Ras/MAPK are all signaling pathways that influence the EMT. These pathways can either activate or decrease the production of EMT-inducing transcription factors, resulting in a change in character from an epithelial to a mesenchymal phenotype.158 TGF-β signaling, for example, is a powerful inducer of EMT, and its activation is linked to increased metastasis in cancer.159 Exosomes are important in cancer because they induce the EMT, which converts noninvasive epithelial tumor cells into invasive mesenchymal-like cells.160 Cancer cells can detach from the main tumor, move through the lymphatic system or through the circulation of blood, and form new metastatic colonies in distant organs as a result of this process.161 Exosomes long noncoding RNA play a significant role in EMT promoting.162 EMT has been linked to increased cell mobility, invasiveness, and metastasis. Exosome-mediated EMT might be averted; exosomal cargo could be modified to cause disruption with EMT signaling systems, and exosomes could be used as diagnostic or prognostic indicators for EMT-driven malignancies. Understanding these pathways has the potential to lead to the development of innovative treatment techniques for preventing metastasis and improving cancer patient outcomes.163 EMT is closely associated with tumor formation, invasion, metastasis, and treatment resistance. Certain cells exhibit both epithelial and mesenchymal EMT markers, forming a hybrid population. The bioactive substances within exosomes43 exert unique regulatory effects that promote a shift toward a mesenchymal cell population, triggering the onset of EMT. Snail1-expressing fibroblasts are present in exosomes derived from cancer-associated fibroblasts (CAFs), which inhibit E-cadherin expression and induce EMT in A549 lung cancer cells. In bladder cancer, CAF-secreted exosome-mediated IL-6 signaling fosters an aggressive cancer pattern.44 In cancer, the fibrosis process is linked with cancer development.137−139 This process is continued via chronic inflammation (20% of cancer related to this phenomenon) or viral infection. Extracellular vesicles (EVs) have strong participation in this process.137 Tumor-derived exosomes (TEXs) promote cancer metastasis (Figure 4).118,132−135
Figure 4.
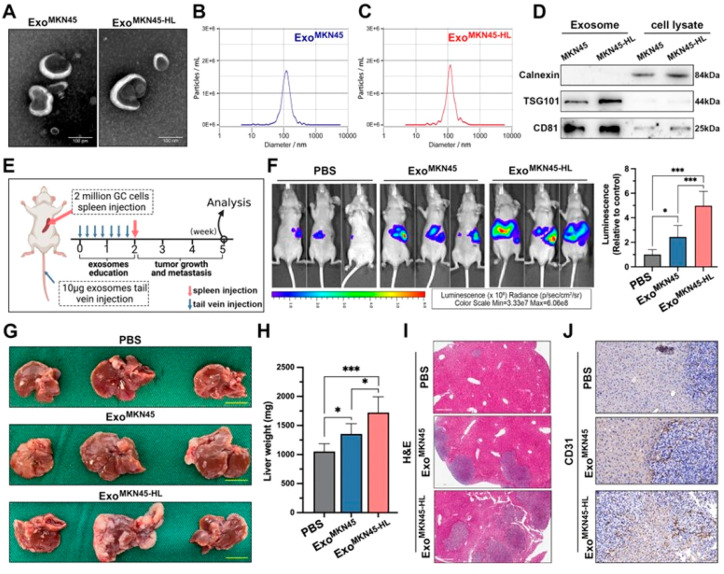
Exosomes associated with liver metastasis in gastric cancer (GC) show distinct characteristics. (A) Transmission electron microscopy (TEM) image of exosomes derived from MKN45 and MKN45-HL cells and (B, C) size distribution analysis of purified exosomes from MKN45 and MKN45-HL cells using NanoSight. (D) Western blot assessment of exosome markers (TSG101 and CD81) in exosomes and lysates from MKN45 and MKN45-HL cells. (E) Diagram illustrating the process of establishing the exosome-informed GC-LM model. (F) Representative in vivo imaging system (IVIS) outcomes in mice injected with luciferase-tagged MKN45 cells into the spleen after being exposed to PBS, MKN45 exosomes, or MKN45-HL exosomes. (G–I) Impact of GC-derived exosomes on liver metastasis in mice, featuring images of liver metastasis, liver weight, and H&E staining. (J) Representative CD31 immunohistochemical staining images of liver metastasis tissues from exosome-educated mice (Adapted with permission from ref (118). The copyright is licensed under a Creative Commons Attribution 4.0 International License 2022, .J Exp. Clin. Cancer Res., Springer Nature).
TEXs carry a small protein called MAP17 between tumor cell subsets to enable horizontal spread and EMT. MiRNA-92a-3p is found in significant concentrations in liver and colon cancer.45 In liver cancer, PTEN expression is suppressed by exosome miRNA-92a-3p and enhances metastasis in colon cancer. In breast cancer, exosome miRNA-181 enhances metastasis. M2 is the cancer-promoting macrophage and suppresses the immune system.46 CAF-derived exosome miRNA-342 plays a role in immune suppression. HOTTIP, an exosome lncRNA, promotes its target HMGA1 in gastric cancer (GC) cells, causing EMT and cisplatin resistance. One of the most exciting facts is that exosomes also play a vital role in radiotherapy resistance.119 Exosomes play a vital role in colorectal cancer EMT.47 Circular RNA (noncoding RNA), another group of exosome RNA, has strong regulatory activity in cancer metastasis. In prostate cancer, circular RNA and miRNA-582 promote EMT. Circular RNA influences several signaling cascades in lung cancer.48,49 The participation of the exosome molecular signature in EMT is explained in Table 1.
Table 1. Exosome-Associated Molecular Cargos Interlink in EMT.
| exosome cargos | molecules | involvement in EMT | references |
|---|---|---|---|
| Proteins | PDL1 | Induce the immune cell’s apoptosis and suppress the immune system | (27) |
| FasL | Induce cytotoxic T cells apoptosis and suppress the immune system | (31) | |
| Tetraspanins (CD9, CD63, CD81, CD151) | Cancer angiogenesis, metastasis, EMT | (100) | |
| CD97 | Premetastatic niche in gastric carcinoma cells | (58) | |
| Annexins, integrin α3, fibronectin, and metalloproteinase | Extracellular matrix remodeling | (34, 35) | |
| Integrin | Organ-specific metastasis | (67) | |
| Heat shock proteins | It reduces NK cell-based anticancer response in cancer | (101) | |
| Lipids | Membrane lipid | Cell signaling | (104, 105) |
| RNA | miRNA-9 and miRNA-181a | Breast cancer progression | (25) |
| miRNA-425-5p, miRNA-130b-3p, and miRNA-25-3p | M2 polarization | (25) | |
| DNA | Genomic DNA, mitochondrial DNA, oncogenic DNA | Reflect on the parental cell mutation status and it also related epigenetic regulation | (102, 9) |
| Carbohydrate | Glycans | Cancer metastasis | (103) |
6. Role of Exosomes in Premetastatic Niche Formation
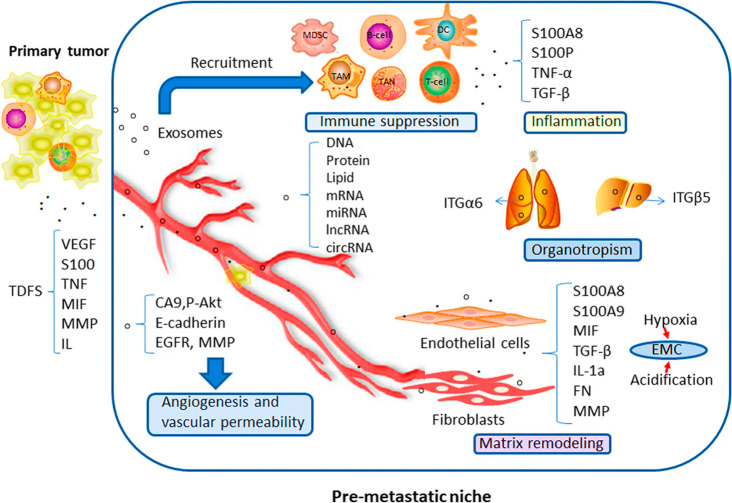
The formation of a premetastatic niche (PMN) (a tumor-driven environment in a distant organ) supports the growth and survival of metastasized tumor cells. The development of secondary lesions significantly contributes to cancer-related deaths. Over the past decades, research has suggested the potential role of tumor-derived extracellular vesicles in regulating PMN.50 Cao58 outlined four critical components that drive metastasis niche formation, including bone-originated cells, stromal cells, immune cell suppression, and tumor-derived secreted factors (TDSFs), such as cytokines, growth factors, interleukin-1, tumor necrosis factor-α, β, and vascular endothelial growth factor (VEGF).51 These factors converge at premetastatic sites before the accumulation of cancer cells, often in organs distinct from the primary tumor site.52 By acting in a paracrine manner on tumor cells, TDSFs promote their migration toward potential PMN formation sites.53 TDSFs may activate host stroma within premetastatic niches to induce the expression of pro-inflammatory components. PMNs are regions where immune cells, such as bone marrow-derived cells (BMDCs), are actively recruited, leading to increased secretion of inflammatory components. Inflammatory elements, transported via the bloodstream, eventually reach PMNs on exosomes isolated from the tumor, transforming the PMN into a tumor-supportive, inflamed microenvironment.54 Both tumor cells and stroma-derived exosomes enhance systemic infiltration and progression of tumor cells throughout the metastatic cascade,55,56 influencing various cancer hallmarks such as uncontrolled cell growth, angiogenesis, and metastasis. Exosomes can directly impact potential metastatic tissue growth and initiate PMN development by altering local factors like cell population, nutrient availability, and vascularization or by influencing the creation of a permissive microenvironment that allows bone marrow-derived cells, like mesenchymal stem cells (MSCs), to migrate to the tumor site and prime the parenchyma for cancerous cells.57 This evidence highlights the pivotal role of exosomes in cancer metastasis as they are involved in establishing and maintaining PMNs. Notably, exosome CD97 is linked to the formation of a PMN in gastric carcinoma cells,58 while exosome miRNA-21 and miRNA-29a trigger inflammatory responses during this process.59 Exosomes also express PD-L1-mediated immune cell evasion, promoting PMN formation (Figure 5).120
Figure 5.
Exosome involvement in premetastatic niche formation (Adapted with permission from ref (120). The copyright is licensed under a Creative Commons Attribution 4.0 International License 2019, Molecular Cancer, Springer Nature).
7. Role of Exosome in Organ-Specific Metastasis
Metastasis is the most devastating stage in cancer, which when established successfully in a distant vital organ makes the root cause of cancer irreversible to a certain extent. Metastasis is caused when cancer cells migrate to another organ and develop a secondary tumor.60 There are not any known mechanisms that link EMT plasticity to organotropism metastasis, but research suggests that epithelial plasticity governs cancer stemness, and cancer stem cells (CSCs) are what cause organotropism metastatic.61−63 This entire process of metastasis is carried out on the molecular and cellular levels and even on the genomic level. Recent studies have shown the role of extracellular vesicles in giving guidance to circulating cancer cells to migrate to specific organs and form a secondary tumor. Specifically, extracellular vesicle surface molecules (integrins principle one) promoted metastasis.64,65 The role of integrin in recent years has been associated with metastasis due to its property of two-way receptor signaling. It is evident that constitutive activation of integrins by internal stimulation results in better adherence to the ECM and, consequently, a more complex interplay of these adhesion receptors with their substrates. Several worldwide studies recommend that TEX surface integrins are the major ingredient that influences organ-specific metastasis in cancer.66−68 Integrins are the glycoprotein that contracts by two subunits, α and β. Based on this subunit, exosomes guide the circulated cancer cell to migrate to different organs and form a secondary tumor such as bone (α4β1, αVβ6, αVβ3), brain (αVβ3, αVβ5, αVβ8), liver (α5β1, α2β1, αVβ5), lymph node (α4β1, αVβ7), and lung (α6β1, α6β4) (Figure 6).68,69 Tumor-derived exosomes (TEXs) reprogram the immune system to promote cancer progression.58,69,70
Figure 6.
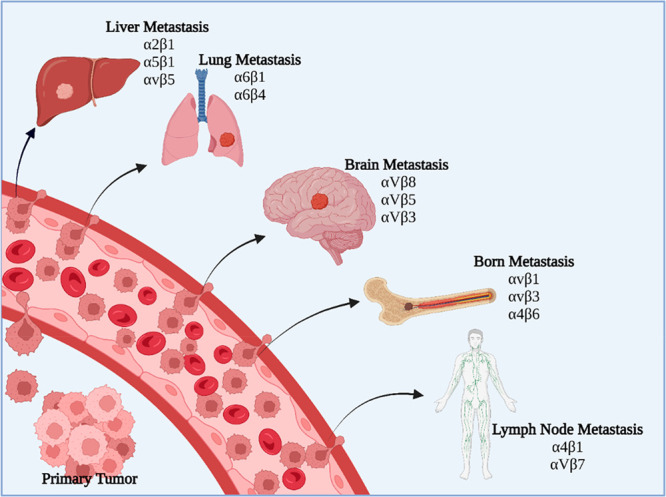
Tumor-derived exosome (TEX) integrins led to organ-specific metastasis (created with BioRender.com).
8. Exosome Source of Cancer EMT Biomarkers
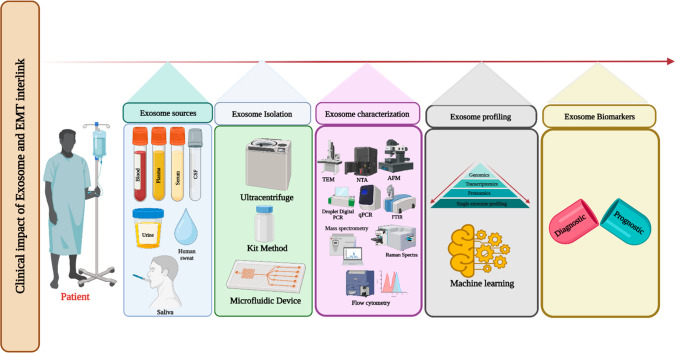
Cancer biomarker research is the most exciting event because this process can only give guidance on proper treatment and understanding of the complication level of the disease. TEXs are a promising source of dynamic biomarkers of cancer (Figure 7). Exosome-derived EMT biomarkers have the potential to reveal ground-breaking insights in cancer research, illuminating the intricate mechanisms driving cancer development. These cutting-edge biomarkers provide a glimpse into the mystifying realm of EMT, setting the stage for remarkable breakthroughs in cancer diagnosis, prognosis, and therapy. By tapping into the capabilities of exosome-related EMT biomarkers, we can transform our understanding of cancer and usher in an era of hope and promise for patients across the globe.
Figure 7.
Clinical importance of exosomes and EMT interlink (created with BioRender.com).
With breast cancer topping global cancer statistics as the most prevalent cancer,71 cutting-edge diagnostic methods such as exosome-associated CD82 are emerging as valuable biomarkers for early detection.72 Exosome research has revealed a treasure trove of essential biomarkers for various cancers, including saliva-derived exosomes in oral cancer122 and plasma-derived exosome miRNA-222 as a prognostic marker for breast cancer.73 Sweat and tear exosomes are also a source of cancer biomarkers.131,136 In lung cancer, serum-derived exosome miRNA-106b74 serves as a diagnostic tool, while plasma-derived exosome miRNA-21 and miRNA-425775 provide prognostic insights. For liver cancer, diagnostics and prognostics are enhanced by serum-derived exosome circular RNA76 and serum-derived exosome miRNA-1262. Research on colon cancer has uncovered exosome-associated biomarkers CD147 (blood-based diagnostic marker)77 and miRNA-486-5p (prognostic marker).78 Diagnostics and prognostics for brain cancer have been advanced by serum-derived exosome miRNA-182-5p79 and miRNA-301a,80 respectively. Table 2 explores EMT-related biomarkers in greater detail, highlighting the incredible potential of exosome research in transforming cancer detection, prognosis, and treatment for the most common cancers worldwide.
Table 2. Exosome-Associated Molecular Significance as an EMT Biomarker.
| exosome-related EMT biomarker | exosome source | molecules | function | references |
|---|---|---|---|---|
| Diagnostic marker | Blood | CD82 | Breast metastasis | (72) |
| Serum | miRNA-106b | It associated with lung lymph node metastasis | (74) | |
| Serum | circRNA-100 | Liver cancer tumor metastasis | (76) | |
| Blood | CD147 | Its high expression in advanced stage colon cancer | (77) | |
| Serum | miRNA-182-5p | High expression in brain cancer | (79) | |
| Prognostic marker | Plasma | miR-222 | High expression led to NF-κB mediated breast cancer lymphatic metastasis | (73) |
| Plasma | miR-21 | Lung cancer | (75) | |
| miRNA-4257 | ||||
| Serum | miRNA-1262 | Lower expression in liver cancer | (102) | |
| Plasma and Serum | miRNA-486-5p | Colon cancer lymph node metastasis | (78) | |
| Serum | miRNA-301a | High expression in brain cancer | (80) |
9. Exosome-Based Therapeutic Approach for the EMT
Epithelial to mesenchymal transition is the key basis of metastasis in cancer progression. During the EMT, the cancer cells attain various properties such as self-renewal, resistance against apoptosis, and initializing of the tumor. All of these help the few cancer cells to colonize a distant organ and transform it into a secondary infection site.81,82 During the EMT, cancer cells develop radiation resistance and chemoresistance which make cancer treatment more challenging.83−85 The ABC transporter protein is the major molecular component that pumps out the drug from the cancer cells, and as a result, it leads to drug resistance in cancer. EMT regulatory several transcription factors alter the apoptosis phenomena in cancer cells.86,87 The signaling pathway targeting may be a promising approach to reducing EMT and tumor resistance to therapy. A variety of signaling pathways are involved in the EMT of tumor cells. The EMT is governed by a well-established signaling system known as TGF-/Smad signaling. Gastric cancer and glioma cells have shown that the TGF-receptor inhibitors LV2109761 and LV364947 impede the EMT brought on by ionizing radiation, increasing tumor cells’ irradiation sensitivity.88,89 Exosome is a promising approach for cancer prevention (Figure 8). The recent era of treatment focuses mainly on plant-derived exosomes for drug delivery. Future medical applications for drug delivery systems could benefit from the specificity of plant-derived exosomes provided by particular orientations as well as their capacity to transport hydrophobic medicines, modify genes for therapeutic purposes, and avoid immunological rejection.90 Plant-derived exosomes (PDXs) are a potential therapeutic tool for cancer with low toxicity.91 Mesenchymal stem cell (MSC) derived exosomes show EMT inhibition properties in lung cancer.92 Several research investigations show that exosome-based cancer therapeutic development is more promising.93 Exosome-based cancer therapy is the beginning of a next generation of cancer treatment.123 Although exosome-based cancer therapeutic approaches show promising results, there are some questions that have not been solved such as exosome heterogeneity, large-scale production, diversity of beach production (individual batches get different exosomes), and therapeutic exosome toxicological investigation.140−143 Stem cell-derived exosome is a potential source of therapeutic exosomes.144 Research evidence indicates that stem cell exosomes also promote cancer;145 if stem cell-derived exosomes are not modified, they can promote cancer or inhibit it.144 TEXs show anticancer activity,146 but their internal oncogenic cargo support plays a dual nature in cancer treatment.147 Plant-derived exosomes show effective anticancer activity against cancer, but more toxicological investigation is required.148 Toxicological effects of CAR-T cell derived exosomes are low, compared to CAR-T cells.149 Artificial chimeric exosomes are another type of exosome that overcomes production limitations with effective anticancer activity with low toxicity.150 Immune cell-derived exosomes are also a promising source of cancer therapy.151 Exosome-based immunotherapy needs more investigation for standard therapeutic development.152 Finally, the exosome-based therapeutic approach needs more time for proper clinical investigation and effective and affordable cancer therapeutic development.
Figure 8.
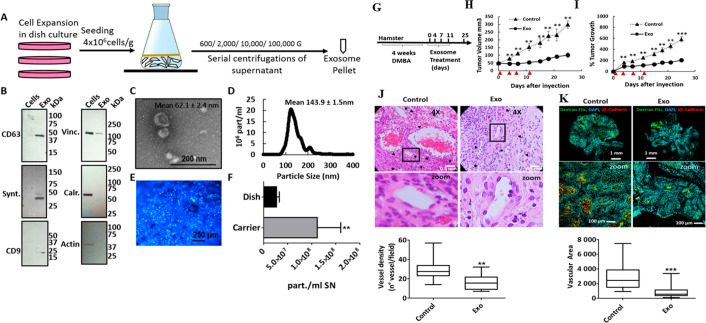
Stem cell derived exosomes inhibit tumor growth and angiogenesis. (A) Exosome isolation protocol, (B) Western blot analysis of exosome biomarkers (negative exosome markers Vinculin (Vinc.), Calreticulin (Calr.), and β-Actin (Actin)). (C) Image of purified exosomes in scanning electron micrograph. (D) Size distribution of exosomes determined by nanosight. (E) Hoechst-stained MenSC on BioNOC II carrier, showing a typical confluence for exosome production. (F) Yield of purified exosomes in PBS as Particles (part)/mL of initial cell culture supernatant (SN) cell lysate (Cells) and exosomes. (G) Experiment plan. Tumors were induced with 4 weeks of DMBA treatment, and four injections of exosomes were administered every 3–4 days. (H, I) Tumor growth in mm3 tumor volume and relative tumor growth after days of exosome treatment. Control tumors are shown as triangles and exosome-treated tumors, as circles. (J) Histological sections of tumors at day 25 (end-point) with Hematoxylin and eosin stain (H&E). Quantification of vessel density based on H&E sections is shown on bottom. (K) Dextran-Fitc (green), VE Cadherin (red), and Hoechst (blue) stained histological sections of tumors at day 25 (end-point). (Adapted with permission from ref (124). The copyright is licensed under a Creative Commons Attribution 4.0 International License 2019, Scientific Reports, Springer Nature).
10. Future Orientation of Exosome-Based Cancer Research
Exosome and cancer association lead to cancer progression and development. It has successfully proven to be a great regulator in the field of oncology due to its dynamic roles in cancer, such as immune cell reprogramming, extracellular matrix remodeling,125 premetastatic niche formation,94 initiation of metastasis,95 and finally organ-specific metastasis,67 and most importantly EMT.96 The clinical impact of exosomes is significant. Exosomes are adding an impactful aspect to liquid biopsy. Circulated exosome is an emerging source of a cancer biomarker (diagnostic and prognostic).38 The therapeutic domain of exosomes has a signature landmark. It is also a potential cancer drug delivery tool. The engineered exosomes97 are showing more effectiveness in therapeutic applications. Exosome research faces some questions such as heterogeneity,126−128 isolation of golden standard protocol, etc.98 The single exosome profiling method decodes this complication (Figure 9). In recent times, muti-omics and machine learning have also come into exosome research.126 The intradisciplinary research approach in exosomes supports the development of efficient and affordable solutions for cancer.129 The worldwide large-scale scientific mind works on its limitations. The exosome is the brightest star in future cancer precision medicine.99,130
Figure 9.
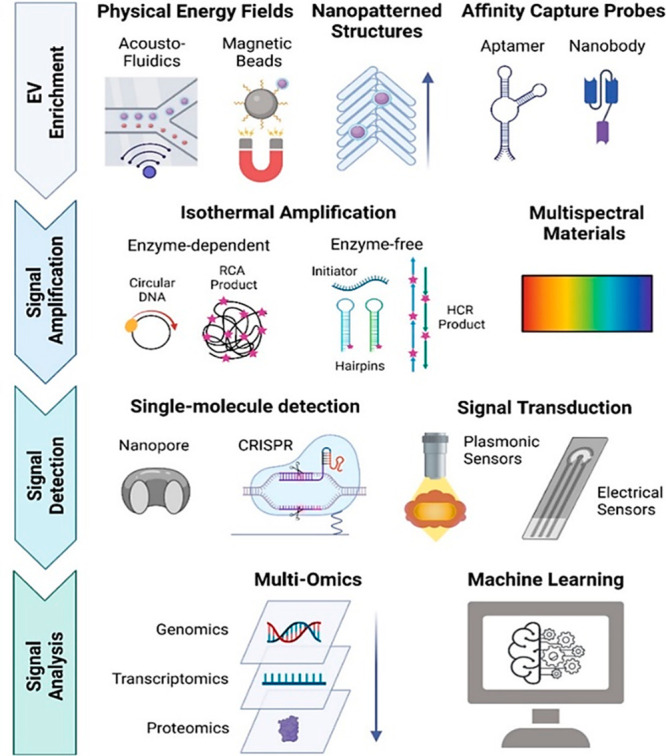
Single exosome profiling (Adapted from ref (126). Copyright 2022 American Chemical Society).
11. Conclusion
Exosome-based cancer research not only is an innovative approach but also holds the key to unlocking the intricate enigma of the EMT in cancer. Tumor-derived exosomes (TEXs) have been instrumental in deciphering the complex concepts and mechanisms underlying cancer progression. Moreover, exosomes are emerging as potent tools in cancer therapy, with applications in drug delivery and harnessing the potential of various therapeutic exosome sources such as stem cell-derived, immune cells derived, chimeric exosomes, modified exosomes and plant-derived exosomes. To fully capitalize on these advancements, we must focus on creating nanotechnology-based smart platforms for efficient exosome isolation and molecular profiling. As we venture into this transformative liquid biopsy era, we edge closer to realizing the dream of precision oncology. By embracing the power of exosomes, we can revolutionize cancer research and treatment, ultimately improving patient outcomes and saving countless lives.
Acknowledgments
N. D. Thorat acknowledges funding under the Science Foundation Ireland and Irish Research Council (SFI-IRC) pathway programme (21/PATH-S/9634).
Author Contributions
○ B.B. and N.M. contributed equally.
The authors declare no competing financial interest.
References
- Siegel R. L.; Miller K. D.; Wagle N. S.; Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- de Martel C.; Georges D.; Bray F.; Ferlay J.; Clifford G. M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global health 2020, 8, e180–e190. 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- Ferlay J.; Colombet M.; Soerjomataram I.; Parkin D. M.; Piñeros M.; Znaor A.; Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021, 149, 778. 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- Xu R.; Rai A.; Chen M.; Suwakulsiri W.; Greening D. W.; Simpson R. J. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nature reviews Clinical oncology. 2018, 15, 617–638. 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- Doyle L. M.; Wang M. Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K.; Breyne K.; Ughetto S.; Laurent L. C.; Breakefield X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms E.; Cabañas C.; Mäger I.; Wood M. J. A.; Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Frontiers in immunology 2018, 9, 738. 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Dhar R.; Kumar L. B. S. S.; Shivji G. G.; Jayaraj R.; Devi A. Theranostic signature of tumor-derived exosomes in cancer. Med. Oncol. 2023, 40, 321. 10.1007/s12032-023-02176-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B.; Dhar R.; Mukherjee S.; Gorai S.; Devi A.; Krishnan A.; Alexiou A.; Papadakis M. Exosome DNA: An untold story of cancer. Clin. Transl. Disc. 2023, 3, e218 10.1002/ctd2.218. [DOI] [Google Scholar]
- Ghosh S.; Dhar R.; Gurudas; Shivji G.; Dey D.; Devi A.; Jha S. K.; Adhikari M. D.; Gorai S. Clinical Impact of Exosomes in Colorectal Cancer Metastasis. ACS Appl. Bio Mater. 2023, 6, 2576. 10.1021/acsabm.3c00199. [DOI] [PubMed] [Google Scholar]
- Dhar R.; Devi A.; Gorai S.; Jha S. K.; Alexiou A.; Papadakis M. Exosome and epithelial-mesenchymal transition: A complex secret of cancer progression. J. Cell Mol. Med. 2023, 27, 1603–1607. 10.1111/jcmm.17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.; Ng A. S.; Cai S.; Li Q.; Yang L.; Kerr D. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncology 2021, 22, e358–e368. 10.1016/S1470-2045(21)00343-0. [DOI] [PubMed] [Google Scholar]
- Mashouri L.; Yousefi H.; Aref A. R.; Ahadi A. M.; Molaei F.; Alahari S. K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular cancer 2019, 18, 75. 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mukerjee N.; Mukherjee D.; Devi A.; Jha S. K.; Gorai S.. Plant-derived exosomes: A new dimension in cancer therapy. Phytother. Res., in press; 10.1002/ptr.7828. [DOI] [PubMed] [Google Scholar]
- Geeurickx E.; Lippens L.; Rappu P.; De Geest B. G.; De Wever O.; Hendrix A. Recombinant extracellular vesicles as biological reference material for method development, data normalization and assessment of (pre-)analytical variables. Nature protocols 2021, 16, 603–633. 10.1038/s41596-020-00446-5. [DOI] [PubMed] [Google Scholar]
- Kotani A.; Ito M.; Kudo K. Non-coding RNAs and lipids mediate the function of extracellular vesicles in cancer cross-talk. Seminars in cancer biology 2021, 74, 121–133. 10.1016/j.semcancer.2021.04.017. [DOI] [PubMed] [Google Scholar]
- van der Koog L.; Gandek T. B.; Nagelkerke A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthcare Mater. 2022, 11, e2100639 10.1002/adhm.202100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Xie F.; Wang L.; Zhang L.; Zhang S.; Fang M.; Zhou F. The function and clinical application of extracellular vesicles in innate immune regulation. Cellular & molecular immunology 2020, 17, 323–334. 10.1038/s41423-020-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratta S.; Tancini B.; Sagini K.; Delo F.; Chiaradia E.; Urbanelli L.; Emiliani C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. International journal of molecular sciences 2020, 21, 2576. 10.3390/ijms21072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. J.; Hao M.; Yeo S. K.; Guan J. L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 2020, 39, 2539–2549. 10.1038/s41388-020-1162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz H. U.; Brotherton D.; Inal J. Communication is key: extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS microbiology reviews 2022, 46, fuab044. 10.1093/femsre/fuab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Liu Y.; Liu H.; Tang W. H. Exosomes: biogenesis, biologic function and clinical potential. Cell & bioscience 2019, 9, 19. 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H.; Im H.; Castro C. M.; Breakefield X.; Weissleder R.; Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Zeng S.; Gong Z.; Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Molecular cancer 2020, 19, 160. 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeratski F. G.; Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS journal 2021, 288, 10–35. 10.1111/febs.15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D. I.; Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology 2009, 9, 162–174. 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. W. A.; Jahangir S.; Ghosh B.; Yesmin F.; Anis A.; Satil S. N.; Anwar F.; Rashid M. H. Exosomes for regulation of immune responses and immunotherapy. Journal of Nanotheranostics 2022, 3, 55–85. 10.3390/jnt3010005. [DOI] [Google Scholar]
- Yang M.; McKay D.; Pollard J. W.; Lewis C. E. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer research 2018, 78, 5492–5503. 10.1158/0008-5472.CAN-18-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Hua W.; Liu J.; Fan L.; Wang H.; Sun G. Exosomes derived from endoplasmic reticulum-stressed liver cancer cells enhance the expression of cytokines in macrophages via the STAT3 signaling pathway. Oncology letters 2020, 20, 589–600. 10.3892/ol.2020.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A.; Zhou W.; Liu L.; Fong M. Y.; Champer J.; Van Haute D.; Chin A. R.; Ren X.; Gugiu B. G.; Meng Z.; Huang W.; Ngo V.; Kortylewski M.; Wang S. E. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci. Rep. 2014, 4, 5750. 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W.; Wieckowski E.; Taylor D. D.; Reichert T. E.; Watkins S.; Whiteside T. L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clinical cancer research: an official journal of the American Association for Cancer Research 2005, 11, 1010–1020. 10.1158/1078-0432.1010.11.3. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach H.; Gahan P. B. Exosomes in immune regulation. Non-coding RNA 2021, 7, 4. 10.3390/ncrna7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L.; Zhang Q.; Cheng Y.; Chen X.; Wang G.; Shi M.; Zhang T.; Cao Y.; Pan H.; Zhang L.; Wang G.; Deng Y.; Yang Y.; Chen G. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother Cancer. 2018, 6, 145. 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mallik S.; Devi A. Exosomal microRNAs (exoMIRs): micromolecules with macro impact in oral cancer. 3 Biotech 2022, 12, 155. 10.1007/s13205-022-03217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A.; Thakur B. K.; Weiss J. M.; Kim H. S.; Peinado H.; Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer cell 2016, 30, 836–848. 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmik M.; Ullmann P.; Rodriguez F.; Haan S.; Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. International journal of cancer 2020, 146, 895–905. 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavets V.; Kochetkova M.; Pitson S. M.; Samuel M. S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Frontiers in oncology 2018, 8, 431. 10.3389/fonc.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami A. B.; Mahjoubin-Tehran M.; Movahedpour A.; Morshedi K.; Sheida A.; Taghavi S. P.; Mirzaei H.; Hamblin M. R. Role of exosomes in malignant glioma: microRNAs and proteins in pathogenesis and diagnosis. Cell communication and signaling: CCS 2020, 18, 120. 10.1186/s12964-020-00623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.; Liu P.; Wu Y.; Meng X.; Wu M.; Han J.; Tan X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer science 2018, 109, 2946–2956. 10.1111/cas.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E.; Wang X.; Gong Z.; Yu M.; Wu H.; Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal transduction and targeted therapy 2020, 5, 242. 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K.; Zhou Q.; Qiao B.; Shao B.; Hu S.; Wang G.; Yuan W.; Sun Z. Exosome-derived noncoding RNAs: Function, mechanism, and application in tumor angiogenesis. Molecular therapy. Nucleic acids 2022, 27, 983–997. 10.1016/j.omtn.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D.; Tamma R.; Annese T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Translational oncology 2020, 13, 100773 10.1016/j.tranon.2020.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskeh M. D. A.; Entezari M.; Mirzaei S.; Zabolian A.; Saleki H.; Naghdi M. J.; Sabet S.; Khoshbakht M. A.; Hashemi M.; Hushmandi K.; Sethi G.; Zarrabi A.; Kumar A. P.; Tan S. C.; Papadakis M.; Alexiou A.; Islam M. A.; Mostafavi E.; Ashrafizadeh M. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol Oncol. 2022, 15, 83. 10.1186/s13045-022-01305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J.; Li M.; Tan Y.; Cao L.; Gu Q.; Yang H.; Hu C. Snail1-expressing cancer-associated fibroblasts induce lung cancer cell epithelial-mesenchymal transition through miR-33b. Oncotarget 2017, 8, 114769–114786. 10.18632/oncotarget.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.; Fawzy A.; Akel S. Y.; Gamal H.; Elshimy R. A. A. Evaluation of microRNA 92a Expression and Its Target Protein Bim in Colorectal Cancer. Asian Pacific journal of cancer prevention: APJCP 2022, 23, 723–730. 10.31557/APJCP.2022.23.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R.; Mitra P.; Gupta N.; Sharma S.; Singh V. K.; Mukerjee N.; Dhasmana A.; Gundamaraju R. Cellular landscaping of exosomal miRNAs in cancer metastasis: From chemoresistance to prognostic markers. Advances in Cancer Biology-Metastasis 2022, 5, 100050 10.1016/j.adcanc.2022.100050. [DOI] [Google Scholar]
- Rocha M. R.; Morgado-Diaz J. A. Epithelial-Mesenchymal Transition in colorectal cancer: Annexin A2 is caught in the crosshairs. Journal of cellular and molecular medicine 2021, 25, 10774–10777. 10.1111/jcmm.16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q.; Zhang C.; Sun X.; Li Q. Circular RNAs function as competing endogenous RNAs in multiple types of cancer. Oncology letters 2017, 15, 23–30. 10.3892/ol.2017.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Luo R.; Wang J.; Luan X.; Wu D.; Chen H.; Hou Q.; Mao G.; Li X. Tumor Cell-Derived Exosomal miR-770 Inhibits M2Macrophage Polarization via Targeting MAP3K1 to Inhibit the Invasion of Non-small Cell Lung Cancer Cells. Frontiers in cell and developmental biology 2021, 9, 679658 10.3389/fcell.2021.679658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. J.; Lima L. G.; Möller A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Seminars in cell & developmental biology 2017, 67, 3–10. 10.1016/j.semcdb.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Mazumdar A.; Urdinez J.; Boro A.; Arlt M. J. E.; Egli F. E.; Niederöst B.; Jaeger P. K.; Moschini G.; Muff R.; Fuchs B.; Snedeker J. G.; Gvozdenovic A. Exploring the Role of Osteosarcoma-Derived Extracellular Vesicles in Pre-Metastatic Niche Formation and Metastasis in the 143-B Xenograft Mouse Osteosarcoma Model. Cancers (Basel). 2020, 12, 3457. 10.3390/cancers12113457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R. N.; Riba R. D.; Zacharoulis S.; Bramley A. H.; Vincent L.; Costa C.; MacDonald D. D.; Jin D. K.; Shido K.; Kerns S. A.; Zhu Z.; Hicklin D.; Wu Y.; Port J. L.; Altorki N.; Port E. R.; Ruggero D.; Shmelkov S. V.; Jensen K. K.; Rafii S.; Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005, 438, 820–7. 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.; Ji X.; Liu J.; Fan D.; Zhou Q.; Chen C.; Wang W.; Wang G.; Wang H.; Yuan W.; Ji Z.; Sun Z. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer. 2019, 18, 39. 10.1186/s12943-019-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.; Liu Y.; Lee H.; Herrmann A.; Zhang W.; Zhang C.; Shen S.; Priceman S. J.; Kujawski M.; Pal S. K.; Raubitschek A.; Hoon D. S. B.; Forman S.; Figlin R. A.; Liu J.; Jove R.; Yu H. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012, 21, 642–654. 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Xu W. T.; Bian Z. Y.; Fan Q. M.; Li G.; Tang T. T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer letters 2009, 281, 32–41. 10.1016/j.canlet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Kidd S.; Spaeth E.; Dembinski J. L.; Dietrich M.; Watson K.; Klopp A.; Battula V. L.; Weil M.; Andreeff M.; Marini F. C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem cells (Dayton, Ohio) 2009, 27, 2614–2623. 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer cell 2016, 30, 668–681. 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Tadokoro H.; Umezu T.; Ohyashiki K.; Hirano T.; Ohyashiki J. H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013, 288, 34343–34351. 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauf A. C.; Massagué J. Surviving at a Distance: Organ-Specific Metastasis. Trends in cancer 2015, 1, 76–91. 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T. T.; Yang M. H. Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness. Molecular oncology 2017, 11, 792–804. 10.1002/1878-0261.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi M.; Nosrati R.; Salmaninejad A.; Dehghani S.; Shahryari A.; Saberi A. Organ-specific metastasis of breast cancer: molecular and cellular mechanisms underlying lung metastasis. Cellular oncology (Dordrecht) 2018, 41, 123–140. 10.1007/s13402-018-0376-6. [DOI] [PubMed] [Google Scholar]
- Ren D.; Zhu X.; Kong R.; Zhao Z.; Sheng J.; Wang J.; Xu X.; Liu J.; Cui K.; Zhang X. H.; Zhao H.; Wong S. T. C. Targeting Brain-Adaptive Cancer Stem Cells Prohibits Brain Metastatic Colonization of Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 2052–2064. 10.1158/0008-5472.CAN-17-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S.; Weinberg R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011, 147, 275–292. 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Li C.; Trojanowicz B.; Li X.; Shi D.; Zhan C.; Wang Z.; Chen L. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2016, 19, 754–766. 10.1007/s10120-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K. K.; Pal S.; Moulik S.; Chatterjee A. Integrins and metastasis. Cell adhesion & migration 2013, 7, 251–261. 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A.; Costa-Silva B.; Shen T.-L.; Rodrigues G.; Hashimoto A.; Tesic Mark M.; Molina H.; Kohsaka S.; Di Giannatale A.; Ceder S.; Singh S.; Williams C.; Soplop N.; Uryu K.; Pharmer L.; King T.; Bojmar L.; Davies A. E.; Ararso Y.; Zhang T.; Zhang H.; Hernandez J.; Weiss J. M.; Dumont-Cole V. D.; Kramer K.; Wexler L. H.; Narendran A.; Schwartz G. K.; Healey J. H.; Sandstrom P.; Jørgen Labori K.; Kure E. H.; Grandgenett P. M.; Hollingsworth M. A.; de Sousa M.; Kaur S.; Jain M.; Mallya K.; Batra S. K.; Jarnagin W. R.; Brady M. S.; Fodstad O.; Muller V.; Pantel K.; Minn A. J.; Bissell M. J.; Garcia B. A.; Kang Y.; Rajasekhar V. K.; Ghajar C. M.; Matei I.; Peinado H.; Bromberg J.; Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015, 527, 329–35. 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mukherjee S.; Mukerjee N.; Mukherjee D.; Devi A.; Ashraf G. M.; Alserihi R. F.; Tayeb H. H.; Hashem A. M.; Alexiou A.; Thorate N. Interrelation between extracellular vesicles miRNAs with chronic lung diseases. J. Cell Physiol. 2022, 237, 4021–4036. 10.1002/jcp.30867. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Ma X.; Yu J. Exosomes and organ-specific metastasis. Molecular therapy Methods & clinical development 2021, 22, 133–147. 10.1016/j.omtm.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao Y. J.; Kim H. S.; Hwang E. H.; Woo J.; Zhang M.; Moon W. K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget 2018, 9, 7398–7410. 10.18632/oncotarget.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L.; Miller K. D.; Fuchs H. E.; Jemal A. Cancer statistics, 2022. CA: a cancer journal for clinicians 2022, 72, 7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Wang H.; Lu Z.; Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J. Hematol. Oncol. 2019, 12, 133. 10.1186/s13045-019-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J.; Xu Z.; Zhang Y.; Tan C.; Hu W.; Wang M.; Xu Y.; Tang J. Exosome-mediated miR-222 transferring: An insight into NF-κB-mediated breast cancer metastasis. Experimental cell research 2018, 369, 129–138. 10.1016/j.yexcr.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Sun S.; Chen H.; Xu C.; Zhang Y.; Zhang Q.; Chen L.; Ding Q.; Deng Z. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life sciences 2020, 244, 117297 10.1016/j.lfs.2020.117297. [DOI] [PubMed] [Google Scholar]
- Dejima H.; Iinuma H.; Kanaoka R.; Matsutani N.; Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncology letters. 2017, 13, 1256–1263. 10.3892/ol.2017.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Y.; Huang Z. L.; Huang J.; Xu B.; Huang X. Y.; Xu Y. H.; Zhou J.; Tang Z. Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 20. 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.; Ma L.; Gong M.; Su G.; Zhu S.; Zhang W.; Wang S.; Li Z.; Chen C.; Li L.; Wu L.; Yan X. Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry. ACS Nano 2018, 12, 671–680. 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- Alves Dos Santos K.; Clemente Dos Santos I. C.; Santos Silva C.; Gomes Ribeiro H.; de Farias Domingos I.; Nogueira Silbiger V. Circulating Exosomal miRNAs as Biomarkers for the Diagnosis and Prognosis of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 346. 10.3390/ijms22010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimkhani S.; Vafaee F.; Hallal S.; Wei H.; Lee M. Y. T.; Young P. E.; Satgunaseelan L.; Beadnall H.; Barnett M. H.; Shivalingam B.; Suter C. M.; Buckland M. E.; Kaufman K. L. Deep sequencing of circulating exosomal microRNA allows non-invasive glioblastoma diagnosis. NPJ Precision Oncol. 2018, 2, 28. 10.1038/s41698-018-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F.; Qing Q.; Pan Q.; Hu M.; Yu H.; Yue X. Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cellular oncology (Dordrecht) 2018, 41, 25–33. 10.1007/s13402-017-0355-3. [DOI] [PubMed] [Google Scholar]
- Das B.; Sarkar N.; Bishayee A.; Sinha D. Dietary phytochemicals in the regulation of epithelial to mesenchymal transition and associated enzymes: A promising anticancer therapeutic approach. Seminars in cancer biology 2019, 56, 196–218. 10.1016/j.semcancer.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Lee C. C.; Cheng Y. C.; Chang C. Y.; Lin C. M.; Chang J. Y. Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption. Sci. Rep. 2018, 8, 17477 10.1038/s41598-018-35392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weadick B.; Nayak D.; Persaud A. K.; Hung S. W.; Raj R.; Campbell M. J.; Chen W.; Li J.; Williams T. M.; Govindarajan R. EMT-Induced Gemcitabine Resistance in Pancreatic Cancer Involves the Functional Loss of Equilibrative Nucleoside Transporter 1. Molecular cancer therapeutics 2021, 20, 410–422. 10.1158/1535-7163.MCT-20-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M.; Wu C.; Guo E.; Peng S.; Zhang L.; Sun W.; Liu D.; Hu G.; Hu G. FOXO3a knockdown promotes radioresistance in nasopharyngeal carcinoma by inducing epithelial-mesenchymal transition and the Wnt/β-catenin signaling pathway. Cancer letters 2019, 455, 26–35. 10.1016/j.canlet.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Takaoka Y.; Konno M.; Koseki J.; Colvin H.; Asai A.; Tamari K.; Satoh T.; Mori M.; Doki Y.; Ogawa K.; Ishii H. Mitochondrial pyruvate carrier 1 expression controls cancer epithelial-mesenchymal transition and radioresistance. Cancer Sci. 2019, 110, 1331–1339. 10.1111/cas.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. S.; Sun Y. Z.; Wang S. M.; Ruan J. S. Epithelial-mesenchymal transition: potential regulator of ABC transporters in tumor progression. Journal of Cancer 2017, 8, 2319–2327. 10.7150/jca.19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberuyi N.; Rahgozar S.; Pourabutaleb E.; Ghaedi K. Selective dysregulation of ABC transporters in methotrexate-resistant leukemia T-cells can confer cross-resistance to cytarabine, vincristine and dexamethasone, but not doxorubicin. Current research in translational medicine 2021, 69, 103269 10.1016/j.retram.2020.09.003. [DOI] [PubMed] [Google Scholar]
- Hao Y.; Baker D.; Ten Dijke P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. International journal of molecular sciences 2019, 20, 2767. 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.; Huang T.; Zhang D.; Wang M.; Wu B.; Shang Y.; Sattar S.; Ding L.; Liu Y.; Jiang H.; Liang Y.; Zhou F.; Wei Y. TGF-β receptor inhibitor LY2109761 enhances the radiosensitivity of gastric cancer by inactivating the TGF-β/SMAD4 signaling pathway. Aging (Albany NY). 2019, 11, 8892–8910. 10.18632/aging.102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Li S.; Zhang S.; Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian journal of pharmaceutical sciences 2022, 17, 53–69. 10.1016/j.ajps.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.; Zhang L.; Zhang Y.; Lu R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics 2022, 14, 822. 10.3390/pharmaceutics14040822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Su Y.; Fa X. Restoration of BRG1 inhibits proliferation and metastasis of lung cancer by regulating tumor suppressor miR-148b. OncoTargets and therapy 2015, 8, 3603–3612. 10.2147/OTT.S95500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqil F.; Gupta R. C. Exosomes in Cancer Therapy. Cancers 2022, 14, 500. 10.3390/cancers14030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil A. A.; Rhee W. J. Exosomes: biogenesis, composition, functions, and their role in pre-metastatic niche formation. Biotechnology and Bioprocess Engineering 2019, 24, 689–701. 10.1007/s12257-019-0170-y. [DOI] [Google Scholar]
- Wu M.; Wang G.; Hu W.; Yao Y.; Yu X. F. Emerging roles and therapeutic value of exosomes in cancer metastasis. Molecular cancer 2019, 18, 53. 10.1186/s12943-019-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Lee S.; Shin E.; Seong K. M.; Jin Y. W.; Youn H.; Youn B. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells 2020, 9, 861. 10.3390/cells9040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P. H. L.; Xiang D.; Tran T. T. D.; Yin W.; Zhang Y.; Kong L.; Chen K.; Sun M.; Li Y.; Hou Y.; Zhu Y.; Duan W. Exosomes and Nanoengineering: A Match Made for Precision Therapeutics. Adv. Mater. 2020, 32, e1904040 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- Li X.; Corbett A. L.; Taatizadeh E.; Tasnim N.; Little J. P.; Garnis C.; Daugaard M.; Guns E.; Hoorfar M.; Li I. T. S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL bioengineering 2019, 3, 011503 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goričar K.; Dolžan V.; Lenassi M. Extracellular Vesicles: A Novel Tool Facilitating Personalized Medicine and Pharmacogenomics in Oncology. Frontiers in pharmacology 2021, 12, 671298 10.3389/fphar.2021.671298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla R. R.; Pandrangi S.; Kumari S.; Gavara M. M.; Badana A. K. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia-Pacific journal of clinical oncology 2018, 14, 383–391. 10.1111/ajco.12869. [DOI] [PubMed] [Google Scholar]
- Lv L. H.; Wan Y. L.; Lin Y.; Zhang W.; Yang M.; Li G. L.; Lin H. M.; Shang C. Z.; Chen Y. J.; Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012, 287, 15874–15885. 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Tian L.; Lu J.; Ng I. O. Exosomes and cancer - Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. 10.1038/s41389-022-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltész B.; Buglyó G.; Németh N.; Szilágyi M.; Pös O.; Szemes T.; Balogh I.; Nagy B. The Role of Exosomes in Cancer Progression. International journal of molecular sciences 2022, 23, 8. 10.3390/ijms23010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. T.; Wang Y. K.; Tseng Y. J. Exosomal Proteins and Lipids as Potential Biomarkers for Lung Cancer Diagnosis, Prognosis, and Treatment. Cancers 2022, 14, 732. 10.3390/cancers14030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland T.; Sandvig K.; Llorente A. Lipids in exosomes: Current knowledge and the way forward. Progress in lipid research 2017, 66, 30–41. 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Huda M. N.; Nafiujjaman M.; Deaguero I. G.; Okonkwo J.; Hill M. L.; Kim T.; Nurunnabi M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater Sci. Eng. 2021, 7, 2106–2149. 10.1021/acsbiomaterials.1c00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A.; Wysoczynski M.; Kijowski J.; Marquez-Curtis L.; Machalinski B.; Ratajczak J.; Ratajczak M. Z. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer. 2005, 113, 752–760. 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- Hakulinen J.; Sankkila L.; Sugiyama N.; Lehti K.; Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell Biochem. 2008, 105, 1211–1218. 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- Han K. Y.; Dugas-Ford J.; Seiki M.; Chang J.H.; Azar D. T. Evidence for the Involvement of MMP14 in MMP2 Processing and Recruitment in Exosomes of Corneal Fibroblasts. Invest Ophthalmol Vis Sci. 2014, 56, 5323–5329. 10.1167/iovs.14-14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy J. W.; Sedgwick A.; Rosse C.; Muralidharan-Chari V.; Raposo G.; Method M.; Chavrier P.; D’Souza-Schorey C. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat. Commun. 2015, 6, 6919. 10.1038/ncomms7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runz S.; Keller S.; Rupp C.; Stoeck A.; Issa Y.; Koensgen D.; Mustea A.; Sehouli J.; Kristiansen G.; Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007, 107, 563–71. 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- Li C. J.; Liu Y.; Chen Y.; Yu D.; Williams K. J.; Liu M. L. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am. J. Pathol. 2013, 182, 1552–62. 10.1016/j.ajpath.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G.; D’Ascenzo S.; Borsotti P.; Giavazzi R.; Pavan A.; Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002, 160, 673–80. 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y.; Shan Y.; Chen J.; Yue H.; You B.; Shi S.; Li X.; Cao X. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015, 106, 1669–77. 10.1111/cas.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y.; You B.; Shi S.; Shi W.; Zhang Z.; Zhang Q.; Gu M.; Chen J.; Bao L.; Liu D.; You Y. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis. 2018, 9, 382. 10.1038/s41419-018-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. D.; Koo B. H.; Kim Y. H.; Jeon O. H.; Kim D. S. Exosome release of ADAM15 and the functional implications of human macrophage-derived ADAM15 exosomes. FASEB J. 2012, 26, 3084–95. 10.1096/fj.11-201681. [DOI] [PubMed] [Google Scholar]
- Karampoga A.; Tzaferi K.; Koutsakis C.; Kyriakopoulou K.; Karamanos N. K. Exosomes and the extracellular matrix: a dynamic interplay in cancer progression. Int. J. Dev Biol. 2022, 66, 97–102. 10.1387/ijdb.210120nk. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Xie L.; Lu C.; Gu C.; Xia Y.; Lv J.; Xuan Z.; Fang L.; Yang J.; Zhang L.; Li Z.; Wang W.; Xu H.; Li B.; Xu Z. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J. Exp Clin Cancer Res. 2022, 41, 296. 10.1186/s13046-022-02499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque S.; Dhar R.; Kar R.; Mukherjee S.; Mukherjee D.; Mukerjee N.; Nag S.; Tomar N.; Mallik S. Cancer stem cells (CSCs): key player of radiotherapy resistance and its clinical significance. Biomarkers. 2023, 28, 139–151. 10.1080/1354750X.2022.2157875. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Ji X.; Liu J.; Fan D.; Zhou Q.; Chen C.; Wang W.; Wang G.; Wang H.; Yuan W.; Ji Z.; Sun Z. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer. 2019, 18, 39. 10.1186/s12943-019-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. The concept of immune surveillance against tumors. first theories. Oncotarget. 2017, 8, 7175–7180. 10.18632/oncotarget.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A.; Bhattacharya B.; Mandal D.; Dhar R.; Muthu S. Salivary exosomes: A theranostics secret of oral cancer - Correspondence. Int. J. Surg. 2022, 108, 106990 10.1016/j.ijsu.2022.106990. [DOI] [PubMed] [Google Scholar]
- Kar R.; Dhar R.; Mukherjee S.; Nag S.; Gorai S.; Mukerjee N.; Mukherjee D.; Vatsa R.; Chandrakanth Jadhav M.; Ghosh A.; Devi A.; Krishnan A.; Thorat N. D. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater Sci. Eng. 2023, 9, 577–594. 10.1021/acsbiomaterials.2c01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger L.; Ezquer M.; Lillo-Vera F.; Pedraza P. L.; Ortúzar M. I.; González P. L.; Figueroa-Valdés A. I.; Cuenca J.; Ezquer F.; Khoury M.; Alcayaga-Miranda F. Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci. Rep. 2019, 9, 663. 10.1038/s41598-018-36855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J.; Abisoye-Ogunniyan A.; Metcalf K. J.; Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R. T.; Ko J. Future of Digital Assays to Resolve Clinical Heterogeneity of Single Extracellular Vesicles. ACS Nano 2022, 16, 11619–11645. 10.1021/acsnano.2c04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Gorai S.; Devi A.; Muthusamy R.; Alexiou A.; Papadakis M. Decoding of exosome heterogeneity for cancer theranostics. Clin Transl Med. 2023, 13, e1288 10.1002/ctm2.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Devi A.; Patil S.; Tovani-Palone M. R. Exosomes in cancer therapy: Advances and current challenges. Electron J. Gen Med. 2023, 20, em524 10.29333/ejgm/13456. [DOI] [Google Scholar]
- Dhar R.; Bhattacharya B.; Mandal D.; Devi A.; Thorat N. D. Exosome-based cancer vaccine: A cutting-edge approach - Correspondence. Int. J. Surg. 2022, 108, 106993 10.1016/j.ijsu.2022.106993. [DOI] [PubMed] [Google Scholar]
- Dhar R.; Gorai S.; Devi A.; Jha S. K.; Rahman M. A.; Alexiou A.; Papadakis M. Exosome: A megastar of future cancer personalized and precision medicine. Clin Transl Disc. 2023, 3, e208 10.1002/ctd2.208. [DOI] [Google Scholar]
- Mirgh D.; Krishnan A.; Gorai S. Sweat exosomes: A new horizon of liquid biopsy in cancer. Journal of Liquid Biopsy. 2023, 2, 100122 10.1016/j.jlb.2023.100122. [DOI] [Google Scholar]
- Dey D.; Ghosh S.; Mirgh D.; Panda S. P.; Jha N. K.; Jha S. K. Role of exosomes in prostate cancer and male fertility. Drug Discov Today. 2023, 28, 103791 10.1016/j.drudis.2023.103791. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; Nag S.; Mukerjee N.; Maitra S.; Muthusamy R.; Fuloria N. K.; Fuloria S.; Adhikari M. D.; Anand K.; Thorat N.; Subramaniyan V.; Gorai S. Unlocking Exosome-Based Theragnostic Signatures: Deciphering Secrets of Ovarian Cancer Metastasis. ACS Omega. 2023, 8, 36614–36627. 10.1021/acsomega.3c02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S.; Bhattacharya B.; Dutta S.; Mandal D.; Mukherjee S.; Anand K.; Eswaramoorthy R.; Thorat N.; Jha S. K.; Gorai S. Clinical Theranostics Trademark of Exosome in Glioblastoma Metastasis. ACS Biomater Sci. Eng. 2023, 9, 5205–5221. 10.1021/acsbiomaterials.3c00212. [DOI] [PubMed] [Google Scholar]
- Dey A.; Ghosh S.; Bhuniya T.; Koley M.; Bera A.; Guha S.; Chakraborty K.; Muthu S.; Gorai S.; Vorn R.; Vadivalagan C.; Anand K. Clinical Theragnostic Signature of Extracellular Vesicles in Traumatic Brain Injury (TBI). ACS Chem. Neurosci. 2023, 14, 2981–2994. 10.1021/acschemneuro.3c00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inubushi S.; Kawaguchi H.; Mizumoto S.; Kunihisa T.; Baba M.; Kitayama Y.; Takeuchi T.; Hoffman R. M.; Tanino H.; Sasaki R. Oncogenic miRNAs Identified in Tear Exosomes From Metastatic Breast Cancer Patients. Anticancer Res. 2020, 40, 3091–3096. 10.21873/anticanres.14290. [DOI] [PubMed] [Google Scholar]
- Tang R.; Zhou Y.; Mei S.; Xu Q.; Feng J.; Xing S.; Gao Y.; Qin S.; He Z. Fibrotic extracellular vesicles contribute to mechanical ventilation-induced pulmonary fibrosis development by activating lung fibroblasts via JNK signalling pathway: an experimental study. BMJ. Open Respir Res. 2023, 10, e001753 10.1136/bmjresp-2023-001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldo C.; Terri M.; Riccioni V.; Battistelli C.; Bordoni V.; D’Offizi G.; Prado M. G.; Trionfetti F.; Vescovo T.; Tartaglia E.; Strippoli R.; Agrati C.; Tripodi M. Fibrogenic signals persist in DAA-treated HCV patients after sustained virological response. J. Hepatol. 2021, 75, 1301–1311. 10.1016/j.jhep.2021.07.003. [DOI] [PubMed] [Google Scholar]
- Chandler C.; Liu T.; Buckanovich R.; Coffman L. G. The double edge sword of fibrosis in cancer. Transl Res. 2019, 209, 55–67. 10.1016/j.trsl.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H.; Liu H.; Sun T.; Wang H.; Zhang X.; Xu W. Exosome derived from stem cell: A promising therapeutics for wound healing. Front Pharmacol. 2022, 13, 957771 10.3389/fphar.2022.957771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbo S.; Maione R.; Tripodi M.; Battistelli C. Next RNA Therapeutics: The Mine of Non-Coding. Int. J. Mol. Sci. 2022, 23, 7471. 10.3390/ijms23137471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou M.; Huang L.; Yang J.; Chiang Z.; Chen S.; Liu J.; Guo L.; Zhang X.; Zhou X.; Xu X.; Yan X.; Wang Y.; Zhang J.; Xu A.; Tse H. F.; Lian Q. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool?. Cell Death Dis. 2022, 13, 580. 10.1038/s41419-022-05034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussen B. M.; Faraj G. S. H.; Rasul M. F.; Hidayat H. J.; Salihi A.; Baniahmad A.; Taheri M.; Ghafouri-Frad S. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 2022, 22, 323. 10.1186/s12935-022-02743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi B.; Dayeri B.; Zahedi E.; Khoshbakht S.; Nezamabadi; Pour N.; Ranjbar H.; Davari Nejad A.; Noureddini M.; Alani B. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022, 29, 1105–1116. 10.1038/s41417-022-00427-8. [DOI] [PubMed] [Google Scholar]
- Vallabhaneni K. C.; Penfornis P.; Dhule S.; Guillonneau F.; Adams K. V.; Mo Y. Y.; Xu R.; Liu Y.; Watabe K.; Vemuri M. C.; Pochampally R. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015, 6, 4953–4967. 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei R.; Baghaei K.; Hashemi S. M.; Zali M. R.; Ghanbarian H.; Amani D. Tumor-Derived Exosomes Enriched by miRNA-124 Promote Anti-tumor Immune Response in CT-26 Tumor-Bearing Mice. Front Med. (Lausanne). 2021, 8, 619939 10.3389/fmed.2021.619939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.; Luo J. D.; Jiang H.; Duan D. D. Tumor exosomes: a double-edged sword in cancer therapy. Acta Pharmacol Sin. 2018, 39, 534–541. 10.1038/aps.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dad H. A.; Gu T. W.; Zhu A. Q.; Huang L. Q.; Peng L. H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W.; Lei C.; Liu S.; Cui Y.; Wang C.; Qian K.; Li T.; Shen Y.; Fan X.; Lin F.; Ding M.; Pan M.; Ye X.; Yang Y.; Hu S. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. L.; Wang Y. J.; Sun J.; Zhou J.; Xing C.; Huang G.; Li J.; Yang H. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem. Sci. 2019, 10, 1555–1561. 10.1039/C8SC03224F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn N. L.; Wang L.; Chow E. K.; Lim C. T.; Goh B. C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Lai X.; Li Q.; Wu F.; Lin J.; Chen J.; Zheng H.; Guo L. Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons From Somatic Cell Reprogramming. Front Cell Dev Biol. 2020, 8, 760. 10.3389/fcell.2020.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amack J. D. Cellular dynamics of EMT: lessons from live in vivo imaging of embryonic development. Cell Commun. Signal. 2021, 19, 79. 10.1186/s12964-021-00761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M. K.; Endo K.; Itoh Y.; Osada A. H.; Kimura Y.; Ueki K.; Yoshizawa K.; Miyazawa K.; Saitoh M. Ets family proteins regulate the EMT transcription factors Snail and ZEB in cancer cells. FEBS Open Bio. 2022, 12, 1353–1364. 10.1002/2211-5463.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh C. Y.; Chai J. Y.; Tang T. F.; Wong W. F.; Sethi G.; Shanmugam M. K.; Chong P. P.; Looi C. Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells. 2019, 8, 1118. 10.3390/cells8101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drápela S.; Bouchal J.; Jolly M. K.; Culig Z.; Souček K. ZEB1: A Critical Regulator of Cell Plasticity, DNA Damage Response, and Therapy Resistance. Front Mol. Biosci. 2020, 7, 36. 10.3389/fmolb.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath P.; Huirem R. S.; Dutta P.; Palchaudhuri S. Epithelial-mesenchymal transition and its transcription factors. Biosci Rep. 2022, 42, 42. 10.1042/BSR20211754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.; Lin W.; Long Y.; Yang Y.; Zhang H.; Wu K.; Chu Q. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022, 7, 95. 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R.; Weinberg R. A. EMT and Cancer: More Than Meets the Eye. Dev Cell. 2019, 49, 313–316. 10.1016/j.devcel.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.; Li J.; Zhou X.; Zhao X.; Huang B.; Qin Y. Exosomes Regulate the Epithelial-Mesenchymal Transition in Cancer. Front Oncol. 2022, 12, 864980 10.3389/fonc.2022.864980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A. W.; Pattabiraman D. R.; Weinberg R. A. Emerging Biological Principles of Metastasis. Cell. 2017, 168, 670–691. 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Ni Y. Q.; Xu H.; Xiang Q. Y.; Zhao Y.; Zhan J. K.; He J. Y.; Li S.; Liu Y. S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct Target Ther. 2021, 6, 383. 10.1038/s41392-021-00779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares J.; Fares M. Y.; Khachfe H. H.; Salhab H. A.; Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020, 5, 28. 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]