Abstract
Excess abdominal fat reduces carcass yield and feed conversion ratio, thereby resulting in significant economic losses in the poultry industry. Our previous study demonstrated that dietary addition of folic acid reduced fat deposition and changed gut microbiota and short-chain fatty acid. However, whether folic acid regulating abdominal fat deposition was mediated by gut microbiota was unclear. A total of 210 one-day-old broiler chickens were divided into 3 groups including the control (CON), folic acid (FA), and fecal microbiota transplantation (FMT) groups. From 14th day, broiler chickens in CON and FA groups were given perfusion administration with 1 mL diluent daily, while 1 mL fecal microbiota transplantation suspension from FA group prepared before was perfusion in FMT group receiving control diets. The result showed that abdominal fat percentage was significantly lower in FA and FMT groups when compared with CON group (P < 0.05). Morphology analysis revealed that the villus height of jejunum and ileum were significantly higher in FMT group (P < 0.05), and the villus height of jejunum was also significantly higher in FA group (P < 0.05), while the diameter and cross-sectional area (CSA) of adipocytes were significantly decreased in FA and FMT groups when compared with CON group (P < 0.05). Western blot results indicated that the expression levels of FOXO1 and PLIN1 in FMT group were significantly increased (P < 0.05), whereas the expression levels of PPARγ, C/EBPα, and FABP4 were significantly decreased (P < 0.05). Additionally, the Chao1, Observed-species, Shannon and Simpson indexes in FA and FMT groups were significantly higher (P < 0.05), but the microbiota were similar between FMT and FA groups (P < 0.05). LEfSe analysis determined that Lactobacillus, Clostridium and Dehalobacterium were found to be predominant in FA group, while Oscillospira, Shigella, and Streptococcus were the dominant microflora in FMT group. Furthermore, these cecal microbiota were mostly involved in infectious disease, cellular community prokaryotes, cell motility and signal transduction in FA group (P < 0.05), whereas functional capacities involved in signal transduction, cell motility, infectious disease and environment adaptation were enriched significantly of cecal microbiota in FMT group (P < 0.05). In summary, both fecal microbiota transplantation from the broiler chickens of dietary added folic acid and dietary folic acid addition effectively reduced abdominal fat deposition, indicating that the regulatory effect of folic acid on abdominal fat deposition was mediated partly by gut microbiota in broiler chickens.
Key words: abdominal fat, broiler chicken, cecal microbiota, fecal microbiota transplantation, folic acid
INTRODUCTION
With the improvement of growth performance in broiler chickens, the accumulation of excess fat also boosts, subsequently increasing slaughter and processing costs. Consequently, addressing the issue of reducing abdominal fat has become a major challenge for the poultry industry. It was reported that probiotics could enhance host lipid metabolism by regulating and maintaining the dynamic balance of gut microbiota (Wang et al., 2017b; Balaguer et al., 2022). Therefore, it is particularly urgent to identify probiotics that are more effective in reducing lipid deposition under the condition of antibiotics prohibition.
Gut microbiota could directly or indirectly taken part in regulating lipid metabolism, and change individual energy balance and body weight (Wen et al., 2019; Xiang et al., 2021). Although the mechanism by which microbiota regulate lipid metabolism is unclear, it has been certified that gut microbiota played an indispensable role. Existing research indicated that gut flora and its metabolism (e.g., small organic acid, bile acid, vitamin, choline metabolite and lipid) were crucial mediators of dietary and genetic interaction, meanwhile they were closely related to energy metabolism (Heiss and Olofsson, 2018; Radziejewska et al., 2020) elaborated the relationship between single carbon unit metabolism and lipid metabolism from the aspects of nutrition, microbiology and genetics, and indicated that gut microbiota could regulate single carbon unit metabolism to affect folic acid and choline content of the host, which played a crucial part in lipid metabolism and transport. Furthermore, Mardinoglu certified that dietary intervention could affect folic acid production by altering the composition of gut microbiota, thereby reducing body fat content (Mardinoglu et al., 2018). Han found that folic acid could alleviate obesity resulted from high-fat diet by changing gut microbiota in mice (Han et al., 2023).
A large of evidence suggested that fecal microbiota transplantation (FMT) could regulate fat deposition by reshaping gut microbiota. For instances, the FMT alleviated HFD-induced steatohepatitis in mice by its beneficial effects on gut microbiota (Zhou et al., 2017). Conversely, when the fecal microbiota from obese human or mice was transplanted to germ-free (GF) or antibiotic-treated mice, increased body fat deposition was found in recipient animals (Ridaura et al., 2013; Ellekilde et al., 2014). In addition, the fecal microbiota transplantation from low abdominal fat deposition chicken reshaped the cecal microbiota of the recipients, ultimately reducing abdominal fat deposition (Chen et al., 2023). The above studies have collectively underscored the viability of addressing issues related to lipid metabolism in livestock and poultry production by using fecal microbiota transplantation to intervene or reconstruct gut flora. Our previous study revealed that dietary folic acid supplementation reduced abdominal fat deposition, and affected gut microbiota and SCFAs production (Liu et al., 2023b), while whether the regulation folic acid regulating of abdominal fat deposition was mediated by gut microbiota was unclear. In the current study, the fecal microbiota from folic acid group prepared before were used to perform FMT, aiming to elucidate the role of gut microbiota in folic acid regulating fat deposition in broiler chickens. It is expected that our findings could provide valuable guidance for the development of probiotics in poultry production.
MATERIALS AND METHODS
Animals and Experimental Design
A total of 210 one-day-old healthy Arbor Acres broiler chickens were used in this study and randomly divided into 3 groups with 7 replicates per group. From the 14 d of age, the broiler chickens in CON group and FA group were perfusion administration with 1 mL fecal microbiota working solution daily, while 1 mL fecal microbiota suspension was used as substitute in FMT group continued to 28 d of age. The dietary arrangements were as follows: birds in CON and FMT group were provided with basal diet (1.3 mg/kg FA); while 13 mg/kg FA was contained in the diet of FA group. The detailed formulation and composition of basal diet were provided in Supplemental Table S1, which were referred to our previous study (Liu et al., 2023b). All birds were obtained from the Xian Dacheng Poultry Industry Co., Ltd. (Xianyang, China). The experimental protocol for all broiler chickens in the study received approval from the Animal Ethics Committee of the Northwest A&F University.
Preparation of Fecal Microbiota Transplantation Suspension
Cecal chymus form broiler chickens in FA group in our previous study (Liu et al., 2023b) were collected and suspended in saline solution. Following agitation and subsequent centrifugation at 800 rpm for 5 min, the fecal microbiota suspension was obtained and stored at −80℃ for future use.
Growth Performance and Sample Collection
At 28 d of age, feed intake and body weight were recorded for each replicate, then the average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were calculated. Simultaneously, 1 bird per replicate close to the average BW of the replicate was selected and humanely euthanized by cervical dislocation, then abdominal fat was removed and weighed to calculate abdominal fat percentage relative to body weight (g of organ/g of body weight). Additionally, fresh cecal chyme was collected for subsequent gut microbiota analysis and obtained samples from the duodenum, jejunum and ileum for intestinal morphology analysis. Following the sampling process, abdominal fat and cecal chyme were frozen in liquid nitrogen and stored at -80°C for further analysis.
Intestinal Morphology Analysis
Duodenum, jejunum and ileum carefully repeated rinsing with normal saline, approximately 2 cm intestine tissue pieces of the middle part were fixed 24 h with 4% formaldehyde for hematoxylin-eosin (H&E) histological analysis, which was performed by Wuhan Service bio Technology Co., Ltd. (Wuhan, China). Then intestine tissue sections were examined with light microscope (BH-2, Olympus, Japan), while the villus height (VH) and crypt depth (CD) were recorded, and the rate of villus height to crypt depth (VH/CD) was calculated.
Abdominal Fat Morphology Analysis
About 1 cm3 abdominal fat tissue pieces were fixed 48 h with 4% formaldehyde for H&E histological analysis by Wuhan Service bio Technology Co., Ltd. (Wuhan, China). Abdominal adipose tissue sections were examined and photographed with light microscope (BH-2, Olympus, Japan). Under 10×20 microscope, every H&E stained sections of abdominal fat were randomly select 3 visual fields for the image acquisition. Then the diameter and area of adipocytes were determined using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring) based on the previously reported method (Chen and Farese, 2002).
Western Blot Analysis
Approximately 30 ug abdominal fat tissue was lysed and homogenized in RIPA lysis buffer with protease and phosphatase inhibitor (PL001, Shanxi Zhonghui Hecai Biomedical Technology Co., Ltd.) on ice for 15 min, followed by centrifugation (12,000 × g, 4℃, 5 min) to collect supernatant. BCA kit (AP0011M, Shaanxi AccuRef Scientific Biomedical Technology Co., Ltd.) was employed to detect the total protein concentration. Proteins were size fractionated by CFAS any KD PAGE (PE008, Shaanxi Zhonghui Hecai Biomedical Technology Co., Ltd.) gels and transferred into a PVDF membrane (IPVH00011, Solarbio Life Science Co. Ltd). Then the membranes were blocked in 5% BSA and incubated with GAPDH (AB0037, Abways), FOXO1 (CY7234, Abways), PPARγ (WL01800, Wanleibio), C/EBPα (CY5723, Abways), PLIN1 (PTM7147, PTMBIO), and FABP4 (PTM6416, PTMBIO) overnight at 4℃, which were diluted at a ratio of 1:1,000. Afterward, the secondary antibody incubation was carried out for 90 min at room temperature following 1:2,000 dilutions. Finally, the blotted bands were imaged in the gel imaging system and visualized based on gray values with Image-Pro Plus (Media Cybernetics, Silver Spring).
DNA Extraction and 16s rRNA Analysis
The entirety of genomic DNA was obtained from cecal contents using the OMEGA Soil DNA Kit (Omega Bio-Tek, Norcross, GA). Subsequently, the V3V4 variable regions of bacterial were amplified by PCR with the following primers, F: ACTCCTACGGGAGGCAGCA, R: GGACTACHVGGGTWTCTAAT. The PCR amplification program consisted of an initial denaturation phase at 94℃ to 96℃ for 1 min, succeeded by annealing at 50℃ to 60℃ for 30 s, and extension at 72℃ for 1 min, which was repeated for 35 cycles. The PCR amplified products were subsequently purified using the AxyPrep DNA gel extraction kit (Axygen, Arizona). Finally, 16S rRNA sequencing was performed on the Illumina platform. All the aforementioned laboratory procedures and data analyses were performed by Shanghai Personal Biotechnology Co., Ltd.
The quality of reads and the effect of merging were quality-controlled filtered by PE reads splicing, tags filtering and removal of chimeras. Then, the Alpha diversity analysis (Rarefaction curve, Alpha diversity index) was performed with QIIME2 software (http://qiime.org/index.html) to compare the similarity of species diversity and richness in different groups. In addition, LEfSe analysis was used to determine the microbiota differences among different groups according to the following parameters: linear discriminant analysis (LDA) score >2 and P < 0.05. Finally, the Kyoto Encyclopedia of Genes and Genomes (KEGG) database of the sequence were performed, then microbiota functional differences were visualized by STAMP software. The concrete method was referred to previous study (Zhang et al., 2022; Liu et al., 2023a).
Statistical Analysis
The experimental data were analyzed using the SPSS 20.0 and plotted using GraphPad Prism 8. One-way ANOVA analysis was performed to compare the data results between groups and Duncan's multiple comparisons test was used to determine significance. Difference was considered significant at P value <0.05.
RESULTS
Growth Performance
As shown in Table 1, no differences were found about BW, ADFI, ADG and FCR of broiler chickens in CON, FA and FMT groups (P > 0.05). However, the abdominal fat percentage was significantly lower in FA and FMT groups when compared with CON group (P < 0.05).
Table 1.
Effects of folic acid on growth performance in broiler chickens based on fecal microbiota transplantation.
| Days | Items | CON | FA | FMT | SEM | P value |
|---|---|---|---|---|---|---|
| 1–14 d | BW14 (g) | 384.24 | 380.76 | 380.62 | 7.72 | 0.979 |
| ADFI (g) | 28.42 | 28.51 | 28.16 | 0.91 | 0.988 | |
| ADG (g) | 24.29 | 24.06 | 24.07 | 0.54 | 0.984 | |
| FCR | 1.16 | 1.18 | 1.18 | 0.03 | 0.967 | |
| 14–28 d | BW28 (g) | 1167.86 | 1179.71 | 1201.57 | 24.05 | 0.858 |
| ADFI (g) | 82.68 | 83.73 | 81.53 | 1.61 | 0.868 | |
| ADG (g) | 55.98 | 57.07 | 58.64 | 1.53 | 0.794 | |
| FCR | 1.50 | 1.47 | 1.40 | 0.04 | 0.542 | |
| 1–28 d | ADFI (g) | 55.55 | 56.12 | 54.84 | 1.05 | 0.894 |
| ADG (g) | 40.13 | 4.56 | 41.36 | 0.85 | 0.853 | |
| FCR | 1.39 | 1.38 | 1.33 | 0.02 | 0.444 | |
| Abdominal fat (%) | 0.98a | 0.76b | 0.85b | 0.03 | 0.001 |
BW = body weight; ADFI = average daily feed intake; ADG = average daily gain; FCR = feed conversion ratio. Different letter indicate significant correlation (P < 0.05) and the same letter or no letter indicates no significance within a row (P > 0.05). The same below.
Intestinal Morphology
As shown in Table 2, the morphology of duodenum in FA and FMT groups remained unaffected when compared with CON group (P > 0.05). Nevertheless, the villus height of jejunum was significantly higher in FMT group compared to the CON and FA groups (P < 0.05). Furthermore, the villus height of ileum was significantly higher in FA group compared to the CON group, while only a discernibly increasing trend in FMT group (P < 0.05).
Table 2.
Effects of folic acid on intestinal morphology in broiler chickens based on fecal microbiota transplantation.
| Intestinal segment | Items | CON | FA | FMT | SEM | P value |
|---|---|---|---|---|---|---|
| Duodenum | Villus height/um | 1502.89 | 1640.91 | 1882.21 | 78.67 | 0.128 |
| Crypt depth/um | 211.90 | 230.68 | 305.29 | 19.23 | 0.091 | |
| VH/CD | 7.21 | 7.14 | 6.26 | 0.32 | 0.448 | |
| Jejunum | Villus height/um | 898.73b | 865.66b | 1225.07a | 70.01 | 0.036 |
| Crypt depth/um | 232.62 | 219.74 | 257.20 | 8.55 | 0.202 | |
| VH/CD | 3.87 | 3.90 | 4.84 | 0.26 | 0.233 | |
| Ileum | Villus height/um | 576.75b | 886.73a | 724.29ab | 54.83 | 0.037 |
| Crypt depth/um | 164.35 | 182.65 | 150.36 | 12.32 | 0.627 | |
| VH/CD | 3.49 | 5.14 | 4.98 | 0.41 | 0.213 |
Abdominal Fat Morphology Analysis
The anatomical results indicated that there was less abdominal fat deposition in FA and FMT groups when compared with CON group (Figure 1A). Correspondingly, H&E staining demonstrated that the adipocytes looked smaller (Figure 1B), and the diameter and cross-sectional area (CSA) of adipocytes were significantly decreased in FA and FMT groups when compared with CON group (P < 0.05; Figure 1C and D).
Figure 1.
The phenotypic observation and morphological analysis of abdominal fat. (A) Abdominal fat anatomy observation and H&E staining for abdominal fat tissue (magnification: 10 × 40; scale bar 50 um); (B) adipocyte diameter statistics; (C) adipocyte CSA statistics.
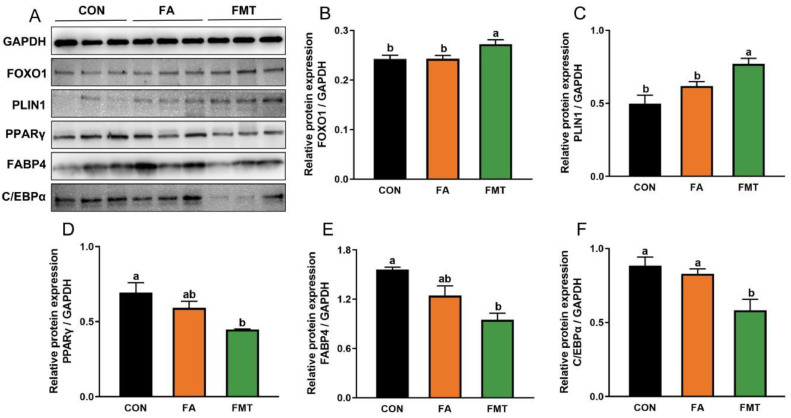
Expression of Adipogenesis-Related Protein
Protein expression related to adipocyte proliferation and differentiation was showed in Figure 2. The protein expression of FOXO1 and PLIN1 were elevated in FMT group when compared with Con and FA groups. However, PPARγ, C/EBPα and FABP4 were significantly reduced in FMT group when compared with CON group (P < 0.05). Similarly, there existed reduction trend for PPARγ and FABP4 protein expression in FA group.
Figure 2.
Expression of adipogenesis-related protein in abdominal fat tissue. (A) Western blotting of adipocyte proliferation and differentiation; (B) Protein gray values were determined with Image-Pro Plus software and normalized to GAPDH (n = 3 for per group).
Alpha Diversity Analysis and Species Composition of Cecum Microbiota
The rarefaction curves of Chao1, Shannon, Observed-species and Simpson index were higher in FA and FMT groups than CON group under the same sequencing depth (Figure 3A–D). Correspondingly, the alpha diversity indices including Chao1, Observed-species, Simpson and Shannon index also indicated consistent changes (Figure 3E–H). Bacterial abundance and diversity showed significant similarity between FA and FMT groups. UPGMA clustering analysis was displayed to illustrate the alterations of bacterial abundance between groups in the heat map (Figure 3I). The abundance of Helicobacter, Shigella, Enterococcus, Sutterella, Oscillospira, Dorea, Clostridium, Ruminococcus, and Coprobacillus significantly increased in FMT group. A comparable trend was observed such as the abundance of Lactobacillus, Akkermansia, and Blautia in FA group.
Figure 3.
Alpha diversity analysis and species composition of cecum microbiota. Rarefaction curve of Chao (A), Shannon (B), Observed-species (C) and Simpson (D) index; Chao1 (E), Shannon (F), Observed-species (G) and Simpson (H) index. The asterisk (*) indicated statistically significant differences (2-tailed unpaired test, *P < 0.05, **P < 0.01); Cluster analysis heat map of the top 20 genus (I), and different colors represented the size of the correlation coefficient.
Differential Bacteria and Functional Prediction Analysis
LEfSe analysis was used to identify dominant microbiota of each group. Lactobacillus, Clostridium, and Dehalobacterium were found to be predominant in FA group (Figure 4A), whereas Oscillospira, Shigella, and Streptococcus were dominant microflora in the FMT group (Figure 4B). Additionally, the functional prediction was performed using PICRUSt method. Infectious disease, cellular community prokaryotes, cell motility and signal transduction were enriched in FA group (P < 0.05; Figure 4C), while functional capacities involved in signal transduction, cell motility, infectious disease and environment adaptation were found in FMT group (P < 0.05; Figure 4D).
Figure 4.
Differential bacteria and functional prediction analysis of cecum microbiota. LEfSe analysis the microbiota abundances difference at genus level between 2 groups (A and B). The default parameters were LDA Score >2 and P < 0.05. Comparisons of functional pathways associated with changes in the gut microbiota (C and D).
DISCUSSION
Excessive abdominal fat deposition not only diminished carcass yield and feed utilization efficiency, but also affected the metabolic health of broiler chickens. A large of evidence suggested that FMT could regulate fat deposition by reshaping gut microbiota. Chen observed that FMT from the low abdominal fat deposition chicken significantly reduced abdominal fat weight and index (Chen et al., 2023). Consistent with the results of previous studies, our results in the current study showed that FMT from lower abdominal fat mediated by folic acid also reduced abdominal fat percentage in broiler chickens. Notably, both fecal bacterial solution perfusion and dietary folic acid addition exhibited similar effects on broiler growth and development, while the abdominal fat deposition could be effectively reduced through fecal microbiota transplantation. Furthermore, the small intestine was an important digestive and immune organ of animals, which had the function of digesting and absorbing nutrients and resisting the invasion from external pathogens. The villus height and crypt depth were vital indicators for evaluating the gut health of animals (Farahat et al., 2021). In our study, the villus height of jejunum in FMT group and a corresponding increase in ileum villus height of in FA group. This phenomenon could be attributed to the microbiota and their metabolites adhered to the intestinal mucosa and formed a protective layer, which prevented some irritating substances form damaging the intestinal mucosa and provided a safer growth environment for intestinal villi and intestinal epithelial cells. On the other hand, it also implied that FMT did not cause intestinal damage.
The excessive deposition of fat tissue was mainly attributed to adipocyte hypertrophy rather than hyperplasia (Longo et al., 2019; Ma et al., 2020). Therefore, the fat mass reduction was primarily caused by decrease in adipocyte size rather than adipocyte number. In this study, we observed that the perfusion of fecal microbiota transplantation suspension could effectively reduce the diameter of adipocyte. Gut had the potential to impact the host fat metabolism by regulating the expression of genes related to lipid synthesis and decomposition.
Gut microbes play a pivotal role in influencing the host fat metabolism by regulating the expression of genes related to fat metabolism (Zhao et al., 2018). The essential transcription factors related to lipid metabolism and deposition included FOXO1, PPARγ, C/EBPα and FABP4 (Fu et al., 2014). FOXO1 serves as a pivotal transcription factor responsible for regulating a range of cellular functions, with a predominant role in fat metabolism and cell cycle regulation (Chen et al., 2019). The activation of FOXO1 could suppress PPARγ activity, consequently leading to the inhibition of adipocyte differentiation. PPARγ predominantly expressed in adipocyte, which combined with C/EBPα to participate in adipocyte development and stimulating the expression of genes related to lipid metabolism and adipocyte differentiation in broiler chickens (Wang et al., 2017a; Nematbakhsh et al., 2021). C/EBPα was expressed in liver and fat tissue, which played a crucial role in the transcriptional activation of adipocyte differentiation by stimulating lipid-specific genes required for synthesis, uptake and storage of long-chain fatty acids (Nematbakhsh et al., 2021). FABP4 induced hypertrophy by mediating the sequestration of fatty acids for triglyceride synthesis. Additionally, it regulated PPARγ expression and participated in fatty acid transport and metabolism (Wang et al., 2017a). In this study, it was observed that the intervention with fecal microbiota suspension significantly inhibited the expression of PPARγ, FABP4 and C/EBPα in abdominal fat tissue, suggesting the accumulation of lipid droplets was restrained in FMT group. PLIN1 was a lipid droplet associated protein in adipocytes, and has a dual regulatory effect on lipid decomposition (Granneman et al., 2009). It was found that PLIN1 expression was upregulated in abdominal fat by fecal microbiota suspension perfusion, which might be related to reduced basal lipolysis by preventing lipase from approaching lipid droplets. These results declared that fecal microbiota suspension perfusion suppressed adipocytes differentiation, aligning with the finding from our previous study on dietary folic acid addition (Liu et al., 2023b).
Approximately one-tenth of the host transcriptome was under the regulation of microorganisms (Sommer et al., 2015). Therefore, any changes in microbial composition could lead to alterations in the host phenotype. Microbiota was the most significant component of fecal bacteria suspension, and long-term fecal microbiota transplantation had the potential to alter the phenotype of abdominal fat. In this study, the alpha diversity indices were higher in FMT group than CON group, indicating that the fecal microbiota suspension perfusion changed the community structure of gut flora and improved gut microbiota diversity. More interestingly, notable similarities were observed in both gut microbiota diversity and richness between FA group and FMT group which was given fecal microbiota suspension from FA group.
Cornejo-Pareja observed that fat deposition was associated with alterations in microbial community composition (Cornejo-Pareja et al., 2019). To pinpoint pivotal microbial community members potentially linked to fat deposition, we used LEfSe analysis to evaluate the differences in bacterial genera between FA and FMT groups in comparison to CON group. The LEfSe analysis found that Oscillospira, Shigella, and Streptococcus as the primary microbes in broiler chickens of FMT group. Notably, Oscillospira exhibited lower abundance in gut of obesity and the early study involving monozygotic twins revealed a strong inverse correlation between Oscillospira and obesity (Tims et al., 2013). A substantial of evidence suggested that Oscillospira played a significant role in metabolic activities related to obesity (Gophna et al., 2017), while Oscillospira was capable of producing various short-chain fatty acids (SCFAs) dominated by butyric acid (Gophna et al., 2017; Jiao et al., 2018) and was significantly associated with adipose and inflammatory diseases (Konikoff and Gophna, 2016; Gophna et al., 2017; Yang et al., 2020). In addition, it is crucial that gut bacteria could produce large amounts of folic acid and other B vitamins for body utilize (Morowitz et al., 2011). Recently, genomic studies have indicated that bacterial genera Streptococcus and Lactobacillus common in the distal intestine were capable of synthesizing folic acid (Engevik et al., 2019). Ulteriorly, numerous studies have underscored the potential advantages of folic acid in lipid metabolism and obesity prevention. Previous study reported that low-carbohydrate diet could decrease fat by improving intestinal Streptococcus abundance and serum folic acid levels (Mardinoglu et al., 2018). In the current study, Streptococcus and Lactobacillus were the dominants microbe in FMT and FA groups, thus we speculated that reduced abdominal fat deposition might be related to folic acid synthesis in the intestine of broiler chickens. As a type of amylolytic bacteria, Oscillospira not only broken-down complex carbohydrates but also reduced the synthesis of proinflammatory interleukins, participated in motor regulation and even had detoxification functions (Kashtanova et al., 2018). Consistent with previous studies, function prediction of gut microbiota revealed that more complex functions such as cell motility and infectious disease in FMT group. Accordingly, we also found similar microbiota functions in FA group, this might because although the specific bacterial genera were different, they all belong to Firmicutes and performed similar functions in the host. In the present study, we confirmed the phenotype that dietary folic acid addition reduced abdominal fat deposition mediated by gut microbiota based on fecal microbiota transplantation. In addition, Oscillospira and Streptococcus have been identified as a candidate for regulating abdominal fat deposition. Forthcoming research needs to be carried out to verify whether these candidate bacteria could develop functions on reducing abdominal fat in broiler chickens.
CONCLUSIONS
In conclusion, this study elucidated that the intervention with dietary supplementation of folic acid and fecal microbiota transplantation from the folic acid group effectively reduced fat deposition (as shown in Figure 5), indicating that the regulatory influence of folic acid on abdominal fat deposition was indeed mediated by the gut microbiota alteration in broiler chickens. Furthermore, the Streptococcus and Oscillospira were found to be potential probiotic candidates for reducing fat deposition in poultry reduction fat.
Figure 5.
Schematic diagram for dietary folic acid addition reducing abdominal fat deposition in broilers based on FMT methods. The green and red arrow indicates decreasing and increasing respectively. The proliferation and differentiation of adipocyte was inhibited by detecting genes expression.
ACKNOWLEDGMENTS
We sincerely thank members of the Innovative Research Team of Animal Nutrition & Healthy Feeding (Northwest A&F University, Shaanxi, China) for their help in sample harvesting. This work was funded by the National Science Foundation of China (32372910 and 32102567), National Key Research & Development Program of China (2023YFF1001900 and 2023YFD1301400), and the Program for Shaanxi Science & Technology (2022KJXX-13, 2023-YBNY-144, and 2022GD-TSLD-46-0302).
Author Contributions
Xiaoying Liu and Yanli Liu conceived and planned the research; Chaohui Wang, Yun Li, Yumeng Wang, Xi Sun, Qianggang Wang, Jiarui Luo, and Wen Lv carried out the investigation and methodology; Xiaoying Liu drafted the manuscript; Xiaojun Yang and Yanli Liu contributed to manuscript revision and funding acquisition.
DISCLOSURES
The authors declare that they have no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103392.
Appendix. Supplementary materials
REFERENCES
- Balaguer F., Enrique M., Llopis S., Barrena M., Navarro V., Álvarez B., Chenoll E., Ramón D., Tortajada M., Martorell P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: a novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2022;15:805–816. doi: 10.1111/1751-7915.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Akhtar M., Ma Z., Hu T., Liu Q., Pan H., Zhang X., Nafady A.A., Ansari A.R., Abdel-Kafy E.-S.M., Shi D., Liu H. Chicken cecal microbiota reduces abdominal fat deposition by regulating fat metabolism. NPJ Biofilms Microbiom. 2023;9:28. doi: 10.1038/s41522-023-00390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.C., Farese R.V. Determination of adipocyte size by computer image analysis. J. Lipid Res. 2002;43:986–989. [PubMed] [Google Scholar]
- Chen J., Lu Y., Tian M., Huang Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J. Mol. Endocrinol. 2019;62:239–253. doi: 10.1530/JME-18-0178. [DOI] [PubMed] [Google Scholar]
- Cornejo-Pareja I., Muñoz-Garach A., Clemente-Postigo M., Tinahones F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019;72:26–37. doi: 10.1038/s41430-018-0306-8. [DOI] [PubMed] [Google Scholar]
- Ellekilde M., Selfjord E., Larsen C.S., Jakesevic M., Rune I., Tranberg B., Vogensen F.K., Nielsen D.S., Bahl M.I., Licht T.R., Hansen A.K., Hansen C.H.F. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci. Rep. 2014;4:5922. doi: 10.1038/srep05922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik M.A., Morra C.N., Röth D., Engevik K., Spinler J.K., Devaraj S., Crawford S.E., Estes M.K., Kalkum M., Versalovic J. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Front. Microbiol. 2019;10:2305–2322. doi: 10.3389/fmicb.2019.02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat M., Ibrahim D., Kishawy A.T.Y., Abdallah H.M., Hernandez-Santana A., Attia G. Effect of cereal type and plant extract addition on the growth performance, intestinal morphology, caecal microflora, and gut barriers gene expression of broiler chickens. Animal. 2021;15:100056–100062. doi: 10.1016/j.animal.2020.100056. [DOI] [PubMed] [Google Scholar]
- Fu R.Q., Liu R.R., Zhao G.P., Zheng M.Q., Chen J.L., Wen J. Expression profiles of key transcription factors involved in lipid metabolism in Beijing-You chickens. Gene. 2014;537:120–125. doi: 10.1016/j.gene.2013.07.109. [DOI] [PubMed] [Google Scholar]
- Gophna U., Konikoff T., Nielsen H.B. Oscillospira and related bacteria – from metagenomic species to metabolic features. Environ. Microbiol. 2017;19:835–841. doi: 10.1111/1462-2920.13658. [DOI] [PubMed] [Google Scholar]
- Granneman J.G., Moore H.-P.H., Krishnamoorthy R., Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J. Biol. Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W., Li M., Yang M., Chen S., Lu Y., Tang T., Wang R., Zhang C., Qi K. Dietary folic acid supplementation inhibits highfat dietinduced body weight gain through gut microbiota-associated branched-chain amino acids and mitochondria in mice. J. Nutr. Sci. Vitaminol. 2023;69:105–120. doi: 10.3177/jnsv.69.105. [DOI] [PubMed] [Google Scholar]
- Heiss C.N., Olofsson L.E. Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao N., Baker S.S., Nugent C.A., Tsompana M., Cai L., Wang Y., Buck M.J., Genco R.J., Baker R.D., Zhu R., Zhu L. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol. Genom. 2018;50:244–254. doi: 10.1152/physiolgenomics.00114.2017. [DOI] [PubMed] [Google Scholar]
- Kashtanova D.A., Tkacheva O.N., Doudinskaya E.N., Strazhesko I.D., Kotovskaya Y.V., Popenko A.S., Tyakht A.V., Alexeev D.G. Gut microbiota in patients with different metabolic statuses: moscow study. Microorganisms. 2018;6:98–107. doi: 10.3390/microorganisms6040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konikoff T., Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016;24:523–524. doi: 10.1016/j.tim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang C., Wang Y., Wang C., Sun X., Zhu Y., Yang X., Zhang L., Liu Y. Age-associated changes in the growth development of abdominal fat and their correlations with cecal gut microbiota in broiler chickens. Poult. Sci. 2023;102:102900–102909. doi: 10.1016/j.psj.2023.102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang J., Liu X., Liu R., Wang Y., Huang X., Li Y., Liu R., Yang X. Dietary folic acid addition reduces abdominal fat deposition mediated by alterations in gut microbiota and SCFA production in broilers. Anim. Nutr. 2023;12:54–62. doi: 10.1016/j.aninu.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M., Zatterale F., Naderi J., Parrillo L., Formisano P., Raciti G.A., Beguinot F., Miele C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019;20:2358–2369. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Sun J., Zhu S., Du Z., Li D., Li W., Li Z., Tian Y., Kang X., Sun G. MiRNAs and mRNAs analysis during abdominal preadipocyte differentiation in chickens. Animals. 2020;10:468–478. doi: 10.3390/ani10030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinoglu A., Wu H., Bjornson E., Zhang C., Hakkarainen A., Räsänen S.M., Lee S., Mancina R.M., Bergentall M., Pietiläinen K.H., Söderlund S., Matikainen N., Ståhlman M., Bergh P.-O., Adiels M., Piening B.D., Granér M., Lundbom N., Williams K.J., Romeo S., Nielsen J., Snyder M., Uhlén M., Bergström G., Perkins R., Marschall H.-U., Bäckhed F., Taskinen M.-R., Borén J. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metab. 2018;27:559–571.e5. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz M.J., Carlisle E.M., Alverdy J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. North Am. 2011;91:771–785. doi: 10.1016/j.suc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematbakhsh S., Pei Pei C., Selamat J., Nordin N., Idris L.H., Abdull Razis A.F. Molecular regulation of lipogenesis, adipogenesis and fat deposition in chicken. Genes. 2021;12:414–443. doi: 10.3390/genes12030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziejewska A., Muzsik A., Milagro F.I., Martínez J.A., Chmurzynska A. One-carbon metabolism and nonalcoholic fatty liver disease: the crosstalk between nutrients, microbiota, and genetics. Lifestyle Genom. 2020;13:53–63. doi: 10.1159/000504602. [DOI] [PubMed] [Google Scholar]
- Ridaura V.K., Faith J.J., Rey F.E., Cheng J., Duncan A.E., Kau A.L., Griffin N.W., Lombard V., Henrissat B., Bain J.R., Muehlbauer M.J., Ilkayeva O., Semenkovich C.F., Funai K., Hayashi D.K., Lyle B.J., Martini M.C., Ursell L.K., Clemente J.C., Van Treuren W., Walters W.A., Knight R., Newgard C.B., Heath A.C., Gordon J.I. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214–1241226. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F., Nookaew I., Sommer N., Fogelstrand P., Bäckhed F. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 2015;16:62–77. doi: 10.1186/s13059-015-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tims S., Derom C., Jonkers D.M., Vlietinck R., Saris W.H., Kleerebezem M., de Vos W.M., Zoetendal E.G. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707–717. doi: 10.1038/ismej.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Kim W.K., Cline M.A., Gilbert E.R. Factors affecting adipose tissue development in chickens: a review. Poult. Sci. 2017;96:3687–3699. doi: 10.3382/ps/pex184. [DOI] [PubMed] [Google Scholar]
- Wang H., Ni X., Liu L., Zeng D., Lai J., Qing X., Li G., Pan K., Jing B. Controlling of growth performance, lipid deposits and fatty acid composition of chicken meat through a probiotic, Lactobacillus johnsonii during subclinical Clostridium perfringens infection. Lipids Health Dis. 2017;16:38–48. doi: 10.1186/s12944-017-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Yan W., Sun C., Ji C., Zhou Q., Zhang D., Zheng J., Yang N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019;13:1422–1436. doi: 10.1038/s41396-019-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H., Gan J., Zeng D., Li J., Yu H., Zhao H., Yang Y., Tan S., Li G., Luo C., Xie Z., Zhao G., Li H. Specific microbial taxa and functional capacity contribute to chicken abdominal fat deposition. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.643025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.J., Pham M.T., Rahim A.R., Chuang T.-H., Hsieh M.-F., Huang C.-M. Mouse abdominal fat depots reduced by butyric acid-producing leuconostoc mesenteroides. Microorganisms. 2020;8:1180–1192. doi: 10.3390/microorganisms8081180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Akhtar M., Chen Y., Ma Z., Liang Y., Shi D., Cheng R., Cui L., Hu Y., Nafady A.A., Ansari A.R., Abdel-Kafy E.-S.M., Liu H. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome. 2022;10:107–126. doi: 10.1186/s40168-022-01299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X., Fu H., Xue X., Lu C., Ma J., Yu L., Xu C., Ren Z., Xu Y., Xu S., Shen H., Zhu X., Shi Y., Shen Q., Dong W., Liu R., Ling Y., Zeng Y., Wang X., Zhang Q., Wang J., Wang L., Wu Y., Zeng B., Wei H., Zhang M., Peng Y., Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- Zhou D., Pan Q., Shen F., Cao H.-X., Ding W.-J., Chen Y.-W., Fan J.-G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.