Abstract
Background
High ultra-processed food (UPF) consumption is associated with the development of various diet-related non-communicable diseases, especially obesity and type 2 diabetes. The present study aimed to systematically review the association between UPF consumption and non-alcoholic fatty liver disease (NAFLD) and its leading risk factors; metabolic syndrome (MetS) and insulin resistance (IR).
Methods
A comprehensive search was conducted in PubMed, Scopus, Embase, Web of Science, CINAHL, and Cochrane (March 2023), and references of the identified articles were checked. The search keywords were defined through an exploratory investigation in addition to MeSH and similarly controlled vocabulary thesauruses. Observational and interventional studies were included. Studies that focused only on specific groups of processed foods or overlapping dietary patterns were excluded. The quality assessment was conducted using the Joanna Briggs Institute’s critical appraisal tools for observational studies and Cochrane’s risk of bias 2 tool for randomized-control trials. A narrative synthesis was employed to report the results.
Results
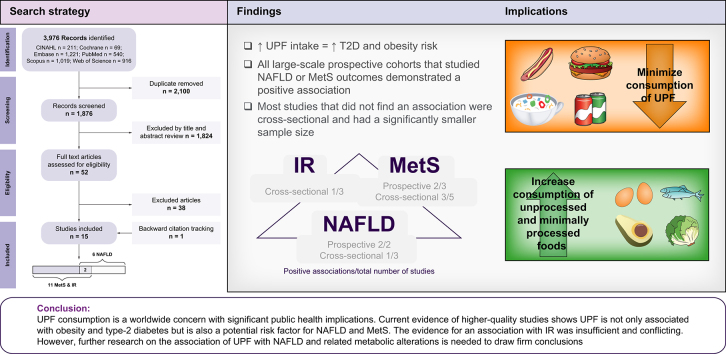
Fifteen studies were included, with a total of 52,885 participants, one randomized-controlled trial, and fourteen observational studies (nine cross-sectional and five prospective). The review has shown a significant association between UPF consumption and NAFLD in three studies out of six, MetS in five out of eight, and IR in one out of three. All large-scale prospective cohorts that studied NAFLD or MetS outcomes demonstrated a positive association. In contrast, studies that did not demonstrate significant associations were mostly cross-sectional and small. The evidence for an association with IR was insufficient and conflicting.
Conclusion
The included studies are few, observational, and based upon self-reported dietary assessment tools. However, current evidence indicates that UPF is not only associated with obesity and type 2 diabetes but may also be a risk factor for NAFLD and MetS. UPF is a worldwide concern deserving further longitudinal research.
Impact and implications
Overconsumption of ultra-processed food (UPF) may lead to the development of obesity and type 2 diabetes, but the association with non-alcoholic fatty liver disease (NAFLD) is not well established. The present systematic review shows that UPF may be associated with NAFLD, although more large prospective studies are needed. These findings emphasize the importance of minimizing the consumption of UPF to prevent NAFLD and other metabolic diseases among the general adult population. This systematic review and further prospective studies, epidemiological or interventional, can help physicians provide patients with evidence-based nutritional recommendations and will support policymakers in restricting the marketing of UPF as well as promoting affordable, healthy, and minimally processed foods.
Keywords: ultra-processed food, fatty liver, metabolic syndrome, insulin resistance, systematic review
Graphical abstract
Highlights
-
•
UPF consumption is associated with obesity and type 2 diabetes.
-
•
We systematically reviewed the association of UPF with NAFLD, MetS and IR.
-
•
All large-scale prospective cohorts found an association of UPF with NAFLD and MetS.
-
•
These associations were independent of BMI and energy intake.
-
•
Limiting consumption of UPF may be advised for NAFLD prevention.
Introduction
Non-alcoholic fatty liver disease (NAFLD), recently renamed metabolic dysfunction-associated steatotic liver disease (MASLD),1 is the most common chronic liver disease worldwide, and according to a recent meta-analysis, it has an estimated overall global prevalence of 32.4% (95% Cl 29.9-34.9%) among the adult population.2 The NAFLD spectrum includes hepatic steatosis and progression to non-alcoholic steatohepatitis (NASH), fibrosis, and cirrhosis.3 Moreover, it is a multi-system disease that interacts with many metabolic pathways and is closely associated with metabolic syndrome (MetS),4,5 insulin resistance (IR), type 2 diabetes (T2D), and obesity.[6], [7], [8] The global prevalence of NAFLD, according to two meta-analyses, was estimated at 75.3% (95% CI 70.9-79.2%) among the obese population9 and 55.5% (95% CI 47.3-63.7%) among patients with T2D.10 Moreover, while no meta-analysis was conducted, a recent systematic review found that NAFLD prevalence was significantly higher among people with MetS and increased with the number of MetS criteria.11 Other well-established key lifestyle factors associated with NAFLD include lack of physical activity, high sedentary behavior,12 and poor nutritional intake.13 Moreover, current strategies for treating and preventing NAFLD focus on lifestyle changes, weight reduction, and controlling comorbid conditions associated with NAFLD pathogenesis, such as IR, dyslipidemia, and T2D.14 A large number of studies have been conducted to assess the relationship between NAFLD and overall dietary patterns,[15], [16], [17] as well as different nutritional components,[18], [19], [20] implicating saturated fatty acids (SFAs)[21], [22], [23] and added fructose, mainly in the form of sucrose (i.e. table sugar) and high-fructose corn syrup (HFCS),24,25 as major risk factors. Among the leading sources of these nutrients in the modern diet are ultra-processed foods and drinks (UPF), made of contents derived from foods and additives, undergoing multiple industrial processes to create the final product.26 UPF contain characteristic ingredients of no or rare other culinary use (such as HFCS and hydrogenated oils) and tend to be high in energy, salt, sugars (mainly fructose or HCFS), and fat (in particular SFAs), with low nutritional value.27 Furthermore, UPF is usually very easy to use, durable, and hyper-palatable.28 These characteristics, among other things, have led to a significant increase in UPF consumption over the last few decades,[29], [30], [31] accounting for over 50% of mean energy intake in the UK32 and the US,33 30% in Mexico,34 and 21.5% in Brazil35 for both children and adults. The association between the dietary share of UPF and the risk of various diet-related non-communicable diseases (NCDs) was broadly investigated, indicating an association with T2D, cardiovascular diseases, cancer, as well as MetS, obesity and all-cause mortality.27,36 The association of UPF with obesity and T2D is well established and was recently systematically reviewed.[36], [37], [38], [39], [40], [41] While the evidence is gradually increasing, there remains a need for further high-quality studies on the association between UPF consumption and NAFLD, as well as its major risk factors, MetS and IR. Therefore, a systematic review on the association between UPF consumption and NAFLD, MetS, and IR was conducted. Moreover, in order to cover a broader perspective, a comprehensive literature summary was conducted on the association between UPF consumption and obesity and T2D.
Materials and methods
This study was submitted to the International Prospective Register of Systematic Reviews – PROSPERO (CRD42023397579) and conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines42 (for the full PRISMA 2020 checklist, see Table S1).
Search strategy
The systematic literature search was completed in March 2023, with the assistance of the University of Haifa library service for systematic reviews. The search was conducted in the following databases: PubMed, Scopus, Embase, Web of Science, CINAHL, and Cochrane (Table S2). Further studies were searched non-systematically on Google Scholar and by checking the references of the identified articles. The systematic search strategy was related to exposure to UPF and outcomes of NAFLD, MetS, and IR. The search keywords were defined through an exploratory investigation in addition to MeSH (Medical Subject Headings; PubMedCochrane) and similarly controlled vocabulary thesauruses (CINAHL Subject Headings, CINAHL; Emtree, Embase) to locate relevant articles (Table S3). We restricted the results to the terms contained only in the title or title and abstract.
The non-systematic section of this review was related to exposure to UPF and outcomes of obesity and T2D. A thorough literature search was conducted in Google Scholar and PubMed and by checking the references of identified articles.
Eligibility criteria
Different food classification systems have been proposed to categorize foods regarding food processing. A systematic review has shown that of the main five systems compared, NOVA is the most specific, coherent, clear, comprehensive, and workable.43 Furthermore, as of today, the NOVA food classification is the most common system applied worldwide.27,28 Thus, we included studies that used the NOVA food classification system and studies that did not use the NOVA food classification system but classified foods based on their processing level similarly to NOVA. Since UPF as a whole has been shown to be related to various NCDs,27 we chose to include only studies that assessed UPF as a whole to elaborate on its association with NAFLD. In addition, studies focusing only on specific subgroups of processed foods (such as sugar-sweetened beverages or processed meat) were excluded since these subgroups have already been demonstrated to be related to NAFLD.24,44,45 Additional inclusion criteria were: adult participants (aged >18 years); observational studies (cross-sectional and prospective); interventional studies; results reported as odds ratios (ORs), relative risks (RRs), hazard ratios (HRs), or β, with 95% CIs. In the systematic section, we assessed the association with NAFLD, MetS, and IR as diagnosed using any recognized diagnostic tools and criteria. Importantly, for NAFLD outcome, we excluded studies that included populations with viral hepatitis, autoimmune or inherited (Wilson disease and hemochromatosis) liver diseases, alcohol-related fatty liver disease, and fatty liver suspected to be secondary to hepatotoxic drugs or inflammatory bowel diseases. In addition, for MetS outcomes, we excluded studies focusing only on specific metabolic parameters, which are part of the MetS criteria (e.g., fasting glucose and lipid profile). In the non-systematic section, we assessed the association with obesity and T2D, diagnosed according to standard criteria. In particular, for the obesity outcome, we included studies that estimated overweight, obesity, and abdominal obesity. No restrictions were applied on years, language, or quality of publication.
Data extraction
Two independent reviewers (S.Z.S and L.G) assessed the eligibility of the selected papers based on previously defined inclusion and exclusion criteria. In case of disagreement, reviewers resolved it by consensus. The decision to include the studies was conducted in two stages; first, based on the study title and abstract screening, and next, by full-text reading. Finally, the following information was extracted from each selected study: author (year, location); source of data (setting, length); study population and participant demographics; exposure variables and details of the intervention; adjustment for potentially confounding variables; main outcome variables and results; information for the assessment of the risk of bias.
Quality assessment
The quality of each included study identified through the systematic search was assessed by at least two independent reviewers (from the following: S.Z.S, L.G, S.E.A, D.I.W., and M.G.K) by using the Joanna Briggs Institute’s critical appraisal tools for observational studies46 and the Cochrane Collaboration’s tool47 for risk of bias in randomized-controlled trials (RCTs) (RoB 2 tool). In case of disagreement, reviewers resolved it by consensus.
Data synthesis
In light of the variability in the assessment methods of UPF consumption and the studied clinical outcomes, and due to a relatively low number of studies for each of the outcomes, a narrative synthesis approach was chosen to report the results. We tabulated study characteristics and classified studies into groups according to the different outcomes and study designs. The findings were synthesized to provide extended insights regarding the associations of interest.
Results
Study selection
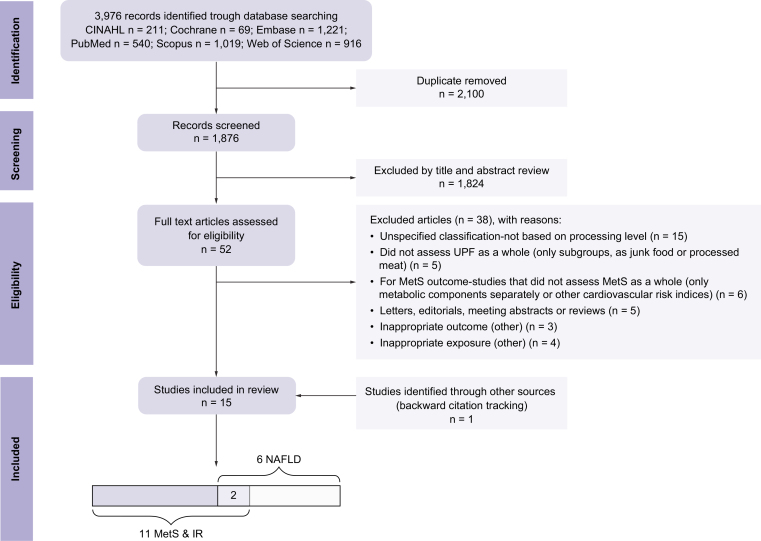
The selection process is shown in Fig. 1. A total of fifteen eligible studies were included, fourteen studies were included at the end of the selection process, and one additional study was identified through backward citation tracking (six studies with NAFLD outcome, eleven studies with MetS or IR outcomes [two studies examined several outcomes]). Of the final eligible studies selected, one was an RCT, and fourteen were observational studies (nine cross-sectional and five prospective).
Fig. 1.
Flowchart of the study selection process for the present systematic review.
Fifteen studies were included at the end of the selection process, one RCT, and fourteen observational studies (nine cross-sectional and five prospective). IR, insulin resistance; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; RCT, randomized-controlled trial.
A list of full-text articles that did not meet the inclusion criteria, including the detailed reasons for their exclusion, is presented in the supplementary material (Table S4).
Systematic evaluation of the association between UPF consumption and the risk of NAFLD
A summary of all studies is depicted in Table 1. Despite the similarity in the method used to evaluate UPF consumption (based on the NOVA classification system), there was inconsistent evidence among the few observational studies examining the associations between UPF consumption as a whole and NAFLD. The study with the largest sample size is a recent prospective cohort of Chinese adults (n = 16,168),48 with NAFLD evaluated by abdominal ultrasound (AUS), in which participants in the upper quartile of UPF consumption (calculated as nutrient density, g/1,000 kCal per day) had an 18% higher risk of developing NAFLD compared to those in the lower quartile. Furthermore, an increment of 62.7 g/1,000 kCal per day in UPF consumption (equal to one SD) was associated with a 6% increased risk of NAFLD in the fully adjusted model, taking into account BMI and principal risk factors contributing to NAFLD among other confounders. Moreover, supporting results were found in two other observational studies in which NAFLD was evaluated by biomarkers.49,50 The first, a cross-sectional study among US adults (n = 6,545) with NAFLD evaluated by the fatty liver index.50 The second, a prospective analysis nested in an RCT among Spanish adults (1 year of follow-up, n = 5,867), with NAFLD evaluated by the fatty liver index and hepatic steatosis index.49
Table 1.
Ultra-processed food consumption and NAFLD (n = 6).
| Author (year, location) | Source of data (mean/median follow-up time) | Population age-range and/or mean ± SD (%women) | Dietary questionnaire UPF assessment method | Adjustment | Main results |
|---|---|---|---|---|---|
| Intervention | |||||
| Hall KD. (2020, USA)53 | Inpatient, crossover RCT | n = 20 31.2±1.6 y (50%) n = 13 sub-sample with liver MRS |
UPF diet vs. unprocessed diet for 2 weeks, followed by the alternate diet for the next 2 weeks. All meals provided at an amount of 1.6∗EER, to consume ad libitum during 60 min. NOVA classification |
Randomization | Baseline liver fat (by MRS) was 1.2±0.1%. Liver fat was not significantly changed after the unprocessed diet (0.95±0.1%, p = 0.24) or the UPF diet (1.1±0.2%; p = 0.74) |
| Prospective | |||||
| Zhang S. (2022, China)48 | The Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) (4.2 y) | n = 16,168 18-90 y 38.3±0.2 y (57.4%) | Quantitative 81/100-items FFQ (in the past month, previously validated) NOVA classification (nutrient density, g/1,000 kCal per day) |
Age, gender, BMI, smoking status, alcohol consumption, educational level, occupation, household income, physical activity, family history of diseases, depressive symptoms, energy intake, healthy diet score, hypertension, diabetes, and hyperlipidemia | Participants with the highest UPF consumption (4th quartile vs. 1st quartile) had 18% relatively higher risk of developing NAFLD (by AUS) (HR 1.18, 95% CI 1.07-1.30; p for trend <0.0001). HR (95% CI) for one standard deviation increment in UPF consumption, equivalent to 62.7 g/1,000kCal per day, was 1.06 (1.03-1.09) |
| Konieczna J. (2022, Spain)49 | Sub-sample from the Spanish Prevention with Mediterranean Diet (PREDIMED-Plus trial) Prospective analysis nested in RCT (1 y, first year) | n = 5,867 55-75 y 65.0±4.9 y (47.8%) | Semi-quantitative 143-items FFQ at baseline, 6- and 12-month follow-up NOVA classification (% of total food weight) (UPF coded as continuous and sex-specific quintiles) |
Age, gender, study arm, educational level, smoking status, height, physical activity, sedentary behavior, alcohol consumption, and follow-up time. (also sensitivity analysis for dietary factors, obesity measures, and related diseases) | A 10% increment in UPF consumption was associated with greater levels of NAFLD-related biomarkers; FLI score (β = 1.60, 95% CI 1.24-1.96) and HSI score (β = 0.43, 95% CI 0.29-0.57). FLI- estimates for Q5 vs. Q1; β = 3.73, 95% CI 3.10-4.35. HSI- estimates for Q5 vs. Q1; β = 0.93, 95% CI 0.67-1.18; p for trend <0.001) |
| Cross-sectional | |||||
| Liu Z. (2022, USA)50 | National Health and Nutrition Examination Survey (NHANES) | n = 6,545 >20 y mean 49.3 y (0.34ySE) (53.5%) | 24-hour dietary recall NOVA classification (% of total food weight) |
Age, gender, race/ethnicity, educational level, family income to poverty ratio, marital status, smoking status, BMI, serum ALT, fasting triglycerides, total cholesterol, and uric acid | Higher UPF consumption (4th quartile of >68.3% vs. 1st quartile of <41.6%) was associated with higher odds for probable NAFLD, as evaluated by FLI ≥30 (OR 1.83, 95% CI 1.33–2.53). A 10% increment in UPF consumption was associated with 15% higher odds for probable NAFLD (OR 1.15, 95% CI 1.09-1.22; p for trend <0.001) |
| Friden M. (2022, Sweden)52 | Prospective investigation of Obesity, Energy, and Metabolism (POEM) | n = 286 50 y (all participants) (53%) | Semi-quantitative 140-items FFQ (a shorter version of a previously validated FFQ) NOVA classification (% of total kCal) |
Gender, BMI, educational level, physical activity, smoking status, alcohol consumption, and dietary factors (protein, fiber, total sugar, saturated and polyunsaturated fat intake) | Intake of UPF was positively associated with liver fat (by MRI) in crude linear regression models (β = 0.02, p = 0.006). However, the association was attenuated after further adjustments. A 10% increment in UPF consumption was not associated with the prevalence of NAFLD (OR 1.32, 95% CI 0.84–2.09) |
| Ivancovsky-Wajcman D. (2021, Israel)51 | Hepatic screening study | n = 789 40-70 y 58.8±6.6 y (47.4%) |
Semi-quantitative 117-items FFQ NOVA classification (% of total kCal) |
Age, gender, BMI, saturated fat intake, protein intake, physical activity, coffee consumption, and fiber intake | UPF consumption (above median of 28% vs. under median) had no association with NAFLD (by AUS), NASH, and significant fibrosis biomarkers (FibroMax, BioPredictive). Higher UPF consumption among subjects with NAFLD was associated with higher odds for NASH (OR 1.89, 95% CI 1.07-3.38). Higher UPF consumption among ever smokers in the entire sample and those with NAFLD was associated with significant fibrosis (OR 1.89, 95% CI 1.03-3.45 and OR 2.85, 95% CI 1.14-7.14, respectively) |
ALT, alanine aminotransferase; AUS, abdominal ultrasound; FFQ, food frequency questionnaire; FLI, fatty liver index; HSI, hepatitis steatosis index; MRS, magnetic resonance spectroscopy; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OR, odds ratio; RCT, randomized-controlled trial; UPF, ultra-processed food; WC, waist circumference.
In contrast, two smaller-scale cross-sectional studies51,52 that examined the associations between UPF consumption and the prevalence of NAFLD found no significant independent associations. In the first study among Swedish adults (n = 286), which evaluated liver fat by magnetic resonance imaging (MRI), intake of UPF was positively associated with liver fat in crude linear regression models. However, the association was attenuated following adjustments.52 In the second study held in Israel (n = 789),51 no association was found with NAFLD (by AUS). Still, participants with NAFLD who consumed more than 28.4% of their daily energy from UPF (above sample median) had almost two-fold higher odds for NASH according to serum markers (NASHtest) (OR 1.89, 95% CI 1.07-3.38). Moreover, stratification by smoking status revealed a positive association between high UPF consumption and significant fibrosis marker (FibroTest) only among past or current smokers.
Only a small-sample sub-analysis (n = 13) within a crossover RCT examined the effect of 14-day UPF food intake on liver fat measured by magnetic resonance spectroscopy (MRS), showing no significant impact of unprocessed or UPF diet interventions.53
Systematic evaluation of the association between UPF consumption and the risk of metabolic syndrome and insulin resistance
A summary of all studies is depicted in Table 2. All studies that examined the association between UPF and MetS or IR used NOVA food classification and were observational. Most studies pointed towards a positive association. The two most recent and extensive prospective cohorts took place in Brazil and China (n = 8,065 and n = 5,147, respectively) and examined the association between UPF intake and the risk for MetS.54,55 Despite considerable differences in the study population as well as in the amount of UPF consumed (median UPF consumption – Brazil 366 g/day; China 16.3 g/day), higher UPF consumption was associated with increased risk for MetS in both cohorts (4th vs. 1st quartile; RR 1.19, 95% CI 1.07-1.32 and HR 1.17, 95% CI 1.01-1.35, in Brazil and China, respectively). In contrast, another study in the Brazilian population (n = 896),56 with a prolonged follow-up time of 14 years, did not find an association. However, this study was conducted before the use of the NOVA food classification, and as emphasized by the authors, it was not initially designed to accurately measure UPF intake.
Table 2.
Ultra-processed food consumption and metabolic syndrome1and insulin resistance (n = 11).
| Author (year, location) | Source of data (mean/median follow-up time) | Population age - range and/or mean ± SD (% women) | Dietary questionnaire UPF assessment method | Adjustment | Main results |
|---|---|---|---|---|---|
| Prospective studies | |||||
| Canhada SL. (2023, Brazil)54 | Brazilian Longitudinal Study of Adult health (ELSA-Brazil) (7.9 y) |
n = 8,065 35-74 y (58.7%) |
Semi-quantitative 114-items FFQ NOVA classification (g/day) |
Age, gender, BMI, center, race/color, income level, school achievement, smoking status, physical activity, alcohol consumption, energy intake | Higher UPF consumption (4th quartile of >552 g/day vs. 1st quartile of <234 g/day) was associated with 19% increased risk of incident MetS (RR 1.19, 95% CI 1.07–1.32). A 150 g increase in UPF consumption a day was associated with a 4% higher risk of incident MetS (RR = 1.04, 95% CI 1.02–1.06) |
| Pan F. (2023, China)55 | China Nutrition and Health Survey (CNHS) (6 y) |
n = 5,147 >18 y (50.0%) |
24-hour dietary recall of 3 consecutive days at each survey Cumulative mean UPF intake NOVA classification (g/day) |
Gender, age, BMI, educational level, place of residence, regions, income level, smoking status, drinking status, metabolic equivalents, urbanicity, energy intake, and dietary factors (protein, total fat, carbohydrate, and sodium intake) | Higher UPF consumption (4th quartile of >36.1 g/day vs. 1st quartile of <6.5 g/day(was associated with 17% increased risk for MetS (HR 1.17, 95% CI 1.01–1.35; p for trend = 0.047) |
| Magalhães EIDS. (2022, Brazil)56 | The Ribeirão Preto birth cohort (14 y) |
n = 896 23-25 y (55.7%) |
Semi-quantitative 83-item FFQ (non-validated) NOVA classification (% of total kCal) NOVA classification (% of total food weight) |
Gender, age, skin color, educational level, marital status, household income, alcohol consumption, smoking status, physical activity, and energy intake | UPF consumption had no association with MetS (% of kCal RR 1.00, 95% CI 0.99-1.01; % of weight RR 1.00, 95% CI 0.99-1.01) |
| Cross-sectional studies | |||||
| Bezerra Barbosa L. (2023, Brazil)59 | Quilombos community-based survey | n = 895 19-59 y (100%) |
24-hour dietary recall NOVA classification (% of total kCal) NOVA score (ranging from 0 to 23) |
Model 3. Excess weight and neck circumference, plus variables from model 1 that showed p < 0.05 in the analysis for the aforementioned model - age and household income | Higher UPF consumption (4th quartile of 40.5% vs. 1st quartile of 0.0%) was not associated with a higher prevalence of MetS (PR 1.09, 95% CI 0.89-1.32). None of NOVA score categories were associated with higher prevalence of MetS |
| Liu Z. (2022, USA)50 | National Health and Nutrition Examination Survey (NHANES) | n = 6,545 >20 y mean 49.3 y (0.34 y SE) (53.5%) |
24-hour dietary recall NOVA classification (% of total food weight) |
Age, gender, race/ethnicity, educational level, family income to poverty ratio, marital status, smoking status, BMI, serum ALT, fasting triglycerides, total cholesterol, and uric acid | Higher UPF consumption (4th quartile of >68.3% vs. 1st quartile of <41.6%) was associated with higher odds for IR (OR 1.52, 95% CI 1.12–2.07), and a 10% increment in UPF consumption was associated with 11% higher odds for IR (OR 1.11, 95% CI 1.05–1.18; p for trend <0.002). IR was defined as the upper quartile (>Q4) of the study sample’s HOMA levels (>4.37) |
| Silva Meneguelli T. (2022, Brazil)62 | The Cardiovascular Health Care Program of the University Federal of Vicosa (PROCARDIO-UFV) | n = 325 ≥20 y (58.5%) |
24-hour dietary recall NOVA classification (% of total kCal) |
Gender, age, schooling, marital status, smoking status, and physical activity | No association was found between UPF and IR (PR 1.01, 95% CI 0.99-1.02). IR was defined as the upper quartile (>Q4) of the study sample’s TyG index (exact threshold not specified) |
| Hosseininasab D. (2022, Iran)61 | The community health center of the Tehran University of Medical Sciences (TUMS) | n = 391 18-48 y 36.7±9.1 y (100%) |
Semi-quantitative 147-items FFQ NOVA classification (g/day) |
Model 1. Age, BMI, physical activity, energy intake, supplement intake, job status | In adjusted linear regression models, an increase in one gram of UPF consumption was not significantly associated with QUICKI (β = -4.306, 95% CI -0.001-0.001) nor HOMA (β = -2.096, 95% CI -0.002-0.002) in the main multivariable model |
| Ivancovsky-Wajcman D. (2021, Israel)51 | Hepatic screening study | n = 789 40-70 y 58.8±6.6 y (47.4%) |
Semi-quantitative 117-items FFQ NOVA classification (% of total kCal) |
Age, gender, BMI, saturated fat intake, protein intake, physical activity, coffee consumption, and fiber intake | Higher UPF consumption (above median of 28%) was associated with higher odds for MetS (OR 1.88, 95% CI 1.31-2.71) |
| Martínez Steele E. (2019, USA)57 | National Health and Nutrition Examination Survey (NHANES) | n = 6,385 >20 y (50.8%) |
24-hour dietary recall NOVA classification (% of total kCal) |
Gender, age, race/ethnicity, family income to poverty ratio, educational attainment, smoking status, and physical activity | A 10% increase in UPF consumption was associated with a 4% higher prevalence of MetS (PR 1.04, 95% CI 1.02-1.07). Higher UPF consumption (5th quintile of >71% vs. 1st quintile of <40%) was associated with a higher prevalence of MetS (PR 1.28, 95% CI 1.09-1.50). The association was stronger in young adults (PR 1.94, 95% CI 1.39-2.72) and decreased with age |
| Lavigne-Robichaud M. (2018, Canada)58 | Nituuchischaayihititaau Aschii Environment-and-Health Study | n = 811 ≥18 y (58.7%) |
24-hour dietary recall NOVA classification (% of total kCal) |
Age, gender, area of residence, smoking status, alcohol consumption, and energy intake | Higher UPF consumption (5th quintile of 83% vs. 1st quintile of 21%) was associated with higher prevalence of MetS (OR 1.90, 95% CI 1.14-3.17; p for trend = 0.04) |
| Nasreddine L. (2018, Lebanon)60 | Community-based survey | n = 302 ≥18 y 39.4±13.8 y (61.2%) |
Semi-quantitative 80-items FFQ NOVA classification (% of total kCal) followed by dietary pattern analysis. The ultra-processed dietary pattern consisted mainly of fast foods, snacks, and sweets, while also including meat, roasted nuts, and liquor |
Age, gender, BMI, marital status, area of residence, educational level, income level, smoking status, physical activity, and energy intake | The ultra-processed dietary pattern had no association with MetS (OR 1.11, 95% CI 0.26-4.65) |
AHA, American Heart Association; ALT, alanine aminotransferase; FFQ, food frequency questionnaire; HOMA-IR, homeostatic model assessment for insulin resistance; HR, hazard ratio; IR, insulin resistance; MetS, metabolic syndrome; OR, odds ratio; PR, prevalence ratio; QUICKI, quantitative insulin-sensitivity check index; RR, relative risk; TyG, triglyceride-glucose (index); UPF, ultra-processed food.
MetS definition as accepted, recommended by several statements and guidelines of medical associations109,110 and with modification for use in the Asian population:111 the presence of at least three of five criteria; impaired fasting glucose (fasting glucose ≥100 mg/dl and/or medication), hypertension (systolic blood pressure/diastolic blood pressure ≥130/80-85 mmHg and/or medication), hypertriglyceridemia (triglycerides ≥150 mg/dl and/or medications), low levels of high-density lipoprotein cholesterol <40/50 mg/dl (among men and women, respectively), and abdominal obesity (elevated waist circumference among men and women, population- and country-specific definitions).
Positive associations were also observed in three cross-sectional studies,51,57,58 including a large-scale examination survey conducted among 6,385 US adults,57 in which higher UPF consumption (5th quintile of >71% vs. 1st quintile of <30%) was associated with a higher prevalence of MetS (prevalence ratio 1.28, 95% CI 1.09-1.50), and a dose-response association for every 10% increase in UPF consumption was observed.
On the other hand, two smaller-scale cross-sectional surveys found no significant association between UPF consumption and the prevalence of MetS.59,60 Both studies have limitations. The first study was conducted among a very specific Quilombos community and solely among women (Brazil, n = 895).59 The second study was conducted among a representative population but used NOVA food classification followed by dietary pattern analysis (Lebanon, n = 302),60 in which the ‘ultra-processed’ dietary pattern also included non-UPF products such as roasted nuts and low-fat dairy, which may have led to an inadequate exposure assessment.
Only three cross-sectional studies examined the association between UPF and IR, using NOVA food classification and a variety of indices to estimate IR. The first, by Liu et al. (n = 6,545, US population),50 found that higher UPF consumption (4th vs. 1st quartile) was associated with higher odds for IR (OR 1.52, 95% CI 1.12-2.07), as evaluated by HOMA-IR (homeostatic model assessment for IR). Furthermore, a 10% increment in UPF consumption was associated with 11% higher odds for IR. Conversely, another study (Iran, n = 391, 100% women) did not demonstrate a significant association with HOMA nor with the QUICKI (quantitative insulin-sensitivity check index).61 Similarly, no association was found in the third study conducted among 325 Brazilian adults, which evaluated IR using the triglyceride-glucose index.62
The association between UPF consumption and primary risk factors for NAFLD
Overweight, obesity, and abdominal obesity
A summary of all studies is depicted in Table 3. The vast majority of the observational studies found a positive association between UPF consumption and several anthropometric measures: BMI, overweight, obesity, and abdominal obesity. All but one of the studies evaluated UPF consumption using the NOVA classification system. In an extensive prospective study based on the French NutriNet-Santé cohort (n = 110,260),63 UPF intake was positively associated with BMI gain and risk of overweight and obesity. In a recent cohort study of the UK Biobank (n = 18,218), UPF consumption was related to a higher risk of multiple indicators of obesity.64 A 79% and 30% higher risk of developing obesity and abdominal obesity was observed among those in the upper quartile compared to the lower quartile of UPF consumption. Similar results were observed in several other cohort studies.55,[65], [66], [67], [68], [69], [70]
Table 3.
Ultra-processed food consumption and overweight, obesity, and abdominal obesity (n = 21).
| Author (year, location) | Source of data (mean/median follow-up time) | Population age - range and/or mean ± SD (% women) | Dietary questionnaire UPF assessment method | Main results |
|---|---|---|---|---|
| Intervention | ||||
| Hall KD. (2020, USA)53 |
Inpatient, crossover RCT | n = 20 31.2±1.6 y (50%) |
UPF diet vs. unprocessed diet for 2 weeks, followed by the alternate diet for the next 2 weeks. All meals were provided at an amount of 1.6∗EER, to consume ad libitum during 60 min. NOVA classification | Energy intake was greater during the UPF diet (508±106 kCal/day; p = 0.0001), with increased consumption of carbohydrate (280±54 kCal/day; p <0.0001) and fat (230±53 kCal/day; p = 0.0004). Participants gained 0.8±0.3 kg (p = 0.01) during the UPF diet and lost 1.1±0.3 kg (p = 0.001) during the unprocessed diet |
| Prospective studies | ||||
| Pan F. (2023, China)55 | China Nutrition and Health Survey (CNHS) (6 y) | n = 5,147 >18 y (50.0%) |
24-hour dietary recall of 3 consecutive days at each survey Cumulative mean UPF intake NOVA classification (g/day) |
Higher UPF consumption (4th quartile of >36.1 g/day vs. 1st quartile of <6.5 g/day) was associated with 33% increased risk of abdominal obesity (WC ≥90 for men and ≥80 for women) (HR = 1.33, 95% CI 1.18–1.51; p for trend <0.001) |
| Magalhães EIDS. (2022, Brazil)56 | The Ribeirão Preto birth cohort (14 y) |
n = 896 23-25 y (55.7%) |
Semi-quantitative 83-items FFQ (non-validated) NOVA classification (% of total kCal) NOVA classification (% of total food weight) |
Only in women, UPF consumption was associated with increased risk of abdominal obesity (% of kCal RR = 0.57, 95% CI 0.38-0.85; % of weight RR = 0.57, 95% CI 0.43-0.77) |
| Li M. (2021, China)68 | China Nutrition and Health Survey (CNHS) (NA) | n = 12,451 >20 y 43.7±14.7 y (51.3%) |
24-hour dietary recall of 3 consecutive days at each survey Cumulative mean UPF intake NOVA classification (g/day) categorized into four levels: non-consumers, 1–19 g/day, 20–49 g/day, ≥50 g/day |
Higher UPF consumption (levels: 1-19 g/day, 20-49 g/day, ≥50 g/day vs. non-consumers) was associated with increased risk of overweight/obesity (BMI ≥25 kg/m2) (OR (95% CI) 1.45 (1.26-1.65), 1.34 (1.15-1.57), and 1.45 (1.21-1.74), respectively, p for trend = 0.015), and of central obesity (OR (95% CI) 1.54 (1.38-1.72), 1.35 (1.19-1.54), and 1.50 (1.29-1.74), respectively) |
| Cordova R. (2021, 9 European countries)69 | European Prospective Investigation into Cancer and Nutrition (EPIC) study (5 y) |
n = 348,748 25-70 y (73%) |
Quantitative dietary questionnaires or semi-quantitative FFQ, or a combination of semi-quantitative FFQ and 7- and 14-day records. NOVA classification (quintiles of energy-adjusted UPF consumption, g/day, using the residual method) | Energy-adjusted UPF consumption quintiles (176±102, 221±117, 270±129, 364±133, 686±303 g/day). Higher consumption of UPF (per 1 SD increment) was positively associated with weight gain (0.12 kg/5 years, 95% CI 0.09-0.15). Higher UPF consumption (5th quintile vs. 1st quintile) was associated with greater risk of becoming overweight/obese in normal weight participants (RR = 1.15, 95% CI 1.11-1.19), and with greater risk (RR = 1.16, 95% CI 1.09-1.23) of becoming obese in participants who were overweight at baseline |
| Konieczna J. (2021, Spain) (67) | Sub-sample from the Spanish Prevention with Mediterranean Diet (PREDIMED-Plus trial) Prospective analysis nested in RCT (1 y, first year) |
n = 1,485 55-75 y 65.3±5 y (47.5%) |
Semi-quantitative 143-items FFQ at baseline, 6- and 12-month follow-up (face-to-face) NOVA classification (% of total food weight) |
A 10% increment in UPF consumption was associated with greater accumulation of visceral fat measured with DXA (β 0.09 z-score, 95% CI 0.05-0.13), android-to-gynoid fat ratio (0.05, 0.00-0.09, and total fat (0.09, 0.06-0.13). Comparison of the highest UPF consumption (3rd tertile vs. 1st tertile) related to increased regional and overall adiposity during follow-up, with a significant dose-response relationship (p for trend <0.05) |
| Rauber F. (2021, UK)64 |
Sub-sample of the UK Biobank cohort (5 y) |
n = 18,218 40-69 y 55.9±7.4 y (52.1%) |
24-hour dietary recall (web-based, self-administered) NOVA classification (% of total kCal) (Quartiles of UPF, sex-specific cut-offs) |
Higher UPF consumption (4th quartile; men 71.7% and women 70.3% vs. 1st quartile; men 26.3% and women 24.1%) was associated with higher risk for obesity (HR = 1.79, 95%Cl 1.06-3.03), abdominal obesity (HR = 1.30, 95%Cl 1.13-1.48), ≥5% increase in BMI (HR = 1.31, 95% Cl 1.20-1.43), WC (HR = 1.35, 95%Cl 1.25-1.45) and %BF (HR = 1.14, 95% Cl 1.03-1.25) |
| Sandoval-Insausti H. (2020, Spain)70 |
Seniors-ENRICA-1 cohort (6 y) |
n = 652 ≥60 y 67.1±5.8 y (44.3%) |
Face-to-face dietary history (DH-ENRICA), recording all food consumed in a typical week in the preceding year. NOVA classification (% of total kCal) (tertiles of UPF, sex-specific cut-offs) |
Participants with a higher UPF consumption (3rd tertile of 28.7% vs. 1st tertile of 7.3%) were more likely to develop abdominal obesity (OR = 1.62, 95%Cl 1.04–2.54; p for linear trend = 0.037). The food groups that contributed the most to this association were non-alcoholic beverages and meat products |
| Beslay M. (2020, France)63 | NutriNet-Santé cohort (4.1 y) |
n = 110,260 ≥18 y 43.1±14.6 y (78.2%) |
24-hour dietary recall (web-based) of 3 non-consecutive days, randomly assigned over two weeks NOVA classification (% of total food weight) |
A positive association between an increment of 10% of UPFs and gain in BMI (β time × UPF continuous = 0.02 (0.01–0.02), p <0.001). UPF intake was associated with a higher risk of overweight (HR for an absolute increase of 10% of UPF = 1.11, 95% CI 1.08-1.14, p<0.001), and for obesity (HR for an absolute increment of 10% of UPF = 1.09, 95% CI 1.05-1.13, p<0.001) |
| Canhada SL. (2020, Brazil)66 | Brazilian Longitudinal Study of Adult health (ELSA-Brazil) (3.8 y) |
n = 11,827 35-74 y 51.3±8.7 y (55%) |
Semi-quantitative 114-items FFQ NOVA classification (% of total kCal) |
Highest quartile (>30.8%) vs. the lowest quartile (<17.8%) of UPF consumption was associated with a greater risk of large weight gain and waist gain, defined as annual gain ≥90th percentile (RR = 1.27, 95% CI 1.07-1.50 and RR = 1.33, 95% CI 1.12-1.58 respectively), overweight/obesity incidence (RR = 1.20, 95% CI 1.03-1.40) and obesity incidence (RR = 1.02, 95% CI 0.85-1.21) |
| Mendonça RD. (2016, Spain)65 | The Seguimiento University of Navarra (SUN-project) (8.9 y) |
n = 8,451 37.6±11.0 y (65%) |
Self-administered semi-quantitative 136-items FFQ NOVA classification (servings/day) |
Participants in the highest quartile vs. the lowest (6.1 vs. 1.5 servings/day of UPF) had a higher risk of developing overweight or obesity (HR = 1.26, 95% CI 1.10-1.45; p for trend = 0.001). A higher incidence of overweight and obesity with increasing baseline quartiles of UPF |
| Cross-sectional | ||||
| Silva Meneguelli T. (2022, Brazil)62 | The cardiovascular health care program of the Federal University of Vicosa (PROCARDIO-UFV) | n = 325 ≥20 y (58.5%) |
24-hour dietary recall NOVA classification (% of total kCal) |
Positive associations were found between UPF consumption and excessive body weight (adults with BMI ≥25 kg/m2 and elderlies with BMI ≥ 28kg/m2) (PR = 1.004, 95% CI 1.00-1.01), and abdominal obesity (WC ≥90 for men and ≥80 for women) (PR = 1.004, 95% CI 1.00-1.01). UPF consumption was not associated with percent body fat |
| Martinez-Perez C. (2021, Spain)105 | The PREDIMED-Plus trial | n = 5,636 65.1±4.9 y (48.5%) | Semi-quantitative 143-items FFQ NOVA, IARC, IFIC, and UNC classification (g/day) |
UPF consumption in all the classification systems was associated with weight and WC, NOVA classification showed the highest value. A 5% increment in UPF consumption was associated with 0.11 higher BMI (95% CI 0.05-0.18) |
| Machado PP. (2020, Australia)75 | The National Nutrition and Physical Activity Survey (NNPAS) | n = 7,411 20-85 y (48.3%) |
24-hour dietary recall NOVA classification (% of total kCal) |
UPF consumption was associated with higher BMI and WC and a greater prevalence of obesity and abdominal obesity (p trend ≤ 0.001 for all outcomes). Dose-response associations between UPF consumption and BMI/obesity. Higher UPF consumption (5th quintile of 74.2% vs. 1st quintile of 12.7%) was associated with higher odds of obesity (OR 1.61, 95% CI 1.27-2.04) and abdominal obesity (OR 1.38, 95% CI 1.10-1.72). The association between UPF consumption and BMI/WC was stronger among people aged ≥40 years, females, and inactive people |
| Nardocci M. (2021, Canada)106 | Canadian Community Health Survey (CCHS)-nutrition | n = 13,608 >19 y (50%) |
24-hour dietary recall NOVA classification (% of total kCal) |
A 10% increase in UPF consumption was associated with 6% higher odds of obesity (OR 1.06, 95% CI 1.02-1.11). Higher UPF consumption (3rd tertile of ≥58.7% kCal/day vs. 1st of ≤ 38.5% kCal/day) was associated with higher odds of obesity (OR 1.31, 95% CI 1.06-1.60). Stratification by education revealed an association among high-education university participants (OR 2.17, 95% CI 1.45-3.24); with no association for adults who graduated high school or less |
| Rauber F. (2020, UK)74 | The UK National Diet and Nutrition Survey Rolling Programme (NDNS) |
n = 6,143 19-96 y (51.6%) |
Food diary - 4 days NOVA classification (% of total kCal) |
Higher UPF consumption (4th quartile of 74% vs. 1st quintile of 35%) was associated with 1.66 kg/m2 higher BMI (95% CI 0.96-2.36), 3.56 cm higher WC (95% CI 1.79-5.33) and higher odds for being obese (OR 1.90, 95% CI 1.39-2.61). The association was stronger among women. A dose-response relationship was observed in both sexes. A 10% increase in UPF consumption was associated with 0.38 kg/m2 higher BMI (95% CI 0.20-0.55), 0.87 cm higher WC (95% CI 0.40-1.33), and higher odds of being obese (OR 1.18, 95% CI 1.08-1.28). No association was observed for abdominal obesity (in both sexes) |
| Asma' A. (2019, Malaysia)72 | Kuala Nerus, a district in Terengganu | n = 200 18-59 y median 33 y (75%) |
24-hour dietary recall – 2 days NOVA classification (% of total kCal) |
UPF consumption was not associated with BMI, WC, and %body fat |
| Nardocci M. (2019, Canada)107 | Canadian Community Health Survey (CCHS) |
n = 19,363 ≥18 y 46±0.13 y (49%) |
24-hour dietary recall NOVA classification (% of total kCal) |
UPF consumption was associated with BMI (the mean BMI across quintiles of UPF consumption was 26.6; 27.0; 26.8; 27.3; 27.4 kg/m2; p for trend < 0.001). A 10% point increase in UPF consumption was associated with higher prevalence of obesity (OR 1.05, 95% CI 1.01- = 1.08) and overweight (OR 1.03, 95% CI 1.01-1.07) Higher UPF consumption (5th quintile of 76.0% vs. 1st quintile of 20.1%) was associated with higher odds of obesity (OR 1.32, 95% CI 1.05-1.57) |
| Juul F. (2018, USA)73 | National Health and Nutrition Examination Survey (NHANES) | n = 15,977 20-64 y 41.9±0.2 y (50.6%) |
24-hour dietary recall NOVA classification (% of total kCal) |
Higher UPF consumption (5th quintile of >74.2% vs. 1st quintile of <36.5%) was associated with 1.61 units higher BMI (95% CI 1.11-2.10), 4.07 cm higher WC (95% CI 2.94-5.19) and higher odds for being overweight (OR 1.48, 95% CI 1.25-1.76), obese (OR 1.53, 95% CI 1.29-1.81) and abdominal obesity (OR 1.62, 95% CI 1.39-1.89); p for trend <0.001 for all outcomes. The association was stronger among women |
| Silva FM. (2018, Brazil)108 | Brazilian Longitudinal Study of Adult health (ELSA-Brazil) |
n = 8,977 35-64 y (51.9%) |
Semi-quantitative 114-items FFQ NOVA classification (% of total kCal) |
Higher UPF consumption (4th quartile >29% vs. 1st quintile <16%) was associated with a higher BMI (β = 0.80, 95% CI 0.53-1.07 kg/m2), WC (β = 1.71, 95% CI 1.02-2.40 cm) and higher odds for being overweight (OR 1.31, 95% CI 1.13-1.51), obese (OR 1.41, 95% CI 1.18-1.69) and increased WC (OR 1.41, 95% CI 1.20-1.66) |
| Adams J. (2015, UK)71 | UK National Diet and Nutrition Survey (NDNS) | n = 2,174 >18 y (51.4%) |
Food diary - 4 days Classification: unprocessed or minimally processed; processed ingredients; and UPF (% of total kCal) |
UPF consumption had no association with markers of body weight. Higher intake of processed ingredients was associated with lower BMI [-0.09, 95% CI −0.16-(-0.03)], and reduced odds of overweight/obesity (OR 0.97, 95% CI 96-99), and obesity (OR 0.98, 95% CI 97-99) |
DXA, dual-energy X-ray absorptiometry; EER, estimated energy requirements; FFQ, food frequency questionnaire; HR, hazard ratio; IARC, International Agency for Research on Cancer; IFIC, International Food Information Council; OR, odds ratio; PR, prevalence ratio; RCT, randomized-controlled trial; RR, relative risk; UNC, University of North Carolina; UPF, ultra-processed food; WC, waist circumference.
On the other hand, two cross-sectional studies found no significant associations between intake of UPF and measures of body weight. However, it should be noted that the first study (published in 2015, n = 2,174) used a previous three-group NOVA classification that is no longer in use,71 and the more recent study was a relatively small-scale study with a convenience sample of 200 adults.72
A few studies indicated gender differences. A cross-sectional national study of 15,977 US adults found a significant interaction between gender and UPF consumption for BMI and waist circumference, with a more pronounced association among women.73 Two other cross-sectional studies (UK, n = 6,143; Australia, n = 7,411) found a stronger association between UPF and obesity measures among women,74,75 and one additional Brazilian cohort (n = 896) demonstrated an increased risk for abdominal obesity only among women (RR 0.57, 95% CI 0.43-0.77) with no association among men.56
A 2021 meta-analysis of observational studies (twelve cross-sectional and two prospective) supported the association between UPF consumption and increased risk of overweight (OR 1.36, 95% CI 1.23-1.51), obesity (OR 1.51, 95% CI 1.34-1.70), and abdominal obesity (OR 1.49, 95% CI 1.34-1.66).37 Similar findings were found in three other meta-analyses conducted between 2020-2021,[37], [38], [39], [40] with one reporting a linear dose-response association.40
Only a single crossover RCT examined the effect of UPF food intake on obesity,53 in which twenty adults were given an ad libitum UPF diet vs. an unprocessed diet for 2 weeks each. During the UPF diet, the energy intake was greater by about 500 kCal/day, correlating to weight gain and resulting in about 1 kg weight gain vs. 1 kg weight reduction in those receiving the unprocessed diet.
Type 2 diabetes
A summary of all studies is depicted in Table 4. All the studies that examined the association between UPF consumption and the risk of T2D found a strong and positive association using the NOVA food classification. All but one of the studies were prospective cohorts, with the first published at the beginning of 2020 by Srour B. and colleges (NutriNet-Sante, France).76 In this study (n = 104,707, follow-up time of 6 years), a 10% increment in UPF consumption was associated with a higher risk of T2D (HR 1.13, 95% CI 1.03-1.23). This association was later confirmed by four other large prospective cohort studies conducted in different settings.41,[77], [78], [79] Notably, one of them is a recent study that demonstrated an association between UPF intake and T2D risk among three large US cohorts (n = 71,871, n = 87,918, and n = 38,847; follow-up of 5,187,678 person-years). This study also conducted a meta-analysis of five prospective cohort studies (including the present US cohort analysis), which further supported a positive association between UPF and T2D (pooled RR for each 10% increment of total UPF consumption was 1.12, 95% CI 1.10-1.13).41
Table 4.
Ultra-processed food consumption and type-2 diabetes (n = 6).
| Author (year, location) | Source of data (mean/median follow-up time) | Population age – range and/or mean ± SD (% women) | Dietary questionnaire UPF assessment method | Main results |
|---|---|---|---|---|
| Prospective studies | ||||
| Chen Z. (2023, USA)41 | The Nurses’ Health Study (NHS), The NHSII, and The Health Professionals’ Follow-up Study (HPFS) (NA) |
NHS, n = 71,871 30-55 y (100%) NHSII, n = 87,918 25-42 y (100%) HPFS, n = 38,847 40-75 y (0%) |
Semi-quantitative 116-130-items FFQ NOVA classification (servings/day) (sensitivity analysis for total kCal/day, % of total kCal, % of total food weight, and energy-adjusted servings/day) |
Higher UPF consumption (5th quintile vs. 1st quintile), as servings/day, was associated with higher risk of T2D (pooled HR 1.28, 95% CI 1.21-1.36). Each one-serving increment in UPF consumption was associated with higher risk for T2D (pooled HR 1.03, 95% CI 1.02-1.03). Higher UPF consumption (5th quintile vs. 1st quintile), as % of total food weight, was associated with higher risk of T2D (pooled HR 1.46, 95% CI 1.391.54). A 10% increment in UPF consumption was associated with a higher risk for T2D (pooled HR 1.12, 95% CI 1.10-1.13) |
| Duan MJ. (2022, The Netherlands)77 | The Lifelines cohort study (41 months) |
n = 70,421 35-70 y (58.6%) |
Semi-quantitative 110-items FFQ NOVA classification (% of total food weight) and dietary pattern |
A 10% increment in UPF consumption was associated with a higher risk for T2D (OR 1.25, 95% CI 1.16-1.34). Higher risk also for a pattern high in cold savory snacks (OR 1.16, 95% CI 1.09-1.22) and a pattern high in warm savory snacks (OR 1.15, 95% CI 1.08-1.21) |
| Levy RB. (2021, UK)79 | UK Biobank (5.4 y) |
n = 21,730 40-69 y 55.8+7.4 y (52.9%) |
24-hour dietary recall NOVA classification (% of total food weight) |
A 10% points increment in UPF consumption was associated with a higher risk for T2D (HR 1.12, 95% CI 1.04-1.20). Higher UPF consumption (4th quartile of 49.1% vs. 1st quintile of 7.7%) was associated with higher risk of T2D (HR 1.44, 95% CI 1.04-2.02; p for trend <0.028) |
| Llavero-Valero M. (2021, Spain)78 | The Seguimiento University of Navarra (SUN-project) (12 y) |
n = 20,060 37.4±12.2 y (61.5%) |
Semi-quantitative 136-items FFQ NOVA classification (g/day) |
Higher UPF consumption (3rd tertile >323.3 g/day vs. 1st tertile <214.6 g/day) was associated with higher risk of T2D (HR 1.53, 95% CI 1.06-2.22), with a significant dose-response relationship (p for linear trend = 0.024) |
| Srour B. (2020, France)76 | NutriNet-Santé cohort (6 y) |
n = 104,707 >18 y (79.2%) |
24-hour dietary recall NOVA classification (% of total food weight and g/day) |
A 10% increment in UPF consumption was associated with a higher risk for T2D (HR 1.13, 95% CI 1.03-1.23). For a 100 g/day increment in UPF consumption (HR 1.05, 95% CI 1.02-1.08) |
| Cross-sectional studies | ||||
| Nardocci M. (2021, Canada)106 | Canadian Community Health Survey (CCHS)-nutrition | n = 13,608 >19 y (50%) |
24-hour dietary recall NOVA classification (% of total kCal) |
Higher UPF consumption (3rd tertile ≥58.7% kCal/day vs. 1st ≤38.5% kCal/day) was associated with higher odds of T2D (OR 1.37, 95% CI 1.01-1.85) |
FFQ, food frequency questionnaire; HR, hazard ratio; OR, odds ratio;T2D, type 2 diabetes; UPF, ultra-processed food.
Quality assessment
The quality assessment of the included studies identified through the systematic search is depicted in Table S5. According to Joanna Briggs Institute’s critical appraisal tools for observational studies, cohort studies were scored 9 to 11, and cross-sectional studies were scored 6 to 8. Three cohorts48,54,55 and four cross-sectional50,51,57,58 studies represented the highest quality (with a maximum of 11 and 8 points assigned to them, respectively). The most common risk of bias was regarding the reliability and validity of the methods used to evaluate the outcomes. The single crossover RCT included was evaluated separately using Cochrane’s RoB 2 tool for RCTs. Accordingly, the study was evaluated as having ‘some concern for bias’, stemming from the limitation that NAFLD was tested only among a small sub-sample (see Table S6).
Discussion
The present study systematically reviewed the association between UPF consumption and NAFLD and its leading risk factors; MetS and IR. Furthermore, it covered a broader perspective by comprehensively reviewing the association between UPF and other primary risk factors for NAFLD: obesity, and T2D.
While increased UPF consumption is strongly and consistently associated with obesity and T2D in the vast majority of the studies reviewed, pointing at a dose-response relationship, evidence concerning NAFLD, MetS, and IR outcomes is less robust. Specifically, the analysis carried out in this current systematic review found an increased risk of NAFLD in three studies out of six,[48], [49], [50] MetS in five out of eight,51,54,55,57,58 and IR in one out of three.50 Most studies that demonstrated an association with NAFLD were prospective, including a study in which NAFLD was evaluated by AUS, showing a significant association between high consumption of UPF and the incidence of NAFLD.48 A substantial drawback is that half of the studies were cross-sectional and thus did not allow causal inference. In addition, the only interventional study had a very small sample size and was not explicitly designed to study NAFLD.53 Furthermore, very few studies examined additional outcomes of NAFLD, such as NASH and fibrosis markers. Importantly, all studies adjusted for BMI and considered total daily energy intake (either by adjustment or as part of the UPF assessment method). Thus, in those demonstrating an association between UPF and NAFLD, the association seems independent of BMI, obesity, or energy intake.
Most studies that demonstrated an association with MetS were large-scale examination surveys, including two recent and extensive prospective cohorts.54,55 At the same time, most studies that did not find an association were cross-sectional and had a significantly smaller sample size.59,60 There are no interventional studies in this field. In addition, there were differences in the extent of adjustment between the studies, with some of them[56], [57], [58], [59], [60] not adjusting for known risk factors for MetS,80 such as BMI, physical activity, and alcohol consumption. Lastly, there is little data regarding the association between UPF and IR. All three studies were cross-sectional, and two of them did not focus on IR as the primary outcome.61,62
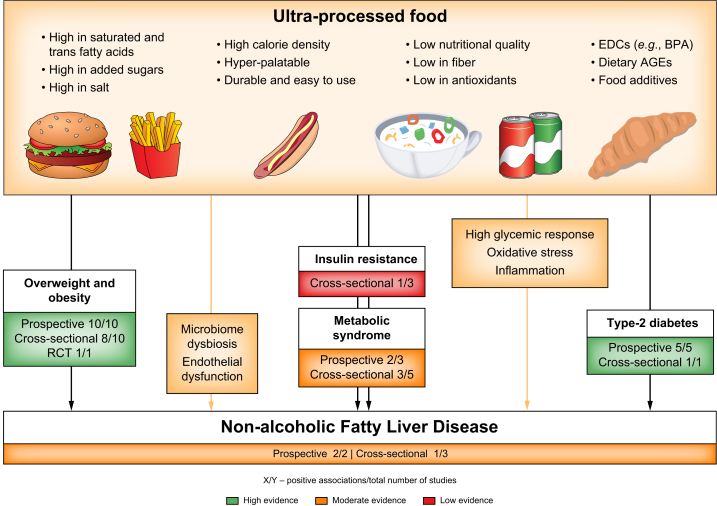
The harmful effects of UPF on NAFLD can be explained through several pathways (Fig. 2). First, the harmful effect may be partly explained by the poor nutritional value of UPF.27 The majority of UPF has a high caloric density and large amounts of added sugar and SFAs, which are well-known risk factors for NAFLD. Moreover, a high UPF diet may indicate decreased consumption of foods with high nutritional quality, known as protective factors for NAFLD, such as fruits and vegetables that contribute vitamins and fibers (among other nutrients). However, most studies adjusted for nutritional factors other than UPF, such as SFA intake, fiber intake, and overall adherence to a healthy diet, but the association between UPF and NAFLD persisted.48,49
Fig. 2.
Ultra-processed food consumption and NAFLD, its major risk factors, and plausible mechanisms underlying the associations.
The effect of UPF on NAFLD can be explained through several pathways. First, the poor nutritional value of UPF. Second, non-nutritional food compounds or additives within UPF (e.g., EDCs and AGEs) through their pro-oxidative and proinflammatory properties. Lastly, dietary factors common in UPF were found to alter gut microbiome composition (dysbiosis), including saturated fatty acids, fructose, and food additives. AGEs, advanced glycated end products; BPA, bisphenol A; EDCs, endocrine-disrupting chemicals; NAFLD, non-alcoholic fatty liver disease; UPF, ultra-processed food.
Second, non-nutritional food compounds or additives within UPF may contribute to NAFLD, possibly through their pro-oxidative and proinflammatory properties such as endocrine-disrupting chemicals (EDCs)81,82 and advanced glycation end products (AGEs).83,84 EDCs are a large group of diverse chemical compounds that can be classified as natural or synthetic. Exposure to the latter is increasing due to the development of industrialized areas, with pesticides, phthalates, and bisphenol A (BPA) being some of the most characterized EDCs to date.81 BPA exposure is very common and has been studied in relation to NAFLD. It is a building block of plastics and of the lining in food and beverage containers and disrupts pancreatic B-cells function and whole-body glucose homeostasis.85 A recent study examining the association of BPA with histological diagnosis of NAFLD found significantly higher BPA plasma levels among 60 individuals with NAFLD compared to matched controls, with even higher BPA plasma levels among individuals with NASH.85 While some studies demonstrated a link between UPF consumption and increased exposure to various EDCs,86,87 further studies are needed to confirm an association with NAFLD.
In addition, UPF can significantly contribute to the intake of AGEs due to its high sugar, fructose, and fat content and high heat preparation methods during industrial processing.88 A diet high in AGEs results in increased serum concentrations of AGEs,89 and in turn, serum AGEs play a role in NAFLD pathogenesis. The activation of liver RAGE (AGE receptor) by AGEs leads to a cascade of downstream signaling, including oxidative stress and hepatocyte ballooning.88,89 A case-control study among 675 individuals in Iran found that a diet high in AGEs was associated with higher odds of NAFLD.84 Furthermore, a meta-analysis of observational studies, including 1,844 participants, found an association between different types of serum AGEs and NAFLD.83 Regarding T2D and MetS, a meta-analysis of thirteen RCTs showed that a diet low in AGEs reduced serum insulin, total cholesterol, and LDL levels.90 Another meta-analysis of seventeen RCTs further found that a diet low in AGEs reduced serum leptin and inflammatory and oxidative stress markers and increased adiponectin.91
Lastly, alterations in microbiome composition and the abundance of specific taxa have repeatedly been related to the pathophysiology of NAFLD and NASH.[92], [93], [94] Multiple factors influence gut microbiome composition, with a leading role for environmental factors, including those associated with lifestyle and diet.95,96 Dietary factors common in UPF were found to negatively influence and alter gut microbiome composition (dysbiosis), including SFAs, fructose, food additives, and AGEs.97,98 These dietary factors contribute to the pathogenesis of obesity-related disorders, including MetS and NAFLD.99 Only a few studies examined the association between UPF consumption and gut microbiota. Among elderly individuals with obesity and MetS, there were associations between higher UPF consumption and different taxa abundances (Alloprevotella, Negativibacillus, Prevotella, and Sutterella).100 Moreover, alpha diversity was significantly lower in males who consumed more than five servings/day of UPF.101 None of those studies found significant results in terms of beta diversity and Firmicutes-Bacteroidetes ratio.
The present systematic review has several limitations that should be addressed. First, as mentioned above, the included studies were mainly observational and thus prone to reverse causality (in the cross-sectional studies) and residual confounding. However, RCTs with “hard endpoints”, such as NAFLD incidence, are very difficult or impossible to conduct. Second, in reported nutritional intake, there is a possibility of a recall bias, although it is non-differential in prospective studies and thus can only lead to an underestimation of the associations. In fact, all of the tools applied for dietary assessment were based on self-reported intake (food frequency questionnaire, 24-hour recalls, and food diaries) and were not previously validated or specifically designed to capture dietary data at the level of food processing and specifically for NOVA classification. New technology-based dietary assessment methods should be adapted (web-based and mobile device applications), allowing participants to choose specific products from various market products available, enabling a better evaluation of the product’s specific industrial processing, additives used, packing method, etc. Third, none of the studies determined NAFLD and IR with their gold standard diagnostic tools – liver biopsy and glucose clamp, respectively. Nevertheless, the non-invasive methods used in the included studies are validated and accepted diagnostic tools appropriate for epidemiological studies.102,103 Finally, a possible publication bias might be expected in favor of significant findings regarding UPF consumption and health outcomes.
In turn, our study presents several strengths. Among them is an extensive exploratory investigation for the widest possible range of accurate keywords, followed by a rigorous literature search and selection process. Additionally, as opposed to a parallel systematic review and meta-analysis that examined the association between UPF consumption and NAFLD,104 special attention was made to evaluate the exposure to UPF precisely and to distinguish the relevant studies that focus on the assessment of UPF as a whole and not on overlapping dietary patterns as “Western” and “fast-food” or on specific groups of processed food (e.g., processed meat). Another strong point was the variability of the populations studied, strengthening the external validity of the findings and highlighting their worldwide relevance. In particular, the various countries studied have diverse consumption of UPF. According to a 2021 systematic review,31 worldwide consumption of UPF (as a % of total energy intake) ranges from 8-51.2% in Brazil to 19-24% and 17-33% in Spain and France, with the highest percentages in the UK and the US (>50%). To our knowledge, no study evaluated the proportion of UPF from total energy intake in China. Still, according to the China Health and Nutrition Survey results,68 the mean UPF consumption increased almost four times from 1997 to 2011, with a daily food weight proportion of UPF from 1.0-3.6%.
In conclusion, research on the association of UPF with NAFLD and related metabolic alterations was mostly conducted in recent years, with insufficient studies to draw a firm conclusion. However, current evidence from higher-quality studies shows that UPF is a potential risk factor for NAFLD and MetS. These findings are in accordance with previous systematic reviews that assessed the association of UPF consumption with other diet-related NCDs, mostly obesity and T2D, as was also comprehensively demonstrated in our study. Taken together, it would seem reasonable to advise minimizing UPF consumption, implemented by increasing awareness of its harmful health effects among the general public and patients, using mandatory front-of-pack food labeling, as well as applying policy measures of taxation of UPF, restricting its advertising and marketing while subsidizing minimally processed foods. Since the growing UPF consumption is a worldwide concern with significant public health implications, elaborating on its effects on liver disease and other NCDs is a priority. Future research should have a prospective design, epidemiological or interventional, use dietary assessment tools designed specifically for food processing evaluation, assess validated clinical liver outcomes, and be supported by mechanistic studies to establish causality.
Financial support
The authors did not receive any financial support to produce this manuscript.
Authors' contributions
Laura Sol Grinshpan and Shira Zelber-Sagi; conceived the research question, developed the methods, conducted data collection and analysis, and drafted the manuscript. Sigal Eilat-Adar and Michal Gillon-Keren; conceived the research question and provided input on data analysis, interpretation, and manuscript drafting. Dana Ivancovsky-Wajcman and Revital Kariv; critically reviewed the manuscript. All authors approved the final version of the manuscript.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Conflict of interest
The authors of this study declare that they do not have any conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
We would like to express our appreciation to Amy Lauren Shapira (BPT, MA) and Ronit Marco (BSc, MA) from the University of Haifa library service for systematic reviews for their invaluable guidance and support throughout the search process.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100964.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Rinella M.E., Lazarus J.V., Ratziu V., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–1986. doi: 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riazi K., Azhari H., Charette J.H., et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 3.Sayiner M., Koenig A., Henry L., et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20(2):205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Adams L.A., Anstee Q.M., Tilg H., et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 5.Smits M.M., Ioannou G.N., Boyko E.J., et al. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol. 2013;28(4):664–670. doi: 10.1111/jgh.12106. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis H., Craig D., Barker R., et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marusic M., Paic M., Knobloch M., et al. NAFLD, insulin resistance, and diabetes mellitus type 2. Can J Gastroenterol Hepatol. 2021;2021 doi: 10.1155/2021/6613827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L., Liu D.W., Yan H.Y., et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17(6):510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 9.Quek J., Chan K.E., Wong Z.Y., et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(1):20–30. doi: 10.1016/S2468-1253(22)00317-X. [DOI] [PubMed] [Google Scholar]
- 10.Younossi Z.M., Golabi P., de Avila L., et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Zohara Z., Adelekun A., Seffah K.D., et al. The prospect of non-alcoholic fatty liver disease in adult patients with metabolic syndrome: a systematic review. Cureus. 2023;15(7) doi: 10.7759/cureus.41959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallsworth K., Thoma C., Moore S., et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Front Gastroenterol. 2015;6(1):44–51. doi: 10.1136/flgastro-2014-100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ullah R., Rauf N., Nabi G., et al. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int J Biol Sci. 2019;15(2):265–276. doi: 10.7150/ijbs.30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelber-Sagi S., Grinshpan L.S., Ivancovsky-Wajcman D., et al. One size does not fit all; practical, personal tailoring of the diet to NAFLD patients. Liver Int. 2022;42(8):1731–1750. doi: 10.1111/liv.15335. [DOI] [PubMed] [Google Scholar]
- 16.Semmler G., Datz C., Reiberger T., et al. Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int. 2021;41(10):2249–2268. doi: 10.1111/liv.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassani Zadeh S., Mansoori A., Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36(6):1470–1478. doi: 10.1111/jgh.15363. [DOI] [PubMed] [Google Scholar]
- 18.Ivancovsky-Wajcman D., Fliss-Isakov N., Grinshpan L.S., et al. High meat consumption is prospectively associated with the risk of non-alcoholic fatty liver disease and presumed significant fibrosis. Nutrients. 2022;14(17):3533. doi: 10.3390/nu14173533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomone F., Ivancovsky-Wajcman D., Fliss-Isakov N., et al. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep. 2020;2(2) doi: 10.1016/j.jhepr.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vancells Lujan P., Vinas Esmel E., Sacanella Meseguer E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients. 2021;13(5):1442. doi: 10.3390/nu13051442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosqvist F., Iggman D., Kullberg J., et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63(7):2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez E.A., Kahl S., Seelig A., et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J Clin Invest. 2017;127(2):695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luukkonen P.K., Sadevirta S., Zhou Y., et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41(8):1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He K., Li Y., Guo X., et al. Food groups and the likelihood of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Br J Nutr. 2020;124(1):1–13. doi: 10.1017/S0007114520000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen T., Abdelmalek M.F., Sullivan S., et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteiro C.A., Cannon G., Levy R.B., et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteiro C.A., Cannon G., Lawrence M., et al. 2019. Ultra-processed foods, diet quality, and health using the NOVA classification system. [Google Scholar]
- 28.Monteiro C.A., Cannon G., Moubarac J.C., et al. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popkin B. 2030 Food, Agriculture and rural development in Latin America and the Caribbean. 2019. Ultra-processed foods’ impacts on health. [Google Scholar]
- 30.Monteiro C.A., Moubarac J.C., Levy R.B., et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21(1):18–26. doi: 10.1017/S1368980017001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marino M., Puppo F., Del Bo C., et al. A systematic review of worldwide consumption of ultra-processed foods: findings and criticisms. Nutrients. 2021;13(8):2778. doi: 10.3390/nu13082778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rauber F., Louzada M., Martinez Steele E., et al. Ultra-processed foods and excessive free sugar intake in the UK: a nationally representative cross-sectional study. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2018-027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraldi L.G., Martinez Steele E., Canella D.S., et al. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marron-Ponce J.A., Flores M., Cediel G., et al. Associations between consumption of ultra-processed foods and intake of nutrients related to chronic non-communicable diseases in Mexico. J Acad Nutr Diet. 2019;119(11):1852–1865. doi: 10.1016/j.jand.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Costa Louzada M.L., Martins A.P., Canella D.S., et al. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev Saude Publica. 2015;49:38. doi: 10.1590/S0034-8910.2015049006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delpino F.M., Figueiredo L.M., Bielemann R.M., et al. Ultra-processed food and risk of type 2 diabetes: a systematic review and meta-analysis of longitudinal studies. Int J Epidemiol. 2022;51(4):1120–1141. doi: 10.1093/ije/dyab247. [DOI] [PubMed] [Google Scholar]
- 37.Lane M.M., Davis J.A., Beattie S., et al. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021;22(3):1–19. doi: 10.1111/obr.13146. [DOI] [PubMed] [Google Scholar]
- 38.Askari M., Heshmati J., Shahinfar H., et al. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes (Lond) 2020;44(10):2080–2091. doi: 10.1038/s41366-020-00650-z. [DOI] [PubMed] [Google Scholar]
- 39.Pagliai G., Dinu M., Madarena M.P., et al. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125(3):308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moradi S., Entezari M.H., Mohammadi H., et al. Ultra-processed food consumption and adult obesity risk: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. 2023;63(2):249–260. doi: 10.1080/10408398.2021.1946005. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z., Khandpur N., Desjardins C., et al. Ultra-processed food consumption and risk of type 2 diabetes: three large prospective US cohort studies. Diabetes Care. 2023;46(7):1335–1344. doi: 10.2337/dc22-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moubarac J.C., Parra D.C., Cannon G., et al. Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep. 2014;3(2):256–272. doi: 10.1007/s13679-014-0092-0. [DOI] [PubMed] [Google Scholar]
- 44.Chen H., Wang J., Li Z., et al. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: an updated systematic review and dose-response meta-analysis. Int J Environ Res Public Health. 2019;16(12):2192. doi: 10.3390/ijerph16122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng X., Li J., Zhao H., et al. Lifestyle as well as metabolic syndrome and non-alcoholic fatty liver disease: an umbrella review of evidence from observational studies and randomized controlled trials. BMC Endocr Disord. 2022;22(1):95. doi: 10.1186/s12902-022-01015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moola S., Munn Z., Tufanaru C., et al. In: JBI manual for evidence synthesis. Aromataris E., Munn Z., editors. JBI; 2020. Chapter 7: systematic reviews of etiology and risk. Available from: [DOI] [Google Scholar]
- 47.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S., Gan S., Zhang Q., et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin chronic low-grade systemic inflammation and health cohort study. Int J Epidemiol. 2022;51(1):237–249. doi: 10.1093/ije/dyab174. [DOI] [PubMed] [Google Scholar]
- 49.Konieczna J., Fiol M., Colom A., et al. Does consumption of ultra-processed foods matter for liver health? Prospective analysis among older adults with metabolic syndrome. Nutrients. 2022;14(19):4142. doi: 10.3390/nu14194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z., Huang H., Zeng Y., et al. Association between ultra-processed foods consumption and risk of non-alcoholic fatty liver disease: a population-based analysis of NHANES 2011-2018. Br J Nutr. 2023;130(6):996–1004. doi: 10.1017/S0007114522003956. [DOI] [PubMed] [Google Scholar]
- 51.Ivancovsky-Wajcman D., Fliss-Isakov N., Webb M., et al. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021;41(11):2635–2645. doi: 10.1111/liv.14996. [DOI] [PubMed] [Google Scholar]
- 52.Friden M., Kullberg J., Ahlstrom H., et al. Intake of ultra-processed food and ectopic-, visceral- and other fat depots: a cross-sectional study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.774718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall K.D., Ayuketah A., Brychta R., et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2020;32(4):690. doi: 10.1016/j.cmet.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Canhada S.L., Vigo Á., Luft V.C., et al. Ultra-processed food consumption and increased risk of metabolic syndrome in adults: the ELSA-Brasil. Diabetes Care. 2023;46(2):369–376. doi: 10.2337/dc22-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan F., Wang Z., Wang H., et al. Association between ultra-processed food consumption and metabolic syndrome among adults in China-results from the China health and nutrition survey. Nutrients. 2023;15(3):752. doi: 10.3390/nu15030752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magalhaes E., de Oliveira B.R., Rudakoff L.C.S., et al. Sex-dependent effects of the intake of NOVA classified ultra-processed foods on syndrome metabolic components in Brazilian adults. Nutrients. 2022;14(15):3126. doi: 10.3390/nu14153126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez Steele E., Juul F., Neri D., et al. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev Med. 2019;125:40–48. doi: 10.1016/j.ypmed.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Lavigne-Robichaud M., Moubarac J.C., Lantagne-Lopez S., et al. Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in northern Quebec, Canada. Public Health Nutr. 2018;21(1):172–180. doi: 10.1017/S136898001700115X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbosa L.B., Vasconcelos N.B.R., Dos Santos E.A., et al. Ultra-processed food consumption and metabolic syndrome: a cross-sectional study in Quilombola communities of Alagoas, Brazil. Int J Equity Health. 2023;22(1):14. doi: 10.1186/s12939-022-01816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasreddine L., Tamim H., Itani L., et al. A minimally processed dietary pattern is associated with lower odds of metabolic syndrome among Lebanese adults. Public Health Nutr. 2018;21(1):160–171. doi: 10.1017/S1368980017002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosseininasab D., Shiraseb F., Noori S., et al. The relationship between ultra-processed food intake and cardiometabolic risk factors in overweight and obese women: a cross-sectional study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.945591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meneguelli T.S., Juvanhol L.L., Leite A.D., et al. Minimally processed versus processed and ultra-processed food in individuals at cardiometabolic risk. Br Food J. 2022;124(3):811–832. [Google Scholar]
- 63.Beslay M., Srour B., Mejean C., et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Sante cohort. PLoS Med. 2020;17(8):1–19. doi: 10.1371/journal.pmed.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rauber F., chang K., vamos E.P., et al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr. 2021;60(4):2169–2180. doi: 10.1007/s00394-020-02367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendonca R.D., Pimenta A.M., Gea A., et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104(5):1433–1440. doi: 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 66.Canhada S.L., Luft V.C., Giatti L., et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Public Health Nutr. 2020;23(6):1076–1086. doi: 10.1017/S1368980019002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Konieczna J., Morey M., Abete I., et al. Contribution of ultra-processed foods in visceral fat deposition and other adiposity indicators: prospective analysis nested in the PREDIMED-Plus trial. Clin Nutr. 2021;40(6):4290–4300. doi: 10.1016/j.clnu.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Li M., Shi Z. Ultra-processed food consumption associated with overweight/obesity among Chinese adults-results from China health and nutrition survey 1997-2011. Nutrients. 2021;13(8):2796. doi: 10.3390/nu13082796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cordova R., Kliemann N., Huybrechts I., et al. Consumption of ultra-processed foods associated with weight gain and obesity in adults: a multi-national cohort study. Clin Nutr. 2021;40(9):5079–5088. doi: 10.1016/j.clnu.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Sandoval-Insausti H., Jimenez-Onsurbe M., Donat-Vargas C., et al. Ultra-processed food consumption is associated with abdominal obesity: a prospective cohort study in older adults. Nutrients. 2020;12(8):2368. doi: 10.3390/nu12082368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams J., White M. Characterisation of UK diets according to degree of food processing and associations with socio-demographics and obesity: cross-sectional analysis of UK National Diet and Nutrition Survey (2008-12) Int J Behav Nutr Phys Act. 2015;12(1):160. doi: 10.1186/s12966-015-0317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asma A., Gan H.J., Hayati M.Y., et al. Food classification system based on food processing and its relationship with nutritional status of adults in Terengganu, Malaysia. Food Res. 2019;4(2):539–546. [Google Scholar]
- 73.Juul F., Martinez-Steele E., Parekh N., et al. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018;120(1):90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 74.Rauber F., Steele E.M., Louzada M., et al. Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008-2016) PLoS One. 2020;15(5):1–15. doi: 10.1371/journal.pone.0232676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Machado P.P., Steele E.M., Levy R.B., et al. Ultra-processed food consumption and obesity in the Australian adult population. Nutr Diabetes. 2020;10(1):39. doi: 10.1038/s41387-020-00141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srour B., Fezeu L.K., Kesse-Guyot E., et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-santé prospective cohort. JAMA Intern Med. 2020;180(2):283–291. doi: 10.1001/jamainternmed.2019.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan M.J., Vinke P.C., Navis G., et al. Ultra-processed food and incident type 2 diabetes: studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. 2022;20(1):7. doi: 10.1186/s12916-021-02200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Llavero-Valero M., Escalada-San Martin J., Martinez-Gonzalez M.A., et al. Ultra-processed foods and type-2 diabetes risk in the SUN project: a prospective cohort study. Clin Nutr. 2021;40(5):2817–2824. doi: 10.1016/j.clnu.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 79.Levy R.B., Rauber F., Chang K., et al. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clin Nutr. 2021;40(5):3608–3614. doi: 10.1016/j.clnu.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 80.Jemal A., Girum T., Kedir S., et al. Metabolic syndrome and its predictors among adults seeking medical care: a trending public health concern. Clin Nutr ESPEN. 2023;54:264–270. doi: 10.1016/j.clnesp.2023.01.034. [DOI] [PubMed] [Google Scholar]
- 81.Cano R., Perez J.L., Davila L.A., et al. Role of endocrine-disrupting chemicals in the pathogenesis of non-alcoholic fatty liver disease: a comprehensive review. Int J Mol Sci. 2021;22(9):4807. doi: 10.3390/ijms22094807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ravichandran G., Lakshmanan D.K., Arunachalam A., et al. Food obesogens as emerging metabolic disruptors; A toxicological insight. J Steroid Biochem Mol Biol. 2022;217 doi: 10.1016/j.jsbmb.2021.106042. [DOI] [PubMed] [Google Scholar]
- 83.Litwinowicz K., Waszczuk E., Gamian A. Advanced glycation end-products in common non-infectious liver diseases: systematic review and meta-analysis. Nutrients. 2021;13(10):3370. doi: 10.3390/nu13103370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jahromi M.K., Tehrani A.N., Teymoori F., et al. Dietary advanced glycation end products are associated with an increased risk of non-alcoholic fatty liver disease in Iranian adults. BMC Endocr Disord. 2023;23(1):111. doi: 10.1186/s12902-023-01365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dallio M., Masarone M., Errico S., et al. Role of bisphenol A as environmental factor in the promotion of non-alcoholic fatty liver disease: in vitro and clinical study. Aliment Pharmacol Ther. 2018;47(6):826–837. doi: 10.1111/apt.14499. [DOI] [PubMed] [Google Scholar]
- 86.Buckley J.P., Kim H., Wong E., et al. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US national health and nutrition examination survey, 2013-2014. Environ Int. 2019;131 doi: 10.1016/j.envint.2019.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steele E.M., Khandpur N., da Costa Louzada M.L., et al. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS ONE. 2020;15(7) doi: 10.1371/journal.pone.0236738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernando D.H., Forbes J.M., Angus P.W., et al. Development and progression of non-alcoholic fatty liver disease: the role of advanced glycation end products. Int J Mol Sci. 2019;20(20):5037. doi: 10.3390/ijms20205037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kellow N.J., Savige G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur J Clin Nutr. 2013;67(3):239–248. doi: 10.1038/ejcn.2012.220. [DOI] [PubMed] [Google Scholar]
- 90.Sohouli M.H., Fatahi S., Sharifi-Zahabi E., et al. The impact of low advanced glycation end products diet on metabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2021;12(3):766–776. doi: 10.1093/advances/nmaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baye E., Kiriakova V., Uribarri J., et al. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-02268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roychowdhury S., Selvakumar P.C., Cresci G.A.M. The role of the gut microbiome in nonalcoholic fatty liver disease. Med Sci (Basel) 2018;6(2):47. doi: 10.3390/medsci6020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharpton S.R., Schnabl B., Knight R., et al. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 2021;33(1):21–32. doi: 10.1016/j.cmet.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharpton S.R., Ajmera V., Loomba R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin Gastroenterol Hepatol. 2019;17(2):296–306. doi: 10.1016/j.cgh.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahn J., Hayes R.B. Environmental influences on the human microbiome and implications for noncommunicable disease. Annu Rev Public Health. 2021;42:277–292. doi: 10.1146/annurev-publhealth-012420-105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rothschild D., Weissbrod O., Barkan E., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 97.Jennison E., Byrne C.D. The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease. Clin Mol Hepatol. 2021;27(1):22–43. doi: 10.3350/cmh.2020.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phuong-Nguyen K., McNeill B.A., Aston-Mourney K., et al. Advanced glycation end-products and their effects on gut health. Nutrients. 2023;15(2):405. doi: 10.3390/nu15020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leung C., Rivera L., Furness J.B., et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 100.Atzeni A., Martínez M., Babio N., et al. Association between ultra-processed food consumption and gut microbiota in senior subjects with overweight/obesity and metabolic syndrome. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.976547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cuevas-Sierra A., Milagro F.I., Aranaz P., et al. Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients. 2021;13(8):2710. doi: 10.3390/nu13082710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muniyappa R., Madan R., Varghese R.T., et al. MDText.com, Inc.; South Dartmouth (MA): 2020. Assessing insulin sensitivity and resistance in humans.https://www.ncbi.nlm.nih.gov/sites/books/NBK278954/ [Updated 2021 Aug 9] Available from: [Google Scholar]
- 103.Zhou J.H., Cai J.J., She Z.G., et al. Noninvasive evaluation of nonalcoholic fatty liver disease: current evidence and practice. World J Gastroenterol. 2019;25(11):1307–1326. doi: 10.3748/wjg.v25.i11.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henney A.E., Gillespie C.S., Alam U., et al. Ultra-processed food intake is associated with non-alcoholic fatty liver disease in adults: a systematic review and meta-analysis. Nutrients. 2023;15(10):2266. doi: 10.3390/nu15102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez-Perez C., San-Cristobal R., Guallar-Castillon P., et al. Use of different food classification systems to assess the association between ultra-processed food consumption and cardiometabolic health in an elderly population with metabolic syndrome (PREDIMED-Plus cohort) Nutrients. 2021;13(7):2471. doi: 10.3390/nu13072471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nardocci M., Polsky J.Y., Moubarac J.C. Consumption of ultra-processed foods is associated with obesity, diabetes and hypertension in Canadian adults. Can J Public Health. 2021;112(3):421–429. doi: 10.17269/s41997-020-00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nardocci M., Leclerc B.S., Louzada M.L., et al. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health. 2019;110(1):4–14. doi: 10.17269/s41997-018-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silva F.M., Giatti L., de Figueiredo R.C., et al. Consumption of ultra-processed food and obesity: cross sectional results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort (2008–2010) Public Health Nutr. 2018;21(12):2271–2279. doi: 10.1017/S1368980018000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alberti K.G., Eckel R.H., Grundy S.M., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 110.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heng D., Ma S., Lee J.J., et al. Modification of the NCEP ATP III definitions of the metabolic syndrome for use in Asians identifies individuals at risk of ischemic heart disease. Atherosclerosis. 2006;186(2):367–373. doi: 10.1016/j.atherosclerosis.2005.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.