Abstract
Background
Effective use of Danio rerio as a preclinical model requires standardization of macronutrient sources to achieve scientific reproducibility across studies and labs.
Objective
Our objective was to evaluate a bacterial-based single-cell protein (SCP) for the production of open-source standardized diets with defined health characteristics for the zebrafish research community.
Methods
We completed a 16-wk feeding trial using juvenile D. rerio 31 d postfertilization (10 tanks per diet and 14 D. rerio per tank) with formulated diets containing either a typical fish protein ingredient [standard reference (SR) diet] or a novel bacterial SCP source [bacterial protein (BP) diet]. At the end of the feeding trial, growth metrics, body composition, reproductive success, and bulk transcriptomics of the liver (RNAseq on female D. rerio with confirmatory rtPCR) were performed for each diet treatment.
Results
D. rerio fed the BP diet had body weight gains equivalent to the D. rerio fed fish protein, and females had significantly lower total carcass lipid, indicating reduced adiposity. Reproductive success was similar between treatments, suggesting normal physiological function. Genes differentially expressed in female D. rerio fed the BP diet compared with females fed the SR diet were overrepresented in the gene ontologies of metabolism, biosynthesis of cholesterol precursors and products, and protein unfolding responses.
Conclusion
Protein source substantially affected body growth metrics and composition as well as gene expression. These data support the development of an open-source diet utilizing an ingredient that correlates with improved health profiles and reduced variability in notable outcomes.
Keywords: nutrition, gene expression, body composition, animal nutrition, alternative protein sources, obesity
Introduction
Zebrafish (Danio rerio) have emerged as an increasingly important model organism for biomedicine and other scientific disciplines [1]. However, as the field matures, it is becoming increasingly apparent that the nutritional goals for optimal use of this model should evolve beyond basic production and reproduction to the establishment and maintenance of clinically healthy research subjects. We recognize that rigor and reproducibility in biomedical research have become a major focus at the NIH and within the research community at large [2]. Commercial diets used in the zebrafish research community lack ingredient transparency, standardization, and control of macro- and micro-nutrient composition. Feed variability has been shown to lead to variable outcomes among studies [3], yet researchers are often unable to mitigate these issues because the quantitative and qualitative ingredient information is not readily available [4].
Among the various ingredient and nutrient profiles fed to D. rerio in research colonies, D. rerio growth is largely affected by dietary protein levels, with a positive linear correlation between weight gain and protein content when the latter is increased up to the predicted daily dietary requirement (∼38%–45% of dry matter for juvenile D. rerio when fish meal is used as the protein source [5]). Commercial diets typically contain even higher levels of protein, reporting 50%–65% of dry matter protein. In addition to quantity, the quality of protein sources varies substantially among sources. Typical animal protein sources in fish diets include fish meal, squid meal, krill meal, casein, whey, gelatin, and feather and poultry meal [6]. Widely used plant sources include soy, wheat or corn products, cottonseed meal, and oats. Several plant-based protein sources currently used in D. rerio and Atlantic salmon diets (soy and gluten, respectively) can contain or mimic anti-nutritional factors that lead to alterations in immune function [7,8]. Consequently, a sustainably sourced quality protein is recommended for inclusion in experimental and reference diets.
For most animal diets, the protein sources should ensure that amino acid profiles and other associated bioactive compounds are sufficient to promote growth and health. Recent studies have introduced novel bacteria and yeast single-cell protein (SCP) sources. Technology-enhanced SCP production allows manufacturing of these protein sources in large quantities with high levels of purity and consistency [9]. SCP appears to be an excellent fish meal alternative in several food fish and other aquatic species, indicating SCP can serve as a sufficient replacement of typical fish protein sources [10,11]. SCP appears free of anti-nutritional factors, making SCP a higher value protein source than many derived from plants. To evaluate the value of SCP as a standardized fish diet, our study compares health outcomes (survival, weight gain, adiposity, and reproductive success) when replacing typical fish diet protein (fish meal) for a bacterial SCP source. Furthermore, we employed transcriptomic analysis to interrogate whether substitution of SCP into the diet leads to changes in gene expression within the liver that could indicate positive or negative influences of SCP on metabolic health. Results from this study will assist in the creation of defined open-source diets for the zebrafish community.
Methods
Experimental housing and husbandry
All procedures were approved by the University of Alabama Insitutional Care and Use Committee (UAB IACUC) and adhere to standard D. rerio husbandry requirements for housing and euthanasia. D. rerio embryos (AB strain) were randomly collected from a mass spawning of males and females. Embryos were transferred to Petri dishes (n = 50 per dish) and incubated at 28.5°C until 5 d postfertilization (dpf). At 5 dpf, hatched larvae were polycultured in 6-L static tanks (n = 100 larvae per tank) with the rotifer Branchionus plicatilis at a salinity of 4 ppt, and enriched with Nannochloropsis (RotiGrow Omega, Reed Mariculture). At 11 dpf, all tanks were fed Artemia until 28 dpf. At 28 dpf, all 6-L tanks were combined, and D. rerio were randomly distributed into 2.8-L tanks at n = 14 fish per tank. Each tank was then randomly assigned to one of the dietary treatments (n = 10 tanks per treatment) and the 16-wk feeding trial was initiated. To obtain initial weights and lengths, a subsample of D. rerio (n = 50) were individually weighed and photographed before experiment implementation. For the first 2 wk of the trial, D. rerio were provided a ratio of 10% of the initial body weight per day of powdered feeds. Daily ratios were weighed for individual tanks. Rations were adjusted based on weight gain and food conversion ratios every 2 wk. D. rerio were fed at 09:00 and 17:00 each day.
All tanks were maintained at ∼28°C and 1500 μS/cm conductivity in a commercial recirculating system (Aquaneering), with 5.4 L exchanged from each tank per hour. Municipal tap water was passed through mechanical filtration (5 μm sediment filter and charcoal), reverse osmosis, and a cation/anion exchange resin. Synthetic sea salts (Crystal Sea, Marine Enterprises International) were added to adjust conductivity for the system water source. Sodium bicarbonate was added as needed to maintain the pH of the system water at 7.4. Total ammonia nitrogen, nitrite, and nitrate were measured colorimetrically once weekly. To help sustain adequate water quality, a minimal water exchange of 20% was performed on the recirculating system once per week. Tanks were maintained on the same recirculating system throughout the duration of the experiment. To reduce environmental confounding effects from noise, light, vibration, or other unidentified sources, tanks were cleaned and returned to a new position on the recirculating rack system every 2 wk. Tanks were siphoned every other day to remove any excess uneaten feed or debris. Experimental animals were maintained under a 14-h light/10-h dark cycle with lights turned on at 07:00 local time. At the end of the study, D. rerio were killed by rapid submersion in ice-cold water for a minimum of 10 min after opercular motion had ceased. Carcasses were stored at −80°C until analysis.
Diet preparation
Each diet contained cholesterol, menhaden oil, corn oil, vitamin (custom vitamin mixture MP Biomedicals) and mineral premixes (MP Biomedicals 290284), and alginate binders (ingredients and catalog numbers listed in Table 1). Protein sources were casein (MP Biomedicals, Cat. No. 0296012805) and either fish protein hydrolysate (Cat. No. CPSP90, Scoular) or SCP sources provided by Meridian Biotech (The Woodlands,) consisting of bacteria (composition in TABLE 2, TABLE 3). Diets were identical in macronutrient profiles and ingredients were adjusted to provide comparable lipid (n6:n3 ratio) and total amino acid content (Table 4). Protein ingredient adjustment by amino acid content was done instead of crude protein because of the large non-protein nitrogen content of the BP source. All ingredients were weighed on an analytical balance (Mettler-Toledo New Classic MF Model MS8001S or Model PG503-S Mettler-Toledo, LLC.) and mixed using a Kitchen Aid Professional 600 Orbital Mixer (Kitchen Aid). The diets were cold extruded into strands to preserve nutrient content using a commercial food processor (Cuisinart) and strands were air-dried for 24 h on wire trays. Diets were labeled as standard reference diet (SR diet that contains fish protein hydrolysate) and bacterial protein diet (BP diet, bacterial SCP-containing diet).
TABLE 1.
Ingredient vendors and catalog numbers
| Ingredient | Vendor | Catalog number |
|---|---|---|
| Fish protein hydrolysate | The Scoular Company | CPSP90 |
| Dextrin type III | MP Biomedicals | 0216005790 |
| Mineral mix AIN93G | MP Biomedicals | 0296040002 |
| Casein low-trace metals | MP Biomedicals | 0296012805 |
| Soy protein isolated | MP Biomedicals | 0290545605 |
| Corn oil | MP Biomedicals | 0290141401 |
| Safflower oil | MP Biomedicals | 0210288890 |
| Menhaden fish oil | Omega Protein | Virginia Prime Gold |
| Vitamin diet fortification mixture | MP Biomedicals | 0290465401 |
| Diatomaceous earth, acid washed | Andwin Scientific | D3877 |
| Alphacel non-nutritive bulk | MP Biomedicals | 0290045305 |
| D-(+)-glucosamine hydrochloride | MP Biomedicals | 0210178225 |
| Cholesterol NF | MP Biomedicals | 02101380-CF |
| Lecithin, soy, refined | MP Biomedicals | 0210214790 |
| Ascorbyl palmitate | MP Biomedicals | 0210078180 |
| Potassium phosphate monobasic | MP Biomedicals | 02195453.5 |
| Wheat starch | MP Biomedicals | 0290295225 |
| Alginate | TIC Gums | TICA-Algin 400 |
| Betaine | MP Biomedicals | 150461 |
| Canthaxanthin | DSM | Carophyll Red |
TABLE 2.
Composition of diets used for feeding trial
| Ingredients (g/kg) | SR | BP |
|---|---|---|
| Casein—low-trace metals | 350.00 | 350.00 |
| Fish protein hydrolysate | 200.00 | 0.00 |
| MRD Pro Batch 2 | 0.00 | 317.90 |
| Wheat starch | 56.50 | 56.50 |
| Dextrin type III | 16.10 | 16.10 |
| Alpha cellulose | 10.00 | 10.00 |
| Diatomaceous earth | 125.70 | 0.00 |
| Menhaden fish oil (ARBP) Virginia Prime Gold | 26.00 | 39.00 |
| Safflower oil | 45.50 | 40.30 |
| Alginate | 20.00 | 20.00 |
| Soy lecithin (refined) | 40.00 | 40.00 |
| Vit Pmx (MP Vit Diet Fortification Mixture)1 | 40.00 | 40.00 |
| Mineral Pmx aka premix (AIN 93G)2 | 30.00 | 30.00 |
| Canthaxanthin (10%) | 23.10 | 23.10 |
| Potassium phosphate monobasic | 11.50 | 11.50 |
| Glucosamine | 2.50 | 2.50 |
| Betaine | 1.50 | 1.50 |
| Cholesterol | 1.20 | 1.20 |
| Ascorbylpalmitate | 0.40 | 0.40 |
| Total | 1000.00 | 1000.00 |
Abbreviations: BP, bacterial protein; SR, standard reference.
MP Biomedicals 904654: Vitamin A Acetate (500,000 IU/gm) 1.80000, Vitamin D2 (850,000 IU/gm) 0.12500, DL-a-Tocopherol Acetate 22.00000, Ascorbic Acid 45.00000, Inositol 5.00000, Choline Chloride 75.00000, Menadione 2.25000, p-Aminobenzoic Acid 5.00000, Niacin 4.25000, Riboflavin 1.00000, Pyridoxine Hydrochloride 1.00000, Thiamine Hydrochloride 1.00000, Calcium Pantothenate 3.00000, Biotin 0.02000, Folic Acid 0.09000, and Vitamin B12 0.00135, measures are mg/g.
AIN 93 mineral mix for Envigo: Sucrose, fine ground 209.496, Calcium Carbonate 357.0, Sodium Chloride 74.0, Potassium Phosphate, monobasic 250.0, Potassium Citrate, monohydrate 28.0, Potassium Sulfate 46.6, Magnesium Oxide 24.3, Manganese Carbonate 0.63, Ferric Citrate 6.06, Zinc Carbonate 1.65, Cupric Carbonate 0.31, Potassium Iodate 0.01, Sodium Selenite 0.0103, Chromium Potassium Sulfate, dodecahydrate 0.275, Lithium Chloride 0.0174, Boric Acid 0.0815, Sodium Fluoride 0.0635, Nickel Carbonate Hydroxide, tetrahydrate 0.0318, and Ammonium Meta-Vanadate 0.0066 measures are mg/g.
TABLE 3.
Amino acid content of protein sources (as fed)
| Fish protein hydrolysate | MRD Pro Batch 2 | Casein | |
|---|---|---|---|
| Aspartic acid | 6.09 | 4.69 | 5.07 |
| Threonine | 2.16 | 1.55 | 4.10 |
| Serine | 3.29 | 1.58 | 5.28 |
| Glutamic acid | 9.72 | 6.96 | 16.50 |
| Proline | 4.37 | 1.98 | 7.78 |
| Glycine | 7.68 | 2.85 | 1.17 |
| Alanine | 5.10 | 4.81 | 2.02 |
| Cystine | 1.17 | 0.29 | 2.80 |
| Valine | 3.88 | 3.18 | 5.51 |
| Methionine | 2.12 | 0.96 | 2.63 |
| Isoleucine | 2.92 | 2.31 | 3.93 |
| Leucine | 5.66 | 4.02 | 7.75 |
| Tyrosine | 5.26 | 1.60 | 6.36 |
| Phenylalanine | 3.06 | 2.14 | 4.14 |
| Lysine | 6.20 | 2.86 | 6.19 |
| Histidine | 1.71 | 0.64 | 3.18 |
| Arginine | 5.99 | 2.04 | 2.75 |
| Tryptophan | 0.70 | 0.70 | 1.25 |
Numbers represent percent of each amino acid in each protein source with moisture present.
TABLE 4.
Macronutrient and energy content
| SR | BP | |
|---|---|---|
| Calculated protein level by amino acids (%) as fed | 45.44 | 45.44 |
| Calculated protein level by amino acids (%) dry | 50.49 | 50.49 |
| Calculated lipid level (%) as fed | 11.04 | 10.98 |
| Calculated lipid level (%) dry | 12.27 | 12.20 |
| Calculated soluable digestible carbohydrate level (%) as fed1 | 17.13 | 25.83 |
| Calculated energy level (cal/g) as fed | 4326 | 4714 |
| Protein:Energy ratio as fed2 | 0.645 | 0.660 |
| Ash (%) as fed | 15.06 | 5.91 |
| Fiber (%) as fed | 1.33 | 1.83 |
Abbreviations: BP, bacterial protein; SR, standard reference.
Calculation used for soluble digestible carbohydrate: carbohydrate = 100 – (moisture % + protein % + lipid % + ash % + fiber %).
Calculation used for Protein:Energy ratio: Energy from protein (cals)/Energy from lipid, soluble digestible carbohydrate, and protein (cals).
Growth and body composition parameters
After random assignment of tanks to the dietary treatments, D. rerio were weighed together as a group from each treatment tank to 0.001 g and photographed from above in a clean 1-L breeding tank using a D70 camera (Nikon) every 2 wk throughout the experiment. At the termination of the feeding trial, all D. rerio were sexed and weighed individually to 0.001 g and photographed. All photographs were analyzed with NIS Elements 3.1 software to determine the standard length (measured from the tip of the snout to the distal end of the caudal peduncle) to 1 mm. Total lipid for females (n = 10) and males (n = 9–10) of each diet was determined using the Folch lipid extraction protocol optimized for D. rerio.
Egg production and viability
At the end of the 16-wk feeding trial, males and females from each diet treatment were separated, placed in different 2.8-L tanks at n = 10, and maintained on the treatment diets for an additional 4 wk for breeding analysis. Maintenance conditions and feeding regime continued as described. For each diet, egg production and embryo viability [at 4 and 24 h postfertilization (hpf)] were assessed. Females and males were randomly selected from each tank and paired with Artemia-fed females and males from the UAB Aquatic Animal Resource Core. Breeding pairs (1 male and 1 female) were transferred to 1-L breeding tanks (Aquaneering) with a divider separating the pair on the evening before breeding. Dividers were removed when the lights were turned on the next morning for a 2-h period of spawning, after which each male and female were returned to their respective tanks. Successful spawning was recorded and females from unsuccessful spawning events were removed from the study and killed as described below. Immediately after spawning, eggs/embryos from successful breeding pairs were collected, cleaned, counted, and scored as viable embryos or non-viable eggs. After counting, viable embryos were divided into Petri dishes (n = 50) and incubated overnight at 28.5°C in fresh Embryo Medium (1500 μS/cm conductivity). At 24 hpf, viable embryos were counted and assessed for normal development based on their morphology. The 10 random breeding pairs for each diet were set up once every other week for 4 wk with females bred twice and males once, resulting in 17–20 total breeding events per diet for females and 9–10 breeding events for males.
RNA isolation
At termination of the feeding trial, livers from 5–7 males and 8 females from each dietary treatment were dissected out, flash frozen in nitrogen and transferred to −80°C for storage. Subsequently, RNA was isolated from these livers using RNeasy Lipid Tissue Mini Kit (Qiagen) per the manufacturer’s instructions. Purified RNA was subjected to quantification and purity assessment via NanoDrop.
RNA sequencing and analysis
Four female liver RNA samples from each dietary treatment were sent to the UAB Genomics Core Laboratory, Heflin Center for Genomic Sciences. From this RNA, poly-A selected indexed RNA libraries were prepared using the Ultra II RNA Library Prep kit and sequenced on the Illumina NextSeq 500 platform to achieve a minimum of 30 million, single end, 75 bp reads per sample. Bioinformatic analysis of the RNA sequencing reads was evaluated for sequence quality using FastQC (ver. 0.11.8). On average, the Phred quality score across samples was >35 indicating a high level of confidence in the accuracy of individual base calls (>99.9%). Next, the sequences were aligned to the Ensembl D. rerio reference genome (GRCz11) and individual gene counts were obtained for each sample using the quantMode feature of the STAR aligner (ver. 2.5.2b). Differential expression analysis was performed using DESeq2 (ver. 1.22.2) and methods similar to those published previously [12]. Gene Ontology (GO) analysis (http://geneontology.org, accessed 24 March, 2023) analysis was performed on differentially expressed genes (DEGs) determined via DESeq2 (ver. 1.22.2) [[13], [14], [15]]. The DeSeq analysis was run utilizing the default setting and produced a full list of genes that can be found in Supplemental Data 1. The data were filtered to obtain a concise list of highly DEGs, using the following criteria: BaseMean > 500, log2FC > |1.5| and a P value < 0.1. A number of DEGs with a BaseMean < 500 and relating to oocyte biology and oocyte metabolism were observed and these are attributed to potential oocyte contamination during liver dissections. The liver and oocytes in female fish are proximal to each other anatomically. Filtering based on the parameters discussed above.
rtPCR
For rtPCR analysis, a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used to synthesize cDNA as per the manufacturer’s instructions with a starting amount of 5 μg of total RNA in a 100 uL reactions run on a SimpliAmp™ Thermal Cycler (Applied Biosystems). A volume of 5 uL of cDNA was diluted to 1:20 and used for a 20 uL total reaction using TaqMan™ Fast Advanced Master Mix (Applied Biosystems) and MicroAmp™ Fast Optical 96-Well Reaction Plates (Applied Biosystems). Gene-specific TaqMan primers were purchased from Applied Biosystems and were designed by the manufacturer (b2m-Dr03432699_m1, rpl7-Dr03114687_g1, ldlra-Dr03109730_m1, pdia4-Dr03080709_m1, hspa5-Dr03107861_m1, and hmgcs1-Dr03107117_m1). A total of 40 rtPCR cycles were run on a QuantStudio 3 Real-Time PCR System and results were analyzed with QuantStudio™ Design & Analysis Software v1.5.1 using (Applied Biosystems). b2m and rpl7 as were used for normalization by a geometric average [16].
Statistical modeling and analysis
Data from this study were analyzed with RStudio Statistical Software (R Core Team, 2016, v0.99.896), and graphs generated with Statistical Package for Social Science (SPSS) ver.2.3 (IBM). All data were analyzed for normality and equal variances. Any datasets with a non-normal distribution were log-transformed. All terminal analyses for continuous outcomes were stratified by sex. Terminal wet body weight and total body length were compared separately by linear random effects model with tank as a random variable. Total body moisture was analyzed by analysis of variance, and fat mass was analyzed with analysis of covariance, adjusting for dry body weight as a covariate. Since unequal variances were observed in rtPCR results, these data were analyzed using 1-tailed Welch's unequal variances t-test to validate RNAseq. For total embryos produced, previous examination of similar datasets revealed overdispersion with excessive truncated zeroes (non-successful breeding events), indicating that these data were well suited for a hurdle negative binomial model [17]. Data for total embryo production were fitted to a hurdle negative binomial model with the help of the pscl package of the R language [18]. Diet and week of breeding were included as predictors in the model and analyzed for main effects on total embryo production. The outcome for embryo viability is a proportion between 0 and 1, with 2 types of zeroes present: truncated (non-successful breeding events) and sampling (0 viable embryos produced). For this reason, a zero-inflated beta regression model (BEZI) is selected as the most appropriate model. The first component of the BEZI model uses logistic regression and the parameter nu (controls the probability that a 0 occurs) to analyze the 0 counts and determine the probability of 0 viable embryos produced. The second component analyzes the positive counts by fitting a beta regression to compare the expected proportion of viable embryos and includes the parameters mu (mean) and sigma (variance) (John Dawson, Deptartment of Biostatistics, personal communication). The best-fit model usually includes all 3 parameters and is selected with the help of the GAMLSS package of the R language [19].
Results
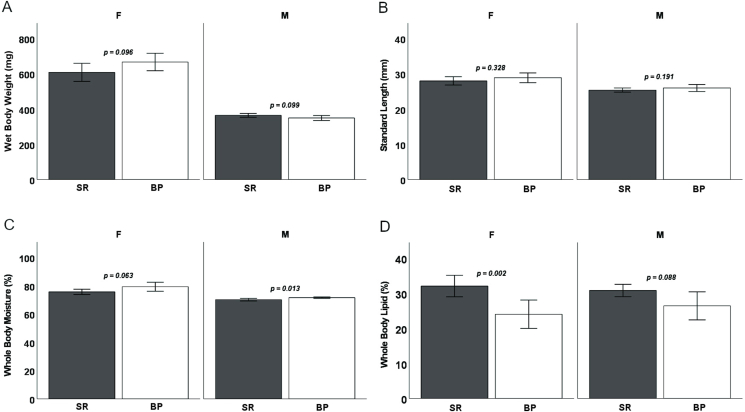
Both diets sustained D. rerio growth and development over the 16-wk feeding trial (Figure 1). For terminal wet body weights, when the sexes were separated, males and females showed no differences in terminal body weight (P = 0.099 and P = 0.096, respectively) (Figure 2A). Male and female D. rerio fed the SR and BP diets showed no significant differences in standard body length (P = 0.191 and P = 0.328, respectively) (Figure 2B). Females had no significant differences in total body moisture (P = 0.063), but males fed the BP had a higher total body moisture compared with males fed the SR diet (P = 0.0129) (Figure 2C). For total body lipids, female D. rerio fed the BP diet had less adiposity than D. rerio fed SR (P = 0.002), but males show no significant difference (P = 0.088) (Figure 2D). In regard to reproduction, there were no differences in spawning success for males and females fed the diet treatments paired with the opposite sex fed Artemia (P = 0.900 and P = 0.597, respectively; Table 5). Total egg production between female D. rerio fed the SR and BP diets was not significantly different (P = 0.597, Figure 3A). Eggs fertilized by males and females fed the BP diet had no significant differences in viability than those fed the SR diet at either 4 hpf (P = 0.774 and P = 0.838, respectively) (Figure 3B) or 24 hpf (P = 0.284 and P = 0.850, respectively) (Figure 3C). For female egg viability, the first or second breeding event had no impact on egg viability at either 4 or 24 hpf (P = 0.598 and P = 0.199, respectively).
FIGURE 1.
Total body weight average for tanks of fish (mg) for male and female zebrafish (combined) fed either BP (black line) or SR (dashed line) protein diets measured every 2 wk from week 2 to week 14 on the assigned diets (n = 10 tanks, 14 fish per tank for each diet treatment). P value represents linear regression of the trend line. BP, bacterial protein; SR, standard reference.
FIGURE 2.
Terminal wet body weight (mg; A), standard length (mm; B), total body moisture (%; C), and dry body lipid (%; D) averages for individual fish for male and female zebrafish (separated) measured at termination at 14 wk on the assigned diets (n = 10 tanks, 14 fish per tank for each diet treatment).
TABLE 5.
Male and female breeding events
| Success breeding | Attempted breeding | |
|---|---|---|
| Male | ||
| SR | 3 | 9 |
| BP | 3 | 10 |
| Female | ||
| SR | 12 | 20 |
| BP | 7 | 20 |
Abbreviations: BP, bacterial protein; SR, standard reference.
Attempts are pairings of males from the diet study with stock females or females from the diet study with stock males. Success breeding represents bred pairs that resulted in eggs being released.
FIGURE 3.
Total egg produced (A), egg viability at 4 hpf (B), and egg viability at 24 hpf (C) averages for breeding of female zebrafish after 14–18 wk on the assigned diets crossed with males fed Artemia (n = 20 breeding events for female of the dietary treatments with a 2-wk gap).
To evaluate molecular changes specifically associated with a complete substitution of fish protein hydrolysate (SR diet) with bacterial SCP (BP diet), we assessed changes in liver transcriptomics of female D. rerio using RNAseq. Principal component analysis of the regularized log-transformed count values (rlog) for each sample revealed that the majority of the biological replicates within an individual diet clustered together, and the differences between the diets were explained by the variance observed across principal component 2 (PC2, 15%). Two samples from each condition exhibited unusual variance across PC1 (72%); however, because these 2 samples clustered with their diet as expected across PC2, they were not excluded from the analysis (Figure 4). Comparing the normalized gene expression between D. rerio fed either the SR or the BP diet revealed 267 DEGs (BaseMean > 500, log2FC > |1.5|, and a P value < 0.1). The top 10 up- and downregulated genes are presented in Figure 5. Using GO analysis on all 267 DEGs, we determined key biological pathways associated with these genes. The top 15 pathways were selected based on the largest fold enrichment, and a false discovery rate (FDR) < 0.05 (Figure 6). A complete table of all GO pathways outputted is presented in Supplemental Data 2. We found that the majority of these 267 DEGs clustered into major biological processes such as terpenoid biosynthetic process (GO: 0016114 and FDR = 2.35E-02) (gene ratio 3/11), secondary alcohol biosynthetic process (GO: 1902653 and FDR = 7.21E-04) (gene ratio 5/22), sterol biosynthetic process (GO: 0016126 and FDR = 1.74E-05) (gene ratio 7/32), steroid biosynthetic process (GO: 0006694 and FDR = 6.86E-04) (gene ratio 7/66), and cellular response to unfolded protein (GO: 0034620 and FDR = 9.13E-04) (gene ratio 6/45). Our GO analysis included several lipid-related pathways, which may reflect the decrease in adiposity observed among female D. rerio fed the BP diet. These included sterol biosynthetic bioprocess (GO: 0016126 and FDR = 1.74E-05) (gene ratio 7/32), cellular response to lipid (GO: 0071396 and FDR = 3.65E-03) (gene ratio 7/159 fatty acid metabolic process (GO: 0006631 and FDR = 1.30E-02) (gene ratio 5/57), sterol metabolic process (GO: 0016125 and FDR = 1.69E-04) (gene ratio 7/32), lipid metabolic process (GO: 0006629 and FDR = 2.50E-06) (gene ratio 28/873), fatty acid catabolic process (GO: 0009062 and FDR = 2.16E-02) (gene ratio 5/57), and lipid biosynthetic process (GO: 0008610 and FDR = 9.25E-04) (gene ratio 15/415). Genes involved in these pathways can be seen in Supplemental Data 3 (Figure 7).
FIGURE 4.
Principal component analysis (PCA) among samples, red dots represent the female D. rerio fed with the fish protein hydrolysate diet [standard reference (SR)], and the blue dots represent the female D. rerio fed with the single-cell bacterial protein (BP) diet. The PCA plot was generated via DeSeq2 (ver. 1.22.2).
FIGURE 5.
The top 10 upregulated and top 10 downregulated differentially expressed genes (DEGs). The genes were selected after differential expression analysis (DeSeq2), and were filtered based on a BaseMean > 500, log2FC > |1.5| and a P value < 0.1.
FIGURE 6.
Top 15 most significantly enriched gene ontologies (Gene Ontology, released 3 January, 2023) based on the highest fold enrichment, and a FDR < 0.05, were plotted in R, using ggplot2 (v3.4.0). FDR, false discovery rate.
FIGURE 7.
Genes associated with lipid-related pathways determined via GO analysis. These pathways were: “sterol biosynthetic bioprocess,” “response to lipid,” “fatty metabolic process,” sterol metabolic process,” “lipid metabolic process,” “fatty acid catabolic process,” and “lipid biosynthetic process.” Genes with a log2foldchange > |1.5|, and a P value < 0.1 were plotted via a ggplot2 (v.3.4.0). GO, gene ontology.
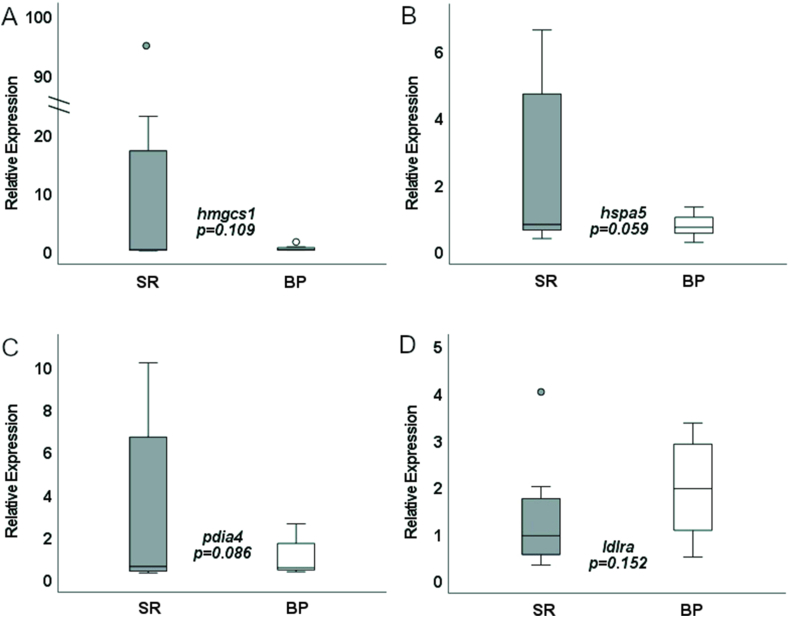
Validation of the RNAseq data was performed using quantitative PCR and tested DEGs related to 2of the major GO pathways: cholesterol homeostasis genes hmgcs1 and ldlra, and the unfolded protein response genes hspa5 and pdia4 (FIGURE 8, FIGURE 9). The expression levels of hmgcs1, pdia4, and hspa5 in female D. rerio given either the SR or BP diet all trended in the same direction as seen in the transcriptomic analysis with decreased expression of all 3 genes in the BP-fed females compared with the SR-fed females. However, sample variation was high and these differences in expression were only significant at an alpha near 0.1 (P = 0.109, 0.059, and 0.086, respectively). Expression of ldlra in female livers again was in the same direction as detected by RNAseq with increased expression in BP-fed females compared with SR-fed females, yet high sample variation led to differences that were not significant (P = 0.152). Notably, unlike the transcriptomic and quantitative PCR results observed in female D. rerio, expression of hmgcs1, pdia4, and ldlra were not differentially expressed in male D. rerio fed the same diets suggesting an impact of diet by sex effects (P = 0.217, 0.172, and 0.303, respectively). Male hspa5 expression difference was not in the same direction as what was seen in the transcriptome analysis and was therefore excluded from the 1-tailed analysis.
FIGURE 8.
rtPCR liver expression of SR- and BP-fed females (n = 8 livers). BP, bacterial protein; SR, standard reference.
FIGURE 9.
rtPCR liver expression of SR- and BP-fed males (n = 5–7 livers). BP, bacterial protein; SR, standard reference.
Discussion
For SCPs, it is notable that the total replacement of a high-quality fish protein hydrolysate with bacterial SCP fully supported growth metrics of body mass weight gain and total length. Markers of fecundity were also fully supported by bacterial SCP. Remarkably, the relative lack of adiposity concomitant with weight gain suggests that females fed bacterial SCP maintained or increased body lean matter (fat-free mass), the majority of which is most likely protein [20]. Similar trends were observed in males. In addition to the body composition changes, in the livers of these D. rerio, we observed DEGs associated with gene ontologies related to metabolism (GO: 190263, GO: 0015804), biosynthesis of cholesterol precursors and products (GO: 0016125, GO: 0006695, GO: 0016126, and GO:0008299), and unfolded protein responses (GO: 0034620, GO: 0030968, and GO: 000692653) [13,14].
The body composition changes observed are related to gene expression changes in gene ontologies associated with metabolism. A substantial number of DEGs related to lipid metabolism were present in our most altered gene ontologies via diet in female livers. Hamidoghli et al. [21] evaluated the impact of bacterial SCP in replacing fish meal in whiteleg shrimp Litopenaeus vannamei, and observed similar body composition outcomes, including an increased crude protein and decreased crude lipid in tail muscle after a 9-wk feeding trial. Replacement of fish meal with bacterial SCP in the rainbow trout Oncorhynchus mykiss resulted in increased weight gain over a 60 d feeding trial at a 50% replacement, but limited weight gain at 100% replacement (which authors attributed to a reduced feed intake related to palatability) [22]. Muscle analysis of the 100% SCP replacement fed O. mykiss correlated with decreased crude protein, lipid content, and n3:n6 fatty acid ratio. In addition, SCP inclusion decreased enzyme activity related to digestion, including bile salt-activated lipase, which is important for TAG (triglycerides) and cholesterol absorption. These metabolic changes these authors observed could potentially have contributed to observed differences in body composition. The Nile tilapia Oreochromis niloticus fed diets with yeast SCP replacement of fish meal had decreased carcass lipid content with no differences in weight gain or carcass protein in a 12-wk feeding trial [23]. Consequently, these data suggest that changes in gene expression related to lipid metabolism are associated with decreased whole-body lipid content without weight (fat-free mass) loss, and are a positive attribute of the inclusion of SCP products. Future studies should also include metabolic profiles associated with other tissues including the adipose and skeletal muscle.
Several of the gene ontologies altered by the SCP diet are related to cholesterol metabolism. SCPs of bacterial origin have been reported to have prebiotic and probiotic properties, both of which influence cholesterol homeostasis and related genes via bile acid biosynthesis pathways [24,25]. Hmgcs1, which was down regulated in livers of female D. rerio fed the bacterial SCP, is a part of the cholesterol biosynthesis pathway in D. rerio and other animals [26]. Oczkowicz et al. [27] found that pigs fed a SCP derived from corn-dried distiller’s grains exhibited a decrease in expression of hmgcs1 (mammalian hmgc1 is homologous in D. rerio [28]). ldlra was upregulated in female D. rerio fed the SCP diet, and is responsible for the uptake of LDL cholesterol particles from blood circulation into organ tissues [29]. We hypothesize upregulation of ldlra will lower cholesterol in blood circulation in D. rerio and this may be compensatory to lower de novo cholesterol production.
SCPs contain beta-glucan, a potent prebiotic. Beta-glucan decreased circulating total cholesterol, HDL, LDL, and triglycerides and increased the HDL:total cholesterol ratio in rats [30]. Rats provided beta-glucan or spent brewers yeast diets also had lower liver total cholesterol with no differences in weight from rats fed a standard commercial feed. Carneiro et al. [31] replaced dietary fish meal (5% of the total diet) for D. rerio with a SCP composed of microalgae. This resulted in higher body weight gain over a 60-d feeding period with lower triglycerides, LDL, and total cholesterol and increased HDL. Combined, these studies suggest that D. rerio is a novel model for the study of cholesterol metabolism and its impact on liver health, and could be an excellent model for developing preclinical treatment and preventative strategies such as those provided by the use of statins [32]. Continued use of the D. rerio model can have a profound impact on our understanding of macronutrients in regulating health benefits.
In addition to changes in lipid and cholesterol metabolism, the expression of endoplasmic reticulum unfolded protein response genes was also influenced by the inclusion of SCP in the diet. Unfolded protein responses are highly conserved among mammals and teleost species [33]. Pdia4 and hspa5 exhibited decreased liver expression in female D. rerio fed the bacterial SCP. Pdia4 modulates inflammatory responses related to insulin signaling in a mouse model of genetic insulin resistance fed a high-fat diet [34]. Hspa5 expression in D. rerio was increased on a high-fat diet and high-fat diets with supplemented cholesterol [32]. The expression changes were concomitant with increased TAG and free cholesterol in the liver. Pdia4 and hspa5 have also been shown to have increased liver expression in mice with normal insulin sensitivity on a high-fat diet [35,36]. The altered expression of genes in this unfolding protein response ontology suggests that the bacterial SCP diet impacts endoplasmic reticulum (ER) stress commonly seen with obese phenotypes [37]. Future work is needed to determine lipid and cholesterol metabolism changes and their relation to animal health and SCP diets.
As suggested previously, bacterial SCP is an effective substitute for fish protein hydrolysate and may positively influence physiological outcomes because of its comparable amino acid content. In addition, bacterial SCP production is cost effective compared with live harvest of fish meal sources. Bacterial SCP may also function as a prebiotic and/or probiotic. We speculate that physiological and transcriptional effects measured in the current study are corroborated in the gut microbiome. It is possible that dietary effects are mediated by the gut microbiome, exerting microbial influences through altered nutrient processing, allocation, and signaling. Future work will focus on the overall understanding of the interactions of the dietary macronutrients, the microbiome and resulting metabolome, resource partitioning, and the gut–brain signaling axis. The zebrafish model shows great promise in elucidating changes in fundamental metabolic networks underlying dietary influence on tissue and organismal health. These data put us one step closer to the goal of establishing alternative protein ingredients for open-source diets for use in the D. rerio model, increasing the utility of this species as a preclinical research model.
Author contributions
The authors’ responsibilities were as follows – MBW: was involved in the initial inception of the project and wrote the primary draft of the manuscript, provided statistical analysis, creation of figures and tables, conducted lipid extractions, animal dissection, RNA extraction and rtPCR, and assisted in animal care; GBHG: manuscript creation, editing, RNAseq analysis, created associated figures, and data management; JWP: performed the RNAseq analysis and created associated figures; CXF: RNAseq analysis, created associated figures, data analysis, and manuscript editing; SBC: performed primary care of animals, and animal dissection; ALL: contributed to initial diet formulation and experimental design; RJB: contributed to the formulation and production of the diets; MLH, MLP: provided expertise in experimental design and manuscript composition; SAW: provided mentorship, project supervision, and manuscript writing assistance; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This study was supported by Meridian Biotech, the NIH STTR R41 DK131698-01 to SAW, and the UAB NORC Lab Animal Nutrition Core (P30DK056336).
Data availability
Bulk RNA sequencing datasets of D. rerio samples are publicly available on the BioSample Submission Portal (https://www.ncbi.nlm.nih.gov/bioproject/) under the BioProject ID PRJNA973118. Additional data sets are available from authors upon request.
Acknowledgments
We acknowledge the participation of members of the Watts laboratory at UAB for assistance during this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdnut.2023.102057.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Choi T.-Y., Choi T.-I., Lee Y.-R., Choe S.-K., Kim C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021;53(3):310–317. doi: 10.1038/s12276-021-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landis S.C., Amara S.G., Asadullah K., Austin C.P., Blumenstein R., Bradley E.W., et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler L.A., Williams M.B., Dennis-Cornelius L.N., Farmer S., Barry R.J., Powell M.L., et al. Influence of commercial and laboratory diets on growth, body composition, and reproduction in the zebrafish Danio rerio. Zebrafish. 2019;16(6):508–521. doi: 10.1089/zeb.2019.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes H., Peres H., Carvalho A.P. Dietary protein requirement during juvenile growth of zebrafish (Danio rerio) Zebrafish. 2016;13(6):548–555. doi: 10.1089/zeb.2016.1303. [DOI] [PubMed] [Google Scholar]
- 6.Young V.R., Pellett P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J.Clin. Nutr. 1994;59(5):1203S. doi: 10.1093/ajcn/59.5.1203S. –1212S. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes-Appelgren P., Opazo R., Barros L., Feijoó C.G., Urzúa V., Romero J. Effect of the dietary inclusion of soybean components on the innate immune system in zebrafish. Zebrafish. 2014;11(1):41–49. doi: 10.1089/zeb.2013.0934. [DOI] [PubMed] [Google Scholar]
- 8.Chikwati E.M., Venold F.F., Penn M.H., Rohloff J., Refstie S., Guttvik A., et al. Interaction of soyasaponins with plant ingredients in diets for Atlantic salmon, Salmo salar L. Br. J. Nutr. 2012;107(11):1570–1590. doi: 10.1017/S0007114511004892. [DOI] [PubMed] [Google Scholar]
- 9.Drejer A., Ritschel T., Jørgensen S.B., Jørgensen J.B. Economic optimizing control for single-cell protein production in a U-loop reactor. Comput. Aided Chem. Eng. 2017;40:1759–1764. [Google Scholar]
- 10.Jones S.W., Karpol A., Friedman S., Maru B.T., Tracy B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020;61:189–197. doi: 10.1016/j.copbio.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Williams M.B., Lawrence A.L., Chehade S.B., Yuan Y., Fowler A.L., Barry R.J., et al. Zebrafish (Danio rerio) exhibit positive growth profiles when fed dietary yeast and bacterial-based single-cell protein as a replacement for fish protein hydrolysate. N. Am. J. Aquacult. 2023;85(3):252–261. [Google Scholar]
- 12.Harris M.L., Fufa T.D., Palmer J.W., Joshi S.S., Larson D.M., Incao A., et al. A direct link between MITF, innate immunity, and hair graying. PLOS Biol. 2018;16(5) doi: 10.1371/journal.pbio.2003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi H., Muruganujan A., Ebert D., Huang X., Thomas P.D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbon S., Ireland A., Mungall C.J., Shu S.Q., Marshall B., Lewis S., et al. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009;25(2):288–289. doi: 10.1093/bioinformatics/btn615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day-Richter J., Harris M.A., Haendel M. Gene Ontology OBO-Edit Working Group, S. Lewis, OBO-Edit—an ontology editor for biologists. Bioinformatics. 2007;23(16):2198–2200. doi: 10.1093/bioinformatics/btm112. [DOI] [PubMed] [Google Scholar]
- 16.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 18.Zeileis A., Kleiber C., Jackman S. Regression models for count data in R. J. Stat. Softw. 2008;27(8):1–25. [Google Scholar]
- 19.Rigby R.A., Stasinopoulos D.M. Generalized additive models for location, scale and shape. J. R. Stat. Soc. C Appl. Stat. 2005;54(3):507–554. [Google Scholar]
- 20.Kaushik S., Georga I., Koumoundouros G. Growth and body composition of zebrafish (Danio rerio) larvae fed a compound feed from first feeding onward: toward implications on nutrient requirements. Zebrafish. 2011;8(2):87–95. doi: 10.1089/zeb.2011.0696. [DOI] [PubMed] [Google Scholar]
- 21.Hamidoghli A., Yun H., Won S., Kim S., Farris N.W., Bai S.C. Evaluation of a single-cell protein as a dietary fish meal substitute for whiteleg shrimp Litopenaeus vannamei. Fish. Sci. 2019;85:147–155. [Google Scholar]
- 22.Zamani A., Khajavi M., Nazarpak M.H., Gisbert E. Evaluation of a bacterial single-cell protein in compound diets for rainbow trout (Oncorhynchus mykiss) fry as an alternative protein source. Animals (Basel) 2020;10(9):1676. doi: 10.3390/ani10091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bob-Manuel F., Alfred-Ockiya J. Evaluation of yeast single cell protein (SCP) diets on growth performance, feed conversion and carcass composition of Tilapia Oreochromis niloticus (L.) fingerlings Afr . J. Biotechnol. 2011;10(46):9473. 7478. [Google Scholar]
- 24.Sivamaruthi B.S., Fern L.A., Ismail D.S.N. Rashidah Pg Hj, Chaiyasut C. The influence of probiotics on bile acids in diseases and aging. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110310. [DOI] [PubMed] [Google Scholar]
- 25.Rondanelli M., Opizzi A., Monteferrario F. The biological activity of beta-glucans. Minerva Med. 2009;100(3):237–245. [PubMed] [Google Scholar]
- 26.Brown A.J., Coates H.W., Sharpe L.J. Biochemistry of lipids, lipoproteins and membranes. Elsevier; 2021. Cholesterol synthesis; pp. 317–355.https://www.sciencedirect.com/science/article/pii/B9780128240489000055 [Google Scholar]
- 27.Oczkowicz M., Szmatoła T., Świątkiewicz M., Pawlina-Tyszko K., Gurgul A., Ząbek T. Corn dried distillers grains with solubles (cDDGS) in the diet of pigs change the expression of adipose genes that are potential therapeutic targets in metabolic and cardiovascular diseases. BMC Genom. 2018;19(1):1–14. doi: 10.1186/s12864-018-5265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourain V., Armant O., Lübke L., Diotel N., Rastegar S. Uwe Strähle, Multi-dimensional transcriptome analysis reveals modulation of cholesterol metabolism as highly integrated response to brain injury. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.671249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go G.-w., Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012;85(1):19. [PMC free article] [PubMed] [Google Scholar]
- 30.Waszkiewicz-Robak B., Bartnikowska E. Effects of spent brewer’s yeast and biological β-glucans on selected parameters of lipid metabolism in blood and liver in rats. J. Anim. Feed Sci. 2009;18(4):699–708. [Google Scholar]
- 31.Carneiro W.F., Castro T.F.D., Orlando T.M., Meurer F., de Jesus Paula D.A., do Carmo Rodrigues Virote B., et al. Replacing fish meal by Chlorella sp. meal: effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture. 2020;528 [Google Scholar]
- 32.Dai W., Wang K., Zheng X., Chen X., Zhang W., Zhang Y., et al. High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: a novel model for screening anti-hepatic steatosis drugs. Nutr. Metab. (Lond). 2015;12(1):1–11. doi: 10.1186/s12986-015-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa T., Taniguchi Y., Okada T., Takeda S., Mori K. Vertebrate unfolded protein response: mammalian signaling pathways are conserved in medaka fish. Cell Struct. Funct. 2011;36(2):247–259. doi: 10.1247/csf.11036. [DOI] [PubMed] [Google Scholar]
- 34.Chien-Hsing L., Chiang C.-F., Lin F.-H., Kuo F.-C., Su S.-C., Huang C.-L., et al. PDIA4, a new endoplasmic reticulum stress protein, modulates insulin resistance and inflammation in skeletal muscle. Front. Endocrinol. 2022;13:3417. doi: 10.3389/fendo.2022.1053882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collison K.S., Maqbool Z.M., Inglis A.L., Makhoul N.J., Saleh S.M., Bakheet R.H., et al. Effect of dietary monosodium glutamate on HFCS-induced hepatic steatosis: expression profiles in the liver and visceral fat. Obesity (Silver Spring) 2010;18(6):1122–1134. doi: 10.1038/oby.2009.502. [DOI] [PubMed] [Google Scholar]
- 36.Figueiredo L.S., Oliveira K.M., Freitas I.N., Silva J.A., Silva J.N., Favero-Santos B.C., et al. Bisphenol-A exposure worsens hepatic steatosis in ovariectomized mice fed on a high-fat diet: role of endoplasmic reticulum stress and fibrogenic pathways. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.118012. [DOI] [PubMed] [Google Scholar]
- 37.Cnop M., Foufelle F., Velloso L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012;18(1):59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bulk RNA sequencing datasets of D. rerio samples are publicly available on the BioSample Submission Portal (https://www.ncbi.nlm.nih.gov/bioproject/) under the BioProject ID PRJNA973118. Additional data sets are available from authors upon request.