Abstract
This study examines B-cell immunoglobulin (Ig) class-switching events in the context of parasite antigen-specific Th-cell responses in experimental African trypanosomiasis. Inbred mice were infected with Trypanosoma brucei rhodesiense, and the coordinate stimulation of Th-cell cytokine responses and B-cell responses to the trypanosome variant surface glycoprotein (VSG) was measured. The cytokines produced by T cells in response to VSG, at both the transcript and protein levels, were gamma interferon and interleukin-2 (IL-2) but not IL-4 or IL-5. Isotype profiles of antibodies specific for VSG showed that IgG1, IgG2a, and IgG3 switch responses predominated; no VSG-specific IgE responses were detected. To determine whether cryptic IL-4 responses played a role in promoting the unexpected IgG1 switch response, IL-4 knockout mice were infected; the cytokine responses and Ig isotype profiles of IL-4 knockout mice were identical to those of the wild-type control mice except for dramatically reduced IgG1 levels in response to VSG. Thus, these results revealed an IL-4-dependent component of the VSG-driven B-cell Cμ-to-Cγ1 switch. We speculate that an IL-4 response is mediated primarily by cells other than T lymphocytes since IL-4-secreting but parasite antigen-unresponsive, “background” cells were detected in all infected mice and since infected nude mice also displayed a detectable IgG1 switch response. Overall, our results suggest that B-cell clonal stimulation, maturation, and Ig class switching in African trypanosomiasis may be partially regulated by unusual mechanisms that do not include antigen-specific Th1 or Th2 cells.
B-cell responses are tightly regulated by mechanisms that control clonal expansion and cellular differentiation. Mature T-dependent B-cell responses to complex antigens normally are controlled by signals derived from antigen engagement of the B-cell receptor complex, by signals from interactions between CD40 and CD40 ligand on antigen-specific B and Th cells, and by cytokines released from antigen-activated Th cells (1, 6, 13, 19, 38, 48, 54, 58, 66, 70). Following B-cell activation, the spectrum of B-cell immunoglobulin (Ig) isotype responses that develop is regulated primarily by cytokines released by antigen-stimulated Th cells at the B-cell–T-cell interface. Classical studies of Ig isotype regulation have determined that specific subsets of cytokines direct specific Ig CH gene rearrangements that lead to isotype switching; the result is expression of different Ig isotypes within antigen-driven B-cell clones (64). Much of our knowledge of Ig class-switching events has been derived from carefully controlled and elegant in vitro studies of B cells stimulated with an array of antigens, mitogens, or other activating agents. Relatively fewer studies of Ig isotype switching have been performed in infectious-disease systems in which Th-cell and B-cell responses to defined microbial antigens have been examined or in which modulations of host immune responses occur as the result of an infection.
In the present study, we examined parasite antigen-specific antibody (Ab) isotype responses of mice infected with African trypanosomes. The plasma membrane of trypanosomes is covered by variant surface glycoprotein (VSG) homodimers that form a dense molecular surface coat (9, 71). VSG molecules are immunodominant antigens which serve as potent stimulators of the immune system, and Abs to exposed VSG determinants destroy parasites expressing a specific VSG phenotype; however, trypanosomes have the capacity to evade the host immune system by undergoing extensive antigenic variation in which different VSG genes are expressed (8, 28, 40). In addition to VSG-specific B-cell responses, Trypanosoma brucei rhodesiense-infected mice mount Th1-cell responses to VSG molecules displayed by variant antigenic types (VATs) of the LouTat 1 serodeme (28, 29, 46). These T-cell responses are mediated by αβ TCR+ CD4+ cells that recognize VSG peptides in an antigen-presenting cell-dependent and major histocompatibility complex class II-dependent manner (46). Since infected mice make prominent T-cell-dependent B-cell responses to VSG (11, 14, 26, 27, 45), the relationship between parasite antigen-driven Th-cell cytokine responses and Ab class-switching responses could be evaluated.
This study characterizes for the first time the cytokine basis for Ig class switching in African trypanosomiasis. The results reveal that unexpected B-cell Ab isotype switch responses can occur during infection in the presence of polarized Th-cell responses and suggest that cryptic cytokine responses and/or alternative Ig class-switching mechanisms may be activated during African trypanosome infections.
(Portions of this work were completed as part of the requirements for the Ph.D. degree [L. Schopf] and M.S. degree [X.-J. Bi] of the University of Wisconsin.)
MATERIALS AND METHODS
Animals.
Mice with the B10.BR and C57BL/6 genetic backgrounds are robust B-cell responders to the VSG molecule and are relatively resistant when infected with trypanosomes of the LouTat 1 serodeme (11, 14, 26, 27). For this reason, adult male B10.BR/SgSnJ and C57BL/6J mice as well as athymic nude, interleukin-4 (IL-4) knockout, and gamma interferon (IFN-γ) knockout mice with the C57BL/6 genetic background were used for experimental infections in this investigation; all the animals were obtained from The Jackson Laboratory (Bar Harbor, Maine). Outbred Swiss mice obtained from Harlan Sprague-Dawley (Madison, Wis.) were used for expanding trypanosome stabilates in vivo. All animals were housed in University-approved facilities and were handled strictly according to National Institutes of Health and University of Wisconsin Research Animal Resource Center guidelines.
Trypanosomes.
Frozen stabilates of T. b. rhodesiense VATs LouTat 1 and LouTat 1.5 were thawed and used for infection. To expand trypanosome stabilates for the purposes of establishing experimental infections, Swiss mice were immunosuppressed with cyclophosphamide (Cytoxan; 300 mg/kg of body weight [Mead Johnson and Co., Evansville, Ind.]) before being infected with the desired VAT. This treatment effectively eliminates B-cell responses to the VSG molecule and prevents immune selection for any minor VATs present (53). Trypanosomes subsequently were isolated from the blood of cyclophosphamide-treated mice by a modified procedure (25). Briefly, animals were exsanguinated from the retrobulbar sinus into heparinized tubes. The blood was diluted with PBSG (phosphate-buffered saline [PBS], 1% glucose [pH 8.0, I = 0.217]) and passed over a DEAE-cellulose column (Whatman, Clifton, N.J.) equilibrated with PBSG; under these conditions, cellular blood components adhere to the column matrix whereas trypanosomes pass through. Trypanosomes isolated in this manner were subsequently washed with PBSG by centrifugation at 1,000 × g for 10 min at 4°C and counted. Confirmation of the VSG phenotype was made by VSG-specific monoclonal Ab (MAb) typing, as we have described previously (67). To initiate experimental infections, mice received an intraperitoneal injection of 105 trypanosomes. All infections were monitored at routine intervals by examining mouse tail blood for the presence of trypanosomes.
VSG purification and characterization.
The soluble form of VSG from LouTat 1 and LouTat 1.5 was purified from viable trypanosomes by established procedures (43, 46, 67). Briefly, purified washed trypanosomes in PBSG were concentrated by centrifugation. The trypanosomes were resuspended to 109 cells/ml in PBSG, and 1-ml aliquots were incubated in siliconized tubes at 4°C overnight. The trypanosome cell suspensions were subsequently shaken at 200 rpm for 90 min at 25°C and then centrifuged at 1,000 × g for 20 min at 4°C. The resultant supernatant fluid was concentrated and dialyzed against PBS in a Centriprep-30 tube (Amicon Corp., Danvers, Mass.) by centrifugation. LouTat 1 VSG concentrates were then passed over a DEAE-cellulose column to select and purify the VSG. LouTat 1.5 concentrates were passed over an Affi-Gel (Bio-Rad, Melville, N.Y.) VSG-specific MAb affinity column equilibrated with PBS (pH 7.5); LouTat 1.5 VSG molecules were eluted with 5× PBS (pH 7.5), and the eluate was diluted to 1× PBS (pH 7.5) and concentrated by centrifugation in a Centriprep-30 tube. All VSG samples were assessed for purity by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels run under reducing conditions; a single band was seen which corresponded to an apparent molecular mass of 62 kDa for the LouTat 1 monomer or 61 kDa for the LouTat 1.5 VSG monomer.
Cell cultures.
Single-cell suspensions were prepared from the spleen (SPC), peripheral lymph nodes (LNC; mesenteric, inguinal and brachial), and peritoneum (PEC; nonelicited washout cells) of animals, at different times of infection, by routine methods and procedures described previously (7, 46, 73). Cell suspensions were made in ice-cold tissue culture medium (RPMI 1640 containing 10% fetal bovine serum, 16 mM HEPES buffer, 0.3 mg of l-glutamine per ml, and 50 μg of gentamicin per ml). Erythrocytes were removed by hypotonic lysis and passage over glass wool to remove cell debris. Cell viability, as measured by trypan blue exclusion, was routinely greater than 95% viable cells. All cell cultures were incubated at 37°C in an atmosphere of 5% CO2 in air.
Cytokine assays.
SPC, LNC, and PEC from normal and infected mice were cultured for 12 to 48 h in the presence of medium alone, concanavalin A (ConA), or VSG; in selected instances, trypanosome whole-cell extracts (WCE) prepared by sonication of sterile viable trypanosomes were added to lymphocyte cultures (30). Cytokines present in the culture supernatant fluids following stimulation were assessed by enzyme-linked immunosorbent assay (ELISA). Immulon-4 (Dynatech) ELISA plates were coated with cytokine-specific capture Ab overnight at 4°C, washed four times with PBS–0.05% Tween 20, and blocked for 30 min with PBS–2% bovine serum albumin at 37°C. After a subsequent washing step, the cytokine standards or supernatant fluids were added to the wells, which were then incubated for 2 h at 37°C or overnight at 4°C. The wells were washed four times and incubated with secondary (IFN-γ ELISA) or detector Ab (IL-2, IL-4, and IL-5 ELISAs). IFN-γ plates were washed again and incubated with detector Ab. All the ELISA plates were developed, after a final wash step, with the Vectastain ABC-AP kit (Vector Laboratories, Burlingame, Calif.) as specified by the manufacturer, and the optical density reading for each well was determined with an automated plate reader at 405 nm. Reagents for the IFN-γ ELISA were R46A2 ascites (capture Ab) and polyclonal rabbit anti-IFN-γ (secondary Ab; kindly provided by Colleen Hayes, University of Wisconsin). These reagents were used at optimal dilutions of 1:3,000 and 1:1,000, respectively. The detector Ab used was biotinylated goat anti-rabbit Ig at a 1:20,000 dilution (Sigma Chemical Co., St. Louis, Mo.). The reagents for the IL-2 and IL-4 ELISAs were obtained from Pharmingen (San Diego, Calif.); both the capture and detector Abs for each cytokine were used at 0.5 μg/ml. Additionally, the biological activity of IL-2 and IL-4 was monitored by the CTLL-2 cell stimulation assay in the presence or absence of inhibitory concentrations of MAb to IL-2 or IL-4 (S4B6 and 11B11, respectively) (7). A commercially available ELISA kit from Genzyme (Cambridge, Mass.) was used, as specified by the manufacturer, for the detection of IL-5 in culture supernatant fluids. Enzyme-linked immunospot (ELISPOT) assays were performed by standard methods (7). Different concentrations of cells from 12-h cultures in tissue culture medium, medium plus VSG, or rat anti-CD3 (Pharmingen) as described previously (47) were plated into the wells of 96-well microtiter plates containing a nitrocellulose base (Millititer, HA, Millipore Corp.); these wells were pretreated with the relevant capture Abs used above for the cytokine ELISAs. After a 20-h incubation period at 37°C under 5% CO2 in air, the cells were removed, the wells were washed, and relevant detector Abs and reagents were added as above. The cytokine spots were counted under a dissecting microscope.
Antibody isotype ELISAs.
Sera from normal and infected mice were used in Ab isotype-specific ELISAs. Immulon-4 plates were coated with purified VSG antigen at 4 μg/ml overnight at 4°C. The plates then were washed, blocked, and washed again as described for the cytokine ELISAs. A dilution series of individual serum samples from different time points of infection was added to wells in triplicate, the wells were incubated for 2 h at 37°C, and the samples were given another wash. The VSG-specific ELISA plates then were incubated with horseradish peroxidase (HRP)-conjugated rat anti-mouse IgM, IgG1, IgG2b, IgG3, or IgA (Zymed), HRP-conjugated sheep anti-mouse IgG2a (Sigma), or biotinylated rat anti-mouse IgE (provided by Colleen Hayes) for 1 h at 37°C. The plates were washed four times, the o-phenylenediamine substrate (Sigma Chemical Co.) for the HRP conjugates was added, and the assay mixture was developed for 10 min at 25°C in the absence of light. The optical density reading at 490 nm for each well was determined with an automated plate reader.
QC-PCR.
Total RNA was purified from freshly isolated and 12- to 48-h-cultured SPC, LNC, and PEC (with or without VSG or ConA) from uninfected and infected mice by using a protocol optimized for Ultraspec RNA (Biotecx Laboratories, Inc., Houston, Tex.). Single-stranded cDNA was generated from RNA by using oligo(dT) priming and avian myeloblastosis virus reverse transcriptase (Promega, Corp., Madison, Wis.) by a standard protocol. The resulting single-stranded cDNA provided a template for gene-specific quantitative competitive PCR (QC-PCR) amplification (41, 42, 51, 52) with commercial 5′ and 3′ oligonucleotide primers obtained from Clontech (Palo Alto, Calif.) or synthesized by the University of Wisconsin Biotechnology Center. The primers used were G3PDH (upper) (5′ TGA AGG TCG GTG TGA ACG GAT TTG GC 3′), G3PDH (lower) (5′ CAT GTA GGC CAT GAG GTC CAC CAC 3′), IFN-γ (upper) (5′ CAT CTT GGC TTT GCA GCT CTT CCT CAT GGC 3′), IFN-γ (lower) (5′ TGG ACC TGT GGG TTG TTG ACC TCA AAC TTG GC 3′), IL-2 (upper) (5′ ATG TAC AGC ATG CAG CTC GCA TC 3′), IL-2 (lower) (5′ GGC TTG TTG AGA TGA TGC TTT GAC A 3′), IL-4 (upper) (5′ GAG ATC ATC GGC ATT TTG AAC 3′), and IL-4 (lower) (5′ GCT CTT TAG GCT TTC CAG GAA GTC 3′). To competitively quantitate the mRNA levels, a dilution series of PCR MIMICS (Clontech) was added to the PCR amplification mixtures containing the experimental cDNA samples. The PCR MIMICS used were nonhomologous, GC-neutral DNA molecules containing relevant cytokine primer sites at each end; because the molar quantity of the competitive MIMICS at the start of the PCR reaction was known, the actual number of target DNA molecules in the cDNA preparations could be determined. To control for experimental variability, each calculation was normalized to levels of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (G3PDH), in each mRNA sample. Briefly, cDNA samples, MIMICS, reaction buffer, and PCR primers were mixed and heated at 94°C for 3 min and cooled to 40°C, AmpliTaq DNA polymerase (Perkin-Elmer, Branchburg, N.J.) was added, and the products were amplified under the following conditions: initial denaturation at 94°C for 1 min followed by 25 to 35 cycles of denaturation (94°C for 1 min), annealing (60°C for 2 min [55°C for 2 min for IL-4]), and extension (72°C for 3 min), with a final extension at 72°C for 7 min. The reaction products were evaluated by agarose gel electrophoresis (1.2% agarose) and ethidium bromide staining.
RESULTS
Th1-cell cytokine responses to VSG.
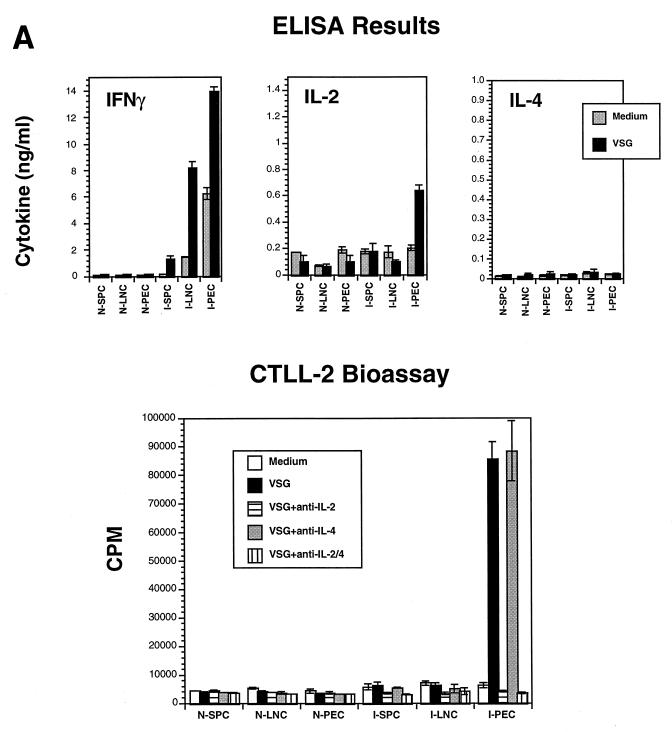
Recent studies in our laboratory showed for the first time that VSG-specific T-cell responses occur during trypanosome infections (29, 46, 47). The present study extends this work to the B-cell compartment by examining T-dependent B-cell responses to VSG in the context of VSG-specific Th-cell responses. As shown in Fig. 1, a prominent VSG-induced IFN-γ response was made during infection of mice with T. b. rhodesiense LouTat 1.5. This cytokine response was seen as early as day 4 of infection, primarily in the SPC population. As the time of infection progressed, the IFN-γ response declined in the SPC cultures but increased within the PEC and LNC compartments. VSG-induced IFN-γ responses peaked between days 7 and 14 of infection (Fig. 1A), at time points after the infecting VAT expressing the relevant VSG had been cleared, but gradually diminished thereafter to undetectable levels. Therefore, infection induced a VSG-specific T-cell response that was characterized in part by IFN-γ production; these responses, as well as the IL-2 responses (see below), were mediated by CD4+ T cells (46). At some time points of infection, significant levels of IFN-γ were spontaneously released by cells cultured in medium alone (Fig. 1A) and significant amounts of IFN-γ were detectable in the sera of infected mice (12, 46); these observations may be explained by the ongoing stimulation of Th cells by VSG as well as other trypanosome antigens. Furthermore, trypanosome-derived T-lymphocyte triggering factor (TLTF) may cause CD8+ T cells to secrete IFN-γ in an antigen-nonspecific manner during infection (3, 36, 37).
FIG. 1.
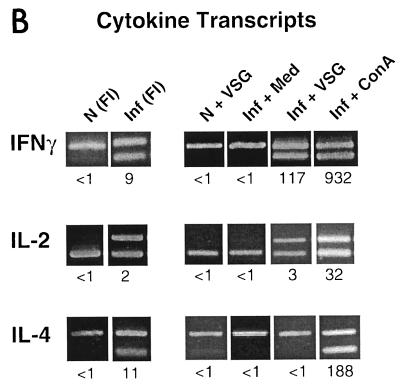
Th1-cell pattern of cytokines detectable after VSG stimulation of lymphocytes from T. b. rhodesiense LouTat 1.5-infected B10.BR mice. (A) ELISA and bioassay for cytokines secreted by SPC, LNC, and PEC harvested from control (N) and 2-week-infected (I) mice; the cells were stimulated with LouTat 1.5 VSG (10 μg/ml), and the culture supernatant fluids were harvested at 24 hr. (B) QC-PCR analysis of cytokine transcripts expressed in PEC of normal (N) or 2-week-infected (Inf) mice, either before culture (freshly isolated [FI]) or after 24-h culture stimulation with medium, LouTat 1.5 VSG (10 μg/ml), or ConA (0.5 μg/ml). The MIMICS amplification product is the upper ethidium bromide-stained band in the IFN-γ and IL-4 samples and the lower band in the IL-2 samples; the numbers represent the numbers of cytokine transcripts detectable per 1,000 G3PDH transcripts. Stimulation of cells with heterologous VSG or other unrelated antigens gave no detectable response in either the serological tests or the transcript assays. The results shown are from a single representative experiment of more than 10 separate experiments performed over a 4-year period.
Another Th1-cell cytokine, IL-2, is also produced by VSG-stimulated T cells during infection with trypanosomes (Fig. 1); however, unlike IFN-γ, IL-2 synthesis was compartmentalized largely to the peritoneal T-cell population, as we have described previously (46). Figure 1A shows IL-2 levels detectable in cultures derived from normal and LouTat 1.5-infected mice on day 14 postinfection. Only T cells from the infected-mouse peritoneal compartment produced IL-2 in response to VSG stimulation; the highest level of IL-2 was detected on day 14, as shown. The inclusion of blocking Ab to the IL-2 receptor (IL-2R) in culture medium did not reveal any differences in the observed compartmentalization; therefore, IL-2 utilization by activated cells was not a factor (data not shown). We have previously reported that the appearance of systemically emerging VATs later in infection also triggers compartmentalized IL-2 responses to VSG (46); in all results obtained to date, neither the cytokine patterns nor the Ig isotype patterns observed (below) were dependent upon the route of infection or infection with any particular VAT of the LouTat 1 serodeme.
Absence of Th2-cell cytokine responses to VSG during infection.
Lymphocyte supernatant fluids from cultures of normal and infected mice were evaluated by ELISA for Th2-cell-associated cytokines. In contrast to IFN-γ and IL-2 production, no significant IL-4 production in response to VSG stimulation was detected in SPC, LNC, or PEC populations at any time during infection; all values were below the accurate detection limits of 25 pg/ml in the ELISA (Fig. 1A). The addition of blocking Ab to the IL-4R during lymphocyte culture stimulation with VSG failed to reveal the presence in supernatant fluids of any IL-4 that may have otherwise been consumed by cells in culture (data not shown). The testing of supernatant fluids for biologically active IL-2 and IL-4 was also done in CTLL-2 bioassays; as with results from ELISAs, there was evidence for IL-2- but not IL-4-mediated stimulation of cells (Fig. 1A). These results were also confirmed by ELISPOT assays (below).
Since IL-5 secretion is also characteristic of Th2-cell responses and IL-5 is a cytokine that influences B-cell differentiation (6), cells from infected mice were examined for IL-5 production as well. However, culture supernatant fluids from VSG-stimulated cells revealed no detectable IL-5 response to VSG (all values were below 18 pg/ml [data not shown]). Additionally, VSG-inducible IL-10 is not detected during infections, although IL-10 is detectable in macrophage populations both early and late in infection, presumably as part of the normal counterregulatory response to IFN-γ activation of macrophages (data not shown). To determine if IL-4 or IL-5 responses to trypanosome antigens other than VSG were being made, lymphocytes from infected animals were cultured with WCE of heterologous VATs in which the antigenic makeup differed only in terms of the VSG. In no cultures were IL-4 or IL-5 responses to WCE detectable; however, similar to the response to purified VSG (Fig. 1A), IFN-γ and IL-2 were secreted in response to WCE.
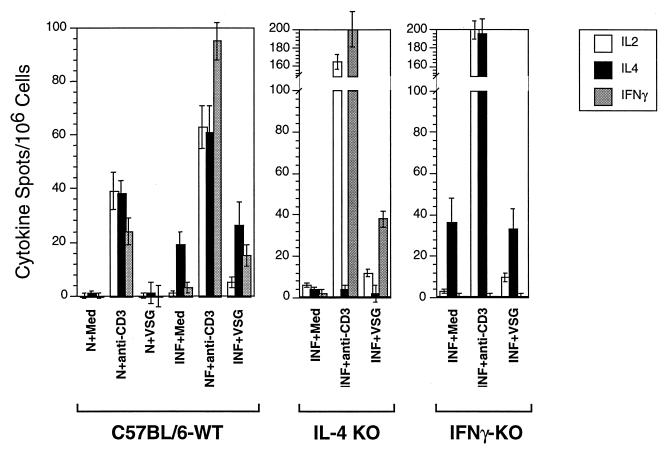
ELISPOT assay results (Fig. 2) largely paralleled the cytokine secretory responses noted above, with one exception: infection-induced “background” IL-4 cytokine spots were detected in all medium control cell cultures within a few days of infection. These IL-4 responses were not further upregulated following stimulation with VSG (Fig. 2) or with WCE. Therefore, while an increase in the background numbers of IL-4-secreting cells was detectable during trypanosome infection, these cells did not respond to parasite antigens and appear to be distinct from T cells responding to VSG.
FIG. 2.
ELISPOT assays of cells harvested from C57BL/6 wild-type (WT), C57BL/6 IL-4 knockout (KO), and C57BL/6 IFN-γ knockout mice infected with T. b. rhodesiense LouTat 1 for 2 weeks. PEC were stimulated with LouTat 1 VSG (10 μg/ml) or anti-CD3 (0.5 μg/well) for 12 h and then examined by ELISPOT tests for cytokine secretion. SPC and LNC responses were similar to PEC responses except for an absence of antigen-inducible IL-2, similar to the ELISA and bioassay results (Fig. 1A); stimulation with heterologous VSG or other unrelated antigens gave no detectable responses above those of medium controls. The results shown are from a single representative experiment of more than four such complete experiments performed over a 2-year period.
Determination of cytokine mRNA levels by QC-PCR.
Cytokine transcript levels during infection were evaluated by a sensitive method (Fig. 1B). Total RNA was extracted from freshly isolated SPC and PEC populations after they were harvested from normal and infected mice, as well as from cells that had been stimulated for a further 24 h with medium, ConA, or VSG. QC-PCR analyses of cytokine transcripts were subsequently performed. Freshly isolated SPC and PEC populations from infected mice exhibited detectable levels of IFN-γ, IL-2, and IL-4 transcripts compared to the same cells from normal mice (Fig. 1B); however, these basal cytokine transcript levels disappeared during a 24-h culture period in the presence of medium. In contrast, cells from infected PEC populations revealed a marked increase in the levels of both IFN-γ and IL-2 transcripts when stimulated with ConA or VSG (Fig. 1B), while SPC revealed a VSG-specific upregulation in IFN-γ transcript levels only. An examination of IL-4 transcript levels in VSG or WCE-stimulated cells during infection revealed that there was no detectable antigen-induced upregulation of transcript in any of the cell populations (Fig. 1B).
Thus, the aggregate cytokine results presented here demonstrate that a predominant Th1-cell response to VSG occurs during trypanosome infection. These results, which are in agreement with those of our earlier studies (28, 29, 46), reveal an absence of serologically detectable and biologically active IL-4 or IL-5 following antigenic stimulation. However, basal levels of IL-4 transcript and IL-4-secreting cells in ELISPOT were detected in cells from infected mice the absence of stimulation, suggesting that some cells may have the capability to produce perhaps small amounts of IL-4 (e.g., undetectable by ELISA or bioassay) in situ. Although basal IL-4 responses were detected, these responses were not further upregulated by incubation of cells with VSG or other trypanosome antigens.
VSG-specific Ab isotype responses.
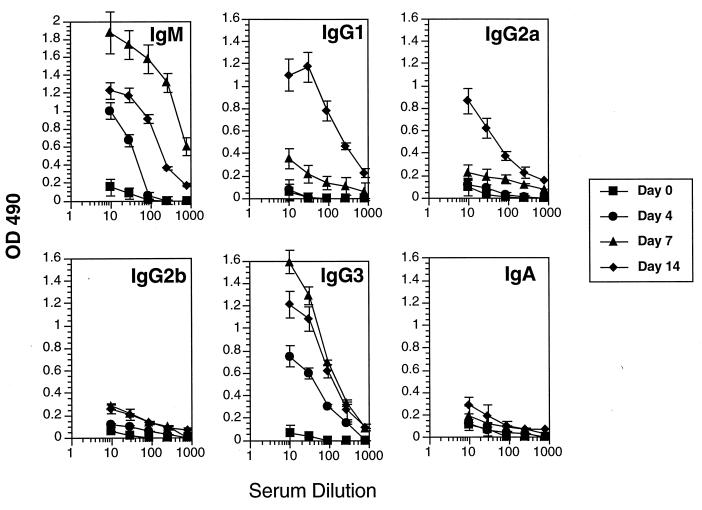
Having demonstrated that Th-cell stimulation by the VSG molecule during infection produced a predominant Th1-cell cytokine response, we examined the emerging Ab response to VSG in the context of helper-cell cytokine responses. Ab to VSG appeared rapidly following infection with trypanosomes of the T. b. rhodesiense LouTat 1 serodeme, as we have previously described (14, 27, 28, 44, 45, 68); as in earlier studies, the peak VSG-specific Ab responses occurred by days 7 to 14 and then the responses gradually declined (Fig. 3). When we determined the isotypes of VSG-specific Ab, we found that the predominant VSG-specific Ab isotypes made during infection were IgM, IgG1, IgG2a, and IgG3; no IgE was detectable (<10 ng/ml) and only minimal amounts of IgG2b and IgA were detectable. The switch to IgG1 was unexpected because high levels of IFN-γ are present in infected mice and this cytokine is known to inhibit a switch to IgG1 and IgE, in addition to inducing a switch to IgG2a (1, 19, 21, 23, 38, 48, 58, 66). Furthermore, IL-4 is known to be involved in blocking IgG2a production (23); however, no detectable IL-4 or other Th2 cytokines were secreted during infection in response to VSG stimulation.
FIG. 3.
VSG-specific Ab isotype responses of B10.BR mice infected with T. b. rhodesiense LouTat 1. The LouTat 1 VSG-specific Ab responses are shown only for the first 14 days of infection, after which time all the Ab responses gradually declined. Similar results were obtained with C57BL/6 mice, and heterologous VSGs did not react above background levels of sera from uninfected mice. These representative results are from one experiment of more than 10 separate experiments performed over a 4-year period. OD 490, optical density at 490 nm.
Ig isotype responses and T-cell cytokine profiles of athymic mice and IL-4 and IFN-γ knockout mice.
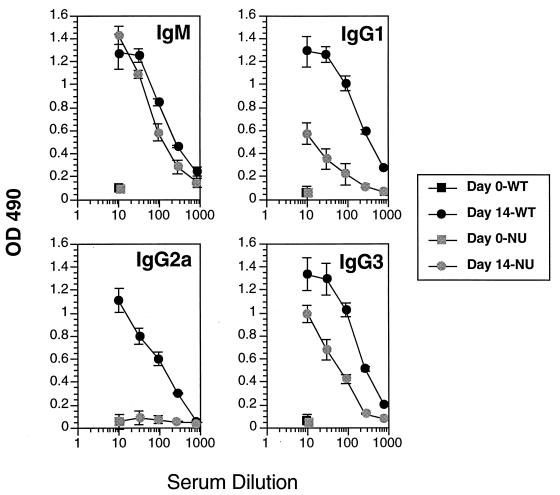
To determine the extent of T-cell involvement in the IgG1 switch, C57BL/6 athymic nude mice were infected and examined for their B-cell responses. All nude mice made prominent IgM and IgG3 but not IgG2a Ab responses to VSG (Fig. 4). These results show that the IgM and IgG3 responses are relatively T independent but that the IgG2a response is strictly T dependent. The nude mice also made a detectable IgG1 response, although the magnitude was lower than that of the thymus-intact mice (Fig. 4). This result shows that the IgG1 response is relatively T-cell independent, in contrast to the IgG2a response, and suggests that the IgG1 switch factors(s) is produced in animals lacking significant numbers of peripheral Th cells. Although we could not detect VSG-inducible IFN-γ, IL-2, or IL-4 responses in infected nude mice, the observation of a T-independent IgG1 switch is tempered by the fact that small numbers of peripherally maturing T cells present in nude mice may influence B-cell maturation (49).
FIG. 4.
VSG-specific Ab isotype responses of C57BL/6 wild-type (WT) and C57BL/6 nu/nu (NU) mice infected with T. b. rhodesiense LouTat 1. The peak LouTat 1 VSG-specific Ab responses are shown for day 14 of infection. Heterologous VSGs did not react above background levels of sera from uninfected mice. These results are from one representative experiment of four separate experiments performed over a 2-year period. OD 490, optical density at 490 nm.
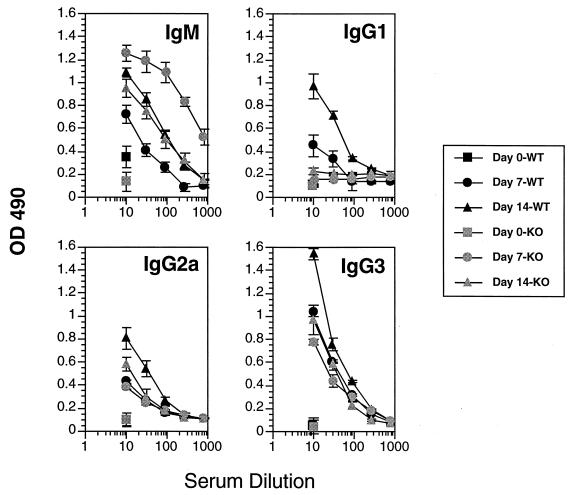
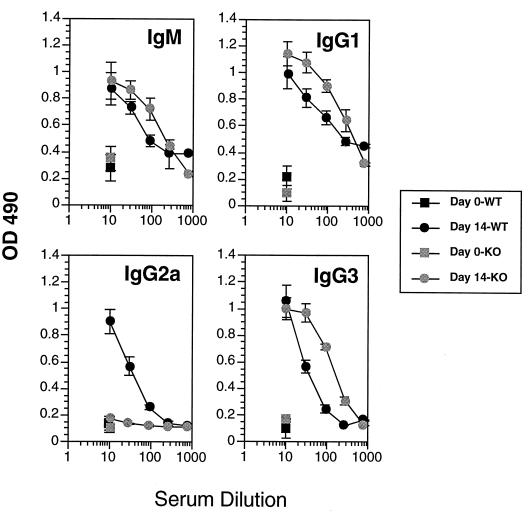
Subsequently, since no detectable IL-4 was made in response to trypanosome antigens in infected mice but basal increases in IL-4 transcript levels and small numbers of IL-4-secreting, antigen-unresponsive cells were detectable (Fig. 1 and 2), we examined the VSG-specific immune responses of IL-4 knockout mice. Infected IL-4 knockout mice made IFN-γ and IL-2 responses to VSG like the wild-type control mice (Fig. 2); however, the Ab isotype profile differed from that of wild-type mice in that there was a loss of the IgG1 switch response to VSG (Fig. 5). This result clearly demonstrates the IL-4-dependent nature of the IgG1 switch responses of trypanosome-infected mice to VSG. In a related experiment, the immune responses of IFN-γ knockout mice were also examined; these animals made IL-2 but not IFN-γ or IL-4 responses to VSG (Fig. 2); the Ig isotype pattern revealed loss of the IgG2a response to VSG but no major modulations in any of the other isotypes (Fig. 6). This result clearly shows the dependence of the IgG2a switch response on IFN-γ.
FIG. 5.
VSG-specific Ab isotype responses of C57BL/6 wild-type (WT) and C57BL/6 IL-4 knockout (KO) mice infected with T. b. rhodesiense LouTat 1. The LouTat 1 VSG-specific Ab responses are shown on days 7 and 14 of infection. Heterologous VSGs did not react above background levels of sera from uninfected mice. These results are from one representative experiment of four separate experiments performed over a 2-year period. OD 490, optical density at 490 nm.
FIG. 6.
VSG-specific Ab isotype responses of C57BL/6 wild-type (WT) and C57BL/6 IFN-γ knockout (KO) mice infected with T. b. rhodesiense LouTat 1. The peak LouTat 1 VSG-specific responses are shown on day 14 of infection. Heterologous VSGs did not react above background levels of sera from uninfected mice. These results are from one representative experiment of two separate experiments performed over a 2-year period. OD 490, optical density at 490 nm.
DISCUSSION
The mechanisms that regulate B-cell responses during African trypanosomiasis have not been previously elucidated. In other model systems, Th-cell responses are known to play a central role in the regulation of B-cell growth and differentiation by providing both membrane-activating signals and cytokines that regulate the Ig class switch responses of antigen-activated B cells (1, 6, 13, 19, 38, 50, 54, 70). However, until recently, Th-cell responses during African trypanosomiasis were thought to be completely suppressed (10, 28, 39, 65, 72, 73). Therefore, we had examined other immune mechanisms that might regulate B-cell responses to parasite antigens, including studies showing that the downregulation of VSG-specific B-cell responses was associated with anti-idiotypic responses (67, 68). There was no substantive evidence, however, for an idiotypic network operating during upregulation of VSG-specific B-cell responses in infection.
Subsequent studies in our laboratory revealed qualitative and quantitative differences in the VSG-specific Ab responses of infected athymic and thymus-intact mice (29, 45), suggesting that stimulation of the VSG-specific B-cell response involved both T-independent and T-dependent mechanisms, despite the apparent evidence for the suppression of T-cell responses to parasite antigens (39). Another clue that Th-cell responses occurred during trypanosome infections came from VSG sequence analysis (4, 43). The tertiary and secondary structures of VSG molecules have been shown by X-ray crystallography studies to be highly conserved (4). However, this conservation is not reflected at the primary structural level, since VSG molecules, including LouTat 1 serodeme VSGs, exhibit extensive amino acid sequence variation throughout the molecule (4, 43). Sequence analysis also revealed that “hypervariable” regions were dispersed throughout LouTat 1 and other VSG molecules and included those regions predicted not to be exposed to VSG-specific Abs (43); it was proposed that such variable regions in non-Ab-accessible sites may have been selected for by hypothetical Th-cell responses to processed VSG determinants (4, 29). However, these types of analyses provided only indirect evidence for Th-cell responses to the VSG molecule during infection. A major advance in terms of understanding T-dependent mechanisms of immune system stimulation in trypanosomiasis came from recent work in which it was demonstrated that, despite active suppression of T-cell proliferative responses by products of activated macrophages (10, 47, 65), cytokines were produced in response to VSG stimulation (46). These results provided the first direct evidence for VSG-specific Th-cell responses during infection. We hypothesized, therefore, that Th-cell cytokines may play a crucial role in the upregulation of T-dependent B-cell responses to the VSG molecule (29).
In the present study, we examined host B-cell responses to VSG by using a mouse model system and VATs of the T. b. rhodesiense LouTat 1 serodeme. The B10.BR/SgSnJ and C57BL/6J mouse strains make a strong VSG-specific Ab response to VSG molecules displayed by the infecting trypanosome VAT; this response, coupled with subsequent B-cell responses to VSG molecules displayed by emerging VATs, permits infected mice to control parasitemias (28). The work presented here provides a further characterization of Th-cell responses to the VSG molecule by defining the VSG-specific B-cell responses of trypanosome-infected mice at the isotype level in the context of defined Th-cell cytokine responses. Taken together, the data provide compelling evidence for a predominant VSG-specific Th1-cell cytokine response and the apparent absence of a Th2-cell response to VSG during trypanosome infections. The differentiation of Th0 cells to the Th1 phenotype is not due to any intrinsic characteristics of the VSG molecule itself, since some T-cell lines derived from animals immunized with VSG in the absence of infection produced both Th1- and Th2-cell-associated cytokines (46); rather, the polarization appears to be dependent upon a strong and early IFN-γ-independent stimulation of macrophage IL-12 production, coupled with an inhibitory effect directly on Th2-cell outgrowth, within the first few days of infection (30a).
One unusual feature of the work presented here is the unexpected Ab isotype profile generated in response to the VSG molecule. Much of the current information regarding Ig class-switching mechanisms has been derived from in vitro studies with lipopolysaccharide-activated or Ab-stimulated B cells in the presence of defined cytokines. Specific cytokines appear to activate Ig gene site-specific recombinases that splice out intervening CH gene sequences, producing rearrangements giving new VH-CH combinations (64). Citing classical examples (5, 6, 15, 16, 20, 55, 59, 60, 64), it is well known that IL-4 promotes Cμ-to-Cγ1 and -Cɛ switching events within Ig CH genes of activated B-cell clones while IFN-γ, in contrast, promotes Cμ-to-Cγ2a and -Cγ3 switching events; furthermore, IL-4 inhibits Cγ2a isotype switching while IFN-γ inhibits Cγ1 and Cɛ switching events. Thus, the cytokine products of antigen-stimulated Th1 and Th2 cells have been shown to exert distinct regulatory and counterregulatory effects on the target B cells with which they react. While this work has provided an excellent foundation for our understanding of the molecular mechanisms of class switching, results presented here and elsewhere in the context of infectious-disease model systems (17, 31, 32, 35, 56–58, 61) have modified earlier hypotheses based on classical approaches. Considering the complexities of antigens presented by pathogens and the intricate immunoregulatory events triggered by certain infections, it is not surprising that observations concerning Ig class switching are different from those derived from earlier, better-defined in vitro systems. The data presented here, in which we see a polarized antigen-specific Th1-cell response and antigen-specific B-cell IgG1, IgG2a, and IgG3 switch responses, provide an interesting comparison to some of the current models of Ig class-switching events. First, it was apparent that the trypanosome VSG surface coat, coupled with other factors, provides T-independent signals to B cells, as we have described previously (29, 45); these signals were sufficient to drive not only an IgM response but also part of the IgG1 switch response (see the discussion on IL-4, below), as well as the IgG3 response (Fig. 4). It has long been established by in vitro and in vivo studies that IFN-γ is responsible for the IgG2a switch response. In trypanosome infections, high levels of IFN-γ were secreted in response to the VSG molecule and other parasite antigens (Fig. 1 and 2) (46) and very high levels of IFN-γ are detectable in the sera of infected mice (12); as expected, high levels of IFN-γ-dependent and VSG-specific IgG2a were detectable (Fig. 3, 4, and 6). Studies suggesting that IFN-γ is also responsible for IgG3 switching events (6) are not directly supported by our results, since both nude and IFN-γ knockout mice exhibited strong IgG3 responses to VSG (Fig. 4 and 6). IL-4 has been shown to promote a switch to IgG1 and IgE as well as to suppress IgG2a production; although no IL-4 secreted in response to trypanosome antigens was detected in our system, there clearly was an IL-4-dependent, but partially T-independent, component to the VSG-specific IgG1 switch, as shown by the IL-4 knockout and nude mouse studies (Fig. 4 and 5). Of interest is that even though the IgG1 response was IL-4 dependent, no detectable IgE was produced; furthermore, the levels of IFN-γ present during infection appeared to exhibit no apparent downregulatory influence on the IgG1 switch response (Fig. 3 and 6). This is surprising since the inhibitory effect of IFN-γ is believed to predominate even in the presence of Th2 cytokines (22). In a similar vein, the low levels of cryptic IL-4 produced were insufficient to downregulate the IgG2a responses of infected mice (Fig. 3 and 5).
The cellular source of IL-4 that drives the IgG1 switch in trypanosome-infected mice is not clear. We could not detect an increase in the level of IL-4 transcripts, serologically detectable IL-4 protein, or biologically active IL-4 after stimulation with VSG or other trypanosome antigens (Fig. 1 and 2). However, IL-4 transcripts were detectable in freshly isolated, but not culture-stimulated, cells from infected mice (Fig. 1B), and IL-4-secreting “background” cells were detectable by the ELISPOT assay, in which the numbers of such cells did not increase following antigenic stimulation (Fig. 2), suggesting either that the cells did not express receptors for trypanosome antigens or that the cells were unable to undergo clonal expansion when stimulated. Thus, VSG-nonresponsive cells appear to be the source of low levels of IL-4 that are sufficient to drive the Cγ1 switch in VSG-specific B cells. In all likelihood, this cellular source may be non-Th2 cells such as γδ T cells or mast cells, basophils, or NK cells (24, 49, 62, 63, 74). Although we cannot formally rule out a failure to detect very small numbers of VSG- or other parasite antigen-reactive Th2 cells as the source of IL-4, further support for a role of non-T cells is based on the detection of IL-4 transcripts in trypanosome-infected scid and RAG-2 knockout mice (30a). Another contributing source of IL-4 may be CD8+ T cells stimulated by TLTF (3, 36, 37). A paper that appeared while this study was in progress showed that IL-4- and IFN-γ-secreting cells appear after the stimulation of nonimmune cells with TLTF, which binds to the CD8 molecule and triggers cytokine synthesis (2). Thus, there may be a CD8+ T-cell component that nonspecifically releases IL-4 after exposure by infection to TLTF and contributes to the IL-4-driven IgG1 switch; this supposition is supported by the nude mouse data in the present study, in which there is an identifiable T-dependent as well as a T-independent component to the switch (Fig. 4). That we found no perturbation in IL-4-secreting VSG-nonresponsive cells in IFN-γ knockout mice (Fig. 2) suggests that, if TLTF is involved, the ability to induce IL-4 production is neither linked to the production of IFN-γ nor negatively regulated by it.
Another hypothesis to explain the IgG1 switch response to VSG is that precommitted VSG-specific memory Th2 cells exist in uninfected mice and that these cells may, upon VSG exposure during an infection, interact with VSG-specific B cells and secrete IL-4 to cause the switch in the absence of further significant clonal expansion. There are data from other model systems that support this contention (69), and the early appearance of parasite-specific IgG responses relative to the appearance of IgM in experimental trypanosomiasis has been described previously (18). Furthermore, we have recently demonstrated the presence of VSG-cross-reactive natural autoantibodies in the sera of uninfected animals, including germ-free mice, suggesting that there may be VSG-primed Th and B cells in normal mice (33, 34). We have demonstrated that VSG-stimulated T cells undergo limited clonal proliferation during infection due to inhibitory effects of nitric oxide and prostaglandins (47) and that high levels of IL-12 and IFN-γ are released early in infection and can inhibit the outgrowth of Th2 cells (30a); it is conceivable, therefore, that if precommitted Th cell populations exist and if they express the Th2 cytokine phenotype and recognize epitopes shared by host cytoskeletal antigens and VSG (33, 34), they may be able to provide limited IL-4-mediated signals to VSG-specific B cells but be unable to proliferate to the point of being detectable in culture. However, more recent data from our laboratory showed that mature Ig transcripts containing VSG-specific VH plus Cγ1 or Cγ2a sequences were detectable only after 1 week of infection; these transcripts represented dominant Ig VH family responses to VSG determinants in infected animals, and there was no difference in the time of appearance of antigen-specific Cγ1 transcripts compared to Cγ2a transcripts (3a). Thus, the potential presence of an early (or precommitted) Th2- or B-cell response does not seem to skew the timing of appearance of the Cγ1 switch response relative to other more predictable switching events.
In summary, this paper provides important new insights into the regulation of VSG-specific B-cell responses in African trypanosomiasis and extends our previous work on VSG-specific Th1 cell stimulation during infection (29, 46). The most surprising observation was the production of VSG-specific IgG1 in an IL-4-dependent manner, in the absence of demonstrable antigen-specific Th2-cell responses. Based on the aggregate evidence presented here, it is probable that non-α/β TCR+ T cells are the source of IL-4 in trypanosome-infected nimals and that these cells are stimulated to produce IL-4 in a manner that is antigen independent and insensitive to negative regulation by IFN-γ. Overall, our results provide a foundation for studying the alternative regulation of Ig class-switching mechanisms in African trypanosomiasis by non-antigen-specific cells that may be part of the innate immune system.
ACKNOWLEDGMENTS
This study was supported by funds from the National Institutes of Health (AI-22441).
We thank Jim Schrader and John Barkei for their excellent technical assistance.
REFERENCES
- 1.Abbas A K, Burstein H J, Bogen S A. Determinants of helper T cell-dependent antibody production. Semin Immunol. 1993;5:441–447. doi: 10.1006/smim.1993.1050. [DOI] [PubMed] [Google Scholar]
- 2.Bakhiet M, Jansson L, Buscher P, Holmdahl R, Kristensson K, Olsson T. Control of parasitemia and survival during Trypanosoma brucei brucei infection is related to strain-dependent ability to produce IL-4. J Immunol. 1996;157:3518–3526. [PubMed] [Google Scholar]
- 3.Bakhiet M, Olsson T, Edlund C, Hojeberg B, Holmberg K, Lorentzen J, Kristensson K. A Trypanosoma brucei brucei-derived factor that triggers CD8+ lymphocytes to interferon-gamma secretion: purification, characterization and protective effects in vivo by treatment with a monoclonal antibody against the factor. Scand J Immunol. 1993;37:165–178. doi: 10.1111/j.1365-3083.1993.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 3a.Bi, X.-J., and J. M. Mansfield. Unpublished data.
- 4.Blum J L, Down J A, Gurnett A M, Carrington M, Turner M J, Wiley D C. A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature. 1993;362:603–609. doi: 10.1038/362603a0. [DOI] [PubMed] [Google Scholar]
- 5.Coffman R L, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-γ. J Immunol. 1986;136:949. [PubMed] [Google Scholar]
- 6.Coffman R L, Lebman D A, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 7.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1991. [Google Scholar]
- 8.Cross G A. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- 9.Cross G A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975;71:393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- 10.Darji A, Beschin A, Sileghem M, Heremans H, Brys L, Debaetselier P. In vitro simulation of immunosuppression caused by Trypanosoma brucei: active involvement of gamma interferon and tumor necrosis factor in the pathway of suppression. Infect Immun. 1996;64:1937–1943. doi: 10.1128/iai.64.6.1937-1943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Gee A L, Levine R F, Mansfield J M. Genetics of resistance to the African trypanosomes. VI. Heredity of resistance and variable surface glycoprotein-specific immune responses. J Immunol. 1988;140:283–288. [PubMed] [Google Scholar]
- 12.De Gee A L, Sonnenfeld G, Mansfield J M. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J Immunol. 1985;134:2723–2726. [PubMed] [Google Scholar]
- 13.DeKruyff R H, Rizzo L V, Umetsu D T. Induction of immunoglobulin synthesis by CD4+ T cell clones. Semin Immunol. 1993;5:421–430. doi: 10.1006/smim.1993.1048. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey W L, Mansfield J M. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983;130:405–411. [PubMed] [Google Scholar]
- 15.Dunnick W, Elenich L, Berry J, Albrecht D, Stavnezer J, Claflin J L. Regulation of switch recombination to the murine gamma 1 gene. Curr Top Microbiol Immunol. 1992;182:143–147. doi: 10.1007/978-3-642-77633-5_17. [DOI] [PubMed] [Google Scholar]
- 16.Elenich L A, Ford C S, Collins J T, Dunnick W A. γ1 heavy chain transgenes are responsive to IFN-γ repression and CD40 ligation. J Immunol. 1997;158:4564–4573. [PubMed] [Google Scholar]
- 17.Fernandez-Lago L, Santoyo F J, Vizcaino N, Chordi A. Class-specific immune response to Yersinia enterocolitica serotype O9 antigens as determined by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:1243–1248. doi: 10.1128/jcm.29.6.1243-1248.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finerty J F, Gasbarre L, Kendrick L P. Kinetics of immunoglobulin and specific antibody responses of CBA mice infected with Trypanosoma rhodesiense. Parasite Immunol. 1984;6:13–22. doi: 10.1111/j.1365-3024.1984.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 19.Finkelman F D, Holmes J, Katona I M, Urban J, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 20.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 21.Finkelman F D, Katona I M, Mosmann T R, Coffman R L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- 22.Finkelman F D, Urban J., Jr Cytokines: making the right choice. Parasitol Today. 1992;8:311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 23.Finkelman F D, Urban J F, Jr, Beckmann M P, Schooley K A, Holmes J M, Katona I M. Regulation of murine in vivo IgG and IgE responses by a monoclonal anti-IL-4 receptor antibody. Int Immunol. 1991;3:599–607. doi: 10.1093/intimm/3.6.599. [DOI] [PubMed] [Google Scholar]
- 24.Gauchat J F, Henchoz S, Mazzei G, Aubry J P, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 25.Lanham S M, Godfrey D G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970;28:521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 26.Levine R F, Mansfield J M. Genetics of resistance to African trypanosomes: role of the H-2 locus in determining resistance to infection with Trypanosoma rhodesiense. Infect Immun. 1981;34:513–518. doi: 10.1128/iai.34.2.513-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine R F, Mansfield J M. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J Immunol. 1984;133:1564–1569. [PubMed] [Google Scholar]
- 28.Mansfield J M. Immunobiology of African trypanosomiasis: a revisionist view. In: Boothroyd J C, Komuniecki R, editors. Molecular approaches to parasitology. New York, N.Y: Wiley-Liss; 1995. pp. 477–496. [Google Scholar]
- 29.Mansfield J M. T-cell responses to the trypanosome variant surface glycoprotein: a new paradigm? Parasitol Today. 1994;10:267–270. doi: 10.1016/0169-4758(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield J M, Craig S A, Stelzer G T. Lymphocyte function in experimental African trypanosomiasis: mitogenic effects of trypanosome extracts in vitro. Infect Immun. 1976;14:976–981. doi: 10.1128/iai.14.4.976-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Mansfield, J. M., et al. Unpublished data.
- 31.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 32.Mond J J, Vos Q, Lees A, Snapper C M. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 33.Muller N, Imboden M, Detmer E, Mansfield J M, Seebeck T. Cytoskeleton-associated antigens from African trypanosomes are recognized by self-reactive antibodies of uninfected mice. Parasitology. 1993;107:411–417. doi: 10.1017/s0031182000067767. [DOI] [PubMed] [Google Scholar]
- 34.Muller N, Mansfield J M, Seebeck T. Trypanosome variant surface glycoproteins are recognized by self-reactive antibodies in uninfected hosts. Infect Immun. 1996;64:4593–4597. doi: 10.1128/iai.64.11.4593-4597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen L, Knipe D M, Finberg R W. Mechanism of virus-induced Ig subclass shifts. J Immunol. 1994;152:478–484. [PubMed] [Google Scholar]
- 36.Olsson T, Bakhiet M, Hojeberg B, Ljungdahl A, Edlund C, Andersson G, Ekre H P, Fung-Leung W P, Mak T, Wigzell H, Fiszer U, Kristensson K. CD8 is critically involved in lymphocyte activation by a T. brucei brucei-released molecule. Cell. 1993;72:715–727. doi: 10.1016/0092-8674(93)90400-k. [DOI] [PubMed] [Google Scholar]
- 37.Olsson T, Bakhiet M, Kristensson K. Interactions between Trypanosoma brucei and CD8+ T cells. Parasitol Today. 1992;8:237–239. doi: 10.1016/0169-4758(92)90124-k. [DOI] [PubMed] [Google Scholar]
- 38.Parker D. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 39.Paulnock D M, Smith C, Mansfield J M. Antigen presenting cell function in African trypanosomiasis. New York, N.Y: Alan R. Liss, Inc.; 1989. pp. 135–144. [Google Scholar]
- 40.Pays E. Genetics of antigenic variation in African trypanosomes. Res Microbiol. 1991;142:731–735. doi: 10.1016/0923-2508(91)90088-r. [DOI] [PubMed] [Google Scholar]
- 41.Piatak M, Jr, Luk K C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–81. [PubMed] [Google Scholar]
- 42.Piatak M, Jr, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 43.Reinitz D M, Aizenstein B D, Mansfield J M. Variable and conserved structural elements of trypanosome variant surface glycoproteins. Mol Biochem Parasitol. 1992;51:119–132. doi: 10.1016/0166-6851(92)90207-z. [DOI] [PubMed] [Google Scholar]
- 44.Reinitz D M, Mansfield J M. Independent regulation of B cell responses to surface and subsurface epitopes of African trypanosome variable surface glycoproteins. J Immunol. 1988;141:620–626. [PubMed] [Google Scholar]
- 45.Reinitz D M, Mansfield J M. T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice. Infect Immun. 1990;58:2337–2342. doi: 10.1128/iai.58.7.2337-2342.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schleifer K W, Filutowicz H, Schopf L R, Mansfield J M. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J Immunol. 1993;150:2910–2919. [PubMed] [Google Scholar]
- 47.Schleifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 48.Schultz C L, Coffman R L. Control of isotype switching by T cells and cytokines. Curr Opin Immunol. 1991;3:350–354. doi: 10.1016/0952-7915(91)90037-2. [DOI] [PubMed] [Google Scholar]
- 49.Seder R A, Paul W E, Ben-Sasson S Z, LeGros G S, Kagey-Sobotka A, Finkelman F D, Pierce J H, Plaut M. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991;94:137–140. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- 50.Sher A, Coffman R L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 51.Siebert P D, Larrick J W. Competitive PCR. Nature. 1992;359:557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- 52.Siebert P D, Larrick J W. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. BioTechniques. 1993;14:244–249. [PubMed] [Google Scholar]
- 53.Smith C J, Levine R F, Mansfield J M. Cloning of African trypanosomes in mice immunosuppressed by cyclophosphamide treatment. Am J Trop Med Hyg. 1982;31:1098–1102. doi: 10.4269/ajtmh.1982.31.1098. [DOI] [PubMed] [Google Scholar]
- 54.Snapper C M, Finkelman F D. Immunoglobulin class switching. In: Paul W E, editor. Fundamental Immunology. 3rd ed. New York, N.Y: Raven Press; 1993. pp. 837–864. [Google Scholar]
- 55.Snapper C M, Finkelman F D, Paul W E. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 56.Snapper C M, Marcu K B, Zelazowski P. The immunoglobulin class switch: beyond “accessibility”. Immunity. 1997;6:217–223. doi: 10.1016/s1074-7613(00)80324-6. [DOI] [PubMed] [Google Scholar]
- 57.Snapper C M, Mond J J. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 58.Snapper C M, Mond J J. Towards a comprehensive view of immunoglobulin synthesis by CD4+ T cell clones. Immunol Today. 1993;12:15. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 59.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 60.Snapper C M, Peschel C, Paul W E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 61.Snapper C M, Rosas F R, Jin L, Wortham C, Kehry M R, Mond J J. Bacterial lipoproteins may substitute for cytokines in the humoral immune response to T cell-independent type II antigens. J Immunol. 1995;155:5582–5589. [PubMed] [Google Scholar]
- 62.Snapper C M, Yamaguchi H, Moorman M A, Mond J J. An in vitro model for T cell-independent induction of humoral immunity. A requirement for NK cells. J Immunol. 1994;152:4884–4892. [PubMed] [Google Scholar]
- 63.Snapper C M, Yamaguchi H, Moorman M A, Sneed R, Smoot D, Mond J J. Natural killer cells induce activated murine B cells to secrete Ig. J Immunol. 1993;151:5251–5260. [PubMed] [Google Scholar]
- 64.Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 65.Sternberg J M, Mabbott N A. Nitric oxide-mediated suppression of T cell responses during Trypanosoma brucei infection—soluble trypanosome products and interferon-gamma are synergistic inducers of nitric oxide synthase. Eur J Immunol. 1996;26:539–543. doi: 10.1002/eji.1830260306. [DOI] [PubMed] [Google Scholar]
- 66.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 67.Theodos C M, Mansfield J M. Regulation of B cell responses to the variant surface glycoprotein molecule in trypanosomiasis. II. Down-regulation of idiotype expression is associated with the appearance of lymphocytes expressing antiidiotypic receptors. J Immunol. 1990;144:4022–4029. [PubMed] [Google Scholar]
- 68.Theodos C M, Reinitz D M, Mansfield J M. Regulation of B cell responses to the variant surface glycoprotein (VSG) molecule in trypanosomiasis. I. Epitope specificity and idiotypic profile of monoclonal antibodies to the VSG of Trypanosoma brucei rhodesiense. J Immunol. 1990;144:4011–4021. [PubMed] [Google Scholar]
- 69.Toellner K-M, Gulbranson-Judge A, Taylor D R, Sze D M, MacLennan C M. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Kooten C, Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 71.Vickerman K, Luckins A G. Localization of variable antigens in the surface coat of Trypanosoma brucei using ferritin conjugated antibody. Nature. 1969;224:1125–1126. doi: 10.1038/2241125a0. [DOI] [PubMed] [Google Scholar]
- 72.Wellhausen S R, Mansfield J M. Characteristics of the splenic suppressor cell-target cell interaction in experimental African trypanosomiasis. Cell Immunol. 1980;54:414–424. doi: 10.1016/0008-8749(80)90221-x. [DOI] [PubMed] [Google Scholar]
- 73.Wellhausen S R, Mansfield J M. Lymphocyte function in experimental African trypanosomiasis. II. Splenic suppressor cell activity. J Immunol. 1979;122:818–824. [PubMed] [Google Scholar]
- 74.Wen B L, Pao W, Wong F S, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen M J, Hayday A C. Germinal center formation, immunoglobulin class switching and autoantibody production driven by “non α/β” T cells. J Exp Med. 1996;183:2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]