Abstract
Soft tissue sarcomas accounts for 1–2% of adult malignancies. Undifferentiated pleomorphic sarcoma (UPS) is a rare subtype that lack immunohistochemical markers for a specific definition. About 18% of sarcomas are at a locally advanced stage, often requiring several cycles of chemotherapy and radiotherapy, in addition to surgery. For a young woman, this can mean delaying pregnancies with a high risk of therapy-induced ovarian damage. For this reason, proper counseling on fertility preservation plays a key role. In addition, all women of childbearing age with cancer, should be informed about the importance of planning a pregnancy to improve maternal and neonatal outcomes. We report a rare case of a 40-year-old woman with a UPS who, during CT scan after chemotherapy to decide on surgery, find out she was pregnant. After counseling, the patient decides to go ahead with the pregnancy.
Keywords: Soft tissue sarcoma, Metastasis, Pregnancy, Pregnancy associated Cancer, Fertility
Introduction
Cancer during pregnancy, although rare, is an important ethical and biological issue for the appropriate care of both mother and fetus. Despite being a growing public health problem, details on its epidemiology are scarce and conflicting due to lack of publications and data. [1].
The most common types of pregnancy associated cancer (PAC) are breast cancer, melanoma, cervical cancer, lymphomas and leukemias [2].
The estimated overall incidence of pregnancy-associated cancer is 1/1000 pregnancies. [3].
However, the real incidence of PAC is probably underestimated because not all of cases are recorded in databases: spontaneous abortion and voluntary termination of pregnancy are often overlooked.
Several studies showed that the incidence of PAC is increasing [3], [4]. Although delayed childbearing appears to be a risk factor because the development of cancer is associated with older age, advanced diagnostic techniques and greater interaction with healthcare services during pregnancy could also be factors contributing to the increased incidence rates [4].
Eibye S. et al. estimated a rise from 5,4% to 8,3% the incidence of pregnancy-associated cancer during a 30-year period in Denmark [5].
Similarly, Lee YY et al. showed, over a 14-year period, the same increasing trend, especially for mothers older than 35 years [4].
In addition, the probability of initiating and managing a successful pregnancy in metastatic cancer is scarcely reported in literature [6].
In this article we present the case of a woman with a metastatic undifferentiated pleomorphic sarcoma (UPS) who started and conduced her pregnancy combined with a literature review to discuss the etiology, clinical manifestations, diagnosis, treatment and prognosis of pregnancy complicated with STS.
Case-report
A 40-year-old G2P0 woman presented at 14 + 4 weeks gestation to the High-Risk Pregnancy Unit of Vittore Buzzi University Hospital in Milan, Italy, in November 2020.
Regarding her obstetrical history, in 2016 she underwent an urgent caesarean section with longitudinal hysterotomy for previa placental abruption at 24 + 4 gestational weeks. The newborn, weighing 699 g, died of septic shock on day 4.
In 2018 she was diagnosed with a grade III undifferentiated pleomorphic sarcoma (UPS WHP 2016) involving the left thigh without a specific phenotype. No genetically transmitted diseases or malignancies were present in her family history. She underwent three cycles of chemotherapy with Adriamycin and Ifosmamide and radiotherapy for a dose of 50 Gy in 25 fractions with VMAT technique in view of surgery to remove soft parts in the left obturator region. Surgery was performed in July 2018 and histological examination showed undamaged surgical resection margins. In March 2019, the patient underwent double thoracotomy for lung metastasectomy with two further cycles of chemotherapy with Adriamycin and Ifosfamide.
Planned surgery for an increased size of the focal lobar lesion had to be postponed due to the occasional CT scan finding of an evolving pregnancy at the 9th gestational week.
The probability of stochastic and genetic effects on the unborn child, given the estimated in utero dose of 6.3 mSv, was calculated as < 0.028% and < 0.0001%, respectively (ICRP publication 103).
The pregnancy, monitored every fortnight, developed physiologically. At 22 + 4 weeks’ gestation, the MRI detected an increased in volume of the focal apical lesion of the left lobe, so the patient underwent left lower pulmonary lobectomy: postponing the surgery to postpartum would have resulted in an increase in the volume of the lesion such that it was no longer surgically treatable. Nevertheless, the procedure had to be delayed two weeks later than planned due to an asymptomatic COVID-19 infection. The surgery was performed without intra- and post-operative complications.
Histological examination confirmed lung metastases of undifferentiated pleomorphic spindle cell sarcoma with massive infiltration of the visceral pleura but without peribronchial lymph nodes involvement.
Subsequently, the pregnancy was complicated by gestational diabetes at 25 weeks, which was treated with diet alone. At the beginning of the third trimester, a diagnosis of fetal growth restriction (FGR) was made with an estimated fetal weight at the 6 th percentile according to the growth curves.
Weekly fetal Doppler velocimetry monitoring was always regular, but the mean pulsatility index (mPI) of both uterine arteries was always above the 95th percentile.
At 34 weeks the patient was hospitalized for hypertension and was diagnosed preeclampsia. The 24-hour proteinuria collection was negative (308 mg/lt) and blood tests were normal with no signs of organ damage.
At 35 weeks and 5 days gestation, the patient underwent an urgent caesarean section due to altered computerized cardio-tocography (cCTG), reduced fetal movements, fetal growth arrest, preeclampsia and rhythmic uterine contractile activity.
During the caesarean section, which was performed without any complications, both peritoneal lavage and fetal blood from the umbilical artery were sent for cytological analysis to assess the presence of further metastases.
A live male baby weighing 2180 g (12th centile) was delivered; APGAR scores at one minute and five minutes were 9 and 10, respectively. Arterial blood gas analysis of the umbilical cord blood showed no signs of acidosis: pH 7.29, pCO2 57,4 mmHg, base excess – 1.0 mmol/L, Lac 1.44 mmol/L.
The infant was transferred to the neonatal intensive care unit for monitoring due to prematurity: he had no complications and was discharged after 6 days.
Breastfeeding was started and the mother's postoperative course was uncomplicated, she was discharged with the baby.
Cytologic examination of the peritoneal washings performed before and after opening the uterine cavity did not show any malignant tumor cells. Cytologic examination of umbilical cord blood with “buffy coat” preparation was negative for malignant tumor cells. [7].
Histological placental examination did not reveal the presence of neoplastic lesions.
The histological and cytological reports of mother, foetus and placenta are shown in Table 1.
Table 1.
Histological and cytological examination report of mother, foetus and placenta.
| Mother | Foetus | Placenta | |
|---|---|---|---|
| Type of Tumor | Undifferentiated pleomorphic sarcoma, grade III | No tumor detected | No tumor detected |
| Metastases | Lung metastases of undifferentiated spindle cell sarcoma | No metastasi detected | No metastases detected |
| Cytologic examination of peritoneal washing | Negative for malignant tumor cells | ||
| Cytologic examination of umbelical cord blood | Negative for malignant tumor cells |
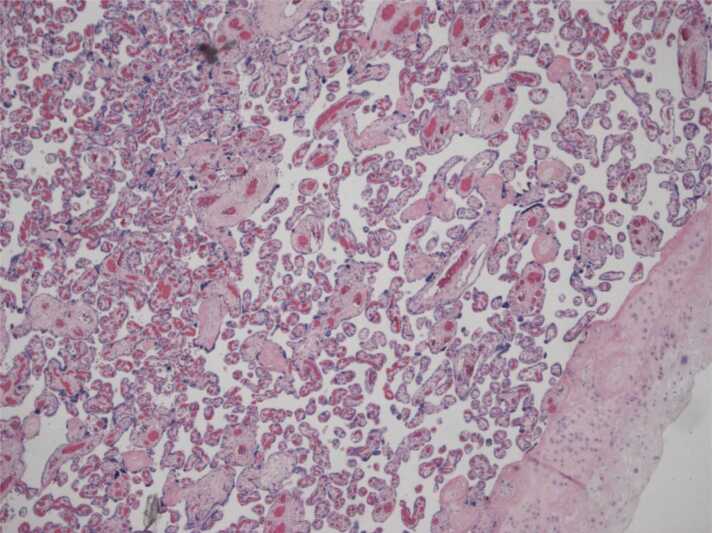
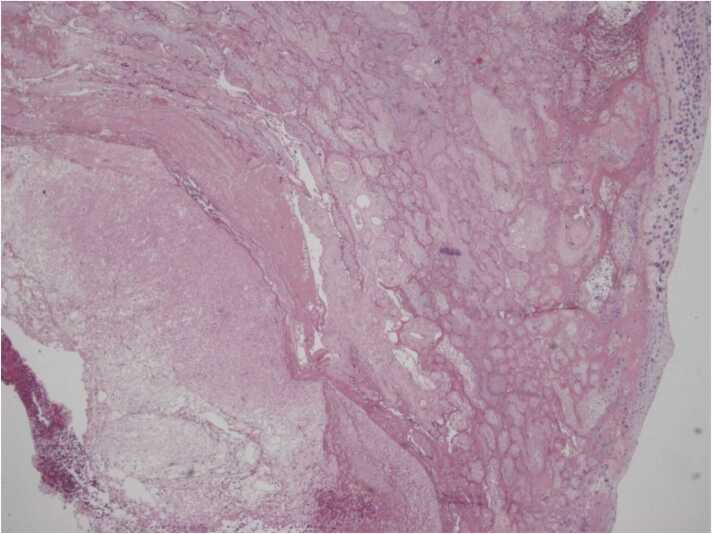
The placenta showed areas of maternal vascular malperfusion: distal villous hypoplasia with a huge intervillous space (Fig. 1) and collapsed ghost villi diagnostic of an old infarct (Fig. 2), consistent with FGR.
Fig. 1.
4 × 0001: a field with distal villous hypoplasia, short villi and a huge intervillous space.
Fig. 2.
4 × 0003: collapsed ghost villi diagnostic of an old infarct.
Although the amounts of ischemic areas are not in itself sufficient for a diagnosis of maternal vascular malperfusion, the combination of areas of old infarction and distal villous hypoplasia is suggestive of maternal vascular malperfusion.
The patient died 18 months after the caesarean delivery due to complications from metastatic disease.
Soft tissue sarcomas (STS)
STS are tumor arising from muscles, tendons, synovial, adipose tissue and connective tissues that affect all ages and gender. They represent 1–2% of adult malignancies. [8].
About 16% of sarcomas are found in a locally advanced stage, with a 5-year relative survival rate of 17% and a median survival rate close to 18 months. The main site of metastasis are lungs. [9], [10].
STS include more than 80 different histological subtypes with specific biological characterizations [11]. Those reported in literature associated with pregnancy include osteosarcoma, liposarcoma, Ewing’s sarcoma, rhabdomyosarcoma, chondrosarcoma, gastrointestinal stromal tumor (GIST), fibromyxoid sarcoma, angiomyxoma, synovial sarcoma, fibrosarcoma, leiomyosarcoma, endometrial stromal sarcoma and angiosarcoma [12] and malignant fibrous histiocytoma (MFH) [13].
MFH has been permanently removed from the 2013 World Health Organization (WHO) classification of soft tissue tumors and reclassified as undifferentiated pleomorphic sarcoma (UPS) [14].
Pregnancies associated to Soft Tissue Sarcomas (STS) are rare [15] and data on pregnancies in women with histologically documented metastatic sarcomas are limited.
Yazigi A et al. reported four cases of women with metastatic sarcomas who carried on their pregnancies after stopping systemic cancer treatment with good maternal-fetal outcomes and prolonged maternal survival. Three histotypes were involved in the report: epithelioid hemangioendothelioma, low-grade fibromyxoid sarcoma and GIST.
The only case of malignant fibrous MFH in pregnancy reported in literature concerns a 38-year-old woman who received the inauspicious diagnosis in the immediate postnatal period and died after 3 months. [13].
Diagnosis of STS
In most cases, the diagnosis of STS is already known at the time of pregnancy. A recent case series conducted in Toronto reported 48 women diagnosed with STS during a 10-year period: only 10 patients (20.8%) were diagnosed with cancer during pregnancy. [12].
The guiding symptom is pain and the main sign is a mass localized mainly to the abdominal-pelvic region and to the upper and lower limbs. Sometimes hemorrhagic syndromes are present due to involvement of large retroperitoneal vessels. [12].
Diagnostic imaging methods include both CT scan and magnetic resonance imaging (MRI). Ionizing diagnostic imaging during pregnancy should be avoided or used only when essential for the management of the pregnancy. [16].
The cumulative fetal dose radiation should not exceed 100 mGY. [17].
Nonionizing imaging procedures such as ultrasound and MRI are safe during pregnancy except for the use of gadolinium. [18].
STS diagnosis with biopsy to define the histological subtype is difficult with pathologists' discordance rate sometimes reaching 30%. [11].
Treatment of STS
Surgery
Surgery is the standard treatment in case of localized adult-type STS. [19] The American College of Obstetricians and Gynecologists' Committee on Obstetric Practice has stated that there are no data to make specific recommendations for non-obstetric surgery during pregnancy. However, at any gestational age, teratogenic have never been demonstrated by anesthetic agents when used in standard concentrations, nor is there evidence that fetal exposure to anesthetic drugs affects neurodevelopment. Therefore, a pregnant woman should never be denied a necessary and unpostponable surgery, regardless of trimester. It is reasonable to state that the first part of the second trimester should be preferred to limit miscarriage.
Laparoscopy appears to be safer than laparotomy if the surgery is performed by experienced surgeons, also because it allows a better vision of the abdominal cavity. [20].
Post-operative radiotherapy
ESMO - EURACAN Clinical Practice Guidelines recommend postoperative radiotherapy as the standard treatment in tumor with unfavorable prognostic factors such as high-grade, tissue invasion or tumor diameter > 5 cm. [10].
In clinical practice, it is usual to postpone treatment to the post-partum period to avoid fetal harm, unless there is an urgent clinical need and the irradiation site is sufficiently distant from the uterus. [21], [22].
Several adverse effects have been reported for the fetus after gestational radiotherapy, including intrauterine growth restriction (IUGR), risk of childhood cancer (solid tumor and/or leukemia) and subaverage intellectual functioning. The severity of adverse effects depends on the extent of the irradiation field, time of radiation exposure and gestation period. [23].
Chemotherapy
Regarding chemotherapy, its role remains controversial in both neoadjuvant and adjuvant settings: in case of metastatic disease, surgical treatment is recommended as the first choice in lung disease with a limited number of metastases without other extrapulmonary localizations. [10].
If the patient undergoes chemotherapy, both preoperatively and postoperatively, anthracycline and/or ifosfamide seem to be the most appropriate choice. Miller et al. conducted a multi- institutional retrospective study on 13 patients, to evaluate the administration of anthracyclines and/or ifosfamide in pregnancy-associated sarcoma. They found a lower rate of live births in patients receiving a combination of doxorubicin and ifosfamide during pregnancy (5/9, 55.6%) compared to patients treated with anthracycline-based regimens without ifosfamide (4/4, 100%). Besides, they showed that combination therapies with doxorubicin and ifosfamide may have higher risks of fetal harm when given early in the second trimester as compared with later in pregnancy. [24].
In case of inoperable metastatic disease, chemotherapy is palliative and does not affect survival.
Metastasectomies
In case of metastatic disease, surgical treatment is recommended as the first choice in lung disease with a limited number of metastases without other extrapulmonary localizations.
There is consensus in repeating metastasectomies in case of relapse of disease, always respecting the above-mentioned criteria of radicality and patient selection. [11].
Management of pregnancy complicated by STS
The aim is to carry the pregnancy to term. However, if the disease is severe and requires immediate intervention, early termination of pregnancy may be advised especially in the first trimester. [25].
2-weekly ultrasound monitoring of fetal growth is strongly recommended: in maternal cancer population poor general health, malnutrition and chemotherapy or radiotherapy (if any) are risk factors for intrauterine growth restriction (IUGR). [26], [27].
Delivery is usually planned. Iatrogenic preterm delivery should be avoided in order not to incur the long-term comorbidities of preterm infants: from 37 weeks of pregnancy delivery can be considered unless there are major life-threatening complications for both mother and fetus. [28].
Regarding delivery, several studies show that vaginal delivery could be the first choice unless there are contraindications. Nevertheless, other studies show an increased percentage (30%) of caesarean sections [4] mainly due to psychophysical stress and tumor mass effect with limited joint mobility, especially in STS. [12].
Placental histology is recommended in assessing the risk of fetal metastases, especially in patients with metastatic tumors. Metastases, if present, are usually found in the intervillous space. If metastases are present, they should also be investigated in the newborn by clinical examination and initially by ultrasounds. Although there is limited evidence, it seems that the transfer of mother-to-fetus metastases only occurs if metastases are found at the villus level. [26].
Discussion
STS represent less than 1% of all tumors [29] and UPS is a rare subtype that lack immunohistochemical markers for a specific definition.
According to our knowledge, our is the first case reported in literature of a pregnancy that occurred and was successfully carried to term in a woman diagnosed with metastatic UPS.
The rarity of this case is not only tied to the uncommon type of tumor, but also to the low probability of the patient to get pregnant after her clinical history.
The first finding in literature on the effects of chemotherapy drugs on the female reproductive system dates back to the 1970 s and is related to cyclophosphamide therapy, which was linked to amenorrhea and follicular destruction. [30].
As chemotherapy treatments usually involves combination of several drugs, it is not easy to understand the effects of a single drug on the female reproductive system but is certain that the most severe long-term outcome of exposure to cytotoxic drug treatment is infertility due to premature ovarian failure (POF) or insufficiency (POI) [31].
Radiation treatment, on the other hand, can damage the uterine vasculature and the structure of the endometrium and myometrium. Although it is unclear whether this is a consequence of ovarian damage or the result of direct damage to the uterus, fertility may decline. [32].
Considering all the cancer treatment protocols the patient performed without undergoing any methods of prevention and reduction of ovarian damage induced from chemotherapy and radiotherapy, the probability of pregnancy was very low.
Given the relationship between chemo-radiotherapy, miscarriage, impaired organogenesis and unplanned pregnancy, the birth of a child without anatomical defects is to be considered an extraordinary event.
It cannot be ruled out if IUGR was a consequence of the cancer treatment the woman underwent in previous years: damaged uterine tissue could alter placentation and trophoblastic invasion. Consequently, as is well known, aberrant spiral arteries remodeling leads to altered maternal-fetal blood flow and thus fetal growth restriction. [33].
The patient’s placenta was histologically analyzed: villous immaturity suggested a decreased utero-placental blood flow, which probably contributed to IUGR.
The issue of sarcoma’s growth acceleration during pregnancy is debated in literature. In our case cancer progression was evident during pregnancy. However, it's still unclear whether this progression was due to pregnancy-related factors when compared to similar tumors in women who are not pregnant or if it is a direct consequence of stopping chemotherapy or radiotherapy. However, in previous reports about STS during pregnancies, enhancement of tumor was evident in several cases. [34], [35].
Since a general treatment strategy for pregnant women with sarcoma cannot be outlined because it is a rare condition, each case should be discussed by a multidisciplinary team and the diagnostic and therapeutic approaches should be tailored for every woman.
Conclusions
The rarity of this clinical case lays the groundwork for discussing the important ethical dilemma of cancer in pregnancy and in particular the complexity of managing pregnancy-associated STS.
The poor prognosis of UPS and its tailored therapies seemed to be incompatible with this pregnancy that, contrary to expectations, was carried through and ended without any severe maternal-fetal complications.
This unplanned pregnancy placed the patient in front of two options: termination of pregnancy or delaying cancer treatment once the vitality of the fetus is reached. After a meticulous counseling to the couple, the patient chose to go through with the pregnancy. It is very hard for a woman to choose the best deal and for the doctor to make the best approach guiding the patient in her decisions.
A multidisciplinary approach involving oncologists, obstetricians, neonatologists, and other specialists is essential to navigate the intricate decisions required to balance maternal oncological care with fetal well-being.
Author agreement statement
All the authors declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. They confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. They understand that the Corresponding Author is the sole contact for the Editorial process. She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
CRediT authorship contribution statement
Rossi Roberta Simona: Formal analysis. Cetin Irene: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. Nebuloni Manuela: Formal analysis. Quarenghi Aida: Data curation, Supervision. Lubrano Chiara: Writing – review & editing. Di Simone Giuliana: Conceptualization, Data curation, Writing – original draft. Sala Valentina: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft.
Declaration of Competing Interest
None.
References
- 1.Cottreau C.M., Dashevsky I., Andrade S.E., et al. Pregnancy-associated cancer: A U.S. population-based study. J Women’s Health. 2019;28:250–257. doi: 10.1089/jwh.2018.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galati F., Magri V., Arias-Cadena P.A., et al. Pregnancy-associated breast cancer: a diagnostic and therapeutic challenge. Diagnostics. 2023;13(4):604. doi: 10.3390/diagnostics13040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardonick E. Cancer occurs in approximately 1 per 1, 000 pregnancies. Oncol (Williston Park) 2008;22(8 Suppl Nurse Ed):22–23. [PubMed] [Google Scholar]
- 4.Lee Y.Y., Roberts C.L., Dobbins T., et al. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994–2008: a population- based linkage study. BJOG. 2012;119(13):1572–1582. doi: 10.1111/j.1471-0528.2012.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eibye S., Kjaer S.K., Mellemkjaer L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet Gynecol. 2013;122(3):608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- 6.Zagouri F., Dimitrakakis C., Marinopoulos S., Tsigginou A., Dimopoulos M.A. Cancer in pregnancy: disentangling treatment modalities. ESMO Open. 2016;1(3) doi: 10.1136/esmoopen-2015-000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlidis N., Pentheroudakis G. Metastatic involvement of placenta and foetus in pregnant women with cancer. Recent Results Cancer Res. 2008;178:183–194. doi: 10.1007/978-3-540-71274-9_16. [DOI] [PubMed] [Google Scholar]
- 8.Hui J.Y.C. Epidemiology and etiology of sarcomas. Surg Clin North Am. 2016;96(5):901–914. doi: 10.1016/j.suc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Howlader N., Noone A.M., Krapcho M., et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019.
- 10.Casali P.G., Abecassis N., Aro H.T., et al. Soft tissue and visceral sarcomas: ESMO–EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv168–iv269. doi: 10.1093/annonc/mdy321. [DOI] [PubMed] [Google Scholar]
- 11.AIOM (Associazione Italiana di Oncologia Medica), Linee guida sarcoma tessuti molli. Edizione 2020. https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Sarcomi.pdf.
- 12.Figueiro-Filho E.A., Al-Sum H., Parrish J., Wunder J.S., Maxwell C. Maternal and fetal outcomes in pregnancy affected by Bone and Spft Tissue Tumors. AJP Rep. 2018;8(4):e343–e348. doi: 10.1055/s-0038-1676289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H.W., Hsu C.S., Lin Y.H., Hsu M.I., Chiang H.K., Chou S.Y. Malignant fibrous histiocytoma during pregnancy: a case report. Taiwan J Obstet Gynecol. 2006;45(1):86–88. doi: 10.1016/S1028-4559(09)60201-1. [DOI] [PubMed] [Google Scholar]
- 14.Robles-Tenorio A., Solis-Ledesma G. StatPearls. StatPearls Publishing,; Treasure Island (FL): 2023. Undifferentiated Pleomorphic Sarcoma. April 10. [PubMed] [Google Scholar]
- 15.Zarkavelis G., Petrakis D., Fotopoulos G. Bone and soft tissue sarcomas during pregnancy: a narrative review of the literature. J Adv Res. 2016;7:581–587. doi: 10.1016/j.jare.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain C. ACOG Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol. 2019;133(1):186. doi: 10.1097/AOG.0000000000003049. [DOI] [PubMed] [Google Scholar]
- 17.Yoon I., Slesinger T.L. StatPearls. StatPearls Publishing,; Treasure Island (FL): 2023. Radiation Exposure In Pregnancy. May 1. [PubMed] [Google Scholar]
- 18.ACR (American Colege of Radiology) Committee on Drugs and Constrast Media. ACR Manual on Contrast Media.Version 2020. 97–100.
- 19.Gronchi A., Lo Vullo S., Colombo C., et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–511. doi: 10.1097/SLA.0b013e3181cf87fa. [DOI] [PubMed] [Google Scholar]
- 20.ACOG committee opinion no. 775: Nonobstetric Surgery During Pregnancy. Obstet Gynecol.2019;13384): e285-e286. [DOI] [PubMed]
- 21.Maggern C., Van Gerwen M., Van Calsteren K., et al. Management of cancer during pregnancy and current evidence of obstetric, neonatal and pedriatric outcome: a review article. Int J Gynecol Cancer. 2019;29:404–416. doi: 10.1136/ijgc-2018-000061. [DOI] [PubMed] [Google Scholar]
- 22.Azim H.A., Jr, Pavlidis N., Peccatori F.A. Treatment of the pregnant mother with cancer: a systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part I Solid: Tumors Cancer Treat Rev. 2010;36(2):101–109. doi: 10.1016/j.ctrv.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Peccatori F.A., Azim H.A., Jr, Orecchia R., et al. Cancer, pregnancy and fertility: ESCMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi160–vi170. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 24.Miller D., Livingston J.A., Park Y., et al. Pregnancy outcomes related to the treatment of sarcomas with anthracyclines and/or ifosfamide during pregnancy. Cancer Med. 2022;11(18):3471–3478. doi: 10.1002/cam4.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han S.N., Kesic V.I., Van Calsteren K., Petkovic S., Amant F. ESGO ‘Cancer in Pregnancy’ Task Force. Cancer in pregnancy: a survey of current clinical practice. Eur J Obstet Gynecol Reprod Biol. 2013;167(1):18–23. doi: 10.1016/j.ejogrb.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Wolters V., Heimovaara J., Maggen C., et al. Management of pregnancy in women with cancer. Int J Gynecol Cancer. 2021;31(3):314–322. doi: 10.1136/ijgc-2020-001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Haan J., Verheecke M., Van Calsteren K., et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 28.Amant F., Berveiller P., Boere I.A., et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol. 2019;30(10):1601–1612. doi: 10.1093/annonc/mdz228. [DOI] [PubMed] [Google Scholar]
- 29.Doyle L.A. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120(12):1763–1774. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 30.Koyama H., Wada T., Nishizawa Y., Iwanaga T., Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39(4):1403–1409. doi: 10.1002/1097-0142(197704)39:4<1403::aid-cncr2820390408>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Roness H., Kashi O., Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril. 2016;105(1):20–29. doi: 10.1016/j.fertnstert.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths M.G., Winship A., Hutt K. Do cancer therapies damage the uterus and compromise fertility? Hum Reprod Update. 2020;26(2):161–173. doi: 10.1093/humupd/dmz041. [DOI] [PubMed] [Google Scholar]
- 33.van der Kooi A.L.F., Kelsey T.W., van den Heuvel-Eibrink M.M., Laven J.S.E., Wallace W.H.B., Anderson R.A. Perinatal complications in female survivors of cancer: a systematic review and meta-analysis. Eur J Cancer. 2019;111:126–137. doi: 10.1016/j.ejca.2019.01.104. [DOI] [PubMed] [Google Scholar]
- 34.Merimsky O., Le Cesne A. Soft tissue and bone sarcomas in association with pregnancy. Acta Oncol. 1998;37(7-8):721–727. doi: 10.1080/028418698430106. [DOI] [PubMed] [Google Scholar]
- 35.Merimsky O., Inbar M., Issakov J., et al. Gestation-related malignant musculoskeletal tumors. Isr Med Assoc J. 2003;5(4):264–267. [PubMed] [Google Scholar]