Abstract
Objective
The dorsal vagal complex (DVC) of the hindbrain is a major point of integration for central and peripheral signals that regulate a wide variety of metabolic functions to maintain energy balance. The REV-ERB nuclear receptors are important modulators of molecular metabolism, but their role in the DVC has yet to be established.
Methods
Male REV-ERBα/β floxed mice received stereotaxic injections of a Cre expressing virus to the DVC to create the DVC REV-ERBα/β double knockout (DVC RDKO). Control littermates received stereotaxic injections to the DVC of a green fluorescent protein expressing virus. Animals were maintained on a normal chow diet or a 60% high-fat diet to observe the metabolic phenotype arising from DVC RDKO under healthy and metabolically stressed conditions.
Results
DVC RDKO animals on high-fat diet exhibited increased weight gain compared to control animals maintained on the same diet. Increased weight gain in DVC RDKO animals was associated with decreased basal metabolic rate and dampened signature of brown adipose tissue activity. RDKO decreased gene expression of calcitonin receptor in the DVC and tyrosine hydroxylase in the brown adipose tissue.
Conclusions
These results suggest a previously unappreciated role of REV-ERB nuclear receptors in the DVC for maintaining energy balance and metabolic rate potentially through indirect sympathetic outflow to the brown adipose tissue.
Keywords: REV-ERBα/β, Dorsal vagal complex, Diet-induced obesity, Brown adipose tissue, Metabolic rate
Highlights
-
•
REV-ERBα/β double knockout (RDKO) in the dorsal vagal complex (DVC) elicits hypersensitivity to high-fat diet (HFD).
-
•
DVC RDKO lowers basal metabolic rate and gene markers for brown adipose tissue activity on HFD.
-
•
DVC RDKO may influence sensitivity to HFD by indirectly regulating sympathetic outflow.
1. Introduction
Obesity is a major public health issue with myriad treatment options but a lack of highly effective, long-term therapies [1,2]. Although development of obesity is complex and multifactorial, obesity is a disease of energy balance dysregulation in the brain [3,4]. While the neural controls of energy balance are distributed across the brain [5,6], the dorsal vagal complex (DVC) of the hindbrain is a major integrator of peripheral and central metabolic signals [5,7]. The DVC is comprised of the area postrema (AP), nucleus of the solitary tract (NTS), and the dorsal motor nucleus of ten (DMX). The AP and NTS receive afferent information regarding macronutrient composition of meals and gastric distention while the DMX sends efferent signals to peripheral organs ranging from the heart to the reproductive tract via the vagus nerve [[8], [9], [10], [11]]. Thus, the DVC as a collective structure is an integral gatekeeper of regulatory flow between central and peripheral sources of homeostatic energy signals.
Due to this, the DVC has garnered attention as a central area of action for weight management pharmaceuticals. Metabolic signals such as glucagon-like peptide, melanocortin 3 and 4, leptin, insulin, and calcitonin are highly expressed in the DVC and are targets of interest for obesity therapeutics [7,[12], [13], [14], [15], [16]]. However, the molecular mechanisms underpinning DVC control of energy balance remains poorly understood.
REV-ERB α and β nuclear receptors are important for maintaining tissue-specific molecular metabolism [17]. They are transcriptional repressors that constitute a critical negative arm of the mammalian circadian clock [17] and directly repress thousands of genes [[18], [19], [20]]. In peripheral tissues, REV-ERBs modulate lipid and glucose handling and their depletion produces metabolic maladies such as hepatosteatosis, dyslipidemia, and impaired glucose and insulin tolerance [18,[21], [22], [23]]. The central roles of REV-ERBs are primarily behavioral such that loss of expression in the whole body or brain induces disturbances in sleep, memory, and social behaviors [[24], [25], [26]]. However, in specific groups of neurons or brain areas REV-ERBs control centrally mediated metabolic functions and it is unknown whether the DVC is one of these areas [23,27,28].
Therefore, a critical unmet need of the field is to determine how REV-ERBs function in the DVC. Therefore, we knocked out REV-ERBα/β in the DVC (DVC RDKO) of male mice to determine the effect of RDKO on metabolic homeostasis. Here we report that DVC RDKO exacerbates high-fat diet-induced obesity (DIO) by decreasing basal metabolic rate. Our results suggest this may be due to decreased brown adipose tissue (BAT) activity arising potentially from indirect DVC control of sympathetic nervous system (SNS) outflow.
2. Materials and methods
2.1. Animals and diet
All animal work was approved by the University of Pennsylvania Perelman School of Medicine Institutional Animal Care and Use Committee (IACUC protocol number 804747 issued to Mitchell Lazar). Male Nr1d1fl/fl/Nr1d2fl/fl [29] mice were maintained on a C57Bl6/J background (Jackson Labs, Stock 008661). Mice were bred and maintained at 20–22 °C on a 12:12 h light:dark cycle with ad libitum access to food and water unless otherwise noted. The normal chow diet (NCD, LabDiet, 5010) consisted of 12.7% kcal fat, 28.7% kcal protein, and 58.5% kcal carbohydrates. The high-fat diet (HFD, Research Diets, D12492) consisted of 60% kcal fat from lard and soybean oil, 20% kcal protein, and 20% kcal carbohydrates with 5.24 kcal/g. Surgical procedures were performed on 6–8-week-old male littermates. Sacrifice and tissue collection were performed 7 h after lights on to capture the peak time of REV-ERB expression [28].
2.2. Stereotaxic surgery
To perform injections, animals were anesthetized with isoflurane and secured into the stereotaxis (Stoelting Instruments, 51730U). A surgical plane of anesthesia was confirmed by toe pinch. Depilatory cream was used to remove animals' fur on the dorsal aspect of the head and neck before alternating EtOH and betadine swabs and application of a surgical drape. Subcutaneous, pre-operative analgesic (5 mg/kg meloxicam) was administered. A ∼1.5 cm incision was made in the skin on the dorsal aspect of the head and neck and the neck muscles were carefully retracted. A small window was drilled into the ventral aspect of the interparietal bone, and the head was positioned at a 5–10° ventral angle to visualize the DVC. Injections were performed using Hamilton Neuros syringes (Hamilton, 65460-02) and a Quintessential Stereotaxic Injector (Stoelting, 53311) using the following coordinates: ML: 0.3 mm, AP: −7.5 mm, DV: 4.3 mm. REV-ERBα/β were knocked out in the DVC of male Nr1d1fl/fl/Nr1d2fl/fl mice via bilateral intraparenchymal injection of 50 nL of 5 × 107 GC of AAV8-CMV-Cre (Addgene, 105538-AAV8) to create DVC RDKO. For the control group, Nr1d1fl/fl/Nr1d2fl/fl mice received bilateral injections of the same volume and titer of AAV8-CMV-EGFP (Addgene, 105530-AAV8). Needles remained in both injection sites for ∼30 s to ensure penetrance of the injection and prevent backflow. After needles were removed from the second injection, neck muscles were repositioned, and skin was sutured with absorbable thread. Post-operative analgesic (bupivacaine) was administered to the skin around the incision site. Animals were then placed in a clean, warmed cage for recovery and were maintained at 30 °C for three days post-op with soft diet and water gel (ClearH2O, 72-06-5022 and 70-01-5002 respectively) to supplement ad libitum NCD and water. Animals were given subcutaneous 5 mg/kg meloxicam for three days post-op and were weighed and monitored for ten days post-op. To allow for sufficient surgical recovery and viral expression, animals were maintained on ad libitum NCD and water for 2–4 weeks post-op before metabolic phenotyping or beginning HFD.
2.3. Metabolic rate measurement
Metabolic rate was determined by in vivo indirect calorimetry in Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments) cages. Animals were placed singly in CLAMS cages with bedding, food, and water that mimicked the home cage environment. After two days of adaptation, experimental data were collected for three days. Data were acquired every 15 min by Oxymax software.
2.4. Food intake measurement
Food intake was measured by Biological Data Acquisition (BioDAQ, Research Diets) cages. Animals were placed singly in BioDAQ cages with bedding, food, and water that mimicked the home cage environment. After two days of acclimation, experimental data were collected for three consecutive days. Data were acquired at each food hopper interaction and analyzed by BioDAQ Data Analysis software. An average of the three consecutive days was determined for each animal and then summed over the light cycle, dark cycle, and total 24 h.
2.5. RNA extraction, cDNA synthesis, and RTqPCR
For DVC RNA extraction, the area was microdissected from the brainstem and homogenized with 1 mL of QIAzol Lysis Reagent (Qiagen, 79306) using Pellet-Pestel (Kimble, 749540). Intrascapular BAT and inguinal white adipose tissue (iWAT) was homogenized in 500uL of QIAzol Lysis Reagent using a TissueLyser system (Qiagen, 85300). After homogenization, total RNA from both tissues was purified and collected using RNeasy Mini Kits (Qiagen, 74004). RNA quality and quantity was determined by NanoDrop (Thermo Scientific, ND-ONE-W).
cDNA was synthesized using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, 4368814). qPCR was run using Power SYBR Green PCR Master Mix (Applied Biosystems, 4368577) and QuantStudio 6 Flex Real-Time PCR system and software (Applied Biosystems, 4485691). All primers (Appendix) were validated, and qPCR results were analyzed by standard curve and normalization to 18s.
2.6. RNAscope
For quantification of Nr1d1 in the DVC, brains were rapidly removed and fixed overnight in 4% paraformaldehyde at 4C. Brains were then washed 3× with 0.1M phosphate-buffered saline (PBS, pH 7.4) and transferred to 30% sucrose in 0.1M PBS and maintained at 4C until the brains sunk. Brains were then embedded in OCT compound, frozen at −80C, and then sectioned on a cryostat in the coronal plane at 14 μm thickness. Slices were collected on Superfrost Plus slides (VWR) and stored at −80C until further analysis. RNAscope was performed as previously described [28]. Briefly, RNAscope Multiplex Fluorescent Reagent Kit v2 (ACDBio, 323110) was used per the manufacturer's instructions. Detection was carried out using a custom probe produced by ACDBio for Nr1d1 mRNA targeting exons 4 and 5 (Mm-Nr1d1-O2-C3, 1097521-C3). Following a series of amplification steps, sections were mounted with VECTASHIELD PLUS with DAPI (Vector Labs, H-2000). Slides were imaged on an EVOS FL Auto 2 (Thermo Scientific, AMAFD2000) widefield microscope at 10× magnification. Images provided are representative views of control and DVC RDKO sections to confirm lack of robust Nr1d1 expression within the DVC of the RDKO group.
2.7. Brown adipose tissue histology and immunofluorescence
Freshly isolated BAT was fixed overnight in 4% paraformaldehyde at 4 °C before EtOH dehydration. Tissue was embedded in paraffin and sectioned at 7 μm. Hematoxylin and eosin (H&E) staining and immunofluorescence (IF) for tyrosine hydroxylase (TH) was performed by the Institute of Diabetes, Obesity, and Metabolism Histology Core using a standard protocol. The antibody for TH staining was obtained from EMD Millipore (AB1542). Slides were imaged on an EVOS FL Auto 2 (Thermo Scientific, AMAFD2000) widefield microscope. Histological slides were imaged at 20× magnification and IF was imaged at 40× magnification. Cell number and size in histology samples and IF signal were analyzed using FIJI Is Just ImageJ (FIJI, NIH).
2.8. Statistical analysis
Statistical analysis and graphing were performed using GraphPad Prism 7 software. All data were presented as mean standard error measurement (SEM) and statistical significance was set a priori as p < 0.05. Gene expression, final body weight, food intake, and brown adipocyte histology and IF were compared using the Mann–Whitney U-test. Running body weight gain, metabolic rate, and activity were compared using a repeated measures analysis of variance (RM ANOVA) with a Tukey post-hoc test. Correlation analyses were performed using Pearson correlation analysis.
3. Results and discussion
3.1. REV-ERBα/β double knockout in the dorsal vagal complex (DVC RDKO)
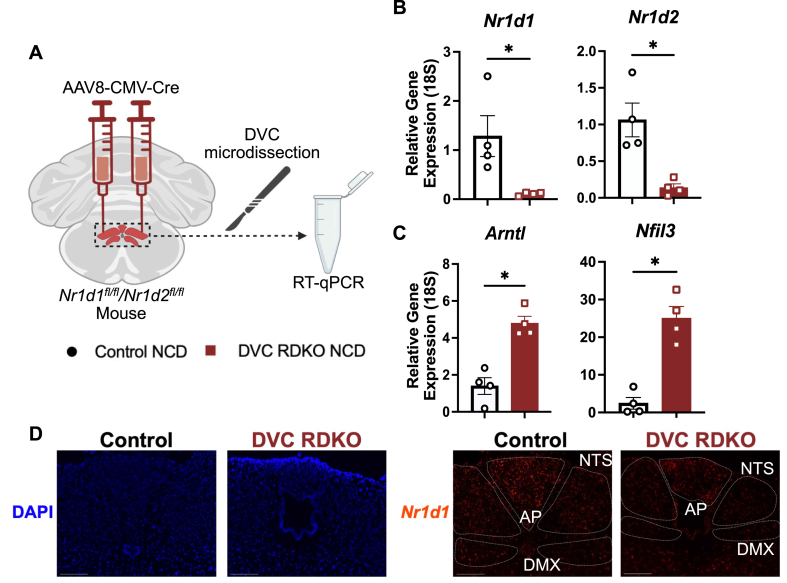
Male Nr1d1fl/fl/Nr1d2fl/fl mice received stereotaxic injections of AAV8-CMV-EGFP or AAV8 CMV-Cre to create the control and DVC RDKO groups, respectively. After viral expression and metabolic phenotyping, the DVC was microdissected from the hindbrain for RTqPCR confirmation of the knockout (Figure 1A). REV-ERBα (Nr1d1) and REV-ERBβ (Nr1d2) exhibited reduced expression in the DVC of DVC RDKO animals (Figures 1B). REV-ERBα/β are constitutive repressors of the circadian clock activator, Arntl (BMAL1) and the accessory circadian clock repressor, Nfil3 (E4BP4) [17]. Consistent with reduced expression of their repressors, Arntl and Nfil3 expression were increased in DVC RDKO animals (Figure 1C). In situ Nr1d1 expression was found to be decreased throughout the DVC (Figure 1D).
Figure 1.
REV-ERBα/β double knockout in the dorsal vagal complex (DVC RDKO). A) Experimental scheme for DVC RDKO generation and tissue processing. B–C) RT-qPCR analysis of Nr1d1 (REV-ERBα), Nr1d2 (REV-ERBβ), Arntl (BMAL1), and Nfil3 (E4BP4) (n = 4–5, mean ± SEM). D) Representative images at 10× magnification from RNAscope of Control and DVC RDKO animals for confirmation of Nr1d1 knockout in the DVC (scale bar 275 μm). B–C) Results were compared by Mann–Whitney U-test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.2. DVC RDKO does not impact food intake or body weight on normal chow diet (NCD)
The DVC is a major regulatory site for food intake, satiety, and energy balance [5]. Therefore, we hypothesized DVC RDKO would result in altered food intake and affect body weight. However, DVC RDKO animals maintained on NCD did not exhibit different food intake when analyzed over 24 h (Figure 2A). DVC RDKO also did not exhibit increased body weight (Figure 2B).
Figure 2.
DVC RDKO does not impact food intake or body weight on normal chow diet (NCD). A) Light cycle, dark cycle, and total 24hr food intake measurements (n = 4–5, mean ± SEM). B) Final body weight measurement 5–7 weeks after stereotaxic injection for Control and DVC RDKO animals on NCD (n = 4–5, mean ± SEM). Data was analyzed by Mann–Whitney U-test.
3.3. DVC RDKO exacerbates diet-induced obesity and dampens metabolic activity
We have previously shown that RDKO in the hypothalamus does not manifest in a metabolic phenotype on NCD [28]. We therefore hypothesized that a metabolic stressor, such as an obesogenic HFD, is necessary to uncover the metabolic deregulatory effects of DVC RDKO. To explore this possibility, control and DVC RDKO mice were placed on a 60% HFD for 12 weeks. We observed increased weight gain in DVC RDKO mice (Figure 3A). The DVC RDKO animals exhibited similar meal size, eating frequency, and time spent eating (Supplementary Figure 1A-E), but displayed a non-significant increase in grams of food consumed (Figure 3B) and kcal of food consumed (Supplementary Figure 1F) as compared to HFD-fed controls. We also observed decreased VO2 in the DVC RDKO animals (Figure 3C) with a negative correlation to body weight when fit to the entire model (Supplementary Figure 2A), but no correlation to body weight when fit to the control or DVC RDKO groups independently (control: r = −0.542, p = 0.635; DVC RDKO: r = 0.986, p = 0.1071). This change was not accompanied with altered locomotor activity (Figure 3D). We conclude that a minor increase in food intake coupled with decreased VO2 over the course of HFD exposure exacerbated the DIO phenotype in DVC RDKO.
Figure 3.
DVC RDKO exacerbates diet-induced obesity and dampens metabolic activity. A) Running body weight gain graph for 12 weeks of HFD (n = 4–5, mean ± SEM). B) Light, dark, and total food intake on HFD (n = 3–4, mean ± SEM). C) Oxygen consumption as a measure for metabolic rate on HFD (n = 3–4, mean ± SEM). D) Activity measurements on HFD (n = 3–4, mean ± SEM). A&C) Results were compared by repeated measures ANOVA. B) Results were compared by Mann–Whitney U-test. ∗∗p < 0.01, ∗∗∗p < 0.001.
3.4. DVC RDKO decreases molecular signatures for brown adipose tissue (BAT) activity
Since decreased VO2 in the DVC RDKO animals occurred independent of changes in locomotor activity, we explored other sources affecting the phenotype. Brown adipose tissue (BAT) quickly oxidizes lipid stores to regulate metabolic rate and its activity is regulated by outflow from the sympathetic nervous system (SNS) [30].
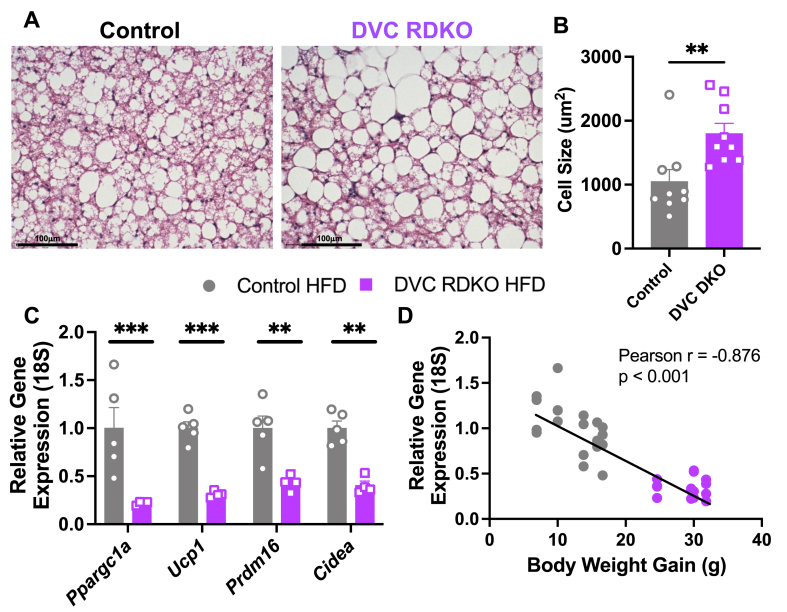
The BAT of DVC RDKO animals had large, unilocular adipocytes compared to the control group (Figure 4A) and was consistent with increased size by quantification (Figure 4B). Gene markers for BAT activity Ppargc1a, Ucp1, Prdm16, and Cidea were decreased in the DVC RDKO group (Figure 4C) with a negative correlation between the relative expression of these genes and grams of body weight gained on HFD (Figure 4D). Changes in BAT gene expression were not observed on NCD suggesting a synergistic effect of DVC RDKO and HFD feeding on BAT pathophysiology (Supplementary Figure 3A).
Figure 4.
DVC RDKO decreases molecular signatures for BAT activity on HFD. A) Representative images of BAT from control and DVC RDKO animals after 12 weeks of 60% HFD feeding. B) Quantification of BAT adipocyte size from 3 technical replicates of 3 biological replicates (n = 9, mean ± SEM). C-D) RTqPCR for BAT activity markers (C) and correlation of their expression with body weight gain at week 12 (D) (n = 4–5, mean ± SEM). B–C) Results were compared by Mann–Whitney U-test. D) Results were analyzed by Pearson correlation. ∗∗p < 0.01, ∗∗∗p < 0.001.
Due to REV-ERBs' role as repressors of the circadian clock, DVC RDKO may also induce exacerbated weight gain due to desynchrony among the DVC, the rest of the body, and the external environment. Indeed, the DVC has been shown to have a robust cell-autonomous clock that can be altered by HFD feeding [[31], [32], [33]]. However, this possibility requires further study.
3.5. DVC RDKO decreases calcitonin receptor gene expression in the DVC and tyrosine hydroxylase expression in the BAT
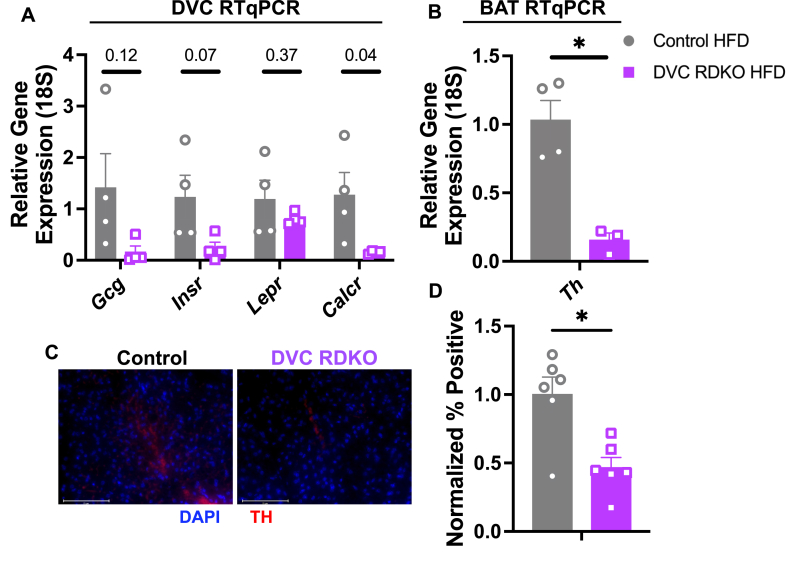
There are established connections between the DVC and BAT [34,35]. Specifically, the NTS of the DVC projects to thalamic regions that regulate activity in the pre-sympathetic dorsal raphe nuclei (DRN) [35]. Through these neural networks, the DVC can modulate BAT activity in response to the humoral and neural feeding signals it receives from the periphery [[36], [37], [38]]. Of the several factors and receptors that mediate DVC control of energy balance, we measured gene expression for Gcg (glucagon-like peptide), Insr (insulin receptor), Lepr (leptin receptor), and Calcr (calcitonin receptor) from microdissected DVCs in control and DVC RDKO animals fed HFD. Gcg and Insr exhibited a non-significant decrease in expression, but Calcr was significantly decreased (Figure 5A). Direct REV-ERBα/β targets exhibit overexpression upon RDKO due to release from REV-ERB repressive activity. The mechanism underlying decreases in Gcg, Insr, and Calcr following RDKO is less clear, but most likely due to loss of REV-ERB repression of direct regulators of these genes. Indeed, in several other tissues including liver and heart, loss of REV-ERBs increases the expression of Nfil3 (E4BP4), a powerful transcriptional repressor whose target genes thus become further repressed in the absence of REV-ERBs [39,40].
Figure 5.
DVC RDKO decreases markers of sympathetic outflow to the BAT. A) DVC RTqPCR for gene markers of energy balance regulation (n = 4, mean ± SEM). B) BAT RTqPCR for Th (n = 3–4, mean ± SEM). C) Representative images at 40× magnification of IF for DAPI and TH from Control and DVC RDKO BAT (scale bar 75 μm). D) Quantification of fluorescent signal in six images per group from three biological replicates (n = 3, mean ± SEM). A,B,D) Results were compared by Mann–Whitney U-test. ∗p < 0.05, ∗∗p < 0.01.
The decrease in Calcr (CTR) gene expression was intriguing because CTRs are G-protein coupled receptors that are often found in complex with a receptor activating modifying protein (RAMP) to form the heterodimerized amylin receptor [41]. Activation of CTRs in the DVC has been found to regulate not only food intake, but energy expenditure through indirect NTS projections to the DRN that modulate SNS-stimulated BAT activity [[36], [37], [38],42,43]. Therefore, we measured BAT expression of tyrosine hydroxylase (Th) to determine disruptions in general SNS outflow and found that Th RNA and TH protein expression were decreased in the DVC RDKO animals (Figure 5B–D). Th was also decreased in inguinal white adipose tissue (iWAT) suggesting that DVC RDKO non-specifically dampens SNS tone under HFD-fed conditions (Supplementary Figure 4A-B). Although full elucidation of this mechanism requires additional testing, our data suggests that DVC RDKO may increase sensitivity to HFD by dampening SNS outflow.
4. Conclusions
DVC RDKO exacerbated DIO due to decreased basal metabolic rate and markers for BAT activity. We postulate that this was caused by de-repression of repressors for the key energy balance regulators in the DVC (Figure 6). This was accompanied by diminished Th expression in the BAT suggesting decreased activity of the SNS (Figure 6). Definitive characterization of CTR in the DVC as a potential mechanism for the DVC RDKO phenotype requires further testing. Follow up studies utilizing next-generation sequencing techniques and rescue studies should be performed to determine whether other signaling pathways are impacted by DVC RDKO. Furthermore, we used a CMV promoter to drive Cre expression thereby deleting REV-ERBs in all cell types. It is unknown how specific cell types within the DVC function to affect DIO, energy expenditure, and BAT activity in our model, and it should be pointed out that all experiments were performed at 22 °C, which is a thermal stressor for mice. As obesity and metabolic disease become increasingly prevalent in Western society, it is crucial to understand the central mechanisms that underlie energy homeostasis. Herein, we uncovered a role for the REV-ERBα/β nuclear receptors in the DVC for maintaining energy balance and metabolic rate potentially through indirect, calcitonin-mediated sympathetic outflow to the BAT in a mouse model.
Figure 6.
Proposed Model. REV-ERBα/β in the dorsal vagal complex (DVC) may indirectly regulate sympathetic nervous system (SNS) outflow to the brown adipose tissue (BAT) potentially by orchestrating calcitonin receptor (Calcr) gene expression. Upon feeding with a 60% high-fat diet, DVC REV-ERBα/β double knockout (DVC RDKO) induced a remarkable exacerbation of diet-induced obesity due to decreased basal metabolic rate and markers for BAT activity. We postulate that this was potentially caused by de-repression of repressors for Calcr in the DVC that indirectly decrease SNS outflow to the BAT.
Author Contributions
LNW and MAL designed the study, interpreted the data, and edited the manuscripts. LNW and SMF performed survival surgeries. LNW performed RTqPCR, statistical analysis, and prepared the manuscript. LNW, LCM, AJA, and CCC performed sacrifice and tissue collection. LNW, LCM, and AJA performed metabolic phenotyping and food intake experiments. MB performed RNAscope experiments. LCM performed breeding and genotyping of Nr1d1fl/fl/Nr1d2fl/fl mice. LNW and SMF designed and optimized surgical procedure for DVC injection. LNW, MRH, and MAL designed studies and interpreted gene expression data in the BAT and DVC. All authors reviewed and edited the manuscript.
Declaration of competing interest
The authors declare the following competing interests:
MAL is on the advisory board and has received research funding from Pfizer and is on the advisory board and is co-founder of Flare Therapeutics. MRH receives additional research funding from Boehringer Ingelheim, Novo Nordisk, Pfizer, Gila Therapeutics, and Eli Lilly & Co. that was not used in support of these studies. AJA, CCC, LNW, LCM, MB, and SMF have no competing interests to declare.
Acknowledgements
The authors would like to thank B. Krusen and T. Acavedo for their invaluable help and discussion. We thank L. Cheng from the Penn Institute for Diabetes, Obesity, and Metabolism Histology core, and the Rodent Metabolic Phenotyping Core of the Penn Diabetes Research Center (P30DK19525). Multiple figures in this manuscript (Figure 1, Figure 6) were made using BioRender. This work was supported by NIH R01DK45596 (MAL), NIH F32DK128984 (LNW), NIH T32DK007314 (LNW), NIH R25MH119043 (CCC), NIH R01DK105155 and the JPB Foundation (MAL).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101861.
Appendix.
| RTqPCR Primers | ||
|---|---|---|
| Forward (5' -> 3') | Reverse (5' -> 3') | |
| Nr1d1 | GTCTCTCCGTTGGCATGTCT | CCAAGTTCATGGCGCTCT |
| Nr1d2 | CGGATCACATGGTCGAGGAG | TGCTCCTCCGAAAGAAACCC |
| Arntl | CTTTATCAGCTGCACATCACTCAGA | GGACATTGCATTGCATGTTGG |
| Nfil3 | GCTCTTTTGTGGACGAGCAT | ACCGAGGACACCTCTGACAC |
| Ppargc1a | GGACATGTGCAGCCAAGACTCT | CACTTCAATCCACCCAGAAAGCT |
| Ucp1 | GGCCAGTTGGTTTTCACAGA | GGATTGGCCTCTACGACTCA |
| Prdm16 | CAGCACGGTGAAGCCATT | CGCGTGCATCCGCTTGTG |
| Cidea | TGCTCTTCTGTATCGCCCAGT | GCCGTGTTAAGGAATCTGCTG |
| Gcg | CCTTCAAGACACAGAGGAGAACC | CTGTAGTCGCTGGTGAATGTGC |
| Insr | AGATGAGAGGTGCAGTGTGGCT | GGTTCCTTTGGCTCTTGCCACA |
| Lepr | CTTTCCTGTGGACAGAACCAGC | AGCACTGAGTGACTCCACAGCA |
| Calcr | AAGATGGACCCTCATGCCAGTG | CTCGTCGGTAAACACAGCCATG |
| Th | TGCACACAGTACATCCGTCATGC | GCAAATGTGCGGTCAGCCAACA |
| 18s | AGTCCCTGCCCTTTGTACACA | CGATCCGAGGGCCTCACTA |
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Continued food intake analysis of Control vs DVC RDKO mice fed a high-fat diet. A) Average meal size in grams, B) average meal size in kcal, C) average meal frequency, D) average within meal eating frequency, E) average time spent eating, and E) light, dark, and total kcal consumed on HFD (n = 4, mean ± SEM). Results were compared by Mann-Whitney U-test.
Pearson correlation for VO2 vs body weight. A) Pearson correlation for VO2 vs body weight of control and DVC RDKO mice fed HFD (n = 3).
DVC RDKO does not change molecular signatures for BAT activity on NCD. A) RTqPCR for markers for BAT activity and SNS activity in the BAT of NCD-fed control and DVC RDKO animals (n = 3, mean ± SEM). Results were compared by Mann-Whitney U-test.
DVC RDKO decreases Th expression in iWAT on HFD and NCD. RTqPCR for Th in iWAT samples from A) HFD and B) NCD fed mice (n = 3-4, mean ± SEM). Results were compared by Mann-Whitney U-test.
Data availability
Data will be made available on request.
References
- 1.Aronne L.J., Hall K.D., Jakicic J.M., Leibel R.L., Lowe M.R., Rosenbaum M., Klein S. Describing the Weight-reduced State: Physiology, Behavior, and Interventions. Obesity. 2021;29:S9–S24. doi: 10.1002/oby.23086. Wiley Online Libr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz M.W., Seeley R.J., Zeltser L.M., Drewnowski A., Ravussin E., Redman L.M., Leibel R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocrine Rev. 2017 doi: 10.1210/er.2017-00111. academic.oup.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall K.D., Farooqi I.S., Friedman J.M., Klein S., Loos R.J.F., Mangelsdorf D.J., et al. The energy balance model of obesity: beyond calories in, calories out. Am J Clin Nutr. 2022;115:1243–1254. doi: 10.1093/AJCN/NQAC031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegelman B.M., Flier J.S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 5.Grill H.J., Hayes M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metabol. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grill H.J., Kaplan J.M. The neuroanatomical Axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig M.Q., Cheng W., Gordian D., Lee J., Paulsen S.J., Hansen S.N., et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat Metab. 2021;3:530–545. doi: 10.1038/s42255-021-00363-1. 2021 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoud H.R., Albaugh V.L., Neuhuber W.L. Gut-brain communication and obesity: understanding functions of the vagus nerve. J Clin Invest. 2021:131. doi: 10.1172/JCI143770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDougle M., Araujo A. de, Vergara M., Singh A., Yang M., Braga I., et al. de Fats and sugars recruit distinct gut-brain circuits to control food intake and reward. Faseb J. 2022:36. doi: 10.1096/FASEBJ.2022.36.S1.00R29. [DOI] [Google Scholar]
- 10.Berthoud H.R. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/J.REGPEP.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughton W.B., Powley T.L. 1987. Localization of Efferent Function in the Dorsal Motor Nucleus of the Vagus; p. 252. [DOI] [PubMed] [Google Scholar]
- 12.Stein L.M., Lhamo R., Cao A., Workinger J., Tinsley I., Doyle R.P., et al. Dorsal vagal complex and hypothalamic glia differentially respond to leptin and energy balance dysregulation. Transl Psychiatry. 2020;10:1–12. doi: 10.1038/s41398-020-0767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng W., Ndoka E., Hutch C., Roelofs K., MacKinnon A., Khoury B., et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI insight. 2020;5 doi: 10.1172/JCI.INSIGHT.134359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berglund E.D., Liu T., Kong X., Sohn J.W., Vong L., Deng Z., et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. 2014 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippi B.M., Bassiri A., Abraham M.A., Duca F.A., Yue J.T.Y.Y., Lam T.K.T. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes. 2014;63:892–899. doi: 10.2337/DB13-1044. [DOI] [PubMed] [Google Scholar]
- 16.Stein L.M., McGrath L.E., Lhamo R., Koch-Laskowski K., Fortin S.M., Skarbaliene J., et al. The long-acting amylin/calcitonin receptor agonist ZP5461 suppresses food intake and body weight in male rats. Am J Physiol Regul Integr Comp Physiol. 2021;321:R250–R259. doi: 10.1152/AJPREGU.00337.2020/ASSET/IMAGES/LARGE/AJPREGU.00337.2020_F006.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adlanmerini M., Lazar M.A. The REV-ERB nuclear receptors: timekeepers for the core clock period and metabolism. Endocrinology. 2023:164. doi: 10.1210/ENDOCR/BQAD069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho H., Zhao X., Hatori M., Yu R.T., Barish G.D., Lam M.T., et al. 2012. Regulation of circadian behaviour and metabolism by REV-ERB-a and REV-ERB-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng D., Liu T., Sun Z., Bugge A., Mullican S.E., Alenghat T., et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/SCIENCE.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Fang B., Emmett M.J., Damle M., Sun Z., Feng D., et al. GENE REGULATION. Discrete functions of nuclear receptor rev-erbα couple metabolism to the clock. Science. 2015;348:1488–1492. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bugge A., Feng D., Everett L.J., Briggs E.R., Mullican S.E., Wang F., et al. Rev-erbα and rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan D., Xiong Y., Trinh T.M., Xiao Y., Hu W., Jiang C., et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types downloaded from. Science. 2020;369:1388–1394. doi: 10.1126/science.aba8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding G., Li X., Hou X., Zhou W., Gong Y., Liu F., et al. Corrections & amendments author correction: REV-ERB in GABAergic neurons controls diurnal hepatic insulin sensitivity check for updates. 2021;595 doi: 10.1038/s41586-021-03654-5. [DOI] [PubMed] [Google Scholar]
- 24.Mang G.M., La Spada F., Emmenegger Y., Chappuis S., Ripperger J.A., Albrecht U., et al. Altered sleep homeostasis in rev-erbα knockout mice. Sleep. 2016;39:589–601. doi: 10.5665/SLEEP.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jager J., Timothy O'Brien W., Manlove J., Krizman E.N., Fang B., Gerhart-Hines Z., et al. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor rev-erbα. Mol Endocrinol. 2014;28:490–498. doi: 10.1210/ME.2013-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H., Zhao X., Hatori M., Yu R.T., Barish G.D., Lam M.T., et al. Regulation of circadian behavior and metabolism by rev-erbα and rev-erbβ. Nature. 2012;485:123. doi: 10.1038/NATURE11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adlanmerini M., Krusen B.M., Nguyen H.C.B., Teng C.W., Woodie L.N., Tackenberg M.C., et al. REV-ERB nuclear receptors in the suprachiasmatic nucleus control circadian period and restrict diet-induced obesity. Sci Adv. 2021;7 doi: 10.1126/SCIADV.ABH2007/SUPPL_FILE/SCIADV.ABH2007_TABLE_S2.ZIP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adlanmerini M., Nguyen H.C.B., Krusen B.M., Teng C.W., Geisler C.E., Peed L.C., et al. Hypothalamic REV-ERB nuclear receptors control diurnal food intake and leptin sensitivity in diet-induced obese mice. J Clin Invest. 2021:131. doi: 10.1172/JCI140424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dierickx P., Emmett M.J., Jiang C., Uehara K., Liu M., Adlanmerini M., et al. SR9009 has REV-ERB–independent effects on cell proliferation and metabolism. Proc Natl Acad Sci USA. 2019;116:12147–12152. doi: 10.1073/pnas.1904226116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/PHYSREV.00015.2003/ASSET/IMAGES/LARGE/Z9J0010402890022.JPEG. [DOI] [PubMed] [Google Scholar]
- 31.Chrobok L., Klich J.D., Jeczmien-Lazur J.S., Pradel K., Palus-Chramiec K., Sanetra A.M., et al. Daily changes in neuronal activities of the dorsal motor nucleus of the vagus under standard and high-fat diet. J Physiol. 2022;600:733–749. doi: 10.1113/JP281596. [DOI] [PubMed] [Google Scholar]
- 32.Chrobok L., Klich J.D., Sanetra A.M., Jeczmien-Lazur J.S., Pradel K., Palus-Chramiec K., et al. Rhythmic neuronal activities of the rat nucleus of the solitary tract are impaired by high-fat diet – implications for daily control of satiety. J Physiol. 2022;600:751–767. doi: 10.1113/JP281838. [DOI] [PubMed] [Google Scholar]
- 33.Chrobok L., Northeast R.C., Myung J., Cunningham P.S., Petit C., Piggins H.D. Timekeeping in the hindbrain: a multi-oscillatory circadian centre in the mouse dorsal vagal complex. Commun Biol. 2020;3:1–12. doi: 10.1038/s42003-020-0960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland J., Sorrell J., Yates E., Smith K., Arbabi S., Arnold M., et al. A brain-melanocortin-vagus Axis mediates adipose tissue expansion independently of energy intake. Cell Rep. 2019;27:2399–2410.e6. doi: 10.1016/J.CELREP.2019.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao W.H., Madden C.J., Morrison S.F. Inhibition of Brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2010;299 doi: 10.1152/AJPREGU.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skibicka K.P., Grill H.J. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705. doi: 10.1210/EN.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fozzato A., New L.E., Griffiths J.C., Patel B., Deuchars S.A., Filippi B.M. Manipulating mitochondrial dynamics in the NTS prevents diet-induced deficits in Brown fat morphology and glucose uptake. Life Sci. 2023;328 doi: 10.1016/J.LFS.2023.121922. [DOI] [PubMed] [Google Scholar]
- 38.Skibicka K.P., Grill H.J. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology. 2008;149:3605–3616. doi: 10.1210/EN.2007-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dierickx P., Zhu K., Carpenter B.J., Jiang C., Vermunt M.W., Xiao Y., et al. Circadian REV-ERBs repress E4bp4 to activate NAMPT-dependent NAD+ biosynthesis and sustain cardiac function. Nat. Cardiovasc. Res. 2022;1:45. doi: 10.1038/S44161-021-00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang B., Everett L.J., Jager J., Briggs E., Armour S.M., Feng D., et al. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell. 2014;159:1140–1152. doi: 10.1016/J.CELL.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pondel M. Calcitonin and calcitonin receptors: bone and beyond. Int J Exp Pathol. 2000;81:405–422. doi: 10.1046/J.1365-2613.2000.00176.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zakariassen H.L., John L.M., Lutz T.A. Central control of energy balance by amylin and calcitonin receptor agonists and their potential for treatment of metabolic diseases. Basic Clin Pharmacol Toxicol. 2020;127:163–177. doi: 10.1111/BCPT.13427. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes-Santos C., Zhang Z., Morgan D.A., Guo D.F., Russo A.F., Rahmouni K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology. 2013;154:2481–2488. doi: 10.1210/EN.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Continued food intake analysis of Control vs DVC RDKO mice fed a high-fat diet. A) Average meal size in grams, B) average meal size in kcal, C) average meal frequency, D) average within meal eating frequency, E) average time spent eating, and E) light, dark, and total kcal consumed on HFD (n = 4, mean ± SEM). Results were compared by Mann-Whitney U-test.
Pearson correlation for VO2 vs body weight. A) Pearson correlation for VO2 vs body weight of control and DVC RDKO mice fed HFD (n = 3).
DVC RDKO does not change molecular signatures for BAT activity on NCD. A) RTqPCR for markers for BAT activity and SNS activity in the BAT of NCD-fed control and DVC RDKO animals (n = 3, mean ± SEM). Results were compared by Mann-Whitney U-test.
DVC RDKO decreases Th expression in iWAT on HFD and NCD. RTqPCR for Th in iWAT samples from A) HFD and B) NCD fed mice (n = 3-4, mean ± SEM). Results were compared by Mann-Whitney U-test.
Data Availability Statement
Data will be made available on request.