Abstract
BACKGROUND:
Integrating quality-of-life (QOL) outcomes into clinics may assist providers in identifying and responding to problems experienced by cancer survivors. To date, however, patient-reported outcomes (PROs) such as QOL are used infrequently to guide care. We integrated QOL assessments into a prostate cancer survivorship clinic and compared recovery and satisfaction among men managed in the survivorship clinic with those followed with more routine care.
METHODS:
We conducted a before-after study comparing 235 men treated surgically for prostate cancer who received routine follow-up care with 102 men managed in a survivorship clinic characterized by point-of-care QOL reporting and integration of QOL scores (EPIC) following radical prostatectomy. We then assessed baseline and postprostatectomy QOL at 6 and 12 months, as well as patient satisfaction, and compared outcomes between groups.
RESULTS:
Although baseline QOL was comparable, scores were generally higher among the survivorship group at 6 months and 1 year compared with those followed with routine care. In particular, sexual function scores were significantly higher among patients managed in the survivorship clinic (52.2 vs 33.6 at 1 year, P <.01). Satisfaction scores were consistently higher in the survivorship clinic group compared with the routine-care group (all P <.05).
CONCLUSIONS:
Patient QOL and satisfaction were higher among men managed in a survivorship program, suggesting that disease-specific survivorship clinics that integrate QOL reporting into care pathways may yield better outcomes compared with less tailored approaches to patient care following cancer therapy.
Keywords: prostate cancer, survivorship care, quality of life assessment
INTRODUCTION
Although most men treated for localized prostate cancer experience favorable cancer outcomes, treatment is often associated with lasting functional and quality-of-life (QOL) impairments. For example, men treated surgically (prostatectomy) face a 10%−15% risk of problematic urinary incontinence and greater than 50% risk of erectile dysfunction.1,2 Radiation therapy is associated with similar QOL deficits, including sexual dysfunction, exacerbation of obstructive urinary symptoms, and bothersome bowel symptoms.3–5 As a result, there has been long-standing interest in assessing quality-of-life outcomes among this patient population. However, prior efforts in this area have been primarily research oriented, focusing on instrument development, measurement and assessment of treatment-related consequences. Relatively little work has focused on integrating or translating QOL outcomes into clinical settings to guide patient care, in part because of a lack of available point-of-care tools to collect, score, and interpret patient-reported outcomes during clinic visits. Other barriers limiting the use of QOL in clinical care include logistical issues, such as lack of provider time to track functional outcomes or use them to guide interventions. As a result, treatment-related impairments and health problems may go unrecognized or underassessed.
Integrating QOL measurement into clinical settings may positively impact patient care, particularly in preference-sensitive conditions that involve trade-offs in potential complications and health states. These objectives are also concord-ant with Institute of Medicine recommendations to incorporate systematically developed assessment tools into routine clinical care to better identify the late effects of cancer and its treatment.6 The use of computer-based, interactive surveys in the clinic, for example, has the potential to improve provider and patient awareness of treatment-related symptoms and impairments in a timely manner. Previous research has demonstrated a number of benefits of using computerized QOL assessment systems in the clinical setting.7,8 Implementing QOL assessment in clinical practice has been shown to increase physician awareness of QOL issues and improve patient-physician communication during consultation9,10 and has led to better outcomes in other health domains, such as patient well-being and satisfaction.11,12
Despite these encouraging findings, point-of-care QOL assessment is not yet widespread. In addition to practical challenges associated with translating QOL information into clinical settings, the extent to which incorporating quantitative PROs into routine clinical care improves patient care and outcome is not fully known. In this context, we hypothesized that using PROs to guide survivorship care improves clinical outcomes and sought to further study this area by integrating QOL assessments into the routine follow-up care of prostate cancer survivors and adapting survivorship care to patient-reported outcomes QOL assessment.
MATERIALS AND METHODS
Study Design and Cohort
A total of 337 men treated surgically (radical prostatectomy) for localized prostate cancer (stage and Gleason information) were included in the study. A controlled before-after observational cohort study design was used because of practical limitations in performing a randomized controlled trial, such as contamination issues associated with randomizing patients to a clinic-level intervention at a single institution and complexity-related challenges associated with clustered or group randomization across multiple clinics and/or institutions. Quasiexperimental approaches, such as before-after study designs, are often used in settings in which randomization is not practical or possible.13,14 The “before” group consisted of 235 men treated with radical prostatectomy at the University of Michigan between April 2003 and March 2006 who were followed with routine clinical follow-up care. The “after” group consisted of 102 men treated surgically at the University of Michigan between 2008 and 2010. Study patients in the ‘after” group were followed in a dedicated prostate cancer survivorship program that incorporated point-of-care QOL assessment into follow-up clinic visits to guide care, and dedicated nursing and sexual health therapist consultations to address detected incontinence and sexual dysfunction following surgery. Survey panels were administered and collected at regular intervals (baseline, and 6, 12, and 24 months posttreatment) in both groups.
Surveys and Study Outcome Measures
Aspects of functional recovery (urinary continence and erectile dysfunction), and corresponding disease-specific quality of life were assessed using the Expanded Prostate Cancer Index Composite (EPIC).15 EPIC is a reliable and valid health scale consisting of 26 items covering 5 domains (urinary continence, urinary irritation, sexual function, bowel function, and vitality) and is scored on a 0–100 summary scale, with higher scores correspond to higher health states. Patient satisfaction was assessed using a cancer-specific adaptation of the Service Satisfaction Scale (SCa).16,17 The SCa consists of 16 items and measures several aspects of satisfaction, including satisfaction with outcome, provider manner and skill, health information, and access. Responses are scored and converted to a 0–100 scale, with higher scores indicating higher levels of patient satisfaction. Surveys were self-completed by study participants prior to surgery (baseline) and at regular intervals (6, 12, and 24 months in follow-up) after surgery. “Before”-group patients completed surveys in paperpencil format, whereas “after”-group patients completed electronic surveys to facilitate point-of-care scoring. Changes in quality-of-life scores served as the primary outcome, and satisfaction scores served as the secondary study outcome. In addition to absolute scores, time to functional recovery, defined as the time required for scores to return to 75% of baseline, was also examined as an outcome measure.18
Study Setting
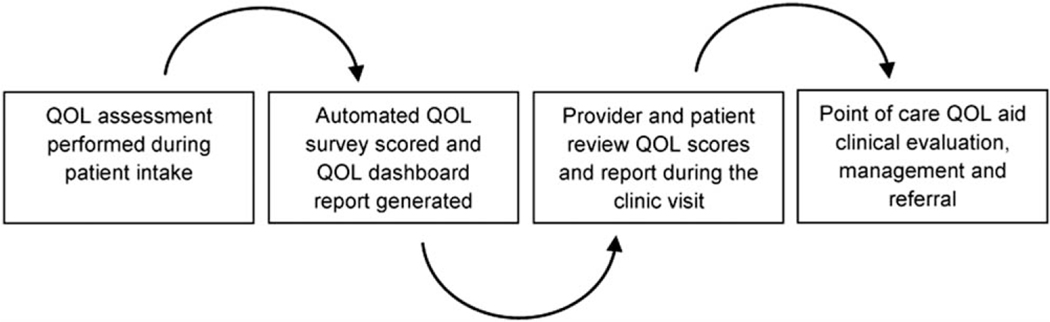
All study patients were treated at a high-volume tertiary referral academic cancer center. “Before”-group patients were managed through standard clinical evaluation and management pathways without PRO integration or use of QOL assessment to guide their subsequent care. In contrast, patients in the “after” group were followed through a dedicated prostate cancer survivorship clinic designed to objectively identify treatment-related dysfunction and deficits through an electronic, point-of-care QOL assessment system. The survivorship program was implemented in 2007 and consisted of 2 distinguishing features compared with the routine care provided through most urology clinics: 1) integration of patient-reported QOL outcome reporting at the point of care; 2) protocol-driven referral to dedicated nursing, pelvic floor physical therapy and sexual health therapist consultations during follow-up visits tailored to reported QOL outcomes. Through this system, QOL surveys were administered, scored, and output into dashboard reports for patient and provider review during clinic visits. Dashboards included prior QOL scores if available, calculated minimally important differences,19 and displayed score trajectories over time to provide objective patient-specific outcomes and changes (increased or declines) in QOL scores. Dashboards were available for providers and patient review during clinic visits with the intent that they be used as a clinical tool to identify areas of concern and prompt referral to rehabilitation services, such as sex therapists, pelvic floor rehabilitation physical therapists, or an erectile dysfunction specialist (Fig. 1, Addendum).
Figure 1.
Survivorship clinic process schema.
Statistical Analysis
EPIC scores in “before” and “after” groups were compared using the Wilcoxon rank-sum test for each time. Adjusted generalized linear models (GLMs) were then used to assess the independent effect of management in the survivorship clinic (“after” group) compared with usual care (“before” group). Variables included in the models were group (“after” vs “before”), age, race (white, black, other), BMI (obese, overweight, normal), Gleason score (≤6, 7, 8–10), stage (T3 vs T2), nerve sparing (yes vs no), and surgical approach (robotic/laparoscopic vs open), as well as patient history of diabetes, hypertension, prior myocardial infarction, or other cancer. All tests were performed with a 2-sided significance level set at .05. This analysis focused on 1-year cross-sectional scores. Functional recovery, defined as recovery of QOL scores to at least 75% of the baseline level for each subject, was also examined and compared between “before” and “after” groups.18 Differences between the groups in patient satisfaction were then assessed at 6 and 12 months using the Wilcoxon rank-sum test. Differences between the “before” and “after” groups in clinical and demographic variables (eg, changes in stage and Gleason score, and differences in percentage of cases managed with nerve-sparing and/or robot-assisted laparoscopic prostatectomy) were explicitly examined and included in models to account and adjust for potential secular trends given the before-after study design. Sample-size estimates using 0.5 standard deviations of a QOL measure as a threshold to determine clinically meaningful differences between groups estimated that a sample of 100 patients per group (based on the size of the “after” group) would provide 0.9 power at a .05 significance level. All tests were performed at the 5% significance level (2 sided) using standard statistical software (SAS, 9.4, Cary, NC). The study was approved and overseen by the University of Michigan Institutional Review Board.
RESULTS
Clinical and demographic variables are shown in Table 1. There were no differences in age or race. Although most men were white in both groups, black men comprised between 5% and 6% of both the “before”-group and the “after”-group cohorts. No differences in clinical stage were noted, although the “after” group was characterized by slightly lower prostate-specific antigen values (median, 6.1 and 4.7 ng/mL for the “before” and “after” groups, respectively). Similarly, there were a greater number of Gleason 6 cases among the “before” group, likely reflecting secular changes in the management of low-risk, early-stage prostate cancer and stricter treatment criteria among the “after”-group patients.
TABLE 1.
Clinical and Demographic Variables Between “Before” and “After” Groups
| Before | After | P | |
|---|---|---|---|
| n | 235 | 102 | |
| Age | 59.3 (38.8–76.2) | 60.8 (43.4–76.2) | .43 |
| BMI | 27.9 (19.2–41.1) | 28.3 (17.8–39.6) | .14 |
| Race | .13 | ||
| White | 217 (93.1) | 91 (89.2) | |
| Black | 13 (5.6) | 6 (5.9) | |
| Other | 3(1.3) | 5 (4.9) | |
| Comorbidities | |||
| Diabetes | 22 (9.4) | 10 (9.8) | .90 |
| Hypertension | 86 (36.6) | 53 (52.0) | .01 |
| CAD/MI | 14 (6.0) | 3 (2.9) | .25 |
| Previous cancer | 30 (12.8) | 9 (8.8) | .30 |
| PSA | 6.1 (0.5–51.0) | 4.7 (0.6–54.3) | <.0001 |
| Gleason score | <.0001 | ||
| ≤6 | 123 (52.3) | 21 (21.0) | |
| 7 | 103 (43.8) | 73 (73.0) | |
| 8–10 | 9 (3.8) | 6 (6) | |
| Stage | .44 | ||
| Stage 2 | 194 (84.0) | 89 (87.3) | |
| Stage 3 | 37 (16.0) | 13 (12.7) | |
| Nerve sparing | 198 (85.7) | 86 (84.3) | .74 |
| Approach | <.0001 | ||
| Open | 140 (59.6) | 6 (5.9) | |
| Laparoscopic/robotic | 95 (40.4) | 96 (94.1) |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; PSA, prostate-specific antigen.
Quality-of-life and patient satisfaction scores at 6 months and 1 year are shown in Table 2. Significant differences were noted in sexual function and vitality domains of EPIC at 1 year, favoring the “after” group (33.6 vs 52.2 for sexual function scores). The “after” group also consistently demonstrated higher satisfaction scores across all 4 domains of satisfaction (outcome, service, information, and access). For example, the “after”-group scores were 95.8 for satisfaction with outcomes and 93.7 for satisfaction with access compared with 89.0 and 89.5, respectively, in the “before” group.
TABLE 2.
Mean QOL and Satisfaction Scores in “Before” and “After” Groups
| Baseline |
6 Months |
1 Year |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | Before | After | P | |
| Urinary incontinence | 94.1 | 94.5 | .70 | 72.4 | 74.6 | .24 | 78.9 | 78.0 | .89 |
| Urinary irritation | 86.3 | 88.5 | .35 | 90.7 | 93.3 | .08 | 92.7 | 94.9 | .34 |
| Sexual function | 77.6 | 71.9 | .30 | 25.3 | 43.2 | <.01 | 33.6 | 52.2 | <.01 |
| Bowel function | 96.0 | 95.2 | .52 | 95.9 | 97.8 | .07 | 95.2 | 96.9 | .19 |
| Hormone function | 91.3 | 94.0 | <.01 | 90.1 | 94.5 | .01 | 90.2 | 95.1 | .03 |
| Satisfaction with outcome | 88.2 | 93.1 | <.01 | 89.0 | 95.8 | <.01 | |||
| Satisfaction with manner/skill | 91.1 | 95.4 | <.01 | 90.5 | 97.0 | <.01 | |||
| Satisfaction with information | 88.1 | 94.8 | <.01 | 87.9 | 96.4 | <.01 | |||
| Satisfaction with access | 89.3 | 93.1 | <.01 | 89.5 | 93.7 | .02 | |||
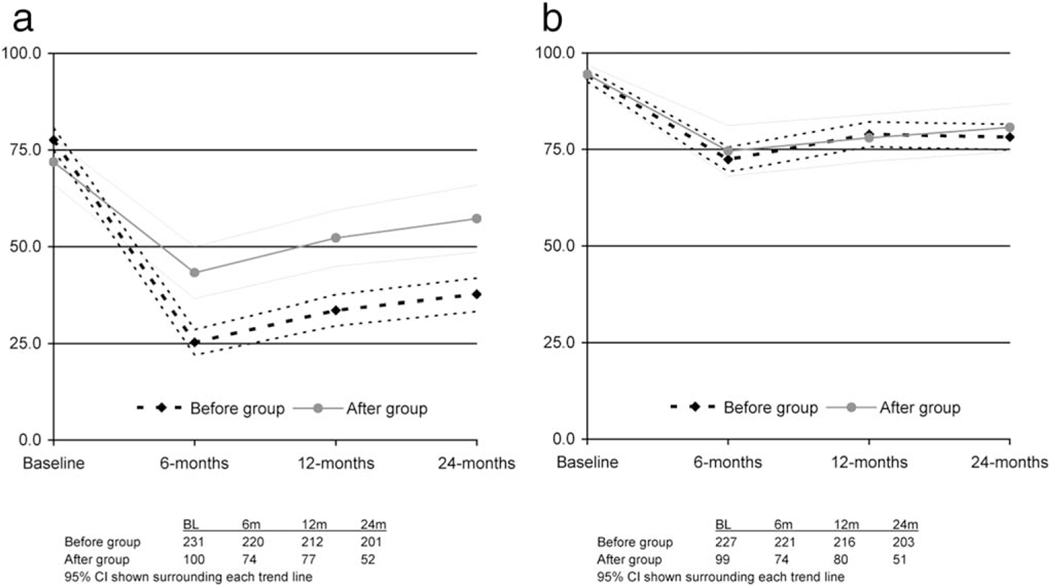
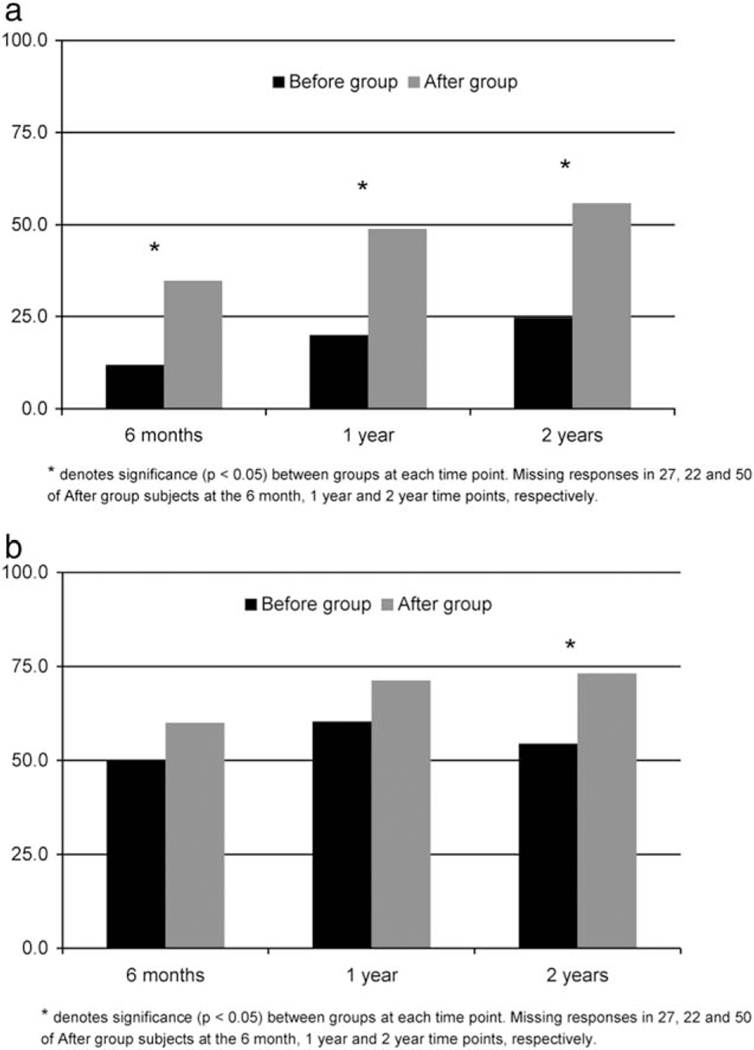
Figures 2 and 3 display recovery of sexual function and urinary continence across assessment points and the proportion of men achieving recovery to 75% of baseline, respectively. Although both groups started with similar baseline scores, sexual function scores were higher at 6, 12, and 24 months among those managed through the survivorship clinic (Fig, 2A), and a greater proportion of men in the “after” group reached 75% of their baseline sexual function score (Fig. 3A). Although urinary continence scores were similar in the groups across all times (Fig. 2B), a high percentage of patients in the “after” group achieved recovery of urinary incontinence scores to 75% of baseline scores (Fig. 3B).
Figure 2.
(A) Mean EPIC sexual function scores in “before” and “after” groups. (B) Mean EPIC urinary continence scores in “before” and “after” groups.
Figure 3.
(A) Recovery to 75% of baseline sexual function scores in “before” and “after” groups, (B) Recovery to 75% of baseline urinary function in “before” and “after” groups.
Results of the adjusted generalized linear regression models are shown in Table 3. Although the survivorship clinic was not associated with better urinary function, the “after” group was independently associated with higher sexual function and satisfaction scores. Older age and diabetes were also independently associated with decreased sexual function scores. Other potential confounding factors, such as disease stage, Gleason score, performance of nerve sparing, and the use of robot-assisted laparoscopic prostatectomy, were not significantly associated with outcomes in the adjusted GLM.
TABLE 3.
Multivariable-Adjusted Modeled Differences in Means for Functional and Satisfaction Scores
| Variable | Sexual Function |
Satisfaction With Outcome |
||||
|---|---|---|---|---|---|---|
| Δ in Mean | SE | P | Δ in Mean | SE | P | |
| Before group | Referent | - | - | Referent | - | - |
| After group | 19.539 | 4.145 | <.0001 | 5.985 | 1.659 | .0004 |
| Patient age | −0.898 | 0.273 | .001 | 0.092 | 0.101 | .366 |
| Diabetes | Referent | - | - | Referent | - | - |
| No diabetes | 12.712 | 5.993 | .035 | −2.988 | 2.165 | .169 |
| Nerve sparing | Referent | - | - | Referent | - | - |
| Non-nerve sparing | −4.117 | 4.913 | .403 | −0.621 | 1.878 | .741 |
| Robotic/laparoscopic | Referent | - | - | Referent | - | - |
| Open | 1.446 | 7.950 | .856 | 0.657 | 2.869 | .819 |
DISCUSSION
A major goal of survivorship research is to identify and remediate functional disabilities related to cancer and its treatment. To move these objectives forward, concerted efforts to integrate assessment tools into routine clinical care are needed. Using point-of-care PRO QOL assessments and dashboards in a dedicated survivorship clinic, we found that functional scores, recovery, and satisfaction improved compared with routine care. Most notably, postprostatectomy sexual function scores and the likelihood of recovering to within 75% of baseline were significantly greater among men in the “after” group than in those in the “before” group. However, only about half of men in the “after” group achieved this level of recovery, indicating that even in survivorship care settings resourced with dedicated functional recovery personnel and programs, substantial progress is still needed. Another key finding in our study was that the survivorship clinic was independently associated with both sexual recovery and patient satisfaction. Although there were differences in group characteristics (eg, Gleason score, use of robot-assisted laparoscopic approach) that may have contributed to differences in functional outcomes, those factors were not significantly associated with better outcomes in adjusted models, and the survivorship program (“after” group) remained the primary predictor of better outcomes in final models. Our results were further bolstered by the extent to which survivorship care improved patient satisfaction; men in the “after” group endorsed significantly higher satisfaction scores across all measured domains (satisfaction with outcome, access, manner/skill, and information). These results suggest that dedicated programs that incorporate PRO into clinical care improve care on a number of fronts.
To date, research efforts among early-stage prostate cancer survivors have focused primarily on measuring and comparing patient-reported QOL outcomes following local therapy.1,5,20,21 Despite the importance of this work, there has been little effort to examine if a better understanding of the consequences that commonly result from managing early-stage prostate cancer translate into better recognition and management of functional and quality-of-life deficits or to improvements in the survivorship experience. A major barrier to achieving this goal has been the failure to incorporate patient-reported QOL widely into clinical practice. Nevertheless, using a computer-based, interactive format has the potential to improve provider awareness of patient symptoms and health impairments at the point-of-care. Previous studies have shown beneficial effects of using computerized QOL assessment systems in the clinical setting. Implementing QOL assessment in clinical practice increases physician awareness of QOL issues, improves patient-physician communication, and can help patient well-being.22 For example, Detmar and colleagues found that quality-of-life issues were discussed significantly more frequently when QOL survey results were available during clinic visits and that physicians were more likely to identify moderate to severe health problems when incorporating PRO into their clinical assessments.9 Velikova and colleagues have shown that point-of-care QOL reports not only broaden issues discussed during clinic visits and focus physician evaluations on patient-reported concerns, but also contribute to patient well-being and improve subsequent QOL.10,12 Similar to our findings, Giesler et al reported long-term improvement in QOL outcomes among men treated for localized prostate cancer who participated in a nurse-driven intervention focused on computer capture and review of serial QOL assessment following treatment compared with those who did not.23 Currently, however, patient-reported outcomes such as quality of life are seldom assessed or integrated in most clinical settings.
The results of our study indicate that clinic-level systems that collect, report, and integrate quality-of-life assessments and outcomes into the management of men treated for early-stage prostate cancer can help to increase recognition of the functional problems associated with prostatectomy (eg, incontinence and erectile dysfunction), direct the evaluation and management of functional deficits, and improve key patient outcomes, such as functional recovery and patient satisfaction. The success of such systems hinges not only on point-of-care PRO assessment, but also on the integration of those outcomes into clinic care through a reporting infrastructure (eg, dashboards), and linking PRO assessment to downstream clinical resources that exist within the framework of a survivorship program, such as dedicated nurses who focus on reviewing concerns and detected deficits with patients, physical therapists specializing in pelvic floor rehabilitation, and sex therapists and counselors. In the setting of the University of Michigan Prostate Cancer Survivorship Program, patients experienced significant benefits. Not only were functional QOL scores higher than patients managed through a dedicated, comprehensive survivorship program, patient satisfaction was consistently higher across several satisfaction domains, including satisfaction with outcomes, provider skill, information, and access. These results likely reflect more responsive, expert, and timely management facilitated by the availability of PROs at the point of care. Higher satisfaction scores, moreover, may reflect better service experiences, in part through ready access to health information and resources among men managed trough the survivorship clinic. More broadly, the results of this study suggest that comprehensive survivorship programs that integrate PRO assessments into the clinical work flow of patient care may translate to more patient-centered care and better health outcomes following cancer treatment.
The findings from our study should be interpreted in the context of several limitations. First, this was a nonrandomized study, so causality between PRO reporting and the survivorship clinic cannot be directly inferred, given potential confounding and selection bias inherent in nonrandomized studies. We used a quasiexperimental before-after approach largely because of pragmatic concerns related to possible contamination resulting from randomizing a clinicwide intervention within a single clinical setting and the inability to implement a larger, multi-institutional clustered randomization scheme, given the initial scope of the project. Furthermore, the before-after study design leveraged existing QOL data from a relatively contemporaneous cohort of men treated with prostatectomy. Second, our study was limited to men treated surgically with radical prostatectomy. Although early-stage prostate cancer can be treated with a number of modalities (surgery, external radiation therapy, interstitial brachytherapy, cryotherapy), we chose to focus on surgery to simplify the study’s implementation and because data from a surgical “before” group was readably accessible. Our approach and findings, however, are likely applicable across treatment modalities. Third, secular trends in surgical approach represent a possible source of confounding. As reflected in Table 1, a larger percentage of men underwent robotic-assisted laparoscopic prostatectomy in the “after” group than in the “before” group. The timeframe of “after”-group accrual coincided with the introduction and more frequent use of robotic prostatectomy; however, most evidence comparing open with robotic prostatectomy has not demonstrated a substantial difference in functional outcomes between the 2 approaches, particularly during the learning phase of robotic prostatectomy.24–26 Further, the use of robotic-assisted surgery was not significantly associated with better QOL scores in our study, suggesting that this potential confounder did not affect our results significantly. Other surgical techniques may have also differed between groups. For example, the use of anterior urethropexy has been used relatively frequently during robotic prostatectomy to decrease urethral mobility and aid in recovery of urinary continence.27 Our findings uncovered more substantial differences in erectile and sexual functional outcomes than for continence outcomes, so the application of such nuanced surgical techniques may not translate to substantial gains in PROs. The larger effect in sexual recovery may have also resulted from relatively low rates of urinary incontinence with current surgical and postoperative care. Men in the “after” group also reported significantly higher satisfaction, which may correlate directly with more patient-centered care or may potentially be influenced by more frequent visits with health care providers. However, the increase in satisfaction scores across all satisfaction domains, including satisfaction with outcome supports the former association. Despite these limitations, the results of this study provide useful information regarding the advantage of integrating and responding to PROs in clinical practice.
Conclusions
Integrating PROs such as QOL into clinical practice through practical point-of-care systems and responding to those outcomes through a comprehensive, structured approach based on dedicated resources improve functional outcomes and are associated with higher patient satisfaction. Although these initial finding support survivorship models of care to address and manage treatment-related deficits among cancer survivors, additional larger-scale studies performed using randomized approaches are necessary to better define the efficacy of survivorship care programs.
Supplementary Material
FUNDING SUPPORT
This study was funded by an AUA Foundation Research Scholarship.
Daniela Wittmann reports a financial interest in Pfizer, Inc. David C. Miller discloses relationships with Arbor Matrix and Blue Cross and Blue Shield of Michigan. Brent K. Hollenbeck discloses a relationship with Ellseiver. John T. Wei discloses financial relationships/interest with Johnson & Johnson, Neotract, Nxthera, and Histosonics, Inc.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
James E. Montie discloses a financial interest in Histosonics, Inc.
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. [DOI] [PubMed] [Google Scholar]
- 2.Penson DF, McLerran D, Feng Z, et al. 5-Year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2005;173:1701–1705. [DOI] [PubMed] [Google Scholar]
- 3.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–135. [DOI] [PubMed] [Google Scholar]
- 4.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. [DOI] [PubMed] [Google Scholar]
- 5.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate cancer survivors. N Engl J Med. 2008;358:1250–1261. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 7.Hanscom B, Lurie JD, Homa K, Weinstein JN. Computerized questionnaires and the quality of survey data. Spine. 2002;27:1797–1801. [DOI] [PubMed] [Google Scholar]
- 8.Detmar SB, Aaronson NK. Quality of life assessment in daily clinical oncology practice: a feasibility study. Eur J Cancer. 1998;81:87–94. [DOI] [PubMed] [Google Scholar]
- 9.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality of life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034. [DOI] [PubMed] [Google Scholar]
- 10.Velikova G, Brown JM, Smith AB, Selby PJ. Computer-based quality of life questionnaires may contribute to doctor-patient interactions in oncology. Br J Cancer. 2002;86:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu J, Huang J, Fung V, Robertson N, Jimison H, Frankel R. Health information technology and physician-patient interactions: impact of computers on communication during outpatient primary care visits. J Am Med Inform Assoc. 2005;12:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice imporves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–724. [DOI] [PubMed] [Google Scholar]
- 13.Meyer BD. Natural and quasi-experiments in economics. J Business Economic Stat. 1995;13:151–161. [Google Scholar]
- 14.Eccles M, Grimshaw J, Campbell M, Ramsay C. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care. 2003;12:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. [DOI] [PubMed] [Google Scholar]
- 16.Greenfield TK, Attkinson CC. Steps towards a multifactorial satisfaction scale for primary care and mental health services. Evaluation and Program Planning. 1989;12:271–278. [Google Scholar]
- 17.Kamo N, Dandapani SV, Miksad RA, et al. Evaluation of the SCA instrument for measuring patient satisfaction with cancer care administered via paper or via the Internet. Ann Oncol. 2011;22:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krupski TL, Saigal CS, Litwon MS. Variation in continence and potency by definition. J Urol. 2003;170(4 Pt 1):1291–1294. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Clinical Significance Consensus Meeting Group. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002; 77:367–370. [DOI] [PubMed] [Google Scholar]
- 20.Johnson TK, Gilliland FD, Hoffman RM, et al. Racial/ethnic differences in functional outcomes in the 5 years after diagnosis of localized prostate cancer. J Clin Oncol. 2004;22:4193–4201. [DOI] [PubMed] [Google Scholar]
- 21.Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: results from CapSURE. J Urol. 2004;171(2 Pt 1):703–707. [DOI] [PubMed] [Google Scholar]
- 22.Berry DL, Blumenstein BA, Halpenny B, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol. 2011;29:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giesler RB, Given B, Given CW, et al. Improving the quality of life of patients with prostate cancer: A randomized trial testing the efficacy of a nurse-driven intervention. Cancer. 2005;104:752–762. [DOI] [PubMed] [Google Scholar]
- 24.Hu JC, Wang Q, Pashos CL, Lipsitz SR, Keating NL. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008;26:2278–2284. [DOI] [PubMed] [Google Scholar]
- 25.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302: 1557–1564. [DOI] [PubMed] [Google Scholar]
- 26.Gandaglia G, Sammon JD, Chang SL, et al. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol. 2014;32:1419–1426. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EK, Hedgepeth RC, He C, Wood DP Jr. The impact of anterior urethropexy during robotic prostatectomy on urinary and sexual outcomes. J Endourol. 2011;25:615–619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.