Abstract
Cancer-related cognitive impairment (CRCI) is a significant problem for patients receiving chemotherapy. While a growing amount of pre-clinical and clinical evidence suggests that inflammatory mechanisms underlie CRCI, no clinical studies have evaluated for associations between CRCI and changes in gene expression. Therefore, the purpose of this study was to evaluate for differentially expressed genes and perturbed inflammatory pathways across two independent samples of patients with cancer who did and did not report CRCI. The Attentional Function Index (AFI) was the self-report measure used to assess CRCI. AFI scores of <5 and of >7.5 indicate low versus high levels of cognitive function, respectively. Data from 182 patients in Sample 1 were analyzed using RNA-seq. Data from 158 patients in Sample 2 were analyzed using microarray. Of the 185 patients in Sample 1, 49.2% had an AFI score of <5 and 50.8% had an AFI score of >7.5. Of the 158 patients in Sample 2, 50.6% had an AFI score of <5 and 49.4% had an AFI score of >7.5. Twelve KEGG signaling pathways were significantly perturbed between the AFI groups, five of which were signaling pathways related to inflammatory mechanisms (e.g., cytokine-cytokine receptor interaction, tumor necrosis factor signaling). This study is the first to describe perturbations in inflammatory pathways associated with CRCI. Findings highlight the role of cytokines both in terms of cytokine-specific pathways, as well as pathways involved in cytokine production and cytokine activation. These findings have the potential to identify new targets for therapeutics and lead to the development of interventions to improve cognition in patients with cancer.
Keywords: cancer-related cognitive impairment, chemotherapy, IL-17 signaling pathway, MAPK signaling pathway, gene expression, neuroinflammation
1. Introduction
While advances in cancer treatments have increased survival rates, they are not without significant adverse effects. Cognitive impairment, which is often associated with chemotherapy and originally referred to as “chemobrain,” is one such adverse effect. However, because recent evidence suggests that cognitive impairment is associated with other types of cancer treatment as well as with the cancer itself, it was renamed cancer-related cognitive impairment (CRCI).1 CRCI includes changes in a wide range of cognitive functions (e.g., memory, learning, attention, concentration, processing, executive function).2 In terms of its impact, patients with CRCI report decrements in job performance and productivity,3, 4 as well as increases in interpersonal and social strain,5 embarrassment,5 and distress.6 Despite the large number of studies that have evaluated a variety of interventions for CRCI (e.g., cognitive training, pharmacologic, exercise; for reviews see7–12), improvements in cognitive function are inconsistent. The development of effective interventions for CRCI is hampered by a poor understanding of its underlying mechanism(s).13 Given that CRCI is reported by as many as 75% of patients undergoing cancer treatment,14 continued research is warranted to determine its underlying mechanism(s).
As noted in several reviews,2, 13, 15–18 one of the most frequently hypothesized mechanisms for CRCI involves the direct and indirect effects of inflammation. Inflammation appears to play a central role in CRCI because this process occurs as a result of the cancer itself and/or cancer treatments.19 Specifically, cytokines are dysregulated in response to the presence of tumor cells,20 chemotherapy,18 radiation therapy,21 and/or stress.22, 23
Only five studies were identified that evaluated for associations between self-reported cognitive impairment and serum or plasma levels of pro- or anti-inflammatory cytokines.24–28 In two studies of patients receiving chemotherapy,24, 28 no associations were found between serum cytokines and self-reported cognitive impairment. However, in a series of three studies from two overlapping cohorts of women with breast cancer receiving chemotherapy,25–27 associations were found between CRCI and altered levels of plasma cytokines. Across these three studies,25–27 while one evaluated for associations with tumor necrosis factor (TNF)-α and interleukin (IL)-6,26 the other two25, 27 included several additional cytokines (i.e., IL-1β, IL-2, IL-4, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor, interferon-γ). In all of these studies, increased levels of IL-6 were associated with higher levels of perceived cognitive impairment.25–27 In the two studies that found associations with higher levels of IL-4,25, 27 while one found decrements27 the other found improvements in self-reported cognitive function.25
Taken together, these findings suggest that inflammatory mechanisms play a role in self-reported changes in cognitive function. However, the positive associations were with a single sample of patients with breast cancer who had CRCI assessed using the Functional Assessment of Cancer Therapy-Cognitive Function scale. The inconsistent findings across the five studies24–28 may be related to the timing of the measures in relationship to the receipt of chemotherapy and the relatively small sample sizes in two of the studies.24, 28 In addition, two of the studies did not report whether they controlled for diurnal variations in serum cytokines.24, 28 Additional studies are needed that use other biomarkers to evaluate for associations between inflammatory mechanisms and CRCI.
Three pre-clinical studies were identified that evaluated for changes in gene expression associated with CRCI.29–31 In a study of mice treated with cyclophosphamide and mitomycin-C,31 perturbations in the cytokine-cytokine receptor interaction pathway were identified in the prefrontal cortex. In another study that evaluated cytokine profiles and gene expression changes in hippocampal tissue of mice treated with doxorubicin,29 upregulation of pro-inflammatory cytokines correlated with decreases in recognition and memory. Subsequent reversal of inflammation correlated with improvements in cognitive function. In a third study that evaluated the role of inflammation and oxidative stress in the hippocampus,30 rats treated with cyclophosphamide and doxorubicin demonstrated elevated cytokine levels and activation of the mitogen-activated protein kinase (MAPK) signaling pathway in hippocampal tissue, similar to changes observed in the aging brain. While the findings from these pre-clinical studies suggest a link between inflammatory pathways and CRCI,29–31 direct comparisons with patients are difficult because all three studies evaluated brain tissue and only one of them29 utilized behavioral testing (i.e., novel object recognition, fear conditioning, object to place tasks) to evaluate cognition. However, given the growing amount of pre-clinical and clinical evidence that suggests that inflammatory mechanisms underlie CRCI and the absence of clinical studies on associations between CRCI and changes in gene expression, we evaluated for differentially expressed genes and perturbed inflammatory pathways across two independent samples of patients with cancer who did and did not report CRCI.
2. METHODS
2.1. Patients and settings
This study is part of a larger, longitudinal study of the symptom experience of oncology outpatients receiving chemotherapy whose details are published elsewhere.32, 33 Eligible patients were ≥18 years of age; had a diagnosis of breast, gastrointestinal, gynecological, or lung cancer; had received chemotherapy within the preceding four weeks; were scheduled to receive at least two additional cycles of chemotherapy; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs.
2.2. Study procedures
The study was approved by the Institutional Review Board at each of the study sites. Of the 2234 patients approached, 1343 consented to participate (60.1% response rate). The major reason for refusal was being overwhelmed with their cancer treatment. Eligible patients were approached in the infusion unit during their first or second cycle of chemotherapy by a member of the research team to discuss study participation and obtain written informed consent. Data from the enrollment assessment (i.e., assessment of cognitive function in the week prior to the patient’s second or third cycle of chemotherapy) were used in this analysis. Blood for ribonucleic acid (RNA) isolation was collected at the enrollment assessment. Medical records were reviewed for disease and treatment information. For this study, a total of 717 patients provided a blood sample for the gene expression analyses (see Supplemental Figure 1).
2.3. Instruments
Demographic and clinical characteristics –
Demographic information was obtained using a self-report questionnaire. Functional status was assessed using the Karnofsky Performance Status (KPS) scale.34 The occurrence, treatment, and functional impact of 13 common medical conditions were assessed using the Self-Administered Comorbidity Questionnaire (SCQ).35 Alcohol consumption, behaviors, and associated problems were measured using the Alcohol Use Disorders Identification test (AUDIT).36
Cancer-related cognitive impairment (CRCI) assessment –
The 16-item Attentional Function Index (AFI) assesses an individual’s perceived effectiveness in performing daily activities that are supported by attention and working memory.37 A higher total mean score on a 0 to 10 numeric rating scale indicates better cognitive function.37 Total scores are grouped into categories of attentional function (i.e., <5 low function, 5.0 to 7.5 moderate function, >7.5 high function).38 The Cronbach’s α for the total AFI score was 0.93.
MAX2 index –
The toxicity of each patient’s chemotherapy regimen was rated using the MAX2 index. Briefly, the MAX2 score is the average of the most frequent grade 4 hematologic toxicity and the most frequent grade 3 to 4 nonhematologic toxicity reported in publications of a regimen and correlates well with the average overall risk of severe toxicity for that regimen.39, 40
2.4. Coding of the emetogenicity of the chemotherapy regimens and antiemetic regimens
The coding of the emetogenicity of the chemotherapy regimens and antiemetic regimens were described previously.41 Briefly, the Multinational Association for Supportive Care in Cancer guidelines42 were used to classify each chemotherapy drug in the regimen based on its emetogenic potential. Each antiemetic regimen was coded into one of four groups (Tables 1 and 2).
Table 1.
Differences in Demographic and Clinical Characteristics Between Patients in Sample 1 (RNA Seq) with Low and High Attentional Function Index Scores
| Characteristic | High AFI (score of >7.5) 50.8% (n=94) |
Low AFI (score of <5) 49.2% (n=91) |
Statistics |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 58.4 (10.0) | 54.6 (13.2) | t = 2.23, p = 0.027 |
| Education (years) | 16.1 (3.1) | 15.7 (3.0) | t = 1.01, p = 0.315 |
| Body mass index (kg/m2) | 26.6 (5.1) | 27.0 (7.1) | t = −0.43, p = 0.665 |
| KPS score | 83.6 (11.6) | 72.0 (11.7) | t = 6.78, p < 0.001 |
| Number of comorbidities | 2.1 (1.2) | 3.1 (1.6) | t = −4.88, p < 0.001 |
| SCQ score | 4.5 (2.5) | 7.5 (4.0) | t = −6.13, p < 0.001 |
| AUDIT score | 2.7 (2.3) | 3.1 (3.1) | t = −0.90, p = 0.369 |
| Time since diagnosis (years) | 1.6 (2.9) | 1.9 (3.2) | U, p = 0.308 |
| Time since diagnosis (median) | 0.44 | 0.45 | |
| Number of prior cancer treatments | 1.5 (1.3) | 1.6 (1.5) | t = −0.32, p = 0.751 |
| Number of metastatic sites including lymph node involvement | 1.3 (1.2) | 1.2 (1.2) | t = 0.65, p = 0.515 |
| Number of metastatic sites excluding lymph node involvement | 0.8 (1.0) | 0.7 (1.0) | t = 0.21, p = 0.837 |
| MAX2 score | 0.17 (0.08) | 0.19 (0.08) | t = −1.85, p = 0.066 |
| % (n) | % (n) | ||
| Male | 31.9 (30) | 15.4 (14) | |
| Hispanic mixed or other | 11.7 (11) | 18.7 (17) | |
| Married or partnered (% yes) | 63.7 (58) | 57.3 (51) | FE, p = 0.446 |
| Lives alone (% yes) | 19.6 (18) | 24.4 (22) | FE, p = 0.476 |
| Childcare responsibilities (% yes) | 17.8 (16) | 24.7 (22) | FE, p = 0.278 |
| Care of adult responsibilities (% yes) | 8.2 (7) | 11.0 (9) | FE, p = 0.606 |
| Currently employed (% yes) | 47.9 (45) | 24.2 (22) | FE, p = 0.001 |
| ≥$100,000 | 37.9 (33) | 28.9 (24) | |
| Rheumatoid arthritis | 2.1 (2) | 6.6 (6) | FE, p = 0.165 |
| Exercise on a regular basis (% yes) |
70.7 (65) | 62.1 (54) | FE, p = 0.268 |
| Smoking current or history (% yes) |
26.1 (24) | 40.4 (36) | FE, p = 0.058 |
| Lung | 5.3 (5) | 15.4 (14) | NS |
| Surgery & CTX & RT | 12.1 (11) | 14.6 (13) | |
| 28 day cycle | 6.4 (6) | 6.6 (6) | |
| High | 13.8 (13) | 22.0 (20) | |
| NK-1 receptor antagonist and two other antiemetics | 23.9 (22) | 33.0 (30) | |
| Mean AFI score at enrollment | 8.3 (0.7) | 3.8 (0.9) | t = 37.89, p < 0.001 |
Abbreviations: AFI = Attentional Function Index; AUDIT = Alcohol Use Disorders Identification Test; CTX = chemotherapy; FE = Fisher’s exact test; kg = kilograms; KPS = Karnofsky Performance Status; m2 = meter squared, n/a = not applicable; NK-1 = neurokinin-1; NS = not significant; RT = radiation therapy; SCQ = Self-administered Comorbidity Questionnaire; U = Mann-Whitney U test
Table 2.
Differences in Demographic and Clinical Characteristics Between Patients in Sample 2 (Microarray) with Low and High Attentional Function Index Scores
| Characteristic | High AFI (score of >7.5) 49.4% (n=78) |
Low AFI (score of <5) 50.6% (n=80) |
Statistics |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 57.9 (10.5) | 53.6 (12.4) | t = 2.33, p = 0.021 |
| Education (years) | 16.6 (2.7) | 16.0 (3.0) | t = 1.44, p = 0.152 |
| Body mass index (kg/m2) | 25.6 (5.3) | 28.1 (6.2) | t = −2.69, p = 0.008 |
| KPS score | 83.8 (10.2) | 74.2 (10.9) | t = 5.72, p < 0.001 |
| Number of comorbidities | 2.1 (1.1) | 2.9 (1.5) | t = −3.33, p = 0.001 |
| SCQ score | 4.7 (2.4) | 6.6 (3.3) | t = −4.16, p < 0.001 |
| AUDIT score | 2.6 (1.8) | 3.2 (3.1) | t = −1.17, p = 0.247 |
| Time since diagnosis (years) | 2.5 (4.2) | 2.5 (3.6) | U, p = 0.143 |
| Time since diagnosis (median) | 0.42 | 0.61 | |
| Number of prior cancer treatments | 1.9 (1.8) | 2.1 (1.6) | t = −0.86, p = 0.393 |
| Number of metastatic sites including lymph node involvement | 1.4 (1.4) | 1.2 (1.1) | t = 0.96, p = 0.336 |
| Number of metastatic sites excluding lymph node involvement | 0.9 (1.2) | 0.7 (1.0) | t = 0.98, p = 0.329 |
| MAX2 score | 0.17 (0.08) | 0.17 (0.08) | t = −0.26, p = 0.793 |
| % (n) | % (n) | ||
| Male | 25.6 (20) | 15.0 (12) | |
| Hispanic, Mixed, or Other | 5.2 (4) | 10.0 (8) | |
| Married or partnered (% yes) | 82.1 (64) | 51.2 (41) | FE, p < 0.001 |
| Lives alone (% yes) | 11.5 (9) | 26.3 (21) | FE, p = 0.025 |
| Childcare responsibilities (% yes) | 20.8 (16) | 26.3 (21) | FE, p = 0.456 |
| Care of adult responsibilities (% yes) |
9.7 (7) | 12.2 (9) | FE, p = 0.792 |
| Currently employed (% yes) | 48.7 (38) | 21.3 (17) | FE, p < 0.001 |
| ≥$100,000 | 62.8 (49) | 26.3 (21) | |
| Rheumatoid arthritis | 1.3 (1) | 3.8 (3) | FE, p = 0.620 |
| Exercise on a regular basis (% yes) |
80.8 (63) | 63.7 (51) | FE, p = 0.021 |
| Smoking current or history (% yes) | 32.1 (25) | 39.7 (31) | FE, p = 0.404 |
| Lung | 17.9 (14) | 7.5 (6) | |
| Surgery & CTX & RT | 18.2 (14) | 20.0 (16) | |
| 28 day cycle | 9.0 (7) | 6.3 (5) | |
| High | 19.2 (15) | 20.0 (16) | |
| NK-1 receptor antagonist and two other antiemetics | 17.6 (13) | 27.6 (21) | |
| Mean AFI score at enrollment | 8.4 (0.7) | 4.0 (0.8) | t = 36.03, p < 0.001 |
Abbreviations: AFI = Attentional Function Index; AUDIT = Alcohol Use Disorders Identification Test; CTX = chemotherapy; FE = Fisher’s exact test; kg = kilograms; KPS = Karnofsky Performance Status; m2 = meter squared; NK-1 = neurokinin-1; RT = radiation therapy; SCQ = Self-administered Comorbidity Questionnaire; U = Mann-Whitney U test
2.5. Acquisition and processing of gene expression data
The methods used for the gene expression analyses are described in detail elsewhere.33 In brief, gene expression of total RNA isolated from peripheral blood of the 717 patients who provided a blood sample was quantified for 357 patients using RNA-sequencing (RNA-seq) (i.e., Sample 1) and for 360 patients using microarray (i.e., Sample 2).
2.6. Data analyses
Demographic and clinical data –
Demographic and clinical data from the two patient samples were analyzed separately using SPSS Version 27 (IBM Computation, Armonk, NY). To evaluate for differences in gene expression using an extreme phenotype approach, patients were classified into two groups based on their AFI scores (i.e., <5 = low cognitive function versus >7.5 = high cognitive function). For each gene expression platform, differences in demographic and clinical characteristics between the two groups were evaluated using parametric and non-parametric tests. Logistic regression analyses were used to determine significant covariates for inclusion in the differential expression analyses.
Differential expression and pathway impact analyses (PIA) –
Differential expression was quantified using generalized linear models that were implemented separately for each sample (i.e., using edgeR43 for Sample 1 and limma44 for Sample 2).33 These analyses were adjusted for demographic and clinical characteristics that differed between patients who did and did not report CRCI, based on their AFI scores. In addition, the models included surrogate variables not associated with CRCI to adjust for potential batch effects.45 The differential expression results were summarized as the log fold-change and p-value for each gene. Only genes that had a common direction of expression (i.e., the same sign for the log fold-change) were retained for subsequent analyses (n=5,235). Sequence loci data were annotated with Entrez gene identifier. The gene symbols were annotated using the HUGO Gene Nomenclature Committee resource database.46 The differential expression results of the two datasets were merged at the gene level using the Entrez gene identifier. Fisher’s Combined Probability test was used to combine the differential gene expression results from both datasets using the uncorrected p-values.47, 48
To evaluate these results and interpret them in the context of CRCI-related mechanisms, we used PIA to test for patterns in higher orders of biology.33 PIA includes potentially important biological factors (e.g., gene-gene interactions, flow signals in a pathway, pathway topologies), the magnitude (i.e., log fold-change), and p-values from the combined differential expression analysis.49 The PIA included the results of the combined differential expression analysis for all genes having a common direction of differential expression (i.e., cutoff free) to determine probability of pathway perturbations (pPERT) using Pathway Express.50 A total of 214 signaling pathways were defined using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.51 Fisher’s Combined Probability test was used to combine the PIA tests from both datasets using the uncorrected p-values.47, 48 The significance of the combined transcriptome-wide PIA analysis was assessed using a family wise error rate (FWER) of 1% under the Bonferroni method.50 Finally, we evaluated these results for inflammatory pathways.
3. RESULTS
3.1. RNA-seq performance
Of the 357 patients whose gene expression was quantified using RNA-seq (i.e., Sample 1), 193 were in the extreme phenotype groups (i.e., AFI <5 = Low group versus AFI >7.5 = High group). Five of these patients were excluded as outliers or for poor RNA quantification (Supplemental Figure 1). Of the remaining 188 evaluable patients, an additional three patients were excluded for missing phenotypic data after imputation. Of the remaining 185 patients whose phenotype data were evaluated (Table 1), three patients were excluded from the gene expression analysis as outliers based on the multidimensional scaling plots. Median library threshold size was 9,273,000 reads. Following quality control filters, 13,301 genes were included in the final analysis. The common dispersion was estimated as 0.179, yielding a biological coefficient of variation of 0.423 well within the expected value for clinical samples.52 Data from 182 patients in Sample 1 (i.e., Low group (n=89), High group (n=93)) were analyzed using RNA-seq (Supplemental Figure 1).
3.2. Microarray performance
Of the 360 patients whose gene expression was quantified using microarray (i.e., Sample 2), 179 were in the extreme phenotype groups (i.e., AFI <5 = Low group versus AFI >7.5 = High group). Three of these patients were identified as outliers using distance array signal intensity distributions with arrayQualityMetrics (Supplemental Figure 1).53 Of the remaining 176 evaluable patients, an additional 18 patients were excluded for missing phenotypic data. The phenotype data for the remaining 158 patients were evaluated (Table 2). All of the samples demonstrated good hybridization performance for biotin, background negative, and positive control assays on the arrays. Limma was used for background correction, quantile normalization, and log2 transformation.44 Of the initial probes evaluated for quality (n = 46,542), 1953 probes had insufficient expression measurements (Illumina detection p-value <0.05) and were excluded, leaving 44,589 probes for analysis. Data from 158 patients in Sample 2 (i.e., Low group (n=80) and High group (n=78)) were analyzed using microarray (Supplemental Figure 1).
3.3. Differences in demographic and clinical characteristics
Of the 185 patients with phenotypic data in Sample 1, 49.2% had an AFI score of <5 and 50.8% had an AFI score of >7.5 (Table 1). Compared to the High group, patients in the Low group were significantly younger, more likely to be female, more likely to have a lower annual income, and less likely to be employed. In addition, patients in the Low group had lower KPS scores; a higher number of comorbidities; higher SCQ scores; were more likely to self-report a diagnosis of lung disease, depression, or back pain; and were less likely to have gastrointestinal cancer.
Of the 158 patients with phenotypic data in Sample 2, 50.6% had an AFI score of <5 and 49.4% had an AFI score of >7.5 (Table 2). Compared to the High group, patients in the Low group were significantly younger, more likely to have a lower annual income, less likely to be employed, less likely to be married or partnered, and more likely to live alone. In addition, patients in the Low group had lower KPS scores; a higher body mass index; a higher number of comorbidities; higher SCQ scores; were less likely to exercise on a regular basis; and were more likely to self-report a diagnosis of depression or back pain.
3.4. Logistic regression analyses
In the logistic regression analysis for Sample 1, seven variables were retained in the final model (i.e., age, ethnicity, current employment status, KPS score, SCQ score, self-reported diagnoses of depression, cancer diagnosis) and were used as covariates in the gene expression analysis (Table 3). Patients who were younger, had a lower KPS score, and a higher SCQ score were more likely to belong to the Low group. In addition, patients who were employed had a 70% decrease in the odds of belonging to the Low group. Patients who reported their ethnicity as Black had a 90% decrease in the odds of belonging to the Low group. Having a diagnosis of depression was associated with a 3.81 times increase in the odds of belonging to the Low group. Patients with gastrointestinal cancer had a 73% decrease in the odds of belonging to the Low group.
Table 3.
Multiple Logistic Regression Analyses Predicting Low Attentional Function Index Group Membership
| Sample 1 (n = 185) | |||
|---|---|---|---|
| Predictors | Odds Ratio | 95% CI | p-value |
| Age | 0.96 | 0.92, 0.99 | 0.041 |
| Hispanic, Mixed, or Other | 1.44 | 0.45, 4.56 | 0.538 |
| Currently employed | 0.30 | 0.12, 0.74 | 0.009 |
| Karnofsky Performance Status score | 0.93 | 0.90, 0.97 | < 0.001 |
| Self-administered Comorbidity Questionnaire score | 1.24 | 1.06, 1.47 | 0.009 |
| Self-reported diagnosis of depression | 3.81 | 1.20, 12.17 | 0.024 |
| Lung cancer | 2.18 | 0.43, 10.99 | 0.343 |
| Overall model fit: df = 11, X2 = 101.00, p < 0.001 | |||
| Sample 2 (n = 158) | |||
| Predictors | Odds Ratio | 95% CI | p-value |
| Married or partnered | 0.29 | 0.12, 0.69 | 0.005 |
| Karnofsky Performance Status score | 0.92 | 0.88, 0.96 | < 0.001 |
| Self-reported diagnosis of depression | 5.22 | 1.86, 14.68 | 0.002 |
| Self-reported diagnosis of back pain | 2.55 | 1.03, 6.33 | 0.043 |
| Overall model fit: df = 4, X2 = 61.49, p < 0.001 | |||
Abbreviations: CI = confidence interval; df = degrees of freedom; OR = odds ratio
With Sample 2, four variables were retained in the final logistic regression model (i.e., married or partnered, KPS score, self-reported diagnoses of depression and back pain) and were used as covariates in the gene expression analysis (Table 3). Patients with a lower KPS score were more likely to belong to the Low group. Patients who were married or partnered had a 71% decrease in the odds of belonging to the Low group. Having a diagnosis of depression was associated with 5.22 times increase in the odds of belonging to the Low group. Patients with a diagnosis of back pain had a 2.55 times increase in the odds of belonging to the Low group.
3.5. Differentially expressed genes and pathways between the two AFI groups
Of the 14 surrogate variables identified for Sample 1, one was associated with AFI scores and was excluded from the final model. The final differential expression model for Sample 1 included 13 surrogate variables and the seven significant demographic and clinical characteristics. Of the 16 surrogate variables identified for Sample 2, two were associated with AFI scores and were excluded from the final model. The final differential expression model for Sample 2 included 14 surrogate variables and the four significant demographic and clinical characteristics.
Fold changes and p-values for the differentially expressed genes were included in the PIA of the 214 KEGG signaling pathways. Using Fisher’s combined probability method, the combined PIA identified 12 KEGG signaling pathways that were significantly perturbed between the AFI groups after correcting for multiple hypothesis testing using a common FWER of 5% (adjusted global perturbation p-value <0.05). Five of these 12 KEGG signaling pathways were related to inflammatory mechanisms (Figure 1, Table 4).
Figure 1.
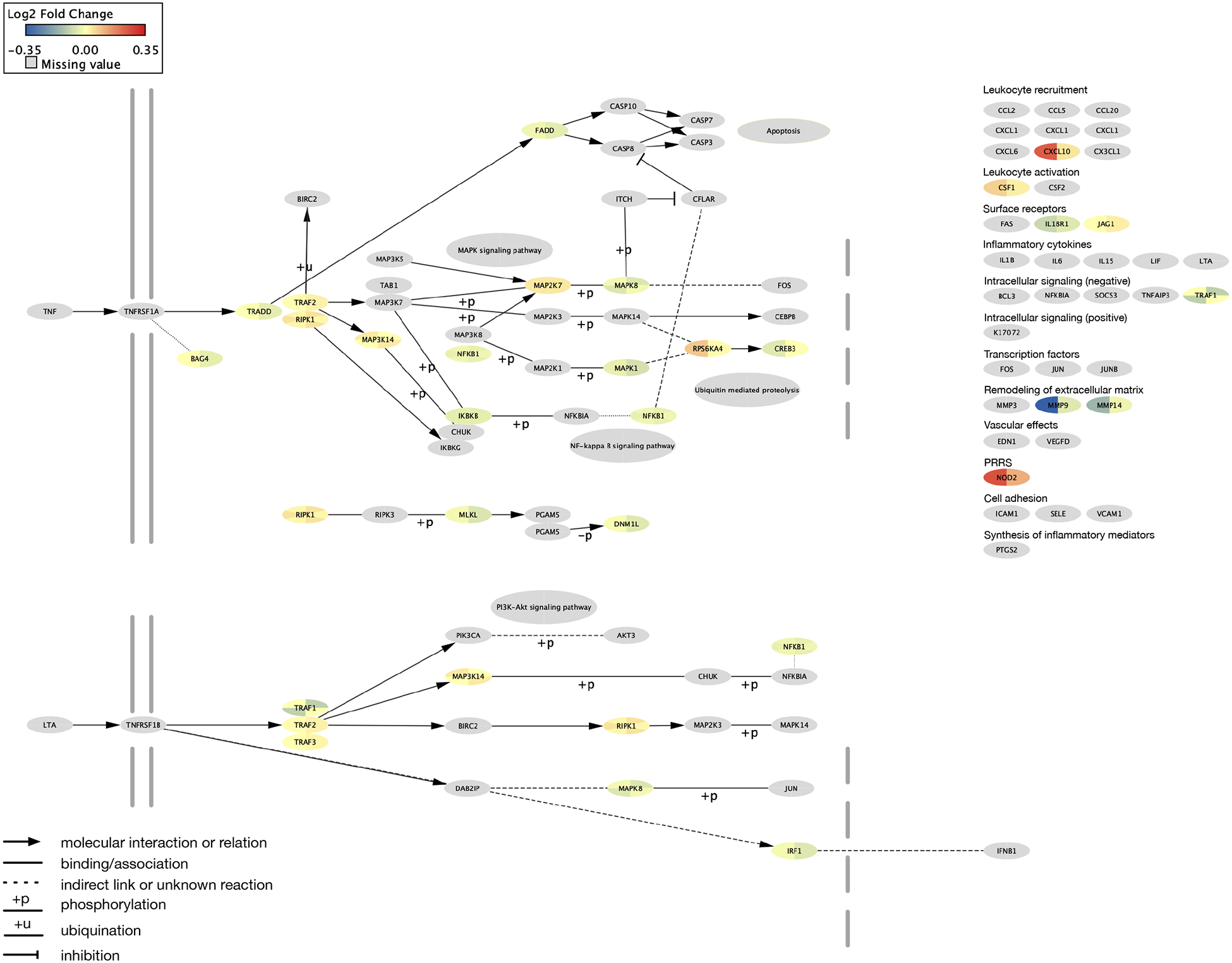
A network representation of the KEGG tumor necrosis factor (TNF) signaling pathway (hsa04668). Genes and their products are depicted as nodes shaped as ellipses. Nodes with missing data are shaded gray. The log2 fold change of differential gene expression between patients with low Attentional Function Index (AFI) as compared to high AFI scores are included in a pie representation for each ellipse. Sample 1 values are on the left side of the pie and Sample 2 are on the right. Edges are depicted by the interaction between the nodes.
Table 4.
Perturbed Inflammatory KEGG Pathways Between Patients with Low and High Attentional Function Index Scores
| Pathway ID | Pathway name | Adjusted Global pPERT |
|---|---|---|
| hsa04060 | cytokine-cytokine receptor interaction | 0.0009 |
| hsa04150 | mTOR signaling pathway | 0.0032 |
| hsa04010 | MAPK signaling pathway | 0.0261 |
| hsa04657 | IL-17 signaling pathway | 0.0340 |
| hsa04668 | TNF signaling pathway | 0.0459 |
Abbreviations: IL-17 = interleukin 17; KEGG = Kyoto Encyclopedia of Genes and Genomes; MAPK = mitogen-activated protein kinase; mTOR = mechanistic target of rapamycin; pPERT = perturbation p-value; TNF = tumor necrosis factor
4. DISCUSSION
This study is the first to describe perturbations in inflammatory pathways that were associated with CRCI in patients with cancer receiving chemotherapy. While no gene expression studies in humans were identified, our findings support previous pre-clinical54 and clinical2, 55, 56 research that suggests that chemotherapy induces inflammatory processes in both the peripheral and central nervous systems. Across both samples, the two common characteristics that were associated with membership to the Low group were a lower functional status and a self-reported diagnosis of depression. Of note, a growing body of literature suggests that changes in cognition and physical function often co-occur, particularly in older adults.57 In addition, as noted in one review,58 findings from both pre-clinical and clinical studies have identified associations between depressive symptoms and changes in inflammatory mediators including cytokines. The remainder of this discussion focuses on the perturbed KEGG signaling pathways associated with inflammation that were identified, namely: cytokine-cytokine receptor interaction, IL-17, TNF, MAPK, and mechanistic target of rapamycin (mTOR).
4.1. Cytokine-cytokine receptor interaction pathway
In general, cytokines serve as intercellular regulators and play a role in a variety of inflammatory processes.59 In terms of cognitive function, cytokines are involved in synaptic plasticity, neuromodulation, and neurogenesis.60 In patients with cancer, chemotherapy stimulates cytokine production through its effects on both normal and tumor cells.61 This increase in cytokine production is hypothesized to compromise the integrity of the blood-brain barrier, which allows for circulating cytokines to enter the brain.61 This cytokine response leads to neuroinflammation and neuronal cell death which manifests as CRCI.2
In one pre-clinical study that explored the effects of cyclophosphamide and mitomycin on the murine brain,31 perturbations in the cytokine-cytokine receptor interaction pathway were found three weeks after chemotherapy. However, measures of cognitive function were not assessed in this study. In another study that evaluated for pathways associated with six neurodegenerative diseases with progressive neuronal loss as a common feature (e.g., Alzheimer’s disease, Huntington’s disease),62 the cytokine-cytokine receptor interaction pathway was common to all six diseases. Due to the complexity of interactions among cytokines and cytokine receptors, their impact on cognition is likely dependent on a combination of mechanisms.59
4.2. IL-17 signaling pathway
The IL-17 signaling pathway consists of the IL-17 family of pro-inflammatory cytokines (i.e., IL-17A-F) that are active in acute and chronic inflammatory responses.63 These cytokines, mainly IL-17A and IL-17F, play key roles in the defense against extracellular pathogens and mediate inflammatory responses in autoimmune and inflammatory conditions.64 IL-17 receptors activate multiple pathways involved in the production of inflammatory products (e.g., MAPK signaling pathway) and trigger the production of multiple chemokines and cytokines.65 In addition, studies of murine and human brain epithelial cells demonstrated that IL-17 can alter the integrity of the blood-brain barrier.66, 67
Additional evidence to support involvement of IL-17 in CRCI comes from a study that evaluated for associations between plasma concentrations of seventeen chemokines and cytokines (i.e., monocyte chemotactic and activating factor (MCP-1), macrophage inflammatory protein (MIPS-1β), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor, interferon-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17) and objective measures of cognitive impairment in women with early stage breast cancer.68 IL-17 concentrations were elevated prior to the initiation of chemotherapy and followed a significant downward trend over the next two years. Prior to the initiation of chemotherapy, lower concentrations of IL-17 were associated with more rapid psychomotor speed. However, at the midpoint of chemotherapy, higher IL-17 concentrations were associated with improvements in psychomotor speed, cognitive flexibility, and executive functioning. While the concentrations of IL-17 and five other chemokines and cytokines (MIPS-1β, MCP-1, IL-6, IL-12, G-CSF) changed significantly over the two years, trends in each of these cytokines were variable. The authors hypothesized that these variations were due to the differential responses of the various cytokines to different aspects of the cancer experience (e.g., cancer, chemotherapy, radiation therapy).68 These results underscore the complex effect of neuroinflammation on CRCI.
4.3. TNF signaling pathway
Tumor necrosis factor is a cytokine that plays a role in a variety of intercellular signaling pathways. The TNF signaling pathway includes processes that are involved in immunity, cell death, and cell survival, as well as inflammatory and immune functions.69 Once TNF is activated, it binds to TNFR1 (i.e., TNF-α) or TNFR2 (i.e., TNF-β). Almost all cells express TNFR1. While TNFR2 is less frequently expressed, it is present on cells in the central nervous system (e.g., microglia, neuron subtypes, oligodendrocytes). TNF inhibition plays a role in neuroinflammatory conditions such as Alzheimer’s disease, traumatic brain injury, and stroke.70 Along with IL-6, TNF-α was implicated as a possible mediator of decreased hippocampal volume and verbal memory difficulties in survivors of breast cancer who were treated with chemotherapy.71 However, in another sample of patients receiving chemotherapy,26 no associations were found between CRCI and levels of TNF-α.
4.4. MAPK signaling pathway
The highly conserved MAPK signaling pathway plays a role in a number of cellular processes (e.g., proliferation, differentiation, migration).72 Expression of varying classes of MAPKs (e.g., p38 MAPKs, extracellular signal-related kinases (ERK1/2), Jun amino-terminal kinases/stress-activated kinases (JNKs/SAPKs)) occur in humans. The JNK and p38 MAPK pathways are activated by several types of stimuli (e.g., DNA-damaging agents/chemotherapy, radiation, oxidative stress) or proinflammatory cytokines (e.g., TNF-α).73 This activation results in neuroinflammation and neuronal apoptosis.74
While research on the association between CRCI and the MAPK signaling pathway is limited,75 a growing body of evidence suggests that MAPKs play a role in various cellular functions associated with both memory and learning. Furthermore, inhibition of JNK, p38, and ERK1/2 MAP kinases reduces brain inflammation and neuronal damage.76 In terms of CRCI, in a recent pre-clinical study,30 increased levels of both JNK and ERK signaling molecules were found in the hippocampus of rats treated with doxorubicin and cyclophosphamide. The authors hypothesized that the upregulation of the JNK and ERK pathways was due in part to inflammatory and oxidative stress responses induced by the chemotherapy.
4.5. mTOR signaling pathway
The mTOR signaling pathway is an evolutionarily conserved serine/threonine protein kinase that has key roles in cell growth, proliferation, metabolism,77 and cytokine production.78 This pathway consists of two complexes (i.e., mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2)) that differ in terms of structure and function.79 In response to the presence of amino acids, growth factors, oxygen, energy, and stress, mTORC1 regulates cellular growth, lipid metabolism, protein synthesis, cell survival, and autophagy.80 In contrast, mTORC2 is mainly stimulated by growth factors;81 mediates cell growth through regulation of the actin cytoskeleton;82 and may play a role in cell metabolism, proliferation, and survival.83 Central to this pathway is rapamycin, a naturally occurring macrolide, that has immunosuppressive and anti-proliferative properties.77 By binding to the 12-kDa FK506-binding protein 12 (FKBP12), rapamycin forms a complex that directly inhibits mTORC1. However, chronic exposure to rapamycin is required to inhibit mTORC2.84
While no studies were found that directly linked the mTOR signaling pathway to CRCI, evidence exists to support a link between mTOR signaling and other conditions that result in decrements in cognitive function. For example, alterations in normal mTOR activity are implicated in the development of various neurological diseases, including Alzheimer’s disease.85 The accumulation of amyloid-beta (Aβ) plaques in the brain is a key characteristic of Alzheimer’s disease and is hypothesized to result from decreased autophagy.86 While pre-clinical and clinical studies have demonstrated that increased levels of Aβ cause mTOR signaling to increase or decrease, both types of alterations in mTOR signaling result in cognitive deficits.85, 86
In addition, mTOR is involved in synaptic plasticity and memory through the regulation of multiple factors involved in protein synthesis.87 While multiple pre-clinical studies have demonstrated that inhibition of mTOR signaling improves spatial learning and social memory85 and protects the integrity of the blood-brain barrier,88 other studies found that alterations in mTOR signaling result in deficits in learning and memory.85 Given these contradictory findings, further research is warranted to better understand the complex role of mTOR signaling in cognition.
4.6. Strengths and limitations
While this study had a relatively large sample size; included rigorous quality controls; utilized two complimentary methods to measure gene expression; set strict criteria for differential expression and pathway perturbation selection; and provided results from independent tests across two samples, some limitations warrant consideration. Because of its cross-sectional design, longitudinal studies are needed that assess for association between cognitive changes before, during, and after chemotherapy and changes in gene expression and pathway perturbations. Second, given that this study is the first evaluation of associations between CRCI and gene expression changes, our findings warrant confirmation in independent samples. Third, this study evaluated for CRCI using a single subjective measure. Future research should explore associations between subjectively and objectively measured CRCI and changes in gene expression. Fourth, because CRCI was assessed during chemotherapy, evaluations are warranted with other types of cancer treatment (e.g., radiation therapy, immunotherapy, surgery).
4.7. Conclusion
This study is the first to describe perturbations in inflammatory pathways associated with CRCI. Consistent with previous research,2, 89 our findings highlight the role of cytokines both in terms of cytokine-specific pathways, as well as pathways involved in cytokine production and cytokine activation. Of note, two of the pathways identified in this study (i.e., IL-17 and TNF signaling pathways) include cytokines that are known to interact synergistically and contribute to neuroinflammation.90 In addition, IL-17 receptors trigger the production of cytokines and chemokines and activate the MAPK signaling pathway.65 Activation of the MAPK signaling pathway, in turn, can trigger increased inflammation. Due to the complex nature of CRCI, significant gaps remain in our understanding of CRCI and the role of neuroinflammation in its development. Given the strength of our findings and the evidence that supports these inflammatory pathways in alterations in memory and learning,75, 85, 87 and neuroinflammatory diseases,62, 70, 76, 85 continued research is warranted. The findings from this research have the potential to identify new targets for therapeutics and lead to the development of interventions to improve cognition in patients with cancer.
Supplementary Material
Funding
This study was funded by a grant from the National Cancer Institute (CA134900). Ms. Oppegaard and Harris are supported by a grant from the National Institute of Nursing Research (T32NR016920). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Miaskowski is an American Cancer Society Clinical Research Professor. Ms. Harris is supported by a grant from the American Cancer Society. Ms. Oppegaard and Ms. Shin are supported by grants from the Oncology Nursing Foundation.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- 1.Horowitz TS, Treviño M, Gooch IM, Duffy KA. Understanding the Profile of Cancer-Related Cognitive Impairments: A Critique of Meta-Analyses. JNCI : Journal of the National Cancer Institute. 2019;111(10):1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren X, Boriero D, Chaiswing L, Bondada S, St. Clair DK, Butterfield DA. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2019;1865(6):1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Ah D, Storey S, Crouch A. Relationship between self-reported cognitive function and work-related outcomes in breast cancer survivors. J Cancer Surviv. 2018;12(2):246–255. [DOI] [PubMed] [Google Scholar]
- 4.Von Ah D, Storey S, Tallman E, Nielsen A, Johns SA, Pressler S. Cancer, cognitive impairment, and work-related outcomes: An integrative review. Oncology Nursing Forum. 2016;43(5):602–616. [DOI] [PubMed] [Google Scholar]
- 5.Myers JS. Cancer- and chemotherapy-related cognitive changes: the patient experience. Seminars in Oncology Nursing. 2013;29(4):300–307. [DOI] [PubMed] [Google Scholar]
- 6.Wu LM, Kuprian N, Herbert K, Amidi A, Austin J, Valdimarsdottir H, Rini C. A mixed methods analysis of perceived cognitive impairment in hematopoietic stem cell transplant survivors. Palliative and Supportive Care. 2019;17(4):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bail J, Meneses K. Computer-Based Cognitive Training for Chemotherapy-Related Cognitive Impairment in Breast Cancer Survivors. Clinical Jourhnal of Oncology Nursing. 2016;20(5):504–509. [DOI] [PubMed] [Google Scholar]
- 8.Vance DE, Frank JS, Bail J, Triebel KL, Niccolai LM, Gerstenecker A, Meneses K. Interventions for cognitive deficits in breast cancer survivors treated with chemotherapy. Cancer Nursing. 2017;40(1):E11–E27. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Y, Cheng AS, Chan CC. Meta-Analysis of the Effects of Neuropsychological Interventions on Cognitive Function in Non-Central Nervous System Cancer Survivors. Integrative Cancer Therapies. 2016;15(4):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Luo Y, Zeng Y. Meta-analysis of meditative/relaxation-based interventions for cognitive impairment in cancer patient. Internatioal Journal of Nursing Sciences. 2017;4(3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh PJ, Kim J. The effects of nonpharmacologic interventions on cognitive function in patients with cancer: A meta-analysis. Oncology Nursing Forum. 2016;43(5):E205–217. [DOI] [PubMed] [Google Scholar]
- 12.Chan RJ, McCarthy AL, Devenish J, Sullivan KA, Chan A. Systematic review of pharmacologic and non-pharmacologic interventions to manage cognitive alterations after chemotherapy for breast cancer. European Journal of Cancer. 2015;51(4):437–450. [DOI] [PubMed] [Google Scholar]
- 13.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International review of psychiatry (Abingdon, England). 2014;26(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Seminars in Oncology. 2011;38(3):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, Winocur G, De Ruiter MB, Castel H. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Annals of Oncology. 2019;30(12):1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson B, Marks DL. Pretreatment Cancer-Related Cognitive Impairment—Mechanisms and Outlook. Cancers. 2019;11(5):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv L, Mao S, Dong H, Hu P, Dong R. Pathogenesis, Assessments, and Management of Chemotherapy-Related Cognitive Impairment (CRCI): An Updated Literature Review. Journal of oncology. 2020;2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XM, Walitt B, Saligan L, Tiwari AF, Cheung CW, Zhang ZJ. Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine. 2015;72(1):86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE. Proposed mechanisms for cancer- and treatment-related cognitive changes. Seminars in Oncology Nursing. 2013;29(4):260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen SR, Schmid MC. Macrophages as key drivers of cancer progression and metastasis. Mediators of inflammation. 2017;2017:9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Experimental hematology. 2007;35(4 Suppl 1):96–104. [DOI] [PubMed] [Google Scholar]
- 22.Lacourt TE, Heijnen CJ. Mechanisms of Neurotoxic Symptoms as a Result of Breast Cancer and Its Treatment: Considerations on the Contribution of Stress, Inflammation, and Cellular Bioenergetics. Current Breast Cancer Reports. 2017;9(2):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5(3):243–251. [DOI] [PubMed] [Google Scholar]
- 24.Hoogland AI, Nelson AM, Gonzalez BD, Small BJ, Breen EC, Sutton SK, Syrjala KL, Bower JE, Pidala J, Booth-Jones M, Jacobsen PB, Jim HSL. Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain Behav Immun. 2019;80:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Lee JA, Fan G, Tan YP, Yong WS, Madhukumar P, Loo SK, Ang SF, Wong M, Chay WY, Ooi WS, Dent RA, Yap YS, Ng R, Chan A. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Annals of Oncology. 2015;26(7):1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chae JW, Ng T, Yeo HL, Shwe M, Gan YX, Ho HK, Chan A. Impact of TNF-alpha (rs1800629) and IL-6 (rs1800795) polymorphisms on cognitive impairment in Asian breast cancer patients. PLoS One. 2016;11(10):e0164204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toh YL, Wang C, Ho HK, Chan A. Distinct cytokine profiles across trajectories of self-perceived cognitive impairment among early-stage breast cancer survivors. Journal of Neuroimmunology. 2020;342:577196. [DOI] [PubMed] [Google Scholar]
- 28.Apple AC, Schroeder MP, Ryals AJ, Wagner LI, Cella D, Shih PA, Reilly J, Penedo FJ, Voss JL, Wang L. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage: Clinical. 2018;20:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen BD, Apodaca LA, Syage AR, Markarian M, Baddour AAD, Minasyan H, Alikhani L, Lu C, West BL, Giedzinski E, Baulch JE, Acharya MM. Attenuation of neuroinflammation reverses Adriamycin-induced cognitive impairments. Acta neuropathologica communications. 2019;7(1):186–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagnall-Moreau C, Chaudhry S, Salas-Ramirez K, Ahles T, Hubbard K. Chemotherapy-induced cognitive impairment Is associated with increased inflammation and oxidative damage in the hippocampus. Molecular neurobiology. 2019;56(10):7159–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovalchuk A, Rodriguez-Juarez R, Ilnytskyy Y, Byeon B, Shpyleva S, Melnyk S, Pogribny I, Kolb B, Kovalchuk O. Sex-specific effects of cytotoxic chemotherapy agents cyclophospha-mide and mitomycin C on gene expression, oxidative DNA damage, and epigenetic alterations in the prefrontal cortex and hippocampus – an aging connection. Aging. 2016;8(4):697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atallah M, Cooper B, Munoz RF, Paul SM, Anguera J, Levine JD, Hammer M, Wright F, Chen LM, Melisko M, Conley YP, Miaskowski C, Dunn LB. Psychological symptoms and stress are associated with decrements in attentional function in cancer patients undergoing chemotherapy. Cancer Nursing. 2019. [DOI] [PubMed] [Google Scholar]
- 33.Singh KP, Dhruva A, Flowers E, Paul SM, Hammer MJ, Wright F, Cartwright F, Conley YP, Melisko M, Levine JD, Miaskowski C, Kober KM. Alterations in Patterns of Gene Expression and Perturbed Pathways in the Gut-Brain Axis Are Associated With Chemotherapy-Induced Nausea. Journal of Pain & Symptom Management. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karnofsky D Performance scale. Kennealey GT, Mitchell MS, editors. New York: Plenum Press; 1977. [Google Scholar]
- 35.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis & Rheumatism. 2003;49(2):156–163. [DOI] [PubMed] [Google Scholar]
- 36.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies in Alcohol. 1995;56:423–432. [DOI] [PubMed] [Google Scholar]
- 37.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology. 2011;20(2):194–202. [DOI] [PubMed] [Google Scholar]
- 38.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14(1):70–78. [DOI] [PubMed] [Google Scholar]
- 39.Extermann M, Bonetti M, Sledge GW, O’Dwyer PJ, Bonomi P, Benson AB 3rd. MAX2--a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. European Journal of Cancer. 2004;40(8):1193–1198. [DOI] [PubMed] [Google Scholar]
- 40.Utne I, Loyland B, Grov EK, Rasmussen HL, Torstveit AH, Cooper BA, Mastick J, Mazor M, Wong M, Paul SM, Conley YP, Jahan T, Ritchie C, Levine JD, Miaskowski C. Distinct attentional function profiles in older adults receiving cancer chemotherapy. European Journal of Oncology Nursing. 2018;36:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh KP, Kober KM, Dhruva AA, Flowers E, Paul SM, Hammer MJ, Cartwright F, Wright F, Conley YP, Levine JD, Miaskowski C. Risk factors associated with chemotherapy-induced nausea in the week before the next cycle and impact of nausea on quality of life outcomes. Journal of Pain & Symptom Management. 2018;56(3):352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M, participants of the MECCC. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Annals of Oncology. 2016;27(suppl 5):v119–v133. [DOI] [PubMed] [Google Scholar]
- 43.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smyth GK. limma: Linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor Statistics for Biology and Health. New York, NY: Springer; 2005. p. 397–420. [Google Scholar]
- 45.Leek JT, Storey JD. Capturing heterogeneity in gene expression studies by surrogate variable analysis. Plos Genetics. 2007;3(9):1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray KA, Daugherty LC, Gordon SM, Seal RL, Wright MW, Bruford EA. Genenames.org: The HGNC resources in 2013. Nucleic Acids Res. 2013;41(Database issue):D545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisher RA. Statistical Methods for Research Workers. Edinburgh: Oliver and Boyd; 1925. [Google Scholar]
- 48.Fisher RA. Questions and answers #14. The American Statistician. 1948;2(5):30–31. [Google Scholar]
- 49.Mitrea C, Taghavi Z, Bokanizad B, Hanoudi S, Tagett R, Donato M, Voichita C, Draghici S. Methods and approaches in the topology-based analysis of biological pathways. Frontiers in Physiology. 2013;4:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Research. 2007;17(10):1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aoki-Kinoshita KF, Kanehisa M. Gene annotation and pathway mapping in KEGG. In: Bergman NH, editor. Comparative Genomics: Humana Press; 2007. p. 71–91. [DOI] [PubMed] [Google Scholar]
- 52.Landau WM, Liu P. Dispersion estimation and its effect on test performance in RNA-seq data analysis: a simulation-based comparison of methods. PLoS One. 2013;8(12):e81415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics--a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009;25(3):415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsos A, Johnston IN. Chemotherapy-induced cognitive impairments: A systematic review of the animal literature. Neuroscience and biobehavioral reviews. 2019;102:382–399. [DOI] [PubMed] [Google Scholar]
- 55.Shi Q, Wang XS, Li G, Shah ND, Orlowski RZ, Williams LA, Mendoza TR, Cleeland CS. Racial/ethnic disparities in inflammatory gene single-nucleotide polymorphisms as predictors of a high risk for symptom burden in patients with multiple myeloma 1 year after diagnosis. Cancer. 2015;121(7):1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson EM, Monje M. Emerging mechanistic underpinnings and therapeutic targets for chemotherapy-related cognitive impairment. Curr Opin Oncol. 2019;31(6):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Utne I, Cooper BA, Ritchie C, Wong M, Dunn LB, Loyland B, Grov EK, Hammer MJ, Paul SM, Levine JD, Conley YP, Kober KM, Miaskowski C. Co-occurrence of decrements in physical and cognitive function is common in older oncology patients receiving chemotherapy. European Journal of Oncology Nursing. 2020;48:101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2015;321:138–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson CJ, Finch CE, Cohen HJ. Cytokines and Cognition—The Case for A Head-to-Toe Inflammatory Paradigm. Journal of the American Geriatrics Society (JAGS). 2002;50(12):2041–2056. [DOI] [PubMed] [Google Scholar]
- 60.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience and biobehavioral reviews. 2009;33(3):355–366. [DOI] [PubMed] [Google Scholar]
- 61.Wardill HR, Mander KA, Van Sebille YZA, Gibson RJ, Logan RM, Bowen JM, Sonis ST. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction: Cytokine-Mediated Blood Brain Barrier Disruption. International journal of cancer. 2016;139(12):2635–2645. [DOI] [PubMed] [Google Scholar]
- 62.Khayer N, Mirzaie M, Marashi SA, Jalessi M. Rps27a might act as a controller of microglia activation in triggering neurodegenerative diseases. PloS one. 2020;15(9):e0239219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64(2):477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monin L, Gaffen SL. Interleukin 17 family cytokines: Signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harbor Perspectives in Biology. 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cipollini V, Anrather J, Orzi F, Iadecola C. Th17 and cognitive impairment: Possible mechanisms of action. Frontiers in Neuroanatomy. 2019;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, Marchi N, van Boxel-Dezaire AHH. IFN-gamma, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochemical and Biophysical Research Communications. 2018;507(1–4):274–279. [DOI] [PubMed] [Google Scholar]
- 67.Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB Journal. 2010;24(4):1023–1034. [DOI] [PubMed] [Google Scholar]
- 68.Lyon DE, Cohen R, Chen H, Kelly DL, McCain NL, Starkweather A, Ahn H, Sturgill J, Jackson-Cook CK. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. Journal of Neuroimmunology. 2016;301:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kollias G, Sfikakis PP, Karger S. TNF pathophysiology: molecular and cellular mechanisms. New York;Basel, Switzerland;: Karger; 2010. [Google Scholar]
- 70.Clark IA, Vissel B. A Neurologist’s Guide to TNF Biology and to the Principles behind the Therapeutic Removal of Excess TNF in Disease. Neural plasticity. 2015;2015:358263–358210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, behavior, and immunity. 2012;30:S109–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. [DOI] [PubMed] [Google Scholar]
- 73.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et Biophysica Acta. 2010;1802(4):396–405. [DOI] [PubMed] [Google Scholar]
- 74.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Archives of Toxicology. 2015;89(6):867–882. [DOI] [PubMed] [Google Scholar]
- 75.Albert-Gasco H, Ros-Bernal F, Castillo-Gomez E, Olucha-Bordonau FE. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. International journal of molecular sciences. 2020;21(12):4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasten-Jolly J, Lawrence DA. CNS Cytokines. In: Blass JP, editor. Neurochemical Mechanisms in Disease. New York, NY: Springer New York; 2011. p. 359–382. [Google Scholar]
- 77.Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Seminars in Cell & Developmental Biology. 2014;36:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dazert E, Hall MN. mTOR signaling in disease. Current Opinion Cell Biology. 2011;23(6):744–755. [DOI] [PubMed] [Google Scholar]
- 79.Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell & Bioscience. 2020;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu W, Hall MN. Regulation of mTORC2 Signaling. Genes (Basel). 2020;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biology. 2004;6(11):1122–1128. [DOI] [PubMed] [Google Scholar]
- 83.Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. Journal of Biological Chemistry. 2010;285(11):7866–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell. 2006;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 85.Bockaert J, Marin P. mTOR in Brain Physiology and Pathologies. Physiological Reviews. 2015;95(4):1157–1187. [DOI] [PubMed] [Google Scholar]
- 86.Talboom JS, Velazquez R, Oddo S. The mammalian target of rapamycin at the crossroad between cognitive aging and Alzheimer’s disease. NPJ Aging & Mechanisms of Disease. 2015;1:15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends in Neurosciences. 2010;33(2):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. American Journal of Physiology Heart and Circulatory Physiology. 2018;314(4):H693–H703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren X, St Clair DK, Butterfield DA. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol Res. 2017;117:267–273. [DOI] [PubMed] [Google Scholar]
- 90.Groves TR, Farris R, Anderson JE, Alexander TC, Kiffer F, Carter G, Wang J, Boerma M, Allen AR. 5-Fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behavioural brain research. 2017;316:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


