Abstract
One strategy for the induction of mucosal immune responses by oral immunization is to administer the antigen in conjunction with cholera toxin. Cholera toxin consists of one A polypeptide (CTA) which is noncovalently linked to five B subunits (CTB) via the A2 portion of the A subunit (CTA2). Coupling of antigens to the nontoxic B subunit of cholera toxin may improve the immunogenicity of antigens by targeting them to GM1 ganglioside on M cells and intestinal epithelial cells. Here, we describe the construction of a translational fusion protein containing the serine-rich Entamoeba histolytica protein (SREHP), a protective amebic antigen, fused to a maltose binding protein (MBP) and to CTA2. When coexpressed in Escherichia coli with the CTB gene, these proteins assembled into a holotoxin-like chimera containing MBP-SREHP-CTA2 and CTB. This holotoxin-like chimera (SREHP-H) inhibited the binding of cholera toxin to GM1 ganglioside. Oral vaccination of mice with SREHP-H induced mucosal immunoglobulin A (IgA) and serum IgG antiamebic antibodies and low levels of mucosal anti-CTB antibodies. Our studies confirm that the genetic coupling of antigens to CTA2 and their coexpression in E. coli can produce holotoxin-like molecules that are mucosally immunogenic without the requirement for supplemental cholera toxin, and they establish the SREHP-H protein as a candidate for evaluation as a vaccine to prevent amebiasis.
The first step in the pathogenesis of intestinal disease caused by the protozoan parasite Entamoeba histolytica is the adherence of the amebic trophozoite to intestinal epithelial cells. Antibodies against several different E. histolytica surface antigens, including the serine-rich E. histolytica protein (SREHP), have been shown to block amebic adherence to mammalian cells in vitro (17–20, 24, 25, 27). These findings suggest that the induction of intestinal mucosal antibodies that blocked amebic adherence to intestinal epithelial cells in humans could potentially prevent amebic infection. One approach for the induction of mucosal antibody responses to a protein antigen has been to orally administer the target antigen in conjunction with cholera toxin (CT) or the heat-labile toxin from Escherichia coli (LT). Both molecules have adjuvant properties, facilitating the induction of mucosal immune responses to antigens which normally are not immunogenic when given by the oral route (9–11, 22). CT is composed of one A subunit (CTA), which contains the active toxin domain (A1) as well as a short sequence (A2) which serves to link the A subunit noncovalently to five B subunits (CTB) (reviewed in reference 2). CTB mediates the binding of the toxin to GM1 ganglioside on the surface of intestinal cells; this binding permits internalization of CTA by cells, with resultant intoxication (4). The mechanisms underlying the adjuvant properties of CT and LT are under study, but the nontoxic B subunit is immunogenic in humans, and antigens coupled to the B subunit will be targeted to GM1 ganglioside on intestinal cells, which may increase the immunogenicity of the coupled antigens (1, 6, 7, 16, 17, 21–23, 30). Previously, it was shown that mice fed a peptide composed of one of the dodecapeptide repeats of the SREHP molecule fused to CTB develop mucosal antiamebic antibody responses (30). However, this approach is limited by the requirement that only small peptides be coupled to CTB to ensure that it retains its ability to pentamerize (8).
A new strategy for the delivery of antigens in conjunction with CTB is to genetically engineer the fusion of the protein of interest to the A2 segment of the CTA moiety (CTA2) (14). Expression of CTB on the same plasmid allows the formation of holotoxin-like chimeras, where assembly of the toxin complex occurs, but the toxic CTA domain is replaced by the antigen of interest. Model holotoxin-like chimeras containing maltose binding protein (MBP), bacterial alkaline phosphatase, or β-lactamase have been successfully engineered (14). These chimeras can bind to GM1 ganglioside, consistent with maintenance of a functional CTB pentamer. Recently, this approach was used to create a holotoxin-like chimera containing the saliva-binding region of Streptococcus mutans AgI/II (12, 13). Here, we describe the construction of a holotoxin-like molecule containing a SREHP-MBP-CTA2 fusion protein, and we show that oral administration of this SREHP holotoxin-like molecule (SREHP-H) can induce mucosal antiamebic antibodies in mice.
MATERIALS AND METHODS
Genetic constructions.
Plasmid pMGJ96, which contains the MBP-encoding gene fused to the CTA2 coding region and the wild-type ctxB gene (14) (which encodes the CTB protein), was used to transform E. coli TX1 for expression of the MBP-CTA2 fusion protein and CTB as previously described (14). Initial attempts to express an SREHP-CTA2 fusion protein proved unsuccessful (data not shown), so an alternative strategy of placing the SREHP sequence between the MBP and CTA2 coding regions was devised. To construct the SREHP-MBP-A2 fusion protein, nucleotides 175 to 726 of the SREHP cDNA from plasmid pLi228 (24) were amplified by PCR with incorporation of SacI and BamHI sites, and the resultant fragment was cloned into pCRII cloning vector (Invitrogen Corporation, San Diego, Calif.). This fragment was then excised by SacI and BamHI digestion and ligated into SacI- and BamHI-digested pMGJ96 to produce the pSS11 plasmid. This inserts the SREHP coding region in frame into the 3′ end of the MBP coding region. The pSS11 plasmid was used to transform E. coli TX1 for expression of the MBP-SREHP-CTA2 fusion protein and CTB as previously described (14).
Expression and purification of recombinant proteins.
Logarithmic-phase cells (A600 = 0.8 to 1.0) were induced to make fusion proteins by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to a concentration of 0.4 mM and then grown overnight. Cultures were concentrated fivefold, resuspended in phosphate-buffered saline (PBS) or Tris-buffered saline (TBS) and then treated with 1 mg of polymyxin B per ml for 30 min at 37°C to produce periplasmic extracts (14). These supernatants were precipitated with 50% ammonium sulfate, and the resulting pellets were resuspended in PBS or TBS and then dialyzed overnight against either 20 mM Tris-Cl (pH 7.4)–200 mM NaCl–1 mM EDTA (for purification of MBP-CTA2-CTB complex, designated MBP-H) or PBS (for purification of the MBP-SREHP-CTA2/CTB complex, designated SREHP-H). Affinity purification of MBP-H was carried out on an amylose resin column (New England Biolabs, Beverly, Mass.) washed with 20 mM Tris-Cl (pH 7.4)–200 mM NaCl–1 mM EDTA, and the protein was eluted with 10 mM maltose. Affinity purification of SREHP-H was carried out on a column containing monoclonal antibody 2D4 (which binds the SREHP molecule) coupled to Sepharose (19, 26). Bound SREHP-H was eluted with 3.2 M MgCl2, and samples were immediately dialyzed against TBS. The amount of holotoxin present in samples of purified SREHP-H and MBP-H used for GM1 ganglioside studies was determined by using scanning densitometry of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-separated SREHP-H and MBP-H to compare the density of CTB bands seen in each preparation to the density of the band seen with a known quantity of SDS-PAGE-separated purified CT holotoxin.
GM1 ganglioside binding.
SREHP-H and MBP-H in periplasmic extracts were detected by a GM1 solid-phase immunoassay. Enzyme-linked immunosorbent assay (ELISA) plates were coated with GM1 ganglioside at 100 ng/well, washed with PBS–0.5% Tween 20, and then incubated overnight with periplasmic extracts diluted 1:5. After the washing, binding of SREHP-H to GM1 ganglioside was detected by the addition of monoclonal antibody 2D4 at 10 μg/ml, followed by washing and then the addition of alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Sigma, St. Louis, Mo.). Binding of MBP-H to GM1 ganglioside was detected by the addition of rabbit anti-MBP serum (New England Biolabs) followed by washing and then the addition of alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma). Binding of both fusion proteins was detected with goat anti-CTB serum (Calbiochem, San Diego, Calif.) (30). A competitive GM1 binding ELISA was performed as previously described (30), with purified SREHP-H, MBP-H, CTA (Sigma), and CT (Sigma) adjusted to equimolar concentrations and diluted with an equal volume of 100 ng of biotinylated CTB (List Biologics, Campbell, Calif.). The washing of samples, the addition of horseradish peroxidase-conjugated strepavidin (Zymed Laboratories, San Francisco, Calif.), and the developing step were performed exactly as previously described (30).
Immunizations and samplings.
Groups of 5 to 10 adult female BALB/c mice were fed 0.5 ml of either 100 μg of SREHP-H, MBP-H, CTB-SREHP-12, or CTB suspended in 0.2 M NaHCO3 by gastric intubation with a blunt-tipped feeding needle. In some initial experiments, mice also received 5 μg of CT. Mice were immunized on days 0, 14, and 28 and sacrificed on day 35. Serum and stool specimens were obtained before immunization and on days 14, 28, and 35. Stool samples were obtained and processed as previously described (31). Mesenteric lymph nodes (MLN) and spleen cells were obtained at sacrifice and processed to prepare erythrocyte-free single-cell suspensions for an enzyme-linked immunospot (ELISPOT) assay as previously described (5).
Detection of antibody-producing cells.
Spleen and MLN cell suspensions were assayed for the number of specific antibody-secreting cells (ASC) in an ELISPOT assay according to a previously reported protocol (30). In brief, wells were coated with either E. histolytica HM1:IMSS lysate (100 μg/ml), GM1 ganglioside (5 μg/ml) to which CTB (3 μg/ml) was bound, or 10% fetal calf serum (FCS). After blocking with 10% FCS, 0.1 ml of a cell suspension containing 105 spleen or MLN cells was added in duplicate to the wells, and the plates were incubated for 3 h in 5% CO2 at 37°C. After a washing, spots were developed by the addition of a 1:200 dilution of horseradish peroxidase-conjugated anti-mouse IgA or anti-mouse IgG (Southern Biotechnology Associates, Birmingham, Ala.) followed by a washing and then addition of 0.1 ml of 3-amino-9-ethylcarbazole-H2O2 substrate. ASC were counted as spots under a dissecting microscope, and numbers were expressed as ASC/106 cells.
ELISA and immunoblotting.
To detect antiamebic IgA and IgG antibodies, samples of serum (diluted 1:100) or stool (diluted 1:5) were reacted overnight at 4°C in 96-well plates coated with E. histolytica HM1:IMSS trophozoites (105/well) according to a previously described protocol (31). Following multiple washes, biotin-labeled goat anti-mouse IgG or IgA (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.) diluted 1:2,000 was added to all wells for a 2-h incubation at 37°C; then the plates were washed numerous times and a 1:4,000 dilution of horseradish peroxidase-conjugated strepavidin (Zymed) was added for a 1-h incubation at room temperature. The plates were washed, and then color was developed with a 1-mg/ml solution of 2,2′-azinobis (3-ethylbenzthiazolinesulfonic acid (ABTS) (Sigma) (31). Detection of serum and stool IgA and IgG anti-CTB antibodies was performed in the same manner, except that ELISA plates coated with GM1 ganglioside (5 μg/ml) to which CTB (3 μg/ml) was bound were used (30, 31).
Immunoblotting to confirm the presence of SREHP in the SREHP-H complex was performed by using an ECL kit (Amersham Life Science) according to the manufacturer’s protocol, with a 1:5000 dilution of monoclonal antibody 2D4 as the primary antibody and a 1:5,000 dilution of the provided horseradish peroxidase-conjugated goat anti-mouse antibody as the secondary antibody. Immunoblotting to confirm the presence of CTB in the SREHP-H and MBP-H complexes was done according to an identical protocol but was performed with a 1:500 dilution of goat anti-CTB serum (Calbiochem) followed by a 1:1,000 dilution of rabbit anti-goat IgG (Kirkegaard and Perry Laboratories) and then horseradish peroxidase-conjugated goat anti-rabbit antibody (Sigma).
RESULTS
Expression, purification, and characterization of the SREHP-H and MBP-H holotoxin-like chimeras.
Periplasmic extracts from E. coli TX1 expressing either the SREHP-H or the MBP-H chimera were analyzed by Coomassie blue staining of SDS-PAGE-separated extracts, and a species at 97 kDa (the predicted size of the MBP-SREHP-CTA2 fusion protein) was detected in the extracts from SREHP-H-expressing bacteria but not in periplasmic extracts from MBP-H-expressing bacteria or in untransformed E. coli TX1 (data not shown). In contrast, a species at 47 kDa (the predicted size of the MBP-CTA2 fusion protein) was seen in periplasmic extracts from MBP-H-expressing bacteria but not in bacteria expressing SREHP-H or in untransformed E. coli TX1 (data not shown). When periplasmic extracts from SREHP-H-expressing bacteria were assayed by GM1 ELISA for SREHP with monoclonal antibody 2D4, significant binding of the antibody was detected, demonstrating that the periplasmic extracts contained SREHP-H chimeras consisting of SREHP associated with CTB (data not shown). When periplasmic extracts from MBP-H-expressing bacteria were assayed by GM1 ELISA for MBP with anti-MBP antibodies, MBP-H chimeras capable of binding to GM1 ganglioside were detected. As expected, the monoclonal anti-SREHP antibody showed no reactivity with periplasmic extracts from MBP-H-expressing bacteria that had been reacted with GM1 ganglioside, but anti-MBP antibodies did react with periplasmic extracts from the SREHP-H chimera (which contains MBP) that had been reacted with GM1 ganglioside (data not shown). SREHP-H and MBP-H bound to GM1 ganglioside were reactive with anti-CTB antibodies, while none of the antibodies reacted with periplasmic extracts from E. coli TX1 in the GM1 ELISA (data not shown).
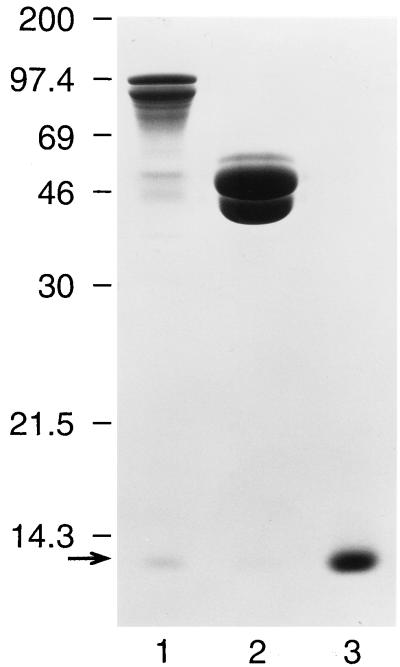
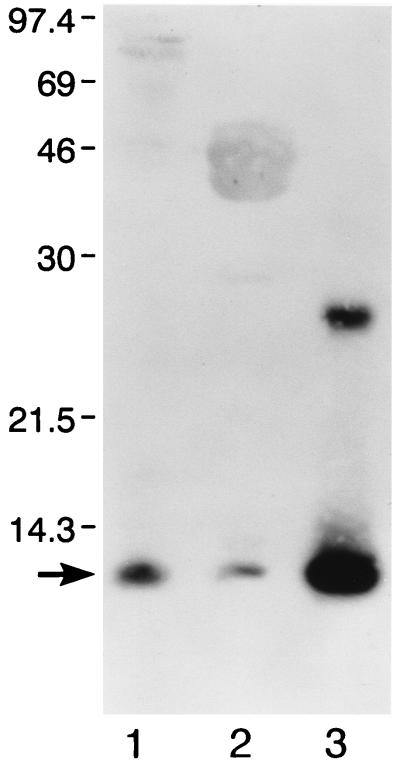
Having demonstrated that periplasmic extracts from SREHP-H- and MBP-H-expressing bacteria contained holotoxin-like molecules capable of binding to GM1 ganglioside, we purified the SREHP-H and MBP-H chimeras as well as free MBP-SREHP-CTA2 and MBP-CTA2 fusion proteins by affinity chromatography. As shown in Fig. 1, following affinity purification of SREHP-H, we obtained a species at 97 kDa, the expected size of the MBP-SREHP-CTA2 fusion protein, and a species at approximately 12 kDa, the predicted size for CTB, consistent with purification of the SREHP-H chimera. The band at 12 kDa was consistently less dense than that seen for the MBP-SREHP-CTA2 fusion protein, suggesting that not all of the purified MBP-SREHP-CTA2 was complexed to CTB. Following purification of MBP-H, we obtained a species at 47 kDa (the predicted size of the MBP-CTA2 fusion protein) and a species at 12 kDa (the predicted size of CTB). Note that although equal amounts of protein were added to each lane, the CTB band seen with purified MBP-H was even less dense than that detected with Coomassie blue-stained SDS-PAGE-separated SREHP-H, suggesting that fewer of the MBP-CTA2 fusion proteins purified were assembled with CTB into holotoxin-like chimeras. The identity of the 97-kDa molecule as MBP-SREHP-CTA2 was confirmed by immunoblotting with monoclonal antibody 2D4, which showed reactivity with the 97-kDa protein in SDS-PAGE-separated SREHP-H lysates but no reactivity with any species in MBP-CTA2 lysates (data not shown). Immunoblotting with anti-MBP antibodies confirmed that the 47-kDa protein was MBP-CTA2, and immunoblotting with anti-CTB antibody confirmed that the 12-kDa species seen in both purified SREHP-H and MBP-H was CTB (Fig. 2).
FIG. 1.
Purification of SREHP-H and MBP-H proteins. Shown is a Coomassie blue stain of SDS-PAGE-separated purified SREHP-H (lane 1), MBP-H (lane 2), and CTB (lane 3). A band at approximately 97 kDa, the expected size of the MBP-SREHP-CTA2 fusion protein, and a band at approximately 12 kDa (arrow), the expected size of the CTB protein, are seen in lane 1. A band at 47 kDa, the expected size of the MBP-CTA2 protein, and a band at 12 kDa are seen in lane 2. The low-molecular-mass bands in lanes 1 and 2 can be seen to comigrate with CTB (lane 3). Molecular masses (in kilodaltons) are shown on the left.
FIG. 2.
CTB is detected in purified SREHP-H and MBP-H complexes. SDS-PAGE-separated purified SREHP-H (lane 1), MBP-H (lane 2), and CTB (lane 3) underwent immunoblotting with polyclonal antiserum to CTB. All three preparations contain immunoreactive CTB (arrow). Molecular masses (in kilodaltons) are shown on the left.
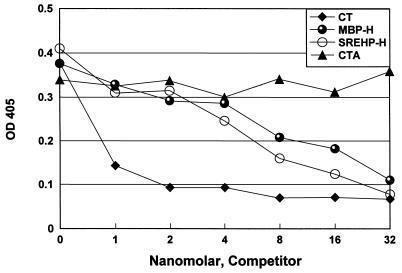
To determine whether the purified SREHP-H and MBP-H molecules had GM1 ganglioside binding activity, we performed a competitive assay for GM1 binding (30). We examined the ability of purified SREHP-H, MBP-H, CT, and CTA to inhibit the binding of biotinylated CTB to GM1 ganglioside. Because the purified SREHP-H and MBP-H preparations contained significant amounts of uncomplexed MBP-SREHP-CTA2 and MBP-CTA2 respectively, concentrations of the holotoxin-like chimeras were calculated on the basis of densitometric comparison of CTB quantities in SREHP-H and MBP-H versus purified CT. As shown in Fig. 3, significant inhibition of CTB binding to GM1 ganglioside was seen with both SREHP-H and MBP-H compared to CTA.
FIG. 3.
SREHP-H can inhibit the binding of CT to GM1 ganglioside. Equimolar concentrations of the purified SREHP-H and MBP-H proteins, CT, and CTA were serially diluted and then mixed with a standard amount of biotinylated CTB and reacted with GM1 ganglioside bound to an ELISA plate. SREHP-H, MBP-H, and CT could inhibit the binding of CTB to GM1 ganglioside, while no inhibition was detected with the CTA preparation. OD 405, optical density at 405 nm.
Mucosal immunogenicity of the SREHP-H and MBP-H chimeras.
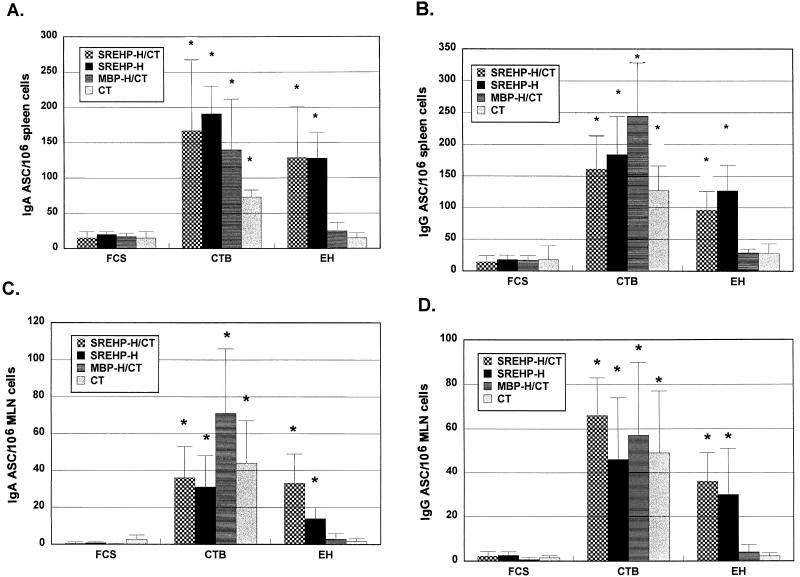
The immunogenicity of the SREHP-H and MBP-H chimeras was assessed in two different experiments. To determine whether SREHP-H was immunogenic and whether the antiamebic and anti-CTB response to SREHP-H was comparable to that seen when SREHP-H was administered with a subclinical dose of CT, we orally immunized groups of five to seven mice with either SREHP-H, SREHP-H and 5 μg of CT (SREHP-H/CT), MBP-H and 5 μg of CT (MBP-H/CT), or 5 μg of CT alone at days 0, 14, and 28. Seven days later (at day 35), the numbers of ASC producing IgG and IgA antiamebic and anti-CTB antibodies in spleen and MLN cells were measured by the ELISPOT assay. As shown in Fig. 4, cells secreting IgA and IgG that bound amebic trophozoite antigens were found in spleen and MLN cells from mice vaccinated with SREHP-H. There were no statistically significant differences in the number of cells secreting IgA or IgG that bound to amebic trophozoites between mice receiving SREHP-H alone or SREHP-H and supplemental CT. Mice vaccinated with MBP-H and supplemental CT or with CT alone did not have significant numbers of cells secreting IgA or IgG that bound to amebic trophozoites. Mice from all four groups (SREHP-H, SREHP-H/CT, MBP-H/CT, and CT) had splenic and MLN cells that secreted IgG or IgA anti-CTB antibodies, and the differences between them were not significant. Few cells secreting IgG or IgA that reacted with FCS were detected in splenic or MLN cells from any of the four groups.
FIG. 4.
Oral immunization with SREHP-H generates ASC in spleen and MLN cells producing IgA and IgG antiamebic antibodies. (A and B) Numbers of IgA and IgG ASC, respectively, from spleen cells obtained from mice immunized with SREHP-H, SREHP-H/CT, MBP-H/CT, or CT alone that produce antiamebic antibodies (EH), anti-CTB antibodies (CTB), or anti-FCS (FCS) antibodies. (C and D) Numbers of IgA and IgG ASC, respectively, in MLN cells obtained from mice immunized with SREHP-H, SREHP-H/CT, MBP-H/CT, or CT alone producing antiamebic antibodies, anti-CTB antibodies, and anti-FCS antibodies. Bars show the mean values ± the standard errors of the means (error bars) for groups of five to seven mice. An asterisk over the bar indicates that the mean value was significantly higher (P < 0.05) than the corresponding FCS (control) value.
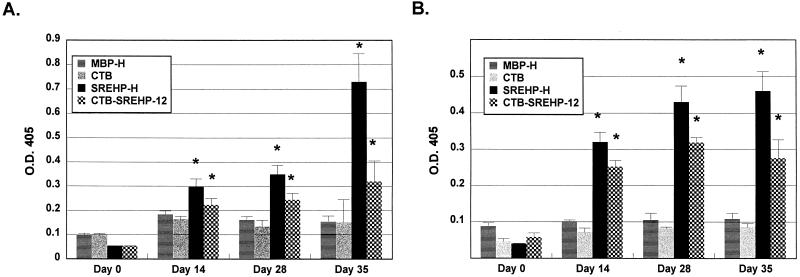
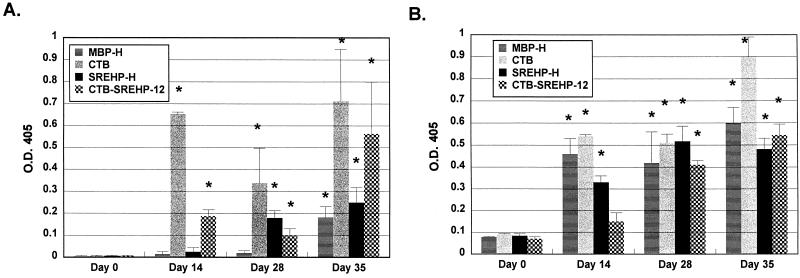
Having established that the SREHP-H chimera was immunogenic, we measured mucosal and humoral IgA and IgG antibody responses to amebic trophozoites after SREHP-H vaccination. For these studies, we also compared the response to SREHP-H with the response to a chimeric protein consisting of a dodecapeptide derived from SREHP fused to the CTB peptide (CTB-SREHP-12) (30). Previous studies showed that oral immunization with CTB-SREHP-12 and supplemental CT could induce mucosal IgA antiamebic responses in bile and stool samples from vaccinated mice, but the ability of CTB-SREHP-12 to induce mucosal immune responses without supplemental CT had not been evaluated (30). In this study, groups of 7 to 10 mice were orally immunized with either SREHP-H, MBP-H, CTB-SREHP-12, or CTB on days 0, 14, and 28, and IgG and IgA antiamebic and anti-CTB antibodies in stool and serum samples were assayed. Mice receiving SREHP-H developed IgA antiamebic antibodies in their stools (Fig. 5A). We did not detect IgG antiamebic responses in the stools of vaccinated mice (data not shown). The IgA antiamebic response in the stools of SREHP-H-immunized mice was significantly higher than preimmunization IgA levels as early as day 14 and was highest at day 35. Mice immunized with CTB-SREHP-12 also had levels of IgA antiamebic antibodies in their stools that were significantly greater than preimmunization levels at day 14, but they were not significantly different from those of MBP-H- and CTB-vaccinated mice until day 35 (Fig. 5A). The IgA antiamebic response in stools was significantly higher in SREHP-H-vaccinated mice than in CTB-SREHP-12-vaccinated mice at day 35 (P < 0.01). Mice immunized with MBP-H or CTB did not develop IgA antiamebic antibodies in stools (Fig. 5A). SREHP-H- and CTB-SREHP-12-vaccinated mice developed IgG antiamebic antibodies that could be detected in serum by day 14 (Fig. 5B). We did not detect IgA antiamebic antibodies in the sera of vaccinated mice (data not shown). There was a trend towards higher levels of IgG antiamebic antibodies in the sera of SREHP-H-vaccinated mice than in the sera of CTB-SREHP-12-vaccinated mice, but a statistically significant difference was detected only at day 35 (P < 0.05). Mice immunized with MBP-H or CTB did not produce IgG antiamebic antibodies in serum (Fig. 5B). All vaccinated mice produced IgA anti-CTB antibodies in their stools by day 35, but the levels of responses were low. Levels of anti-CTB antibodies were highest and detectable earliest in CTB- and CTB-SREHP-12-vaccinated mice (Fig. 6A). This may reflect higher CT concentrations in these preparations than in the MBP-H and SREHP-H preparations. IgG anti-CTB antibodies were not detected in the stools of vaccinated mice (data not shown). IgA anti-CTB responses in the stools of MBP-H-vaccinated mice were not significantly different from preimmunization levels until day 35. All vaccinated mice produced IgG anti-CTB antibodies in serum, and the levels were significantly higher than preimmunization levels for all groups by day 28 (Fig. 6B). The serum IgG anti-CTB response in mice orally immunized with CTB was significantly higher than those of other groups at day 35 (P < 0.05).
FIG. 5.
Oral immunization with SREHP-H induces IgA antiamebic antibodies in the stools and IgG antiamebic antibodies in the sera of vaccinated mice. (A) IgA antiamebic antibodies in stool samples from mice vaccinated with SREHP-H, MBP-H, CTB-SREHP-12, or CTB immediately before vaccination (day 0) and 14, 28, and 35 days after the initial vaccine dose. (B) IgG antiamebic antibodies in sera from the same mice at the identical time points. Bars show the mean values ± the standard errors of the means (error bars) for groups of 7 to 10 mice. An asterisk over the bar indicates that the mean value was significantly higher (P < 0.05) than the preimmunization (day 0) mean. O.D. 405, optical density at 405 nm.
FIG. 6.
Oral immunization with SREHP-H induces IgA anti-CTB antibodies in the stools and IgG anti-CTB antibodies in the sera of vaccinated mice. (A) IgA anti-CTB antibodies in stool samples from mice vaccinated with SREHP-H, MBP-H, CTB-SREHP-12, or CTB immediately before vaccination (day 0) and 14, 28, and 35 days after the initial vaccine dose. (B) IgG anti-CTB antibodies in sera from the same mice at the identical time points. Bars show the mean values ± the standard errors of the means (error bars) for groups of 7 to 10 mice. An asterisk over the bar indicates that the mean value was significantly higher (P < 0.05) than the preimmunization (day 0) mean. O.D. 405, optical density at 405 nm.
DISCUSSION
The generation of a protective immune response at the intestinal mucosal surface may be critical in preventing amebiasis and other enteric infections. Oral vaccines can induce immune responses at mucosal surfaces, but the development of effective vaccines requires the identification of suitable antigens and a mode of delivery that will generate protective responses. Previous studies from our laboratory have identified the amebic SREHP molecule as a candidate for inclusion in a vaccine to prevent amebiasis. Active immunization with recombinant SREHP-MBP (by parenteral immunization or by oral vaccination with attenuated Salmonella typhimurium expressing the SREHP-MBP fusion protein) can protect against amebic liver abscess in animal models, while passive immunization of severe combined immunodeficient (SCID) mice with anti-SREHP antibodies can prevent amebic liver abscess in mice after direct hepatic inoculation of E. histolytica trophozoites (28, 29, 31). One of the promising approaches for the delivery of oral vaccines to induce mucosal immune responses has been the coupling of antigens to the nontoxic B subunit of CT. Chemical coupling of antigens to CTB confers the abilities to bind to GM1 ganglioside and to elicit secretory IgA antibodies (6, 16, 27, 30). Recently, the construction of a fusion protein containing the CTB moiety fused to a dodecapeptide derived from SREHP (CTB-SREHP-12) was described (30). When mice were orally immunized with CTB-SREHP-12 and a subclinical dose of CT, they produced mucosal IgA antiamebic antibodies and IgG antiamebic antibodies in serum. While this approach was successful in generating mucosal IgA antibodies to SREHP and amebas, there are limitations to this strategy, including the ability to fuse only a short portion of the SREHP molecule to CTB (longer peptides have been shown to reduce CTB pentamer formation, thus reducing GM1 ganglioside binding by the complex) (8) and the possible need for supplemental CT to induce antibody responses, an undesirable feature for any vaccine destined for human use.
As an alternative strategy to induce mucosal antibody responses to SREHP, we engineered a holotoxin-like molecule that was produced by coexpressing a MBP-SREHP-CTA2 fusion protein and CTB in E. coli. We established that this SREHP-H molecule in crude extracts could bind to GM1 ganglioside and that the purified SREHP-H complex could inhibit the binding of CT to GM1 ganglioside. SDS-PAGE analysis of SREHP-H that had been affinity purified with an anti-SREHP antibody demonstrated the presence of CTB, indicating that detectable quantities of the MBP-SREHP-CTA2 fusion protein produced were complexed to CTB. We also produced an MBP-H molecule using a previously described construct (14), and lysates from bacteria expressing MBP-H contained complexes of MBP capable of binding to GM1 ganglioside. However, MBP-H that was affinity purified by amylose resin chromatography contained fewer chimeric complexes than were found in SREHP-H, suggesting either that the purification process selected for noncomplexed MBP-CTA2 fusion protein, that the MBP-CTA2 fusion protein was less efficient in forming stable holotoxin molecules with CTB, or that the ratios of fusion protein to CTB produced by E. coli were different for the strains that expressed the SREHP and MBP constructs.
We found that mice orally immunized with the SREHP-H molecule produced ASC in MLN and spleen cells that made IgA and IgG antiamebic antibodies and that the number of antiamebic ASC produced in SREHP-H-vaccinated mice was significantly higher than the number detected in mice vaccinated with MBP-H and CT or with CT alone. While there was a trend towards a higher number of ASC producing antiamebic antibodies in mice orally vaccinated with SREHP-H and supplemental CT than in mice vaccinated with SREHP-H alone, the difference was not statistically significant. These data indicated that SREHP-H can induce antiamebic mucosal immune responses without supplemental CT. Measurement of IgA antibodies in stools confirmed this finding, as SREHP-H-vaccinated mice produced IgA antiamebic antibodies, while no antiamebic antibodies were detected in MBP-H-vaccinated mice. We were also able to detect IgG antiamebic antibodies in the sera of SREHP-H-vaccinated mice but detected no antiamebic antibodies in MBP-H-vaccinated mice. These findings are consistent with the results seen when a chimeric protein containing a portion of S. mutans AgI/II fused to CTA2 was used (12, 13). Mice orally immunized with this construct (without supplemental CT) produced mucosal IgA antibodies and serum IgG antibodies to the streptococcal adhesin (12). Mice immunized with SREHP-H also produced IgA anti-CTB antibodies in their stools and IgG anti-CTB antibodies in their sera, but the level of response was low, and the response developed more slowly and was lower in magnitude than that seen in CTB- or CTB-SREHP-12-vaccinated mice. This may simply reflect less CTB in the SREHP-H preparation than in the CTB-SREHP-12 and CTB preparations. Despite the lower number of holotoxin-like complexes in the MBP-H preparation, mice vaccinated with MBP-H produced anti-CTB antibodies in their stools and sera, indicating that the smaller quantity of CTB present in the MBP-H preparation was still adequate for induction of anti-CTB responses. We were also able to detect IgA antiamebic antibodies in the stools of mice that were vaccinated with the CTB-SREHP-12 fusion protein. While the magnitude of this response was lower than that detected with SREHP-H immunization, these results demonstrate that the CTB-SREHP-12 construct is mucosally immunogenic and does not require supplemental CT for the induction of mucosal antiamebic antibodies. These data indicate that when the SREHP molecule (or a portion of it) is coupled to CTB, via either the A2 linkage or direct fusion, the resultant chimera is mucosally immunogenic. Whether the effect of CTB is due to its targeting function (facilitating binding of the SREHP complex to GM1 ganglioside) or is secondary to some other property of CTB (a recent study has indicated that CTB can bind to T-cell clones and induce interleukin 4 production, thus providing a potential mechanism for adjuvant activity) remains to be determined (15).
Previous successful attempts to induce mucosal immune responses to recombinant E. histolytica antigens have utilized either oral administration of antigen in conjunction with CT or delivery of antigen by attenuated S. typhimurium vectors (3, 31). The ability of SREHP-H to induce mucosal antiamebic antibodies without supplemental CT makes it a viable and attractive candidate for further testing as an oral vaccine to prevent amebic colonization and disease. In summary, our studies confirm that genetic coupling of antigens to CTA2 and their coexpression in E. coli with CTB can result in the formation of holotoxin-like molecules that are mucosally immunogenic without supplemental CT, and they establish that oral vaccination with SREHP-H can induce mucosal IgA antiamebic antibodies.
ACKNOWLEDGMENTS
We thank Tonghai Zhang for help with these studies.
This work was supported in part by grants NIH AI30084 and WHO GPV-15/181/281 to S.L.S. and by grant NIH AI31940 to R.K.H. S.L.S. is the recipient of Research Career Development Award AI01231 from the NIAID.
F. Sultan and L.-L. Jin contributed equally to this work.
REFERENCES
- 1.Bessen D, Fischetti V. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988;56:2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betley M, Miller V, Mekalanos J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1989;40:577–605. doi: 10.1146/annurev.mi.40.100186.003045. [DOI] [PubMed] [Google Scholar]
- 3.Beving D E, Soong C-J G, Ravdin J I. Oral immunization with a recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin elicits an intestinal secretory immunoglobulin A response that has in vitro adherence inhibition activity. Infect Immun. 1996;64:1473–1476. doi: 10.1128/iai.64.4.1473-1476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973;12:3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Nilsson L-A, Nygren H, Ouchterlony O, Tarkowski A. A solid phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 6.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerkinsky, C., A.-M. Svennerholm, and J. Holmgren. 1993. Induction and assessment of immunity at enteromucosal surfaces in humans: implications for vaccine development. Clin. Infect. Dis. 16(Suppl. 2):S106–S116. [DOI] [PubMed]
- 8.Dertzbaugh M T, Elson C O. Reduction in oral immunogenicity of cholera toxin B subunit by N-terminal peptide addition. Infect Immun. 1993;61:384–390. doi: 10.1128/iai.61.2.384-390.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson C O. Cholera toxin and its subunits as potential oral adjuvants. Curr Top Microbiol Immunol. 1989;146:29–33. doi: 10.1007/978-3-642-74529-4_3. [DOI] [PubMed] [Google Scholar]
- 10.Elson C O, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–2897. [PubMed] [Google Scholar]
- 11.Elson C O, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2741. [PubMed] [Google Scholar]
- 12.Hajishengallis G, Hollingshead S K, Koga T, Russell M W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 13.Hajishengallis G, Michalek S M, Russell M W. Persistence of serum and salivary antibody responses after oral immunization with a bacterial protein antigen genetically linked to the A2/B subunits of cholera toxin. Infect Immun. 1996;64:665–667. doi: 10.1128/iai.64.2.665-667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobling M G, Holmes R K. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun. 1992;60:4915–4924. doi: 10.1128/iai.60.11.4915-4924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T K, Fox B S. Cholera toxin B subunit binding to an antigen-presenting cell directly co-stimulates cytokine production from a T-cell clone. Int Immunol. 1996;8:1849–1856. doi: 10.1093/intimm/8.12.1849. [DOI] [PubMed] [Google Scholar]
- 16.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholera and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie S J, Halsey J F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984;133:1818–1824. [PubMed] [Google Scholar]
- 18.Meza I, Cazares F, Rosales-Encina J L, Talamas-Rohana P, Rojkind M. Use of antibodies to characterize a 220-kilodalton surface protein from Entamoeba histolytica. J Infect Dis. 1987;156:798–805. doi: 10.1093/infdis/156.5.798. [DOI] [PubMed] [Google Scholar]
- 19.Myung K, Burch D, Jackson T F H G, Stanley S L., Jr Serodiagnosis of invasive amebiasis using a recombinant Entamoeba histolytica antigen-based ELISA. Arch Med Res. 1992;23:285–288. [PubMed] [Google Scholar]
- 20.Ravdin J I, Petri W A, Jr, Murphy C F, Smith R D. Production of mouse monoclonal antibodies which inhibit in vitro adherence of Entamoeba histolytica trophozoites. Infect Immun. 1986;53:1–5. doi: 10.1128/iai.53.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell M W, Wu H. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991;59:4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez J L, Trofa A F, Taylor D N, Kuschner R A, DeFraites R F, Craig S C, Rao M R, Clemens J D, Svennerholm A-M, Sadoff J C, Holmgren J. Safety and immunogenicity of the oral, whole cell/recombinant B subunit cholera vaccine in North American volunteers. J Infect Dis. 1993;167:1446–1449. doi: 10.1093/infdis/167.6.1446. [DOI] [PubMed] [Google Scholar]
- 23.Snider D P. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15:317–348. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 24.Stanley S L, Jr, Becker A, Kunz-Jenkins C, Foster L, Li E. Cloning and expression of a membrane antigen of Entamoeba histolytica possessing multiple tandem repeats. Proc Natl Acad Sci USA. 1990;87:4976–4980. doi: 10.1073/pnas.87.13.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley S L, Jr, Huizenga H, Li E. Isolation and partial characterization of a surface glycoconjugate of Entamoeba histolytica. Mol Biochem Parasitol. 1992;50:127–138. doi: 10.1016/0166-6851(92)90250-n. [DOI] [PubMed] [Google Scholar]
- 26.Stanley S L, Jr, Tian K, Koester J P, Li E. The serine rich Entamoeba histolytica protein (SREHP) is a phosphorylated membrane protein containing O-linked terminal N-acetylglucosamine (O-GlcNAc) residues. J Biol Chem. 1995;270:4121–4126. doi: 10.1074/jbc.270.8.4121. [DOI] [PubMed] [Google Scholar]
- 27.Svennerholm A-M, Jertborn M, Gothefors L, Karim A M M M, Sack D A, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, Cieslak P R, Foster L, Kunz-Jenkins C, Stanley S L., Jr Antibodies to the serine rich Entamoeba histolytica protein (SREHP) prevent amebic liver abscess in severe combined immunodeficient (SCID) mice. Parasite Immunol. 1994;16:225–230. doi: 10.1111/j.1365-3024.1994.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Cieslak P R, Stanley S L., Jr Protection of gerbils from amebic liver abscess by immunization with a recombinant Entamoeba histolytica antigen. Infect Immun. 1994;62:1166–1170. doi: 10.1128/iai.62.4.1166-1170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T, Li E, Stanley S L., Jr Oral immunization with the dodecapeptide repeat of the serine-rich Entamoeba histolytica protein (SREHP) fused to the cholera toxin B subunit induces a mucosal and systemic anti-SREHP antibody response. Infect Immun. 1995;63:1349–1355. doi: 10.1128/iai.63.4.1349-1355.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Stanley S L., Jr Oral immunization with an attenuated vaccine strain of Salmonella typhimurium expressing the serine-rich Entamoeba histolytica protein (SREHP) induces an anti-amebic immune response and protects gerbils from amebic liver disease. Infect Immun. 1996;64:1526–1531. doi: 10.1128/iai.64.5.1526-1531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]