Abstract
Lipotoxicity is a pivotal factor that initiates and exacerbates liver injury and is involved in the development of metabolic-associated fatty liver disease (MAFLD). However, there are few reported lipotoxicity inhibitors. Here, we identified a natural anti-lipotoxicity candidate, HN-001, from the marine fungus Aspergillus sp. C1. HN-001 dose- and time- dependently reversed palmitic acid (PA)-induced hepatocyte death. This protection was associated with IRE-1α-mediated XBP-1 splicing inhibition, which resulted in suppression of XBP-1s nuclear translocation and transcriptional regulation. Knockdown of XBP-1s attenuated lipotoxicity, but no additional ameliorative effect of HN-001 on lipotoxicity was observed in XBP-1s knockdown hepatocytes. Notably, the ER stress and lipotoxicity amelioration was associated with PLA2. Both HN-001 and the PLA2 inhibitor MAFP inhibited PLA2 activity, reduced lysophosphatidylcholine (LPC) level, subsequently ameliorated lipotoxicity. In contrast, overexpression of PLA2 caused exacerbation of lipotoxicity and weakened the anti-lipotoxic effects of HN-001. Additionally, HN-001 treatment suppressed the downstream pro-apoptotic JNK pathway. In vivo, chronic administration of HN-001 (i.p.) in mice alleviated all manifestations of MAFLD, including hepatic steatosis, liver injury, inflammation, and fibrogenesis. These effects were correlated with PLA2/IRE-1α/XBP-1s axis and JNK signaling suppression. These data indicate that HN-001 has therapeutic potential for MAFLD because it suppresses lipotoxicity, and provide a natural structural basis for developing anti-MAFLD candidates.
Key words: Lipotoxicity, MAFLD, ER stress, IRE-1α, XBP-1s, JNK, PLA2
Graphical abstract
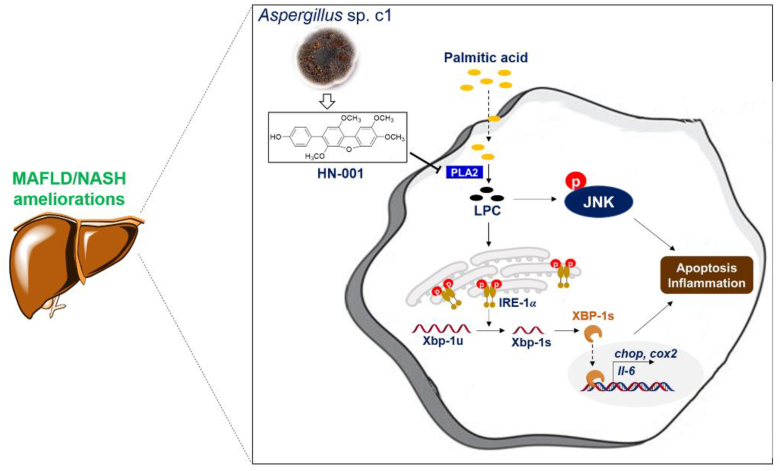
The identified marine fungus metabolite HN-001 ameliorates lipotoxicity by suppressing IRE-1α/XBP-1s axis and JNK pathway for MAFLD treatment with PLA2 as an upstream target.
1. Introduction
Metabolic-associated fatty liver disease (MAFLD) is emerging as the leading chronic liver disease worldwide with excessive lipid ectopic accumulation in liver and is viewed as the hepatic manifestation of metabolic syndrome1. It includes a spectrum of histological features ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), is associated with ballooning and injury of hepatocytes, inflammation and/or deposition of collagen and fibrosis progression; and ultimately drives to liver cirrhosis and hepatocellular carcinoma2,3. At present, MAFLD is considered the most common liver disease, and it has exceeded the hepatitis B virus infection of the size of the affected population, reaching the magnitude of an epidemic. However, the lack of ideal drugs and increasing demands emphasize the importance of developing anti-MAFLD candidates.
MAFLD is a complex disease characterized by contributions of a diverse array of factors to its pathogenesis4,5. The primary instigator of metabolic alterations in hepatocytes is ectopic accumulation of lipids, which subsequently induces hepatocyte damage and death. This phenomenon is commonly referred to as “lipotoxicity”6, 7, 8. Toxic lipids can induce hepatocyte damage through different mechanisms, including modification of intracellular organelle function, direct activation of death receptor signaling pathways, and aberrant activation of stress response genes9. Among these processes, the endoplasmic reticulum (ER) stress response has been demonstrated to play a crucial role in the progression of MAFLD and NASH10. During ER stress, IRE-1α activates proteins involved in either apoptosis or inflammation, including spliced XBP-1 (XBP-1s) and c-Jun N-terminal kinase (JNK). Either XBP-1s or JNK activation is associated with cell death in both liver parenchymal and nonparenchymal cells, thus exacerbating hepatic steatosis11,12. Additionally, JNK interacts with mitochondria to induce reactive oxygen species (ROS) generation and subsequently apoptosis13. Meanwhile, the interplay among lipotoxicity, inflammation, and fibrosis sustains the production of toxic signals, exerting a prolonged influence that drives the progression from MAFLD to NASH6,14. During our search for novel pharmacologic agents to disrupt this mechanism, we identified a natural product called HN-001 (MW: 380.4), derived from the marine fungus Aspergillus sp. C1 and reported its antibacterial15, antioxidant15, antiinflammatory16, and antiausterity17 properties. However, its potential as an anti-MAFLD agent and the underlying molecular mechanism remain unexplored.
The objective of this study was to assess the anti-lipotoxic effect of HN-001 in hepatocytes induced by palmitic acid (PA) and investigate the molecular mechanism underlying the ability of HN-001 to alleviate lipotoxicity and promote hepatocyte survival. Additionally, we aimed to evaluate the hepatoprotective effect of HN-001 in a mouse model of high-fat and high-cholesterol (HFC) diet-induced MAFLD, which closely recapitulates the pathogenesis of MAFLD in clinical settings. The results demonstrated that HN-001 effectively mitigated PA-induced lipotoxicity in hepatocytes by suppressing ER stress through inhibition of the PLA2/IRE-1α/XBP-1s axis. Furthermore, in the chronic HFC diet-fed MAFLD mouse model, HN-001 alleviated all manifestations of MAFLD without any observable signs of toxicity or adverse effects.
2. Results and discussion
2.1. Discovery of HN-001 as a novel natural anti-lipotoxic candidate in PA-treated hepatocytes
Lipotoxicity-induced hepatocyte death plays an essential role in driving liver injury, hepatic inflammation and fibrogenesis initiation7,8. The anti-lipotoxic effect of HN-001 (the chemical structure is listed in Fig. 1A) was determined in PA-treated hepatocytes as previously reported18. The anti-NASH agent and PPARγ agonist rosiglitazone (20 μmol/L) served as a positive control. PA treatment induced excessive lipid accumulation and exacerbated hepatocyte injury, as indicated by increased levels of cellular triglycerides (TGs) and free fatty acids (FFAs), an increased number of apoptotic cells and a decreased number of viable cells (Fig. 1B–F). In addition, caspase 3 activity was significantly higher in the PA-treated group than in the blank group (Fig. 1G). HN-001 treatment dose-dependently reduced the cellular TG content but increased the FFA level, thus reversing PA-induced lipotoxicity in hepatocytes. This protection was evidenced by significant decreases in the number of apoptotic cells and the activity of caspase 3 activity but an increase in the number of viable cells compared with those in the PA-treated control group (Fig. 1B–G). Interestingly, rosiglitazone treatment failed to decrease cellular TG levels or reverse lipotoxicity. We also determined the time dependency of HN-001. Interestingly, HN-001 treatment displayed time-dependent inhibition of hepatocyte apoptosis and enhancement of cell survival (Fig. 1H–J). This hepatocyte-protective effect was observed as early as 8 h upon PA induction. It is worth noting that the anti-lipotoxic effect of HN-001 was not related to cell toxicity (Supporting Information Fig. S1).
Figure 1.
Discovery of HN-001 as a novel natural anti-lipotoxic candidate in hepatocytes. (A) Chemical structure of the compound HN-001. (B–C) Determination of cellular TG and FFA levels. (D) Imaging of cellular TG levels using the specific lipid dye Nile red. Scale bar, 50 μm. (E–F) Apoptotic and viable hepatocyte counting by flow cytometry analysis. (G) Caspase 3 activity determination. (H–J) Time-dependent effect of HN-001 in protecting hepatocytes against lipotoxicity induced by PA. (H) Representative images of the contrast field in hepatocytes. (I) Apoptotic cell quantification. (J) Viable cell quantification. n = 5 independent experiments. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with blank group (PA untreated) cells; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with PA group cells.
2.2. HN-001 dose-dependently eliminates lipotoxicity and ameliorates ER stress
Induction of ER stress involves the activation of three transmembrane ER-resident stress sensors: inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor (ATF6). We then determined the effect of HN-001 on ER stress signaling. HN-001 treatment dose-dependently reversed PA treatment-induced ER stress activation, as indicated by decreases in the total protein level of IRE-1α and ATF6, and the levels of phosphorylated IRE-1αSer724 and PERKThr980 (Fig. 2A). Immunofluorescence analysis of phosphorylated IRE-1αSer724 (p-IRE-1αSer724) further confirmed the inhibitory effect of HN-001 on IRE-1α activation (Fig. 2B). An interesting study has reported that IRE-1α is an endogenous substrate of ER-associated degradation (ERAD) and that GRP78 plays a key role in retaining IRE-1α for ERAD degradation by assembling an IRE-1α/GRP78/SEL1L complex19. HN-001 treatment increased the protein levels of GRP78 and the ERAD marker SEL1L as well as the interactions between IRE-1α, GRP78, and SEL1L (Supporting Information Fig. S2A and S2B). Additionally, we treated PA-induced hepatocytes with the ERAD inhibitor Eeyarestatin I in the presence or absence of HN-001 and found that Eeyarestatin I treatment activated the IRE-1α/XBP-1s axis, as indicated by increases in the levels of IRE-1α, XBP-1s and phosphorylated IRE-1α, thus exacerbating hepatocyte death (Fig. S2C and S2D). Cotreatment with Eeyarestatin I and HN-001 weakened the suppressive effect of HN-001 on the IRE-1α/XBP-1s axis and lipotoxicity in PA-induced hepatocytes (Fig. S2C and S2D), indicating that HN-001-mediated downregulation of IRE-1α may be involved in improving ERAD.
Figure 2.
HN-001 treatment inhibits the IRE-1α/XBP-1s axis in PA-treated hepatocytes. (A) Expression levels of ER stress signaling pathway related proteins. (B) Immunofluorescence analysis of p-IRE-1α (Ser724). Scale bar, 50 μm. (C) Effect of HN-001 on XBP-1 mRNA splicing. The resultant cDNA was digested with PstI and analyzed by 3% agarose gel to visualize the activated spliced (XBP-1s) and unspliced (XBP-1u) products. β-actin was used as a control. Gene expression levels were quantified and normalized to those of β-actin. The average levels of XBP-1s/XBP-1u in the blank group from 5 independent experiments were set as 1, and the relative fold changes were determined. (D) Protein levels of ER stress biomarkers and apoptosis-related proteins. (E) Determination of cellular ROS levels by flow cytometry analysis. (F) Imaging of cellular ROS using a DCFH-DA probe. Scale bar, 50 μm. n = 5 independent experiments. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with blank group (PA untreated) cells; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with PA-treated cells.
A detailed study has demonstrated that IRE-1α activation-mediated XBP-1 mRNA splicing is indispensable for ER stress activation20. Splicing activation of XBP-1s has been shown to promote lipogenesis in the liver by directly modulating the expression of lipogenic genes21,22. Regarding the lipid-decreasing effect, we then determined the effect of HN-001 on XBP-1 splicing. Interestingly, HN-001 treatment inhibited PA-induced XBP-1 splicing, as indicated by an increase in the level of XBP-1u and decreases in the level of XBP-1s and the ratio of XBP-1s to XBP-1u (Fig. 2C). Immunoblot analysis of XBP-1s confirmed the inhibitory effect on XBP-1s translation (Fig. 2D).
Our and other studies have recently revealed that persistent activation of ER stress is a key instigator of hepatocyte apoptosis, leading to liver injury12,23. HN-001 addition dose-dependently decreased the protein levels of the ER stress-induced apoptosis marker CAAT enhancer binding protein homologous protein (CHOP) and apoptosis markers, including cleaved caspase 7 and caspase 3 (Fig. 2D). These data were consistent with the inhibition of caspase 3 activity and the promotion of cell survival. ROS, a representative biomarker of oxidative stress, are thought to be upstream regulators of apoptosis. As shown in Fig. 2E and F, PA treatment markedly increased cellular ROS levels, while HN-001 treatment dose-dependently reversed this stimulation.
2.3. HN-001 inhibits lipotoxicity by suppressing ER stress
PA-induced hepatocyte apoptosis is a sequential biological process involved in ER stress and proapoptotic signaling. To further reveal whether HN-001 prevented hepatocyte death in association with ER stress attenuation, we performed a time dependency examination after PA treatment with or without HN-001 treatment. As shown in Fig. 3, PA treatment activated the IRE-1α/XBP-1s axis in a time-dependent manner, as indicated by increases in the total and phosphorylated levels of IRE-1α (Fig. 3A). This activation occurred as early as 1 h upon PA induction, leading to XBP-1 splicing and subsequent hepatocyte apoptosis at 4–8 h. Examination of the protein level and enzyme activity of caspase 3 revealed that PA induction increased the protein level of cleaved caspase 3 and the activity of caspase 3 starting at 8 h after PA treatment (Fig. 3B–D). Interestingly, HN-001 treatment time-dependently inhibited IRE-1α activation in PA-treated hepatocytes as early as 1 h after PA treatment, correspondingly blocking XBP-1 splicing and subsequent apoptosis. Compared with those in counterpart PA-treated control cells, XBP-1 splicing and hepatocyte apoptosis were inhibited at each time point (Fig. 3A–D). In addition, examination of ROS levels revealed that HN-001 treatment time dependently reversed PA-induced increases in ROS levels (Fig. 3E and F).
Figure 3.
HN-001 suppresses lipotoxicity in a time-dependent manner alongside IRE-1α/XBP-1 axis inhibition. Hepatocytes were treated with PA for 0, 1, 4, 8, 12, or 24 h with or without HN-001 treatment (25 μmol/L), after which the cells were harvested and subjected to the indicated examinations. (A) Expression levels of IRE-1α/XBP-1 axis proteins. (B) Protein levels of CHOP and the apoptosis marker cleaved caspase 3. (C) Immunofluorescence analysis of cleaved caspase 3. Scale bar, 50 μm. (D) Caspase 3 activity determination. (E) Determination of cellular ROS levels by flow cytometry analysis. (F) Imaging of cellular ROS using a DCFH-DA probe. Scale bar, 50 μm. (G) Proposed mechanism by which HN-001 ameliorates lipotoxicity in PA-induced hepatocytes. n = 5 independent experiments. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with blank (PA-untreated) cells; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with PA-treated cells.
To further elucidate the relationship between ER stress inhibition and lipotoxicity amelioration, the reported IRE-1α/XBP-1s axis inhibitor TUDCA24 was used as a positive control. Notably, TUDCA treatment efficiently downregulated the protein levels of ER stress signaling proteins and apoptosis markers in PA-treated hepatocytes, thus preventing apoptosis (Supporting Information Fig. S3A–S3C). Furthermore, we treated dithiothreitol (DTT, ER stress inducer)-induced hepatocytes with HN-001 or TUDCA. Interestingly, DTT incubation activated ER stress and induced hepatocyte apoptosis, while these effects were reversed upon TUDCA or HN-001 addition (Fig. S3D and S3E). Immunoblot analysis revealed that either HN-001 or TUDCA treatment suppressed DTT-mediated ER stress activation and decreased the protein level of cleaved caspase 3 (Fig. S3F). These data indicate that inhibition of the IRE-1α/XBP-1 axis contributes to the lipotoxicity amelioration of HN-001, at least in part (Fig. 3G).
2.4. HN-001 inhibits XBP-1s transcriptional regulation
Spliced XBP-1 plays essential roles in activating ER stress by directly binding to the promoters of genes, including Chop. Additionally, XBP-1 has been reported to modulate inflammation by controlling the transcription of Cox2 and Il-6 (Fig. 4A)25,26. We next determined the effect of HN-001 in blocking XBP-1s nuclear translocation and its ability to control downstream genes. As shown in Fig. 4B, HN-001 treatment decreased the levels of XBP-1s in the nucleus and cytosol as well as the ratio of nuclear XBP-1s (nXBP-1s) to cytosolic XBP-1s (cXBP-1s). Immunofluorescence analysis further confirmed the inhibitory effect of HN-001 on nuclear XBP-1s levels (Fig. 4C). Moreover, we observed a time-dependent effect on XBP-1s nuclear translocation and, as expected, found that PA treatment time-dependently increased the total and nuclear levels of XBP-1s but that these increases were reversed by addition of HN-001 (Fig. 4D). By performing chromatin immunoprecipitation (ChIP) assays, we found that PA treatment significantly enriched the protein XBP-1s in the promoters of genes involved in ER stress and inflammation, including Chop, Cox2, and Il-6, while this enrichment was blocked upon HN-001 treatment (Fig. 4E). Consistent with these results, HN-001 treatment dose-dependently reversed the PA-stimulated increases in the transcription levels of Chop, Cox2 and Il-6 (Fig. 4F–H).
Figure 4.
HN-001 inhibits XBP-1s nuclear translocation and binding to target genes. (A) Schematic diagram of XBP-1s in regulating downstream genes. (B) Determination of XBP-1s levels in the cytosol and nucleus. Protein levels were quantified and normalized to those of the loading control (GAPDH). The average levels of proteins in the blank group from 5 independent experiments were set as 1, and the relative fold changes were determined. (C) Immunofluorescence analysis of XBP-1s. Scale bar, 50 μm. (D) Quantification of the time-dependent effect of PA on XBP-1s nuclear translocation with or without HN-001 (25 μmol/L) treatment. Scale bar, 30 μm. (E) XBP-1s recruitment to the target gene promoter region as analyzed by ChIP. IgG was used as a negative control. The average level in the blank group from 5 independent experiments was set as 1, and relative fold changes were calculated. (F–H) Determination of the mRNA levels of Chop, Cox2, and Il-6 by qRT‒PCR assay. The gene expression levels were calculated via normalization to those of the loading control (β-actin). The average levels of genes in the blank group from 5 independent experiments were set as 1, and the relative fold changes were determined. n = 5 independent experiments. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with blank group (PA-untreated) cells; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with PA-treated cells.
2.5. Knockdown of XBP-1s reduces lipotoxicity
To reveal the role of the IRE-1α/XBP-1s axis in the lipotoxicity amelioration of HN-001, we knocked down XBP-1s expression in HepG2 cells by transfecting the cells with control or XBP-1s siRNA (Fig. 5A). Both XBP-1s knockdown and HN-001 treatment effectively downregulated the expression of downstream genes, including Chop, Cox2, and Il-6 (Fig. 5B). Immunoblot analysis of apoptosis-related proteins, including CHOP and cleaved caspase 3 (C-caspase 3) revealed that suppression of XBP-1s inhibited PA-induced hepatocyte apoptosis (Fig. 5C). Determination of caspase 3 activity also confirmed that apoptosis was inhibited when XBP-1s expression was downregulated (Fig. 5D). Correspondingly, PA-induced lipotoxicity was weakened in both HN-001-treated and Xbp-1s-knockdown cells (Fig. 5E). Notably, there were no additional ameliorative effects on lipotoxicity after HN-001 treatment in XBP-1s-knockdown hepatocytes (Fig. 5). These data indicate that HN-001 ameliorates lipotoxicity in hepatocytes is associated with the IRE-1α/XBP-1s axis suppression.
Figure 5.
Inhibition or knockdown of XBP-1s protects hepatocytes against lipotoxicity. HepG2 cells were transfected with control or XBP-1s siRNA for 12 h and then exposed to PA in the presence or absence of HN-001 (25 μmol/L) for another 24 h. (A) Schematic diagram of control/XBP-1s siRNA or HN-001 treatment in HepG2 cells. (B) mRNA levels of Xbp-1s, Chop, Cox2, and Il-6. (C) Protein levels of downstream genes of XBP-1s and cleaved caspase 3 and quantification. The average protein levels in the blank group from 5 independent experiments were set as 1, and relative fold changes were calculated. (D) Caspase 3 activity determination. (E) Viable cell counting. n = 5 independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001, compared with PA-treated, siRNA-transfected control cells.
2.6. HN-001 ameliorates ER stress mediated lipotoxicity by inhibiting PLA2
The lysophosphatidylcholine (LPC) class is a class of lipids derived from phosphatidylcholine by partial hydrolysis through phospholipase A2 (PLA2), which appears to act as a key instigator of lipotoxicity by triggering ER stress and activating the proapoptotic JNK pathway27,28 (Fig. 6A). PA treatment markedly increased cellular PLA2 enzyme activity and the PLA2 protein level, leading to the accumulation of LPC (Fig. 6B–D). These increases were correlated with activation of the JNK pathway, as indicated by increased levels of phosphorylated JNK (p-JNKThr183+Tyr185) (Fig. 6D). Immunofluorescence analysis of PLA2 further confirmed the ability of PA to increase PLA2 levels (Fig. 6E). Notably, HN-001 treatment dose-dependently reversed the increases in the level and enzyme activity of PLA2 (Fig. 6B–D). This reversal resulted in LPC level reduction (Fig. 6C). Correspondingly, HN-001 treatment blocked the activation of the JNK pathway induced by PA (Fig. 6D and E). We also determined the time-dependent effects of HN-001 in blocking the PLA2 and JNK pathways. As shown in Fig. 6F and G, PA treatment time-dependently increased PLA2 enzyme activity as early as 1 h, while it enhanced the PLA2 protein level and subsequent JNK pathway activity starting at 4 h. HN-001 addition efficiently inhibited PLA2, including its enzyme activity and protein expression, and the JNK pathway.
Figure 6.
PLA2 is supposed to be an upstream regulator of ER stress and lipotoxicity. (A) Proposed mechanism by which PLA2 regulates ER stress and hepatocyte death. (B‒C) Determination of cellular PLA2 enzyme activity and LPC levels. (D) Protein levels of the PLA2 and JNK pathways. (E) Immunofluorescence analysis of protein PLA2. Scale bar, 50 μm. (F–G) Time-dependent effects of HN-001 in suppressing PLA2 activity (F) and the PLA2/JNK axis (G). (H–M) Hepatocytes were treated with the PLA2 inhibitor MAFP (3 μmol/L) for 24 h in the presence or absence of PA, and then cells were harvested and subjected to the indicated examinations. (H) Cellular PLA2 enzyme activity. (I) Cellular LPC level. (J) mRNA level of Xbp-1s. (K) ROS level. (L) Caspase 3 activity determination. (M) Viable cell counting. n = 5 independent experiments. (N) Molecular docking analysis between HN-001 and the protein PLA2. The crystal structure of human PLA2 (PDB ID: 1BCI) was selected. The hydrogen bond and hydrophobic force are displayed as blue and yellow dashed lines, respectively. The image was generated in PyMOL. (O) Determination of the binding affinity of HN-001 for the protein PLA2. (P) PLA2 enzyme activity determination. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with blank group (PA-untreated) cells; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with PA-treated cells.
To further verify whether HN-001-mediated inhibition of ER stress is correlated with the inhibition of PLA2, we administered the PLA2 inhibitor MAFP to hepatocytes. As expected, MAFP addition reversed PA-induced increases in cellular PLA2 activity and LPC content, leading to reductions in Xbp-1s transcription levels and ROS levels, thus decreasing caspase 3 activity and enhancing hepatocyte survival (Fig. 6H–M). Moreover, we overexpressed PLA2 protein in PA-induced hepatocytes and found that PLA2 overexpression activated ER stress and exacerbated hepatocyte death. Notably, PLA2 overexpression weakened the lipotoxicity-ameliorating effects of HN-001 (Supporting Information Fig. S4), suggesting that PLA2 plays a crucial role in the lipotoxicity-ameliorating effects of HN-001. Furthermore, molecular docking analysis was performed to explore the potential interaction between HN-001 and the human protein PLA2 (PDB ID: 1BCI). As shown in Fig. 6N, the skeleton of HN-001 matched protein PLA2 well, occupying multiple interaction sites due to its unique molecular structure. MeO-10 and phenol hydroxyl groups formed hydrogen bonds with Asn64 and Asp99. The benzene ring attached to the dibenzofuran interacted with residue Phe63 through π‒π stacking. Surface plasmon resonance (SPR) assays revealed that HN-001 interacted with PLA2 protein with a binding affinity of 6.8 μmol/L (Fig. 6O). Furthermore, HN-001 dose-dependently inhibited PLA2 enzymatic activity with a half-maximal inhibitory concentration (IC50 value) of 15.2 μmol/L (Fig. 6P). These data demonstrate that HN-001 is a potential natural PLA2 inhibitor and that HN-001 ameliorates lipotoxicity and protects hepatocyte survival by inhibiting IRE-1α/XBP-1s signaling with PLA2 as an upstream target.
2.7. HN-001 attenuates the manifestations of MAFLD in HFC diet-induced mice
Male C57BL/6J mice were fed with a chow (CH) diet or HFC diet for 19 weeks, and HN-001 (10 or 20 mg/kg) or its vehicle was administrated each other day at last 8 weeks (Fig. 7A). As expected, HFC diet feeding induced significant increases in liver weight and the ratio of liver weight to body weight (Fig. 7B and C). Quantification of liver TG levels showed an eight-fold increase in the liver TG content in MAFLD mice compared with that in CH diet-fed mice (Fig. 7D). Determination of liver enzyme levels in plasma, including AST, ALT and ALP, demonstrated that chronic HFC diet feeding induced liver breakdown (Fig. 7E). Pathological examinations, including H&E and oil red O staining, revealed that the MAFLD mice displayed severe hepatic steatosis and liver injury (Fig. 7F). Notably, HN-001 treatment reversed HFC-diet feeding-induced hepatic steatosis and liver injury in mice. Compared with MAFLD control mice, HN-001-treated mice exhibited dose-dependent attenuation of HFC diet feeding-induced increases in liver weight, liver TG, and plasma AST, ALT, and ALP levels (Fig. 7B–E). Pathological examinations also confirmed the therapeutic effect of HN-001 against MAFLD in vivo (Fig. 7F). Quantification of the activity and protein level of caspase 3 demonstrated that HN-001 treatment efficiently prevented HFC diet feeding-induced hepatocyte damage and death, as indicated by increases in caspase 3 activity and protein expression in the liver (Fig. 7G and H). Inflammation is a typical feature of MAFLD/NASH induced by liver macrophages following liver injury. We therefore next examined the anti-inflammatory effect of HN-001. Immunofluorescence analysis of the macrophage marker CD68 in the liver demonstrated that HN-001 administration suppressed the activation of CD68 in the livers of MAFLD mice (Fig. 7H).
Figure 7.
HN-001 ameliorates MAFLD in HFC diet-fed mice. Eight-week-old male C57BL/6J mice were fed a HFC diet for 19 weeks, and HN-001 (10, 20 mg/kg) was administered orally for the last 8 weeks every other day. After treatment, plasma and livers were collected for the indicated examinations. (A) Schematic diagram of HN-001 treatment in mice. (B–C) Mouse liver weight and ratio of liver to body weight. (D) Determination of liver TG levels. (E) Plasma AST, ALT, and ALP levels. (F) Pathological examination of H&E (for liver injury) and oil red O (for steatosis) and NAFLD Activity Score (NAS) evaluation. Scale bar, 200 μm. (G) Caspase 3 activity determination. (H) Immunofluorescence analysis of cleaved caspase 3 (C-caspase 3) and CD68 in the liver. Scale bar, 200 μm. (I–K) mRNA levels of genes related to lipogenesis, ER stress, apoptosis, and inflammation. (L) Quantification of the protein levels of C-caspase 3, IL-6, and TNF-α. The average levels of genes or proteins in the chow diet control group were set as 1, and the relative fold changes were calculated. (M) Sirius red and Masson staining for liver fibrosis evaluation. Scale bar, 200 μm. (N) mRNA level of Cola1a. n = 9–10 mice per group. The data shown are individual values with means ± SEMs. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with vehicle-treated CH diet-fed mice; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with vehicle-treated MAFLD mice.
Consistent with the reductions in liver TGs, liver injury and inflammation, HN-001 treatment suppressed HFC diet feeding-induced activation of genes involved in lipogenesis (including Srebp-1c, Acc1, Fasn, Fatp1, and Fatp4), ER stress and apoptosis (including Chop, Trail, Dr5, and Bcl-2), and inflammation (including lipopolysaccharide receptor [Lspr], interleukin-6 [Il-6], and tumor necrosis factor-alpha [Tnf-α]) (Fig. 7I–K). Immunoblot analysis also confirmed the suppressive effects of HN-001 on the expression levels of cleaved caspase 3, IL-6, and TNF-α (Fig. 7L). Notably, HN-001 administration decreased fiber accumulation in the liver, as indicated by Sirius red and Masson staining (Fig. 7M). Consistent with these results, gene expression analysis demonstrated that HN-001 treatment in mice downregulated the transcript level of the fibrosis marker Col1a1 (Fig. 7N). Notably, treating CH diet-fed mice for 8 weeks with HN-001 marginally affected all examined parameters of the liver (Fig. 7), suggesting that HN-001 is well tolerated in mice at pharmacological doses and that HN-001 is a potential therapeutic candidate for MAFLD treatment.
2.8. Suppression of the PLA2/IRE-1α/XBP-1s axis in the livers of HN-001-treated MAFLD mice
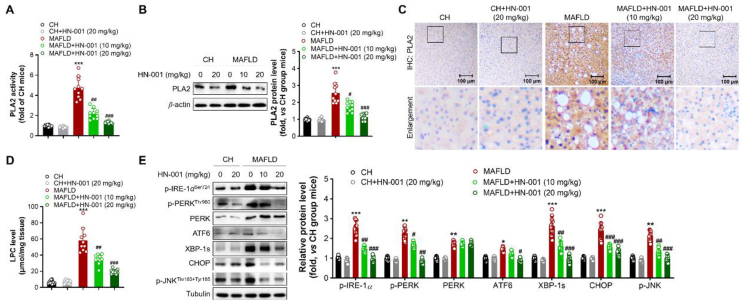
We then determined the enzyme activity and expression level of PLA2 in the livers of mice. Compared with CH diet feeding, HFC diet feeding induced great increases in the enzyme activity and protein level of PLA2 (Fig. 8A–C), leading to a significant increase in the level of LPC and thus activating the IRE-1α/XBP-1s axis and JNK pathway in the livers of MAFLD mice (Fig. 8D and E). HN-001 treatment significantly reversed the increases in PLA2 activity and protein expression and decreased LPC levels in the liver, resulting in suppression of the IRE-1α/XBP-1s axis and JNK pathway, as indicated by decreases in the levels of phosphorylated IRE-1α, PERK, and JNK and the total protein levels of ATF6, XBP-1s and CHOP (Fig. 8). These data coincided with our cellular findings, suggesting that HN-001 ameliorates MAFLD in mice by suppressing the PLA2/IRE-1α/XBP-1s axis and JNK pathway.
Figure 8.
HN-001 inhibits the PLA2/IRE-1α/XBP-1s axis and JNK signaling in the liver. (A) Determination of PLA2 enzyme activity. (B) Protein level of PLA2 and quantification. (C) Immunohistochemistry (IHC) analysis of PLA2. Scale bar, 100 μm. (D) Quantification of LPC levels. (E) Expression of ER stress signaling- and JNK pathway-related proteins and quantification. The average levels of proteins in CH diet-fed mice were set as 1, and the relative fold changes were calculated. n = 10 mice per group. The data shown are individual values with means ± SEMs. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared with vehicle-treated CH diet-fed mice; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with vehicle-treated MAFLD mice.
3. Discussion
Lipotoxicity is a key instigator of hepatocyte damage and plays an essential role in the development of MAFLD and NASH. In the present study, we identified a natural product (HN-001) isolated from the marine fungus Aspergillus sp. C1 as a novel antilipotoxicity candidate. Its hepatoprotective and MAFLD-ameliorating effects as well as its molecular action were studied. Our results demonstrated that HN-001 protected hepatocytes against PA-induced lipotoxicity by inhibiting the IRE-1α/XBP-1s axis and its transcriptional regulation involved in apoptosis and inflammation. These were correlated with PLA2 inhibition. The observed therapeutic effects of HN-001 in ameliorating MAFLD, including suppression of lipotoxicity and improvement of liver pathologies, largely fulfilled the recommended criteria for the treatment of NASH. Notably, our evaluation of plasma parameters related to liver function indicated that HN-001 is safe and does not exhibit any detectable toxicity or adverse effects. These findings suggest that HN-001 holds promise as a potential novel structure for the development of anti-MAFLD/NASH agents.
Multiple theories have emerged to explain the underlying mechanisms of the pathologies of MAFLD. Recently, a large body of evidence has supported the idea that perturbation of ER homeostasis contributes to dysfunction of hepatic lipid metabolism and chronic liver diseases, such as MAFLD10. ER stress engages an evolutionarily conserved pathway termed the unfolded protein response (UPR). IRE-1α, the most conserved ER stress transducer, participates in the UPR through autophosphorylation and then splices the mRNA of XBP-1 (the only characterized target of IRE-1α) through its RNase activity29, which further activates ER stress and enhances lipid synthesis and apoptosis through transcriptional regulation. Although ER stress serves at first to re-establish ER homeostasis and promotes cell survival by directing unfolded or misfolded proteins toward degradation30, prolonged activation of ER stress triggers apoptotic pathways causing cell death23. Previous studies have demonstrated that IRE-1α RNase activity increases under sustained conditions of ER stress and enhances proapoptotic signaling31. Presently, we found that incubation of hepatocytes with PA (a saturated fatty acid) time-dependently increased the total and phosphorylated levels of IRE-1α and spliced XBP-1, leading to augmentation of ER stress and hepatocyte death. Notably, activation of the IRE-1α/XBP-1s axis was observed as early as 1 h after PA treatment. Meanwhile, the ER stress inducer DTT induced hepatocyte death after a short period of 4 h of incubation. These findings indicate that activation of ER stress is an essential factor in hepatocyte death induced by PA. The observed ER stress-associated lipotoxicity was reversed by adding HN-001. Treating hepatocytes with HN-001 dose- and time-dependently blocked ER stress activation in as little as 1–4 h, while hepatocyte apoptosis occurred at approximately 8 h after PA treatment. Additionally, knockdown of XBP-1s efficiently attenuated lipotoxicity, while no additional antilipotoxic effects were observed when HN-001 was added to XBP-1s-knockdown hepatocytes. Furthermore, treatment with either the IRE-1α/XBP-1s axis inhibitor TUDCA or HN-001 efficiently inhibited ER stress and hepatocyte death in PA- or DTT-treated hepatocytes. These data suggest that HN-001 ameliorates lipotoxicity and MAFLD by suppressing the IRE-1α/XBP-1s axis, at least in part.
In hepatocytes, apoptosis can occur via intrinsic pathways activated by multiple intracellular metabolic or organelle stresses. PA induces apoptosis by metabolizing PA into LPC, which is catalyzed by PLA28. LPC has been implicated in lipotoxicity and hepatocyte death through caspase 3, and its levels are increased in the livers or plasma of both human NASH patients32 and animal models of MAFLD/NASH33. Studies have also revealed that LPC triggers apoptosis through activation of downstream JNK or induction of CHOP34. Consistent with this finding, we found that either PA induction or chronic HFC diet feeding significantly increased LPC levels and activated the JNK axis. These effects were in parallel with substantial hepatocyte death and enhancements in the enzyme activity and expression level of PLA2. Treatment with HN-001 inhibited PLA2 enzyme activity and decreased its protein level in a dose- and time-dependent manner, leading to a reduction in LPC level, thus inhibiting ER stress and the JNK pathway and promoting hepatocyte survival. HN-001 inhibited PLA2 enzyme activity in PA-treated hepatocytes as early as 1 h prior to hepatocyte apoptosis. Furthermore, overexpression of PLA2 overwhelmed IRE-1α/XBP-1s axis activation, exacerbating hepatocyte death. PLA2 overexpression weakened the antilipotoxic effect of HN-001. These data imply that PLA2 is an essential regulator of IRE-1α/XBP-1s axis activation and that PLA2 is an upstream therapeutic target for HN-001 to counter lipotoxicity and MAFLD. By performing binding kinetics, enzyme inhibition, and molecular docking analysis, we identified PLA2 as a potential target protein of HN-001.
Over the past 30 years, 50% of the 1881 approved drugs have directly or indirectly originated from natural products35. Marine-derived fungi are the main sources of marine microbial natural products due to their complex genetic background, chemodiversity and high yield of natural products. According to a survey of marine microbial natural products from 2015 to 2021, marine fungi of the genus Aspergillus have received the most attention from chemists and pharmacologists for their structurally diverse, abundant biologically active secondary metabolites and broad bioactivities36. Presently, we report for the first time the anti-MAFLD action of a natural product isolated from the fungal genus Aspergillus. Our study sheds new light on metabolites from marine fungi with activity against chronic metabolic diseases. This study may provide a novel structural basis for the development of new anti-MAFLD candidates.
4. Conclusions
Overall, our data collectively demonstrate that HN-001 attenuates MAFLD by reducing hepatic steatosis, lipotoxicity, liver injury, inflammation, and fibrogenesis in the liver. These therapeutic effects are in accordance with the recommended criteria for potential new MAFLD/NASH drugs37. The molecular mode of action involves inhibition of lipotoxicity, consistent with the proposed mechanism of the pathogenesis of NASH38. These beneficial effects are driven by inhibition of the IRE-1α/XBP-1s axis and JNK pathway, with PLA2 as an upstream target (Fig. 9).
Figure 9.
Proposed mechanism by which HN-001 protects against MAFLD in mice. As exemplified by compound HN-001, inhibition of PLA2 in hepatocytes or in mice decreases LPC content, thus blocking the JNK pathway and ameliorating hepatocyte apoptosis. On the other hand, a reduction in LPC inhibits IRE-1α activation, leading to XBP-1 splicing inhibition and transcriptional regulation blockade and ameliorating ER stress, hepatocyte apoptosis, and inflammation. As a result, MAFLD is alleviated.
5. Experimental
5.1. Fungal cultivation and compound isolation
The fungus Aspergillus sp. c1 was isolated by one of the authors (Zhi-kai Guo) from coral reef in the South China Sea in 2018. The isolate was identified as Aspergillus sp. by its morphological characteristics and the voucher specimen was deposited in Hainan Key Laboratory of Tropical Microbe Resources, Institute of Tropical Bioscience and Biotechnology, Haikou, China. The strain was cultivated on PDA agar plate at 28 °C for 5 days. Then small agar plugs with mycelia were inoculated into 1 L erlenmeyer flasks, each containing 200 mL MEA liquid medium, which was cultivated at 28 °C with 160 rpm/min. After 4 days of fermentation, 15 mL of the seed cultures were inoculated into 180 L erlenmeyer flasks, each containing 30 g rice, and fermented at 28 °C for 45 days under static conditions.
The fermentation material was extracted with EtOAc at room temperature for four times to yield an extract (120.0 g). Then the extract was fractionated by silica gel CC eluting with a gradient of petroleum ether-EtOAc mixtures (v/v, 100:0, 25:1, 10:1, 5:1, 2:1, 1:1, 0:1) to give 8 fractions (Fr.A–Fr.H). The Fr.F (petroleum ether/EtOAc, v/v, 2:1) was separated by a C18 reversed-phase column, eluted with aqueous methanol (40%–100%), to yield the Fr.F-6 (70% aqueous methanol), which was further purified by Sephadex LH-20 CC with 100% MeOH to give compound HN-001. HN-001 was identified as 4,5-dimethoxycandidusin A by comparing its 1H NMR and HR-ESIMS spectroscopic data (Supporting Information Figs. S5–S6) with those described in the literature39.
HN-001: greyish white powder; 1H NMR (500 MHz, DMSO-d6) 7.46 (1H, s, H-3), 3.87 (3H, s, 4-OMe), 3.86 (3H, s, 5-OMe), 7.46 (1H, s, H-6), 3.78 (3H, s, 3′-OMe), 6.76 (1H, s, H-5″), 4.00 (3H, s, 6′-OMe), 7.43 (1H, d, J = 8.5 Hz, H-2″), 6.85 (1H, d, J = 8.5 Hz, H-3″), 9.54 (1H, s, 4″-OH), 6.85 (1H, d, J = 8.5 Hz, H-5″), 7.43 (1H, d, J = 8.5 Hz, H-6″); HR-ESIMS m/z 381.1333 [M + H]+ (Calcd. for C22H21O6, 381.1333). Purity: 96.47% (Supporting Information Fig. S7).
5.2. Cell culture and PA induction
Human hepatoma cell line HepG2 (ATCC, Cat#HB-8065, RRID: CVCL_0027) was cultured within DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin (100 U/mL) and streptomycin (100 mg/mL). For induction, HepG2 cells were exposed to 0.5 mmol/L PA (Sigma, Cat#P0500, China) induction for 24 h with or without HN-001 treatment at indicated concentrations.
5.3. TG quantification and imaging
Cellular TG was extracted as described previously18. Briefly, the liver tissue or cells were homogenized and extracted with equal volumes of chloroform/methanol. The chloroform phase was removed to a new tube and dried and was then resuspended in isopropyl alcohol as a total lipid extract sample. The quantities of total TG (Jiancheng Bio Cat# A110-2, Nanjing, China) in the livers were then assayed according to the manufacturer's protocols. 100 μL of TG working reagents were mixed with 10 μL of ddH2O, TG, or DAG standards and the samples respectively. The reaction was incubated at 37 °C for 15 min. Absorbance (A) of blank, standards, and samples were recorded at 510 nm. TG concentrations were calculated as (Asample−Ablank)/(Astandard−Ablank) × concentration of standards and normalized to tissue weight or protein content.
For cellular TG imaging, cells were stained with 5 μmol/L Nile red dye at 37 °C for 30 min in dark. Then washed with culture medium and representative images were captured using confocal microscopy (Zeiss, Germany) under an excitation wavelength of 555 nm.
5.4. Viable and apoptotic cell counting
After treatment, cells were collected and re-suspended in 1 mL culture medium. The suspension was mixed with 0.4% trypan blue at 1:1 (v/v) and incubated at room temperature for 5 min. The hemocytometer was filled with 10 μL cell mixture for cell counting under a light microscope (Olympus, Germany). The stained (death hepatocyte) and non-stained cells (viable hepatocyte) were counted, respectively. For viable and apoptotic counting, after treatment, cells were collected and washed with PBS (0.1 mol/L) for twice, then stained with Annexin V/propidium iodide (PI) (Multi Science, #AT107, China) in 1 × binding buffer at dark for 15 min. Cells were washed with PBS for twice and then subjected to flow-cytometry analysis (Beckman, Germany). Each sample was loaded with 15,000 cells for the analysis in a flow cytometer at the auto-setting. Data were analyzed by Expo 32 Software (Beckman, Germany). According to the instructions of the Annexin V/PI, either Annexin V or PI/Annexin V labelled cells were defined as apoptotic cells. Viable cells were not labelled by either PI or Annexin V.
5.5. Caspase 3 activity determination
The activity of caspase 3 (Biovision Cat# K105-100, Wuhan, China) in cultured cells was measured by a fluorescence-based assay. Approximately 10 μg cell lysate was added to the wells and incubated with the fluorogenic substrate rhodamine 110 bis-(N-CBZ-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide; Z-DEVD-R110; 25 μmol/L) at room temperature. Substrate cleavage and rhodamine 110 accumulation was measured fluorometrically at 499 nm (excitation) and at 521 nm (emission) in a multiplate reader (Spectromax Gemini, Molecular Devices). The protein content was determined by BCA Assay Kit (Thermo Cat# 23227, Guangzhou, China) with human serum albumin as a standard.
5.6. ROS detection and imaging
Intracellular ROS was measured using a DCFH-DA probe (Beyotine, Cat# S0033, Chengdu, China). Briefly, after treatment, the treated cells were collected and re-suspended in culture medium containing 5 μmol/L DCFH-DA, and incubated at 37 °C for 30 min and then immediately analyzed by flow cytometry (Beckman, Germany). Data were collected from at least 10,000 cells at a flow rate of 250–300 cells per second. For cellular ROS imaging, treated cells were stained with 10 μmol/L DCFH-DA at 37 °C for 15 min in dark. Then washed with culture medium and representative images were captured using confocal microscopy (Zeiss, Germany) under an excitation wavelength of 488 nm.
5.7. Immunoblotting, immunofluorescence and immunohistochemistry analysis
Cell or livers were lysed with RIPA lysis buffer containing protease and phosphatase inhibitor cocktail and total protein was extracted and quantified by using a protein determination kit (Thermo Fisher Scientific, Cat#23227, China). Protein samples were prepared by centrifuging at the speed of 12,000 × g for 30 min at 4 °C, the supernatant was boiled at 95 °C for 10 min and then subjected to SDS-PAGE. 15 μg of total protein was separated by electrophoresis in a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane (Thermo Fisher Scientific, Cat#88520, China). After blocking, the full membranes were cropped according to the molecular weight of examined proteins and incubated with antibodies at 4 °C overnight. The used antibodies were listed in Supporting Information Table S1. The membranes were washed to remove unbound antibodies and then incubated with HRP-conjugated secondary antibodies at room temperature for 45 min. Protein bands were visualized one by one with an ECL kit (Millipore, Cat#64-201BP, China). The uncropped data of immunoblot were uploaded as a supplemented file. Densitometry analysis was performed using Image J Software (Rawak Software Inc., Stuttgart, Germany) relative to the loading control β-actin or GAPDH or tubulin. The average protein levels of control group cells or control mice from each independent experiment or mice were set as 1, relative changes were calculated. For immunofluorescence assay, cells were fixed with pre-cold ethanol at −20 °C, then blocked and incubated with cleaved caspase 3 or p-IRE-1αSer724 or XBP-1s at 4 °C overnight. After washing, cells were co-stained with fluorescent labelled secondary antibody (Cell Signaling Technology, Cat#7076, RRID:AB_330924, China) and 2 μg/mL DAPI (for nucleus, Thermo Fisher Scientific, Cat#R37606, China) at 37 °C for 1 h. Fluorescence images were acquired using confocal microscope (Olympus Confocal Microscope).
5.8. Total RNA extraction, PCR and qRT-PCR
Total RNA from cells or mouse livers was isolated using the TRIzol method (Invitrogen, Cat# 15596018, Guangzhou, China) and the results were analyzed on an ABI StepOne Plus real time PCR system (Applied Biosystems, USA, RRID:SCR_015805) using the 2−ΔΔCt method as our previous report40. β-Actin was used as a loading control, and relative mRNA levels were normalized to loading control β-actin. Primer sequence was listed in Supporting Information Table S2. The average gene levels in control cells or chow diet (CH) fed mice set as 1, relative folds were calculated by comparing with control group.
5.9. Chromatin immunoprecipitation
ChIP assay was performed using a ChIP assay kit (Thermo Fisher Scientific, Cat#26157, Guangzhou, China) according to manufacturer's instructions as our previous reported18. HepG2 cells were exposed to PA induction in the presence or absence of HN-001 (25 μmol/L) treatment for 24 h. Proteins bound to DNA were cross-linked by adding formaldehyde to a final concentration of 1% for 15 min at 37 °C and the cross-linking was stopped addition of glycine to a final concentration of 0.125 mol/L. Cells were then washed twice with ice-cold PBS containing protease inhibitors, pelleted, resuspended in 100 μL Lysis buffer I and typsined by MNase, the isolated nucleus then lysis with 100 μL Lysis buffer II and obtained approximate DNA size of 150–400 bp. Samples were pre-cleared with protein G/A agarose beads and then immunoprecipitated with anti-XBP-1s (Cell signaling Technology, Cat#27901, China) or IgG overnight at 4 °C. Chromatin protein/DNA complexes were eluted from the agarose beads twice by adding 100 μL of elution buffer at room temperature for 15 min. Cross-linking was reversed by heating to 65 °C for 2 h. DNA fragments were purified and the targeted DNA was amplified by real-time PCR with the ABI PRISM 7900HT sequence detection system using SYBR green (Roche, Cat#04913914001, Guangzhou, China). The sheared chromatin from each tube was diluted 10×, with 10% saved as input DNA and the rested were prepared for targeted genes.
5.10. siRNA or PLA2 plasmid transfection
HepG2 cells were transfected with 75 nmol/L control siRNA or XBP-1s siRNA (Ribobio Co., Ltd., Guangzhou, China) for 12 h using lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, Cat#L3000015, Guangzhou, China). For PLA2 overexpression, HepG2 cells were transfected with 5 μg PLA2 plasmid (MG51562-UT, Sino biologic, China) for 12 h using lipofectamine 3000 transfection reagent. Then cells were exposed to PA induction with or without compound treatment for another 24 h, and cells were harvested and subjected to indicated analysis.
5.11. PLA2 activity determination
Cellular and liver phospholipase A2 (PLA2) activity was measured using a colorimetric cytosolic PLA2 (cPLA2) assay kit (Cat #765021, Cayman Chemical, USA) following the manufacturer's instructions. For PLA2 enzyme activity determination in vitro, the full length recombinant PLA2 (51562-M08H, Sino biological, China) were incubated with HN-001 at 37 °C for 15 min, then the PLA2 enzyme activity was measured using PLA2 ELISA kit.
5.12. LPC level detection
The levels of LPC (mmBio Cat#MM-50888H1, China) in cells or livers (prepared as described before) were measured by ELISA assays according to the manufacturer's instructions. The cell lysate or liver homogenate was collected after a designated treatment and centrifuged at 4000 rpm for 10 min at 4 °C to remove cell debris. Briefly, 10 μg cell or liver lysate, saline, and standard were added to the ELISA plate and incubated at 37 °C for 90 min. Wells were washed four times with elution buffer and then incubated with biotin-labelled antibodies at 37 °C in the dark for another 30 min followed by reaction with the corresponding substrate. The color produced was proportional to the concentration of LPC and was measured at 450 nm and quantified against the standard curve from the known amount of LPC.
5.13. Surface plasmon resonance (SPR) assay
All experiments were carried out on a Biacore 8K instrument equilibrated at 25 °C in PBS-T buffer (PBS 0.05% Tween 20 and 0.1% DMSO). CM5 sensor chip was activated using NHS/EDC amine coupling reaction. Purified protein PLA2 was immobilized on CM5 sensor chip in 10 mmol/L sodium acetate buffer. To determine the interaction, HN-001 was diluted with running buffer (12 mmol/L HEPES, 4 mmol/L Tris, 1 mmol/L EDTA, 1.5 mmol/L MgCl2 and 0.005% Tween-20) ranging from 0.3125 to 20 μmol/L and injected sequentially onto the PLA2 immobilized surface. The ligand was injected at a flow rate of 25 μL/min for 150 s during the association phase, which was followed by a 300 s disassociation phase at 25 °C. The equilibrium dissociation constant (KD) was analyzed by fitting the dose–response curve using BIAcore evaluation software. KD was calculated from ka and kd, KD = kd/ka.
5.14. Animal study
All animal care and experimental procedures were approved by the GuangDong Medical College Committee on Animal Ethics for the Use of Laboratory Animals in accordance with the Animal Welfare Legislation of China. Every effort was made to minimize the use of the animals and their discomfort. Animal studies are reported in compliance with the ARRIVE guidelines. Male C57BL/6J mice aged 8–10 weeks (18–20 g) bred at the Laboratory Animal Centre of GuangDong Medical College (Dongguan, China) were used for the study. The mice were housed under specific pathogen free and reared in line with standardized methods at 22 ± 1 °C on a 12/12 h light/dark cycle with free access to food and water. After 7 days acclimatization, mice were fed with either a chow diet (CH, with 70% calories from starch and provided by the Animal Center) or the high-fat and high-cholesterol (HFC, 60% fat and 1.2% cholesterol) diet (Research Diets, Cat#D12492, USA) ad libitum for up to 18 weeks. The CH or HFC mice were randomly divided into two subgroups at the beginning of week 11 to receive HN-001 (20 mg/kg) or its vehicle (5% castor oil + 0.5% PEG400; control group) intraperitoneal injection (i.p.) every other day. The used vehicle is safe and selected according to our previous and other reports41. HN-001 was administrated in the last 8 weeks.
5.15. Plasma chemicals and liver TGs determination
At the end of the study, mice were fasted for 6 h (08:00–14:00) and anaesthetized by an i.p. injection of ketamine/xylazine. After the mice were fully anaesthetized, the eyeball was removed to collect blood samples into a tube containing 1 mmol/L EDTA. After the blood samples were collected, the anaesthetized mice were killed by cervical decapitation. Livers were weighed, freeze-clamped or fixed in 4% formaldehyde solution. Plasma levels of alkaline phosphatase (ALP), glutamic-pyruvic transaminase (ALT) and aspartate aminotransferase (AST) were determined by an Olympus AU 600 auto-analyser (Olympus, Japan). Liver TGs were extracted by the method of Folchand and determined by a Peridochrom triglyceride GPO-PAP kit as previously described18.
5.16. Histological analysis and definition of the scoring system
Dissected tissues were fixed in 4% formaldehyde and embedded in paraffin according to standard procedures. Tissue sections of 4 μm thickness were stained with hematoxylin and eosin (H&E, for liver injury determination), oil red O staining (for steatosis determination), sirius red and masson staining (for fibrosis determination). The sections for histological examination were coded before assessment. The scores of steatosis, inflammation, ballooning, and fibrosis in the liver were evaluated by a pathologist, without knowledge of the treatments, using the non-alcoholic fatty liver disease (NAFLD) activity scoring system described by Kleiner et al.42.
5.17. Immunohistochemistry (IHC) analysis
The paraformaldehyde embedded livers were sectioned (4 μm thick) with a rotary microtome (Leica, Germany). Sections were deparaffinized and hydrated. Heat-mediated antigen was retrieved with 10 mmol/L citrate buffer pH 6.0 (Thermo Scientific, Cat#005000, Guangzhou, China). Endogenous peroxide was inhibited by incubating with a freshly prepared 3% H2O2 solution in MeOH. Non-specific antigens were blocked by incubating sections for 1 h with IHC blocking buffer (Thermo Scientific, Cat#00-4953-54, Guangzhou, China). Then liver sections were stained with PLA2 (Affinity Biosciences Cat# AF6329, Guangzhou, China) followed by a goat anti-rabbit IgG HRP conjugate (Thermo Scientific, Cat#65-6120, Guangzhou, China). Color was developed after incubation with 3,3′-diaminobenzidine (DAB) substrate (Thermo Scientific, Cat#SK34065, Guangzhou, China), followed by haematoxylin counterstaining and mounting (Vector laboratories, Cat#H-5000, China). All sections were viewed with a light microscope (Olympus, Germany) and photographed at × 200 magnification. The positive (stained) area for each marker was imaged with a minimum of 10 random liver sections per sample. Images are presented in the Figures, showing the animal with the median value for each group. The immunoblotting or immunohistochemistry has been conducted the experimental detail provided conforms with Pharmacological Research Guidelines.
5.18. Molecular docking
The crystal structure of human PLA2 protein (PDB code: 1BCI) was selected for molecular docking utilized by a Surflex-dock method embedded in the software Tripos Sybyl X2.0. The CHARMM force field method and the Momany−Rone partial charge method were used to add hydrogen atom and charge to the system, respectively. All ionizable residues in the system were set to protonation states at a neutral pH, crystal water molecules were removed, and other parameters were set to default values.
5.19. Data and statistical analysis
For cellular experiments, data were obtained from 5 biologically independent experiments. For animal study, data were obtained from 10 independent mice in each group as presented in Figure legends. The statistical analysis with parametric variables was performed using the original experiment data (non-normalized data) unless otherwise specific stated in the Fig. legends. For the optical density analysis of immunoblotting assay, the average level of control group from 5 independent experiments or mice of control group were set as 1, relative folds were calculated by comparing with the control group. Data are included in data analysis and presentation and expressed as the mean ± SEM. Differences between two groups were analysed by Student's t-test using Graphpad Prism (Graphpad Software Inc., CA, USA, RRID:SCR_002798). Statistical analysis for multiple groups was performed by one-way ANOVA followed by Tukey's HSD post hoc-tests. The statistical analysis of NAS score was performed by using non-parametric analysis. A P value of ≤0.05 was considered statistically significant.
5.20. Materials
Aspergillus sp. c1 is a symbiotic fungus isolated from hard coral collected in the South China Sea in 2018. Compound HN-001 were isolated with purity over >95%. It was dissolved in DMSO solution to form a storage solution of 10 mmol/L and diluted with culture medium to a final concentration for cell experiments. PLA2 inhibitor MAFP was purchased from Selleck (MedChemExpress, Cat#HY-103334, China).
Acknowledgments
This study was supported by National Natural Science Foundation of China (82260674 to Yong Rao, 82160653 to Ling Huang); Fundamental Research Funds for Hainan University (KYQD(ZR)-21114 to Yong Rao; KYQD(ZR)-21089 to Ling Huang, China), Hainan Provincial Natural Science Foundation of China (822MS054 to Yong Rao), Natural Science Foundation of Guangdong Province (2021A1515010488 to Yong Rao, China), Central Public-interest Scientific Institution Basal Research Fund for CATAS-ITBB (1630052022016, 1630052019011, China).
Author contribution
This study was initiated and designed by Ling Huang and Yong Rao. Yong Rao, Rui Su, Xingxing Chai, Chenyan Wu, Guanyu Yang, and Junjie Wu performed biological evaluation and mechanism study of HN-001. Jinjian Li, Congjun Xu, Zhongping Jiang and Zhikai Guo performed compound isolation and characterization. Tingting Fu performed molecular docking analysis. Yong Rao and Ling Huang provided reagents, materials and analysis tools. Data were analyzed and interpreted by Yong Rao and Rui Su. The manuscript was written by Yong Rao and Ling. Huang with input from all authors.
Conflicts of interest
The authors declare no competing interests.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2023.08.032.
Contributor Information
Yong Rao, Email: raoyong@hainanu.edu.cn.
Congjun Xu, Email: congjunxu@hainanu.edu.cn.
Ling Huang, Email: Linghuang@hainanu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell E.E., Wong V.W., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 3.Rinella M.E., Sanyal A.J. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- 4.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Svegliati-Baroni G., Pierantonelli I., Torquato P., Marinelli R., Ferreri C., Chatgilialoglu C., et al. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2019;144:293–309. doi: 10.1016/j.freeradbiomed.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut‒liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Dong J., Viswanathan S., Adami E., Singh B.K., Chothani S.P., Ng B., et al. Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat Commun. 2021;12:66–80. doi: 10.1038/s41467-020-20303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mota M., Banini B.A., Cazanave S.C., Sanyal A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.Y., Garcia-Carbonell R., Yamachika S., Zhao P., Dhar D., Loomba R., et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell. 2018;175:133–145. doi: 10.1016/j.cell.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebeaupin C., Vallée D., Hazari Y., Hetz C., Chevet E., Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69:927–947. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Sasako T., Ohsugi M., Kubota N., Itoh S., Okazaki Y., Terai A., et al. Hepatic Sdf2l1 controls feeding-induced ER stress and regulates metabolism. Nat Commun. 2019;10:947–962. doi: 10.1038/s41467-019-08591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan H., Gao Y., Zhang Y. Inhibition of JNK suppresses autophagy and attenuates insulin resistance in a rat model of nonalcoholic fatty liver disease. Mol Med Rep. 2017;15:180–186. doi: 10.3892/mmr.2016.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan L.T., Van Rooyen D.M., Koina M.E., Mccuskey R.S., Teoh N.C., Farrell G.C. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol. 2014;61:1376–1384. doi: 10.1016/j.jhep.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vellingiri M.M., Ashwin J., Soundari A., Sathiskumar S., Priyadharshini U., Paramasivam D., et al. Mycofabrication of AgONPs derived from Aspergillus terreus FC36AY1 and its potent antimicrobial, antioxidant, and anti-angiogenesis activities. Mol Biol Rep. 2021;48:7933–7946. doi: 10.1007/s11033-021-06824-w. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X., Peng X., Lin J., Zhang Y., He H., Zhao G. Lipoxin A4 activates ALX/FPR2 to attenuate inflammation in Aspergillus fumigatus keratitis. Int Immunopharm. 2021;96:107785–107794. doi: 10.1016/j.intimp.2021.107785. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Naime W.A., Kimishima A., Setiawan A., Fahim J.R., Fouad M.A., Kamel M.S., et al. Mitochondrial targeting in an anti-austerity approach involving bioactive metabolites isolated from the marine-derived fungus Aspergillus sp. Mar Drugs. 2020;18:555–567. doi: 10.3390/md18110555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao Y., Li C., Hu Y.T., Xu Y.H., Song B.B., Guo S.Y., et al. A novel HSF1 activator ameliorates non-alcoholic steatohepatitis by stimulating mitochondrial adaptive oxidation. Br J Pharmacol. 2022;179:1411–1432. doi: 10.1111/bph.15727. [DOI] [PubMed] [Google Scholar]
- 19.Sun S., Shi G., Sha H., Ji Y., Han X., Shu X., et al. IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nat Cell Biol. 2015;17:1546–1555. doi: 10.1038/ncb3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong L., Tan C.W., Feng P.J., Liu F.B., Liu D.X., Zhou J.J., et al. Activation of TREM-1 induces endoplasmic reticulum stress through IRE-1α/XBP-1s pathway in murine macrophages. Mol Immunol. 2021;135:294. doi: 10.1016/j.molimm.2021.04.023. 03. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y.C., Zeng X.Y., He Y., Liu H., Wang B., Zhou H., et al. Rutaecarpine analogues reduce lipid accumulation in adipocytes via inhibiting adipogenesis/lipogenesis with AMPK activation and UPR suppression. ACS Chem Biol. 2015;10:1570–1571. doi: 10.1021/acschembio.5b00242. [DOI] [PubMed] [Google Scholar]
- 22.Lake A.D., Novak P., Hardwick R.N., Flores-Keown B., Zhao F., Klimecki W.T., et al. The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicol Sci. 2014;137:26–35. doi: 10.1093/toxsci/kft230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao Y., Lu Y.T., Li C., Song Q.Q., Xu Y.H., Xu Z., et al. Bouchardatine analogue alleviates non-alcoholic hepatic fatty liver disease/non-alcoholic steatohepatitis in high-fat fed mice by inhibiting ATP synthase activity. Br J Pharmacol. 2019;176:2877–2893. doi: 10.1111/bph.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latif M.U., Schmidt G.E., Mercan S., Rahman R., Gibhardt C.S., Stejerean-Todoran I., et al. NFATc1 signaling drives chronic ER stress responses to promote NAFLD progression. Gut. 2022;71:2561–2573. doi: 10.1136/gutjnl-2021-325013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew Q.J., Chu K.L., Lee J., Koh P.L., Rajasegaran V., Teo J.Y., et al. PCAF interacts with XBP-1S and mediates XBP-1S-dependent transcription. Nucleic Acids Res. 2011;39:429–439. doi: 10.1093/nar/gkq785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N.H., Arias C., et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Kakisaka K., Cazanave S.C., Fingas C.D., Guicciardi M.E., Bronk S.F., Werneburg N.W., et al. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:77–84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauber K., Bohn E., Kröber S.M., Xiao Y.J., Blumenthal S.G., Lindemann R.K., et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 29.Sidrauski C., Cox J.S., Walter P. TRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87:405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 30.Loi M., Molinari M. Mechanistic insights in recov-ER-phagy: micro-ER-phagy to recover from stress. Autophagy. 2020;16:385–386. doi: 10.1080/15548627.2019.1709767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhi H., Kaufman R.J. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han M.S., Park S.Y., Shinzawa K., Kim S., Chung K.W., Lee J.H., et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Pathil A., Mueller J., Warth A., Chamulitrat W., Stremmel W. Ursodeoxycholyl lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology. 2012;55:1369–1378. doi: 10.1002/hep.25531. [DOI] [PubMed] [Google Scholar]
- 34.Lee D.H., Jung Y.S., Yun J., Han S.B., Roh Y.S., Song M.J., et al. Peroxiredoxin 6 mediates acetaminophen-induced hepatocyte death through JNK activation. Redox Biol. 2020;32:101496. doi: 10.1016/j.redox.2020.101496. 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 36.Zhao C., Liu H., Zhu W. New natural products from the marine-derived Aspergillus fungi―a review. Weishengwu Xuebao. 2016;56:331–362. [PubMed] [Google Scholar]
- 37.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 38.Ashraf N.U., Sheikh T.A. Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Free Radic Res. 2015;49:1405–1418. doi: 10.3109/10715762.2015.1078461. [DOI] [PubMed] [Google Scholar]
- 39.Guo Z.K., Yan T., Guo Y., Song Y.C., Jiao R.H., Tan R.X., et al. P-Terphenyl and diterpenoid metabolites from endophytic Aspergillus sp. YXf3. J Nat Prod. 2012;75:15–21. doi: 10.1021/np200321s. [DOI] [PubMed] [Google Scholar]
- 40.Li C., Xu Y.H., Hu Y.T., Zhou X., Huang Z.S., Ye J.M., et al. Matrine counteracts obesity in mice via inducing adipose thermogenesis by activating HSF1/PGC-1α axis. Pharmacol Res. 2022;177:106136–106148. doi: 10.1016/j.phrs.2022.106136. [DOI] [PubMed] [Google Scholar]
- 41.Rao Y., Yu H., Gao L., Lu Y.T., Xu Z., Liu H., et al. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br J Pharmacol. 2017;174:2457–2470. doi: 10.1111/bph.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.