Abstract
Neuropathic pain is a debilitating pathological condition that presents significant therapeutic challenges in clinical practice. Unfortunately, current pharmacological treatments for neuropathic pain lack clinical efficacy and often lead to harmful adverse reactions. As G protein-coupled receptors (GPCRs) are widely distributed throughout the body, including the pain transmission pathway and descending inhibition pathway, the development of novel neuropathic pain treatments based on GPCRs allosteric modulation theory is gaining momentum. Extensive research has shown that allosteric modulators targeting GPCRs on the pain pathway can effectively alleviate symptoms of neuropathic pain while reducing or eliminating adverse effects. This review aims to provide a comprehensive summary of the progress made in GPCRs allosteric modulators in the treatment of neuropathic pain, and discuss the potential benefits and adverse factors of this treatment. We will also concentrate on the development of biased agonists of GPCRs, and based on important examples of biased agonist development in recent years, we will describe universal strategies for designing structure-based biased agonists. It is foreseeable that, with the continuous improvement of GPCRs allosteric modulation and biased agonist theory, effective GPCRs allosteric drugs will eventually be available for the treatment of neuropathic pain with acceptable safety.
Key words: Neuropathic pain, Allosteric modulators, G protein-coupled receptors, Analgesia
Graphical abstract
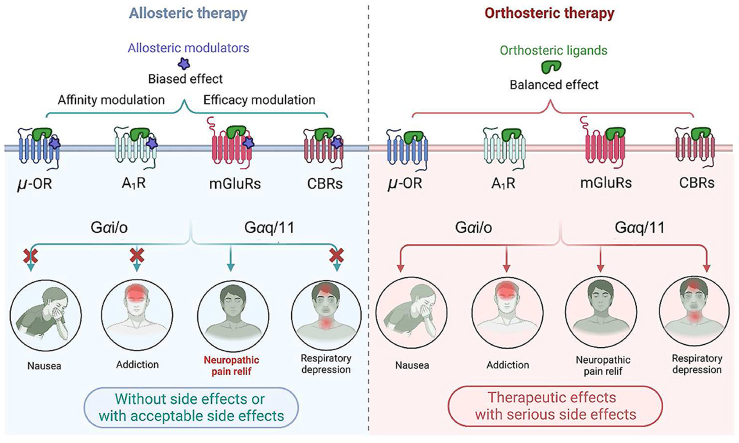
The allosteric modulation based on four key GPCRs (μ-OR, A1R, mGluRs and CBRs) shows promise as a therapeutic strategy for neuropathic pain. Allosteric modulators effectively mitigate non-specific side effects by modulating the affinity or efficacy of the orthosteric ligands, or through biased signaling effects.
1. Introduction
Neuropathic pain (NP) is a prevalent chronic pain condition that results from injury, lesion or disease of the somatosensory nervous system1. It is a rapidly progressing and indefinite condition characterized by persistent sensory abnormalities and increased nociceptive sensitization. Furthermore, it is often accompanied by mood disorders such as anxiety and depression, which significantly impact the quality of life of patients and impose a considerable economic burden on individuals and society2,3. Epidemiological studies have revealed that neuropathic pain is a common health problem, affecting 6.9%–10% of the general population4. The incidence of neuropathic pain is anticipated to increase due to the increased survival rate of cancer patients, the aging population, and the rapidly expanding prevalence of diabetes mellitus5.
The first-line drugs for managing neuropathic pain are anticonvulsants (e.g., gabapentin and pregabalin), tricyclic antidepressants (TCAs) (e.g., amitriptyline), and serotonin-norepinephrine reuptake inhibitors (SNRIs) (e.g., duloxetine and venlafaxine)6, 7, 8, 9. Opioid analgesics are approved as second-line medications for the management of neuropathic pain in patients with moderate to severe pain. However, current medications for neuropathic pain have limited clinical efficacy10, with only about 30%–50% of patients experiencing pain relief after treatment. Moreover, relief from neuropathic pain often comes at the cost of unpleasant side effects. A meta-analysis indicated that pregabalin considerably raised the risk of adverse events, such as somnolence, ataxia, and euphoria11. Adverse effects associated with opioid use include central nervous system (CNS) symptoms such as sedation, respiratory depression, delirium, tolerance, addiction, and physical dependence12. Additionally, long-term use of TCAs and SNRIs can cause dry mouth, visual abnormalities, constipation, postural hypotension, dizziness, drowsiness, nausea, and vomiting, among other adverse effects13. The lack of efficacy and inevitable side effects of drugs for neuropathic pain treatment highlights the urgency of developing new effective and safe analgesics.
G protein-coupled receptors (GPCRs) are transmembrane proteins that convert extracellular chemical signals into intracellular responses. There are approximately 800 GPCRs encoded in the human genome which respond to a wide range of signaling molecules such as odors, hormones, neurotransmitters, chemokines, and more. These molecules encompass photons, amines, carbohydrates, lipids, peptides, and proteins14,15. GPCRs are classified into six classes based on their amino acid sequences, with only classes A, B, C, and D present in humans. GPCRs are the most sought-after drug targets, with almost 40% of FDA-approved drugs targeted toward GPCRs. These receptors are also involved in pain perception and modulation pathways, such as the opioid and cannabinoid receptors. Clinical and preclinical data suggest that agonists or inhibitors targeting these receptors can provide therapeutic benefits for neuropathic pain, but they can also result in adverse effects unrelated to analgesia due to the simultaneous activation of multiple downstream signaling transductions. Therefore, the development of allosteric modulators targeting the allosteric sites in GPCRs shows potential in treating neuropathic pain. This review highlights the involvement of several GPCRs, including the μ-opioid, cannabinoid, metabotropic glutamate receptors, and adenosine receptors, in the modulation and processing of nociceptive information in neuropathic pain. The article also describes the molecular mechanisms underlying their analgesic and pro-nociceptive effects. Additionally, we summarize recent advances in the preclinical of allosteric modulators targeting GPCRs for the treatment of neuropathic pain (Fig. 1 and Table 1). Lastly, we present examples of biased drug development based on GPCR crystal structures, as structure-based allosteric drug discovery is expected to accelerate with the resolution of more GPCR crystal structures, especially those of class A GPCRs.
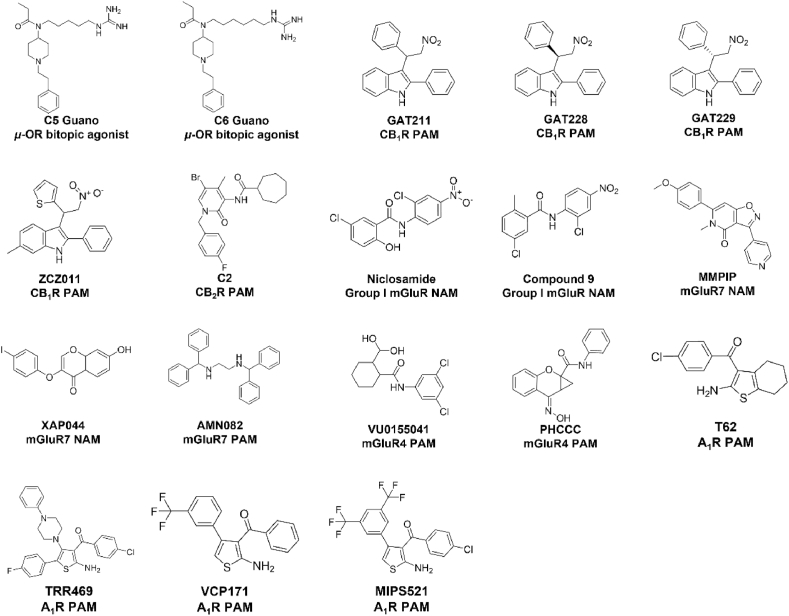
Figure 1.
Chemical structures of allosteric ligands of GPCRs discussed in the main text.
Table 1.
Compounds targeting GPCRs with analgesic effects for neuropathic pain.
| Allosteric target | Compd. | Allosteric feature | Subject | Model, dose and administration | Pharmacological effect | Ref. |
|---|---|---|---|---|---|---|
| μ-OR | C6 guano | Bitopic agonist | Male CD-1 mice | CCI (10, 30, or 100 nmol; icv.) | ↓Mechanical allodynia | 118 |
| CB1R | GAT211 | PAM | Male C57BL/6J mice | Paclitaxel-induced neuropathic pain (0.1, 1, 2.5, 5, 10, 20, 30 mg/kg; i.p.) | ↓Paclitaxel-induced mechanical and cold allodynia | 129 |
| ZCZ011 | PAM | Male C57BL/6 J mice | CCI (10, 20, 40 mg/kg; i.p.) | ↓CCI-induced mechanical and cold allodynia | 133 | |
| CB2R | C2 | PAM | Male CD-1 mice | Oxaliplatin-induced neuropathic pain (1, 5, 10, 20 mg/kg; p.o.) | ↓Oxaliplatin-induced Hypersensitivity | 137 |
| Group I mGluR | Niclosamide | NAM | Male SD rats | SNL (0.15 μmol/L, i.t.; 60 mg; i.p.; 300 mg; p.o.) | ↓Mechanical allodynia | 140 |
| Compound 9 | NAM | Male SD rats | SNL (75 mg; i.p.) | ↓Mechanical allodynia | 140 | |
| mGluR7 | MMPIP | NAM | Male CD1 mice | SNI (20 mg/kg, s.c.) | ↓Mechanical allodynia and thermal hyperalgesia | 141 |
| XAP044 | NAM | Male CD1 mice | SNI (30 mg/kg, s.c.) | ↓Mechanical allodynia and thermal hyperalgesia | 141,159 | |
| AMN082 | PAM | Male SD rats | Paclitaxel-induced neuropathic pain (10, 30, 100 nmol/L; i.t.) | ↓Mechanical allodynia and thermal hyperalgesia | 142 | |
| mGluR4 | VU0155041 | PAM | Male SD rats | SNL (125, 250, 500 nmol/L; i.t.) | ↓Mechanical allodynia | 144 |
| PHCCC | PAM | Male SD rats | CCI (10, 20, 40 μg; i.t.) | ↓Mechanical allodynia | 145 | |
| A1R | T62 | PAM | Male SD rats | SNL (50, 100 mg/kg, p.o.; 10 μg, i.t) | ↓Mechanical allodynia | 154,160 |
| TRR469 | PAM | Male CD1 mice | STZ-induced neuropathy (0.01, 0.3, 3 mg/kg; i.p.) | ↓Mechanical allodynia →Free of locomotor disturbance |
161 | |
| VCP171 | PAM | Male SD rats | SNL (1, 3, 10, 30 μg; i.t.) | ↓Mechanical allodynia | 103,104 | |
| MIPS521 | PAM | Male and female SD rats | SNL (1, 3, 10, 30 μg; i.t.) | ↓Mechanical allodynia | 103 |
μ-OR, μ-opioid receptor; A1R, Adenosine receptor A1; CB1R, Cannabinoid receptor 1; CB2R, Cannabinoid receptor 2; mGluR, metabotropic glutamate receptor; PAM, positive allosteric modulator; NAM, negative allosteric modulator; CCI, chronic constriction injury; SNI, spared-nerve injury; SNL, spinal nerve ligation; STZ, streptozotocin; icv., intracerebroventricular; i.p., intraperitoneal administration; p.o., oral administration; s.c., subcutaneous administration; i.t., intrathecal administration; ↑: Increased; ↓: Attenuate; →No effect.
2. Allosteric modulation of GPCRs
GPCRs are an exemplary allosteric regulatory system that adopts different conformations in the dynamic equilibrium between inactive and active states. These pre-existing conformational states are present within an ensemble, and the specific conformation adopted depends on the extracellular stimuli received by the GPCRs, leading to the activation of downstream signaling pathways in different orders. Specific residues, called “micro-switches”16, extend from the extracellular to the intracellular G protein binding site throughout the GPCR landscape and control the conformational transition upon GPCR activation17. The allosteric effect is induced by the formation and disruption of hydrophobic and hydrogen bond interactions between the surrounding residue groups triggered by ligand binding sites. This effect is then propagated to the next layer of “micro-switches” until the G protein binding site is reached, ultimately regulating the dynamic equilibrium between inactive and active conformations of the receptor.
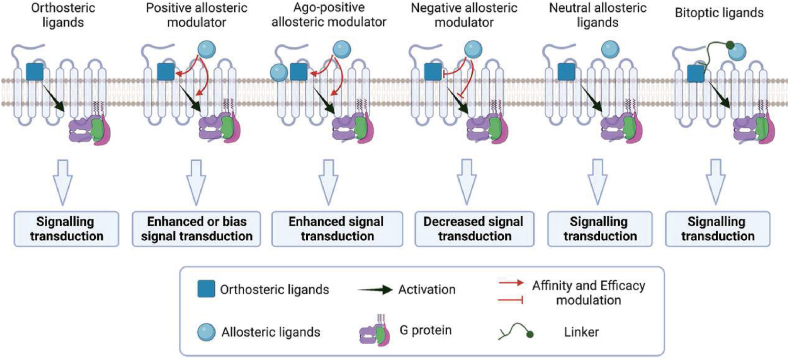
Studies have shown that a single ligand for GPCRs can only induce one active conformation. However, in some cases, ligands can bind to both orthosteric and allosteric sites simultaneously, leading to a novel receptor conformation and selectivity among downstream signals18, 19, 20, 21. Allosteric ligands are categorized as positive allosteric modulators (PAMs), negative allosteric modulators (NAMs), agonist-positive allosteric modulators (ago-PAMs), neutral allosteric ligands (NALs), and bitopic ligands, based on the receptor function modulated by the allosteric ligand in the presence of the endogenous ligand22,23 (Fig. 2). PAMs and NAMs stabilize receptors in specific conformational states and can change the intrinsic effectiveness of an orthosteric agonist to engage downstream signaling processes by modulating the affinity of the orthosteric binding pocket for the orthosteric ligands. PAMs and NAMs regulate downstream signal activation through affinity modulation and efficacy modulation24. Wu et al.15 suggested that PAMs can enhance downstream signal transduction through four mechanisms: i) promoting the affinity of agonist binding without affecting signal transduction, ii) directly enhancing signal transduction without affecting agonist binding, iii) increasing the affinity of agonist binding while enhancing signal transduction, and iv) reducing the affinity of agonist binding while increasing signal transduction (Fig. 2). Allosteric modulators within a structural class can have diverse effects on receptors, including positive or negative modulation and inverse agonism. Furthermore, both PAMs and NAMs require the presence of an orthosteric ligand to exert their effects. Generally, PAMs and NAMs exhibit probe dependence, meaning they display differential modulation of receptor signaling characteristics in the presence of different orthosteric ligands25, which is attributed to the distinct conformational changes induced by different orthosteric ligands upon receptor activation. However, there are some PAMs that lack probe dependence, as they activate the receptor to the same extent and exhibit similar signaling characteristics in the presence of different orthosteric ligands. One possible reason for this is the receptor's selectivity for downstream effector proteins or its preference for a specific binding conformation. Additionally, when PAMs modulate multiple signaling pathways of the receptor, the allosteric modulation of one pathway might be excessively strong, overshadowing other weaker allosteric effects. Some ligands, called ago-PAMs, have a dual conformational modulator and conformational agonist functions, allowing them to possess the properties of both orthosteric and allosteric ligands without losing PAM selectivity (Fig. 2). The ago-PAMs can induce receptor activation independent of orthosteric ligands, suggesting that the binding site of ago-PAMs represents a novel active switch distinct from the orthosteric site. The discovery of drugs targeting this site may be beneficial for investigating the unconventional molecular mechanisms underlying receptor activation26, 27, 28, 29. NALs bind to allosteric sites but do not influence orthosteric ligand responses; however, they can compete with PAMs and NAMs for allosteric site binding and limit their effects24,30. NALs are useful tools for validating binding sites, even if they are not explored as drug candidates (Fig. 2). Bitopic ligands are another relatively new concept in allosteric ligand design, which involves connecting two pharmacophores with orthosteric and allosteric affinities with a linker to create a single ligand with high selectivity between receptor subtypes and sufficient receptor affinity (Fig. 2)22,31,32. Celine Valant et al. synthesized a bitopic ligand by connecting adenosine and VCP746 through N-hexylbenzamide, which demonstrated complete in vitro stimulation and a pronounced signal bias towards the cAMP signaling pathway33.
Figure 2.
Modulation of GPCRs by orthosteric and biased allosteric modulators. (A) The binding of orthosteric ligands to GPCRs stabilizes the receptor conformation in multiple active states that activate different downstream effector proteins, including heterotrimers of Gαβγ (e.g., Gαs, Gαi/o, Gαq/11, Gα12/13) and β-arrestin. These effector proteins induce downstream signaling pathways (e.g., cAMP accumulation, Ca2+ localization, and activation of Erk) that regulate various physiological activities of the cell. (B) In contrast, biased conformation modulators stabilize only one active conformation of the GPCR, producing a more limited physiological effect than orthosteric ligands. While most biased conformation modulators require an orthosteric ligand to regulate the active conformation of the receptor, there are also biased conformation modulators that induce receptor activation alone.
Compared to orthosteric sites, allosteric sites in GPCRs are under less evolutionary pressure, resulting in lower conservation within the same protein subtype. Allosteric modulators of GPCRs represent a hallmark of allostery, allowing selective modulation of highly homologous protein subtypes and avoiding non-specific targeting of homologous proteins, which may cause adverse effects, thus having practical therapeutic implications34,35. The primary advantage of allosteric modulators of GPCRs lies in their ability to align their effects with the temporal and spatial release patterns of endogenous ligands, thereby preserving the natural rhythmic signaling of endogenous ligands, preventing excessive receptor activation and rapid desensitization, ultimately reducing the development of drug tolerance36. This feature is a concrete expression of the synergistic nature between the allosteric modulators and the orthosteric ligands. It presupposes that the allosteric modulators exhibit a very low direct activation effect. On the other hand, the allosteric modulatory effect generally has an upper limit37, making allosteric modulators acceptable with tolerable side effects. Allosteric modulators may cooperate with orthosteric ligands to stabilize different functional conformations of the same GPCR, generating biased signaling38, 39, 40. This effect has attracted great attention from structural biologists, cell biologists, and drug developers, as it has the potential to manipulate signal transduction of receptors with drugs precisely. While the possibility of achieving purely biased signaling is low, it can still improve drug safety to some extent. Furthermore, biased ligands’ development can serve as chemical probes to study the conformational changes during receptor activation. It is worth noting that the same GPCRs may have different orthosteric ligands, including endogenous ligands and chemical molecules discovered through medicinal chemistry41, amplifying the diversity of downstream signal control of receptors. The artificial selection of GPCR signal transduction can be achieved by combining different orthosteric ligands and allosteric modulators42.
3. Allosteric target for neuropathic pain
Central sensitization is the pathophysiological mechanism underlying neuropathic pain. This phenomenon is due to disturbances in the release and absorption of neurotransmitters, as well as in neuronal potentials. To alleviate neuropathic pain, it is essential to correct the underlying neurological disorder. Recent advances in our understanding of the neurophysiology of the nociceptive perception pathway in neuropathic pain have identified new protein targets for the development of novel analgesics. These targets mainly comprise GPCRs, ion channels, enzymes, and transporter proteins.
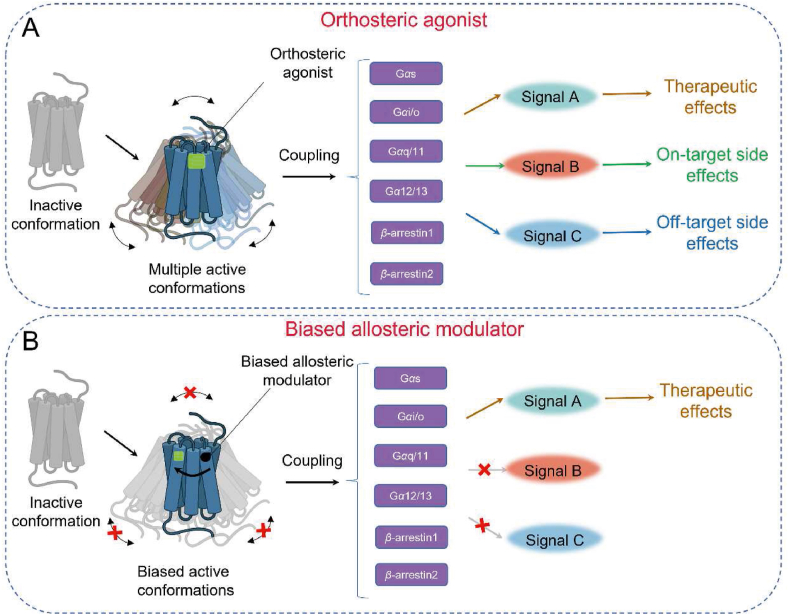
GPCRs are the largest superfamily of cell surface proteins in the human genome and are still a prime target for developing analgesics. Over one-third of all FDA-approved medications have GPCRs as their therapeutic target43. GPCRs play a crucial role in the onset and maintenance of neuropathic pain in the central nervous system, including adenosine receptors, cannabinoid receptors, and metabotropic glutamate receptors. The structural architecture of these receptors consists of seven transmembranes (7TMs) helices connected by three extracellular and three intracellular loops44. Orthosteric sites, located at the center of the 7TMs in the extracellular region, possess conserved structures that serve as switches for extracellular signaling by GPCR signaling. The binding of orthosteric ligands to the conserved orthosteric binding site of GPCRs facilitates the transition of the receptor from an inactive conformation to an active conformation. Endogenous ligands typically induce multiple active conformations of GPCRs, which are already present in the receptor's conformational ensemble. As a result, they activate various downstream effectors, including G proteins (such as Gαs, Gαi/o, Gαq, Gα12/13) and β-arrestin (such as β-arrestin1, β-arrestin2)45. For example, the activation of neurotensin receptor 146 and vasopressin receptor 248 by their respective endogenous ligands leads to the activation of multiple G protein effectors. It is noteworthy that GPCRs still exhibit a preference for specific active conformations among multiple active states. This ensures that upon activation, most receptors adopt a particular active conformation, enabling interaction with downstream Gα proteins. NTSR1 primarily promotes Gαq dissociation48, while V2R mainly facilitates Gαs dissociation47. These activation properties of orthosteric ligands confer diverse physiological functions to the receptors. However, the therapeutic use of orthosteric ligands can induce both desired therapeutic effects and on-target side effects. Moreover, the orthosteric binding site is highly conserved, particularly among subtypes of the same GPCR, which can contribute to the occurrence of off-target effects of orthosteric ligands (Fig. 3A). Strategies for discovering drugs that target GPCRs mainly involve the development of ligands that interact with allosteric binding sites, which are distinct from the orthosteric site in terms of topography49. Allosteric ligands, such as PAM or NAM, fine-tune the activation conformation of GPCRs by binding to their allosteric sites in synergy with endogenous ligands, thereby precisely regulating downstream signal transduction50. The practical advantage of allosteric ligands is their ability to selectively enhance therapeutically relevant signaling while minimizing adverse effects on the target (Fig. 3B).
Figure 3.
Five types of allosteric modulators modulate the function of GPCRs. Both PAMs and NAMs can modulate the affinity of the orthosteric binding pocket for an orthosteric ligand or affect the intrinsic efficacy of an orthosteric agonist to engage downstream signalling mechanisms. Ago-PAMs possess the properties of both orthosteric and allosteric ligands. NALs bind to allosteric sites and have no influence on orthosteric ligand responses; nonetheless, they compete with PAMs and NAMs for allosteric site binding and inhibit their effects. Bitopic ligands are a class of compounds that exhibit high selectivity and affinity for specific target receptors. They are formed by connecting orthosteric and allosteric ligands via specific linker molecules. The use of such linkers results in a distinct and selective binding mode of the ligands to the receptor.
3.1. Mechanism of μ-OR-mediated analgesic effects and adverse effects on neuropathic pain
The human opioid receptors are considered the optimal receptors in pain management and are expressed in various pathways responsible for pain perception, transmission, and regulation, including sensory neurons, synaptic neurons in the spinal cord, the brainstem, midbrain, and cortical layer. The opioid system in humans consists of μ-opioid receptors (μ-OR), δ-opioid receptors, κ-opioid receptors, and nociceptin receptors51, 52, 53, 54. These opioid receptors trigger intracellular signaling events by activating Gαi/o proteins, inhibiting adenylate cyclase, decreasing intracellular Ca2+ influx, increasing K+ influx, and promoting postsynaptic membrane hyperpolarization, ultimately preventing signal transmission of secondary projection neurons55. The μ-opioid receptor has been the most extensively studied opioid receptor, and drug development targeting μ-opioid receptors is a popular topic in pain medication research56,57. Most of the currently available opioid drugs are agonists of μ-OR, and classical opioid analgesics such as morphine and fentanyl demonstrate high selectivity for μ-OR. Nonetheless, the adverse effects related to μ-opioid drugs, such as respiratory depression and tolerance, have limited their use in various chronic pain conditions, including neuropathic pain.
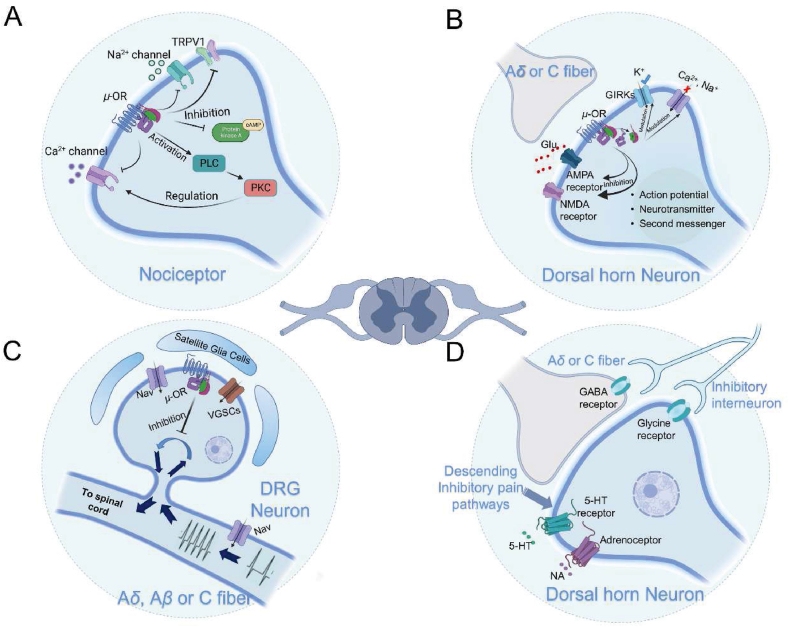
When opioid peptides bind to the μ-opioid receptor, the pre-coupled heterotrimeric Gαi/Gβ/Gγ rapidly responds to the stimulation and dissociates into Gαi and Gβ/Gγ subunits. This process involves conformational changes in the Gαi protein and GDP/GTP exchange in the cytoplasm. As a result, adenylate cyclase (AC), located on the membrane, is inhibited, preventing the conversion of intracellular ATP to cyclic adenosine monophosphate (cAMP). Furthermore, the Gβ/Gγ subunits bind to free Gαs in the cytoplasm, further inhibiting the accumulation of cAMP. Opioid receptors couple with various Ca2+ channels located on the cell membrane, such as N-type, T-type, and P/Q-type channels. This coupling suppresses the entry of intracellular Ca2+, thereby reducing multiple intracellular signaling events initiated by Ca2+ as a second messenger, including neurotransmitter release and membrane depolarization. It is worth noting that some research suggested that μ-OR activation exhibits a dual effect on AC inhibition by both reducing the conductance of voltage-gated Ca2+ channels and activating the PLC/PKC pathway, thereby modulating the activity of Ca2+ channels on the plasma membrane58. This regulatory mechanism appears contradictory and currently lacks a definitive conclusion, but the majority of research still supports the notion that μ-OR activation leads to a decrease in intracellular cAMP levels (Fig. 4A). Activating μ-opioid receptors on the postsynaptic membrane can induce the opening of G-protein-coupled inwardly rectifying potassium channels (GIRKs) coupled with G proteins, causing K+ inflow and membrane hyperpolarization. This blocks the transmission of action potentials that encode pain information. The spinal dorsal horn, where the region modulates the nociceptive information from the periphery, is the first station for the transmission of peripheral nociceptive information into the central nervous system before transmitting it to higher centers. μ-OR can directly modulate NMDA receptors and AMPA receptors, both of which play important roles in the transmission of pain information and long-term potentiation in neuropathic pain (Fig. 4B). The mechanism by which the opioid receptor regulates these two types of ionotropic glutamate receptors to mediate analgesic effects on neuropathic pain is not yet understood. In addition, the activation of μ-opioid receptors can also inhibit TTX-resistant Na+ channels and TRPV1 channels in nociceptors and dorsal root ganglion neurons (Fig. 4C). Since the opioid receptor is widely distributed in the nervous system, these signaling events occur synchronously at all levels of neurons involved in pain perception, transmission, and modulation pathways after receiving opioid receptor agonist treatment. μ-OR are also involved in the descending inhibitory system for pain modulation. The descending inhibitory system originates from the periaqueductal gray (PAG) region of the midbrain, which contains abundant opioidergic neurons that secrete various endogenous opioids, including enkephalin and endorphins. Neuronal projections from the PAG nucleus descend to the midline raphe magnus nucleus located in the ventral aspect of the medullary rostral region, which is rich in serotoninergic neurons and serves as a crucial relay station for the descending inhibition from the PAG. Furthermore, within the adjacent medulla spinal system, there are descending fibers abundant in noradrenaline, opioid peptides, and serotonin, which terminate in the spinal dorsal horn. These fibers exert endogenous analgesic effects through the release of noradrenaline and serotonin under the multi-level control of the PAG (Fig. 4D).
Figure 4.
Mechanisms of analgesic action of μ-opioid receptor in the peripheral nervous system (PNS) and central nervous system (CNS). (A) In nociceptive receptors, the activation of μ-opioid receptor (μ-OR) reduces the release of nociceptive substances and decreases Ca2+ production following nerve injury by interacting with TRPV1, H1R, and NK1R. (B) In dorsal root ganglion neurons, μ-OR inhibits the transmission of nociceptive information to the central nervous system by blocking Nav and VGSCs. (C) In spinal dorsal horn neurons, μ-OR induces cell membrane hyperpolarization by inhibiting Nav and VGSCs-mediated Ca2+ influx and activating GIRKs-mediated K+ influx. Moreover, μ-OR modulates ionotropic glutamate receptors, resulting in central analgesic effects. (D) The downstream pain inhibitory pathway activated by μ-OR inhibits the upstream transmission of nociceptive information by modulating 5-HT and norepinephrine receptors, as well as glycine receptors, on spinal dorsal horn neurons.
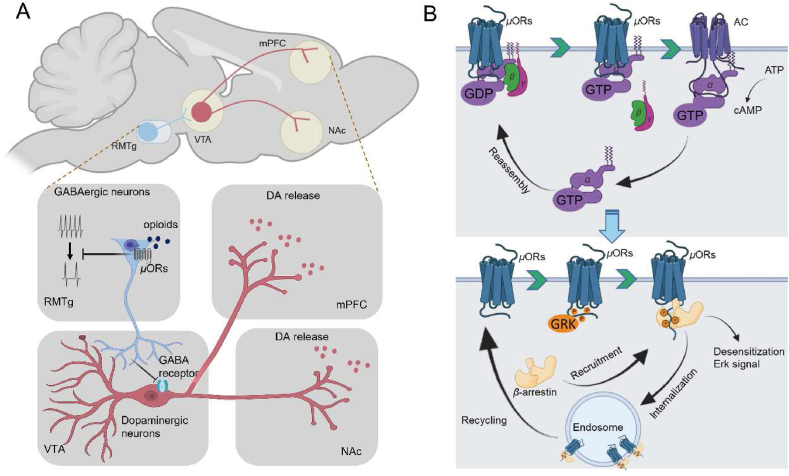
Opioid drugs have a strong rewarding effect, which contributes to their addiction potential, thus limiting their clinical use. In recent years, extensive research has been conducted on the neural mechanisms underlying opioid drug dependence. The results have identified the key brain regions and neural circuits involved in opioid addiction, with the reward circuit being considered the primary neural circuit59. This circuit comprises multiple cortical and subcortical areas that process reward-related behavior, including reward valence encoding, reward expectation errors, and motivation regulation. Drug addiction mediated by μ-opioid receptors is primarily linked to the positive reward circuit, and the midbrain dopamine system in the mesolimbic pathway serves as the center of this circuit60. Dopaminergic neurons in the ventral tegmental area (VTA) project to several critical components of the positive reward circuit, including the nucleus accumbens (NAc), medial prefrontal cortex (mPFC), and basolateral amygdala (BLA). The VTA plays a crucial role in regulating reward function and contains mainly dopaminergic neurons (55%–65%) and around 30% GABAergic neurons. The VTA is rich in dopaminergic neurons, which are the main area regulating reward function. Dopaminergic neurons in the VTA project to the NAc to encode reward valence and motivation highlighting61, project to the mPFC to mainly regulate executive control, and the project to the BLA to enhance associative learning of reward background62. Opioids activate μ-opioid receptors to inhibit GABAergic neurons, which disinhibits dopaminergic neurons in the VTA and increases dopamine release (Fig. 5A). Recent studies have highlighted the role of the rostromedial tegmental nucleus (RMTg), located in the tail of the VTA, in opioid addiction. Although RMTg and VTA share anatomical and functional heterogeneities, pre-induced GABAergic inhibitory synaptic currents in interneurons of VTA, NAc, and RMTg brain regions did not affect the VTA, while inhibitory synaptic currents generated in RMTg were significantly reduced. The RMTg is predominantly composed of GABAergic neurons (>75%), with high expression levels of μ-opioid receptors on these neurons63, 64, 65. Therefore, opioids mainly act on μ-opioid receptors in the RMTg, which may open GABAA-controlled chloride ion channels and subsequently inhibit GABAergic neurons (Fig. 5A).
Figure 5.
Mechanisms of μ-opioid receptor-mediated addiction and analgesic tolerance. (A) The neural circuit mechanisms underlying opioid-induced addiction mainly involve the inhibition of GABAergic neurons within the ventral tegmental area (VTA) through sustained activation of the μ-opioid receptor (μ-OR) in the rostromedial tegmental nucleus (RMTg), as well as the induction of the medial prefrontal cortex (mPFC) and sustained dopamine release in the nucleus accumbens (NAc). (B) The activation process of μ-OR primarily involves the conversion of GDP to GTP on Gα proteins, leading to the dissociation of activated Gα proteins from Gβγ and the conversion of intracellular ATP to cAMP, and the internalization process of μ-OR occurs through the recruitment of β-arrestin under the action of GRK phosphorylation, resulting in the depression of the plasma membrane at the receptor site to form an endosome. A portion of the receptor located on the endosome is subsequently re-inserted into the plasma membrane.
Analgesic tolerance is another common adverse reaction to opioid drugs. Repeated use of the same dose of opioid drugs can decrease the analgesic effect, and a higher dose may be required to produce the same effect. In fact, all effects caused by opioid drugs, such as respiratory depression and constipation, can potentially develop tolerance. For a long time, receptor internalization has been considered the main mechanism by which μ-opioid receptors mediate analgesic tolerance. After receptor activation, intracellular GPCR kinases are rapidly recruited to the C-terminal tail of the opioid receptor, phosphorylating specific amino acid sites. The phosphorylated GPCRs enhance its affinity for intracellular β-arrestin, which, under the action of clathrin, promotes receptor internalization and forms early endosomes66. The GPCR on the late endosome membrane is degraded through the lysosome pathway or recycled and inserted back into the membrane (Fig. 5B). Only a small fraction of internalized μ-opioid receptors are degraded, and most are dephosphorylated and transported back to the membrane67. Therefore, the mechanism of μ-opioid receptor internalization is considered significant in reducing opioid tolerance. This type of tolerance was initially thought to be due to adaptive changes in the body to prevent excessive receptor activation and the risk of death. Although agonist-induced μ-receptor internalization can reduce opioid tolerance, controversy still exists about the mechanism by which receptor internalization delays opioid tolerance68, 69, 70. Some scholars believe that the β-arrestin pathway mediates the adverse effects of opioid drugs, such as respiratory depression. The experimental basis of this theory is mainly derived from Raehal et al.’s71 discovery that morphine's analgesic effect in mice with β-arrestin2 defects is significantly prolonged, and the side effects of respiratory depression and acute constipation are significantly reduced. This theory has led to the development of opioid receptor biased drugs for several decades. However, recent studies have challenged this theory, as different research groups have found that fentanyl can still induce respiratory depression and constipation in mice lacking β-arrestin function. Therefore, it is necessary to consider the different theories on the mechanisms of the adverse reactions of opioid receptors. The biological regulatory process of opioid drugs in the body is extensive, and even the currently widely proven mechanisms are quite complex. Therefore, the adverse reactions mediated by opioid drugs may result from multifaceted effects.
3.2. CBR-mediated analgesic effects on neuropathic pain
The endocannabinoid system (ECS) plays a crucial role in pain modulation and neuropathic pain pathophysiology. ECS is composed of cannabinoid receptors, endogenous ligands, and enzymes involved in their synthesis and degradation. CB1 receptor (CB1R) and CB2 receptor (CB2R) are the two main types of seven-transmembrane cannabinoid receptors that couple with inhibitory Gαi/o protein. CB1R is highly expressed in presynaptic terminals of both inhibitory and excitatory neurons in the CNS72,73, while CB2R is primarily found in immune cells74. N-Arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) are the most important endogenous ligands for cannabinoid receptors75. These endocannabinoids are retrograde messengers synthesized mainly in postsynaptic membrane phospholipids that regulate presynaptic neurotransmitter release76. The ECS plays a crucial role in analgesia by inhibiting pain stimuli transmission. CB1R located on pain receptors in the peripheral nervous system inhibits pain stimuli transmission. At critical nodes such as the dorsal root ganglia and spinal dorsal horn, CB1R activation inhibits neurotransmitter release and pain information transmission. CB2R activation in immune cells can also inhibit inflammatory factors that promote pain information transmission and sensitization in both peripheral and central nervous systems77,78. Moreover, CB1R located on the spinal cord activates the descending inhibitory pathway by inhibiting GABA release in the periaqueductal gray and rostral ventromedial medulla78. Furthermore, CB1R expressed in the prefrontal cortex and hypothalamus can regulate the affective and emotional components related to pain79.
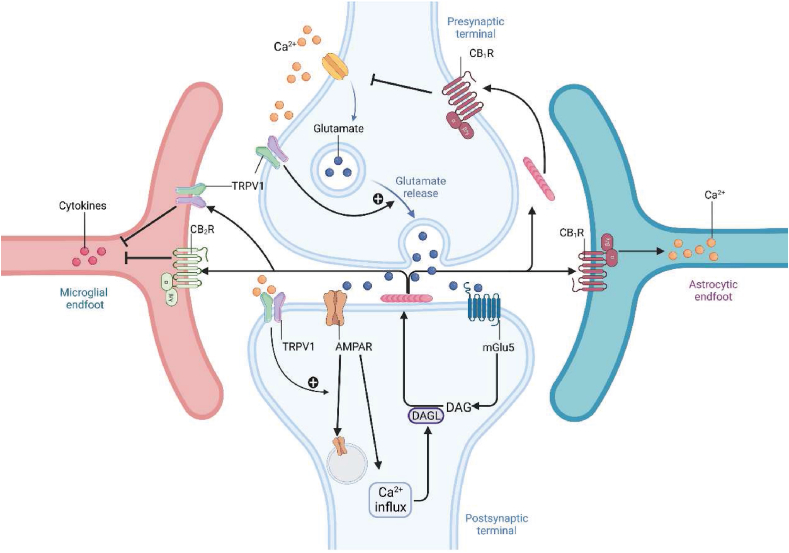
CBR primarily couples with inhibitory Gαi/o proteins, and Gαq/11, which mediates intracellular calcium signaling, is also coupled with CBR. In neuropathic pain, CBR regulates synaptic plasticity related to chronic pain. Endogenous cannabinoids respond to glutamate release from the presynaptic membrane induced by noxious stimuli, which opens voltage-gated calcium channels and TRPV1-mediated calcium influx in the presynaptic membrane, leading to a large amount of glutamate release80. Glutamate activates mGlu5 on the postsynaptic membrane, which in turn activates phospholipase C (PLC) and diacylglycerol lipase (DAGL) required for 2-AG synthesis while inducing inositol triphosphate (IP3), which mobilizes intracellular calcium (Fig. 6). Furthermore, glutamate activates AMPA receptors, enhancing intracellular calcium signaling (Fig. 6). Interestingly, the postsynaptic TRPV1 can reduce excitatory synaptic transmission by increasing AMPA receptor reuptake and mediating TRPV1 long-term suppression81 (Fig. 6). Synthesized after mGlu5 activation, 2-AG can achieve retrograde long-term depression of synaptic transmission through presynaptic CB1R82. Moreover, 2-AG can amplify the Ca2+ influx in astrocytes through CB1R, promoting the release of gliotransmitters (such as glutamate) into the synaptic cleft and amplifying the signal of 2-AG83,84. Binding with CB2R or TRPV1 on microglia, 2-AG induces the release of related cytokines85 (Fig. 6).
Figure 6.
Synaptic and immune suppressive actions mediated by cannabinoid receptors in neurons and glial cells. The activation of CB1R reduces Ca2+ release from presynaptic neurons by inhibiting VGSCs at the presynaptic membrane, resulting in decreased Ca2+ influx in postsynaptic neurons. CB1R activation localized on astrocytes induces Ca2+ release by the Gαq pathway. The activation of CB2R localized on microglia reduces cytokine release, indicating its role in immune suppression.
Preclinical studies have established the significance of the ECS in the treatment of neuropathic pain, paving the way for new and effective analgesic strategies. As previously mentioned, the activation of CB1Rs in the peripheral nervous system impedes the transmission of pain signals to the central nervous system. Selective deletion of CB1Rs in nociceptors enhances pain responses in mice with neuropathic pain, and the analgesic effects of systemic cannabinoid agonists are significantly reduced86. On the contrary, constitutive deletion of CB1Rs has no significant impact on the pain characteristics of mice with neuropathic pain, but anxiety- and depression-like behaviors are markedly increased. Pharmacological studies of receptors have also revealed that non-selective cannabinoid agonists and selective CB1R and CB2R agonists can produce antinociceptive effects in various animal models of neuropathic pain. In addition, the anti-nociceptive effects of blocking the reuptake of endogenous cannabinoids and pharmacological inhibition of FAAH do not cause tolerance in neuropathic pain models87. Evidence suggests that constitutive deletion of CB2R can significantly enhance neuropathic pain behavior, which might involve the inflammatory response mediated by CB1R activation in microglia88. While preclinical studies have extensively demonstrated the beneficial effects of cannabinoid receptor agonists in neuropathic pain, there are still differences between preclinical animal models and clinical stage research data supporting the use of cannabinoid agonists in the treatment of neuropathic pain. Clinical data indicate that several cannabinoid preparations, such as oral dronabinol and nabiximols, as well as cannabis extracts administered via the oral mucosa, inhalation, and vaporization, can be employed to treat neuropathic pain. Furthermore, studies have shown that cannabinoids have moderate analgesic effects and can improve sleep without serious adverse effects when compared to placebo89. However, a review of six cannabis trials involving 325 patients with neuropathic pain concluded that it may be beneficial but important side effects, such as addiction and worsening of psychiatric disorders, should be considered90. In conclusion, cannabinoid receptor agonists have therapeutic benefits in neuropathic pain, but different doses and routes of administration may produce different effects on neuropathic pain, mainly influenced by the tissue localization of cannabinoid receptor subtypes and functional differences in presynaptic and postsynaptic sites. Combining various routes of administration and the effects of cannabinoid agonists is more conducive to the study of the analgesic activity of cannabinoid receptors.
3.3. mGluRs-mediated analgesic effects on neuropathic pain
In neuropathic pain, increased glutamate input to spinal dorsal horn neurons is a key mechanism inducing central sensitization and an essential factor in the development and maintenance of neuropathic pain syndromes after nerve injury91, 92, 93, 94. Glutamate exerts its effects through two major classes of receptors: ionotropic glutamate receptors and metabotropic glutamate receptors (mGluRs). The mGluRs belong to the C class of the G protein-coupled receptor family and regulate glutamate activity between synapses in the nervous system, thereby modulating synaptic transmission. Eight mGluRs have been cloned to date and divided into three groups based on their sequence homology, signal transduction mechanisms, subcellular localization, and pharmacological characteristics95,96. Group I mGluRs, including mGluR1 and mGluR5, located on the postsynaptic membrane, are mainly coupled to Gαq/11 proteins. They stimulate phosphoinositide hydrolysis, induce intracellular calcium mobilization, and play an essential role in regulating neuronal excitability and current modulation through ionotropic glutamate receptors. Conversely, Group II (mGluR2, mGluR3) and Group III (mGluR4, mGluR6, mGluR7, or mGluR8) mGluRs located on the presynaptic membrane are mainly coupled to Gαi/o proteins97. Their activation is negatively correlated with adenylate cyclase activation and is primarily responsible for reducing glutamate transmission between neurons and participating in reducing GABA release at inhibitory synapses through heterosynaptic facilitation. Although the subcellular localization and expression abundance of some mGluR subtypes in the nervous system remains unclear, it is important to note that the physiological functions mediated by presynaptic and postsynaptic mGluRs differ significantly, even if they are of the same subtype. This limitation limits the application of agonists or antagonists to specific brain regions and nuclei to examine their analgesic effects in neuropathic pain.
Adequate evidence suggests that activation of group I mGluRs results in heightened excitability of dorsal horn neurons, leading to the development of pain hypersensitivity and hyperalgesia. This may be associated with the upregulation of group I mGluRs expression in the dorsal horn of the spinal cord after neuropathic pain. Studies have shown that the expression of group I mGluRs in the dorsal horn of the spinal cord varies spatially and temporally after NP. After spinal cord injury (SCI), the expression of mGluR1 is increased in the surrounding area of the lesion and chronically increased in the deep and superficial layers of the spinal cord98. This increase in mGluR1 expression may cause excessive excitation of spinal dorsal horn projection neurons in the pain pathway, resulting in persistent pain transmission, pain hypersensitivity, and hyperalgesia. The temporal changes in mGluR1 expression parallel the development of NP features, such as mechanical and thermal hyperalgesia. Similarly, the expression of mGluR5 increases in the spinal dorsal horn layers I and II and mediates thermal hyperalgesia but not mechanical hyperalgesia after spinal nerve ligation99. Selective pharmacological inhibition of mGluR5 weakens the development of thermal hyperalgesia but has no effect on mechanical hyperalgesia, while a selective mGluR1 antagonist weakens the development of mechanical hyperalgesia but enhances that of thermal hyperalgesia. This is because the superficial layers (I and II) of the dorsal horn receive signal input from small myelinated (Aδ) and unmyelinated (C) fibers carrying information from temperature receptors, while the deeper laminae (III–V) receive input from layers I and II and larger myelinated fibers such as Aβ fibers, which carry information from muscle, bone, skin, and subcutaneous mechanical receptors. In summary, the spinal cord injury model shows that mGluR1 is expressed in both deep and superficial layers of the dorsal horn of the spinal cord, while mGluR5 is significantly upregulated only in the superficial layer. However, whether the upregulation of both receptors occurs in glial cells or neurons remains to be confirmed. Notably, different preclinical animal models of NP may lead to markedly different mechanisms of NP, and the similarity between these models and human NP requires further investigation. Thus, it is crucial to comprehensively investigate the changes in mGluRs in various preclinical NP models and determine their roles in the occurrence and maintenance of neuropathic pain.
The antinociceptive effect mediated by activation of group III mGluRs is mainly due to the modulation of spinal glutamate levels. This leads to a decrease in spontaneous and evoked spinal synaptic transmission, resulting in a reduced discharge of spinal dorsal horn projection neurons100. This, in turn, reduces mechanical hypersensitivity without modulating brief nociceptive stimuli. Both mGlu4 and mGlu7 are present at the presynaptic terminals of spinal dorsal horn afferent fibers, and their activation can downregulate pathological glutamatergic activity in the pain environment101. This suggests that drugs targeting group III mGluRs may be a promising target for controlling neuropathic pain. However, under basal conditions, the glutamate concentration at spinal synaptic clefts is insufficient to activate mGlu4 and mGlu7. This is a characteristic of group III mGluRs in pain modulation. In conclusion, mGluRs are significant in the occurrence and development of neuropathic pain, and the industry and academia widely accept them as the most promising target for neuropathic pain treatment.
3.4. A1R-mediated analgesic effects on neuropathic pain
Adenosine is a widely distributed biological molecule in the body and can be generated through the metabolism of adenosine triphosphate. It exerts its biological effects by acting on four types of adenosine receptors (A1, A2A, A2B, and A3). Adenosine and adenosine receptors have gained widespread attention in nociception and central injury processing, mainly due to the anti-nociceptive effects of A1R activation. A1Rs were first identified in trigeminal neurons in the peripheral nervous system102 and subsequently in the dorsal root ganglia by immunopositive reaction, serving as a primary modulation center for sensory information. Local administration of A1R agonists has been shown to significantly reduce heat hyperalgesia after nerve injury in rats103. In vitro experiments have demonstrated that A1R activation primarily reduces cAMP via preferential coupling to the Gαi/o pathway, which is the main mechanism for A1R-mediated analgesia102. Moreover, A1R activation can reduce calcium influx and decrease the release of calcitonin gene-related peptide (CGRP)102.
In the model of neuropathic pain, injury to the sciatic nerve significantly increases the endogenous adenosine tone in the rat spinal dorsal horn. This conclusion is supported by the significant enhancement of eEPSC amplitude in the spinal dorsal horn superficial layers (I and II) after pharmacological inhibition of A1R104. Although A1R is mainly expressed on spinal neurons in the superficial layers of the spinal dorsal horn, it is also expressed on glial cells105,106. Intrathecal administration of A1R agonists induces a significant analgesic effect in nerve-injured animals, which is significantly stronger than that of systemic administration. This highlights the prominent role of intrathecal A1R activation in pain modulation. Central administration of adenosine does not induce an increase in pain threshold or other pain-related biological activities in normal animals, but it can relieve pain hypersensitivity in nerve-injured animals, with effects lasting up to 24 h. This sustained analgesic effect also exists in patients with chronic neuropathic pain, as intrathecal injection of adenosine can reduce their pain hypersensitivity and allodynia106. However, the existing theoretical basis cannot explain this effect since the number of A1R in the spinal cord after nerve injury has not changed, nor has the downstream G protein coupling degree107. Gomes et al.108 proposed that the analgesic effect of adenosine may depend on the spinal noradrenergic mechanism after nerve injury. Other major mechanisms involving spinal A1R-mediated analgesia include inducing neuronal hyperpolarization by K+ inward currents in spinal dorsal horn intrinsic neurons, inhibiting peptide release, and inhibiting glutamate release. In conclusion, activation of adenosine A1R has a definite analgesic effect in chronic pain and is one of the most promising targets for treating neuropathic pain109,110.
Although adenosine has shown anti-nociceptive effects in humans, and numerous drugs targeting adenosine receptors have been studied clinically over the past few decades, adenosine and adenosine receptor agonists have not yet been utilized to treat patients with neuropathic pain. This is partially due to uncertainty regarding adenosine's therapeutic effect on spontaneous pain, as multiple clinical studies have found that adenosine effectively relieves thermal hyperalgesia, but lacks efficacy in treating spontaneous pain—the most common characteristic of neuropathic pain111. Additionally, intrathecal injection of adenosine causes powerful but transient neurological side effects, such as headaches, possibly due to activation of adenosine A2R112,113. Although peripheral injection is also ineffective, it produces pain and vasodilation effects114. The primary challenge for using adenosine or adenosine receptor agonists as analgesics is the cardiovascular side effects that occur with systemic administration. Adenosine A1R is mainly expressed in the cardiac atrioventricular and sinoatrial nodes, and activation of A1R in these areas causes bradycardia. Therefore, complete activation of A1R is unsuitable for pain control under systemic administration because it can cause a severe atrioventricular block. On the other hand, using partial agonists for A1R may be a viable option, but it still faces the challenge of cardiovascular risk and insufficient efficacy. From a receptor perspective, maximizing the reduction of adverse reactions while maintaining efficacy is a challenge because the four subtypes of adenosine receptors have high homology in their orthosteric site sequence, making it difficult to develop selective agonists. Combining agonists of A1R's allosteric site with endogenous adenosine can achieve disease-specific selectivity. Recently, several studies on positive allosteric modulators of A1R have been published, which have revitalized this analgesic target. In the following sections, we will describe the progress of positive allosteric modulators of A1R in detail, and propose future research directions based on this.
4. Allosteric therapies for neuropathic pain
4.1. Allosteric modulators targeting the μ-OR alleviate neuropathic pain
Early studies indicate that the analgesic effects of opioids are mediated through the G-protein signaling pathway of μOR, while their side effects are caused by the arrestin signaling pathway71,115. In 2020, the FDA approved Oliceridine (TRV130), the first analgesic drug designed to target μOR based on the concept of G-protein bias, for the treatment of moderate to severe pain. Oliceridine exhibited 6.25-fold higher analgesic efficacy than morphine. However, the β-arrestin2 recruitment efficacy induced by oliceridine is only 14% of that induced by morphine116. Mori et al.122 re-examined the pharmacological profile of oliceridine and demonstrated that it relieved CCI-induced thermal nociceptive hyperalgesia without producing tolerance after being administered only four times intermittently over a 28-day period. However, data on the efficacy and side effects of oliceridine in patients with clinical neuropathic pain are lacking.
The molecular mechanisms responsible for the biased signaling induced by biased ligands in most GPCRs have remained unclear, hampering progress in the discovery of biased molecular structures for therapeutically important targets like opioid receptors. Cryo-EM single particle analysis was used by Xu et al.117 to obtain the structures of DAMGO, morphine, fentanyl, SR17018, oliceridine and PZM21 binding μOR. Comparative analysis revealed that SR17018 and PZM21, which are two ligands with almost no blocker recruitment activity, weakly interact with TM6 residues and adopt a pose away from TM7 without any direct interaction with TM7 residues. Oliceridine, which retains the weak β-arrestin signaling ability of the ligand, adopts a binding pose similar to SR17018 and PZM21, and its pyridine ring forms an additional hydrophobic interaction with TM6/7. DAMGO, fentanyl, and morphine-binding μ-OR structures exhibit stable contacts with TM6 and TM7. W295A in TM6 and W320A in TM7 mutations have a significant impact on G protein signaling, while cAMP inhibition and G protein recruitment were only minimally or partially affected. Fentanyl, morphine and DAMGO-induced β-arrestin recruitment were almost abolished. A rational design based on the fentanyl structure indicated that ligand and TM6/7 interactions contribute to β-arrestin recruitment, with FBD1 and FBD3 both showing a substantial reduction in β-arrestin recruitment activity after propyl or isopropyl being used to replace the ortho-aniline portion of fentanyl that forms interactions with TM6/7.
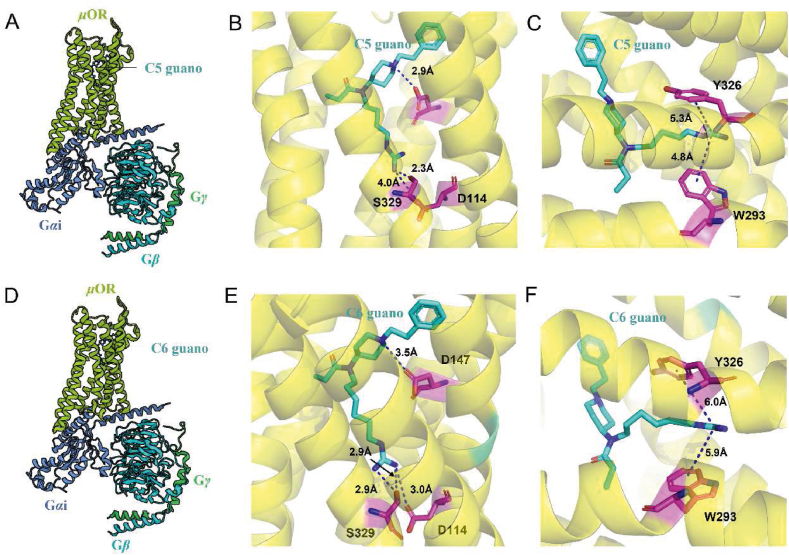
Brian K. Kobilka's team designed C5 guano and C6 guano118, the first-class bitopic ligand for the μ opioid receptor, using the fentanyl backbone to target the conserved sodium-binding site in μOR (Fig. 7A, D). The Cryo-EM structure also reveals that the shorter C5 linker affords only limited interaction (distance 4.0 Å) between the basic guanidine warhead of C5 guano and the major anchor of the Na+ binding site, the acidic D114 side chain. The absence of interaction with D114 is compensated by the strong hydrogen bond formed between the guanidine warhead and S329 side chain, which is another key residue in the Na-binding site (Fig. 7B). In comparison to the μOR bound with C5 guano, the Cryo-EM structure of μOR bound with C6 guano exhibits subtle local differences. In the structure of C6 guanidine-bound μOR, the distance between D114 and the guanidine warhead is 3.0 Å, enabling the formation of a direct salt bridge (Fig. 7E). In addition to interactions with polar residues in the Na-binding site, the positive charge of the guanidine warhead of both C5 and C6 guanidine may also engage in weak cation‒π interactions with aromatic residues located in the polar channel, further stabilizing the binding pose (Fig. 7C, F). In vitro activity assays showed that this bitopic ligand acts as a full agonist of μOR, with a significant reduction in its β-arrestin 2 recruitment capacity. C6 guano dose-dependently improved mechanical abnormalities of pain and blunted the aversive mood associated with neuropathic pain in CCI-exposed mice. However, this compound exhibited an accelerated respiratory rate response compared to morphine, and although the mechanism of this effect is unclear, μ-OR knockdown experiments suggest that it is an off-target effect unrelated to μ-OR. All in vivo antinociception experiments with C6 guano required intracerebroventricular administration, presumably because the presence of guanidine groups made it difficult to cross the blood‒brain barrier. Nevertheless, this discovery may be applicable to the development of bitopic allosteric modulators for other class A GPCRs, as the Na+-binding conformational site is highly conserved in the majority of class A GPCRs119.
Figure 7.
The bitopic ligand, C5 guano and C6 guano, occupies both the orthosteric binding site and the conserved Na + binding site of the μ-opioid receptor (PDB ID: 7U2L, 7U2K). (A) Schematic diagram depicting the C5 guano‒μ-OR-Gαβγ protein complex. (B) and (C) depict the binding modes of the bitopic ligand C5 guano and μ-OR, respectively. (D) Schematic diagram depicting the C6 guano‒μ-OR-Gαβγ protein complex. (E) and (F) depict the binding modes of the bitopic ligand C6 guano and μ-OR, respectively. Hydrogen bond interactions are shown in blue dashed lines, while cation‒π interactions are depicted in black dashed lines.
Several recent studies have challenged the “Arrestin-adverse reaction” hypothesis and suggest that neurotoxic side effects, such as respiratory depression, are not related to arrestin signaling but rather depend on the degree of G protein activation120. Evidence supporting this challenge comes from transgenic mice established by Kliewer et al.121 who mutated all phosphorylation sites on the C-tail of the μ-opioid receptor to alanine, rendering it unable to be phosphorylated by GRK and recruit β-arrestin. They found that fentanyl still induced respiratory depression, constipation and opioid withdrawal symptoms. Surprisingly, fentanyl's analgesic effect was strongly enhanced while analgesic tolerance was greatly diminished. Furthermore, μ-OR did not show any form of internalization under in vitro treatment with 10 μmol/L123. However, oliceridine, which is also a μ-OR agonist, produced adverse effects of constipation and respiratory depression compared to the reduced adverse effects of morphine in clinical trials. These results suggest that the β-arrestin pathway does not fully mediate the adverse reactions produced by μ opioid receptor agonists. For μ-OR, a weakness of biased agonism is that the molecular mechanism of β-arrestin2-mediated respiratory depression has not been demonstrated. The activation of μ-OR by DAMGO or fentanyl drives GIRKs activation through Gαi/o protein signaling, leading to the inhibition of rhythmic respiration123. Other evidence suggests that mice with GIRK2 protein deficiency in the ventral lateral medulla do not show respiratory depression after systemic administration of fentanyl or local administration of DAMGO120,124. In most GPCRs, including the μ-OR, the numerous physiological functions have not been mapped to clear signaling pathways for G proteins or β-arrestin, and ongoing studies of molecular signaling in GPCRs remain a challenge.
4.2. Allosteric modulators targeting the CBR alleviate neuropathic pain
The endocannabinoid system comprises of cannabinoid receptors, CB1R and CB2R, endocannabinoids, such as anandamide and 2-arachidonic acid glycerol, and enzymes responsible for endocannabinoid synthesis and degradation125. This system plays a significant role in regulating neurotransmitter release in the CNS. Activating cannabinoid receptors can be a promising therapeutic strategy for treating a variety of diseases, including neuropathic pain. However, drugs targeting the orthosteric site of the cannabinoid receptor are associated with psychoactive side effects, physical dependence, and abuse. There is a crucial need to develop safer and more effective therapeutic strategies to exploit the benefits of cannabinoid receptor activation. One such strategy is to use PAMs that target allosteric sites on cannabinoid receptors, as they provide an opportunity to enhance the therapeutic potential of cannabinoid receptor activation126,127.
Laprairie et al.128 synthesized GAT211 to alleviate paclitaxel-induced mechanical and cold allodynia in mice with neuropathic pain in a dose-dependent manner. GAT211 did not inhibit paclitaxel-induced mechanical and cold allodynia in CB1R−/− mice. Moreover, the application of CB1R antagonists following a 19-day long-term treatment with GAT211 did not produce tolerance, withdrawal syndrome, or physical dependence caused by orthosteric cannabinoid agonists. Intriguingly, GAT211 exhibited a synergistic effect with morphine in producing anti-allodynia effects and helped prevent the development of morphine tolerance129. Radioligand receptor binding assays conducted on mouse brain membranes showed that GAT211 did not displace human CB1R or CB1R-bound [3H]CP55940, but rather slowed the dissociation kinetics of [3H]CP55940 binding, indicating that it behaves as a PAM128. GAT211 is a racemic compound derived from 2-phenylindole with a chiral center. The enantiomers, GAT228 (R-(+) enantiomer) and GAT229 (S-(−) enantiomer), exhibit distinct allosteric regulatory properties towards CB1R in vitro. GAT211 exhibited properties consistent with both positive allosteric modulation and partial agonist activity of CB1R through an allosteric site, acting as an ago-PAM. Pharmacological profiling of the chiral separated enantiomers exclusively attributed the allosteric agonist activity to GAT228 (R-(+) enantiomer), while GAT211's potent PAM activity was attributed to GAT229 (S-(−) enantiomer)130. Jose Mitjavila and colleagues131 demonstrated that the application of GAT229 alone did not elicit excitatory postsynaptic currents mediated by CB1R, whereas GAT228 did. Moreover, in the presence of an endogenous agonist, GAT229 induces stronger excitatory postsynaptic currents than GAT211. These findings further indicate that GAT228 functions as an ago-PAM (positive allosteric modulator) and GAT229 acts as a PAM for CB1R regulation. It is noteworthy that GAT229 does not exhibit probe dependence, which is relatively uncommon since previously reported more potent modulators of CB1R, such as Org27569 and PSNCBAM-1, both displayed probe dependence132. This intriguing observation warrants further investigation into the molecular mechanism of GAT229 as a PAM lacking probe dependence, which could potentially lead to the development of additional molecular probes or drugs targeting CB1R. From a drug development perspective, PAMs that exhibit stable synergistic effects in the presence of different endogenous ligands may be more easily characterized in future in vivo experiments. ZCZ011, derived from GAT211, is a selective CB1R PAM, that alleviates CCI-induced mechanical and cold allodynia in a dose-dependent manner, without inducing cannabis-like side effects (e.g., rigidity, hypothermia, thermal analgesia, or motor retardation)133. Moreover, in vitro β-arrestin recruitment and ERK phosphorylation assays showed that ZCZ011 enhances AEA-stimulated CB1R signaling133. The chemical structure of ZCZ011 suggested that it is also in the racemic compound. Moreover, there is a possibility to differentiate the PAM properties of ZCZ011 from its ago-PAM properties. Despite the absence of studies testing the allosteric regulatory properties of the enantiomers following the chiral separation of ZCZ011, conducting such a test would yield meaningful results. Several studies have demonstrated that CB2R ligands lack the ability of CB1R agonists to produce psychoactive effects or of selective CB1R antagonists to produce other serious adverse effects, making them promising agents for the treatment of several diseases134, 135, 136. Selective CB2R agonists lacking psychoactive properties could represent another promising treatment option under certain conditions. Gado et al.137 designed and synthesized C2, the first CB2R PAM, which did not affect CB2R receptor signaling in the absence of a CB2R agonist. After oral administration, C2 dose-dependently reversed the oxaliplatin-induced cold allodynia, and pharmacological antagonism demonstrated that this effect was mainly mediated by CB2R.
4.3. Allosteric modulators targeting the mGluRs alleviate neuropathic pain
Numerous studies have confirmed that the dysregulated signaling of group I mGluRs, which contain mGluR1 and mGluR5, is observed in many pathological neural processes, including neuropathic pain. Industry and academic groups have made extensive efforts to identify variant modulators of these two receptors as therapeutic agents for relevant diseases138,139. Niclosamide, characterized as a negative allosteric modulator of Group I mGluRs, exhibits superior selectivity for group I over group III mGluRs, with significant activity in blocking mechanical nociceptive sensitization associated with nerve injury140. Interestingly, Compound 9, the carbamate derivative of niclosamide, produced anti-mechanical nociceptive hypersensitivity in vivo but was inactive against both mGluR1 and mGluR5 in vitro. Compound 9 is released by hydrolysis reactions to produce biologically active niclosamide in vivo140.
Gentaroh Suzuki and colleagues first characterized MMPIP in vitro as a negative allosteric modulator of mGluR7. Subsequently, Palazzo et al.141 tested MMPIP's pharmacological activity in vivo by administering subcutaneous injections of the compound to SNI mice. This resulted in reduced thermal hyperalgesia, mechanical allodynia, anxiety and depression-like behavior, and improved cognitive performance, without altering pain thresholds or affective and cognitive performance in control mice. When MMPIP is delivered systemically, other sites of action should be considered, as they may affect the overall impact of the treatment, depending on the density, location, and cell expression of mGluR7. In SNI mice, another selective mGluR7 NAM, XAP044, also reduced thermal hyperalgesia and mechanical allodynia, further supporting the use of systemic mGluR7 blockers for analgesia in neuropathic pain141. MMPIP also demonstrated antidepressant and anxiolytic-like effects in SNI mice. However, it is difficult to distinguish whether the affective responses are secondary to analgesia because pain and affective disorders share common neural pathways and neurotransmitters. In contrast to MMPIP and XAP044, intrathecal administration of AMN082 (a mGluR7 PAM) had anti-mechanical allodynia and anti-thermal hyperalgesia in paclitaxel-induced neuropathic pain142. The contradictory findings may be explained by several factors, including the under-characterization of the allosteric modulation of either the NAM or PAM of mGluR7 and variations in the estimates of the functional affinity of the radioligand receptor binding assay for allosteric modulators, which may depend on the signaling pathway being measured (i). Another possibility is that AMN082 can promote the internalization of mGluR7, leading to results similar to the antagonistic effect (ii). Additionally, the AMN082 metabolite inhibits serotonin and norepinephrine transporters143, suggesting that its in vivo effect should be interpreted with caution because it may involve mechanisms other than mGluR7 stimulation.
Hua Wang and colleagues discovered that the expression of mGluR4 was significantly reduced in the superficial dorsal horn of the spinal cord 7 days after spinal nerve ligation (SNL) surgery, but not mGluR7144. This might be due to increased glutamate release from primary afferent nerves after nerve ligation, which could lead to the internalization or degradation of mGluR4 expression at presynaptic sites in response to overactivation. In their study, the authors found that intrathecal administration of VU0155041, a PAM of mGluR4, dose-dependently alleviated SNL-induced mechanical nociceptive hypersensitivity, with no effect observed in healthy animals143. Interestingly, the use of AMN082, a PAM of mGluR7, showed no significant analgesic effect, which contradicts the study by Dolan and colleagues. The scientific explanation for this contradiction has been described previously. A known PAM of mGluR4, PHCCC, has demonstrated therapeutic effects in a dose-dependent manner in CCI-induced mechanical hypersensitivity, although it does not produce complete analgesic effects145. Importantly, PHCCC does not alter the baseline thresholds in healthy rodents145, which may involve expression changes of mGluR4 in the occurrence and development of neuropathic pain. Further research is needed to investigate this disease-adaptive alteration of mGluR4 in the process of neuropathic pain, which could contribute to the discovery of drugs targeting mGluR4.
4.4. Allosteric modulators targeting the A1R alleviate neuropathic pain
The purinergic molecule adenosine is a crucial cytoprotective agent that has the potential to modulate various types of pain, especially through its interaction with the A1 receptor (A1R). A1R is a member of the GPCRs superfamily, which is preferentially coupled to Gαi/Go proteins146,147. While prototypical A1R agonists have analgesic effects, the development of drugs targeting adenosine receptors has failed clinically148. This is due to the difficulties in selectively targeting the A1R subtype as the orthosteric binding sites are highly conserved among the four adenosine receptor subtypes (A1, A2A, A2B, and A3)147. In addition, A1R is widely expressed not only in the CNS but also in the heart and adipose tissue, which may cause dose-limiting side effects such as bradycardia when A1R agonists are used149. To overcome the limitations of orthosteric A1R agonists, the allosteric binding site can be targeted. The allosteric site can exhibit higher selectivity between GPCRs isoforms due to greater sequence variability150,151. Additionally, allosteric drugs offer an additional advantage as the direction and magnitude of the effect exerted by the allosteric ligand on the endogenous orthosteric agonist will also be exerted by the agonist on the modulator due to the reciprocity of communication between the two binding domains150.
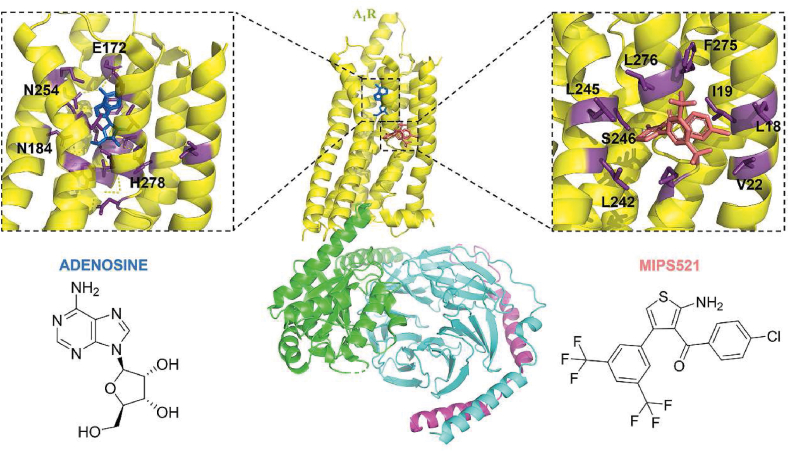
T62 was characterized as a PAM of A1R more than 20 years ago152, 153, 154, and it showed anti-mechanical allodynia in the SNL model. However, a recent study found that oral administration of 100 mg/kg of T62 for four consecutive weeks produced analgesic tolerance and downregulation of A1R in the spinal cord154. On the other hand, Fabrizio Vincenzi et al.160 characterized TRR469 as a PAM for A1R. Radioligand receptor binding experiments showed that TRR469 enhanced agonist radioligand [3H]CCPA binding and induced a dramatic increase in adenosine affinity in mouse spinal cord membranes. TRR469 dose-dependently attenuated STZ-induced mechanical allodynia in mice with neuropathic pain and fully restored pain thresholds to baseline levels. Importantly, TRR469 did not induce motor deficits and tonic responses in mice. Neuropathic pain is associated with increased endogenous adenosine tone in the dorsal horn of the spinal cord, and a significant rise in extracellular adenosine in lamina I. VCP171 was found to inhibit SNL-induced mechanical allodynia in both layer I and layer II neurons. In vivo investigations revealed that intrathecal injection of VCP171 dose-dependently relieved SNL-induced mechanical allodynia without causing sedation. To degrade any extracellular adenosine, adenosine deaminase (ADA) was used in vitro. When VCP171 was administered in the presence of ADA, the evoked excitatory postsynaptic potentials (eEPSCs) were not significantly affected. AMPAR-mediated eEPSCs returned to baseline levels 15 min after ADA perfusion was stopped. This result characterizes VCP171 as a PAM that significantly alleviates mechanical nociceptive hypersensitivity in the SNL model104. Importantly, VCP171 demonstrated a higher affinity for the allosteric site on the A1R than other PAMs, such as T62 and TRR469. MIPS521, on the other hand, has a lower affinity for A1R variant sites than VCP171, but it was more effective than VCP171 in reducing eEPSCs in the spinal cord of nerve-injured animals103. They characterized MIPS521 as a PAM, and cryoelectronic microscopy revealed its binding to a novel allosteric binding pocket on A1R. The novel metastable binding site occupied by the PAM is a shallow pocket formed by the side chains of L242, L245, S246, L276, M283, F275, V22, L18, I19, and is exposed to the lipid interface. This also explains its relatively low affinity (Fig. 8). This is the first PAM of A1R to be characterized by both structural biology and in vitro biological activity.
Figure 8.
Cryo-EM structure of the human A1R-Gαi2 protein complex bound to adenosine (Endogenous agonist) and MIPS521 (PAM) (PDB ID: 7LD3). The black squares showed the orthosteric binding site of adenosine in the central of TMD (left). Adenosine is shown as blue sticks. Residues interacting with adenosine are shown as purple sticks. The black squares showed the allosteric binding site of MIPS521 in the intersection of the TMD and lipid interface (right).
5. Discussion and future direction
The discovery and characterization of allosteric modulators are largely dependent on the precise resolution of protein structures, and identification of allosteric sites is critical for structure-based allosteric drug design. Eutectic structures of some NP-associated proteins and allosteric molecules have been revealed to understand the molecular basis of allosteric modulation. For instance, cryo-electron microscopy discovered a unique allosteric pocket at the extrahelical interface of TM 1,6 and 7 of the A1R103, which may encourage more structure-based, rational allosteric medication designs for neuropathic pain in the future. Therefore, future studies should focus on identifying unique allosteric sites and processes by which modulators and endogenous ligands synergistically stabilize protein dynamics in allosteric modulators and proteins associated with neuropathic pain. The discovery of GPCRs allosteric sites primarily relies on allosteric probes. However, it is worthwhile to explore the accurate identification of allosteric sites that can regulate orthosteric sites from the structure of GPCRs without the use of allosteric probes. Additionally, bitopic allosteric modulators can effectively connect allosteric sites, orthosteric sites, or conserved ion binding sites, resulting in surprising and unpredictable regulatory effects. Summarizing and revealing differences in structural characteristics and signal regulation of existing bitopic allosteric modulators may provide some regularities, but this review does not cover this scope.
The regulation of GPCRs signaling bias is primarily achieved through a unique allosteric mechanism. This allosteric site, also known as a cryptic allosteric modulation site155,156, allows for disease-context-specific selectivity. To understand the mechanism of allosteric regulation and discover the conformational landscape in which proteins are biased to activate, various techniques such as molecular dynamics simulations, site-directed mutagenesis157, and conformational biosensors from computational biology, cell biology, and structural biology must work together158. Although the complexities of signal bias present a challenge for allosteric modulator medicinal-chemistry campaigns, pharmacology must take on the challenge of translating allosteric modulators into the most effective and minimally toxic therapeutics.
As neuropathic pain is a continuously progressing condition that requires long-term management, the therapeutic advantages and safety profiles of any treatment alternatives are equally important. Therefore, the safety of allosteric modulators reported in preclinical trials needs further investigation in human clinical trials. While the current allosteric treatment of neuropathic pain has been successful in laboratory settings, transitioning from in vitro/in vivo laboratory experiments to human trials requires a significant step forward. This includes the development of pharmacokinetics, pharmacology, toxicology, and formulation of allosteric drugs in preclinical trials.
This review summarized the efficacy profiles of pharmacotherapeutic candidates currently in preclinical trials for the recent advancements in allosteric therapy of neuropathic pain. Allosteric drugs targeting nociceptive pathway proteins have demonstrated efficacy and lack of significant off-target adverse effects. This provided a new paradigm for the development of novel drugs for neuropathic pain.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (No. 81925034 and No. 22237005), the Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-01-E00036, China), the Key Research and Development Program of Ningxia Hui Autonomous Region (No. 2022CMG01002, China), the innovative research team of high-level local universities in Shanghai (Nos. SSMU-ZLCX20180702 and SHSMU-ZDCX20212700, China), the Natural Science Foundation of Ningxia (Nos. 2022AAC02029 and 2021AAC03139, China), the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (No. SN-ZJU-SIAS-007, China), the open fund of state key laboratory of Pharmaceutical Biotechnology, Nanjing University (No. KF-202204, China).
Author contributions
Chunhao Zhu contributed to the conception and preparation of the manuscript; Xiaobing Lan participated in written the manuscript; Zhiqiang Wei jointly drafted the manuscript via a literature survey and made the figures; Jianqiang Yu revised the manuscript; Jian Zhang supervised the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Zhiqiang Wei, Email: weizhiqiang@ouc.edu.cn.
Jianqiang Yu, Email: yujq910315@163.com.
Jian Zhang, Email: jian.zhang@sjtu.edu.cn.
References
- 1.IASP-Pain.org [homepage on the Internet]. Washington: International Association for th-e Study of Pain; [updated 2021; cited 2022 June 9]. Available from: http://www.iasp-pain.org/Education/Content.aspx? ItemNumber=1698&navItemNumber=576. 2014.
- 2.Attal N., Lanteri-Minet M., Laurent B., Fermanian J., Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–2843. doi: 10.1016/j.pain.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Doth A.H., Hansson P.T., Jensen M.P., Taylor R.S. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149:338–344. doi: 10.1016/j.pain.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 4.van Hecke O., Austin S.K., Khan R.A., Smith B.H., Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhu C., Liu N., Tian M., Ma L., Yang J., Lan X., et al. Effects of alkaloids on peripheral neuropathic pain: a review. Chin Med. 2020;15:106. doi: 10.1186/s13020-020-00387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attal N., Cruccu G., Baron R., Haanpää M., Hansson P., Jensen T.S., et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113. doi: 10.1111/j.1468-1331.2010.02999.x. e88. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin R.H., O'Connor A.B., Backonja M., Farrar J.T., Finnerup N.B., Jensen T.S., et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Moulin D., Boulanger A., Clark A.J., Clarke H., Dao T., Finley G.A., et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–335. doi: 10.1155/2014/754693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu A., Weinberg E., Moulin D.E., Clarke H. Pharmacologic management of chronic neuropathic pain: review of the canadian pain society consensus statement. Can Fam Physician. 2017;63:844–852. [PMC free article] [PubMed] [Google Scholar]
- 10.Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onakpoya I.J., Thomas E.T., Lee J.J., Goldacre B., Heneghan C.J. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labianca R., Sarzi-Puttini P., Zuccaro S.M., Cherubino P., Vellucci R., Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Invest. 2012;32(Suppl 1):53–63. doi: 10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Varrassi G., Müller-Schwefe G., Pergolizzi J., Orónska A., Morlion B., Mavrocordatos P., et al. Pharmacological treatment of chronic pain―the need for CHANGE. Curr Med Res Opin. 2010;26:1231–1245. doi: 10.1185/03007991003689175. [DOI] [PubMed] [Google Scholar]
- 14.Yang D., Zhou Q., Labroska V., Qin S., Darbalaei S., Wu Y., et al. G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduct Targeted Ther. 2021;6:7. doi: 10.1038/s41392-020-00435-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y., Tong J., Ding K., Zhou Q., Zhao S. GPCR allosteric modulator discovery. Adv Exp Med Biol. 2019;1163:225–251. doi: 10.1007/978-981-13-8719-7_10. [DOI] [PubMed] [Google Scholar]
- 16.Nygaard R., Frimurer T.M., Holst B., Rosenkilde M.M., Schwartz T.W. Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci. 2009;30:249–259. doi: 10.1016/j.tips.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Ballesteros J., Weinstein H. Integrated methods for modeling G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 18.Dror R.O., Arlow D.H., Maragakis P., Mildorf T.J., Pan A.C., Xu H., et al. Activation mechanism of the β2-adrenergic receptor. Proc Natl Acad Sci U S A. 2011;108:18684–18689. doi: 10.1073/pnas.1110499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatakrishnan A.J., Deupi X., Lebon G., Heydenreich F.M., Flock T., Miljus T., et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature. 2016;536:484–487. doi: 10.1038/nature19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F., Guo L., Li Y., Wang G., Wang J., Zhang C., et al. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021;600:164–169. doi: 10.1038/s41586-021-04077-y. [DOI] [PubMed] [Google Scholar]
- 21.Xiao P., Yan W., Gou L., Zhong Y.N., Kong L., Wu C., et al. Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell. 2021;184:943–956. doi: 10.1016/j.cell.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christopoulos A., Changeux J.P., Catterall W.A., Fabbro D., Burris T.P., Cidlowski J.A., et al. International Union of Basic and Clinical Pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev. 2014;66:918–947. doi: 10.1124/pr.114.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenakin T., Strachan R.T. PAM-Antagonists: a better way to block pathological receptor signaling? Trends Pharmacol Sci. 2018;39:748–765. doi: 10.1016/j.tips.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Conn P.J., Christopoulos A., Lindsley C.W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D., Immadi S.S., Wu Z., Kendall D.A. Translational potential of allosteric modulators targeting the cannabinoid CB(1) receptor. Acta Pharmacol Sin. 2019;40:324–335. doi: 10.1038/s41401-018-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill J.K., Dhankher P., Sheppard T.D., Sher E., Millar N.S. A series of α7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol Pharmacol. 2012;81:710–718. doi: 10.1124/mol.111.076026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thakur G.A., Kulkarni A.R., Deschamps J.R., Papke R.L. Expeditious synthesis, enantiomeric resolution, and enantiomer functional characterization of (4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-TQS): an allosteric agonist-positive allosteric modulator of α7 nicotinic acetylcholine receptors. J Med Chem. 2013;56:8943–8947. doi: 10.1021/jm401267t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antony J., Kellershohn K., Mohr-Andrä M., Kebig A., Prilla S., Muth M., et al. Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. Faseb J. 2009;23:442–450. doi: 10.1096/fj.08-114751. [DOI] [PubMed] [Google Scholar]
- 29.Valant C., Gregory K.J., Hall N.E., Scammells P.J., Lew M.J., Sexton P.M., et al. A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J Biol Chem. 2008;283:29312–29321. doi: 10.1074/jbc.M803801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez A.L., Nong Y., Sekaran N.K., Alagille D., Tamagnan G.D., Conn P.J. A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol Pharmacol. 2005;68:1793–1802. doi: 10.1124/mol.105.016139. [DOI] [PubMed] [Google Scholar]
- 31.Mohr K., Tränkle C., Kostenis E., Barocelli E., De Amici M., Holzgrabe U. Rational design of dualsteric GPCR ligands: quests and promise. Br J Pharmacol. 2010;159:997–1008. doi: 10.1111/j.1476-5381.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valant C., Robert Lane J., Sexton P.M., Christopoulos A. The best of both worlds? Bitopic orthosteric/allosteric ligands of g protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2012;52:153–178. doi: 10.1146/annurev-pharmtox-010611-134514. [DOI] [PubMed] [Google Scholar]
- 33.Valant C., May L.T., Aurelio L., Chuo C.H., White P.J., Baltos J.A., et al. Separation of on-target efficacy from adverse effects through rational design of a bitopic adenosine receptor agonist. Proc Natl Acad Sci U S A. 2014;111:4614–4619. doi: 10.1073/pnas.1320962111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu S., Zhang J. Small molecule allosteric modulators of G-protein-coupled receptors: drug‒target interactions. J Med Chem. 2019;62:24–45. doi: 10.1021/acs.jmedchem.7b01844. [DOI] [PubMed] [Google Scholar]
- 35.Lu S., Chen Y., Wei J., Zhao M., Ni D., He X., et al. Mechanism of allosteric activation of SIRT6 revealed by the action of rationally designed activators. Acta Pharm Sin B. 2021;11:1355–1361. doi: 10.1016/j.apsb.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazareno S., Dolezal V., Popham A., Birdsall N.J. Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol Pharmacol. 2004;65:257–266. doi: 10.1124/mol.65.1.257. [DOI] [PubMed] [Google Scholar]
- 37.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 38.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 39.Leach K., Sexton P.M., Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kenakin T., Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 41.Wootten D., Christopoulos A., Sexton P.M. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov. 2013;12:630–644. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 42.Gentry P.R., Sexton P.M., Christopoulos A. Novel allosteric modulators of G protein-coupled receptors. J Biol Chem. 2015;290:19478–19488. doi: 10.1074/jbc.R115.662759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauser A.S., Attwood M.M., Rask-Andersen M., Schiöth H.B., Gloriam D.E. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatakrishnan A.J., Deupi X., Lebon G., Tate C.G., Schertler G.F., Babu M.M. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 45.Flock T., Ravarani C.N.J., Sun D., Venkatakrishnan A.J., Kayikci M., Tate C.G., et al. Universal allosteric mechanism for Gα activation by GPCRs. Nature. 2015;524:173–179. doi: 10.1038/nature14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slosky L.M., Bai Y., Toth K., Ray C., Rochelle L.K., Badea A., et al. β-Arrestin-biased allosteric modulator of NTSR1 selectively attenuates addictive behaviors. Cell. 2020;181:1364–1379.e14. doi: 10.1016/j.cell.2020.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heydenreich F.M., Plouffe B., Rizk A., Milić D., Zhou J., Breton B., et al. Michaelis-menten quantification of ligand signaling bias applied to the promiscuous vasopressin V2 receptor. Mol Pharmacol. 2022;102:139–149. doi: 10.1124/molpharm.122.000497. [DOI] [PubMed] [Google Scholar]
- 48.Besserer-Offroy É., Brouillette R.L., Lavenus S., Froehlich U., Brumwell A., Murza A., et al. The signaling signature of the neurotensin type 1 receptor with endogenous ligands. Eur J Pharmacol. 2017;805:1–13. doi: 10.1016/j.ejphar.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 49.Changeux J.P. The concept of allosteric interaction and its consequences for the chemistry of the brain. J Biol Chem. 2013;288:26969–26986. doi: 10.1074/jbc.X113.503375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seyedabadi M., Ghahremani M.H., Albert P.R. Biased signaling of G protein coupled receptors (GPCRs): molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol Ther. 2019;200:148–178. doi: 10.1016/j.pharmthera.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y., Mestek A., Liu J., Hurley J.A., Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- 52.Kieffer B.L., Befort K., Gavériaux-Ruff C., Hirth C.G. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1994;91:1193. doi: 10.1073/pnas.91.3.1193-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minami M., Toya T., Katao Y., Maekawa K., Nakamura S., Onogi T., et al. Cloning and expression of a cDNA for the rat kappa-opioid receptor. FEBS Lett. 1993;329:291–295. doi: 10.1016/0014-5793(93)80240-u. [DOI] [PubMed] [Google Scholar]
- 54.Cox B.M., Christie M.J., Devi L., Toll L., Traynor J.R. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol. 2015;172:317–323. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zöllner C., Stein C. Opioids. Handb Exp Pharmacol. 2007;177:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 56.Valentino R.J., Volkow N.D. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018;43:2514–2520. doi: 10.1038/s41386-018-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darcq E., Kieffer B.L. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19:499–514. doi: 10.1038/s41583-018-0028-x. [DOI] [PubMed] [Google Scholar]
- 58.Rubovitch V., Gafni M., Sarne Y. The mu opioid agonist DAMGO stimulates cAMP production in SK-N-SH cells through a PLC-PKC-Ca++ pathway. Brain Res Mol Brain Res. 2003;110:261–266. doi: 10.1016/s0169-328x(02)00656-3. [DOI] [PubMed] [Google Scholar]
- 59.Kaufling J., Veinante P., Pawlowski S.A., Freund-Mercier M.J., Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 60.Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fields H.L., Margolis E.B. Understanding opioid reward. Trends Neurosci. 2015;38:217–225. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasserman D.I., Tan J.M., Kim J.C., Yeomans J.S. Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine-induced locomotion. Eur J Neurosci. 2016;44:1761–1770. doi: 10.1111/ejn.13237. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Catalan M.J., Kaufling J., Georges F., Veinante P., Barrot M. The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience. 2014;282:198–216. doi: 10.1016/j.neuroscience.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 65.Taussig M.J., Kirk D., Wang M.W., Ellis S., Meek K., Marvel J., et al. Idiotype-anti-idiotype interactions of VHIX-coded anti-progesterone and anti-arsonate antibodies. Comparison of passive haemagglutination and radioimmunoassays. Scand J Immunol. 1987;26:267–276. doi: 10.1111/j.1365-3083.1987.tb02260.x. [DOI] [PubMed] [Google Scholar]
- 66.Gondin A.B., Halls M.L., Canals M., Briddon S.J. GRK Mediates μ-Opioid receptor plasma membrane reorganization. Front Mol Neurosci. 2019;12:104. doi: 10.3389/fnmol.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanowitz M., von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- 68.He L., Fong J., von Zastrow M., Whistler J.L. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 69.Grecksch G., Bartzsch K., Widera A., Becker A., Höllt V., Koch T. Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology (Berl) 2006;186:177–184. doi: 10.1007/s00213-006-0365-8. [DOI] [PubMed] [Google Scholar]
- 70.He L., Whistler J.L. An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol. 2005;15:1028–1033. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 71.Raehal K.M., Walker J.K., Bohn L.M. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Therapeut. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 72.Katona I., Freund T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 73.Mátyás F., Urbán G.M., Watanabe M., Mackie K., Zimmer A., Freund T.F., et al. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galve-Roperh I., Chiurchiù V., Díaz-Alonso J., Bari M., Guzmán M., Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013;52:633–650. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P., Di Marzo V., Elphick M.R., et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB₁ and CB₂. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson R.I., Nicoll R.A. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 77.La Porta C., Bura S.A., Aracil-Fernández A., Manzanares J., Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain. 2013;154:160–174. doi: 10.1016/j.pain.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Racz I., Nadal X., Alferink J., Baños J.E., Rehnelt J., Martín M., et al. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J Neurosci. 2008;28:12125–12135. doi: 10.1523/JNEUROSCI.3400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaughan C.W., Connor M., Bagley E.E., Christie M.J. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- 80.Marrone M.C., Morabito A., Giustizieri M., Chiurchiù V., Leuti A., Mattioli M., et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat Commun. 2017;8 doi: 10.1038/ncomms15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chávez A.E., Chiu C.Q., Castillo P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson R.I., Nicoll R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 83.Cassano T., Calcagnini S., Pace L., De Marco F., Romano A., Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aymerich M.S., Aso E., Abellanas M.A., Tolon R.M., Ramos J.A., Ferrer I., et al. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol. 2018;157:67–84. doi: 10.1016/j.bcp.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Stampanoni Bassi M., Gentile A., Iezzi E., Zagaglia S., Musella A., Simonelli I., et al. Transient receptor potential vanilloid 1 modulates central inflammation in multiple sclerosis. Front Neurol. 2019;10:30. doi: 10.3389/fneur.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlosburg J.E., Blankman J.L., Long J.Z., Nomura D.K., Pan B., Kinsey S.G., et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Racz I., Nadal X., Alferink J., Baños J.E., Rehnelt J., Martín M., et al. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J Neurosci. 2008;28:12136–12145. doi: 10.1523/JNEUROSCI.3402-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boychuk D.G., Goddard G., Mauro G., Orellana M.F. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29:7–14. doi: 10.11607/ofph.1274. [DOI] [PubMed] [Google Scholar]
- 90.Hill K.P. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 91.Chiechio S., Copani A., Melchiorri D., Canudas A.M., Storto M., Calvani M., et al. Metabotropic receptors as targets for drugs of potential use in the treatment of neuropathic pain. J Endocrinol Invest. 2004;27:171–176. [PubMed] [Google Scholar]
- 92.Xie J.D., Chen S.R., Pan H.L. Presynaptic mGluR5 receptor controls glutamatergic input through protein kinase C-NMDA receptors in paclitaxel-induced neuropathic pain. J Biol Chem. 2017;292:20644–20654. doi: 10.1074/jbc.M117.818476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vincent K., Wang S.F., Laferrière A., Kumar N., Coderre T.J. Spinal intracellular metabotropic glutamate receptor 5 (mGluR5) contributes to pain and c-fos expression in a rat model of inflammatory pain. Pain. 2017;158:705–716. doi: 10.1097/j.pain.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 94.Li J.Q., Chen S.R., Chen H., Cai Y.Q., Pan H.L. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem. 2010;112:162–172. doi: 10.1111/j.1471-4159.2009.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nicoletti F., Bockaert J., Collingridge G.L., Conn P.J., Ferraguti F., Schoepp D.D., et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niswender C.M., Conn P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hermans E., Challiss R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mills C.D., Fullwood S.D., Hulsebosch C.E. Changes in metabotropic glutamate receptor expression following spinal cord injury. Exp Neurol. 2001;170:244–257. doi: 10.1006/exnr.2001.7721. [DOI] [PubMed] [Google Scholar]
- 99.Dogrul A., Ossipov M.H., Lai J., Malan T.P., Jr., Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000;292:115–118. doi: 10.1016/s0304-3940(00)01458-0. [DOI] [PubMed] [Google Scholar]
- 100.Cui L., Kim Y.R., Kim H.Y., Lee S.C., Shin H.S., Szabó G., et al. Modulation of synaptic transmission from primary afferents to spinal substantia gelatinosa neurons by group III mGluRs in GAD65-EGFP transgenic mice. J Neurophysiol. 2011;105:1102–1111. doi: 10.1152/jn.00108.2010. [DOI] [PubMed] [Google Scholar]
- 101.Vilar B., Busserolles J., Ling B., Laffray S., Ulmann L., Malhaire F., et al. Alleviating pain hypersensitivity through activation of type 4 metabotropic glutamate receptor. J Neurosci. 2013;33:18951–18965. doi: 10.1523/JNEUROSCI.1221-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carruthers A.M., Sellers L.A., Jenkins D.W., Jarvie E.M., Feniuk W., Humphrey P.P. Adenosine A(1) receptor-mediated inhibition of protein kinase a-induced calcitonin gene-related peptide release from rat trigeminal neurons. Mol Pharmacol. 2001;59:1533–1541. doi: 10.1124/mol.59.6.1533. [DOI] [PubMed] [Google Scholar]
- 103.Draper-Joyce C.J., Bhola R., Wang J., Bhattarai A., Nguyen A.T.N., Cowie-Kent I., et al. Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature. 2021;597:571–576. doi: 10.1038/s41586-021-03897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imlach W.L., Bhola R.F., May L.T., Christopoulos A., Christie M.J. A positive allosteric modulator of the adenosine A1 receptor selectively inhibits primary afferent synaptic transmission in a neuropathic pain model. Mol Pharmacol. 2015;88:460–468. doi: 10.1124/mol.115.099499. [DOI] [PubMed] [Google Scholar]
- 105.Dickenson A.H., Suzuki R., Reeve A.J. Adenosine as a potential analgesic target in inflammatory and neuropathic pains. CNS Drugs. 2000;13:77–85. [Google Scholar]
- 106.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 107.Bantel C., Li X., Eisenach J.C. Intraspinal adenosine induces spinal cord norepinephrine release in spinal nerve-ligated rats but not in normal or sham controls. Anesthesiology. 2003;98:1461–1466. doi: 10.1097/00000542-200306000-00024. [DOI] [PubMed] [Google Scholar]
- 108.Gomes J.A., Li X., Pan H.L., Eisenach J.C. Intrathecal adenosine interacts with a spinal noradrenergic system to produce antinociception in nerve-injured rats. Anesthesiology. 1999;91:1072–1079. doi: 10.1097/00000542-199910000-00028. [DOI] [PubMed] [Google Scholar]
- 109.Lao L.J., Kumamoto E., Luo C., Furue H., Yoshimura M. Adenosine inhibits excitatory transmission to substantia gelatinosa neurons of the adult rat spinal cord through the activation of presynaptic A(1) adenosine receptor. Pain. 2001;94:315–324. doi: 10.1016/S0304-3959(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 110.Patel M.K., Pinnock R.D., Lee K. Adenosine exerts multiple effects in dorsal horn neurones of the adult rat spinal cord. Brain Res. 2001;920:19–26. doi: 10.1016/s0006-8993(01)02844-x. [DOI] [PubMed] [Google Scholar]
- 111.Sawynok J., Sweeney M.I. The role of purines in nociception. Neuroscience. 1989;32:557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- 112.Lind G., Schechtmann G., Winter J., Linderoth B. Drug-enhanced spinal stimulation for pain: a new strategy. Acta Neurochir Suppl. 2007;97:57–63. doi: 10.1007/978-3-211-33079-1_7. [DOI] [PubMed] [Google Scholar]
- 113.Eisenach J.C., Hood D.D., Curry R., Sawynok J., Yaksh T.L., Li X. Intrathecal but not intravenous opioids release adenosine from the spinal cord. J Pain. 2004;5:64–68. doi: 10.1016/j.jpain.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 114.Taiwo Y.O., Levine J.D. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38:757–762. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- 115.DeWire S.M., Yamashita D.S., Rominger D.H., Liu G., Cowan C.L., Graczyk T.M., et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Therapeut. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 116.Markham A. Oliceridine: first approval. Drugs. 2020;80:1739–1744. doi: 10.1007/s40265-020-01414-9. [DOI] [PubMed] [Google Scholar]
- 117.Zhuang Y., Wang Y., He B., He X., Zhou X.E., Guo S., et al. Molecular recognition of morphine and fentanyl by the human μ-opioid receptor. Cell. 2022;185:4361–4375.e19. doi: 10.1016/j.cell.2022.09.041. [DOI] [PubMed] [Google Scholar]
- 118.Faouzi A., Wang H., Zaidi S.A., DiBerto J.F., Che T., Qu Q., et al. Structure-based design of bitopic ligands for the μ-opioid receptor. Nature. 2023;613:767–774. doi: 10.1038/s41586-022-05588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zarzycka B., Zaidi S.A., Roth B.L., Katritch V. Harnessing ion-binding sites for GPCR pharmacology. Pharmacol Rev. 2019;71:571–595. doi: 10.1124/pr.119.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kliewer A., Gillis A., Hill R., Schmiedel F., Bailey C., Kelly E., et al. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol. 2020;177:2923–2931. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kliewer A., Schmiedel F., Sianati S., Bailey A., Bateman J.T., Levitt E.S., et al. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun. 2019;10:367. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mori T., Takemura Y., Arima T., Iwase Y., Narita M., Miyano K., et al. Further investigation of the rapid-onset and short-duration action of the G protein-biased μ-ligand oliceridine. Biochem Biophys Res Commun. 2021;534:988–994. doi: 10.1016/j.bbrc.2020.10.053. [DOI] [PubMed] [Google Scholar]
- 123.Montandon G., Ren J., Victoria N.C., Liu H., Wickman K., Greer J.J., et al. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology. 2016;124:641–650. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levitt E.S., Abdala A.P., Paton J.F., Bissonnette J.M., Williams J.T. μ Opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J Physiol. 2015;593:4453–4469. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu H.C., Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatr. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dopart R., Lu D., Lichtman A.H., Kendall D.A. Allosteric modulators of cannabinoid receptor 1: developing compounds for improved specificity. Drug Metab Rev. 2018;50:3–13. doi: 10.1080/03602532.2018.1428342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alaverdashvili M., Laprairie R.B. The future of type 1 cannabinoid receptor allosteric ligands. Drug Metab Rev. 2018;50:14–25. doi: 10.1080/03602532.2018.1428341. [DOI] [PubMed] [Google Scholar]
- 128.Slivicki R.A., Xu Z., Kulkarni P.M., Pertwee R.G., Mackie K., Thakur G.A., et al. Positive allosteric modulation of cannabinoid receptor type 1 suppresses pathological pain without producing tolerance or dependence. Biol Psychiatr. 2018;84:722–733. doi: 10.1016/j.biopsych.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Slivicki R.A., Iyer V., Mali S.S., Garai S., Thakur G.A., Crystal J.D., et al. Positive allosteric modulation of CB(1) cannabinoid receptor signaling enhances morphine antinociception and attenuates morphine tolerance without enhancing morphine-induced dependence or reward. Front Mol Neurosci. 2020;13:54. doi: 10.3389/fnmol.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laprairie R.B., Kulkarni P.M., Deschamps J.R., Kelly M.E.M., Janero D.R., Cascio M.G., et al. Enantiospecific allosteric modulation of cannabinoid 1 receptor. ACS Chem Neurosci. 2017;8:1188–1203. doi: 10.1021/acschemneuro.6b00310. [DOI] [PubMed] [Google Scholar]
- 131.Mitjavila J., Yin D., Kulkarni P.M., Zanato C., Thakur G.A., Ross R., et al. Enantiomer-specific positive allosteric modulation of CB1 signaling in autaptic hippocampal neurons. Pharmacol Res. 2018;129:475–481. doi: 10.1016/j.phrs.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baillie G.L., Horswill J.G., Anavi-Goffer S., Reggio P.H., Bolognini D., Abood M.E., et al. CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol Pharmacol. 2013;83:322–338. doi: 10.1124/mol.112.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ignatowska-Jankowska B.M., Baillie G.L., Kinsey S., Crowe M., Ghosh S., Owens R.A., et al. A cannabinoid CB1 receptor-positive allosteric modulator reduces neuropathic pain in the mouse with no psychoactive effects. Neuropsychopharmacology. 2015;40:2948–2959. doi: 10.1038/npp.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jourdan T., Szanda G., Rosenberg A.Z., Tam J., Earley B.J., Godlewski G., et al. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A. 2014;111:E5420–E5428. doi: 10.1073/pnas.1419901111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gatta-Cherifi B., Cota D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int J Obes. 2016;40:210–219. doi: 10.1038/ijo.2015.179. [DOI] [PubMed] [Google Scholar]
- 136.Mazier W., Saucisse N., Gatta-Cherifi B., Cota D. The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metabol. 2015;26:524–537. doi: 10.1016/j.tem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 137.Gado F., Di Cesare Mannelli L., Lucarini E., Bertini S., Cappelli E., Digiacomo M., et al. Identification of the first synthetic allosteric modulator of the CB(2) receptors and evidence of its efficacy for neuropathic pain relief. J Med Chem. 2019;62:276–287. doi: 10.1021/acs.jmedchem.8b00368. [DOI] [PubMed] [Google Scholar]
- 138.Trabanco A.A., Cid J.M., Lavreysen H., Macdonald G.J., Tresadern G. Progress in the development of positive allosteric modulators of the metabotropic glutamate receptor 2. Curr Med Chem. 2011;18:47–68. doi: 10.2174/092986711793979706. [DOI] [PubMed] [Google Scholar]
- 139.Li G., Jørgensen M., Campbell B.M. Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009-July 2013) Pharm Pat Anal. 2013;2:767–802. doi: 10.4155/ppa.13.58. [DOI] [PubMed] [Google Scholar]
- 140.Ai N., Wood R.D., Yang E., Welsh W.J. Niclosamide is a negative allosteric modulator of group I metabotropic glutamate receptors: implications for neuropathic pain. Pharm Res (N Y) 2016;33:3044–3056. doi: 10.1007/s11095-016-2027-9. [DOI] [PubMed] [Google Scholar]
- 141.Palazzo E., Romano R., Luongo L., Boccella S., De Gregorio D., Giordano M.E., et al. MMPIP, an mGluR7-selective negative allosteric modulator, alleviates pain and normalizes affective and cognitive behavior in neuropathic mice. Pain. 2015;156:1060–1073. doi: 10.1097/j.pain.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 142.Wang J., Jiang C., Ba X., Yang S., Wu J., Huang Z., et al. Selective activation of metabotropic glutamate receptor 7 blocks paclitaxel-induced acute neuropathic pain and suppresses spinal glial reactivity in rats. Psychopharmacology (Berl) 2021;238:107–119. doi: 10.1007/s00213-020-05662-1. [DOI] [PubMed] [Google Scholar]
- 143.Sukoff Rizzo S.J., Leonard S.K., Gilbert A., Dollings P., Smith D.L., Zhang M.Y., et al. The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise?. J Pharmacol Exp Therapeut. 2011;338:345–352. doi: 10.1124/jpet.110.177378. [DOI] [PubMed] [Google Scholar]
- 144.Wang H., Jiang W., Yang R., Li Y. Spinal metabotropic glutamate receptor 4 is involved in neuropathic pain. Neuroreport. 2011;22:244–248. doi: 10.1097/WNR.0b013e3283453843. [DOI] [PubMed] [Google Scholar]
- 145.Goudet C., Chapuy E., Alloui A., Acher F., Pin J.P., Eschalier A. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain. 2008;137:112–124. doi: 10.1016/j.pain.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 146.Poon A., Sawynok J. Antinociception by adenosine analogs and inhibitors of adenosine metabolism in an inflammatory thermal hyperalgesia model in the rat. Pain. 1998;74:235–245. doi: 10.1016/s0304-3959(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 147.Ribeiro J.A., Sebastião A.M., de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 148.Zylka M.J. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011;17:188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Funakoshi H., Chan T.O., Good J.C., Libonati J.R., Piuhola J., Chen X., et al. Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation. 2006;114:2240–2250. doi: 10.1161/CIRCULATIONAHA.106.620211. [DOI] [PubMed] [Google Scholar]
- 150.May L.T., Leach K., Sexton P.M., Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 151.Christopoulos A., Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 152.Musser B., Mudumbi R.V., Liu J., Olson R.D., Vestal R.E. Adenosine A1 receptor-dependent and -independent effects of the allosteric enhancer PD 81,723. J Pharmacol Exp Therapeut. 1999;288:446–454. [PubMed] [Google Scholar]
- 153.Kourounakis A., Visser C., de Groote M., Ap I.J. Differential effects of the allosteric enhancer (2-amino-4,5-dimethyl-trienyl)[3-trifluoromethyl) phenyl]methanone (PD81,723) on agonist and antagonist binding and function at the human wild-type and a mutant (T277A) adenosine A1 receptor. Biochem Pharmacol. 2001;61:137–144. doi: 10.1016/s0006-2952(00)00536-0. [DOI] [PubMed] [Google Scholar]
- 154.Li X., Bantel C., Conklin D., Childers S.R., Eisenach J.C. Repeated dosing with oral allosteric modulator of adenosine A1 receptor produces tolerance in rats with neuropathic pain. Anesthesiology. 2004;100:956–961. doi: 10.1097/00000542-200404000-00028. [DOI] [PubMed] [Google Scholar]
- 155.Lu S., He X., Yang Z., Chai Z., Zhou S., Wang J., et al. Activation pathway of a G protein-coupled receptor uncovers conformational intermediates as targets for allosteric drug design. Nat Commun. 2021;12:4721. doi: 10.1038/s41467-021-25020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu S., Qiu Y., Ni D., He X., Pu J., Zhang J. Emergence of allosteric drug-resistance mutations: new challenges for allosteric drug discovery. Drug Discov Today. 2020;25:177–184. doi: 10.1016/j.drudis.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 157.Ni D., Li X., He X., Zhang H., Zhang J., Lu S. Drugging K-Ras(G12C) through covalent inhibitors: mission possible?. Pharmacol Ther. 2019;202:1–17. doi: 10.1016/j.pharmthera.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 158.Shao L., Chen Y., Zhang S., Zhang Z., Cao Y., Yang D., et al. Modulating effects of RAMPs on signaling profiles of the glucagon receptor family. Acta Pharm Sin B. 2022;12:637–650. doi: 10.1016/j.apsb.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gee C.E., Peterlik D., Neuhäuser C., Bouhelal R., Kaupmann K., Laue G., et al. Blocking metabotropic glutamate receptor subtype 7 (mGlu7) via the Venus flytrap domain (VFTD) inhibits amygdala plasticity, stress, and anxiety-related behavior. J Biol Chem. 2014;289:10975–10987. doi: 10.1074/jbc.M113.542654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pan H.L., Xu Z., Leung E., Eisenach J.C. Allosteric adenosine modulation to reduce allodynia. Anesthesiology. 2001;95:416–420. doi: 10.1097/00000542-200108000-00025. [DOI] [PubMed] [Google Scholar]
- 161.Vincenzi F., Targa M., Romagnoli R., Merighi S., Gessi S., Baraldi P.G., et al. TRR469, a potent A(1) adenosine receptor allosteric modulator, exhibits anti-nociceptive properties in acute and neuropathic pain models in mice. Neuropharmacology. 2014;81:6–14. doi: 10.1016/j.neuropharm.2014.01.028. [DOI] [PubMed] [Google Scholar]