Abstract
Bioactive compounds derived from herbal medicinal plants modulate various therapeutic targets and signaling pathways associated with cardiovascular diseases (CVDs), the world's primary cause of death. Ginkgo biloba, a well-known traditional Chinese medicine with notable cardiovascular actions, has been used as a cardio- and cerebrovascular therapeutic drug and nutraceutical in Asian countries for centuries. Preclinical studies have shown that ginkgolide B, a bioactive component in Ginkgo biloba, can ameliorate atherosclerosis in cultured vascular cells and disease models. Of clinical relevance, several clinical trials are ongoing or being completed to examine the efficacy and safety of ginkgolide B-related drug preparations in the prevention of cerebrovascular diseases, such as ischemia stroke. Here, we present a comprehensive review of the pharmacological activities, pharmacokinetic characteristics, and mechanisms of action of ginkgolide B in atherosclerosis prevention and therapy. We highlight new molecular targets of ginkgolide B, including nicotinamide adenine dinucleotide phosphate oxidases (NADPH oxidase), lectin-like oxidized LDL receptor-1 (LOX-1), sirtuin 1 (SIRT1), platelet-activating factor (PAF), proprotein convertase subtilisin/kexin type 9 (PCSK9) and others. Finally, we provide an overview and discussion of the therapeutic potential of ginkgolide B and highlight the future perspective of developing ginkgolide B as an effective therapeutic agent for treating atherosclerosis.
Key words: Cardiovascular disease, Atherosclerosis, Ginkgo biloba, Ginkgolide B, Endothelial dysfunction, LOX-1, PCSK9, PAF-R antagonist
Graphical abstract
Ginkgolide B possesses the antiatherosclerotic advantage of “multitarget and multipathway”, which is closely linked to its regulating endothelial dysfunction, thrombosis, leukocyte action, and risk factors.
1. Introduction
Atherosclerotic cardiovascular diseases (CVDs) are the major cause of human morbidity and mortality globally1, 2, 3. Atherosclerosis is a chronic inflammatory disease induced by interactions between endothelial cells, smooth muscle cells, various kinds of circulating blood cells (e.g., platelets, neutrophils, and monocytes), the immune system, and the peripheral nervous system4, 5, 6, 7, 8, 9, 10, 11, 12, 13; these interactions lead to altered vascular cell function and the development of life-threatening atherosclerotic plaques14. Initially, endothelial dysfunction allows low-density lipoprotein cholesterol particles (LDL-C) to infiltrate into the vascular neointima, where they are trapped and retained by atherogenic proteoglycans with modified (hyper-elongated) glycosaminoglycan chains15,16. The trapped LDL-C undergoes oxidative modifications to become oxidized low-density lipoprotein (ox-LDL)17. Monocyte-derived macrophages take up those lipids to form foam cells and transition into the intima to form “fatty streaks” lesions18,19. Finally, with the formation of a necrotic core and a fibrous cap, the lesion develops into an atherosclerotic plaque; plaques can be labile or stable depending upon their composition. Once the high-risk, vulnerable plaque ruptures, it can form a fatal arterial thrombus, eventually provoking tissue ischemia diseases due to insufficient blood supply to vital organs20. Because of the aging of the population worldwide and unhealthy lifestyles, the prevalence of atherosclerosis is increasing21,22. Therefore, there is a great medical need to identify potential drugs to prevent and treat atherosclerosis.
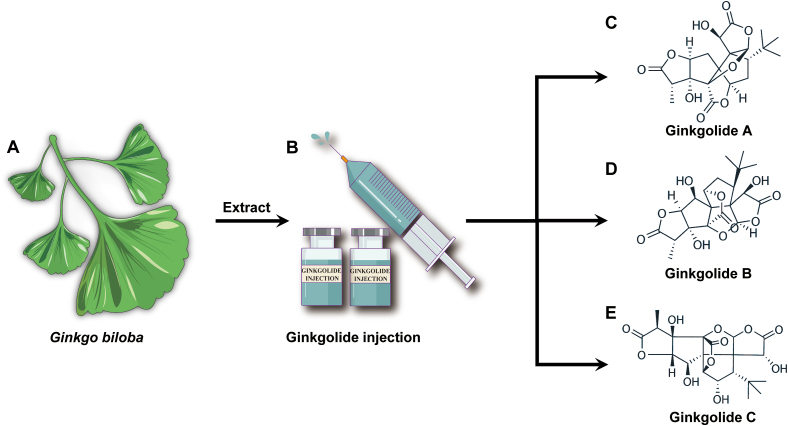
Ginkgo biloba L. (Fig. 1), a valuable herbal preparation from the dried leaves of ginkgo trees, has a medicinal history of over 2000 years and has been considered a “living fossil”. Its extract, which has been commonly used in preventing and treating multiple cardiovascular diseases clinically, has the traditional effects of promoting blood circulation, activating meridians to relieve pain, melting turbidity, and lowering lipids23, 24, 25, 26. Ginkgo biloba standardized extract (EGb 761) mainly contains ginkgo flavonoids, biflavonoids, and ginkgolides, with the latter being the most characteristic component of Ginkgo biloba. At present, ten ginkgolides have been discovered from Ginkgo biloba27, 28, 29, 30, 31, 32, among which ginkgolide A (GA, Fig. 1C), ginkgolide B (GB, Fig. 1D), and ginkgolide C (GC, Fig. 1E) are the primary substances regulating cardiovascular effects in Ginkgo biloba. As one of the most pharmacologically active and representative compounds in ginkgolides, GB can prevent or slow atherosclerosis progression in preclinical in vitro and in vivo models of atherosclerosis, suggesting its therapeutic potential in cardiovascular and cerebrovascular diseases that involve atherosclerosis as the pathological basis33, 34, 35, 36, 37, 38, 39, 40, 41, 42.
Figure 1.
Ginkgo biloba L. and its major active ginkgolide compounds' chemical structures mediate cardiovascular protection actions. (A) Ginkgo biloba is a valuable herbal medicine from the dried leaves of ginkgo trees. (B–E) Ginkgolide injection is a traditional remedy for CVDs, and its main bioactive components are ginkgolide A (PubChem CID# 9909368), ginkgolide B (PubChem CID# 6324617) and ginkgolide C (PubChem CID# 24721502).
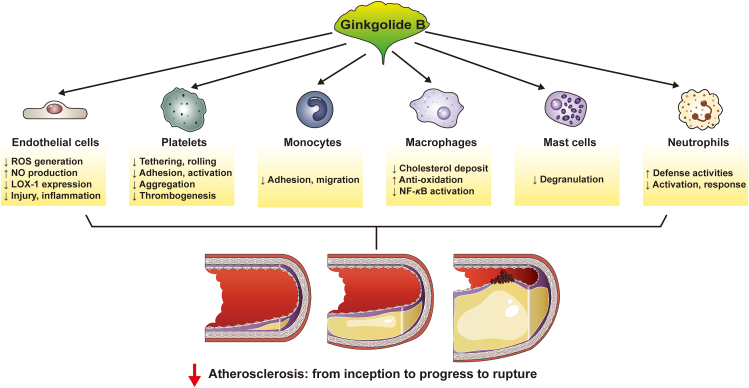
Considering the expanding appreciation of the pharmacological profile of GB in cardio- and cerebrovascular medicine, especially in atherosclerosis, a comprehensive review of the antiatherosclerotic impacts and mechanisms of action of GB is needed. The purpose of this review is to highlight the therapeutic potential of GB in reducing atherosclerosis and its clinical sequelae (ischemia and strokes) by providing an overview of the protective benefits and molecular mechanisms of action of GB in preventing or alleviating atherosclerosis (Fig. 2).
Figure 2.
Ginkgolide B regulates important cellular processes in atherosclerosis. Ginkgolide B inhibits the progression of atherosclerosis via a variety of mechanisms. Ginkgolide B, in particular, corrects endothelial dysfunction, inhibits thrombin and platelet function, inhibits inflammation, reduces macrophage-derived foam cell formation, and so on.
2. Emerging mechanisms of action and cellular targets for the protection against atherosclerotic vascular disorders by GB
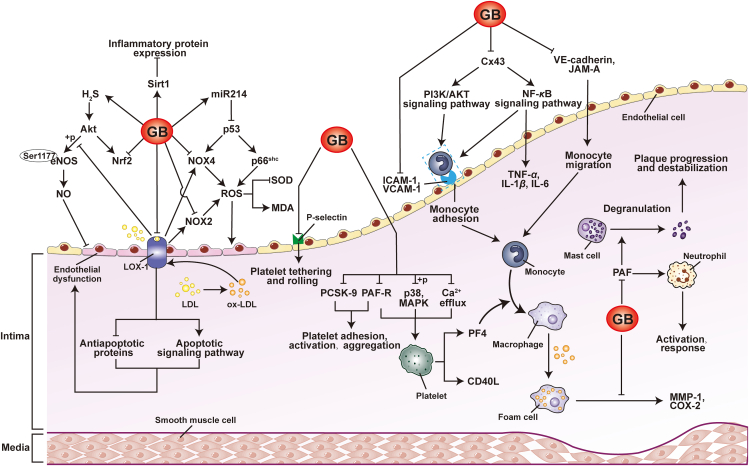
Accumulating evidence has indicated that GB, the natural substance from Ginkgo biloba, possesses atheroprotective actions33,34. In particular, GB exerts antiatherosclerotic effects in various relevant models, including umbilical vein endothelial cells (HUVECs)37,39, human platelets40 and ApoE−/− mice33,34. GB reduces atherosclerosis by altering the functions of multiple vascular cells (Fig. 2) and modulating molecular targets (Fig. 3) associated with atherogenesis. It is noteworthy that GB also has pharmacological effects on myocardial ischemia/reperfusion injury-provoked inflammatory responses in rats by modulating the A20–NF-κB signaling pathway43, suggesting that GB possibly has extra therapeutic advantages in treating coronary atherosclerotic disorders.
Figure 3.
Novel vascular targets of ginkgolide B. Ginkgolide B regulates a range of therapeutic targets during atherosclerosis. Endothelial cells, platelets, monocytes, macrophages, neutrophils, and other cells targeted by ginkgolide B are summarized. The regulatory actions of ginkgolide B on various cellular targets indicate its therapeutic promise in atherosclerotic vascular disorders.
The disease process of atherosclerosis is complex and involves several interconnected processes, such as endothelial cell dysfunction, platelet adhesion and activation, and leukocyte function and activation14,44, 45, 46, 47. Upon biochemical insults to endothelial cells, the expression of inflammatory cytokines is enhanced, which attracts monocytes to congregate at the injury site48, 49, 50. By interacting with the endothelium, monocytes attach to the endothelium surface and promote further inflammation51. After moving into the neointima from the endothelium, monocytes develop into macrophages. By ingesting ox-LDL, these macrophages transform into foam cells, a key component in the development of atherosclerotic plaques52. Additionally, von Willebrand factor (vWF) and collagen, which trigger platelet adhesion, are exposed in injured endothelial cells53,54. For platelets to adhere to the endothelium, vWF and collagen bind to GPIIb/IIIa receptors on the surface of platelets45. Once attached, platelets are activated and then release growth factors and proinflammatory substances while aggregating to form blood clots55. In addition to monocytes, other leukocytes in the circulation are also recruited into the vascular wall at sites of lipid accumulation, which further exacerbates plaque formation and worsens the development of atherosclerosis56,57. Therefore, multiple biochemical and cellular events can be addressed in the treatment and prevention of atherosclerosis to intervene in these steps and mitigate or even prevent the potentially devastating consequences of atherosclerosis and its clinical sequelae. It is worth noting that GB not only plays a protective role in endothelial injury, an early event in atherosclerosis, but also inhibits other links that promote the further development of atherosclerosis, including the adhesion and activation of platelets and the activation of leukocyte function. A key point herein is that GB could orchestrate the complex crosstalk between the injured endothelium, platelets, and leukocytes against atherosclerotic vascular disorders. To demonstrate the orchestration ability of GB more clearly, the parts that follow break these interconnected processes down and provide further details.
2.1. GB corrects endothelial dysfunction
The vascular endothelium, the innermost layer of blood vessels, not only plays a crucial role in regulating endothelium-dependent vasodilation, but also hinders platelet and leukocyte adherence to the artery wall and regulates thrombosis and inflammation58. Endothelial dysfunction, caused by free radicals such as reactive oxygen species (ROS), transforming growth factor-β48,59, high ox-LDL levels, and other risk factors, is an early stage in the development of atherosclerosis.
One central mechanism of alleviating endothelial dysfunction is decreasing the expression and/or activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs)60,61. Abnormal NOX activity results in the overproduction of ROS, specifically superoxide (O2•–) and hydrogen peroxide (H2O2), which impair vascular function by damaging endothelial cells and subsequently promote further endothelial dysfunction62. Since therapeutic strategies based on scavenging ROS using antioxidants have been ineffective in delaying the development of arteriosclerosis in humans, blocking their enzymatic production by inhibiting NOXs may represent an effective strategy for restoring endothelial function and ultimately preventing or treating atherosclerosis61. These findings highlight the critical role of NOXs in the development of endothelial dysfunction and offer a promising avenue for new therapeutic approaches to combat atherosclerosis. Notably, due to its highly conserved histidine residue, NOX4 produces H2O2 rather than O2•– 63,64, and it is expressed at a higher level than the other four members of the NOX family65. In addition, a recent study66 showed that endothelial NOX4 dysfunction aggravates atherosclerosis via endoplasmic reticulum stress, and more importantly, NOX4 was thought to be involved in the incidence and progression of human coronary atherosclerotic disease by increasing intracellular oxidative stress67. Furthermore, NOX4 overexpression was linked to smooth muscle cell growth inhibition, apoptosis, and senescence, factors that contribute to plaque destabilization68. Given these findings, the targeting of NOXs, especially NOX4, with innovative therapeutic agents may hold significant promise for the prevention and treatment of coronary artery diseases. Two independent studies35,42 have demonstrated that GB has a pharmacological effect on intracellular oxidative stress in combination with a reduction in ROS by reducing NOX4 expression. Additionally, GB also significantly elevated SOD activity and decreased the expression of GPX1 protein and NOX269, indicating that GB has potential cardiovascular benefits conferred by its antioxidant effect. Further mechanistic studies indicated that GB suppressed apoptosis and lowered superoxide production by reducing the expression of p53-regulated NOX4 and p66Shc 70. Notably, NOX4-derived H2O2 could activate NOX2, boosting mitochondrial ROS (mtROS) generation via pSer36-p66Shc 71. These findings suggest that the antioxidative mechanism of GB may operate through a complex interplay of the NOX4/NOX2/pSer36-p66Shc/mtROS axis, which highlights the intricate and multifaceted nature of its therapeutic potential for cardiovascular disease. Although the precise antioxidant mechanism of GB is unclear, current research indicates that GB may help mitigate the pathological effects of oxidative stress by targeting this pathway, ultimately offering new avenues for the prevention and treatment of atherosclerotic vascular disorders.
NO, as a dissolved gas, is a key vascular protective factor of the endothelium and regulates many domains of atherosclerotic processes72. Thus, endothelial NO synthase (eNOS)-dependent NO production is another essential mechanism that counteracts endothelial dysfunction and atherosclerosis73, 74, 75. The expression of eNOS can be regulated by different mechanisms, principally through kinase-mediated multisite phosphorylation76,77. Among various phosphorylation sites, Ser1177 is one of the most studied sites associated with endothelial dysfunction44,78,79, and inhibition of its phosphorylation attenuated the H2S-stimulated increase in endothelial NO production80. Wang et al.69 found that GB alleviated endothelial dysfunction in diabetic rats by increasing NO bioavailability and H2S generation, the mechanism of which might be linked to eNOS Ser1177 phosphorylation. Additionally, GB had protective effects on the revascularization of blood vessels by promoting the proliferation and activity of endothelial progenitor cells from bone marrow via activation of the Akt/eNOS and mitogen-activated protein kinase (MAPK)/p38 signaling pathways81, further confirming the regulatory effects of GB on eNOS-dependent endothelial function. These findings provide additional valuable insight into the molecular mechanisms underlying the counteracting actions of GB on endothelial dysfunction and atherosclerosis.
In addition to regulating the generation of ROS and NO, GB also protects the endothelium by downregulating lectin-like oxidized LDL receptor-1 (LOX-1) expression41. LOX-1 is an ox-LDL scavenger receptor with a critical role in endothelial dysfunction and the progression of atherosclerosis82,83. In vitro, ox-LDL-treated increased LOX-1 expression triggers endothelial cell apoptosis by reducing anti-apoptotic proteins (including c-IAP-1 and Bcl-2) and activating pro-apoptotic pathways (containing caspase-3 and caspase-9)84, thereby causing a direct injurious biochemical effect on the endothelium. In addition to this direct effect on endothelial cells, LOX-1 knockdown dramatically lowered ROS levels, which was linked to decreased NOX2 and NOX4 expression85. Moreover, endothelial cell-specific overexpression of LOX-1 blunted eNOS-activating phosphorylation in ApoE−/− mice, suggesting that LOX-1 might induce endothelial dysfunction by reducing NO production82. Thus, targeting LOX-1 may be explored as a novel therapeutic method to correct endothelial dysfunction by influencing multiple targets. Interestingly, GB is capable of decreasing LOX-1 expression. A recent study41 indicated that GB supplementation inhibited ROS production in ox-LDL-activated HUVECs by suppressing LOX-1 and NOX4 expression. In line with this result, Feng et al.35 reported that GB improved endothelial dysfunction by targeting LOX-1, NOX4, monocyte chemotactic protein-1, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and inflammatory cascade-associated markers. In ox-LDL-treated endothelial cells, GB not only suppressed LOX-1 expression but also increased the expression of Sirtuin-1 (Sirt1), a deacetylase with antiaging properties whose mechanism is tied to the inhibition of Akt activation38. Notably, the upstream kinase cyclin-dependent kinase 5 (CDK5) phosphorylates Sirt1, which promotes endothelial cell senescence and atherosclerosis86. Therefore, it will be intriguing to further study whether GB suppresses Sirt1 function in senescent endothelial cells and inhibits atherogenesis by affecting CDK5-mediated phosphorylation of Sirt1.
Although the protective effects of GB on the endothelium have not been extensively investigated, it is possible to speculate that it could act as a multitargeting agent to regulate endothelial function through a variety of mechanisms, including diminished ROS production derived from NOXs, enhanced eNOS-derived NO production, decreased expression of LOX-1, and upregulation of Sirt1 expression. The net result of these actions would be the maintenance of endothelial cell homeostasis. These initial findings concerning the regulation of endothelial function by GB shed new light on the molecular mechanisms that underpin the antiatherosclerotic potential of GB.
2.2. GB alleviates thrombosis by inhibiting platelet activation
Early atherosclerosis is caused by endothelial cell malfunction, and platelets adhere to these cells in vulnerable places45,87. After platelet adherence and aggregation, platelets are activated and secrete chemokines and cytokines, which leads to further aggravation of endothelial cell dysfunction and inflammation88,89. Of utmost significance, platelets are involved in atherosclerotic thrombosis, increasing the likelihood of vascular occlusion that may provoke life-threatening atherosclerotic complications90, 91, 92, 93, such as myocardial infarction and angina pectoris. In summary, the role of platelets in atherosclerosis is to promote endothelial dysfunction, inflammation, and thrombosis. The process by which platelets adhere to the vascular endothelium and promote thrombus formation can be divided into four stages: platelet tethering and rolling, platelet firm adhesion and activation, platelet aggregation, and ultimately thrombus stabilization. GB is an interesting molecule because it can inhibit all of these steps. Below, we explore these actions in detail.
2.2.1. The effect of GB on platelet tethering and rolling
The first step in thrombus formation is mostly regulated by activated platelets- and endotheliocyte-expressed P-selectin (CD62P), whose ligands are P-selectin glycoprotein ligand-1 (PSGL-1) and glycoprotein Ib (GPIb)94, 95, 96, 97. P-selectin is involved in platelet aggregation as well as platelet and leukocyte rolling on activated endothelium98, 99, 100. Besides regulating platelet-endothelial cell interactions, P-selectin also has a major impact on platelet-leukocyte interactions101. Platelet-P-selectin binds to leukocyte-PSGL-1, forming platelet-leukocyte complexes that encourage the production of chemokines (including CCL2 and CCL5) and cytokines [such as interleukin-1β (IL-1β)], ultimately further activating leukocytes and promoting atherosclerosis102.
Despite the complex nature of thrombus formation, there is promise in treating thrombosis and atherosclerosis by blocking P-selectin/PSGL-1 interactions. For example, injection of monoclonal antibodies against P-selectin or PSGL-1 holds potential for treating thrombosis and atherosclerosis103. In aortic plaques in ApoE−/− mice, GB attenuated atherosclerotic lesions and P-selectin by possibly regulating the PI3K/Akt pathway, and interestingly, its effect was similar to that of aspirin, the most promising antiatherosclerosis agent for preventing thrombosis33. A study104 has shown that GB is an inhibitor of P-selectin translocation, which suggests that GB inhibits the transfer of P-selectin from Weibel-Palade bodies of ECs to the cell surface during inflammation. The studies reveal a crucial new insight into the effects of GB on thrombosis, specifically that GB possesses an inhibitory effect on the initial step of thrombosis—the tethering and rolling of platelets—by attenuating P-selectin translocation. These results shed light on one of the mechanisms of action of GB in alleviating thrombosis. Furthermore, there exists a gap in knowledge in this area regarding the potential inhibitory effect of GB on platelet-leukocyte interactions through its regulation of P-selectin translocation. Further research in this area of vascular biology would provide a deeper understanding of the pharmacological and potential therapeutic actions of GB.
2.2.2. The effect of GB on platelet adhesion and activation
Platelet adhesion to endothelial cells requires activation of integrins (crucially platelet αIIbβ3 integrin) on the surface of both cell types105, 106, 107, and adhesion leads to platelet activation. As the major integrin in platelets, αIIbβ3 is referred to as the glycoprotein GPIIb/IIIa (CD41/CD61) complex, which can bind to vWF, fibrinogen, and other ligands108, showing that platelet activation is a multifactorial process. Notwithstanding the strong inhibitory effects of GB on platelet adhesion33,39,109, its mechanism of action is unclear. Since GBE preparations can reduce the level of vWF in plasma110,111, it can be speculated that GB might inhibit platelet adhesion by reducing the binding of vWF to αIIbβ3. Nevertheless, it remains to be explored whether GB can modulate vWF in vivo and whether such regulation can inhibit platelet adhesion in animal models of relevant vascular diseases.
Known as a natural product antagonist of platelet-activating factor receptor (PAF-R)112, GB exerts a strong inhibitory effect on inflammation and platelet activation by inhibiting the elevation of PAF levels and reducing the interaction/binding of PAF with PAF-R113, for which the IC50 value of GB is 0.273 μmol/L114. PAF is a powerful inflammatory mediator that is involved in a series of diseases caused by inflammatory pathways in combination with PAF-R115,116. Other biomolecules, such as ox-LDL and lipopolysaccharide, can exhibit PAF-like activity by serving as ligands/activators of PAF-R117,118, indicating that ox-LDL formed in the endothelium could bind PAF-R and induce inflammation. Consistent with this notion, GB inhibits the PAF-induced cascade in inflammatory reactions119 and substantially suppresses inflammation caused by lipopolysaccharide by acting on the PAF-R downstream signaling pathway120. Additionally, PAF promotes platelet activation121, while GB pretreatment antagonizes this effect122,123. Multiple pathways contribute to platelet activation124, and a recent study55 demonstrated that proprotein convertase subtilisin/kexin 9 (PCSK9) promotes platelet activation and thrombosis in combination with CD36, an effect that is abolished by PCSK9 inhibitors or aspirin. PCSK9 expression was increased in ox-LDL-exposed HUVECs, whereas GB downregulated this elevation in mRNA and protein levels, which was verified both in vitro and in silico41. This evidence implies that GB might also suppress platelet activation and thrombosis by inhibiting PCSK9 levels in addition to PAF blockade. Overall, GB demonstrates potential as a multitarget therapeutic agent with the ability to inhibit PAF-induced inflammatory injuries, platelet activation, and thrombosis through its multifunctional mechanisms.
2.2.3. The effect of GB on platelet aggregation and thrombogenesis
GB-mediated inhibition of platelet aggregation is another key point in thrombus prevention. PAF exerts a stimulatory effect on platelet aggregation in rabbits and dogs, leading to changes in circulatory flow at the site of arterial stenosis and endothelial injury, and PAF-stimulated platelet aggregation is inhibited by GB treatment125. A previous study40 showed that GB inhibited platelets from releasing platelet factor 4 (PF4) and CD40 ligand (CD40L), the mechanism of which is related to the suppression of Syk and p38 MAPK phosphorylation, as well as calcium efflux, and the blocking of PAF-R40. Platelet-released PF4 plays a role in thrombogenesis, and PF4 neutralization is a major part of the anticoagulant action of heparin126. Studies127,128 have shown that PF4 acts as a monocyte chemokine to induce monocytes to differentiate into macrophages. Furthermore, platelet CD40L mediates thrombogenesis and inflammation in atherosclerosis by enhancing platelet-leukocyte and leukocyte–endothelial interactions, apart from platelet–platelet interactions129. Given the abovementioned evidence, it is feasible that GB, as well as a direct inhibitory effect on platelet aggregation and thrombosis, could have an indirect suppressive effect on atherogenesis by inhibiting leukocyte–endothelial interactions, monocyte differentiation into macrophages, and subsequent foam cell formation.
2.3. GB inhibits leukocyte action in atherosclerosis
Increasing evidence130,131 has shown that leukocyte subsets aggregate in different stages of atherosclerotic lesions and take part in pathological reactions during the formation of atherosclerosis, supporting the concept that atherosclerosis is a chronic inflammatory disorder. Endothelial damage and dysfunction lead directly to leukocyte infiltration. Once bound to endothelial cells, leukocytes migrate from the endothelium to atherosclerotic plaques under the action of various chemokines132. During the process of leukocyte recruitment, the expression of ICAM-1 and VCAM-1 are two major determinants133, which participate jointly in multiple steps that mediate leukocyte–endothelial cell interactions, including slow rolling, crawling and arrest134. Many studies135, 136, 137 have indicated that blocking the adhesion molecules ICAM-1 and VCAM-1 reduces atherosclerotic lesions, and these two molecules provide potentially valuable targets for therapeutic interventions in atherosclerosis. Interestingly, evidence shows that GB is effective in inhibiting both ICAM-135,38 and VCAM-141,138. The effect of GB on ICAM-1 action has been confirmed in other studies139, 140, 141, which showed that GB reduced ICAM-1, TNF-α, IL-1β, and IL-6, probably related to blocking NF-κB activation36. However, although GB has been shown to downregulate VCAM-1 levels in TNF-α-treated HUVECs, the underlying mechanism of action has not yet been further explored. Overall, the study results above revealed a new mechanism of action of GB in protecting against leukocyte invasion and inflammation by suppressing both ICAM-1 and VCAM-1 under diseased conditions. Not only does GB regulate both of these targets, but it has also been shown to impede the entry of leukocytes into the intima or directly regulate a variety of leukocytes, such as monocytes, macrophages, mast cells, and neutrophils, via action on different molecular targets (see Table 1). Given the critical role of leukocyte subsets in the beginning and developing stages of atherosclerotic lesions, it is possible that GB has an impact on atherosclerosis by favorably modulating leukocyte functions.
Table 1.
The effects of GB on leukocyte function.
| Leukocyte subset | Target modulated by GB | Influence of GB on leukocyte subsets in atherosclerosis |
|---|---|---|
| Monocytes | VCAM-1, VE-cadherin, Cx43 | GB suppressed monocyte adhesion in TNF-α-stimulated HUVECs under varied degrees of laminar shear stress39 |
| JAM-A, Cx43, and VE-cadherin | GB inhibited junction protein expression and decreased monocyte transmigration in ox-LDL-treated HUVECs37 | |
| VCAM-1, ICAM-1 | GB suppressed THP-1 monocyte adhesion to TNF-α-induced HUVECs in a dose-dependent manner142 | |
| Macrophages | LOX-1, NOX4, MMP-1, COX-2 | GB decreased cholesterol deposits and showed atherosclerotic protective property35 |
| iNOS, NO | GB antagonized homocysteine-stimulated iNOS-mediated NO production in macrophages through antioxidation and attenuating NF-κB activation143 | |
| Mast cells | PAF-R | GB pretreatment significantly reduced the increase of mast cell degranulation in the rat isolated omentum stimulated by adenosine analogue144 |
| PAF-R | Pretreatment with GB observably decreased PAF's degranulating actions in omental mast cells while significantly reducing PAF's histamine-releasing impact on peritoneal mast cells145 | |
| Neutrophils | PAF-R | GB completely inhibited PAF-induced neutrophil response and subsequent neutrophil-mediated endothelial injury146 |
| [Ca2+]i, PAF-R | GB stimulated intracellular signaling events in neutrophils through tyrosine phosphorylation of proteins, phosphorylation of P38 map kinase, calcium transience, and phospholipase D activity, thus enhancing neutrophils' defense activities147 | |
| PAF-R, CD18, CDllb, superoxide anion | Neutrophils pretreated with GB reduced plasma-mediated neutrophil stimulation after myocardial infarction in the presence of high plasma PAF levels148 | |
| PAF-R | GB specifically suppressed neutrophil activation by blocking the binding of PAF to its receptor on neutrophil149 | |
| PAF-R | GB inhibited PAF-induced chemotaxis of neutrophils150 |
2.3.1. The effects of GB on monocytes
In the process of atherogenesis, after adhering to endothelial cells, monocytes migrate to the intima and differentiate into macrophages. Subsequently, monocyte-derived macrophages take up ox-LDL, leading to foam cell development. As shown in Table 2, GB had a suppressive impact on the TNF-α/ox-LDL-stimulated elevation of ICAM-1 and VCAM-1 in HUVECs. In addition to these two targets, GB can also affect monocytes by regulating other entities, such as connexin 43 (Cx43), junctional adhesion molecule (JAM-A), and vascular endothelial cadherin (VE-cadherin). As one of the most significant major initiators of atherosclerosis, monocyte-endothelial cell adhesion can not only be modulated by VCAM-1 and ICAM-1 expression, which is regulated by endotheliocyte-expressed Cx43151 but also be rapidly decreased by monocytic Cx43 channel function through ATP release and conversion to adenosine152. In 2019, a study found that Cx43 expression in monocytes could attenuate cell adhesion by activating the PI3K/AKT/NF-κB signaling pathway153. In terms of JAM-A, a previous study154 reported that inhibition of endothelial JAM-A expression inhibited the inflow of monocytes into the artery wall and limited the development of atherosclerotic lesions. Furthermore, Allingham et al.155 discovered that ICAM-1-mediated VE-cadherin phosphorylation on tyrosines 658 and 731 contributes to leukocyte transendothelial migration. These findings highlight junction proteins as critical control targets of leukocyte adhesion and migration, underscoring their therapeutic promise as potential targets to treat various inflammation-related diseases. Interestingly, recent evidence37 has shown that GB substantially reduced monocyte migration by inhibiting JAM-A, Cx43, and VE-cadherin expression, and the mechanism of GB-induced inhibition of these proteins was connected with inhibition of the PI3K/Akt pathway. These studies provide yet another basis for the potential of GB in the treatment of atherosclerotic CVDs and reveal the importance of targeting monocyte adhesion and migration in the treatment of inflammation-related diseases, including atherosclerosis.
Table 2.
Pharmacokinetic properties of GB and its preparations.
| Species | Dose | Pharmacokinetic parameter of GB | Ref. |
|---|---|---|---|
| Humans | 3 EGb 761™ tablets (the amount of GB is 836.46 μg) |
Cmax = 10.90 ± 5.59 ng/mL Tmax = 2.00 ± 1.63 h AUClast = 37.54 ± 17.35 ng/mL·h AUCtot = 71.73 ± 26.98 ng/mL·h %AUCextra = 38.10 ± 12.20 t1/2 = 3.94 ± 1.42 h |
Karin et al.187 |
| 90 drops (2.73 mL) Geriaforce™ tincture (the amount of GB is 147.45 μg) |
Cmax = 1.45 ± 0.98 ng/mL Tmax = 0.88 ± 0.38 h AUClast = 2.99 ± 3.22 ng/mL·h AUCtot = 6.17 ± 3.77 ng/mL·h %AUCextra = 20.53 ± 12.05 t1/2 = 2.34 ± 0.81 h |
||
| 4 Ginkgo fresh plant extract tablets (the amount of GB is 524.56 μg) |
Cmax = 4.61 ± 2.87 ng/mL Tmax = 2.35 ± 2.27 h AUClast = 15.45 ± 13.44 ng/mL·h AUCtot = 29.86 ± 25.08 ng/mL·h %AUCextra = 43.48 ± 27.64 t1/2 = 4.01 ± 2.58 h |
||
| Beagle dogs | 20 mg/kg conventional GB tablet | AUC0–t = 236.2 ± 81.14 μg/L·h AUC0-∞ = 246.8 ± 92.08 μg/L·h MRT0–t = 5.65 ± 3.42 h MRT0–∞ = 6.58 ± 3.54 h t1/2 = 4.45 ± 3.04 h Tmax = 3.73 ± 1.21 h CLz/F = 90.94 ± 33.97 L/h/kg Vz/F = 560.2 ± 405.4 L/kg Cmax = 66.64 ± 29.30 μg/L |
Zhao et al.188 |
| 20 mg/kg GB capsule made from hot-melt extrusion technology | AUC0–t = 606.7 ± 125.03 μg/L·h AUC0–∞ = 672.0 ± 59.89 μg/L·h MRT0–t = 4.18 ± 1.55 h MRT0-∞ = 8.75 ± 7.63 h T1/2 = 4.14 ± 1.81 h Tmax = 0.80 ± 0.18 h CLz/F = 29.96 ± 2.70 L/h/kg Vz/F = 405.7 ± 398.5 L/kg Cmax = 309.2 ± 106.0 μg/L |
||
| 20 mg/kg GB pellet created using liquid layer technology | AUC0–t = 419.1 ± 55.58 μg/L·h AUC0–∞ = 438.9 ± 71.14 μg/L·h MRT0–t = 3.53 ± 1.77 h MRT0–∞ = 4.30 ± 2.38 h t1/2 = 3.14 ± 1.80 h Tmax = 1.20 ± 1.04 h CLz/F = 46.68 ± 8.65 L/h/kg Vz/F = 216.1 ± 132.4 L/kg Cmax = 192.4 ± 84.22 μg/L |
||
| Beagle dogs | 80 mg/kg EGb |
Cmax = 16.23 ± 8.39 ng/mL tmax = 0.25 ± 0.37 h AUC0–t = 19.86 ± 6.33 ng/mL·h AUC0–∞ = 20.49 ± 6.26 ng/mL·h MRT = 2.03 ± 0.26 h F = 33.70% |
Chai et al.191 |
| Rats | 40 mg/kg EGb |
Cmax = 13447.10 ± 4137.57 ng/mL Tmax = 2.08 ± 1.02 h t1/2 = 3.86 ± 3.53 h AUC0–t = 82680.76 ± 22792.87 ng/mL·h |
Guan et al.192 |
| Rats | 3 mg/kg total ginkgolides for 7 days |
C5 min = 4608.3 ± 755.6 ng/mL T1/2 = 2.0 ± 0.7 h AUC0–6t = 2016.9 ± 671.9 μg/L·h AUC0–∞ = 2117.7 ± 739.5 μg/L·h |
Wang et al.189 |
Cmax: maximum plasma concentration; Tmax: time of maximum plasma concentration; AUC: area under the plasma concentration–time curve; %AUCextra: percentage of the area extrapolated for calculation of AUC from time zero to infinity; AUCtot: the total AUC; AUClast: AUC from the time of dosing to the time of the last measurable concentration; t1/2: elimination half-time; CL: clearance; MRT: mean residence time; Vz: apparent volume of distribution; F: bioavailability.
2.3.2. The effect of GB on macrophages
As a major inflammatory cell type, macrophages derived from monocytes are critical in the progression of atherosclerosis. The progression of atherosclerosis is marked by two main events, namely, macrophage infiltration and macrophage-derived foam cell production156,157. Therefore, pharmacological intervention, which targets the inhibition of macrophage inflammation and its behavior and function, should be regarded as a potential therapy for atherosclerosis. An earlier study143 showed that homocysteine, an independent risk factor for atherosclerotic disease158, stimulated iNOS-mediated NO production in macrophages at pathophysiological concentrations, while GB blocked this action through antioxidant properties and reduced NF-κB activation. Subsequently, Feng et al.35 reported that GB also exhibited atheroprotective properties by inhibiting the upregulation of MMP-1 and cyclooxygenase-2 (COX-2) expression in RAW264.7 macrophages. The increased expression of MMP-1, contributing to plaque expansion and fibrous cap destruction, has been associated with intraplaque hemorrhage in macrophages in the plaque area159. For COX-2, an earlier study160 provided pharmacological and genetic proof that COX-2 stimulated the early formation of atherosclerotic lesions in LDL receptor-deficient mice. The aforementioned research illuminates the potential of GB as a promising therapeutic strategy for inhibiting the conversion of monocytes into macrophages, as well as for modulating macrophage inflammation and behavior, thus highlighting its potential as an effective intervention for preventing the progression of atherosclerosis. The relationship between atherosclerotic macrophages and exosomes has attracted significant attention in recent years. These small vesicles released by macrophages play a critical role in atherosclerosis by facilitating intercellular communication and regulating several cellular processes, including cell proliferation, apoptosis, the inflammatory response, and hematopoiesis161,162. This would provide evidence that GB might regulate intercellular communication to affect different vascular cells in atherosclerosis if GB modulates the production of macrophage-derived exosomes.
2.3.3. The effect of GB on mast cells
Activated mast cells release various biochemical mediators, such as proteases, histamines, and proinflammatory agents163,164. These mediators facilitate plaque progression and instability by stimulating cholesterol uptake, inhibiting cholesterol efflux, increasing leukocyte infiltration (including monocytes, neutrophils, and T cells), and inducing the apoptosis of plaque cells (such as endothelial cells, macrophages, and smooth muscle cells), which may eventually contribute to plaque rupture with an acute thrombotic event164. In omental mast cells, two independent studies144,145 consistently showed that GB pretreatment markedly reduced PAF-mediated mast cell degranulation. Although the finding that GB is a prospective drug for decreasing mast cell degranulation is encouraging, it is still unknown whether a similar effect of GB on mast cells exists in the intima and how GB acts on mast cells and their released granules, areas that deserve further investigation.
2.3.4. The effect of GB on neutrophils
Neutrophils have diverse effects on different stages of atherosclerosis, such as causing early atherosclerosis, promoting the instability of plaques, and facilitating the shedding of endothelial cells in atherosclerotic plaques165. It has been reported in the earlier literature166 that PAF activates neutrophils either directly or by enhancing TNF-α and neutrophil chemoattractant production, implying that PAF antagonists have an inhibitory effect on neutrophils. In line with this evidence, in plasma samples taken from patients with acute myocardial ischemia, GB, utilized as a specific PAF antagonist, showed a strong inhibitory effect on neutrophil activation148,149. Moreover, trace amounts of PAF substantially enhance the neutrophil response to subsequent activation by agonists, including respiratory bursts characterized by enhanced superoxide release and degranulation as measured by elastase release. These actions can be ameliorated by GB by inhibiting superoxide production, elastase release and cell aggregation146. In addition to inhibition of neutrophil activation, GB also suppressed PAF-stimulated chemotaxis of neutrophils and blocked the specific binding of [3H]-PAF to eosinophils and neutrophils150. Given the importance of the neutrophil extracellular trap in atherosclerosis and atherothrombosis56, the potential role of GB in the neutrophil extracellular traps should be further investigated.
Taken together, the evidence above points to the ability of GB to mitigate the deleterious vascular effects of inflammation caused by leukocytes. Considering the various protective roles of GB in a range of leukocyte types, including monocytes, macrophages, mast cells, and neutrophils, it is reasonable to suggest that GB has potential as a therapeutic agent for treating atherosclerotic CVDs.
3. The influence of GB on risk factors for atherosclerosis and cardiovascular disease
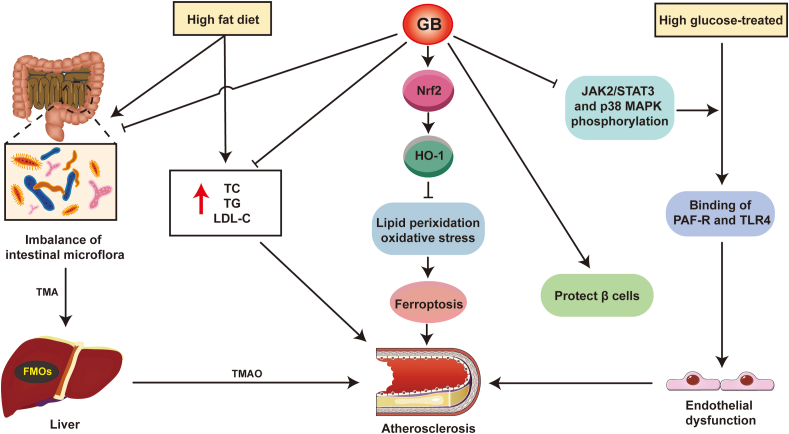
In the initial stages of vascular disease, many risk factors, such as the biochemical parameters of hyperlipidemia and hyperglycemia, can affect the critical role of the endothelium in maintaining vascular quiescence and homeostasis. Addressing hyperlipidemia with appropriate medical treatments can slow the development and progression of atherosclerosis and resultant cardiovascular disease167,168. In patients with diabetes and hence hyperglycemia, broad alterations in vascular homeostasis are the main feature of vasculopathy, eventually leading to atherothrombosis169,170. Since these are two important parameters of the multitude of risk factors for atherosclerosis and cardiovascular disease, GB could mitigate the progression of atherosclerosis by ameliorating these risk factors (Fig. 4).
Figure 4.
Diagram of the mechanism of GB on independent risk factors for atherosclerosis. Trimethylamine N-oxide (TMAO) levels in the blood can be utilized as a predictor of early atherosclerosis. Trimellitic anhydride (TMA) is derived from digested and absorbed foods containing choline or trimethylamine ingredients and then oxidized to TMAO by flavin monooxygenases (FMOs) secreted by the liver. This process could be inhibited by regulating the intestinal flora after GB treatment. In addition, a high-fat diet (HFD) resulted in higher levels of TG, TC, and LDL-C in serum, which were positively correlated with atherosclerosis. GB significantly attenuated the abnormal lipid and cholesterol metabolism induced by HFD. On the other hand, GB can improve resistance to ferroptosis through the Nrf2/HO-1 signaling pathway, thereby improving the pathological process of atherosclerosis. In addition to ameliorating HFD-induced atherosclerosis, GB also reduces endothelial dysfunction after high glucose treatment by inhibiting PAF-R and TLR4 binding, and it can treat diabetes mellitus by protecting β cells.
3.1. Hyperlipidemia
According to statistics by the International Atherosclerosis Society, those with the highest levels of LDL-C are at higher risk for atherosclerotic CVD171. Additionally, Nordestgaard et al.167 also observed that patients with familial hypercholesterolemia reached the LDL-C burden threshold earlier and developed premature atherosclerotic diseases. This evidence points to LDL playing a causative role in atherosclerosis. Thus, computed plasma LDL-C has become a promising therapeutic strategy for atherosclerosis and the emphasis is on appraising the risk and therapeutic effect of atherosclerotic CVDs172,173. As reported, GB could significantly decrease the levels of TC, TG, and LDL-C, as well as raise the level of high-density lipoprotein in hyperlipidemic mouse serum, an action that is very interestingly related to the manipulation of gut microbiota by GB34. A recent paper174 reported that the gut microbiota is associated with atherosclerosis, and hence, this emerging factor needs to be included as a risk factor for cardiovascular disease. Of note, one of the pathways by which the gut microbiota has a beneficial impact on the progression of atherosclerosis is to modulate host metabolism (such as cholesterol and lipid metabolism)175, which implies that GB-regulated gut microbiota might affect atherosclerotic plaque formation by reducing cholesterol and lipid levels in the body. Furthermore, Yang et al.176 discovered that fat droplet deposition, occurring in hepatic steatosis, and the number of necrotic liver cells were reduced after consuming GB, showing that GB affects lipid accumulation, liver injury, and fatty acid metabolism. They also found that GB effectively upregulated HO-1 expression via the Nrf2 pathway, thereby increasing resistance to ferroptosis176. Ferroptosis is an iron-dependent necrosis that causes oxidative damage to phospholipids. It has been reported that disturbances in intracellular iron can injure macrophages, vascular smooth muscle cells, and vascular endothelial cells and alter atherosclerosis via lipid peroxidation, oxidative stress, inflammation, dyslipidemia, and other risk factors or pathological processes177. The evidence above indicates that GB, by regulating the gut microbiota and increasing resistance to ferroptosis, can have an impact on atherosclerosis by reducing hyperlipidemia.
3.2. Hyperglycemia
The hallmarks of the dysmetabolic state of diabetes (type 2 diabetes) are insulin resistance and hyperglycemia, which cause endothelial and smooth muscle cell dysfunction through inflammatory responses, oxidative stress, and platelet-activating mechanisms, which are drivers for CVDs in diabetes mellitus. Some research has indicated the therapeutic potential of GB in glucose metabolism disorders. For instance, the protective effect of GB on pancreatic β cells was dose-dependent, and GB significantly reduced the likelihood of severe insulitis in rats, suggesting that GB could be used as a PAF antagonist in the treatment of insulin-dependent diabetes mellitus178. In addition to preventing endothelial dysfunction in diabetic model rats69, GB also has a protective effect on high glucose-treated endothelial cells by inhibiting the binding of PAF-R and TLR4179. The potential mechanism was linked to the suppression of JAK2/STAT3 and p38 MAPK phosphorylation179. Notably, regulating the JAK/STAT signaling pathway contributes to improving the progression of obesity and diabetes180,181, which not only reveals the therapeutic significance of targeting the JAK/STAT signaling pathway by GB in the treatment of obesity and diabetes but also provides a new strategy to ameliorate hyperlipidemia and hyperglycemia to treat atherosclerosis. In addition, the activation of p38 MAPK is a driving factor of atherosclerosis in humans and is involved in multiple cell types that influence atherosclerosis (such as endotheliocytes182, smooth muscle cells183, and various immune cells184,185), and targeting p38 MAPK by GB or other medicinal plant-derived bioactive compounds is expected to be an option for future therapeutic intervention in atherosclerosis. The potential effects of GB on micro- and macrovascular complications associated with hyperglycemia require further evaluation, especially considering that 40% of deaths in patients with diabetes arise from CVDs in China186.
4. Pharmacokinetic characteristics of GB and its preparations
The pharmacokinetic parameters of GB in both animals and humans have been investigated. As indicated in Table 2, several investigations have demonstrated that GB and its preparations have potential therapeutic value. Woelkart et al.187 studied volunteers who consumed three different GB-containing formulations and discovered that GB was of low bioavailability in humans, but its bioavailability could be improved by preparing it in a variety of dosage forms. The reason for the limited oral bioavailability of GB, as for many drugs, is its poor solubility, which impacts its absorption, distribution, metabolism and elimination. The developments of pharmaceutical sciences and the development of new GB formulations can overcome this problem. A study on beagle dogs discovered that GB in plasma has good stability within 4 h and that GB preparations prepared by hot-melt extrusion or liquid layer technology can significantly improve the AUC and plasma concentrations of GB in vivo188, implying that the development of novel oral GB formulations that possess high bioavailability is possible. Distribution studies show that GB is present in many organs and tissues, with the largest concentrations in the lungs, heart, spleen, kidney, liver, and gut and the lowest concentrations in the brain189,190. However, interestingly, borneol-modified ginkgolide liposomes can contribute to promoting the transport of ginkgolides across the blood–brain barrier and increasing the drug concentration of GB in the brain190, implying that GB could become a therapeutic drug for the treatment of cerebrovascular disease with the assistance of borneol.
5. Clinical trials of ginkgolide B-related drug preparations in the prevention of acute ischemic cerebrovascular disease
A wide variety of pharmaceutical preparations that contain GB are commercially available. To date, mounting clinical evidence has demonstrated the therapeutic value of GB-related drug preparations for stroke or ischemia (Table 3). Notably, two clinical trials specifically focusing on safety have demonstrated the positive impact of GB injection on ischemic stroke disorders, providing promising evidence of the potential of GB as a medicinal agent.
Table 3.
Clinical trials of a GB-related product for acute ischemic cerebrovascular disease.
| Registration No. | Recruitment status | Study title | Target disease | Phase | Interventions |
|---|---|---|---|---|---|
| CTR20200390 | Completed | A phase I clinical trial to evaluate the safety and tolerability of single and multiple intravenous ginkgolide B injections in healthy Chinese subjects | Acute ischemic stroke | Ⅰ | Ginkgolide B injection |
| CTR20200487 | Recruiting | A phase I clinical study to evaluate the safety, tolerability, and pharmacokinetics of ginkgolide B injection in healthy Chinese volunteers | Acute ischemic stroke | Ⅰ | Ginkgolide B injection |
| ChiCTR-IPR-17012310 | Recruiting | Ginkgolide in ischemic stroke patients with large artery atherosclerosis (GISAA)193 | Stroke | IV | Ginkgolide injection + oral aspirin |
| NCT03772847 | Completed | Ginkgolide with intravenous alteplase thrombolysis in acute ischemic stroke neurological improving trial200 | Intravenous alteplase thrombolysis Neurological improving |
IV | Ginkgolide plus alteplase |
| ChiCTR-ONC–13003247 | Completed | A multicenter clinical trial assessment of Baiyu Ginkgolide injection, in the treatment of ischemic stroke with blood stasis syndrome | Ischemic stroke | IV | Baiyu Ginkgolide injection |
| NCT01958957 | Completed | A safety study of ginkgolides meglumine injection in the treatment of ischemic stroke | Ischemic stroke | IV | Ginkgolides Meglumine Injection |
| CTR20160610 | Completed | Single and multiple administration of dimethylaminoethyl ginkgolide B mesylate tablets in a randomized double-blind placebo-controlled PK/PD study and the effect of eating on PK/PD | Acute ischemic cerebrovascular disease | Ⅰ | Dimethylaminoethyl ginkgolide B mesylate tablets |
| CTR20140805 | Completed | Research protocol of multiple administration, dose-escalation, randomized, double-blind, placebo-controlled phase I clinical trial of dimethylaminoethyl ginkgolide B mesylate injection | Acute ischemic stroke | Ⅰ | Dimethylaminoethyl ginkgolide B mesylate injection |
| CTR20210113 | Recruiting | A phase I clinical study on the safety, tolerability and pharmacokinetics/pharmacodynamics (PK/PD) of a single dose of dimethylaminoethyl ginkgolide B mesylate injection in healthy adult subjects. | Acute ischemic cerebrovascular disease | Ⅰ | Dimethylaminoethyl ginkgolide B mesylate injection |
| CTR20210115 | Recruiting | Phase I clinical study of safety, tolerability, and pharmacokinetics/pharmacodynamics (PK/PD) of multidose dimethylaminoethyl ginkgolide B mesylate tablets in healthy adult subjects. | Acute ischemic cerebrovascular disease | Ⅰ | Dimethylaminoethyl ginkgolide B mesylate tablets |
Ginkgolide injection is one representative GB preparation. Assessed from the composition of raw materials that have been marketed in China, GA and GB are the main components of ginkgolide injection (the content of both is greater than 95%), of which GB has the strongest pharmacological activity193,194; it appears that the contribution of GA and GC provides a synergistic effect with GB. Based on the aforementioned evidence, it can be inferred that GB likely plays a crucial role as the primary therapeutic agent in ginkgolide injections. Currently, several ginkgolide injections have completed clinical phase IV trials that investigated the impact of ginkgolide injections on the treatment of ischemic stroke. Additionally, an ongoing clinical study is actively recruiting patients to evaluate the impact of ginkgolide injections on ischemic stroke patients with large artery atherosclerosis, which is expected to be concluded shortly. Furthermore, two small-sample studies195,196 demonstrated that the combination of rosuvastatin and ginkgo biloba extract tablets has demonstrated clear clinical efficacy in treating cerebral infarction patients with dyslipidemia or diabetes. This combination therapy effectively reduces carotid atherosclerotic plaque, alleviates inflammation, improves blood lipid profiles, and exhibits a favorable safety profile. These clinical studies have confirmed the therapeutic effect of GB-related drug preparations in the treatment of stroke or ischemia and have also suggested their potential for reducing atherosclerotic plaques. However, it remains unclear whether the administration of GB-related drug preparations alone, especially the isolated GB compound, can effectively reduce atherosclerotic plaques or even treat atherosclerosis in clinical practice.
In addition, a larger percentage of the total were clinical trials involving dimethylaminoethyl ginkgolide B mesylate (XQ-1H), a derivative of GB, in ischemic cerebrovascular disorders. This might be because GB is a hexacyclic cage diterpenoid molecule with high structural stiffness, low water solubility, and low bioavailability, all of which restrict its potency and efficacy187,197. XQ-1H has superior water solubility and bioavailability and a higher safety threshold than GB, allowing it to be used in more clinical settings198,199. It is possible to propose that if the issue of the poor solubility of GB can be resolved by structural changes, it may considerably increase its efficacy and broaden its therapeutic value. As a result, structural changes in GB to improve water solubility are a key direction for advancing clinical research in this field.
Unfortunately, there have been no clinical studies specifically addressing the prevention and treatment of atherosclerosis using GB monomer or XQ-1H. However, with increased focus on GB monomers and structurally modified compounds, more in-depth preclinical investigations and clinical trials are anticipated to define their role in atherosclerosis.
6. Concluding remarks and future perspectives
GB is a ginkgolide with favorable pharmacological and therapeutic effects on preventing atherosclerosis. Multiple studies have demonstrated that GB has antiatherosclerotic actions that merit further exploration. The functions of endothelial cells, platelets, and other immunological cells, all of which are involved in atherosclerosis, can be regulated by GB, thus reducing the formation and development of atherosclerotic plaques. GB could also be beneficial in the management of hyperlipidemia and hyperglycemia, both of which are important risk factors in atherosclerosis. In short, GB possesses the therapeutic advantage of “multitarget and multipathway”, which is closely linked to its modulation of lipid and glucose metabolism, inflammation, oxidative stress, endothelial function, leukocyte accumulation, platelet aggregation, thrombosis, and other processes. This scenario is therapeutically important in light of the complex pathological mechanisms of atherosclerosis and the intricate interaction among various vascular cells.
Atherosclerosis has the potential to precipitate additional types of CVDs, the most alarming of which are heart and brain damage. The regulation of the PI3K/AKT/NF-κB, p38 MAPK, JAK/STAT, and PAF-R signaling pathways by GB is not only beneficial for the attenuation of atherosclerosis but also protective against myocardial ischemia/reperfusion injury43,201, ischemic stroke202, 203, 204 and cerebral ischemia/reperfusion injury205,206. It is also of interest that GB acts as a PAF antagonist affecting multiple proatherosclerotic cellular processes, including inhibiting thrombosis, suppressing neutrophil activation and reducing mast cell degranulation. As an early contributor to atherosclerosis, PAF promotes atherosclerotic plaque formation through inflammation207,208, endothelial dysfunction146, oxidative stress209, and platelet activity210, which indicates that PAF antagonists might help prevent and cure atherosclerosis. There is evidence that GB exhibits potent PAF antagonism by hindering the binding of PAF to its receptor. For example, GB reduced the incidence of ventricular fibrillation by 40% during ischemia and reperfusion by inhibiting the binding of PAF to PAF-R receptors on platelets123. Furthermore, activated platelets stimulated smooth muscle cell proliferation by releasing PAF, but this proliferation declined by approximately 50% after GB treatment211. Since VSMCs are a key component in the genesis and establishment of atherosclerosis, their proliferation may be helpful as the disease progresses212. It has been reported that GB can also reduce VSMC growth in a concentration-dependent manner213,214, which implies that GB might improve VSMC function to treat atherosclerosis. While GB affects a variety of cells involved in the atherosclerotic process, it is uncertain whether it can reverse atherosclerosis by affecting proliferation or phenotype on VSMCs.
In the last decade, our knowledge of the atheroprotective benefits of GB and biological targets has continued to expand. However, there are many limitations and challenges to the clinical application of GB in atherosclerosis. For example, although mounting clinical evidence has demonstrated the therapeutic value of GB-related drug preparations for acute ischemic cerebrovascular disease, mainly focusing on the safety and tolerability of GB, there is still no clinical trial that proves the effectiveness of GB for the treatment of atherosclerosis, which needs to be conducted in the future. Besides, the low oral bioavailability of GB has been addressed by nanoparticle encapsulation215,216 or structural molecular modifications217. Nevertheless, those strategies are currently only used to study the prevention of Parkinson's disease215,216 or ischemic stroke218,219 and have not been studied in atherosclerosis models, which might be the future direction for the GB study area. In view of a study220 showing that loading GB into liposomes boosted the therapeutic efficacy in the cerebral ischemia-reperfusion injury model, it is plausible that liposome formulations for GB will possess improved antiatherosclerosis efficacy. It is unknown if recombination with other vectors, particularly nanomaterials with distinct ROS-regulating capacities that may precisely cure atherosclerosis221, can improve GB's therapeutic effectiveness. Despite the fact that we have some initial understanding of the actions of GB in the prevention of atherosclerosis, many fundamental issues remain to be addressed. For instance, are structurally modified GB analogues more efficacious in preventing and treating atherosclerosis? Do GB and its derivatives affect the activation of NLRP3-mediated inflammasomes222 and the cGAS–STING pathway223 in the context of atherosclerosis? Is there a function for GB in controlling other apoptotic and nonapoptotic programs of death of vascular cells or immune cells, such as pyroptosis, necroptosis, or copper-dependent death224, in response to diverse pathogenic stimuli within the atherosclerotic microenvironment, in addition to ferroptosis?
In summary, in vivo and in vitro research demonstrates that GB has the potential to prevent and treat atherosclerosis by acting via a variety of targets and pathways. It can also help to mitigate the impact of other risk factors that contribute to atherosclerosis. GB regulates a range of cell functions and risk factors, providing considerable vascular protection and serving as a candidate medication to prevent and treat atherosclerosis. Future considerations can center on improving GB bioavailability, intestinal absorption, and in vivo stability, which would provide a valuable drug template for advanced clinical applications.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Suowen Xu, Email: sxu1984@ustc.edu.cn.
Wencai Ye, Email: chywc@aliyun.com.
Zhiping Liu, Email: zhiping0414@163.com.
Acknowledgments
This work is supported by National Natural Science Foundation of China (82270500, 81870324, 82203304, 82070464, U1401225, U21A20419); National Mega-Project for Innovative Drugs (2019ZX09735002); Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y036, 2017BT01Y093, China); National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province, 2017B090903004, China).
Author contributions
Weile Ye, Jiaojiao Wang and Zhiping Liu conceived and designed the manuscript. Weile Ye, Jiaojiao Wang, Jiami Zou and Zhihua Zheng prepared figures and tables and wrote the manuscript. Peter J Little, Jing Lu, Dongmei Zhang, Peiqing Liu, Yanjun Yin, Hao Liu, Suowen Xu, Wencai Ye and Zhiping Liu provided the idea and revised the manuscript. Wencai Ye and Zhiping Liu directed the study. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflict of interests.
References
- 1.Weir H.K., Anderson R.N., Coleman King S.M., Soman A., Thompson T.D., Hong Y., et al. Heart disease and cancer deaths—trends and projections in the United States, 1969–2020. Prev Chronic Dis. 2016;13:E157. doi: 10.5888/pcd13.160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 3.Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-Cabo F., Fuster V., Silla-Castro J.C., González G., Lorenzo-Vivas E., Alvarez R., et al. Subclinical atherosclerosis and accelerated epigenetic age mediated by inflammation: a multi-omics study. Eur Heart J. 2023;44:2698–2709. doi: 10.1093/eurheartj/ehad361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng B., Yin W.N., Suzuki T., Zhang X.H., Zhang Y., Song L.L., et al. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Wang J., Uryga A.K., Reinhold J., Figg N., Baker L., Finigan A., et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132:1909–1919. doi: 10.1161/CIRCULATIONAHA.115.016457. [DOI] [PubMed] [Google Scholar]
- 7.Farina F.M., Serio S., Hall I.F., Zani S., Cassanmagnago G.A., Climent M., et al. The epigenetic enzyme DOT1L orchestrates vascular smooth muscle cell-monocyte crosstalk and protects against atherosclerosis via the NF-κB pathway. Eur Heart J. 2022;43:4562–4576. doi: 10.1093/eurheartj/ehac097. [DOI] [PubMed] [Google Scholar]
- 8.Orecchioni M., Kobiyama K., Winkels H., Ghosheh Y., McArdle S., Mikulski Z., et al. Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production. Science. 2022;375:214–221. doi: 10.1126/science.abg3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amadori L., Calcagno C., Fernandez D.M., Koplev S., Fernandez N., Kaur R., et al. Systems immunology-based drug repurposing framework to target inflammation in atherosclerosis. Nat Cardiovasc Res. 2023;2:550–571. doi: 10.1038/s44161-023-00278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauersberger C., Sager H.B., Wobst J., Dang T.A., Lambrecht L., Koplev S., et al. Loss of soluble guanylyl cyclase in platelets contributes to atherosclerotic plaque formation and vascular inflammation. Nat Cardiovasc Res. 2022;1:1174–1186. doi: 10.1038/s44161-022-00175-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Momi S., Falcinelli E., Petito E., Ciarrocca Taranta G., Ossoli A., Gresele P. Matrix metalloproteinase-2 on activated platelets triggers endothelial PAR-1 initiating atherosclerosis. Eur Heart J. 2022;43:504–514. doi: 10.1093/eurheartj/ehab631. [DOI] [PubMed] [Google Scholar]
- 12.Schumski A., Ortega-Gómez A., Wichapong K., Winter C., Lemnitzer P., Viola J.R., et al. Endotoxinemia accelerates atherosclerosis through electrostatic charge-mediated monocyte adhesion. Circulation. 2021;143:254–266. doi: 10.1161/CIRCULATIONAHA.120.046677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanta S.K., Peng L., Li Y., Lu S., Sun T., Carnevale L., et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature. 2022;605:152–159. doi: 10.1038/s41586-022-04673-6. [DOI] [PubMed] [Google Scholar]
- 14.Xu S., Ilyas I., Little P.J., Li H., Kamato D., Zheng X., et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73:924–967. doi: 10.1124/pharmrev.120.000096. [DOI] [PubMed] [Google Scholar]
- 15.Little P.J., Osman N., O'Brien K.D. Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol. 2008;19:448–454. doi: 10.1097/MOL.0b013e32830dd7c4. [DOI] [PubMed] [Google Scholar]
- 16.Tsiantoulas D., Eslami M., Obermayer G., Clement M., Smeets D., Mayer F.J., et al. APRIL limits atherosclerosis by binding to heparan sulfate proteoglycans. Nature. 2021;597:92–96. doi: 10.1038/s41586-021-03818-3. [DOI] [PubMed] [Google Scholar]
- 17.Sakata N., Uesugi N., Takebayashi S., Nagai R., Jono T., Horiuchi S., et al. Glycoxidation and lipid peroxidation of low-density lipoprotein can synergistically enhance atherogenesis. Cardiovasc Res. 2001;49:466–475. doi: 10.1016/s0008-6363(00)00262-5. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Qu L., Matz A.J., Murphy P.A., Liu Y., Manichaikul A.W., et al. AtheroSpectrum reveals novel macrophage foam cell gene signatures associated with atherosclerotic cardiovascular disease risk. Circulation. 2022;145:206–218. doi: 10.1161/CIRCULATIONAHA.121.054285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y., Duan H., Qian Y., Feng L., Wu Z., Wang F., et al. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. 2017;27:352–372. doi: 10.1038/cr.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong M.K., Mintz G.S., Lee C.W., Kim Y.H., Lee S.W., Song J.M., et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110:928–933. doi: 10.1161/01.CIR.0000139858.69915.2E. [DOI] [PubMed] [Google Scholar]
- 21.Bergström G., Persson M., Adiels M., Björnson E., Bonander C., Ahlström H., et al. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144:916–929. doi: 10.1161/CIRCULATIONAHA.121.055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzhova I., Fuster V., Fernández-Ortiz A., Ordovás J.M., Sanz J., Fernández-Friera L., et al. The importance of breakfast in atherosclerosis disease: insights from the PESA study. J Am Coll Cardiol. 2017;70:1833–1842. doi: 10.1016/j.jacc.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Lyu M., Cui Y., Zhao T., Ning Z., Ren J., Jin X., et al. Mediated atherosclerosis signaling and inflammatory response as a common protection mechanism of shuxuening injection against both myocardial and cerebral ischemia–reperfusion injuries. Front Pharmacol. 2018;9:312. doi: 10.3389/fphar.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y., Li S., Cui W., Zu X., Du J., Wang F. Ginkgo biloba extract improves coronary blood flow in healthy elderly adults: role of endothelium-dependent vasodilation. Phytomedicine. 2008;15:164–169. doi: 10.1016/j.phymed.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y.Z., Li S.Q., Zu X.G., Du J., Wang F.F. Ginkgo biloba extract improves coronary artery circulation in patients with coronary artery disease: contribution of plasma nitric oxide and endothelin-1. Phytother Res. 2008;22:734–739. doi: 10.1002/ptr.2335. [DOI] [PubMed] [Google Scholar]
- 26.Xiao G., Lyu M., Wang Y., He S., Liu X., Ni J., et al. Ginkgo flavonol glycosides or ginkgolides tend to differentially protect myocardial or cerebral ischemia–reperfusion injury regulation of TWEAK–Fn14 signaling in heart and brain. Front Pharmacol. 2019;10:735. doi: 10.3389/fphar.2019.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngan N.T., Quang T.H., Tai B.H., Song S.B., Lee D., Kim Y.H. Anti-inflammatory and PPAR transactivational effects of components from the stem bark of Ginkgo biloba. J Agric Food Chem. 2012;60:2815–2824. doi: 10.1021/jf204768d. [DOI] [PubMed] [Google Scholar]
- 28.Li M., Xia Z.M., Li B., Tian Y., Zhang G-j, Xu C., et al. Chemical constituents from Ginkgo biloba L. male flowers and their biological activities. Med Chem Res. 2019;28:1557–1566. [Google Scholar]
- 29.Bolshakov S., Dzyuba S.V., Decatur J., Nakanishi K. A concise synthesis of ginkgolide M, a minor component of a terpene trilactone fraction from Ginkgo biloba roots. J Nat Prod. 2006;69:429–431. doi: 10.1021/np050403i. [DOI] [PubMed] [Google Scholar]
- 30.Liao H.J., Zheng Y.F., Li H.Y., Peng G.P. Two new ginkgolides from the leaves of Ginkgo biloba. Planta Med. 2011;77:1818–1821. doi: 10.1055/s-0030-1271153. [DOI] [PubMed] [Google Scholar]
- 31.Weinges K., Hepp M., Jaggy H. Chemie der ginkgolide, II. Isolierung und strukturaufklärung eines neuen ginkgolids. Liebigs Ann Chem. 1987;1987:521–526. [Google Scholar]
- 32.Ma S., Liu X., Xu Q., Zhang X. Transport of ginkgolides with different lipophilicities based on an hCMEC/D3 cell monolayer as a blood–brain barrier cell model. Life Sci. 2014;114:93–101. doi: 10.1016/j.lfs.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Liu X., Zhao G., Yan Y., Bao L., Chen B., Qi R. Ginkgolide B reduces atherogenesis and vascular inflammation in ApoE–/– mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv Z., Shan X., Tu Q., Wang J., Chen J., Yang Y. Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE mice. Biomed Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111100. [DOI] [PubMed] [Google Scholar]
- 35.Feng Z., Yang X., Zhang L., Ansari I.A., Khan M.S., Han S., et al. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother Res. 2018;32:2417–2427. doi: 10.1002/ptr.6177. [DOI] [PubMed] [Google Scholar]
- 36.Li R., Chen B., Wu W., Bao L., Li J., Qi R. Ginkgolide B suppresses intercellular adhesion molecule-1 expression via blocking nuclear factor-kappaB activation in human vascular endothelial cells stimulated by oxidized low-density lipoprotein. J Pharmacol Sci. 2009;110:362–369. doi: 10.1254/jphs.08275fp. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Sun W., Zhao Y., Chen B., Wu W., Bao L., et al. Ginkgolide B inhibits JAM-A, Cx43, and VE-Cadherin expression and reduces monocyte transmigration in oxidized LDL-stimulated human umbilical vein endothelial cells. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/907926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L., Liu X., Zhao Y., Chen B., Li X., Qi R. Ginkgolide B reduces LOX-1 expression by inhibiting Akt phosphorylation and increasing Sirt1 expression in oxidized LDL-stimulated human umbilical vein endothelial cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M., Sun J., Chen B., Zhao Y., Gong H., You Y., et al. Ginkgolide B inhibits platelet and monocyte adhesion in TNFα-treated HUVECs under laminar shear stress. BMC Compl Alternative Med. 2018;18:220. doi: 10.1186/s12906-018-2284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X., Yan Y., Bao L., Chen B., Zhao Y., Qi R. Ginkgolide B inhibits platelet release by blocking Syk and p38 MAPK phosphorylation in thrombin-stimulated platelets. Thromb Res. 2014;134:1066–1073. doi: 10.1016/j.thromres.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Wang G., Liu Z.B., Li M.H., Li Y., Alvi S.S., Ansari I.A., et al. Ginkgolide B mediated alleviation of inflammatory cascades and altered lipid metabolism in HUVECs via targeting PCSK-9 expression and functionality. BioMed Res Int. 2019;2019 doi: 10.1155/2019/7284767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S., Chen B., Wu W., Bao L., Qi R. Ginkgolide B reduces inflammatory protein expression in oxidized low-density lipoprotein-stimulated human vascular endothelial cells. J Cardiovasc Pharmacol. 2011;57:721–727. doi: 10.1097/FJC.0b013e31821a50a8. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R., Xu L., Zhang D., Hu B., Luo Q., Han D., et al. Cardioprotection of ginkgolide B on myocardial ischemia/reperfusion-induced inflammatory injury via regulation of A20-NF-κB pathway. Front Immunol. 2018;9:2844. doi: 10.3389/fimmu.2018.02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng S., Li Z., Song J., Wang P., Xu J., Hu W., et al. Endothelial METRNL determines circulating METRNL level and maintains endothelial function against atherosclerosis. Acta Pharm Sin B. 2023;13:1568–1587. doi: 10.1016/j.apsb.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theilmeier G., Michiels C., Spaepen E., Vreys I., Collen D., Vermylen J., et al. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–4493. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 46.Popa M., Tahir S., Elrod J., Kim S.H., Leuschner F., Kessler T., et al. Role of CD40 and ADAMTS13 in von Willebrand factor-mediated endothelial cell–platelet–monocyte interaction. Proc Natl Acad Sci U S A. 2018;115:E5556–E5565. doi: 10.1073/pnas.1801366115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frodermann V., Rohde D., Courties G., Severe N., Schloss M.J., Amatullah H., et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25:1761–1771. doi: 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen P.Y., Qin L., Li G., Wang Z., Dahlman J.E., Malagon-Lopez J., et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat Metab. 2019;1:912–926. doi: 10.1038/s42255-019-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S., Xu Y., Liu P., Zhang S., Liu H., Slavin S., et al. The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis. Eur Heart J. 2019;40:2398–2408. doi: 10.1093/eurheartj/ehz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anisimov A., Fang S., Hemanthakumar K.A., Örd T., van Avondt K., Chevre R., et al. The angiopoietin receptor Tie2 is atheroprotective in arterial endothelium. Nat Cardiovasc Res. 2023;2:307–321. doi: 10.1038/s44161-023-00224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia M., Li Q., Guo J., Shi W., Zhu L., Huang Y., et al. Deletion of BACH1 attenuates atherosclerosis by reducing endothelial inflammation. Circ Res. 2022;130:1038–1055. doi: 10.1161/CIRCRESAHA.121.319540. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z., Zhu H., Dai X., Wang C., Ding Y., Song P., et al. Macrophage liver kinase B1 inhibits foam cell formation and atherosclerosis. Circ Res. 2017;121:1047–1057. doi: 10.1161/CIRCRESAHA.117.311546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozawa K., Muller M.A., Varlamov O., Tavori H., Packwood W., Mueller P.A., et al. Proteolysis of Von Willebrand factor influences inflammatory endothelial activation and vascular compliance in atherosclerosis. JACC Basic Transl Sci. 2020;5:1017–1028. doi: 10.1016/j.jacbts.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Mansi S., Robinson C.L., Kostelnik K.B., McCormack J.J., Mitchell T.P., Lobato-Márquez D., et al. Proximity proteomics identifies septins and PAK2 as decisive regulators of actomyosin-mediated expulsion of von Willebrand factor. Blood. 2023;141:930–944. doi: 10.1182/blood.2022017419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi Z., Hu L., Zhang J., Yang W., Liu X., Jia D., et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) enhances platelet activation, thrombosis, and myocardial infarct expansion by binding to platelet CD36. Circulation. 2021;143:45–61. doi: 10.1161/CIRCULATIONAHA.120.046290. [DOI] [PubMed] [Google Scholar]
- 56.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willems S., Vink A., Bot I., Quax P.H., de Borst G.J., de Vries J.P., et al. Mast cells in human carotid atherosclerotic plaques are associated with intraplaque microvessel density and the occurrence of future cardiovascular events. Eur Heart J. 2013;34:3699–3706. doi: 10.1093/eurheartj/eht186. [DOI] [PubMed] [Google Scholar]
- 58.Landmesser U., Hornig B., Drexler H. Endothelial function: a critical determinant in atherosclerosis?. Circulation. 2004;109:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 59.Dong Y., Wang B., Du M., Zhu B., Cui K., Li K., et al. Targeting epsins to inhibit fibroblast growth factor signaling while potentiating transforming growth factor-beta signaling constrains endothelial-to-mesenchymal transition in atherosclerosis. Circulation. 2023;147:669–685. doi: 10.1161/CIRCULATIONAHA.122.063075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schürmann C., Rezende F., Kruse C., Yasar Y., Löwe O., Fork C., et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drummond G.R., Selemidis S., Griendling K.K., Sobey C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang J., Huang K., Xu S., Garcia J.G.N., Wang C., Cai H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helmcke I., Heumüller S., Tikkanen R., Schröder K., Brandes R.P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxidants Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 64.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H., Goettsch C., Xia N., Horke S., Morawietz H., Förstermann U., et al. Differential roles of PKCalpha and PKCepsilon in controlling the gene expression of Nox4 in human endothelial cells. Free Radic Biol Med. 2008;44:1656–1667. doi: 10.1016/j.freeradbiomed.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Yu W., Li S., Wu H., Hu P., Chen L., Zeng C., et al. Endothelial Nox4 dysfunction aggravates atherosclerosis by inducing endoplasmic reticulum stress and soluble epoxide hydrolase. Free Radic Biol Med. 2021;164:44–57. doi: 10.1016/j.freeradbiomed.2020.12.450. [DOI] [PubMed] [Google Scholar]
- 67.Sorescu D., Weiss D., Lassègue B., Clempus R.E., Szöcs K., Sorescu G.P., et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 68.Xu S., Chamseddine A.H., Carrell S., Miller F.J. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014;2:642–650. doi: 10.1016/j.redox.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G.G., Chen Q.Y., Li W., Lu X.H., Zhao X. Ginkgolide B increases hydrogen sulfide and protects against endothelial dysfunction in diabetic rats. Croat Med J. 2015;56:4–13. doi: 10.3325/cmj.2015.56.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma W., Li J., Hu J., Cheng Y., Wang J., Zhang X., et al. miR214-regulated p53-NOX4/p66shc pathway plays a crucial role in the protective effect of ginkgolide B against cisplatin-induced cytotoxicity in HEI-OC1 cells. Chem Biol Interact. 2016;245:72–81. doi: 10.1016/j.cbi.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Kim Y.M., Kim S.J., Tatsunami R., Yamamura H., Fukai T., Ushio-Fukai M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol. 2017;312:C749–C764. doi: 10.1152/ajpcell.00346.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Z., Yang Y., Xiong K., Li X., Qi P., Tu Q., et al. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials. 2015;63:80–92. doi: 10.1016/j.biomaterials.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 73.Casas J.P., Bautista L.E., Humphries S.E., Hingorani A.D. Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation. 2004;109:1359–1365. doi: 10.1161/01.CIR.0000121357.76910.A3. [DOI] [PubMed] [Google Scholar]
- 74.Ozaki M., Kawashima S., Yamashita T., Hirase T., Namiki M., Inoue N., et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim T.K., Jeon S., Park S., Sonn S.K., Seo S., Suh J., et al. 2′–5′ Oligoadenylate synthetase-like 1 (OASL1) protects against atherosclerosis by maintaining endothelial nitric oxide synthase mRNA stability. Nat Commun. 2022;13:6647. doi: 10.1038/s41467-022-34433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mount P.F., Kemp B.E., Power D.A. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 77.Jin Y.J., Chennupati R., Li R., Liang G.Z., Wang S.P., Iring A., et al. Protein kinase N2 mediates flow-induced endothelial NOS activation and vascular tone regulation. J Clin Invest. 2021;131 doi: 10.1172/JCI145734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hambrecht R., Adams V., Erbs S., Linke A., Kränkel N., Shu Y., et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 79.Jing C., Zhang G., Liu Z., Xu Q., Li C., Cheng G., et al. Peroxidasin promotes diabetic vascular endothelial dysfunction induced by advanced glycation end products via NOX2/HOCl/Akt/eNOS pathway. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Predmore B.L., Julian D., Cardounel A.J. Hydrogen sulfide increases nitric oxide production from endothelial cells by an Akt-dependent mechanism. Front Physiol. 2011;2:104. doi: 10.3389/fphys.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang Y., Huang B., Sun L., Peng X., Chen X., Zou X. Ginkgolide B promotes proliferation and functional activities of bone marrow-derived endothelial progenitor cells: involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell Mater. 2011;21:459–469. doi: 10.22203/ecm.v021a34. [DOI] [PubMed] [Google Scholar]
- 82.Akhmedov A., Rozenberg I., Paneni F., Camici G.G., Shi Y., Doerries C., et al. Endothelial overexpression of LOX-1 increases plaque formation and promotes atherosclerosis in vivo. Eur Heart J. 2014;35:2839–2848. doi: 10.1093/eurheartj/eht532. [DOI] [PubMed] [Google Scholar]
- 83.Mehta J.L., Basnakian A.G. Interaction of carbamylated LDL with LOX-1 in the induction of endothelial dysfunction and atherosclerosis. Eur Heart J. 2014;35:2996–2997. doi: 10.1093/eurheartj/ehu122. [DOI] [PubMed] [Google Scholar]
- 84.Chen J., Mehta J.L., Haider N., Zhang X., Narula J., Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 85.Zhu T.T., Zhang W.F., Luo P., Qian Z.X., Li F., Zhang Z., et al. LOX-1 promotes right ventricular hypertrophy in hypoxia-exposed rats. Life Sci. 2017;174:35–42. doi: 10.1016/j.lfs.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 86.Bai B., Liang Y., Xu C., Lee M.Y., Xu A., Wu D., et al. Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation. 2012;126:729–740. doi: 10.1161/CIRCULATIONAHA.112.118778. [DOI] [PubMed] [Google Scholar]
- 87.Gerdes N., Seijkens T., Lievens D., Kuijpers M.J., Winkels H., Projahn D., et al. Platelet CD40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler Thromb Vasc Biol. 2016;36:482–490. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 88.Huo Y., Schober A., Forlow S.B., Smith D.F., Hyman M.C., Jung S., et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 89.Barrett T.J., Schlegel M., Zhou F., Gorenchtein M., Bolstorff J., Moore K.J., et al. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aax0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yeung J., Li W., Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 2018;70:526–548. doi: 10.1124/pr.117.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King S.M., McNamee R.A., Houng A.K., Patel R., Brands M., Reed G.L. Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation. 2009;120:785–791. doi: 10.1161/CIRCULATIONAHA.108.845461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeFilippis A.P., Trainor P.J., Thanassoulis G., Brumback L.C., Post W.S., Tsai M.Y., et al. Atherothrombotic factors and atherosclerotic cardiovascular events: the multi-ethnic study of atherosclerosis. Eur Heart J. 2022;43:971–981. doi: 10.1093/eurheartj/ehab600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saha P., Gutmann C., Kingdon J., Dregan A., Bertolaccini L., Grover S.P., et al. Venous thrombosis accelerates atherosclerosis in mice. Circulation. 2023;147:1945–1947. doi: 10.1161/CIRCULATIONAHA.123.064268. [DOI] [PubMed] [Google Scholar]
- 94.Frenette P.S., Johnson R.C., Hynes R.O., Wagner D.D. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frenette P.S., Moyna C., Hartwell D.W., Lowe J.B., Hynes R.O., Wagner D.D. Platelet-endothelial interactions in inflamed mesenteric venules. Blood. 1998;91:1318–1324. [PubMed] [Google Scholar]
- 96.Frenette P.S., Denis C.V., Weiss L., Jurk K., Subbarao S., Kehrel B., et al. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet–endothelial interactions in vivo. J Exp Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romo G.M., Dong J.F., Schade A.J., Gardiner E.E., Kansas G.S., Li C.Q., et al. The glycoprotein Ib–IX–V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jalkanen S., Karikoski M., Mercier N., Koskinen K., Henttinen T., Elima K., et al. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood. 2007;110:1864–1870. doi: 10.1182/blood-2007-01-069674. [DOI] [PubMed] [Google Scholar]
- 99.Merten M., Chow T., Hellums J.D., Thiagarajan P. A new role for P-selectin in shear-induced platelet aggregation. Circulation. 2000;102:2045–2050. doi: 10.1161/01.cir.102.17.2045. [DOI] [PubMed] [Google Scholar]
- 100.Massberg S., Enders G., Leiderer R., Eisenmenger S., Vestweber D., Krombach F., et al. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood. 1998;92:507–515. [PubMed] [Google Scholar]
- 101.Yokoyama S., Ikeda H., Haramaki N., Yasukawa H., Murohara T., Imaizumi T. Platelet P-selectin plays an important role in arterial thrombogenesis by forming large stable platelet–leukocyte aggregates. J Am Coll Cardiol. 2005;45:1280–1286. doi: 10.1016/j.jacc.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 102.Dole V.S., Bergmeier W., Mitchell H.A., Eichenberger S.C., Wagner D.D. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phillips J.W., Barringhaus K.G., Sanders J.M., Hesselbacher S.E., Czarnik A.C., Manka D., et al. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107:2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- 104.Garland R.C., Sun D., Zandi S., Xie F., Faez S., Tayyari F., et al. Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury. Faseb J. 2011;25:1284–1294. doi: 10.1096/fj.10-160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coller B.S. αIIbβ3: structure and function. J Thromb Haemostasis. 2015;13(Suppl 1):S17–S25. doi: 10.1111/jth.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gawaz M., Neumann F.J., Dickfeld T., Reininger A., Adelsberger H., Gebhardt A., et al. Vitronectin receptor (αvβ3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation. 1997;96:1809–1818. doi: 10.1161/01.cir.96.6.1809. [DOI] [PubMed] [Google Scholar]
- 107.Reininger A.J., Agneskirchner J., Bode P.A., Spannagl M., Wurzinger L.J. c7E3 Fab inhibits low shear flow modulated platelet adhesion to endothelium and surface-absorbed fibrinogen by blocking platelet GP IIb/IIIa as well as endothelial vitronectin receptor—results from patients with acute myocardial infarction and healthy controls. Thromb Haemostasis. 2000;83:217–223. [PubMed] [Google Scholar]
- 108.Huang J., Li X., Shi X., Zhu M., Wang J., Huang S., et al. Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol. 2019;12:26. doi: 10.1186/s13045-019-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shang Q., Zhou X., Yang M.R., Lu J.G., Pan Y., Zhu G.Y., et al. Amide derivatives of ginkgolide B and their inhibitory effects on paf-induced platelet aggregation. ACS Omega. 2021;6:22497–22503. doi: 10.1021/acsomega.1c01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X.S., Zheng W.Y., Lou S.X., Lu X.W., Ye S.H. Effect of Ginkgo leaf extract on vascular endothelial function in patients with early stage diabetic nephropathy. Chin J Integr Med. 2009;15:26–29. doi: 10.1007/s11655-009-0026-8. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H., Cao Y., Pei H., Wang H., Ma L., Wang Z., et al. Shenmayizhi formula combined with ginkgo extract tablets for the treatment of vascular dementia: a randomized, double-blind, controlled trial. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/8312347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee C.M., Jung W.K., Na G., Lee D.S., Park S.G., Seo S.K., et al. Inhibitory effects of the platelet-activating factor receptor antagonists, CV-3988 and Ginkgolide B, on alkali burn-induced corneal neovascularization. Cutan Ocul Toxicol. 2015;34:53–60. doi: 10.3109/15569527.2014.903573. [DOI] [PubMed] [Google Scholar]
- 113.Xia S.H., Hu C.X., Zhao Z.L., Xia G.D., Di Y. Significance of platelet activating factor receptor expression in pancreatic tissues of rats with severe acute pancreatitis and effects of BN52021. World J Gastroenterol. 2007;13:2992–2998. doi: 10.3748/wjg.v13.i21.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kondratskaya E.L., Pankratov Y.V., Lalo U.V., Chatterjee S.S., Krishtal O.A. Inhibition of hippocampal LTP by ginkgolide B is mediated by its blocking action on PAF rather than glycine receptors. Neurochem Int. 2004;44:171–177. doi: 10.1016/s0197-0186(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 115.Hyland I.K., O'Toole R.F., Smith J.A., Bissember A.C. Progress in the development of platelet-activating factor receptor (PAFr) antagonists and applications in the treatment of inflammatory diseases. ChemMedChem. 2018;13:1873–1884. doi: 10.1002/cmdc.201800401. [DOI] [PubMed] [Google Scholar]
- 116.Tjoelker L.W., Wilder C., Eberhardt C., Stafforini D.M., Dietsch G., Schimpf B., et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374:549–553. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- 117.Heery J.M., Kozak M., Stafforini D.M., Jones D.A., Zimmerman G.A., McIntyre T.M., et al. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakamura M., Honda Z., Waga I., Matsumoto T., Noma M., Shimizu T. Endotoxin transduces Ca2+ signaling via platelet-activating factor receptor. FEBS Lett. 1992;314:125–129. doi: 10.1016/0014-5793(92)80957-i. [DOI] [PubMed] [Google Scholar]
- 119.Ji R.L., Xia S.H., Di Y., Xu W. Mechanism and dose-effect of gginkgolide B on severe acute pancreatitis of rats. World J Gastroenterol. 2011;17:2241–2247. doi: 10.3748/wjg.v17.i17.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xia S.H., Xiang X.H., Chen K., Xu W. Roles of BN52021 in platelet-activating factor pathway in inflammatory MS1 cells. World J Gastroenterol. 2013;19:3969–3979. doi: 10.3748/wjg.v19.i25.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Montrucchio G., Bergerone S., Bussolino F., Alloatti G., Silvestro L., Lupia E., et al. Streptokinase induces intravascular release of platelet-activating factor in patients with acute myocardial infarction and stimulates its synthesis by cultured human endothelial cells. Circulation. 1993;88:1476–1483. doi: 10.1161/01.cir.88.4.1476. [DOI] [PubMed] [Google Scholar]
- 122.Chakrabarty S., Thomas P., Sheridan D.J. Contribution of platelets and platelet-activating factor (PAF) to the arrhythmogenic, haemodynamic and necrotic effects of acute myocardial ischaemia. Eur Heart J. 1991;12:583–589. doi: 10.1093/oxfordjournals.eurheartj.a059944. [DOI] [PubMed] [Google Scholar]
- 123.Wainwright C.L., Parratt J.R., Bigaud M. The effects of PAF antagonists on arrhythmias and platelets during acute myocardial ischaemia and reperfusion. Eur Heart J. 1989;10:235–243. doi: 10.1093/oxfordjournals.eurheartj.a059471. [DOI] [PubMed] [Google Scholar]
- 124.Estevez B., Du X. New concepts and mechanisms of platelet activation signaling. Physiology. 2017;32:162–177. doi: 10.1152/physiol.00020.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Golino P., Ambrosio G., Ragni M., Pascucci I., Triggiani M., Oriente A., et al. Short-term and long-term role of platelet activating factor as a mediator of in vivo platelet aggregation. Circulation. 1993;88:1205–1214. doi: 10.1161/01.cir.88.3.1205. [DOI] [PubMed] [Google Scholar]
- 126.Eslin D.E., Zhang C., Samuels K.J., Rauova L., Zhai L., Niewiarowski S., et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104:3173–3180. doi: 10.1182/blood-2003-11-3994. [DOI] [PubMed] [Google Scholar]
- 127.Nassar T., Sachais B.S., Akkawi S., Kowalska M.A., Bdeir K., Leitersdorf E., et al. Platelet factor 4 enhances the binding of oxidized low-density lipoprotein to vascular wall cells. J Biol Chem. 2003;278:6187–6193. doi: 10.1074/jbc.M208894200. [DOI] [PubMed] [Google Scholar]
- 128.Scheuerer B., Ernst M., Dürrbaum-Landmann I., Fleischer J., Grage-Griebenow E., Brandt E., et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 129.Lievens D., Zernecke A., Seijkens T., Soehnlein O., Beckers L., Munnix I.C., et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weber C., Zernecke A., Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 131.Liu W., Yalcinkaya M., Maestre I.F., Olszewska M., Ampomah P.B., Heimlich J.B., et al. Blockade of IL-6 signaling alleviates atherosclerosis in Tet2-deficient clonal hematopoiesis. Nat Cardiovasc Res. 2023;2:572–586. doi: 10.1038/s44161-023-00281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hinterdobler J., Schott S., Jin H., Meesmann A., Steinsiek A.L., Zimmermann A.S., et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur Heart J. 2021;42:4077–4088. doi: 10.1093/eurheartj/ehab371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sans M., Panés J., Ardite E., Elizalde J.I., Arce Y., Elena M., et al. VCAM-1 and ICAM-1 mediate leukocyte–endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116:874–883. doi: 10.1016/s0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 134.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 135.Collins R.G., Velji R., Guevara N.V., Hicks M.J., Chan L., Beaudet A.L. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huo Y., Hafezi-Moghadam A., Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 137.Leisegang M.S., Bibli S.I., Günther S., Pflüger-Müller B., Oo J.A., Höper C., et al. Pleiotropic effects of laminar flow and statins depend on the Krüppel-like factor-induced lncRNA MANTIS. Eur Heart J. 2019;40:2523–2533. doi: 10.1093/eurheartj/ehz393. [DOI] [PubMed] [Google Scholar]
- 138.Zhou T., You W.T., Ma Z.C., Liang Q.D., Tan H.L., Xiao C.R., et al. Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation. Acta Pharmacol Sin. 2016;37:177–186. doi: 10.1038/aps.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akyürek N., Salman B., Irkörücü O., Tezcaner T., Azili C., Erdem O., et al. The effect of platelet activating factor antagonist BN 52021 on bacterial translocation and ICAM-I expression in experimental obstructive jaundice. J Invest Surg. 2005;18:247–256. doi: 10.1080/08941930500248805. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y.G., Li F.J., Wang J., Wang X.D. Effects of ginkgolide B on inflammation induced by cerebral ischemia–reperfusion in rats. J Chin Med Mater. 2010;33:578–580. [PubMed] [Google Scholar]
- 141.Ozturk H., Ozturk H., Yagmur Y. PAF antagonist BN-52021 reduces intercellular adhesion molecule-1 expression and oxidative stress in rats with reperfusion damage due to unilateral testicular torsion. Pediatr Surg Int. 2006;22:191–196. doi: 10.1007/s00383-005-1621-4. [DOI] [PubMed] [Google Scholar]
- 142.Sneddon A.A., McLeod E., Wahle K.W., Arthur J.R. Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: suppression by conjugated linoleic acid. Biochim Biophys Acta. 2006;1761:793–801. doi: 10.1016/j.bbalip.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 143.Woo C.W.H., Cheung F., Chan V.W.H., Siow Y.L., O K Homocysteine stimulates inducible nitric oxide synthase expression in macrophages: antagonizing effect of ginkgolides and bilobalide. Mol Cell Biochem. 2003;243:37–47. doi: 10.1023/a:1021601512058. [DOI] [PubMed] [Google Scholar]
- 144.Northover A.M., Northover B.J. Degranulation of rat omental mast cells by A1 receptor agonists in vitro. Mediat Inflamm. 1996;5:341–345. doi: 10.1155/S096293519600049X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Northover A.M., Northover B.J. Inhibition of NO-synthase and degranulation of rat omental mast cells in vitro. Mediat Inflamm. 1996;5:257–261. doi: 10.1155/S0962935196000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vercellotti G.M., Yin H.Q., Gustafson K.S., Nelson R.D., Jacob H.S. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988;71:1100–1107. [PubMed] [Google Scholar]
- 147.Lenoir M., Muntaner O., Pedruzzi E., Roch-Arveiller M., Tissot M., Drieu K., et al. Ginkgolide B stimulates signaling events in neutrophils and primes defense activities. Biochem Biophys Res Commun. 2005;335:1149–1154. doi: 10.1016/j.bbrc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 148.Siminiak T., Egdell R.M., O'Gorman D.J., Dye J.F., Sheridan D.J. Plasma-mediated neutrophil activation during acute myocardial infarction: role of platelet-activating factor. Clin Sci (Lond) 1995;89:171–176. doi: 10.1042/cs0890171. [DOI] [PubMed] [Google Scholar]
- 149.Földes-Filep E., Braquet P., Filep J. Inhibition by BN 52021 (ginkgolide B) of the binding of [3H]-platelet-activating factor to human neutrophil granulocytes. Biochem Biophys Res Commun. 1987;148:1412–1417. doi: 10.1016/s0006-291x(87)80289-9. [DOI] [PubMed] [Google Scholar]
- 150.Kurihara K., Wardlaw A.J., Moqbel R., Kay A.B. Inhibition of platelet-activating factor (PAF)-induced chemotaxis and PAF binding to human eosinophils and neutrophils by the specific ginkgolide-derived PAF antagonist, BN 52021. J Allergy Clin Immunol. 1989;83:83–90. doi: 10.1016/0091-6749(89)90480-6. [DOI] [PubMed] [Google Scholar]
- 151.Yuan D., Sun G., Zhang R., Luo C., Ge M., Luo G., et al. Connexin 43 expressed in endothelial cells modulates monocyte–endothelial adhesion by regulating cell adhesion proteins. Mol Med Rep. 2015;12:7146–7152. doi: 10.3892/mmr.2015.4273. [DOI] [PubMed] [Google Scholar]
- 152.Yuan D., Wang Q., Wu D., Yu M., Zhang S., Li L., et al. Monocyte–endothelial adhesion is modulated by Cx43-stimulated ATP release from monocytes. Biochem Biophys Res Commun. 2012;420:536–541. doi: 10.1016/j.bbrc.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 153.Ji H., Qiu R., Gao X., Zhang R., Li X., Hei Z., et al. Propofol attenuates monocyte–endothelial adhesion via modulating connexin43 expression in monocytes. Life Sci. 2019;232 doi: 10.1016/j.lfs.2019.116624. [DOI] [PubMed] [Google Scholar]
- 154.Schmitt M.M., Megens R.T., Zernecke A., Bidzhekov K., van den Akker N.M., Rademakers T., et al. Endothelial junctional adhesion molecule—a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 155.Allingham M.J., van Buul J.D., Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 156.Tabas I., Bornfeldt K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zong P., Feng J., Yue Z., Yu A.S., Vacher J., Jellison E.R., et al. TRPM2 deficiency in mice protects against atherosclerosis by inhibiting TRPM2–CD36 inflammatory axis in macrophages. Nat Cardiovasc Res. 2022;1:344–360. doi: 10.1038/s44161-022-00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Loscalzo J. Homocysteine-mediated thrombosis and angiostasis in vascular pathobiology. J Clin Invest. 2009;119:3203–3205. doi: 10.1172/JCI40924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nikkari S.T., O'Brien K.D., Ferguson M., Hatsukami T., Welgus H.G., Alpers C.E., et al. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995;92:1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- 160.Burleigh M.E., Babaev V.R., Oates J.A., Harris R.C., Gautam S., Riendeau D., et al. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation. 2002;105:1816–1823. doi: 10.1161/01.cir.0000014927.74465.7f. [DOI] [PubMed] [Google Scholar]
- 161.Bouchareychas L., Duong P., Phu T.A., Alsop E., Meechoovet B., Reiman R., et al. High glucose macrophage exosomes enhance atherosclerosis by driving cellular proliferation & hematopoiesis. iScience. 2021;24 doi: 10.1016/j.isci.2021.102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu G., Zhang J., Zhao Q., Zhuang W., Ding J., Zhang C., et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem Int Ed Engl. 2020;59:4068–4074. doi: 10.1002/anie.201913700. [DOI] [PubMed] [Google Scholar]
- 163.Sun J., Sukhova G.K., Wolters P.J., Yang M., Kitamoto S., Libby P., et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 164.Shi G.-P., Bot I., Kovanen P.T. Mast cells in human and experimental cardiometabolic diseases. Nat Rev Cardiol. 2015;12:643–658. doi: 10.1038/nrcardio.2015.117. [DOI] [PubMed] [Google Scholar]
- 165.Silvestre-Roig C., Braster Q., Ortega-Gomez A., Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17:327–340. doi: 10.1038/s41569-019-0326-7. [DOI] [PubMed] [Google Scholar]
- 166.Serizawa A., Nakamura S., Suzuki Baba S., Nakano M. Involvement of platelet-activating factor in cytokine production and neutrophil activation after hepatic ischemia–reperfusion. Hepatology. 1996;23:1656–1663. doi: 10.1002/hep.510230649. [DOI] [PubMed] [Google Scholar]
- 167.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478. doi: 10.1093/eurheartj/eht273. 390a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ridker P.M., Bhatt D.L., Pradhan A.D., Glynn R.J., MacFadyen J.G., Nissen S.E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401:1293–1301. doi: 10.1016/S0140-6736(23)00215-5. [DOI] [PubMed] [Google Scholar]
- 169.Paneni F., Beckman J.A., Creager M.A., Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kanter J.E., Averill M.M., Leboeuf R.C., Bornfeldt K.E. Diabetes-accelerated atherosclerosis and inflammation. Circ Res. 2008;103:e116–e117. doi: 10.1161/CIRCRESAHA.108.182642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Santos R.D., Gidding S.S., Hegele R.A., Cuchel M.A., Barter P.J., Watts G.F., et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4:850–861. doi: 10.1016/S2213-8587(16)30041-9. [DOI] [PubMed] [Google Scholar]
- 172.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang J.K., Li Y., Zhao X.L., Liu Y.B., Tan J., Xing Y.Y., et al. Ablation of plasma Prekallikrein decreases low-density lipoprotein cholesterol by stabilizing low-density lipoprotein receptor and protects against atherosclerosis. Circulation. 2022;145:675–687. doi: 10.1161/CIRCULATIONAHA.121.056491. [DOI] [PubMed] [Google Scholar]
- 174.Xue H., Chen X., Yu C., Deng Y., Zhang Y., Chen S., et al. Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ Res. 2022;131:404–420. doi: 10.1161/CIRCRESAHA.122.321253. [DOI] [PubMed] [Google Scholar]
- 175.Jonsson A.L., Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 176.Yang Y., Chen J., Gao Q., Shan X., Wang J., Lv Z. Study on the attenuated effect of ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445 doi: 10.1016/j.tox.2020.152599. [DOI] [PubMed] [Google Scholar]
- 177.Ouyang S., You J., Zhi C., Li P., Lin X., Tan X., et al. Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis. 2021;12:782. doi: 10.1038/s41419-021-04054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beck J.C., Goodner C.J., Wilson C., Wilson D., Glidden D., Baskin D.G., et al. Effects of ginkgolide B, a platelet-activating factor inhibitor on insulitis in the spontaneously diabetic BB rat. Autoimmunity. 1991;9:225–235. doi: 10.3109/08916939109007648. [DOI] [PubMed] [Google Scholar]
- 179.Chen K., Sun W., Jiang Y., Chen B., Zhao Y., Sun J., et al. Ginkgolide B suppresses TLR4-mediated inflammatory response by inhibiting the phosphorylation of JAK2/STAT3 and p38 MAPK in high glucose-treated HUVECs. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9371602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bako H.Y., Ibrahim M.A., Isah M.S., Ibrahim S. Inhibition of JAK–STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.117045. [DOI] [PubMed] [Google Scholar]
- 181.Kim H., Lim J.J., Shin H.Y., Suh H.J., Choi H.S. Lactobacillus plantarum K8-based paraprobiotics suppress lipid accumulation during adipogenesis by the regulation of JAK/STAT and AMPK signaling pathways. J Funct Foods. 2021;87 [Google Scholar]
- 182.Li D.X., Chen W., Jiang Y.L., Ni J.Q., Lu L. Antioxidant protein peroxiredoxin 6 suppresses the vascular inflammation, oxidative stress and endothelial dysfunction in angiotensin II-induced endotheliocyte. Gen Physiol Biophys. 2020;39:545–555. doi: 10.4149/gpb_2020029. [DOI] [PubMed] [Google Scholar]
- 183.Miyabe M., Nakamura N., Saiki T., Miyabe S., Ito M., Sasajima S., et al. Porphyromonas gingivalis lipopolysaccharides promote proliferation and migration of human vascular smooth muscle cells through the MAPK/TLR4 pathway. Int J Mol Sci. 2022;24:125. doi: 10.3390/ijms24010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheng F., Twardowski L., Fehr S., Aner C., Schaeffeler E., Joos T., et al. Selective p38α MAP kinase/MAPK14 inhibition in enzymatically modified LDL-stimulated human monocytes: implications for atherosclerosis. FASEB J. 2017;31:674–686. doi: 10.1096/fj.201600669R. [DOI] [PubMed] [Google Scholar]
- 185.Liang X., Wang L., Wang M., Liu Z., Liu X., Zhang B., et al. MicroRNA-124 inhibits macrophage cell apoptosis via targeting p38/MAPK signaling pathway in atherosclerosis development. Aging (Albany NY) 2020;12:13005–13022. doi: 10.18632/aging.103387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bragg F., Holmes M.V., Iona A., Guo Y., Du H., Chen Y., et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA. 2017;317:280–289. doi: 10.1001/jama.2016.19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Woelkart K., Feizlmayr E., Dittrich P., Beubler E., Pinl F., Suter A., et al. Pharmacokinetics of bilobalide, ginkgolide A and B after administration of three different Ginkgo biloba L. preparations in humans. Phytother Res. 2010;24:445–450. doi: 10.1002/ptr.3074. [DOI] [PubMed] [Google Scholar]
- 188.Zhao J., Geng T., Wang Q., Si H., Sun X., Guo Q., et al. Pharmacokinetics of ginkgolide B after oral administration of three different ginkgolide B formulations in Beagle dogs. Molecules. 2015;20:20031–20041. doi: 10.3390/molecules201119678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang S., Ouyang B., Aa J., Geng J., Fei F., Wang P., et al. Pharmacokinetics and tissue distribution of ginkgolide A, ginkgolide B, and ginkgolide K after intravenous infusion of ginkgo diterpene lactones in a rat model. J Pharm Biomed Anal. 2016;126:109–116. doi: 10.1016/j.jpba.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 190.Lv Z., Yang Y., Wang J., Chen J., Li J., Di L. Optimization of the preparation conditions of borneol-modified ginkgolide liposomes by response surface methodology and study of their blood brain barrier permeability. Molecules. 2018;23:303. doi: 10.3390/molecules23020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chai Z., Song Y., Gao C., Wang J. Relative bioavailability of terpene lactones in Beagle dogs after ig administration of domestic and imported Ginkgo Leaf Tables. Chin Tradit Herb Drugs. 2018;49:2840–2844. [Google Scholar]
- 192.Guan H., Qian D., Ren H., Zhang W., Nie H., Shang E., et al. Interactions of pharmacokinetic profile of different parts from Ginkgo biloba extract in rats. J Ethnopharmacol. 2014;155:758–768. doi: 10.1016/j.jep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 193.Dong Y., Zhang J., Wang Y., Zhao L., Li R., Wei C., et al. Effect of ginkgolide in ischemic stroke patients with large artery atherosclerosis: results from a randomized trial. CNS Neurosci Ther. 2021;27:1561–1569. doi: 10.1111/cns.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nishimon S., Yamaguchi M., Muraki H., Sakai N., Nishino S. Intraperitoneal injection of ginkgolide B, a major active compound of Ginkgo biloba, dose-dependently increases the amount of wake and decreases non-rapid eye movement sleep in C57BL/6 mice. Neurosci Lett. 2020;722 doi: 10.1016/j.neulet.2020.134832. [DOI] [PubMed] [Google Scholar]
- 195.Yan-yan L., Jun T. Effect of rosuvastatin combined with Ginkgo Biloba Leaves extract tablets on carotid atherosclerotic plaques and dyslipidemia in patients with cerebral infarction. J Xinxiang Med Univ. 2016;33:133–135. [Google Scholar]
- 196.Ke S., Qingcheng Y. The impact of rosuvastatin combined with Ginkgo biloba extract tablets on carotid atherosclerotic plaque and inflammatory response in patients with ischemic stroke and diabetes. Chin J Gerontol. 2017;37:1933–1935. [Google Scholar]
- 197.Kressmann S., Biber A., Wonnemann M., Schug B., Blume H.H., Müller W.E. Influence of pharmaceutical quality on the bioavailability of active components from Ginkgo biloba preparations. J Pharm Pharmacol. 2002;54:1507–1514. doi: 10.1211/002235702199. [DOI] [PubMed] [Google Scholar]
- 198.Qin Y., Chen T., Jin Q., Guo K., Feng H., Heller D., et al. Pharmacokinetics, metabolism and disposition of [14C]XQ-1H after intravenous administration to male rats. Drug Metabol Lett. 2017;10:228–239. doi: 10.2174/1872312811666170118162931. [DOI] [PubMed] [Google Scholar]
- 199.Yang Q., Fang W., Lv P., Geng X., Li Y., Sha L. Therapeutic neuroprotective effects of XQ-1H in a rat model of permanent focal cerebral ischemia. Pharmacology. 2012;89:1–6. doi: 10.1159/000334625. [DOI] [PubMed] [Google Scholar]
- 200.Zhang X., Zhong W., Ma X., Zhang X., Chen H., Wang Z., et al. Ginkgolide with intravenous alteplase thrombolysis in acute ischemic stroke improving neurological function: a multicenter, cluster-randomized trial (GIANT) Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.792136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhu P.C., Tong Q., Zhuang Z., Wang Z.H., Deng L.H., Zheng G.Q., et al. Ginkgolide B for myocardial ischemia/reperfusion injury: a preclinical systematic review and meta-analysis. Front Physiol. 2019;10:1292. doi: 10.3389/fphys.2019.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li X., Huang L., Liu G., Fan W., Li B., Liu R., et al. Ginkgo diterpene lactones inhibit cerebral ischemia/reperfusion induced inflammatory response in astrocytes via TLR4/NF-κB pathway in rats. J Ethnopharmacol. 2020;249 doi: 10.1016/j.jep.2019.112365. [DOI] [PubMed] [Google Scholar]
- 203.Shu Z.M., Shu X.D., Li H.Q., Sun Y., Shan H., Sun X.Y., et al. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neurosci Ther. 2016;22:729–739. doi: 10.1111/cns.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang X., Zheng T., Hong H., Cai N., Zhou X., Sun C., et al. Neuroprotective effects of Ginkgo biloba extract and ginkgolide B against oxygen–glucose deprivation/reoxygenation and glucose injury in a new in vitro multicellular network model. Front Med. 2018;12:307–318. doi: 10.1007/s11684-017-0547-2. [DOI] [PubMed] [Google Scholar]
- 205.Gu J.H., Ge J.B., Li M., Wu F., Zhang W., Qin Z.H. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharmaceut Sci. 2012;47:652–660. doi: 10.1016/j.ejps.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 206.Zheng P.D., Mungur R., Zhou H.J., Hassan M., Jiang S.N., Zheng J.S. Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both and. Neural Regen Res. 2018;13:1204–1211. doi: 10.4103/1673-5374.232476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raheem S.G. Investigating platelet-activating factor as a potent proinflammatory mediator in coronary atherosclerotic patients. Cell Mol Biol. 2021;67:1–4. doi: 10.14715/cmb/2021.67.3.1. [DOI] [PubMed] [Google Scholar]
- 208.Sumita C., Yamane M., Matsuda T., Maeda M., Nariai T., Fujio Y., et al. Platelet activating factor induces cytoskeletal reorganization through Rho family pathway in THP-1 macrophages. FEBS Lett. 2005;579:4038–4042. doi: 10.1016/j.febslet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 209.Quiroga J., Alarcón P., Manosalva C., Taubert A., Hermosilla C., Hidalgo M.A., et al. Glycolysis and mitochondrial function regulate the radical oxygen species production induced by platelet-activating factor in bovine polymorphonuclear leukocytes. Vet Immunol Immunopathol. 2020;226 doi: 10.1016/j.vetimm.2020.110074. [DOI] [PubMed] [Google Scholar]
- 210.Gavriil L., Detopoulou M., Petsini F., Antonopoulou S., Fragopoulou E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: a randomised, double-blind and placebo-controlled trial. Br J Nutr. 2019;121:982–991. doi: 10.1017/S0007114519000308. [DOI] [PubMed] [Google Scholar]
- 211.Cirillo P., Golino P., Ragni M., Battaglia C., Pacifico F., Formisano S., et al. Activated platelets and leucocytes cooperatively stimulate smooth muscle cell proliferation and proto-oncogene expression via release of soluble growth factors. Cardiovasc Res. 1999;43:210–218. doi: 10.1016/s0008-6363(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 212.Wu G., Cai J., Han Y., Chen J., Huang Z.P., Chen C., et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mao Y.J., Wang L., Wang W.J. Effect of ginkgolide B on the function of rat aorta smooth cells and U937 cells stimulated by oxLDL. Acta Pharm Sin. 2006;41:36–40. [PubMed] [Google Scholar]
- 214.Wei E.H., Rao M.R., Ji N.D., Chen X.Y., Chen Q. Inhibitory effects of ginkgolide B on proliferation of bovine aortic smooth muscle cells. Acta Pharm Sin. 2002;37:90–93. [PubMed] [Google Scholar]
- 215.Liu Y., Liu W., Xiong S., Luo J., Li Y., Zhao Y., et al. Highly stabilized nanocrystals delivering ginkgolide B in protecting against the Parkinson's disease. Int J Pharm. 2020;577 doi: 10.1016/j.ijpharm.2020.119053. [DOI] [PubMed] [Google Scholar]
- 216.Zhao Y., Xiong S., Liu P., Liu W., Wang Q., Liu Y., et al. Polymeric nanoparticles-based brain delivery with improved therapeutic efficacy of ginkgolide B in Parkinson's disease. Int J Nanomed. 2020;15:10453–10467. doi: 10.2147/IJN.S272831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu W., Li P., Feng F., Mao L. Isolation and structure characterization of related impurities in 10-O-(N,N-dimethylaminoethyl)-ginkgolide B methanesulfonate (XQ-1H) bulk drug and quantitation by a validated RP-LC. J Pharm Biomed Anal. 2010;52:603–608. doi: 10.1016/j.jpba.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 218.Fei Y., Zhao B., Zhu J., Fang W., Li Y. XQ-1H promotes cerebral angiogenesis via activating PI3K/Akt/GSK3β/β-catenin/VEGF signal in mice exposed to cerebral ischemic injury. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119234. [DOI] [PubMed] [Google Scholar]
- 219.Xu D., Hou K., Li F., Chen S., Fang W., Li Y. XQ-1H alleviates cerebral ischemia in mice through inhibition of apoptosis and promotion of neurogenesis in a Wnt/β-catenin signaling dependent way. Life Sci. 2019;235 doi: 10.1016/j.lfs.2019.116844. [DOI] [PubMed] [Google Scholar]
- 220.Li Y., Zhang M., Li S., Zhang L., Kim J., Qiu Q., et al. Selective ischemic-hemisphere targeting ginkgolide B liposomes with improved solubility and therapeutic efficacy for cerebral ischemia–reperfusion injury. Asian J Pharm Sci. 2023;18 doi: 10.1016/j.ajps.2023.100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liang X., Li H., Li X., Tian X., Zhang A., Luo Q., et al. Highly sensitive H2O2-scavenging nano-bionic system for precise treatment of atherosclerosis. Acta Pharm Sin B. 2023;13:372–389. doi: 10.1016/j.apsb.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jia X., Bai X., Yang X., Wang L., Lu Y., Zhu L., et al. VCAM-1-binding peptide targeted cationic liposomes containing NLRP3 siRNA to modulate LDL transcytosis as a novel therapy for experimental atherosclerosis. Metabolism. 2022;135 doi: 10.1016/j.metabol.2022.155274. [DOI] [PubMed] [Google Scholar]
- 223.Li X., Chen X., Zheng L., Chen M., Zhang Y., Zhu R., et al. Non-canonical STING–PERK pathway dependent epigenetic regulation of vascular endothelial dysfunction via integrating IRF3 and NF-κB in inflammatory response. Acta Pharm Sin B. 2023;13:4765–4784. doi: 10.1016/j.apsb.2023.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tsvetkov P., Coy S., Petrova B., Dreishpoon M., Verma A., Abdusamad M., et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]