ABSTRACT.
Chagas disease (CD) is a parasitic infection caused by the parasite Trypanosoma cruzi. Reports of CD cases associated with oral transmission have increased, particularly in Colombia, Brazil, and Venezuela. In this investigation, parasitological, serological, and molecular tests were conducted on samples obtained from humans, mammal reservoirs, and hosts involved in the assessment of a suspected oral transmission outbreak in Cubara, Boyaca, Colombia. Seropositivity was observed in 60% (3 of 5) of index patients and 6.4% (5 of 78) of close contacts. Trypanosoma cruzi DNA was detected by quantitative polymerase chain reaction in 100% of index cases, 6.4% (5 of 78) of close contacts, 60% (6 of 10) of canines, and 100% (5 of 5) of opossums. In all index cases, the TcI lineage was identified, along with two cases of mixed infection (TcI/TcII–TcVI). Hemoculture revealed a flagellate presence in 80% of opossums, whereas all triatomine bugs tested negative. Our findings suggest a potential oral transmission route through contamination with opossum secretions.
Trypanosoma cruzi is the causative agent of Chagas disease (CD), which affects approximately 6 to 7 million people worldwide.1 This parasite has been classified into six discrete typing units (DTUs)—TcI through TcVI—and the TcBat genotype.2 Although the main transmission mechanism of CD is vector borne, occurring through contact of humans with feces of insects from the Triatominae subfamily, there has been an increase in the number of acute cases of CD associated with possible oral transmission, mainly in Brazil, Venezuela, and Colombia, often with high mortality rates and case numbers.3 In Colombia, approximately 64 outbreaks of acute cases of CD with possible oral transmission have been reported, with contaminated food and beverages identified as sources of infection, contaminated with feces and/or residues from triatomines or secretions from marsupials infected with T. cruzi. These outbreaks have been associated mainly with infection by the TcI DTU.4 Despite the increasing number of acute cases of CD with possible oral transmission in Colombia, studies documenting the detection of T. cruzi DNA and genetic variability of the parasite are limited, despite their usefulness in investigating the sources of infection and generating control strategies to mitigate the impact of this significant transmission scenario.3,4 The objective of this study is to describe and analyze an outbreak of acute CD with possible oral transmission through the application of parasitological and molecular diagnostic techniques, and T. cruzi genotyping in a municipality in Colombia.
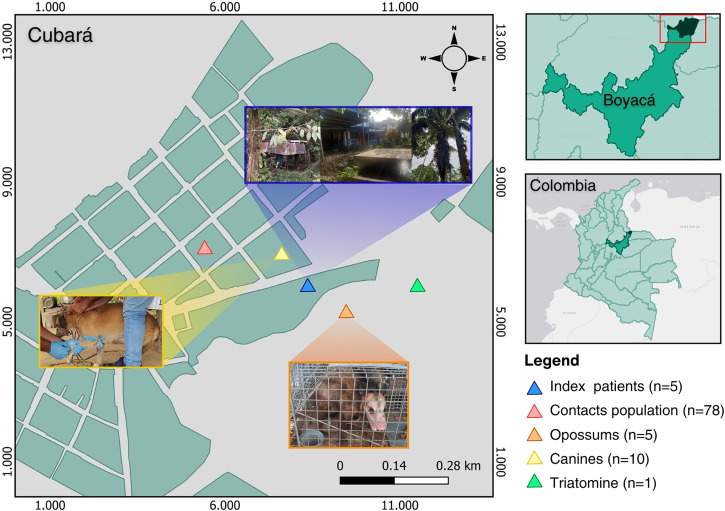
In this study, 99 human serum samples were collected in the municipality of Cubara, Boyaca, Colombia (Figure 1) December 16 to 17, 2021. Five samples were from index cases and 78 were from close contacts that were included based on epidemiological links. As part of the associated field investigation, 15 blood samples were collected from potential reservoirs (four from Didelphis marsupialis, one of Caluromys spp., and 10 from canines), through active searching of triatomines in the intra-, peri-, and extra-domiciliary areas of the outbreak site and 21 adjacent houses. Only one specimen of Panstrongylus geniculatus was collected from a rural community of associated surveillance. Parasitological diagnoses using microhematocrit values and serological diagnosis using ELISA (CHAGATEK ELISA, Microelisa system) were acquired from human samples. All samples were subjected to DNA detection using quantitative polymerase chain reaction (qPCR) targeting satellite DNA, and genotyping was performed using conventional PCR targeting the mini-exon gene.5 In addition, hemocultures were conducted on opossum samples, and triatomine feces were cultured.6
Figure 1.
Geographic location of patients, reservoirs, and triatomines in the municipality of Cubara.
The index cases of the outbreak corresponded to a resident family in Cubara. On December 16, one of the index cases presented with the following symptoms: fever, muscle and joint pain, mild splenomegaly, and diffuse abdominal pain. Suspecting CD, on December 17, 2021, infection with T. cruzi was confirmed in the symptomatic patient and the four family members residing in the same household using microhematocrit levels, and samples were taken for serological and molecular testing, confirming the infection in all index cases. Subsequently, a field investigation was carried out on the same date, and samples were collected from close contacts (Table 1).
Table 1.
Results of the parasitological, serological, and molecular tests
| Sample source | No. of samples | Positive by parasitological test, % (n/N) | Positive by serological diagnosis, % (n/N) | Positive by molecular diagnosis, % (n/N) | |
|---|---|---|---|---|---|
| Microhematocrit | Hemoculture | ||||
| Index cases | 5 | 100 (5/5) | – | 60 (3/5) | 100 (5/5) |
| Epidemiological nexus contacts | 78 | – | – | 6.41 (5/78) | 6.41 (5/78) |
| Didelphis marsupialis | 5 | – | 80 (4/5) | – | 100 (5/5) |
| Canis familiaris lupus | 10 | – | – | – | 90 (9/10) |
| Panstrongylus geniculatus | 1 | – | 0 (0/1) | – | – |
The median parasite load in the index cases was 3.4 parasite equivalents (Par. Eq.)/mL, and DTU TcI was detected in all cases, with two cases showing mixed infection with other DTUs (TcI/TcII–TcVI). The parasite loads in the index cases identified through epidemiological links, positive by qPCR, could not be quantified because they were less than the quantification limit of 1.5 Par. Eq./mL.7 In didelphids, the presence of the DTU TcI and one case of mixed infection (TcI/TcII–TcVI) was identified. In canines, T. cruzi DNA was detected in 90% (9 of 10) of the cases. TcI infection was observed in 50% (5 of 10) of the cases, and 40% (4 of 10) showed infection by other DTUs (TcII–TcVI). In addition, 80% (4 of 5) of opossums tested positive by hemoculture, whereas the only specimen of triatomine tested negative for all tests.
In this study, we observed low parasite loads (3.4 Par. Eq./mL) in the index patients, compared with the parasitemias reported in previous studies8–10 of outbreaks of possible oral transmission in Colombia, Venezuela, and Brazil. This difference could be explained by the long interval between the possible infection with the parasite and the confirmation of the cases, as described in previous oral outbreaks in Colombia.4 Parasite concentration serves as a significant indicator in diagnosing patients with acute oral-acquired CD infection, as the most common clinical manifestations such as generalized malaise, anorexia, fever, abdominal pain, and cardiomegaly are linked closely to a high initial parasitemia.4,11 Most reported cases demonstrate a rapid progression and increasing severity of the disease, accompanied by systemic signs of infection and accelerated cardiac involvement. Hence, it is of the utmost importance to maintain a high level of clinical suspicion during the diagnostic process as a result of elevated mortality rates.11
In the group of close contacts, patients who tested positive through serological tests and qPCR were characterized by having null parasite loads and being asymptomatic. Considering that Cubara presents a high risk of active vector transmission, we presume these patients might be in the chronic phase.12 However, it is important to highlight the need for biomarkers that can identify patients in the acute phase, who may potentially become asymptomatic, to confirm the disease stage in endemic areas where multiple transmission scenarios can occur.
All sampled opossums were found to be infected with T. cruzi, possibly as a result of their high consumption of triatomine insects with elevated parasite loads.13 However, this assumption holds true specifically in the sylvatic scenario. Previous studies in Cubara have reported low rates of infestation with Rhodnius prolixus at the household level, but a greater presence in sylvatic areas, where these synanthropic mammals can serve as food sources, thereby sustaining the active transmission of the parasite in domestic and peridomestic environments.12,14 In addition, these synanthropic mammals migrate to peridomestic areas as a result of the effects of deforestation and urbanization, which have altered their natural habitats, and they are attracted to garbage, where they find food sources.11,13
Despite active searching for triatomines, only one specimen of P. geniculatus was found in the outbreak area. This can be explained by the recent increase in vector control activities, such as house fumigation in rural and urban areas of Cubara, prompted by previous reports in the area that showed high infestation rates with R. prolixus between 2016 and 2018.12 These efforts are part of the ongoing vector transmission interruption program in Colombia and the department of Boyaca.15 In addition, there has been a growing number of reported cases of oral transmission of CD in areas with low endemicity, and successful interruption of domestic vector transmission, as seen in the Brazilian Amazon after the interruption of vector transmission by Triatoma infestans.16
It has been reported17 that canines become infected with T. cruzi in the wild and are considered sentinels of the disease, although few reports indicate their involvement in outbreaks of orally transmitted infection. Our results demonstrate an active circulation of the parasite in these hosts being a possible indicator of the risk of infection of other nearby dwellings and of an outbreak involving humans–opposums–dogs.4,18
In conclusion, we present an outbreak of CD in Cubara, which we presume to be possibly orally transmitted, given that 1) the presence of the parasite was detected in all sampled mammals reservoirs, 2) we identified the same circulating T. cruzi DTUs found in the index patients and opossums, 3) entomological surveillance results revealed a limited presence of vector insects in the outbreak area, 4) the outbreak area has opossum circulation resulting from high deforestation rates and the presence of attractants such as garbage, and 5) there was a high risk because of the presence of opportunistic species such as Didelphis marsupialis, which exhibited mixed infection according to several potential active foci in the outbreak area, as well as weak barriers that do not prevent their intra-domiciliary mobilization. Our results primarily support the hypothesis of possible oral T. cruzi infection, which may have occurred through water or food contamination with opossum secretions, and may be a result of changing transmission dynamics generated by recent control efforts. These findings highlight the need for new foci in the epidemiological surveillance of CD in Colombia.
ACKNOWLEDGMENTS
We specially thank the Programa de Control de ETV de Boyacá, Secretaría Departamental de Salud de Arauca, and Grupo de Vigilancia en Salud Pública de Boyacá for their access to and support with the index patient samples and entomological and reservoirs fieldwork.
REFERENCES
- 1. World Health Organization , 2023. Chagas Disease (American Trypanosomiasis). Available at: https://www.who.int/health-topics/chagas-disease. Accessed September 5, 2023.
- 2. Zingales B, 2018. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop 184: 38–52. [DOI] [PubMed] [Google Scholar]
- 3. Bruneto EG. et al. , 2021. Case-fatality from orally-transmitted acute Chagas disease: a systematic review and meta-analysis. Clin Infect Dis 72: 1084–1092. [DOI] [PubMed] [Google Scholar]
- 4. Hernández C. et al. , 2016. High-resolution molecular typing of Trypanosoma cruzi in 2 large outbreaks of acute Chagas disease in Colombia. J Infect Dis 214: 1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernández C, Cucunubá Z, Flórez C, Olivera M, Valencia C, Zambrano P, León C, Ramírez JD, 2016. Molecular diagnosis of Chagas disease in Colombia: parasitic loads and discrete typing units in patients from acute and chronic phases. PLoS Negl Trop Dis 10: e0004997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcon GEB, Ferreira JJG, de Almeida EA, Delicio AM, Pereira MB, Wanderley JDS, Martins LC, Andrade PD, de Lima RG, Costa SCB, 2022. Parasite load evaluation by qPCR and blood culture in Chagas disease and HIV co-infected patients under antiretroviral therapy. PLoS Negl Trop Dis 16: e0010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duffy T. et al. , 2013. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis 7: e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramírez JD, Montilla M, Cucunubá ZM, Floréz AC, Zambrano P, Guhl F, 2013. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis 7: e2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muñoz-Calderón A, Silva-Gomes NL, Apodaca S, Alarcón de Noya B, Díaz-Bello Z, Souza LRQ, Costa ADT, Britto C, Moreira OC, Schijman AG, 2021. Toward the establishment of a single standard curve for quantification of Trypanosoma cruzi natural populations using a synthetic satellite unit DNA sequence. J Mol Diagn 23: 521–531. [DOI] [PubMed] [Google Scholar]
- 10. Esper HR, de Freitas VLT, Assy JGPL, Shimoda EY, Berreta OCP, Lopes MH, França FOS, 2019. Fatal evolution of acute Chagas disease in a child from northern Brazil: factors that determine poor prognosis. Rev Inst Med Trop São Paulo 61: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franco-Paredes C, Villamil-Gómez WE, Schultz J, Henao-Martínez AF, Parra-Henao G, Rassi A, Jr., Rodríguez-Morales AJ, Suarez JA, 2020. A deadly feast: elucidating the burden of orally acquired acute Chagas disease in Latin America: public health and travel medicine importance. Travel Med Infect Dis 36: 101565. [DOI] [PubMed] [Google Scholar]
- 12. Cantillo-Barraza O, Medina M, Zuluaga S, Blanco MI, Caro R, Jaimes-Dueñez J, Beltrán V, Xavier SC, Triana-Chavez O, 2021. Distribution and natural infection status of synantrophic triatomines (Hemiptera: Reduviidae), vectors of Trypanosoma cruzi, reveals new epidemiological scenarios for chagas disease in the highlands of Colombia. PLoS Negl Trop Dis 15: e0009574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alarcón de Noya B, Díaz-Bello Z, Ruiz-Guevara R, Noya O, 2022. Chagas disease expands its epidemiological frontiers from rural to urban areas. Front Trop Dis 3: 799009. [Google Scholar]
- 14. Zingales B, Bartholomeu DC, 2022. Trypanosoma cruzi genetic diversity: impact on transmission cycles and Chagas disease. Mem Inst Oswaldo Cruz 117: e210193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luquetti A, 2019. Evaluación Internacional de la Situación Epidemiológica y de Control de Chagas en 34 Municipios de los Departamentos de Arauca, Boyacá, Casanare, Norte Santander, Santander y Vichada. Colombia: Organización Panamericana de la Salud & Organización Munidal de la Salud. [Google Scholar]
- 16. Shikanai-Yasuda MA, Carvalho NB, 2012. Oral transmission of Chagas disease. Clin Infect Dis 54: 845–852. [DOI] [PubMed] [Google Scholar]
- 17. Jaimes-Dueñez J, Jiménez-Leaño ÁP, Esteban-Mendoza M, Moreno-Salcedo LA, Triana-Chávez O, Cantillo-Barraza O, 2020. Epidemiological and clinical characteristics of Trypanosoma cruzi infection in dogs (Canis lupus familiaris) from a Chagas disease-endemic urban area in Colombia. Prev Vet Med 182: 105093. [DOI] [PubMed] [Google Scholar]
- 18. Jaimes-Dueñez J, Triana-Chávez O, Cantillo-Barraza O, Hernández C, Ramírez JD, Góngora-Orjuela A, 2017. Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute Chagas disease outbreaks in Colombia. Prev Vet Med 141: 1–6. [DOI] [PubMed] [Google Scholar]