ABSTRACT.
Erythema nodosum (EN) is characterized by rapidly developing, painful, erythematous subcutaneous nodules, most of which are located in the pretibial areas. This cutaneous finding can be caused by a variety of conditions, however Burkholderia pseudomallei is rarely the cause. This particular patient presented with a high-grade fever with characteristic EN on both pretibial areas. All of the typical EN causes were investigated, but the findings were all negative. The lesions progressed to severe hemorrhagic bleb features, and because the patient resided in Northeast Thailand, a melioidosis-endemic region, testing for B. pseudomallei was performed. Because a high level of melioidosis serology of more than 1:10,240 was detected, melioidosis therapy was started. At the 12-week follow-up after melioidosis therapy, the titer had declined to 1:1,280, indicating that melioidosis-related severe, cutaneous EN symptoms were the most likely diagnosis in this patient. We discovered a case of EN with severe hemorrhagic bleb features as a unique clinical manifestation of melioidosis. When a patient resides in an endemic area, B. pseudomallei should always be considered as a possible causative organism.
INTRODUCTION
Melioidosis is a disease caused by the gram-negative bacillus Burkholderia pseudomallei. The organism is found primarily in tropical countries such as Thailand, India, and northern Australia. The majority of cases occur during the rainy season, and transmission is thought to occur mostly by inhalation, percutaneous injection, or ingestion. There are some known risk factors for melioidosis, including diabetes, excessive alcohol consumption, chronic kidney diseases, chronic lung disorders, and immunosuppression.1 However, children who develop melioidosis may not have identifiable risk factors and are immunocompetent.
Melioidosis has a wide range of clinical manifestations, ranging from localized infections,2 diffuse visceral abscesses, pneumonia, and sepsis. Cutaneous findings of B. pseudomallei infection typically present in the form of abscesses or chronic skin ulceration.3–5 Only a few studies6,7 have noted a presentation of erythema nodosum (EN) caused by B. pseudomallei. Herein, we reported a case of EN with features of severe hemorrhagic blebs as a unique clinical manifestation of melioidosis in a child living in Thailand, a melioidosis-endemic region.
CASE REPORT
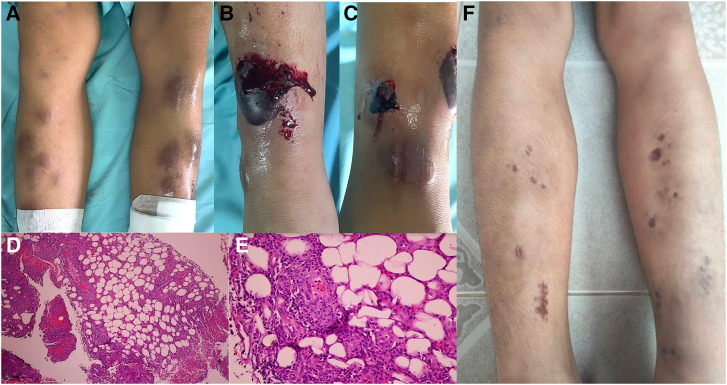
A 14-year-old boy presented with a 1-week high-grade fever with multiple painful, erythematous nodules on his lower limbs (Figure 1A). Other localizing symptoms were unremarkable. No previous history of sore throat, chronic cough, weight loss, or night sweats was noted. The patient had no underlying disease and revealed previous good health. A physical examination revealed that the patient had a body temperature of 39°C. There were unremarkable chest and abdominal symptoms. The lymph nodes were impalpable. Erythema nodosum was diagnosed based on its typical presentation and location. A skin biopsy was performed, and histology (Figure 1D and E) indicated septal panniculitis in the absence of vasculitis, confirming the presence of EN lesions in this patient.
Figure 1.
(A) Photograph showing multiple, tender erythematous nodules on the anterior aspect of bilateral legs. (B, C) Large hemorrhagic blebs on the previously identified erythema nodosum. Microphotograph of a histological section showing septal panniculitis in hematoxylin–eosin (H&E) stain at ×40 magnification (D) and ×400 magnification (E). (F) Healed lesions with atrophic scarring and postinflammatory hyperpigmentation.
To determine the etiology of EN, numerous investigations were performed, including antistreptolysin O, anti-DNase levels, mycoplasma serology, throat swab culture, and hemoculture, all of which were negative. A chest X-ray showed no infiltration or abnormal lymph nodes. All of the antinuclear antibodies, extractable nuclear antigens, antineutrophil cytoplasmic antibodies, anti-double–stranded DNA, and angiotensin-converting enzyme tests were negative.
The skin lesions worsened over 3 days while the patient was in the hospital, with extensive hemorrhagic blebs appearing on the EN sites (Figure 1B and C). The contents of hemorrhagic bullae were cultured on routine laboratory media (MacConkey and Blood agar), but no organisms were detected. Eventually, a high, positive melioidosis serology (> 1:10,240) was identified. As a result, the patient was given a presumptive diagnosis of melioidosis and was treated with 1.5 g intravenous ceftazidime every 8 hours.
The patient received intravenous ceftazidime for 5 days during hospitalization, achieving favorable clinical outcomes of decreasing fever and nonprogressive skin lesions. Thereafter, trimethoprim–sulfamethoxazole (TMP-SMX) (80 mg/400 mg), two tablets orally twice a day was prescribed as maintenance therapy. The patient returned for a 12-week follow-up after the initial therapy. Skin lesions had healed and demonstrated atrophic scarring and postinflammatory hyperpigmentation (Figure 1F). At the 12-week follow-up, the melioidosis titer had declined from 1:10,240 to 1:1,280. Trimethoprim–sulfamethoxazole was given orally for 12 weeks, resulting in full disease recovery.
DISCUSSION
Erythema nodosum manifests as rapidly developing, painful, erythematous subcutaneous nodules located primarily in the pretibial regions. Lesions are often bilateral and symmetric, with diameters ranging from 1 to 5 cm. Erythema nodosum can be caused by a number of disorders, including infection, drugs, sarcoidosis, pregnancy, inflammatory bowel disease, immunization, autoimmune illness, cancer, and others.8 Streptococcus has been the most common organism associated with EN, followed by Mycoplasma spp., Mycobacterium tuberculosis, and Chlamydia, and histoplasmosis.8
Our patient presented with characteristic painful, erythematous nodules in the pretibial regions, indicating a clinical diagnosis of EN, which was confirmed by finding evidence of septal panniculitis in the patient’s skin histopathology. All typical EN causes were examined to determine the cause of EN; however, all yielded negative findings. Although cutaneous manifestations of melioidosis as EN lesions are uncommon, B. pseudomallei was suggested as a potential cause of the differential diagnosis because the patient lived in a melioidosis-endemic region of Thailand.
A few cases of suspected EN caused by melioidosis have been documented previously in Malaysia and Australia. The diagnosis of melioidosis in reported instances in Malaysia was confirmed by hemoculture,7 whereas the reported cases from Australia6 were identified using serology rather than tissue or blood culture.
The culture of B. pseudomallei from any specimen remains the gold standard for diagnosis of melioidosis. Samples of blood and urine, and a throat swab should be obtained for culture, together with respiratory secretions, and pus and wound swabs when relevant.9 All specimens from patients with suspected melioidosis should be handled with the appropriate safety precautions. The organism grows well on most routine laboratory media, but specimens from nonsterile sites can benefit from the use of selective media (such as Ashdown’s agar and selective broth), which permit the growth of B. pseudomallei and suppress other organisms.10 However, bacteremia with positive cultures occurs in just 73% of instances,11,12 and some clinical microbiological laboratories do not have selective media, with the risk that any such organisms may be given an alternative identification. As a result, serology can assist occasionally in determining a diagnosis, particularly when significant levels of melioidosis are observed.
In this case, blood and hemorrhagic bullae content were collected for culture. However, no organism was found in either specimen. Unidentified B. pseudomallei in the culture of the hemorrhagic pus might be explained by the use of a regular culture medium (MacConkey and Blood agar) in our patient. Even though the culture results were negative in our patient, the elevated melioidosis serology titer (1:10,240), which is the highest titer compared with prior studies,6–8 indicated melioidosis infection presumptively. Furthermore, at the 12-week follow-up, the titer had decreased to 1: 1,280 after sufficient melioidosis therapy, indicating that melioidosis was the cause of the presenting EN lesion.
The finding of severe hemorrhagic blebs on the implicated EN site is a unique presentation. It was intriguing to discover hemorrhagic bleb without any histopathological evidence of vasculitis or substantial infiltration of inflammatory cells. In this case, hemorrhagic blebs on the involved EN site may have been caused by the pathogenic organism (B. pseudomallei) itself. Burkholderia pseudomallei may cause cellular deformation, local thrombotic microangiopathy, and local microcirculation impairment. Upon disruption of the integrity of the vessel wall, rapid and multifaceted interactions between circulating platelets, endothelial cells, and subendothelial structures occur. As a result, platelets adhere to the vessel wall, are activated, and form aggregates with each other, leading to microvascular obstruction and cessation of bleeding.13,14
Melioidosis treatment is divided into two phases: intravenous intense and oral eradication. The duration of the intravenous intense phase is determined by the severity of the infection. Patients with pneumonia, bacteremia, or osteomyelitis may require a prolonged period of intravenous intensive care.15 Because our patient had a limited infection with a negative blood culture, he required a short duration of intravenous therapy. For oral eradication maintenance therapy, oral TMP-SMX was administered for 12 weeks. This treatment is in accordance with the current practice guidelines for melioidosis with localized infection.16
CONCLUSION
We present a case of EN with severe hemorrhagic blebs as a unique clinical manifestation in melioidosis in Thai endemic region. With the clinical characteristic of severe hemorrhagic blebs on the relevant EN site and with patients who reside in a melioidosis-endemic location, B. pseudomallei should always be considered as a possible causative organism.
REFERENCES
- 1. Panamonta O, Lumbiganon P, 1997. Diabetic ketoacidosis and melioidosis in a child. J Med Assoc Thai 80: 671–674. [PubMed] [Google Scholar]
- 2. Fertitta L, Monsel G, Torresi J, Caumes E, 2019. Cutaneous melioidosis: a review of the literature. Int J Dermatol 58: 221–227. [DOI] [PubMed] [Google Scholar]
- 3. Lumbiganon P, Kosalaraksa P, 2013. Uncommon clinical presentations of melioidosis in children: 2 cases with sore throat and 1 case with urticarial rash. Southeast Asian J Trop Med Public Health 44: 862–865. [PubMed] [Google Scholar]
- 4. Lumbiganon P, Viengnondha S, 1995. Clinical manifestations of melioidosis in children. Pediatr Infect Dis J 14: 136–140. [DOI] [PubMed] [Google Scholar]
- 5. Lumbiganon P, Chotechuangnirun N, Kosalaraksa P, Teeratakulpisarn J, 2011. Localized melioidosis in children in Thailand: treatment and long-term outcome. J Trop Pediatr 57: 185–191. [DOI] [PubMed] [Google Scholar]
- 6. Diolombi M, Seneviratne M, Norton R, 2020. Case report: erythema nodosum and melioidosis: an unreported association. Am J Trop Med Hyg 103: 1841–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan PY, Goh JY, 2022. Erythema nodosum: an atypical presentation of melioidosis. Rev Soc Bras Med Trop 55: e00362022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung AKC, Leong KF, Lam JM, 2018. Erythema nodosum. World J Pediatr 14: 548–554. [DOI] [PubMed] [Google Scholar]
- 9. Limmathurotsakul D, Wuthiekanum V, Wongsuvan G, Pangmee S, Amornchai P, Teparrakkul P, Teerawattanasook N, Day NPJ, Peacock SJ, 2011. Repeat blood culture positive for B. pseudomallei indicates an increased risk of death from melioidosis. Am J Trop Med Hyg 84: 858–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stavrou C, Veraitch O, Morris-Jones S, Walker SL, 2021. Leg ulceration due to cutaneous melioidosis in a returning traveller. BMJ Case Rep 14: e241490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charoenwong P, Lumbiganon P, Puapermpoonsiri S, 1992. The prevalence of the indirect hemagglutination test for melioidosis in children in an endemic area. Southeast Asian J Trop Med Public Health 23: 698–701. [PubMed] [Google Scholar]
- 12. Karunanayake P, 2022. Melioidosis: clinical aspects. Clin Med (Lond) 22: 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levi M, Scully M, Singer M, 2018. The role of ADAMTS‐13 in the coagulopathy of sepsis. J Thromb Haemost 16: 646–651. [DOI] [PubMed] [Google Scholar]
- 14. Panuncialman J, Falanga V, 2010. Unusual causes of cutaneous ulceration. Surg Clin North Am 90: 1161–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sullivan RP, Marshall CS, Anstey NM, Ward L, Currie BJ, 2020. 2020 Review and revision of the 2015 Darwin melioidosis treatment guideline: paradigm drift not shift. PLoS Negl Trop Dis 14: e0008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anunnatsiri S. et al. , 2021. A comparison between 12 versus 20 weeks of trimethoprim-sulfamethoxazole as oral eradication treatment for melioidosis: an open-label, pragmatic, multicenter, non-inferiority, randomized controlled trial. Clin Infect Dis 73: e3627–e3633. [DOI] [PMC free article] [PubMed] [Google Scholar]